Abstract
Objective:
Resective surgery is the most effective treatment option for patients with refractory epilepsy; however identification of patients who will benefit from epilepsy surgery remains challenging. Synthetic aperture magnetometry and excess kurtosis mapping (SAM(g2)) of magnetoencephalography (MEG) is a non-invasive tool that warrants further examination in the pediatric epilepsy population. Here, we examined the utility of MEG with SAM(g2) to determine if MEG epileptiform foci correlates with surgical outcome and to develop a predictive model incorporating MEG information to best assess likelihood of seizure improvement/freedom from resective surgery.
Methods:
564 subjects who had MEG at the Children’s Hospital of Philadelphia between 2010-2015 were screened. Clinical epilepsy history and prior electrographic records were extracted and reviewed and correlated with MEG findings. MEG assessments were made by both a neurologist and neuroradiologist. Predictive models were developed to assess the utility of MEG in determining Engel class at one year and five years after resective epilepsy surgery.
Results:
The number of MEG spike foci was highly associated with Engel class outcome at both one year and five years; however, using MEG data in isolation was not significantly predictive of 5 year surgical outcome. When combined with clinical factors; scalp EEG (single ictal onset zone), MRI (lesional or not), age and sex in a logistic regression model MEG foci was significant for Engel class outcome at both 1 year (p=0.03) and 5 years (0.02). The percent correctly classified for Engel class at one year was 78.43% and the positive predictive value was 71.43.
Significance:
MEG using SAM(g2) analysis in an important non-invasive tool in the identification of those patients who will benefit most from surgery. Integrating MEG data analysis into pre-surgical evaluation can help to predict epilepsy outcome after resective surgery in the pediatric population if utilized with skilled interpretation.
Introduction
Pharmacoresistant epilepsy remains a prevalent public health problem, leading to significant disability and mortality (England et al., 2012). Surgical options are central to the approach to medication-resistant focal epilepsy; yet despite extensive pre-surgical workup, desired outcomes disappoint providers and patients with up to 60% of subjects not obtaining seizure freedom (Englot et al., 2016; Hyunmi Kim et al., 2013). A number of potential reasons for failed epilepsy surgery exist, including the inability to correctly identify which patients will respond to surgery, failure to remove the entire seizure focus due to presence of eloquent cortex in the epileptic zone, or misaligned presurgical workup. Improved non-invasive methods are needed both to identify which patients will benefit from epilepsy surgery and to precisely define the ictal onset zone. Towards this end, we hypothesized that synthetic aperture magnetometry and excess kurtosis mapping (SAM(g2)) of magnetoencephalography (MEG) in conjunction with defined clinical features will improve patient selection for epilepsy surgery and ultimately improve surgical outcomes.
Magnetoencephalography measures magnetic fields generated by the electrical currents produced by synchronized neuronal activity. Unlike EEG, which directly measures volume-conducted electric currents originating from the cortex, magnetic fields are minimally distorted by the variable conductivity of tissue overlying the brain. Therefore the resolution of the signal provides more precise localization of spike discharges (Tovar-Spinoza et al., 2008). When magnetic field recordings undergo source modeling and the identified sources are overlaid onto structural MRI images, MEG can be used to help define the epileptogenic zone, to plan for invasive (intracranial) EEG monitoring, and to plan for subsequent resection of tissue involved in seizure generation. Source localization is most commonly done using equivalent current dipole (ECD) modeling, which describes a theoretical electrical discharge which best explains the measured magnetic fields at a specific time point. Beamforming methods such as synthetic aperture magnetometry (SAM) use spatial filters to estimate the sources of magnetic fields in pre-defined sub centimeter volumes. SAM(g2) combines SAM with statistical methods to identify high kurtosis (g2) as a way of identifying and localizing spike discharges (Robinson et al., 2004; Ukai et al., 2004). Additionally, SAM(g2) analysis offers potential benefits over ECD including improved resolution of multifocal foci, adaptive spatial filtering and automated spike detection (Robinson et al., 2004; Sugiyama et al., 2009). Although prior studies have found SAM(g2) analysis to be consistent with equivalent current dipole analysis (Robinson et al., 2004; Ukai et al., 2004) in which there is a unifocal locus, there are few studies that have investigated its direct utility in pre-surgical evaluation (Mohamed et al., 2013a, 2018). One recent study of 22 adults found that the concordance of kurtosis beamformer localization and the resection cavity was similar to that of using equivalent current dipole fitting (Hall et al., 2018). The use of SAM(g2) type of analysis in the pediatric population has not been previously well-studied. Given that pediatric patients may have limited participation with a MEG or may require sedation in order to perform MEG, specific examination of this population is warranted.
Prior studies have validated MEG for pre-surgical epilepsy evaluation. MEG data compares favorably to invasive pre-surgical techniques, with MEG typically localizing in similar locations to both intracranial EEG/ECoG (Knowlton, 2008) and stereo-EEG abnormalities (Murakami et al., 2016). Studies vary in regard to MEG-EEG concordance; some studies demonstrate better localization of MEG, while others suggest the opposite (Fischer et al., 2005; Paulini et al., 2007). In fact, sensitivity for detecting EEG epileptiform activity by MEG in adults was estimated at 70% from a large cohort of 455 subjects (Stefan et al., 2011). As much of the literature has focused on MEG’s concordance with other assessment modalities, further research is needed on determining the ultimate clinical utility of this method.
On this point the literature is sparse, with one study of 35 pediatric patients with neocortical epilepsy finding that MEG focal beamforming localization did not correlate with surgical outcomes (Mohamed et al., 2013b). Pediatric patients represent a distinctive epilepsy group because seizure etiology is different from that of adults and is more likely to be extra-temporal in origin. The developing brain differs from the adult brain in the basic mechanisms of epileptogenesis and propagation of seizures (Rho et al., 2006; Whiting and Duchowny, 1999). Given these differences in focus localization, epilepsy etiology, and mechanisms of epileptogenesis further examination of the utility of MEG amongst pediatric patients is needed.
