Abstract
Serum bone turnover markers show diurnal variation in humans, suggesting that circadian rhythms contribute to normal bone physiology. This conclusion is corroborated by bone phenotypes in mice with genetic disruption of the circadian molecular clock mechanism, for instance via deletion of the transcription factor Brain and Muscle Arntl-like 1 (Bmal1). To dissect the contribution of circadian molecular clocks in individual bone cell types, we generated mice with conditional deletion of Bmal1 in osteoclasts (Ctsk-cre) and in mesenchymal cells of the limbs (Prx1-cre). We report that deletion of Bmal1 in osteoclasts had no effect on trabecular or cortical bone parameters in vivo or on osteoclast differentiation in vitro. In contrast, Bmal1f/f.Prx1-cre mice had significantly less trabecular and cortical bone than Bmal1f/f littermate controls, recapitulating the bone phenotype of Bmal1 germline deficient mice. The number of osteoblast precursors in the bone marrow of Bmal1f/f.Prx1-cre mice was similar to wild-type controls, while the in vitro differentiation capacity of Bmal1-deficient osteoblast precursors, measured as induction of alkaline phosphatase activity, was significantly lower. Despite this, serum procollagen type 1 N-terminal propeptide (P1NP), a measure of bone formation in vivo, was higher in Bmal1f/f.Prx1-cre mice than in Bmal1f/f mice. Consistent with a high bone turnover state in the mutant mice, the bone resorption marker serum C-terminal telopeptides of Type I collagen (CTX-I) was also elevated, and Bmal1f/f.Prx1-cre mice had a higher number of tartrate resistant acid phosphatase (TRAP) positive osteoclasts than Bmal1f/f controls. These results demonstrate that adult bone mass in mice is controlled by the intrinsic circadian molecular clock in mesenchymal cells but not osteoclasts. The effect of the mesenchymal cell clock on bone turnover appears to involve osteoblast-osteoclast cross-talk.
Keywords: Genetic animal models, bone, osteoclasts, osteoblasts, transcription factors, microCT
1. Introduction
Many biological processes follow circadian rhythms which, in humans and other mammals, are generated by cell-intrinsic molecular clocks that generate diurnal changes in gene expression, affecting about 10 % of the transcriptome in any given cell type [1]. At the molecular level, the mammalian molecular clock is a transcriptional-translational feedback mechanism. Positive regulators (Bmal1, Clock, Npas2) drive the expression of negative feedback regulators (Per and Cry genes, Nr1d1), which in turn inhibit the expression and activity of the positive regulators in a cycle that lasts approximately 24 hours [2]. A central clock in the hypothalamus receives sensory input from the eyes and communicates with peripheral tissues via neuronal and hormonal pathways to synchronize peripheral tissue clocks with the environment. Disruption of the synchrony between environmental and endogenous circadian rhythms, for instance in shift workers, or dysfunction of the molecular clock mechanism in older people, has been linked to disease. Understanding the molecular mechanisms underlying circadian control may also be important for optimal drug therapy [3].
Several serum bone turnover markers in humans show diurnal variation [4], suggesting a role for circadian molecular clocks in bone physiology. This conclusion is corroborated by bone phenotypes in mice with genetic disruption of the circadian molecular clock mechanism. Mice with germline deletion of Cry1/Cry2 or Per1/Per2, transcription factors that form the negative feedback loop of the circadian molecular clock, were shown to have a high bone mass phenotype thought to be mediated via effects of leptin and the sympathetic nervous system on osteoblast function [5]. Bmal1 is the major positive regulator of the molecular clock. Published bone phenotypes for mice with germline deletion of Bmal1 have varied, reporting either low bone mass [6] or no bone phenotype [5, 7]. We found that Bmal1 germline mutant mice have a low bone mass phenotype. To further clarify the role of circadian molecular clocks in bone physiology we generated mice with conditional deletion of Bmal1 in osteoclasts and mesenchymal cells, respectively. Our results confirm a critical function for circadian molecular clocks in mesenchymal cells, as conditional deletion of Bmal1 in mesenchymal cells but not in osteoclasts recapitulated the low bone mass phenotype seen in germline Bmal1 deficient mice. Moreover, our data suggest that the development of low bone mass in these mice is the result of increased bone turnover and involves cross-talk between osteoblasts and osteoclast lineage cells.
2. Material and Methods
2.1. Mice
Bmal1f/f mice (B6.129S4(Cg)-Arntltm1Weit/J) [8], Prx1-cre mice (B6.Cg-Tg(Prrx1-cre)1Cjt/J)[9] and ROSA26mT/mG mice (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) [10], all on a C57BL/6 background, were purchased from the Jackson Laboratory. Ctsk-cre mice [11] were a gift of Shigeaki Kato, University of Tokyo, Japan and have been backcrossed to C57BL/6 for 20 generations. The Bmal1− allele arose by Cre-mediated germline deletion in offspring of a female Bmal1f/f.Prx1-cre mouse [9]. Bmal1−/− mice are infertile [7]. Bmal1−/− and Bmal1+/+ littermates were generated by crossing Bmal1+/− parents. Bmal1f/f.Ctsk-cre, Bmal1f/+.Ctsk-cre, and Bmal1f/f littermates were generated by mating Bmal1f/+.Ctsk-cre and Bmal1f/f parents. Bmal1f/f.Prx1-cre and Bmal1f/f controls were derived by mating Bmal1f/f.Prx1-cre males with Bmal1f/f females. Age and sex of the mice included in each experiment are specified in the text. In most cases, 8-week-old male knockout mice and control littermates were pooled from multiple litters for analysis. Consecutive animals were analyzed without selection. Mice were housed in microisolator cages with up to 5 mice per cage in specific pathogen-free animal facilities at the Harvard T.H. Chan School of Public Health or at Brigham and Women’s Hospital under standard 12 hour light - 12 hour dark conditions, the beginning of the light period corresponding to circadian time (CT) 0 hours. Animals had access to standard mouse chow (PicoLab Mouse Diet 20, #5058, LabDiet) and water ad libitum. All studies were performed according to institutional and NIH guidelines for care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committees of Harvard Medical School and Brigham and Women’s Hospital.
