Abstract
Background
Early diagnosis of leptospirosis may contribute to the effectiveness of antimicrobial therapy and early outbreak recognition. Nucleic acid and antigen detection tests have the potential for early diagnosis of leptospirosis. With this systematic review, we assessed the sensitivity and specificity of nucleic acid and antigen detection tests.
Objectives
To determine the diagnostic test accuracy of nucleic acid and antigen detection tests for the diagnosis of human symptomatic leptospirosis.
Search methods
We searched electronic databases including MEDLINE, Embase, the Cochrane Library, and regional databases from inception to 6 July 2018. We did not apply restrictions to language or time of publication.
Selection criteria
We included diagnostic cross‐sectional studies and case‐control studies of tests that made use of nucleic acid and antigen detection methods in people suspected of systemic leptospirosis. As reference standards, we considered the microscopic agglutination test alone (which detects antibodies against leptospirosis) or in a composite reference standard with culturing or other serological tests. Studies were excluded when the controls were healthy individuals or when there were insufficient data to calculate sensitivity and specificity.
Data collection and analysis
At least two review authors independently extracted data from each study. We used the revised Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS‐2) to assess risk of bias. We calculated study‐specific values for sensitivity and specificity with 95% confidence intervals (CI) and pooled the results in a meta‐analysis when appropriate. We used the bivariate model for index tests with one positivity threshold, and we used the hierarchical summary receiver operating characteristic model for index tests with multiple positivity thresholds. As possible sources of heterogeneity, we explored: timing of index test, disease prevalence, blood sample type, primers or target genes, and the real‐time polymerase chain reaction (PCR) visualisation method. These were added as covariates to the meta‐regression models.
Main results
We included 41 studies evaluating nine index tests (conventional PCR (in short: PCR), real‐time PCR, nested PCR, PCR performed twice, loop‐mediated isothermal amplification, enzyme‐linked immunosorbent assay (ELISA), dot‐ELISA, immunochromatography‐based lateral flow assay, and dipstick assay) with 5981 participants (1834 with and 4147 without leptospirosis). Methodological quality criteria were often not reported, and the risk of bias of the reference standard was generally considered high. The applicability of findings was limited by the frequent use of frozen samples. We conducted meta‐analyses for the PCR and the real‐time PCR on blood products.
The pooled sensitivity of the PCR was 70% (95% CI 37% to 90%) and the pooled specificity was 95% (95% CI 75% to 99%). When studies with a high risk of bias in the reference standard domain were excluded, the pooled sensitivity was 87% (95% CI 44% to 98%) and the pooled specificity was 97% (95% CI 60% to 100%). For the real‐time PCR, we estimated a summary receiver operating characteristic curve. To illustrate, a point on the curve with 85% specificity had a sensitivity of 49% (95% CI 30% to 68%). Likewise, at 90% specificity, sensitivity was 40% (95% CI 24% to 59%) and at 95% specificity, sensitivity was 29% (95% CI 15% to 49%). The median specificity of real‐time PCR on blood products was 92%. We did not formally compare the diagnostic test accuracy of PCR and real‐time PCR, as direct comparison studies were lacking. Three of 15 studies analysing PCR on blood products reported the timing of sample collection in the studies included in the meta‐analyses (range 1 to 7 days postonset of symptoms), and nine out of 16 studies analysing real‐time PCR on blood products (range 1 to 19 days postonset of symptoms). In PCR studies, specificity was lower in settings with high leptospirosis prevalence. Other investigations of heterogeneity did not identify statistically significant associations. Two studies suggested that PCR and real‐time PCR may be more sensitive on blood samples collected early in the disease stage. Results of other index tests were described narratively.
Authors' conclusions
The validity of review findings are limited and should be interpreted with caution. There is a substantial between‐study variability in the accuracy of PCR and real‐time PCR, as well as a substantial variability in the prevalence of leptospirosis. Consequently, the position of PCR and real‐time PCR in the clinical pathway depends on regional considerations such as disease prevalence, factors that are likely to influence accuracy, and downstream consequences of test results. There is insufficient evidence to conclude which of the nucleic acid and antigen detection tests is the most accurate. There is preliminary evidence that PCR and real‐time PCR are more sensitive on blood samples collected early in the disease stage, but this needs to be confirmed in future studies.
Plain language summary
How accurate are nucleic and antigen detection tests in diagnosing leptospirosis?
What was studied in this review?
Leptospirosis is an infectious disease, caused by bacteria called Leptospira that can be found in soil, freshwater, or in the infected urine of certain animals. It is mainly a problem in humid, tropical countries in Southeast Asia, and Central and South America, but it can also occur in temperate regions.
Leptospirosis causes fever and headache, and in some cases kidney, lung, or heart problems. Often, the symptoms are not unique for the disease, which makes it difficult to diagnose, and is therefore frequently missed.
Laboratory tests confirm diagnosis. These tests are based on demonstration of the presence of Leptospira, its DNA, or antibodies against Leptospira. Nucleic acid and antigen detection tests, such as conventional polymerase chain reaction (PCR) and real‐time PCR, identify the bacterium or its DNA directly in blood or urine. Nucleic acid and antigen detection tests may detect Leptospira better in the early days of an infection, so that people can be treated earlier with antibiotics – resulting in better outcomes – and can provide useful information in outbreak situations. In outbreak situations, nucleic acid and antigen detection tests could serve as early warning systems.
What was the aim of this review?
The aim was to assess how well nucleic acid and antigen tests perform in detecting leptospirosis. In other words, to assess how many mistakes these tests make by either missing people with leptospirosis or misidentifying people without leptospirosis (healthy people or people with another disease).
What were the main results in this review?
The review included information from 41 studies with 5981 participants. We identified nine nucleic acid and antigen detection tests, of which PCR and real‐time PCR were most often investigated.
An important finding was that the accuracy of both PCR and real‐time PCR varied strongly between studies. We presented average accuracies for both tests, but there was great uncertainty around these averages. PCR often correctly identified people without leptospirosis (averaging 95 in 100 people), but frequently missed people with leptospirosis (averaging 30 in 100 people). The accuracy of the real‐time PCR depended on the cut‐off value for a positive test result. At a cut‐off value where real‐time PCR often correctly identified people without leptospirosis (averaging 95 in 100 people), it also frequently missed people with leptospirosis (averaging 71 in 100 people). If a person tests positive or negative for PCR or real‐time PCR, the chance of the person actually having the disease depends on whether the suspicion of leptospirosis in that person was already high before taking the test. So, when interpreting the results of any of these tests, one must consider the strength of suspicion of leptospirosis in an individual, and how often leptospirosis occurs in the setting in which the test will be used.
It was uncertain whether PCR or real‐time PCR performed better in detecting leptospirosis, since studies directly comparing these two tests were lacking. The results of other nucleic and antigen detection tests are described in the main text of the review.
How reliable were the results of the studies in this review?
Not all studies were conducted according to the highest scientific standards. This means that the results of some studies may have been overestimated or underestimated. Furthermore, the tests used to verify whether a person truly had leptospirosis or not (called the reference standard) may not accurately distinguish people with or without leptospirosis. For these reasons, more high‐quality studies are needed to confirm the reliability of these results.
Who do the results of this review apply to?
The results may apply to people who may have leptospirosis. However, the performance of the PCR and real‐time PCR vary considerably among studies and it is yet unclear what causes this difference in performances. It is probable that the test performs better or worse depending on how prevalent leptospirosis is in the region, and depending on the time between the onset of symptoms and time of testing. Therefore, it is difficult to generalise the results of this review to all settings.
How up‐to‐date is this review?
The review authors searched for and used studies published up to 6 July 2018.
Summary of findings
Background
Target condition being diagnosed
Leptospirosis is a worldwide prevalent zoonosis caused by the pathogenic spirochaetes of the bacterial genus Leptospira (Farr 1995). Humans acquire the infection through direct contact with the infected urine of carrier animals, or by contact with the environment contaminated with pathogenic leptospires. In recent years, leptospirosis has been identified as a common public health problem, illustrated by outbreaks in Southeast Asia, and Central and South America. Furthermore, the incidence of leptospirosis in both low‐income and middle‐ to high‐income countries appears to be increasing (Pappas 2008; Vijayachari 2008; Pijnacker 2016; Duarte 2019; Warnasekara 2019), causing substantial morbidity and mortality (Costa 2015). The disease is most frequently found in tropical and subtropical climates with incidences ranging from 10 to 100 per 100,000 people in endemic regions. Pathogenic leptospires also persist in more temperate regions, such as Denmark, Greece, Portugal, France, Germany, and the Netherlands, where it is an important cause of illness in returning travellers (Lau 2010; Jensenius 2013). Factors contributing to higher levels of prevalence are local agricultural practices, close proximity to mammalian reservoirs, poor sanitation, soil contact, and high rainfall (Mwachui 2015). Flooding associated with heavy seasonal rainfall and natural disasters may increase incidence to epidemic proportions, to more than 100 per 100,000 people (WHO 2003). It is thought that the emergence of leptospirosis is aggravated by global climate change, increasing contact between humans and wild animal populations, and the exponential expansion of urban slums (McBride 2005; Guerra 2013).
The clinical manifestations of leptospirosis are diverse; symptoms range from a mild undifferentiated fever syndrome including myalgia and headaches, to the severest form that may involve renal failure and jaundice (classically known as Weil's disease), and other complications such as pulmonary haemorrhages, aseptic meningitis, and myocarditis (Bharti 2003). Fatality rates for severe forms range from 5% to 50% (WHO 2003; McBride 2005). The non‐specific clinical presentation of leptospirosis makes it challenging to distinguish from infections such as malaria, dengue, influenza, hepatitis, and yellow fever (Bharti 2003). Consequently, laboratory tests are essential to confirm the diagnosis. These tests are based on either demonstration of leptospires, antibodies against leptospires, or their DNA.
The current reference standard for the diagnosis of leptospirosis is based on antibody detection by the microscopic agglutination test (MAT), with or without culture. Since anti‐Leptospira antibodies appear only in the later stage of the disease, MAT and other serological tests, such as the immunoglobulin M (IgM) enzyme‐linked immunosorbent assay (ELISA), are impractical in establishing an early diagnosis (Picardeau 2014). In addition, the culture of leptospires does not contribute to an early diagnosis, due to their slow growth (WHO 2003). Nucleic acid tests, such as the polymerase chain reaction (PCR) and antigen detection tests, can detect leptospiral DNA or antigens directly in blood in the first days of the disease and are thus capable of yielding an early diagnosis (WHO 2003). This type of early detection test may facilitate early outbreak warnings and make the administration of early microbial treatment possible. Additionally, leptospires appear in the urine after a few days, on which nucleic acid detection methods can be applied as well (WHO 2003). Early administration of treatment is generally considered to improve a person's outcome compared to treatment at a later disease stage, although more studies are needed to confirm this (Brett‐Major 2012).
Index test(s)
This review evaluated nucleic acid and antigen detection tests for pathogenic leptospires. Commonly used nucleic acid tests for the diagnosis of human leptospirosis are the PCR, its variants, and isothermal amplification tests such as loop‐mediated isothermal amplification (LAMP). Nucleic acid tests can be used to test blood, cerebrospinal fluid (CSF), aqueous humour, and urine samples. Other antigen detection tests include ELISA and fluorescent antibody testing (FAT) for the detection of Leptospira antigens, silver staining, and immunohistochemistry.
Substantial variation can be expected between laboratories on how the index tests are performed with regard to the timing of sample collection and threshold values. The timing of sample collection may greatly affect the test's accuracy, as leptospires are known to (dis)appear in different sample types as the disease progresses. For example, it is recommended that nucleic acid and antigen detection tests are performed on blood between one and 10 days postonset (DPO) of symptoms, as leptospiraemia declines rapidly until below detection after 10 DPO (WHO 2003). Tests performed on blood samples collected after 10 DPO may lead to false‐negative findings. Tests in urine are expected to be positive after 10 to 14 DPO (Picardeau 2014).
Reference standard
MAT is the most widely used serological test for leptospirosis. It is considered to be the reference standard, often used in combination with other serological tests (such as IgM ELISA), and with or without culture of leptospires from blood or urine.
MAT is considered an imperfect reference standard. It has a high diagnostic specificity, as the observation of seroconversion or a titre rise confirms current leptospirosis, but a negative MAT does not rule out the possibility of leptospirosis. Limmathurotsakul and colleagues used a Bayesian latent class analysis (LCA) to estimate the accuracy of MAT, which was 49.8% sensitive and 98.8% specific (Limmathurotsakul 2012). The LCA assumes that there is no reference standard, and estimates disease prevalence by taking the results of multiple tests into account (Rutjes 2007).
In another study to estimate the accuracy of MAT, Goris and colleagues selected culture‐positive people as being infected (proof of leptospirosis) and people with other known diseases and unknown disease as controls, and performed MAT on both groups (Goris 2012). In this study, the sensitivity of MAT was estimated at 81.7% and specificity of MAT was estimated at 100%.
Using a reference standard with low sensitivity to compare against the index test may result in biased estimates of specificity. However, when the case definition in the Goris 2012 study was changed to include people who were IgM ELISA positive, the sensitivity increased to 93.3% without sacrificing the specificity (Goris 2012). This indicates that combining multiple tests with high specificity as a composite reference standard can yield increased sensitivity. Therefore, we decided to include studies with only MAT as the reference standard, and studies that used other serological tests, or culturing, or both, alongside MAT as the reference standard.
Variability in MAT performance between laboratories exists and may affect test accuracy. MAT requires a panel of live Leptospira serovars (group of micro‐organisms characterised by specific set of antigens) that occur in the region, supplemented with a panel of globally standardised serovars when people present with a travelling history (Goris 2012). Determining and maintaining such panels are major, but essential, tasks; inadequate panels may lead to false‐negative results. The timing of sample collection may also influence sensitivity or specificity; antibodies are usually detectable from five to seven DPO onwards. MAT‐case definitions may vary between laboratories; a four‐fold rise in titre in paired sera or seroconversion is indicative of current infection, but some laboratories may use a high titre in a single serum sample (seropositivity) as a case definition for people who do not return for follow‐up. Seropositivity is not necessarily evidential of a current infection, since antibodies may persist after a previous infection, or cross‐reactivity with other diseases may occur (such as legionellosis, hepatitis, and autoimmune diseases) (WHO 2003). Therefore, the desirable cut‐off titres for the single‐sample MAT are higher in regions where leptospirosis and similar infectious diseases are highly prevalent.
Leptospires can be cultured from blood, CSF, dialysate fluid, and (postmortem) tissue, often within 10 DPO. Culture of urine is useful after 10 DPO. Leptospires are slow‐growing, fastidious bacteria. Cultures have to be maintained for at least four months before being regarded negative. Culturing provides evidence for leptospirosis but lacks sensitivity and does not contribute to an early diagnosis. The sensitivity of culture is estimated not to exceed 23%, according to an analysis of people with leptospirosis from 1925 to 2008 in the Netherlands (Goris 2013).
Clinical pathway
Figure 1 shows a diagnostic pathway, as suggested by Goris and colleagues (Goris 2012). A person with symptoms compatible with leptospirosis (such as fever, headaches, myalgia, conjunctival effusion, and vomiting) is evaluated for likelihood by assessing risk factors, and consequently classified as an 'early presentation' (DPO 10 or fewer) or a 'late presentation' (DPO greater than 10). Real‐time PCR is recommended as the test of choice for early presentations as it can detect leptospiral DNA in blood. Blood culture is conducted alongside real‐time PCR to confirm leptospirosis as well as to provide insight in locally occurring serovars. MAT and IgM ELISA are recommended for later presentations since antibodies are expected to appear in serum after five to seven DPO. The person is considered to have leptospirosis if any of the test results is positive.
1.
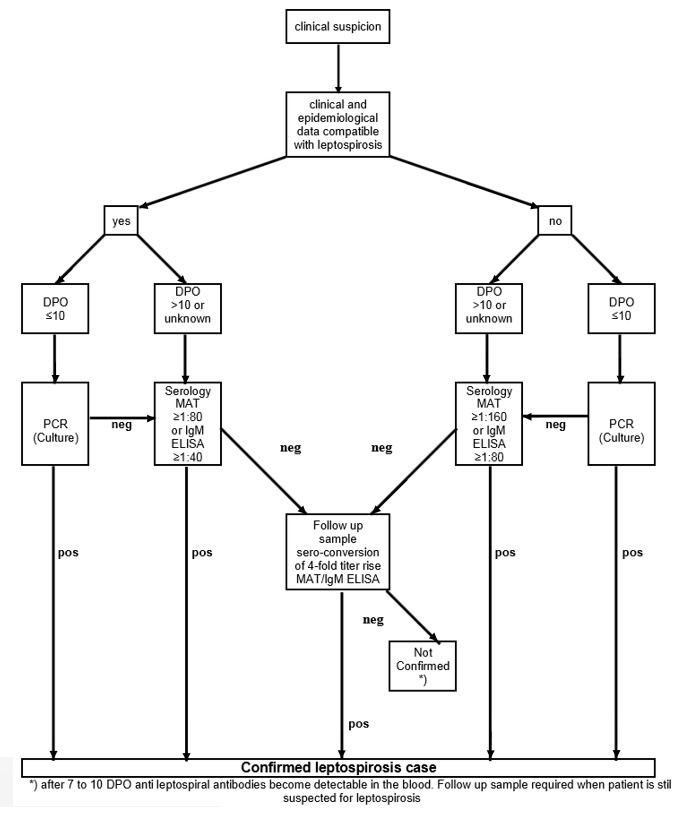
Algorithm, assisting with interpretations and conclusions on the outcome of laboratory testing (adapted from Goris 2012). Antibody titres shown in this figure are optimised for leptospirosis cases in the Netherlands. DPO: days postonset of symptoms; IgM ELISA: immunoglobulin M enzyme‐linked immunosorbent assay; MAT: microscopic agglutination test; neg: negative; PCR: real‐time polymerase chain reaction; pos: positive.
If a person tests positive with either an antibody or an antigen test, this person will be treated with antibiotics. If the tests return negative, then the recommendation is to test again in two weeks' time. However, if the person is very ill, clinicians will in some cases decide to treat with antibiotics anyway.
Rationale
The aim of this systematic review was to assess the diagnostic test accuracy of nucleic acid and antigen detection tests for human symptomatic leptospirosis. A similar diagnostic accuracy review on serology tests (antibody detection tests) for leptospirosis is being conducted by Goris and colleagues (Goris 2011).
Nucleic acid and antigen detection tests may serve several purposes based on their ability for early detection. First, and most important, an accurate test in the early stage of the disease may improve patient outcomes by facilitating timely administration of effective antibiotics. Although the limited available evidence presented by the latest Cochrane Review on antimicrobial therapy was inconclusive (Brett‐Major 2012), one study reported a shortened duration of illness in early‐stage leptospirosis (McClain 1984), while three studies that studied advanced leptospirosis yielded conflicting results (Edwards 1988; Watt 1988; Costa 2003). This raises the possibility that antibiotic therapy may have a greater effect when delivered earlier. Second, an early test may be useful in participant recruitment for studies evaluating antibiotics in early‐stage leptospirosis. Third, it may facilitate early warning of leptospirosis outbreaks and yield more reliable estimates of leptospirosis incidence in the affected region. Not all antigen tests may be applicable as early detection tests, but they are nevertheless good candidates for assessment since accurate, low‐cost, simple, and convenient point‐of‐care tests are urgently needed.
Objectives
To determine the diagnostic test accuracy of nucleic acid and antigen detection tests for the diagnosis of human symptomatic leptospirosis.
Secondary objectives
To investigate the comparative accuracy of nucleic acid and antigen detection tests.
To assess the influence of potential sources of heterogeneity on the diagnostic test accuracy of nucleic acid and antigen detection tests, namely:
timing of sample collection for the index test;
disease prevalence in the study population;
blood sample type for the index test (whole blood, plasma, or serum);
primers or target genes for the PCR and other nucleic acid tests;
threshold of the index test;
real‐time PCR visualisation method;
brand of the test.
