Abstract
Lung transplant immunosuppression regimens generally include the calcineurin inhibitor tacrolimus. We hypothesized that mean residual expression (MRE) of calcineurin-dependent genes assesses rejection and infection risk better than tacrolimus trough. We prospectively followed 44 lung allograft recipients 2 to 18 months post-transplant and measured changes in whole blood IL-2, interferon-γ, and GM-CSF gene expression following a tacrolimus dose. Post-transplant duration, immunosuppressive medication levels, and bronchoscopic rejection and infection assessments were compared with MRE using generalized-estimating equation-adjusted models. Prednisolone effect on MRE was assessed ex vivo in blood samples from non-transplanted controls. Tacrolimus concentration inhibiting 50% of cytokine production (IC50) was measured in a pre-transplant subset. Results showed that MRE did not change with diagnosis of rejection, but that airway infection was associated with a 20% absolute decrease (95% CI 11% – 29%). MRE increased with time following transplantation but was not associated with tacrolimus trough. Interestingly, MRE correlated inversely with corticosteroid dose in the study cohort and ex vivo. Pre-transplant tacrolimus IC50 depended on the cytokine measured and varied between individuals, suggesting a range in baseline responses to tacrolimus. We conclude that MRE identifies infection risk in lung allograft recipients, potentially integrating calcineurin inhibitor and steroid effects on lymphocyte effector function.
INTRODUCTION:
Tacrolimus is a critical component of most post-lung transplant maintenance immunosuppression regimens (1). Following initial observations of highly variable efficacy and side-effect profiles with fixed dosing regimens, dosing by trough has become the standard of care (2). However, variation in clinical effects between individuals with similar trough levels has led to the concern that tacrolimus trough levels inaccurately reflect degree of immunosuppression. Several clinical studies have investigated an assay based on the mean residual gene expression (MRE) at peak versus trough calcineurin inhibitor concentrations following a single dose. The three genes assayed are downstream of the nuclear factor of activated T-cells (NFAT) family of transcription factors that are activated by calcineurin, and so this gene expression ratio may assess the degree of immunosuppression attributable to a given dose of calcineurin inhibitor (3, 4). It is hoped that tacrolimus titration based on this assay could lead to decreased incidence of rejection, infection, and cancer in renal allograft recipients (5, 6).
Optimizing immunosuppression in lung allograft recipients remains challenging. In the first-year after transplantation, 35% of lung allograft recipients have at least one episode of acute rejection requiring treatment (7). Acute cellular rejection is a risk factor for chronic lung allograft dysfunction (CLAD), which affects nearly 50% of patients at 5 years and is the most significant cause of death after the first year (7, 8). Acute cellular rejection and CLAD occur despite high doses of tacrolimus combined with mycophenolate mofetil and prednisone. At the same time, these immunosuppression regimens have been linked with increased rates of infection and malignancy (9).
The difficulties tailoring immunosuppressive therapy for lung transplant recipients have fueled demand for a blood-based assay to monitor the immune system. The ImmuKnow assay measures CD4+ T cell production of adenosine triphosphate (ATP) in response to stimulation with phorbol 12-myristate 13-acetate (PMA). However, enthusiasm for this assay diminished after studies demonstrated poor test characteristics for diagnosing infection and rejection in lung transplant recipients (10). The MRE assay is thought to be more specific to tacrolimus because the comparison of peak and trough gene expression can mitigate impacts of peripheral blood mononuclear cell (PBMC) lymphocyte count and of other immunosuppressive medications (11, 12). The use of ionomycin to trigger calcium-dependent NFAT signaling and the measurement of the expression of genes under the NFAT promoter should further enhance the specificity for calcineurin inhibitors.
This MRE assay has shown early success in monitoring kidney, liver, and heart allograft recipients (3, 13, 14). However, the specificity of this assay for tacrolimus dosing and performance characteristics in identifying infection and rejection risk previously has not been evaluated in lung allograft recipients. We hypothesized that MRE levels would be associated with the level of tacrolimus-based immunosuppression and could identify lung transplant recipients at increased risk for infection or rejection.
