Abstract
Background
Assisted reproductive technologies (ART) including in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), combine gametes to enhance the probability of fertilisation and pregnancy. Advanced sperm selection techniques are increasingly employed in ART, most commonly in cycles utilising ICSI. Advanced sperm selection techniques are proposed to improve the chance that structurally intact and mature sperm with high DNA integrity are selected for fertilisation. Strategies include selection according to surface charge; sperm apoptosis; sperm birefringence; ability to bind to hyaluronic acid; and sperm morphology under ultra‐high magnification. These techniques are intended to improve ART outcomes.
Objectives
To evaluate the effectiveness and safety of advanced sperm selection techniques on ART outcomes.
Search methods
We conducted a systematic search of electronic databases (Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL via the Cochrane Register of Studies Online, MEDLINE, Embase, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL); trials registers (ClinicalTrials.gov, Current Controlled Trials, and the World Health Organization International Clinical Trials Registry Platform); conference abstracts (Web of Knowledge); and grey literature (OpenGrey) for relevant randomised controlled trials (RCTs). We handsearched the reference lists of included studies and similar reviews. The search was conducted in June 2018.
Selection criteria
We included RCTs comparing advanced sperm selection techniques versus standard IVF, ICSI, or another technique. We excluded studies of intracytoplasmic morphologically selected sperm injection (IMSI), as they are subject to a separate Cochrane Review. Primary outcomes measured were live birth and miscarriage per woman randomly assigned. Secondary outcome measures included clinical pregnancy per woman randomly assigned. Secondary adverse events measured included miscarriage per clinical pregnancy and foetal abnormality.
Data collection and analysis
Two review authors independently assessed study eligibility and risk of bias and extracted data. Any disagreements were resolved by consultation with a third review author. We consulted study investigators to resolve queries. Risk ratios (RRs) were calculated with 95% confidence intervals (CIs). We combined studies using a fixed‐effect model. We evaluated the quality of the evidence using GRADE methods.
Main results
We included eight RCTs (4147 women). The quality of evidence ranged from very low to low. The main limitations were imprecision, performance bias, and attrition bias.
Hyaluronic acid selected sperm‐intracytoplasmic sperm injection (HA‐ICSI) compared to ICSI
Two RCTs compared the effects of HA‐ICSI versus ICSI on live birth. The quality of the evidence was low. There may be little or no difference between groups: 25% chance of live birth with ICSI versus 24.5% to 31% with HA‐ICSI (RR 1.09, 95% CI 0.97 to 1.23, 2903 women, I2 = 0%, low‐quality evidence). Three RCTs reported on miscarriage. HA‐ICSI may decrease miscarriage per woman randomly assigned: 7% chance of miscarriage with ICSI versus 3% to 6% chance with HA‐ICSI (RR 0.61, 95% CI 0.45 to 0.83, 3005 women, I2 = 0%, low‐quality evidence) and per clinical pregnancy: 20% chance of miscarriage with ICSI compared to 9% to 16% chance with HA‐ICSI (RR 0.62, 95% CI 0.46 to 0.82, 1065 women, I2 = 0%, low‐quality evidence). Four RCTs reported on clinical pregnancy. There may be little or no difference between groups: 37% chance of pregnancy with ICSI versus 34% to 40% chance with HA‐ICSI (RR 1.00, 95% CI 0.92 to 1.09, 3492 women, I2 = 0%, low‐quality evidence).
HA‐ICSI compared to SpermSlow
One RCT compared HA‐ICSI to SpermSlow. The quality of the evidence was very low. We are uncertain whether HA‐ICSI improves live birth compared to SpermSlow (RR 1.13, 95% CI 0.64 to 2.01, 100 women) or clinical pregnancy (RR 1.05, 95% CI 0.66 to 1.68, 100 women). We are uncertain whether HA‐ICSI reduces miscarriage per woman (RR 0.80, 95% CI 0.23 to 2.81, 100 women) or per clinical pregnancy (RR 0.76, 95% CI 0.24 to 2.44, 41 women).
Magnetic‐activated cell sorting (MACS) compared to ICSI
One RCT compared MACS to ICSI for live birth; three reported clinical pregnancy; and two reported miscarriage. The quality of the evidence was very low. We are uncertain whether MACS improves live birth (RR 1.95, 95% CI 0.89 to 4.29, 62 women) or clinical pregnancy (RR 1.05, 95% CI 0.84 to 1.31, 413 women, I2 = 81%). We are also uncertain if MACS reduces miscarriage per woman (RR 0.95, 95% CI 0.16 to 5.63, 150 women, I2 = 0%) or per clinical pregnancy (RR 0.51, 95%CI 0.09 to 2.82, 53 women, I2=0)
Zeta sperm selection compared to ICSI
One RCT evaluated Zeta sperm selection. The quality of the evidence was very low. We are uncertain of the effect of Zeta sperm selection on live birth (RR 2.48, 95% CI 1.34 to 4.56, 203 women) or clinical pregnancy (RR 1.82, 95% CI 1.20 to 2.75, 203 women). We are also uncertain if Zeta sperm selection reduces miscarriage per woman (RR 0.73, 95% CI 0.16 to 3.37, 203 women) or per clinical pregnancy (RR 0.41, 95% CI 0.10 to 1.68, 1 RCT, 62 women).
MACS compared to HA‐ICSI
One RCT compared MACS to HA‐ICSI. This study did not report on live birth. The quality of the evidence was very low. We are uncertain of the effect on miscarriage per woman (RR 1.52, 95% CI 0.10 to 23.35, 78 women) or per clinical pregnancy (RR 1.06, 95% CI 0.07 to 15.64, 37 women). We are also uncertain of the effect on clinical pregnancy (RR 1.44, 95% CI 0.91 to 2.27, 78 women).
Authors' conclusions
The evidence suggests that sperm selected by hyaluronic acid binding may have little or no effect on live birth or clinical pregnancy but may reduce miscarriage. We are uncertain of the effect of Zeta sperm selection on live birth, clinical pregnancy, and miscarriage due principally to the very low quality of the evidence for this intervention. We are uncertain of the effect of the other selection techniques on live birth, miscarriage, or pregnancy.
Further high‐quality studies, including the awaited data from the identified ongoing studies, are required to evaluate whether any of these advanced sperm selection techniques can be recommended for use in routine practice.
Keywords: Humans; Male; Sperm Retrieval; Apoptosis; Apoptosis/physiology; Birefringence; Hyaluronic Acid; Hyaluronic Acid/metabolism; Randomized Controlled Trials as Topic; Sperm Injections, Intracytoplasmic; Sperm Injections, Intracytoplasmic/methods; Spermatozoa; Spermatozoa/physiology
Plain language summary
Advanced sperm selection techniques for assisted reproduction
Review question
We sought to determine if any advanced sperm selection techniques used for assisted reproduction, except for ultra‐high magnification, alter the rates of live birth, clinical pregnancy, miscarriage, or foetal abnormalities.
Background
In vitro fertilisation (IVF) with or without intracytoplasmic sperm injection (ICSI) is a commonly used treatment for subfertile couples. It is thought that the selection of high‐quality sperm may improve outcomes for these couples. Advanced sperm selection techniques use complex methods to select healthy, mature, and structurally sound sperm for fertilisation. Despite the use of these techniques in many centres worldwide, their effectiveness is unclear.
Study characteristics
We included eight randomised controlled trials (a type of study in which participants are assigned to one of two or more treatment groups using a random method) with a total of 4147 women. Four studies evaluated sperm selection by their ability to bind to hyaluronic acid during the ICSI process (HA‐ICSI) against ICSI. One study compared HA‐ICSI versus SpermSlow. One study compared HA‐ICSI versus magnetic‐activated cell sorting (MACS) versus ICSI. Three studies compared MACS versus ICSI. One study compared sperm selection by surface charge Zeta potential versus ICSI. Six of the included studies reported rates of live birth; seven reported clinical pregnancy; six reported miscarriage per clinical pregnancy and per woman randomly assigned; and none reported on foetal abnormalities.
Key results
The current evidence suggests that advanced sperm selection strategies in assisted reproductive technologist (ART) may not result in an increase in the likelihood of live birth. The only sperm selection technique that potentially increases live birth and clinical pregnancy rates is Zeta sperm selection, yet these results were of very low quality and derived from a single study, therefore we are uncertain of the effect. There is low‐quality evidence that HA‐ICSI decreases miscarriage rates when compared with ICSI. We are uncertain whether the other sperm selection techniques alter clinical pregnancy or miscarriage rates. No studies reported on foetal abnormalities, and further studies of suitable quality are required before any of these advanced sperm selection techniques can be recommended for use in clinical practice.
Evidence quality
The evidence gathered was of very low to low quality. The main limitations were imprecision associated with low numbers of participants or events and high risk of performance bias. Data on important clinical outcomes such as foetal abnormalities were absent.
Summary of findings
Summary of findings for the main comparison. Hyaluronic acid‐selected sperm (HA‐ICSI) compared to ICSI for assisted reproduction.
| Hyaluronic acid‐selected sperm (HA‐ICSI) compared to ICSI for assisted reproduction | |||||
| Patient or population: assisted reproduction Setting: IVF unit Intervention: hyaluronic acid‐selected sperm (HA‐ICSI) Comparison: ICSI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with ICSI | Risk with HA‐ICSI | ||||
| Live birth per woman randomly assigned | Study population | RR 1.09 (0.97 to 1.23) | 2903 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,3 | |
| 253 per 1000 | 276 per 1000 (245 to 311) | ||||
| Miscarriage per woman randomly assigned | Study population | RR 0.61 (0.45 to 0.83) | 3005 (3 RCTs) | ⊕⊕⊝⊝ LOW 2,3 | |
| 70 per 1000 | 43 per 1000 (31 to 58) | ||||
| Miscarriage per clinical pregnancy | Study population | RR 0.62 (0.46 to 0.82) | 1065 (3 RCTs) | ⊕⊕⊝⊝ LOW 2,3 | |
| 197 per 1000 | 122 per 1000 (90 to 161) | ||||
| Clinical pregnancy per woman randomly assigned | Study population | RR 1.00 (0.92 to 1.09) | 3492 (4 RCTs) | ⊕⊕⊝⊝ LOW 1,3 | |
| 370 per 1000 | 370 per 1000 (341 to 404) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level due to imprecision; direction of effect inconsistent. 2Downgraded one level due to low event rate. 3Downgraded one level due to risk of bias; performance bias of largest trial may significantly affect outcome.
Summary of findings 2. Hyaluronic acid‐selected sperm (HA‐ICSI) compared to viscous medium containing HA (SpermSlow) for assisted reproduction.
| Hyaluronic acid‐selected sperm (HA‐ICSI) compared to viscous medium containing HA (SpermSlow) for assisted reproduction | |||||
| Patient or population: assisted reproduction Setting: IVF unit Intervention: hyaluronic acid‐selected sperm (HA‐ICSI) Comparison: viscous medium containing HA (SpermSlow) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with viscous medium containing HA (SpermSlow) | Risk with HA‐ICSI | ||||
| Live birth per woman randomly assigned | Study population | RR 1.13 (0.64 to 2.01) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 300 per 1000 | 339 per 1000 (192 to 603) | ||||
| Miscarriage per woman randomly assigned | Study population | RR 0.80 (0.23 to 2.81) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 100 per 1000 | 80 per 1000 (23 to 281) | ||||
| Miscarriage per clinical pregnancy | Study population | RR 0.76 (0.24 to 2.44) | 41 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 250 per 1000 | 190 per 1000 (60 to 610) | ||||
| Clinical pregnancy per woman randomly assigned | Study population | RR 1.05 (0.66 to 1.68) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 400 per 1000 | 420 per 1000 (264 to 672) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded three levels due to very serious imprecision; data derived from a single RCT, low numbers, broad Cl.
Summary of findings 3. Magnetic‐activated cell sorting (MACS) compared to ICSI for assisted reproduction.
| Magnetic‐activated cell sorting (MACS) compared to ICSI for assisted reproduction | |||||
| Patient or population: assisted reproduction Setting: IVF unit Intervention: magnetic‐activated cell sorting (MACS) Comparison: ICSI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with ICSI | Risk with MACS | ||||
| Live birth per woman randomly assigned | Study population | RR 1.95 (0.89 to 4.29) | 62 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 212 per 1000 | 414 per 1000 (189 to 910) | ||||
| Miscarriage per woman randomly assigned | Study population | RR 0.95 (0.16 to 5.63) | 150 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 34 per 1000 | 32 per 1000 (5 to 192) | ||||
| Miscarriage per clinical pregnancy | Study population | RR 0.51 (0.09 to 2.82) | 53 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 130 per 1000 | 67 per 1000 (12 to 368) | ||||
| Clinical pregnancy per woman randomly assigned | Study population | RR 1.05 (0.84 to 1.31) | 413 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 | |
| 408 per 1000 | 429 per 1000 (343 to 535) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded three levels due to very serious imprecision; data derived from a single RCT, low numbers, broad CI. 2Downgraded three levels due to very serious imprecision; data derived from two RCTs, low numbers, broad CI. 3Downgraded three levels due to very serious unexplained heterogeneity.
Summary of findings 4. Zeta sperm selection compared to ICSI for assisted reproduction.
| Zeta sperm selection compared to ICSI for assisted reproduction | |||||
| Patient or population: assisted reproduction Setting: IVF unit Intervention: Zeta sperm selection Comparison: ICSI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with ICSI | Risk with Zeta sperm selection | ||||
| Live birth per woman randomly assigned | Study population | RR 2.48 (1.34 to 4.56) | 203 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 119 per 1000 | 295 per 1000 (159 to 542) | ||||
| Miscarriage per woman randomly assigned | Study population | OR 0.73 (0.16 to 3.37) | 203 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 40 per 1000 | 29 per 1000 (7 to 122) | ||||
| Miscarriage per clinical pregnancy | Study population | RR 0.41 (0.10 to 1.68) | 62 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 182 per 1000 | 75 per 1000 (18 to 305) | ||||
| Clinical pregnancy per woman randomly assigned | Study population | RR 1.82 (1.20 to 2.75) | 203 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 238 per 1000 | 432 per 1000 (285 to 653) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Data derived from a single RCT. 2Downgraded three levels due to very serious imprecision; low numbers, broad CI.
