Abstract
Conflicting data regarding the ability of hydrogen sulfide (H2S), which reaches high levels in the large intestine owing to biosynthesis in the intestinal cells and intestinal bacteria, to promote or inhibit colorectal cancer cell proliferation have been reported recently. In the present study, the effect of H2S on the proliferation of the human colorectal cancer cell line Caco-2 was examined by using the H2S donor GYY4137. At concentrations of 0.5 mM and 1.0 mM, GYY4137 significantly inhibited Caco-2 cell viability. Cell cycle analysis, and apoptosis and necrosis detection revealed that the anti-proliferative effect of GYY4137 was partially attributable to the induction of S-G2/M cell cycle arrest, apoptosis and necrosis. These results suggest that H2S has the potential to suppress human colorectal cancer cell proliferation by influencing both cell cycle and cell death.
Keywords: Food chemistry, Nutrition, Environmental toxicology, Biochemistry, Molecular biology, Cancer research, Toxicology, Diet, Public health, GYY4137, Sodium hydrosulfide, Hydrogen sulfide, Colorectal cancer, Proliferation
1. Introduction
Colorectal cancer is one of the leading causes of death worldwide, being responsible for approximately 10% of total cancer-related mortality [1]. About 3–5% of colorectal cancers may be due to inherited genetic defects, and up to 25% of patients may have some degree of hereditary predisposition for this disease, although the majority of colorectal cancers occur in a sporadic manner, in the absence of documented family history [2].
Hydrogen sulfide (H2S) is synthesized naturally from cysteine by several enzymes, including cystathionine γ-lyase, cystathionine β-synthetase, and 3-mercaptosulfurtransferase, in a wide range of mammalian and non-mammalian cells both in vitro and in vivo. In the last two decades, numerous physiological and pathophysiological roles have been proposed for this gas along with a plethora of cellular and molecular targets, including a range of ion channels, enzymes, and transcription factors [3]. In the large intestine, H2S is mainly produced by endogenous sulfate-reducing bacteria, and its concentration reaches up to 3.4 mM in human colon [3, 4, 5]. It has also been reported that in colon cancer patients, the amount of H2S is increased, because the expression of H2S-metabolizing enzymes is reduced [6, 7]. Moreover, fecal sulfide level is elevated in patients with ulcerative colitis, a condition associated with an increased risk of colon cancer [8].
To date, many studies have been undertaken using sulfide salts such as sodium hydrosulfide (NaHS), which release H2S instantaneously into aqueous solution. However, the release of endogenous H2S from cells is likely to occur in lesser amounts and at a much slower rate than that from sulfide salts, and therefore, NaHS may not accurately mimic the biological effects of naturally produced H2S. Recognizing the need for organic molecules capable of releasing H2S over extended periods of time, Li et al. [9] reported that morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate (GYY4137) releases H2S slowly both in vitro and in vivo.
The present study was undertaken to investigate the effect of GYY4137, a slow-releasing H2S donor, on the proliferation of the colorectal cancer cell line Caco-2. Further, a possible mechanism of the H2S effect was evaluated by analyzing cell cycle and cell death characteristics.
2. Materials and methods
2.1. Materials
GYY4137 was obtained from Dojindo Molecular Technologies, Inc. (Rockville, MD, USA). NaHS was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Annexin V-FLUOS Staining Kit and In Situ Cell Death Detection Kit, fluorescein were purchased from Roche Diagnostics (Indianapolis, IN, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), ribonuclease A (RNase A), and propidium iodide (PI) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). All other reagents were of analytical grade.
