Abstract
Introduction:
Online Diabetes Prevention Programs (DPPs) can be scaled-up and delivered broadly. However, little is known about real-world effectiveness and how outcomes compare with in-person DPP. This study examined online DPP weight loss and participation outcomes and secondarily compared outcomes among participating individuals with parallel in-person interventions.
Study design:
A large non-randomized trial supplemented by a comparative analysis of participating individuals from a concurrent trial of two parallel in-person programs: in-person DPP and the Veterans Administration’s standard of care weight loss program (MOVE!).
Setting/participants:
Obese/overweight Veterans with prediabetes enrolled in online DPP (n=268) between 2013 and 2014. Similar eligibility criteria were used to enroll in-person participants between 2012 and 2014 (n=273 in-person DPP, n=114 MOVE!) within a separate trial.
Intervention:
Online DPP included a virtual group format, live e-coach, weekly modules delivered asynchronously and wireless home scales. In-person programs included eight to 22 group-based, face-to-face sessions.
Main outcomes measures:
Weight change at 6 and 12 months, using wirelessly uploaded home scale data or electronic medical record weights from clinical in-person visits. Outcomes were analyzed between 2015 and 2017.
Results:
From 1,182 invitations, 268 (23%) participants enrolled in online DPP. Among these, 158 (56%) completed eight or more modules; mean weight change was –4.7 kg at 6 months and –4.0 kg at 12 months. In a supplemental analysis of participants completing one or more sessions/modules, online DPP participants were most likely to complete eight or more sessions/modules (87% online DPP vs 59% in-person DPP vs 55% MOVE!, p<0.001). Online and in-person DPP participants lost significantly more weight than MOVE! participants at 6 and 12 months; there was no significant difference in weight change between online and in-person DPP.
Conclusions:
An intensive, multifaceted online DPP intervention had higher participation but similar weight loss compared to in-person DPP. An intensive, multifaceted online DPP intervention may be as effective as in-person DPP and help expand reach to those at risk.
INTRODUCTION
Prediabetes affects 84 million, or one in three, U.S. adults.1 About 15%–30% of adults with prediabetes will transition to type 2 diabetes within 5 years,2,3 and lifetime risk can be upwards of 70%.4,5 However, incident diabetes risk can be lowered by 58% with intensive lifestyle interventions, such as the Diabetes Prevention Program (DPP).6 Because DPP is a critical diabetes prevention strategy with potential for significant public health impact,7 many translational studies have been conducted. A systematic review of 53 comprehensive lifestyle programs showed these interventions to be effective in reducing diabetes incidence, weight, and fasting blood glucose, with more intensive programs (more available sessions) leading to better outcomes.8 Another systematic review of 38 DPPs found more than half demonstrated at least 2.4 kg of weight loss.9 However, three quarters of DPPs delivered in-person had low reach (33% or less of eligible participants participated).9
Lack of reach significantly attenuates population impact in real-world settings.10,11 Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet.8,12,13 This trial tested an intensive, multifaceted online DPP for its effect on 6- and 12-month participation and weight outcomes in a real-world clinical setting. Because direct head-to-head comparisons of online versus in-person DPP are lacking, a supplementary analysis compares outcomes for individuals who completed one or more sessions between online DPP and two in-person programs using data from a parallel, two-arm trial, known as the Veterans Affairs(VA)–DPP Trial.14,15
METHODS
The study design is a non-randomized Veterans Health Administration (VHA) online DPP trial conducted between 2013 and 2016. Eligible Veterans with prediabetes self-selected into a 12-month online DPP developed by OmadaHealth. The primary analysis examined participation and weight outcomes at 6 and 12 months. The IRB approved study activities at all VHA sites, and informed consent was obtained from all participants.
Study Population
Participants were obese (BMI≥30 kg/m2) or overweight (BMI≥25 kg/m2) with one or more obesity-related conditions and prediabetes (laboratory confirmed in prior 6 months; HbA1c, 5.7%–6.4% or fasting blood glucose, 100–125 mg/dL). Individuals with: (1) history of diabetes in VA electronic medical records (EMRs), or (2) laboratory evidence of diabetes (HbA1c ≥6.5% or fasting blood glucose ≥126 mg/dL), or (3) anti-glycemic medication use other than metformin, documented in VA pharmacy records or self-reported within prior 6 months were excluded. Participants in the VA-DPP Trial, a parallel, pragmatic, two-arm trial comparing in-person DPP (VA-DPP) with VA’s standard of care weight loss program (MOVE!), were ineligible.
