Abstract
Current treatment in late-life cognitive impairment and dementia is still limited, and there is no cure for brain tissue degeneration or reversal of cognitive decline. Physical activity represents a promising non-pharmacological interventional approach in many diseases causing cognitive impairment, but its effect on brain integrity is still largely unknown. Especially research of cerebral alterations in disease state that goes beyond observations of clinical improvement is crucial to understand disease processes and possible effective treatments. In this systematic review, we address the question how physical activity and fitness in mild cognitive impairment (MCI) and Alzheimer's disease (AD) influences brain architecture compared to cognitively healthy elderly. We review both interventional studies comprising aerobic, coordinative and resistance exercises and observational studies on fitness and physical activity combined with Magnetic Resonance imaging (MRI). Different MRI approaches were included such as volumetric and structural analyses, Diffusion Tensor Imaging (DTI), functional MRI and Cerebral Blood Flow (CBF). We evaluate MRI results for different exercise modalities and performed a methodological evaluation of interventional studies in cognitive decline compared to normal aging. According to our results, among 12 interventions in AD/MCI, aerobic exercise is most frequently applied (9 studies). Interventions in AD/MCI altogether reveal a higher methodological quality compared to interventions in healthy elderly (8.33 ± 2.19 vs. 6.25 ± 2.36 out of 13 points), with most frequent missing aspects related to descriptions of complications, lack of intention-to-treat and statistical power analyses. Effects of aerobic exercise and fitness seem to mainly impact brain structures sensitive to neurodegeneration, which especially comprise frontal, temporal and parietal regions, such as the hippocampal/parahippocampal region, precuneus, anterior cingulate and prefrontal cortex, which are reported by several studies. General fitness measured via an objective fitness assessment and questionnaires seems to have a more global cerebral effect, probably due to its long-term application, whereas distinct intervention effects of durations between 3 and 6 months seem to concentrate on more local brain regions as the hippocampus, which can also be influenced by region of interest analyses. There is still a lack of evidence on other or combined types of intervention modalities, such as resistance, coordinative as well as multicomponent exercise during cognitive decline, and complex interventions as dancing. Future research should examine their beneficial effect on brain integrity, since several non-MRI studies already point to their advantageous impact. As a further future prospect, combination and application of newly developed imaging methods such as metabolic imaging should be envisaged to understand physical activity and its cerebral influence under its many-sided facets.
Keywords: Physical activity, Exercise, Fitness, MRI, Neuroimaging, Dementia
Abbreviations: ACC, Anterior Cingulate Cortex; AD, Alzheimer's Disease; BDNF, Brain-derived-neurotrophic-factor; CBF, Cerebral Blood Flow; CCT, Computerized Cognitive Training; CSF, Cerebrospinal Fluid; DTI, Diffusion-Tensor-Imaging; IGF-1, Insulin-like-Growth-Factor; MCI, Mild cognitive impairment; MLTPA, Minnesota Leisure Time Physical Activity; MoCA, Montreal Cognitive Assessment; MRI, Magnetic Resonance Imaging; MTL, Medial Temporal Lobe; PET, Positron Emission Tomography; PFC, Prefrontal Cortex; PRT, Progressive Resistance Training; RCT, Randomized Controlled Trial; ROI, Region of Interest; SBAS, Stanford Brief Activity Survey; SCI, Subjective Cognitive Impairment; VBM, Voxel-Based Morphometry
Highlights
-
•
23 studies on physical activity in cognitive decline applying MRI were included.
-
•
Exercise in cognitive decline impacts brain regions sensitive to neurodegeneration.
-
•
These regions mainly comprise parietal, frontal and temporal brain regions.
-
•
Interventions in MCI show higher methodological quality than in healthy aging.
-
•
More studies on alternative intervention forms and metabolic imaging are needed.
1. Introduction
In our growing elderly population, Alzheimer's disease (AD) is the central form of dementia with a massive socio-economic impact, representing one of the most expensive diseases for our health systems (Gustavsson et al., 2011). Despite being highly relevant for our society, medical treatment for preventing cognitive decline is still sparse (Moniz-Cook et al., 2011). Therefore, alternative forms of treatment, which can be well implemented in patients' daily routine, are gaining the attention of current research. In this context, physical activity is a viable promising low-cost, low-risk, individual and widely available option, which is already known for its reduction impact in health risks, such as cardiovascular diseases, cancer and mental health problems (Nelson et al., 2007). Accordingly, beneficial effects of exercise and fitness on cognition and brain structure have also been described and offer a promising tool for preventing cognitive decline during the aging process (Van Der Borght et al., 2009; Eadie et al., 2005; Kronenberg et al., 2003; Van Praag et al., 1999; Redila et al., 2006; Norton et al., 2014). Several randomized controlled trials (RCTs) in young, as well as elderly, healthy humans showed that physical exercise lead to an improvement in cognition, especially in spatial and executive functioning (Gates et al., 2013; Hess et al., 2014; Zheng et al., 2016). Some studies also report a reduced risk of development of dementia (Burns et al., 2008; Lautenschlager et al., 2012; 2008; Ngandu et al., 2015; Tolppanen et al., 2015; Vidoni et al., 2012b). There are further indications that physical activity might slow down progression of dementia and that cardiorespiratory fitness can help reducing the detrimental effects of cerebral amyloid on cognition in AD (Schultz, 2015) and could decrease the amount of amyloid beta 1–42 in cerebrospinal fluid (CSF) (Baker et al., 2010).
Despite such evidence of the advantageous effect of exercise in many aspects, the structural changes at the cerebral level in neurodegenerative diseases are still poorly understood, especially when comparing to cognitively healthy older adults. This understanding, however, is crucial for offering an optimized treatment adapted to disease state. In this context, MRI represents a neuroimaging tool, easy to implement in clinical routine, to further examine alterations on brain level corresponding to cognitive improvements in neurodegenerative disease. Next to structural changes, such as regional volume alterations, MRI can further serve to detect functional alterations, network shifting and even metabolic alterations in neurodegenerative disease progression (Reetz et al., 2012; Romanzetti et al., 2014). It is also able to monitor intervention effects (Hohenfeld et al., 2017) and disease progression, which makes it a most valuable biomarker. So far, longitudinal MRI studies on MCI have shown that initial degeneration focusses on substructures of the temporal lobe, spreading to the parietal lobe and finally extending to frontal lobe regions when converting to AD (Chételat et al., 2005; Whitwell et al., 2007). Recent findings via diffusion-tensor-imaging (DTI) have proved that not only gray matter, but also white matter is affected in MCI and AD, leading to changes in the connections of hippocampus (Fellgiebel et al., 2004, Fellgiebel et al., 2005), posterior cingulum (Fellgiebel et al., 2004; Medina et al., 2006), thalamus (Rose, 2006) and regions in the posterior white matter in MCI, also correlating with cognitive impairment. In functional MRI (fMRI) studies on MCI, there have been several discrepant results. On the one hand, studies showed decreased activity in the medial temporal lobe (MTL) in AD and their genetic-at-risk population (Johnson et al., 2006; Machulda et al., 2003; Mondadori et al., 2007; Petrella et al., 2006; Ringman and Coppola, 2013). On the other hand, other studies reported an increase of activity in temporal regions, especially in very early MCI (Kircher et al., 2007; Lenzi et al., 2011), which is discussed as a possible compensatory increase of activity brain response, reflecting recruitment of supplementary neural resources to counteract the effects of AD pathology, or could in contrary, as indicated in a pharmacological intervention study, represent a dysfunctional condition (Bakker et al., 2012).
When taking these described brain alterations into account, several relevant questions arise when considering the effects of physical exercise on brain integrity in MCI/AD: What is the specific effect of physical exercise on the brain? Are only certain brain regions/networks responsive to physical activity, regions which are mainly affected by disease state, as described above? The aim of this systematic review is to give an overview of studies examining the brain changes detectable by MRI after physical exercise intervention of individuals with MCI and/or AD, which may support planning of future interventional studies. In the first section of this review, we describe our search methods. In a second part, we rate the quality of RCTs according to criteria from the Cochrane Library, the PEDro Scale and the Evidence-based Medicine Working Group (Forbes et al., 2008; Guyatt et al., 1993, Guyatt et al., 1994; Liu and Latham, 2009; Maher et al., 2003) introduced by Pitkälä et al., 2013. We additionally visualize the rating results, ordered by interventions, and compare cerebral changes induced by exercise between cognitively healthy older adults and patients with cognitive impairment. Finally, we discuss potential study designs and provide an outlook of future work.
2. Materials and methods
2.1. Search methods
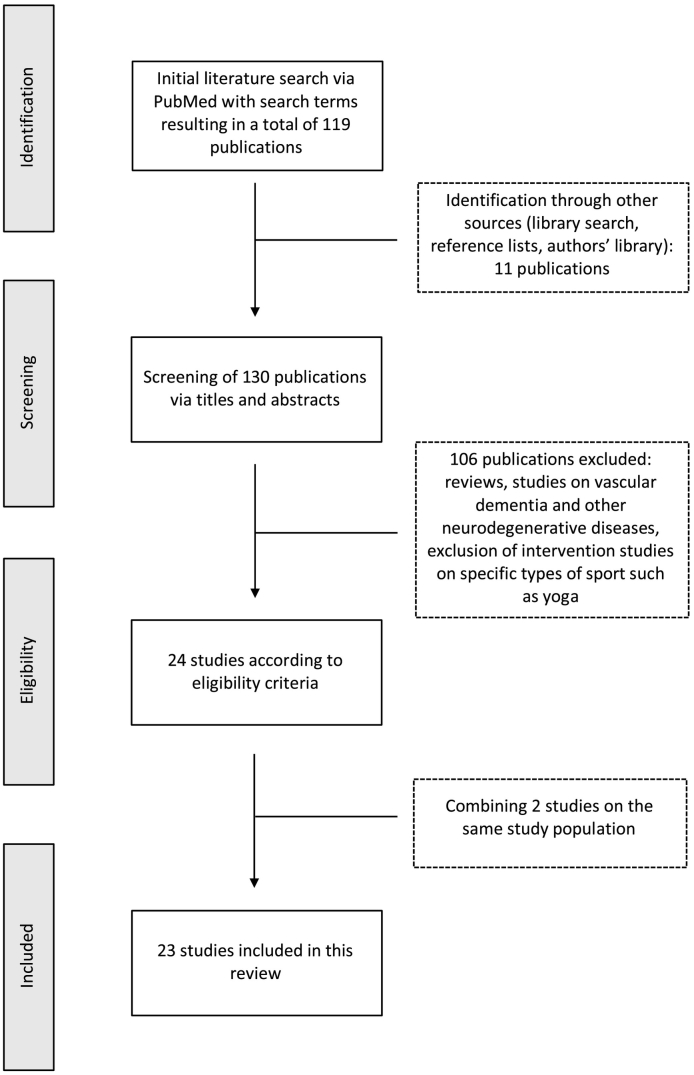
A comprehensive search of PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) was performed from inception to and including October 2018. Reference lists of included articles and author's personal libraries were manually searched for further publications. Only articles in English were selected. The following search term composed of the relevant keywords was used for search: (“MCI” OR “Alzheimer” OR “Dementia” OR “Cognitive impairment”) AND (“Fitness” OR “Exercise” OR “Physical activity”) AND “MRI”. For comparison purposes, studies including older subjects were selected via keywords “MRI” AND (“Exercise” OR “Fitness”) AND (“Age” OR “Elderly”) and were further searched manually in reference lists and authors' libraries. The main focus on this review lies on cerebral alterations induced by physical exercise in disease state. Studies were included when (1) the state of fitness and physical activity of subjects were examined via objective techniques or via questionnaires or via interventions, such as aerobic, resistance, coordinative training/multicomponent exercise, in (2) individuals with MCI and/or AD. We included studies where (3) MRI was performed to detect changes in cerebral structures, comprising studies using structural MRI with Voxel-based-morphometry (VBM) or similar volume analyses and cortical thickness, functional MRI and resting state MRI and connectivity analyses via DTI and cerebral blood flow (CBF). We further report on cognitive outcomes. We excluded interventions relying on specific sport arts (e.g. yoga, Thai Chi) and interventions, such as dancing, which represents a complex combination of activity, music involvement, interaction with a partner and cognition. This was done to facilitate interpretation and comparability of induced MRI alterations. We further excluded studies on vascular dementia/vascular alterations and other neurodegenerative diseases. Randomized, non-randomized controlled, and cohort study designs were included. The review was structured according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) recommendations (http://www.prisma-statement.org/). A schematic flow chart of the inclusion process is given in Fig. 1.