2.2. Measurement of H2S
After incubation with GYY4137 (1.0 mM, 100 μL) or NaHS (1.0 mM, 100 μL) in Minimum Essential Medium (MEM; Life Technologies Corporation, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Nichirei Biosciences Inc., Tokyo, Japan) and 1% non-essential amino acids (Life Technologies Corporation, Carlsbad, CA, USA), aliquots were mixed with a solution of 0.85% zinc acetate (w/v) and 3% NaOH (1:1 ratio, 100 μL). Methylene blue was then formed by the addition of N,N-dimethyl-p-phenylenediamine-dihydrochloride dye and FeCl3 at final concentrations of 2.5 and 3.3 mM, respectively, and absorbance was subsequently monitored at 670 nm. The concentration of H2S was determined using a standard curve of NaHS (0–400 μM).
2.3. Cell culture
Caco-2 human colon cancer cell line was purchased from the European Collection of Cell Cultures (Salisbury, Wiltshire, UK) and cultured in MEM supplemented with 10% fetal bovine serum and 1% non-essential amino acids. The cells were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
2.4. Cell viability assay
Cell viability was measured by the MTT assay, as described previously [10, 11]. Briefly, the cells at a density of 2.5 × 104 cells/2 mL/9.5 cm2 well were incubated with the test reagents for 72 h. After incubation, the medium was removed, and the cells were incubated with 1.1 mL of MTT solution (0.1 mL of 5 mg/mL MTT in 1 mL of the medium) for 4 h. The product was eluted from the cells by the addition of 20% sodium dodecyl sulphate/0.01 M HCl, and absorbance at 595 nm was determined using an SH-1200 Lab microplate reader (Corona Electric Co., Ltd, Ibaraki, Japan). Cell viability was calculated according to the following equation: cell viability (%) = (absorbance of experiment group/absorbance of control group) × 100.
2.5. Cell cycle analysis
Cell cycle analysis was performed by flow cytometry as reported previously [10, 11]. Briefly, the cells at a density of 1.0 × 106 cells/28 cm2 dish were incubated with the test reagents for 72 h and then collected by centrifugation (4 °C, 200 × g, 5 min). The pellet was fixed with 70% ethanol cooled at −20 °C on ice for 30 min. Following fixation, the cells were incubated with 100 μg/mL RNase A at 37 °C for 30 min. The cells were treated with PI (50 μg/mL) in a dark place on ice for 30 min. The samples were filtrated through a nylon mesh (35 μm) and examined in a FACScanTM flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, US).
2.6. Determination of cell apoptosis and/or necrosis
DNA fragmentation in cell nucleus, an indicator of apoptosis, was detected by using an in situ Cell Death Detection Kit and fluorescein, and confocal laser scanning microscope (Carl Zeiss Co., Ltd., LSM-510; excitation at 495 nm and emission at 530 nm), as reported previously [12]. Briefly, the cells at a density of 3.0 × 105 cells/0.8 cm2 of Nunc™ Lab-Tek™ Chamber Slide (Thermofisher Scientific K.K., Tokyo, Japan) were incubated with the test reagents for 48 h. Blue coloring indicates cell nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI). TUNEL-positive nuclei (apoptotic cells) were visualized by green coloring. Clear light blue coloring (a mixture of blue coloring and green coloring) shows DNA fragmentation in the nuclei.
In addition, apoptosis or necrosis was detected by flow cytometry using an annexin V-fluorescein staining kit. Briefly, the cells were incubated with the test reagents at a density of 1.0 × 106 cells/28 cm2 dish for 48 h, and then collected by centrifugation. The cell pellets were incubated with staining solution containing annexin V-fluorescein and PI at room temperature for 15 min. After adequate dilution according to the cell density, the samples were filtrated through a nylon mesh (35 μm), and subjected to a FACS AriaTM III flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ), as reported previously [13].
2.7. Statistics
Results are the means ± SEM. The significance of differences between two groups was assessed using the Student's t test, and differences between multiple groups were assessed by one-way analysis of variance (ANOVA), followed by Scheffe's multiple range test. P values less than 0.05 were considered significant.
