Abstract
Various pesticide nanocarriers have been developed. However, their pest-control applications remain limited in laboratories. Herein, we developed silica nanocapsules encapsulating fipronil (SNC) and their engineered form, poly(ethyleneimine)-coated SNC (SNC-PEI), based on recombinant catalytic modular protein D4S2 and used them against termite colonies Coptotermes lacteus in fields. To achieve this, an integrated biomolecular bioprocess was developed to produce D4S2 for manufacturing SNC containing fipronil with high encapsulation efficiency of approximately 97% at benign reaction conditions and at scales sufficient for the field applications. PEI coating was achieved via electrostatic interactions to yield SNC-PEI with a slower release of fipronil than SNC without coating. As a proof-of-concept, bait toxicants containing varied fipronil concentrations were formulated and exposed to nine termite mounds, aiming to prolong fipronil release hence allowing sufficient time for termites to relocate the baits into and distribute throughout the colony, and to eliminate that colony. Some baits were relocated into the mounds, but colonies were not eliminated due to several reasons. We caution others interested in producing bait toxicants to be aware of the multilevel resistance mechanisms of the Coptotermes spp. “superorganism”.
Keywords: Chemical engineering, Nanotechnology, Nanocapsules, Sustained release, Pesticide delivery system, Fipronil, Subterranean termites, Composite materials, Encapsulation technology, Molecular engineering, Nanoparticles
1. Introduction
Nanotechnology promises a great impact on agricultural fields. Among many applications of nanotechnology in agriculture, development of nanoscale carriers for efficient and sustainable utilities of pesticides has received a lot of attention [1, 2, 3]. Specifically, encapsulation of pesticidal active ingredients within nanocarriers enhances their deliverability to target pests and enables control over their release kinetics, while at the same time protects them from premature degradation caused by direct exposure to ultraviolet light, low pH or heat [4, 5, 6]. These integrated controlled-release and protective properties of nanocarriers are expected to facilitate better efficacy of the encapsulated pesticide by prolonging its release on a target site, as compared to non-encapsulated formulations. In this way, the application rates of pesticides can be potentially reduced, minimizing pest resistance development and adverse environmental impacts. This has been one of the key drivers for the application of nanotechnology in agriculture [1].
Progress in the development of nanocarriers has created a library of delivery systems for various pesticides like insecticides, herbicides, and fungicides [7, 8, 9]. In this regard, nanocarriers synthesized based on lipids (e.g., liposome [10], nanoemulsions [11], and solid lipid nanoparticles [12, 13, 14]) and polymers (nanospheres [15, 16], nanogels [17, 18], and micelles [19, 20]) have been widely used. Recently, agrochemical nanocarriers based on silica have been increasingly viewed as an attractive alternative [21, 22, 23, 24, 25, 26]. Their structural properties can be engineered from solid to mesoporous nanoparticles and even liquid-filled mesoporous nanoparticles (core–shell) to facilitate high-capacity loading [27]. Furthermore, due to their rich surface chemistry [27, 28], their surfaces can be physically or chemically functionalized with small molecules (e.g., sulfonate [29] or amine [30]) and/or biomacromolecules (e.g., α-cyclodextrin [31], pectin [32], chitosan [33] or alginate [34, 35]) for further sustaining and/or controlling the release of agrochemicals.
Many of the aforementioned nanocarriers have been tested and compared to conventional formulations for their efficacy against various types of pests. Cao et al. loaded pyraclostrobin into silica nanoparticles and then surface-coated the nanoparticles with chitosan to sustain its release against Phomopsis asparagi, and only half dose of the technical recommendation was required to demonstrate effective fungicidal activities [25]. Mattos et al. improved the stability and prolonged the release of neem bark extract by encapsulating in silica nanoparticles, so that the nanoformulation was able to eliminate worker ants, Acromyrmex crassispinus [26]. We have previously synthesized biocompatible silica nanocapsules based on SurSi peptide [36] and demonstrated the release of encapsulated fipronil in a time-controlled manner through control of the silica-shell thickness to eliminate worker and soldier termite colonies, Coptotermes acinaciformis [24]. Overall, efficacies of pesticide-loaded nanoparticles can be enhanced by up to 30% relative to conventional products [1].
Despite the efforts in developing nanoformulations of pesticides aforementioned, the evaluation of their performances has been limited to the laboratory environment. Application of pesticide-loaded nanocarriers in actual field conditions, to the best of our knowledge, are yet available in the literature. Herein, we reported the efficacy tests of our silica nanocapsules encapsulating fipronil against termite colonies of Coptotermes lacteus (C. lacteus) (Froggatt) at their natural feeding-sites in tropical northern Queensland, Australia. Termites are prevalent throughout the tropics and subtropics [37] where they are the most problematic pests [38]. The economic impact of termites exceeds US$40 billion annually worldwide [39]. Therefore, controlling termite pest populations are urgent. However, elimination of termite pests in fields remains challenging due to, for example, their sophisticated behaviors [40], seasonal weather patterns [41], density and age of the colonies [42], and also different susceptibility of different castes of termites (e.g., queen, king, soldier, worker) within the nest to pesticides [43].
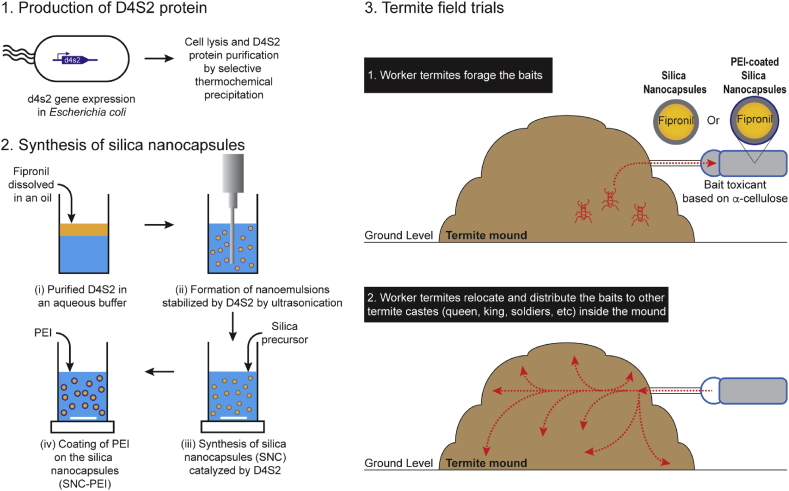
In this study, we utilized bait toxicant systems comprising a mixture of fipronil-loaded silica nanocapsules and α-cellulose, and aimed for eliminating termite colonies of C. lacteus in the fields (Fig. 1). To achieve this, recombinant catalytic modular protein D4S2 was produced in microbial cell factory and used [44], in lieu of SurSi peptide we previously used [24], to enable cost-effective manufacture of fipronil-loaded silica nanocapsules at scales sufficient for the field trials. Moreover, the use of protein D4S2 enabled the formation and stabilization of nanoemulsions and subsequently catalyzed the synthesis of nanoemulsion-templated silica nanocapsules at neutral pH, room temperature and without using any toxic reagents. Surface chemistry of the silica nanocapsules was further modified to prolong the sustained release of the encapsulated fipronil. We hypothesized that worker termites would forage for the bait toxicants and relocate the baits into their mounds, and the sustained release of fipronil would provide a sufficient time for the worker termites to distribute the baits to the other termite castes within the mound and subsequently eliminate the colonies (Fig. 1).
Fig. 1.
Application of silica nanocapsules as fipronil nanocarriers against termite colonies in field. (1) Production of D4S2 protein in microbial cell factories. (2) Synthesis of silica nanocapsules (SNC) and poly(ethyleneimine) (PEI)-coated silica nanocapsules (SNC-PEI) using the D4S2 protein-stabilized nanoemulsions as the template. (3) Hypothetical schemes of worker termites foraging the bait toxicants, then relocating the baits into and distributing them to other termite castes (e.g., queen, king, soldiers, etc.) inside the mound. The sustained release properties of silica nanocapsules would provide sufficient time for bait distribution, hence, colony elimination. Note: the red dotted lines illustrate the movement of worker termites, and the arrow illustrates their moving direction.