MEG may improve clinical practice in multiple ways. MEG can screen patients with nonlocalizing EEG and MRI for focal findings. Second, MEG can tailor intracranial EEG placement to ensure coverage of EEG abnormities. Lastly, MEG can be used to avoid intracranial EEG altogether if concordance between MEG, MRI and scalp EEG is sufficient (Sutherling et al., 2008). Thus, potential benefits of a non-invasive assessments such as MEG are significant, particularly in the pediatric population in which invasive techniques are often more challenging to perform and can have greater associated risks (Anderson et al., 2014; Hunmin Kim et al., 2013; Kim et al., 2012).
Despite its growing use, our understanding of the value of MEG with SAM(g2) in pre-surgical assessment for epilepsy surgery in the pediatric population is limited. Therefore, we assessed the utility of MEG in pre-surgical assessment to determine if MEG localization correlates with surgical outcome after resective epilepsy surgery and to develop a predictive model incorporating MEG information to best assess likelihood of seizure improvement/freedom from resective surgery.
Methods
We conducted a single-center retrospective cohort study examining the utility of MEG in pediatric patients prior to epilepsy surgery. This investigation was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia (CHOP). Subjects underwent MEG performed at CHOP between 2010 and 2016. Additional inclusion criteria were age <18 years at the time of MEG and subsequent resective surgery for epilepsy. Subjects were excluded if inadequate clinical or imaging information was available in the medical record before or after surgery. Subjects were required to have at least 6 months of post-operative follow-up at CHOP.
Demographics, epilepsy history, surgical history and information regarding pre-surgical assessments were extracted from the electronic medical record (EPIC systems). Study data were collected and managed using Redcaps (Research Electronic Data Capture) electronic data capture tools hosted at The Children’s Hospital of Philadelphia (Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, 2009). Data included, age, sex, age of seizure onset, epilepsy syndrome (if any), presence of developmental/intellectual disorder, seizure types, epilepsy etiology, past treatments, including number of trialed anti-epileptic treatments (anti-epileptic drugs (AEDs), steroids, and the ketogenic diet) and approximate seizure burden (seizures/month) at time of MEG. MRI imaging reports were reviewed by J.G. and categorized as lesional or non-lesional. MRI categorization was re-reviewed by a board-certified radiologist with added qualification in neuroradiology (E.S.S.) who confirmed classification and clarified equivocal report findings. Data extracted from EEG reports (both scalp EEG and intracranial EEG) included: irritative zone (areas with occasional-frequent epileptiform abnormalities), ictal onset zone, and number of seizures captured. Data extracted from surgical reports and follow-up clinical visit notes included: resected area, surgical complications, deficits occurring post-operatively and persistence of lesional tissue post-operatively.
In following previously published methods, estimation of sources with excess kurtosis was determined using adaptive spatial filtering by synthetic aperture magnetometry SAM(g2) (CTF v5.4) software tools (Robinson et al., 2004). SAM(g2) allows for the detection of excess kurtosis associated with interictal or ictal spikes, simultaneously mapping the putative generators and reconstructing the source waveforms from the local maxima associated with these generators (Canuet et al., 2008; Ishii et al., 2008; Kirsch et al., 2006; Oishi et al., 2006; Robinson et al., 2004; Ukai et al., 2004; Xiao et al., 2006; Zhang et al., 2011). The reconstructed source waveforms can be considered to be a virtual depth electrode in that the source waveform provide a continuous estimate of the neuromagnetic activity arising from the voxel with good similarity to the neural activity detected by invasive monitoring (Oishi et al., 2006).
MEG recordings were performed at the Lurie Family Foundations’ MEG Imaging Center of the Department of Radiology at the Children’s Hospital of Philadelphia in a magnetically shielded room using a whole-cortex 275-channel MEG system (VSM MedTech Inc., Coquitlam, BC). Fiducial coils were placed on the nasion and left and right preauricular points for each patient to permit continuous head localization during the recordings, and for co-registration with each patient’s volumetric brain MRI. Head position was recorded continuously to ensure that head movements did not exceed 1 cm over the duration of the MEG recordings. Patients who could not remain still for the recording period (typically 30-50 minutes were anesthetized by the pediatric anesthesiology service. Anesthesia was typically provided using dexmedetomidine alone and propofol was avoided. Notation of state and presence/absence of anesthesia was noted for every patient.
Spontaneous MEG data were processed offline after synthetic third order gradient correction. At least ten, 2-minute recordings were collected per patient. Each 2-minute recording was reviewed manually with significant artifacts (muscle, head motion etc.) identified and removed (bad segment DataEditor) prior to SAM(g2) analysis. For each patient, a source space grid (5 mm resolution) was computed for a source model encompassing the entire brain (local spheres based on the inner skull contour). Beamformer weights were then constructed and virtual electrodes representing each location in source space were computed.
Prior to SAM(g2) analysis, the MEG data were filtered from 3 to 70 Hz with a notch filter at 60 Hz. SAM(g2) excess kurtosis was then calculated within the frequency band of 20-70 Hz for each 2-min epoch separately (also used to calculate covariance). All local maxima with an inte-rpeak spacing >10 mm in each map for g2 > 1 were saved, and SAM(g2) source waveforms were computed for each location for viewing source activity in the 3 to 70 Hz range and aligned with each patient’s volumetric structural MRI scan using common fiducial landmarks. The SAMg2 threshold of g2 > 1 was selected to localize kurtotic signals from both sharply contoured healthy background rhythms as well as epilepsy. This choice was made for a variety of reasons, including to observe how the background rhythms change during sleep and to show how rhythms change with specific medications. This lower threshold than what is typically seen in the literature results in more features being analyzed than those relating specifically to epileptic sharps and spikes. Thus, the determination of a reportable kurtotic event in our clinical work depends on both 1. a SAMg2 peak and 2. A virtual sensor waveform showing characteristic epileptogenic activity. If the event shows features consistent with sharply contoured resting brain rhythms (mu, alpha, beta, etc), it is simply ignored.
