Abstract
In the intensive care unit, alcohol intake above the NIAAA recommendations regardless of the existence of an Alcohol Use Disorder (AUD), was associated with an increased risk of death and longer time on ventilator. This rises the hypothesis that unhealthy alcohol use may lead to specific issues when weaning the mechanical ventilation (i.e. agitation or its related complications) regardless of AUD or withdrawal syndrome. Thus, we proposed to use baclofen off-label to avoid agitation. The data presented in this article is related to the research article entitled: “Pharmacokinetics and toxicity of high-dose baclofen in ICU patients” Vourc'h et al., 2019 Data provided in this submission includes 1) the detailed methods for baclofen assay by mass spectrometric detection, 2) the supplementary population pharmacokinetic analysis presenting observed concentration vs. population or individual predicted concentration (raw data of the latter is also available), and 3) the algorithm for the adaptation of baclofen daily doses according of the renal clearance to assess the risk of toxicity in critically ill patients.
Keywords: Baclofen, Pharmacokinetic, Intensive care unit, Agitation, Alcohol use disorder
Specifications table
| Subject area | Pharmacokinetic, unhealthy alcohol use, off-label use of baclofen, acute kidney injury, renal failure, alcohol use disorder, alcohol withdrawal syndrome |
| More specific subject area | Prevention of agitation in unhealthy alcohol user in the ICU |
| Type of data | Table, text and figures |
| How data was acquired | Liquid chromatography – tandem mass spectrometry assay 3200 QTRAP® (SCIEX, Villebon-sur-Yvette, France). |
| Data format | Analyzed data |
| Experimental factors | In 20 patients under mechanical ventilation in the intensive care unit, blood samples were taken following enteral administration of baclofen (feeding tube) and analyzed by chromatography to determine drug concentration over a 8-h period after the intake. |
| Experimental features | Mass spectrometric detection was performed in positive ion mode using selected reactant monitoring |
| Data source location | Nantes University Hospital, Clinical pharmacology department, Nantes, FRANCE. |
| Data accessibility | Pharmacokinetic data are hosted with the article. Additional de-identified data collected for the study, including individual participant data will be made available to others. The study protocol, statistical analysis plan, ethics committee approval will be made available on reasonable request by addressing an e-mail to the corresponding author. |
| Related research article | Vourc'h, M., Dailly, E., Hourmant, Y., Bellouard, R., Mahe, P. J., Deslandes, G. et al. (2019). Pharmacokinetics and toxicity of high-dose baclofen in ICU patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry. http://doi.org/10.1016/j.pnpbp.2019.02.016[1] |
Value of the data
|
1. Data
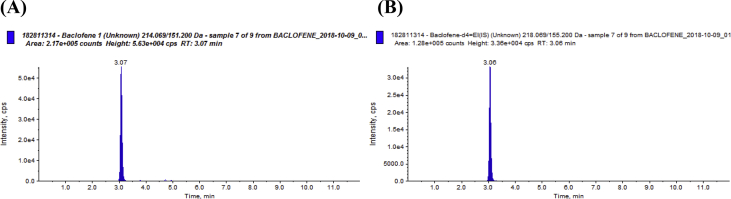
In a surgical intensive care, unhealthy alcohol users received off-label baclofen to prevent agitation during the awakening phase, trying to ease mechanical ventilation weaning. We created an algorithm for baclofen dose adaptation according to estimated glomerular filtration rate. The starting day of baclofen is referred as “day 1”: Patients received a loading dose of baclofen via an enteral feeding tube on day 1. Then daily doses were divided into 3 intakes until the weaning of mechanical ventilation. The pharmacokinetic study started on day 3: Samples were taken just before administration of baclofen and then from 30 min to 8 hours after administration (total of 8 samples). This article comprises: the method used to treat and analyze the samples (see Methods for baclofen assay), data files generated with the NONMEM software version 7.3 (Icon Development Solutions, Hanover, USA) based on baclofen assays (see a chromatogram example in Fig. 1 and additional results of the population pharmacokinetic analysis in Fig. 2. Finally, it includes the algorithm for baclofen doses adjustment according to the renal function (see Table 2).
Fig. 1.
Chromatograms of a patient (A) plasma sample (0,17 mg/L) and the internal standard (B). These chromatograms with a high intensity signal for a low baclofen plasma concentration show the specificity and the high sensitivity of the assay. This assay is specific because no interfering chromatographic peak was found with a retention time close to the retention time of the baclofen peak (3.07 min). This assay is sensitive because the peak intensity (about 5.0 × 104 cps) is highly superior to the background noise of the baseline although the baclofen plasma concentration (0,17 mg/l) is low.