2. Materials and methods
2.1. Materials
Analytical grade fipronil (C12H4Cl2F6N4OS, M 437.15 g/mol, powder) was kindly provided by Accensi Pty. Ltd. (Narangba, Australia). Miglyol 812 was purchased from AXO Industry S.A. (Wavre, Belgium). Prior to use, it was passed through heat-activated silica gel (Sigma-Aldrich, Castle Hill, Australia). A stock solution of poly(ethyleneimine) (PEI) (#P3143, Sigma-Aldrich) was prepared at 5% (w/v), pH 8 in water (using hydrochloric acid to adjust the pH). Water with >18.2 MΩ cm resistivity was used (a Milli-Q system, Merck Millipore, Bayswater, Australia). Other chemicals were of analytical grade, and used as received. They were purchased from either Sigma-Aldrich or Merck.
2.2. Protein production from Escherichia coli (E. coli)
E. coli strain BL21(DE3) containing plasmid pET-14b(+) with the inserted DNA encoding D4S2 protein was cultivated as previously described [45]. Briefly, a single colony was selected from a freshly streaked Luria Bertani (LB) agar plate, and was then inoculated and incubated overnight at 30 °C, 180 rpm. The resulting overnight pre-cultures were inoculated to give main cultures with a starting OD600 of 2.5 × 10−3. The main cultures were grown at 37 °C, 180 rpm for about 4 h to reach an OD600 of 0.5, then induced with 1 mM isopropyl-β-D-thiogalactopyranoside and allowed to grow at 37 °C for additional 4 h. Final OD600 of 2 was routinely obtained. All media was supplemented with kanamycin sulfate at 15 μg/mL.
To purify the recombinant D4S2 protein, the cells were harvested and then disrupted by sonication (Branson Sonifier 250, Branson Ultrasonics, CT, USA) using an energy output of 60 W (4 times, each for 30 s). The crude cell-extract was added with 0.5% PEI and stirred at 4 °C for 60 min to precipitate nucleic-acid contaminants. Na2SO4 (solid) was added to the supernatant obtained after centrifugation (48,000 × g, 4 °C, 20 min) to a concentration of 1 M, and then incubated at 90 °C for 30 min to precipitate protein contaminants. After centrifugation (48,000 × g, 4 °C, 20 min), the supernatants were mixed with Na2SO4 (solid) to a final concentration of 1.8 M by stirring at 30 °C for 60 min to isolate D4S2 protein. The D4S2 precipitates were washed three times using a rinsing buffer containing 25 mM Tris-HCl, 1 M NaCl and 1.8 M Na2SO4 at pH 8, and then resolubilized in 25 mM Tris-HCl, 1 M NaCl, pH 8 before dialysis against 25 mM sodium 4-(2-hydroxyethyl)-1-piperazine ethanesulfonate (HEPES) buffer at pH 7.5.
2.3. Silica nanocapsules synthesis
An oil solution of Miglyol 812 containing 10 g/L fipronil in was prepared, and was then added to 1.53 g/L D4S2 protein in 25 mM HEPES buffer pH 7.5 at a volume ratio of 1:9 (10% oil, v/v). The mixture was then sonicated using a Branson Sonifier 450 ultrasonicator for four 30 s bursts at 40 W. The resulting fipronil-loaded nanoemulsions were diluted five times, and then mixed with the tetraethoxysilane (TEOS, 160 mM) at room temperature for 24 h to produce oil-core silica shell nanocapsules (SNC) with fipronil loaded. The silica nanocapsules were collected subsequently after washing with ethanol and then water. To form PEI-coated silica nanocapsules (SNC-PEI), the suspensions were mixed with 0.5% PEI at a volume ratio of 1:1 by stirring at room temperature for 24 h before washing thrice with water. Prior to field trial, the pellets of either SNC or SNC-PEI stored at 4 °C were re-suspended in water and re-characterized for ensuring quality consistency.
2.4. Material characterization
2.4.1. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Protein samples was analyzed qualitatively using SDS-PAGE. NuPAGE 4–12% Bis-Tris Precast Gels with an XCell SureLockTM Mini-Cell Electrophoresis system and 2-(N-morpholino) ethanesulfonic acid (MES) buffer were used (Life Technologies, Mulgrave, Australia). Novex BenchMark pre-stained Protein Ladder (Life Technologies, Mulgrave, Australia) was used as the standard.
2.4.2. Reversed-phase high-performance liquid chromatography (RP-HPLC)
Concentrations of fipronil were analyzed by RP-HPLC using a Shimadzu system (Kyoto, Japan) with a Jupiter C18 column (5 μm, 300 Å, 150 mm × 4.6 mm) (Torrance, CA). The mobile phase A was 0.1% H3PO4 aqueous solution, and the phase B was 90% acetonitrile and 0.1% H3PO4. The elution gradient increased from 50 to 70% B over 20 min at a wavelength of 220 nm.
2.4.3. Dynamic light scattering (DLS)
Size, size distributions and zeta potentials of fipronil-loaded nanoemulsions, SNC and SNC-PEI were measured by DLS using Zetasizer Nano ZS (Malvern Panalytical Ltd., Malvern, U.K.). Samples were diluted by a factor of 100 to avoid multiple scattering effects.
2.4.4. Transmission electron microscopy (TEM)
A drop of silica nanocapsules (2 μL) was deposited onto grids (ProSciTech Pty. Ltd., Kirwan, Australia) and was then observed under TEM (JEOL 1010, Tokyo, Japan) at 100 kV. The size of the nanocapsules was determined using iTEM software equipped with the TEM.
2.5. Encapsulation efficiency
To extract fipronil from the oil core, acetonitrile was added to the silica-nanocapsule suspensions to a final concentration of 60% (v/v) and then mixed through an overnight stirring at room temperature. After centrifugation, the concentration of fipronil in the supernatant was determined using RP-HPLC as described above. The encapsulation efficiency (%EE) was calculated as the ratio of the amount of fipronil in the silica nanocapsules to the initial amount of fipronil in the oil.
2.6. Fipronil release in vitro
The release of fipronil from the SNC and SNC-PEI were tested and compared. 100 μL nanocapsules containing 200 μg fipronil were each sealed in dialysis tubings having a cellulose's membrane size of 3.5 kDa (Merck Millipore, Bayswater, Australia), and then placed into a beaker containing10 mL of water while being shaken at room temperature for 19 days. At different time intervals, 200 μL aliquots of the solution in the beaker were taken, replaced with water, and then analyzed using RP-HPLC.
2.7. Termite field trial
Colonies of C. lacteus (epigeous nesting, mound building) occur near Beerburrum (26.9686° S, 152.9713° E), southeast Queensland, Australia, where the work was conducted. C. lacteus is very common in eastern Australia from Victoria to southern Queensland [46]. Mound-building termites such as C. lacteus are useful to verify the effects of bait toxicants on the termite colonies due to easy access to their mounds [47, 48]. The mounds were characterized by an outer earthen wall enclosing a dense woody mass molded reproductive, eggs and larvae, soldiers, and workers [48]. The mounds we used were about 0.5 m (height) × 1 m (diameter).
A control-α-cellulose bait comprised of α-cellulose and water at a weight ratio of 1:4 was presented in a plastic commercial drink bottle. The bottle was connected to a plastic conduit, 400 mm (length) × 18 mm (diameter), containing a corrugated cardboard wick (400 mm × 20 mm) and secured with a hose clamp (control assembly). A 25 mm-diameter borehole was made about 300 mm above ground level horizontally into a mound through the external crust into the carton material [48]. The conduit was inserted into the borehole to a depth of 200 mm. Fire-blanket material (500 mm in a square) and then a black plastic bag was wrapped around the bottle and conduit and secured with wire. A treated-α-cellulose bait comprised of α-cellulose and nanocapsule suspension at a weight ratio of 1:4 was similarly presented, connected to the mound and secured (treated assembly). The bottle varied in size according to the amount of fipronil required for delivery. A control- and a treated-assembly were both installed into each of nine mounds at different timelines (Table 1). Inspections were conducted fortnightly and each assembly was inspected visually for termite activity. Where the opaque bait or termite muddying inside the bottle prevented clear vision, a TermatracT3i device (Termatrac Pty Ltd., Australia) was used to detect termite movement in the conduit and inside the bottle. Estimates of bait removal were problematic and tended to be qualitative.