Methods
Criteria for considering studies for this review
Types of studies
We included diagnostic test accuracy studies, that is, any study that evaluated the sensitivity and specificity of a nucleic acid or antigen detection test in comparison with a reference standard. In this review, we discerned three types of eligible diagnostic test accuracy studies based on their method of participant selection: the cross‐sectional study, the single‐gate case‐control study, and the two‐gate case‐control study. Their respective characteristics are summarised in Table 5 and illustrated in Figure 2.
1. Study designs.
| Design type | Design name | Description |
| Single‐gate | Cross‐sectional study | Recruitment of a consecutive series of participants in whom leptospirosis is suspected. The index test and the reference standard is done on all participants and the results of the 2 tests are compared with each other. |
| Case‐control study | Recruitment of participants with a positive reference standard result and participants with a negative reference standard result who are randomly selected from the same cohort of participants with the suspicion of leptospirosis. The index test is subsequently applied to all participants. | |
| 2‐gate | Case‐control study | Recruitment of participants with a positive reference standard result and participants who are diagnosed with alternative conditions that resemble the clinical presentation of leptospirosis. The index test is subsequently applied to all participants. |
2.
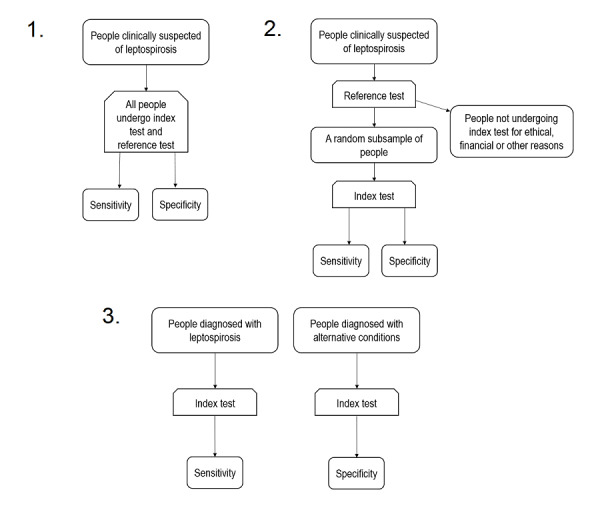
Eligible study designs. 1. Cross‐sectional study; 2. single‐gate case‐control study; 3. two‐gate case‐control study.
In cross‐sectional studies, people with clinical suspicion of leptospirosis are consecutively enrolled and undergo both the index test and reference standard. In the similar single‐gate case‐control study, usually all people with positive reference standard results and a subsample of people with negative reference standard results from an original clinically suspected cohort are subsequently tested with the index test. We referred to these two study designs simply as 'single‐gate' designs (i.e. having a single inclusion criteria for clinical presentation) (Rutjes 2005). The main difference between these two designs is that the prevalence of the target condition in the single‐gate case‐control study is artificial, whereas in a cross‐sectional study, a true prevalence can be estimated.
In a two‐gate case‐control design, people with positive reference standard results and people who do not have leptospirosis are enrolled to subsequently undergo the index test. Since the participants with and without the target condition are selected from two separate cohorts, this study design is at a higher risk of bias in comparison to the single‐gate designs. The two‐gate case‐control designs can be further separated into studies in which the controls have an alternative condition resembling leptospirosis (two‐gate with alternative diagnoses controls), and studies in which the controls are healthy (two‐gate with healthy controls).
We excluded two‐gate case‐control designs with healthy controls because these studies are known to produce inflated estimates of diagnostic accuracy (Rutjes 2005).
We placed no restrictions on language and publication date. When studies met our eligibility criteria but reported insufficient data for the construction of two‐by‐two tables, we excluded them. In cases where the full‐text article was not retrievable or in case of meeting abstracts, we included the study if the abstract reported data for the construction of two‐by‐two tables. We excluded studies when both abstract and the full‐text article were not retrievable. We contacted study authors to obtain the full‐text article or study data prior to exclusion, but we excluded studies when no answer was obtained after a lengthy interval. Finally, we excluded studies with fewer than 10 participants, as they would add little value to the review.
Participants
Eligibility of participants depended on the study design.
Cross‐sectional studies: people with clinical suspicion of leptospirosis were eligible. Compatible symptoms were, but were not limited to, fever, myalgia, headaches, malaise, conjunctival suffusion, rash, nausea/vomiting, anorexia, and cough.
Single‐gate case‐control studies: eligible were cases with a positive reference standard result and controls derived from the same clinically suspected group as the cases, but with a negative reference standard result.
Two‐gate case‐control studies: eligible were cases with a positive reference standard result and controls with a different known disease that resembled the clinical presentation of leptospirosis.
We excluded studies that screened asymptomatic people for leptospirosis.
Index tests
All diagnostic tests that used nucleic acid and antigen detection methods were included. Tests eligible for inclusion were, but were not limited to, PCR and its variants, that is, LAMP, ELISA, FAT, silver staining, or immunohistochemistry. We included index tests with any sample type (e.g. blood products, urine, CSF), any timing of sample collection (recorded as DPO), any variation in laboratory processing, and any threshold for tests on a continuous scale. We excluded studies that did not analyse different sample types separately, as it would be unclear which sample should be tested by the clinician in order to obtain a similar test accuracy.
Target conditions
This review was restricted to human symptomatic leptospirosis. We excluded studies of ocular and neurological manifestations of leptospirosis, as it was unclear whether MAT was a valid reference standard for these target conditions.
Reference standards
We considered several types of reference standards, which are summarised in Table 6. We elaborate the inclusion criteria separately for single‐gate and two‐gate designs.
2. Reference standards.
| Study design | Eligible reference standards | Case definition |
| Single gate | 1. MAT only | MAT positive |
| 2. MAT and culture | ≥ 1 of the tests positive | |
| 3. MAT and ELISA (or other serological tests) | ≥ 1 of the tests positive | |
| 4. MAT and culture and ELISA | ≥ 1 of the tests positive | |
| 2 gate | 5. MAT only | MAT positive |
| 6. Culture only | Culture positive |
All reference standards eligible for inclusion. Tests 2, 3, and 4, which are composite reference standards, are intended to increase sensitivity, provided that each reference standard has been applied to all participants. In two‐gate designs, the sensitivity of the reference standard is irrelevant, as controls are not reference standard negatives. ELISA: enzyme‐linked immunosorbent assay; MAT: microscopic agglutination test.
For single‐gate designs, we considered studies that used MAT, with or without culture or other serological tests such as IgM ELISA. We included these tests alongside MAT in order to compensate for the imperfect sensitivity of MAT as a reference standard. Since these tests have high specificity, we considered any positive result from this composite reference standard as a leptospirosis case. If a study used MAT as a sole reference standard, we considered the risk of bias to be high. We excluded single‐gate designs with culture as the sole reference standard, since culture has a very low sensitivity (Goris 2013).
In two‐gate designs, the people without leptospirosis are not necessarily reference standard negatives, but they are diagnosed with an alternative condition. Hence, we only required a reference standard that ruled in leptospirosis in the case of a positive result. Reference standards considered eligible for studies with this design were those with a high specificity: MAT used alone, or culture used alone.
In order to avoid incorporation bias (the reference standard uses or incorporates the index test), we excluded studies which contained a nucleic acid or antigen detection test in the reference standard.
Search methods for identification of studies
Electronic searches
We searched the following 16 electronic databases: the Cochrane Library (6 July 2018), MEDLINE Ovid (1946 to 6 July 2018), Embase Ovid (1974 to 6 July 2018), Web of Science (1975 to 6 July 2018), CINAHL (1937 to 6 July 2018), BIOSIS Previews (1993 to 8 February 2015 due to terminated institutional subscription), PubMed (for publications not yet included in MEDLINE; 1946 to 8 February 2015), Google Scholar, African Index Medicus (1993 to 6 July 2018), African Journals Online (from inception to 8 February 2015), LILACS (Literature in the Health Sciences in Latin America and the Caribbean, 1982 to 6 July 2018), KoreaMed (from inception to 8 February 2015), IMSEAR (Index Medicus for the South‐East Asian Region, from inception to 6 July 2018), IMEMR (Index Medicus for the Eastern Mediterranean Region, from inception to 8 February 2015), WPRIM (Western Pacific Region Index Medicus, from inception to 6 July 2018), and IndMed (from inception to 8 February 2015). For each database, we identified subject headings or free‐text terms and synonyms (or both) related to: leptospirosis, antigen, nucleic acids, PCR, LAMP, hybridisation, immunohistochemistry, silver staining, and dot blot. Appendix 1 shows the search strategies for each database.
Searching other resources
Additionally, we scanned the reference lists of included articles and we searched the World Health Organization's (WHO) International Clinical Trial Registry Platform (www.who.int/ictrp) for ongoing or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (BY, MG) independently screened the titles and abstracts of all records, and excluded records with no relevance to the review question (first sift). We retrieved the full‐text of the remaining records, and three review authors (BY, MG, SdV) independently checked the full‐text articles for eligibility, using a full‐text assessment checklist, with each record being assessed by at least two review authors (second sift). Studies that were excluded during data extraction, excluded meeting abstracts and studies with irretrievable full‐texts, are listed in the Characteristics of excluded studies tables. We resolved disagreements between review authors by consensus or by consulting a senior author (ML).
Data extraction and management
From each study, two out of three review authors (BY, MG, SdV) independently extracted data by using a specially designed data collection form. The data collection form contained the following items.
Study ID.
Study design.
Study region.
Regional prevalence.
Participants: selection methods, sex and age distribution, symptoms, risk factors.
Index tests: threshold values, timing of sample collection (defined as DPO of symptoms where 1 DPO was 0 to 24 hours after onset of symptoms); type of sample.
Reference standards: threshold values, timing of sample collection.
Two‐by‐two contingency table for sensitivity and specificity calculations.
Each of the three review authors first piloted the form on two included studies to check for applicability. We resolved discrepancies between the authors by discussion and consensus. We contacted study authors for missing information.
Assessment of methodological quality
We assessed the quality of included studies using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool (Whiting 2011). The QUADAS‐2 tool helps quality assessment by assessment of risk of bias and applicability of results across four domains: participant selection, index test, reference standard, and flow and timing. We custom‐tailored QUADAS‐2 to the needs of our review by adding additional signalling questions where needed. We also piloted the tool on two included studies and refined it accordingly. See Appendix 2 for the signalling questions and review‐specific guidance.
Statistical analysis and data synthesis
We arranged results from each study in two‐by‐two contingency tables in which we compared people with confirmed leptospirosis (as defined by a positive MAT or other serological test or culture result) and people without leptospirosis (none of the reference standard tests were positive, or people having another disease than leptospirosis) to the binary test results from the index tests. From these tables, we calculated sensitivity and specificity for each study. As previously described, we excluded studies reporting insufficient data for the construction of two‐by‐two tables.
Some studies reported two thresholds for MAT, where the higher threshold was considered 'confirmed leptospirosis', and the lower threshold was considered 'probable leptospirosis'. In these studies, we chose the higher threshold dataset for the primary analysis. This was because we considered the specificity of the reference standard to be more important than its sensitivity. The lower threshold dataset was analysed in a sensitivity analysis.
We presented individual study results graphically by plotting estimates of sensitivity and specificity in forest plots and the summary receiver operating characteristic (SROC) space. We conducted a random‐effects meta‐analysis using the bivariate model to estimate summary values for sensitivity and specificity when little variation in threshold values was presumed. If studies used multiple thresholds for the index test, we constructed a SROC curve using the hierarchical summary receiver operating characteristic (HSROC) model. All analyses were done in SAS 9.4 (Cary Inc.).
We separately described studies that reported head‐to‐head comparisons of index tests (or index test characteristics) in the same study population, but did not perform meta‐analyses to formally compare these index tests due to the lack of a sufficient number of studies.
Investigations of heterogeneity
We assessed heterogeneity initially by visually inspecting the forest plots and the ROC plot. The following covariates were investigated as potential sources of heterogeneity.
Timing of sample collection for the index test. We planned to analyse this based on how study authors reported the timing: as a continuous variable using medians or means, or as a categorical variable using timing intervals (e.g. 1 DPO to 4 DPO versus 5 DPO to 10 DPO).
Prevalence in the study population (continuous variable). This was computed using two‐by‐two table data from cross‐sectional studies. If a case‐control study reported prevalence data of the original cohort, we also used these data.
Blood sample type for the index test (categorical variable; whole blood, plasma, or serum).
Primers or target genes for the PCR and other nucleic acid tests (categorical variable). Since two PCRs with the same target gene could use different primers, we also specified the original reference of the technique.
Threshold of the index test, if applicable (continuous variable; e.g. threshold cycles (Ct) for the real‐time PCR).
Real‐time PCR visualisation method (categorical variable; TaqMan probe; or SYBR green).
Brand of the test, if applicable (categorical variable).
Sensitivity analyses
To examine the robustness of the results to the decisions we made in the review process, we conducted analyses with the following alternative decisions.
Exclusion of studies with only abstracts.
Exclusion of studies with high risk of bias for the 'patients' domain.
Exclusion of studies with high risk of bias for the 'reference standard' domain.
Exclusion of studies that used antibiotics before the index test.
The choice of the lower MAT threshold dataset for the analysis, in studies that reported two thresholds for MAT.
Results
Results of the search
We conducted the final electronic search on 6 July 2018 and identified 6880 records (see Figure 3). After title and abstract screening, and after inclusion of one additional record, which we identified by contacting one of the authors (Destura 2007), we included 181 records for full‐text assessment. We excluded 127 records; 102 records due to clear irrelevance and 25 records for other reasons (see Characteristics of excluded studies table). At this stage, we identified and included one full‐text publication of a meeting abstract (Denipitiya 2016).
3.
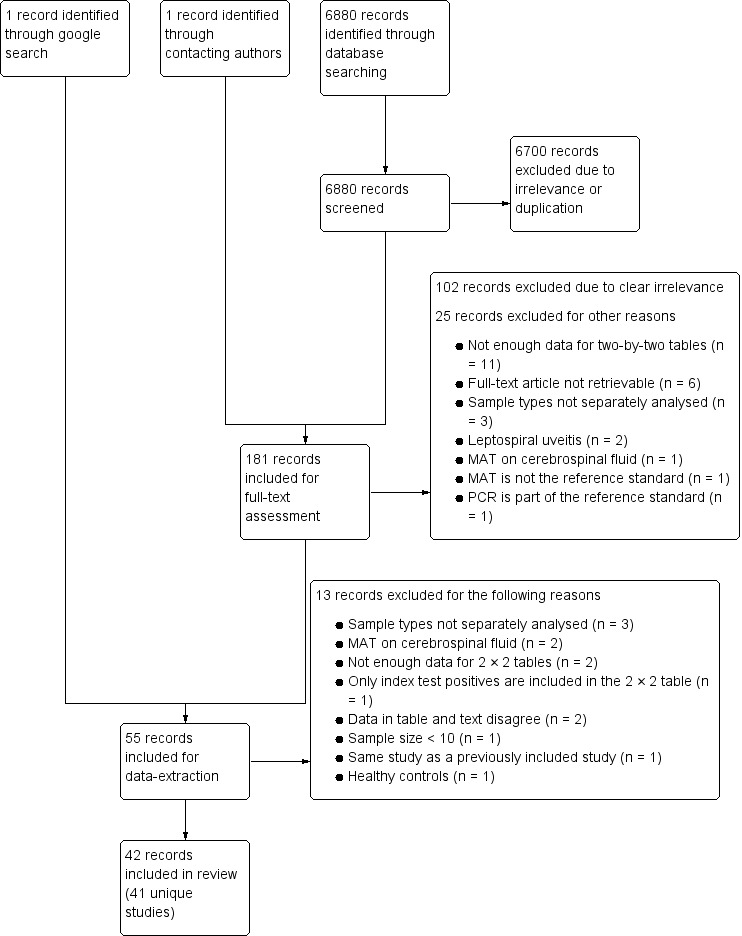
Study flow diagram. MAT: microscopic agglutination test; n: number of records; PCR: polymerase chain reaction.
We included the remaining 55 records for data collection, of which 13 were excluded for various reasons.
The review included 42 records, corresponding to 41 unique studies. We regarded four publications as two studies because they included the same population (Thaipadungpanit 2011 and Sonthayanon 2011 are grouped under Thaipadungpanit 2011; Waggoner 2014 (published in Journal of Clinical Microbiology) and Waggoner 2014 (published in PloS One) are grouped under Waggoner 2014). We considered one publication as two studies because two different populations were included (Villumsen 2012 BC; Villumsen 2012 U). Searching the WHO International Clinical Trial Registry Platform yielded no relevant records.
Description of studies
Included studies
The Characteristics of included studies table and Table 7 give an overview of all included studies. Forty‐one studies included 5981 participants, of whom 1834 were classified as having leptospirosis, and 4147 as not having leptospirosis. Thirty studies were cross‐sectional, five were single‐gate case‐control, four were two‐gate case‐control studies, and study design was dubious in two studies (Zhang 1992; Gravekamp 1993), with Zhang 1992 being most likely either a cross‐sectional or single‐gate case‐control study, but not a two‐gate study.
