Abstract
Objective.
An intimate association exists between oxidative stress and inflammation. Because adipose tissue (AT) inflammation is intricately linked to metabolic disorders, we hypothesized that reducing oxidative stress will be effective in ameliorating AT inflammation in obesity.
Methods.
Wild type mice were fed a high fat diet (HF) for 8 wk followed by 2 wk treatment with nanoformulated copper/zinc superoxide dismutase (NanoSOD). The mice were divided into: 1) Chow diet, 2) HF, and 3) HF+NanoSOD.
Results.
The HF+NanoSOD-treated mice showed a significant decrease in plasma and liver triglycerides (TGs) compared to HF-fed mice. Interestingly, NanoSOD reduced the expression of macrophage and inflammatory markers in visceral AT (VAT) and stromal cells derived from VAT. Moreover, the activation of pro-inflammatory signaling pathways, in particular, the extracellular signal-regulated kinases, was blunted in VAT upon NanoSOD treatment. However, markers of oxidative stress were not altered significantly in HF+NanoSOD group in our experimental conditions. Pre-treatment of either macrophages or adipocytes significantly reduced the inflammatory response invoked in an in vitro co-culture system further supporting the role of NanoSOD in inhibiting obesity-linked inflammation.
Conclusion.
Our data suggest that NanoSOD is effective in reducing AT macrophage accumulation and AT inflammation, and also in promoting TG metabolism in obesity.
Introduction
Adipose tissue (AT) inflammation characterized by macrophage accumulation and secretion of inflammatory mediators is an underlying factor leading to obesity-linked metabolic disorders (1, 2). Oxidative stress is a prevalent condition in obesity. When obesity persists for a prolonged period of time, antioxidant sources can be depleted, decreasing the activity of enzymes such as superoxide dismutase (SOD) and catalase (CAT) in erythrocytes (3). The activity of SOD and glutathione peroxidase in individuals with obesity is significantly lower compared with that in healthy persons (4). In addition to erythrocyte levels, AT levels of the antioxidant enzymes are reduced in obesity (5). The levels of carbonylated proteins, a marker of oxidative stress, have been shown to be increased in the subcutaneous fat in obese human subjects (6). These studies indicate that oxidative stress is closely associated with obesity and that targeting this process may be effective in alleviating obesity-linked metabolic disorders.
Despite the strong association between oxidative stress and obesity, there is a paucity of evidence suggesting the potential of superoxide dismutase in ameliorating obesity-associated metabolic disorders. Pires et al. have shown that treatment with MnTBAP, a MnSOD mimetic, reduces visceral adiposity and AT inflammation (7). The effectiveness of antioxidant therapy still remains questionable due to the failure in clinical trials that addressed the benefits of using antioxidant vitamins in treating human diseases (8, 9). The lack of potent and highly bioavailable antioxidants is considered to be a major challenge in antioxidant therapy. With the advent of nanotechnology, research on antioxidant enzyme therapy has gained increased momentum.
Several types of nanoformulated antioxidant enzymes, in particular, SOD1, have been designed and studied for their efficacy in ameliorating various diseases. For example, liposome-encapsulated Cu/ZnSOD (SOD1) reduced angiotensin II (Ang II)-induced hypertension (10). Rosenbaugh et al. have reported the benefits of polyethyleneimine-polyethylene glycol (PEI-PEG)-conjugated Cu/ZnSOD nanozyme in attenuating angiotensin II-induced pressor response in rabbits (11). Recently, a novel formulation of Cu/ZnSOD was developed wherein SOD1 is covalently stabilized and cross-linked to improve the stability and efficacy of SOD1 in vivo. Manickam et al. have reported that this formulation of Cu/ZnSOD (hereafter referred to as NanoSOD) is very effective in scavenging superoxide in cultured endothelial and neuronal cells in vitro and in reducing ischemic brain injury in rats (12). In the current study, we sought to determine the effectiveness of NanoSOD in reducing AT inflammation in obesity. Because oxidative stress is prevalent in obesity and because agents with antioxidant properties can reduce inflammation, we hypothesized that NanoSOD will be effective in reducing AT inflammation and systemic metabolic homeostasis in a model of diet-induced obesity.
Materials and Methods
Mice and Diet.
Male wild type C57BL/6 mice were purchased from Jackson lab and fed a chow diet (CD, Purina Lab Diet #5001) or a high fat diet (HF, Research Diets Inc. #D12451) for 8 wk. The diet provides 20% protein, 35% carbohydrates and 45% fat in terms of calories. Lard was used as a fat source.
NanoSOD preparation.
NanoSOD was synthesized and characterized as reported earlier (12). Briefly, native bovine CuZnSOD protein (Sigma–Aldrich) was mixed with poly L-lysine–polyethylene glycol copolymer (PLL–PEG). The Cu/ZnSOD and PLL–PEG complex was covalently stabilized using a reducible cross-linker, 3,3′-dithiobis(sulfosuccinimidylproprionate) (DTSSP; Thermo Fisher Scientific). The size of NanoSOD was estimated to be ~35 nm.
NanoSOD treatment.
Eight week post-diet, the mice were divided into 3 groups: 1) chow diet (CD), 2) high fat diet (HF), and 3) high fat diet + NanoSOD (HF+NanoSOD). The HF-fed mice were weight-matched before the treatment. A cohort of HF diet-fed mice were injected intraperitoneally (i.p.) with NanoSOD once every other day for a period of 15 days (8 injections total). The mice were continued on respective diets until sacrifice. Lean and fat mass were measured by EchoMRI before sacrifice. Twenty four hour post-injection of the last dose of NanoSOD, the mice were sacrificed after 5 h fasting. All animal care procedures were carried out with approval from the Institutional Animal Care and Use Committee of VA Nebraska-Western Iowa Health Care System.
