Abstract
This paper outlines corrosion thresholds for different environmental conditions of metallic materials commonly used in the tower, foundation, and nacelle/gearbox of an offshore wind turbine. These threshold values were derived from laboratory corrosion testing employing electrochemical analysis techniques, using the media/solvents that are representative to the operating environment of those wind turbine parts, such as seawater, grease, oils/lubricants, or their combination, at room temperature and at 328K. These values can provide an indication when general/local corrosion or protective film/surface damages have occurred. They can thus be utilised for detecting and monitoring corrosion at certain locations in the wind turbine structure. The presented data have been verified and validated to ensure their repeatability and reliability by means of numerous laboratory tests in accordance to the relevant engineering test standards and an extensive literature/published data review.
Keywords: Offshore, Wind turbines, Detection, Monitoring, Corrosion sensor, Electrochemical analysis, OCP, ZRA, EIS and PPC
Specifications table
| Subject area | Chemistry |
| More specific subject area | Corrosion of Metals |
| Type of data | Tables |
| How data was acquired | Electrochemical analysis methods: Open Circuit Potential (OCP), Zero Resistance Ammeter (ZRA), Electrochemical Impedance Spectroscopy (EIS) and PotentiodynamicPolarisation Curve (PPC) Facilities: Potentio/galvanostat, model GillAC, made by ACM Instruments Software: Gill AC serial no 600 |
| Data format | Raw Data and Analysed |
| Experimental factors | In accordance to the recommended and relevant international test standards [1], [2], [3], [4], [5] |
| Experimental features |
Test samples: Metallic materials include low carbon structural steel S235 and S355, stainless steels SS316L and SS430, Aluminium alloys AA1010, AA3103, AA5052 and AA6061. Corrosion testing: OCP utilised a two electrodes cell. ZRA, EIS and PPC utilised a three electrodes cell. The test samples were corroded artificially by PPC. OCP, ZRA and EIS were conducted on non-corroded and corroded test samples at room temperature (RT) and at 328K. Environment/Solutions/Media:
|
| Data source location | School of Mechanical and Design Engineering (SMDE), University of Portsmouth, Hampshire, United Kingdom |
| Data accessibility | The data is with this article |
| Related research article | J.I. Ahuir-Torres, N.Bausch, A. Farrar, S. Webb, S. Simandjuntak, a. Nash, B. Thomas, J. Muna, C.Jonsson, D. Mathew, Benchmarking parameters for remote electrochemical corrosion detection and monitoring of offshore wind turbine structures, Wind Energy, 22–6 (2019), 857–876. |
Value of the data
|
1. Data
The investigated metallic materials commonly used in the foundation, tower and nacelle/gearbox of an offshore WT with their typical environments are listed in Table 1.
Table 1.
Investigatedmetallic materials commonly used in the offshore WT tower, foundation and nacelle/gearbox and their typical operating conditions/environment.
| WT Parts | Environment | Metallic Materials/Alloys |
|---|---|---|
| Foundation, Tower | Seawater | Stainless steel (SS) 316L Structural steel (S) 355 Aluminium Alloys (AA) 3103, AA5052 |
| Nacelle/Gearbox | Semi solid lubricants (Grease) with added corrosion inhibitor | SS430 S235 AA1010 AA6061 |
| Oil/lubricant (e.g. Poly-Alpha-Olefin) | ||
| Mixed environment (Seawater/Grease/Oil) |
The Open Circuit Potential (OCP), Zero Resistance Ammeter (ZRA), Electrochemical Impedance Spectroscopy (EIS) and PotentiodynamicPolarisation Curve (PPC) are the electrochemical analysis techniques utilised in conjunction with the conducted corrosion testing. Table 2 highlights the characteristics of each of these techniques and the relationships between their relevant corrosion parameters and outputs. The nomenclatures of these parameters are outlined in Table 3.
Table 2.
Electrochemical analysis techniques.
| Techniques | Characteristics | Equations | Outputs |
|---|---|---|---|
|
|
Potential (E), Units: Voltage (V) | |
|
|
Current density (I), Units: Amps/square centimetres (A/cm2) | |
|
|
|
Impedance (Z), Units: Ohm per square centimetres (Ω/cm2) |
|
|
|
Potential (E), Unit: Voltage (V) Current density (I), Unit: Amps/square centimetres (A/cm2) |
Table 3.