Summary of findings 5. Magnetic‐activated cell sorting (MACS) compared to hyaluronic acid‐selected sperm (HA‐ICSI) for assisted reproduction.
| Magnetic‐activated cell sorting (MACS) compared to hyaluronic acid‐selected sperm (HA‐ICSI) for assisted reproduction | |||||
| Patient or population: assisted reproduction Setting: IVF unit Intervention: magnetic‐activated cell sorting (MACS) Comparison: hyaluronic acid‐selected sperm (HA‐ICSI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with HA‐ICSI | Risk with MACS | ||||
| Miscarriage per woman randomly assigned | Study population | RR 1.52 (0.10 to 23.35) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 21 per 1000 | 32 per 1000 (2 to 497) | ||||
| Miscarriage per clinical pregnancy | Study population | RR 1.06 (0.07 to 15.64) | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 53 per 1000 | 56 per 1000 (4 to 823) | ||||
| Clinical pregnancy per woman randomly assigned | Study population | RR 1.44 (0.91 to 2.27) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 404 per 1000 | 582 per 1000 (368 to 918) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded three levels due to serious imprecision; data derived from a single RCT, low numbers, broad CI.
Background
Description of the condition
In vitro fertilisation (IVF) is a form of assisted reproductive technology (ART) that is used for treating infertility, a condition that affects an estimated 15% of the population. In vitro fertilisation usually involves controlled ovarian hyperstimulation, surgical oocyte retrieval, in vitro fertilisation, and embryo transfer. Intracytoplasmic sperm injection (ICSI) involves injecting a single sperm into the cytoplasm of each oocyte to achieve fertilisation. Intracytoplasmic sperm injection is commonly used as a treatment for male factor infertility when semen parameters are poor; when sperm have been surgically retrieved; or when repeated fertilisation with standard IVF has failed (Palermo 1992).
Despite technological advances, pregnancy rates remain relatively low, hence the drive for researchers to seek out other modifiable aetiologies such as sperm dysfunction. Successful embryo development and subsequent pregnancy outcome are likely to be impacted by the quality of the sperm that fertilise an oocyte (Sakkas 2000). Ideally, only sperm with a high chance of successful fertilisation and subsequent embryo growth would be used for ART. These sperm would be viable and mature, have high DNA integrity, and be structurally sound.
Sperm preparation and selection in IVF are limited to semen washing, density gradient centrifugation, and use of swim‐up techniques (Boomsma 2007). In ICSI, routine sperm selection is based on motility and gross morphology (sperm are examined under a microscope at 200× to 400× magnification) after one or more of the above methods of semen preparation has been applied. Advanced sperm selection techniques based on alternative characteristics might enable further selection of the most appropriate sperm for use in ART.
Description of the intervention
Advanced sperm selection techniques have developed as a means of improving ART outcomes in certain clinical scenarios. Techniques can be categorised as follows.
Surface charge selection
Electrophorectic sperm selection and sperm Zeta potential are surface charge selection protocols utilised in both IVF and ICSI. The Zeta potential of the sperm is the electrical potential between the sperm membrane and its surroundings. The Zeta potential decreases with capacitation, and normally differentiated sperm are charged electronegatively. Semen is placed into an electrophoretic device and a current applied. Normally differentiated negatively charged sperm are rapidly separated and collected from an adjacent chamber (Ainsworth 2005).
Sperm apoptosis
Selection of non‐apoptotic sperm for use in ART is based on the presence of phosphatidylserine on the external surface of the sperm membrane in the early stages of apoptosis. Magnetic‐activated cell sorting (MACS) and glass wool separation columns utilise the magnetic properties of phosphatidylserine to separate apoptotic sperm from non‐apoptotic sperm (Grunewald 2001).
Hyaluronic acid binding
Hyaluronic acid (HA) is the main component of the extracellular matrix of the cumulus oophorus. Hyaluronic acid binding sites on the sperm plasma membrane indicate sperm maturity. Mature sperm bind to and digest HA and thus have a better chance of reaching the oocyte for fertilisation. In vitro, HA is utilised as a 'physiological selector' of mature intact sperm.
Two systems for HA sperm selection are currently available. Physiological intracytoplasmic sperm injection (PICSI; Origio, Måløv, Denmark) is a plastic culture dish with spots of HA attached to its base. Sperm are bound by the head to HA and are selected for microinjection (Huszar 2007). SpermSlow is a viscous medium containing HA. Appropriate sperm appear 'slowed' and are selected.
Sperm birefringence
The mature sperm nucleus has high intrinsic birefringence due to longitudinally orientated subacrosomal protein filaments. With the use of polarised light microscopy, sperm birefringence can be evaluated and a mature sperm selected (Gianaroli 2008).
Sperm morphology (intracytoplasmic morphologically selected sperm injection)
Subtle defects in sperm morphology (acrosome, nucleus, mitochondria, tail, postacrosoma lamina and neck) can be observed using ultra‐high magnification (6000×) microscopy (motile sperm organelle morphology examination (MSOME)) (Bartoov 2002). Intracytoplasmic morphologically selected sperm injection (IMSI) is a modification of ICSI that uses this technique (Bartoov 2003). This review did not evaluate IMSI, as it is the subject of another Cochrane Review (Teixeira 2013).
How the intervention might work
Each sperm selection modality utilises different characteristics of sperm structure, physiology, or function to promote selection of the most normal sperm. Selection of the most appropriate sperm for fertilisation in vitro may help improve fertilisation and the quality of embryos created, therefore there is a better chance of healthy pregnancy. Advanced sperm selection protocols aim to improve ART outcomes and may limit possible deleterious effects on offspring of using sperm with defective DNA (Aitken 2007).
Why it is important to do this review
Advanced sperm selection techniques are hypothesised to improve ART outcome through the selection of sperm with a variety of 'beneficial characteristics'. Although individual small studies have suggested that these techniques have clinical benefit (Sakkas 2013), there remains no comprehensive review of randomised controlled trials (RCTs) in this area. The current review includes only RCTs, so the results can better guide clinical practice and further research efforts.
Objectives
To evaluate the effectiveness and safety of advanced sperm selection techniques on ART outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished RCTs investigating the impact of advanced sperm selection techniques in ART were eligible for inclusion. We excluded non‐randomised studies due to high risk of bias. Cross‐over studies are inappropriate in this context and were excluded.
Types of participants
Women or couples undergoing ART.
Types of interventions
Trials comparing an advanced sperm selection technique with either another advanced sperm selection technique or an advanced sperm selection technique with standard sperm preparation techniques (e.g. semen washing, density gradient centrifugation, swim‐up techniques).
Advanced sperm selection techniques include the following.
Surface charge selection.
Sperm apoptosis.
Hyaluronic acid binding.
Sperm birefringence.
We excluded sperm selection by sperm morphology using ultra‐high magnification (IMSI), as this is the subject of another Cochrane Review (Teixeira 2013).
Types of outcome measures
Primary outcomes
Effectiveness
Live birth per woman randomly assigned.
(Live birth is defined as the delivery of a live foetus beyond 20 completed weeks' gestation.)
Adverse events
Miscarriage per woman randomly assigned.
(Miscarriage is defined as pregnancy loss at less than 20 completed weeks' gestation, or when the foetus weighs less than 500 g. Miscarriage must be confirmed by ultrasound and pregnancy test or histology and includes partial loss of multiple pregnancies.)
Secondary outcomes
Effectiveness
Clinical pregnancy per woman randomly assigned.
(Clinical pregnancy is defined as identification of a gestational sac on ultrasound at equal to or greater than seven weeks' gestation.)
Adverse events
Foetal abnormalities per woman randomly assigned.
Miscarriage, foetal abnormalities per clinical pregnancy.
Fertilisation rates, implantation rates, and outcomes related to embryo development and quality are of importance to this review and are described in the Characteristics of included studies section. These outcomes were not included in the meta‐analysis because standardised grading systems for morphology are lacking, and denominators for fertilisation and implantation rates differ.
Search methods for identification of studies
The search included no language restriction and was designed and conducted by SM, BK, and SL, in consultation with the Information Specialist from the Cochrane Gynaecology and Fertility Group. The search was conducted in June 2018.
Electronic searches
We searched the following electronic databases, trial registers, and websites.
Cochrane Gynaecology and Fertility Specialised Register; ProCite platform, searched 14 June 2018 (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO); web platform, searched 14 June 2018 (Appendix 2).
MEDLINE; Ovid platform, searched from 1946 to 14 June 2018 (Appendix 3).
Embase; Ovid platform, searched from 1980 to 14 June 2018 (Appendix 4).
PsycINFO; Ovid platform, searched from 1806 to 14 June 2018 (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL); EBSCO platform, searched from 1961 to 14 June 2018 (Appendix 6).
Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials:
Current Controlled Trials (www.controlled‐trials.com);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/Default.aspx).
Citation indexes (scientific.thomson.com/products/sci/).
Conference abstracts in the Web of Knowledge (wokinfo.com/)
Latin American and Caribbean Health Science Information Database (LILACS), for trials from the Portuguese‐ and Spanish‐speaking world (bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F)
PubMed (www.ncbi.nlm.nih.gov/pubmed/).
Open System for Information on Grey Literature in Europe (OpenSIGLE) database (opensigle.inist.fr/) and Google Scholar for grey literature.
Searching other resources
We searched the reference lists of articles retrieved by the search.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (SL and LS) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. Any disagreements as to study eligibility were resolved by discussion or by consultation with a third review author (SM). We documented the selection process using a PRISMA flow chart.
Data extraction and management
Two review authors independently extracted data from the eligible studies. Any disagreements were resolved by discussion or by consultation with a third review author. Data extracted included study characteristics and outcome data and details of methods, participants, setting, context, interventions (sperm selection protocols), outcomes, results, and publications. We attempted to contact study investigators via email to obtain additional information. No replies were received from any of the study authors.
Assessment of risk of bias in included studies
Two review authors (SL and SM) independently assessed the included studies for risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2011). This instrument assesses random sequence generation and allocation concealment (selection bias); blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective reporting; and other bias. Any disagreements were resolved by discussion or by consultation with a third review author (AY). We have provided support for our judgements and presented our conclusions in the 'Risk of bias' table, which we planned to incorporate into the interpretation of review findings by means of sensitivity analyses.
We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes or reporting outcomes in insufficient detail to allow inclusion. We sought published protocols and compared outcomes between the protocol and the final published study.
Measures of treatment effect
The extracted data were dichotomous (e.g. live‐birth rate, miscarriage rate). Using Review Manager 5 software (RevMan 2011), we entered the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). We presented 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
The primary analysis was performed per woman randomly assigned. Per‐pregnancy data were included for some miscarriage outcomes. We briefly summarised data that did not allow valid analysis (e.g. 'per‐cycle' data), but did not meta‐analyse these data. If studies reported only per‐cycle data, we attempted to contact the study authors to obtain 'per‐woman randomised' data.
We counted multiple live births (e.g. twins, triplets) as a single live‐birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis to the greatest degree possible and made attempts to obtain missing data from the original trial authors. When the data could not be obtained, we assumed that the outcome measure (e.g. live birth, clinical pregnancy) did not occur. For other outcomes, we analysed available data. We planned to subject any imputation undertaken to sensitivity analysis (see Sensitivity analysis).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We planned to assess statistical heterogeneity using the I2 statistic, with an I2 measurement greater than 50% indicating moderate heterogeneity and an I2 greater than 60% representing substantial heterogeneity (Higgins 2003; Higgins 2008). If substantial heterogeneity was apparent, we planned to explore possible explanations for it using a sensitivity analysis (see Sensitivity analysis) and to consider subgroup analyses. We planned to take any statistical heterogeneity into account when interpreting the results, especially if any variation in the direction of effect was noted.
Assessment of reporting biases
Reporting bias was minimised by ensuring a comprehensive search for eligible studies. We planned that if 10 or more studies were included in an analysis, we would use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
Where studies were sufficiently similar, we combined data using a fixed‐effect model for the following comparisons.
ICSI versus advanced sperm selection technique, stratified by individual sperm selection technique (refer to Description of the intervention for details).
Advanced sperm selection technique versus another advanced sperm selection technique.
We displayed an increase in the risk of a particular outcome graphically, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse effects), in the meta‐analysis to the right of the centre line and a decrease in the risk of a particular outcome to the left of the centre line.
We planned to calculate number needed to treat for an additional beneficial outcome (NNTB) if we identified significant findings.
Subgroup analysis and investigation of heterogeneity
We planned that if sufficient data were available, we would conduct subgroup analyses to identify separate evidence within the following subgroups.
Sperm morphology: when the Kruger score is equal to or less than 4%.
Increased DNA fragmentation index (according to the study cut‐off).
Surgically retrieved sperm.
Female participants over 38 years of age.
We did not ultimately perform subgroup analysis once data were extracted and reviewed.
Sensitivity analysis
We planned to conduct sensitivity analyses for primary outcome measures to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses would include consideration of whether the review conclusions would have differed if:
eligibility were restricted to studies without high risk of bias;
a random‐effects model had been adopted; or
alternative imputation strategies had been implemented.
A sensitivity analysis was not performed.
Overall quality of the body of evidence: 'Summary of findings' table
We generated a 'Summary of findings' table using GRADEpro GDT software. This table evaluates the overall quality of the body of evidence for the main review outcomes (live birth, clinical pregnancy, miscarriage) using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias). We justified, documented, and incorporated into the reporting of results for each outcome our judgements about the quality of evidence (high, moderate, low, or very low).
