Abstract
Background
Toxoplasmosis, a protozoan parasitic disease caused by Toxoplasma gondii, has been a serious human and veterinary medicine problem with global distribution. In the current study, we assessed immunogenicity and protective efficiency of a novel dual-promoter DNA vaccine, harboring SAG1 and GRA7 genes, from RH strain of Toxoplasma gondii (T. gondii) with or without CpG-ODN as adjuvant in a murine model.
Methods
BALB/c mice were immunized intramuscularly with pVitro-SAG1-GRA7 alone and pVitro-SAG1-GRA7 with CpG-ODN three times at three-week intervals. Enzyme-linked immunosorbent assay (ELISA) was used to assess total IgG, IgG1 and IgG2a antibodies and gamma interferon (IFN-γ) and interleukin-10 (IL-10) cytokines in mice sera. Four weeks post final vaccination, MTT assay and lethal challenge-infection with 1×103 tachyzoites of T. gondii RH strain were carried out to assess stimulation index (SI) and mice survival time, respectively.
Results
The IgG levels in mice immunized with multicomponent vaccines, including pVitro-SAG1–GRA7 alone and pVitro-SAG1–GRA7 with CpG-ODN, were significantly higher than those in control mice or single-gene DNA-vaccinated ones (P<0.05). Furthermore, level of IgG2a in mice receiving pVitro-SAG1–GRA7 with CpG-ODN was significantly higher than that in mice receiving pVitro-SAG1-GRA7 alone (P<0.05). The Toxoplasma lysate antigen (TLA)-stimulated lymphocytes from pVitro-SAG1-GRA7 with CpG-ODN group responded more dramatically than those from control groups or single-gene DNA-vaccinated groups (P<0.001). The pVitro-SAG1-GRA7 with CpG-ODN-vaccinated mice developed high levels of IgG2a and IFN-γ (P<0.001) and low levels of IgG1 and IL-10, compared to control groups, suggesting a modulated immune response type Th1. In addition, survival time of the mice immunized with pVitro-SAG1-GRA7 with CpG-ODN was significantly extended, compared to controls (P<0.05); however, all mice died.
Conclusion
The multivalent pVitro-SAG1-GRA7 DNA vaccine with CpG-ODN adjuvant is a promising vaccine candidate against toxoplasmosis.
Keywords: Toxoplasma gondii, RH strain, SAG1, GRA7, pVitro2-neo-mcs, DNA vaccine, BALB/c mice, CpG-ODN adjuvant
Introduction
Toxoplasma gondii (T. gondii), the agent of toxoplasmosis, is an obligate intracellular parasite capable of infecting a wide range of warm-blooded animals, including livestock and humans.1 Approximately, one-third of the human populations are infected with T. gondii due to its numerous sources of infection across the world.2 Toxoplasmosis is often asymptomatic in immunocompetent patients; however, it results in significant morbidity and mortality in patients who receive immunosuppressive drugs, suffer from acquired immuno deficiency syndrome (AIDS) and are congenitally infected.3–5 Furthermore, T. gondii infection causes economic losses in livestock, especially sheep and goats, due to abortion and neonatal loss.6 Human can be infected by ingestion of undercooked or raw meat containing viable tissue cysts or ingestion of food or water contaminated with oocysts.7 Chemotherapy does not seem to be successful in long terms since the drugs of choice, sulphadiazine and pyrimethamine fail to completely eliminate the parasite; while including toxic side effects.8 Therefore, search for a new effective vaccine against T. gondii is a priority in prevention of toxoplasmosis. The current candidate antigens involved in protective immunity against toxoplasmosis include surface antigens (SAGs), rhoptry proteins (ROPs), microneme proteins (MICs) and dense granule antigens (GRAs), which have been widely assessed for their immunological effects in animal models.9–12 SAG1 is believed to be the most promising vaccine candidate within these antigens since it is regulated jointly by humoral and cellular immune responses that only occur in tachyzoites. Therefore, researchers are more likely to choose SAG1 in development of recombinant or DNA vaccines.13 These unique secretory products play important roles in parasite adhesion, invasion, establishment of parasitophorous vacuole (PV), survival and replication inside PV.14 Of these products, GRA proteins are secreted during or after parasite invasion inside the PV. Up-to-date, more than 40 genetically distinct GRA proteins have been identified which immunogenicity and correlation with virulence are being studied.15 In this study, GRA7 was selected as an ideal choice due to its high antigenicity, which can lead to significant humoral and cellular immune responses to toxoplasmosis.16,17 Despite most GRA proteins secreted in tachyzoites and bradyzoites, GRA7 is produced by all three T. gondii infective stages including sporozoites. It has been proven that a further prominent correlation exists between steady-state levels of GRA7 and T. gondii virulence compared to GRA6, a GRA protein located on the parasitophorous vacuole membrane (PVM).18 Indicating PVM localization of GRA proteins does not necessarily make one GRA protein a further favorable vaccine candidate. It has also been proven that DNA vaccine, encoding GRA7, induces specific humoral and cellular immune responses more efficiently than that GRA1, GRA4 and GRA6 DNA vaccines do.14
In recent decades, studies have been carried out to develop a safe and effective vaccine against toxoplasmosis; from inactivated or attenuated to protein vaccines in subunits or multiantigenic cocktails and DNA vaccines.19 However, only one vaccine is available in the market, namely Toxovax, which is exclusively produced for veterinary use. Toxovax is a live-attenuated vaccine with the possibility of reverting to its wild type and hence not appropriate for human use.20 From various strategies of vaccination, DNA vaccines seem further promising as they can elicit specific Th1 immune responses which is crucial in eliminating intracellular organisms.21 In case of inactivated vaccines such as DNA vaccines, it is highly recommended to simultaneously use an appropriate adjuvant to increase the magnitude and duration of induced immune responses unless they might be ineffective. Therefore, CpG-ODN adjuvant, a synthetic oligodeoxynucleotide containing unmethylated CpG motifs, was selected in this study. It elicits native and adaptive immune responses such as other pathogen-associated molecular patterns (PAMPs) by attaching toll-like receptor 9 (TLR-9) and triggering MyD88 signaling pathway that involves up-regulation of nuclear factor-κB (NF-κB). This eventually results in production of various cytokines (especially IL-12 and IL-10) and co-stimulatory ligands such as B7-1 and B7-2.22,23 It is well documented that use of CpG-ODN adjuvant results in Th1-dominated type immunity; therefore, it could be a promising choice in the rational design of efficient vaccines against toxoplasmosis. Furthermore, this adjuvant has been shown to be safe for human use with the benefit of improving immunity even in neonates, elderly people and immunocompromised patients.24 In the current study, immune responses and protections of three eukaryotic expression constructs were assessed against challenge infections of T. gondii, RH strain, in a murine model. The challenges were carried out using a dual-promoter vector pVitro2-neo-mcs, including pVitro-SAG1, pVitro-GRA7 and pVitro-SAG1-GRA7 with or without CpG-ODN as adjuvant.