3. Overview of characteristics of included studies.
| Study ID | Study design | Region | Sample size | Prevalence | Sensitivity | Specificity | Index testa | Original reference of index test method | Target gene/primera | Threshold | Timing (DPO)a | Sample typea | Reference standard | Sample for MAT |
| Agampodi 2012 | CC1 | Sri Lanka | 105 | 21.7% | 51.0% | 98.2% | qPCR | Smythe 2002 | rrs | U | 1–10 | Whole blood | MAT | Paired only |
| 18.4% | 98.2% | qPCR | Smythe 2002 | rrs | U | 1–10 | Serum | |||||||
| Agampodi 2016 | CS | Sri Lanka | 96 | 43.8% | 27.3% | 25.0% | qPCR | Smythe 2002 | rrs | U | 3–7 | Blood/serum | MAT | Single + paired |
| Ahmed 2009 | CS | Netherlands | 75 | 19.5% | 100% | 100% | qPCR | Ahmed 2009 | secY | 35 Ct | 1–4 | Blood/serum | MAT OR IgM ELISA OR Culture | Single + paired |
| 62 | 68.8% | 100% | qPCR | Ahmed 2009 | secY | 35 Ct | 5–10 | Blood/serum | ||||||
| 133 | 88.5% | 100% | qPCR | Ahmed 2009 | secY | 35 Ct | 1–10 | Blood/serum | ||||||
| Ananyina 2000 | CC2 | Russia and China | 158 | U | 68.0% | 100% | cPCR | Gravekamp 1993 | G1/G2 and B64‐I/B64‐II primers | NA | U | Serum | MAT | Single + paired |
| Backstedt 2015 | CS | Brazil | 25 | 72.0% | 27.8% | 71.4% | qPCR | Stoddard 2009 | lipL32 | U | U | Whole blood | MAT OR culture | Single + paired |
| 55.6% | 14.3% | qPCR | Backstedt 2015 | rrs | U | U | Whole blood | |||||||
| Biscornet 2017 | CS | Seychelles | 223 | 13.9% | 35.5% | 90.1% | qPCR | Smythe 2002 | rrs | 35 Ct | U | Serum | MAT OR IgM ELISA | Single + paired |
| Blanco 2014 | CS | Brazil | 521 | 5.4% | 14.3% | 100% | cPCR | Merien 1992 | rrs | NA | U | Serum | MAT | Single + paired |
| 85.7% | 100% | N PCR | Merien 1992 | rrs | NA | U | Serum | |||||||
| Cardona 2008 | CS | Venezuela | 73 | 27.4% | 20.0% | 77.4% | cPCR | Gravekamp 1993 | G1/G2 and B64‐I/B64‐II primers | NA | U | Serum | MAT | Single + paired |
| 45.0% | 71.7% | cPCR | Gravekamp 1993 | G1/G2 and B64‐I/B64‐II primers | NA | U | Urine | |||||||
| Céspedes 2007 | CS | Peru | 118 | 22.0% | 55.4% | 100% | cPCR | Merien 1992 | rrs | NA | 1–7 | Whole blood | MAT OR IgM ELISA OR culture | Single + paired |
| Chandrasiri 2010 | CS | Sri Lanka | 59 | 11.8% | 14.3% | 86.5% | cPCR | U | G1/G2 primers | NA | U | Whole blood | MAT | Single |
| Chaurasia 2018 | CS | India | 29 | 79.3% | 100% | 66.7% | ELISA (LipL32) | Chaurasia 2018 | NA | U | U | Urine | MAT | Single |
| 91.3% | 50.0% | ELISA (Fla1) | Chaurasia 2018 | NA | U | U | Urine | |||||||
| 78.3% | 83.3% | ELISA (LipL41) | Chaurasia 2018 | NA | U | U | Urine | |||||||
| 91.3% | 66.7% | ELISA (HbpA) | Chaurasia 2018 | NA | U | U | Urine | |||||||
| 100% | 66.7% | ELISA (SphCD210) | Chaurasia 2018 | NA | U | U | Urine | |||||||
| 91.3% | 66.7% | ELISA (Sph2) | Chaurasia 2018 | NA | U | U | Urine | |||||||
| 39.1% | 83.3% | ELISA (Sph4) | Chaurasia 2018 | NA | U | U | Urine | |||||||
| De Abreu Fonseca 2006 | CC2 | Brazil | 80 | U | 38.3% | 100% | cPCR | Gravekamp 1993/Kee 1994 | G1/G2 and LP1/LP2 primers | NA | U | Whole blood | MAT OR culture | Single + paired |
| 36.7% | 100% | cPCR | Gravekamp 1993/Kee 1994 | G1/G2 and LP1/LP2 primers | NA | U | Urine | |||||||
| Denipitiya 2016 | CS | Sri Lanka | 111 | 58.6% | 67.7% | 91.3% | qPCR | Ahmed 2009 | secY | 35 Ct | 1–5 | Whole blood | MAT | Single + paired |
| Fan 1999 | CS | China | 15 | 33.3% | 100% | 80.0% | cPCR | Fan 1999 | rrs | NA | U | Serum | MAT | U |
| Gokmen 2016 | CS | Turkey | 47 | 44.7% | 90.5% | 42.3% | N PCR | Bomfim 2008 | lipL32 | NA | U | Serum | MAT | Single |
| 95.2% | 42.3% | N PCR | Merien 1992 | rrs | NA | U | Serum | |||||||
| Gonzalez 2013 | CS | Uruguay | 183 | 46.4% | 30.6% | 100% | qPCR | Stoddard 2009 and Bourhahy 2001 | lipL32 | U | U | Serum | MAT | Paired only |
| Gravekamp 1993 | U | Netherlands and Barbados | 119 | U | 49.4% | 100% | cPCR | Gravekamp 1993 | G1/G2 and LP1/LP2 primers | NA | U | Serum | MAT OR IgM ELISA | U |
| Kitashoji 2015 | CS | Philippines | 287 | 46.0% | 14.4% | 83.2% | LAMP | Koizumi 2012 | rrs | NA | 6.5 | Plasma | MAT | Single + paired |
| 14.1% | 90.6% | LAMP | Koizumi 2012 | rrs | NA | U | Urine | |||||||
| Koizumi 2009 | CS | Sri Lanka | 107 | 24.3% | 0.0% | 96.3% | N PCR | Kawabata 2001/mod:Koizumi 2008 | flaB | NA | 7 | Serum | MAT | Single only |
| Merien 2005 | CS | Oceania | 51 | 33.3% | 70.6% | 61.8% | N PCR | Merien 1992 | rrs | NA | 5 | Serum | MAT | Single + paired |
| 70.6% | 61.8% | qPCR | Merien 2005 | LFB1‐F/LFB1‐R primers | U | 5 | Serum | |||||||
| Ooteman 2006 | CS | Brazil | 125 | 37.6% | 36.2% | 70.5% | cPCR | Gravekamp 1993 | G1/G2 primers | NA | U | Serum | MAT | Single + paired |
| Pakoa 2018 | CS | Vanuatu | 130 | 11.5% | 0% | 97.4% | qPCR | Stoddard 2009 | lipL32 | U | U | Serum | MAT | Single |
| Riediger 2007 | CS | U | 66 | 22.7% | 46.7% | 76.5% | cPCR | Gravekamp 1993 | G1/G2 and B64‐I/B64‐II primers | NA | U | Whole blood | MAT | Single + paired |
| 40.0% | 80.4% | cPCR | Gravekamp 1993 | G1/G2 and B64‐I/B64‐II primers | NA | U | Urine | |||||||
| Riediger 2017 | CS | Brazil | 150 | 84.7% | 60.6% | 56.2% | qPCR | Stoddard 2009 | lipL32 | 40 Ct | U | Whole blood | MAT OR culture | Single + paired |
| 29.1% | 87.0% | qPCR | Stoddard 2009 | lipL32 | 40 Ct | U | Serum | |||||||
| Saengjaruk 2002 | CC2 | Thailand | 43 | U | 64.0% | 100% | dot‐ELISA | Saengjaruk 2002 | NA | NA | 5 | Urine | Culture | NA |
| Samsonova 1997 | CC2 | China and Russia | 75 | U | 66.0% | 96.4% | cPCR | Gravekamp 1993 | G1/G2 and B64‐I/B64‐II primers | NA | U | Serum | MAT | U |
| Seng 2007 | CS | Cambodia | 121 | 3.3% | 75.0% | 94.0% | cPCR 2× | U | rrl (23S) | NA | (1) 14, (2) 35 | Serum | MAT OR culture | Single + paired |
| Sonthayanon 2013 | CC1 | Thailand | 250 | 31.8% | 59.0% | 92.0% | qPCR | Slack 2007 | rrs | U | U | Whole blood | MAT OR culture | Single + paired |
| Sukmark 2018 | CS | Thailand | 202 | 42.6% | 22.1% | 77.6% | cPCR | Stoddard 2009 | lipL32 | NA | Cases 4, controls 3 | Urine | MAT OR culture | Single + paired |
| Thaipadunpanit/ Sonthayanon 2011 | CC1 | Thailand | 266 | 31.8% | 55.6% | 89.5% | qPCR | Slack 2007 | rrs | U | 5 | Whole blood | MAT OR culture | Paired only |
| 42.9% | 93.2% | qPCR | Stoddard 2009 | lipL32 | U | 5 | Whole blood | |||||||
| 43.6% | 83.5% | LAMP | Sonthayanon 2011 | rrs | NA | 5 | Whole blood | |||||||
| 37.6% | 90.2% | LAMP | Lin 2009 | lipL41 | NA | 5 | Whole blood | |||||||
| Vanasco 2016 | CC1 | Argentina | 188 | 35.5% | 29.9% | 81.0% | qPCR | Stoddard 2009 | lipL32 | 40 Ct | 5 | Serum/blood | MAT OR IgM ELISA | Single + paired |
| 13.4% | 88.4% | cPCR | Stoddard 2009 | lipL32 | NA | 5 | Serum/blood | |||||||
| Villumsen 2012 BC | CS | Denmark | 29 | 24.1% | 85.7% | 100% | qPCR | Villumsen 2012 | lipL32 | U | U | Blood culture | MAT | Single + paired |
| 100% | 95.5% | qPCR | Smythe 2002 | rrs | U | U | Blood culture | |||||||
| Villumsen 2012 U | CS | Denmark | 54 | 5.6% | 100% | 98.0% | qPCR | Villumsen 2012 | lipL32 | U | U | Urine | MAT | Single + paired |
| 100% | 98.0% | qPCR | Smythe 2002 | rrs | U | U | Urine | |||||||
| Waggoner 2014 | CS | Brazil | 55 | 10.9% | 100% | 4.1% | qPCR | Waggoner 2014 | rrs | 45 Ct | 8 | Plasma/serum | MAT | Single only |
| 100% | 0.0% | qPCR (UFI Assay) | Waggoner 2014 | rrs | 45 Ct | Range 1–19 | Plasma/serum | |||||||
| Waggoner 2015 | CC1 | Brazil | 478 | NA | 9.1% | 92.8% | qPCR (UFI Assay) | Waggoner 2014 | rrs | 45 Ct | U | Serum | MAT | Single only |
| Wangroongsarb 2005 | CS | Thailand | 93 | 16.1% | 80.0% | 96.2% | cPCR | U/Kawabata 2001 | rrs / flaB | NA | U | Whole blood | MAT OR culture | Paired |
| Widiyanti 2013 | CS | Philippines | 44 | 63.6% | 57.1% | 56.3% | cPCR | Kawabata 2001 | flaB | NA | 5.5 | Urine | MAT | Single only |
| 89.3% | 62.5% | Dipstick | Widyanti 2013 | NA | NA | 5.5 | Urine | |||||||
| 96.4% | 56.3% | ICG‐LFA | Widiyanti 2013 | NA | NA | 5.5 | Urine | |||||||
| Woods 2018 | CS | Laos | 766 | 4.4% | 9.4% | 98.5% | qPCR | Slack 2007 | rrs | 40 Ct | 5 | Serum | MAT OR culture | Single + paired |
| 3.0% | 99.0% | qPCR | Slack 2007 | rrs | 40 Ct | 5 | Buffy coat | |||||||
| 17.2% | 90.1% | qPCR | Slack 2007 | rrs | 40 Ct | 5 | Urine | |||||||
| 9.4% | 98.8% | qPCR | Woods 2018 | rrs/lipL32 | 45 Ct | 5 | Serum | |||||||
| 12.1% | 99.0% | qPCR | Woods 2018 | rrs/lipL32 | 45 Ct | 5 | Buffy coat | |||||||
| 13.8% | 99.0% | qPCR | Woods 2018 | rrs/lipL32 | 45 Ct | 5 | Urine | |||||||
| Wu 1996 | CS | China | 19 | 47.4% | 100% | 0.0% | cPCR | Wu 1993 | rrs | NA | U | Serum | MAT OR culture | U |
| Yersin 1998 | CS | Seychelles | 112 | 53.6% | 46.7% | 96.2% | cPCR 2× | Merien 1995 | rrs | NA | (1) 3.9–4.5 (2) ≥ 14 |
Serum | MAT | Paired only |
| Zhang 1992 | U | China | 175 | 75.4% | 100% | 32.6% | cPCR | Zhang 1992 | rrl (23S) | NA | 1–5 | Serum | MAT OR Culture | Paired only |
Summary table of included studies. 95% confidence intervals are not shown. Timing of sample collection (DPO of symptoms) is presented as median numbers or range or interquartile range. aUnderlined are the direct comparisons of index tests. CC1: single‐gate case‐control study; CC2: two‐gate case‐control study; CS: cross‐sectional study; Ct: threshold cycle; DPO: days postonset; EDTA: ; ICG‐LFA: immunochromatography‐based lateral flow assay; IgM ELISA: immunoglobulin G enzyme‐linked immunosorbent assay; LAMP: loop‐mediated isothermal amplification; MAT: microscopic agglutination test; MAT OR … OR …: a positive result of any one of these tests is considered a leptospirosis case; PCR: polymerase chain reaction; cPCR: conventional PCR; N PCR: nested PCR; NA: not applicable; cPCR 2x: conventional PCR performed twice at different moments in time; qPCR: real‐time PCR; U: unknown.
The index tests evaluated were conventional PCR (henceforth PCR; 17 studies), real‐time PCR (18 studies), nested PCR (four studies), PCR performed twice (performed twice on each participant at different DPO and regarded as positive if at least one result was positive; two studies), LAMP (two studies), ELISA (one study), dot‐ELISA (one study), immunochromatography‐based lateral flow assay (ICG‐based LFA; one study), and dipstick assay (one study) (see Table 8). Five studies directly compared tests in the same population: PCR versus real‐time PCR (Vanasco 2016), PCR versus nested PCR (Blanco 2014), nested PCR versus real‐time PCR (Merien 2005), real‐time PCR versus LAMP (Thaipadungpanit 2011), and ICG‐based IFA versus dipstick assay versus PCR (Widiyanti 2013).
4. Overview of index tests included in the review.
| Index test | Studies | Samplesa | Target genes/primers (original reference)a | Timing of sample collection, # days post onset of symptomsa | Threshold |
| Conventional PCR | 17 | Whole bloodb or serum (1) Serum (9) Whole bloodb (4) Blood product, unspecified (1) Urine (5) |
Testing on blood products
Testing on urine
|
Testing on blood products
Testing on urine
|
NA |
| Real‐time PCR | 18 | Blood or serum (3) Serum or plasma (9) Whole bloodb (6) Blood culture (1) Buffy coat (1) Urine (2) |
Testing on blood products
Testing on urine
Testing on blood culture
Testing on buffy coat
|
Testing on blood products
Testing on urine
Testing on blood culture
Testing on buffy coat
|
Testing on blood products:
Testing on urine:
Testing on blood culture:
Testing on buffy coat:
|
| Nested PCR | 4 | Serum (4) |
rrs (Merien 1992) (3) flaB (Kawabata 2001 /Koizumi 2008) (1) lipL32 (Bomfim 2008) (1) |
All samples 7 (1) Median 5 (1) Not reported (2) |
NA |
| PCR 2× | 2 | Serum (2) |
rrl (23S) (unknown) (1) rrs (Merien 1995) (1) |
Median of 1st: 14, 2nd: 35 (1) 1st: 4, 2nd: 18 (1) |
NA |
| LAMP | 2 | Whole bloodb (1) Plasma (1) Urine (1) |
rrs (Sonthayanon 2011) (1) rrs (Koizumi 2012) (1) lipL41 (Lin 2009) (1) |
Cases: median 4, controls: median 6 (1) Median 6.5 (1) |
NA |
| ELISA | 1 | Urine (1) | NA | Not reported (1) | Not reported (1) |
| Dot‐ELISA | 1 | Urine (1) | NA | Not reported (1) | NA |
| ICG‐based LFA | 1 | Urine (1) | NA | Median 5.5 (1) | NA |
| Dipstick | 1 | Urine (1) | NA | Median 5.5 (1) | NA |
aNumbers between parentheses indicate the number of studies. bFor whole blood, EDTA blood was used in all studies. Ct: threshold cycle; ELISA: enzyme‐linked immunosorbent assay; ICG‐based LFA: immunochromatography‐based lateral flow assay; LAMP: loop‐mediated isothermal amplification; NA: not applicable; PCR: polymerase chain reaction; PCR 2×: PCR performed twice at different moments in time;
We observed high heterogeneity regarding the characteristics of the participants, the execution of the index tests, and the choice of the reference standards. Most of the participants were from (sub)tropical countries, and prevalence of leptospirosis in the study population ranged from 3.3% (Seng 2007; Cambodia) to 84.7% (Riediger 2017; Brazil) (median 32.5%; interquartile range (IQR) 18.7 to 46.7; computed from only cross‐sectional studies or with data from the original cohort studies). All participants were reported to be suspect of having leptospirosis, but symptoms were often not reported. Most commonly reported symptoms consisted of fever, myalgia, headaches, malaise, and jaundice. Some studies were reportedly conducted in an outbreak setting (Samsonova 1997; Ananyina 2000; Céspedes 2007; Agampodi 2012; Kitashoji 2015; Agampodi 2016). Antibiotic use was often unreported, but eight studies gave antibiotics to some participants before the index test (Yersin 1998; Ananyina 2000; Seng 2007; Koizumi 2009; Thaipadungpanit 2011; Sonthayanon 2013; Kitashoji 2015; Woods 2018).
Regarding the index test, timing of sample collection was often not reported, and reported DPOs differed substantially between studies (Table 8). We also identified a large variety of primers or target genes used in the PCR, real‐time PCR, and LAMP. None of the nucleic acid or antigen detection tests included in this review were commercially available. The variation in the choice of reference standard and its methodological significance will be discussed in methodological quality of included studies.
Excluded studies
We excluded 127 records after full‐text assessment. One hundred and two records were excluded for one of five main reasons: not a diagnostic test accuracy study, animal studies, inclusion of healthy controls, use of only culture as reference standard, and no distinction between different sample types for the index test. Twenty‐five records were considered potentially eligible but were excluded for the following main reasons: no two‐by‐two table data (11 records), full‐text article not retrievable (six records), sample types were not separately analysed (three records), target condition being leptospiral uveitis (two records), MAT was tested with CSF (one record; we were uncertain whether this was an appropriate reference standard), MAT was not the reference standard (one record), and PCR was part of the reference standard (one record).
We excluded an additional 13 studies after data collection for one of the following reasons: sample types were not separately analysed (three studies), MAT was tested with CSF (one study), no two‐by‐two table data (two studies), only index test positives being included in the two‐by‐two table (one study), data in table and text disagree (two studies), sample size fewer than 10 (one study), same study as a previously included study (one study), and healthy controls (one study) (see Characteristics of excluded studies table).
Methodological quality of included studies
We assessed methodological quality using the QUADAS‐2 tool. See Figure 4 and Figure 5 for quality assessment results of PCR, Figure 6 and Figure 7 for real‐time PCR, and Figure 8 and Figure 9 for all other tests. Overall, the reporting of quality items was poor; therefore, it remained difficult to quantify the risk of bias in included studies.
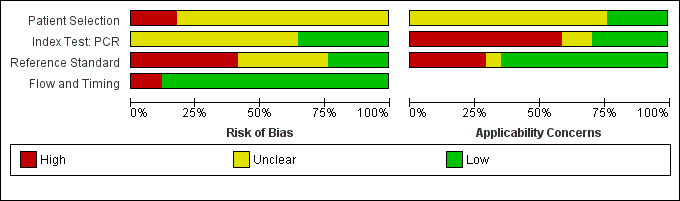
4.
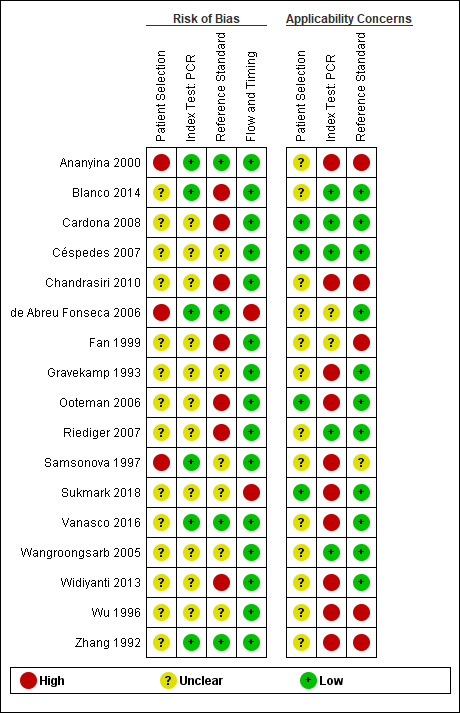
All conventional polymerase chain reaction (PCR) studies: risk of bias and applicability concerns. Sukmark 2018 and Widiyanti 2013 were not part of the PCR (blood products) meta‐analysis.
5.
All conventional polymerase chain reaction (PCR) studies: risk of bias and applicability concerns graph.
6.
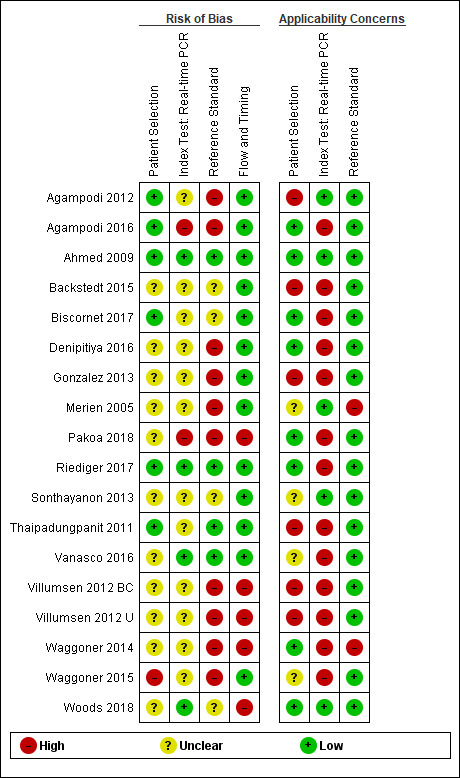
All real‐time polymerase chain reaction (PCR) studies: risk of bias and applicability concerns. Villumsen 2012 BC and Villumsen 2012 U were not part of the real‐time PCR (blood products) meta‐analysis.