Recruitment occurred between September 2013 and June 2014 at four geographically diverse VA sites. Recruitment integrated with established clinical processes related to obesity screening and MOVE! referrals. One site recruited from women’s primary care; the three remaining sites recruited from general primary care. Eligible participants were identified using EMR data and invited to participate by letter and phone calls (i.e., no in-person assessments required).
Online DPP was a 12-month intensive lifestyle intervention with weekly modules (educational materials on healthy eating and exercise) delivered asynchronously through a web-based platform. Participants were assigned to virtual, closed groups using a proprietary algorithm with variables including age, BMI, and geographic location. Appendix Figure 1 includes an online DPP screen shot and Appendix Table 1 lists program components.
All participants received cellular-enabled (wireless) scales to remotely collect weights and were encouraged to maintain physical activity and diet logs. A human coach monitored online group interactions and provided individualized feedback. Participants interacted with coaches by phone or private online messaging or both. Participants could also post online messages for group members.
The main login page showed a weight loss metric tracking the entire group’s progress toward their combined weight loss goal. Each participant also had an individual progress bar showing their progress across program requirements (completion of weekly modules, weigh-ins).
Measures
Primary outcomes were weight change (kg) at 6 and 12 months because weight loss is a significant predictor of diabetes risk reduction.16–18 Weight change was assessed objectively using cellular-enabled scales. Clinical weights were extracted from the VA’s Corporate Data Warehouse, a national data repository comprising data from local VHA EMRs, for individuals who did not use their cellular-enabled scales.
Participation was assessed via completion of weekly online modules using modified the Centers for Disease Control and Prevention Standards for the Diabetes Prevention Recognition Program.19 Demographic data were obtained from VA’s Corporate Data Warehouse. Race and ethnicity data were self-reported at enrollment; Corporate Data Warehouse data supplemented missing data.
Statistical Analysis
Online DPP participants with two or more weight assessments, including a baseline weight (via cellular-enabled scale or EMR), during the study window were included in the primary analysis. A multilevel mixed effects regression model was used with all available changes in baseline weight during 12 months follow-up as the dependent variable. Sites and participants nested within sites were included as random intercepts to adjust for within-participant and within-site correlations of the outcomes and moving average was used for the within-participant errors from repeated measures. Participants using cellular-enabled scales had, on average, significantly more weight data than participants for whom only VA EMR weights were available. To reduce potential biases resulting from differences in the number of weight assessments per participant, responses were treated as if they were sampled with different sampling probabilities and weighted by the inverse of the number of weight assessments for each participant. The model was adjusted for baseline weight, gender, race (African American versus not), and program day since enrollment. Because of non-linear trajectories of individuals’ weights, the model also included a days-squared term where days were centered at 180 days before squaring to remove collinearity between days and days-squared term. A dummy variable indicating type of weight data (cellular-enabled scale or EMR) was included to account for potential confounding by different measurement methods, and interaction terms of days X days-squared by the indicator for the type of weight data were added to account for differential weight trends in those who uploaded the data versus those who did not. From the model, predicted 6- and 12-month changes in baseline weight were obtained. An additional analysis used percentage weight loss as the outcome of interest.
A secondary comparative analysis of online DPP included data from a parallel, non-randomized VA-DPP Trial of two in-person groups: (1) DPP and (2) VA’s standard of care weight loss program (MOVE!).14,15 DPP consisted of 22 face-to-face sessions focusing on 7% weight loss and ≥150 minutes of moderate physical activity. Participants were assigned to one group with the same trained facilitator over 12 months (i.e., closed group). MOVE! consisted of eight to 12 face-to-face core healthy lifestyle sessions followed by monthly maintenance sessions. MOVE! participants could attend different groups, facilitators varied between sessions, and there were no specified group lifestyle goals. Appendix Table 1 compares intervention components for online DPP, VA-DPP, and MOVE!.
In-person DPP and MOVE! participants were overweight/obese Veterans with prediabetes (i.e., similar eligibility criteria as online DPP); recruitment occurred at three of the four same sites and began 13 months prior to the online DPP trial (August 2012 to January 2014). In-person cohorts were part of a quality improvement study with a research component; a subset provided consent as approved by the VA IRB.