Table 1.
Details of treatment of 9 Coptotermes lacteus mounds used in the field study.
| Mound No. | Initiation date | Fipronil conc. (ppm) | Fipronil mass (g) | Bottle size (L) | Monitoring concluded | Duration (weeks) |
|---|---|---|---|---|---|---|
| 1 | 25/01/2016 | 1000* | 0.2 | 0.6 | 11/06/2016 | 19 |
| 2 | 16/05/2016 | 1000* | 0.2 | 0.6 | 30/08/2016 | 15 |
| 3 | 1/07/2016 | 1000* | 0.2 | 0.6 | 15/09/2016 | 11 |
| 4 | 21/02/2017 | 10 | 0.006 | 1.25 | 7/06/2017 | 15 |
| 5 | 21/02/2017 | 20 | 0.012 | 1.25 | 7/06/2017 | 15 |
| 6 | 21/02/2017 | 100 | 0.06 | 1.25 | 7/06/2017 | 15 |
| 7 | 22/07/2017 | 100 | 0.25 | 3.0 | 3/11/2017 | 14 |
| 8 | 22/07/2017 | 250 | 0.25 | 1.25 | 3/11/2017 | 14 |
| 9 | 22/07/2017 | 500 | 0.25 | 1.25 | 3/11/2017 | 14 |
Note: * Fipronil-loaded nanocapsules not coated with poly (ethyleneimine) (PEI).
To monitor colony health, two boreholes with 25 mm in diameter were made into each mound into the carton material and left empty. The boreholes were observed a week later and re-drilled if plugged to the outer edge of the crust by termites. Where a borehole was not plugged to the outer crust, a metal rod was inserted to determine if the borehole had been blocked at the inner edge of the crust: if so, it was re-drilled. Control bottles with apparently little α-cellulose bait remaining were replaced (without removing the conduit from the mound) from time-to-time, allowing for continuous monitoring of colony health. Mounds 1, 2 and 3 were destructively sampled with a mattock on 11th June, 2016, 30th August, 2016 and 15th September 2016, respectively. The mounds were progressively destroyed and visually inspected for live termites to just below ground level. Two boreholes were drilled into the remaining mounds 4–9 on 30th July 2018 and inspected on 3rd August 2018 to monitor colony health many months after treatment. Mound 4 was destructively sampled on 9th August 2018.
3. Results and discussion
3.1. Production of recombinant catalytic modular proteins
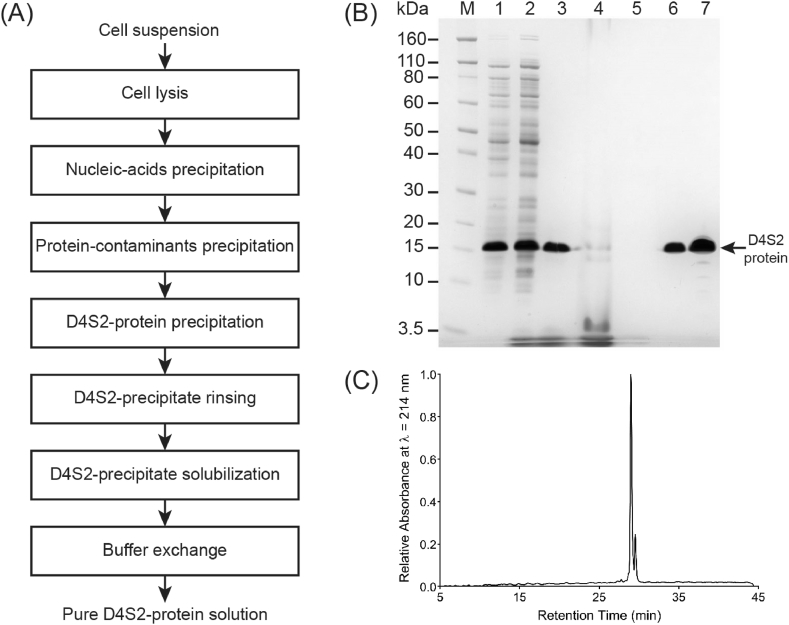
We previously used SurSi peptide (M 3.6 kDa) to synthesize silica nanocapsules for termite control in a laboratory setting [24]. However, peptides are too costly for large-scale production unless they are re-designed as recombinant proteins to enable production in microbial cell factories [49]. In this paper, we produced and used recombinant catalytic modular D4S2 protein (M 13.3 kDa) [44], in lieu of SurSi peptide, as the key ingredient to catalyze the formation of silica nanocapsules for termite field trials. We had designed D4S2 by genetically combining both the surface-active module DAMP4 protein [50] and the silicification module of the SurSi peptide [36]. In this way, D4S2 can be produced renewably and cost-effectively in industrially-relevant bacteria Escherichia coli (E. coli) [44, 45]. Furthermore, the modular design allows D4S2 to be isolated and purified from E. coli using a scalable, non-chromatographic approach, that is, selective thermochemical precipitation [45, 51, 52, 53] (Fig. 2A). This is because of the inclusion of four-helix bundled DAMP4 protein within D4S2 that enables D4S2 to retain its stability and solubility at 90 °C in the presence of 1 M sodium sulfate while most protein contaminants are precipitated [54]. Compared to chromatography-based methods that are widely used for purifying high-value biopharmaceutical products [49], chromatography-free methods offer cheaper, shorter cycle period and higher purification yield. We obtained up to 0.39 g of D4S2 protein with high purity in one purification cycle (Fig. 2A) after scaling up the purification procedure to almost 14 times more than that of our smaller-scale attempt [45]. Additionally, the resultant D4S2 yield was 4 times higher than that of the chromatography-based method [44]. The purified D4S2 was in a correct molecular weight and high purity as demonstrated qualitatively in the SDS-PAGE (Fig. 2B). RP-HPLC analyses confirmed the quality of the purified D4S2 (Fig. 2C). Such amount of D4S2 was sufficient to synthesize up to 250 mL suspension of silica nanocapsules encapsulating approximately 0.25 g fipronil for the treatment of one termite mound (Table 1).
Fig. 2.
Production of recombinant catalytic modular protein D4S2 based on selective thermochemical precipitation. (A) Process-flow diagram of the purification of D4S2 protein. (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the supernatant samples obtained after: (1) cell lysis; (2) nucleic-acids precipitation; (3) protein-contaminants precipitation; (4) D4S2-protein precipitation; (5) D4S2 protein-precipitate rinsing; (6) D4S2 protein-precipitate solubilization; and (7) buffer exchange. M: Marker. (C) The purity of final product D4S2 protein as characterized using a reversed-phase high-performance liquid chromatography (RP-HPLC).