Results
Description of studies
Results of the search
The search strategy for the initial review identified 1007 studies. Thirty studies were potentially eligible and were retrieved in full text. Following publication of our protocol, a Cochrane Review was published regarding sperm selection by sperm morphology under ultra‐high magnification (Teixeira 2013). After discussion with the Cochrane Gynaecology and Fertility Group, we amended the scope of our review to exclude the use of ultra‐high magnification for sperm selection. Two studies met the inclusion criteria for the original review (Parmegiani 2012; Worrilow 2013), both of which evaluated hyaluronic acid binding for ICSI. We excluded 17 studies.
The 2019 update included a further 416 abstracts from a search date limited from 1 January 2014 until 14 June 2018; one study initially classified as ongoing reached completion in January 2019 and was thus included. After duplicates were removed, 332 remained and 21 full‐text articles were assessed. We excluded seven studies, identified eight ongoing studies, and included six studies in the review (Esfahani 2016; Majumdar 2013; Miller 2019; Romany 2014; Troya 2015; Ziarati 2018). A total of eight trials were thus included in the review (see PRISMA flow chart in Figure 1). We identified no suitable studies regarding sperm selection by birefringence. See Characteristics of included studies and Characteristics of excluded studies.
1.

PRISMA study flow diagram.
Included studies
Study design and setting
We included eight parallel‐design RCTs in the review (Esfahani 2016; Majumdar 2013; Miller 2019; Parmegiani 2012; Romany 2014; Troya 2015; Worrilow 2013; Ziarati 2018). Six were single‐centre studies conducted in Italy, India, Iran (two studies), Peru, and Spain, and the other two were a multicentre studies performed at 10 IVF units in the USA, Worrilow 2013, and 16 IVF units in the UK (Miller 2019). We identified two further conference abstracts that contained data from the above two trials, which we have listed as secondary references.
Participants
Parmegiani 2012 included 49 women in the HA‐ICSI group and 50 in the SpermSlow group. No study arm received standard ICSI only. Couples were included if the woman was ≤ 41 years of age; ICSI treatment was to be utilised; total sperm number was ≥ 1 million; and sperm motility was ≥ 5%. Couples using sperm collected surgically or with severe oligoasthenoteratozoospermia were excluded.
Worrilow 2013 included 240 women in the intervention group (HA‐ICSI) and 242 in the control group (standard ICSI). Couples were included if they were receiving ICSI as part of their ART treatment. Participants were excluded if the woman was > 40 years old or if testicular sperm was used. Participants were divided into cohorts on the basis of the proportion of sperm bound to hyaluronan in the unprocessed sample. Participants were further excluded if hyaluronan binding was < 2%. Participants were divided into those with hyaluronan‐bound sperm between 2% and 65% or > 65%, and then were further divided into study groups (intervention or control).
Esfahani 2016 included 102 women in the group with sperm selection by Zeta potential and 101 in the ICSI control group. Both groups utilised density gradient centrifugation. Couples were included if the female partner was below 40 years of age with adequate follicle count, good oocyte quality, and endometrial thickness below 8 mm, and the male partner had at least one semen parameter below WHO 2010 criteria.
Majumdar 2013 included 71 women in the HA‐ICSI group and 80 in the standard ICSI group. Couples were included if they had unexplained infertility and were undergoing their first ICSI cycle with normal semen parameters; age < 39; no uterine abnormalities, hydrosalpinx, moderate/severe endometriosis; and at least 4 oocytes retrieved.
Miller 2019 included 1381 women in the HA‐ICSI group and 1371 women in the ICSI control group. Women were included if age 18 to 43, body mass index (BMI) 19 to 35 kg/m2, follicle‐stimulating hormone (FSH) 3 to 20 mIU/mL or anti‐Müllerian hormone (AMH) at least 1.5 pmol/L. Men were included if they had not had a vasovasostomy or been treated for cancer and had been abstinent for at least three days.
Romany 2014 included 138 women in the MACS group and 125 in the ICSI control group. Females were included if 30 to 45 years of age, BMI < 30 kg/m2, first ICSI cycle, absence of uterine pathology, and no history of recurrent miscarriage. All men enrolled in the study presented more than 10% of motile sperm in raw sperm and had more than 1 million motile spermatozoa per ejaculate after swim‐up. An altered apoptotic profile and increased DNA fragmentation were not included as selection criteria.
Troya 2015 included 47 women in the HA‐ICSI group, 33 women in the MACS group, and 55 women in the ICSI control group. Patients were included if there were normal semen parameters and were excluded on the basis of a history of endometriosis.
Ziarati 2018 included 29 women in the MACS group and 33 in the conventional ICSI group. Both groups used density gradient centrifugation. Couples were included if there was male factor infertility and at least two semen parameters below WHO 2010 criteria. Exclusion criteria were evidence of seminal infection, history of crypto‐orchidism, autoantibodies, orchitis, systemic or endocrine diseases. Females were excluded if they were over 42 years of age or had fewer than six matured oocytes or poor‐quality oocyte.
Interventions
One study compared HA‐ICSI versus SpermSlow.
Four studies compared HA‐ICSI versus standard ICSI.
Three studies compared MACS versus standard ICSI.
One study compared Zeta potential selection versus standard ICSI.
One study compared HA‐ICSI versus MACS.
Outcomes
Six studies reported live birth.
Six studies reported miscarriage rate.
Seven studies reported clinical pregnancy rate.
No studies reported on foetal anomalies.
Excluded studies
We excluded 24 studies from the review for the following reasons.
Eight studies were not RCTs (Berkovitz 2006; Casciani 2014; Charehjooy 2014; Fleming 2007; Ghosh 2007; Parmegiani 2010b; San Carchenilla 2013; Stimpfel 2017).
Three studies were pseudo‐randomised (Gianaroli 2008; Gianaroli 2010; Jin 2015).
Eleven studies did not analyse a relevant intervention (Antinori 2008; Balaban 2011; Blanchard 2010; Figueira 2011; Kim 2014; Knez 2011; Knez 2012; Mahmoud 2011; Setti 2011; Setti 2012a; Setti 2012b).
One study analysed participants per treatment randomly assigned (Parmegiani 2010a), and despite attempts to contact the study investigators, we were unable to obtain 'per‐woman' data.
One study did not evaluate a relevant outcome (Liu 2017).
We excluded nine studies pertaining to IMSI, as this intervention is the subject of a separate Cochrane Review (Teixeira 2013).
Risk of bias in included studies
For details see Characteristics of included studies; Figure 2; Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence allocation
Five studies utilising computer‐generated randomisation were at low risk of bias related to sequence generation (Majumdar 2013; Miller 2019; Romany 2014; Worrilow 2013; Ziarati 2018). Two studies were at unclear risk of bias, as the method of random sequence generation was not reported (Parmegiani 2012; Troya 2015). One study was at high risk of bias related to random sequence generation as it utilised block randomisation (Esfahani 2016).
Allocation concealment
Four studies were at low risk of bias as the investigators performing randomisation had no involvement in the trial (Miller 2019; Romany 2014; Troya 2015; Worrilow 2013), whilst information was insufficient to permit a judgement for the remaining four trials (Esfahani 2016; Majumdar 2013; Parmegiani 2012; Ziarati 2018).
Blinding
Blinding ‐ performance bias
In four studies participants and personnel were blinded (Esfahani 2016; Romany 2014; Troya 2015; Worrilow 2013); in two studies this was unclear (Majumdar 2013; Ziarati 2018); and two studies were high risk of bias for this domain (Miller 2019; Parmegiani 2012).
Blinding ‐ detection bias
In four studies outcome assessors were blinded (Esfahani 2016; Miller 2019; Romany 2014; Troya 2015), whilst in the other four studies this was unclear (Majumdar 2013; Parmegiani 2012; Worrilow 2013; Ziarati 2018). However, lack of blinding of outcome assessment is unlikely to affect any of the outcome measures.
Incomplete outcome data
In one study the risk of attrition bias was high, as it could not be determined to which study group participants with incomplete data belonged (Worrilow 2013). Data were incomplete for 4 out of 482 participants. We assessed five other studies as at high risk of attrition bias due to loss of follow‐up and postrandomisation exclusion (Esfahani 2016; Majumdar 2013; Romany 2014; Troya 2015; Ziarati 2018). We deemed the remaining two studies as at low risk of bias for this domain as all data were analysed by intention‐to‐treat (Miller 2019; Parmegiani 2012).
Selective reporting
We considered one study to be at high risk of reporting bias as data were not available for all outcome measures (Worrilow 2013). We assessed the remaining studies as at low risk of reporting bias (Esfahani 2016; Majumdar 2013; Miller 2019; Parmegiani 2012; Romany 2014; Troya 2015; Ziarati 2018). We found no evidence to suggest that specific outcomes were reported on the basis of statistical significance.
Other potential sources of bias
One study was potentially biased, as it was stopped prematurely due to financial constraints and a slower‐than‐expected recruitment time (Worrilow 2013).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
See: Table 1 (Conventional sperm selection (ICSI) versus hyaluronic acid‐selected sperm (HA‐ICSI); Table 2 (HA‐ICSI versus viscous medium containing HA (SpermSlow); Table 3 (Magnetic‐activated cell sorting (MACS) versus ICSI); Table 4 (Zeta sperm selection versus ICSI); Table 5 (MACS versus HA‐ICSI).
1. HA‐ICSI versus ICSI
Primary outcomes
1.1 Live birth per woman randomly assigned (effectiveness)
Two studies reported live birth (Majumdar 2013; Miller 2019). There may be little or no difference between interventions for this outcome. If ICSI leads to a 25% live‐birth rate, HA‐ICSI leads to a live‐birth rate ranging from 24% to 31% (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.97 to 1.23, 2 RCTs, 2903 women, I2 = 0%, low‐quality evidence; Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1 Hyaluronic acid sperm selection (HA‐ICSI) versus ICSI, Outcome 1 Live birth per woman randomly assigned.
4.

Forest plot of comparison: 1 Hyaluronic acid sperm selection (HA‐ICSI) versus ICSI, outcome: 1.1 Live birth per woman randomly assigned.
1.2 Miscarriage per woman randomly assigned (adverse event)
Three included studies reported on miscarriage that were suitable for meta‐analysis (Majumdar 2013; Miller 2019; Troya 2015). There was evidence of a decreased rate of miscarriage in the intervention group. If ICSI leads to a 7% miscarriage rate per woman, HA‐ICSI leads to a rate ranging from 3% to 6% (RR 0.61, 95% CI 0.45 to 0.83, 3 RCTs, 3005 women, I2 = 0%, low‐quality evidence; Analysis 1.2).
1.2. Analysis.
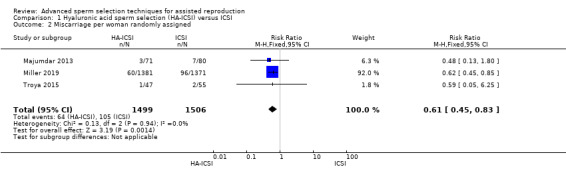
Comparison 1 Hyaluronic acid sperm selection (HA‐ICSI) versus ICSI, Outcome 2 Miscarriage per woman randomly assigned.
Pregnancy loss rate was also reported in one study (Worrilow 2013), however the data were not suitable for meta‐analysis. From the data provided, we were unable to determine to which treatment group a miscarriage pertained.
Secondary outcomes
1.3 Miscarriage per clinical pregnancy (adverse event)
Three included studies reported on miscarriage per clinical pregnancy that were suitable for meta‐analysis (Majumdar 2013; Miller 2019; Troya 2015). There was evidence of a decreased rate of miscarriage in the intervention group. If ICSI leads to a 20% miscarriage rate per clinical pregnancy, HA‐ICSI leads to a rate ranging from 9% to 16% (RR 0.62, 95% CI 0.46 to 0.82, 3 RCTs, 1065 women, I2 = 0%, low‐quality evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1 Hyaluronic acid sperm selection (HA‐ICSI) versus ICSI, Outcome 3 Miscarriage per clinical pregnancy.
1.4 Clinical pregnancy per woman randomly assigned (effectiveness)
Four included studies reported on clinical pregnancy (Majumdar 2013; Miller 2019; Troya 2015; Worrilow 2013). There may be little or no difference between interventions for this outcome. If ICSI leads to a 37% clinical pregnancy rate, HA‐ICSI leads to a clinical pregnancy rate ranging from 34% to 40% (RR 1.00, 95% CI 0.92 to 1.09, 4 RCTs, 3492 women, I2 = 0%, low‐quality evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1 Hyaluronic acid sperm selection (HA‐ICSI) versus ICSI, Outcome 4 Clinical pregnancy per woman randomly assigned.
Foetal abnormality (adverse event)
None of the included studies reported on foetal abnormality.
2. HA‐ICSI versus viscous medium containing HA (SpermSlow)
Primary outcomes
2.1 Live birth per woman randomly assigned (effectiveness)
One included study reported on live birth (Parmegiani 2012). We are uncertain of the effect of the intervention on live‐birth rates. If SpermSlow leads to a 30% live‐birth rate, HA‐ICSI leads to a live‐birth rate ranging from 19% to 60% (RR 1.13, 95% CI 0.64 to 2.01, 1 RCT, 100 women, very low‐quality evidence; Analysis 2.1; Figure 5).
2.1. Analysis.

Comparison 2 HA‐ICSI versus viscous medium containing HA (SpermSlow), Outcome 1 Live birth per woman randomly assigned.
5.

Forest plot of comparison: 2 Hyaluronic acid sperm selection (HA‐ICSI) versus viscous medium containing HA (SpermSlow), outcome: 2.1 Live birth per woman randomly assigned.