A focus of abnormality was initially identified by an area where the pseudo t-statistic for kurtosis exceeded the cutoff threshold (50% of peak whole brain) and confirmed by viewing the beamformer virtual depth electrode (source activity-time trace) to confirm that the discharge stood out from background activity and had a morphology consistent with a spike wave discharge. The clinical reporting was performed by E.S.S. and W.G. or T.R. independent of this study. If patients had more than one MEG performed in the course of their evaluation, the study closest to the time of surgical resection was included in the analysis. Scalp EEG was not routinely used to verify MEG findings. For this study, MEG findings were re-evaluated retrospectively by the neuroradiologist (E.S.S.), and blinded to the surgical outcome of the patients to allow for standardized characterization of the findings for the planned analysis. In addition, a neurologist re-evaluated the spike foci using set criteria. A focus was labelled as distinct from another foci if the two areas were at least 3 gyri apart. Each recording was then categorized as having one MEG focus of abnormality (category 1), 2-3 foci of abnormality (category 2), or greater than 3 foci of abnormality (category 3). Finally, the MEG findings were categorized as being unifocal (category 1) or multifocal (category 2 or 3).
Seizure outcomes were graded using the Engel classification as described by the assessment subcommittee and quality standards subcommittee of the American Academy of Neurology and American Epilepsy Society (French et al., 2004; Wheeler et al., 2011). Engel class was assessed both one year post-surgery and at time of last follow-up or 5 years post-surgery (whichever time point was first), and stratified into good (class I or II) or poor outcome (class III, IV). This grouping captures clinically useful outcomes categories (a significant reduction in seizure vs. no reduction or worsening of seizures and has been utilized in other prior MEG studies (Agarwal et al., 2018; Mohamed et al., 2013a; Zhang et al., 2011). Other outcomes data collected included number of antiepileptic treatments after surgery, additional medication or increasing dose of medications, and seizures per month at both one year and two years post-surgery.
All statistical analysis was performed using STATA 15 (StataCorp). Logistic regression was used to evaluate factors associated with good (Engel class 1 or 2) or poor (Engel class 3 or 4) outcomes at one year post-surgery and 5 years post-surgery (for subjects where this data was available). Univariate models exploring the association of MEG variables with outcome were evaluated first, followed by multivariate models which included factors known to be associated with surgical outcome, such as presence of a MRI-visible lesion.
Results
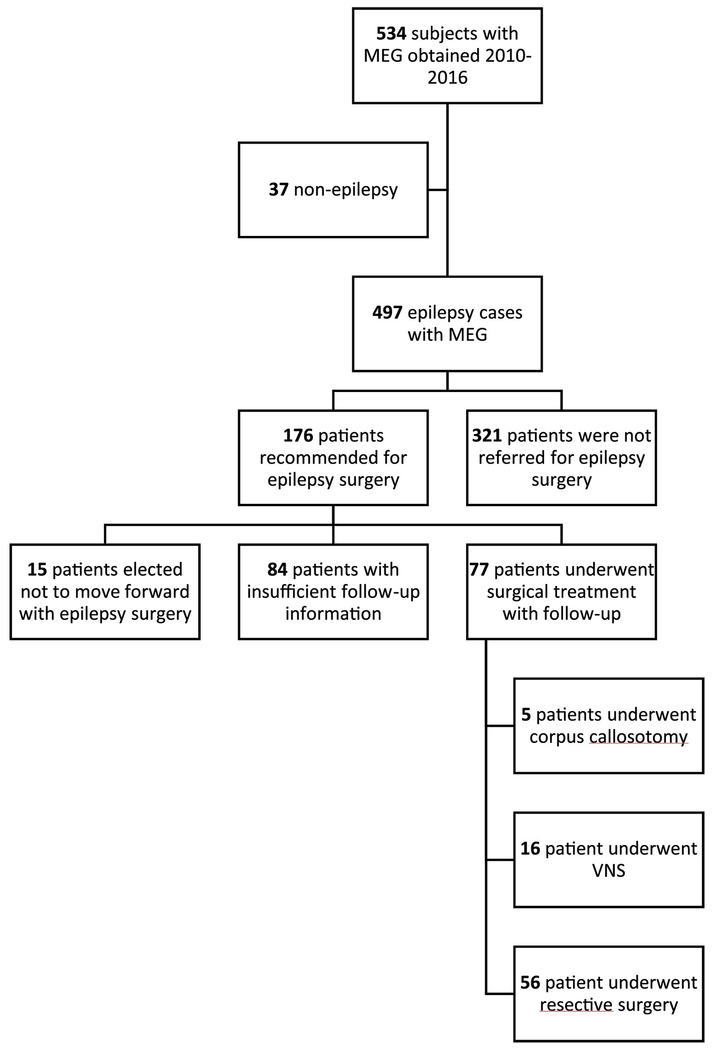
Of the 562 screened subjects who had an MEG at CHOP between 2010 and 2016, 176 were deemed candidates for resective epilepsy surgery, and 56 met study inclusion criteria (Figure 1). Demographic characteristics of this study population are summarized in Table 1. Overall, males and females were evenly represented. Mean age of epilepsy diagnosis was 62.7 months (95% CI 48.60-76.79 months). Focal epilepsy was appropriately the most common epilepsy type 53/56 (94.6%). Subjects had been treated with a mean of 5.31 (95% CI 4.61-6.01) AEDs prior to surgery and 5.4 anti-epileptic treatments (AETs), which included ketogenic diet and other alternative or immunomodulatory therapies (95% CI 4.65-6.24). None of the patients had a prior history of resective surgery. Seizure types were primarily focal motor with impairment of consciousness (29/56, 51.79%) and focal motor without impairment of consciousness 19/56 (33.93%). The remaining seizure types included focal without motor with and without impairment of consciousness, generalized with and without impairment of consciousness and spasms. Epilepsy etiologies included focal cortical dysplasia, mass, stroke, polymicrogyria, heterotopia, and genetic, with focal cortical dysplasia being the most common.
Fig. 1.
Patient screening.