Fig. 1.
Flow chart of the inclusion process of literature according to the PRISMA criteria. A total of 23 studies is included in this review.
2.2. Methodological quality
We performed an evaluation of the methodological quality of the studies using a modified rating system established by Pitkälä et al., 2013. This rating system combines criteria for randomized intervention trials developed by the Cochrane library modified by Cochrane collaborators (Forbes et al., 2008; Liu and Latham, 2009), as well as the PEDro scale, which is a tool for evaluating the methodological quality of clinical trials related to physiotherapy (Maher et al., 2003) and criteria developed by the Evidence-based Medicine Working Group (Guyatt et al., 1993, Guyatt et al., 1994). Criteria are listed according to Pitkälä and colleagues in Table 1. Each criterion represents one point. If the study fulfills only parts of the criterion or one criterion is not reported in the manuscript, zero points are given. Classification into high, medium and low quality was performed using the total rating score (high quality: >10 points, medium quality 7–10 points, poor quality <7 points). Two researchers (AH, ASC) evaluated the studies independently and a consensus was met, when assessments differed.
Table 1.
Criteria for evaluation of studies methodological quality (Pitkälä et al., 2013). The criteria selection were drawn from several sources (Forbes et al., 2008; Guyatt et al., 1993, Guyatt et al., 1994; Liu and Latham, 2009; Maher et al., 2003) as described in Pitkälä et al. Each criterion was evaluated with one point.
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3. Comparison of cerebral alterations in patients Versus older adults
For a determination and comparison between groups and studies of the impact of physical activity and fitness on MCI and AD, anatomical masks of brain regions, flagged as significantly associated with exercise and fitness, using a threshold of p < 0.05, were selected from the wfu-pickatlas toolbox for SPM (version 3.0.4) (Maldjian et al., 2003) and illustrated accounting for methodological quality in intervention studies or for sample size in non-intervention studies. Only studies presenting data on regional volume and/or cortical thickness were included to facilitate direct comparison between studies and subject populations. Results are reported in the results section for the different physical activity modalities. Comparisons were calculated between intervention studies, objective fitness assessment and questionnaires about physical activity. Cerebral sub-regions of superordinate brain regions affected by exercise are illustrated in respect to sample sizes and reported frequency of occurrence.
3. Results
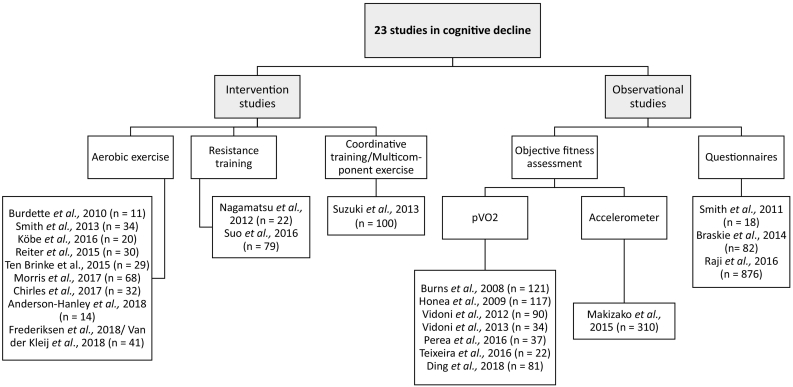
From our literature search, a total of 23 MRI studies on physical activity and cognitive decline met inclusion criteria (Table 3, Table 4, Supplementary Table 5, 6). Thirteen studies report on participants with MCI, 8 studies on participants with early AD and one study on participants with subjective memory loss. One study included both participants with AD and MCI (Raji et al., 2016). There were twelve intervention studies of different durations (range 3–6 months) and frequency (range 2–5 sessions per week). Among the intervention studies, 9 studies applied aerobic exercise, 2 applied resistance training, one applied a multicomponent exercise (Fig. 2). From the studies with aerobic exercise, two studies used combined interventions with cognitive stimulation (Anderson-Hanley et al., 2018; Köbe et al., 2016) and additional nutritional supplementation (Köbe et al., 2016). Suo et al., 2016, used a combination of progressive resistance training and cognitive stimulation. Two publications were categorized together since they examined the same sample (Frederiksen et al., 2018; van der Kleij et al., 2018). There were three publications performing analyses from the same sample pool (Chirles et al., 2017; Reiter et al., 2015; Smith et al., 2013) and Nagamatsu, 2012 and Ten Brinke et al., 2015, performed analyses from the same intervention.
Table 3.
Overview of intervention studies in cognitive impairment.
| Study | Description of sample |
Intervention/Content |
Results |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Included sample size | Mean age in years (SD) | Description | Duration in weeks | Frequency per week | Session duration (min) | Outcome measures MRI and cognition | Main MRI Results | Cognitive results | Follow- up | Methodological quality | |
| Burdette et al., 2010 | 11 SCI | 74.0 (2.5) | I: AT (mainly walking); C: stretching | 16 | 4 | Total of 150 min/week | Postinterventional structural MRI, ASL, Resting state | I: Increase of hippocampal CBF Increase of hippocampal connectivity |
Not performed | N | 7 |
| Smith et al., 2013 | 35 (17 MCI, 18 HC), 34 with MRI | MCI 78.7 (7.5), HC (76.0 (7.3) | Supervised treadmill walking of moderate intensity | 12 | Gradual increase to 4 sessions | 30 | fMRI Famous Name Discrimination Task Neuropsychology |
Decrease in semantic memory retrieval-related activation | Improved Learning on the AVLT in MCI and HC | N | 8 |
| Köbe et al., 2016 | 22 MCI, 13 in intervention, 9 in control condition; 20 with MRI | 70 (7.2) in intervention | I: Omega-3 FA, aerobic exercise, cognitive stimulation; C: Omega-3 FA, stretching and toning | 24 | 2 | 45 | MRI Carotid int. media SNP genotyping Neuropsychology Serology Anthropometrics |
I: Increase in GM volume in middle frontal cortex, frontal pole, angular cortex, pre-cuneus, post. Cingulate cortex | No effect on cognitive results | N | 7 |
| Reiter et al., 2015 (see also Smith et al., 2013) | 30 (14 MCI, 16 HC) | MCI 78.85 (7.75), HC: 75.87 (6.90) | Supervised treadmill walking of moderate intensity | 12 | Gradual increase to 4 sessions | 30 | CT Neuropsychology |
Association between larger fitness and changes of CT in bilat. Insula, precentr. Gyri, precuneus, post. Cingulate, inf. + sup. Frontal cortices and temporal gyrus in MCI | See Smith et al., 2013 | N | 8 |
| Ten Brinke et al., 2015 | 86 MCI (all F) (29 with MRI) |
AT 76.07 (3.43), RT 73.75(3.72), BAT 75.46 (3.93) | AT, RT, BAT | 24 | 2 | 60 | MRI Neuropsychology Functional and physical battery |
AT: increased hippoc. Volume | Association between increased left hippoc. Volume and reduced verbal memory | N | 11 |
| Morris et al., 2017 | 68 with probable AD | 72.9 (7.7) | Supervised moderate AT vs. stretching and toning | 24 | 3–5 | Total of 150 min/week | Hippocampal and total GM volume Neuropsychology |
Association of change in cardiorespiratory fitness with bilateral hippoc. Volume | Association of change in cardioresp. Fitness with change in memory | N | 12 |
| Chirles et al., 2017 (see Smith et al., 2013) | 32 (16 MCI, 16 HC) | MCI 79.6 (6.8), HC 76.1 (7.2) | Supervised treadmill walking of moderate intensity | 12 | Gradual increase to 4 sessions | 30 | Resting state Neuropsychology (see Smith et al., 2013) |
Increased functional connectivity of PCC/precuneus; Postcentral gyrus with decreased connectivity in HC | See Smith et al., 2013 | N | 7 |
| Anderson-Hanley et al., 2018 | 14 MCI for 6 months analysis (46 in 3 months analysis) | 78.1 (9.9) | I: virtual reality bike rides | 24 | Gradual increase from 2 to at least 3–5 sessions | 45 | GM volume Neuropsychology Functional assessment Salivary biomarkers (BDNF, CRP, IGF-1, IL- 6, and VEGF) |
Association between greater exercise dose and increasing PFC and ACC; Inverse correlation between verbal memory errors and DLPFC volume | I: Improvement in immediate verbal memory, self-report of everyday cognitive function + physical ability | N | 7 |
| Frederiksen et al., 2018; van der Kleij et al., 2018 | 41 (mild to moderate AD) | I: 67.8 (7.7); C: 69.8 (7.7) | I: Aerobic exercise of moderate-to-high intensity | 16 | 3 | 60 | Regional Volume ASL Neuropsychology |
Positive correlation of exercise load with hippocampal volume change and frontal CT No effect on CBF |
Association between volume changes in frontal CT and mental speed + attention | N | 8 |
| Nagamatsu, 2012 | 77 with MCI (RT = 26, AT = 24, BAT = 27); 22 with MRI | 74.9 (3.5) | RT, AT, BAT | 24 | 2 | 60 | fMRI during associative memory Neuropsychology |
Functional changes in RT group in right lingual, occipital-fusiform gyri, right frontal pole during encoding and recall of associations | Improvement of RT group during Stroop and associative memory test | N | 5 |
| Suo et al., 2016 | 86 MCI (79 with MRI) | 70.1 (6.7) | PRT + CCT; PRT + CCC; CCT+ stretching; stretching + CCC | 26 | 2 | 90 | CT Resting state Neuropsychology |
Increase in CT of PC in all PRT groups Change in GM of PC correlated with improvement in ADAS-Cog |
Reduced decline of overall memory performance in CCT, but not PRT; Improvement on ADAS-Cog in PRT groups | Y | 8 |
| Suzuki et al., 2013 | 100 (50 amnestic MCI, 50 other MCI) | I: 74.8 (7.4) C: 75.8 (6.1) | I: Multicomponent exercise; C: education control group | 24 | 2 | 90 | VSRAD Neuropsychology Serology |
Reduced whole brain atrophy in MCI | I: Improvement of MMSE and WMS-LM in aMCI | N | 12 |
Abbreviations: ACC = Anterior Cingulate Cortex, AD = Alzheimer’s Disease, ADAS-Cog = Alzheimer's Disease Assessment Scale – Cognitive Subscale, aMCI = amnestic Mild Cognitive Impairment, ASL = Arterial Spin Labelling, AT = Aerobic Training, AVLT = Rey Auditory Verbal Learning Test, BAT = Balance and Tone Training, BDNF = Brain-Derived Neurotrophic Factor, C = Control condition, CBF = Cerebral Blood Flow, CCC = Cognitive Control Condition, CCT = Computerized Cognitive Training, CT = Cortical Thickness, DLPFC = Dorsolateral Prefrontal Cortex, GM = Gray Matter, HC = Healthy Controls, I = Intervention, IGF-1 = Insulin-Like-Growth Factor, IL-6 = Interleukin 6, MCI = Mild Cognitive Impairment, MMSE = Mini-Mental State Examination, N = No; Omega-3-FA = Omega-3-Fatacids, PA = Physical activity, PC = Posterior cingulate, PCC = Posterior Cingulate Cortex, PFC = Prefrontal Cortex, PRT = Progressive Resistance Training, RT = Resistance Training, SCI = Subjective Cognitive Impairment, SD = Standard Deviation, SNP = Single Nucleotide Polymorphism, VEGF = Vascular Endothelial Growth Factor, VSRAD = voxel-based specific regional analysis system for Alzheimer's disease, WM = white matter, WMS-LM = Wechsler Memory Scale – Logical Memory, Y = Yes.