2.2. Micro-computed tomography
A Scanco Medical μCT 35 system with an isotropic voxel size of 7 μm was used for micro-computed tomographic (microCT) analysis of femurs. All microCT analysis was performed blinded to the genotype of the analyzed mice. Bones were scanned in 70% ethanol using an X-ray tube potential of 55 kVp, an X-ray intensity of 0.145 mA and an integration time of 600 ms. A region beginning approximately 0.28 mm proximal to the growth plate and extending 1.1 mm proximally was selected for trabecular bone analysis. A second region 0.6 mm in length centered at the midpoint of the femur was used to measure cortical bone parameters. A semi-automated contouring approach was used to distinguish cortical and trabecular bone. The region of interest was thresholded using a manually determined global threshold set at 368 mg HA/cm3 for trabecular bone and 708 mg HA/cm3 for cortical bone. 3-D microstructural properties of bone, including bone volume fraction (BV/TV), trabecular number (Tb N), trabecular thickness (Tb Th), trabecular separation (Tb Sp), trabecular bone mineral density (Tb BMD), cortical thickness (Cort Th), and cortical bone mineral density (Cort BMD) were calculated using software supplied by the manufacturer.
2.3. Histology
For osteoclast quantification, femurs from 8-week-old Bmal1f/f and Bmal1f/f.Prx1-cre mice were fixed in 10% neutral buffered formalin (NBF) for 24 hours followed by decalcification with 14% EDTA for 14 days and paraffin embedding. 5 μm sections were stained for TRAP activity [12] and counterstained with hematoxylin. The OsteoMeasure Analysis System (OsteoMetrics) was used to determine the osteoclast surface per bone surface within a region of interest 100 μm proximal to the growth plate. Two sections from each of 6 mice per group were analyzed. Slides were imaged on a Leica DM2000 LED microscope. For deletion analysis, femurs from 4-week-old Bmal1f/f.ROSA26mT/mG and Bmal1f/f.Ctsk-cre.ROSA26mT/mG mice were fixed in 4% paraformaldehyde (PFA) and decalcified with 14% EDTA for 2 days. Cryosections (16 μm) were stained with DAPI and imaged with a Leica TCS SP8 confocal microscope.
2.4. In vitro osteoclast assays
Total bone marrow cells were cultured on suspension dishes (Corning) in α-MEM with ribonucleosides (Corning) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin G, 1 mg/ml streptomycin (both Cellgro), and 40 ng/ml macrophage colony-stimulating factor (M-CSF, R&D). After 3 days, non-adherent cells were replated at a density of 6x104 cells/well in 24 well plates or 1x104 cells/well in 96 well plates and cultured in medium with 10 ng/ml RANKL (R&D) and 20 ng/ml M-CSF for 5-7 days. Osteoclasts were then stained for TRAP activity or harvested for quantitative polymerase chain reaction (qPCR). For reporter analysis, Bmal1f/f.Ctsk-cre.ROSA26mT/mG and Bmal1f/f.ROSA26mT/mG control osteoclasts were grown on 1 mm glass coverslips (Sigma) coated in FBS, fixed in 4% PFA and stained with DAPI. An Olympus FSX100 Inverted Microscope was used to evaluate EGFP expression.
2.5. In vitro osteoblast assays
Long bones were isolated from 7-10 day old mice, crushed and digested with 2 mg/ml dispase II (Roche) and 1 mg/ml collagenase type II (Worthington Biochemicals) in α-MEM for 30 minutes at 37 °C with agitation, whereupon the supernatant was collected (Fraction 1) and replaced with fresh digestion medium. Two subsequent digestions were performed identically (Fraction 2, 3). For colony forming unit (CFU) assays, Fraction 1 was plated at 1.5×105 cells/well in medium onto 6 wells plates, expanded for 4 days and then differentiated for 21 days in α-MEM medium without ribonucleosides (Corning) supplemented with 10 % FBS, 100 U/ml penicillin G, 1 mg/ml streptomycin, 50 μg/ml ascorbic acid and 5 mM β-glycerophosphate. Cultures were fixed in 10% neutral buffered formalin (NBF) and stained for alkaline phosphatase activity. Colonies were counted by two independent investigators blinded to genotype. Fraction 2 and 3 were pooled, plated onto 10 cm dishes and passaged every other day for 10 days. The expanded population was then plated at 1.1×104 cells/cm2, differentiated for 28 days as described and stained with alizarin red and von Kossa. Cultures were also stained and analyzed for alkaline phosphatase activity as described [13]. The alkaline phosphatase index (AP index) was derived by normalizing alkaline phosphatase activity to Alamar Blue (Invitrogen) fluorescence as a measure of cell viability. All cultures were performed with cells from individual mice.
2.6. Quantification of Bmal1 deletion
Genomic DNA was extracted from primary osteoclast or osteoblast cultures using a standard protocol for tail DNA isolation, i.e. digestion with proteinase K (Sigma) followed by isopropanol precipitation. Real-time quantitative PCR analysis of DNA samples was done on a StepOnePlus Real-Time PCR System (Applied Biosystems) with Fast SYBR Green Master Mix (Applied Biosystems) and the following primer pairs: FL5 - GCTCACAGGCTGCAGAGG, TTCCAGACGACCAGGTTTGG; D3Mit319 - TCTCCCTCACTTTTTCCTTCC, AACAGCCAGTCCAGCAAATC; D7Mit97 - CTTCCACACATCCACACTTACA, TCTTGGTCTCCAGCCTCTGT. The FL5 primer pair flanks the upstream loxP site in the floxed Bmal1 locus yielding a PCR product of ~170 base pairs (bp). Cre-mediated excision of the floxed DNA segment abrogates the reverse primer site and results in loss of the PCR product. The satellite markers D3Mit319 and D7Mit97 were used for quantification of total DNA, with the primers above yielding PCR products of 197 bp and 138 bp, respectively, for C57BL/6 genomic DNA. Abundance of the floxed Bmal1 allele was measured by normalizing the cT value for FL5 over the mean cT value for D3Mit319 and D7Mit97 using the ΔcT method. Data were further normalized by dividing each individual data point by the mean of the Bmal1f/f samples in a given experiment.