Fig. 2.
Population pharmacokinetic analysis of baclofen (color). Diagnostic plots for population pharmacokinetic analysis of baclofen: observed concentration [DV (mg/L)] versus population predicted concentration [PRED (mg/L)], weighted residuals (WRES) versus PRED in the final model (Figs. 2-1 and 2-2) and the base model (Figs. 2-3 and 2-4).
Table 2.
Protocol of sedation with baclofen dose adjustment according to renal function.
| eGFR (ml/min/1.73m2) | Day 1, Baclofen loading dose (mg) | Day 2 and following days until extubation/tracheotomy (mg) |
|---|---|---|
| >90 | 150 | 50-50-50 |
| 89–60 | 100 | 30-20-50 |
| 30–59 | 70 | 20-20-30 |
| <30 ou CVVHFa | 50 | 20-10-20 |
| IHDb | 50 | 50 mg before each session |
| <15 without RRTc | 50 | No administration |
eGFR: estimation glomerular filtration rate by the CKD-EPI equation. aCVVHF: continuous veno-venous hemofiltration, bIHD: intermittent hemodialysis, cRRT: renal replacement therapy.
2. Experimental design, materials, and methods
2.1. Methods for baclofen assay
Plasma samples were mixed with acetonitrile containing the internal standard (baclofen-d4) and centrifuged. The supernatant was transferred into glass tubes and then evaporated at 37 °C under a stream of nitrogen. Samples were recovered with mobile phase and then injected into the chromatographic system Agilent 1200 series (Agilent, Les Ulis, France). Separations were performed on an Aquasil® 3 μm C18 50 mm × 2.1 mm column (Thermo Fisher Scientific, USA). The (MS/MS) system used was a 3200 QTRAP® (SCIEX, Villebon-sur-Yvette, France). Mass spectrometric detection was performed in positive ion mode using selected reactant monitoring [baclofen m/z 214.07→151.2, baclofen-d4 (internal standard) m/z 218.07→155.2]. The method was found to be accurate (inaccuracy <10%) and showed good precision (imprecision <10%) The limit of quantitation is 0.01 mg/L. A chromatograph corresponding to a patient plasma sample is presented in Fig. 1.
2.2. Additional analysis for the pharmacokinetic model
The population pharmacokinetic analysis was performed using NONMEM software version 7.3 (Icon Development Solutions, Hanover, USA). The diagnostic plots corresponding to the final and base pharmacokinetic models are presented in Fig. 2. The raw data used to produce the graphs of the final model are presented in Table 1.
Table 1.
Raw data to produce diagnostic plots of the final model.
| PATIENT | DV | PRED | WRES | PATIENT | DV | PRED | WRES |
|---|---|---|---|---|---|---|---|
| 1 | 0,1 | 0,24 | −1,29 | 6 | 0,25 | 0,27 | 0,13 |
| 1 | 0,47 | 0,34 | −0,06 | 6 | 0,35 | 0,37 | 0,33 |
| 1 | 0,91 | 0,54 | 2,17 | 6 | 0,48 | 0,57 | −0,33 |
| 1 | 0,7 | 0,58 | −0,29 | 6 | 0,48 | 0,45 | 0,09 |
| 1 | 0,56 | 0,56 | −0,71 | 6 | 0,48 | 0,6 | −0,68 |
| 1 | 0,36 | 0,43 | 0,29 | 6 | 0,4 | 0,47 | −0,03 |
| 1 | 0,23 | 0,32 | −0,15 | 6 | 0,31 | 0,36 | −0,02 |
| 1 | 0,16 | 0,24 | 0,16 | 6 | 0,23 | 0,27 | −0,33 |