Table 1:
Baseline Characteristics broken down by Engel class at one year
| Variable | All | Low Engel class | High Engel class | p value |
|---|---|---|---|---|
| Sex | 0.52(0.38-0.65) | 0.5(0.33-0.66) | 0.56(0.31-0.81) | 0.70 |
| Age of epilepsy onset (months) | 62.69(48.60-76.79) | 66.82(47.46-86.19) | 53.33(37.09-69.58) | 0.38 |
| Number of prior AEDs | 5.31(4.82-8.36) | 4.74(4.11-5.36) | 6.59(4.82-8.36) | 0.01 |
| Number of prior AETs | 5.44(4.65-6.23) | 4.84(4.15-5.54) | 6.88(4.78-8.98) | 0.02 |
| History of motor delay | 0.43 (0.29-0.56) | 0.45(0.28-0.61) | 0.39(0.14-0.64) | 0.69 |
| History of cognitive delay | 0.54(0.40-0.67) | 0.5(0.33-0.67) | 0.61(0.36-0.86) | 0.44 |
| Age at time of surgery | 11.75 (10.65-12.84) | 12.39(11.13-13.65) | 10.39(8.20-12.57) | 0.09 |
| Current AED # (at time of surgery) | 2.11(1.86-2.35) | 2.0(1.71-2.29) | 2.33(1.85-2.82) | 0.20 |
| Ketogenic diet prior to surgery | 0.14(0.04-0.24) | 0.08(−0.01-0.19) | 0.27(0.01-0.52) | 0.10 |
| MRI lesional | 0.82(0.72-0.92) | 0.82(0.68-0.94) | 0.83(0.64-1.02) | 0.88 |
| Single ictal EEG focus | 0.57(0.43-0.71) | 0.47(0.21-0.73) | 0.62(0.45-0.79) | 0.32 |
| Single interictal EEG focus | 0.38(0.24-0.51) | 0.46(0.28-0.63) | 0.22(0.01-0.43) | 0.09 |
Interictal discharges on scalp EEG were limited to a single region in 37.74% (20/56) of patients, and a single seizure onset zone was recorded in 56.86% (29/56). MRI was lesional in 46/56 (84.1%) of subjects. Focal cortical dysplasia was identified on MRI imaging in 25/56(44.6%) of subjects as the most common lesional abnormality. Twenty-eight subjects (50.0%) went onto have phase 2 intracranial EEG evaluation.
MEG was performed without anesthesia in 49/56 (87%) of subjects. MEG showed epileptiform activity in all subjects. Multifocal MEGs (as defined by 3 non-contiguous foci involving bilateral hemispheres) were seen in 34/56 (60.7%) of subjects, though 35/56(62.5%) had a predominant focus.
Mean age at surgery was 11.75 years (IQR 8-14 years). Mean time from diagnosis of epilepsy to epilepsy surgery was 6.5 years. Resection areas involved included (n, % of total): frontal (25, 44.6%), temporal (33, 58.9%), parietal (14, 25.0%), occipital (5, 8.9%). Most surgeries did not have any complications (such as infection or stroke) (41/56 73.2%). However, resulting neurologic deficits were seen in 20 subjects (35.7%) following surgery, the most common being a visual field cut.
At one-year post-resection, 67.8% of subjects had an Engel class 1 or 2 outcome, and at 5 years 53.6% continued to be in the good Engel class category. There was no significant difference in Engel class at one and five years (p=0.12). Baseline characteristics including age, sex, prior number of AEDs, MRI lesion, and area of resection were not significantly different between those with good Engel class outcomes compared to those with poor Engel class outcomes (Table 1). Mean number of AEDs at the time of MEG was 2.11 and one-year post-surgery was 1.71 (p=0.02) and 2 years post-surgery was 1.53 (p=0.004). Significantly fewer subjects required dose escalation or additional AEDs in the low Engel class group compared to the high Engel class group (0 subjects vs. 18 subjects) (p<0.001) at one year.
The number of MEG spike foci was highly associated with Engel class outcome (Table 2). Compared to patients with unifocal MEG spike discharges, the odds ratio of a poor outcome at one year was 7.6 (95% CI 1.47-39.29, p=0.01) in those with category 2 MEG findings (2-3 separate foci) and 9.5 (95% CI 1.27-10.96, p=0.01) in those with category 3 MEG findings (>3 separate foci). At 5 years, this trend persisted but no longer reached clinical significance with the odds of a poor outcome being 3.12 times greater (95% CI0.92-10.51, p=0.10) in those with 2-3 foci, and 4.17 (95% CI 0.75-23.18, p=0.10) times greater if the number of foci was greater than 3. Regardless of the foci evaluator (radiologist or neurologist), MEG foci were associated with Engel class outcome.
Table 2:
Predictive Model for Engel class at one and five years using age, sex, lesional MRI, single ictal scalp EEG focus, and MEG foci
| Engel class (low vs. high) at one year p=0.02 | ||
|---|---|---|
| Positive Predictive Value | 78.43% | |
| Sensitivity | 58.82% | |
| Specificity | 88.24% | |
| Correctly Classified | 78.43% | |
| Characteristic | Odds Ratio | 95% Confidence Interval |
| Age | 0.85 | 0.71-1.02 |
| Sex | 1.60 | 0.4-6.38 |
| Lesional MRI (Yes/No) | 0.55 | 0.08-3.98 |
| Single ictal scalp EEG focus | 0.71 | 0.07-13.38 |
| Number of foci reevaluation (2-3) | 7.54 | 1.16-49.10 |
| Number of foci reevaluated (>3) | 39.98 | 2.55-600.07 |
| Engel class (low vs. high) at five years: p=0.03 | ||
| Positive Predictive Value | 66.67% | |
| Sensitivity | 72.00% | |
| Specificity | 65.38% | |
| Correctly Classified | 68.63% | |
| Characteristic | Odds Ratio | 95% Confidence Interval |
| Age | 0.83 | 0.69-1.00 |
| Sex | 0.31 | 0.08-1.18 |
| Lesional MRI (Yes/No) | 0.50 | 0.09-2.92 |
| Single ictal scalp EEG focus | 0.62 | 0.17-2.31 |
| Number of foci reevaluated (2-3) | 4.80 | 1.01-22.73 |
| Number of foci reevaluated (>3) | 10.52 | 0.94-117.93 |
After demonstrating that the number of MEG SAM(g2) regions correlates with outcome, we set out to determine if this information could be combined with other clinical features to predict surgery outcome. Although MEG SAM(g2) regions and foci reevaluated were associated with Engel classification, using MEG data in isolation was not significantly predictive of 5-year surgical outcomes. Furthermore, MEG alone lacked sufficient positive predictive value (50.0%) and thus would not be clinically useful in isolation of other patient factors. Therefore, a logistic regression model to predict outcome at both one and five years was generated utilizing additional clinically applicable assessments.