Materials and methods
Animal
In the current study, 6–8-weeks-old female BALB/c mice weighing 20–25 g were used. The animals were hosted in standard laboratory conditions (light-dark cycle conditions, controlled temperatures of 22°C ±2) with food and water supplies ad libitum. Animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health of the United States and approved for the use of laboratory animals by the Ethical Committee of the Tehran University of Medical Sciences, Tehran, Iran. Study was approved by the Ethics Committee of Tehran University of Medical Sciences (Approval No. 21648) and efforts were made to minimize animal distress.
Parasites and soluble tachyzoite antigen preparation
Antigens have been prepared as described previously.25 Briefly, tachyzoites of T. gondii RH strain were collected from peritoneal cavity of the infected mice. Tachyzoites were washed with cold phosphate buffer saline (PBS, pH 7.2) twice and were sonicated and centrifuged at 14,000× g for 1 hr at 4°C. Supernatant was then collected as soluble tachyzoite antigen (STAg) and stored at −70°C until use.
Construction of recombinant plasmids
Three eukaryotic expression constructs were produced using a dual-promoter vector pVitro2-neo-mcs (Invivogen, USA) as follows: pVitro-SAG1, pVitro-GRA7 and pVitro-SAG1-GRA7. Full length of SAG1 and GRA7 (molecular weight of approximately 30 and 26 kDa, respectively) were expressed in HeLa cells by the corresponding constructs. HeLa cell line was purchased commercially from the National Cell Bank of Iran (NCBI, Pasteur Institute of Iran). The whole protocol is fully described in a previous study by the authors.26 Briefly, genomic DNA was extracted from tachyzoites using QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Full-length coding region of SAG1 and GRA7 genes were amplified using PCR and specific primers. Amplified fragments were cloned into pTZ57R/T vectors (Thermo Scientific InsTAclone PCR Cloning Kit, USA) according to the manufacturer’s instructions. The recombinant construct, pTZ-SAG1, was digested with BglII and NheI, whereas pTZ-GRA7 was digested with BamHI and ClaI to prepare ligands with proper sticky ends. Then, ligations were subcloned into empty pVitro2-neo-mcs vectors, previously digested by corresponding restriction enzymes. Subcloning was confirmed using colony PCR, enzymatic digestion and sequencing. Ability of all three constructs to produce the corresponding antigens in mammalian HeLa cells was verified using Western blot analysis.
BALB/c mice immunization and challenge
In general, 84 BALB/c mice were divided randomly into seven groups of 12 mice. Negative control groups, including Groups I to III, received 100 µL of PBS, 100 µg of empty pVitro2-neo-mcs vector in 100 µL of PBS and 30 µg of CpG-ODN in 100 µL of PBS, respectively. Experimental groups of IV to VII were injected with 100 µg of pVitro-SAG1 plasmid in 100 µL of PBS, 100 µg of pVitro-GRA7 plasmid in 100 µL of PBS, 100 µg of pVitro-SAG1-GRA7 plasmid in 100 µL of PBS and 100 µg of pVitro-SAG1-GRA7 plasmid with 30 µg of CpG-ODN in 100 µL of PBS, respectively. Mice were immunized intramuscularly (IM) thrice on days 1, 21 and 42. Four weeks after the last immunization, nine immunized BALB/c mice from each group were randomly selected and involved in infection experiments. Each mouse was inoculated intraperitoneally (IP) with a single dose of 1×103 tachyzoites of the virulent T. gondii, RH strain. Mice were monitored twice daily and the time of death was recorded until all mice were dead. Other mice (three mice per group) were used for removing spleens under sodium pentobarbital anesthesia and further assessments include MTT and cytokine assays.
Assessment of antibodies using enzyme-linked immunosorbent assay (ELISA)
Effects of vaccination on humoral response levels were assessed by measuring total antigen-specific IgG, IgG1 and IgG2a using sera collected on days 0, 20, 41 and three weeks after the final immunization (day 62) using ELISA. Briefly, STAg of the T. gondii RH strain was diluted to 10 μg/mL with coating buffer (pH 9.6) and divided into Costar 96-well flat-bottom microtiter plates (Thermo Fisher Scientific, USA) using 100 μL per well. After an overnight incubation at 4°C, 100 μL of blocking buffer (PBST containing 2.5% of skim milk) was added to each well to block non-specific binding sites and plates were incubated at 37°C for 1 hr. Then, plates were washed thrice with PBS buffer containing 0.05% of Tween-20 (PBST). Then, 100 µL of diluted mouse sera (1:100 dilution) was added to each well and incubated at 37°C for 1 hr. After washing the plates, 100 µL of 1:750 diluted horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1 or IgG2a (Abcam abID#97265, 97240, 97245, UK) was added to each well and then plates were incubated at 37°C for 1 hr. After incubation and wash steps, substrate of ortho-phenylenediamine (OPD) (Sigma-Aldrich, USA) was added to the wells. Reactions were stopped using 2M H2SO4 solution and the optical density (OD) was measured using automated ELISA reader (Model LX800; Biotek, Winooski, USA) at 490 nm. All samples were tested in triplicate.
MTT assay
Homogenous splenocyte suspensions were prepared based on published methods.27 The MTT assay was carried out according to the manufacturer’s instructions. Briefly, splenocytes were cultured in Costar 96-well plates (1×105 cells/well) in RPMI 1640 Media containing 10% of FCS (Gibco, UK) and stimulated with 50 µg/mL of STAg or 5 µg/mL of concanavalin A (Con A) (Sigma, USA) as positive control or media alone as negative control and incubated at 37°C for 72 hrs with 5% CO2. Supernatant was discarded and 10 µL of methyl thiazolyl tetrazolium (Sigma, USA) in RPMI 1640 and 10% of FCS media were added to each well and incubated at 37°C for 4 hrs. Then, 100 µL of dimethyl sulfoxide solution (Merck KGaA, Darmstadt, Germany) was added to each well to dissolve the formazan crystals. The absorbance was read at 540 nm using automated ELISA reader (Model LX800; Biotek, Winooski, USA) and the stimulation index (SI) was calculated for each group. All tests were repeated thrice.