MATERIALS AND METHODS:
Study population
This prospective observational cohort study was approved by the University of California, San Francisco (UCSF) institutional review board under protocol #14–13221 and registered on clinicaltrials.gov (identifier ). The study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. Assay optimization and post-hoc analyses were performed on anonymized whole blood samples from healthy, non-transplanted volunteers obtained under protocol #15–16072. Lung transplant recipients at UCSF were recruited 1–2 months after transplantation and followed for 6 to 18 months after transplantation. Subjects not treated with tacrolimus were excluded. All subjects provided written informed consent.
Immunosuppression practices followed institutional protocols. Induction regimens included basiliximab and methylprednisolone. In the absence of rejection, prednisone was administered at 20 mg daily for the first 3 months, tapered to 0.2 mg/kg daily over the next 3 months, and then gradually tapered to 0.1 mg/kg by 12 months. Targeted tacrolimus troughs were 10–14 ng/ml in the first 3 months, 10–12 ng/ml in the next 3 months, and 8–10 ng/ml thereafter. Mycophenolate mofetil was started in the immediate post-operative period targeting 2 g/day in divided doses but could be reduced or stopped in the setting of leukopenia, skin cancer, or other side effects.
Predictor variables: MRE and flow cytometric assays.
MRE assays were performed within one day of a bronchoscopy. Briefly, residual expression of IFN-γ, IL-2 and GM-CSF in stimulated whole blood samples taken at 90–120 minutes after a tacrolimus dose versus trough were determined by quantitative PCR, as previously described (3, 4). Detailed descriptions of the MRE assay and of flow cytometric measurement of tacrolimus IC50 are contained in the supplement.
Outcome measures
Acute cellular rejection and bronchoscopically-detected infection were the primary outcomes. Acute cellular rejection was determined based on clinical interpretation of transbronchial biopsy specimens graded for rejection according to International Society for Heart and Lung Transplantation (ISHLT) guidelines, predominantly by a single thoracic transplant pathologist (15), who was not aware of MRE values. Infection was defined based on the presence of a potentially pathogenic organism in bronchoalveolar lavage (BAL) fluid and one or more of the following: symptoms consistent with acute infection, CT findings suggesting active infection, or moderate or greater organism burden on semi-quantitative culture. This study definition was based on the 2010 ISHLT definitions of pneumonia and trachobronchitis. Since moderate or greater burdens of pathogenic organisms are typically treated with antibiotics per UCSF protocols, we added high organism burden as a criteria (16).
Daily mycophenolate mofetil, prednisone, and tacrolimus doses were abstracted from clinical charts. Tacrolimus troughs were assayed on whole blood by the clinical lab using the Architect Immunoassay (Abbott Park, IL). The tacrolimus trough value closest in time to the study visit was used.
Sample Size and Statistical Analysis
The target enrollment was 50 subjects, which was estimated to provide 97% power to identify subjects at risk for acute cellular rejection and 89% power to identify subjects at risk for infections, assuming a 5% incidence of ≥ A2-grade rejection and an 18% incidence of infection. These power calculations were performed based on two samples t-tests (17) with effective sample sizes adjusted for intracluster correlation (18).
Reproducibility of MRE measurements was determined by Pearson’s product moment correlation. Associations between MRE, weeks after transplantation and tacrolimus trough level were visualized using generalized additive modeling. Associations with infection and rejection status, time post-transplant, and immunosuppressive agent doses and trough levels were tested using separate linear models adjusted with generalized estimating equations (GEE) to account for repeated measures on individual subjects. The local hazard ratios for infection versus MRE and tacrolimus trough level were determined using a logistic generalized additive mixed model, with subject identifier as a random effect and MRE and tacrolimus trough level as a fixed effect. Statistical analyses were performed in R (version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria) using the “pwr,” “gee,” “mgcv,” and “nplr” packages (17, 19, 20).