3. Results
3.1. Release of H2S from GYY4137
As shown in Fig. 1, incubation in culture medium containing either 1 mM GYY4137 or 1 mM NaHS resulted in the release of micromolar concentrations of H2S detected in collected aliquots by the methylene blue formation assay. Release of H2S from NaHS was rapid, peaking at or before 20 min (Fig. 1A) and declining to undetectable levels by 48 h (Fig. 1B). In contrast, peak H2S release from GYY4137 was much lower (about one third of that observed with NaHS) (Fig. 1A), but it was sustained for up to 72 h (Fig. 1B). A similar result was observed by Lee et al. [14]. The results illustrated in Fig. 1 confirm that NaHS and GYY4137 are rapid-releasing and slow-releasing H2S donors, respectively.
Fig. 1.
Release of H2S by GYY4137 (1.0 mM, ●) and NaHS (1.0 mM, ▲) into culture medium. (A) H2S release was determined at time intervals up to 2 h. (B) H2S release was determined at time intervals up to 72 h. Data are expressed as the mean ± SEM (n = 4).
3.2. GYY4137 suppresses the proliferation of Caco-2 cells more potently than NaHS
Table 1 shows effects of GYY4137 and NaHS at concentrations of 0.5 and 1.0 mM on Caco-2 cell viability. Caco-2 cells were incubated with either GYY4137 or NaHS for 72 h and then, changes in cell viability were measured by the MTT assay. GYY4137 significantly decreased cell viability both at 0.5 mM and 1.0 mM by 30.9% and 52.9%, respectively (P < 0.01 vs. Control). Although NaHS suppressed the cell growth (0.5 and 1.0 mM, 23.4 and 31.0% inhibition), the inhibitory efficacy of 1.0 mM GYY4137 was significantly more than that of 1.0 mM NaHS. These results mean that GYY4137 inhibited Caco-2 cell growth more potently than NaHS.
Table 1.
Effects of GYY4137 and NaHS on the proliferation of Caco-2 cells.
| Treatment | Cell viability |
|
|---|---|---|
| (% of control) | ||
| Control | 100.0 ± 3.4 | |
| GYY4137 | 0.5 mM | 69.1 ± 5.0α |
| 1.0 mM | 47.1 ± 4.3αβ | |
| NaHS | 0.5 mM | 76.6 ± 4.7α |
| 1.0 mM | 69.0 ± 4.2α |
Caco-2 cells were treated with GYY4137 or NaHS for 72 h. Proliferation was assayed by the MTT assay. Data are expressed as the mean ± SEM (n = 9). αP < 0.01 vs. Control; βP<0.01 vs. 1.0 mM NaHS.
3.3. GYY4137 induces S-G2/M phase arrest in Caco-2 cells
To determine whether GYY4137-induced cell growth inhibition involved cell cycle changes, we examined distribution of cells in cell cycle phases by flow cytometry (Fig. 2). The treatment of Caco-2 cells with 0.5 and 1.0 mM GYY4137 for 72 h induced an accumulation of cells in the S and G2/M phases of cell cycle. Concomitantly with these increases in the population of cells in the S and G2/M phases, a decrease in the population of cells in the G0/G1 phase was observed. This result implies that GYY4137 induced an arrest of Caco-2 cells in the S-G2/M phase.
Fig. 2.
Effect of GYY4137 on the distribution of Caco-2 cells in different phases of cell cycle. Caco-2 cells were treated with 0.5 and 1.0 mM GYY4137 for 72 h. (A) Representative flow cytometry charts. (B) The effect of 0.5 and 1.0 mM GYY4137 on the percentages of Caco-2 cells in the G0/G1, S, and G2/M phases. Data are expressed as the mean ± SEM (n = 7). αP < 0.05, βP < 0.01; significantly different from the corresponding value in the cells that were not exposed to GYY4137.