7.
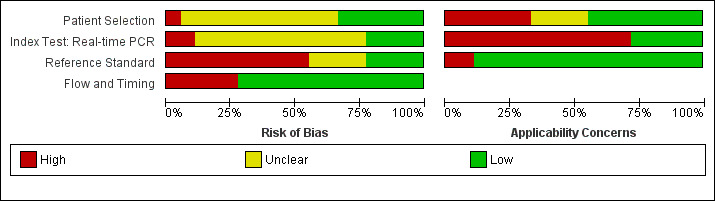
All real‐time polymerase chain reaction (PCR) studies: risk of bias and applicability concerns graph.
8.
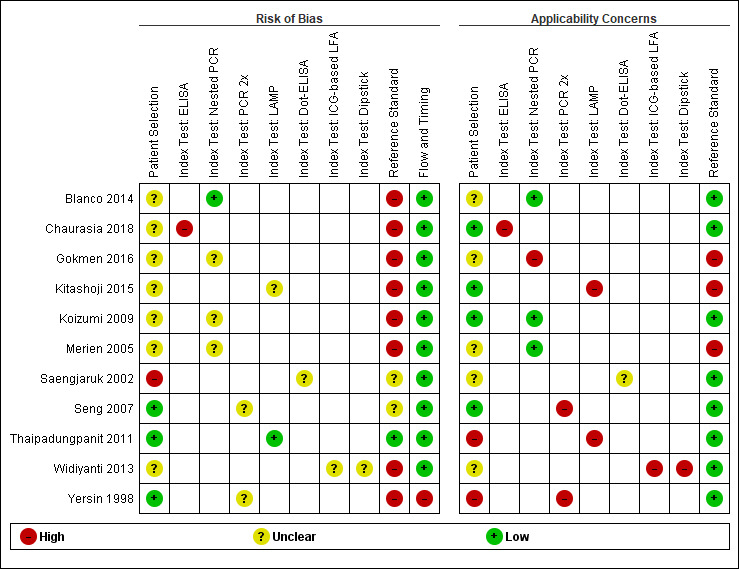
Studies of nested polymerase chain reaction (PCR), PCR performed twice (PCR 2×), loop‐mediated isothermal amplification (LAMP), enzyme‐linked immunosorbent assay (ELISA), dot‐ELISA, immunochromatography‐based lateral flow assay (ICG‐based LFA), and dipstick assay: risk of bias and applicability concerns.
9.
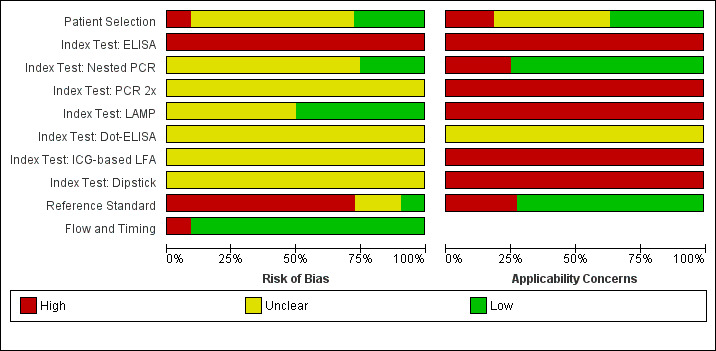
Studies of nested polymerase chain reaction (PCR), PCR performed twice (PCR 2×), loop‐mediated isothermal amplification (LAMP), enzyme‐linked immunosorbent assay (ELISA), dot‐ELISA, immunochromatography‐based lateral flow assay (ICG‐based LFA), and dipstick assay: risk of bias and applicability concerns
Risk of bias
Patient selection
Eight studies had a single‐gate design (six cross‐sectional studies, two single‐gate case‐control studies) with consecutive or random enrolment, and therefore, they were rated at low risk (Yersin 1998; Seng 2007; Ahmed 2009; Thaipadungpanit 2011; Agampodi 2012; Agampodi 2016; Biscornet 2017; Riediger 2017). However, in most studies, participant selection process was not or only very briefly described, leading to frequent 'unclear risk' judgements. Four studies employed a two‐gate design and the risk of bias was therefore considered high (Samsonova 1997; Ananyina 2000; Saengjaruk 2002; de Abreu Fonseca 2006).
Index test
Studies seldom reported blinding of index test interpreters for reference standard results. Eight studies ensured adequate blinding (Samsonova 1997; Ananyina 2000; de Abreu Fonseca 2006; Ahmed 2009; Thaipadungpanit 2011; Blanco 2014; Vanasco 2016; Woods 2018), and two studies did the index test before the reference standard (Zhang 1992; Riediger 2017). No study reported that interpreters were unblinded. We considered the positivity threshold to be prespecified if readout methods for the index test led to a binary outcome (i.e. yes or no). This was the case for all index tests except real‐time PCR and ELISA. Eight of 18 studies prespecified Cts for the real‐time PCR (Ahmed 2009; Waggoner 2014; Waggoner 2015; Denipitiya 2016; Vanasco 2016; Biscornet 2017; Riediger 2017; Woods 2018).
Reference standard
We considered the risk of bias of the reference standard to be high in 22 studies. Only seven of 41 studies were at low risk for this domain (Zhang 1992; Ananyina 2000; de Abreu Fonseca 2006; Ahmed 2009; Thaipadungpanit 2011; Vanasco 2016; Riediger 2017). Following QUADAS‐2, we judged this domain based on two aspects: choice of reference standard and blinding of interpreters to index test results.
MAT was the sole reference standard in 22 studies with a single‐gate design (17 cross‐sectional studies, five single‐gate case‐controls studies) (Yersin 1998; Fan 1999; Merien 2005; Ooteman 2006; Riediger 2007; Cardona 2008; Koizumi 2009; Chandrasiri 2010; Agampodi 2012; Villumsen 2012 BC; Villumsen 2012 U; Gonzalez 2013; Widiyanti 2013; Blanco 2014; Waggoner 2014; Kitashoji 2015; Waggoner 2015; Agampodi 2016; Denipitiya 2016; Gokmen 2016; Chaurasia 2018; Pakoa 2018), which we regarded as high risk due to its imperfect sensitivity. Ten single‐gate studies used a composite reference standard: two studies used MAT and IgM ELISA (Vanasco 2016; Biscornet 2017), 10 studies used MAT and culturing (Zhang 1992; Wu 1996; Wangroongsarb 2005; Seng 2007; Thaipadungpanit 2011; Sonthayanon 2013; Backstedt 2015; Riediger 2017; Sukmark 2018; Woods 2018), and two studies used all three (Céspedes 2007; Ahmed 2009).
As an additional criterion, we required MAT to include paired samples for the judgement 'low risk'. All but one study (Wu 1996) with a composite reference standard fulfilled this criterion. When MAT alone or culture alone was used as the reference standard in two‐gate designs, risk of bias was considered low.
Two studies reported blinding of the reference standard interpreters, in which the blinding was adequate (Ahmed 2009; Riediger 2017). We also considered blinding to be adequate in nine studies in which the reference standard was done before the index test (Samsonova 1997; Ananyina 2000; de Abreu Fonseca 2006; Thaipadungpanit 2011; Villumsen 2012 BC; Villumsen 2012 U; Waggoner 2014; Waggoner 2015; Vanasco 2016).
Flow and timing
Risk of bias for flow and timing was low for 32 studies and unclear for one study (Riediger 2007). Eight studies were considered high risk, as they did not include all patients in the analysis, with reasons varying from decisions by clinicians not to request MAT to exclusion based on inadequate urine samples (Yersin 1998; de Abreu Fonseca 2006; Villumsen 2012 BC; Villumsen 2012 U; Waggoner 2014; Pakoa 2018; Sukmark 2018; Woods 2018). All two‐gate studies did not apply the same reference standards for cases and controls (Samsonova 1997; Ananyina 2000; Saengjaruk 2002; de Abreu Fonseca 2006). However, we did not consider this as differential verification bias, as differential verification bias implies that the choice of reference standard depended on the result of the index test, which was not the case in these studies.
Concerns regarding applicability of results to clinical practice
Concerns regarding the representativeness of the patient population
As studies were largely heterogeneous in their population, our standard for a representative patient population was low. We considered the patient population to be representative if patients with both single and paired samples were included, and if the patient characteristics did not differ significantly from the expected recipients of the test in practice (e.g. not all patients were female, or not all had severe renal failure). However, 17 studies did not provide sufficient description regarding patient selection methods or characteristics, leading to frequent 'unclear concern' judgements (Zhang 1992; Gravekamp 1993; Wu 1996; Samsonova 1997; Fan 1999; Ananyina 2000; Saengjaruk 2002; Merien 2005; Wangroongsarb 2005; de Abreu Fonseca 2006; Riediger 2007; Chandrasiri 2010; Widiyanti 2013; Blanco 2014; Waggoner 2015; Gokmen 2016; Vanasco 2016). We had high concerns for four studies that excluded patients with only a single blood sample instead of paired samples (Yersin 1998; Thaipadungpanit 2011; Agampodi 2012; Gonzalez 2013). Although verification by MAT is more accurate with paired samples, excluding patients with single samples may not reflect a representative clinical population, as they may have been patients with a severe disease course who did not survive until the second blood sampling. We also had high concerns for one study that included only patients with a strong suspicion for advanced severe leptospirosis (Backstedt 2015), and two studies that excluded patients that had used antibiotics (Villumsen 2012 BC; Villumsen 2012 U).
Concerns regarding the representativeness and reproducibility of the index test
Defining representativeness was difficult for the index test, since all of the included tests were inhouse tests. Since we assumed that only fresh patient samples would be used for testing in clinical practice, we defined the concern as being high when the studies used frozen samples. This was the case for 22 studies (Gravekamp 1993; Samsonova 1997; Yersin 1998; Ooteman 2006; Seng 2007; Thaipadungpanit 2011; Agampodi 2012; Villumsen 2012 BC; Villumsen 2012 U; Gonzalez 2013; Widiyanti 2013; Waggoner 2014; Backstedt 2015; Kitashoji 2015; Waggoner 2015; Agampodi 2016; Denipitiya 2016; Gokmen 2016; Vanasco 2016; Biscornet 2017; Riediger 2017; Sukmark 2018). We also had concerns regarding applicability in one study, which added salt buffer to patient samples (Wu 1996). Five studies failed to provide detailed descriptions of the execution of the index test, leading us to have high concern whether repetition would be possible (Zhang 1992; Ananyina 2000; Chandrasiri 2010; Chaurasia 2018; Pakoa 2018).
Concerns regarding the reproducibility of the reference standard
We also applied 'high concern' judgements for the reference standard when studies failed to provide detailed description of the execution of the reference standard (Zhang 1992; Wu 1996; Fan 1999; Ananyina 2000; Merien 2005; Chandrasiri 2010; Waggoner 2014; Kitashoji 2015; Gokmen 2016).
Findings
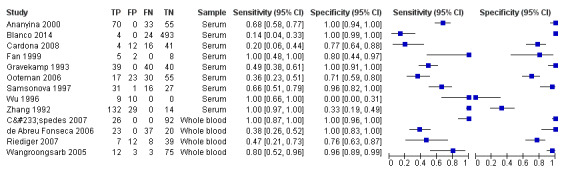
Conventional polymerase chain reaction
Seventeen studies reported test accuracy data for the PCR. Fifteen studied PCR on blood products (serum: nine studies: whole blood (ethylenediaminetetraacetic acid (EDTA)): four studies; blood or serum: one study; unspecified blood product: one study) and of them, three also studied PCR on urine. Two studies included exclusively urine samples. The sensitivity of PCR on blood products ranged from 13% to 100%, and the specificity from 0% to 100% (see Figure 10). The 12 studies analysing PCR on blood products did not report the timing of sample collection for the index test (further referred to as DPO). Three studies did report the DPO: one reported a mean of five days (Vanasco 2016), one reported a range of one to five days (Zhang 1992), and one a range of one to seven days (Céspedes 2007).
10.
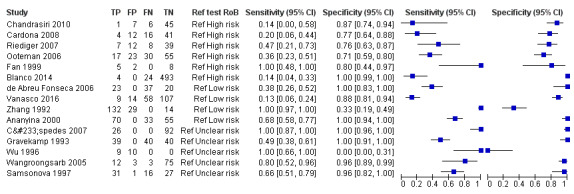
Forest plot of conventional polymerase chain reaction (PCR) on blood products. Ref test RoB: risk of bias for the 'reference standard' domain.
The sensitivity of PCR on urine ranged from 22% to 57%, and the specificity from 56% to 100% (see Figure 11).
11.
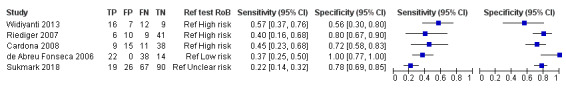
Forest plot of conventional polymerase chain reaction (PCR) on urine. Ref test RoB: risk of bias for the 'reference standard' domain.
Overall meta‐analysis
We conducted a meta‐analysis of PCR on blood products (see Figure 12). Using the bivariate model based on 15 studies (1884 participants, 660 with and 1224 without leptospirosis), the pooled sensitivity of PCR on blood products was 70% (95% CI 37% to 90%) and the pooled specificity was 95% (95% CI 75% to 99%). Based on a median prevalence of leptospirosis of 32.5%, the positive post‐test probability (PPP) was 87% (95% CI 53% to 97%) and the negative post‐test probability (NPP) was 87% (95% CI 71% to 95%). The positive likelihood ratio was 13.56 (95% CI 2.61 to 70.29) and the negative likelihood ratio was 0.32 (95% CI 0.12 to 0.82). There were too few studies for the PCR on urine to conduct a meaningful meta‐analysis.
12.
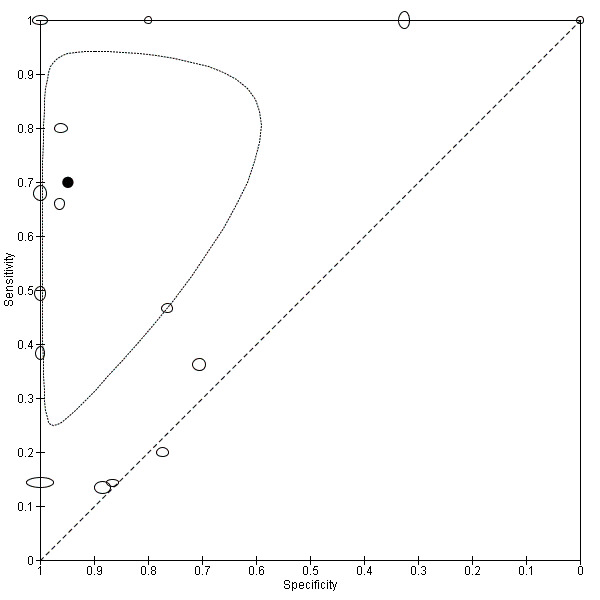
Summary ROC plot for conventional polymerase chain reaction (PCR) on blood products.
Transparent dots indicate the test accuracy of the individual studies included in the analysis; the black dot indicates the pooled test accuracy. The ellipse around the pooled test accuracy is the 95% confidence region. The size of the transparent dots represents the sample size, with the vertical diameter representing the number of cases and horizontal diameter representing the number of non‐cases.
Investigations of heterogeneity
We assessed heterogeneity only for PCR on blood products. We planned to investigate the following sources of heterogeneity: timing of sample collection, prevalence, blood sample type, target gene/primer, and brand of test.
Timing of sample collection: timing of sample collection was usually unreported (12 studies) and the subgroups were too small to investigate heterogeneity.
Prevalence: we investigated whether prevalence of leptospirosis was associated with test accuracy. Studies with a higher prevalence of leptospirosis had a significantly lower specificity (P = 0.0004). Prevalence was not associated with sensitivity (P = 0.2).
Blood sample type for the index test: we included four studies using whole blood and nine studies using serum (see Figure 13). The pooled sensitivity of whole blood was 78% (95% CI 22% to 98%) and pooled specificity was 99% (95% CI 61% to 100%), and the pooled sensitivity of serum was 78% (95% CI 37% to 96%) and pooled specificity was 93% (95% CI 50% to 100%), meaning that these subgroups did not differ significantly from each other.
Target gene/primers: a large variety of target genes and primers were used for the PCR (see Table 8), but the subgroups were too small to investigate heterogeneity.
Brand of the index test: all tests were inhouse tests.
13.
Forest plot of conventional polymerase chain reaction (PCR) on whole blood versus conventional PCR on serum.
Sensitivity analyses
See Table 9 for an overview of the analyses.
5. Pooled sensitivity and specificity of the conventional polymerase chain reaction (PCR) overall meta‐analysis and the sensitivity analyses.
| Analysis | Sensitivity (95% CI) | Specificity (95% CI) |
| Overall meta‐analysis PCR | 70% (37% to 90%) | 95% (75% to 99%) |
| Exclusion of studies at 'high risk of bias' for patient selection | 76% (31% to 96%) | 91% (60% to 98%) |
| Exclusion of studies at 'high risk of bias' for reference standard | 87% (44% to 98%) | 97% (60% to 100%) |
| Exclusion of studies that reported the use of antibiotics | 71% (34% to 92%) | 93% (71% to 99%) |
| Meta‐analysis with the lower MAT threshold dataset | 70% (36% to 90%) | 95% (75% to 99%) |
| Exclusion of studies that were only reported as abstracts | 74% (40% to 93%) | 96% (74% to 99%) |
CI: confidence intervals; MAT: microscopic agglutination test.
Risk of bias: we excluded three studies that were at high risk of bias in the 'patient selection' domain of QUADAS‐2 (Samsonova 1997; Ananyina 2000; de Abreu Fonseca 2006). This resulted in a pooled sensitivity of 76% (95% CI 31% to 96%) and pooled specificity of 91% (95% CI 60% to 98%), showing no important difference from the overall meta‐analysis. Likewise, we excluded six studies with high risk of bias in the 'reference standard' domain (Fan 1999; Ooteman 2006; Riediger 2007; Cardona 2008; Chandrasiri 2010; Blanco 2014). The resulting pooled sensitivity was 87% (95% CI 44% to 98%) and pooled specificity was 97% (95% CI 60% to 100%). While the sensitivity of the PCR increased, the CIs were very wide, with substantial overlap with the results of the overall meta‐analysis.
Antibiotic use: we excluded one study that reported the use of antibiotics in the patient population (Ananyina 2000). Sensitivity analysis with the 14 remaining studies (13 did not report on antibiotic use, and one study reported that antibiotics were not used (Chandrasiri 2010)) resulted in a pooled sensitivity of 71% (95% CI 34% to 92%) and pooled specificity of 93% (95% CI 71% to 99%). These results did not differ from the overall meta‐analysis.
Lower MAT threshold: two studies reported each two threshold values for the MAT (Ooteman 2006; Cardona 2008). For the overall analyses, we selected the higher threshold dataset. Sensitivity analysis with the lower threshold dataset in these two studies made no difference to the findings (pooled sensitivity: 70%, 95% CI 36% to 90%; pooled specificity: 95%, 95% CI 75% to 99%).
Abstract‐only study: we repeated the analysis excluding one study that was only reported as an abstract (Chandrasiri 2010). The pooled sensitivity was 74% (95% CI 40% to 93%) and the pooled specificity was 96% (95% CI 74% to 99%), demonstrating no important change from the overall meta‐analysis.
Comparison of different conventional polymerase chain reaction methods
Four studies reported direct comparisons (i.e. comparisons between different conventional PCR methods studied in the same study population): different timing of sample collection (one study) and different sample types for the PCR (three studies).
-
Timing of sample collection: Céspedes 2007 compared the results of PCR on whole blood when samples from three different time frames were tested: 1 DPO to 7 DPO, 8 DPO to 9 DPO and 1 DPO to 9 DPO.
For 1 DPO to 7 DPO, sensitivity of PCR was 100% (95% CI 87% to 100%) and specificity was 100% (95% CI 96% to 100%).
For 8 DPO to 9 DPO, sensitivity was 30% (95% CI 18% to 45%) and specificity was 100% (95% CI 74% to 100%).