Metabolic variables.
Blood glucose was measured using the Accuchek Aviva glucometer. Plasma free fatty acids were measuring using a kit (Wako). Plasma total cholesterol and TGs were measured using kits from Raichem. Plasma insulin was measured using kits from Mercodia.
In vivo metabolic studies.
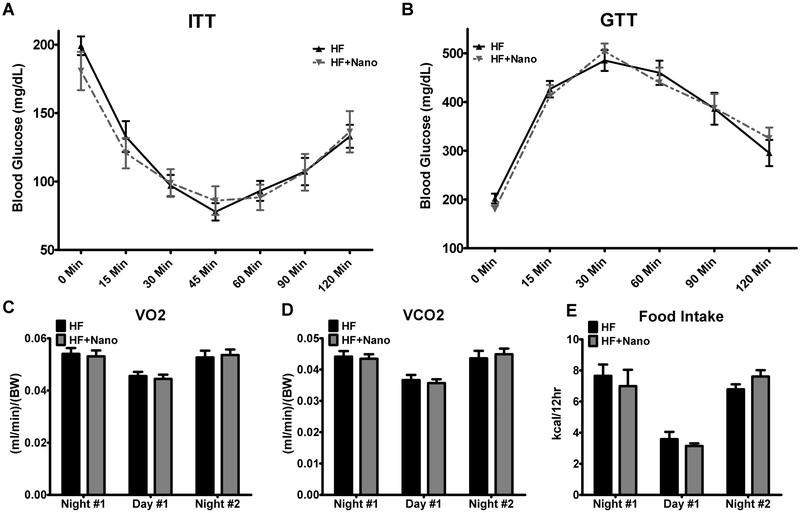
A separate set of mice were used for energy expenditure and, intraperitoneal insulin (ITT) and glucose tolerance tests (GTT). Because of the short duration of the treatment, we measured energy expenditure after 2 or 4 injections, ITT after 5 injections, and GTT after 7 injections of NanoSOD. For energy expenditure studies, mice were acclimatized for 2 days and housed individually in Promethion cages (Sable Systems Int). We recorded VO2, VCO2, and food intake for 36 h. Measurements for 12 h light and 12 h dark cycles are reported separately. For ITT and GTT, mice were fasted for 5 h and injected (i.p.) with insulin (0.75 units/kg body weight) and glucose (1g/kg body weight), respectively. Changes in blood glucose levels were recorded at various time points.
Isolation of stromal vascular cells (SVCs).
SVCs were isolated from VAT as we described earlier (13).
Real-time PCR analysis.
Real-time PCR analysis was carried out using primers from Applied Biosystems. A ΔΔCT method was used to calculate gene expression and the values were normalized to 18S.
Western blot analysis.
Antibodies against ERK and phospho ERK, were purchased from Cell Signaling Technologies. Antibodies for SOD1, MCP-1, and GAPDH were from Santa Cruz Biotechnology. IRDye 680 and 800 conjugated secondary antibodies were obtained from LI-COR Biosciences.
Histology.
Liver sections were stained with hematoxylin and eosin (H&E). For immunofluorescence, the VAT sections were incubated with anti-F4/80 primary antibody (AbD Serotec) followed by incubation with Alexa Fluor 568 conjugated secondary antibody (Life Technologies). The sections were mounted with antifade gold containing DAPI (4’,6-diamidino-2-phenylindole, Invitrogen). The pictures were taken at 10X or 20X magnification using a Nikon eclipse 80i inverted fluorescence microscope.
Macrophage and adipocyte co-culture.
Thioglycollate-elicited mouse primary peritoneal macrophages were collected as we described previously (14). Differentiation of 3T3-L1 cells to adipocytes was performed as we reported earlier with minor modifications (15). We obtained hypertrophic adipocytes (adipocytes with large lipid droplets) by maintaining them in culture for 4–6 wk before they were used for the co-culture experiments. In Experiment 1, macrophages were pre-treated with NanoSOD for 3h followed by rinsing in PBS. Macrophages were then added onto adipocytes at 40,000 cells/well in 12-well culture dishes. We collected media after 24 h of co-culture and cell lysates after 48 h. In Experiment 2, adipocytes were pre-treated with NanoSOD for 6 h followed by rinsing in PBS. Untreated control macrophages were then added onto adipocytes and media and cell lysates were collected. The media was analyzed for MCP-1 by enzyme-linked immunosorbent assay (ELISA) and cell lysates for SOD1 content by western blot analysis.
ELISA.
MCP-1 protein in cell culture supernatant was measured by sandwich ELISA. Briefly, diluted cell culture supernatant was added to a 96-well plate that was coated with hamster anti-mouse MCP-1 antibody (BD Biosciences). After careful washes, biotin-conjugated hamster anti-mouse MCP-1 antibody was added (BD Biosciences). Next, avidin peroxidase was added followed by color developing reagents.
Gas chromatography.
Hepatic tryglycerides were analyzed by gas chromatography at the Lipid Core Laboratory of Vanderbilt University (SV 2006).
Statistical analysis.
Results are presented as mean ± SEM. Statistical significance was determined by the one-way analysis of variance followed by Bonferroni’s post-hoc analysis. In experiments involving 2 groups, a one-tailed Student’s t test was performed. Graph-Pad Prism software was used to determine statistical significance (P<0.05 was considered significant). Outliers were detected using the Grubb’s test or box plot analysis (values ± 1.5 times the interquartile range) and were removed from the data set.