2.7. Gene expression in cortical bone
Diaphyseal bone from femurs was flushed to remove the marrow. RNA was isolated from the remaining cortical bone with Trizol reagent (Life Technologies) using a bullet blender (Next Advance) to homogenize the tissue. 500 ng of RNA was used to generate cDNA (Affinity Script RT-PCR cDNA Synthesis Kit, Agilent Technology). Real-time quantitative PCR (qPCR) was performed in duplicate using Sybr green reagent (Life Technologies). Primer sequences were Opg – TGTCCAGATGGGTTCTTCTCA, CGTTGTCATGTGTTGCATTTCC; Rankl – CAGCATCGCTCTGTTCCTGTA, CTGCGTTTTCATGGAGTCTCA; Hprt – GTTAAGCAGTACAGCCCCAAA, AGGGCATATCCAACAACAAAC.
2.8. Serum bone turnover markers
Blood was collected from mice by cardiac puncture following euthanasia at the time points indicated in the text. Serum was isolated using serum separator tubes (BD Biosciences) and analyzed using commercial ELISA kits for CTX-I and P1NP (IDS), or RANKL and OPG (R&D).
2.9. Statistical Analysis
Statistical analysis and graphing were done with Prism 7 (Graphpad Software). Two experimental groups were compared using the Student’s t test and three groups by one-way ANOVA. The bars in all graphs represent mean and standard deviation (SD).
3. Results
3.1. Adult Bmal1 germline knockout mice have a low bone mass phenotype
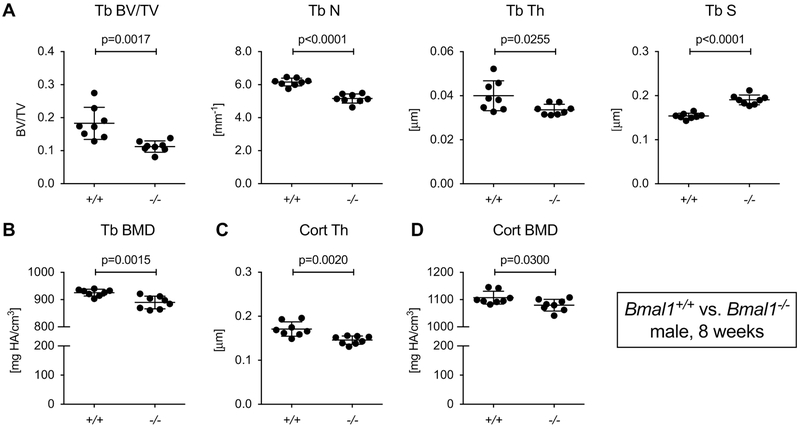
We analyzed femurs from 8-week-old male mice with germline deletion of Bmal1 (Bmal1−/−) by microCT. This age was selected for analysis to avoid confounding of the results by the progressive arthropathy in Bmal1−/− mice [7], which might restrict mobility at later time points. At 8 weeks of age, the trabecular bone mass in male Bmal1−/− mice was about 40% lower than in Bmal1+/+ male littermates (Fig. 1A). Corresponding changes were observed for trabecular number, thickness and separation. Trabecular BMD was reduced by 5% (Fig. 1B). Bmal1−/− mice also showed a slight but statistically significant reduction of cortical thickness and cortical BMD (Fig. 1C and D).
Fig.1. Bmal1−/− mice have reduced bone mass.
Femurs from 8-week-old male Bmal1+/+ and Bmal1−/− littermates were analyzed by microCT. (A) Trabecular bone parameters, (B) trabecular BMD, (C) cortical thickness, and (D) cortical BMD. Bars are mean and SD; n=8 animals per group; p values by Student’s t test. Tb, trabecular; Cort, cortical; BV/TV, bone volume/tissue volume; N, number; Th, thickness; S, separation; BMD, bone mineral density.
3.2. Osteoclast-specific deletion of Bmal1 has no effect on microCT bone parameters
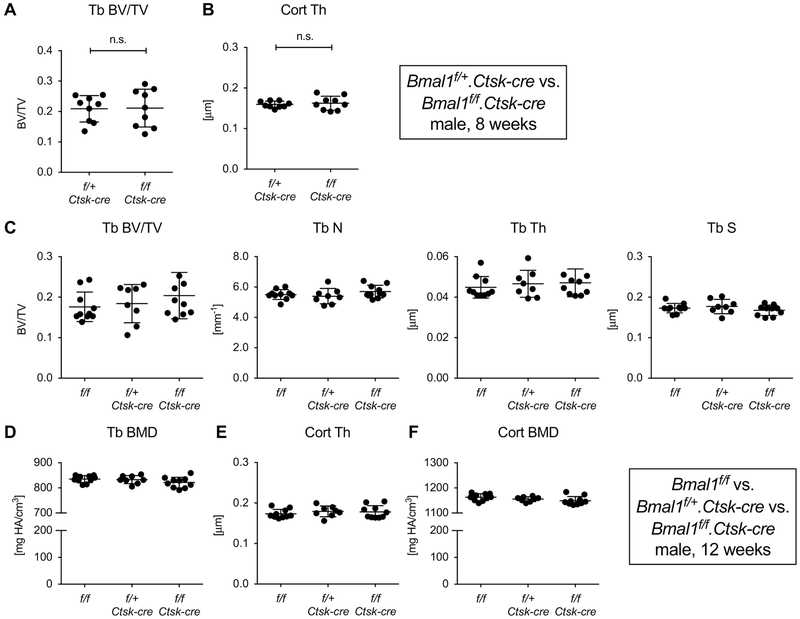
To address whether the reduction in trabecular bone mass is a function of Bmal1 deficiency in osteoclast or osteoblasts, we generated mice with conditional deletion of Bmal1 using a mouse strain with a floxed Bmal1 allele [8] that has been widely used to analyze the effects of tissue-specific Bmal1 deletion. In this strain, Cre recombinase activity excises the exon encoding the helix-loop-helix domain of Bmal1 resulting in a functional null mutant [8]. First, we generated mice with osteoclast-specific deletion of Bmal1 using the Ctsk-cre driver strain [11] comparing 8-week-old Bmal1f/f.Ctsk-cre mice with Bmal1f/+.Ctsk-cre littermates. None of the standard trabecular or cortical bone microCT parameters demonstrated a trend in the direction of the phenotype observed in Bmal1 germline mutants (Fig. 2A and B, Fig. S1).
Fig. 2. Mice with conditional deletion of Bmal1 in osteoclasts lack a bone phenotype.