To account for differences in recruitment and enrollment processes between online and in-person trials, which may have impacted motivation to enroll, the comparative analytic cohort was restricted to individuals who completed one or more sessions/modules. A multilevel mixed effects regression model was used to test the association between study arms and change in baseline weight at 6 and 12 months. Baseline was set as the date of first session/module completion. Weights were routinely documented in the EMR at clinical visits, including in-person DPP and MOVE! sessions. The model adjusted for the same variables as the primary online DPP analytic model and was similarly inversely weighted by the number of weight assessments per participant. Additionally, the model included indicators for different intervention groups and interaction terms between days X days-squared by intervention group indicators. From the model, predicted 6- and 12-month changes in baseline weight were obtained by intervention group and tested for differences across groups. An additional analysis used percentage weight loss as the outcome of interest.
All analyses were conducted between 2015 and 2017 using Stata, version 14.1.
RESULTS
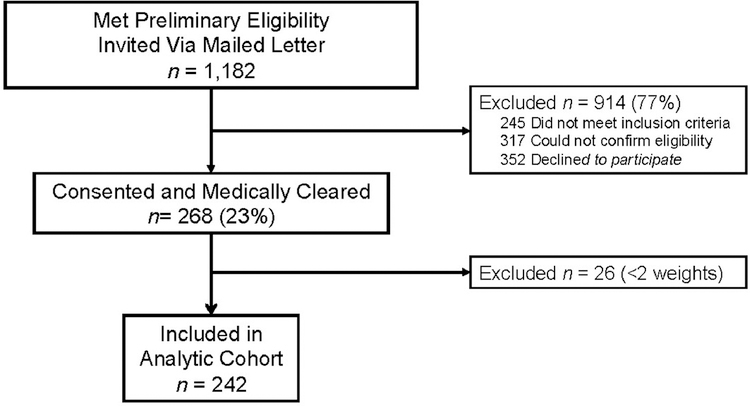
Between September 2013 and June 2014, a total of 1,182 patients were assessed for eligibility and invited to enroll (Figure 1).20,21 A total of 268 (23%) participants consented to participate. Online DPP participants had a mean age of 60 years (SD=11.3), BMI of 32.6 kg/m2 (SD=5.5), and HbA1c 6.0% (SD=0.2); 31% were female, 12% were Hispanic and 24% were African American (Table 1). Sixty-four percent completed one or more online modules (n=172), 59% completed four or more sessions (n=158) and 56% completed eight or more sessions (n=149; Table 2).
Figure 1.
CONSORT diagram of participant flow through online DPP.
aDiabetes was defined by HbA1c>6.4% or FPG>125 mg/dL, diabetes medications or diagnosis.
VA-DPP, VA Diabetes Prevention Program; BL, baseline; FPG, fasting blood glucose.
Table 1.
Baseline Characteristics of Online DPP Participants.
| Characteristic | Consented for online DPP N=268 | Included in primary analysis N=244 | Excluded from primary analysis N=26 | p-value Included vs excluded |
|---|---|---|---|---|
| Female, n (%) | 82 (30.6) | 63 (36.6) | 6 (23.1) | 0.38 |
| Age, years, mean (SD) | 60.3 (11.3) | 60.1 (10.8) | 62.7 (11.2) | 0.26 |
| Weight, kilograms, mean (SD) | 98.5 (18.7) | 99.5 (19.3) | 94.5 (6.6) | 0.67 |
| BMI, kg/m2, mean (SD) | 32.6 (5.5) | 33.3 (5.6) | 31.6 (3.3) | 0.70 |
| Ethnicity, n (%)a | ||||
| Hispanic | 31 (11.6) | 21 (12.2) | 1 (4.0) | 0.21 |
| Race, n (%)a | 0.18 | |||
| Black | 65 (24.3) | 32 (18.6) | 7 (26.9) | |
| White | 191 (71.3) | 133 (77.3) | 18 (69.2) | |
| Other | 10 (3.7) | 7 (4.1) | 0 (0.0) | |
| Missing | 2 (0.7) | 0 (0.0) | 1 (3.8) | |
| Comorbidities, n (%) | ||||
| HTN | 175 (65.3) | 112 (65.1) | 15 (57.7) | 0.39 |
| CAD | 33 (12.3) | 19 (11.0) | 3 (11.5) | 0.90 |
| Mental health | 120 (44.8) | 73 (42.4) | 11 (42.3) | 0.79 |
| HbA1c %, mean (SD)b | 6.0 (0.2) | 6.0 (0.2) | 6.0 (0.2) | 0.39 |
22 missing values.