Clinically applicable assessments were selected a priori to reflect those factors typically emphasized in pre-surgical evaluation; namely scalp EEG (single ictal onset zone) and MRI (lesional or not), age and sex. When analyzing these additional assessments without MEG data in a logistic regression model, they were not predictive of Engel class outcome. The odds of a good outcome were increased by 0.55 (95% CI 0.17-1.79, p=0.3) with a single ictal onset zone and 1.13 (95% CI 0.25-5.00, p= 0.87) with a lesional MRI. With the addition of MEG spike foci logistic regression was significant for Engel class outcome at both 1 year (p=0.03) and 5 years (0.02). The sensitivity, specificity, positive predictive value, and percentage correctly classified were calculated for each model. The results using the MEG foci are shown in Table 2. The results were similar if using the radiologist re-evaluation of the MEG foci or the defined (3 gyri) criteria. The percent correctly classified for Engel class at one years if age, sex, MRI lesional yes/no, single ictal focus yes/no, and MEG foci input was 78.43% and the positive predictive value was 71.43.
These results demonstrate that MEG data is a useful tool that can be predictive of outcome in pediatric epilepsy patients, both at 1 and 5 years, Although MEG data can be independently predictive of Engel class outcome, it is best utilized in conjunction with other key clinical characteristics including EEG and MRI data. Furthermore, the MEG data needs to be interpreted to include focality and overlap of regions of spikes. Without this spatial interpretation of the data, the MEG does not predict outcome. Finally, adding clinical characteristics to the MEG data improves prediction of outcome for pediatric epilepsy surgery patients.
Discussion
Here, we demonstrated that MEG using SAM(g2) analysis can aid in the identification of those patients who will benefit most from surgery. Furthermore, integrating MEG data analysis into pre-surgical evaluation can help to predict epilepsy outcome after resective surgery in the pediatric population.
Our patient cohort represents a highly refractory patient group; the majority of patients were on at least 4 AEDs with an early onset of epilepsy (3 years) of diverse etiologies. As well, a significant proportion of our patients (n=31) did not have a single ictal onset zone on scalp EEG or did not have a lesional MRI (n=11). Thus many of the patients in this cohort showed what have previously been described as poor prognostic signs including non-lesional MRI, long epilepsy duration, and scalp EEG without a single ictal onset zone (Ryvlin and Rheims, 2016; Sun et al., 2015). Interestingly, having a lesional MRI was not associated with a better Engel class outcome, in contrast to previous demonstrations in the adult literature. This is likely due to the small number of non lesional patient who underwent surgery in our cohort (Gaínza-Lein et al., 2018).
Despite the identified risk factors among this patient cohort, overall most patients showed improvement after surgery as illustrated by a lower Engel class and by the significant reduction of AED treatment at two years post-surgery. Although parental satisfaction measures were not specifically obtained in this study, significant reduction in seizures has been shown to correlate with patient and parent satisfaction with surgery in prior studies (Endler lachinski et al., 2013; Gilliam et al., 1997; Hosoyama et al., 2017). Reduction in AED treatment is an important aspect of epilepsy surgery outcomes to capture because it is clear that higher numbers of AEDs are associated with poorer quality of life and increased side effects (St. Louis, 2009; St Louis, 2009).
To the best of our knowledge, this study is the first to specifically evaluate the utility of using MEG and the SAM(g2) analysis method in pre-surgical evaluation of pediatric patients with refractory epilepsy in association with Engel class outcomes. Prior MEG studies have focused primarily on determining the utility of MEG in adult epilepsy populations, so a gap exists in defining MEG utility in pediatric epilepsy. Pediatric epilepsy is distinctive from that present in adults, with a lower frequency of temporal lobe onset epilepsy and more disparate etiologies. MEG has been used successfully in pediatrics (Gaetz et al., 2014; Oishi et al., 2006), and here we demonstrate that SAM(g2) analysis can highly correlate with outcome in patients undergoing epilepsy surgery. Importantly, MEG was able to identify and localize epileptiform activity in all patients with non-lesional MRIs. This is particularly useful because typically, patients without an MRI lesion have poorer surgical outcome (Brodbeck et al., 2010; Gaínza-Lein et al., 2018; Krsek et al., 2009). Without a clear lesion on MRI, MEG provides invaluable localization to help guide resections.
Interestingly, MEG source localization was shown to be more predictive of surgical outcomes at one and 5 years than was a lesional MRI. This may be, in part, due to the inherent advantage of MEG in incorporating both functional and localization. MEG is unique because it provides both structural and functional information concomitantly in a non-invasive method when used in conjunction with MRI (Fischer et al., 2005; RamachandranNair et al., 2007). SAM(g2) analysis acts as a spatial filter that allows for the localization of the epileptic zone with improved precision and resolution (Robinson et al., 2004). The integration of localization and functional information provided by MEG with SAM(g2) analysis may be one reason why MEG is uniquely useful in identifying those patients who will benefit most from resective surgery.
The strengths of this study include its relatively large size, which enabled analysis of the associations between MEG findings and surgical outcome. Our study also had the advantage of lengthy post-surgical follow-up. This is important because “relapses”, or return of seizures, post-surgery can occur over one year after the resection, as has been previously illustrated (McIntosh et al., 2004). This would give false impression about the power of a method to predict or delineate outcome. The need for anesthesia to facilitate imaging for the majority of patients in this pediatric cohort did not appear to limit the utility of MEG – epileptiform activity was identified in all of the patients studied. We therefore provide support that MEG with SAMg2 analysis is a plausible tool to be used in the pediatric population, as previously demonstrated (Gaetz et al., 2014; Schwartz et al., 2010). Furthermore, we have proposed a clinically applicable and readably useable predictive model for Engel class outcome, integrating MEG data with other key pre-surgical data including scalp EEG and MRI findings.