Results
Effect of NanoSOD on metabolic variables
The body weight, body weight gain, and fat mass were significantly higher in HF and HF+NanoSOD groups compared to CD controls (Table 1). The weights of individual fat pads such as VAT, subcutaneous AT and perirenal AT were significantly higher in HF and HF+NanoSOD groups compared to CD controls. However, no significant difference was noted between HF and HF+NanoSOD treated mice. The plasma total cholesterol was increased significantly (P<0.0001) in both HF and HF+Nano groups compared to CD controls (Figure 1 A). The plasma FFAs were not altered whereas plasma triglycerides (TGs) were significantly reduced (P<0.01) in HF+Nano group (P<0.05) compared to HF-fed mice (Fig. 1B&C). The fasting blood glucose level was significantly increased in HF (P<0.01) but not in HF+NanoSOD mice compared to CD-fed mice. The plasma insulin level was significantly higher (P<0.01) in HF+Nano group compared to CD controls. HOMA-IR, a measure of insulin resistance, was significantly increased in both HF (P<0.01) and HF+NanoSOD (P<0.05) treated mice (Fig. 1D-F). Insulin and glucose tolerance tests revealed that the response of HF+Nano mice to acute insulin or glucose exposure was not altered compared to HF controls (Fig. 2A&B). Analysis of energy expenditure revealed that VO2 and VCO2 did not vary between HF and HF+Nano groups after 2 or 4 injections with NanoSOD (Fig 2C&D). Also, food intake was not altered between HF and HF+Nano treated mice (Fig. 2E). Together, these data suggest that NanoSOD treatment for 2 wk resulted in reduced plasma TG without improvements in glucose handling.
Table 1:
Effect of NanoSOD on metabolic parameters
| Measurement | n | CD | HF | HF+Nano |
|---|---|---|---|---|
| Body Wt. (g) | 20-22 | 26.3 ± 0.5 | 34.9 ± 0.8c | 34.2 ± 0.8c |
| Body Wt. Gain (g) | 20-22 | 5.3 ± 0.3 | 12.5 ± 0.7c | 12.6 ± 0.6c |
| Fat Mass (g) | 14-16 | 2.35 ± 0.13 | 9.69 ± 0.6c | 9.48 ± 0.59c |
| Lean Mass (g) | 14-16 | 22.5 ± 0.7 | 24.2 ± 0.7 | 23.5 ± 0.5 |
| Liver Wt. (g) | 20-22 | 1.12 ± 0.04 | 1.17 ± 0.06 | 1.13 ± 0.03 |
| VAT Wt. (g) | 20-22 | 0.39 ± 0.02 | 1.94 ± 0.08c | 1.73 ± 0.12c |
| Subcutaneous AT Wt. (g) | 6-8 | 0.08 ± 0.01 | 0.40 ± 0.07a | 0.43 ± 0.05b |
| Peri-Renal AT Wt. (g) | 6-8 | 0.08 ± 0.02 | 0.55 ± 0.09a | 0.78 ± 0.10b |
P<0.01
P<0.001
P<0.0001 vs. CD. Values are mean ± SEM for each group.
Fig.1.
Effect of NanoSOD on metabolic variables in mice exhibiting diet-induced obesity. Total cholesterol (A), free fatty acids (B), triglycerides (C), fasting blood glucose (D), insulin (E), and insulin resistance via HOMA-IR (F) were recorded. Values are expressed as mean ± SEM of 12-14 samples in each group. *P<0.05, **P<0.01, & ****P<0.0001 vs CD; ^P<0.05 vs HF. CD, chow diet; HF, high fat diet; and Nano, NanoSOD.
Fig.2.
Effect of NanoSOD on insulin and glucose tolerance, energy expenditure, and food intake. Fasted mice were intraperitoneally injected with insulin (A) and glucose (B), and blood glucose was recorded at select time points. Energy expenditure in the form of VO2 (C) and VCO2 (D) and Food intake (E) was measured using a Promethion metabolic system. Energy expenditure was measured after 2 or 4 injections and values are expressed as mean ± SEM of 7-8 samples in each group. Food intake was measured after 4 or 5 injections and values are mean ± SEM of 4-5 samples in each group. HF, high fat diet; and Nano, NanoSOD.