Nomenclatures.
| Symbol | Significance |
|---|---|
| V/V | Volume/Volume |
| Wt/Wt | Weight/Weight |
| Ecel | Cell potential |
| Eo | Reference potential |
| K | Gas constant |
| T | Temperature |
| n | Number of the transferred electrons in the corrosion reaction |
| F | Faraday constant |
| [Prod] | Molar concentration of the products |
| [React] | Molar concentration of the reactants |
| P | Stoichiometric factor of the products |
| R | Stoichiometric factor of the reactants |
| In | Current density for each readings |
| N | Number of readings |
| IR.M.S | Root Mean Square of the current density |
| C.R. | Corrosion rate |
| M | Molar mass |
| d | Density of the material |
| Z(f) | Impedance according to the frequency |
| Eo | Amplitude of the potential |
| Io | Current density amplitude |
| f | Frequency |
| t | time |
| θ | Angle of phase |
| R(f) | Resistance |
| C(f) | Capacitance |
| fmax | Frequency at maximum angle of phase |
| R(fmax) | Resistance at maximum angle of phase |
| L | Thickness of the corrosion product or process |
| εr | Relative permittivity |
| εo | Permittivity of the vacuum |
| A | Area |
| Eapplied | Applied potential |
| Ecorr | Corrosion potential |
| βc | Cathodic slope |
| βa | Anodic slope |
| Ic | Cathodic current density |
| Ia | Anodic current density |
| Icorr | Corrosion current density |
| Rp | Polarization resistance |
| E | Potential |
| I | Current density |
| R | Resistance |
| C | Capacitance |
| D.A. | Data Acquisition |
| tTotal | Total time of the experiments |
| Δf | Frequencies range |
| ΔV(R.M.S) | Root mean square amplitude of the potential |
| S.R | Sweep Rate |
| Eini | Initial potential |
| Eocp | Potential to open circuit |
| Efinal | Final potential |
| ERef | Potential of the reference electrode |
| Ilim | Limit current density |
| Zreal | Real Impedance |
| Zimag | Imaginary Impedance |
| Zmod | Impedance Modulus |
The corrosion threshold ranges or values for various different environmental conditions of the investigated alloys are therefore essentially of the four mentioned electrochemical analysis techniques’ parameters. These values tabulated in Table 4, Table 5 are compiled with regards to the types of corrosion i.e. uniform/general or localised corrosion. The table also includes the selected references that are used to verify the presented data. The extensive literature/published data review indicated a large variability in the methods/procedures of testing and data generation. Therefore, those references containing only work performed in accordance to the international standards were considered in the review and for the data verification. In addition, Table 6 represents PPC analysed data (βa and βc) of the metallic materials from the corrosion testing conducted at different environments. These parameters can be used to evaluate the corrosion rate, C.R. (their relationship is shown in Table 2), thus for life prediction.
Table 4.
Corrosion threshold ranges or values for different environment conditions in association with the uniform/general corrosion of the commonly used metallic materials in foundation, tower and nacelle of an offshore WT.