This study also has several limitations, including limitations inherent to retrospective collection of clinical data. Data was limited to that which was reported in patient’s electronic charts. Many patients were excluded from the study because of limited data either prior to or after surgery (Figure 1). It is important to note that the patient cohort recommended for resective surgery represents a biased sample, as these were typically patients with more concordant data during their pre-surgical workup. Additionally, identification of foci number using gyral distance is inherently flawed. Depending upon the directionality of a given area of cortex, this method is subject to interpretation and therefore may vary with each reader. Although it is imprecise, this methodology was utilized in order to provide a gross measure of spatially distinctive foci. Further study validating this method is needed. Further validation is also needed for the predictive model put forth here. Predictive modeling was developed using retrospective data and thus additional future prospective studies validating this model are needed.
Identification of patients who will have the best outcomes from resective surgery remains challenging, and is currently insufficient. MEG with SAM(g2) analysis is an important tool that provides key information on likelihood of seizure freedom or reduced seizure frequency after surgery. Here we demonstrated that fewer number of foci detected in MEG using SAM(g2) analysis is associated with a better surgical outcome. This supports other studies in the adult population in which kurtosis beamforming in MEG was useful in localization of the resection area (Hall et al., 2018). It is important to note that in both methods of foci identification, MEG SAM(g2) analysis needs to be interpreted to include regional and spatial context of the data, not just absolute location, in order to maximize clinical utility. Priorities for further research include testing this predictive model prospectively to validate its use. Further research examining the use of MEG in non-lesional MRI cases in particular is needed. This study suggests that MEG is an essential tool in pre-surgical analysis and its addition to MRI and scalp EEG allows for significantly better predictive value of post-surgical outcomes. As surgical technologies, including stereo EEG, RNS and DBS continue to expand, better tools to guide clinicians in surgical decision making are needed.
Highlights:
MEG using SAM(g2) analysis can be successfully used amongst pediatric patients with refractory epilepsy during presurgical workup.
MEG with SAM(g2) analysis is provides significant information regarding likelihood of seizure freedom or reduced seizure frequency after surgery.
Amongst pediatric patients without a lesional MRI, MEG with SAM(g2) analysis may be particularly useful in assessment for resective epilepsy surgery.
Acknowledgments:
The authors would like to acknowledge support from the NIH-National Institute of Neurological Disorders and Stroke, National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders. Additional support was received from the Intellectual and Developmental Disabilities Research Center of Children’s Hospital of Philadelphia Research Institute. We would also like to thank the MEG techs, the neurologists who referred patients for MEG, and the patients and their families
Disclosures: Dr. Roberts has served on the advisory board or as a consultant with Prism Clinical Imaging, CTF, Ricoh, Spago Nanomedical and Avexis. Additionally, he discloses intellectual property related to MEG as a biomarker for pharmaceutical therapy.
Dr. Marsh is a site PI for company sponsored trials for Greenwich Pharma, Zogenix Pharma, and Marinus Pharmaceuticals. He has received grant support from Zogenix Pharma and Greenwich pharma. He is a consultant for Stoke Therapeutics, Takeda Pharmaceuticals. None of this support is related to this work. The remaining authors have no conflicts of interest.
Declaration of Interests
Dr. Gofshteyn is supported by the NIH/National Institute of Neurological Disorders and Stroke NSADA-K12 Career Development Award (#NS5250799523). Additionally Dr. Marsh received grant support from Rett Syndrome Research Trust, RettSyndrome.org, International CDKL5 foundation, NIH/National Institute of Neurological Disorders (grant number R01NS099348) and Stroke, NIH/National Institute of Child Health and Human Development, and the state of Pennsylvania. Dr. Roberts received support from the Intellectual and Developmental Disabilities Research Center of Children’s Hospital of Philadelphia Research Institute (grant #U54-HD086984) and the National Institute on Deafness and Other Communication Disorders (grant #R01-DC008871).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal N, Krishnan B, Burgess RC, Prayson RA, Alexopoulos AV, Gupta A, 2018. Magnetoencephalographic Characteristics of Cortical Dysplasia in Children. Pediatr. Neurol. 78, 13–19. 10.1016/j.pediatrneurol.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Anderson CT, Carlson CE, Li Z, Raghavan M, 2014. Magnetoencephalography in the preoperative evaluation for epilepsy surgery. Curr. Neurol. Neurosci. Rep. 14, 446 10.1007/s11910-014-0446-8 [DOI] [PubMed] [Google Scholar]
- Brodbeck V, Spinelli L, Lascano AM, Polio C, Schaller K, Vargas MI, Wissmeyer M, Michel CM, Seeck M, 2010. Electrical source imaging for presurgical focus localization in epilepsy patients with normal MRI. Epilepsia 51, 583–591. 10.1111/j.1528-1167.2010.02521. [DOI] [PubMed] [Google Scholar]
- Canuet L, Ishii R, Iwase M, Kurimoto R, Ikezawa K, Azechi M, Wataya-Kaneda M, Takeda M, 2008. Tuberous sclerosis: localizing the epileptogenic tuber with synthetic aperture magnetometry with excess kurtosis analysis. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 15, 1296–1298. 10.1016/j.jocn.2007.03.030 [DOI] [PubMed] [Google Scholar]