Effect of NanoSOD on VAT inflammation
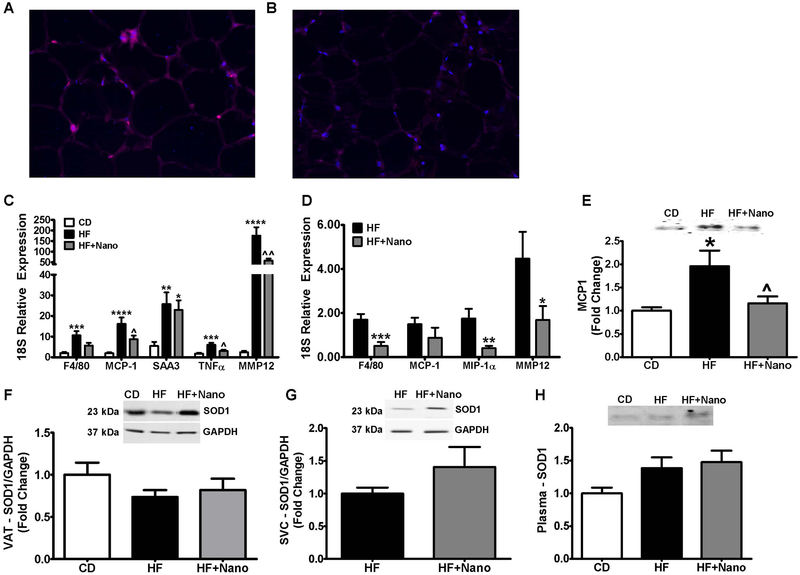
Because macrophages accumulate in AT in obesity and secrete inflammatory mediators, we next analyzed macrophage and inflammatory markers in whole VAT. Immunofluorescence analysis revealed that F4/80, a macrophage marker, is lower in HF+Nano group compared to HF-fed mice (Fig. 3A & B). Real-time PCR analysis of VAT for the mRNA expression of F4/80 showed a profound increase only in HF-fed mice whereas HF+Nano treated mice showed a reduction (Fig. 3C). The markers of inflammation including MCP1, TNFα, and MMP12 were significantly increased in HF diet-fed mice compared to CD controls whereas treatment with NanoSOD greatly reduced the expression of these genes. We next analyzed the mRNA levels of macrophage and/or inflammatory markers in macrophage-rich SVCs collected from VAT. As shown in Fig. 3D the mRNA level of F4/80 was significantly reduced upon treatment with NanoSOD compared to HF controls. Moreover, we noted that markers of inflammation such as MIP1α and MMP12 were significantly lower in NanoSOD-treated mice compared to HF controls.
Fig.3.
Effect of NanoSOD on VAT and systemic inflammation, and SOD1 bioavailability. VAT sections from HF (A) and HF+Nano (B) were assessed for F4/80 expression and fluorescent images obtained at 20X magnification. Markers of inflammation were determined in the VAT (C), and SVC (D) via real-time PCR. MCP1 protein levels were also analyzed in the plasma (E). SOD1 bioavailability was determined in VAT (F), SVCs (G), and plasma (H) via western blot analysis. All mRNA values were normalized to 18S, and protein normalized to GAPDH. Values are expressed as mean ± SEM of 10-14 samples in each group. *P<0.05, **P<0.01, ***P<0.001, & ****P<0.0001 vs CD; ^P<0.05 & ^^P<0.01 vs HF. CD, chow diet; HF, high fat diet; and Nano, NanoSOD.
We also noted that the plasma level of MCP1 was significantly lower in HF+NanoSOD treated mice compared to HF group (Fig. 3E). We next determined whether reduction in inflammation is associated with an increase in SOD1 protein in HF+Nano treated mice (Fig. 3F-H). Although the inflammatory markers were reduced, the protein level of SOD1 was not altered significantly in VAT, SVCs, and plasma samples of HF+Nano treated mice. Together, these data show that NanoSOD treatment is highly effective in reducing AT and systemic inflammation; however, the protein levels of SOD1 are not altered significantly when mice are sacrificed 24 h post-treatment.
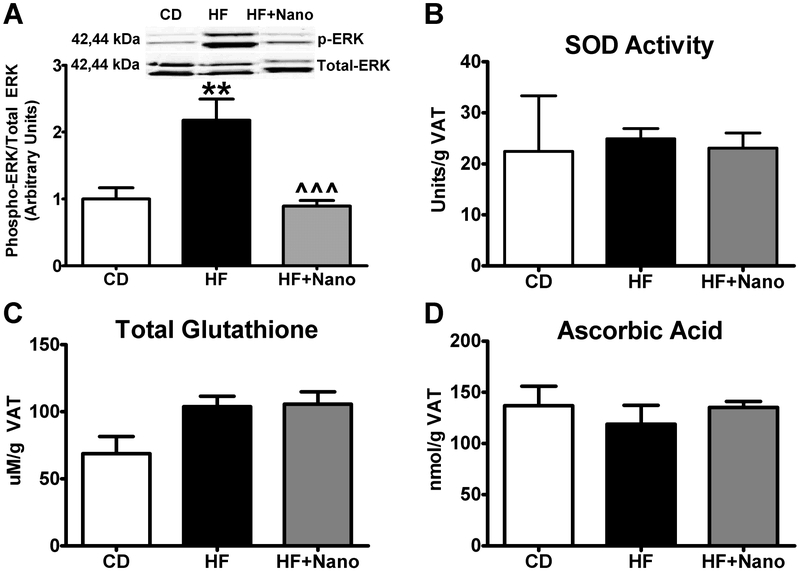
Western blot analysis of VAT for signaling pathways activated by oxidative stress, in particular, ERK1/2 showed that the phosphorylation of this protein was significantly increased in HF compared to CD controls. On the other hand, NanoSOD treatment leads to a profound decrease in phospho ERK1/2 compared to HF diet-fed mice (Fig. 4A). However, markers of oxidative stress/antioxidant response such as SOD activity, GSH depletion, and ascorbate levels were not altered significantly (Fig. 4B-D).
Fig.4.
Effect of NanoSOD on VAT oxidative stress. Phosphorylation of ERK1/2 (A), as well as markers of antioxidant defense such as superoxide dismutase activity (B), total glutathione (C), and ascorbic acid (D) were analyzed. P-ERK was normalized to total ERK. Values are expressed as mean ± SEM of 6-14 samples in each group. **P<0.01 vs CD; ^^^P<0.001 vs HF. CD, chow diet; HF, high fat diet; and Nano, NanoSOD.
Pretreatment of macrophages or adipocytes with NanoSOD reduces co-culture induced inflammatory response.