| Material/Alloy | WT Parts | General Corrosion |
*Notes | ||||
|---|---|---|---|---|---|---|---|
| Environment | Corrosion Data |
||||||
| E (V) | I (A/cm2) | R (Ω*cm2) | C(F/cm2) | ||||
| SS316L | Foundation, Tower | Seawater at RT and pH = 8.2 | >-0.140, 0.400< | <1.500*10−7 | >6.245*104 | <1.739*10−5 | [10], [11], [12]/Oxidised Surface |
| >6.928*105 | <4.149*10−5 | [10], [11], [12]/Bare Surface | |||||
| SS430 | Nacelle/Gearbox | Grease at RT and pH = 5.2 | >-0.040, 3.000< | <3.452*10−9 | <4.255*106 | <1.266*10−11 | a/Lubricant |
| <2.258*108 | >1.619*10−8 | a/Bare Surface | |||||
| Grease & Seawater (30:70 wt/wt) at RT and pH = 4.3 | >-0.063, 3.000< | <3.005*10−8 | <1.023*106 | >3.020*10−11 | a/Lubricant | ||
| >7.000*107 | <1.721*10−8 | a/Bare Surface | |||||
| Grease & Seawater (30:70 wt/wt) at 328K and pH = 6.8 | >-0.180, 1.102< | <1.261*10−7 | <2.820*104 | >1.260*10−10 | a/Lubricant | ||
| <1.590*106 | <3.920*10−6 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | <-0.237, 1.375< | <2.484*10−10 | <4.597*106 | >8.805*10−12 | a/Lubricant | ||
| >3.810*108 | >6.938*10−9 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | >-0.042, 3.000< | <1.521*10−9 | <6.036*104 | >6.721*10−11 | a/Lubricant | ||
| >1.880*106 | <4.755*10−6 | a/Bare Surface | |||||
| S235 | Grease at RT and pH = 5.2 | >-0.060, 3.000< | <3.354*10−9 | <2.121*106 | <7.834*10−12 | a/Lubricant | |
| >2.990*108 | >7.441*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | >-0.160, 3.000< | <2.506*10−9 | >1.314*106 | >3.426*10−11 | a/Lubricant | ||
| >7.640*107 | <1.885*10−8 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | >-0.220, 0.990< | <1.249*10−7 | <1.270*104 | <5.664*10−11 | a/Lubricant | ||
| <6.720*105 | <6.637*10−7 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | <1.400, 3.000< | <3.615*10−10 | <6.234*106 | <9.074*10−12 | a/Lubricant | ||
| <6.810*108 | >1.262*10−8 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | >0.070, 3.000< | <8.254*10−10 | <3.700*104 | >1.079*10−10 | a/Lubricant | ||
| >4.490*106 | >9.727*10−6 | a/Bare Surface | |||||
| AA1010 | Grease at RT and pH = 5.2 | >-0.506, 3.000< | <3.546*10−9 | <4.968*106 | >3.795*10−11 | a/Lubricant | |
| >3.360*108 | <1.822*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | >-0.760, −0.400< | <2.163*10−8 | >2.178*106 | <2.235*10−11 | a/Lubricant | ||
| >3.530*107 | <9.919*10−9 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | >-0.700, 3.000< | <1.443*10−7 | >5.476*104 | <1.142*10−10 | a/Lubricant | ||
| >6.300*105 | <2.067*10−6 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | >-0.190, 0.600< | <1.038*10−10 | >7.362*106 | <9.515*10−12 | a/Lubricant | ||
| >4.855*108 | <2.830*10−9 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | >-0.250, 3.000< | <2.940*10−10 | <4.388*104 | <9.422*10−11 | a/Lubricant | ||
| >2.896*106 | <2.861*10−6 | a/Bare Surface | |||||
| AA6061 | Grease at RT and pH = 5.2 | >-0.290, 3.000< | <1.190*10−10 | <4.633*106 | <1.609*10−11 | a/Lubricant | |
| >4.633*108 | <1.346*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | >-0.546, 0.840< | <1.000*10−8 | >2.150*106 | <2.405*10−11 | a/Lubricant | ||
| >4.434*107 | <1.129*10−8 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | >-0.741, 0.230< | <1.678*10−7 | <2.462*104 | >2.593*10−10 | a/Lubricant | ||
| <7.741*105 | <4.345*10−7 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | >0.718, 3.000< | <4.383*10−10 | >1.370*107 | >1.549*10−11 | a/Lubricant | ||
| >4.580*108 | >9.389*10−9 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | >-0.129, 3.000< | <5.669*10−9 | <7.402*106 | <3.908*10−11 | a/Lubricant | ||
| >4.250*106 | <1.205*10−6 | a/Bare Surface | |||||
*Notes:
Numbers indicate references of the reviewed literatures/documents that were used to verify the data.
Indicates data validated by in-house (repetitive) testing.
Table 5.
