Abstract
Non-tuberculous mycobacterial species are uncommon human pathogens. They are divided into slow and rapid growing mycobacteria (RGM) with Mycobacterium smegmatis group as an uncommon pathogen among the RGM.
A 19 years old male presented with a 1 month history of dyspnea, orthopnea, unintentional weight loss, palpitation, flu-like symptoms and dry cough. Physical examination revealed tachycardia, distended superficial chest veins with a decrease in breath sounds at the right lower lung with fine crepitations. CT of the chest showed a large anterior mediastinal mass infiltrating the pericardium and three chambers of the myocardium that was confirmed using echocardiography. Despite negative workup for tuberculosis, the patient was treated successfully using first-line anti-TB treatment, which was begun before the tissue culture grew M. smegmatis.
To our knowledge, this is the first case in the literature of M. smegmatis infection mimicking cardiomediastinal tuberculoma, and RGM should be suspected in similar presentations with negative TB workup, even in an immunocompetent patient. This is also the first patient to be treated using only first-line anti-tuberculous treatment successfully in the literature.
Keywords: Mycobacterium smegmatis, Cardiac, Granuloma, Saudi Arabia
Introduction
Non-tuberculous mycobacterium (NTM) refers to more than 150 species that are not Mycobacterium tuberculosis (MTB) or M. leprae [1]. These species are mostly nonmotile aerobic organisms, with rigid and thick cell walls [2]. Hence, they are environmental organisms that are found in all habitats and households [1,2]. Non-tuberculous mycobacterium can be classified to two major classes based on the speed of growth with 7 days as a cutoff, slowly growing mycobacterium (SGM) like Mycobacterium avium complex (MAC) and M. kansasii, and rapidly growing mycobacterium (RGM) like M. abscessus complex (MABC), M. fortuitum complex and M. smegmatis group [1]. The RGM are sub-classified to 6 taxonomic groups, each group is identified based on pigmentation and DNA analysis [3]. Organisms of the M. smegmatis group are not among the most clinically relevant RGM, instead, M. fortuitum, M. chelonae, and M. abscessus comprise more than 80% of reported cases [3]. Out of the 75 species identified as RGM, the M. smegmatis group are uncommon and consist of two species only M. smegmatis and M. goodii [3]. To our knowledge, there has been no previously documented case of M. smegmatis group infection presenting as a granulomatous cardiomediastinal mass in the literature; and we present this case of a cardiomediastinal mass caused by M. smegmatis that was successfully treated medically.
Case report
A 19 years old male, previously healthy, presented to the emergency department with 1 month history of progressively increasing exertional dyspnea that was associated with orthopnea, paroxysmal nocturnal dyspnea, and palpitations. These symptoms were associated with influenza-like symptoms without a fever. He also had a dry cough and unintentional weight loss of 16 kg over nine months before this presentation. Physical examination revealed dyspnea and tachycardia but normal blood pressure measurements. His cardiac examination revealed an audible third heart sound with normal jugular venous pressure and no lower limb edema. There were distended superficial veins all over the right upper part of the chest, with a decrease in breath sounds at the right lower lung area with fine crepitations.
Initial electrocardiogram confirmed sinus tachycardia and right axis deviation. His lab work revealed microcytic hypochromic anemia without any evidence of bleeding, leukocytosis of 18.9 K/μL that was mainly neutrophilic with lymphopenia, thrombocytosis 446 K/μL, isolated hyponatremia of 131 mmol/L, elevated erythrocyte sedimentation rate of 55 mm/h, C-reactive protein of 63.50 mg/L, and normal cardiac enzymes, liver profile and renal function tests. HIV test was negative.