Table 4.
Overview of observational studies evaluating physical activity via fitness assessment or questionnaires in cognitive impairment.
| Study | Description of sample |
Content |
Results |
|||
|---|---|---|---|---|---|---|
| Included sample size; gender (F/M) | Mean age in years (SD) | Description | Outcome measures MRI and cognition | Main MRI results | Cognitive results | |
| Burns et al., 2008 | 121 (64 HC, 57 early AD) | 73.5 | pVO2 via treadmill test | Whole brain volume, WM, GM Neuropsychology PASE |
Association of fitness with whole brain, gray and white matter volume in AD; Association between increased fitness and increased brain volume in AD |
In AD association between pVO2 and performance on Logical Memory II, Trail Making B and Digit symbol test |
| Honea et al., 2009 | 117 (56 HC, 61 early AD) | 73.8 (6.3) | pVO2 via treadmill test | VBM analysis, whole brain volume WM, GM volume PASE ApoE4 status |
Association between fitness and WM in bilateral inferior parietal cortex, and after SVC in hippocampus/parahippocampus in AD In HC no association |
– |
| Smith et al., 2011a | 18 aMCI (9 high PA, 9 low PA) | Low PA: 73.6 (8.3); High PA 75.0 (5.5) | High PA and Low PA group according to SBAS | 3 T fMRI with semantic memory task Neuropsychology |
Increased activation in left caudate in High-PA aMCI No difference in volume of caudate nucleus |
No difference in neuropsychological or discrimination performance |
| Vidoni et al., 2012a | 90 (37 early AD, 53 HC) | HC 73.2 (6.7), AD 73.8 (5.8) | pVO2 over 2 years via treadmill test | VBM Neuropsychology (Burns et al., 2008) |
Association between fitness and atrophy mainly in left parahippocampus in AD | Association between fitness and increased progression of dementia severity |
| Vidoni et al., 2013 | 34 (18 HC, 16 early AD) | HC 72.2 (7.2), AD 74.9 (7.4) | pVO2 via treadmill test | fMRI Stroop task PPT |
Association between fitness and increased activation in ACC only in HC | – |
| Braskie et al., 2014 | 82 (43 HC; 39 ADCE) | Controls 79.3 (4.8) AD 81.9 (5.1) | MLTPA Walking questionnaire |
Whole brain volume at year 9 Neuropsychology TNFα |
Association between PA and total brain volume after 9 years | Association between lower PA and risk of AD |
| Perea et al., 2016 | 37 ADCE | 72.35 (7.9) | pVO2 in cardio-pulm. Exercise test | DTI Neuropsychology |
Association between fitness and increased white matter integrity in right IFOF | No sign. Results on UDS |
| Raji et al., 2016 | 876 (213 MCI or AD) | 78.3 (3.9) | MLTPA | VBM Neuropsychology |
Association between caloric expenditure and precuneus, posterior cingulate, vermis in MCI/AD Interaction between increasing caloric expenditure and cognitive impairment in left hippocampus and cerebellar vermis |
Not reported |
| Teixeira et al., 2016 | 22 aMCI | 68.5 (5.3) | pVO2 via treadmill test | VBM, DTI Neuropsychology CSF |
Correlation of aerobic fitness with gray matter in frontal and parietal areas Positive correlation with FA + negative correlation with MD, RD, and AxD in multiple tracts |
Not reported |
| Ding et al., 2017 | 81 (26 HC, 55 aMCI) | 65 (7) | VO2max | DTI Neuropsychology |
Positive correlation of fitness with FA + negative correlation with MD, RD in multiple tracts | Association between DTI results and executive performance in MCI |
| Makizako et al., 2015 | 310 MCI | 71.3 (4.4) | Triaxial accelerometer for 2 weeks | MRI Neuropsychology |
Association between moderate PA and hippocampal volume | No association between PA and memory performance |
Abbreviations: ACC = Anterior Cingulate Cortex, AD = Alzheimer’s Disease, aMCI = amnestic mild cognitive impairment, AxD = axial diffusivity, CSF = Cerebrospinal Fluid, DTI = Diffusion-Tensor Imaging, FA = Fractional Anisotropy, DTI = Diffusion-tensor-imaging, GM = Gray Matter, HC = Healthy Controls, IFOF = inferior fronto-occipital fasciculus, MCI = Mild Cognitive Impairment, MD = Mean diffusivity, MLTPA = Minnesota Leisure Time Physical Activity questionnaire, PA = Physical Activity, PASE = Physical Activity Scale for the Elderly, PPT = Physical performance test, RD = Radial diffusivity, SBAS = Stanford Brief Activity Survey, SVC = Small Volume Correction, TNFα = Tumor Necrosis Factor, UDS = uniform data set, VBM = voxel-based-morphometry, WM = White Matter.
Fig. 2.
Overview of included studies in MCI and AD patients with number of included subjects who underwent MRI.
Aside from intervention studies, observational studies applying objective evaluations of fitness levels were also included. In this context, objective measurement of peak VO2 consumption is a widespread technique. It represents the oxygen uptake during peak exercise (such as on a treadmill) and was used in seven of the studies in cognitive impairment. In one study, fitness was assessed by wearing a triaxial accelerometer for 2 weeks (Makizako et al., 2015).
There were three studies using questionnaires to measure physical activity, either using the Minnesota Leisure Time Physical Activity (MLTPA) questionnaire (Braskie et al., 2014; Raji et al., 2016), or the Stanford Brief Activity Survey (SBAS) (Smith et al., 2011a).
3.1. Methodological quality
Methodological quality was rated for all intervention studies (Table 2). Quality of studies in cognitive decline mostly ranged between moderate and high values. Among the most frequent missing points was the lack of description on complications during intervention, missing intention-to-treat-analyses and lack of statistical power analyses and description. From all intervention studies in disease state, Suzuki et al., 2013 had the highest sample size with MRI (n = 100) in their multicomponent exercise intervention, followed by Morris et al., 2017 with 68 participants undergoing MRI in an aerobic exercise intervention, with both studies being of high methodological quality. When comparing studies on cognitive decline with studies on cognitively healthy elderly via students' t-test, the quality was higher in the patients' group (t(30) = 2.48; p = 0.019; 8.33 ± 2.19 points vs. 6.25 ± 2.36 points), primarily considering missing descriptions of randomization and blinding, description of complications during intervention, dropouts and statistical power analyses. When comparing intervention studies using aerobic exercise, which represented the majority, the higher methodological quality of studies in cognitive decline compared to healthy controls became even more prominent (t(21) = 3.95; p = 0.00073, 8.33 ± 1.87 points vs. 5.33 ± 1.69 points).
Table 2.