Cytokine assay
To assess capability of splenic lymphocytes of DNA vaccinated mice to produce cytokines when stimulated by T. gondii antigens, splenocytes were plated in Costar 24-well plates (Thermo Fisher Scientific, USA) at the density of 3.5×106 cells/well and stimulated with 50 µg/mL of STAg or 5 µg/mL of Con A (Sigma, USA) as positive control or media alone as negative control. After 72 hrs of incubation at 37°C in presence of 5% CO2, supernatants were collected in separate microtubes and centrifuged for 10 mins at 3000× rpm and then concentrations of IFN-γ and IL-10 were measured using commercial kits according to the manufacturer’s instructions (Mouse IFN-gamma Platinum ELISA and Mouse IL-10 Platinum ELISA; ebioscience, USA). All tests were repeated thrice.
Statistical analysis
Statistical analysis was performed using SPSS Software v.20 (IBM Corporation, Armonk, NY, USA). The Kaplan-Meier method was used to analyze and compare the survival time between the groups. One-way ANOVA Bonferroni post hoc test was used to compare the mean of each variable (total IgG, IgG1, IgG2a, IL-10 and IFN-γ) in different groups.28 Values of P<0.05 were considered statistically significant.
Results
Results of the eukaryotic recombinant plasmid constructions and expression of their genes in HeLa cells have been reported previously by the authors.26
Humoral immune responses
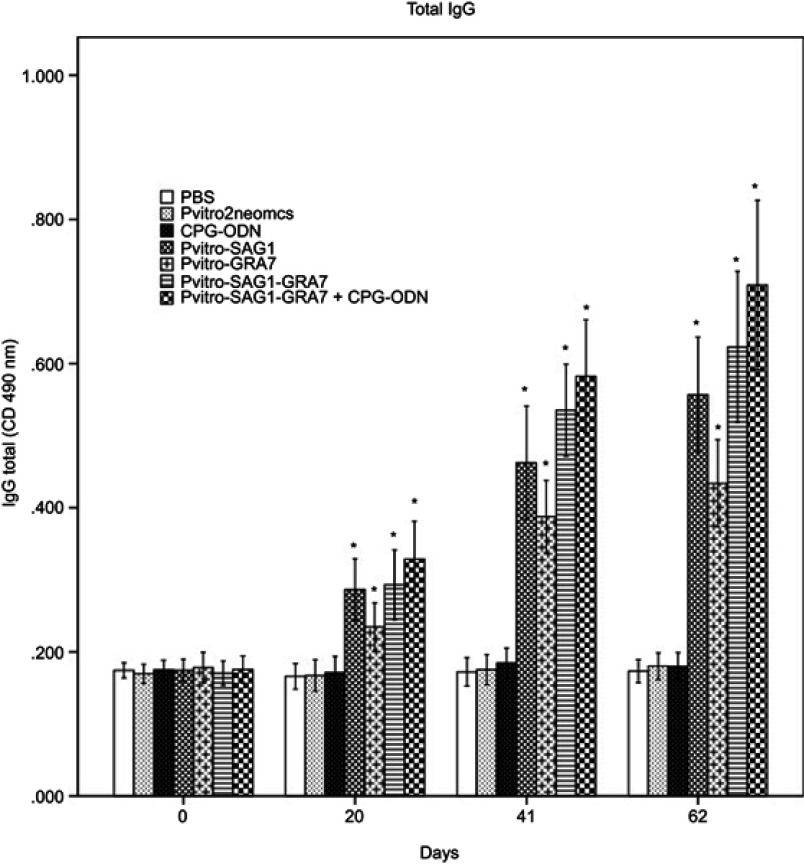
As shown in Figure 1, a significant difference was seen between the IgG levels of all experimental groups compared to control groups 20 days after the first immunization (P<0.05). The IgG levels gradually increased over time in all experimental groups with successive immunization. The IgG levels in mice immunized with multicomponent vaccines of pVitro-SAG1-GRA7 and pVitro-SAG1-GRA7 with CpG-ODN were significantly higher (P<0.05) than those in mice vaccinated with single-gene DNA vaccines of pVitro-SAG1 and pVitro-GRA7; with an exception of pVitro-SAG1 and pVitro-SAG1-GRA7 on Day 62. The levels of total IgG in pVitro-SAG1 group were significantly higher than those in pVitro-GRA7 group (P<0.05), although anti T. gondii total IgG antibody values were higher in sera of mice receiving pVitro-SAG1-GRA7 with CpG-ODN vaccine, compared to those in sera of mice vaccinated with pVitro-SAG1-GRA7 alone. However, the difference was not statistically significant (P>0.05).
Figure 1.
Total IgG antibodies in the collected sera of BALB/c mice immunized with PBS, pVitro2neo-mcs, CpG-ODN, pVitro-SAG1, pVitro-GRA7, pVitro-SAG1GRA7 and pVitro-SAG1GRA7 with CpG-ODN on days 0, 20, 41 and 62 using ELISA. Results are expressed as means of OD 490± SD and statistically significant differences (P<0.05) are indicated by an asterisk (*), compared to control groups.
Abbreviations: PBS, phosphate buffer saline; ELISA, enzyme-linked immunosorbent assay; OD, optical density; SD, standard deviation.