3.4. GYY4137 induces apoptosis and necrosis in Caco-2 cells
To test whether the GYY4137-induced inhibition of Caco-2 cell proliferation was due to apoptosis and/or necrosis, apoptotic cells were visualized by the TUNEL assay, using a confocal laser scanning microscope (Fig. 3). Blue coloring indicates cell nuclei stained by DAPI. TUNEL-positive nuclei were visualized by green coloring. Clear light blue coloring (a mixture of blue coloring and green coloring) shows DNA fragmentation in the nuclei, as indicated by arrows. The addition of 0.5 μM actinomycin D as positive control caused DNA fragmentation in the nuclei, i.e., hallmarks of apoptotic cells. Similarly to actinomycin D, 0.5 mM GYY4137 also increased the number of apoptotic cells in a small extent.
Fig. 3.
Fluorescence image of DNA fragmentation in Caco-2 cells treated with GYY4137. Caco-2 cells were treated with 0.5 mM GYY4137 for 72 h. Apoptotic cells were visualized by the TUNEL assays using a confocal laser scanning microscope. Blue coloring indicates cell nuclei stained by DAPI. TUNEL-positive nuclei were visualized by green coloring. Clear light blue coloring (a mixture of blue coloring and green coloring) shows DNA fragmentation in the nuclei, as indicated by arrows. Data were collected from at least 10 random sections per sample. The data are representative of 3 experiments.
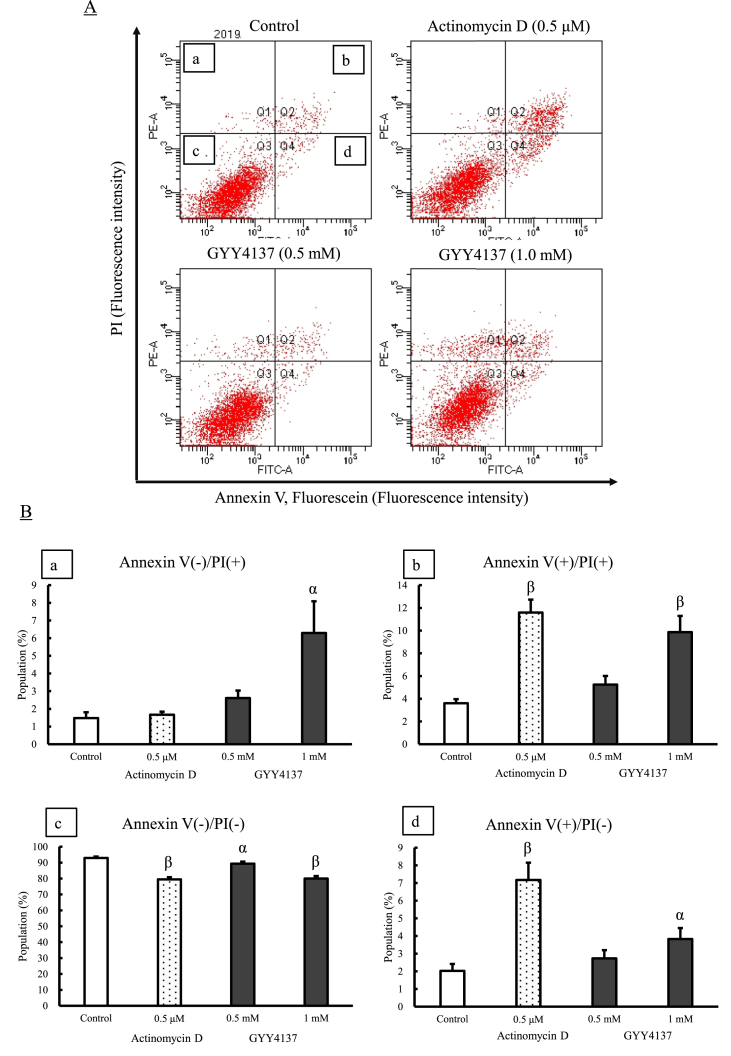
Fig. 4 also illustrates the percentages of apoptotic or necrotic cells, measured by the annexin V-fluorescein/PI assay using flow cytometry, when Caco-2 cells were exposed to 0.5 and 1.0 mM GYY4137 at 48 h. Positive staining with annexin V-fluorescein correlates with a loss of membrane polarity (apoptosis). In contrast, PI can only enter cells after the complete loss of membrane integrity (necrosis). The fraction of the cell population in different quadrants was analyzed using quadrant statistics [13]. Cells in the upper left quadrant [a: annexin V(–)/PI(+)] represented necrotic cells. Cells in the lower right quadrant [d: annexin V(+)/PI(–)] represented early apoptotic cells, and cells in the upper right quadrant [b: annexin V(+)/PI(+)] represented late apoptotic cells. Analytic data of the control, actinomycin D (0.5 μM), and 0.5 and 1.0 mM GYY4137 is shown Fig. 4A. The experiment using actinomycin D (0.5 μM), which is a known inducer of apoptosis, verified the validity of the cell analysis; it significantly increased both the early apoptotic and late apoptotic cell population [Fig. 4B(b,d)]. The treatment of Caco-2 cells with 1.0 mM GYY4137 induced a significant increase in both the early apoptotic and late apoptotic cell population [Fig. 4B(b,d)] in the same way as actinomycin D. Additionally it enhanced the necrotic cell population [Fig. 4B(a)]. The results imply that GYY4137 has the potential to induce both apoptosis and necrosis in Caco-2 cells.
