Abstract
Background/Aims
The aim of the present study was to investigate the effects of the combination treatment of pentasa and probiotics on the microflora composition and prognosis in patients with inflammatory bowel disease (IBD).
Materials and Methods
A total of 40 patients with IBD (19 control group and 21 observation group) were randomized. Patients in the control group were given pentasa, and patients in the observation group were given probiotics along with pentasa. The microflora composition, biochemical indices, inflammatory markers, and activity scores of the two groups were analyzed.
Results
After treatment, the number of enterobacteria, enterococci, saccharomyces, and bacteroides; the levels of fecal lactoferrin, 1-antitrypsin, β2-microglobulin, high-sensitivity C-reactive protein, and interleukin (IL)-6; activity scores; and recurrence rate in the observation group were significantly lower than those in the control group. Bifidobacterium and lactobacillus counts and IL-4 levels were significantly higher in the observation group than in the control group.
Conclusion
The combination of probiotics and pentasa can improve microflora composition in patients with IBD and reduce the level of inflammatory cytokines; therefore, it is worthy of further clinical validation.
Keywords: Pentasa, probiotics, inflammatory bowel disease, microflora structure, prognosis
INTRODUCTION
Inflammatory bowel disease (IBD) refers to diseases involved in chronic gut inflammation. The two main types of IBD are ulcerative colitis (UC) and Crohn’s disease (CD), which are similar in etiology, pathogenesis, and clinical manifestations (1). Epidemiological studies show that the incidence of IBD is on the rise, mainly in Europe and North America, and a large number of patients are young and often with a lifelong risk of recurrence; there have been a few reports of IBD incidences in China (2). In recent years, researches on IBD have increasingly focused on the role of the intestinal flora (3,4). The main treatment strategy for IBD is immunosuppression via corticosteroids and biological agents, such as monoclonal antibodies; however, despite the short-term alleviation of symptoms, immunosuppressive treatments show poor long-term maintenance of intestinal integrity and considerable adverse effects that affect disease progression (5). Pentasa, a controlled-release form of the anti-inflammatory drug mesalazine, is recommended for patients with mild to moderate UC. It inhibits the synthesis and release of prostaglandin, blocks the inflammatory cascade, and relieves intestinal mucosal inflammation; however, its long-term maintenance is still poor (6). Moreover, there are many reports on probiotic therapy for IBD, some of which have good function, and mainly contain lactobacillus, bifidobacterium, and saccharomyces (7). Compared with immunosuppressive agents, probiotics have good effect on repairing the intestinal mucosa but do not work well on extensive mucosal inflammation (8). Therefore, patients with IBD were treated with a combination of the anti-inflammatory pentasa and restorative probiotics, and then the clinical outcomes were evaluated to provide stronger evidence for clinical application.
MATERIALS AND METHODS
General data
A total of 40 patients with IBD who visited The First Affiliated Hospital of Fujian Medical University from January 2015 to June 2016 were selected and randomized into the control group (n=19) and observation group (n=21). The study was approved by the ethics committee of The First Affiliated Hospital of Fujian Medical University.
Inclusion criteria were (1) confirmed IBD diagnosis with mild and moderate symptoms as per the current standards in China, (2) no previous probiotic treatment, (3) no allergy to drugs used in the present study, and (4) cognizance of the purpose of the present study and willingness to sign an informed consent.
Exclusion criteria were (1) severe heart, liver, kidney, and other systemic diseases; (2) pregnancy or lactation; (3) unresponsive to medical treatment and with complications; and (4) immune system disorders.
Treatment regimens
Both groups were given the basic treatment for improving electrolyte balance and proper diet. The control group was given 1–2 pentasa (mesalazine extended action tablet) tablets once and three times a day and a maintenance dose of 1 tablet once and three times a day. The observation group was given 2 probiotics tablets (Bifico) once and three times a day and a largely liquid-based high nutrition diet, in addition to the pentasa regimen.
Observation indices
In the colony counts of gut microbes, a 0.5 g fresh feces was obtained from the subjects and serially diluted to 10−8. Specific medium was inoculated with 10 μL of the fecal diluent and cultured for 48 h at 37°C. The morphology of the resulting colonies was noted, the colonies were counted, and the bacteria were identified by Gram staining and biochemical tests. The number of bacteria in each gram of feces (CFU/g) was calculated, and the results were expressed in logarithmic form.