11 had baseline A1c <5.7% and 6 had baseline A1c>6.4%.
DPP, Diabetes Prevention Program; HTN, hypertension; CAD, coronary heart disease.
Table 2.
Primary Outcome: Participation and Weight Change in Online DPP Cohort at 6 and 12-Months
| Outcomes | Online DPP participants |
|---|---|
| Participation in first 6 months, n (%) | N=268 |
| Completed 1+ sessions | 172 (64.1) |
| Completed 4+ sessions | 158 (59.0) |
| Completed 8+ sessions | 149 (56.0) |
| Predicted mean change in weight (kg) | N=242a |
| Kg weight loss at 6 months (95% CI) | −4.7 (−6.5, −2.8) |
| Kg weight loss at 12 months (95% CI) | −4.0 (−4.9, −3.0) |
| Predicted mean change in weight (%) | N=242a |
| % weight loss at 6 months (95% CI) | −4.4 (−5.7, −3.1) |
| % weight loss at 12 months (95% CI) | −3.7 (−4.2, −3.2) |
Note: All predicted mean changes in weight reported were significantly different from zero (p<0.001).
26 patients who did not have >2 available weights were excluded from the analysis.
DPP, Diabetes Prevention Program.
Of consented individuals, 172 (71%) uploaded two or more weights via a cellular-enabled scale and 70 (29%) did not upload weights but had two or more EMR weights. The remaining 26 (10%) did not have weights from either source; the primary analysis included 242 individuals. There were no significant differences between excluded individuals (n=26) and those included (n=242) in the primary analysis (Table 1). Compared with participants with cellular-uploaded weights, those with EMR weights were more likely to be male, a racial minority, and have lower BMI (Appendix Table 2).
Online DPP resulted in significant mean weight change of –4.7 kg (95% CI= –6.5, –2.8) at 6 months and –4.0 kg (95% CI= –4.9, – 3.0) at 12 months follow-up (Table 2). Mean percentage weight change was also significant at –4.4% (95% CI= –5.7, –3.1) at 6 months and –3.7% (95% CI= –4.2, –3.2) at 12 months. Appendix Figure 2 shows the trajectory of 12-month weight change. Enrollees completing one or more modules lost significantly more weight at 6 months (–6.4 kg) than individuals who did not (–0.3 kg, p=0.004). By 12 months, the difference between those completing one or more modules and those who did not was similar in magnitude, although marginally significant (–5.7 vs 0.5 kg, p=0.068).
The separate VA-DPP Trial was conducted in three of the four online study sites with recruitment initiated 13 months prior to the online DPP (between August 2012 and January 2014). Among 1,830 patients assessed for eligibility, 387 eligible individuals were systematically assigned to in-person DPP (n=273) or MOVE! (n=114, details available elsewhere).15 Among these, 262 participants (n=198 VA-DPP, n=64 MOVE!) completed one or more sessions and were included in a three-arm comparative analysis (Appendix Figure 3).
Compared with in-person DPP and MOVE! participants, online DPP participants were more likely to be female (36% [online DPP] vs 13% [in-person DPP] vs 6% [MOVE!], p<0.001), white (78% vs 52% vs 47%, p<0.001), Hispanic (11.7% vs 3.5% vs 9.2%, p=0.01), and had lower mean BMI (33 vs 35 vs 34, p<0.001; Appendix Table 3). A significantly higher proportion of online DPP participants completed eight or more sessions/modules (87% vs 59% in-person DPP, p<0.001 and vs 55% MOVE!, p<0.001; Table 3). The eight or more sessions cut off was part of the pragmatic VA-DPP evaluation design because one MOVE! site included eight core sessions. At 6 and 12 months, there were no significant differences in weight change (kg or percentage) between online and in-person DPP (Table 3). Online DPP participants had significantly greater weight change (percentage and kg) compared with MOVE! participants at 6 and 12 months (Table 3). Appendix Figure 4 shows the trajectory of 12-month weight change across the three arms.
Table 3.