Corrosion threshold ranges or values for different environment conditions in association with the localised corrosion and passive film damage of the commonly used metallic materials in foundation, tower and nacelle of an offshore W.
| Material/Alloy | WT Parts | Localised Corrosion |
*Notes | ||||
|---|---|---|---|---|---|---|---|
| Environment | Corrosion Data |
||||||
| E (V) | I (A/cm2) | R (Ω*cm2) | C(F/cm2) | ||||
| SS316L | Foundation, Tower | Seawater at RT and pH = 8.2 | <-0.140, 0.400< | >2.500*10−7 | <6.245*104 | >1.739*10−5 | [10], [11], [12]/Oxidised Surface |
| <6.928*105 | >4.149*10−5 | [10], [11], [12]/Bare Surface | |||||
| S355 | <-0.680, −0.650< | >1.456*10−5 | <1.420*103 | >7.906*10−4 | a/Oxidised Surface | ||
| <2.660*102 | <3.597*10−4 | a/Bare Surface | |||||
| ≤9.475*103 | ≥3.722*10−4 | a/Diffusion | |||||
| AA5052 | <-0.650, −0.570< | >4.560*10−6 | ≥4.890*103 | >7.353*10−6 | a/Oxidised Surface | ||
| >3.671*103 | >1.751*10−5 | a/Bare Surface | |||||
| ≤1.538*104 | ≥4.736*10−4 | a/Diffusion | |||||
| <-0.960, −0.750< | – | – | – | [13] | |||
| AA3103 | <-0.650, −0.630< | >1.560*10−6 | >4.200*103 | >8.340*10−6 | a/Oxidised Surface | ||
| >2.756*103 | ≥1.500*10−5 | a/Bare Surface | |||||
| ≤2.538*104 | ≥1.423*10−4 | a/Diffusion | |||||
| <-1.060, −0.510< | – | – | – | [14], [15] | |||
| SS430 | Nacelle/Gearbox | Grease at RT and pH = 5.2 | <-0.040, 3.000< | >3.452*10−9 | >4.255*106 | >1.266*10−11 | a/Lubricant |
| >2.258*108 | <1.619*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | <-0.063, 3.000< | >3.005*10−8 | >1.023*106 | <3.020*10−11 | a/Lubricant | ||
| <7.000*107 | >1.721*10−8 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | <-0.180, 1.102< | >1.261*10−7 | >2.820*104 | <1.260*10−10 | a/Lubricant | ||
| >1.590*106 | >3.920*10−6 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | >-0.237, 1.375< | >2.484*10−10 | >4.597*106 | <8.805*10−12 | a/Lubricant | ||
| <3.810*108 | <6.938*10−9 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | <-0.042, 3.000< | >1.521*10−9 | >6.036*104 | <6.721*10−11 | a/Lubricant | ||
| <1.880*106 | >4.755*10−6 | a/Bare Surface | |||||
| S235 | Grease at RT and pH = 5.2 | <-0.060, 3.000< | >3.354*10−9 | >2.121*106 | >7.834*10−12 | a/Lubricant | |
| <2.990*108 | <7.441*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | <-0.160, 3.000< | >2.506*10−9 | <1.314*106 | <3.426*10−11 | a/Lubricant | ||
| <7.640*107 | >1.885*10−8 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | <-0.220, 0.990< | >1.249*10−7 | >1.270*104 | >5.664*10−11 | a/Lubricant | ||
| >6.720*105 | >6.637*10−7 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | <1.400, 3.000< | >3.615*10−10 | >6.234*106 | >9.074*10−12 | a/Lubricant | ||
| >6.810*108 | <1.262*10−8 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | <0.070, 3.000< | >8.254*10−10 | >3.700*104 | <1.079*10−10 | a/Lubricant | ||
| <4.490*106 | <9.727*10−6 | a/Bare Surface | |||||
| AA1010 | Grease at RT and pH = 5.2 | <-0.506, 3.000< | >3.546*10−9 | >4.968*106 | <3.795*10−11 | a/Lubricant | |
| <3.360*108 | >1.822*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | <-0.760, −0.400< | >2.163*10−8 | <2.178*106 | >2.235*10−11 | a/Lubricant | ||
| <3.530*107 | >9.919*10−9 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | <-0.700, 3.000< | >1.443*10−7 | <5.476*104 | >1.142*10−10 | a/Lubricant | ||
| <6.300*105 | >2.067*10−6 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | <-0.190, 0.600< | >1.038*10−10 | <7.362*106 | >9.515*10−12 | a/Lubricant | ||