2.2 Miscarriage per woman randomly assigned (adverse event)
One included study reported on miscarriage (Parmegiani 2012). We are uncertain of the effect of the interventions on miscarriage rates. If SpermSlow leads to a 10% miscarriage rate per woman, HA‐ICSI leads to a rate ranging from 2% to 28% (RR 0.80, 95% CI 0.23 to 2.81, 1 RCT, 100 women, very low‐quality evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2 HA‐ICSI versus viscous medium containing HA (SpermSlow), Outcome 2 Miscarriage per woman randomly assigned.
Secondary outcomes
2.3 Miscarriage per clinical pregnancy (adverse event)
We are uncertain of the effect of the interventions on miscarriage per clinical pregnancy. If SpermSlow leads to a 25% miscarriage rate per clinical pregnancy, HA‐ICSI leads to a rate ranging from 6% to 61% (RR 0.76, 95% CI 0.24 to 2.44, 1 RCT, 41 women, very low‐quality evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2 HA‐ICSI versus viscous medium containing HA (SpermSlow), Outcome 3 Miscarriage per clinical pregnancy.
2.4 Clinical pregnancy per woman randomly assigned (effectiveness)
One included study reported on clinical pregnancy (Parmegiani 2012). We are uncertain of the effect of the interventions on clinical pregnancy rates. If SpermSlow leads to a 40% clinical pregnancy rate, HA‐ICSI leads to a rate ranging from 26% to 67% (RR 1.05, 95% CI 0.66 to 1.68, 1 RCT, 100 women, very low‐quality evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2 HA‐ICSI versus viscous medium containing HA (SpermSlow), Outcome 4 Clinical pregnancy per woman randomly assigned.
Foetal abnormality (adverse event)
None of the included studies reported on foetal abnormality.
3. MACS versus ICSI
Primary outcomes
3.1 Live birth per woman randomly assigned (effectiveness)
One included study reported on live birth (Ziarati 2018). The evidence was insufficient to establish whether there is a difference between interventions for this outcome. If ICSI leads to a 21% live‐birth rate, MACS leads to a live‐birth rate ranging from 19% to 91% (RR 1.95, 95% CI 0.89 to 4.29, 1 RCT, 62 women, very low‐quality evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3 Magnetic‐activated cell sorting (MACS) versus ICSI, Outcome 1 Live birth per woman randomly assigned.
3.2 Miscarriage per woman randomly assigned (adverse event)
Two included studies reported on miscarriage (Troya 2015; Ziarati 2018). We are uncertain of the effect of the interventions on miscarriage. If ICSI leads to a 3% miscarriage rate, MACS leads to a rate ranging from 1% to 19% (RR 0.95, 95% CI 0.16 to 5.63, 2 RCTs, 150 women, I2 = 0%, very low‐quality evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3 Magnetic‐activated cell sorting (MACS) versus ICSI, Outcome 2 Miscarriage per woman randomly assigned.
Secondary outcomes
3.3 Miscarriage per clinical pregnancy (adverse event)
We are uncertain of the effect of the interventions on miscarriage per clinical pregnancy. If ICSI leads to a 13% miscarriage rate per clinical pregnancy, MACS leads to a rate ranging from 1% to 37% (RR 0.51, 95% CI 0.09 to 2.82, 2 RCTs, 53 women, I2 = 0%, very low‐quality evidence; Analysis 3.3).
3.3. Analysis.

Comparison 3 Magnetic‐activated cell sorting (MACS) versus ICSI, Outcome 3 Miscarriage per clinical pregnancy.
3.4 Clinical pregnancy per woman randomly assigned (effectiveness)
Three included studies reported on clinical pregnancy (Romany 2014; Troya 2015; Ziarati 2018). We are uncertain of the effect of the interventions on clinical pregnancy. If ICSI leads to a 41% clinical pregnancy rate, MACS leads to a rate ranging from 34% to 54% (RR 1.05, 95% CI 0.84 to 1.31, 3 RCTs, 413 women, I2 = 81%, very low‐quality evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3 Magnetic‐activated cell sorting (MACS) versus ICSI, Outcome 4 Clinical pregnancy per woman randomly assigned.
Foetal abnormality (adverse event)
None of the included studies reported on foetal abnormality.
4. Zeta sperm selection versus ICSI
Primary outcomes
4.1 Live birth per woman randomly assigned (effectiveness)
One included study reported on live birth (Esfahani 2016). We are uncertain if Zeta sperm selection improves live‐birth rates. If ICSI leads to a 12% live‐birth rate, Zeta sperm selection leads to a rate ranging from 16% to 54% (RR 2.48, 95% CI 1.34 to 4.56, 1 RCT, 203 women, very low‐quality evidence; Analysis 4.1; Figure 6).
4.1. Analysis.

Comparison 4 Zeta sperm selection versus ICSI, Outcome 1 Live birth per woman randomly assigned.
6.

Forest plot of comparison: 4 Zeta sperm selection versus ICSI, outcome: 4.1 Live birth per woman randomly assigned.
4.2 Miscarriage per woman randomly assigned (adverse event)
One included studiy reported on miscarriage (Esfahani 2016). We are uncertain of the effect of the interventions on miscarriage. If ICSI leads to a 4% miscarriage rate, Zeta sperm selection leads to a rate ranging from 1% to 12% (RR 0.73, 95% CI 0.16 to 3.37, 1 RCT, 203 women, very low‐quality evidence; Analysis 4.2).
4.2. Analysis.

Comparison 4 Zeta sperm selection versus ICSI, Outcome 2 Miscarriage per woman randomly assigned.
Secondary outcomes
4.3 Miscarriage per clinical pregnancy (adverse event)
We are uncertain of the effect of the interventions on miscarriage per clinical pregnancy. If ICSI leads to an 18% miscarriage rate per clinical pregnancy, Zeta sperm selection leads to a rate ranging from 2% to 31% (RR 0.41, 95% CI 0.10 to 1.68, 1 RCT, 62 women, very low‐quality evidence; Analysis 4.3).
4.3. Analysis.

Comparison 4 Zeta sperm selection versus ICSI, Outcome 3 Miscarriage per clinical pregnancy.
4.4 Clinical pregnancy per woman randomly assigned (effectiveness)
One included study reported on clinical pregnancy (Esfahani 2016). We are uncertain if Zeta sperm selection improves clinical pregnancy rate. If ICSI leads to a 24% clinical pregnancy rate, Zeta sperm selection leads to a rate ranging from 29% to 65% (RR 1.82, 95% CI 1.20 to 2.75, 1 RCT, 203 women, very low‐quality evidence; Analysis 4.4).
4.4. Analysis.

Comparison 4 Zeta sperm selection versus ICSI, Outcome 4 Clinical pregnancy per woman randomly assigned.
Foetal abnormality (adverse event)
None of the included studies reported on foetal abnormality.
5. MACS versus HA‐ICSI
Primary outcomes
Live birth per woman randomly assigned (effectiveness)
None of the included studies reported on live birth.
5.1 Miscarriage per woman randomly assigned (adverse event)
One included study reported on miscarriage (Troya 2015). We are uncertain whether there is a difference between interventions for this outcome. If HA‐ICSI leads to a 2% miscarriage rate, MACS leads to a rate ranging from 0% to 50% (RR 1.52, 95% CI 0.10 to 23.35, 1 RCT, 78 women, very low‐quality evidence; Analysis 5.1).
5.1. Analysis.

Comparison 5 Magnetic‐activated cell sorting (MACS) versus HA‐ICSI, Outcome 1 Miscarriage per woman randomly assigned.
Secondary outcomes
5.2 Miscarriage per clinical pregnancy (adverse event)
We are uncertain whether there is a difference between interventions for this outcome. If HA‐ICSI leads to a 5% miscarriage rate per clinical pregnancy, MACS leads to a rate ranging from 0% to 82% (RR 1.06, 95% CI 0.07 to 15.64, 1 RCT, 37 women, very low‐quality evidence; Analysis 5.2).
5.2. Analysis.

Comparison 5 Magnetic‐activated cell sorting (MACS) versus HA‐ICSI, Outcome 2 Miscarriage per clinical pregnancy.
5.3 Clinical pregnancy per woman randomly assigned (effectiveness)
One included study reported on clinical pregnancy (Troya 2015). We are uncertain whether there is a difference between interventions for this outcome. If HA‐ICSI leads to a 40% clinical pregnancy rate, MACS leads to a rate ranging from 37% to 92%. (RR 1.44, 95% CI 0.91 to 2.27, 1 RCT, 78 women, very low‐quality evidence; Analysis 5.3).
5.3. Analysis.

Comparison 5 Magnetic‐activated cell sorting (MACS) versus HA‐ICSI, Outcome 3 Clinical pregnancy per woman randomly assigned.
Foetal abnormality (adverse event)
None of the included studies reported on foetal abnormality.
Secondary analyses
Data were insufficient to conduct any subgroup analyses or to construct a funnel plot to assess reporting bias. We did not perform sensitivity analyses since no imputations were required, and for the main outcome of live birth I2 = 0, so a random‐effects model would not have altered the effect of interventions.
Discussion
Summary of main results
Six included trials reported on live‐birth rate, and evidence indicated there may be little or no difference in effectiveness between HA‐ICSI and ICSI. Due to the low quality of the evidence we are uncertain about the results for the comparisons HA‐ICSI versus SpermSlow and MACS versus ICSI. Very low‐quality evidence from a single study showed that there may be an increased rate of live birth with the use of Zeta sperm selection compared to ICSI.
Six included studies reported on miscarriage. We found low‐quality evidence that HA‐ICSI may be associated with a decreased risk of miscarriage when compared to conventional ICSI per woman randomised and per clinical pregnancy. Due to the very low quality of the evidence, we are uncertain whether there is a difference for these outcomes for HA‐ICSI versus SpermSlow, MACS versus ICSI, MACS versus HA‐ICSI, and Zeta sperm selection versus ICSI.
All eight included trials reported on clinical pregnancy. Very low‐quality evidence from one trial showed that Zeta sperm selection may be associated with a higher likelihood of clinical pregnancy. Due to the very low quality of the evidence, we are uncertain whether there is a difference in clinical pregnancy rates for HA‐ICSI versus standard ICSI, HA‐ICSI versus SpermSlow, MACS versus ICSI, and MACS versus HA‐ICSI.
None of the included studies reported on foetal abnormality outcomes.
No suitable studies were identified that would have permitted evaluation of the effect of sperm selected by sperm birefringence. None of the included studies reported a subgroup suitable for analysis. For details see Table 1; Table 2; Table 3; Table 4; and Table 5.
Assisted reproductive technologies have drastically modified the fertility potential for countless couples since their introduction. As these technologies develop, there appear to be decreasing gains in improving outcomes. Although there exists a good theoretical basis behind the sperm selection techniques analysed here, we did not find a strategy that demonstrates a sizeable improvement to the chances of successful live birth for infertility patients. As such, we cannot recommend drastic change to the counselling or management of such couples at present. It is possible this situation may change as more evidence comes to light. The evidence does suggest that sperm selected by hyaluronic acid binding may reduce miscarriage but may have little or no effect on live birth or clinical pregnancy, however we are uncertain of the effects of the other technologies studied on these outcomes.
Even though we included a very large trial comparing HA‐ICSI with ICSI that found a reduction in miscarriage with HA‐ICSI, the trial was not powered to evaluate the outcome of miscarriage (Miller 2019). As miscarriage is a much less common outcome than live birth, the confidence interval for the absolute risk difference was smaller for miscarriage than for live birth. This might explain why the absolute improvement in live birth was not significant, even though it was similar to the absolute reduction in miscarriage. An absolute risk difference of around 2.5% has a greater effect on outcomes with a low prevalence such as miscarriage than on outcomes with a much higher prevalence like live birth (Miller 2019).
Overall completeness and applicability of evidence
The objectives of this review were addressed by the included studies, which analysed relevant participants and outcomes and most of the investigations of potential relevance to clinicians. There were controlled data available to address the primary outcome measure of live birth per allocated couple, and for the secondary outcomes of clinical pregnancy and miscarriage, for some of the advanced sperm selection techniques described. Data on other important clinical outcomes such as foetal abnormalities were lacking, and no studies on the sperm selection technique of birefringence were found.
Quality of the evidence
Robust conclusions on these techniques to augment successful assisted reproduction technologies are not possible given the quality limitations of the evidence. We assessed the quality of the evidence for the reported outcomes as low or very low. The main limitations were poor reporting of study methods, attrition bias, potential performance bias, and imprecision due to low event rates and low participant numbers. Regarding the outcomes of live birth and clinical pregnancy, the 95% confidence intervals could be compatible with benefit, harm, or with no effect for all interventions except Zeta sperm selection. Zeta sperm selection may improve these outcomes, yet the evidence was of very low quality and data were derived from a single trial. We assessed the evidence for miscarriage as of low quality for the comparison HA‐ICSI versus ICSI, but the evidence did show a possible benefit for miscarriage. For all other interventions, the 95% confidence intervals were compatible with substantial benefit or harm from the intervention, or with no effect. The available trial data for some interventions were sparse in general. We were unable to assess the risk of reporting bias.
Potential biases in the review process
We identified no potential biases in the review process. There always exists the small possibility of incomplete detection of all available RCTs pertaining to the review question which could bias the results of a systematic review. We made every effort to limit this in line with prescribed Cochrane search strategies.
Agreements and disagreements with other studies or reviews
Two other systematic reviews have addressed effects of advanced sperm selection on sperm quality and ART outcomes (Craciunas 2015; Said 2007). Sperm selection techniques similar to those described in this review were investigated. A total of 11 and 44 studies, respectively, were identified, but few of these studies were strictly randomised, and most were deemed unsuitable for inclusion in this review. However, the authors' conclusions were in concordance with our findings. Further clinical trials are required before advanced sperm selection techniques can be recommended in routine practice.