RESULTS:
Subject characteristics of the included subject population are shown in Table 1. Of enrolled subjects, interstitial lung diseases and double lung transplants are more frequent than in ISHLT registry populations (7). A flow diagram of subject enrollment and study sample collection is shown in the Supplement. There were 117 MRE assessments included on 44 subjects. Of these assessments, 17 were at the time of bronchoscopically-detected infection and 11 were at the time of acute cellular rejection, including two events with concurrent infection and rejection.
Table 1 –
Subject Characteristics
| Subjects included, N | 44 |
|---|---|
| Age, mean (SD) | 58 (11) |
| Male gender, N (%) | 34 (77%) |
| Transplant type, N (%) | |
| Double lung | 42 (95%) |
| Single lung | 2 (5%) |
| Ethnicity, N (%) | |
| White, non-Hispanic | 32 (73%) |
| Hispanic | 9 (20%) |
| Black | 3 (7%) |
| Diagnosis group, N (%) | |
| A – Obstructive | 8 (18%) |
| B – Pulmonary vascular | 1 (2%) |
| C – Cystic fibrosis | 2 (5%) |
| D – Restrictive | 33 (75%) |
To assess MRE assay reproducibility in our laboratory, we compared MRE values determined from a single study visit using two different quantitative PCR devices. After correcting for the small bias between the two devices described in Supplemental Figure 2, we found a correlation coefficient of 0.98 (95% CI 0.94–0.99) for 17 paired MRE measurements, suggesting high reproducibility. There were no statistically significant associations between MRE and the time intervals between the tacrolimus doses or between tacrolimus dose and peak level blood draw (Supplemental Figure 3).
Immunosuppression effects on MRE
MRE is expected to vary inversely with immunosuppression; thus, we predicted that MRE will increase over time post-transplant because of protocoled reductions in tacrolimus trough targets. As shown in Figure 1A, we did observe a positive correlation with time post-transplant, with MRE increasing an average of 0.35% (95% CI 0.001 – 0.69%) per week. While one might expect MRE levels to decrease with increasing trough tacrolimus levels, previous studies showed an association with tacrolimus peak but not with trough levels (6). Similarly, we did not observe a significant association between MRE and tacrolimus trough (P = 0.11, Figure 1B) or daily dose (P = 0.47, not shown). There was also no association between MRE and daily mycophenolate mofetil dose (P = 0.46, Figure 1C). However, MRE decreased 1.7% (95% CI 3.3 – 0.1%) per additional mg of daily prednisone (Figure 1D).
Figure 1: Mean Residual Expression (MRE) values as a function of time post-transplant and tacrolimus trough, prednisone dose, and mycophenolate mofetil dose.
MRE increased by 0.35% (95% CI 0.001 – 0.69%, P = 0.049) per week following transplant (A). There was no statistically significant association between MRE and tacrolimus trough value (B, P = 0.11) or mycophenolate mofetil dose (C, P = 0.46), however MRE decreased by 1.7% (95% CI 0.1 – 3.3%, P = 0.04) per mg of daily prednisone dose (D). Local estimated mean values (solid lines) with 95% confidence intervals (dashed lines) were determined by generalized additive modeling.
We sought to determine if prednisone could synergize with tacrolimus in repressing NFAT-dependent cytokine signaling, as suggested by Figure 1B. To model this ex vivo, we incubated whole blood from 5 non-transplanted control subjects with variable concentrations of prednisolone and 0 or 10 ng/ml of tacrolimus and calculated the MRE. Oral prednisone is converted into biologically active prednisolone, and transplant recipient plasma prednisolone concentrations are approximately 10 ng/ml per 1 mg of oral prednisone (21). As shown in Figure 2, we found that MRE peaked at the very low prednisolone concentration of 20 ng/ml, which is roughly equivalent to that produced by a 2 mg dose of prednisone. MRE was decreased at prednisolone levels equivalent to those seen in our study cohort of 100 and 200 ng/ml, corresponding to prednisone doses of 10 and 20 mg, respectively, compared with 0 or 20 ng/ml of prednisolone (P ≤ 0.005). The effect of prednisone on each gene was similar in both the cohort and ex vivo studies.