In biochemical tests, a 10 mL fasting venous blood was collected from each subject early in the morning into heparinized tubes. The blood samples were centrifuged at 5000 rpm for 5 min, and the supernatant was separated for immediate analysis or stored at −80°C for later use. The levels of 1-antitrypsin (ml027560), β2-microglobulin (ml027518), high-sensitivity C-reactive protein (hs-CRP) (ml027874), IL-6 (ml038115), and IL-4 (ml027384) were detected by enzyme-linked immunosorbent assay (ELISA; Shanghai Enzyme-linked Biotechnology Co. Ltd., Shanghai, China) according to the manufacturer’s instructions. To measure fecal lactoferrin levels, 50–120 mg of sample was mixed with an extraction solution (1:100 w/v). After shaking vigorously, 1 mL of the solution was centrifuged, and then 0.5 mL supernatant was obtained for ELISA (ml024507). The activity scores of the two groups were calculated, and the incidence of adverse reactions and the recurrence rate after 1 year were recorded (Table 1).
Table 1.
Activity scores of patients with IBD (score).
| Items | UCAI | CDAI |
|---|---|---|
| Stomachache | No, 0; slight, 1; medium, 2; severe, 3 | Same with UCAI |
| Diarrhea | 0–2 times, 0; 3–4 times, 1; 5–6 times, 2; 7–9 times, 3; >10 times, 4 | Once daily loose stool counts for 1 |
| Others | Gross blood stool: no, 0; <50%, 1; >50%, 2; 100%, 3 Fecal incontinence: no, 0; yes, 1 Abdominal haphalgesia: no, 0; slight, 1; medium resistance, 2; severe or rebound pain, 3 |
Abdominal mass: no, 0; doubtful, 1; confirmed, 2; with haphalgesia, 3 Complication: every one count for 1 score |
IBD: inflammatory bowel disease; CDAI: Crohn’s disease activity index; UCAI: ulcerative colitis activity index.
Statistical analysis
Statistical Package for Social Sciences version 21.0 software (IBM Corp.; Armonk, NY, USA) was used for statistical analysis. Measurement data were expressed as mean±SD. Count data were expressed as percentage and compared by the χ2 test and Fisher’s exact probability test as expressed by chi-square. t-Test was used to compare data with normal distribution, and the rank sum test was used for data with non-normal distribution. A p value <0.05 was considered statistically significant.
RESULTS
Patient data
There were no differences between the two groups with respect to gender, age, duration of disease, and the number of disease types (all p>0.05; Table 2).
Table 2.
Comparison of general information (±SD; n, %).
| Group | Control group (n=19) | Observation group (n=21) | t/χ2 | p |
|---|---|---|---|---|
| Gender (male/female) | 10/9 | 10/11 | 0.1003 | 0.7515 |
| Age (year) | 39.97±8.68 | 42.56±7.58 | 1.0512 | 0.1495 |
| Course of disease (year) | 4.87±1.58 | 5.03±1.64 | 0.3333 | 0.3703 |
| Types of disease | 0.0435 | 0.8348 | ||
| UC | 15 (78.95) | 16 (76.19) | ||
| CD | 4 (21.05) | 5 (23.81) |
UC: ulcerative colitis; CD: Crohn’s disease.
Microflora composition in the two groups
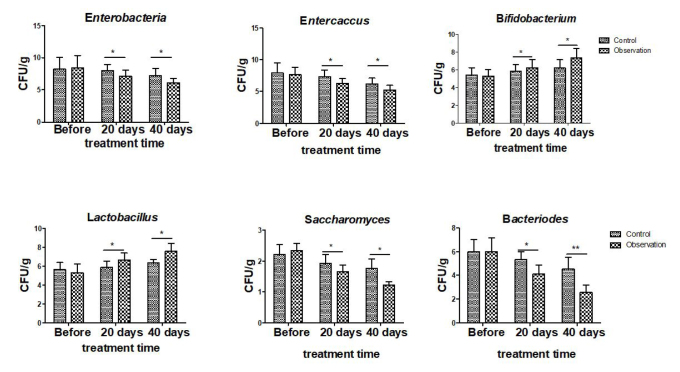
Before treatment, there was no difference in the fecal bacterial counts between the two groups (all p>0.05). After treatment, the number of enterobacteria, enterococci, saccharomyces, and bacteroides decreased significantly in the patients’ feces of both groups, and the observation group had significantly lower counts than the control group. In contrast, the number of bifidobacteria and lactobacilli was significantly increased after treatment and was higher in the observation group than in the control group (all p<0.05; Figure 1).