3.2. Synthesis and characterization of silica nanocapsules
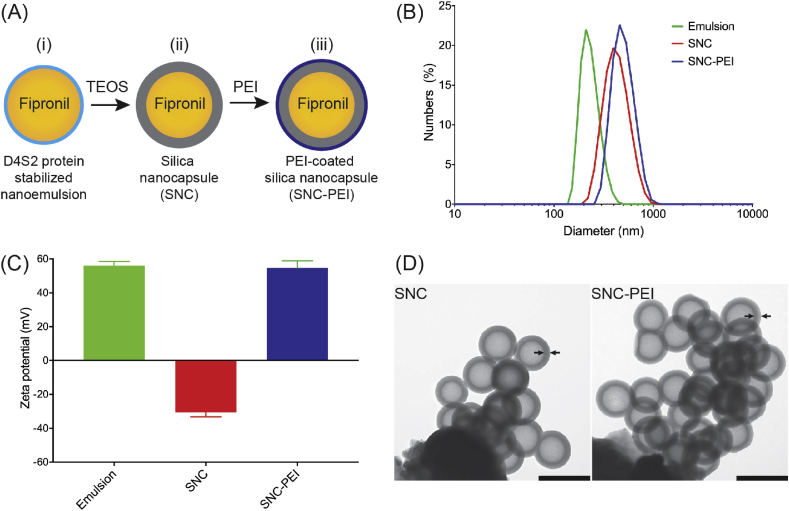
For termite field trials, we synthesized and used both uncoated silica nanocapsules (SNC) and poly(ethyleneimine) (PEI)-coated silica nanocapsules (SNC-PEI) which were pre-loaded with fipronil insecticide (Fig. 3A). The purified D4S2 protein aforementioned was used as the key ingredient for producing the nanoemulsion-templated silica nanocapsules as it has two important functionalities [44]: (1) the protein could facilitate the formation of oil-in-water (O/W) nanoemulsions and subsequently stabilize the nanoemulsions by its adsorption on the nanoemulsion surfaces; and (2) the protein attached on the nanoemulsion surfaces could then attract silica precursor available in bulk solutions and catalyze the formation of silica shells surrounding the nanoemulsions. The use of protein D4S2 enabled the silicification reaction to occur at neutral pH, room temperature and without using any toxic reagents, which is in contrast to the silica nanocapsules synthesized using chemical-based surfactants [27]. To synthesize these SNCs, oil-in-water (O/W) nanoemulsions were first generated and used as a template for silica nanocapsules. Fipronil was solubilized in Miglyol oil at its maximum solubility 10 g/L and then mixed with D4S2 protein solution by ultrasonication to form nanoemulsions containing 10% (v/v) oil phase, enabled by high surface activity of D4S2 [44]. Diameters of the resultant nanoemulsions were 220 ± 19 nm with a size dispersity (Ð) of 0.210 ± 0.05 (Fig. 3B) and positive zeta potential (56 ± 3 mV) (Fig. 3C) which was due to the positively-charged lysine and arginine residues within D4S2 that projected toward bulk solutions. These cationic and polar properties of nanoemulsion surfaces attracted the silica precursor TEOS available in the bulk solutions and catalyzed the formation of silica shell encapsulating the nanoemulsion templates [44]. Spherical oil-core silica-shell nanocapsules with a diameter of 368 ± 20 nm and shell thickness of 60 ± 3 nm were formed (Fig. 3D (i)). The nanocapsules had negative zeta potentials (−31 ± 3 mV) due to the deprotonation of interfacial silanol group (SiO−) at near-neutral pH. To coat silica nanocapsules, branched-chain polymer PEI, which is positively charged, was added to the nanocapsule suspension at near-neutral pH and allowed to self-assemble onto the nanocapsules through electrostatic interactions. The excess, unadsorbed PEI was removed by centrifugation of the nanocapsule suspension, removing the supernatant, and repeated washing with a HEPES buffer solution. At this stage, the zeta potential of the silica nanocapsules was reversed to positive (55 ± 4 mV) (Fig. 3C), demonstrating the successful adsorption of PEI which led to the formation of PEI-coated silica nanocapsules. Reversal of surface charges as indicated by zeta potentials aforementioned is characteristic of polyelectrolyte layer growth on colloidal templates which had also been observed by others [55]. Additional layer of PEI slightly increased the nanocapsule diameter (Fig. 3B) to 372 ± 13 nm with the total shell thickness of 65 ± 3 nm (Fig. 3D (ii)). Both SNC and SNC-PEI were stable in water as there were no significant changes in size and dispersity for over 20 days. This physical stability was likely contributed by high zeta potentials of the nanocapsules (Fig. 3C) which facilitated repulsive forces, hence, preventing aggregation.
Fig. 3.
Synthesis and characterization of silica nanocapsules using oil-in-water nanoemulsions as a template. (A) Fipronil-loaded nanoemulsions stabilized by D4S2 protein (i); addition of tetraethoxysilane (TEOS) formed oil-core silica-shell nanocapsules (SNC) (ii); and addition of poly(ethyleneimine) (PEI) produced PEI-coated silica nanocapsules (SNC-PEI) (iii). (B) Size distribution as measured by dynamic light scattering (DLS). (C) Changes of zeta potentials. (D) Morphology of SNC and SNC-PEI as visualized by transmission electron microscopy (TEM) (scale bars are 500 nm). The arrows are drawn as an example showing the boundary of the silica shells.
3.3. Sustained release of fipronil in water
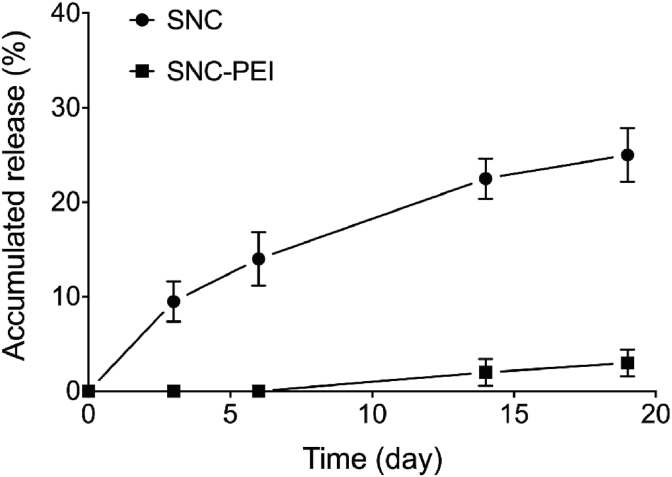
The dissolution of fipronil in the oil phase prior to the formation of nanoemulsion core and subsequent shell formation led to the high efficiency of fipronil encapsulation. Approximately 97% of initial fipronil in the oil phase can be encapsulated in either SNC or SNC-PEI. Further, the cumulative release of fipronil from both SNC and SNC-PEI in water was investigated and compared for a period of 19 days (Fig. 4). The release of fipronil from the silica nanocapsules can fit well to the Higuchi model [4]. Fipronil release from SNC was considerably slow with 10 ± 2% on day 3 and followed by a gradual release to 25 ± 3% on day 19. As we demonstrated previously, the presence of silica shells provided a diffusional barrier to fipronil release [4, 24, 36]. Compared to SNC, fipronil released from SNC-PEI was much slower with only 3 ± 1% on day 19. The additional layer of branched polymer like PEI on silica shells seems to further inhibit the diffusion of fipronil out from the silica nanocapsules. The role of PEI at nanoparticle interfaces to suppress the release of encapsulated actives has also been observed mainly in the area of drug delivery [56, 57]. The delayed release of the encapsulated fipronil was expected to provide ample time for the worker termites to forage the bait toxicants, carry them back into the mound, and then distribute them to the other termite castes (e.g., soldier, queen, etc) within the mound. If the worker termites were drop-dead directly after foraging the baits, the living termites could sense the presence of dead termites and, as a result, they would seal off or avoid the treated area and protect themselves [58]. Therefore, it was hypothesized that all termites within the mound could have time to receive the baits and thus continuously being exposed to fipronil at gradually increased concentrations until colony elimination could be achieved.
Fig. 4.
Release profile of fipronil from (A) uncoated silica nanocapsules (●SNC) and (B) poly(ethyleneimine)-coated silica nanocapsules (■SNC-PEI) in to water.