Figure 2: Ex-vivo MRE as a function of prednisolone level in healthy control subjects.
Whole blood from non-immunosuppressed healthy controls (N = 5) was incubated with or without 10 ng/ml tacrolimus and 0, 20, 100, or 200 ng/ml of prednisolone. Grey lines indicate individual subjects. Mean and robust standard errors are shown in black.
Association between MRE and primary outcomes
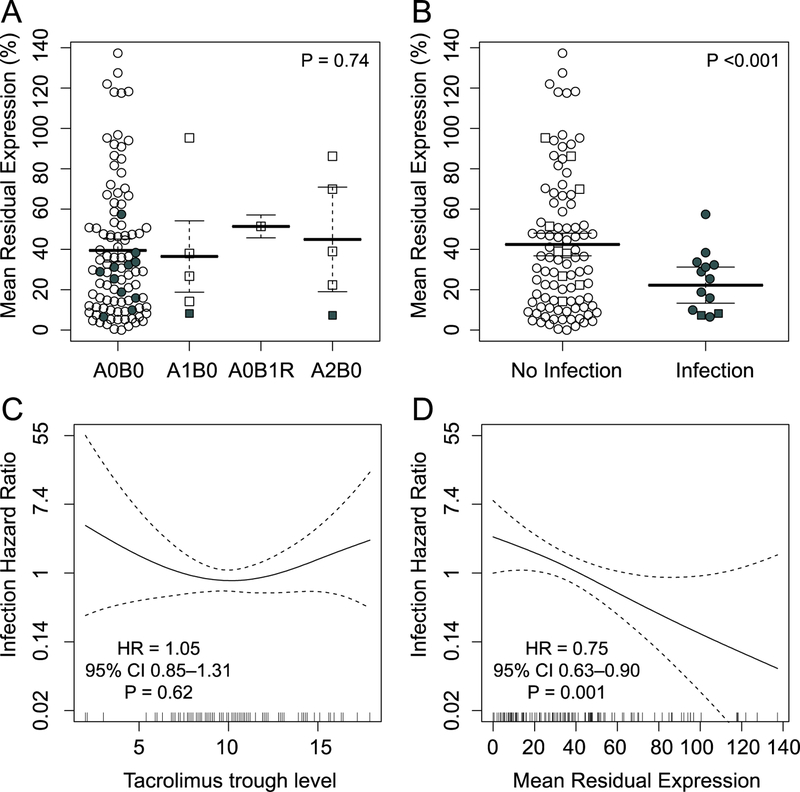
Our primary aim was to assess for an association between MRE and acute cellular rejection and bronchoscopically-detected infection, analogous to what has been seen in the context of other solid organ transplants. As shown in Figure 3A, we did not observe differences in the distribution of MRE when transbronchial biopsies showed acute cellular rejection pathology as compared with biopsies showing no rejection (P = 0.74). However, we did observe significantly lower MRE during infection compared with no infection, with a difference in means of 20% (95% CI 11% – 29%, P < 0.001, Figure 3B). This difference remained when time post-transplant, tacrolimus trough, and prednisone dose were included in the GEE-adjusted model (β = 19%, 95% CI 9% – 29%, P < 0.001). Interestingly, subjects with concurrent rejection and infection at the time of MRE analysis had some of the lowest MRE values. We compared tacrolimus trough and MRE for an association with bronchoscopically-detected infection risk by multivariable modeling (Figure 3C–D), finding MRE to be superior to tacrolimus trough in assessing infection risk. The hazard ratio for infection per 10% increase in MRE was 0.75 (95% CI 0.63 – 0.90, P = 0.001), while there was no statistically significant association between tacrolimus trough and infection risk (HR 1.06, 95% CI 0.85 – 1.31, P = 0.62). There were no statistically significant interactions between week after transplant, infection, and prednisolone dose when these terms considered in pairwise manner in association with MRE. However, a trend towards a negative interaction between week post-transplant and infection (P = 0.06) suggests that MRE might be lower in late infections. The hazard for infection appeared linear over a range of MRE values (Figure 3D). Of the three cytokines that make up the MRE value, IL-2 had the strongest association with infection risk.