Fig. 4.
Effect of GYY4137 on apoptosis or necrosis in Caco-2 cells. Caco-2 cells were treated with 0.5 and 1.0 mM GYY4137 for 48 h. Apoptotic or necrotic cells were measured by the annexin V-fluorescein/PI using a flow cytometry. Data are expressed as the mean ± SEM (n = 4). αP < 0.05, βP < 0.01; significantly different from the corresponding value in the cells that were not exposed to GYY4137.
4. Discussion
There have been conflicting reports regarding the role of H2S in colorectal cancer. Therefore, we have examined here the effect of H2S on the proliferation of human colorectal cancer Caco-2 cells and showed that the H2S donor GYY4137 significantly attenuated Caco-2 cell viability in part due to the induction of S-G2/M cell cycle arrest, apoptosis, and necrosis.
As shown in Fig. 1 and Table 1, the suppression of Caco-2 cell proliferation by H2S at a low concentration following the exposure to GYY4137 occurred over a period of several days under the indicated experimental conditions, i.e., at a comparatively slow rate. In contrast, the exposure of cells to NaHS generated H2S at a higher concentration, and Caco-2 cell proliferation was inhibited over a shorter time frame (fast rate). Thus, it is likely that both the duration of exposure to H2S and its concentration are critical in determining the ability of this gas to inhibit cell proliferation. It is generally accepted that the release of endogenous H2S from cells occurs in low concentrations, and therefore, the consequences of H2S generation at the lower rate after the exposure to GYY4137 more closely mimic biological effects of naturally produced H2S.
Next, some experiments were undertaken to determine the mechanism by which GYY4137 reduced Caco-2 cell proliferation. Flow cytometry analysis of the distribution of treated cultured cells in different phases of cell cycle revealed that both 0.5 and 1.0 mM GYY4137 induced S-G2/M phase cell cycle arrest with an accompanying decrease in the number of cells in the G0/G1 phase (Fig. 2). This confirmed that GYY4137 inhibited DNA synthesis and induced a block at the S-G2/M boundary. The confocal laser scanning microscopy and flow cytometry observations using of the TUNEL and annexin V-fluorescein/PI assays, respectively (Figs. 3 and 4) revealed that GYY4137 at 0.5 or 1.0 mM induced both apoptosis and necrosis in Caco-2 cells.