For 1 DPO to 9 DPO, sensitivity was 55% (95% CI 43% to 67%) and specificity was 100% (95% CI 97% to 100%).
-
Sample types for PCR: the reported direct comparisons were serum versus urine (Cardona 2008) and whole blood versus urine (de Abreu Fonseca 2006; Riediger 2007).
In Cardona 2008, the sensitivity of serum PCR was 20% (95% CI 6% to 44%) and specificity was 77% (95% CI 64% to 88%), and sensitivity of urine PCR was 45% (95% CI 23% to 68%) and specificity was 72% (95% CI 58% to 83%).
In de Abreu Fonseca 2006, the sensitivity of whole blood PCR was 38% (95% CI 26% to 52%) and specificity was 100% (95% CI 83% to 100%), and sensitivity of urine PCR was 37% (95% CI 25% to 40%) and specificity was 100% (95% CI 77% to 100%).
In Riediger 2007, the sensitivity of whole blood PCR was 47% (95% CI 21% to 73%) and specificity was 76% (95% CI 63% to 87%), and sensitivity of urine PCR was 40% (95% CI 16% to 68%) and specificity was 80% (95% CI 67% to 90%).
Real‐time polymerase chain reaction
Eighteen studies assessed the accuracy of the real‐time PCR. Sixteen studies used blood products as sample type: serum or plasma (nine studies), whole blood (six studies), serum or whole blood (three studies), blood culture samples (one study), and buffy coat samples (one study). Two studies used urine samples. Six studies each reported two sets of data: Agampodi 2012 and Riediger 2017 reported data for whole blood and serum, while Backstedt 2015, Thaipadungpanit 2011, and Woods 2018 reported data for two real‐time PCRs, each using a different target gene (rrs, lipL32, or rrs/lipL32). Waggoner 2014 reported data for a monoplex and multiplex (detecting also dengue and malaria) real‐time PCR. Ahmed 2009 reported three sets of data, each evaluating the test at different DPOs (1 DPO to 4 DPO, 5 DPO to 10 DPO, and 1 DPO to 10 DPO). Because we considered 1 DPO to 10 DPO to be the most representative time of sample collection, we included the dataset of 1 DPO to 10 DPO in the meta‐analysis. Seven studies of real‐time PCR on blood products did not report the DPO (Gonzalez 2013; Sonthayanon 2013; Backstedt 2015; Waggoner 2015; Biscornet 2017; Riediger 2017; Pakoa 2018). The other studies all reported DPOs of the index test under 10 days.
The sensitivity of real‐time PCR on blood products ranged from 0% to 100%, and the specificity ranged from 0% to 100% (Figure 14).
14.
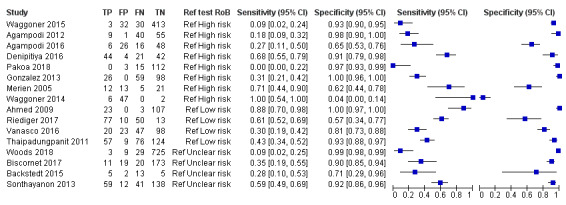
Forest plot of real‐time polymerase chain reaction (PCR) on blood products. Ref test RoB: risk of bias for the 'reference standard' domain.
Two studies assessed real‐time PCR on urine (Villumsen 2012 U; Woods 2018). One study assessed real‐time PCR on blood culture samples (Villumsen 2012 BC), and one study assessed real‐time PCR on buffy coat samples (Woods 2018). Due to the lack of enough studies for urine, blood culture, and buffy coat real‐time PCR, we did not perform a meta‐analysis. The sensitivities and specificities of these PCRs are displayed in Table 10.
6. Sensitivity and specificity of real‐time polymerase chain reaction (PCR) on urine, blood culture, and buffy coat samples.
| Study ID | Sample type | Target gene | Sensitivity | Lower CI | Upper CI | Specificity | Lower CI | Upper CI |
| Woods 2018 | Urine | rrs (Slack 2007) | 17% | 6% | 36% | 90% | 87% | 92% |
| Woods 2018 | Urine | rrs/lipL32 (Woods 2018) | 14% | 4% | 32% | 99% | 98% | 100% |
| Villumsen 2012 U | Urine | rrs (Smythe 2002) | 100% | 29% | 100% | 98% | 90% | 100% |
| Villumsen 2012 U | Urine | lipL32 (Villumsen 2012) | 100% | 29% | 100% | 98% | 90% | 100% |
| Villumsen 2012 BC | Blood culture | rrs (Smythe 2002) | 100% | 59% | 100% | 95% | 77% | 100% |
| Villumsen 2012 BC | Blood culture | lipL32 (Villumsen 2012) | 86% | 42% | 100% | 100% | 85% | 100% |
| Woods 2018 | Buffy coat | rrs (Slack 2007) | 3% | 0% | 16% | 99% | 98% | 100% |
| Woods 2018 | Buffy coat | rrs/lipL32 (Woods 2018) | 12% | 3% | 28% | 99% | 98% | 100% |
CI: 95% confidence intervals.
Overall meta‐analysis
We conducted a meta‐analysis only for real‐time PCR on blood products using the HSROC model. As described previously, seven studies reported multiple data sets. However, each study may only contribute a single data set to the meta‐analysis to prevent the study from being over‐represented. Therefore, we randomly excluded the serum dataset of Agampodi 2012, the rrs dataset of Backstedt 2015, the rrs dataset of Thaipadungpanit 2011, the serum dataset of Riediger 2017, the rrs dataset of Woods 2018, and the multiplex PCR dataset of Waggoner 2014. For reasons mentioned earlier, we included only one of the datasets of Ahmed 2009 (1 DPO to 10 DPO) in the analysis.
The analysis included 16 studies with 3210 participants (826 with and 2384 without leptospirosis) (Figure 14; Figure 15). Because we anticipated that the thresholds of the real‐time PCRs in the included studies would differ, we refrained from estimating a summary point. Instead, we constructed a summary curve. The summary curve is a graph of the values of sensitivity and specificity that are obtained by varying the threshold across all possible values. To illustrate, we estimated the accuracy for three fixed specificity values of 85%, 90% and 95%. At 85% specificity, pooled sensitivity was 49% (95% CI 30% to 68%); at 90% specificity, pooled sensitivity was 40% (95% CI 24% to 59%); and at 95% specificity, pooled sensitivity was 29% (95% CI 15% to 49%). The median specificity of real‐time PCR on blood products was 92%. The CIs were wide due to the heterogeneity of included studies. We did not estimate post‐test probabilities or likelihood ratios for the real‐time PCR as it would be unclear to which threshold values these estimates would correspond.
15.
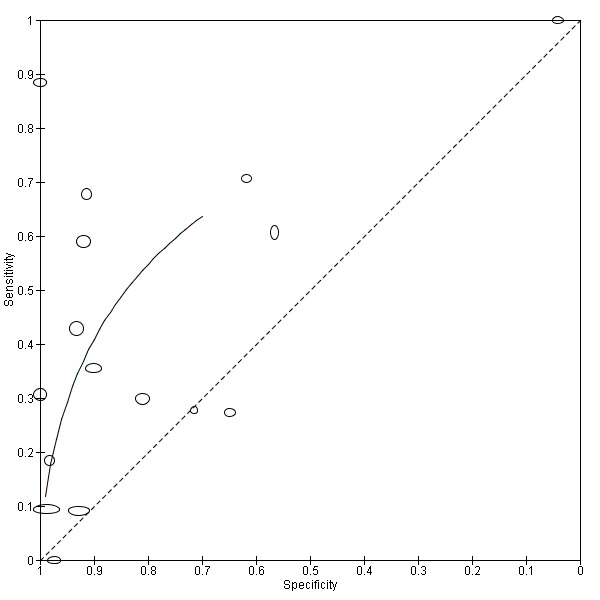
Summary ROC plot for real‐time polymerase chain reaction (PCR) on blood products.
Transparent dots indicate the test accuracy of the individual studies included in the analysis. The solid black line (summary ROC curve) is a graph of the values of sensitivity and specificity that are obtained by varying the threshold across all possible values.The size of the transparent dots represents the sample size, with the vertical diameter representing the number of cases and horizontal diameter representing the number of non‐cases.
Investigations of heterogeneity
We restricted assessment of heterogeneity to real‐time PCR on blood products. In summary, none of the analyses yielded a statistically significant difference.
Timing of sample collection: we could not investigate timing of sample collection as a source of heterogeneity; although it was reported in nine studies, the reporting was too heterogeneous to form adequate subgroups (Table 8Merien 2005; Ahmed 2009; Thaipadungpanit 2011; Agampodi 2012; Waggoner 2014; Agampodi 2016; Denipitiya 2016; Vanasco 2016; Woods 2018).
Prevalence: prevalence was not associated with test accuracy (P = 0.96).
Blood sample type for the index test: nine studies used 'serum or plasma' (Merien 2005; Agampodi 2012; Gonzalez 2013; Waggoner 2014; Waggoner 2015; Biscornet 2017; Riediger 2017; Pakoa 2018; Woods 2018), and six studies used whole blood (Thaipadungpanit 2011; Agampodi 2012; Sonthayanon 2013; Backstedt 2015; Denipitiya 2016; Riediger 2017). There was no statistically significant association between sample type and accuracy (P = 0.42).
Target gene/primer: Table 8 shows an overview of the target genes for the real‐time PCR. Due to the small number of studies in each subgroup, we refrained from analysing the effect of different primers.
Threshold: reported thresholds for the real‐time PCR were 35 Ct, 40 Ct, and 45 Ct. Due to the small number of studies in each subgroup, we refrained from analysing their effect. However, we used the HSROC model to take the threshold effect into account.
Real‐time PCR visualisation method: there was no statistically significant difference (P = 0.058) in the accuracy between studies of real‐time PCR using SYBR green (five studies: Merien 2005; Ahmed 2009; Gonzalez 2013; Backstedt 2015; Denipitiya 2016), and studies of real‐time PCR using TaqMan probes (12 studies: Thaipadungpanit 2011; Agampodi 2012; Sonthayanon 2013; Waggoner 2014; Backstedt 2015; Waggoner 2015; Agampodi 2016; Vanasco 2016; Biscornet 2017; Riediger 2017; Pakoa 2018; Woods 2018). The pooled diagnostic odds ratio (DOR) for the SYBR green real‐time PCR was 46.2 (95% CI 0.89 to 2383.68), while the pooled DOR for the TaqMan real‐time PCR was 3.09 (95% CI 1.25 to 7.63).
Brand of the index test: there were no variations among studies regarding the brand of the test, as all were inhouse tests.
Sensitivity analyses
Risk of bias: according to our QUADAS‐2 judgements, one study had high risk of bias for the 'patient selection' domain (Waggoner 2015). Exclusion of this study from the meta‐analysis yielded a pooled sensitivity of 33% (95% CI 18% to 52%) at a fixed specificity of 95% (Table 11). When we excluded eight studies that had high risk of bias for the 'reference standard' domain (Merien 2005; Agampodi 2012; Gonzalez 2013; Waggoner 2014; Waggoner 2015; Agampodi 2016; Denipitiya 2016; Pakoa 2018), the pooled sensitivity was 37% (95% CI 15% to 66%) at a fixed specificity of 95%. Both analyses did not introduce important changes to the overall result.
Alternative datasets for the overall meta‐analysis: we repeated the analyses with datasets which were previously randomly excluded from the overall meta‐analysis. These are the serum dataset of Agampodi 2012 and Riediger 2017; the rrs dataset of Backstedt 2015, Thaipadungpanit 2011, and Woods 2018; and the multiplex PCR dataset of Waggoner 2014. The repeated analyses with these datasets made no difference to the findings (pooled sensitivity 32%, 95% CI 17% to 52% at a fixed specificity of 95%).
Antibiotic use: we excluded three studies in which participants used antibiotics before the index test (Thaipadungpanit 2011; Sonthayanon 2013; Woods 2018). The pooled sensitivity was 28% (95% CI 12% to 53%) at a fixed specificity of 95%, demonstrating no important change from the overall meta‐analysis.
Lower MAT threshold: sensitivity analysis with the lower MAT threshold dataset in two studies did not lead to different results (Waggoner 2015; Denipitiya 2016). The pooled sensitivity was 29% (95% CI 16% to 47%) at a fixed specificity of 95%.
7. Pooled sensitivity and specificity of the real‐time polymerase chain reaction (PCR) overall meta‐analysis and the sensitivity analysis.
| Analysis | Sensitivity (95% CI) | Specificity (fixed at 95%) |
| Overall meta‐analysis real‐time PCR | 29% (15% to 49%) | 95% |
| Exclusion of studies at 'high risk of bias' for patient selection | 33% (18% to 52%) | 95% |
| Exclusion of studies at 'high risk of bias' for reference standard | 37% (15% to 66%) | 95% |
| Alternative datasets for the overall meta‐analysis | 32% (17% to 52%) | 95% |
| Exclusion of studies in which participants used antibiotics | 28% (12% to 53%) | 95% |
| Meta‐analysis with the lower MAT threshold dataset | 29% (16% to 47%) | 95% |
CI: confidence intervals; MAT: microscopic agglutination test
Comparison of different real‐time polymerase chain reaction methods
We identified several direct comparisons pertaining to sample type, timing of sample collection, and target genes.
-
Timing of sample collection: Ahmed 2009 compared samples collected at 1 DPO to 4 DPO, 5 DPO to 10 DPO, and 1 DPO to 10 DPO.
At 1 DPO to 4 DPO, sensitivity was 100% (95% CI 74% to 100%) and specificity was 100% (95% CI 94% to 100%).
At 5 DPO to 10 DPO, sensitivity was 69% (95% CI 41% to 89%) and specificity was 100% (95% CI 92% to 100%).
At 1 DPO to 10 DPO, sensitivity was 88% (95% CI 70% to 98%) and specificity was 100% (95% CI 97% to 100%).
Sample type: Agampodi 2012 and Riediger 2017 compared whole blood samples with serum samples. In Agampodi 2012, sensitivity of whole blood real‐time PCR was 18% (95% CI 9% to 32%) and specificity was 98% (95% CI 90% to 100%), and sensitivity of serum was 51% (95% CI 36% to 66%) and specificity was 98% (95% CI 90% to 100%). In Riediger 2017, sensitivity of whole blood was 61% (95% CI 52% to 69%) and specificity was 57% (95% CI 34% to 77%), and sensitivity of serum was 29% (95% CI 21% to 38%) and specificity was 87% (95% CI 66% to 97%). Woods 2018 reported direct comparisons of serum, buffy coat, and urine samples in two different real‐time PCRs (one targeting rrs (Slack 2007) and one targeting rrs/lipL32 (Woods 2018)). For clarity, the six pairs of sensitivity and specificity of Woods 2018 are shown in Table 12.
Target gene/primer: four studies compared rrs and lipL32 target genes in the same population (Thaipadungpanit 2011; Villumsen 2012 BC; Villumsen 2012 U; Backstedt 2015). The results of the four studies are displayed in Table 13. Woods 2018 also reported comparisons of rrs (Slack 2007) and rrs/lipl32 (Woods 2018) real‐time PCRs on serum, buffy coat, and urine samples, which are shown in Table 12.
Waggoner 2014 compared two types of real‐time PCR in the same population, namely a multiplex real‐time PCR for leptospirosis, dengue, and malaria, and a monoplex real‐time PCR which used the same primer from the multiplex assay combined with a new probe for pathogenic leptospires. The sensitivity of the multiplex real‐time PCR was 100% (95% CI 54% to 100%) and specificity was 0% (95% CI 0% to 7%) and the sensitivity of the monoplex real‐time PCR was 100% (95% CI 54% to 100%) and specificity was 4% (95% CI 0% to 14%).
8. Woods 2017: direct comparison of serum, buffy coat, and urine real‐time polymerase chain reaction (PCR).
| Sample type | Target gene | Sensitivity | Lower CI | Upper CI | Specificity | Lower CI | Upper CI |
| Serum | rrs/lipl32 (Woods 2018) | 9% | 2% | 25% | 99% | 98% | 99% |
| Buffy coat | rrs/lipl32 (Woods 2018) | 12% | 3% | 28% | 99% | 98% | 100% |
| Urine | rrs/lipl32 (Woods 2018) | 14% | 4% | 32% | 99% | 98% | 100% |
| Serum | rrs (Slack 2007) | 9% | 2% | 25% | 99% | 97% | 99% |
| Buffy coat | rrs (Slack 2007) | 3% | 0% | 16% | 99% | 98% | 100% |
| Urine | rrs (Slack 2007) | 17% | 6% | 36% | 90% | 87% | 92% |
CI: 95% confidence intervals.
9. Studies that report direct comparisons of rrs and lipL32 real‐time polymerase chain reaction (PCR).
| Study ID | Target gene | Sample type | Sensitivity | Lower CI | Upper CI | Specificity | Lower CI | Upper CI |
| Thaipadungpanit 2011 | rrs (Slack 2007) | Whole blood | 56% | 47% | 64% | 89% | 83% | 94% |
| lipL32 (Stoddard 2009) | Whole blood | 43% | 34% | 52% | 93% | 88% | 97% | |
| Villumsen 2012 BC | rrs (Smythe 2002) | Blood culture | 100% | 59% | 100% | 95% | 77% | 100% |
| lipL32 (Villumsen 2012) | Blood culture | 86% | 42% | 100% | 100% | 85% | 100% | |
| Villumsen 2012 U | rrs (Smythe 2002) | Urine | 100% | 29% | 100% | 98% | 90% | 100% |
| lipL32 (Villumsen 2012) | Urine | 100% | 29% | 100% | 98% | 90% | 100% | |
| Backstedt 2015 | rrs (Backstedt 2015) | Whole blood | 56% | 31% | 78% | 14% | 0% | 58% |
| lipL32 (Stoddard 2009) | Whole blood | 28% | 10% | 53% | 71% | 29% | 96% |
CI: 95% confidence intervals.
Nested polymerase chain reaction
Four studies reported accuracy data for the nested PCR (Merien 2005; Koizumi 2009; Blanco 2014; Gokmen 2016). All were cross‐sectional studies using serum as the sample type, and all studies used MAT as the reference standard. The reported mean timing of sample collection was 5 DPO (Merien 2005) and 7 DPO (Koizumi 2009), but was not reported for Blanco 2014 and Gokmen 2016. The sensitivity of nested PCR ranged from 0% (95% CI 0% to 13%) to 95% (95% CI 76% to 100%) and the specificity ranged from 42% (95% CI 23% to 63%) to 100% (95% CI 99% to 100%) (see Figure 16). Since only four studies were available, we did not conduct a meta‐analysis or formal assessments of heterogeneity.
16.
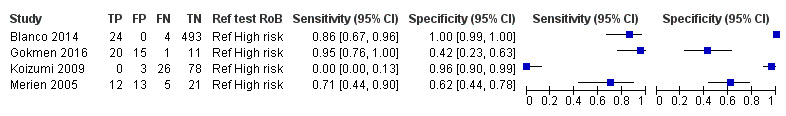
Forest plot of nested polymerase chain reaction (PCR) on serum. Ref test RoB: risk of bias for the 'reference standard' domain.
Comparison of different nested polymerase chain reaction methods
One study compared the rrs (Merien 1992) nested PCR to the lipL32 (Bomfim 2008) nested PCR (Gokmen 2016). The sensitivity of rrs nested PCR was 95% (95% CI 76% to 100%) and specificity was 42% (95% CI 23% to 63%). The sensitivity of the lipL32 nested PCR was 90% (95% CI 70% to 99%) and specificity was 42% (95% CI 23% to 63%).
Conventional polymerase chain reaction performed twice
Two studies reported data for PCR done on serum samples taken at two different times (Yersin 1998; Seng 2007). The PCR was considered positive if one of the two samples was positive. The first sample was taken at admission and the second sample approximately 14 days later. Both studies were cross‐sectional. One study used a composite reference standard (MAT and culturing; Seng 2007), while the other used only MAT (Yersin 1998). Seng 2007 reported sensitivity of 75% (95% CI 19% to 99%) and specificity of 94% (95% CI 88% to 98%), while Yersin 1998 reported sensitivity of 47% (95% CI 34% to 60%) and specificity of 96% (95% CI 87% to 100%).