Evaluation of quality criteria in intervention studies among people with cognitive impairment (A) and cognitively healthy elderly (B) in descending order of total scoring: + = fulfills criteria; − = does not fulfill criteria; ± = fulfills criteria only partly; ? = cannot be concluded from the study report.
| Study | Randomization described and acceptable | Valid definition of diagnosis | Inclusion and exclusion criteria described | Adequate statistical power described with power analyses | Valid measurements and outcome measures | Baseline characteristics in groups described and groups comparable | Drop-out described and included in analysis | Intention to treat analysis | Comparison of differences in changes between the groups in outcome variables | Blinding used | Description of intervention | Compliance described | Complications described | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A) | ||||||||||||||
| Morris et al., 2017 | + | + | + | + | + | + | + | − | + | + | + | + | + | 12 |
| Suzuki et al., 2013 | + | + | + | + | + | + | + | ? | + | + | + | + | + | 12 |
| Ten Brinke et al., 2015 | + | − | + | + | + | + | ± | + | + | + | + | + | + | 11 |
| Smith et al., 2013 | − | + | + | − | + | + | ± | − | + | + | + | + | − | 8 |
| Reiter et al., 2015 | − | + | + | − | + | + | − | − | + | + | + | + | − | 8 |
| Frederiksen et al., 2018; van der Kleij et al., 2018 | + | + | + | − | + | + | ± | − | + | + | + | − | − | 8 |
| Suo et al., 2016 | + | + | − | + | + | − | ± | + | + | + | + | − | − | 8 |
| Burdette et al., 2010 | − | − | + | − | + | + | − | − | + | + | + | + | − | 7 |
| Köbe et al., 2016 | − | + | + | − | + | + | ± | − | + | − | + | + | − | 7 |
| Chirles et al., 2017 | − | + | + | − | + | + | − | − | + | + | ± | + | − | 7 |
| Anderson-Hanley et al., 2018 | ± | − | + | ± | + | + | ± | − | + | ? | + | + | + | 7 |
| Nagamatsu, 2012 | ± | − | − | − | + | + | ± | − | + | + | ± | + | − | 5 |
| B) | ||||||||||||||
| Liu-Ambrose et al., 2010 | + | + | + | + | + | + | ? | + | + | + | + | + | + | 12 |
| Liu-Ambrose et al., 2012 | + | + | + | − | + | + | − | − | + | + | + | + | − | 9 |
| Best et al., 2015 | − | ± | + | − | + | + | + | + | + | + | + | + | − | 9 |
| Nishiguchi et al., 2015 | + | + | + | ± | + | + | ? | − | + | + | + | − | + | 9 |
| Matura et al., 2017 | ± | + | + | + | + | + | ± | − | + | + | + | + | − | 9 |
| Chapman et al., 2013 | − | + | + | − | + | + | + | ? | + | ? | + | + | − | 8 |
| Ruscheweyh et al., 2011 | − | + | + | − | + | + | − | − | + | ± | + | − | − | 6 |
| Maass et al., 2015 | − | + | ± | − | + | + | + | − | + | ? | + | − | − | 6 |
| Kleemeyer et al., 2016 | − | + | + | ± | + | + | ± | − | + | ? | + | ± | − | 6 |
| Godde and Voelcker-Rehage, 2017 | − | + | + | − | + | + | − | − | + | − | + | − | − | 6 |
| Colcombe et al., 2006 | + | ± | ± | − | + | + | − | − | + | ? | + | − | − | 5 |
| Erickson et al., 2011 | − | ± | + | ± | + | + | − | − | + | ? | + | ± | − | 5 |
| Voss et al., 2010 | − | + | ± | − | + | ± | − | − | + | ? | + | + | − | 5 |
| Voss et al., 2013a | − | + | + | − | + | − | − | − | + | ? | + | − | − | 5 |
| Tamura et al., 2015 | − | + | + | ± | + | ± | ± | − | + | ? | + | − | − | 5 |
| Nagamatsu et al., 2016 | − | + | + | − | ± | + | − | − | + | ? | + | − | − | 5 |
| Rosano et al., 2017 | ± | ± | ± | − | + | + | − | − | + | + | ± | + | − | 5 |
| Holzschneider et al., 2012 | − | + | ± | − | + | ± | − | − | + | ? | + | − | − | 4 |
| Flodin et al., 2017; Jonasson et al., 2016 | − | + | ± | ± | + | + | ± | − | + | ? | ± | − | − | 4 |
| Colcombe et al., 2004 | − | ± | ± | − | + | ± | − | − | + | ? | ± | − | − | 2 |
3.2. MRI results by type of intervention in interventional studies
3.2.1. MRI results induced by aerobic exercise
There are nine intervention studies using aerobic exercise as intervention (Table 3). One of the central regions of interest (ROI) targeted in these studies is the hippocampus, as a structure which is early affected in AD. One study reported a direct increase in hippocampal volume in a group of MCI after 6 months intervention of aerobic exercise (Ten Brinke et al., 2015), which was not observed in their resistance training part, though. Two other studies found no direct hippocampal increase after intervention but detected an association between relation of fitness increase and exercise load with hippocampal volume in AD (Morris et al., 2017; Frederiksen et al., 2018 and van der Kleij et al., 2018). The latter study further examined hippocampal CBF and did not find an increase of CBF induced by their 16-week aerobic exercise intervention. In contrast to their findings, Burdette et al., 2010, in a very small sample with subjective cognitive impairment (SCI), detected an increase of CBF in hippocampus and even a stronger connectivity between hippocampus and anterior cingulate. The authors interpreted the increase of connectivity between these two regions as a possible neuropsychological change in executive processes, as the anterior cingulate cortex (ACC) is involved in episodic memory tasks requiring cognitive control (de Chastelaine et al., 2007; Kompus et al., 2009; Fleck et al., 2006). Unfortunately, no corresponding cognitive outcomes are reported in their study and MRI was only performed post-intervention.
However, not only the hippocampus seems to be related to fitness effects: In a 12-week treadmill walking program by Reiter et al., 2015, 14 individuals with MCI and 15 controls demonstrated a significant association between changes in fitness and cortical thickness in several mainly parietal regions. MCI subjects showed even stronger effects in the left insula and left superior temporal gyrus compared to the healthy controls. A possible explanation might be that previous studies suggest a vulnerability of these structures to neurodegeneration (Xie et al., 2012) which might mean they are able to compensate more under intervention when compared to controls. This leads to the conclusion, however, that such structures would be potential targets for future interventions. In the same sample, Smith et al., 2013 performed an fMRI famous name discrimination paradigm and reported a decrease in semantic memory related fMRI activation both in MCI participants and cognitively intact older adults after intervention, reflecting a possible reduced neural workload and an improved neural efficiency. MCI patients also showed new areas of activation compared to controls as in frontal, occipital and temporal regions, which was discussed as reflecting the recruitment of new neural circuits in the context of cognitive improvement. Accordingly, the study by Chirles et al., 2017 reported after the same intervention an increased connectivity in ten regions, comprising the frontal, temporal, parietal and insular lobes in the MCI group compared to the control group during resting state fMRI.
There were also studies combining aerobic exercise with other types of intervention: One study applied aerobic exercise, cognitive stimulation and Omega-3-Fatacid supplementation and reported preserved or increased gray matter volume in frontal, parietal and cingulate cortex after intervention (Köbe et al., 2016). However, no cognitive impact was found and the individual contributions of each of the intervention components cannot be separated. Similarly, a study by Anderson-Hanley et al., 2018 combined exercise with a mental task (during a videogame pedaling exercise with scoring compared to a virtual reality biking tour) in 14 subjects with MCI. Exercise dose was associated with changes in gray matter volume in ACC and prefrontal cortex (PFC), but both conditions led to an improvement in verbal and executive memory.
3.2.2. MRI results induced by resistance training
Only two studies applied resistance training in cognitive decline (Nagamatsu, 2012; Suo et al., 2016; see also Table 3). In the first study, 86 women with probable MCI defined by subjective memory complaints and reduced scores on Montreal Cognitive Assessment (MoCA) participated either in a 6 months resistance training two times a week, aerobic training or balance and tone training as control condition. A sub-sample of 22 participants additionally performed an associative memory task (memorizing face-scene pairs) during fMRI. The resistance group (n = 7) significantly improved in the Stroop task, as well as in the associative memory task, compared to the stretching and toning control group. Resistance training also led to functional changes in three regions of cortex, the right lingual, occipital-fusiform gyri and the right frontal pole, during the encoding and recall of associations. In a study conducted by Suo et al., 2016, 100 older subjects with MCI were randomized to four intervention arms encompassing progressive resistance training (PRT) and computerized cognitive training (CCT). PRT lead to improvement in global cognition and increased gray matter in the posterior cingulate. Interestingly, Suo et al. did not find an additional therapeutic benefit from combining resistance and cognitive training.
3.2.3. MRI results induced by coordinative training
There was no intervention study in cognitive impairment solely performing coordinative training as intervention. In a study by Suzuki et al., 2013, a multicomponent exercise program was performed, including coordinative training twice a week for 6 months, compared to an educational intervention control group. MCI patients in the multicomponent group showed stable whole brain atrophy levels and an improvement in logical memory and general cognitive function. The specific contribution of coordinative training on the outcome is not possible to establish in this study. Nonetheless, studies in cognitively healthy elderly have indicated that coordinative training, using exercises such as balance, eye–hand coordination, leg–arm coordination, lead to an increase in hippocampal volume, similar to aerobic exercise (Boyke et al., 2008; Niemann et al., 2014). In other neurodegenerative diseases such as progressive ataxia due to cerebellar degeneration, coordinative training led to an improvement in motor performance and ataxia possibly reducing disease progression by 2 or more years (Ilg et al., 2009).