Participation and Weight Change at 6 and 12 Months for Individuals Completing >1 Module/Sessions in Online DPP vs In-person DPP (VA-DPP) or MOVE!.
| Outcomes | Online DPP 1+ session N=180a | In-person DPP 1+ visit N=198 | MOVE! 1+ visit N=64 | p-value Online vs in-person DPP | p-value Online vs MOVE! |
|---|---|---|---|---|---|
| Participation in first 6 months, n (%) | |||||
| Completed 4+ sessions | 165 (91.7) | 157 (79.3) | 48 (75.0) | 0.001 | 0.001 |
| Completed 8+ sessionsb | 156 (86.7) | 116 (58.6) | 35 (54.7) | <0.001 | <0.001 |
| Predicted mean change in weight (kg) | |||||
| Kg weight loss at 6 months (95% CI) | −4.8 (−5.5, −4.2) | −4.0 (−5.9, −2.2) | −1.1 (−2.8, 0.6) | 0.53 | 0.002 |
| Kg weight loss at 12 months (95% CI) | −4.1 (−4.9, −3.3) | −3.9 (−5.4, −2.3) | 0.10 (−1.7, 1.9) | 0.84 | <0.001 |
| Predicted mean change in weight (%) | |||||
| % weight loss at 6 months (95% CI) | −4.8 (−5.5, −4.2) | −3.7 (−5.4, −2.0) | −0.8 (−2.3, 0.7) | 0.35 | <0.001 |
| % weight loss at 12 months (95% CI) | −4.1 (−4.8, −3.4) | −3.5 (−4.8, −3.4) | 0.3 (−1.6, 2.2) | 0.54 | <0.001 |
Note: Boldface indicates statistical significance (p<0.05).
The baseline date for this three-arm analysis was the date of completion of the first session/module. There are eight additional online participants in comparative cohort (Table 3) than in online only analytic cohort (Table 2). This is because eight additional persons did not have weight at study enrollment (baseline), but had weights by the time of their first online session (baseline for comparative cohort).
The CDC DPRP includes a milestone focusing on nine or more sessions attended but >8 sessions was part of the pragmatic evaluation design because one MOVE! site included eight core sessions.
VA-DPP, VA Diabetes Prevention Program; CDC, Centers for Disease Control and Prevention; DPRP, Diabetes Prevention Recognition Program.
DISCUSSION
Participants enrolled in an intensive, multifaceted online DPP intervention had significant weight change of –4.7 kg at 6 months and – 4.0 kg at 12 months follow-up. On average, online DPP participants lost 3.7% of their baseline weight at 12 months. This estimate falls within the 3.3%–7.5% weight loss reported by prior online DPP studies.13,22–29 However, in this study outcomes are reported for 90% of consented individuals, including dropouts and those who did not participate at all because EMR weights were used if cellular-enabled scale weights were missing. For context, a novel secondary analysis compared outcomes for participants in online DPP versus in-person DPP and MOVE!. Online DPP had significantly higher participation than in-person DPP and MOVE!. Direct head-to-head comparisons of online versus in-person DPP are lacking but studies of online DPP studies have generally reported high rates of participation. For example in one prior online DPP study, the number of participants completing four or more and nine or more modules was 95% and 92% respectively.28 In addition, a meta-analysis of DPP translation studies has shown the number of DPP sessions completed strongly correlates with the number of sessions offered (r =0.90, p<0.01) so it is not entirely surprising that this online DPP intervention, which included weekly modules over 12 months, led to higher rates of participation.30 At 6 and 12 months, weight change (kg and percentage) was not significantly different between online and in-person DPP. Online and in-person DPP participants had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants.
Several features distinguish this study from prior online DPP trials. First, weight loss outcomes among dropouts, or those without cellular-enabled scale data, were assessed using EMR data, which enabled inclusion of 90% of consented participants. To date, most published online DPP trials have included outcomes only for the subsample of participants with available follow-up weights (i.e., a motivated subgroup not lost to follow-up).22,23,28,29,31 In comparison to three online DPP studies where loss to follow-up was addressed or attrition was minimized, dropout rates ranged between 10% and 16% and weight loss outcomes ranged between 3.3 and 4.7 kg at 6–12 months follow-up.24,30,32 Thus, the mean weight loss of 4.0 kg at 12 months reported in this trial is comparable to outcomes in prior trials where loss to follow-up was addressed or attrition was minimized. Overall, rigorous studies of online DPP have been relatively sparse. Tracking EMR or population-level data lowers the risk of bias from attrition bias and missingness and increases the robustness of findings reported in this study.