Caco-2 cells are one of cancer cell lines that are deficient in p53 protein expression [15, 16]. However, Tsai et al [17] have reported that, independent of p53, activation of extracellular signal-regulated kinase (ERK) leads to both cell cycle arrest and apoptotic cell death via upregulation of p21 and downregulation of Bcl-2, respectively. Yang et al. [18, 19] have also reported that treatment of human aorta smooth muscle cells with H2S elicits an increase in ERK signaling. Therefore, a certain role of ERK activation in inhibiting Caco-2 cell proliferation by GYY4137 may be one possible mechanism.
There have been disparate observations of the effect of H2S on colorectal cancer cell growth in vitro. Rose et al. [20] have reported that 0.25–1.0 mM NaHS protected HCT-116 colorectal cancer cells from apoptosis caused by the chemoprotective agent β-phenylethyl isothiocyanate. Cai et al. [21] have also shown that NaHS at 0.2–1.0 mM promoted the proliferation of human colorectal cancer cell lines HCT-116 and SW480. In contrast, Cao et al. [22] reported that NaHS at millimolar concentrations inhibited cell growth of WiDr colorectal cancer cells. Wu et al. [23] further showed that NaHS at 0.4–1.0 mM inhibited growth of the colorectal cancer cell lines HT-29, SW1116, and HCT-116 via G1 phase cell cycle arrest. In the present study, we showed that GYY4137 inhibited Caco-2 cell growth by the induction of both S-G2/M cell cycle arrest and cell death. These discrepant observations are difficult to reconcile. However, one explanation may be given for the choice of colon cancer cell line: we used Caco-2 cells in the present study, whereas cancer cell lines other than Caco-2 were used in the previous studies [20, 21, 22, 23]. Further studies are needed for the ultimate reconciliation of these two opposite observations.
In conclusion, we found that GYY4137 exhibited anti-proliferative activity against Caco-2 cells. We propose that GYY4137 breaks down slowly to yield H2S, which by simultaneously inducing cell cycle arrest, apoptosis and necrosis, inhibits cell growth. The previous findings by Lee et al. [14] that cancer cells, but not non-cancer cells, can be killed selectively when exposed to relatively small amounts of H2S over a relatively long time period support the significance of the present observations. We believe that our study makes a significant contribution to the literature because, to the best of our knowledge, this is the first time that GYY4137 was shown to reduce Caco-2 cell growth, possibly by inducing both S-G2/M cell cycle arrest, apoptosis and necrosis.
Declarations
Author contribution statement
Satoru Sakuma: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Saaya Minamino, Maya Takase, Yoshitaka Ishiyama, Hiroyuki Hosokura, Yukino Ikeda: Performed the experiments.
Tetsuya Kohda: Contributed reagents, materials, analysis tools or data.
Yohko Fujimoto: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Stelzner F. Spontaneous change of malignancy of solid malignant tumors: statistical investigations of colorectal and pancreatic carcinoma. Chirurg. 2012;83:726–731. doi: 10.1007/s00104-011-2236-z. [DOI] [PubMed] [Google Scholar]
- 2.Link A., Balaguer F., Shen Y., Lozano J.J., Leung H.C., Boland C.R., Goel A. Curcumin modulates DNA methylation in colorectal cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L., Rose P., Moore P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 4.Hu L., Wong P.T., Moore P.K., Bian J.S. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J. Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 5.Fiedler N., Kipen H., Ohman-Strickland P., Zhang J., Weisel C., Laumbach R., Kelly-McNeil K., Olejeme K., Lioy P. Sensory and cognitive effects of acute exposure to hydrogen sulfide. 2008;116:78–85. doi: 10.1289/ehp.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy S., Singh S., Taniere P., Langman M.J., Eggo M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G288–296. doi: 10.1152/ajpgi.00324.2005. [DOI] [PubMed] [Google Scholar]