Femurs from 8-week-old male Bmal1f/+.Ctsk-cre and Bmal1f/f.Ctsk-cre littermates were analyzed by microCT. (A) Trabecular BV/TV, (B) cortical thickness. Subsequently, a three-way analysis of 12-week-old male Bmal1f/f, Bmal1f/+.Ctsk-cre and Bmal1f/f.Ctsk-cre mice was performed comparing (C) trabecular bone parameters, (D) trabecula BMD, (E) cortical thickness, and (F) cortical BMD. Bars are mean and SD; n=8-10 animals per group. There were no statistically significant differences between groups by Student’s t test (A, B) or one-way ANOVA (C-F).
We had chosen this experimental setup to avoid potential Cathepsin K gene-dosage effects caused by the Ctsk-cre knock-in allele. Subsequent experiments revealed that there was no significant difference in microCT parameters between male Ctsk-cre positive mice and wild-type C57BL/6 littermates at 8 weeks (Fig. S2), and we confirmed that Bmal1 haploinsufficiency does not result in a discernable bone phenotype (Fig. S3). We thus concluded that Bmal1 deficiency in osteoclasts has no effect on bone. However, contrary to our findings, a recent report suggested that mice with osteoclast-specific Bmal1 deletion have higher bone mass than Bmal1f/f mice at 12 weeks of age [14]. To address this discrepancy, we performed an additional in vivo study. Bmal1f/+.Ctsk-cre mice were crossed with Bmal1f/f mice to generate littermates with the three genotypes Bmal1f/f, Bmal1f/+.Ctsk-cre and Bmal1f/f.Ctsk-cre, which were analyzed at 12 weeks (Fig. 2C-F). Again, there were no statistically significant differences between male Bmal1f/f.Ctsk-cre mice and littermate controls (Fig. 2C-F) confirming our initial observation.
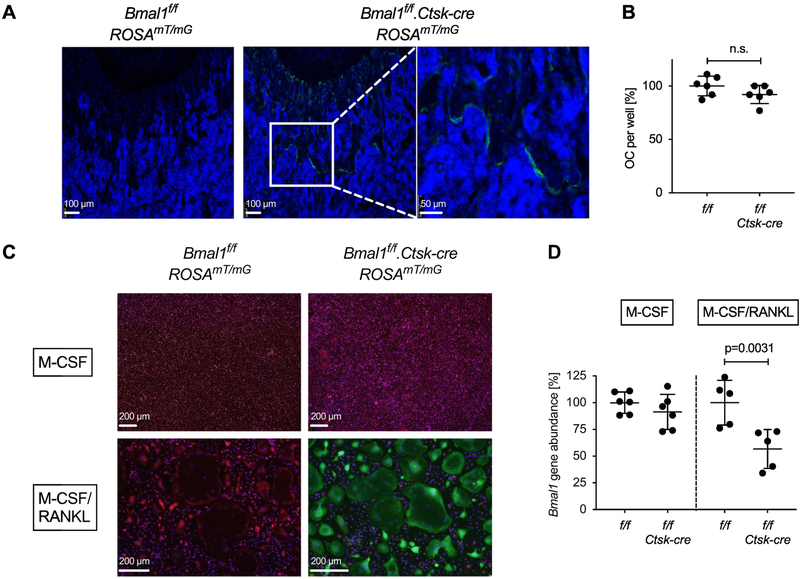
We verified that Ctsk-cre was functional in our mice by crossing the ROSAmT/mG reporter allele into the Bmal1f/f.Ctsk-cre line. Analysis of 4-week-old Bmal1f/f.Ctsk-cre.ROSAmT/mG mice demonstrated green fluorescence near the growth plate in an osteoclast-like distribution (Fig. 3A). No statistically significant difference in osteoclast differentiation from the bone marrow of Bmal1f/f.Ctsk-cre vs. Bmal1f/f bone marrow was observed in vitro (Fig. 3B), despite documented osteoclast Cre-activity demonstrated using the ROSAmT/mG reporter (Fig. 3C) and RANKL-induced Bmal1 deletion measured by qPCR (Fig. 3D). This qPCR assay quantifies abundance of the floxed Bmal1 allele using a primer pair flanking the upstream loxP site. Cre-mediated excision of the floxed gene segment removes the reverse primer binding site and results in loss of the PCR product (Fig. S4). In conclusion, the analysis of Bmal1f/f.Ctsk-cre mice provided no evidence for an effect of osteoclast-specific deletion of Bmal1 on bone parameters that would mimic the bone phenotype observed in mice with germline deletion of Bmal1.
Fig. 3. Deletion of Bmal1 in osteoclasts from Bmal1f/f.Ctsk-cre mice.
(A) Sections of the distal femur from representative 4-week-old Bmal1f/f.ROSA26mT/mG and Bmal1f/f.Ctsk-cre.ROSA26mT/mG mice analyzed by fluorescence microscopy. Green = EGFP, Blue = DAPI. Positive EGFP fluorescence documents a history of Cre activity. (B) Osteoclasts were differentiated in vitro from Bmal1f/f and Bmal1f/f.Ctsk-cre bone marrow of individual mice in the presence of M-CSF and RANKL, and the number of TRAP positive osteoclasts per well was counted. Pooled data from two experiments are shown with each symbol representing one animal. Data were normalized to the mean number of Bmal1f/f osteoclasts in each experiment (100%). (C) Bone marrow cells from Bmal1f/f.ROSA26mT/mG and Bmal1f/f.Ctsk-cre.ROSA26mT/mG mice were differentiated into osteoclasts as in (B), control macrophages were generated by culture with M-CSF. Green = EGFP, Red = tdTomato. (D) Genomic DNA was prepared from Bmal1f/f and Bmal1f/f.Ctsk-cre macrophage (M-CSF) and osteoclast (M-CSF + RANKL) cultures. Deletion of Bmal1 was assessed by real-time quantitative PCR analysis of genomic DNA. Data were normalized to the Bmal1 content in macrophage cultures (100%). Each data point represents one animal. Pooled data from two experiments are shown. Bars are mean and SD; p values by Student’s t test.