3.2.4. Comparison of structural MRI results induced by intervention studies
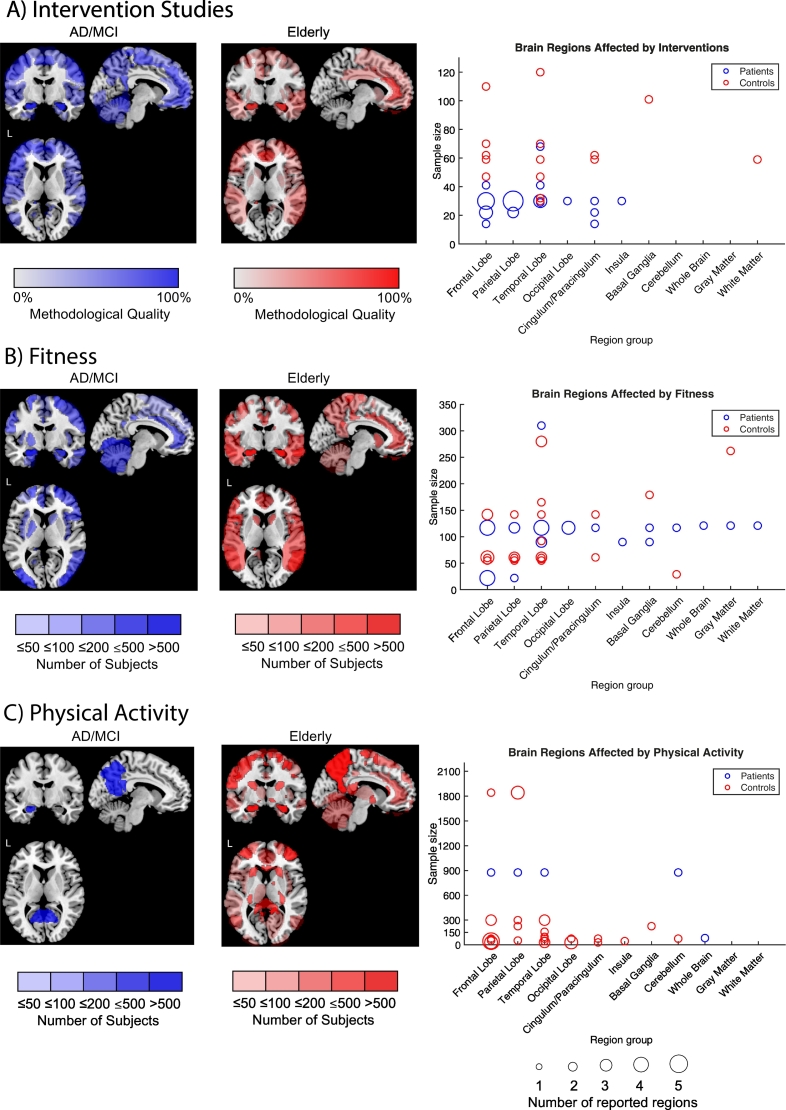
Fig. 3A illustrates effects of intervention studies applying volumetric and cortical thickness MRI analyses in MCI and/or dementia (blue) versus healthy cognitive controls (red). Interventions have a protective effect on hippocampal structures in both groups. In cognitively healthy subjects the effects of exercise show over the whole temporal lobe, whereas in subjects with AD/MCI effects seem to happen more locally and concentrated on certain ROIs which are especially vulnerable to neurodegeneration as the hippocampus, substructures of the frontal lobe and parietal lobe as the precuneus (see also Fig. 3A right). The apparent sensitivity of effects from interventions in brain regions affected by AD pathology is in concordance with previous findings on physical exercise intervention studies using PET and the positive effect of exercise on amyloid (Baker et al., 2010; Okonkwo et al., 2014; Schultz, 2015). We did not find a significant association between intervention duration, number of sessions, session duration and number of regions reported neither for patients nor controls. Both groups did not significantly differ in the duration, frequency or session duration of intervention. Interventions on cognitive decline presented in this comparison had a mean duration of 21 (±5.3) weeks, a frequency of 3.5 (±1.4) sessions and mean duration of 45 (±13.4) minutes per session.
Fig. 3.
Overview of brain regions affected by intervention studies (A), fitness (B) and physical activity evaluated via questionnaires (C) for MCI/AD patients (blue) and cognitively healthy elderly (red). The color grading (overlay transparency) is shown for a single study for reported brain regions. Therefore, an accumulation resulting in increased color grading will occur when certain ROIs are reported by several studies. For intervention studies (A), relevance of brain regions is weighted by methodological quality (0 to 100% of criteria fulfilled). For observational studies (B and C) total sample size is taken for weighting (categorization into samples of <50 subjects, 50–100, 100–200, 200–500, >500 subjects). For comparison purposes, only results from volume and cortical thickness analyses were included. On the right, corresponding illustration of brain regions reported for intervention and observational studies in relation to sample size and reported number of brain regions belonging to superordinate brain regions is given. These are illustrated via circle size (1–5 reported sub-regions). Overview of the names of the brain regions included in this graphical illustration in detail are listed in the supplementary (volume and cortical thickness). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. MRI results by type of fitness assessment in observational studies
3.3.1. Objective assessment of cardiorespiratory fitness
Eight studies assessed fitness in cognitive decline by determining peak oxygen consumption or via accelerometers (see Fig. 2). Several of these studies report a general reduced fitness in AD compared to controls (Burns et al., 2008; Honea et al., 2009; Vidoni et al., 2012a). Fitness status was associated with whole brain, white matter (Burns et al., 2008) and gray matter volume (Honea et al., 2009), with higher correlations in cognitive decline when compared to healthy aging, a finding where a possible higher heterogeneity in the AD group could be discussed. Vidoni et al., 2012a, report that in early AD, lower baseline fitness was associated with a more severe disease progression. Another study examining volume effects, as well as white matter integrity, was conducted by Teixeira et al., 2016. They found an association of aerobic fitness with gray matter morphology in frontal brain areas and integrity of tracts connecting frontal, temporal, occipital and parietal areas via DTI. These results are in line with findings by Perea et al., 2016 and baseline data from Vidoni et al., 2012a. Similarly, DTI studies in older healthy adults showed especially effects on interconnections with frontal regions vulnerable to aging processes (Johnson et al., 2012; Abe et al., 2002; Nusbaum et al., 2001).
There was only one fMRI study using a Stroop paradigm. Vidoni et al., 2013, examining 18 control individuals and 16 early AD patients, reported that fitness was associated with increased middle frontal, superior parietal lobe and decreased anterior cingulate activity in control subjects, but not in AD subjects. The authors discussed a possible diminished fitness effect due to the diagnosis of AD and refer to Nagamatsu, 2012 where improvement in the Stroop task, as well as during associative memory, with increased activity in lingual and temporal regions was positively correlated with improved memory.
In one study with a large sample of 310 MCI subjects fitness was assessed during 2 weeks via a triaxial accelerometer (Makizako et al., 2015). Interestingly, only moderate physical activity, but not intensive physical activity, was associated with hippocampal volume. This is in agreement with findings by Geda et al., 2010, where moderate, but not light or vigorous exercise, performed in midlife or late life was associated with a reduced risk of MCI.
3.3.2. Subjective assessment of cardiorespiratory fitness
There were three studies only using questionnaires for evaluating physical activity in subjects with MCI and/or Alzheimer's disease (Braskie et al., 2014; Raji et al., 2016; Smith et al., 2011a). In Smith et al., 2011a, higher leisure time physical activity, according to the Stanford Brief Activity Survey (SBAS), was associated with an increased activation in the left caudate during a famous name discrimination fMRI task. The authors discussed the involvement of the caudate, not only as a reflection of motor function, but also in the augmentation and facilitation of cognitive processes (see also Crosson et al., 2007; Haeger et al., 2015) and its involvement in the progression of MCI (Hakamata et al., 2010). This is in concordance with findings from Verstynen et al., 2012, where higher cardiorespiratory fitness predicted better cognitive flexibility in older cognitively healthy adults, through greater gray matter volume in the dorsal striatum.
In Braskie et al., 2014, 43 controls and 39 subjects with AD completed the Minnesota Leisure Time Physical Activity (MLTPA) questionnaire, the walking questionnaire, and performed a whole brain volume analysis at year 9 compared to baseline. They stated that physical activity was associated with greater whole brain volume. Lower physical activity was also associated with a higher risk of developing AD in later age. Another study using the MLTPA was performed with a larger sample by Raji et al., 2016, where 876 subjects were examined, among those 213 subjects with either MCI or AD. For the whole sample, higher physical activity was associated with increased bifrontal, bitemporal and biparietal volumes, whereas in the MCI or AD group, higher physical activity was positively associated with left hippocampal volume and the cerebellar vermis.
3.3.3. Comparison of structural MRI alterations induced by observational studies
When comparing fitness effects between cognitive decline and physiological aging it seems that the main focus of fitness in cognitive decline lies on hippocampal and other temporal structures for both groups. (Fig. 3B). Similar to intervention studies, especially frontal, temporal and parietal regions are influenced by fitness. Furthermore, fitness seems to have global effects on gray, white matter and even whole brain volumes, which might be due to the fact that fitness is developed over longer periods compared to relatively short interventions, where there is possibly a more concentrated and regional effect due to the restricted time of application. Fig. 3C shows the comparison of regions influenced by physical activity assessed via questionnaires in the patient group versus the control group where most regions are reported in the temporal lobe. However, there is still a lack of studies applying questionnaires for evaluating physical activity in the context of MRI alterations during cognitive decline. Therefore, the interpretation has to be done with caution and more studies on physical activity assessment combined with MRI are needed.
4. Discussion
The aim of this review is to present an overview of MRI studies on exercise in MCI and AD patients to define the impact of physical activity on brain architecture. We compared different physical activity modalities, both in patients and in cognitively healthy elderly. Altogether, 23 studies with a total of 2268 subjects with MRI on cognitive impairment were included in this review which used different methods concerning the type of intervention and how fitness was objectively assessed. We subdivided the studies based on the design into a) interventional studies and b) observational studies. Altogether, interventional studies in cognitive decline were of higher methodological quality than studies on cognitively healthy elderly. Here, the lack of description on complications such as injuries during intervention is a very important issue in intervention studies in elderly patients. Furthermore, intention-to-treat-analyses are crucial for evaluation of possible future efficient therapeutic intervention methods. Another common issue is the lack of statistical power analyses and description which is still very important given also the relatively small sample sizes in the interventions. Most intervention studies during cognitive decline applied aerobic exercise. When quantitively analyzing and visualizing the effects of aerobic exercise on brain volume and cortical thickness between patients' group and cognitively healthy elderly, the main influence of aerobic exercise in disease state seems to be on distinct temporal, frontal and parietal brain regions. The effects on frontal brain regions matches neuropsychological findings of the positive impact of aerobic exercise mainly on executive functions (Cammisuli et al., 2017; Farina et al., 2014), which might be due to the vulnerability of brain regions involved in executive functioning during aging (Baker et al., 2010; Scherder et al., 2005). This is supported by several studies showing effect of higher physical fitness on larger frontal brain volume (Bugg and Head, 2011; Colcombe et al., 2003, Colcombe et al., 2006; Erickson et al., 2010; Flöel et al., 2010; Gordon et al., 2008; Ruscheweyh et al., 2011; Weinstein et al., 2012) which was not found in young adults (Peters et al., 2009). These findings are also in concordance with studies suggesting that individuals with cognitive impairment activate additional frontoparietal regions during executive tasks compared to cognitively healthy subjects (Rosano et al., 2005; Vidoni et al., 2013; Yetkin et al., 2006; Kaufmann et al., 2008).
Another brain region sensitive to aerobic exercise is the hippocampus, as one of the central regions affected by AD pathology (Fjell and Walhovd, 2010). The responsiveness of temporal substructures to exercise might be explained by their vulnerability to neurodegeneration. In contrast, cognitively healthy subjects seem to be more globally responsive, which might be explained by a higher neuroplasticity of the healthy aging brain compared to disease state, as shown by our illustrations in the results part of this manuscript.