Figure 1.
Changes in microflora composition at different stages in the two groups. The control group was given pentasa (1 tablet once and three times a day), and the observation group was given probiotics (2 tablets once and three times a day) along with pentasa. *p<0.05, **p<0.01.
Biochemical indices of both groups
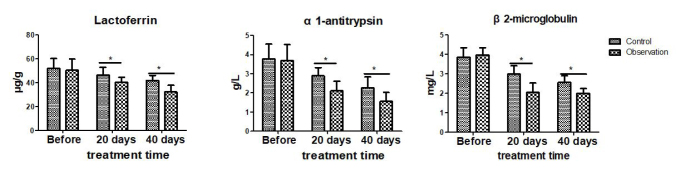
Both groups showed similar pretreatment levels of fecal lactoferrin, serum 1-antitrypsin, and β2-microglobulin (all p>0.05). After treatment, the above indices were significantly lower in observation group than in the control group (all p<0.05; Figure 2).
Figure 2.
Comparison of biochemical indices of both groups. The control group was given pentasa (1 tablet once and three times a day), and the observation group was given probiotics (2 tablets once and three times a day) along with pentasa. *p<0.05.
Inflammatory markers in both groups
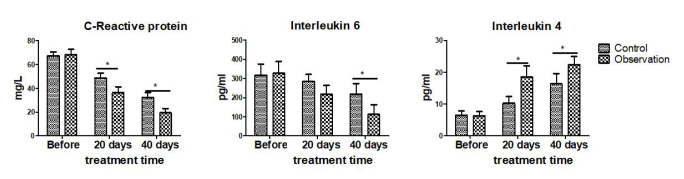
The levels of inflammatory markers, such as hs-CRP, IL-6, and IL-4 levels, were similar in both groups prior to treatment (all p>0.05). After treatment during the same period, the levels of hs-CRP and IL-6 were significantly lower, and the level of IL-4 was significantly higher in the observation group than in the control group (all p<0.05; Figure 3).
Figure 3.
Comparison of inflammatory markers before and after treatment in the two groups. The control group was given pentasa (1 tablet once and three times a day), and the observation group was given probiotics (2 tablets once and three times a day) along with pentasa. *p<0.05.
Prognosis of the two groups
After treatment, the Crohn’s disease activity index (CDAI) and UC activity index (UCAI) scores, as well as the recurrence rate, in the observation group were significantly lower than those in the control group (all p<0.05). There was no difference in the cases with adverse reactions between the two groups (p>0.05; Table 3).
Table 3.
Comparison of prognosis of the two groups (±SD; n, %).
| Group | Control group (n=19) | Observation group (n=21) | t/χ2 | p |
|---|---|---|---|---|
| Activity scores | ||||
| CDAI | 5.29±2.48 | 3.86±2.16 | 2.0587 | 0.0233 |
| UCAI | 8.54±3.28 | 5.38±2.76 | 3.4889 | 0.0006 |
| Recurrence rate | 6 (31.58) | 1 (4.76) | 3.2848 | 0.0395 |
| Adverse reaction | 0.8087 | 0.3284 | ||
| Nausea | 3 (15.79) | 3 (14.29) | ||
| Emesis | 2 (10.53) | 1 (4.76) | ||
| Others | 4 (21.05) | 2 (9.52) | ||
CDAI: Crohn’s disease activity index; UCAI, ulcerative colitis activity index.