- Endler lachinski R, Sousa de Meneses M, Andréia Simão C, Rocha S, de Oliveira Braga F, André Kowacs P, 2013. Patient satisfaction with temporal lobectomy/selective amygdalohippocampectomy for temporal lobe epilepsy and its relationship with Engel classification and the side of lobectomy, Epilepsy & behavior: E&B. 10.1016/j.yebeh.2013.09.022 [DOI] [PubMed] [Google Scholar]
- England MJ, Liverman CT, Schultz AM, Strawbridge LM, 2012. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 25, 266–276. 10.1016/j.yebeh.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Rolston JD, Wang DD, Kirsch HE, Nagarajan SS, Chang EF, 2016. 206 Spikes, Slowing, and Functional Connectivity: Multimodal Magnetoencephalography in Epilepsy Surgery. Neurosurgery 63 Suppl 1, 181 10.1227/01.neu.0000489775.61051.9c [DOI] [Google Scholar]
- Fischer MJM, Scheler G, Stefan H, 2005. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain 128, 153–157. 10.1093/brain/awh333 [DOI] [PubMed] [Google Scholar]
- French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, Theodore WH, Bazil C, Stern J, Schachter SC, Bergen D, Hirtz D, Montouris GD, Nespeca M, Gidal B, Marks WJJ, Turk WR, Fischer JH, Bourgeois B, Wilner A, Faught REJ, Sachdeo RC, Beydoun A, Glauser TA, 2004. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epile. Neurology 62, 1261–1273. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TPL, 2014. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage 86, 1–9. 10.1016/j.neuroimage.2013.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaínza-Lein M, Fernández IS, Jackson M, Abend NS, Arya R, Nicholas Brenton J, Carpenter JL, Chapman KE, Gaillard WD, Glauser TA, Goldstein JL, Goodkin HP, Kapur K, Mikati MA, Peariso K, Tasker RC, Tchapyjnikov D, Topjian AA, Wainwright MS, Wilfong A, Williams K, Loddenkemper T, Loddenkemper T, Gaínza-Lein M, Tasker RC, Sánchez Fernández I, Clark J, Jackson M, Kapur K, Abend NS, Topjian AA, Gaillard WD, Carpenter J, Wainstein S, Dean N, Sperberg K, Glauser TA, Peariso K, Arya R, Clark P, Chapman KE, Mikati MA, Tchapyjnikov D, Helseth A, Cornet K, Turner D, Wainwright MS, Goldstein JL, Rusie A, Payne E, Williams K, Wilfong A, Burrows B, Anderson A, Lai YC, Nayak A, Goodkin HP, Sacco M, Zhu H, Nicholas Brenton J, 2018. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 75, 410–418. https://doi.orq/10.1001/iamaneurol.2017.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam F, Wyllie E, Kashden J, Faught E, Kotagal P, Bebin M, Wise M, Comair Y, Morawetz R, Kuzniecky R, 1997. Epilepsy surgery outcome: comprehensive assessment in children. Neurology 48, 1368–1374. [DOI] [PubMed] [Google Scholar]
- Hall MBH, Nissen IA, van Straaten ECW, Furlong PL, Witton C, Foley E, Seri S, Hillebrand A, 2018. An evaluation of kurtosis beamforming in magnetoencephalography to localize the epileptogenic zone in drug resistant epilepsy patients. Clin. Neurophysiol 10.1016/j.clinph.2017.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama H, Matsuda K, Mihara T, Usui N, Baba K, Inoue Y, Tottori T, Otsubo T, Kashida Y, lida K, Hirano H, Hanaya R, Arita K, 2017. Long-term outcomes of epilepsy surgery in 85 pediatric patients followed up for over 10 years: a retrospective survey. J. Neurosurg. Pediatr. 19, 606–615. 10.3171/2016.12.PEDS16197 [DOI] [PubMed] [Google Scholar]
- Ishii R, Canuet L, Ochi A, Xiang J, Imai K, Chan D, Iwase M, Takeda M, Snead OC 3rd, Otsubo H, 2008. Spatially filtered magnetoencephalography compared with electrocorticography to identify intrinsically epileptogenic focal cortical dysplasia. Epilepsy Res. 81, 228–232. 10.1016/j.eplepsyres.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Kim H, Chung CK, Hwang H, 2013. Magnetoencephalography in pediatric epilepsy. Korean J. Pediatr. 56, 431–438. 10.3345/kjp.2013.56.10.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kankirawatana P, Killen J, Harrison A, Oh A, Rozzelle C, Blount J, Knowlton R, 2013. Magnetic source imaging (MSI) in children with neocortical epilepsy: Surgical outcome association with 3D post-resection analysis. Epilepsy Res. 106, 164–172. 10.1016/j.eplepsyres.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Kim H, Lim BC, Jeong W, Kim JS, Chae J-H, Kim KJ, Chung CK, Hwang YS, Hwang H, 2012. Magnetoencephalography in pediatric lesional epilepsy surgery. J. Korean Med. Sci. 27, 668–673. 10.3346/jkms.2012.27.6.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch HE, Robinson SE, Mantle M, Nagarajan S, 2006. Automated localization of magnetoencephalographic interictal spikes by adaptive spatial filtering. Clin. Neurophysiol. 117, 2264–2271. 10.1016/j.clinph.2006.06.708 [DOI] [PubMed] [Google Scholar]
- Knowlton RC, 2008. Can magnetoencephalography aid epilepsy surgery? Epilepsy Curr. 8, 1–5. 10.1111/j.1535-7511.2007.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsek P, Maton B, Jayakar P, Dean P, Korman B, Rey G, Dunoyer C, Pacheco-Jacome E, Morrison G, Ragheb J, Vinters HV, Resnick T, Duchowny M, 2009. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology 72, 217–223. 10.1212/01.wnl.0000334365.22854.d3 [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GCA, Briellmann RS, Berkovic SF, 2004. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 127, 2018–2030. 10.1093/brain/awh221 [DOI] [PubMed] [Google Scholar]
- Mohamed IS, Bouthillier A, Berube A, Cossette P, Finet P, Saint-Hilaire J-M, Robert M, Nguyen DK, 2018. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 79, 34–41. 10.1016/j.yebeh.2017.10.036 [DOI] [PubMed] [Google Scholar]
- Mohamed IS, Gibbs SA, Robert M, Bouthillier A, Leroux J-M, Khoa Nguyen D, 2013a. The utility of magnetoencephalography in the presurgical evaluation of refractory insular epilepsy. Epilepsia 54, 1950–1959. 10.1111/epi.12376 [DOI] [PubMed] [Google Scholar]