Loop‐mediated isothermal amplification
Two studies using single‐gate designs evaluated the test accuracy of the LAMP (Thaipadungpanit 2011; Kitashoji 2015; Figure 17). LAMP was done on whole blood, plasma, or urine samples. The median timing of sample collection was 6.5 DPO for Kitashoji 2015 (plasma samples only, unreported for urine), but for Thaipadungpanit 2011 the timing was separately reported for cases (median 4 DPO) and non‐cases (median 6 DPO). Kitashoji 2015 reported results for LAMP on plasma (sensitivity 14%, 95% CI 9% to 22%; specificity 83%, 95% CI 76% to 89%) and LAMP on urine samples (sensitivity 14%, 95% CI 7% to 24%; specificity 91%, 95% CI 83% to 95%). Thaipadungpanit 2011 reported results for LAMP targeting rrs (Sonthayanon 2011) (sensitivity 44%, 95% CI 35% to 52%; specificity 83%, 95% CI 76% to 89%) and LAMP targeting lipL41 (Lin 2009) (sensitivity 38%, 95% CI 29% to 46%; specificity 90%, 95% CI 84% to 95%).
17.
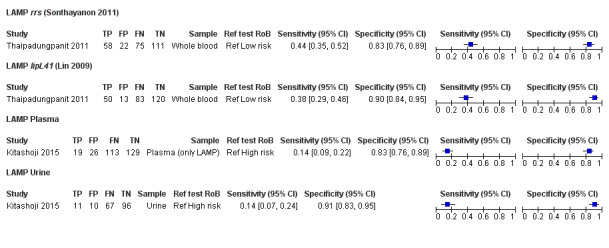
Forest plot of loop‐mediated isothermal amplification (LAMP), on whole blood, plasma or urine. Ref test RoB: risk of bias for the 'reference standard' domain.
Comparison of different loop‐mediated isothermal amplification methods
Thaipadungpanit 2011 compared rrs and lipL41 as target genes and Kitashoji 2015 compared plasma and urine samples for the LAMP (Figure 17).
Enzyme‐linked immunosorbent assay
One cross‐sectional study conducted in India assessed the test accuracy of ELISA on urine samples (Chaurasia 2018), The timing of sample collection and the threshold were not reported. The study used single‐sample MAT as reference standard to classify 23 participants as cases and six as non‐cases. The ELISA was done using seven different target antigens (LipL32, Fla1, LipL41, HbpA, SphCD210, Sph2, and Sph4). For clarity, the sensitivities and specificities of these tests are shown in Table 14.
10. Chaurasia 2018: direct comparison of target antigens for enzyme‐linked immunosorbent assay (ELISA).
| Target antigen | Sensitivity | Lower CI | Upper CI | Specificity | Lower CI | Upper CI |
| LipL32 | 100% | 85% | 100% | 67% | 22% | 96% |
| Fla1 | 91% | 72% | 99% | 50% | 12% | 88% |
| LipL41 | 78% | 56% | 93% | 83% | 36% | 100% |
| HbpA | 91% | 72% | 99% | 67% | 22% | 96% |
| SphCD210 | 100% | 85% | 100% | 67% | 22% | 96% |
| Sph2 | 91% | 72% | 99% | 67% | 22% | 96% |
| Sph4 | 39% | 20% | 61% | 83% | 36% | 100% |
CI: 95% confidence intervals.
Dot‐enzyme‐linked immunosorbent assay
One two‐gate case‐control study evaluated the test accuracy of a monoclonal antibody‐based dot‐ELISA for the detection of leptospiral antigens in urine samples (Saengjaruk 2002). The study was conducted in Thailand with 42 participants, of which 25 were leptospirosis cases confirmed by culture and 17 were people with other illnesses. Timing of sample collection was not reported. The sensitivity of dot‐ELISA was 64% (95% CI 43% to 82%) and specificity was 100% (95% CI 81% to 100%).
Immunochromatography‐based lateral flow assay
One cross‐sectional study assessed the accuracy of an ICG‐based LFA using monoclonal antibodies specific to the Leptospira lipopolysaccharide (Widiyanti 2013). The study tested the urine samples of 44 participants with suspected leptospirosis, and classified 28 as cases based on MAT alone. The mean timing of sample collection was 5.5 DPO. The sensitivity of LFA was 96% (95% CI 82% to 100%) and specificity was 56% (95% CI 30% to 80%).
Dipstick assay
Widiyanti 2013 evaluated a monoclonal antibody‐based dipstick assay on urine, specific to the Leptospira lipopolysaccharide. The same urine samples were tested, with a mean timing of sample collection of 5.5 DPO. The sensitivity of the dipstick assay was 89% (95% CI 72% to 98%) and specificity was 63% (95% CI 35% to 85%).
Comparison of index tests
Several studies performed a comparison of index tests in the same patient population. Since each comparison contained only one study, we could not perform a meta‐analysis.
PCR versus real‐time PCR: Vanasco 2016 reported a comparison of PCR and real‐time PCR, both performed on whole blood or serum samples. PCR had a sensitivity of 13% (95% CI 6% to 24%) and a specificity of 88% (95% CI 81% to 94%). Real‐time PCR had a sensitivity of 30% (95% CI 19% to 42%) and a specificity of 81% (95% CI 73% to 88%).
PCR versus nested PCR: Blanco 2014 reported a comparison of PCR and nested PCR. PCR had a sensitivity of 14% (95% CI 4% to 33%) and a specificity of 100% (95% CI 99% to 100%). Nested PCR had a sensitivity of 86% (95% CI 67% to 96%) and a specificity of 100% (95% CI 99% to 100%).
Real‐time PCR versus nested PCR: Merien 2005 compared real‐time PCR with nested PCR. The study reported identical results for the two tests: sensitivity was 71% (95% CI 44% to 90%) and specificity was 62% (95% CI 44% to 78%).
-
PCR versus ICG‐based IFA versus dipstick assay: Widiyanti 2013 reported a comparison between PCR, ICG‐based IFA, and dipstick assay, with all three tests performed on urine samples.
For PCR on urine, sensitivity was 57% (95% CI 37% to 76%) and specificity was 56% (95% CI 30% to 80%).
For ICG‐based IFA, sensitivity was 96% (95% CI 82% to 100%) and specificity was 56% (95% CI 30% to 80%).
For dipstick assay, sensitivity was 89% (95% CI 72% to 98%) and specificity was 63% (95% CI 35% to 85%).
-
Real‐time PCR versus LAMP: Thaipadungpanit 2011 reported a comparison of rrs (Slack 2007) andlipL32 (Stoddard 2009) real‐time PCR and rrs (Sonthayanon 2011) and lipL41 (Lin 2009) LAMP.
For real‐time PCR targetingrrs, sensitivity was 56% (95% CI 47% to 64%) and specificity was 89% (95% CI 83% to 94%).
For real‐time PCR targeting lipL32, sensitivity was 43% (95% CI 34% to 52%) and specificity was 93% (95% CI 88% to 97%).
For LAMP targeting rrs, sensitivity was 44% (95% CI 35% to 52%) and specificity was 83% (95% CI 76% to 89%).
For LAMP targeting lipL41, sensitivity was 38% (95% CI 29% to 46%) and specificity was 90% (95% CI 84% to 95%).
Discussion
Summary of main results
In this systematic review, we summarised the diagnostic test accuracy of nucleic acid and antigen detection tests for human symptomatic leptospirosis, verified by (a combination of) currently established tests – MAT, culture, and IgM ELISA. We identified 41 studies in the literature evaluating nine index tests, of which conventional PCR and real‐time PCR were the most frequently evaluated tests. While we have performed a meta‐analysis for PCR and real‐time PCR on blood products, individual study results suggested very high between‐study heterogeneity (PCR sensitivity ranging from 13% to 100% and PCR specificity from 0% to 100%). Therefore readers should interpret the meta‐analytic result as a weighted mean of all the heterogeneous settings in which the index tests were evaluated, rather than an estimate that is applicable across settings. Table 1, Table 2, Table 3, and Table 4 give an overview of the most important findings.
Summary of findings 1. Conventional polymerase chain reaction (PCR).
| Conventional polymerase chain reaction (PCR) | ||||
| Population: people suspected of leptospirosis in different stages of disease (early to late), excluding those with solely ocular problems or aseptic meningitis | ||||
| Setting: worldwide, primary to tertiary care facilities, outbreak as well as non‐outbreak settings | ||||
| Index test: conventional PCR on blood samples (whole blood, serum), all inhouse tests | ||||
| Reference standard: MAT on serum alone, or MAT on serum alongside culturing, or MAT on serum alongside IgM ELISA, or MAT on serum alongside culturing and IgM ELISA | ||||
| Number of cases/non‐cases (studies): 660/1224 (15) | Pooled sensitivity: 70% (95% CI 37 to 90) | Pooled specificity: 95% (95% CI 75 to 99) | Consequences in a cohort of 1000 | |
| Prevalence: | Positive post‐test probability: | Negative post‐test probability: | Missed diseased: | Falsely diagnosed: |
| 32.5% (median of all studies) | 87 (95% CI 53 to 97) | 87 (95% CI 71 to 95) | 98 (95% CI 32 to 205) | 35 (95% CI 6 to 168) |
| 9.7% | 59 (95% CI 20 to 89) | 97 (95% CI 92 to 99) | 29 (95% CI 9 to 61) | 47 (95% CI 8 to 225) |
| Positive likelihood ratio: 13.56 (95% CI 2.61 to 70.29) | Negative likelihood ratio: 0.32 (95% CI 0.12 to 0.82) | |||
| Quality of evidence: none of the studies scored 'low risk of bias' on all domains. 6/15 studies used an unreliable reference standard. Risk of spectrum bias was unclear to high. | ||||
| Investigations of heterogeneity: readers should note that the results are very heterogeneous between studies. Specificity declined with increasing leptospirosis prevalence. The choice of PCR blood sample type was not associated with test accuracy. | ||||
| Sensitivity analysis: sensitivity increased to 87% (95% CI 44% to 98%) when studies at high risk of bias for the 'reference standard' domain were excluded. However, the CIs were extremely wide with substantial overlap with the results of the overall meta‐analysis. | ||||
CI: confidence intervals; IgM ELISA: immunoglobulin M enzyme‐linked immunosorbent assay; MAT: microscopic agglutination test.
Summary of findings 2. Conventional polymerase chain reaction (PCR) sensitivity analysis, excluding high risk of bias (reference standard domain).
| Conventional polymerase chain reaction (PCR) sensitivity analysis, excluding studies at high risk of bias (reference standard domain) | ||||
| Population: people suspected of leptospirosis in different stages of disease (early to late), excluding those with solely ocular problems or aseptic meningitis | ||||
| Setting: worldwide, primary to tertiary care facilities, outbreak as well as non‐outbreak settings | ||||
| Index test: conventional PCR on blood samples (whole blood, serum), all inhouse tests | ||||
| Reference standard: MAT on serum alone (2‐gate studies), or MAT on serum alongside culturing, or MAT on serum alongside IgM ELISA, or MAT on serum alongside culturing and IgM ELISA (single‐gate studies) | ||||
| Number of cases/non‐cases (studies): 538/487 (9) | Pooled sensitivity: 87% (95% CI 44% to 98%) | Pooled specificity: 97% (95% CI 60% to 100%) | Consequences in a cohort of 1000 | |
| Prevalence: | Positive post‐test probability: | Negative post‐test probability: | Missed diseased | Falsely diagnosed |
| 32.5% (median of all studies) | 94% (95% CI 41% to 100%) | 94% (95% CI 70% to 99%) | 42 (95% CI 5 to 183) | 17 (95% CI 1 to 272) |
| 9.7% | 78% (95% CI 13% to 99%) | 99% (95% CI 91% to 100%) | 13 (95% CI 2 to 55) | 23 (95% CI 1 to 363) |
| Positive likelihood ratio: 33.86 (95% CI 1.59 to 719.39) | Negative likelihood ratio: 0.13 (95% 0.02 to 0.85) | |||
| Quality of evidence: none of the studies scored 'low risk of bias' on all domains. Risk of bias for the 'reference standard' domain was unclear to low. Risk of bias for the 'patient selection' domain was unclear to high. | ||||
CI: confidence intervals; IgM ELISA: immunoglobulin M enzyme‐linked immunosorbent assay; MAT: microscopic agglutination test; NA: not applicable.
Summary of findings 3. Real‐time polymerase chain reaction (PCR).
| Real‐time polymerase chain reaction (PCR) | ||
| Population: people suspected of leptospirosis in different stages of disease (early to late), excluding those with solely ocular problems or aseptic meningitis | ||
| Setting: worldwide, primary to tertiary care facilities, outbreak as well as non‐outbreak settings | ||
| Index test: real‐time PCR on blood samples (whole blood, plasma, serum), all inhouse tests, using unknown thresholds | ||
| Reference standard: MAT on serum alone, or MAT on serum alongside culturing, or MAT on serum alongside IgM ELISA, or MAT on serum alongside culturing and IgM ELISA | ||
| Number of cases/non‐cases (studies): 826/2384 (16) | Sensitivity at fixed value: 49% (95% CI 30% to 68%) | Specificity at fixed value: 85% (fixed, unknown threshold) |
| Sensitivity at fixed value: 40% (95% CI 24% to 59%) | Specificity at fixed value: 90% (fixed, unknown threshold) | |
| Sensitivity at fixed value: 29% (95% CI 15% to 49%) | Specificity at fixed value: 95% (fixed, unknown threshold) | |
| Prevalence: | Positive post‐test probability: | Negative post‐test probability: |
| 32.5% (median of all studies) | NA | NA |
| 9.7% | NA | NA |
| Positive likelihood ratio: NA | Negative likelihood ratio: NA | |
| Quality of evidence: only 2 studies scored 'low risk of bias' on all domains. 8/16 studies used an unreliable reference standard. Risk of bias for the 'patient selection' domain was generally unclear, with 9/16 studies not reporting clear selection processes. | ||
| Investigations of heterogeneity: readers should note that the results are very heterogeneous between studies. The choice of blood sample type, real‐time PCR visualisation method and prevalence were not associated with test accuracy. | ||
| Sensitivity analysis: when low‐quality studies were excluded, there was no important change in test accuracy. | ||
We refrained from estimating post‐test probabilities and likelihood ratios because the thresholds for the pooled sensitivities and specificities were unknown.
CI: confidence intervals; IgM ELISA: immunoglobulin M enzyme‐linked immunosorbent assay; MAT: microscopic agglutination test; NA: not applicable.
Summary of findings 4. Nested polymerase chain reaction (PCR), conventional PCR performed twice, loop‐mediated isothermal amplification (LAMP), immunochromatography‐based lateral flow assay (ICG‐based LFA) enzyme‐linked immunosorbent assay (ELISA), dot‐ELISA, and dipstick assay.
| Nested polymerase chain reaction (PCR), conventional PCR performed twice, loop‐mediated isothermal amplification (LAMP), immunochromatography‐based lateral flow assay (ICG‐based LFA) enzyme‐linked immunosorbent assay (ELISA), dot‐ELISA, and dipstick assay | |||||||
| Population: people suspected of leptospirosis in unknown stages of disease, excluding those with solely ocular problems or aseptic meningitis | |||||||
| Setting: worldwide, primary to tertiary care facilities, outbreak as well as non‐outbreak settings | |||||||
| Index test: nested PCR (on serum samples, all inhouse tests), conventional PCR performed twice (on serum samples, all inhouse tests), LAMP (on whole blood, plasma, or urine samples, all inhouse tests), ICG‐based LFA (on urine samples, inhouse test), ELISA (on urine samples, inhouse test), dot‐ELISA (on urine samples, inhouse test), dipstick assay (on urine samples, inhouse test) | |||||||
| Reference standard: MAT on serum alone or MAT on serum alongside culturing | |||||||
| Quality of evidence: none of the studies scored 'low risk of bias' on all domains. 8/11 studies were rated 'high risk of bias' for the 'reference standard' domain. Risk of bias for the 'patient selection' domain was generally unclear, with 7/11 studies not reporting clear selection processes. | |||||||
| Study ID | Number of cases/non‐cases | Sensitivity (95% CI) | Specificity (95% CI) | Positive post‐test probability (95% CI) | Negative post‐test probability (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI): |
| Nested PCR (4 studies)a | |||||||
| Blanco 2014 | 28/493 | 86% (67% to 96%) | 100% (99% to 100%) | 100% (100% to 100%) | 99% (98% to 100%) | 834.69 (52.05 to 13,384.42)b | 0.14 (0.06 to 0.35) |
| Gokmen 2016 (lipL32)c | 21/26 | 90% (70% to 99%) | 42% (23% to 63%) | 56% (47% to 64%) | 85% (58% to 96%) | 1.57 (1.10 to 2.24) | 0.23 (0.06 to 0.91) |
| Koizumi 2009 | 26/81 | 0% (0% to 13%) | 96% (90% to 99%) | 13% (0% to 70%)b | 75% (74% to 76%) | 0.43 (0.02 to 8.13)b | 1.04 (1.00 to 1.08) |
| Merien 2005 | 17/34 | 71% (44% to 90%) | 62% (44% to 78%) | 48% (35% to 61%) | 81% (66% to 90%) | 1.85 (1.09 to 3.12) | 0.48 (0.22 to 1.04) |
| Conventional PCR performed twice (2 studies)a | |||||||
| Seng 2007 | 4/117 | 75% (19% to 99%) | 94% (88% to 98%) | 30% (15% to 52%) | 99% (95% to 100%) | 12.54 (5.02 to 31.28) | 0.27 (0.05 to 1.45) |
| Yersin 1998 | 60/52 | 47% (34% to 60%) | 96% (87% to 100%) | 93% (78% to 98%) | 61% (55% to 67%) | 12.13 (3.04 to 48.50) | 0.55 (0.44 to 0.71) |
| LAMP (2 studies)a | |||||||
| Thaipadungpanit 2011 (lipL41)c | 133/133 | 38% (29% to 46%) | 90% (84% to 95%) | 79% (69% to 87%) | 59% (56% to 63%) | 3.85 (2.20 to 6.74) | 0.69 (0.60 to 0.80) |
| Kitashoji 2015 (plasma)c | 132/155 | 14% (9% to 22%) | 83% (76% to 89%) | 42% (30% to 56%) | 53% (51% to 56%) | 0.86 (0.50 to 1.48) | 1.03 (0.93 to 1.14) |
| ICG‐based LFA (1 study)a | |||||||
| Widiyanti 2013 | 28/16 | 96% (82% to 100%) | 56% (30% to 80%) | 79% (69% to 87%) | 90% (56% to 98%) | 2.20 (1.26 to 3.86) | 0.06 (0.01 to 0.46) |
| ELISA (1 study)a | |||||||
| Chaurasia 2018 (LipL32)c | 23/6 | 100% (85% to 100%) | 67% (22% to 96%) | 92% (79% to 97%) | 90% (38% to 100%)b | 3.00 (0.97 to 9.30) | 0.03 (0.00 to 0.58)** |
| Dot‐ELISA (1 study)a | |||||||
| Saengjaruk 2002 | 25/18 | 64% (43% to 82%) | 100% (81% to 100%) | 97% (73% to 100%)b | 67% (54% to 77%) | 24.12 (1.54 to 377.45)b | 0.36 (0.21 to 0.61) |
| Dipstick assay (1 study)a | |||||||
| Widiyanti 2013 | 28/16 | 89% (72% to 98%) | 63% (35% to 85%) | 81% (69% to 89%) | 77% (52% to 91%) | 2.38 (1.25 to 4.54) | 0.17 (0.06 to 0.53) |
aNo meta‐analyses were conducted for these index tests. bZero cell correction by applying 0.5 to each cell cRandomly chosen dataset out of multiple two‐by‐two tables
CI: confidence intervals; MAT: microscopic agglutination test.