Second, a comparison of online versus in-person DPP has not previously been reported. The secondary comparative analysis included two in-person comparators (in-person DPP and MOVE!) as parallel control groups, whereas most prior online DPP studies have reported single group, pre–post comparisons.22,23,24,28,29,31 Third, study participants were predominantly men, who are traditionally underrepresented in both DPP translational30,33,34 and weight loss studies.35 About 24% of online DPP participants were African Americans and 11% were Hispanic; participants were recruited from four geographically different U.S. sites. Because participants were Veterans receiving care in VHA, they were likely to have a lower SES and higher comorbidity burden, particularly mental health comorbidities.36,37 Thus, findings from this study are applicable to segments of the population less frequently included in previously published trials of online interventions.33 This is also the largest trial of online DPP conducted in a non-Medicare population.
These results indicate the effectiveness of the online DPP intervention tested in this study. This intervention included several distinct features, such as individualized feedback from trained human coaches and frequent self-monitoring using cellular-enabled scales, previously associated with improved outcomes in prior online weight loss trials.27,32,38–47 The online DPP also included social media features, which allowed participants to interact with one another in restricted (i.e., closed) online groups to help improve program retention.48,49 Each of these features increases the frequency of potential “touches” participants receive over time. The breadth and diversity of these potential touches may help enhance appeal across a broader population. For example, prior work has shown that some online DPP participants were more motivated after receiving a wireless scale to self-monitor weight (i.e., accountability to the scale), whereas others were more motivated by group-based weight loss goals (i.e., accountability to the group).50 These distinct features can be readily combined in an online DPP intervention to engage a higher proportion of individuals across a population. In addition, the user interface was appealing and user friendly, which is an additional feature of successful online weight loss programs.27 Future studies examining how online DPP intervention components can work together to impact participation and engagement are key. Overall, asynchronous delivery may help increase convenience and provide needed flexibility to help address some of the known patient-level barriers of in-person DPP to expand reach among eligible participants.50
Limitations
There are several important limitations to consider. First, this study included Veterans receiving care in the VHA, which may limit generalizability. However, the sample includes less studied groups, such as men, racial/ethnic minorities, and those with lower SES. Second, trial participants were not randomized and recruitment process differences may have biased the three-arm comparison results. However, the risk of bias was mitigated by only comparing participants who were motivated enough to complete one or more sessions of their program and by adjusting the model for differences in baseline characteristics. Third, there were two weight assessment methods: cellular-enabled scales (online DPP only) and in-person clinical visits. Therefore, all analytic models adjusted for weight assessment type to reduce the potential impact of bias. Seasonal variation in weight may have impacted 6-month results but are unlikely to explain 12-month weight differences. Additionally, objective physical activity data was not collected so impact of the programs on physical activity could not be assessed. Lastly, it is important to note that one site unique to the online DPP trial recruited from the women’s health clinic, which likely contributed to the higher proportion of women who participated in the online program.
CONCLUSIONS
An intensive, multifaceted online DPP intervention with human coaches, objective self-monitoring, individualized feedback, and social online groups led to a mean weight loss of 4.7 kg and 4.0 kg at 6 and 12 months respectively. Compared with in-person DPP, online DPP participants who completed one or more sessions/modules had significantly higher participation but comparable weight change. This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in-person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all of the leadership, coaches, and staff members within the multiple organizations who were involved with this study: Veterans Health Administration National Center for Health Promotion and Disease Prevention, Veterans Affairs (VA) MOVE!, VA Health Services Research and Development Service, VA Diabetes Quality Enhancement Research Initiative (QUERI), Diabetes Prevention Support Center (Group Lifestyle Balance program), VA Ann Arbor Center for Clinical Management Research, Durham VA Medical Center, VA Baltimore Medical Center, VA Greater Los Angeles Healthcare System, and VA Minneapolis Healthcare System.
This work was funded through a VA research grant (SDP 13–230). TM received support from VA QUERI (QUE 15–272) and the Centers for Disease Control and Prevention/National Institute of Diabetes and Digestive and Kidney Diseases (U18DP006128) and NIH/National Institute of Diabetes and Digestive and Kidney Diseases (1R18DK105464–01) and the VA Office of Academic Affiliations through the VA Health Services Research and Development Advanced Fellowship Program (TPM65–010, 2011–2014). MAY received support through the VA Health Services Research and Development Advanced Postdoctoral Fellowship Program, VA Ann Arbor. MLM reported receiving support from the VA (RCS 10–391).
Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this manuscript have been presented at the Society of Behavioral Medicine Annual Meetings in 2015, 2016, and 2017.