- 7.Picton R., Eggo M.C., Langman M.J., Singh S. Impaired detoxication of hydrogen sulfide in ulcerative colitis? Dig. Dis. Sci. 2007;52:373–378. doi: 10.1007/s10620-006-9529-y. [DOI] [PubMed] [Google Scholar]
- 8.Levine J., Ellis C.J., Furne J.K., Springfield J., Levitt M.D. Fecal hydrogen sulfide production in ulcerative colitis. Am. J. Gastroenterol. 1998;93:83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- 9.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.H., Moore P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 10.Kohda T., Sakuma S., Abe M., Fujimoto Y. Monochloramine suppresses the proliferation of colorectal cancer cell line Caco-2 by both apoptosis and G2/M cell cycle arrest. Cell Biochem. Funct. 2014;32:188–193. doi: 10.1002/cbf.2992. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma S., Abe M., Kohda T., Fujimoto Y. Hydrogen peroxide generated by xanthine/xanthine oxidase system represses the proliferation of colorectal cancer cell line Caco-2. J. Clin. Biochem. Nutr. 2015;56:15–19. doi: 10.3164/jcbn.14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuma S., Negoro M., Kitamura T., Fujimoto Y. Xanthine oxidase-derived reactive oxygen species mediate 4-oxo-2-nonenal-induced hepatocyte cell death. Toxicol. Appl. Pharmacol. 2010;249:127–131. doi: 10.1016/j.taap.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Su M.Y., Huang H.Y., Li L., Lu Y.H. Protective effects of 2’4’-dihydroxy-6’-methoxy-3’5’-dimethylchalcone to PC12 cells against cytotoxicity induced by hydrogen peroxide. J. Agric. Food Chem. 2011;59:521–527. doi: 10.1021/jf104408d. [DOI] [PubMed] [Google Scholar]
- 14.Lee Z.W., Zhou J., Chen C.S., Zhao Y., Tan C.H., Li L., Moore P.K., Deng L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes-Zurita F.J., Rufino-Palomares E.E., García-Salguero L., Peragón J., Medina P.P., Parra A., Cascante M., Lupiáñez J.A. Maslinic acid, a natural Triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Štefaniková A., Klačanová K., Pilchová I., Hatok J., Račay P. Cyclin-dependent kinase 2 inhibitor SU9516 increases sensitivity of colorectal carcinoma cells Caco-2 but not HT29 to BH3 mimetic ABT-737. Gen. Physiol. Biophys. 2017;36:539–547. doi: 10.4149/gpb_2017030. [DOI] [PubMed] [Google Scholar]
- 17.Tsai S.C., Huang W.W., Huang W.C., Lu C.C., Chiang J.H., Peng S.F., Chung J.G., Lin Y.H., Hsu Y.M., Amagaya S., Yang J.S. ERK-modulated intrinsic signaling and G(2)/M phase arrest contribute to the induction of apoptotic death by allyl isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int. J. Oncol. 2012;41:2065–2072. doi: 10.3892/ijo.2012.1640. [DOI] [PubMed] [Google Scholar]
- 18.Yang G., Sun X., Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 19.Yang G., Wu L., Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 20.Rose P., Moore P.K., Ming S.H., Nam O.C., Armstrong J.S., Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 2005;11:3990–3997. doi: 10.3748/wjg.v11.i26.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai W.J., Wang M.J., Ju L.H., Wang C., Zhu Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol. Int. 2010;34:565–572. doi: 10.1042/CBI20090368. [DOI] [PubMed] [Google Scholar]
- 22.Cao Q., Zhang L., Yang G., Xu C., Wang R. Butyrate-stimulated H2S production in colon cancer cells. Antioxidants Redox Signal. 2010;12:1101–1109. doi: 10.1089/ars.2009.2915. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y.C., Wang X.J., Yu L., Chan F.K., Cheng A.S., Yu J., Sung J.J., Wu W.K., Cho C.H. Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037572. [DOI] [PMC free article] [PubMed] [Google Scholar]