Figure 3: Association between MRE and tacrolimus levels and rejection and infection outcomes.
MRE is shown stratified by (A) rejection grade and (B) the presence of infection, with bars indicating mean and 95% confidence intervals derived from GEE-adjusted models. Open circles indicate neither infection nor rejection, square symbols indicate rejection, and filled symbols indicate infection. The presence of any type of rejection on biopsy was not associated with MRE (P = 0.74), however mean values for MRE were 20% lower (95% CI 11% – 29%, P <0.001) at the time of infection. The hazard ratios for infection as a function of tacrolimus trough level (C) and MRE (D) are shown, with overall hazard ratios calculated by GEE-adjusted linear models. The hazard ratio for infection was 0.75 (95% CI 0.63 – 0.89) per 10% increase in MRE after adjusting for tacrolimus trough level.
Pre-transplant tacrolimus IC50
We sought to determine the extent to which intrinsic subject characteristics contributed to the variability in immunosuppression as measured by MRE. In a post hoc analysis, we determined tacrolimus IC50 values using flow cytometry for 21 of the subjects in our study for whom pre-transplant PBMC samples were available. In addition to the IL-2 and interferon-γ cytokine production measured in the MRE assay, we also included TNF-α. As shown in Figure 4A, we observed 2- to 4-fold differences across individuals in the IC50 for these cytokines pre-transplant. Interestingly, there were also significant differences between cytokines, with IL-2 having the lowest IC50 and TNF-α having the greatest. There were also statistically significant, positive correlations across individuals for most of the tested cytokine IC50 values, suggesting that some individuals were generally more sensitive to tacrolimus than others. Contrary to our hypothesis, we did not observe an increase in mean MRE across subjects with increasing pre-transplant tacrolimus IC50 (Figure 3B). We also did not find positive correlations between IC50 and mean residual expression of individual cytokines IL-2 and interferon-γ (not shown).
Figure 4: Variability in T cell inhibition by tacrolimus.
(A) The concentration of tacrolimus required to reduce stimulated IL-2, Interferon-γ, and TNF-α expression by 50% (IC50) was determined for CD4+ and CD8+ T cells isolated pre-transplant from study subjects, where available. Lines indicate individual subjects. Significant heterogeneity was observed between subjects and cytokines, with inhibition IL-2 production requiring the least tacrolimus. The significance of unadjusted paired t-test comparisons between groups are indicated as *, P <0.05; ***, P <0.001. (B) Pre-transplant tacrolimus IC50 for all three cytokines is shown as a function of average value of MRE for each subject, with regression lines. There was no significant correlation between IC50 and mean MRE for CD4+ or CD8+ T cells.
DISCUSSION:
We found that suppressed MRE values were associated with bronchoscopically-detected infections, independent of tacrolimus trough levels. MRE levels increased over time as immunosuppression was weaned and decreased with increasing prednisone doses. Finally, MRE values and pre-transplant sensitivity to tacrolimus varied widely across the cohort, demonstrating the important variations in individual responses immunosuppressive medications.
Previous studies in renal transplantation identified an MRE cutoff of around 30% to discriminate between high and low risk of infection (6). Similarly, we observed here that bronchoscopically-detected infection was rare above an MRE value of 40%. Because of the repeat measure study design, we could not test for an association between MRE and infections not detected at the time of bronchoscopy, including extra-pulmonary infections. These are potentially relevant as cytomegalovirus, for example, has been shown to lower MRE (22).