Authors' conclusions
Implications for practice.
The evidence suggests that sperm selected by hyaluronic acid binding may have little or no effect on live birth or clinical pregnancy but may reduce miscarriage. We are uncertain of the effect of Zeta sperm selection on live birth, clinical pregnancy, and miscarriage due principally to the very low quality of the evidence for this intervention. We are uncertain of the effect of the other selection techniques on live birth, miscarriage, or pregnancy.
Further high‐quality studies, including the awaited data from the identified ongoing studies, are required to evaluate whether any of these advanced sperm selection techniques can be recommended for use in routine practice.
Implications for research.
Suitable randomised controlled trials (RCTs) are needed to evaluate the effects of sperm selection based on sperm birefringence on live birth, clinical pregnancy, and miscarriage. There is a paucity of RCT data on congenital abnormality in pregnancies utilising advanced sperm selection techniques, which represents an avenue for future RCTs. It remains unclear whether certain patient subgroups, such as those with high sperm DNA fragmentation or other aetiologies of subfertility, might benefit from these advanced sperm selection techniques, which bears investigating. Trials should use intention‐to‐treat analysis and should report outcomes per woman randomly assigned.
What's new
| Date | Event | Description |
|---|---|---|
| 21 March 2019 | New citation required and conclusions have changed | The addition of 6 new studies has led to changes in conclusions. |
| 21 March 2019 | New search has been performed | Review updated. We included 6 new studies (Esfahani 2016; Majumdar 2013; Miller 2019; Romany 2014; Troya 2015; Ziarati 2018). |
Acknowledgements
We acknowledge the valuable help and support provided by the Cochrane Gynaecology and Fertility Group. Attempts were made to contact the authors of four of the included studies, but none of the authors replied. The authors would like to thank Emily Ford and Ysanne Hook for their contribution to the previous versions of this review. We would like to thank Harry Siristatidis, Deborah Blake, and Jack Wilkinson for their valuable peer review comments.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
ProCite platform
Searched 14 June 2018
Keywords CONTAINS "sperm preparation" or "sperm preparation techniques" or "sperm select" or "sperm selection" or "sperm selection techniques" or "sperm separation" or "sperm sorting" or "birefringent sperm" or "Magnetic Activated Sorting Selection" or "magnetic sperm selection" or "hyaluronan‐bound (HB) sperm" or "hyaluronan bound sperm" or "hyaluronic acid sperm selection" or "hyaluronic acid intracytoplasmic sperm injection" or "IMSI" or "semen preparation" or "membrane properties" or "sperm morphology" or Title CONTAINS "sperm preparation" or "sperm preparation techniques" or "sperm select" or "sperm selection" or "sperm selection techniques" or "sperm separation" or "sperm sorting" or "birefringent sperm" or "Magnetic Activated Sorting Selection" or "magnetic sperm selection" or "hyaluronan‐bound (HB) sperm" or "hyaluronan bound sperm" or "sperm morphology"
(597 hits)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 14 June 2018
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 998
#2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1913
#3 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 497
#4 embryo*: TI,AB,KY 5543
#5 (vitro fertili?ation):TI,AB,KY 2531
#6 ivf:TI,AB,KY 4228
#7 icsi:TI,AB,KY 1937
#8 (intracytoplasmic sperm injection*):TI,AB,KY 1497
#9 blastocyst*:TI,AB,KY 917
#10 infertil* or subfertil*:TI,AB,KY 6562
#11 assisted reproducti*:TI,AB,KY 1019
#12 poor responder*:TI,AB,KY 559
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 12236
#14 (sperm* adj7 selection*):TI,AB,KY 91
#15 (sperm* adj7 separat*):TI,AB,KY 29
#16 (surface charge):TI,AB,KY 17
#17 electrophore*:TI,AB,KY 1120
#18 (zeta adj2 potential):TI,AB,KY 39
#19 (magnetic cell sorting):TI,AB,KY 4
#20 (glass wool):TI,AB,KY 4
#21 (membrane matur*):TI,AB,KY 1
#22 (magnetic activated cell sort*):TI,AB,KY 22
#23 ultramorpholog*:TI,AB,KY 8
#24 (hyaluronic acid adj2 binding):TI,AB,KY 10
#25 (motile sperm* organelle):TI,AB,KY 9
#26 MSOME:TI,AB,KY 12
#27 IMSI:TI,AB,KY 47
#28 (Intracytoplasmic morphologically selected sperm injection*):TI,AB,KY 39
#29 (Raman spectroscopy):TI,AB,KY 55
#30 (scattering adj3 microscopy):TI,AB,KY 3
#31 (polarization microscopy):TI,AB,KY 16
#32 polscope:TI,AB,KY 4
#33 (sperm* adj3 apopto*):TI,AB,KY 12
#34 (nonapoptotic* adj3 sperm*):TI,AB,KY 1
#35 (sperm* adj3 prepar*):TI,AB,KY 102
#36 (semen adj2 prepar*):TI,AB,KY 28
#37 ( hyaluronan bound):TI,AB,KY 5
#38 (hyaluronic acid adj2 bound):TI,AB,KY 1
#39 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 1545
#40 #13 AND #39 228
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 14 June 2018
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (38155) 2 embryo transfer$.tw. (10590) 3 vitro fertili?ation.tw. (20916) 4 ivf‐et.tw. (2147) 5 ivf.tw. (21176) 6 icsi.tw. (7418) 7 intracytoplasmic sperm injection$.tw. (6417) 8 (blastocyst adj2 transfer$).tw. (857) 9 assisted reproduct$.tw. (12838) 10 ovulation induc$.tw. (3913) 11 (ovari$ adj2 stimulat$).tw. (6294) 12 superovulat$.tw. (3247) 13 ovarian hyperstimulation.tw. (4696) 14 COH.tw. (1555) 15 infertil$.tw. (54181) 16 subfertil$.tw. (4585) 17 (ovari$ adj2 induction).tw. (277) 18 exp Reproductive Techniques, Assisted/ (63146) 19 ART.tw. (81080) 20 or/1‐19 (199121) 21 (sperm$ adj7 selection$).tw. (1332) 22 (sperm$ adj7 separat$).tw. (1684) 23 surface charge.tw. (10360) 24 electrophore$.tw. (223565) 25 (zeta adj2 potential).tw. (13572) 26 magnetic cell sorting.tw. (537) 27 glass wool.tw. (461) 28 membrane matur$.tw. (75) 29 magnetic activated cell sort$.tw. (678) 30 ultramorpholog$.tw. (204) 31 (hyaluronic acid adj2 binding).tw. (424) 32 (sperm$ adj5 birefringence).tw. (20) 33 (sperm$ adj3 morphology).tw. (4337) 34 ultra high magnification.tw. (18) 35 motile sperm$ organelle.tw. (50) 36 MSOME.tw. (59) 37 IMSI.tw. (93) 38 Intracytoplasmic morphologically selected sperm injection$.tw. (84) 39 Raman spectroscopy.tw. (17898) 40 confocal light absorption.tw. (6) 41 (scattering adj3 microscopy).tw. (2046) 42 polarization microscopy.tw. (619) 43 polarisation microscopy.tw. (39) 44 polscope.tw. (78) 45 (sperm$ adj3 apopto$).tw. (1135) 46 zeta method.tw. (8) 47 (nonapoptotic$ adj3 sperm$).tw. (11) 48 sperm$ preparation.tw. (419) 49 (sperm$ adj3 prepar$).tw. (1491) 50 (semen adj2 prepar$).tw. (252) 51 (sperm$ adj5 chemotaxis).tw. (198) 52 hyaluronan bound.tw. (20) 53 (hyaluronic acid adj2 bound).tw. (47) 54 or/21‐53 (276014) 55 randomized controlled trial.pt. (463543) 56 controlled clinical trial.pt. (92478) 57 randomized.ab. (414968) 58 randomised.ab. (82886) 59 placebo.tw. (195123) 60 clinical trials as topic.sh. (184035) 61 randomly.ab. (292437) 62 trial.ti. (184087) 63 (crossover or cross‐over or cross over).tw. (76806) 64 or/55‐63 (1215102) 65 exp animals/ not humans.sh. (4470062) 66 64 not 65 (1119196) 67 20 and 54 and 66 (301)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 14 June 2018
1 (sperm$ adj7 selection$).tw. (1727) 2 (sperm$ adj7 separat$).tw. (1864) 3 surface charge.tw. (10527) 4 electrophore$.tw. (222804) 5 (zeta adj2 potential).tw. (16985) 6 magnetic cell sorting.tw. (902) 7 glass wool.tw. (494) 8 membrane matur$.tw. (82) 9 magnetic activated cell sort$.tw. (1112) 10 ultramorpholog$.tw. (196) 11 (hyaluronic acid adj2 binding).tw. (468) 12 (sperm$ adj5 birefringence).tw. (33) 13 (sperm$ adj3 morphology).tw. (5529) 14 ultra high magnification.tw. (48) 15 motile sperm$ organelle.tw. (109) 16 MSOME.tw. (137) 17 IMSI.tw. (250) 18 Intracytoplasmic morphologically selected sperm injection$.tw. (166) 19 Raman spectroscopy.tw. (13680) 20 confocal light absorption.tw. (5) 21 (scattering adj3 microscopy).tw. (1928) 22 polarization microscopy.tw. (614) 23 polarisation microscopy.tw. (50) 24 polscope.tw. (130) 25 (sperm$ adj3 apopto$).tw. (1440) 26 zeta method.tw. (11) 27 (nonapoptotic$ adj3 sperm$).tw. (19) 28 sperm$ preparation.tw. (577) 29 (sperm$ adj3 prepar$).tw. (1791) 30 (semen adj2 prepar$).tw. (346) 31 (sperm$ adj5 chemotaxis).tw. (216) 32 hyaluronan bound.tw. (27) 33 (hyaluronic acid adj2 bound).tw. (48) 34 or/1‐33 (276949) 35 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (60325) 36 embryo$ transfer$.tw. (18427) 37 in vitro fertili?ation.tw. (27018) 38 icsi.tw. (14182) 39 intracytoplasmic sperm injection$.tw. (8497) 40 (blastocyst adj2 transfer$).tw. (1967) 41 ivf.tw. (35592) 42 assisted reproduct$.tw. (19536) 43 ovulation induc$.tw. (5292) 44 (ovari$ adj2 stimulat$).tw. (9782) 45 superovulat$.tw. (3601) 46 ovarian hyperstimulation.tw. (6863) 47 COH.tw. (2167) 48 infertil$.tw. (74760) 49 subfertil$.tw. (6280) 50 (ovari$ adj2 induction).tw. (334) 51 exp infertility therapy/ (88510) 52 or/35‐51 (169835) 53 Clinical Trial/ (964372) 54 Randomized Controlled Trial/ (502372) 55 exp randomization/ (78538) 56 Single Blind Procedure/ (31519) 57 Double Blind Procedure/ (147719) 58 Crossover Procedure/ (55600) 59 Placebo/ (312269) 60 Randomi?ed controlled trial$.tw. (182415) 61 Rct.tw. (28691) 62 random allocation.tw. (1787) 63 randomly allocated.tw. (29761) 64 allocated randomly.tw. (2322) 65 (allocated adj2 random).tw. (797) 66 Single blind$.tw. (20921) 67 Double blind$.tw. (182676) 68 ((treble or triple) adj blind$).tw. (786) 69 placebo$.tw. (268948) 70 prospective study/ (452730) 71 or/53‐70 (1910555) 72 case study/ (54903) 73 case report.tw. (355252) 74 abstract report/ or letter/ (1039688) 75 or/72‐74 (1441148) 76 71 not 75 (1862227) 77 34 and 52 and 76 (674)
Appendix 5. PsycINFO search strategy
Ovid platform
Searched from 1806 to 14 June 2018
1 (sperm$ adj7 selection$).tw. (128) 2 (sperm$ adj7 separat$).tw. (27) 3 surface charge.tw. (19) 4 electrophore$.tw. (1162) 5 (zeta adj2 potential).tw. (13) 6 magnetic cell sorting.tw. (5) 7 glass wool.tw. (4) 8 membrane matur$.tw. (2) 9 magnetic activated cell sort$.tw. (5) 10 ultramorpholog$.tw. (2) 11 (hyaluronic acid adj2 binding).tw. (0) 12 (sperm$ adj5 birefringence).tw. (0) 13 (sperm$ adj3 morphology).tw. (47) 14 ultra high magnification.tw. (1) 15 motile sperm$ organelle.tw. (0) 16 MSOME.tw. (0) 17 IMSI.tw. (3) 18 Intracytoplasmic morphologically selected sperm injection$.tw. (0) 19 Raman spectroscopy.tw. (20) 20 confocal light absorption.tw. (0) 21 (scattering adj3 microscopy).tw. (10) 22 polarization microscopy.tw. (1) 23 polarisation microscopy.tw. (0) 24 polscope.tw. (0) 25 (sperm$ adj3 apopto$).tw. (3) 26 zeta method.tw. (0) 27 (nonapoptotic$ adj3 sperm$).tw. (0) 28 sperm$ preparation.tw. (0) 29 (sperm$ adj3 prepar$).tw. (7) 30 (semen adj2 prepar$).tw. (1) 31 (sperm$ adj5 chemotaxis).tw. (6) 32 hyaluronan bound.tw. (1) 33 (hyaluronic acid adj2 bound).tw. (0) 34 or/1‐33 (1452) 35 exp reproductive technology/ (1703) 36 in vitro fertili?ation.tw. (695) 37 ivf‐et.tw. (17) 38 (ivf or et).tw. (128313) 39 icsi.tw. (68) 40 intracytoplasmic sperm injection$.tw. (52) 41 (blastocyst adj2 transfer$).tw. (4) 42 assisted reproduct$.tw. (862) 43 ovulation induc$.tw. (28) 44 (ovari$ adj2 stimulat$).tw. (56) 45 ovarian hyperstimulation.tw. (11) 46 COH.tw. (108) 47 superovulat$.tw. (6) 48 infertil$.tw. (3238) 49 subfertil$.tw. (83) 50 (ovari$ adj2 induction).tw. (7) 51 or/35‐50 (132618) 52 random.tw. (52982) 53 control.tw. (408738) 54 double‐blind.tw. (21508) 55 clinical trials/ (10937) 56 placebo/ (5103) 57 exp Treatment/ (713120) 58 or/52‐57 (1110496) 59 34 and 51 and 58 (9)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 to 14 June 2018
| # | Query | Results |
| S50 | S37 AND S49 | 15 |
| S49 | S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 | 1,237,935 |
| S48 | TX allocat* random* | 8,756 |
| S47 | (MH "Quantitative Studies") | 19,721 |
| S46 | (MH "Placebos") | 10,785 |
| S45 | TX placebo* | 51,158 |
| S44 | TX random* allocat* | 8,756 |
| S43 | (MH "Random Assignment") | 48,536 |
| S42 | TX randomi* control* trial* | 149,491 |
| S41 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 960,215 |
| S40 | TX clinic* n1 trial* | 224,902 |
| S39 | PT Clinical trial | 86,339 |
| S38 | (MH "Clinical Trials+") | 240,945 |
| S37 | S8 AND S36 | 57 |
| S36 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 | 8,251 |
| S35 | TX (hyaluronic acid N2 bound) | 3 |
| S34 | TX (hyaluronan bound) | 2 |
| S33 | TX zeta method | 9 |
| S32 | TX (sperm* N3 apopto*) | 42 |
| S31 | TX polscope | 2 |
| S30 | TX (polarisation microscopy) | 27 |
| S29 | TX (polarization microscopy) | 27 |
| S28 | TX (scattering N3 microscopy) | 35 |
| S27 | TX confocal light absorption | 1 |
| S26 | TX Raman spectroscopy | 238 |
| S25 | TX MSOME | 5 |
| S24 | TX (motile sperm* organelle) | 4 |
| S23 | TX (ultra high magnification) | 3 |
| S22 | TX (sperm* N3 morphology) | 169 |
| S21 | TX (sperm* N5 birefringence) | 7 |
| S20 | TX (sperm* N5 birefringence) | 7 |
| S19 | TX (hyaluronic acid N2 binding) | 13 |
| S18 | TX ultramorpholog* | 9 |
| S17 | TX (magnetic activated cell sort*) | 41 |