| 2 | 0,26 | 0,33 | −1,48 | 7 | 0,35 | 0,38 | −0,34 |
| 2 | 0,42 | 0,43 | −0,84 | 7 | 0,97 | 0,47 | 3,96 |
| 2 | 0,78 | 0,64 | 0,28 | 7 | 1,08 | 0,68 | −0,94 |
| 2 | 0,88 | 0,67 | 1,55 | 7 | 1,04 | 0,72 | 1,38 |
| 2 | 0,72 | 0,62 | −0,44 | 7 | 0,87 | 0,71 | 1,23 |
| 2 | 0,63 | 0,53 | 0,56 | 7 | 0,49 | 0,58 | −1,7 |
| 2 | 0,47 | 0,42 | 0,68 | 7 | 0,39 | 0,47 | −1,41 |
| 2 | 0,35 | 0,33 | 0,62 | 7 | 0,34 | 0,38 | −0,5 |
| 3 | 0,21 | 0,2 | −0,69 | 8 | 0,62 | 0,45 | 1,05 |
| 3 | 0,32 | 0,3 | −0,68 | 8 | 0,62 | 0,48 | 0,61 |
| 3 | 0,62 | 0,5 | 1,06 | 8 | 0,64 | 0,56 | 0,1 |
| 3 | 0,59 | 0,54 | 0,04 | 8 | 0,64 | 0,58 | −0,06 |
| 3 | 0,52 | 0,52 | −0,85 | 8 | 0,6 | 0,58 | −1 |
| 3 | 0,45 | 0,38 | 0,64 | 8 | 0,6 | 0,52 | −0,4 |
| 3 | 0,36 | 0,28 | 0,92 | 8 | 0,6 | 0,47 | 0,28 |
| 3 | 0,26 | 0,2 | 0,48 | 8 | 0,6 | 0,43 | 0,95 |
| 4 | 0,42 | 0,26 | 0,42 | 9 | 0,64 | 0,35 | 2,36 |
| 4 | 0,5 | 0,46 | −0,04 | 9 | 0,7 | 0,45 | 2,11 |
| 4 | 0,63 | 0,56 | 0,55 | 9 | 0,61 | 0,65 | −0,82 |
| 4 | 0,66 | 0,6 | −0,55 | 9 | 0,6 | 0,69 | −0,64 |
| 4 | 0,67 | 0,59 | −0,6 | 9 | 0,6 | 0,68 | −0,27 |
| 4 | 0,73 | 0,45 | 2,77 | 9 | 0,53 | 0,55 | −1,26 |
| 4 | 0,5 | 0,34 | 0,15 | 9 | 0,52 | 0,51 | 0,27 |
| 4 | 0,37 | 0,26 | −0,67 | 9 | 0,5 | 0,35 | −0,44 |
| 5 | 0,43 | 0,52 | −0,11 | 10 | 0,22 | 0,24 | 0,12 |
| 5 | 0,44 | 0,55 | −0,25 | 10 | 0,23 | 0,34 | 0,38 |
| 5 | 0,48 | 0,64 | −0,81 | 10 | 0,24 | 0,54 | −0,46 |
| 5 | 0,57 | 0,66 | 0,57 | 10 | 0,24 | 0,58 | −1,4 |
| 5 | 0,52 | 0,66 | −0,67 | 10 | 0,25 | 0,56 | −1,62 |
| 5 | 0,51 | 0,62 | −0,42 | 10 | 0,31 | 0,43 | 0,36 |
| 5 | 0,48 | 0,58 | −0,24 | 10 | 0,22 | 0,3 | −0,36 |
| 5 | 0,48 | 0,55 | 0,39 | 10 | 0,21 | 0,22 | 0,25 |
| PATIENT | DV | PRED | WRES | PATIENT | DV | PRED | WRES |
| 11 | 0,11 | 0,29 | −1,04 | 16 | 0,78 | 0,2 | 2,9 |
| 11 | 0,33 | 0,38 | −0,12 | 16 | 0,79 | 0,44 | 0,39 |
| 11 | 0,62 | 0,59 | −0,4 | 16 | 0,87 | 0,51 | 0,86 |
| 11 | 0,72 | 0,63 | 1,3 | 16 | 0,92 | 0,56 | 0,48 |
| 11 | 0,63 | 0,61 | 0,35 | 16 | 0,95 | 0,54 | 0,63 |
| 11 | 0,36 | 0,48 | −0,99 | 16 | 0,97 | 0,44 | 1,39 |
| 11 | 0,24 | 0,37 | −0,3 | 16 | 0,92 | 0,33 | 1,87 |
| 11 | 0,17 | 0,28 | 0,2 | 16 | 0,84 | 0,26 | 1,6 |
| 12 | 0,11 | 0,56 | −1,21 | 17 | 0,85 | 0,98 | −0,51 |
| 12 | 0,33 | 0,65 | −0,34 | 17 | 1,07 | 1,04 | 1,92 |
| 12 | 0,62 | 0,86 | −0,68 | 17 | 1,03 | 1,11 | −0,09 |
| 12 | 0,72 | 0,9 | 1,07 | 17 | 0,97 | 1,12 | −0,79 |
| 12 | 0,63 | 0,89 | 0,1 | 17 | 0,94 | 1,12 | −1,14 |
| 12 | 0,36 | 0,77 | −1,51 | 17 | 0,93 | 1,07 | −0,27 |
| 12 | 0,24 | 0,65 | −0,75 | 17 | 0,91 | 1,03 | −0,22 |
| 12 | 0,17 | 0,55 | 0,04 | 17 | 0,91 | 0,99 | 0,27 |
| 13 | 0,39 | 0,33 | 0,96 | 18 | 0,32 | 0,21 | −0,68 |
| 13 | 0,41 | 0,43 | −1,06 | 18 | 0,82 | 0,31 | 3,61 |
| 13 | 0,74 | 0,63 | 1,21 | 18 | 1,01 | 0,52 | 0,1 |
| 13 | 0,68 | 0,67 | −0,31 | 18 | 1,01 | 0,56 | 0,24 |
| 13 | 0,65 | 0,66 | −0,29 | 18 | 0,97 | 0,56 | 0,84 |
| 13 | 0,53 | 0,53 | −0,09 | 18 | 0,87 | 0,44 | 3,26 |
| 13 | 0,41 | 0,42 | −0,4 | 18 | 0,62 | 0,34 | 0,78 |