TM, LDL, MA, KH, KE, JEW, NIS, MH, FM, BY, RGH, HMK, LSK, and CRR have no financial disclosures. MLM reported ownership of Amgen stock because of his spouse’s employment. CB reported personal fees from Novo Nordisk and personal fees from EnteroMedics outside of the submitted work.
CRR serves as an Associate Editor for the American Journal of Preventive Medicine. She had no involvement in the peer review and decision-making processes for this paper.
REFERENCES
- 1.CDC. National Diabetes Statistics Report www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed June 28, 2018.
- 2.CDC. Prediabetes - Could It Be You? www.cdc.gov/diabetes/basics/prediabetes.html. Accessed July 17, 2018.
- 3.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78(3):305–312. 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379(9833):2279–2290. 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371(9626):1783–1789. 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med 2013;173(2):113–121. 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015;163(6):437–451. 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci 2015;10:172 10.1186/s13012-015-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care 2014;37(4):943–949. 10.2337/dc13-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cefalu WT, Buse JB, Tuomilehto J, et al. Update and next steps for real-world translation of interventions for type 2 diabetes prevention: reflections from a Diabetes Care Editors’ expert forum. Diabetes Care 2016;39(7):1186–1201. 10.2337/dc16-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Internet Use Over Time PewResearchCenter; 2014. [Google Scholar]
- 13.Bian RR, Piatt GA, Sen A, et al. The effect of technology-mediated diabetes prevention interventions on weight: a meta-analysis. J Med Internet Res 2017;19(3):e76 10.2196/jmir.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damschroder LJ, Moin T, Datta SK, et al. Implementation and evaluation of the VA DPP clinical demonstration: protocol for a multi-site non-randomized hybrid effectiveness-implementation type III trial. Implement Sci 2015;10:68 10.1186/s13012-015-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moin T, Damschroder LJ, AuYoung M, et al. Diabetes Prevention Program translation in the Veterans Health Administration. Am J Prev Med 2017;53(1):70–77. 10.1016/j.amepre.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29(9):2102–2107. 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore LL, Visioni AJ, Wilson PW, D’Agostino RB, Finkle WD, Ellison RC. Can sustained weight loss in overweight individuals reduce the risk of diabetes mellitus? Epidemiology 2000;11(3):269–273. 10.1097/00001648-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Van Gaal LF, Mertens IL, Ballaux D. What is the relationship between risk factor reduction and degree of weight loss? Eur Heart J Suppl 2005;7(suppl L):L21–L26. 10.1093/eurheartj/sui082. [DOI] [Google Scholar]
- 19.CDC. National Diabetes Prevention Program: Diabetes Prevention Recognition Program www.cdc.gov/diabetes/prevention/recognition/. Published January 28, 2015. Accessed June 28, 2018.
- 20.Moin T, Damschroder LJ, Youles B, et al. Implementation of a prediabetes identification algorithm for overweight/obese Veterans. J Rehabil Res Dev 2016;53(6):853–862. 10.1682/JRRD.2015.06.0104. [DOI] [PubMed] [Google Scholar]
- 21.Moin T, Makki F, Weinreb JE, et al. The Burden of Pre-diabetes Among Overweight and Obese Veterans: Understanding Prevalence of Disease and Merits of Routine Screening. Poster session presented at Michigan Center for Diabetes Translational Research Symposium; May 22, 2014; Ann Arbor, MI.. [Google Scholar]
- 22.Sepah SC, Jiang L, Peters AL. Translating the Diabetes Prevention Program into an online social network: validation against CDC standards. Diabetes Educ 2014;40(4):435–443. 10.1177/0145721714531339. [DOI] [PubMed] [Google Scholar]
- 23.Sepah SC, Jiang L, Peters AL. Long-term outcomes of a web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res 2015;17(4):e92 10.2196/jmir.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTigue KM, Conroy MB, Hess R, et al. Using the internet to translate an evidence-based lifestyle intervention into practice. Telemed J E Health 2009;15(9):851–858. 10.1089/tmj.2009.0036. [DOI] [PubMed] [Google Scholar]
- 25.Block G, Azar KM, Block TJ, et al. A fully automated Diabetes Prevention Program, Alive-PD: program design and randomized controlled trial protocol. JMIR Res Protoc 2015;4(1):e3 10.2196/resprot.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res 2015;17(10):e240 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McTigue KM, Conroy MB. Use of the internet in the treatment of obesity and prevention of type 2 diabetes in primary care. Proc Nutr Soc 2013;72(1):98–108. 10.1017/S0029665112002777. [DOI] [PubMed] [Google Scholar]