| S16 | TX (membrane matur*) | 36 |
| S15 | TX (glass wool) | 10 |
| S14 | TX (magnetic cell sorting) | 67 |
| S13 | TX(zeta N2 potential) | 124 |
| S12 | TX electrophore* | 7,379 |
| S11 | TX (surface charge) | 98 |
| S10 | TX(sperm* N3 separat*) | 11 |
| S9 | TX(sperm* N3 selection*) | 35 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 5,521 |
| S7 | TX embryo* N3 transfer* | 1,231 |
| S6 | TX ovar* N3 hyperstimulat* | 474 |
| S5 | TX ovari* N3 stimulat* | 442 |
| S4 | TX IVF or TX ICSI | 2,317 |
| S3 | (MM "Fertilization in Vitro") | 1,940 |
| S2 | TX vitro fertilization | 4,077 |
| S1 | TX vitro fertilisation | 4,077 |
Data and analyses
Comparison 1. Hyaluronic acid sperm selection (HA‐ICSI) versus ICSI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per woman randomly assigned | 2 | 2903 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.23] |
| 2 Miscarriage per woman randomly assigned | 3 | 3005 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.45, 0.83] |
| 3 Miscarriage per clinical pregnancy | 3 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.82] |
| 4 Clinical pregnancy per woman randomly assigned | 4 | 3492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
Comparison 2. HA‐ICSI versus viscous medium containing HA (SpermSlow).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per woman randomly assigned | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.64, 2.01] |
| 2 Miscarriage per woman randomly assigned | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.81] |
| 3 Miscarriage per clinical pregnancy | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.24, 2.44] |
| 4 Clinical pregnancy per woman randomly assigned | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.68] |
Comparison 3. Magnetic‐activated cell sorting (MACS) versus ICSI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per woman randomly assigned | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.89, 4.29] |
| 2 Miscarriage per woman randomly assigned | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.16, 5.63] |
| 3 Miscarriage per clinical pregnancy | 2 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.09, 2.82] |
| 4 Clinical pregnancy per woman randomly assigned | 3 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.31] |
Comparison 4. Zeta sperm selection versus ICSI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per woman randomly assigned | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.34, 4.56] |
| 2 Miscarriage per woman randomly assigned | 1 | 203 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.16, 3.37] |
| 3 Miscarriage per clinical pregnancy | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.10, 1.68] |
| 4 Clinical pregnancy per woman randomly assigned | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.20, 2.75] |
Comparison 5. Magnetic‐activated cell sorting (MACS) versus HA‐ICSI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage per woman randomly assigned | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.10, 23.35] |
| 2 Miscarriage per clinical pregnancy | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 15.64] |
| 3 Clinical pregnancy per woman randomly assigned | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.91, 2.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Esfahani 2016.
| Methods | A total of 203 ICSI cycles studied, 101 cycles were allocated to density gradient centrifugation (DGC)/Zeta, and the remaining 102 were included in the DGC group in this prospective study. | |
| Participants | Women below 40 years who had an adequate number of follicles and at last 1 abnormal semen parameter of their partner | |
| Interventions | Sperm selection based on combined density gradient and the Zeta method (according to the modified protocol of Chan and colleagues) | |
| Outcomes | Clinical pregnancy, miscarriage, live birth | |
| Notes | Funding source: None stated. Declaration of interest: None of the authors has any conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The verified couples were randomly allocated using block design. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided; allocation by "one of the staff who was unaware of the experimental study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reports double‐blind; the embryologist who performed the ICSI procedure was unaware of the individual allocation groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Embryo quality was assessed by a certain staff who was not involved and aware of trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20 randomised participants not included, 5 further cases excluded after randomisation and participation. ITT was not employed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Majumdar 2013.
| Methods | Patients with unexplained infertility having normal semen parameters in accordance with WHO 2010 criteria, undergoing their first IVF‐ICSI cycle, were enrolled during the course of the study. | |
| Participants | 156 participants were prospectively randomised after oocyte retrieval and were assigned to either the ICSI group or the PICSI group. | |
| Interventions | PICSI group had sperm selected by the ability to bind to hyaluronic acid. | |
| Outcomes | Clinical pregnancy, live birth, miscarriage | |
| Notes | Funding source: None stated. Declaration of interest: The authors declare they have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement. Lack of blinding of outcome assessment is unlikely to affect the outcomes measured. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 women were excluded from the analysis since they did not have a fresh transfer; these were probably in the PICSI group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Miller 2019.
| Methods | Parallel, 2‐group, randomised trial of couples undergoing an ICSI procedure with fresh embryo transfer | |
| Participants | Eligible couples were undergoing an ICSI procedure. Women were 18 to 43 years, BMI 19 to 35, FSH 3 to 20, AMH at least 1.5. Men were 18 to 55 years, no vasovasostomy, no cancer, 3 days of abstinence. | |
| Interventions | PICSI plates were obtained and sperm selection was done according to the supplier's instructions and only after local training in the procedure. | |
| Outcomes | Live birth (full term + preterm birth included in our analysis), clinical pregnancy, miscarriage | |
| Notes | Funding source: This study was funded by the Efficacy and Mechanism Evaluation (EME) Programme (MREC 13/YH/0162; UKCRN ID 14845), a partnership of the Medical Research Council (MRC) and National Institute for Health Research (NIHR), and supported by the UK NIHR, the NIHR infrastructure in Leeds, and the UK Clinical Research Network. Declaration of interest: SP is a member of the NIHR EME board, outside of the submitted work. RW is a member of the Health Services and Delivery Research researcher‐led panel and the Public Health Research, Research Funding Board outside of the submitted work. SL is chief executive officer of the University of Belfast spinout company, Examen, outside of the submitted work. DM received a grant from Biocoat, outside of the submitted work, and personal fees from Origio to attend a meeting to report the submitted work (in brief). JK‐B received personal fees from Origio to attend a meeting, outside of the submitted work. DG was an independent scientist on the HABSelect trial steering committee. All other authors declare no competing interests. The choice of the PICSI dish for hyaluronan sperm selection was solely pragmatic; no commercial interest influenced the decision. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned with an online system (1:1) to receive either PICSI or a standard ICSI procedure |
| Allocation concealment (selection bias) | Low risk | Trial participants, research staff collecting outcome data, and all trial team members were masked to treatment allocation. Masking embryologists was impractical. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Embryologists not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3928 excluded; 1323 did not meet eligibility criteria. 484 consented but not randomised, 700 not included for other reasons, 626 no further contact. 5 lost to follow‐up in intervention, 9 in allocation (very small overall percentage lost to follow‐up and sensitivity analysis run with no significant effect). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Parmegiani 2012.
| Methods | Randomised controlled trial conducted in a single private assisted reproduction centre (Italy) between September 2010 and March 2011 | |
| Participants |
Inclusion criteria: all infertile women aged ≤ 41 years; undergoing ICSI treatment; total sperm number ≥ 1 million; sperm motility ≥ 5% Exclusion criteria: testicular spermatozoa; severe oligoasthenoteratozoospermia; total sperm number < 1 million; sperm motility < 5% Randomly assigned: 99 participants |
|
| Interventions | Couples were randomly assigned to the following 2 groups. Intervention 1 (PICSI): A 2‐microlitre droplet suspension of treated spermatozoa is placed near each 5‐microlitre culture (3 microdots in total) medium droplet and is subsequently connected to the droplet using the tip of a Gilson pipette. The PICSI dish is incubated at 37 °C under oil (FertiCult Mineral Oil; FertiPro, Beernem, Belgium); within 5 minutes, the bound spermatozoa attach by their head to the surface of the HA microdots and spin. An ICSI injecting pipette (ICSI Micropipets; Humagen Fertility Diagnostics–Origio, Jyllinge, Denmark) is used to pick HA‐bound sperm and inject them 1 by 1 into each oocyte. Spermatozoa spinning faster are preferred. The ICSI injecting pipette was previously loaded with SpermSlow to facilitate sperm micromanipulation. 49 women Intervention 2 (SpermSlow): On a plastic culture dish (IVF Petri dishes; Nunc, catalogue no. 150255), a 2‐microlitre droplet suspension of treated spermatozoa is connected with a pipette tip to a 5‐microlitre droplet of fresh culture medium (FertiCult Flushing Medium). Simultaneously, a 5‐microlitre droplet of SpermSlow is connected with a pipette tip to a 5‐microlitre droplet of fresh culture medium. The spermatozoa on this culture dish are then incubated for 5 minutes at 37 °C under oil (FertiCult Mineral Oil; FertiPro). Spermatozoa bound to HA are slowed in the junction zone of the 2 droplets; these spermatozoa are selected and collected with an injecting pipette (ICSI Micropipets) and are then injected into oocytes. 50 women Both PICSI and SpermSlow: PICSI and SpermSlow procedures are performed at 400× magnification. The spermatozoa are selected according to their morphology (WHO 2010 guidelines). |
|
| Outcomes | Clinical pregnancy rate; miscarriage rate; live‐birth rate | |
| Notes | Study authors were emailed to clarify aspects of methodology, but no reply was received. Funding source: Not stated Declaration of interest: The authors state no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement: states that randomisation was "by sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants were informed of their allocated treatment. Given the lack of blinding of participants, it can be assumed that other study personnel were unblinded. Lack of blinding is unlikely to affect any of the outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether blinding was performed. Lack of blinding of outcome assessment is unlikely to affect the outcomes measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Omission of a single case is unlikely to have a significant impact. Participant was excluded because of high‐risk ovarian hyperstimulation syndrome. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Romany 2014.
| Methods | A 2‐arm, unicentric, prospective, randomised, triple‐blinded trial at the Instituto Valenciano de Infertilidad in Valencia, Spain | |
| Participants | 263 patients attending centre for infertility that had not previously received treatment | |
| Interventions | MACS versus ICSI. The post swim‐up fraction was incubated using annexin V–conjugated microbeads and fresh medium, loaded into a separation column, and coated with a cell‐friendly matrix containing iron spheres that was fixed with a magnet. The fraction of apoptotic spermatozoa were separated, the remaining fraction were used for ICSI. | |
| Outcomes | Clinical pregnancy rate | |
| Notes | Live‐birth rate and miscarriage rate excluded as done per embryo transfer. Funding source: Not stated Declaration of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..by means of computer‐generated randomization..." |
| Allocation concealment (selection bias) | Low risk | The couples receiving treatment, the medical doctor (who evaluated the primary endpoint), and the statisticians performing the data analysis were unaware of the group to which participants were allocated in order to guarantee the triple‐blind nature of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysed as intention‐to‐treat, 15 lost from MACS, 11 lost from control group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Troya 2015.