3.4. Silica nanocapsules as bait toxicants against termite colonies in fields
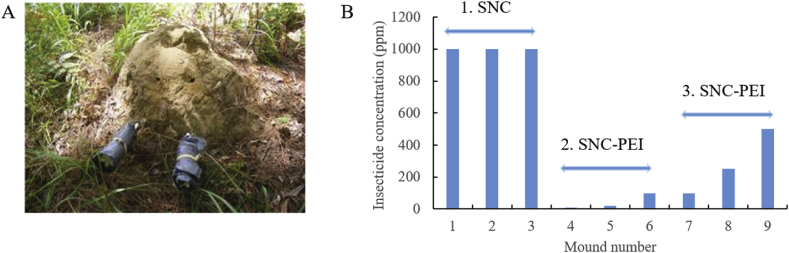
As a proof of concept, efficacies of the fipronil-loaded silica nanocapsules were investigated against termite colonies in fields. To achieve this, either SNC or SNC-PEI containing fipronil were formulated with α-cellulose and then used as bait toxicants for feeding the termite colonies (Table 1). In an individual termite mound, the bait toxicant along with a control bait (α-cellulose and water only) were connected using separate containers to the termite mound via a plastic conduit (Fig. 5A). The use of bait systems takes advantage of the termite eusociality [59] where worker termites, due to their anatomical and behavioral specializations, forage to feed the other termite castes within the colony that do not and cannot forage on their own. The baiting technique is also beneficial as the pesticides are contained in food matrices which are confined within an impervious bait station and then administered in localized sites [60, 61], and any remaining baiting materials can be removed and properly disposed of after the control actions have been completed [62]. In this preliminary field trials, our aims were to: 1) determine whether the bait toxicant could be relocated into a colony of C. lacteus; and 2) eliminate that colony. Different fipronil concentrations of 1,000, 500, 250, 100, 20 and 10 ppm were exposed to 9 mound colonies of C. lacteus (Froggatt) in three trials (Fig. 5B).
Fig. 5.
(A) Representative of the arrangement of untreated and treated α-cellulose baits connected to a termite mound of Coptotermes lacteus in fields near Beerburrum, southeast Queensland, Australia. (B) Three trials subjecting the baits treated with either silica nanocapsules (SNC) or PEI-coated silica nanocapsules (SNC-PEI) containing a varied amount of fipronil insecticide to the nine mounds of termite colonies of C. lacteus in the fields.
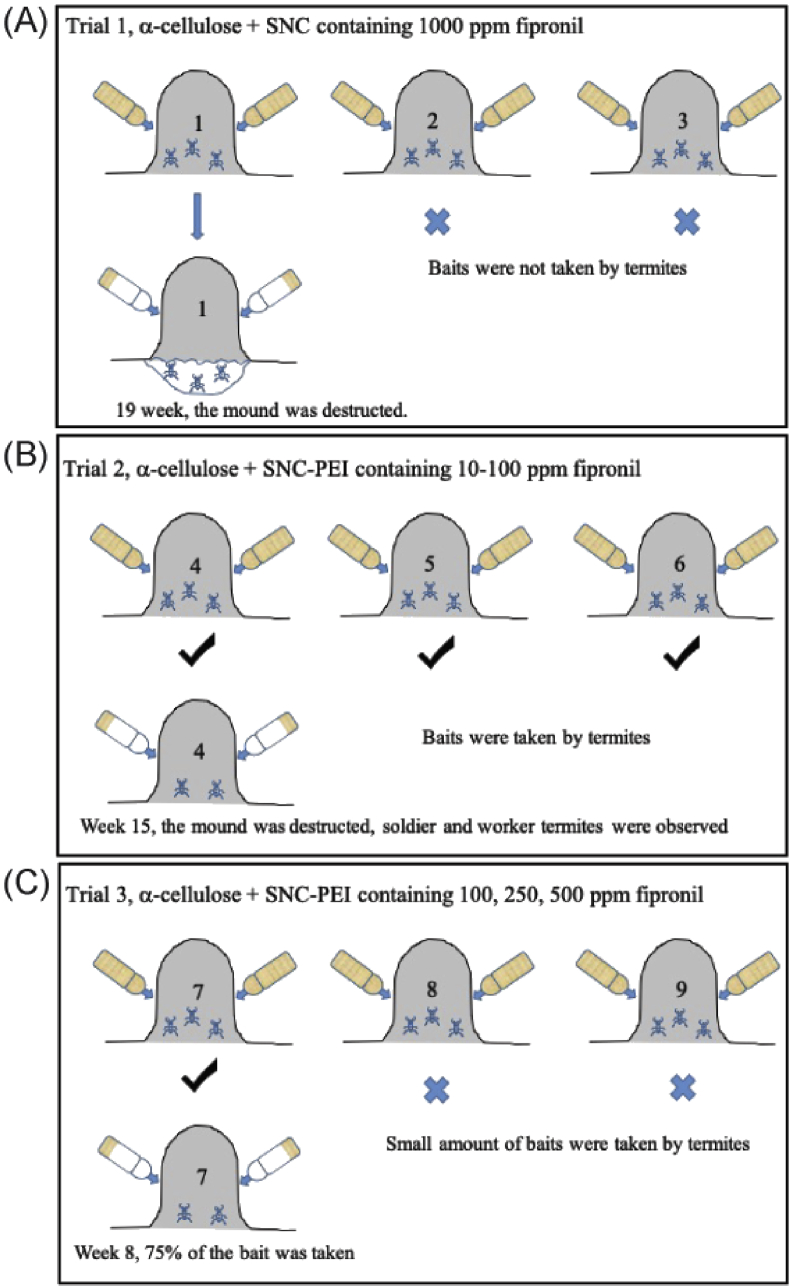
Based on the initial assumption of 1 million termites in one mound, 200 mg of fipronil would be sufficient to treat a mound with a dose of 0.1 μg/termite. Mounds 1–3 were treated with bait toxicants containing SNC loaded with 200 mg of fipronil (≈1000 ppm) (Table 1, Fig. 6A). In contrast to the control baits in all the termite mounds, only treated baits in Mound 1 were relocated by termites into the mound. Two boreholes were made in the mound to monitor the colony health which has been normally applied in Australian mound-building wood-feeding termites [48, 63, 64, 65]. The boreholes into Mound 1 were blocked weekly by the termites until week 18. On week 19, there was no repair on the boreholes in Mound 1, and small black ants (tufted tyrant ants Iridomyrmex sp.) were present in the boreholes. No repair to the mechanical damage on a termite mound usually indicates a decline of termite colony in the mound [48, 63, 64, 65]. Therefore, Mound 1 was then destructively sampled. A putrid stench emanated from the mound with the ants were present throughout the exposed parts of the mound indicating a large number of termites were eliminated, and no live termites were observed in the original mound above the ground level. Initially, we thought that we had successfully eradicated the colony. However, after digging the underground level of Mound 1 on the several weeks following week 19, thousands of healthy-looking termites were found with no ants in this zone. We suspected that live termites sensed the danger when they found accumulated dead termites in the mound, so they took an emergency move and relocated to the underground level. This initial trial taught us two lessons: (1) the fipronil release was not slow enough, so before the bait was distributed to the whole colony, or more accurately to the termite queen, they noticed the danger and left their mound to survive; (2) termites are very intelligent with a highly organized and coordinated society.
Fig. 6.
Summary of the outcome of the termite field trials conducted in this study. (A) Trial 1, α-cellulose + SNC containing 1000 ppm fipronil. (B) Trial 2, α-cellulose + SNC-PEI containing 10–100 ppm fipronil. (C) Trial 3, α-cellulose + SNC-PEI containing 100, 250, 500 ppm fipronil.
Based on the lessons from the first trial, we decided to decrease the fipronil concentration and further slow its release in Trial 2, allowing sufficient time for the worker termites to relocate and distribute the bait toxicants to the whole colony. In this case, we reduced the fipronil concentration from 200 mg (1000 ppm) to 6–60 mg (≈10–100 ppm), and coated the SNC with PEI. Mounds 4–6 were treated with bait toxicants containing SNC-PEI with varying amounts of fipronil at 6, 12, and 60 mg (≈10, 20, 100 ppm), respectively (Table 1, Fig. 6B). In contrast to Trial 1, bait toxicants containing SNC-PEI were relocated into all three mounds in Trial 2, indicating less resistance. However, termite activities were observed in all baits, and boreholes continued to be blocked to the exterior edge of the mound crust until work on these mounds was concluded (week 15) when most of the baits were consumed. Two strikes with the mattock on 9th August 2018 revealed live C. lacteus soldier and worker termites in Mound 4. Termites in Mounds 4–6 were not eliminated by the treatment. This concentration range was too low to exhibit any toxic effect on the termite colonies especially with the very slow fipronil release from SNC-PEI.