Interpretation of the conventional polymerase chain reaction meta‐analysis
From a meta‐analysis of 15 studies evaluating PCR on blood products collected during various stages of disease, the pooled sensitivity was 70% (95% CI 37% to 90%) and the pooled specificity was 95% (95% CI 75% to 99%). This means that in a hypothetical cohort of 1000 people, with a prevalence of 32.5% (325 diseased), 98 (95% CI 32 to 205) cases would be missed by the PCR and 35 (95% CI 6 to 168) non‐diseased people would be incorrectly diagnosed with leptospirosis. If the prior probability of an individual to have leptospirosis is 32.5%, the PPP was 87% (95% CI 53% to 97%) and the NPP was 87% (95% CI 71% to 95%). If the PCR would be used in a setting such as in the Netherlands where the prevalence is lower (9.7% in 2016; Collaborating Centre for Reference and Research on Leptospirosis in Amsterdam, unpublished data), 97/1000 people would have leptospirosis. In such a cohort, 29 (95% CI 9 to 61) diseased people would be missed and 47 (95% CI 8 to 225) non‐diseased people would be incorrectly diagnosed with leptospirosis. If the prior probability of leptospirosis in an individual is 9.7%, the PPP is 59% (95% CI 20% to 89%) and the NPP is 97% (95% CI 92% to 99%). See Table 1.
When the PCR meta‐analysis was repeated without the studies at high risk of bias for the 'reference standard' domain (i.e. only including studies with composite reference standards or two‐gate studies with MAT as reference standard, based on nine studies), the pooled sensitivity was 87% (95% CI 44% to 98%), and the pooled specificity was 97% (95% CI 60% to 100%). See Table 2. This means that in a cohort of 1000 people with 325 leptospirosis patients, 42 (95% CI 5 to 183) cases would be missed and 17 (95% CI 1 to 272) non‐diseased people would be incorrectly diagnosed with leptospirosis. In an individual with 32.5% prior probability of leptospirosis, the PPP is 94% (95% CI 41% to 100%) and the NPP is 94% (95% CI 70% to 99%). If the prevalence is 9.7%, 13 (95% CI 2 to 55) diseased people would be missed and 23 (95% CI 1 to 363) non‐diseased people would be incorrectly diagnosed with leptospirosis. If an individual has 9.7% prior probability of leptospirosis, the PPP is 78% (95% CI 13% to 99%), and the NPP is 99% (95% CI 91% to 100%).
A repeated meta‐analysis of PCR without studies at high risk of bias for the 'patients' domain did not lead to important changes in our results.
Interpretation of the real‐time polymerase chain reaction meta‐analysis
For the real‐time PCR, we estimated an SROC curve instead of a summary sensitivity and specificity, since we expected the positivity threshold (which was often not reported) to vary between studies. The median specificity of real‐time PCR on blood products was 92%. For illustrative purposes, if we were to select a point on the curve with 95% specificity, the pooled sensitivity would be 29% (95% CI 15% to 49%) at an unknown threshold (Table 3). Translating these numbers to a cohort of 1000 people of whom 325 are diseased, would mean that 230 (95% CI 167 to 276) diseased people would be missed and 34 non‐diseased people would be incorrectly diagnosed with leptospirosis. Again, in a setting with a prevalence of 9.7% this would imply that 69 (95% CI 50 to 82) diseased people would be missed and 45 non‐diseased people would be incorrectly diagnosed with leptospirosis. We did not provide PPP and NPP for real‐time PCR, as threshold values needed to produce these estimates were unknown.
When we excluded real‐time PCR studies at high risk of bias for the 'patients' domain or the 'reference standard' domain, there were no important changes in the pooled estimate.
The position of conventional polymerase chain reaction and real‐time polymerase chain reaction in the clinical pathway
Based on the properties of PCR and real‐time PCR, we examined the possible role of these tests in the diagnostic pathway for leptospirosis. Leptospirosis is a potentially life‐threatening disease, meaning that efforts should be undertaken to minimise false‐negative results. In the clinic, while most patients with suspicion of a severe infection are likely to receive broad‐spectrum antibiotics, a false‐negative result impedes the optimisation of the antibiotic therapy and assessment of the prognosis. In an outbreak setting, a missed case of leptospirosis will delay outbreak response and facilitate further dissemination of disease.
For patients presenting in the early disease stage, PCR and real‐time PCR on blood products are preferable as first‐line tests in the clinical pathway based on their ability for early detection. However, whether additional testing is needed to verify a positive or negative test result depends on test accuracy and the prevalence of leptospirosis. While PCR‐based methods have been described as sensitive tests in the literature (Budihal 2014; Hall 2014; Picardeau 2014), our results show that the sensitivity of PCR and real‐time PCR vary greatly between studies, with the CI for PCR sensitivity ranging from 37% to 90%. This can partly be explained by differences in methodological quality, but it is also likely that there is true heterogeneity, such as differences in timing of sample collection. Furthermore, the reliability of a positive or negative test result depends on the prevalence. In our review, prevalence of leptospirosis varied greatly between studies (range 3% to 85%). Consequently, whether PCR‐based methods can be used alone, or together with other follow‐up tests depends on regional considerations such as prevalence, factors that are likely to influence accuracy (e.g. timing of sampling), and downstream consequences of a positive or negative result. For example, in settings with a high prevalence, PCR and real‐time PCR may not have a high enough negative post‐test probability to confidently rule out leptospirosis. In this case, additional testing to verify negative results should be considered.
Comparison between index tests
This review did not find enough evidence to formally compare the diagnostic accuracy of included tests. Direct comparison studies (where two or more index tests are evaluated in the same patient population) are needed to draw valid conclusions about the differences in diagnostic test accuracy between tests, but such studies were lacking. Although the results of the meta‐analyses seem to imply that real‐time PCR has a lower sensitivity than PCR, this is not a valid comparison. The meta‐analysis results are composed of mostly single‐test studies and any differences between real‐time PCR and PCR could arise from other reasons than the differences in the tests themselves, such as differences in study design or spectrum of disease. Therefore, any comparison between the meta‐analytic results of PCR and real‐time PCR must be interpreted with caution.
Heterogeneity of included studies
Substantial heterogeneity, as demonstrated by the wide CIs, complicated the interpretation of our findings.
An important covariate, timing of sample collection, could not be explored in heterogeneity analysis. Three of 15 studies assessing PCR on blood products reported the timing of sample collection (range 1 DPO to 7 DPO of symptoms), and in 9/16 studies assessing real‐time PCR on blood products (range 1 to 19 DPO). However, the subgroups were either too small or the reporting of the timing variable was too heterogeneous for analysis. Two studies comparing test accuracy in patients who presented early with test accuracy in patients who presented later appear to support the hypothesis that the sensitivity of PCR and real‐time PCR is greater in the first few days of illness (Céspedes 2007; Ahmed 2009). This is consistent with the current pathophysiological understanding of leptospirosis, that leptospiraemia declines rapidly and becomes undetectable after 10 DPO (WHO 2003). However, this could also be caused by patients with a higher bacterial load presenting earlier to the clinic due to a more severe clinical presentation than those with a lower bacterial load. Moreover, one study reported that the sensitivity of real‐time PCR was not associated with timing of sample collection in patients presenting with fewer than 10 DPO (P = 0.33) (Agampodi 2012). Ultimately, more studies are needed to confirm the association between timing and test accuracy.
In the case of PCR, statistical heterogeneity may be partly explained by the prevalence of leptospirosis in the study population. Specificity was inversely correlated with prevalence (P = 0.0004). A number of explanations for this association are possible. The prevalence of alternative diagnoses may be higher in places where leptospirosis prevalence is high, causing false‐positive results on the PCR. The PCR may be detecting lower levels of infections that occur more frequently in high‐prevalence settings, that are missed by MAT, and, therefore, recorded as false‐positive results. The laboratories in high‐prevalence settings may be less well‐equipped and more often contaminated, and, therefore, allow more false‐positive results. We did not examine the inverse correlation between prevalence and sensitivity or specificity for real‐time PCR studies, since the HSROC model by default examines the association between prevalence and accuracy (alpha parameter) instead of sensitivity and specificity.
Other covariates that could possibly influence test accuracy, such as real‐time PCR threshold and specific target genes or primers used in PCR‐based methods, have not been ruled out as possible explanations for the significant heterogeneity. It is theoretically possible that the heterogeneity in sensitivity of PCR‐based methods could be explained by differences in Leptospira species, as a primer may not able to detect a particular species. However, this is not very probable as it is usual practice to account for all existing species when developing PCR or its variants (unless a new species emerges). Since all index tests were inhouse tests, there may be other potential sources of heterogeneity (e.g. use of different laboratory equipment or protocols) that cannot be measured reliably or be reported in sufficient detail. For this reason, readers should be cautious when applying summary estimates of test accuracy in their own clinical settings.
Risk of bias
A major point of attention in our review was the use of MAT as reference standard, which is considered to have an imperfect sensitivity. If the reference standard is not sensitive, the specificity of the index test is likely to be underestimated. It is furthermore not inconceivable that some index tests, for example, PCR‐based methods, may be more sensitive than MAT alone. We aimed to address this problem by including composite reference standards and rating the risk of bias as high when MAT was used as the sole reference standard, and when single samples were used (instead of paired). However, only a minority of studies used another reference standard alongside the MAT, leading to a 'high risk of bias' judgement in the majority of studies for this domain. In the case of the PCR, sensitivity increased when studies at 'high risk of bias' for the reference standard were excluded, but specificity was unchanged (Table 9). Other covariates that may be of importance, such as the cut‐off value for MAT, the use of adequate regional panels for MAT, and the differences between composite reference standards, were not taken into account in our review to avoid excessive complexity.
Another issue is the inclusion of four two‐gate case‐control studies (Samsonova 1997; Ananyina 2000; Saengjaruk 2002; de Abreu Fonseca 2006). Cases and controls in these studies are selected separately and do not reflect the spectrum of disease in the clinical population (Rutjes 2005). Another concern in these studies is the possibility of coinfections of leptospirosis and another infectious disease. In two‐gate designs, since controls are not MAT negatives but people with a condition resembling leptospirosis, coinfections with leptospirosis may be present. Treating these people as controls may underestimate the specificity of the index test. These studies have been excluded in the abovementioned sensitivity analyses as they were considered 'high risk' for patient selection (Table 9; Table 11).
Blinding of the index test result to the reference standard result interpreters (or vice versa) was largely unreported. Interpreters were blinded because either blinding methods were used, or by the virtue of their study design (e.g. blinding of the index test interpreter was not needed when the index test was done first). In the index test, eight studies reported to have used a form of blinding in their methods. However, we noted that only one study reported explicit methods for blinding (Samsonova 1997). It was unclear if the remaining studies used proper blinding methods. Considering other possible biases in our review (Figure 4; Figure 6; Figure 8), readers are advised to weigh the results against the quality of evidence.
Other index tests
For other tests included in our review (nested PCR, PCR performed twice, LAMP, ELISA, dot‐ELISA, ICG‐based LFA, and dipstick assay), we could not conduct meta‐analyses or investigations of heterogeneity due to the small number of studies.
Strengths and weaknesses of the review
The strength of our review lies in the fact that we used an extensive search strategy including 16 national and regional databases, without any limitations on languages and without using search filters or keywords containing terms related to diagnostic accuracy. We also contacted authors for full‐text articles in case the studies did not report complete data for the construction of two‐by‐two contingency tables. Furthermore, we aimed to include all nucleic acid tests and antigen detection tests that we could find in the literature. And lastly, we included studies that used MAT with convalescent samples as a reference standard, as well as studies that used MAT with a single, acute sample. This is a strength, because it provides a good reflection of the day‐to‐day reality in clinics and laboratories, but at the same time, it is one of the major limitations of our review. It is known that antibodies appear in the blood only after several days to weeks (Levett 2001). Thus a serological test, such as the MAT, is not applicable for diagnosis of leptospirosis in the early stages. Inclusion of studies using MAT only on acute samples could have led to false‐negative results by the reference standard. This lack of a perfect reference standard implies that the test accuracy of nucleic acid and antigen tests presented here simply reflects the extent of agreement between the index test and MAT, and not necessarily the true test accuracy of the index tests.
Other limitations of our review are as follows. First, studies that potentially satisfy our inclusion criteria could not be included due to lack of clarity or inconsistencies in the full‐text article. Second, due to poor reporting in primary studies, many aspects of the index test and methodological quality remain unclear and limit the potential to generalise our findings. Third, as we have discussed previously, we could not explain the substantial heterogeneity in study results due to the lack of statistical power. Although we have conducted meta‐analyses for PCR and real‐time PCR, it is debatable to what extent the pooled results are applicable to clinical practice, since numerous unexplored covariates are likely to have contributed to the pooled sensitivities and specificities.
Applicability of findings to the review question
We identified some concerns regarding the applicability of the results to our review question when the used research methods differed significantly from clinical practice (Figure 4; Figure 6; Figure 8).
Regarding the selection of participants, four studies excluded participants due to unavailability of convalescent samples for MAT (Yersin 1998; Thaipadungpanit 2011; Agampodi 2012; Gonzalez 2013). Although this is a reasonable decision, since verification by MAT is more accurate with paired acute and convalescent samples, it does not necessarily reflect the patient population in clinical practice, as participants with a fatal course of disease were likely to be excluded from the study as a result. In 19 studies, the patient selection method, inclusion criteria, and characteristics (including the timing of sample collection) were not well reported. These studies stated that the participants were 'clinically suspected for leptospirosis' without stating which signs, symptoms, and risk factors the participants had that made them clinically suspected. Furthermore, with baseline characteristics not reported, it was not possible to determine whether a particular age group or sex was over‐represented in the study.
For the applicability of the index test and the reference standard, we did not only consider whether the method of testing differed from clinical practice, but also whether the execution of the test was reported in such detail that the test could be reproduced in full elsewhere. In four studies, we found that this was not the case for the index test (Ananyina 2000; Chandrasiri 2010; Chaurasia 2018; Pakoa 2018), and in five studies, the procedure (including the cut‐off value) for the MAT was not reported, or there was a reference to an irretrievable study (Samsonova 1997; Ananyina 2000; Merien 2005; Chandrasiri 2010; Waggoner 2014). At least 11 studies used frozen samples for the index test rather than fresh samples. We were uncertain if this could have influenced the test accuracy, so we considered the concern regarding applicability to be high.
Authors' conclusions
Implications for practice.
The validity of review findings are limited by the poor reporting of methodological quality items and the use of suboptimal reference standards. We conclude that there is substantial between‐study variability in the accuracy of conventional polymerase chain reaction (PCR) and real‐time PCR, as well as substantial variability in the prevalence of leptospirosis. Consequently, the position of conventional PCR and real‐time PCR in the clinical pathway depends on regional considerations such as prevalence, factors that are likely to influence accuracy (such as timing of sampling), and downstream consequences of test results. There is insufficient evidence to conclude which of the nucleic acid and antigen detection tests are the most accurate in the early stage of leptospirosis. There is preliminary evidence that conventional PCR and real‐time PCR are more sensitive on blood samples collected early in the disease stage, but this needs to be confirmed in future studies. Evidence regarding other index tests was very limited.
Implications for research.
Our review demonstrates that while there is a wealth of publications on new nucleic acid and antigen detection tests, there is a marked scarcity on well‐designed, well‐performed, and well‐reported validations of such tests. More high‐quality studies are needed with larger samples sizes, especially a larger group of cases to estimate sensitivity more precisely. Future investigators should follow the reporting guidelines of STARD (Standards for the Reporting of Diagnostic Accuracy Studies), to allow the assessment of potential biases in the study, as well as the assessment of the clinical value of the estimated test accuracy. Single‐gate designs, such as the cross‐sectional study, with consecutive enrolment have our recommendation above two‐gate designs because of their lower risk of spectrum bias. The choice of reference standard should not only include MAT, but also culture, and if possible immunoglobulin M enzyme‐linked immunosorbent assay (IgM ELISA), to minimise false‐negative results from occurring. The emphasis should be on paired sampling, to show a possible rise in antibody titres. In order to compare and select the best performing tests, multiple index tests should be evaluated on the same participants so that direct comparison of their accuracy is possible. Last, we encourage future investigators to explore the effects of varying times of sample collection on test accuracy as a potential source of heterogeneity.
Acknowledgements
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
We would like to thank Drs Agostino Colli, Deputy Co‐ordinating editor, and Dimitrinka Nikolova, Managing editor, of the Cochrane Hepato‐Biliary Group, and Cochrane's Diagnostic Test Accuracy Review team, for reviewing our protocol and review, and giving us valuable recommendations. We also thank Dr Patrick Bossuyt for his advice on the interpretation of diagnostic test accuracy. Last, we thank the authors of included studies for their correspondence and sending us valuable study data.
CHBG Editorial Team: Peer reviewers of the protocol and the review: David Brett‐Major, USA Contact editors of the protocol and the review: Agostino Colli, Italy; Giovanni Casazza, Italy Sign‐off editor of the protocol and the review: Agostino Colli, Italy Peer reviewers from The UK Diagnostic Accuracy Reviews Editorial Team: anonymous. Contact editor from The UK Diagnostic Accuracy Reviews Editorial Team: Miriam Brazzelli, UK.