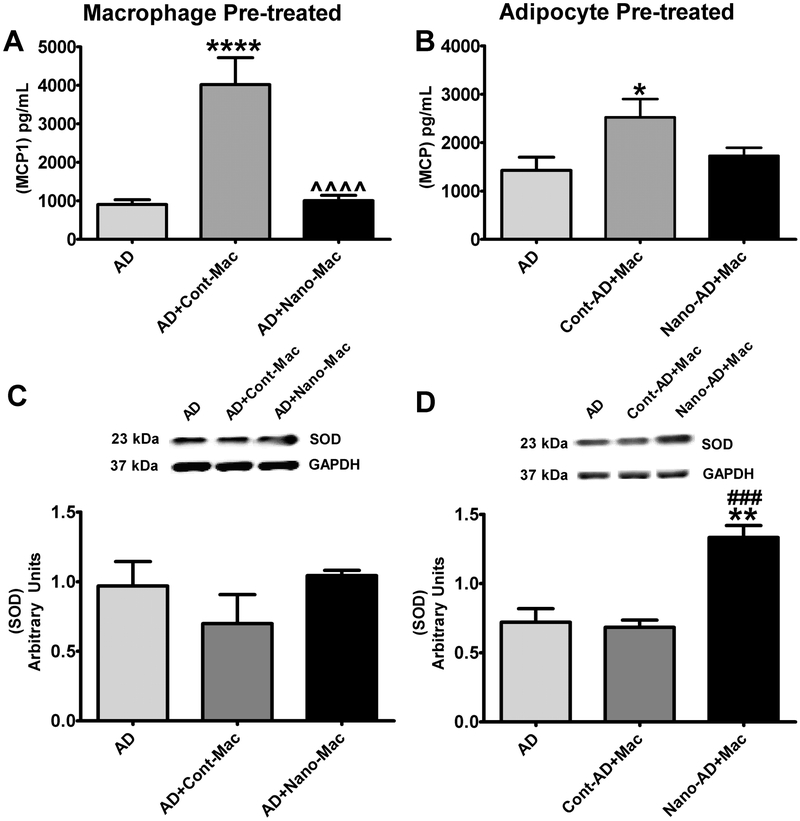
In order to determine the direct effect of NanoSOD in delivering SOD1 to adipocytes and/or macrophages and in reducing their inflammatory response, we performed an in vitro co-culture study (16). In this experiment, we determined the impact of pre-loading macrophages versus adipocytes with NanoSOD in modulating the inflammatory response provoked upon their interaction. Our data show that pre-treatment of either macrophages or adipocytes with NanoSOD attenuates co-culture-induced inflammatory response as detected by MCP-1 secretion (Fig. 5 A&B). Our data also show that pretreatment of macrophages did not increase cellular SOD1 content (Fig. 5C) likely due to the smaller number of macrophages added to the co-culture system (40,000 cells/well). However, pre-treatment of adipocytes with NanoSOD leads to a significant increase in intracellular SOD1 protein (Fig. 5D). These data suggest that nanoformulation of SOD1 actually increases the delivery of SOD1 protein to cells.
Fig.5.
Effect of NanoSOD on inflammatory response in adipocyte-macrophage co-cultures. MCP1 secretion by adipocytes co-cultured with NanoSOD pre-treated macrophages (Nano-mac) (A), and NanoSOD pre-treated adipocytes (Nano-AD) co-cultured with macrophages (B). MCP-1 levels in co-culture media samples at 24 h were analyzed by ELISA. Cell lysates from adipocytes co-cultured with NanoSOD pre-treated macrophages (C), and NanoSOD pre-treated adipocytes co-cultured with control macrophages (D) for 48 h were analyzed for intracellular SOD1 levels by western blotting. Values are expressed as mean ± SEM of 6-12 samples for ELISA (A&B) and 3-6 samples for western blot (C&D) in each group. Protein was normalized to GAPDH. *P<0.05, **P<0.01, & ****P<0.0001 vs AD; ^^^^P<0.0001 vs AD+Cont-Mac; ###P<0.01 vs cont-AD+Mac. AD, adipocytes; AD+Cont-Mac, adipocytes with non-treated macrophages; AD+Nano-Mac, adipocytes with NanoSOD treated macrophages; Cont-AD+Mac, non-treated adipocytes with macrophages; Nano-AD+Mac, NanoSOD treated adipocytes with macrophages.