Results are comparable when referring to objective fitness assessment via pVO2 and accelerometers. Volume of frontal brain regions in disease state seems to be again more responsive to fitness than in cognitively elderly healthy subjects. For assessment of physical activity via questionnaires, there is a small number of studies on individuals with MCI and AD (Braskie et al., 2014; Raji et al., 2016; Smith et al., 2011a). For coordinative and resistance training, as well as multicomponent exercise, there were not enough studies for a direct comparison. In theory, coordinative training demands higher cognitive processes such as attention during balance, eye-hand and leg-arm orientation, as well as spatial orientation or reactions to stimuli. Dancing combines both physical activity with coordinative parts, as well as cognitive activity and social interaction. Not only do elderly with a long-life experience of dancing exhibit increased cognitive performance in fluid intelligence and attention (Kattenstroth et al., 2010, Kattenstroth et al., 2013), but dancing also seems to help rehabilitation in neurological disorders, such as Parkinson's disease and stroke (McGill et al., 2014) and seems to play a preventive role against dementia (Verghese et al., 2003). In a study on cognitively healthy elderly and MCI, subjects with a life-time experience of dance performed better in learning and memory tasks, but showed a trend-level thinner cortex (Porat et al., 2016). More studies on this intervention form are needed to draw conclusions on brain architecture. The effect of resistance training is still poorly understood. In MCI and AD patients, only one study by Nagamatsu, 2012 performed an fMRI associative memory task and in Ten Brinke et al., 2015, resistance training showed no effect on hippocampus but their sample was also small. Interestingly, Suo et al. showed promising results with a positive effect of resistance training on posterior cingulate, whose affection represents an early biomarker in AD, and an increased connectivity of the hippocampus. Another critical point is that it stays unclear if effects of resistance training are gender-specific (Best et al., 2015) since studies on cognitive decline with MRI have mainly focused on female subjects so far (Nagamatsu, 2012; Ten Brinke et al., 2015). Interventions with coordinative and resistance training should therefore move more into focus of research to understand their beneficial effects on brain structure in disease state. Furthermore, an increase of multicomponent intervention studies should be aspired to benefit from the various advantages of different activity forms. There are already indications from studies in mice that the combination of exercise and cognitive enrichment increases protective effects against synaptotoxicity of amyloid in the hippocampus (Nichol et al., 2008). In humans, comparison of aerobic exercise, with and without addition of a cognitive task during exercise, showed an improved cognitive performance in MCI in case of combination (Sacco et al., 2015). Additionally, and related to environmental enrichment, social engagement seen as a protective factor against cognitive decline (Barnes et al., 2004) should be considered in group interventions.
In a further recently published review on the influence of physical activity on AD biomarkers, the authors did not state a clear association between hippocampal volume and physical activity in most of their included studies and also on other AD biomarkers as amyloid beta 1–42, phosphorylated tau, total tau in CSF and FDG- and Amyloid-PET (Frederiksen et al., 2019). In comparison to our review, their focus was on observational studies, though. The authors also discussed the partly low quality and quantity of the observational studies. Our review focusses on overall cerebral alterations induced by exercise interventions and physical activity with a main focus on neurodegeneration detected via MR imaging. Since the spectrum of Alzheimer's continuum is very complex (Jack et al., 2018), more studies are needed to draw conclusions on modification on other biomarkers than MRI in AD.
There are several limitations which need to be considered when interpreting the results of the presented studies on physical activity and cognitive decline. One factor is the risk of reporting and observer bias when focus is put on a priori defined ROIs and not on whole brain analyses, which can especially occur in studies with small sample sizes. Another of the major problems is the large etiological heterogeneity in individuals with MCI, which may account for some of the discrepancy in results. As a risk state for developing dementia (Gauthier et al., 2006) MCI is considered an important timepoint for intervention. There are however individuals with MCI who eventually reverse back into a normal cognitive status. MCI encompasses a variety of etiologies as depression, polymedication and prodromal phases of neurodegenerative diseases. It might even be possible, that effects of different forms of exercise are mediated through different pathways. Some studies point to the assumption that neurogenesis and neuroprotection observed in exercise could be mediated by increases in neurotrophins as Brain-derived-neurotrophic-factor (BDNF) (Erickson et al., 2011; Ruscheweyh et al., 2011; Voss et al., 2013b) and Insulin-like-Growth-Factor (IGF-1) (Cotman et al., 2007). There are indications that increases of neurotrophins and their neuronal effects might result from different exercise modalities (Tsai et al., 2019) but this aspect is still under current research. The exact understanding of underlying cerebral processes might help us in developing new therapeutic concepts in the future.
5. Conclusion
Based on this systematic review in MCI and AD, both physical intervention and observed basal increased fitness status can mediate structural and functional brain alterations, with a main focus on regions sensitive to neurodegeneration during cognitive decline. We did not find an association between rate of whole intervention duration, session duration, frequency of sessions and number of affected brain regions in structural MRI. However, reported whole intervention and session durations as well as frequencies are in concordance with so far recommended values (Blankevoort et al., 2010; Dougherty et al., 2016; Pitkälä et al., 2013). Due to the lack of sufficient studies on resistance training and coordinative training in disease state, more MRI studies of high methodological quality on these modalities should be performed. Furthermore, future studies should aim to include etiologically clearly defined disease groups. According to our results, three further important open questions need to be addressed in the future: A) Do intervention effects in cognitive decline persist after ceasing the intervention and for how long? B) Could intervention via exercise inhibit or slow down conversion from MCI to dementia state? C) Since new neuroimaging methods can help to detect even subtle effects on brain integrity and metabolism in very early stages of AD, future research should focus on newly developed imaging methods as e.g. metabolic imaging via MRI and/or PET or ultra-high-field imaging to better understand the influence of physical activity on brain integrity and neurodegeneration and to facilitate the observation of possible long-term effects in longitudinal studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. AH received a research rotation stipend of RWTH Aachen university.
Declaration of Competing Interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101933.
Appendix A. Supplementary data
Supplementary material
References
- Abe O., Aoki S., Hayashi N., Yamada H., Kunimatsu A., Mori H.…Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol. Aging. 2002;23(3):433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Anderson-Hanley C., Barcelos N.M., Zimmerman E.A., Gillen R.W., Dunnam M., Cohen B.D.…Kramer A.F. The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front. Aging Neurosci. 2018;10(May) doi: 10.3389/fnagi.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A.…Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Krauss G.L., Albert M.S., Speck C.L., Jones L.R., Stark C.E.…Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L.L., Mendes de Leon C.F., Wilson R.S., Bienias J.L., Evans D.A. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. http://www.ncbi.nlm.nih.gov/pubmed/15623694 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Best J.R., Chiu B.K., Liang Hsu C., Nagamatsu L.S., Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J. Int. Neuropsychol. Soc. 2015;21(10):745–756. doi: 10.1017/S1355617715000673. [DOI] [PubMed] [Google Scholar]
- Blankevoort C.G., Van Heuvelen M.J.G., Boersma F., Luning H., De Jong J., Scherder E.J.A. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement. Geriatr. Cogn. Disord. 2010;30(5):392–402. doi: 10.1159/000321357. [DOI] [PubMed] [Google Scholar]
- Boyke J., Driemeyer J., Gaser C., Buchel C., May A. Training-induced brain structure changes in the elderly. J. Neurosci. 2008;28(28):7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie M.N., Boyle C.P., Rajagopalan P., Gutman B.A., Toga A.W., Raji C.A.…Thompson P.M. Physical activity, inflammation, and volume of the aging brain. Neuroscience. 2014;273:199–209. doi: 10.1016/j.neuroscience.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg J.M., Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging. 2011;32(3):506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette J.H., Laurienti P.J., Espeland M.A., Morgan A., Telesford Q., Vechlekar C.D.…Rejeski W.J. Using network science to evaluate exercise-associated brain changes in older adults. Front. Aging Neurosci. 2010;2(Jun):1–10. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J.M., Cronk B.B., Anderson H.S., Donnelly J.E., Thomas G.P., Harsha A.…Swerdlow R.H. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammisuli D.M. Aerobic exercise effects upon cognition in mild cognitive impairment: a systematic review of randomized controlled trials. Arch. Ital. Biol. 2017;155:54–62. doi: 10.12871/000398292017126. [DOI] [PubMed] [Google Scholar]
- Chapman S.B., Aslan S., Spence J.S., DeFina L.F., Keebler M.W., Didehbani N., Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 2013;5(Nov):1–9. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat G., Landeau B., Eustache F., Mézenge F., Viader F., De La Sayette V.…Baron J.C. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. NeuroImage. 2005;27(4):934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chirles T.J., Reiter K., Weiss L.R., Alfini A.J., Nielson K.A., Smith J.C. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J. Alzheimers Dis. 2017;57(3):845–856. doi: 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S.J., Erickson K.I., Raz N., Webb A.G., Cohen N.J., McAuley E., Kramer A.F. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. Ser. A Biol. Med. Sci. 2003;58(2):M176–M180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe S.J., Kramer A.F., Erickson K.I., Scalf P., McAuley E., Cohen N.J.…Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., Mcauley E.…Kramer A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Crosson B., Benjamin M., Levy I. Role of the basal ganglia in language and semantics: supporting cast. In: Hart J., Kraut M.A., editors. Neural Basis of Semantic Memory. Cambridge University Press; Cambridge: 2007. pp. 219–244. [Google Scholar]
- de Chastelaine M., Friedman D., Cycowicz Y.M. The development of control processes supporting source memory discrimination as revealed by event-related potentials. J. Cogn. Neurosci. 2007;19(8):1286–1301. doi: 10.1162/jocn.2007.19.8.1286. [DOI] [PubMed] [Google Scholar]