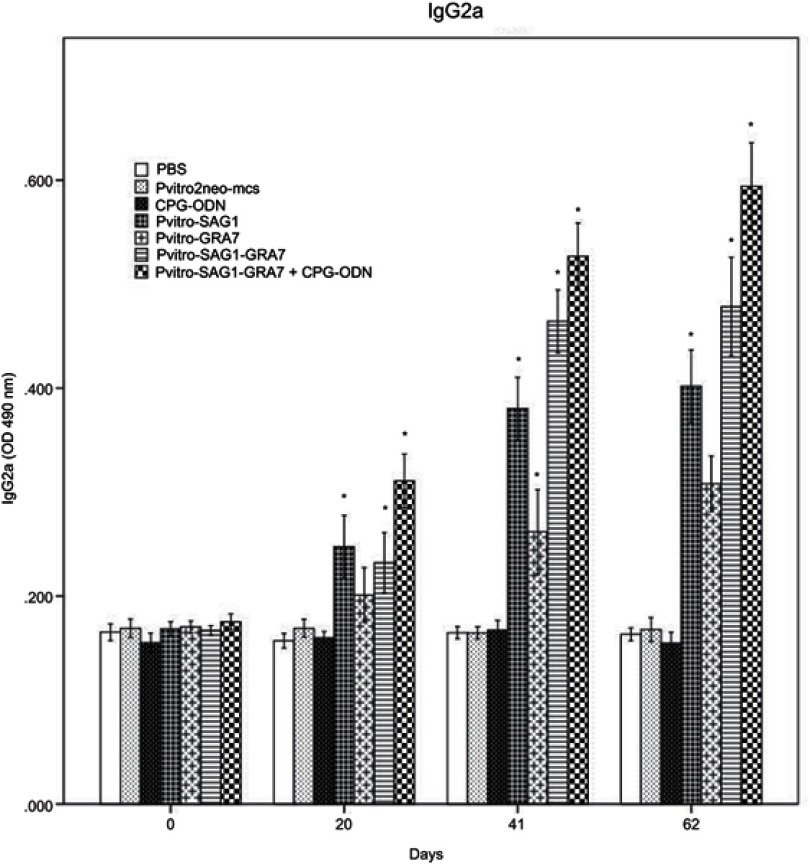
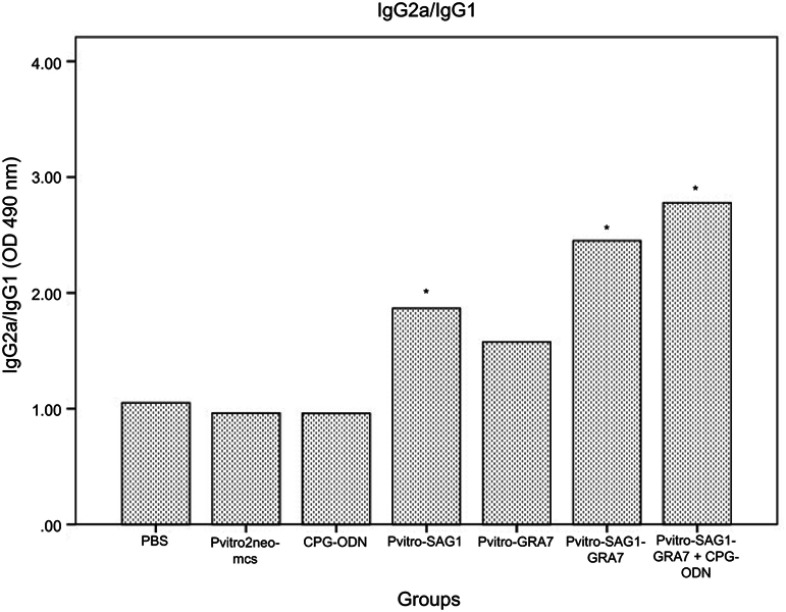
Serum levels of IgG subtypes (IgG1 and IgG2a) were assessed to reveal if elicited immune responses were predominantly toward Th1 or Th2 three weeks after the last inoculation. A mild increase was seen in levels of IgG1, which was rarely significant, compared to control groups (data not shown). Levels of IgG2a significantly increased in all experimental groups, compared to control groups (P<0.001). Moreover, levels of IgG2a in mice receiving pVitro-SAG1-GRA7 with CpG-ODN were significantly higher than those in mice receiving pVitro-SAG1-GRA7 alone, indicating the role of CpG-ODN adjuvant in immune response orientation (Figure 2). The IgG2a/IgG1 variable in pVitro-SAG1-GRA7 group was approximately 2.5 times greater than that in control groups, showing that the vaccine produced Th1-dominant immune responses (Figure 3).
Figure 2.
Levels of IgG2a subtypes in collected sera of BALB/c mice immunized with PBS, pVitro2neo-mcs, CpG-ODN, pVitro-SAG1, pVitro-GRA7, pVitro-SAG1-GRA7 and pVitro-SAG1-GRA7 with CpG-ODN on days 0, 20, 41 and 62 using ELISA. Results are expressed as means of OD 490± SD and statistically significant differences (P<0.05) are indicated by an asterisk (*), compared to control groups.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; OD, optical density; SD, standard deviation.
Figure 3.
The IgG2a/IgG1 ratio in mice sera on Day 62 analyzed using ELISA. Statistically significant differences (P<0.05) are indicated by an asterisk (*), compared to control groups.
Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Lymphocyte proliferation assay
As shown in Table 1, a significant difference was seen between the SI of all experimental groups, compared to control groups (P<0.05). Lymphocytes from pVitro-SAG1-GRA7 and pVitro-SAG1-GRA7 with CpG-ODN groups responded more dramatically than those from control or single-gene groups (P<0.001). Furthermore, difference of SI was statistically significant in mice immunized with pVitro-SAG1-GRA7 with CpG-ODN, compared to that in mice received pVitro-SAG1-GRA7 alone (P<0.001). This indicates the effect of CpG-ODN adjuvant on lymphocyte proliferation. These results also verify that vaccination with pVitro-SAG1-GRA7 can induce cell-mediated immunity.
Table 1.
Stimulation index four weeks post final immunization assessed using ELISA in sera of immunized mice
| Group | Stimulation index ± SD |
|---|---|
| PBS | 0.86±0.09 (d,e,f,g) |
| pVitro2neo-mcs | 0.73±0.13 (d,e,f,g) |
| CpG-ODN | 1.05±0.11 (e,f,g) |
| pVitro-SAG1 | 1.36±0.1 (a,b,f,g) |
| pVitro-GRA7 | 1.58±0.12 (a,b,c,g) |
| pVitro-SAG1-GRA7 | 1.93±0.15 (a,b,c,d,g) |
| pVitro-SAG1-GRA7 with CpG-ODN | 2.84±0.18 (a,b,c,d,e,f) |
| P-value | 0.001 |
Notes: Significant differences (P<0.05) in PBS group (a), pVitro2neo-mcs group (b), CpG-ODN group (c), pVitro-SAG1 group (d), pVitro-GRA7 group (e), pVitro-SAG1-GRA7 group (f) and pVitro-SAG1-GRA7 with CpG-ODN group (g).
Abbreviations: PBS, phosphate-buffered saline; SD, standard deviation; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay.
To analyze the effects of vaccination on cell-mediated immune responses, levels of cytokine (IFN-γ and IL-10) were assessed in different groups. As shown in Table 2, IFN-γ levels in mice vaccinated with pVitro-SAG1-GRA7 alone and pVitro-SAG1-GRA7 with CpG-ODN were significantly higher, compared to those in mice of control or single-gene groups (P<0.001). Low levels of IL-10 in experimental and control groups indicated no statistical differences between all groups (P>0.05). These results verified that pVitro-SAG1-GRA7 vaccine promoted Th1-type immune response; as analysis of subtypes IgG showed.
Table 2.