| <4.855*108 | >2.830*10−9 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | <-0.250, 3.000< | >2.940*10−10 | >4.388*104 | >9.422*10−11 | a/Lubricant | ||
| <2.896*106 | >2.861*10−6 | a/Bare Surface | |||||
| AA6061 | Grease at RT and pH = 5.2 | <-0.290, 3.000< | >1.190*10−10 | >4.633*106 | >1.609*10−11 | a/Lubricant | |
| <4.633*108 | >1.346*10−8 | a/Bare Surface | |||||
| Grease & Seawater at RT and pH = 4.3 | <-0.546, 0.840< | >1.000*10−8 | <2.150*106 | >2.405*10−11 | a/Lubricant | ||
| <4.434*107 | >1.129*10−8 | a/Bare Surface | |||||
| Grease & Seawater at 328K and pH = 6.8 | <-0.741, 0.230< | >1.678*10−7 | >2.462*104 | <2.593*10−10 | a/Lubricant | ||
| >7.741*105 | >4.345*10−7 | a/Bare Surface | |||||
| Oil at RT and pH = 8.8 | <0.718, 3.000< | >4.383*10−10 | <1.370*107 | <1.549*10−11 | a/Lubricant | ||
| <4.580*108 | <9.389*10−9 | a/Bare Surface | |||||
| Oil at 328K and pH = 8.6 | <-0.129, 3.000< | >5.669*10−9 | >7.402*106 | >3.908*10−11 | a/Lubricant | ||
| <4.250*106 | >1.205*10−6 | a/Bare Surface | |||||
*Notes:
Numbers indicate the references of the reviewed literatures/documents that were used to verify the data.
Indicates data validated by in-house (repetitive) testing.
Table 6.
PPC data for the corrosion rate calculation.
| Material/Alloy | WT Parts | Environment | βc (V/decade) | βa (V/decade) | ∗Notes |
|---|---|---|---|---|---|
| SS316L | Foundation, Tower | Seawater at RT and pH = 8.2 | 0.097 | 0.296 | a |
| S355 | 0.034 | 0.163 | a | ||
| AA5052 | 0.585 | 0.072 | a | ||
| AA3103 | 0.055 | 0.044 | a | ||
| SS430 | Nacelle/Gearbox | Grease at RT and pH = 5.2 | 0.540 | 1.256 | a |
| Grease & Seawater at RT and pH = 4.3 | 0.658 | 1.549 | a | ||
| Grease & Seawater at 328K and pH = 6.8 | 0.303 | 1.102 | a | ||
| Oil at RT and pH = 8.8 | 0.385 | 0.992 | a | ||
| Oil at 328K and pH = 8.6 | 0.101 | 0.827 | a | ||
| S235 | Grease at RT and pH = 5.2 | 0.150 | 1.250 | a | |
| Grease & Seawater at RT and pH = 4.3 | 0.648 | 1.500 | a | ||
| Grease & Seawater at 328K and pH = 6.8 | 0.189 | 1.235 | a | ||
| Oil at RT and pH = 8.8 | 0.404 | 0.870 | a | ||
| Oil at 328K and pH = 8.6 | 0.062 | 0.565 | a | ||
| AA1010 | Grease at RT and pH = 5.2 | 0.221 | 0.616 | a | |
| Grease & Seawater at RT and pH = 4.3 | 0.648 | 1.500 | a | ||
| Grease & Seawater at 328K and pH = 6.8 | 0.189 | 1.235 | a | ||
| Oil at RT and pH = 8.8 | – | 1.769 | a | ||
| Oil at 328K and pH = 8.6 | 0.300 | 0.610 | a | ||
| AA6061 | Grease at RT and pH = 5.2 | 0.060 | 0.585 | a | |
| Grease & Seawater at RT and pH = 4.3 | 0.496 | 0.773 | a | ||
| Grease & Seawater at 328K and pH = 6.8 | 0.215 | 1.013 | a | ||
| Oil at RT and pH = 8.8 | 0.337 | 1.059 | a | ||
| Oil at 328K and pH = 8.6 | 0.044 | 0.520 | a |
*Note:
Indicates data validated by in-house (repetitive) testing.
2. Experimental design, materials, and methods
Test samples or coupons of an approximately 2.0cm × 2.0cm × 0.3cm were prepared from the metallic materials listed in Table 1. They were polished using a 1200-grit paper, subsequently in a dissolution comprised of 10% (V/V) colloidal silica gel (0.06 μm colloidal silica gel) and 90% (V/V) distilled water. Following the polishing stage, the metallic samples were washed and cleaned with a commercial detergent and fresh water, then with distilled water and by isopropanol, then dried up using hot air (ASTM E3-11) [6]. A minimum of 0.5 cm2 polished surface area is needed to guarantee a sufficient exposure/contact during the corrosion testing.