In Trial 3, the fipronil concentration was increased to 250 mg but with different amounts of α-cellulose to achieve final fipronil concentrations of 100, 250 and 500 ppm in the bait. Mounds 7–9 were treated with bait toxicants containing SNC-PEI with 100, 250 and 500 ppm fipronil, respectively (Table 1, Fig. 6C). About 75% of the bait toxicants appeared to have been removed into Mound 7 on week 8, whereas only a small amount of baits was relocated into Mounds 8 and 9. We found three dead alates on the inside moist wall of the treated bait container in Mound 8 during week 10. About 100 dead worker termites were seen in the control bait container in Mound 9 on week 12. However, termite wings were also found around Mound 9 which indicated recent swarming demonstrating that the termites behaved normally in the mound despite its exposure to bait toxicants. Overall, termite colonies in Mounds 7–9 were not eliminated by the treatment.
For many years there has been much research conducted seeking biological control of subterranean termites. Whilst the causal organisms tested have been many and varied, certain strains of the entomopathogenic fungus Metarhizium anisopliae (Metsch.) Sorokin have been of particular interest. Experimental work in the laboratory seemed promising, but field studies invariably failed to eliminate the termite colony. These data were reviewed by Chouvenc and Su [66], particularly with regard to Coptotermes spp. They showed that due to multilevel disease resistance mechanisms, the incidence of an epizootic within a group of termites is unlikely: the three major mechanisms were grooming, cellular encapsulation, and gut antifungal activity. Similarly, in extensive laboratory work, Su [67] and Chouvenc [58] showed that Coptotermes formosanus Shiraki and Coptotermes gestroi (Wasmann), respectively, avoided or sealed off areas treated with fipronil. Our fieldwork had similar results in that whilst the bait toxicants were relocated into some of the mounds, colony elimination did not occur. We suspected that the failures to eliminate termite colonies in fields were attributable to the different amounts of bait toxicants consumed by individual termites when the baits were distributed within the colonies, and those who consumed a large quantity of the baits might be drop-dead and thus encouraging other living termites in the mound to seal off. This would be consequently negating the sustained release effects of the silica nanocapsules. As a result of this work, we have shelved further endeavors to use our bait toxicant delivery systems for termite control. We caution others interested in producing a novel termite bait toxicant to be aware of the multilevel resistance mechanisms of the Coptotermes spp. “superorganism” [68].
4. Conclusions
In this study, we have demonstrated the production of recombinant catalytic modular proteins D4S2 for the synthesis of silica nanocapsules at scales sufficient for conducting termite field trials. Silica nanocapsules can be preloaded with fipronil prior to the formation of a silica shell at environmentally friendly reaction conditions, thus achieving the high-loading capacity of almost 97%. In addition, we showed that silica nanocapsules can be coated with poly(ethyleneimine) (PEI) through electrostatic interactions to prolong the sustained release of encapsulated fipronil. Preliminary studies of both silica nanocapsules and PEI-coated silica nanocapsules formulated with α-cellulose as bait toxicants against nine termite colonies in fields were conducted to test their efficacies. Generally, the untreated α-cellulose bait (as control) was more readily relocated into the colony than was the treated α-cellulose bait. Nevertheless, most or all, of the treated bait was relocated from bottles in the Mounds 1, and 4–6, and 75% of the treated bait was relocated in Mound 7. Varying amounts of the baits were removed from the bait containers in the other mounds. Despite achieving relocation of the baits into a mound, we were unable to eliminate a colony. It is possible that termite colonies avoided or sealed off areas treated with the bait toxicants. Further understanding of the behavior of termites and the resistance mechanism will be important for future trial experiment design.
Declarations
Author contribution statement
(*Author deceased) Brenton C. Peters*: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
David Wibowo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Guang-Ze Yang & Yue Hui: Performed the experiments.
Anton P.J. Middelberg: Conceived and designed the experiments; Wrote the paper.
Chun-Xia Zhao: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Australian Research Council (ARC) under Future Fellowship Project (FT140100726) and Discovery Project (DP150100798).
Competing interest statement
The University of Queensland (UQ) filed patent on the use of mineralizing proteins for making silica nanocapsules. Anton P. J. Middelberg, Chun-Xia Zhao, David Wibowo and Brenton C. Peters are named inventors on this patent and through their employment with UQ hold an indirect interest in this intellectual property. Yue Hui and Guang-Ze Yang declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the facilities, and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland. The authors also thank Elliott Peters for providing excellent technical support in the field, and the former termite researcher Dr Michael Lenz for the support over many years, and specifically, for his critical comments on an earlier draft of the manuscript. Termatrac® Pty Ltd (Beenleigh, Australia) and Accensi Pty Ltd (Narangba, Australia) are thanked for providing Termatrac® T3i device and fipronil, respectively.
Contributor Information
Anton P.J. Middelberg, Email: anton.middelberg@adelaide.edu.au.
Chun-Xia Zhao, Email: z.chunxia@uq.edu.au.
References
- 1.Kah M., Kookana R.S., Gogos A., Bucheli T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018;13(8):677–684. doi: 10.1038/s41565-018-0131-1. [DOI] [PubMed] [Google Scholar]
- 2.Yin J., Wang Y., Gilbertson L.M. Opportunities to advance sustainable design of nano-enabled agriculture identified through a literature review. Environ. Sci. Nano. 2018;5(1):11–26. [Google Scholar]
- 3.Kah M., Hofmann T. Nanopesticide research: current trends and future priorities. Environ. Int. 2014;63:224–235. doi: 10.1016/j.envint.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Yang G.-Z., Wibowo D., Yun J.-H., Wang L., Middelberg A.P.J., Zhao C.-X. Biomimetic silica nanocapsules for tunable sustained release and cargo protection. Langmuir. 2017;33(23):5777–5785. doi: 10.1021/acs.langmuir.7b00590. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y., Guo M., Fan C., Dong H., Ding G., Zhang W., Tang G., Yang J., Kong D., Cao Y. Development of novel urease-responsive pendimethalin microcapsules using silica-IPTS-PEI as controlled release carrier materials. ACS Sustain. Chem. Eng. 2017;5(6):4802–4810. [Google Scholar]
- 6.Chevillard A., Angellier-Coussy H., Guillard V., Bertrand C., Gontard N., Gastaldi E. Biodegradable herbicide delivery systems with slow diffusion in soil and UV protection properties. Pest Manag. Sci. 2014;70(11):1697–1705. doi: 10.1002/ps.3705. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S., Nehra M., Dilbaghi N., Marrazza G., Hassan A.A., Kim K.-H. Nano-based smart pesticide formulations: emerging opportunities for agriculture. J. Control. Release. 2019;294:131–153. doi: 10.1016/j.jconrel.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Nuruzzaman M., Rahman M.M., Liu Y., Naidu R. Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J. Agric. Food Chem. 2016;64(7):1447–1483. doi: 10.1021/acs.jafc.5b05214. [DOI] [PubMed] [Google Scholar]