Cytokine production in vaccinated mice splenocyte cultures four weeks post final immunization using ELISA (pg/mL)
| Group | IFN-γ (mean ± SD) | IL-10 (mean ± SD) |
|---|---|---|
| PBS | 67.75±3.83 (d,e,f,g) | 37.75±3.03 |
| pVitro2neo-mcs | 80.75±5.76 (d,e,f,g) | 42.75±2.38 |
| CpG-ODN | 100.75±9.54 (d,e,f,g) | 39±1.581 |
| pVitro-SAG1 | 571.25±24.72 (a,b,c,e,f,g) | 35.75±2.58 |
| pVitro-GRA7 | 315.75±18.15 (a,b,c,d,f,g) | 42.25±4.91 |
| pVitro-SAG1-GRA7 | 802.5±31.66 (a,b,c,d,e,g) | 39.5±5.4 |
| pVitro-SAG1-GRA7 with CpG-ODN | 973.75±66.91 (a,b,c,d,e,f) | 41.25±4.81 |
| P-value | 0.001 | 0.29 |
Notes: Significant differences (P<0.05) in PBS group (a), pVitro2neo-mcs group (b), CpG-ODN group (c), pVitro-SAG1 group (d), pVitro-GRA7 group (e), pVitro-SAG1-GRA7 group (f) and pVitro-SAG1-GRA7 with CpG-ODN group (g).
Abbreviations: PBS, phosphate buffered saline; SD, standard deviation; IFN-γ, gamma interferon; IL-10, interleukin-10; ELISA, enzyme-linked immunosorbent assay.
Survival time analysis against a lethal challenge in BALB/c mice
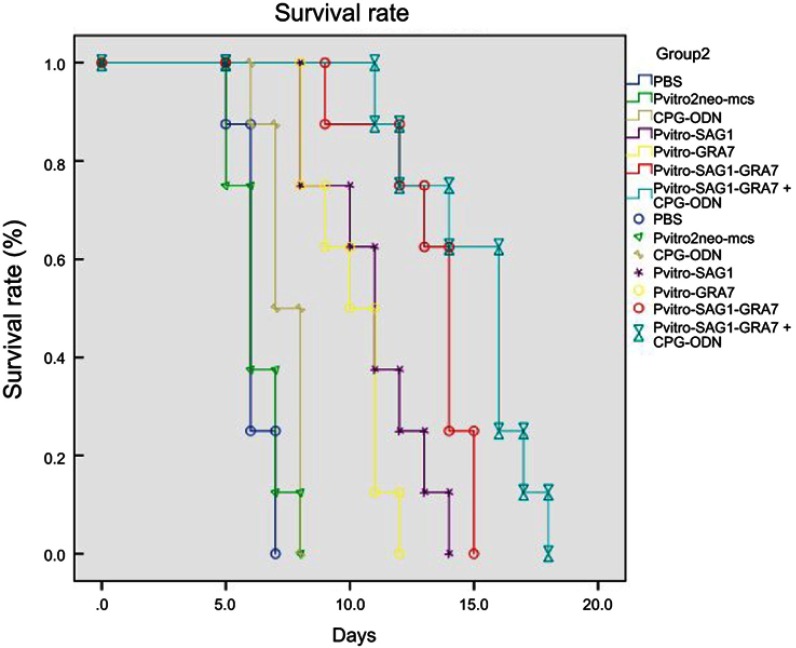
In this study, protective efficiency of DNA vaccines against acute toxoplasmosis was assessed by infecting vaccinated mice with 1×103 tachyzoites of the virulent T. gondii RH strain four weeks after the last immunization and monitoring their survival time until all mice died. Figure 4 shows the percentage of mice survival in different groups. All mice in control groups, including PBS, Pvitro2neo-mcs and CpG-ODN, died within 5–8 days after confronting lethal challenge. The survival time in experimental groups was significantly (P<0.05) longer than that in control groups. Mice immunized with pVitro-SAG1–GRA7 vaccine alone and pVitro-SAG1-GRA7 with CpG-ODN vaccine, respectively, survived 9–15 days (13.25±1.85) and 11–18 days (15.0±2.29) after lethal challenges which were significantly longer than that mice immunized with pVitro-SAG1 or pVitro-GRA7 groups did, including 8–14 (10.87±2.02) and 8–12 (10.0±1.41) days after lethal challenges, respectively. Furthermore, in pVitro-SAG1-GRA7 with CpG-ODN group, a longer survival time was observed, compared to that in pVitro-SAG1-GRA7 group. This indicates that CpG-ODN adjuvant positively affects the protective efficiency of pVitro-SAG1-GRA7 vaccine.
Figure 4.
Survival curves in control and experimental groups after receiving 1×103 T. gondii RH strain tachyzoites four weeks after the last immunization. Each group included nine mice. Survival was monitored daily for 18 days after challenges.
Discussion
Toxoplasmosis is a prevalent, life-threatening infection with adverse economic consequences.19 Up-to-date, no human vaccines have been licensed for toxoplasmosis. DNA vaccination, as an effective immunization method that induces specific cellular and humoral immune responses, seems a promising strategy to prevent toxoplasmosis, especially when designed as a multivalent vaccine.12,19 The capacity to mimic natural antigen processing and presentation in immunized organisms makes DNA vaccine a favorable method to protect humans and animals against intracellular microbial infections such as toxoplasmosis. In general, DNA vaccines are heat stable and relatively easy to produce and cheap.29 In the current study, immunogenicity and protective effectiveness of a newly designed naked DNA vaccine containing full-length SAG1 and GRA7 genes in a dual-promoter plasmid (pVitro2 neo-mcs) were assessed, which can simultaneously produce both antigens as separate proteins in murine models. The pVitro2-neo-mcs plasmid has widely been used in gene therapy. Moreover, it has been used as vector in a few studies on DNA vaccines, including a multi-epitope DNA vaccine containing six antigen segments of T. gondii.30–32 Unlike multivalent cocktail DNA vaccines, the pVitro-SAG1–GRA7 construct ensures the equal delivery of both genes to host cells. Furthermore, the pVitro-SAG1–GRA7 construct, which contains two genes expressed at various stages of the infection, may cause an effective immune response. Furthermore, the construction of this DNA vaccine has provided an opportunity to compare the current dual-expressing DNA vaccine with DNA fusion vaccines producing a single fusion protein.