| Methods | A unicentric, prospective, and randomised study at the Centro de Fertilidad y Reproduccion Asistida | |
| Participants | Patients with infertility having normal sperm concentration parameter in accordance with WHO 2010 criterion who were undergoing ICSI | |
| Interventions | ICSI (control) vs MACS vs PICSI | |
| Outcomes | Miscarriage, clinical pregnancy | |
| Notes | Funding source: Not stated Declaration of interest: No conflicts of interest have been declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Correspondence with author: Randomization based on a single sequence of random assignments which was determined by our personal laboratory before start the study. |
| Allocation concealment (selection bias) | Low risk | Correspondence with author: The reading of the DNA sperm fragmentation slides were done by 1 andrologist at blinding method. After all results were collected there were excluded the cases which not fit according to the policies of the study and then were revealed which group belong. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Correspondence with author: The reading of the DNA sperm fragmentation slides were done by 1 andrologist at blinding method. After all results were collected there were excluded the cases which not fit according to the policies of the study and then were revealed which group belong. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Correspondence with author: The reading of the DNA sperm fragmentation slides were done by 1 andrologist at blinding method. After all results were collected there were excluded the cases which not fit according to the policies of the study and then were revealed which group belong. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 cases from MACS were excluded from the pregnancy rate analysis because they did not have an embryo transfer; instead all embryos were frozen. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Worrilow 2013.
| Methods | Prospective, double‐blind, randomised controlled trial conducted in 10 IVF programmes (USA). Period of enrolment not reported. | |
| Participants |
Inclusion criteria: IVF patients who received ICSI as part of their ART treatment Exclusion criteria: use of testicular sperm; use of donor or cryopreserved gametes; patients receiving pre‐implantation genetic diagnosis (PGD); use of sperm‐sorting procedures; patients for whom only a proportion of oocytes receive ICSI; maternal age > 40 years; < 4 metaphase 2 oocytes at time of oocyte retrieval; initial hyaluronan binding score < 2%; sperm count < 10,000 motile sperm/mL Randomly assigned: 482 participants |
|
| Interventions | Participants were divided into 2 cohorts based on the proportion of HB (hyaluronan bound) sperm in their unprocessed or initial semen (I‐HB). The 2 cohorts were divided based on an I‐HB ≤ 65% or > 65%. Participants with an I‐HB score ≤ 65% were randomly assigned to routine ICSI (control) or sperm selection based on hyaluronan binding. Participants with an I‐HB score > 65% were randomly assigned to 3 groups: control, hyaluronan binding, or non‐participation. The non‐participating group was present to balance the numbers of participants with high and low I‐HB scores. The initial hyaluronan binding score of sperm was evaluated using the HBA Sperm Hyaluronan Binding Assay, a dual‐chambered slide containing an attached layer of hyaluronan located beneath 2 individual coverslips (Biocoat Inc, Horsham, PA, USA). In accordance with the manufacturer's instructions, the HB score was determined by calculating the number of motile HB sperm divided by the number of total motile sperm. Following assessment of I‐HB score, the sperm were subjected to centrifugation on a discontinuous gradient and were washed with sperm‐processing media according to the specific protocol for the participating site. Intervention: The final sperm suspension was placed upon microdots of hyaluronan in the PICSI Sperm Selection Device (Biocoat Inc) and was overlaid with oil. Following a 5‐ to 10‐minute incubation, HB sperm were selected for microinjection. 240 women Control: The final sperm suspension was placed into standard ICSI dishes for selection. 242 women |
|
| Outcomes | Clinical pregnancy rate; pregnancy loss rate | |
| Notes | A longer time than expected was taken to recruit participants, therefore the trial was prematurely closed because of cost implications. Results for the study groups were combined to yield outcome measures regardless of percentage of HB sperm. Study authors were contacted to clarify numerous areas of methodology and results, however no reply was received. Funding source and declaration of interest. This study was funded by Biocoat Inc, Horsham, PA, USA. The statistical analysis plan and subsequent analyses were performed by Sherrine Eid, a biostatistician. The manuscript was prepared by Kathryn C Worrilow, PhD and the study team members. Biocoat Inc was permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the authors. KCW is a scientific advisor to Biocoat Inc. SE is a consultant to Biocoat Inc. DW has nothing to disclose. MP, SS, JW, KI, CK, and TE have nothing to disclose. GDB is a consultant to Cooper Surgical and Unisense. JL is on the scientific advisory board of Origio. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design with a computer random number generator |
| Allocation concealment (selection bias) | Low risk | Investigator performing randomisation has no clinical involvement in the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Apart from embryologists, all participants and personnel were blinded. Non‐blinding of embryologists is unlikely to affect the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement. Lack of blinding of outcome assessment is unlikely to affect the outcomes measured. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results of 4 participants were not reported. It is not clear to which study group the incomplete data belong. |
| Selective reporting (reporting bias) | High risk | The outcome 'pregnancy loss rate' was incompletely reported. Absolute numbers were not given and could not be determined from the information provided. The absolute number of pregnancies given for pregnancy loss was higher than that given for clinical pregnancy. No explanation is given. |
| Other bias | High risk | Participant recruitment took longer than expected, therefore the study was closed prematurely because of higher‐than‐expected costs. |
Ziarati 2018.
| Methods | 2‐group, unicentric, prospective, randomised clinical trial | |
| Participants | 80 infertile couples undergoing ICSI at the Isfahan Fertility and Infertility Center between June 2015 and February 2016. Couples with male factor infertility and at least 2 of their semen parameters below WHO criteria were included. | |
| Interventions | MACS/DGC vs DGC alone Density gradient centrifugation was performed for the control group followed by conventional ICSI. Magnetic‐activated cell sorting was carried out according to Zahedi and colleagues and then followed by DGC and conventional ICSI. |
|
| Outcomes | Clinical pregnancy, miscarriage, live birth | |
| Notes | Funding source: Not stated Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generation list of random numbers with a 1:1 allocation ratio |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement. Lack of blinding of outcome assessment is unlikely to affect the outcomes measured. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 11 in the MACS group and 7 in the control group, not analysed by ITT |
| Selective reporting (reporting bias) | Low risk | Low risk of selective reporting |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
AMH: anti‐Müllerian hormone ART: assisted reproductive technologies BMI: body mass index DGC: density gradient centrifugation FSH: follicle‐stimulating hormone HA: hyaluronic acid HB: hyaluronan binding ICSI: intracytoplasmic sperm injection I‐HB: initial hyaluronan binding ITT: intention‐to‐treat IVF: in vitro fertilisation MACS: magnetic‐activated cell sorting PICSI: physiological intracytoplasmic sperm injection WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antinori 2008 | This study did not meet our inclusion criteria. |
| Balaban 2011 | This study did not meet our inclusion criteria. |
| Berkovitz 2006 | Not an RCT (couples were matched) |
| Blanchard 2010 | This study did not analyse a relevant intervention. |
| Casciani 2014 | Not prospective or randomised |
| Charehjooy 2014 | Not randomised |
| Figueira 2011 | This study did not meet our inclusion criteria. |
| Fleming 2007 | Not an RCT (observational study) |
| Ghosh 2007 | Not an RCT (observational study) |
| Gianaroli 2008 | This study was pseudo‐randomised. |
| Gianaroli 2010 | This study was pseudo‐randomised. |
| Jin 2015 | Pseudo‐randomised (randomised based on odd or even number date of retrieval) |
| Kim 2014 | Comparison made with IMSI (subject of a separate Cochrane Review) |
| Knez 2011 | This study did not meet our inclusion criteria. |
| Knez 2012 | This study did not meet our inclusion criteria. |
| Liu 2017 | Outcome measures were not relevant to this review. |
| Mahmoud 2011 | This study did not meet our inclusion criteria. |
| Parmegiani 2010a | Results were 'per‐treatment randomised' rather than 'per‐woman or couple randomised'. We contacted the study authors to adjust results, but received no reply. |
| Parmegiani 2010b | This study did not meet our inclusion criteria. |
| San Carchenilla 2013 | Not randomised (email sent for further information) |
| Setti 2011 | This study did not meet our inclusion criteria. |
| Setti 2012a | This study did not meet our inclusion criteria. |
| Setti 2012b | This study did not meet our inclusion criteria. |
| Stimpfel 2017 | Not randomised |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01594645.
| Trial name or title | The clinical impact of selecting acrosome reacted spermatozoa for ICSI |
| Methods | |
| Participants | |
| Interventions | Birefringence |
| Outcomes | |
| Starting date | 3 May 2012 |
| Contact information | |
| Notes |
NCT01908569.
| Trial name or title | Application of MACS and time‐lapse technology in good‐prognosis patients |
| Methods | |
| Participants | Good‐prognosis patients undergoing IVF/ICSI |
| Interventions | MACS |
| Outcomes | Embryo quality and pregnancy rates |
| Starting date | 23 July 2013 |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT01908569 |
NCT01916213.
| Trial name or title | The valve of hyaluronic binding selection (PICSI) in improving IVF outcome |
| Methods | |
| Participants | |
| Interventions | PICSI |
| Outcomes | Live birth, miscarriage, clinical pregnancies |
| Starting date | 1 August 2013 |
| Contact information | |
| Notes |
NCT02488434.
| Trial name or title | The effects of using fertile chip in sperm selection for intracytoplasmic sperm injection in unexplained Infertility |
| Methods | |
| Participants | |
| Interventions | Fertile chip |
| Outcomes | |
| Starting date | 26 June 2015 |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT02488434 |
NCT02867111.
| Trial name or title | Sperm selection for infertility treatment (SSA) |
| Methods | |
| Participants | |
| Interventions | Sperm Selection Assay (SSA) |
| Outcomes | Fertilisation, embryo quality, rate of pregnancy and rate of birth |
| Starting date | 1 August 2016 |
| Contact information | |
| Notes |
NCT03085433.
| Trial name or title | Sperm selection by microfluidic separation improves embryo quality in patients with a history of poor embryo quality |
| Methods | |
| Participants | |
| Interventions | Microfluidic sperm sorting chip |
| Outcomes | |
| Starting date | 19 March 2017 |
| Contact information | |
| Notes |
NCT03360526.
| Trial name or title | What is the best sperm source and way of sperm selection in cases with abnormal sORP levels on the day of ICSI? |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | 28 November 2017 |
| Contact information | |
| Notes |
NCT03398317.
| Trial name or title | Sperm selection by either PICSI or MACS in cases with abnormal sperm DNA fragmentation index for ICSI |
| Methods | |
| Participants | |
| Interventions | PICSI vs MACS vs ICSI |
| Outcomes | |
| Starting date | 7 January 2018 |
| Contact information | |
| Notes |
ICSI: intracytoplasmic sperm injection IVF: in vitro fertilisation MACS: magnetic‐activated cell sorting PICSI: physiological intracytoplasmic sperm injection
Differences between protocol and review
Following completion of our protocol, a Cochrane Review titled 'Regular (ICSI) versus ultra‐high magnification (IMSI) sperm selection for assisted reproduction' was published (Teixeira 2013). This publication overlapped considerably with our protocol, and after consultation with the Cochrane Gynaecology and Fertility Group we amended the scope of our review to exclude the use of IMSI for sperm selection. The title of our review was amended accordingly. Following the 2019 update, the adverse outcome of miscarriage per woman randomly assigned was added as a primary outcome.
Contributions of authors
Protocol
SM wrote the first draft of the protocol. BK wrote the revised draft of the protocol. Emily Ford (EF) contributed methodological and statistical expertise to the protocol. AY commented on all drafts of the protocol, as well as on the methods and statistics. DG assisted with revision of the protocol. Ysanne Hook (YH) provided technical expertise and will contribute in the analysis phase of the review.
Full review
SM wrote the draft. BK provided clinical input. EF and AY supplied methodological and statistical expertise. DG commented on the revised draft. YH provided technical input.