We did not observe differences in the risk of rejection based on MRE value. While missed study visits and a higher than expected standard deviation for observed MRE values diminished our statistical power somewhat, the available data suggest that MRE values may not be helpful in identifying lung allograft recipients at increased risk for rejection. Interestingly, the ImmunoKnow assay, which has the practical advantage of requiring only one blood sample, also performed better at assessing infection compared with rejection risk (23). Compared to renal allografts, lung allografts may be more antigenic and require greater immunosuppression (24). It is possible that variation in tacrolimus levels within the targets used early after lung transplantation has little impact on the risk of rejection. Consistent with this notion, we previously reported a plateau in the risk of acute rejection as a function of tacrolimus trough beyond a level of 8–10 ng/mL (25). Indeed, highly potent immunosuppression could even drive rejection though the increased innate immune signaling associated with airway infection.
The finding that MRE decreased with increasing prednisone doses suggests that prednisone may act synergistically with tacrolimus in repressing NFAT-dependent cytokines. Our studies on healthy control samples also demonstrated an effect of steroid dose in the context of the MRE assay. Proinflammatory cytokine gene expression has been shown to increase with low steroid doses and then decrease at the doses typically administered in solid organ transplantation, but these data are the first demonstration of this effect in a context of cytokine suppression by a fixed dose of calcineurin inhibitor (26). The steroid-responsive transcription factor AP-1 also plays an important role in the expression of NFAT-associated genes and so synergistic crosstalk between AP-1 and NFAT signaling pathways may render the MRE assay dependent on other immunosuppressive medications (27). Synergistic effects of steroids and calcineurin inhibitors may limit the specificity of the MRE assay as a measure of tacrolimus-mediated immunosuppression (27, 28).
An important finding of this paper is that immunosuppressive medication sensitivity, whether measured by MRE or by IC50, varies substantially between individuals. Because tacrolimus partitions into red blood cells, serum concentrations can be more than 10-fold lower than whole blood levels, so the observed IC50 values occur within the range of plasma concentrations that are typical in transplant recipients (29). It is intriguing to consider the potential impacts of variable activation of cytokines as tacrolimus concentrations fluctuate throughout the day. While we hypothesized that high IC50 values would predict high average MRE, this was not observed. Although we measured IC50 with interferon-γ, IL-2, and TNF-α production but MRE with interferon-γ, IL-2, and GM-CSF, it is unlikely that this discrepancy accounted for the lack of correlation, as no correlation was observed for the individual cytokines. Indeed, TNF-α is reportedly more narrowly regulated by NFAT subtypes and thus might be a more potent driver of MRE (30). Beyond static patient factors like genotype-determined tacrolimus sensitivity, a number of clinical factors, including age, medical comorbidities, acute and chronic infections, and tacrolimus pharmacokinetics, could contribute to the observed substantial variation in MRE between individuals.
In conclusion, these data demonstrate the potential clinical utility of MRE measurement in lung allograft recipients to identify subjects at increased risk for infection, as well as its limitations. Interestingly, we found that this assay was dependent on prednisone dose, suggesting an interaction between the tacrolimus and prednisone signaling pathways in modulating NFAT-related gene expression. These studies highlight the inadequacies of current methods for lung transplant immunosuppression monitoring and suggest that strategies to characterize the degree of immunosuppression could be of value in lung transplant recipients.
Supplementary Material
• Supplemental Figure 1: Flow diagram of study subject recruitment and sample collection.
• Supplemental Figure 2: Comparison of gene expression measurements across quantitative PCR devices.
• Supplemental Figure 3: Pharmacokinetic effects on MRE measurements
• Supplemental Table 1: Clinical data for bronchoscopically-determined infection cases
• Supplemental Methods: MRE Assay, MRE and Steroid Interaction Assay, and Flow cytometric measurement of tacrolimus 50% inhibitory concentration (IC50)
ACKNOWLEDGEMENTS:
The authors would like to thank Joey Leung and Allison Webber for advice regarding study design and methodology. We thank Charlene Fong, Chiyo Uchida, Edelyn Bautista, Linda Nicola, Mary Beier, Daphne Penaflor, Michelle Devaux, Michelle Ramirez, Fredde Foster, David Prince, AnaMarie Goscila, Mary Heindel, Ivy Sparks, Irene Junejo, Bonnie Slater, Cecilia Lemieux, Paul Tan, Nelson Mercado, Anna Volfson, Karen Neun, Blake Young, Juliet Zabel help with collecting study samples.