Increase of specific IgG levels is vital to eliminate toxoplasmosis, especially in the acute phase. The IgG attaches to T. gondii tachyzoites and prevents their invasion to host cells. They also cause the parasite to be killed by macrophages through opsonization or lysis following activation of the classic complement.33–35 Specific IgG titers have constantly been raised in this study over the time and particularly after the third injection. In the present study, pVitro-SAG1-GRA7 mice showed significantly higher IgG titers than that mice in monovalent or control groups did (P<0.05). Furthermore, co-administration of CpG-ODN adjuvant increased the IgG titer. Although this increase was not statistically significant, it exhibited effects of CpG-ODN on B-cell proliferation. In addition to uncontroversial roles of humoral immune responses that eliminate toxoplasmosis, cell-mediated immunity is crucial to eradicate this mandatory intracellular protozoa.36,37 The IFN-γ and IgG2a are characteristics of Th1 orientation. In fact, Th1 cells stimulate naive CD8+ T-lymphocytes to transform to cytotoxic lymphocyte (CTL) and promote production of IFN-γ that activates NK cells and macrophages. Moreover, Th1 cells play a key role in overcoming toxoplasmosis.36 In the current study, assessment of specific IgG2a levels revealed that all mice in experimental groups produced high levels of this IgG isotype. The pVitro-SAG1-GRA7 vaccine significantly motivated production of higher IgG2a levels than that pVitro-SAG1, pVitro-GRA7 or control groups did. Injection of CpG-ODN adjuvant with pVitro-SAG1-GRA7 vaccine increased the level of IgG2a more significantly than that injection of pVitro-SAG1-GRA7 alone did. This shows the effects of CpG-ODN adjuvant on B-cells to produce IgG2a isotype and co-stimulate the specific immune responses derived from the vaccine. Levels of IgG1 that indicate Th2 responses have slightly been changed. As previously stated, Th1-type cytokines are necessary for the host resistance to T. gondii.38 The IFN-γ, as a major Th1-type cytokine, induces host protection through various mechanisms, including inhibition of T. gondii growth, mediation of nitrogen oxide (NO) biochemical pathway and induction of immune-related GTPase (IRG) accumulation on PV that results in vacuole destruction and hence parasite death.39 In the present study, IFN-γ levels increased dramatically in all experimental groups, especially the multivalent groups. However, this increase was further significant when combined with CpG-ODN adjuvant. A similar finding is seen when comparing IFN-γ levels with IgG2a/IgG1 ratio. These data suggest that the dual gene vaccine (pVitro-SAG1-GRA7) elicits Th1 immune responses efficiently, which is important for a vaccine preventing T. gondii infections. As a proinflammatory cytokine, production of high IFN-γ levels can result in immunopathological damages. The IL-10 is a regulatory cytokine that eliminates Th1 and Th2 effector responses including IFN-γ production through suppression of macrophage and dendritic cell functions. The cytokine enhances B-lymphocyte proliferation and differentiation and antibody production.40 In the present study, IL-10 did not increase. This might affect IgG production and survival time to some extent. IgG level and survival time could be improved if pVitro-SAG1-GRA7 vaccine could induce IL-10 production. In the current study, lymphocyte proliferation demonstrated further cellular immune responses in immunized mice. The SI data showed that splenic T-lymphocytes proliferated exponentially in all experimental groups and increased their clonal population while stimulated by STAg. Results also showed that splenic lymphocytes in pVitro-SAG1-GRA7 group proliferated more significantly, compared to that they did in monovalent or control groups (P<0.05). A further significant proliferation was observed when CpG-ODN adjuvant was co-administered (P<0.05). In this study, the lethal challenge was used to assess immune efficiency of pVitro-SAG1-GRA7 vaccine against the acute infection. Although all mice in experimental groups could survive significantly longer than that mice in control groups could, all died within 8–15 days. Therefore, the vaccine provided a partial protection against toxoplasmosis. Up-to-date, no DNA vaccines have fully protected hosts against intraperitoneal infection of T. gondii RH strain.12,41,42 However, intraperitoneal infection is not the natural entry route for T. gondii RH strain. Furthermore, the most prevalent strain in environment that affects host organisms is the avirulent genotype II strain. Considering the lethal challenge conditions, it is necessary to carry out challenges in further natural conditions for further accurate assessment of pVitro-SAG1-GRA7 vaccine efficiency. The survival time in divalent groups was more significant than that in monovalent groups and co-administration of CpG-ODN adjuvant could significantly extend the survival time, compared to pVitro-SAG1-GRA7 group.
Similar to previous studies, the current data show that SAG1 and GRA7 antigens confer significant immune responses leading to higher survival times, compared to control groups.13,17,43 In fact, studies on T. gondii DNA vaccines do not follow a unified standard protocol to assess vaccine immunogenicity and efficiency. Various parameters affect results of DNA vaccination, including mice strains, vector types, doses and routes of immunization, numbers of injections, vaccination intervals and parasite doses in lethal challenges. Thus, it is difficult to compare the results of various studies. Comparison of pVitro-SAG1-GRA7 vaccine with SAG1-GRA2 or GRA17-GRA23 fusion vaccine demonstrated nearly similar levels of cellular and humoral responses. Moreover, durations of survival time were nearly similar.44,45 In a study, fusion DNA vaccine encoding ROP16 and GRA7 genes could increase the total IgG, IgG2a and IFN-γ levels more significantly than that the pVitro-SAG1-GRA7 vaccine did. Survival time was nearly similar to that of pVitro-SAG1-GRA7 vaccine.43 The divalent pVitro-SAG1-GRA7 vaccine has been shown to more effectively induce immune responses, compared to that monovalent vaccines do. This finding is similar to those reported by nearly all studies on multivalent vaccines.11,12 There is almost a general consensus that multivalent DNA vaccines, including cocktail or fusion DNA vaccines, provide a further effective immune protection against T. gondii than that monovalent DNA vaccines do, regardless of their type. For example, a fusion DNA vaccine, encoding T. gondii SAG1 and GRA2, produced stronger humoral and Th1-type immune responses and considerably longer survival time, compared to that separate SAG1 or GRA2 DNA vaccine did.44 Immune protection has significantly been improved in BALB/c mice immunized with SAG1 or MIC3 cocktail DNA vaccine compared to that in mice immunized with SAG1 plus MIC3 DNA vaccine.46 In another study, ROP16-GRA7 fusion DNA vaccine resulted in higher specific IgG antibodies, higher percentage of CD8+ T-lymphocyte subpopulation and much longer survival time compared to that the monovalent DNA vaccine alone did.37 Therefore, it is concluded that a DNA vaccine encoding antigens from various stages of infection is further powerful. Two reasons of limited quantity of foreign DNA acceptance by eukaryotic cells and inevitable competition in host gene expression system to express foreign genes from various plasmids make pVitro-SAG1-GRA7 vaccine superior to cocktail DNA vaccines. Furthermore, it is further complicated to construct fusion DNA vaccines with possible conformational changes at linking sites.