- Ding K., Tarumi T., Zhu D.C., Tseng B.Y., Thomas B.P., Turner M.…Zhang R. Cardiorespiratory fitness and white matter neuronal fiber integrity in mild cognitive impairment. J. Alzheimers Dis. 2017;61(2):729–739. doi: 10.3233/JAD-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R.J., Ellingson L.D., Schultz S.A., Boots E.A., Meyer J.D., Lindheimer J.B.…Cook D.B. Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer's disease. Alzheimer's Dement. 2016;4:14–17. doi: 10.1016/j.dadm.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie B.D., Redila V.A., Christie B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Raji C.A., Lopez O.L., Becker J.T., Rosano C., Newman A.B.…Kuller L.H. Physical activity predicts gray matter volume in late adulthood: the cardiovascular health study. Neurology. 2010;75(16):1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L.…Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina N., Rusted J., Tabet N. The effect of exercise interventions on cognitive outcome in Alzheimer's disease: a systematic review. Int. Psychogeriatr. 2014;26(1):9–18. doi: 10.1017/S1041610213001385. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A., Wille P., Müller M.J., Winterer G., Scheurich A., Vucurevic G.…Stoeter P. Ultrastructural hippocampal and White matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement. Geriatr. Cogn. Disord. 2004;18(1):101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A., Müller M.J., Wille P., Dellani P.R., Scheurich A., Schmidt L.G., Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol. Aging. 2005;26(8):1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B. Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci. 2010;21(3):187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fleck M.S., Daselaar S.M., Dobbins I.G., Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb. Cortex. 2006;16(11):1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- Flodin P., Jonasson L.S., Riklund K., Nyberg L., Boraxbekk C.J. Does aerobic exercise influence intrinsic brain activity? An aerobic exercise intervention among healthy old adults. Front. Aging Neurosci. 2017;9 doi: 10.3389/fnagi.2017.00267. August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flöel A., Ruscheweyh R., Krüger K., Willemer C., Winter B., Völker K.…Knecht S. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010;49(3):2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Forbes D., Forbes S., Morgan D.G., Markle-Reid M., Wood J., Culum I. Physical activity programs for persons with dementia. In: Forbes D., editor. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd.; Chichester, UK: 2008. [DOI] [PubMed] [Google Scholar]
- Frederiksen K.S., Larsen C.T., Hasselbalch S.G., Christensen A.N., Høgh P., Wermuth L.…Garde E. A 16-week aerobic exercise intervention does not affect hippocampal volume and cortical thickness in mild to moderate Alzheimer's disease. Front. Aging Neurosci. 2018;10(September):1–10. doi: 10.3389/fnagi.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen K.S., Gjerum L., Waldemar G., Hasselbalch S.G. Physical activity as a moderator of Alzheimer pathology: a systematic review of observational studies. Curr. Alzheimer Res. 2019;16(4):362–378. doi: 10.2174/1567205016666190315095151. [DOI] [PubMed] [Google Scholar]
- Gates N., Singh M.A.F., Sachdev P.S., Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatr. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Gauthier S., Reisberg B., Zaudig M., Petersen R.C., Ritchie K., Broich K.…Winblad B. Mild cognitive impairment. Lancet (Lond., Engl.) 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Geda Y.E., Roberts R.O., Knopman D.S., Christianson T.J.H., Pankratz V.S., Ivnik R.J.…Rocca W.A. Physical exercise, aging, and mild cognitive impairment a population-based study. Arch. Neurol. 2010;67(1):80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B., Voelcker-Rehage C. Cognitive resources necessary for motor control in older adults are reduced by walking and coordination training. Front. Hum. Neurosci. 2017;11:156. doi: 10.3389/fnhum.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B.A., Rykhlevskaia E.I., Brumback C.R., Lee Y., Konopack J.F., Mcauley E.…Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45(5):825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson A., Svensson M., Jacobi F., Allgulander C., Alonso J., Beghi E.…Olesen J. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011;21(10):718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Guyatt G.H., Sackett D.L., Cook D.J., Guyatt G., Bass E., Brill-Edwards P.…Wilson M. Users' guides to the medical literature. JAMA. 1993;270(21):2598. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- Guyatt G.H., Sackett D.L., Cook D.J., Guyatt G., Bass E., Brill-Edwards P.…Wilson M. Users' guides to the medical literature. JAMA. 1994;271(1):59. doi: 10.1001/jama.271.1.59. [DOI] [PubMed] [Google Scholar]
- Haeger A., Lee H., Fell J., Axmacher N. Selective processing of buildings and faces during working memory: the role of the ventral striatum. Eur. J. Neurosci. 2015;41(4):505–513. doi: 10.1111/ejn.12808. [DOI] [PubMed] [Google Scholar]
- Hakamata Y., Lissek S., Bar-Haim Y., Britton J.C., Fox N., Leibenluft E.…Pine D.S. Attention Bias modification treatment: a meta-analysis towards the establishment of novel treatment for anxiety. Biol. Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess N.C.L., Dieberg G., Mcfarlane J.R., Smart N.A. The effect of exercise intervention on cognitive performance in persons at risk of, or with, dementia: a systematic review and meta-analysis. Health. Aging Res. 2014:1–10. [Google Scholar]
- Hohenfeld C., Nellessen N., Dogan I., Kuhn H., Müller C., Papa F.…Reetz K. Cognitive improvement and brain changes after real-time functional MRI neurofeedback training in healthy elderly and prodromal Alzheimer's disease. Front. Neurol. 2017;8(384) doi: 10.3389/fneur.2017.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K., Wolbers T., Röder B., Hötting K. Cardiovascular fitness modulates brain activation associated with spatial learning. NeuroImage. 2012;59(3):3003–3014. doi: 10.1016/j.neuroimage.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Honea R.A., Thomas G.P., Harsha A., Anderson H.S., Donnelly J.E., Brooks W.M., Burns J.M. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2009;23(3):188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg W., Synofzik M., Brötz D., Burkard S., Giese M.A., Schöls L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73(22):1823–1830. doi: 10.1212/WNL.0b013e3181c33adf. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B.…Silverberg N. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenstroth Jan-Christoph, Kalisch Tobias, Holt Stephan, Tegenthoff Martin, Dinse Hubert R. Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Front. Aging Neurosci. 2013 doi: 10.3389/fnagi.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.C., Schmitz T.W., Moritz C.H., Meyerand M.E., Rowley H.A., Alexander A.L.…Alexander G.E. Activation of brain regions vulnerable to Alzheimer's disease: the effect of mild cognitive impairment. Neurobiol. Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N.F., Kim C., Clasey J.L., Bailey A., Gold B.T. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. NeuroImage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L.S., Nyberg L., Kramer A.F., Lundquist A., Riklund K., Boraxbekk C.-J. Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front. Aging Neurosci. 2016;8:336. doi: 10.3389/fnagi.2016.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenstroth J.-C., Kolankowska I., Kalisch T., Dinse H.R. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front. Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann L., Ischebeck A., Weiss E., Koppelstaetter F., Siedentopf C., Vogel S.E.…Wood G. An fMRI study of the numerical Stroop task in individuals with and without minimal cognitive impairment. Cortex. 2008;44(9):1248–1255. doi: 10.1016/j.cortex.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Kircher T.T., Weis S., Freymann K., Erb M., Jessen F., Grodd W.…Leube D.T. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J. Neurol. Neurosurg. Psychiatry. 2007;78(8):812–818. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemeyer M.M., Kühn S., Prindle J., Bodammer N.C., Brechtel L., Garthe A.…Lindenberger U. Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. NeuroImage. 2016;131:155–161. doi: 10.1016/j.neuroimage.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Köbe T., Witte A.V., Schnelle A., Lesemann A., Fabian S., Tesky V.A.…Flöel A. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. NeuroImage. 2016;131:226–238. doi: 10.1016/j.neuroimage.2015.09.050. [DOI] [PubMed] [Google Scholar]
- Kompus K., Hugdahl K., Öhman A., Marklund P., Nyberg L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci. Lett. 2009;467(2):76–80. doi: 10.1016/j.neulet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Kronenberg G., Reuter K., Steiner B., Brandt M.D., Jessberger S., Yamaguchi M., Delbru M. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;463:455–463. doi: 10.1002/cne.10945. August. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Lautenschlager N.T., Cox K.L., Flicker L., Foster J.K., van Bockxmeer F.M., Xiao J.…Almeida O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. JAMA. 2008;300(9):1027. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Lautenschlager N.T., Cox K., Cyarto E.V. The influence of exercise on brain aging and dementia. Biochim. Biophys. Acta Mol. basis Dis. 2012;1822(3):474–481. doi: 10.1016/j.bbadis.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Lenzi D., Serra L., Perri R., Pantano P., Lenzi G.L., Paulesu E., Macaluso E. Single domain amnestic MCI: a multiple cognitive domains fMRI investigation. Neurobiol. Aging. 2011;32(9):1542–1557. doi: 10.1016/j.neurobiolaging.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Liu C.-J., Latham N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009;3 doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T., Nagamatsu L.S., Graf P., Beattie B.L., Ashe M.C., Handy T.C. Resistance training and executive functions. Arch. Intern. Med. 2010;170(2):170. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T., Nagamatsu L.S., Voss M.W., Khan K.M., Handy T.C. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol. Aging. 2012;33(8):1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Maass A., Düzel S., Goerke M., Becke A., Sobieray U., Neumann K.…Düzel E. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry. 2015;20(5):585–593. doi: 10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- Machulda M.M., Ward H.A., Borowski B., Gunter J.L., Cha R.H., O'Brien P.C., Petersen R.C., Boeve B.F., Knopman D., Tang-Wai D.F., Ivnik R.J., Smith G.E., Tangalos E.G., Jack C.R. Comparison of memory fMRI response among Normal, MCI, and Alzheimer's patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- Makizako H., Liu-Ambrose T., Shimada H., Doi T., Park H., Tsutsumimoto K.…Suzuki T. Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. J. Gerontol. Ser. A Biol. Med. Sci. 2015;70(4):480–486. doi: 10.1093/gerona/glu136. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matura S., Fleckenstein J., Deichmann R., Engeroff T., Füzéki E., Hattingen E.…Pantel J. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl. Psychiatry. 2017;7(7):e1172. doi: 10.1038/tp.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill A., Houston S., Lee R.Y.W. Dance for Parkinson's: a new framework for research on its physical, mental, emotional, and social benefits. Complement. Ther. Med. 2014;22(3):426–432. doi: 10.1016/j.ctim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Medina D., deToledo-Morrell L., Urresta F., Gabrieli J.D.E., Moseley M., Fleischman D.…Stebbins G.T. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol. Aging. 2006;27(5):663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Mondadori C.R.A., de Quervain D.J.-F., Buchmann A., Mustovic H., Wollmer M.A., Schmidt C.F.…Henke K. Better memory and neural efficiency in young apolipoprotein E 4 carriers. Cereb. Cortex. 2007;17(8):1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Moniz-Cook E., Vernooij-Dassen M., Woods B., Orrell M., Interdem Network Psychosocial interventions in dementia care research: the INTERDEM manifesto. Aging Ment. Health. 2011;15(3):283–290. doi: 10.1080/13607863.2010.543665. [DOI] [PubMed] [Google Scholar]