Funding: Astellas Pharma Global Development, Inc. primarily funded this investigator-initiated study, but had no role in data collection, analysis, interpretation, nor approval of the final manuscript. JRG is also supported by Award Number IK2CX001034 from the Clinical Sciences Research & Development Service of the VA Office of Research and Development.
Abbreviations:
- AP-1
Activator protein 1
- ATP
adenosine triphosphate
- BAL
bronchoalveolar lavage
- CI
Confidence Interval
- CLAD
chronic lung allograft dysfunction
- GEE
generalized estimating equations
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IC50
concentration inhibiting 50%
- IL-2
Interleukin-2
- ISHLT
International Society of Heart and Lung Transplantation
- MRE
Mean residual expression of calcineurin-dependent genes
- N
Number
- NFAT
nuclear factor of activated T-cells
- PBMC
peripheral blood mononuclear cell
- PCR
Polymerase chain reaction
- PMA
phorbol 12-myristate 13-acetate
- SD
Standard deviation
- TNF-α
Tumor necrosis factor alpha
- UCSF
University of California, San Francisco
Footnotes
ClinicalTrials.gov Identifier:
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES:
- 1.Valapour M, Skeans MA, Smith JM et al. OPTN/SRTR 2015 Annual Data Report: Lung. Am J Transplant 2017;17 Suppl 1:357–424. [DOI] [PubMed] [Google Scholar]
- 2.Winkler M, Ringe B, Baumann J et al. Plasma vs whole blood for therapeutic drug monitoring of patients receiving FK 506 for immunosuppression. Clin Chem 1994;40(12):2247–2253. [PubMed] [Google Scholar]
- 3.Giese T, Zeier M, Schemmer P et al. Monitoring of NFAT-regulated gene expression in the peripheral blood of allograft recipients: a novel perspective toward individually optimized drug doses of cyclosporine A. Transplantation 2004;77(3):339–344. [DOI] [PubMed] [Google Scholar]
- 4.Sommerer C, Meuer S, Zeier M et al. Calcineurin inhibitors and NFAT-regulated gene expression. Clin Chim Acta 2012;413(17–18):1379–1386. [DOI] [PubMed] [Google Scholar]
- 5.Sommerer C, Hartschuh W, Enk A et al. Pharmacodynamic immune monitoring of NFAT-regulated genes predicts skin cancer in elderly long-term renal transplant recipients. Clin Transplant 2008;22(5):549–554. [DOI] [PubMed] [Google Scholar]
- 6.Sommerer C, Zeier M, Meuer S et al. Individualized monitoring of nuclear factor of activated T cells-regulated gene expression in FK506-treated kidney transplant recipients. Transplantation 2010;89(11):1417–1423. [DOI] [PubMed] [Google Scholar]
- 7.Yusen RD, Christie JD, Edwards LB et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32(10):965–978. [DOI] [PubMed] [Google Scholar]
- 8.Burton CM, Iversen M, Carlsen J et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant 2009;28(9):888–893. [DOI] [PubMed] [Google Scholar]
- 9.Ng CY, Madsen JC, Rosengard BR et al. Immunosuppression for lung transplantation. Front Biosci (Landmark Ed) 2009;14:1627–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shino MY, Weigt SS, Saggar R et al. Usefulness of immune monitoring in lung transplantation using adenosine triphosphate production in activated lymphocytes. J Heart Lung Transplant 2012;31(9):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webber A, Hirose R, Leung C et al. NFAT-Dependent Cytokine and p70S6 Kinase Activity Assays as Immune Monitoring Tools for Transplant Patients on CNIs and mTOR Inhibitors [abstract]. Am J Transplant 2013;13(suppl 5). [Google Scholar]
- 12.Sommerer C, Morath C, Giese T et al. Activity of nuclear factor of activated T cells is independent of the number of peripheral lymphocytes in FTY720-treated patients. Transplant Proc 2008;40(5):1416–1418. [DOI] [PubMed] [Google Scholar]