Effect of NanoSOD on hepatic lipid accumulation
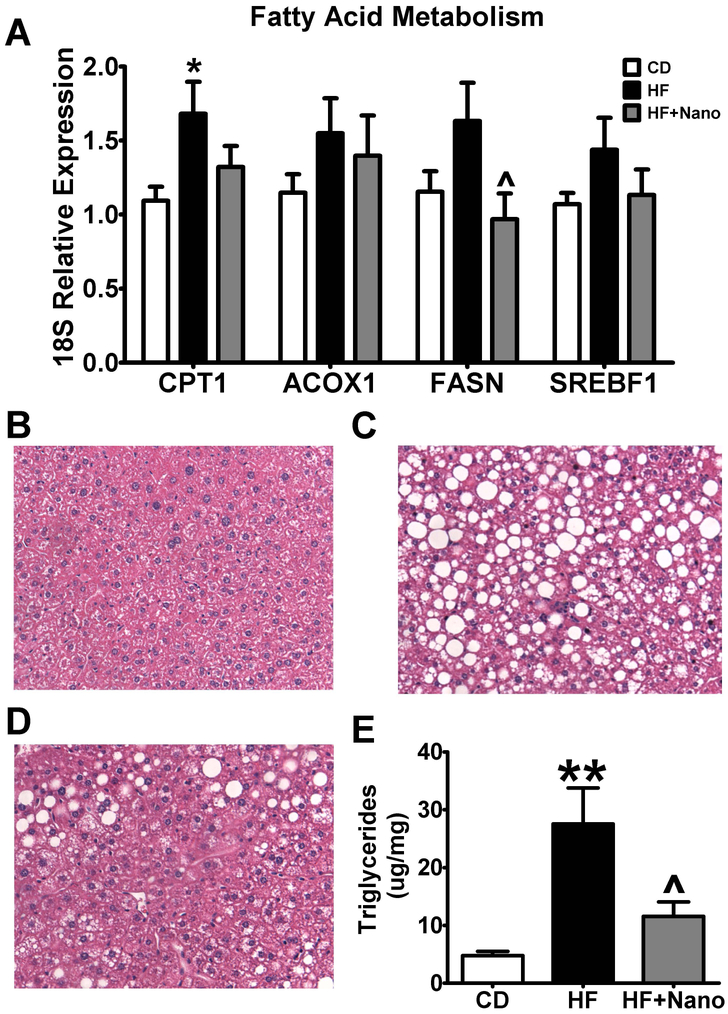
Obesity is associated with excess lipid accumulation in liver leading to hepatic steatosis. The mRNA expression of carnitine palmitoyl transferase (CPT-1), an enzyme involved in fatty acid metabolism was increased only in HF diet-fed mice but not in HF+NanoSOD treated mice (Fig. 6A). The mRNA expression of ACOX-1, an enzyme involved in peroxysomal fatty acid metabolism and, SREBF1, a gene promoting lipogenesis, was not altered among different groups. Interestingly, the expression of fatty acid synthase (FASN), another lipogenic enzyme involved in de novo fatty acid synthesis was reduced in HF+NanoSOD treated mice compared to HF-fed mice. H&E staining of liver sections showed that the lipid accumulation was greatly increased in HF group compared to CD controls. We noted a marked reduction in lipid droplets in HF+NanoSOD treated mice (Fig.6 B-D). Moreover, the levels of hepatic TGs were significantly reduced in HF+Nano compared to HF-fed mice (Fig. 6E). Together, these data show that NanoSOD reduces hepatic lipid accumulation and suggest that inhibition of de novo lipogenesis in liver may be one mechanism for reduced plasma and liver TGs in these mice.
Fig.6.
Effect of NanoSOD on genes modulating fatty acid metabolism and hepatic steatosis. Markers of fatty acid metabolism (A) were assessed in liver samples by real-time PCR. Hepatic lipid accumulation in CD (B), HF (C), and HF+Nano (D) was analyzed using hematoxylin and eosin staining, while hepatic triglycerides (E) were determined using gas chromatography. All mRNA values were normalized to 18S. Values are expressed as mean ± SEM of 10-14 samples in each group. *P<0.05 & **P<0.01 vs CD; ^P<0.05 vs HF. CD, chow diet; HF, high fat diet; and Nano, NanoSOD.
Discussion
In the current study, we have demonstrated that treating obese mice with NanoSOD for only 2 wk results in attenuation of AT inflammation characterized by reduction in macrophage and inflammatory markers in VAT as wells as in VAT stromal cells. This was associated with a reduction in ERK1/2 activation, a signaling pathway activated by both oxidative stress and inflammation. Furthermore, we provide evidence that pre-treatment of macrophages or adipocytes with NanoSOD can attenuate co-culture induced inflammatory response in vitro. Our data also demonstrate that plasma and liver TG levels are reduced upon NanoSOD treatment indicating improvements in lipid homeostasis. However, NanoSOD treatment did not alter markers of oxidative stress in VAT in our experimental condition. Moreover, systemic glucose homeostasis was not altered between HF and HF+NanoSOD treated mice. Taken together, our data suggest that NanoSOD treatment can reduce AT inflammation and systemic and liver TG which, in turn, could reduce obesity-linked lipid disorders including hypertriglyceridemia and non-alcoholic fatty liver disease.
Evidence from animal and human studies suggest that oxidative stress in AT may contribute to the development of inflammation (17, 18) and insulin resistance in obesity (19, 20, 21). An important finding of our study is that NanoSOD treatment leads to a profound reduction in macrophage and inflammatory markers in AT. The in vitro co-culture studies provide further evidence for the direct role of NanoSOD in delivering SOD1 to adipocytes and/or macrophages and reducing macrophage and/or adipocyte inflammation which, in turn, can reduce AT inflammation in obesity. The reduced AT macrophage accumulation and AT inflammation may also be due to improved lipid homeostasis (reduced plasma and liver TGs). This notion is supported by a previous report showing that increased local and circulating free fatty acids in obesity was associated with increased AT macrophage accumulation (22). Therefore, it is very likely that an improvement in systemic and local fatty acid/TG homeostasis can also reduce AT macrophage accumulation and inflammation.
A previous study by Pires et al. has shown that simultaneous exposure of mice to HF diet and MnTBAP, a MnSOD mimetic, reduces AT inflammation and systemic free fatty acid levels (7). In line with this study, we provide evidence that NanoSOD treatment led to a reduction in AT inflammation. Our study differs from that of Pires et al. in several ways. First, we used nanoformulated Cu/ZnSOD whereas Pires et al. used a MnSOD mimetic. Next, we studied the therapeutic effect of NanoSOD by first placing the mice on a HF diet followed by treatment with NanoSOD. In their study, the preventive effect of MnTBAP was assessed. Finally, our data show that in addition to reducing AT inflammation, NanoSOD reduced plasma and liver TG levels. Together, these studies provide critical information regarding the role of superoxide in modulating obesity-linked inflammation and lipid metabolism.