3.3. Conditional deletion of Bmal1 maps the bone phenotype to Bmal1-deficient mesenchymal cells
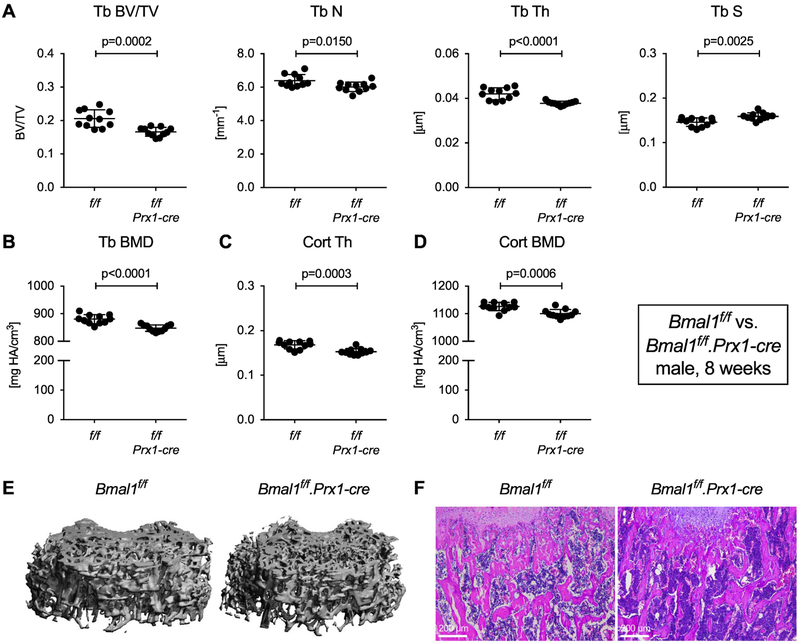
We then analyzed mice with Bmal1 deficiency in mesenchymal cells using the Prx1-cre driver [9]. In contrast to deletion in osteoclasts, mesenchymal cell-specific deletion of Bmal1 in Bmal1f/f.Prx1-cre mice lowered trabecular bone mass in 8-week-old mice with a reduction in BV/TV by ~20% relative to Bmal1f/f littermate controls (Fig. 4A, 4E and F). We observed corresponding statistically significant changes in trabecular number, thickness and separation. Trabecular BMD, cortical thickness and cortical BMD were also decreased (Fig. 4B-D), similar to germline Bmal1 deletion. The same phenotype was present in 8-week-old female mice (Fig. S5). Real-time quantitative PCR data in Fig. S6 provide evidence for Bmal1 deletion and disruption of the circadian molecular clock in the extremities of Bmal1f/f.Prx1-cre mice. Bmal1 deficiency in mesenchymal cells thus recapitulates the bone phenotype observed in Bmal1 germline-knockout mice, mapping the effect of Bmal1 deficiency on bone to a mesenchymal cell lineage derived from Prx1-cre positive precursors.
Fig. 4. Bmal1 deletion in mesenchymal cells reproduces the bone phenotype of Bmal1 germline knockout mice.
Femurs from 8-week-old male Bmal1f/f and Bmal1f/f.Prx1-cre littermates were analyzed by microCT. (A, B) Trabecular bone parameters, (C) cortical thickness, and (D) cortical BMD. Bars are mean and SD; n=10 animals per group; p values by Student’s t test. (E) 3-D rendering of trabecular bone specimens and (F) representative H&E stained sections of trabecular bone from 8-week-old male Bmal1f/f and Bmal1f/f.Prx1-cre mice.
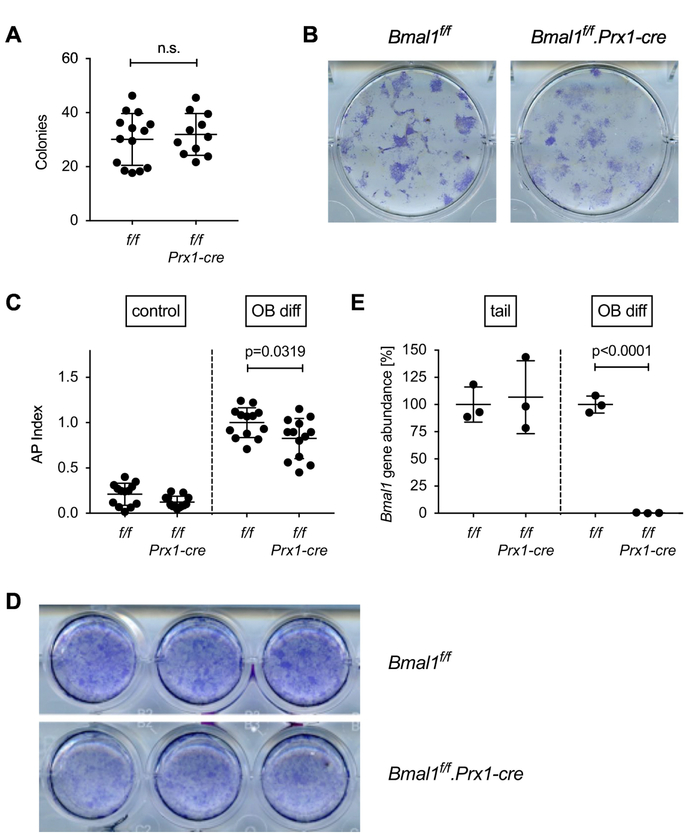
In order to study potential mechanisms, we compared the in vitro osteogenic potential of bone marrow cells from Bmal1f/f and Bmal1f/f.Prx1-cre mice. There was no difference in the number of osteoblast precursors between the two genotypes (Fig. 5A and B). However, we observed a small but statistically significant reduction in osteogenic potential of Bmal1f/f.Prx1-cre bone marrow stromal cell osteoblast precursors measured as induction of alkaline phosphatase activity under osteoblast differentiating conditions (Fig. 5C and D). Panel E in Fig. 5 demonstrates deletion of Bmal1 in osteoblast cultures from Bmal1f/f.Prx1-cre mice, whereas the Bmal1f/f locus is in germline configuration in DNA isolated from the tail, consistent with the Prx1-cre expression pattern [9].
Fig. 5. In vitro osteoblast differentiation from Bmal1f/f.Prx-cre bone marrow.