DISCUSSION
IBD is a common intestinal disease characterized by the loss of intestinal mucosa, congestion, and ulcers (9–11). The etiological factors of IBD mainly include infection, environmental stress, autoimmunity, and genetic factors, in addition to the breakdown of the mucosal immune system that causes immune failure of intestinal tract antigen and microflora imbalance (12–14). When the intestinal tract is colonized by conditioned pathogen and stimulated by exogenous stimuli, the intestinal tract multiplies and releases toxins that trigger an inflammatory reaction and damage the mucosal lining (15,16). However, the gut microflora or probiotics, which compete with these pathogens for space and nutrition, can inhibit their proliferation and maintain a balanced intestinal flora, thereby reducing intestinal tissue damage (17,18). In addition, the prebiotics synthesized by these probiotics can promote their growth, accelerate mucosal repair, restore immune response, and inhibit intestinal inflammation (19). The intestinal microflora is known to change under different physiological conditions. Bjerrum et al. reported that compared with healthy people, the intestinal microflora in patients with IBD is imbalanced, but at the same time, the number of probiotics is also higher in the patients, indicating that probiotics are a defense mechanism against intestinal inflammation (20–23).
Since the intestinal microflora is highly diverse, a single probiotic cannot restore its balance. Current probiotic treatments comprise composite probiotic preparations, such as the Bifico capsule, which is used to regulate the intestinal pH, improve enzyme activity, and antagonize pathogens. In the present study, probiotics treatment was found to reduce the number of Escherichia coli, enterococci, saccharomyces, bacteroides, and other conditional pathogens in the patients’ feces and increase the number of probiotics, such as bifidobacteria and lactobacilli (all p<0.05), thereby restoring the intestinal microflora. This is consistent with the observation that the intestinal flora is correlated with the onset of IBD; the intestinal flora only differs between healthy people and patients with active IBD with respect to species and quantity but has no difference between the active state and the remission state in patients.
The levels of fecal lactoferrin and blood α1-antitrypsin and β2-microglobulin were significantly lower in the observation group than in the control group (all p<0.05), indicating that the local tissue damage has been effectively repaired upon probiotics administration. IL-6 can induce T cell apoptosis, result in abnormal accumulation of intestinal mucosal cells, and aggravate the inflammatory response (24). The levels of proinflammatory hs-CRP and IL-6 were significantly lower, and the level of anti-inflammatory IL-4 was significantly higher in the observation group than in the control group (all p<0.05). The CDAI and UCAI scores in the observation group were significantly lower than those in the control group, whereas the recurrence rate of enteritis in the observation group was significantly lower than that in the control group (all p<0.05). There was no difference in the number of adverse reactions between the two groups (p>0.05).
In summary, the combination treatment of pentasa and probiotics for IBD can effectively re-adjust the composition of the intestinal microflora; reduce intestinal lactoferrin, blood 1-antitrypsin, and β2-microglobulin levels; inhibit inflammatory factors; improve the activity score; and reduce the recurrence rate of enteritis in patients with IBD. The results indicate that our treatment regimen is worthy of further clinical application. However, our study has several limitations. The limitations of the present study include small cohort, short follow-up time, and doubtful patient compliance. In addition, since the specific pathogen of IBD enteritis has not been confirmed, there is uncertainty regarding the correlation between the intestinal microflora balance and IBD. Therefore, the IBD treatment with probiotics needs to be further studied with larger patient cohorts and more controlled conditions.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of The First Affiliated Hospital of Fujian Medical University ((2018)077).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.F., R.G.; Design - H.F., R.G.; Resources - R.G.; Data Collection and/or Processing - H.F., R.G., X.L., Z.H.Z., W.W.Z.; Literature Search - H.F., X.L., W.W.Z.; Writing Manuscript - R.G., H.F., C.D.W., X.L., W.W.Z.; Critical Reviews - H.F., R.G.
Conflicts of Interest: The authors declare that there are no conflicts of interest.
Financial Disclosure: This work was supported by the Fujian Province Natural Science Fund Project (2015J01391).