- 9.Luiz de Oliveira J., Ramos Campos E.V., Fraceto L.F. Recent developments and challenges for nanoscale formulation of botanical pesticides for use in sustainable agriculture. J. Agric. Food Chem. 2018;66(34):8898–8913. doi: 10.1021/acs.jafc.8b03183. [DOI] [PubMed] [Google Scholar]
- 10.Lin L., Cui H., Zhou H., Zhang X., Bortolini C., Chen M., Liu L., Dong M. Nanoliposomes containing Eucalyptus citriodora as antibiotic with specific antimicrobial activity. Chem. Commun. 2015;51(13):2653–2655. doi: 10.1039/c4cc09386k. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen M.-H., Nguyen T.-H.-N., Hwang I.-C., Bui C.-B., Park H.-J. Effects of the physical state of nanocarriers on their penetration into the root and upward transportation to the stem of soybean plants using confocal laser scanning microscopy. Crop Protect. 2016;87:25–30. [Google Scholar]
- 12.Nguyen H.M., Hwang I.C., Park J.W., Park H.J. Enhanced payload and photo-protection for pesticides using nanostructured lipid carriers with corn oil as liquid lipid. J. Microencapsul. 2012;29(6):596–604. doi: 10.3109/02652048.2012.668960. [DOI] [PubMed] [Google Scholar]
- 13.Nakasato D.Y., Pereira A.E.S., Oliveira J.L., Oliveira H.C., Fraceto L.F. Evaluation of the effects of polymeric chitosan/tripolyphosphate and solid lipid nanoparticles on germination of Zea mays, Brassica rapa and Pisum sativum. Ecotoxicol. Environ. Saf. 2017;142:369–374. doi: 10.1016/j.ecoenv.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira J.L., Campos E.V.R., Gonçalves da Silva C.M., Pasquoto T., Lima R., Fraceto L.F. Solid lipid nanoparticles Co-loaded with simazine and atrazine: preparation, characterization, and evaluation of herbicidal activity. J. Agric. Food Chem. 2015;63(2):422–432. doi: 10.1021/jf5059045. [DOI] [PubMed] [Google Scholar]
- 15.Xu X., Bai B., Wang H., Suo Y. A near-infrared and temperature-responsive pesticide release platform through core–shell polydopamine@PNIPAm nanocomposites. ACS Appl. Mater. Interfaces. 2017;9(7):6424–6432. doi: 10.1021/acsami.6b15393. [DOI] [PubMed] [Google Scholar]
- 16.Grillo R., Pereira A.E.S., Nishisaka C.S., de Lima R., Oehlke K., Greiner R., Fraceto L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: an environmentally safer alternative for weed control. J. Hazard Mater. 2014;278:163–171. doi: 10.1016/j.jhazmat.2014.05.079. [DOI] [PubMed] [Google Scholar]
- 17.Karimi A.R., Tarighatjoo M., Nikravesh G. 1,3,5-Triazine-2,4,6-Tribenzaldehyde derivative as a new crosslinking agent for synthesis of pH-thermo dual responsive chitosan hydrogels and their nanocomposites: swelling properties and drug release behavior. Int. J. Biol. Macromol. 2017;105:1088–1095. doi: 10.1016/j.ijbiomac.2017.07.128. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar D.J., Singh A. Base triggered release of insecticide from bentonite reinforced citric acid crosslinked carboxymethyl cellulose hydrogel composites. Carbohydr. Polym. 2017;156:303–311. doi: 10.1016/j.carbpol.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J., Tong X., Morris D., Zhao Y. Toward photocontrolled release using light-dissociable block copolymer micelles. Macromolecules. 2006;39(13):4633–4640. [Google Scholar]
- 20.Ye Z., Guo J., Wu D., Tan M., Xiong X., Yin Y., He G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015;132:520–528. doi: 10.1016/j.carbpol.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 21.Ao M., Zhu Y., He S., Li D., Li P., Li J., Cao Y. Preparation and characterization of 1-naphthylacetic acid–silica conjugated nanospheres for enhancement of controlled-release performance. Nanotechnology. 2012;24(3) doi: 10.1088/0957-4484/24/3/035601. 035601. [DOI] [PubMed] [Google Scholar]
- 22.Popat A., Liu J., Hu Q., Kennedy M., Peters B., Lu G.Q., Qiao S.Z. Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale. 2012;4(3):970–975. doi: 10.1039/c2nr11691j. [DOI] [PubMed] [Google Scholar]
- 23.Sarlak N., Taherifar A., Salehi F. Synthesis of nanopesticides by encapsulating pesticide nanoparticles using functionalized carbon nanotubes and application of new nanocomposite for plant disease treatment. J. Agric. Food Chem. 2014;62(21):4833–4838. doi: 10.1021/jf404720d. [DOI] [PubMed] [Google Scholar]
- 24.Wibowo D., Zhao C.-X., Peters B.C., Middelberg A.P.J. Sustained release of fipronil insecticide in vitro and in vivo from biocompatible silica nanocapsules. J. Agric. Food Chem. 2014;62(52):12504–12511. doi: 10.1021/jf504455x. [DOI] [PubMed] [Google Scholar]
- 25.Cao L., Zhang H., Cao C., Zhang J., Li F., Huang Q. Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. Nanomaterials. 2016;6(7):126. doi: 10.3390/nano6070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattos B.D., Rojas O.J., Magalhães W.L.E. Biogenic silica nanoparticles loaded with neem bark extract as green, slow-release biocide. J. Clean. Prod. 2017;142:4206–4213. [Google Scholar]
- 27.Wibowo D., Hui Y., Middelberg A.P.J., Zhao C.-X. Interfacial engineering for silica nanocapsules. Adv. Colloid Interface Sci. 2016;236:83–100. doi: 10.1016/j.cis.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Hui Y., Wibowo D., Liu Y., Ran R., Wang H.-F., Seth A., Middelberg A.P.J., Zhao C.-X. Understanding the effects of nanocapsular mechanical property on passive and active tumor targeting. ACS Nano. 2018;12(3):2846–2857. doi: 10.1021/acsnano.8b00242. [DOI] [PubMed] [Google Scholar]
- 29.Shan Y., Cao L., Xu C., Zhao P., Cao C., Li F., Xu B., Huang Q. Sulfonate-functionalized mesoporous silica nanoparticles as carriers for controlled herbicide diquat dibromide release through electrostatic interaction. Int. J. Mol. Sci. 2019;20(6):1330. doi: 10.3390/ijms20061330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin X., Xiang X., Sun X., Ni H., Li L. Preparation of nanoscale Bacillus thuringiensis chitinases using silica nanoparticles for nematicide delivery. Int. J. Biol. Macromol. 2016;82:13–21. doi: 10.1016/j.ijbiomac.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Kaziem A.E., Gao Y., He S., Li J. Synthesis and insecticidal activity of enzyme-triggered functionalized hollow mesoporous silica for controlled release. J. Agric. Food Chem. 2017;65(36):7854–7864. doi: 10.1021/acs.jafc.7b02560. [DOI] [PubMed] [Google Scholar]
- 32.Fan C., Guo M., Liang Y., Dong H., Ding G., Zhang W., Tang G., Yang J., Kong D., Cao Y. Pectin-conjugated silica microcapsules as dual-responsive carriers for increasing the stability and antimicrobial efficacy of kasugamycin. Carbohydr. Polym. 2017;172:322–331. doi: 10.1016/j.carbpol.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 33.Cao L., Zhou Z., Niu S., Cao C., Li X., Shan Y., Huang Q. Positive-charge functionalized mesoporous silica nanoparticles as nanocarriers for controlled 2,4-dichlorophenoxy acetic acid sodium salt release. J. Agric. Food Chem. 2018;66(26):6594–6603. doi: 10.1021/acs.jafc.7b01957. [DOI] [PubMed] [Google Scholar]
- 34.Huayao C., Yueshun L., Hongjun Z., Xinhua Z., Sheng G., Hua X. Highly efficient alginate sodium encapsulated chlorpyrifos/copper(II) schiff base mesoporous silica sustained release system with pH and ion response for pesticide delivery. RSC Adv. 2016;6(115):114714–114721. [Google Scholar]