It is interesting to note that the plasma and liver TGs were significantly lower in HF+Nano compared to HF-fed mice suggesting an improved TG metabolism. The link between SOD1 and metabolism is evident from the fact that SOD1 mutant mice with gain of function exhibit hypermetabolism (23, 24) characterized by increased TG metabolism in muscle. Moreover, mice lacking SOD1 have been shown to exhibit increased lipid accumulation in liver via increased lipogenesis (25). Our data show that the expression of fatty acid synthase, a lipogenic enzyme, is significantly reduced in liver upon NanoSOD treatment. Therefore, it is possible that similar mechanisms play a role in reducing TG levels in plasma and liver in our study.
Regarding signaling mechanisms, we noted that activation of ERK1/2 was increased in HF-fed mice whereas NanoSOD attenuated this effect. Evidence suggests that ERK1/2 is activated by free fatty acids and oxidative stress (26), [reviewed in (27)]. Moreover, bioflavonoids and triterpenoids with known anti-oxidant properties have been shown to attenuate inflammation via inhibiting ERK1/2 phosphorylation (28, 29, 30). ERK1/2 activation can lead to activation of NF-κB which, in turn, can activate the inflammatory response. We previously showed that treatment of endothelial cells with linoleic acid, a free fatty acid, can induce oxidative stress and inflammatory response and this was mediated via ERK1/2 and NF-κB (26). Therefore, it is very likely that NanoSOD reduces inflammatory response via blunting ERK1/2 activation.
Despite the promising effects seen with regard to AT inflammation and lipid homeostasis in HF+Nano treated mice, we were not able to show a change in markers of oxidative stress in these mice. One possible reason for this could be our experimental condition. For example, we sacrificed mice 24h after injecting the last dose of NanoSOD. It is possible that NanoSOD was effective in the initial hours and then removed from the system likely via proteolytic degradation. However, the effectiveness of NanoSOD in scavenging superoxide is evident from previous studies. For example, NanoSOD reduced superoxide-induced oxidative stress in cultured endothelial and neuronal cells in vitro (12). Moreover, our in vitro data provide evidence that NanoSOD is effective in increasing SOD1 protein in adipocytes. Therefore, it is possible that improvements seen in AT inflammation and metabolic markers in our study could be due to improved superoxide-scavenging effects of NanoSOD. However, further time course studies will be required to confirm this notion in vivo.
It should be pointed out that clinical trials in humans did not support the benefit of antioxidant therapy against cardiovascular disease [reviewed in (8)]. The failure of these trials could be due the choice of the antioxidants and the endpoints. For example, mostly the antioxidant vitamins were used and vitamin E, in particular, can exert prooxidant effects under certain conditions (31, 32). Moreover, while the role of antioxidant vitamins on cardiovascular disease and cancer has been studied in humans, not much is known regarding their effects on metabolic pathways. Our findings are relevant to human diseases in two ways. First, our findings have implications in considering NanoSOD as a novel therapy to target AT inflammation and lipid disorders in obesity. Second, amyotropic lateral sclerosis (ALS) patients with increased SOD activity exhibit hypermetabolism and a high caloric diet may improve their energy balance and survival (33, 34). Our data suggest the role of reduced de novo fatty acid synthesis as a potential mechanism for improved energy balance in ALS patients upon a high caloric diet. Overall, our study provides a better understanding on the role of NanoSOD in reducing AT inflammation and improving lipid homeostasis in obesity.
What is already known about this subject?
Markers of oxidative stress and inflammation are increased in obesity and are the underlying factors mediating obesity-linked metabolic disorders
Increased SOD1 (Cu/ZnSOD) activity is associated with hypermetabolism in mice and humans
Nanoformulated SOD1 (NanoSOD) is effective in alleviating hypertension and ischemic brain injury in animal models
What does this study add?
The effectiveness of antioxidant enzyme therapy in reducing obesity-linked inflammation is unknown. Our study using NanoSOD provides evidence that short-term treatment with this formulation is effective in reducing adipose tissue (AT) macrophage accumulation and AT inflammation in a mouse model of diet-induced obesity
The reduction in AT inflammation upon NanoSOD treatment is associated with a decrease in ERK1/2 activation in AT
Our study also demonstrates that NanoSOD is effective in reducing plasma and liver triglycerides in high fat diet-fed mice
Acknowledgments
We thank Drs. Tatiana Bronich and Irving Zucker for their support and feedback on this project and in the critical review of this manuscript. Lipid profiles were analyzed at the Lipid Core Laboratory of the Mouse Metabolic Phenotyping Center at Vanderbilt University (DK59637). This paper is the result of work conducted with the resources and the facilities at the VA-Nebraska Western Iowa Health Care System, Omaha.
Funding. This project was supported by the NIH-Nebraska Center for Nanomedicine COBRE Grant (2P20GM103480). AVK and DSM also acknowledge support of The Carolina Partnership, a strategic partnership between the UNC Eshelman School of Pharmacy and the University Cancer Research Fund through the Lineberger Comprehensive Cancer Center.
Footnotes
Disclosure. AVK is the co-inventor of the nanozyme technology at UNMC (Patent number: WO2008141155A1). The technology and products have been exclusively out-licensed to NeuroNano Pharma, a start-up company located in Chapel Hill, NC. AVK is a co-founder, shareholder and director of this company.