- 13.Zahn A, Schott N, Hinz U et al. Immunomonitoring of nuclear factor of activated T cells-regulated gene expression: the first clinical trial in liver allograft recipients. Liver Transpl 2011;17(4):466–473. [DOI] [PubMed] [Google Scholar]
- 14.Konstandin MH, Sommerer C, Doesch A et al. Pharmacodynamic cyclosporine A-monitoring: relation of gene expression in lymphocytes to cyclosporine blood levels in cardiac allograft recipients. Transpl Int 2007;20(12):1036–1043. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Fishbein MC, Snell GI et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26(12):1229–1242. [DOI] [PubMed] [Google Scholar]
- 16.Husain S, Mooney ML, Danziger-Isakov L et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011;30(4):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champely S pwr: Basic Functions for Power Analysis In. 1.2–1 ed., 2017. [Google Scholar]
- 18.Piaggio G, Carroli G, Villar J et al. Methodological considerations on the design and analysis of an equivalence stratified cluster randomization trial. Stat Med 2001;20(3):401–416. [DOI] [PubMed] [Google Scholar]
- 19.Carey VJ, Lumley T, Ripley B. gee: Generalized Estimation Equation Solver In., 2015.
- 20.Wood SN. Generalized Additive Models: An Introduction with R Boca Raton, FL: CRC Press, 2006. [Google Scholar]
- 21.Jusko WJ, Ferron GM, Mis SM et al. Pharmacokinetics of prednisolone during administration of sirolimus in patients with renal transplants. J Clin Pharmacol 1996;36(12):1100–1106. [DOI] [PubMed] [Google Scholar]
- 22.Steinebrunner N, Sandig C, Sommerer C et al. Reduced residual gene expression of nuclear factor of activated T cells-regulated genes correlates with the risk of cytomegalovirus infection after liver transplantation. Transpl Infect Dis 2014;16(3):379–386. [DOI] [PubMed] [Google Scholar]
- 23.Bhorade SM, Janata K, Vigneswaran WT et al. Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant 2008;27(9):990–994. [DOI] [PubMed] [Google Scholar]
- 24.Lee WP, Yaremchuk MJ, Pan YC et al. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg 1991;87(3):401–411. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese DR, Boettger R, Dewey K et al. Impact of CYP3A5, CYP3A4, and ABCB1 Genotypes on Lung Transplant Recipient Early Clinical Outcomes [abstract]. J Heart Lung Transplant 36(4):S151. [Google Scholar]
- 26.Lim HY, Muller N, Herold MJ et al. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 2007;122(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene 2001;20(19):2476–2489. [DOI] [PubMed] [Google Scholar]
- 28.Adcock IM. Molecular mechanisms of glucocorticosteroid actions. Pulm Pharmacol Ther 2000;13(3):115–126. [DOI] [PubMed] [Google Scholar]
- 29.Warty V, Zuckerman S, Venkataramanan R et al. Tacrolimus analysis: a comparison of different methods and matrices. Ther Drug Monit 1995;17(2):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaminuma O, Kitamura F, Kitamura N et al. Differential contribution of NFATc2 and NFATc1 to TNF-alpha gene expression in T cells. J Immunol 2008;180(1):319–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
• Supplemental Figure 1: Flow diagram of study subject recruitment and sample collection.
• Supplemental Figure 2: Comparison of gene expression measurements across quantitative PCR devices.
• Supplemental Figure 3: Pharmacokinetic effects on MRE measurements
• Supplemental Table 1: Clinical data for bronchoscopically-determined infection cases
• Supplemental Methods: MRE Assay, MRE and Steroid Interaction Assay, and Flow cytometric measurement of tacrolimus 50% inhibitory concentration (IC50)