- Morris J.K., Vidoni E., Johnson D.K., Sciver A.V., Mahnken J.D., Honea R.A.…Burns J.M. Aerobic exercise for Alzheimer's disease: a randomized controlled pilot trial. PLoS ONE. 2017;12(2):1–14. doi: 10.1371/journal.pone.0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu L.S. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch. Intern. Med. 2012;172(8):666. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu L.S., Weinstein A.M., Erickson K.I., Fanning J., Awick E.A., Kramer A.F., McAuley E. Exercise mode moderates the relationship between mobility and basal ganglia volume in healthy older adults. J. Am. Geriatr. Soc. 2016;64(1):102–108. doi: 10.1111/jgs.13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.E., Rejeski W.J., Blair S.N., Duncan P.W., Judge J.O. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R.…Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Nichol K.E., Poon W.W., Parachikova A.I., Cribbs D.H., Glabe C.G., Cotman C.W. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C., Godde B., Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front. Aging Neurosci. 2014;6(Jun):1–24. doi: 10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi S., Yamada M., Tanigawa T., Sekiyama K., Kawagoe T., Suzuki M.…Tsuboyama T. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: a randomized controlled trial. J. Am. Geriatr. Soc. 2015;63(7):1355–1363. doi: 10.1111/jgs.13481. [DOI] [PubMed] [Google Scholar]
- Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Nusbaum A.O., Tang C.Y., Buchsbaum M.S., Wei T.C., Atlas S.W. Regional and global changes in cerebral diffusion with normal aging. Am. J. Neuroradiol. 2001;22(1):136–142. http://www.ajnr.org/content/22/1/136 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Okonkwo O.C., Schultz S.A., Oh J.M., Larson J., Edwards D., Cook D.…Sager M.A. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83(19):1753–1760. doi: 10.1212/WNL.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea R.D., Vidoni E.D., Morris J.K., Graves R.S., Burns J.M., Honea R.A. Cardiorespiratory fitness and white matter integrity in Alzheimer's disease. Brain Imaging Behav. 2016;10(3):660–668. doi: 10.1007/s11682-015-9431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Dauvermann M., Mette C., Platen P., Franke J., Hinrichs T., Daum I. Voxel-based morphometry reveals an association between aerobic capacity and grey matter density in the right anterior insula. Neuroscience. 2009;163(4):1102–1108. doi: 10.1016/j.neuroscience.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Petrella J.R., Krishnan S., Slavin M.J., Tran T.-T.T., Murty L., Doraiswamy P.M. Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology. 2006;240(1):177–186. doi: 10.1148/radiol.2401050739. [DOI] [PubMed] [Google Scholar]
- Pitkälä K., Savikko N., Poysti M., Strandberg T., Laakkonen M.L. Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: a systematic review. Exp. Gerontol. 2013;48(1):85–93. doi: 10.1016/j.exger.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Porat S., Goukasian N., Hwang K.S., Zanto T., Do T., Pierce J.…Apostolova L.G. Dance experience and associations with cortical gray matter thickness in the aging population. Dement. Geriatr. Cogn. Disord. Extra. 2016;6(3):508–517. doi: 10.1159/000449130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji C.A., Merrill D.A., Eyre H., Mallam S., Torosyan N., Erickson K.I.…Kuller L.H. Longitudinal relationships between caloric expenditure and gray matter in the cardiovascular health study. J. Alzheimers Dis. 2016;52(2):719–729. doi: 10.3233/JAD-160057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila V.A., Olson A.K., Swann S.E., Mohades G., Webber A.J., Weinberg J., Christie B.R. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Reetz K., Romanzetti S., Dogan I., Saß C., Werner C.J., Schiefer J.…Shah N.J. Increased brain tissue sodium concentration in Huntington's disease - a sodium imaging study at 4T. NeuroImage. 2012;63(1):517–524. doi: 10.1016/j.neuroimage.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Reiter K., Nielson K.A., Smith T.J., Weiss L.R., Alfini A.J., Carson Smith J. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J. Int. Neuropsychol. Soc. 2015;21(10):757–767. doi: 10.1017/S135561771500079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman J.M., Coppola G. New genes and new insights from old genes: update on alzheimer disease. Continuum (Minneap. Minn.) 2013;19(2):358–371. doi: 10.1212/01.CON.0000429179.21977.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanzetti S., Mirkes C.C., Fiege D.P., Celik A., Felder J., Shah N.J. Mapping tissue sodium concentration in the human brain: a comparison of MR sequences at 9.4Tesla. NeuroImage. 2014;96:44–53. doi: 10.1016/j.neuroimage.2014.03.079. [DOI] [PubMed] [Google Scholar]
- Rosano C., Aizenstein H.J., Cochran J.L., Saxton J.A., De Kosky S.T., Newman A.B.…Carter C.S. Event-related functional magnetic resonance imaging investigation of executive control in very old individuals with mild cognitive impairment. Biol. Psychiatry. 2005;57(7):761–767. doi: 10.1016/j.biopsych.2004.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C., Guralnik J., Pahor M., Glynn N.W., Newman A.B., Ibrahim T.S.…Aizenstein H.J. Hippocampal response to a 24-month physical activity intervention in sedentary older adults. Am. J. Geriatr. Psychiatry. 2017;25(3):209–217. doi: 10.1016/j.jagp.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.E. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2006;77(10):1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R., Willemer C., Krüger K., Duning T., Warnecke T., Sommer J.…Flöel A. Physical activity and memory functions: an interventional study. Neurobiol. Aging. 2011;32(7):1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sacco G., Caillaud C., Ben Sadoun G., Robert P., David R., Brisswalter J. Exercise plus cognitive performance over and above exercise alone in subjects with mild cognitive impairment. J. Alzheimers Dis. 2015;50(1):19–25. doi: 10.3233/JAD-150194. [DOI] [PubMed] [Google Scholar]
- Scherder E.J.A., Van Paasschen J., Deijen J.-B., Van Der Knokke S., Orlebeke J.F.K., Burgers I.…Sergeant J.A. Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment. Health. 2005;9(3):272–280. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- Schultz S.A. Cardiorespiratory fitness attenuates the influence of amyloid on cognition. J. Int. Neuropsych. Soc. 2015;21(10):841–850. doi: 10.1017/S1355617715000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C., Nielson K.A., Woodard J.L., Seidenberg M., Verber M.D., Durgerian S.…Rao S.M. Does physical activity influence semantic memory activation in amnestic mild cognitive impairment? Psychiatry Res. 2011;193(1):60–62. doi: 10.1016/j.pscychresns.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C., Nielson K.A., Antuono P., Lyons J.-A., Hanson R.J., Butts A.M.…Verber M.D. Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment. J. Alzheimers Dis. 2013;37(1):197–215. doi: 10.3233/JAD-130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C., Singh M.F., Gates N., Wen W., Sachdev P., Brodaty H., Valenzuela M.J. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry. 2016;21(11):1633–1642. doi: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Ito K.…Kato T. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nemoto K., Kawaguchi A., Kato M., Arai T., Kakuma T.…Asada T. Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int. J. Geriatr. Psychiatr. 2015;30(7):686–694. doi: 10.1002/gps.4205. [DOI] [PubMed] [Google Scholar]
- Teixeira C.V.L., Rezende T.J.R., Weiler M., Nogueira M.H., Campos B.M., Pegoraro L.F.L.…Balthazar M.L.F. Relation between aerobic fitness and brain structures in amnestic mild cognitive impairment elderly. Age. 2016;38(3) doi: 10.1007/s11357-016-9912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Brinke L.F., Bolandzadeh N., Nagamatsu L.S., Hsu C.L., Davis J.C., Miran-Khan K., Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Sports Med. 2015;49(4):248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolppanen A.M., Solomon A., Kulmala J., Kåreholt I., Ngandu T., Rusanen M.…Kivipelto M. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimer's Dement. 2015;11(4):434–443. doi: 10.1016/j.jalz.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Tsai C.-L., Pai M.-C., Ukropec J., Ukropcová B. Distinctive effects of aerobic and resistance exercise modes on neurocognitive and biochemical changes in individuals with mild cognitive impairment. Curr. Alzheimer Res. 2019;16(4):316–332. doi: 10.2174/1567205016666190228125429. [DOI] [PubMed] [Google Scholar]
- Van der Borght K., Kóbor-Nyakas D.É., Klauke K., Eggen B.J.L., Nyakas C., Van der Zee E.A., Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van der Kleij L.A., Petersen E.T., Siebner H.R., Hendrikse J., Frederiksen K.S., Sobol N.A.…Garde E. The effect of physical exercise on cerebral blood flow in Alzheimer's disease. NeuroImage Clin. 2018;20(January):650–654. doi: 10.1016/j.nicl.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Verghese J., Lipton R.B., Katz M.J., Hall C.B., Derby C.A., Kuslansky G.…Buschke H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Verstynen T.D., Lynch B., Miller D.L., Voss M.W., Prakash R.S., Chaddock L.…Erickson K.I. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J. Aging Res. 2012;2012 doi: 10.1155/2012/939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni E.D., Honea R.A., Billinger S.A., Swerdlow R.H., Burns J.M. Cardiorespiratory fitness is associated with atrophy in Alzheimer's and aging over 2 years. Neurobiol. Aging. 2012;33(8):1624–1632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni E.D., Sciver A. Van, Johnson D.K., He J., Honea R., Haines B.…Burns J.M. A community-based approach to trials of aerobic exercise in aging and Alzheimer's disease. Contemp. Clin. Trials. 2012;33(6):1105–1116. doi: 10.1016/j.cct.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni E.D., Gayed M.R., Honea R.A., Savage C.R., Hobbs D., Burns J.M. Alzheimer disease alters the relationship of cardiorespiratory fitness with brain activity during the Stroop task. Phys. Ther. 2013;93(7):993–1002. doi: 10.2522/ptj.20120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Prakash R.S., Erickson K.I., Basak C., Chaddock L., Kim J.S.…Kramer A.F. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010;2(Aug):1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Heo S., Prakash R.S., Erickson K.I., Alves H., Chaddock L.…Kramer A.F. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum. Brain Mapp. 2013;34(11):2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A.M., Voss M.W., Prakash R.S., Chaddock L., Szabo A., White S.M.…Erickson K.I. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav. Immun. 2012;26(5):811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Przybelski S., Weigand S.D., David S. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects Progress from MCI to AD. Brain. 2007;130(Pt 7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Bai F., Yu H., Shi Y., Yuan Y., Chen G.…Li S.-J. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. NeuroImage. 2012;63(1):320–327. doi: 10.1016/j.neuroimage.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin F.Z., Rosenberg R.N., Weiner M.F., Purdy P.D., Cullum C.M. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur. Radiol. 2006;16(1):193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- Zheng G., Xia R., Zhou W., Tao J., Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016;50(23):1443–1450. doi: 10.1136/bjsports-2015-095699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material