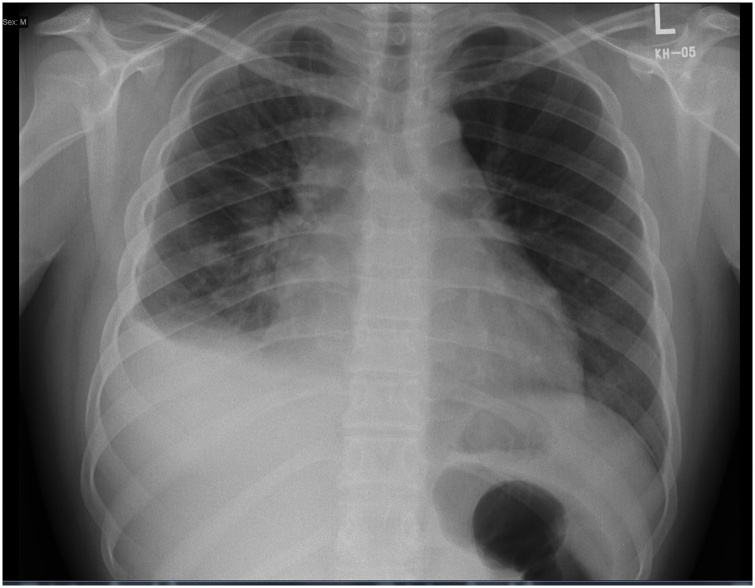
Chest x-ray showed mild right-sided pleural effusion with prominent interstitial markings on the right lung (Fig. 1). Trans-thoracic and trans-esophageal echocardiogram were done revealing a large mass infiltrating the superior vena cava (SVC), both atria, and the mediastinum. The largest part of the mass was occupying the right atrium, and it measured 4.5 × 4.3 × 4.8 cm in its largest diameters. A reduction in the ejection fraction (EF) to 35–45%, moderate to severe global hypokinesia, pericardial thickening with small pericardial effusion were also noted. Contrast-enhanced echo using perflutren lipid microspheres showed no enhancement of the mass, suggesting a non-vascular mass. He was admitted to the intensive care unit for observation and treated as a case of heart failure with possible myopericarditis and SVC syndrome.
Fig. 1.
Posterior-Anterior chest x-ray, showing small right sided pleural effusion with interstitial reticular infiltrate, kerley B-lines and prominent right pulmonary fissure.
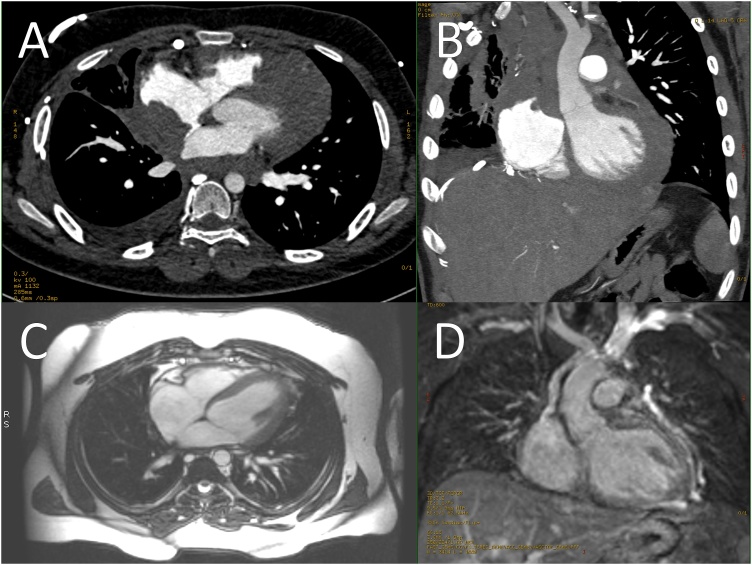
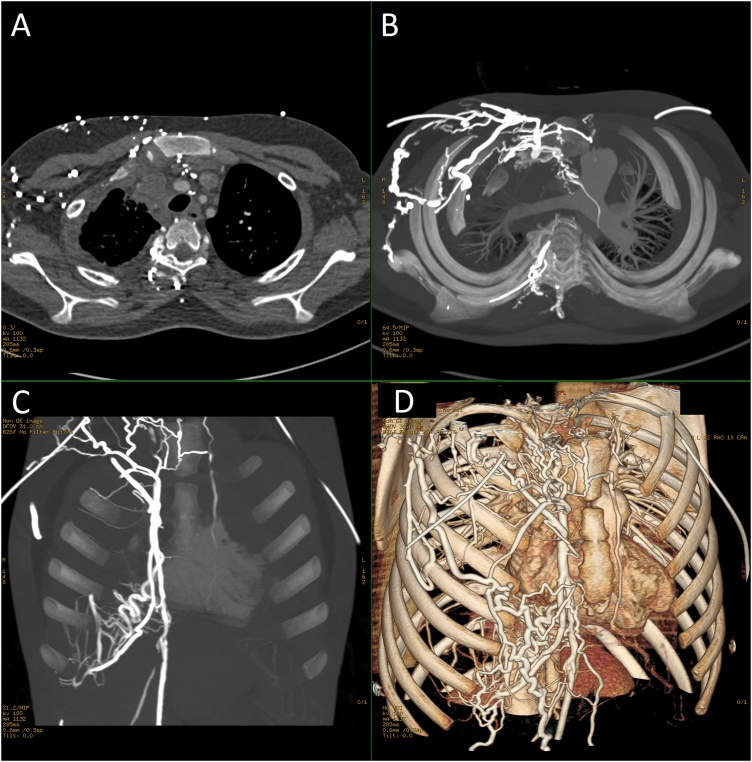
Chest computed tomography (CT) with contrast showed a large anterior mediastinal mass infiltrating the pericardium and myocardium in both atria and left ventricle, engulfing multiple vascular components, involving multiple lymph node groups (Fig. 2, Panel A, B), and obstructing others with thrombus formation causing multiple venous collaterals over the right chest wall (Fig. 3).