The set-up and conditions for the corrosion testing in a substitute ocean water environment (from this point onward is referred to as ‘Seawater’) are in accordance with ASTM D1141 [5]. Meanwhile, the corrosion testing to simulate the conditions and environments in the nacelle/gearbox follows the ASTM D6547 [3] recommendation when using semi solid lubricants with added corrosion inhibitor (from this point onward is referred to as ‘Grease’), the ASTM D665 [4] when using a mixture of 30% (Wt/Wt) Grease and 70% (Wt/Wt) Seawater, and the ASTM D6547 [3] for testing using oils at RT and at 328K.
Electrochemical corrosion testing was performed using a potentio/galvanostat (GillAC, ACM Instruments) that was controlled by software Gill AC serial no 600. OCP utilises a two electrodes cell, namely a working and a reference electrode. ZRA, EIS and PPC added a second working (a sacrificial) or counter electrode to construct a three electrodes cell system. Silver/silver chloride potassium chloride saturated (Ag/AgCl Sat. KCl) was used as the reference electrodes and graphite rods as the second working or counter electrodes. The test sample was the other working electrode.
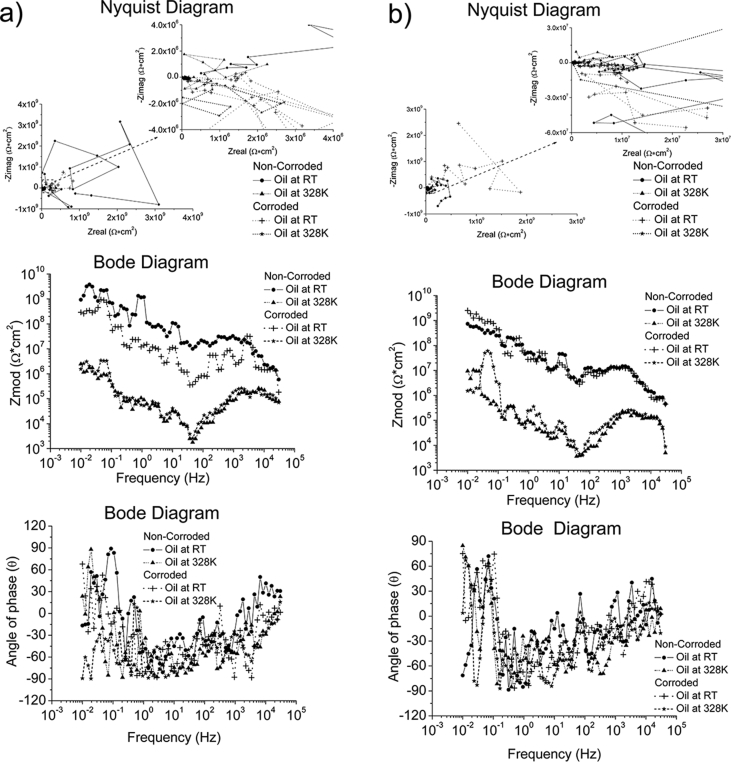
Whilst ZRA and EIS were performed using the same test conditions in all environments, OCP and EIS were conducted using different test conditions depending on the environment. The test conditions used to generate the reported data are specified in Table 7. The complementary information in a format of Nyquist and Bode diagrams to represent the experimental raw data are also presented in Fig. 1, Fig. 2, Fig. 3, Fig. 4.
Table 7.
Test conditions used in conjunction with the four electrochemical analysis techniques.
| Environment | Electrochemical analysis techniques |
|||
|---|---|---|---|---|
| OCP | ZRA | EIS | PPC | |
| Seawater |
f/D.A, 10Hz/0.1s tTotal; 2 hours |
f/D.A, 10Hz/0.1s tTotal; 2 hours |
Δf; 0.01–30000Hz Points; 70 Point/decade; 10 ΔV(R.M.S); 0.01V |
S.R.; 1.67*10−4V/s Eini; Eocp-0.3V Efinal; 3V vs Eref Ilim; 0.01A/cm2 |
| Grease at RT, pH = 5.2 |
f/D.A, 0.3Hz/3s tTotal; 2 hours |
– |
S.R.; 5*10−3V/s Eini; Eocp-1V Efinal; 3V vs Eref Ilim;0.01A/cm2 |
|
| Grease & Seawater at RT, pH = 4.3 | ||||
| Grease & Seawater at 328K, pH = 6.8 | ||||
| Oil at RT, pH = 8.8 | ||||
| Oil at 328K, pH = 8.6 | ||||
Fig. 1.