- 28.Castro Sweet C, Chiguluri V, Gumpina R, et al. Outcomes of a digital health program with human coaching for diabetes risk reduction in a Medicare population. J Aging Health 2018;30(5):692–710. 10.1177/0898264316688791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sepah SC, Jiang L, Ellis RJ, McDermott K, Peters AL. Engagement and outcomes in a digital Diabetes Prevention Program: 3-year update. BMJ Open Diabetes Res Care 2017;5(1):e000422 10.1136/bmjdrc-2017-000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31(1):67–75. 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 31.Chen F, Su W, Becker SH, et al. Clinical and economic impact of a digital, remotely-delivered intensive behavioral counseling program on Medicare beneficiaries at risk for diabetes and cardiovascular disease. PLoS One 2016;11(10):e0163627 10.1371/journal.pone.0163627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA 2001;285(9):1172–1177. 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 33.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: a systematic review and meta-analysis. Prev Med 2017;100:194–207. 10.1016/j.ypmed.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40(10):1331–1341. 10.2337/dc16-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damschroder LJ, Lutes LD, Kirsh S, et al. Small-changes obesity treatment among Veterans: 12-month outcomes. Am J Prev Med 2014;47(5):541–553. 10.1016/j.amepre.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Wong ES, Wang V, Liu CF, Hebert PL, Maciejewski ML. Do Veterans Health Administration enrollees generalize to other populations? Med Care Res Rev 2016;73(4):493–507. 10.1177/1077558715617382. [DOI] [PubMed] [Google Scholar]
- 37.Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. military, Veteran, and civilian men. Am J Prev Med 2012;43(5):483–489. 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 38.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med 2010;51(2):123–128. 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyden JR, Zickmund SL, Bhargava TD, et al. Implementing health information technology in a patient-centered manner: patient experiences with an online evidence-based lifestyle intervention. J Healthc Qual 2013;35(5):47–57. 10.1111/jhq.12026. [DOI] [PubMed] [Google Scholar]
- 40.Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of web-based vs. non-web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res 2004;6(4):e40 10.2196/jmir.6.4.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis EA, Szabo-Reed AN, Ptomey LT, et al. Do weight management interventions delivered by online social networks effectively improve body weight, body composition, and chronic disease risk factors? A systematic review. J Telemed Telecare 2017;23(2):263–272. 10.1177/1357633X16630846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas JG, Leahey TM, Wing RR. An automated internet behavioral weight-loss program by physician referral: a randomized controlled trial. Diabetes Care 2015;38(1):9–15. 10.2337/dc14-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev 2010;11(4):306–321. 10.1111/j.1467-789X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 44.Manzoni GM, Pagnini F, Corti S, Molinari E, Castelnuovo G. Internet-based behavioral interventions for obesity: an updated systematic review. Clin Pract Epidemiol Ment Health 2011;7:19–28. 10.2174/1745017901107010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher C, Ferguson M, Vandelanotte C, et al. A web-based, social networking physical activity intervention for insufficiently active adults delivered via Facebook app: randomized controlled trial. J Med Internet Res 2015;17(7):e174 10.2196/jmir.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leahey TM, Fava JL, Seiden A, et al. A randomized controlled trial testing an internet delivered cost-benefit approach to weight loss maintenance. Prev Med 2016;92:51–57. 10.1016/j.ypmed.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey-Berino J, Pintauro SJ, Gold EC. The feasibility of using internet support for the maintenance of weight loss. Behav Modif 2002;26(1):103–116. 10.1177/0145445502026001006. [DOI] [PubMed] [Google Scholar]
- 48.Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res 2014;16(2):e40 10.2196/jmir.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang T, Chopra V, Zhang C, Woolford SJ. The role of social media in online weight management: systematic review. J Med Internet Res 2013;15(11):e262 10.2196/jmir.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moin T, Ertl K, Schneider J, et al. Women Veterans’ experience with a web-based diabetes prevention program: a qualitative study to inform future practice. J Med Internet Res 2015;17(5):e127 10.2196/jmir.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Under Secretary for Health Information Letter - Managing Overweight and/or Obesity for Veteran Everywhere (MOVE!) Program; 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.