Fig. 2.
[A;Axial, B;Coronal oblique] Chest CT images showing a large anterior mediastinal mass extending along the left brachiocephalic vein and SVC resulting in their obstruction, It encircled the ascending aorta and aortic arch. It also extended along the pericardium resulting in infiltration and extension to the posterolateral right atrial wall as well as infiltration of the right atrial appendage, the right atrial mass measured 4.5 × 4.3 × 4.8 cm in its largest diameters, it continued its extension along the pericardium to adhere to and infiltrate the left ventricle. Multiple anterior mediastinal lymph nodes were also appreciated as well as pleural deposits along the right crus of the diaphragm with few small retrocrural lymph nodes. [C; Axial, D; Coronal] CMR images showing complete resolution of the mass with normal chamber sizes, valves and EF, but small size SVC, with dilated IVC and azygous vein.
Fig. 3.
[A; Axial, B; Axial vessels, C; coronal Vessels, D; Coronal Oblique 3D] Contrast enhanced chest CT showing the SVC and right brachiocephalic vein obstructed and thrombosed with extension of the thrombus to the proximal internal jugular vein with many engorged right chest wall venous collaterals.
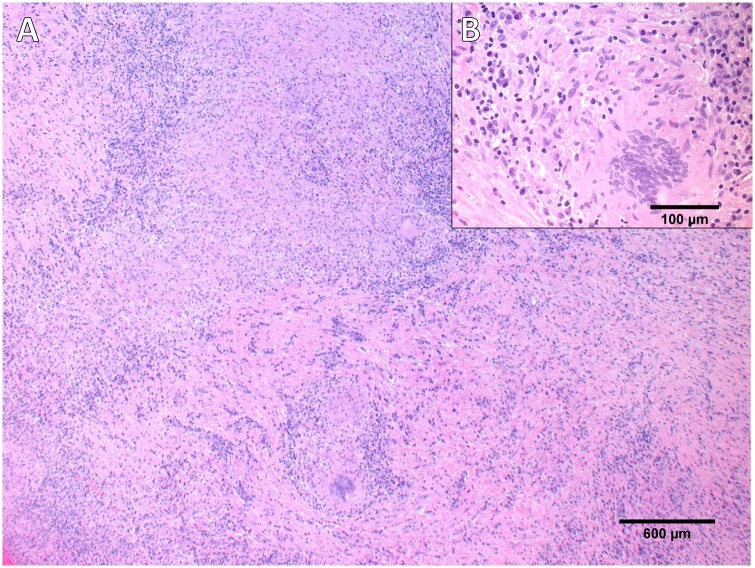
These features were suspicious of an infectious process (fungal infection like aspergillosis or TB), or non-vascular tumors like lymphoma presenting as a heart mass with SVC syndrome. Mediastinotomy and biopsy from the mass were done showing necrotizing granulomatous inflammation and giant cells (Fig. 4).
Fig. 4.
[A; Low power light microscopy picture × 200] showing chronic granulomatous inflammation. [B; high magnification light microscopy picture × 400] showing epithelioid granuloma containing giant cells.
At this point all cultures, acid-fast bacilli smears, and MTB polymerase chain reaction (PCR) from tissue samples were negative for MTB as the top differential diagnosis, but the patient was started empirically on anti-tuberculosis treatment (ATT; Isoniazid 300 mg, Rifampicin 600 mg, Ethambutol 1200 mg, Pyrazinamide 1000 mg) with oral corticosteroids due to high suspicion of TB infection. Tissue culture from the biopsy later on revealed M. smegmatis group. It grew from Löwenstein–Jensen medium as a rough pigmented colony in 6 days and identified using conventional biochemical methods. The patient was discharged on ATT as the patient was clinically improving, along with warfarin and anti-heart failure medications. He continued on ATT for a total of 9 months complicated by atrial flutter that was treated using ablation. A cardiac magnetic resonance was done after three years of treatment for follow-ups instead of CT because the patient developed an allergic reaction to the contrast, it showed complete resolution of the mass (Fig. 2, Panel C, D).