(A) Cells derived by enzymatic digestion of whole long bones from Bmal1f/f and Bmal1f/f.Prx1-cre mice were differentiated in osteogenic medium for 21 days, and the number of alkaline phosphatase positive colonies was counted. Each data point represents the mean of triplicate cultures from the bone marrow of one mouse, bars are mean and SD of data pooled from 4 experiments. Representative wells are shown in (B). (C) Osteoblast precursors were differentiated in osteogenic medium for 28 days and assayed for alkaline phosphatase activity using a colorimetric assay. The AP index was calculated by normalizing alkaline phosphatase activity to cell viability measured by Alamar Blue assay. Data points represent the mean of triplicate cultures from the bone marrow of individual mice; bars are mean and SD of data pooled from 4 experiments; p value by Student’s t test. (D) Representative wells stained with a non-soluble alkaline phosphatase substrate. (E) Genomic DNA isolated from the tail of Bmal1f/f and Bmal1f/f.Prx1-cre mice or osteoblast cultures from Bmal1f/f and Bmal1f/f.Prx1-cre bone marrow was analyzed for deletion of Bmal1 by real-time quantitative PCR. Each data point represents one animal; bars are mean and SD; p value by Student’s t test.
3.4. Evidence for high bone turnover in Bmal1f/f.Prx1-cre mice
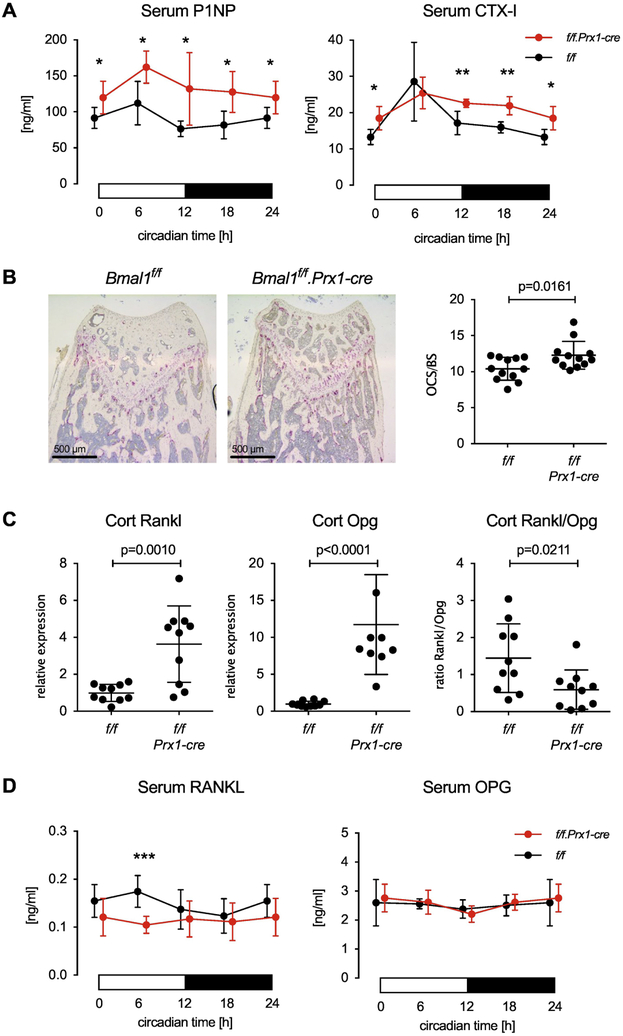
We then analyzed bone turnover markers in the serum of Bmal1f/f and Bmal1f/f.Prx1-cre mice every 6 hours over the course of one day. Contrary to our expectation, serum P1NP as a marker of bone formation was increased in Bmal1f/f.Prx1-cre mice at all time points. Serum CTX-I as a marker of in vivo osteoclast activity was also increased (except at circadian time 6 hours). This suggests that the reduced bone mass in Bmal1f/f.Prx1-cre mice is due to increased bone turnover compared with Bmal1f/f wild-type controls, resulting in an overall negative balance. Consistent with this interpretation we found a small but statistically significant increase in the number of osteoclasts in Bmal1f/f.Prx1-cre mice (Fig. 6B). The in vitro osteoclastogenic potential of Bmal1f/f and Bmal1f/f.Prx1-cre bone marrow did not differ (Fig. S7), suggesting that the effect of Bmal1 deficiency on osteoclast numbers and resorption activity in Bmal1f/f.Prx1-cre mice is the result of osteoblast-osteoclast cross-talk in vivo. Others have reported that Bmal1 controls the expression of RANKL in osteoblasts [15]. Consistent with this, we observed increased expression of Rankl in diaphyseal cortical bone of Bmal1f/f.Prx1-cre mice (Fig. 6C). However, expression of Opg was also increased resulting in a decreased ratio of Rankl/Opg expression in bone (Fig. 6C). We then examined serum protein levels of RANKL and OPG over the course of the 24-hour day. At circadian time 6 hours, serum levels of RANKL in Bmal1f/f.Prx1-cre mice were decreased relative to Bmal1f/f controls, with no significant difference in RANKL at other time points (Fig. 6D). Serum OPG levels were indistinguishable between the two strains (Fig. 6D). Thus, increased local or systemic RANKL activity is unlikely to be the mechanism that explains how Bmal1-deficient osteoblasts promote bone resorption and high bone turnover. Instead, Bmal1 may control secondary pathways by which osteoblasts promote osteoclast formation or activity.
Fig. 6. Enhanced bone turnover in Bmal1f/f.Prx-cre mice.
(A) P1NP and CTX-I measured in the serum of 8-week-old male Bmal1f/f and Bmal1f/f.Prx1-cre littermates collected at 6-hour intervals. Data are mean and SD, n=5 animal per time point and genotype, p values by Student’s t test, * < 0.05, ** < 0.01. (B) Representative TRAP-stained sections of distal femurs from 8-week-old male Bmal1f/f and Bmal1f/f.Prx1-cre littermates. Osteoclast surface (OCS) per bone surface (BS) was measured as described in Methods. 2 slides from each of n=6 mice per genotypes were analyzed, bars are mean and SD; p value by Student’s t test. (C) Rankl, Opg and the ratio of Rankl/Opg gene expression in cortical bone of 8-week-old male Bmal1f/f and Bmal1f/f.Prx1-cre littermates at circadian time 6h, n=10 animals per genotype, p values by Student’s t test. (D) RANKL and OPG serum levels in 8-week-old male Bmal1f/f and Bmal1f/f.Prx1-cre littermates measured in 6-hour intervals, n=7 animals per time point and genotype, p values by Student’s t test, *** < 0.001. In (A) and (D), the 0-hour data are shown at both 0 and 24 hours in order to represent a full 24-hour day.