- Mohamed IS, Otsubo H, Ferrari P, Sharma R, Ochi A, Elliott I, Go C, Chuang S, Rutka J, Snead C 3rd, Cheyne D, 2013b. Source localization of interictal spike-locked neuromagnetic oscillations in pediatric neocortical epilepsy. Clin. Neurophysiol. 124, 1517–1527. 10.1016/j.clinph.2013.01.023 [DOI] [PubMed] [Google Scholar]
- Murakami H, Wang ZI, Marashly A, Krishnan B, Prayson RA, Kakisaka Y, Mosher JC, Bulacio J, Gonzalez-Martinez JA, Bingaman WE, Najm IM, Burgess RC, Alexopoulos AV, 2016. Correlating magnetoencephalography to stereo- electroencephalography in patients undergoing epilepsy surgery. Brain 139, 2935–2947. 10.1093/brain/aww215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Otsubo H, Iida K, Suyama Y, Ochi A, Weiss SK, Xiang J, Gaetz W, Cheyne D, Chuang SH, Rutka JT, Snead OC, 2006. Preoperative simulation of intracerebral epileptiform discharges: synthetic aperture magnetometry virtual sensor analysis of interictal magnetoencephalography data. J. Neurosurg. 105, 41–49. 10.3171/ped.2006.105.1.41 [DOI] [PubMed] [Google Scholar]
- Harris Paul A., Taylor Robert, Thielke Robert, Payne Jonathon, Nathaniel Gonzalez JGC, 2009. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulini A, Fischer M, Rampp S, Scheler G, Hopfengartner R, Kaltenhauser M, Dorfler A, Buchfelder M, Stefan H, 2007. Lobar localization information in epilepsy patients: MEG--a useful tool in routine presurgical diagnosis. Epilepsy Res. 76, 124–130. 10.1016/j.eplepsyres.2007.07.006 [DOI] [PubMed] [Google Scholar]
- RamachandranNair R, Ochi A, Benifla M, Rutka JT, Snead OC 3rd, Otsubo H, 2007. Benign epileptiform discharges in Rolandic region with mesial temporal lobe epilepsy: MEG, scalp and intracranial EEG features. Acta Neurol. Scand. 116, 59–64. 10.1111/j.1600-0404.2006.00759. [DOI] [PubMed] [Google Scholar]
- RamachandranNair R, Otsubo H, Shroff MM, Ochi A, Weiss SK, Rutka JT, Snead OC 3rd, 2007. MEG predicts outcome following surgery for intractable epilepsy in children with normal or nonfocal MRI findings. Epilepsia 48, 149–157. 10.1111/j.1528-1167.2006.00901. [DOI] [PubMed] [Google Scholar]
- Rho J-Y, Yu K, Han J-S, Chae J-I, Koo D-B, Yoon H-S, Moon S-Y, Lee K-K, Han Y-M, 2006. Transcriptional profiling of the developmental^ important signalling pathways in human embryonic stem cells. Hum. Reprod. 21, 405–412. 10.1093/humrep/dei328 [DOI] [PubMed] [Google Scholar]
- Robinson SE, Nagarajan SS, Mantle M, Gibbons V, Kirsch H, 2004. Localization of interictal spikes using SAM(g2) and dipole fit. Neurol. Clin. Neurophysiol. 2004, 74. [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Rheims S, 2016. Predicting epilepsy surgery outcome. Curr. Opin. Neurol. 29, 182–188. 10.1097/WCO.00000000000003Q6 [DOI] [PubMed] [Google Scholar]
- Schwartz ES, Edgar JC, Gaetz WC, Roberts TPL, 2010. Magnetoencephalography. Pediatr. Radiol. 40, 50–58. 10.1007/s00247-009-1451 [DOI] [PubMed] [Google Scholar]
- St. Louis EK, 2009. Minimizing AED Adverse Effects: Improving Quality of Life in the Interictal State in Epilepsy Care. Curr. Neuropharmacol. 10.2174/157015909788848857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Louis EK, 2009. Monotherapy to polytherapy: antiepileptic drug conversions through the spectrum of epilepsy care. Curr. Neuropharmacol. 7, 75–76. 10.2174/157015909788848910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan H, Wu X, Buchfelder M, Rampp S, Kasper B, Hopfengartner R, Schmitt F, Dorfler A, Blumcke I, Zhou D, Weigel D, 2011. MEG in frontal lobe epilepsies: localization and postoperative outcome. Epilepsia 52, 2233–2238. 10.1111/j.1528-1167.2011.03265. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zuo H, Yuan D, Sun Y, Zhang K, Cui Z, Wang J, 2015. Predictors of prognosis in patients with temporal lobe epilepsy after anterior temporal lobectomy. Exp. Ther. Med. 10.3892/etm.2015.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, Lopez N, 2008. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology 71, 990–996. 10.1212/01.wnl.0000326591.29858.1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Spinoza ZS, Ochi A, Rutka JT, Go C, Otsubo H, 2008. The role of magnetoencephalography in epilepsy surgery. Neurosurg. Focus 25, E16 10.3171/FOC/2008/25/9/E16 [DOI] [PubMed] [Google Scholar]
- Ukai S, Kawaguchi S, Ishii R, Yamamoto M, Ogawa A, Mizuno-Matsumoto Y, Robinson SE, Fujita N, Yoshimine T, Shinosaki K, Takeda M, 2004. SAM(g2) analysis for detecting spike localization: a comparison with clinical symptoms and ECD analysis in an epileptic patient. Neurol. Clin. Neurophysiol. 2004, 57. [PubMed] [Google Scholar]
- Wheeler M, De Herdt V, Vonck K, Gilbert K, Manem S, Mackenzie T, Jobst B, Roberts D, Williamson P, Van Roost D, Boon P, Thadani V, 2011. Efficacy of vagus nerve stimulation for refractory epilepsy among patient subgroups: a re-analysis using the Engel classification. Seizure 20, 331–335. 10.1016/j.seizure.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Whiting S, Duchowny M, 1999. Clinical spectrum of cortical dysplasia in childhood: diagnosis and treatment issues. J. Child Neurol. 14, 759–771. 10.1177/088307389901401201 [DOI] [PubMed] [Google Scholar]
- Xiao Z, Xiang J, Holowka S, Hunjan A, Sharma R, Otsubo H, Chuang S, 2006. Volumetric localization of epileptic activities in tuberous sclerosis using synthetic aperture magnetometry. Pediatr. Radiol. 36, 16–21. 10.1007/s00247-005-0013-1 [DOI] [PubMed] [Google Scholar]
- Zhang R, Wu T, Wang Y, Liu H, Zou Y, Liu W, Xiang J, Xiao C, Yang L, Fu Z, 2011. Interictal magnetoencephalographic findings related with surgical outcomes in lesional and nonlesional neocortical epilepsy. Seizure 20, 692–700. 10.1016/j.seizure.2011.06.0 [DOI] [PubMed] [Google Scholar]