REFERENCES
- 1.Rhodes J, Thomas G, Evans BK. Inflammatory Bowel Disease Management. Drugs. 1997;53:189–94. doi: 10.2165/00003495-199753020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Zurek M, Kern I, Manuwald U, et al. Epidemiology and care structures for children and adolescents and young adults up to the 26th year of life with inflammatory bowel diseases (IBD) in Leipzig/Saxony/Germany. J Public Health. 2018:1–6. doi: 10.1007/s10389-017-0884-2. [DOI] [Google Scholar]
- 3.Chadwick VS, Chen W. Medical Importance of the Normal Microflora. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1999. The intestinal microflora and inflammatory bowel disease; pp. 177–221. [DOI] [Google Scholar]
- 4.Bamias G, Pizarro T, Cominelli F. New Paradigms in the Pathogenesis of IBD. Inflamm Bowel Dis. 2011:41–57. doi: 10.1007/978-1-60327-433-3_4. [DOI] [Google Scholar]
- 5.Dong X, Ye X, Chen X, et al. Intestinal and peripheral fibrinogen-like protein 2 expression in inflammatory bowel disease. Dig Dis Sci. 2014;59:769–77. doi: 10.1007/s10620-013-2962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong HJ, Kim J, Yong JS, Kim D, Roh KM, Kang I. pH-Sensitive mesalazine carrier for colon-targeted drug delivery: A two-fold composition of mesalazine with a clay and alginate. Macromolecular Res. 2017;25:1145–52. doi: 10.1007/s13233-017-5150-5. [DOI] [Google Scholar]
- 7.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler M, Wollins E, Toles A, Borum M, Doman DB. The emerging therapeutic role of probiotics in inflammatory bowel disease. Gastroenterol Hepatol. 2008;4:634–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna PV, Shih DQ, Haritunians T, McGovern DP, Targan S. Use of animal models in elucidating disease pathogenesis in IBD. Semin Immunopathol. 2014;36:541–51. doi: 10.1007/s00281-014-0444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamp KJ, Brittain K. Factors that Influence Treatment and Non-treatment Decision Making Among Individuals with Inflammatory Bowel Disease: An Integrative Review. Patient. 2018:1–14. doi: 10.1007/s40271-017-0294-0. [DOI] [PubMed] [Google Scholar]
- 11.Munkholm P, Pedersen N. Evaluation of Quality of Life in Inflammatory Bowel Disease. Crohn’s Disease and Ulcerative Colitis. 2012:333–40. doi: 10.1007/978-1-4614-0998-4_25. [DOI] [Google Scholar]
- 12.Andre MF, Aumaitre O, Piette JC, et al. Analysis of the NOD2/CARD15 gene in patients affected with the aseptic abscesses syndrome with or without inflammatory bowel disease. Dig Dis Sci. 2008;53:490–9. doi: 10.1007/s10620-007-9871-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu ZJ. New progress in the pathogenesis of inflammatory bowel disease. J Intern Med Concepts Prac. 2013;8:5–8. [Google Scholar]
- 14.Ihara S, Hirata Y, Koike K. TGF-beta in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol. 2017;52:777–87. doi: 10.1007/s00535-017-1350-1. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11–21. doi: 10.1007/s00535-015-1098-4. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori S, Sakamoto C. Latest concepts on the association between nonsteroidal anti-inflammatory drug-induced small intestinal injury and intestinal bacterial flora. Clin J Gastroenterol. 2013;6:345–51. doi: 10.1007/s12328-013-0424-8. [DOI] [PubMed] [Google Scholar]
- 17.Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6:14. doi: 10.1186/s13167-015-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokrozub VV, Lazarenko LM, Sichel LM, et al. The role of beneficial bacteria wall elasticity in regulating innate immune response. EPMA J. 2015;6:13. doi: 10.1186/s13167-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He M, Shi B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci. 2017;7:54. doi: 10.1186/s13578-017-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjerrum JT, Wang Y, Hao F, et al. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics. 2015;11:122–33. doi: 10.1007/s11306-014-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med. 2017;95:915–25. doi: 10.1007/s00109-017-1545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Liu Z. Paneth cells: the hub for sensing and regulating intestinal flora. Sci China Life Sci. 2016;59:463–7. doi: 10.1007/s11427-016-5018-5. [DOI] [PubMed] [Google Scholar]
- 23.Hill DA, WAF The Intestinal Immune System during Homeostasis and Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017:15–30. doi: 10.1007/978-3-319-49215-5_2. [DOI] [Google Scholar]
- 24.Curnow SJ, Scheeltoellner D, Jenkinson W, et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173:5290–7. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]