The Bode and Nyquist diagrams of non-corroded and corroded materials immersed in artificial seawater: a) SS316L, b) S355, c) AA5052 and d) AA3103.
Fig. 2.
The Bode and Nyquist diagrams of non-corroded and corroded materials subjected to grease at room temperature: a) SS430, b) S235, c) AA1010 and d) AA6061.
Fig. 3.
The Bode and Nyquist diagram of non-corroded and corroded materials subjected to grease & seawater at room temperature and at 328K: a) SS430, b) S235, c) AA1010 and d) AA6061. Note: The zoom-in area from the Nyquist diagram is shown in the insert plot.
Fig. 4.
The Bode and Nyquist diagram of non-corroded and corroded materials immersed in oil at room temperature and at 328K: a) SS430, b) S235, c) AA1010 and d) AA6061. Note: The zoom-in area from the Nyquist diagram is shown in the insert plot.
Acknowledgments
This work was supported by the Innovate UK iWindCr Project (Grant Number 103504) and co-funded by our industrial partners Avonwood Development Ltd (Co. No. 02570711) and Avanti Communication Plc (Co. No. 03101607). The authors would also like to acknowledge the Faculty of Technology, the School of Mechanical and Design Engineering (SMDE)and the School of Energy and Electronic Engineering (SENE), University of Portsmouth, for their support in this work.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.ASTM . ASTM-D664. 2017. Standard test method for acid number of petroleum products by potentiometric titration. [Google Scholar]
- 2.ASTM . ASTM-D4048. 2016. Standard test method for detection of copper corrosion from lubricanting grease. [Google Scholar]
- 3.ASTM . ASTM-D6547. 2016. Standard test method for corrosiveness of lubricanting fluid to bimetallic couple. [Google Scholar]
- 4.ASTM . ASTM-D665. 2014. Standard test method for rust-preventing characteristics of inhibited mineral oil in the presence of water. [Google Scholar]
- 5.ASTM . ASTM-D1141. 2013. Standard practice for the preparation of substitute ocean water. [Google Scholar]
- 6.ASTM . ASTM-E3. 2009. Standard guide for preparation of metallographic specimens. [Google Scholar]
- 7.Kelly R.G. CRC Press; 2002. Electrochemical Techniques in Corrosion Science and Engineering. Book. [Google Scholar]
- 8.Agarwala V.S., Reed P.L., Ahmad S. CORROSION 2000. NACE International; 2000. Corrosion detection and monitoring-A review. [Google Scholar]
- 10.Jun C. Corrosion and tribocorrosion behaviors of AISI 316 stainless steel and Ti6Al4V alloys in artificial seawater. Trans. Nonferrous Metals Soc. China. 2014;24(4):1022–1031. [Google Scholar]
- 11.Xin S., Li M. Electrochemical corrosion characteristics of type 316L stainless steel in hot concentrated seawater. Corros. Sci. 2014;81:96–101. [Google Scholar]
- 12.Hoseinieh S., Shahrabi T. Influence of ionic species on scaling and corrosion performance of AISI 316L rotating disk electrodes in artificial seawater. Desalination. 2017;409:32–46. [Google Scholar]
- 13.Bonewitz R. An electrochemical evaluation of 1100, 5052, and 6063 aluminum alloys for desalination. Corrosion. 1973;29(6):215–222. [Google Scholar]
- 14.Bonewitz R. An electrochemical evaluation of 3003, 3004, and 5050 aluminum alloys for desalination. Corrosion. 1974;30(2):53–59. [Google Scholar]
- 15.Rowland H.T., DEXTER S.C. Effects of the sea water carbon dioxide system on the corrosion of aluminum. Corrosion. 1980;36(9):458–467. [Google Scholar]