The CpG-ODN adjuvant generates Th1-dominated type, CD8+ T-lymphocyte and CTL immune responses which are necessary for the protective immunity against toxoplasmosis.47,48 Results of the present study have shown that addition of CpG-ODN adjuvant significantly improves the efficiency of pVitro-SAG1-GRA7 vaccines; similarly to results from previous studies.49,50 However, results from a study showed that use of this adjuvant with DNA vaccines against toxoplasmosis could induce impaired immune responses.32 The CpG-ODN adjuvant effects may increase by investigating optimized inoculation timing. It is shown that optimal antigen presentation occurs two or three days after intramuscular DNA vaccination. This is possibly the best time for CpG-ODN adjuvant administration since major histocompatibility complex (MHC) restricted antigen presentation occurs with activation of dendritic cells (DCs), secretion of various cytokines and upregulation of costimulatory molecules.49,51 This may explain the reason for high elevation of IgG levels when CpG-ODN adjuvant is co-inoculated with antigen-base vaccines (3–104 folds), rather than DNA-base vaccines.52 In brief, the current data have demonstrated that the multivalent pVitro-SAG1-GRA7 DNA vaccine, encoding full lengths of T. gondii SAG1 and GRA7, induces strong specific humoral and cellular immune responses and is a novel multivalent DNA vaccine candidate against toxoplasmosis. Furthermore, results have shown that this vaccine is more efficient in immune response and protective efficiency than that the single-gene vaccine is. However, no effective vaccines provide a complete protection against the lethal challenge of T. gondii RH strain tachyzoites; DNA vaccination is still considered as a good strategy for T. gondii control. It is also concluded that CpG-ODN adjuvant addition increases pVitro-SAG1-GRA7 DNA vaccine immunogenicity and immune protection. In conclusion, data from the current study suggest that the multivalent pVitro-SAG1-GRA7 DNA vaccine with CpG-ODN adjuvant is a promising vaccine candidate against toxoplasmosis.
Acknowledgments
This study was supported by the Tehran University of Medical Sciences and Health Services (Project No. 21648), Tehran, Iran. We would like to acknowledge all staff within the Toxoplasmosis Laboratory, Department of Medical Parasitology and Mycology, Tehran University of Medical Sciences, for their useful help.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39(8):877–882. doi: 10.1016/j.ijpara.2009.02.018 [DOI] [PubMed] [Google Scholar]
- 2.Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awan KJ. Congenital toxoplasmosis: chances of occurrence in subsequent siblings. Ann Ophthalmol. 1978;10(4):459–465. [PubMed] [Google Scholar]
- 4.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47(4):554–566. doi: 10.1086/590149 [DOI] [PubMed] [Google Scholar]
- 5.Pereira-Chioccola VL, Vidal JE, Su C. Toxoplasma gondii infection and cerebral toxoplasmosis in HIV-infected patients. Future Microbiol. 2009;4(10):1363–1379. doi: 10.2217/fmb.09.89 [DOI] [PubMed] [Google Scholar]
- 6.Lautenslager JP. Toxoplasmosis as a significant disease in man and animals with special reference to preventive measures by the farm community. Can Vet J. 1987;28(5):261–264. [PMC free article] [PubMed] [Google Scholar]
- 7.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez JB, Szajnman SH. New antibacterials for the treatment of toxoplasmosis; a patent review. Expert Opin Ther Pat. 2012;22(3):311–333. doi: 10.1517/13543776.2012.668886 [DOI] [PubMed] [Google Scholar]
- 9.Caetano BC, Bruna-Romero O, Fux B, Mendes EA, Penido ML, Gazzinelli RT. Vaccination with replication-deficient recombinant adenoviruses encoding the main surface antigens of Toxoplasma gondii induces immune response and protection against infection in mice. Hum Gene Ther. 2006;17(4):415–426. doi: 10.1089/hum.2006.17.415 [DOI] [PubMed] [Google Scholar]
- 10.Wang HL, Wang YJ, Pei YJ, et al. DNA vaccination with a gene encoding Toxoplasma gondii Rhoptry Protein 17 induces partial protective immunity against lethal challenge in mice. Parasite. 2016;23:4. doi: 10.1051/parasite/2016004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang NZ, Chen J, Wang M, Petersen E, Zhu XQ. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev Vaccines. 2013;12(11):1287–1299. doi: 10.1586/14760584.2013.844652 [DOI] [PubMed] [Google Scholar]
- 12.Kur J, Holec-Ga̧sior L, Hiszczyńska-Sawicka E. Current status of toxoplasmosis vaccine development. Expert Rev Vaccines. 2009;8(6):791–808. doi: 10.1586/erv.09.27 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Yin H. Research progress on surface antigen 1 (SAG1) of Toxoplasma gondii. Parasites Vectors. 2014;7(1):180–189. doi: 10.1186/1756-3305-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesbron-Delauw MF, Gendrin C, Travier L, Ruffiot P, Mercier C. Apicomplexa in mammalian cells: trafficking to the parasitophorous vacuole. Traffic. 2008;9(5):657–664. doi: 10.1111/j.1600-0854.2008.00728.x [DOI] [PubMed] [Google Scholar]
- 15.Bai M, Wang JL, Elsheikha HM, et al. Functional characterization of dense granule proteins in Toxoplasma gondii RH strain using CRISPR-Cas9 system. Front Cell Infect Microbiol. 2018;8:300–308. doi: 10.3389/fcimb.2018.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiszczyńska-Sawicka E, Oledzka G, Holec-Gasior L, et al. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii. Vet Parasitol. 2011;177(3–4):281–289. doi: 10.1016/j.vetpar.2010.11.047 [DOI] [PubMed] [Google Scholar]
- 17.Min J, Qu D, Li C, et al. Enhancement of protective immune responses induced by Toxoplasma gondii dense granule antigen 7 (GRA7) against toxoplasmosis in mice using a prime-boost vaccination strategy. Vaccine. 2012;30(38):5631–5636. doi: 10.1016/j.vaccine.2012.06.081 [DOI] [PubMed] [Google Scholar]
- 18.Neudecka A, Stachelhausa S, Nischika N, et al. Expression variance, biochemical and immunological properties of Toxoplasma gondii dense granule protein GRA7. Microbes Infect. 2002;4(6):581–590. doi: 10.1016/S1286-4579(02)01576-9 [DOI] [PubMed] [Google Scholar]
- 19.Jongert E, Roberts CW, Gargano N, Förster-Wald E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem Inst Oswaldo Cruz. 2009;104(2):252–266. doi: 10.1590/S0074-02762009000200019 [DOI] [PubMed] [Google Scholar]
- 20.Buxton D, Thomson K, Maley S, Wright S, Bos HJ. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet Rec. 1991;129(5):89–93. doi: 10.1136/vr.129.5.89 [DOI] [PubMed] [Google Scholar]
- 21.Saha R, Killian S, Donofrio RS. DNA vaccines: a mini review. Recent Patents DNA Gene Sequences. 2011;5(2):92–96. doi: 10.2174/187221511796392114 [DOI] [PubMed] [Google Scholar]