| 13 | 0,36 | 0,34 | 0,22 | 18 | 0,43 | 0,27 | −0,77 |
| 14 | 0,22 | 0,26 | −0,44 | 19 | 0,09 | 0,18 | −0,58 |
| 14 | 0,24 | 0,36 | 0,25 | 19 | 0,15 | 0,28 | −0,31 |
| 14 | 0,24 | 0,56 | −0,52 | 19 | 0,28 | 0,48 | 0,08 |
| 14 | 0,24 | 0,6 | −1,43 | 19 | 0,26 | 0,52 | −1,29 |
| 14 | 0,27 | 0,59 | −1,39 | 19 | 0,25 | 0,5 | −1,31 |
| 14 | 0,3 | 0,45 | −0,42 | 19 | 0,23 | 0,36 | −0,02 |
| 14 | 0,3 | 0,34 | 0,5 | 19 | 0,19 | 0,26 | 0,54 |
| 14 | 0,28 | 0,27 | 0,78 | 19 | 0,1 | 0,18 | −0,32 |
| 15 | 0,06 | 0,23 | −1,56 | 20 | 0,5 | 0,26 | 2,55 |
| 15 | 0,32 | 0,32 | 1,74 | 20 | 0,59 | 0,36 | 0,55 |
| 15 | 0,32 | 0,53 | −2,17 | 20 | 0,87 | 0,57 | 1,52 |
| 15 | 0,5 | 0,56 | 1,03 | 20 | 0,75 | 0,6 | −0,52 |
| 15 | 0,42 | 0,54 | −0,51 | 20 | 0,69 | 0,58 | −0,61 |
| 15 | 0,29 | 0,4 | −0,13 | 20 | 0,66 | 0,45 | 1,98 |
| 15 | 0,18 | 0,3 | −0,47 | 20 | 0,43 | 0,34 | −0,78 |
| 15 | 0,13 | 0,23 | −0,08 | 20 | 0,3 | 0,26 | −1,87 |
DV: observed concentration (mg/L), PRED: population predicted concentration (mg/L), WRES: weighted residuals.
These plots show an improved correlation between observed concentrations (DV) and predicted concentrations (PRED) obtained from the final model including the glomerular filtration rate in comparison with the results obtained from the base model without covariates: the equations established by linear regression between DV versus PRED (DV = 0.775 × PRED +0.128 in the final model versus DV = 0.112 × PRED+0.455 in the base model) and WRES (weighted residuals) versus PRED (WRES = −0.599 × PRED +0.362 in the final model versus WRES = −2.17 × PRED +1.21 in the base model) are respectively closer to the identity line (DV = PRED) and WRES = 0 in the final model (Figs. 2-1 and 2-2) than in the base model (Figs. 2-3 and 2-4).
2.3. Treatment adjustment in patients with renal failure
The following protocol of sedation was applied to adjust baclofen dose to renal function (see Table2).
Acknowledgments
We thank the nurses and the research team of the Intensive Care Unit of Nantes University Hospital. We also thank the team of Clinical Pharmacology Department of Nantes University Hopital.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vourc'h M., Dailly E., Hourmant Y., Bellouard R., Mahe P.J., Deslandes G. Pharmacokinetics and toxicity of high-dose baclofen in ICU patients. Prog. NeuroPsychopharmacol. Biol. Psychiatry. 2019 doi: 10.1016/j.pnpbp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Schulz M., Iwersen-Bergmann S., Andresen H., Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care. 2012;16 doi: 10.1186/cc11441. R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vourc'h M., Feuillet F., Mahe P.J., Sebille V., Asehnoune K. BACLOREA trial group, Baclofen to prevent agitation in alcohol-addicted patients in the ICU: study protocol for a randomised controlled trial. Trials. 2016;17:415. doi: 10.1186/s13063-016-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]