Discussion
Mycobacterium smegmatis group was first isolated from a chancre in a syphilis patient in 1884 followed by a penile secretion in 1885, hence the name “smegma” [4,5]. The first human clinically-relevant cases were reported 100 years after [4]. A recent review article in the Gulf region of the Middle East revealed that 1.5% of all pulmonary infections by NTM were caused by M. smegmatis group. Furthermore, such high RGM distribution follows the trend observed in India and South Korea and in contrary to the high global trend of SGM [2].
Community-acquired infection with M. smegmatis group can present as pneumonia, cellulitis, localized abscesses and/or osteomyelitis after trauma [3,4]. On the other hand, health-care-associated infection includes wound and soft tissue infections with possible osteomyelitis, catheter sepsis, post-cardiac surgery complications including sternal wound infection, prosthetic valve infection, infected prosthesis and cosmetic plastic surgery complications [[4], [5], [6]]. Disseminated infection also has been reported [7].
Non-tuberculous mycobacterium can present as any other granulomatous disease (nodule or mass) with or without caseation [8]. Mycobacterium abscessus has been reported in a recent study presenting as a mass that mimicked lung cancer [8]. Another article suggested that substantial diagnosed cases of pulmonary nodules as MTB are in fact, misdiagnosed NTM [9]. Other reports of suspected cases were treated initially as tuberculoma cases until further workup revealed NTM [10]. Species of the NTM group are considered saprophytes with a high rate of false-positive identification due to colonization rather than active infection [8]. Isolation of M. gordonae and M. terrae from respiratory samples may suggest contamination unlike more virulent species such as MAC and MABC, which may indicate a high probability of active infection [1].
Nowadays, phenotypic methods of identification are used only to correlate with the molecular methods of identification; even high-performance liquid chromatography lacks the accurate separation of certain RGM species within the same group or complex [3]. The most commonly used molecular method is 16 s rRNA gene sequencing with 95.7%–99.7% variation between species [3]. Other molecular methods include PCR restriction fragment analysis, sequencing specific genes (hsp65, rpoB, dnaJ, 23-kDa protein, secA1, recA and sod), 16 s–23 s rRNA internal transcribed spacer, INNO-LiPA multiplex probe assay, MALDI-TOF mass spectrometry and finally whole-genome sequencing [3].
Diagnosing a patient with lung NTM infection does not necessarily require antimicrobials initiation [1]. Most physicians rely on consensus and in vitro antimicrobial susceptibility as no clear guidelines for the treatment of each RGM species is yet published [6]. Species of the RGM group are generally resistant to first-line ATT except for the M. smegmatis group, which are sensitive to ethambutol [5,11,12]. One study recommend against ATT in RGM infections due to poor outcome, but the cases with the poor outcome were not from the M. smegmatis group [13]. The M. smegmatis group are generally among the most susceptible species of RGM along with M. fortuitum group and M. mucogenicum group [3].
In vitro susceptibility patterns guide the antimicrobial regimens choices and guidelines for the RGM antimicrobials testing and MIC breakpoints have been published including recommended antibiotics [3]. The M. smegmatis group have full-to-intermediate susceptibility to amikacin, cefoxitin, imipenem, ciprofloxacin, sulfonamides and moxifloxacin [3]. Interestingly, all isolates of the M. smegmatis group have early-to-late (inducible) resistance to macrolides and clarithromycin specifically [3], which is the cornerstone treatment for some RGM species [5]. This resistance is due to erythromycin ribosomal resistance methylase (erm38) gene that was discovered in M. smegmatis group [3]. A study on a mutated M. smegmatis species showed sensitivity to isoniazid and ethionamide compared to a wild type of the organism [14]. Surgical replacement and removal of an infected prosthesis with NTM may be vital for a curative response as M. goodii, a species from the M. smegmatis group can form a biofilm that may prolong or complicate treatment [15].