- 22.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3(8):849–854. [DOI] [PubMed] [Google Scholar]
- 23.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27(9):2340–2344. doi: 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 24.Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25(3–4):135–154. doi: 10.1080/08830180600743057 [DOI] [PubMed] [Google Scholar]
- 25.Teimouri A, Azami SJ, Keshavarz H, et al. Anti-Toxoplasma activity of various molecular weights and concentrations of chitosan nanoparticles on tachyzoites of RH strain. Int J Nanomedicine. 2018;13:1341–1351. doi: 10.2147/IJN.S177627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayazian Mavi S, Keshavarz H, Modarresi M, et al. Design of a dual-promoter expression vector harboring Sag1 and Gra7 genes from Toxoplasma gondii (RH strain). Trop Biomed. 2018;35(1):126–134. [PubMed] [Google Scholar]
- 27.Wang H, He S, Yao Y, et al. Toxoplasma gondii: protective effect of an intranasal SAG1 and MIC4 DNA vaccine in mice. Exp Parasitol. 2009;122(3):226–232. doi: 10.1016/j.exppara.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 28.Fletcher RH, Fletcher SW, Wagner EH. Clinical Epidemiology: The Essentials. 3rd ed. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 29.Coban C, Koyama S, Takeshita F, Akira S, Ishii KJ. Molecular and cellular mechanisms of DNA vaccines. Hum Vaccin. 2008;4(6):453–457. doi: 10.4161/hv.4.1.4806 [DOI] [PubMed] [Google Scholar]
- 30.Wen Y, Wang CT, Ma TT, et al. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010;101(11):2325–2332. doi: 10.1111/j.1349-7006.2010.01605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Trop S, Baer S, et al. Dynamics of the major histocompatibility complex class i processing and presentation pathway in the course of malaria parasite development in human hepatocytes: implications for vaccine development. PLoS One. 2013;8(9):e75321. doi: 10.1371/journal.pone.0075321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Shi L, Cheng YB, Fan GX, Ren HX, Yuan YK. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol Res. 2009;105(1):267–274. doi: 10.1007/s00436-009-1393-1 [DOI] [PubMed] [Google Scholar]
- 33.Hammouda NA, Abo el-Naga I, Hussein ED, Rashwan EA. Opsonization and intracellular killing of Toxoplasma gondii by human mononuclear phagocytes. J Egypt Soc Parasitol. 1995;25(1):11–17. [PubMed] [Google Scholar]
- 34.Vercammen M, Scorza T, El Bouhdidi A, et al. Opsonization of Toxoplasma gondii tachyzoites with nonspecific immunoglobulins promotes their phagocytosis by macrophages and inhibits their proliferation in nonphagocytic cells in tissue culture. Parasite Immunol. 1999;21(11):555–563. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber RD, Feldman HA. Identification of the activator system for antibody to toxoplasma as the classical complement pathway. J Infect Dis. 1980;141(3):366–369. doi: 10.1093/infdis/141.3.366 [DOI] [PubMed] [Google Scholar]
- 36.Ismail N, Olano JP, Feng HM, Walker DH. Current status of immune mechanisms of killing of intracellular microorganisms. FEMS Microbiol Lett. 2002;207(2):111–120. doi: 10.1111/j.1574-6968.2002.tb11038.x [DOI] [PubMed] [Google Scholar]
- 37.Jongert E, Lemiere A, Van Ginderachter J, De Craeye S, Huygen K, D’Souza S. Functional characterization of in vivo effector CD4+ and CD8+ T Lymphocyte responses in acute Toxoplasmosis: an interplay of IFN-γ and cytolytic T cells. Vaccine. 2010;28(13):2556–2564. doi: 10.1016/j.vaccine.2010.01.031 [DOI] [PubMed] [Google Scholar]
- 38.Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14(2):109–121. doi: 10.1038/nri3598 [DOI] [PubMed] [Google Scholar]
- 39.Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol. 2012;34(6):793–813. doi: 10.1007/s00281-012-0339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771 [DOI] [PubMed] [Google Scholar]
- 41.Meng M, Zhou A, Lu G, et al. DNA prime and peptide boost immunization protocol encoding the Toxoplasma gondii GRA4 induces strong protective immunity in BALB/c mice. BMC Infect Dis. 2013;13(1):494–503. doi: 10.1186/1471-2334-13-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ching XT, Fong MY, Lau YL. Evaluation of immunoprotection conferred by the subunit vaccines of GRA2 and GRA5 against acute toxoplasmosis in BALB/c mice. Front Microbiol. 2016;7:609–619. doi: 10.3389/fmicb.2016.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Q, Wang F, Wang G, et al. Toxoplasma gondii: immune response and protective efficacy induced by ROP16/GRA7 multicomponent DNA vaccine with a genetic adjuvant B7-2. Hum Vaccin Immunother. 2014;10(1):184–191. doi: 10.4161/hv.26703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou H, Min J, Zhao Q, et al. Protective immune response against Toxoplasma gondii elicited by a recombinant DNA vaccine with a novel genetic adjuvant. Vaccine. 2012;30(10):1800–1806. doi: 10.1016/j.vaccine.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 45.Zhu W, Wanga JL, Chena K, et al. Evaluation of protective immunity induced by DNA vaccination with genes encoding Toxoplasma gondii GRA17 and GRA23 against acute toxoplasmosis in mice. Exp Parasitol. 2017;179:20–27. doi: 10.1016/j.exppara.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 46.Fang R, Feng H, Hu M, et al. Evaluation of immune responses induced by SAG1 and MIC3 vaccine cocktails against Toxoplasma gondii. Vet Parasitol. 2012;187(1–2):140–146. doi: 10.1016/j.vetpar.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi H, Horner AA, Takabayashi K, et al. Immunostimulatory DNA prepriming: a novel approach for prolonged Th1-biased immunity. Cell Immunol. 1999;198(1):69–75. doi: 10.1006/cimm.1999.1572 [DOI] [PubMed] [Google Scholar]
- 48.Krieg AM. Immune effects and mechanisms of action of CpG motifs. Vaccine. 2000;19(6):618–622. doi: 10.1016/S0264-410X(00)00249-8 [DOI] [PubMed] [Google Scholar]
- 49.Kojima Y, Xin KQ, Ooki T, et al. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine. 2002;20(23–24):2857–2865. doi: 10.1016/S0264-410X(02)00238-4 [DOI] [PubMed] [Google Scholar]
- 50.Jiang M, Yao J, Feng G. Protective effect of DNA vaccine encoding pseudomonas exotoxin A and PcrV against acute pulmonary P. aeruginosa infection. PLoS One. 2014;9(5):e96609. doi: 10.1371/journal.pone.0096609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Askew D, Chu RS, Krieg AM, Harding CV. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J Immunol. 2000;165(12):6889–6895. doi: 10.4049/jimmunol.165.12.6889 [DOI] [PubMed] [Google Scholar]
- 52.Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009;61(3):248–255. doi: 10.1016/j.addr.2008.12.012 [DOI] [PubMed] [Google Scholar]