4. Discussion
The low bone mass phenotype we observed in Bmal1−/− and Bmal1f/f.Prx1-cre mice mirrors reports of increased bone mass in mice with germline deletion of either Per1/Per2 or Cry1/Cry2 [5]. Bmal1 drives the expression of these transcription factors, which then oppose the activity of Bmal1 [16]. Deletion of Bmal1 thus disrupts the molecular clock at a different point in the circadian cycle than deletion of Per1/Per2 or Cry1/Cry2. This has been likened to the removal of either the thermostat or the furnace from a heating system [17] resulting in opposing changes in the expression of clock-controlled genes and, correspondingly, divergent phenotypic outcomes when either the clock driver Bmal1 or the negative regulators Per1/Per2 or Cry1/Cry2 are deleted. We cannot rule out that Bmal1 has additional functions outside of its role as a core regulator of the circadian molecular clock. Nevertheless, our results identify mesenchymal cells as the driver of the bone phenotype in Bmal1−/− mice, with osteoclast-specific Bmal1 deletion having no effect on bone mass, cortical thickness or BMD. Whether Per1/Per2 or Cry1/Cry2 deletion similarly acts through effects in mesenchymally derived cells remains to be shown.
Our results are consistent with those of Samsa et al., who demonstrated a low bone mass phenotype in Bmal1−/− mice compared with wild-type controls using microCT [6]. In contrast, Bunger et al., in their original description of Bmal1−/− mice [7] did not find a significant decrease in areal bone mineral density in Bmal1−/− mice using Piximus densitometry, although there was a trend toward decreased femoral bone mineral density. Our results also contrast with those of Xu et al. who reported an osteoclast-intrinsic role for Bmal1 in controlling bone mass [14]. Possible explanations for these diverging results include differences in sensitivity of microCT compared to densitometry, genetic background of the knockout mice, stringency of using littermate controls, and possibly effects of local microbiota [18].
Studies of serum bone turnover markers in humans and mice have demonstrated robust circadian variations of osteoclast markers [19, 20]. Our data show that Bmal1 deficiency in the progeny of Prx1 expressing cells results in a small increase in the number of osteoclasts. Additionally, it is likely that Bmal1 deficiency in mesenchymal cells affects osteoclast function in vivo, as the circadian curve of serum CTX-I in Bmal1f/f.Prx1-cre mice is flattened compared with Bmal1f/f controls. The lack of a bone phenotype in mice with osteoclast-specific deletion of Bmal1 suggests that circadian osteoclast activity is mostly under extrinsic control by non-osteoclast clocks. These data suggest that osteocytes, osteoblasts or other mesenchymally derived cells regulate osteoclast activity over the course of a 24-hour day. It has been reported that Bmal1 directly controls the expression of RANKL in osteoblasts [15], and we found increased Rankl expression in cortical bone from Bmal1f/f.Prx1-cre mice. However, we also measured increased Opg expression with a decreased Rankl/Opg ratio in Bmal1f/f.Prx1-cre mice compared to Bmal1f/f animals. Furthermore, serum RANKL levels in Bmal1f/f.Prx1-cre mice were not elevated compared with wild-type littermate controls, at least at 8 weeks of age. How the circadian molecular clock in mesenchymal cells impacts the number and/or function of osteoclasts thus remains to be determined.
Although osteoblast differentiation from Bmal1f/f.Prx1-cre precursors was modestly decreased in vitro, serum markers of bone formation were actually higher in Bmal1f/f.Prx1-cre mice. This is consistent with the findings of Fu et al., who reported that 8-week-old Bmal1−/− mice had an increased bone formation rate but also accelerated bone resorption, as demonstrated by increased urine deoxypyridinoline levels in their study [5]. Similarly, bone resorption activity as measured by serum CTX-I was higher inBmal1f/f.Prx1-cre mice during the majority of the circadian cycle. Taken together, our data suggest that the low bone mass in Bmal1f/f.Prx1-cre mice is due to increased bone turnover. Although we do not have dynamic histomorphometry data, we hypothesize that Bmal1 deficiency in mesenchymal cells results in increased bone resorption mediated by wild-type osteoclasts and secondary stimulation of bone formation. However, this increase in bone formation in Bmal1f/f.Prx1-cre mice is not sufficient to fully compensate for the loss due to cell-intrinsic defects in Bmal1-deficient osteoblasts.
Limitations of our study include the typical issues encountered when analyzing conditional knockout mice. Gene deletion is an artificial disruption of cellular function, which may impact the translatability of such studies. Moreover, results are critically dependent on the deletion characteristics of the specific cre strains used including the potential for gene deletion in cells other than the targeted cell lineage. We also did not analyze the impact of disrupting environmental circadian rhythms on bone.
Interestingly, circadian disruption by shift work is associated with increased markers of bone resorption in women [21]. Furthermore, in a small study, nurses working rotating shifts were found to have lower BMD and a higher prevalence of osteopenia than day time workers in the same hospital [22]. The negative consequences of circadian disruption on bone health was confirmed in the Nurses’ Health Study, where nurses working night shifts for 20 or more years had a relative fracture risk of 1.37 compared to nurses who had never worked shifts [23]. And in the Osteoporotic Fractures in Men (MrOS) study, there was a modest association of the daytime to nighttime activity ratio with areal BMD and change in areal BMD over 4 years [24]. Alternations in diurnal hormone patterns, particularly of reproductive hormones, have been proposed as the mechanism explaining the effect of circadian rhythm disruption on bone [21, 23]. Our work and that of others, using mouse models, demonstrate that mesenchymal cell-intrinsic circadian molecular clocks are major drivers of diurnal variation in bone turnover. Extrinsic circadian rhythm disruptions may affect bone health by interfering with physiological circadian osteoblast-osteoclast interactions.
Supplementary Material
Highlights.
Bmal1−/− mice with genetic disruption of the circadian molecular clock have a low bone mass phenotype.
The bone phenotype of Bmal1−/− mice is recapitulated in mice with conditional Bmal1 deletion in mesenchymal cells (Bmal1f/f.Prx1-cre), but not in mice with conditional Bmal1 deletion in osteoclasts (Bmal1f/f.Ctsk-cre).
Bmal1f/f.Prx1-cre mice exhibit high bone turnover with enhanced bone resorption and bone formation.
Acknowledgements
We thank Jacobo Ramirez for excellent animal care.