The patient in this case was immunocompetent but living in an endemic area with MTB (Saudi Arabia), which is an established risk for NTM infections [2]. The patient’s presentation resembled reported myocardial tuberculoma cases in the clinical, radiological and histopathological pattern [16]. Mycobacterium smegmatis group infection mimicking MTB involving the myocardium has not been documented before in literature, hence, it is an expected complication as almost all NTMs are known to mimic masses and solitary granulomatous nodules [8]. In the presented case, we used first-line ATT only and the patient had a full recovery, which can be attributed to the ethambutol sensitivity and possibly another drug of the ATT.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written consent was not obtained, as all the submitted figures are non-identifiable images without any marks.
CRediT authorship contribution statement
Moayad M. Alqurashi: Conceptualization, Writing - original draft. Ahmad Alsaileek: Visualization, Resources. Ahmad Aljizeeri: Supervision. Hana S. Bamefleh: Resources. Thamer H. Alenazi: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgment
None.
References
- 1.Koh W.-J. Nontuberculous mycobacteria-overview. Microbiol Spectr. 2017;5(1) doi: 10.1128/microbiolspec.TNMI7-0024-2016. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghafli H., Al-Hajoj S. Nontuberculous mycobacteria in Saudi Arabia and Gulf countries: a review. Can Respir J. 2017;2017 doi: 10.1155/2017/5035932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown-Elliott B.A., Philley J.V. Rapidly growing mycobacteria. Microbiol Spectr. 2017;5(1) doi: 10.1128/microbiolspec.TNMI7-0027-2016. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu F. Mycobacterium smegmatis soft tissue infection. Int J Dermatol. 2012;51(12):1518–1520. doi: 10.1111/j.1365-4632.2010.04835.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Elliott B.A., Wallace R.J. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saffo Z., Ognjan A. Mycobacterium smegmatis infection of a prosthetic total knee arthroplasty. IDCases. 2016;5:80–82. doi: 10.1016/j.idcr.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierre-Audigier C., Jouanguy E., Lamhamedi S., Altare F., Rauzier J., Vincent V. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin Infect Dis. 1997;24(5):982–984. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- 8.Hong S.J., Kim T.J., Lee J.-H., Park J.-S. Nontuberculous mycobacterial pulmonary disease mimicking lung cancer: clinicoradiologic features and diagnostic implications. Medicine (Baltimore) 2016;95(26):e3978. doi: 10.1097/MD.0000000000003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon Y.S., Koh W.-J., Kwon O.J., Lee N.Y., Han J., Lee K.S. Mycobacterium abscessus pulmonary infection presenting as a solitary pulmonary nodule. Intern Med. 2006;45(3):169–171. doi: 10.2169/internalmedicine.45.1398. [DOI] [PubMed] [Google Scholar]
- 10.Hahm C.R., Park H.Y., Jeon K., Um S.-W., Suh G.Y., Chung M.P. Solitary pulmonary nodules caused by Mycobacterium tuberculosis and Mycobacterium avium complex. Lung. 2010;188(1):25–31. doi: 10.1007/s00408-009-9203-1. [DOI] [PubMed] [Google Scholar]
- 11.Wallace R.J., Nash D.R., Tsukamura M., Blacklick Z.M., Silcox V.A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- 12.Kumar K.J., Chandra J., Mandal R.N. Fatal pulmonary infection caused by Mycobacterium smegmetis in an infant. Indian J Pediatr. 1995;62(5):619–621. doi: 10.1007/BF02761893. [DOI] [PubMed] [Google Scholar]
- 13.Eid A.J., Berbari E.F., Sia I.G., Wengenack N.L., Osmon D.R., Razonable R.R. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687–694. doi: 10.1086/520982. [DOI] [PubMed] [Google Scholar]
- 14.Elitas M. Isoniazid killing of Mycobacterium smegmatis NADH Pyrophosphatase mutant at single-cell level using microfluidics and time-lapse microscopy. Sci Rep. 2017;7(1):10770. doi: 10.1038/s41598-017-11503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh R.B., Grant M. Mycobacterium goodii endocarditis following mitral valve ring annuloplasty. Ann Clin Microbiol Antimicrob. 2017;16(1):14. doi: 10.1186/s12941-017-0190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.du Toit-Prinsloo L., Saayman G. “Death at the wheel” due to tuberculosis of the myocardium: a case report. Cardiovasc Pathol. 2016;25(4):271–274. doi: 10.1016/j.carpath.2016.03.003. [DOI] [PubMed] [Google Scholar]