References
- 1.Bai Y, Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev 2015;16: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revelo XS, Luck H, Winer S, Winer DA. Morphological and inflammatory changes in visceral adipose tissue during obesity. Endocrine pathology 2014;25: 93–101. [DOI] [PubMed] [Google Scholar]
- 3.Amirkhizi F, Siassi F, Djalali M, Foroushani AR. Assessment of antioxidant enzyme activities in erythrocytes of pre-hypertensive and hypertensive women. J Res Med Sci 2010;15: 270–278. [PMC free article] [PubMed] [Google Scholar]
- 4.Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, et al. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem 2002;35: 627–631. [DOI] [PubMed] [Google Scholar]
- 5.Uthus EO, Picklo MJ Sr. Obesity reduces methionine sulphoxide reductase activity in visceral adipose tissue. Free Radic Res 2011;45: 1052–1060. [DOI] [PubMed] [Google Scholar]
- 6.Frohnert BI, Sinaiko AR, Serrot FJ, Foncea RE, Moran A, Ikramuddin S, et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011;19: 1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pires KM, Ilkun O, Valente M, Boudina S. Treatment with a SOD mimetic reduces visceral adiposity, adipocyte death, and adipose tissue inflammation in high fat-fed mice. Obesity (Silver Spring) 2014;22: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation 2002;105: 2107–2111. [DOI] [PubMed] [Google Scholar]
- 9.Pashkow FJ. Oxidative Stress and Inflammation in Heart Disease: Do Antioxidants Have a Role in Treatment and/or Prevention? Int J Inflam 2011;2011: 514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 1997;95: 588–593. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaugh EG, Roat JW, Gao L, Yang RF, Manickam DS, Yin JX, et al. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials 2010;31: 5218–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manickam DS, Brynskikh AM, Kopanic JL, Sorgen PL, Klyachko NL, Batrakova EV, et al. Well-defined cross-linked antioxidant nanozymes for treatment of ischemic brain injury. J Control Release 2012;162: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saraswathi V, Ramnanan CJ, Wilks AW, Desouza CV, Eller AA, Murali G, et al. Impact of hematopoietic cyclooxygenase-1 deficiency on obesity-linked adipose tissue inflammation and metabolic disorders in mice. Metabolism: clinical and experimental 2013;62: 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraswathi V, Hasty AH. Inhibition of long-chain acyl coenzyme A synthetases during fatty acid loading induces lipotoxicity in macrophages. Arterioscler Thromb Vasc Biol 2009;29: 1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murali G, Desouza CV, Clevenger ME, Ramalingam R, Saraswathi V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prostaglandins, leukotrienes, and essential fatty acids 2014;90: 13–21. [DOI] [PubMed] [Google Scholar]
- 16.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005;25: 2062–2068. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. Journal of physiology and biochemistry 2012;68: 701–711. [DOI] [PubMed] [Google Scholar]
- 19.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440: 944–948. [DOI] [PubMed] [Google Scholar]
- 20.Grimsrud PA, Picklo MJ Sr., Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Molecular & cellular proteomics : MCP 2007;6: 624–637. [DOI] [PubMed] [Google Scholar]
- 21.Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 2015;58: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 22.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 2010;120: 3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A 2004;101: 11159–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fergani A, Oudart H, Gonzalez De Aguilar JL, Fricker B, Rene F, Hocquette JF, et al. Increased peripheral lipid clearance in an animal model of amyotrophic lateral sclerosis. J Lipid Res 2007;48: 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Jiang Z, Lei XG. Knockout of SOD1 alters murine hepatic glycolysis, gluconeogenesis, and lipogenesis. Free Radic Biol Med 2012;53: 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennig B, Lei W, Arzuaga X, Ghosh DD, Saraswathi V, Toborek M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem 2006;17: 766–772. [DOI] [PubMed] [Google Scholar]
- 27.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxidants & redox signaling 2006;8: 1775–1789. [DOI] [PubMed] [Google Scholar]
- 28.Sahu BD, Kumar JM, Sistla R. Baicalein, a Bioflavonoid, Prevents Cisplatin-Induced Acute Kidney Injury by Up-Regulating Antioxidant Defenses and Down-Regulating the MAPKs and NF-kappaB Pathways. PloS one 2015;10: e0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karuppagounder V, Arumugam S, Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, et al. Naringenin ameliorates daunorubicin induced nephrotoxicity by mitigating AT1R, ERK1/2-NFkappaB p65 mediated inflammation. International immunopharmacology 2015;28: 154–159. [DOI] [PubMed] [Google Scholar]
- 30.Ma JQ, Ding J, Zhang L, Liu CM. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-kappaB pathway. Environmental toxicology and pharmacology 2014;37: 975–983. [DOI] [PubMed] [Google Scholar]
- 31.Ouchi A, Ishikura M, Konishi K, Nagaoka S, Mukai K. Kinetic study of the prooxidant effect of alpha-tocopherol. Hydrogen abstraction from lipids by alpha-tocopheroxyl radical. Lipids 2009;44: 935–943. [DOI] [PubMed] [Google Scholar]
- 32.Stocker R The ambivalence of vitamin E in atherogenesis. Trends in biochemical sciences 1999;24: 219–223. [DOI] [PubMed] [Google Scholar]
- 33.Genton L, Viatte V, Janssens JP, Heritier AC, Pichard C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clinical nutrition 2011;30: 553–559. [DOI] [PubMed] [Google Scholar]
- 34.Wills AM, Hubbard J, Macklin EA, Glass J, Tandan R, Simpson EP, et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014;383: 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]