This work was supported by NIH grants R03 AR066357 (JE) and K08 AR062590 (JFC), the Maldari Fund, the Bettina Looram Fund, and the Center for Skeletal Research Core (NIH P30 AR066261).
Abbreviations
- Bmal1
Brain and Muscle Arntl-like 1
- P1NP
Procollagen type 1 N-terminal Propeptide
- CTX-I
C-terminal Telopeptides of Type I Collagen
- TRAP
Tartrate Resistant Acid Phosphatase
- Cry
Cryptochrome
- Per
Period
- CT
Circadian Time
- microCT
micro-computed tomography
- BV/TV
Bone Volume/Tissue Volume
- Tb N
Trabecular Number
- Tb Th
Trabecular Thickness
- Tb Sp
Trabecular Separation
- Tb BMD
Trabecular Bone Mineral Density
- Cort Th
Cortical Thickness
- Cort BMD
Cortical Bone Mineral Density
- NBF
Neutral Buffered Formalin
- RANKL
Receptor Activator of Nuclear factor Kappa-B Ligand
- OPG
Osteoprotegerin
- Ctsk
Cathepsin K
- Prx1
Paired Related Homeobox 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kelly Tsang, Email: ktsang2@bwh.harvard.edu.
Haoming Liu, Email: liuhaoming1992@gmail.com.
Yen Yang, Email: Yen.Yang@umassmed.edu.
Julia F. Charles, Email: jfcharles@bwh.harvard.edu.
Joerg Ermann, Email: jermann@bwh.harvard.edu.
References
- [1].Zvonic S, Ptitsyn AA, Kilroy G, Wu X, Conrad SA, Scott LK, Guilak F, Pelled G, Gazit D, Gimble JM, Circadian oscillation of gene expression in murine calvarial bone, J Bone Miner Res 22(3) (2007) 357–65. [DOI] [PubMed] [Google Scholar]
- [2].Bass J, Takahashi JS, Circadian integration of metabolism and energetics, Science 330(6009) (2010) 1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaur G, Phillips C, Wong K, Saini B, Timing is important in medication administration: a timely review of chronotherapy research, Int J Clin Pharm 35(3) (2013) 344–58. [DOI] [PubMed] [Google Scholar]
- [4].Joseph F, Chan BY, Durham BH, Ahmad AM, Vinjamuri S, Gallagher JA, Vora JP, Fraser WD, The circadian rhythm of osteoprotegerin and its association with parathyroid hormone secretion, J Clin Endocrinol Metab 92(8) (2007) 3230–8. [DOI] [PubMed] [Google Scholar]
- [5].Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G, The molecular clock mediates leptin-regulated bone formation, Cell 122(5) (2005) 803–15. [DOI] [PubMed] [Google Scholar]
- [6].Samsa WE, Vasanji A, Midura RJ, Kondratov RV, Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype, Bone 84 (2016) 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA, Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus, Genesis 41(3) (2005) 122–32. [DOI] [PubMed] [Google Scholar]
- [8].Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ, Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information, Cell 130(4) (2007) 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ, Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer, Genesis 33(2) (2002) 77–80. [DOI] [PubMed] [Google Scholar]
- [10].Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L, A global double-fluorescent Cre reporter mouse, Genesis 45(9) (2007) 593–605. [DOI] [PubMed] [Google Scholar]
- [11].Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S, Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts, Cell 130(5) (2007) 811–23. [DOI] [PubMed] [Google Scholar]
- [12].Erlebacher A, Derynck R, Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype, J Cell Biol 132(1-2) (1996) 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, Arthur S, Xie M, Schneider MD, Zhai B, Gygi S, Davis R, Glimcher LH, The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice, J Clin Invest 120(7) (2010) 2457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu C, Ochi H, Fukuda T, Sato S, Sunamura S, Takarada T, Hinoi E, Okawa A, Takeda S, Circadian Clock Regulates Bone Resorption in Mice, J Bone Miner Res 31(7) (2016) 1344–55. [DOI] [PubMed] [Google Scholar]
- [15].Takarada T, Xu C, Ochi H, Nakazato R, Yamada D, Nakamura S, Kodama A, Shimba S, Mieda M, Fukasawa K, Ozaki K, Iezaki T, Fujikawa K, Yoneda Y, Numano R, Hida A, Tei H, Takeda S, Hinoi E, Bone Resorption Is Regulated by Circadian Clock in Osteoblasts, J Bone Miner Res 32(4) (2017) 872–881. [DOI] [PubMed] [Google Scholar]
- [16].Dudek M, Meng QJ, Running on time: the role of circadian clocks in the musculoskeletal system, Biochem J 463(1) (2014) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yu EA, Weaver DR, Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes, Aging 3(5) (2011) 479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ermann J, Editorial: Of Mice and Mice: Understanding Conflicting Murine Experimental Data, Arthritis Rheumatol 68(8) (2016) 1801–4. [DOI] [PubMed] [Google Scholar]
- [19].Joseph F, Ahmad AM, Ul-Haq M, Durham BH, Whittingham P, Fraser WD, Vora JP, Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis, J Bone Miner Res 23(5) (2008) 721–9. [DOI] [PubMed] [Google Scholar]
- [20].Srivastava AK, Bhattacharyya S, Li X, Mohan S, Baylink DJ, Circadian and longitudinal variation of serum C-telopeptide, osteocalcin, and skeletal alkaline phosphatase in C3H/HeJ mice., Bone 29(4) (2001) 361–7. [DOI] [PubMed] [Google Scholar]
- [21].Lohstroh PN, Chen J, Ba J, Ryan LM, Xu X, Overstreet JW, Lasley BL, Bone Resorption Is Affected by Follicular Phase Length in Female Rotating Shift Workers, Environmental Health Perspectives 111(4) (2002) 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Quevedo I, Zuniga AM, Low bone mineral density in rotating-shift workers, J Clin Densitom 13(4) (2010) 467–9. [DOI] [PubMed] [Google Scholar]
- [23].Feskanich D, Hankinson SE, Schernhammer ES, Nightshift work and fracture risk: the Nurses' Health Study, Osteoporos Int 20(4) (2009) 537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rogers TS, Harrison S, Swanson C, Cauley JA, Barrett-Connor E, Orwoll E, Stone KL, Lane NE, G. Osteoporotic Fractures in Men Study Research, Rest-activity circadian rhythms and bone mineral density in elderly men, Bone Rep 7 (2017) 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.