2019 update
SL and LS undertook abstract review and study selection. SL and SM performed data extraction. The draft was written by SL and revised by SM. SL, SM, and AY collated response to peer review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Sam Lepine: no conflicts of interest reported Simon McDowell: no conflicts of interest reported Leigh M Searle: no conflicts of interest reported Ben Kroon: no conflicts of interest reported Demián Glujovsky: no conflicts of interest reported Anusch Yazdani: no conflicts of interest reported
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Esfahani 2016 {published data only}
- Esfahani MHN, Deemeh MR, Tavalaee M, Sekhavati MH, Gourabi H. Zeta sperm selection improves pregnancy rates and alters sex ratio in male factor infertility patients: a double‐blind, randomized clinical trial. International Journal of Fertility and Sterility 2016;10:253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Majumdar 2013 {published data only}
- Majumdar G, Majumdar A. A prospective randomized study to evaluate the effect of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. Journal of Assisted Reproduction and Genetics 2013;30:1471‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2019 {published data only}
- Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S, et al. Physiological, hyaluronan‐selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two‐group, randomised trial. Lancet 2019;393:416‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmegiani 2012 {published data only}
- Parmegiani L, Cognigni G, Bernardi S, Taraborrelli S, Troilo E, Ciampaglia W, et al. Sperm‐hyaluronic acid (HA) binding selection: PICSE versus Sperm Slow: P‐077. Human Reproduction 2012;27(Suppl 2):ii121–50. [Google Scholar]
- Parmegiani L, Cognigni G, Bernardi S, Troilo E, Taraborrelli S, Arnone A, et al. Comparison of two ready‐to‐use systems designed for sperm‐hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: a prospective, randomized trial. Fertility and Sterility 2012;98(3):632‐7. [DOI] [PubMed] [Google Scholar]
Romany 2014 {published data only (unpublished sought but not used)}
- Romany L, Garrido N, Motato Y, Aparicio B, Remohí J, Meseguer M. Removal of annexin V‐positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: a controlled and randomized trial in unselected males. Fertility and Sterility 2014;102:1567‐75. [DOI] [PubMed] [Google Scholar]
Troya 2015 {published data only}
- Troya J, Zorilla I. Annexin V‐MACS in infertile couples as method for separation of sperm without DNA fragmentation. JBRA Assisted Reproduction 2015;19:66‐9. [DOI] [PubMed] [Google Scholar]
Worrilow 2013 {published data only}
- Worrilow K, Eid S, Woodhouse D, Matthews J, Khoury C, Witmyer J. Prospective, multi‐center, double‐blind, randomized clinical trial evaluating the use of hyaluronan bound sperm (HBS) in ICSI: statistically significant improvement in clinical outcomes. Fertility and Sterility 2011;96(Suppl 3):S58. [Google Scholar]
- Worrilow K, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes ‐ multicenter, double‐blinded and randomized controlled trial. Human Reproduction 2013;28(2):306‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziarati 2018 {published data only}
- Ziarati N, Tavalaee M, Bahadorani M, Nasr Esfahani MH. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Human Fertility 2018;22(2):118‐125. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antinori 2008 {published data only}
- Antinori M, Licata E, Dani G, Cerusico F, Versaci C, d’Angelo D, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reproductive BioMedicine Online 2008;16(6):835‐41. [DOI] [PubMed] [Google Scholar]
- Antinori S, Licata E, Dani G, Cerusico F, Versaci C, Antinori M. A prospective randomized trial to verify the efficacy of IMSI procedure in daily IVF routine. Human Reproduction 2008;23(Suppl 1):i165. [Google Scholar]
Balaban 2011 {published data only}
- Balaban B, Yakin K, Alatas C, Oktem O, Isiklar A, Urman B. Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: a prospective randomized study. Reproductive BioMedicine Online 2011;22(5):472‐6. [DOI] [PubMed] [Google Scholar]
Berkovitz 2006 {published data only}
- Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, et al. How to improve IVF‐ICSI outcome by sperm selection. Reproductive BioMedicine Online 2006;12(5):634‐8. [DOI] [PubMed] [Google Scholar]
Blanchard 2010 {published data only}
- Blanchard M, Haguenoer K, Apert A, Poret H, Barthelemy C, Royere D, et al. Sperm morphology assessment using David's classification: time to switch to strict criteria? Prospective comparative analysis in a selected IVF population?. International Journal of Andrology 2010;34:145‐52. [DOI] [PubMed] [Google Scholar]
Casciani 2014 {published data only}
- Casciani V, Minasi MG, Fabozzi G, Scarselli F, Colasante A, Lobascio AM, et al. Traditional intracytoplasmic sperm injection provides equivalent outcomes compared with human zona‐pellucida‐bound selected sperm injection. Zygote 2013;22:565‐70. [DOI] [PubMed] [Google Scholar]
Charehjooy 2014 {published data only}
- Charehjooy N, Najafi MH, Tavalaee M, Deemeh MR, Azadi L, Shiravi AH, et al. Selection of sperm based on hypo‐osmotic swelling may improve ICSI outcome: a preliminary prospective clinical trial. International Journal of Fertility and Sterility 2014;8:21‐8. [PMC free article] [PubMed] [Google Scholar]
Figueira 2011 {published data only}
- Figueira RCS, Braga DPAF, Pasqualotto EB, Pasqualotto FF, Iaconelli A, Borges E. The role of morphological nuclear integrity of the sperm cells in preimplantation genetic aneuploidy screening cycles outcome. Journal fur Reproduktionsmedizin und Endokrinologie 2010;7:250‐1. [Google Scholar]
- Figueira RDC, Braga DP, Setti AS, Iaconelli A Jr, Borges E Jr. Morphological nuclear integrity of sperm cells is associated with preimplantation genetic aneuploidy screening cycle outcomes. Fertility and Sterility 2011;95(3):990‐3. [DOI] [PubMed] [Google Scholar]
Fleming 2007 {published data only}
- Fleming S, Ilad R, Ong K, Wu Y, Smith H, Aitken J. First prospective controlled trial of a novel membrane‐based electrophoretic method of isolating spermatozoa for IVF. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(Suppl 1):A1‐34. [Google Scholar]
Ghosh 2007 {published data only}
- Ghosh S, Chattopadhyay R, Bose G, Ganesh A, Das S, Chakravarty B. Selection of birefringent spermatozoa under Polscope: effect on intracytoplasmic sperm injection outcome. Andrologia 2011;44:734‐8. [DOI] [PubMed] [Google Scholar]
Gianaroli 2008 {published data only}
- Gianaroli L, Magli C, Cooldel G, Moretti E, Ferraretti A, Baccetti B. Sperm head’s birefringence: a new criterion for sperm selection. Fertility and Sterility 2008;90(1):104‐12. [DOI] [PubMed] [Google Scholar]
Gianaroli 2010 {published data only}
- Gianaroli L, Magli C, Ferraretti A, Crippa A, Lappi M, Capitani S, et al. Birefringence characteristics in sperm heads allow for the selection of reacted spermatozoa for intracytoplasmic sperm injection. Fertility and Sterility 2010;93(3):807‐13. [DOI] [PubMed] [Google Scholar]
Jin 2015 {published data only}
- Jin R, Bao J, Tang D, Liu F, Wang G, Zhao Y, et al. Outcomes of intracytoplasmic sperm injection using the zona pellucida‐bound sperm or manually selected sperm. Journal of Assisted Reproduction and Genetics 2016;33:597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only (unpublished sought but not used)}
- Kim JW, Yeong SY, Yang SH, Yoon SH, Lim JH. Are hyaluronic acid sperm selection and intracytoplasmic morphologically selected sperm injection effective in patients with teratozoospermia?. Fertility and Sterility 2014;102(3):e349. [Google Scholar]
Knez 2011 {published data only}
- Knez K, Zorn B, Tomazevic T, Vrtacnik‐Bokal E, Virant‐ Klun I. The IMSI procedure improves poor embryo development in the same infertile couples with poor semen quality: a comparative prospective randomized study. Reproductive Biology and Endocrinology 2011;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knez 2012 {published data only}
- Knez K, Tomazevic T, Zorn B, Vrtacnik‐Bokal E, Virant‐Klun I. Intracytoplasmic morphologically selected sperm injection improves development and quality of preimplantation embryos in teratozoospermia patients. Reproductive BioMedicine Online 2012;25(2):168‐79. [DOI] [PubMed] [Google Scholar]
Liu 2017 {published data only}
- Liu Y, Feenan K, Chapple V, Roberts P, Matson P. Intracytoplasmic sperm injection using hyaluronic acid of polyvinylpyrrolidone: a time‐lapse sibling oocyte study. Human Fertility 2017;22:39‐45. [DOI] [PubMed] [Google Scholar]
Mahmoud 2011 {published data only}
- Mahmoud K, Triki‐Hmam C, Terras K, Zhioua F, Hfaiedh T, Ben Aribia MH. How and in which indication the IMSI could improve outcomes?. Human Reproduction 2011;26(Suppl 1):i181. [Google Scholar]
Parmegiani 2010a {published data only (unpublished sought but not used)}
- Parmegiani L, Cognigni G, Bernardi S, Trolio E, Ciampaglia W, Filicori M. "Physiologic ICSI": hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertility and Sterility 2010;93(2):598‐604. [DOI] [PubMed] [Google Scholar]
Parmegiani 2010b {published data only}
- Parmegiani L, Cognigni G, Ciampaglia W, Pocognoli P, Marchi F, Filicori M. Efficiency of hyaluronic acid (HA) sperm selection. Journal of Assisted Reproduction and Genetics 2010;27:13‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
San Carchenilla 2013 {published data only (unpublished sought but not used)}
- San Carchenilla M, Agudo D, Rubio S, Bercerra D, Bronet F, Garcia‐Velasco JA, et al. Magnetic Activated Cell Sorting (MACS) is a useful technique to improved pregnancy rate in patients with high level of sperm DNA fragmentation. Human Reproduction 2013;28 (Suppl 1):i118. [Google Scholar]
Setti 2011 {published data only}
- Setti AS, Figueira RC, Braga DP, Iaconelli A Jr, Borges E Jr. Intracytoplasmic morphologically selected sperm injection benefits for patients with oligoasthenozoospermia according to the 2010 World Health Organization reference values. Fertility and Sterility 2011;95(8):2711‐4. [DOI] [PubMed] [Google Scholar]
Setti 2012a {published data only}
- Iaconelli JA, Figueira RCS, Setti AS, Braga DPAF, Pasqualotto EE, Borges E Jr. Gender incidence on intracytoplasmic morphologically selected sperm injection approach: a prospective randomized study. Human Reproduction 2011;26(Suppl 1):i71. [Google Scholar]
- Setti AS, Figueira RC, Braga DP, Iaconelli A Jr, Borges E Jr. Gender incidence of intracytoplasmic morphologically selected sperm injection‐derived embryos: a prospective randomized study. Reproductive BioMedicine Online 2012;24(4):420‐3. [DOI] [PubMed] [Google Scholar]
Setti 2012b {published data only}
- Setti AS, Figueira RDC, Almeida Ferreira Braga DP, Iaconelli A, Borges E. IMSI is beneficial in cases of advanced maternal age: a prospective randomized study. Reproductive BioMedicine Online 2012;Withdrawn. [DOI] [PubMed] [Google Scholar]
Stimpfel 2017 {published data only}
- Stimpfel M, Fevzer T, Verdenik I, Zorn B, Vrtacnik‐Bokal E, Virant‐Klun I. Magnetic‐activated cell sorting of non‐apoptotic spermatozoa improves the good quality blastocyst rate in women over 30 years old: a prospective study in sibling oocytes. Human Reproduction. Conference 2017;32 (Suppl 1):i199. [Google Scholar]
References to ongoing studies
NCT01594645 {published data only}
- NCT01594645. The Clinical Impact of Selecting Acrosome Reacted Spermatozoa for ICSI. https://clinicaltrials.gov/ct2/show/NCT01594645.
NCT01908569 {published data only}
- NCT01908569. Application of MACS and Time‐lapse Technology in Good‐prognosis Patients. https://clinicaltrials.gov/ct2/show/NCT01908569.
NCT01916213 {published data only}
- NCT01916213. The Valve of Hyaluronic Binding Selection (PICSI) in Improving IVF Outcome. https://clinicaltrials.gov/ct2/show/NCT01916213.
NCT02488434 {published data only}
- NCT02488434. The Effects of Using Fertile Chip in Sperm Selection for Intracytoplasmic Sperm Injection in Unexplained Infertility. https://clinicaltrials.gov/ct2/show/NCT02488434.
NCT02867111 {published data only}
- NCT02867111. Sperm Selection for Infertility Treatment (SSA) (SSA). https://clinicaltrials.gov/ct2/show/NCT02867111.
NCT03085433 {published data only}
- NCT03085433. Sperm Selection by Microfluidic Separation Improves Embryo Quality in Patients With a History of Poor Embryo Quality (SPERM). https://clinicaltrials.gov/ct2/show/NCT03085433.
NCT03360526 {published data only}
- NCT03360526. What is the Best Sperm Source and Way of Sperm Selection in Cases With Abnormal sORP Levels on the Day of ICSI?. https://clinicaltrials.gov/ct2/show/NCT03360526.
NCT03398317 {published data only}
- NCT03398317. Sperm Selection by Either PICSI or MACS in Cases With Abnormal Sperm DNA Fragmentation Index for ICSI. https://clinicaltrials.gov/ct2/show/NCT03398317.
Additional references
Ainsworth 2005
- Ainsworth C, Nixon B, Aitken R. Development of a novel electrophoretic system for the isolation of human spermatozoa. Human Reproduction 2005;20(8):2261‐70. [DOI] [PubMed] [Google Scholar]
Aitken 2007
- Aitken R, lullis G. Origins and consequences of DNA damage in male germ cells. Reproductive BioMedicine Online 2007;14:727‐33. [DOI] [PubMed] [Google Scholar]
Bartoov 2002
- Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real‐time fine morphology of motile human sperm cells is associated with IVF‐ICSI outcome. Journal of Andrology 2002;23(1):1‐8. [DOI] [PubMed] [Google Scholar]
Bartoov 2003
- Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, et al. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertility and Sterility 2003;80(6):1413‐9. [DOI] [PubMed] [Google Scholar]
Boomsma 2007
- Boomsma C, Heineman M, Cohlen B, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD004507.pub3] [DOI] [PubMed] [Google Scholar]
Craciunas 2015
- Craciunas L, Tsampras N, Kollman M, Stirbu L, Raine‐Fenning NJ. Use of hyaluronic acid for sperm immobilisation and selection before intracytoplasmic sperm injection: a systematic review and meta‐analysis. World Journal of Obstetrics and Gynaecology 2015;4:113‐23. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 3 November 2018.
Grunewald 2001
- Grunewald S, Paasch U, Glander H. Enrichment of non‐apoptotic human spermatozoa after cryopreservation by immunomagnetic cell sorting. Cell Tissue Bank 2001;2(3):127‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP. Heterogeneity in meta‐analysis should be expected and appropriately quantified. International Journal of Epidemiology 2008;37:1158‐60. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huszar 2007
- Huszar G, Jakab A, Sakkas D, Ozenci C, Cayli S, Delpiano E, et al. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspect. Reproductive BioMedicine Online 2007;14:650‐63. [DOI] [PubMed] [Google Scholar]
Palermo 1992
- Palermo G, Joris H, Devroey P, Steirteghem A. Pregnancies after intracytoplasmic injection of a single spermatozoon into an oocyte. Lancet 1992;340(8810):17. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Said 2007
- Said T, Land J. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Human Reproduction Update 2007;17(6):719‐33. [DOI] [PubMed] [Google Scholar]
Sakkas 2000
- Sakkas A, Manicardi G, Bizarro D, Bianchi P. Possible consequences of performing ICSI with sperm possessing nuclear DNA damage. Human Fertility 2000;3(1):26‐30. [DOI] [PubMed] [Google Scholar]
Sakkas 2013
- Sakkas D. Novel technologies for selecting the best sperm for in vitro fertilization and intracytoplasmic sperm injection. Fertility and Sterility 2013;99(4):1023‐9. [DOI] [PubMed] [Google Scholar]
Teixeira 2013
- Teixeira DM, Barbosa MAP, Ferriani RA, Navarro PA, Raine‐Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra‐high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010167.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McDowell 2014
- McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD010461.pub2] [DOI] [PubMed] [Google Scholar]