- 35.Chen K., Yu G., He F., Zhou Q., Xiao D., Li J., Feng Y. A pH-responsive emulsion stabilized by alginate-grafted anisotropic silica and its application in the controlled release of λ-cyhalothrin. Carbohydr. Polym. 2017;176:203–213. doi: 10.1016/j.carbpol.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 36.Wibowo D., Zhao C.-X., Middelberg A.P.J. Emulsion-templated silica nanocapsules formed using bio-inspired silicification. Chem. Commun. 2014;50(77):11325–11328. doi: 10.1039/c4cc04904g. [DOI] [PubMed] [Google Scholar]
- 37.Pearce M.J. CAB International; London, U.K.: 1997. Termites: Biology and Pest Management. [Google Scholar]
- 38.Verma M., Sharma S., Prasad R. Biological alternatives for termite control: a review. Int. Biodeterior. Biodegrad. 2009;63(8):959–972. [Google Scholar]
- 39.Rust M.K., Su N.-Y. Managing social insects of urban importance. Annu. Rev. Entomol. 2012;57:355–375. doi: 10.1146/annurev-ento-120710-100634. [DOI] [PubMed] [Google Scholar]
- 40.Gautam B.K., Henderson G. Escape behavior of the formosan subterranean termite (Isoptera: Rhinotermitidae) in response to disturbance. J. Insect Behav. 2012;25(1):70–79. [Google Scholar]
- 41.Forschler B.T., Ryder J.C. Subterranean termite, reticulitermes spp (Isoptera: Rhinotermitidae), colony response to baiting with hexaflumuron using a prototype commercial termite baiting system. J. Entomol. Sci. 1996;31(2):143–151. [Google Scholar]
- 42.Jones S.C. Effects of population density on tunneling by formosan subterranean termite (Isoptera: Rhinotermitidae) through treated soil. J. Econ. Entomol. 1990;83(3):875–878. [Google Scholar]
- 43.Ibrahim S.A., Henderson G., Fei H. Toxicity, repellency, and horizontal transmission of fipronil in the formosan subterranean termite (Isoptera: Rhinotermitidae) J. Econ. Entomol. 2003;96(2):461–467. doi: 10.1093/jee/96.2.461. [DOI] [PubMed] [Google Scholar]
- 44.Wibowo D., Zhao C.-X., Middelberg A.P.J. Interfacial biomimetic synthesis of silica nanocapsules using a recombinant catalytic modular protein. Langmuir. 2015;31(6):1999–2007. doi: 10.1021/la504684g. [DOI] [PubMed] [Google Scholar]
- 45.Wibowo D., Yang G.-Z., Middelberg A.P.J., Zhao C.-X. Non-chromatographic bioprocess engineering of a recombinant mineralizing protein for the synthesis of silica nanocapsules. Biotechnol. Bioeng. 2017;114(2):335–343. doi: 10.1002/bit.26079. [DOI] [PubMed] [Google Scholar]
- 46.Gay F.J., Calaby J.H. Termites of the Australian region. In: Weesner F.M., editor. Vol. 11. Academic Press; NY, U.S.: 1970. pp. 393–448. (Biology of Termites Krishna, K.). [Google Scholar]
- 47.Peters B.C., Fitzgerald C.J. Field evaluation of the effectiveness of three timber species as bait stakes and the bait toxicant hexaflumuron in eradicating Coptotermes acinaciformis (Froggatt) (Isoptera: Rhinotermitidae) Sociobiology. 1999;33:227–238. [Google Scholar]
- 48.Evans T.A. Rapid elimination of field colonies of subterranean termites (Isoptera: Rhinotermitidae) using bistrifluron solid bait pellets. J. Econ. Entomol. 2010;103(2):423–432. doi: 10.1603/ec09067. [DOI] [PubMed] [Google Scholar]
- 49.Wibowo D., Zhao C.-X. Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Appl. Microbiol. Biotechnol. 2019;103(2):659–671. doi: 10.1007/s00253-018-9524-1. [DOI] [PubMed] [Google Scholar]
- 50.Middelberg A.P.J., Dimitrijev-Dwyer M. A designed biosurfactant protein for switchable foam control. ChemPhysChem. 2011;12(8):1426–1429. doi: 10.1002/cphc.201100082. [DOI] [PubMed] [Google Scholar]
- 51.Zhao C.-X., Dwyer M.D., Yu A.L., Wu Y., Fang S., Middelberg A.P.J. A simple and low-cost platform technology for producing pexiganan antimicrobial peptide in E. coli. Biotechnol. Bioeng. 2015;112(5):957–964. doi: 10.1002/bit.25505. [DOI] [PubMed] [Google Scholar]
- 52.Sun B., Wibowo D., Middelberg A.P.J., Zhao C.-X. Cost-effective downstream processing of recombinantly produced pexiganan peptide and its antimicrobial activity. Amb. Express. 2018;8(1):6. doi: 10.1186/s13568-018-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun B., Wibowo D., Sainsbury F., Zhao C.-X. Design and production of a novel antimicrobial fusion protein in Escherichia coli. Appl. Microbiol. Biotechnol. 2018;102(20):8763–8772. doi: 10.1007/s00253-018-9319-4. [DOI] [PubMed] [Google Scholar]
- 54.Dimitrijev Dwyer M., Brech M., Yu L., Middelberg A.P.J. Intensified expression and purification of a recombinant biosurfactant protein. Chem. Eng. Sci. 2014;105:12–21. [Google Scholar]
- 55.Richardson J.J., Cui J., Björnmalm M., Braunger J.A., Ejima H., Caruso F. Innovation in layer-by-layer assembly. Chem. Rev. 2016;116(23):14828–14867. doi: 10.1021/acs.chemrev.6b00627. [DOI] [PubMed] [Google Scholar]
- 56.Wu P.-C., Wang W.-S., Huang Y.-T., Sheu H.-S., Lo Y.-W., Tsai T.-L., Shieh D.-B., Yeh C.-S. Porous iron oxide based nanorods developed as delivery nanocapsules. Chem. Eur. J. 2007;13(14):3878–3885. doi: 10.1002/chem.200601372. [DOI] [PubMed] [Google Scholar]
- 57.Shi J., Zhang H., Wang L., Li L., Wang H., Wang Z., Li Z., Chen C., Hou L., Zhang C., Zhang Z. PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials. 2013;34(1):251–261. doi: 10.1016/j.biomaterials.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 58.Chouvenc T. Comparative impact of chitin synthesis inhibitor baits and non-repellent liquid termiticides on subterranean termite colonies over foraging distances: colony elimination versus localized termite exclusion. J. Econ. Entomol. 2018;111(5):2317–2328. doi: 10.1093/jee/toy210. [DOI] [PubMed] [Google Scholar]
- 59.Buczkowski G., Scherer C.W., Bennett G.W. Toxicity and horizontal transfer of chlorantraniliprole in the eastern subterranean termite. J. Econ. Entomol. 2012;105(5):1736–1745. doi: 10.1603/ec12038. [DOI] [PubMed] [Google Scholar]
- 60.Su N.Y., Scheffrahn R.H. A review of subterranean termite control practices and prospects for integrated pest management programs. IPM Rev. 1998;3:1–13. [Google Scholar]
- 61.Su N.Y. Baits as a tool for population control of the formosan subterranean termite. Sociobiology. 2003;41(1A):177–192. [Google Scholar]
- 62.Schoknecht U., Rudolph D., Hertel H. Termite control with microencapsulated permethrin. Pestic. Sci. 1994;40(1):49–55. [Google Scholar]
- 63.Mcclintock C., Webb G.A. Elimination of the mound-building termite, Nasutitermes exitiosus (Isoptera: Termitidae) in south-eastern Australia using bistrifluron bait. J. Econ. Entomol. 2015;108(6):2702–2710. doi: 10.1093/jee/tov232. [DOI] [PubMed] [Google Scholar]
- 64.Webb G. Elimination of Coptotermes lacteus (Froggatt) (Blattodea: Rhinotemitidae) colonies using bistrifluron bait applied through in-ground bait stations surrounding mounds. Insects. 2017;8(3):98. doi: 10.3390/insects8030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webb G.A. Efficacy of bistrifluron termite bait on Coptotermes lacteus (Isoptera: Rhinotermitidae) in southern Australia. J. Econ. Entomol. 2017;110(4):1705–1712. doi: 10.1093/jee/tox133. [DOI] [PubMed] [Google Scholar]
- 66.Chouvenc T., Su N.-Y. Apparent synergy among defense mechanisms in subterranean termites (Rhinotermitidae) against epizootic events: limits and potential for biological control. J. Econ. Entomol. 2010;103(4):1327–1337. doi: 10.1603/ec09407. [DOI] [PubMed] [Google Scholar]
- 67.Su N.-Y. Response of the formosan subterranean termites (Isoptera: Rhinotermitidae) to baits or nonrepellent termiticides in extended foraging arenas. J. Econ. Entomol. 2005;98(6):2143–2152. doi: 10.1603/0022-0493-98.6.2143. [DOI] [PubMed] [Google Scholar]
- 68.Chouvenc T., Su N.-Y. When subterranean termites challenge the rules of fungal epizootics. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0034484. e34484. [DOI] [PMC free article] [PubMed] [Google Scholar]