Cochrane Abdomen & Endocrine Network: Liz Bickerdike, Associate Editor, UK
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy | Hits |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | July 2018 | #1 (leptospir* or (weil* next disease) or "stuttgart disease" or "infectious icterus" or canicola or "mud fever" or "field fever" or (rat next catcher* next yellow*) or "pretibial fever" or grippotyphosa or icterohaemorrhag* or icterohemorrhag* or hardjo or spirochaetos* spirochetos* or "spirochaetal jaundice" or "spirochetal jaundice"):ti,ab,kw #2 ((molecular and (assay* or amplif* or detect* or diagnos* or technique* or test*)) or antigen* or DNA or RNA or rRNA or 16SRNA or 16SrRNA or (nucleic next acid*) or ("polymerase chain" next reaction*) or PCR* or qPCR* or rtPCR* or NAAT or NASBA or ("self‐sustained sequence" next replicat*) or (isothermal next amplif*) or LAMP or primer or primers or probe or probes or hybridizat* or hybridisat* or ISH or FISH or immunohistochem* or immuno‐histochem* or immunohisto‐chem* or ("fluorescent antibody" next technique*) or antibody‐coated or immunoperoxidase* or immuno‐peroxidase* or immunofluorescen* or immuno‐fluorescen* or immunogold or immuno‐gold or IGSS or (silver next stain*) or dot‐blot* or dotblot* or (complement next fixat*) or cft or conglutinat*):ti,ab,kw #3 #1 AND #2 |
9 |
| MEDLINE Ovid | 1946 to July 2018 | #1 exp Leptospirosis/ #2 exp Leptospiraceae/ #3 (leptospir* or (weil* adj disease) or stuttgart disease or infectious icterus or canicola or mud fever or field fever or (rat catcher* adj yellow*) or pretibial fever or grippotyphosa or icteroh?emorrhag* or hardjo or spiroch?etos* or spiroch?etal jaundice).tw,kf. #4 or/1‐3 #5 Molecular Diagnostic Techniques/ #6 (molecular and (assay* or amplif* or detect* or diagnos* or technique* or test*)).tw,kf. #7 exp Antigens/ #8 antigen*.tw,kf. #9 exp Nucleic Acids/ #10 (DNA or RNA or rRNA or 16SRNA or 16SrRNA or nucleic acid*).tw,kf. #11 exp Nucleic Acid Amplification Techniques/ #12 (polymerase chain reaction* or PCR* or qPCR* or rtPCR* or NAAT or NASBA or self‐sustained sequence replicat* or isothermal amplif* or LAMP or primer or primers).tw,kf. #13 exp Molecular Probes/ #14 exp Molecular Probe Techniques/ #15 (probe or probes).tw,kf.. #16 exp Nucleic Acid Hybridization/ #17 (hybridi#at* or ISH or FISH).tw,kf. #18 exp Immunohistochemistry/ #19 (immunohistochem* or immuno‐histochem* or immunohisto‐chem* or fluorescent antibody technique* or antibody‐coated or immunoperoxidase* or immuno‐peroxidase* or immunofluorescen* or immuno‐fluorescen* or immunogold or immuno‐gold or IGSS).tw,kf. #20 Silver Staining/ or silver stain*.tw,kf. #21 (dot‐blot* or dotblot*).tw,kf.. #22 Complement Fixation Tests/ or (complement fixat* or cft or conglutinat*).tw,kf. #23 or/5‐22 #24 4 and 23 #25 animals/ not humans/ #26 24 not 25 |
2367 |
| Embase Ovid | 1947 to July 2018 | #1 leptospirosis/ #2 exp Leptospira/ #3 (leptospir* or (weil* adj disease) or stuttgart disease or infectious icterus or canicola or mud fever or field fever or (rat catcher* adj yellow*) or pretibial fever or grippotyphosa or icteroh?aemorrhag* or hardjo or spiroch?etos* or spiroch?etal jaundice).tw,kw. #4 or/1‐3 #5 molecular diagnosis/ #6 (molecular and (assay* or amplif* or detect* or diagnos* or technique* or test*)).tw,kw. #7 exp antigen/ #8 antigen*.tw,kw. #9 exp nucleic acid/ #10 (DNA or RNA or rRNA or 16SRNA or 16SrRNA or nucleic acid*).tw,kw. #11 exp nucleic acid analysis/ #12 (polymerase chain reaction* or PCR* or qPCR* or rtPCR* or NAAT or NASBA or self‐sustained sequence replicat* or isothermal amplif* or LAMP or primer or primers).tw,kw. #13 exp molecular probe/ #14 (probe or probes).tw,kw. #15 nucleic acid hybridization/ #16 (hybridi#at* or ISH or FISH).tw,kw. #17 exp immunohistochemistry/ #18 immunofluorescence/ #19 fluorescent antibody technique/ #20 (immunohistochem* or immuno‐histochem* or immunohisto‐chem* or fluorescent antibody technique* or antibody‐coated or immunoperoxidase* or immuno‐peroxidase* or immunofluorescen* or immuno‐fluorescen* or immunogold or immuno‐gold or IGSS).tw,kw. #21 silver staining/ #22 silver stain*.tw,kw. #23 (dot‐blot* or dotblot*).tw,kw. #24 complement fixation test/ #25 (complement fixat* or cft or conglutinat*).tw,kw. #26 or/5‐25 #27 4 and 26 #28 (exp animal/ or animal*.hw.) not human/ #29 27 not 28 #30 limit 29 to (conference abstract or conference paper or conference proceeding or "conference review") #31 29 not 30 |
2414 |
| Web of Science | 1975 to July 2018 | #1 TS=(leptospir* OR (weil* NEAR/1 disease) OR "stuttgart disease" OR "infectious icterus" OR canicola OR "mud fever" OR "field fever" OR (rat‐catcher* NEAR/1 yellow*) OR "pretibial fever" OR grippotyphosa OR icterohaemorrhag* OR icterohemorrhag* OR hardjo OR spirochaetos* OR spirochetos* OR "spirochaetal jaundice" OR "spirochetal jaundice") #2 TS=((molecular AND (assay* OR amplif* OR detect* OR diagnos* OR technique* OR test*)) OR antigen* OR DNA OR RNA rRNA OR 16SRNA OR 16SrRNA OR nucleic‐acid* OR ("polymerase chain" NEAR/1 reaction*) OR PCR* OR qPCR* OR rtPCR* OR NAAT OR NASBA OR ("self‐sustained sequence" NEAR/1 replicat*) OR isothermal‐amplificat* OR LAMP OR primer OR primers OR probe OR probes OR hybridizat* OR hybridisat* OR ISH OR FISH immunohistochem* OR immuno‐histochem* OR immunohisto‐chem* OR ("fluorescent antibody" NEAR/1 technique*) OR antibody‐coated OR immunoperoxidase* OR immuno‐peroxidase* OR immunofluorescen* OR immuno‐fluorescen* OR immunogold OR immuno‐gold OR IGSS OR silver‐stain* OR dot‐blot* OR dotblot* OR complement‐fixat* OR cft OR conglutinat*) #3 #1 AND #2 #4 TI=(animal* OR mammal* OR livestock OR cattle OR horse* OR cow* OR sheep OR goat* OR pig* OR dog* OR canine* OR cat OR cats OR rodent* OR rat OR rats OR mouse OR mice OR murine OR hamster*) NOT TI=human* #5 #3 NOT #4 |
2213 |
| CINAHL Plus with Full Text | 1937 to July 2018 | #1 (MH "Leptospirosis") #2 TI (leptospir* OR "weils disease" OR "weil's disease" OR "weil disease" OR "stuttgart disease" OR "infectious icterus" OR canicola OR "mud fever" OR "field fever" OR "rat catcher yellow" OR "rat catcher's yellow" OR "pretibial fever" OR grippotyphosa OR icterohaemorrhag* OR icterohemorrhag* OR hardjo OR spirochaetos* OR spirochetos* OR "spirochaetal jaundice" "spirochetal jaundice") OR AB (leptospir* OR "weils disease" OR "weil's disease" OR "weil disease" OR "stuttgart disease" OR "infectious icterus" OR canicola OR "mud fever" OR "field fever" OR "rat catcher yellow" OR "rat catcher's yellow" OR "pretibial fever" OR grippotyphosa OR icterohaemorrhag* OR icterohemorrhag* OR hardjo OR spirochaetos* spirochetos* OR "spirochaetal jaundice" OR "spirochetal jaundice") #3 S1 OR S2 #4 TI ((molecular AND (assay* OR amplif* OR detect* OR diagnos* OR technique* OR test*)) OR antigen* OR DNA OR RNA OR rRNA OR 16SRNA OR 16SrRNA OR nucleic‐acid* OR "polymerase chain reaction" OR "polymerase chain reactions" OR PCR* OR qPCR* OR rtPCR* OR NAAT OR NASBA OR "self‐sustained sequence replication" OR isothermal‐amplificat* OR LAMP OR primer OR primers OR probe OR probes OR hybridizat* OR hybridisat* OR ISH OR FISH OR immunohistochem* OR immuno‐histochem* OR immunohisto‐chem* OR "fluorescent antibody technique" OR "fluorescent antibody techniques" OR antibody‐coated OR immunoperoxidase* OR immuno‐peroxidase* OR immunofluorescen* OR immuno‐fluorescen* OR immunogold OR immuno‐gold OR IGSS OR silver‐stain* OR dot‐blot* OR dotblot* OR complement‐fixat* OR cft OR conglutinat*) OR AB ((molecular AND (assay* OR amplif* OR detect* OR diagnos* OR technique* OR test*)) OR antigen* OR DNA OR RNA OR rRNA OR 16SRNA OR 16SrRNA OR nucleic‐acid* OR "polymerase chain reaction" OR "polymerase chain reactions" OR PCR* OR qPCR* OR rtPCR* OR NAAT OR NASBA OR "self‐sustained sequence replication" OR isothermal‐amplificat* OR LAMP OR primer OR primers OR probe OR probes OR hybridizat* OR hybridisat* OR ISH OR FISH OR immunohistochem* OR immuno‐histochem* OR immunohisto‐chem* OR "fluorescent antibody technique" OR "fluorescent antibody techniques" OR antibody‐coated OR immunoperoxidase* OR immuno‐peroxidase* OR immunofluorescen* OR immuno‐fluorescen* OR immunogold OR immuno‐gold OR IGSS OR silver‐stain* OR dot‐blot* OR dotblot* OR complement‐fixat* OR cft OR conglutinat*) #5 S3 AND S4 |
77 |
| BIOSIS Previews | 1993 to February 2015 | #1 (leptospir* or (weil* adj disease) or stuttgart disease or infectious icterus or canicola or mud fever or field fever or (rat catcher* adj yellow*) or pretibial fever or grippotyphosa or icteroh?emorrhag* or hardjo or spiroch?etos* or spiroch?etal jaundice).ti,ab,mi. #2 (molecular and (assay* or amplif* or detect* or diagnos* or technique* or test*)).ti,ab,mi. #3 antigen*.ti,ab,mi. #4 (DNA or RNA or rRNA or 16SRNA or 16SrRNA or nucleic acid*).ti,ab,mi. #5 (polymerase chain reaction* or PCR* or qPCR* or rtPCR* or NAAT or NASBA or self‐sustained sequence replicat* or isothermal amplif* or LAMP or primer or primers).ti,ab,mi. #6 (probe or probes).ti,ab,mi.. #7 (hybridi#at* or ISH or FISH).ti,ab,mi. #8 (immunohistochem* or immuno‐histochem* or immunohisto‐chem* or fluorescent antibody technique* or antibody‐coated or immunoperoxidase* or immuno‐peroxidase* or immunofluorescen* or immuno‐fluorescen* or immunogold or immuno‐gold or IGSS).ti,ab,mi. #9 silver stain*.ti,ab,mi. #10 (dot‐blot* or dotblot*).ti,ab,mi. #11 (complement fixat* or cft or conglutinat*).ti,ab,mi. #12 or/2‐11 #13 1 and 12 #14 animals/ not humans/ #15 13 not 14 |
1061 |
| PubMed (as supplied by publisher‐subset) | 1946 to February 2015 | (leptospir*[tw] OR weils disease[tw] OR weil's disease[tw] OR weil disease[tw] OR stuttgart disease[tw] OR infectious icterus[tw] OR canicola[tw] OR mud fever[tw] OR field fever[tw] OR pretibial fever[tw] OR grippotyphosa[tw] OR icterohaemorrhag*[tw] OR icterohemorrhag*[tw] OR hardjo[tw] OR spirochaetos*[tw] OR spirochetos*[tw] OR spirochaetal jaundice[tw] OR spirochetal jaundice[tw]) AND ((molecular[tw] AND (assay*[tw] OR amplif*[tw] OR detect*[tw] OR diagnos*[tw] OR technique*[tw] OR test[tw] OR tests[tw] OR testing[tw])) OR antigen*[tw] OR DNA[tw] OR RNA[tw] OR rRNA[tw] OR 16SRNA[tw] OR 16SrRNA[tw] OR nucleic‐acid*[tw] OR polymerase chain reaction*[tw] OR PCR*[tw] OR qPCR*[tw] OR rtPCR*[tw] OR NAAT[tw] OR NASBA[tw] OR self‐sustained sequence replicat*[tw] OR isothermal amplificat*[tw] OR LAMP[tw] OR primer[tw] OR primers[tw] OR probe[tw] OR probes[tw] OR hybridizat*[tw] OR hybridisat*[tw] OR ISH[tw] OR FISH[tw] OR immunohistochem*[tw] OR immuno‐histochem*[tw] OR immunohisto‐chem*[tw] OR fluorescent antibody technique*[tw] OR antibody‐coated[tw] OR immunoperoxidase*[tw] OR immuno‐peroxidase*[tw] OR immunofluorescen*[tw] OR immuno‐fluorescen*[tw] OR immunogold[tw] OR immuno‐gold[tw] OR IGSS[tw] OR silver‐stain*[tw] OR dot‐blot*[tw] OR dotblot*[tw] OR complement fixat*[tw] OR cft[tw] OR conglutinat*[tw]) AND publisher[sb] | 24 |
| Google Scholar (without patents and citations) | July 2018 | #1 allintitle: leptospirosis | leptospira antigen | antigens | DNA | RNA | rRNA | 16SRNA | 16SrRNA | "nucleic acid" ‐animal ‐mammal ‐livestock ‐cattle ‐horse ‐horses ‐cow ‐cows ‐sheep ‐goat ‐goats ‐pig ‐pigs #2 allintitle: leptospirosis | leptospira "polymerase chain reaction" | PCR | qPCR | rtPCR | NAAT | NASBA | "self‐sustained sequence replication" | "isothermal amplification" | LAMP | primer | primers | probe | probes ‐bovine ‐bovis ‐cattle ‐sheep ‐goat #3 allintitle: leptospirosis | leptospira hybridization | hybridisation | ISH | FISH | Immunohistochemistry | "fluorescent antibody" | "antibody coated" | immunoperoxidase | immunofluorescence | immunofluorescent |immunogold | IGSS #4 allintitle: leptospirosis | leptospira "silver staining" | dotblot | dot‐blot | "complement fixation" | cft | conglutination ‐animal ‐bovine ‐cattle ‐pigs ‐swine Total of #1 to #4 |
1171 |
| African Index Medicus searched via Global Health Library | 1993 to July 2018 | (leptospir* OR "weils disease" OR "weil's disease" OR "weil disease" OR "stuttgart disease" OR "infectious icterus" OR canicola OR "mud fever" OR "field fever" OR "rat catcher yellow" OR "rat catcher's yellow" OR "pretibial fever" OR grippotyphosa OR icterohaemorrhag* OR icterohemorrhag* OR hardjo OR spirochaetos* OR spirochetos* OR "spirochaetal jaundice" OR "spirochetal jaundice") AND ((molecular AND (assay* OR amplif* OR detect* OR diagnos* OR technique* OR test*)) OR antigen* OR DNA OR RNA OR rRNA OR 16SRNA or 16SrRNA OR nucleic‐acid* OR "polymerase chain reaction" OR "polymerase chain reactions" OR PCR* OR qPCR* OR rtPCR* OR NAAT OR NASBA OR "self‐sustained sequence replication" OR "isothermal amplification" OR LAMP OR primer OR primers OR probe OR probes OR hybridizat* OR hybridisat* OR ISH OR FISH OR immunohistochem* OR immuno‐histochem* OR immunohisto‐chem* OR "fluorescent antibody technique" OR "fluorescent antibody techniques" OR antibody‐coated OR immunoperoxidase* OR immuno‐peroxidase* OR immunofluorescen* OR immuno‐fluorescen* OR immunogold OR immuno‐gold OR IGSS OR silver‐stain* OR dot‐blot* OR dotblot* OR "complement fixation" OR cft OR conglutinat*) | 1 |
| African Journals Online | February 2015 | leptospir* | 17 |
| LILACS (Latin‐American and Caribbean Health Sciences Literature) | 1982 to July 2018 | See African Index Medicus | 284 |
| KoreaMed | February 2015 | Leptospir* | 68 |
| IMSEAR (Index Medicus for the South‐East Asian Region) searched via the Global Health Library | July 2018 | See African Index Medicus | 75 |
| IMEMR (Index Medicus for the Eastern Mediterranean Region) | February 2015 | See African Index Medicus | 5 |
| WPRIM (Western Pacific Region Index Medicus) searched via Global Health Library | July 2018 | See African Index Medicus | 95 |
| IndMed searched via Global Health Library | February 2015 | Leptospirosis OR leprospira | 127 |
Appendix 2. QUADAS‐2 review‐specific guidance
| Domain 1: participant selection | ||
| Risk of bias | Q1. Was a consecutive or random sample of participants enrolled? | "Yes" if a consecutive or random sample of participants was enrolled. "No" if a non‐random (or non‐consecutive) selection method was used. "Unclear" if the procedures are only partially reported and you feel that both 'yes' or 'no' are inadequate. |
| Q2. Was a 2‐gate case‐control design avoided? | "Yes" if the study is a cross‐sectional study or a single‐gate case‐control study, i.e. there was a single set of criteria for study admission, typically defined by the clinical presentation. "No" if a 2‐gate case‐control design was used. "Unclear" if the design is insufficiently reported. |
|
| Q3. Did the study avoid inappropriate exclusions? | "Yes" if the study included all eligible participants. "No" if 'difficult‐to‐diagnose' participants or participants with 'red flags' (symptoms that are specific for leptospirosis, such as conjunctival suffusion and renal insufficiency) were excluded. "Unclear" if the process of enrolment has not been sufficiently reported. |
|
| Summary judgement of risk of bias | Low risk: all 3 answers are 'Yes'. High risk: at least 1 answer is 'No'. Unclear risk: there is no 'No' and at least 1 is 'Unclear'. |
|
| Concerns regarding applicability | Q4. Are there concerns that the included patients and setting do not match the review question? | Low: if participants are the unit of investigation, and if the population characteristics are representative for those who will receive the test in practice. High: if samples are used as the unit of investigation, if either only men or women are enrolled, or when there are other covariates that are reason for concern. Unclear: if answering 'Low' or 'High' concern is inappropriate. |
| Domain 2: index test | ||
| Risk of bias | Q1. Were the index test results interpreted without knowledge of the results of the reference standard? | "Yes" if there is a statement that the index test results were interpreted blind to the results of the reference standard. "No" if this does not appear to be the case. "Unclear" if this information is not reported. |
| Q2. If a threshold was used, was it prespecified? | "Yes" if the threshold values were prespecified before start of the study. "No" if the threshold values were selected based on the collected data. "Unclear" if there is insufficient information to make a judgement. |
|
| Summary judgement of risk of bias | Low risk: both answers are 'Yes'. High risk: at least 1 answer is 'No'. Unclear risk: there is no 'No' and at least 1 is 'Unclear'. |
|
| Concerns regarding applicability | Q3. Does the index test, its conduct, or interpretation match the review question? | "Yes" if fresh samples are used, and when the conduct and interpretation of the test is representative for the same test that is done in practice. "No" if frozen samples are used, or there are other covariates that are reason for concern. "Unclear" when both 'Yes' or 'No' are inappropriate. |
| Q4. Is the execution of the test reported in such detail that reproduction is possible? | "Yes" if the reporting is clear, or when the test is a commercial test kit (all ingredients supplied by a single manufacturer). "No" if the reporting is unclear. |
|
| Summary judgement of concerns regarding applicability | Low concern: both answers are 'Yes'. High concern: at least 1 of the 2 is 'No'. Unclear concern: there is no 'No' and at least 1 is 'Unclear'. |
|
| Domain 3: reference standard | ||
| Risk of bias | Q1. Is this the type of test that is likely to correctly classify the target condition? | "Yes" when a combined reference standard with microscopic agglutination test and another test (serology or culture) is used. "No" if microscopic agglutination test is used as the sole reference standard. "Unclear" if answering 'Yes' or 'No' are inappropriate. |
| Q2. Did the execution of the microscopic agglutination test include paired samples? | "Yes" if acute and convalescent samples were taken; convalescent samples were taken after 5 DPO (WHO 2003), with a gap of at least 2 days (Goris 2012) between the first and second sample. "No" if only single samples were used. "Unclear" if answering 'Yes' or 'No' are both inadequate. |
|
| Q3. Were the reference standard results interpreted without knowledge of the results of the index tests? | "Yes" if there is a statement that the reference standard results were interpreted blind to the results of the index test. "No" if this does not appear to be the case. "Unclear" if this information is not reported. |
|
| Summary judgement of risk of bias | Low risk: all 3 answers are 'Yes'. High risk: at least 1 answer is 'No'. Unclear risk: there is no 'No' and at least 1 is 'Unclear'. |
|
| Concerns regarding applicability | Q4. Does the case definition match the review question? | Almost always 'Yes': we do not consider other case definitions than the 1 proposed in the review question as valid (a composite reference standard). This will be assessed in the 'risk of bias' section. |
| Q5. Is the execution of the test reported in such detail that reproduction is possible? | "Yes" when the reporting is clear. "No" when the reporting is unclear. |
|
| Summary judgement of concerns regarding applicability | Low concern: both answers are 'Yes'. High concern: at least 1 of the 2 is 'No'. Unclear concern: there is no 'No' and at least 1 is 'Unclear'. |
|
| Domain 4: flow and timing | ||
| Risk of bias | Q1. Was there an appropriate interval between index test and reference standard? | This item should be marked as "Unclear" since an appropriate interval between antigen detection tests and the reference standard is not known. 1 of the aims of this review is to investigate the differences in the test accuracy of antigen detection tests when the timing of sample collection varies. |
| Q2. Did all participants receive a reference standard? | "Yes" if it is clear that all (or a random selection of) participants who received the index test also received the reference standard. "No" if participants did or did not receive a reference standard based on the outcome of the index test, or the selection of participants to receive the reference standard was not random. "Unclear" if this information is not reported. |
|
| Q3. Did all participants receive the same reference standard? | "Yes" if all participants had the same reference standard. "No" if this was not the case. "Unclear" if this information is not reported. |
|
| Q4. Were all participants included in the analysis? | "Yes" if all participants entered in the study are included in the analysis. "No" if it appears that some of the participants were excluded from the analysis for whatever reason (e.g. did not complete the study, dubious test results). "Unclear" if it not clear whether all participants were accounted for. |
|
| Summary judgement of risk of bias | Disregarding the question on the appropriate interval. Low risk: all 2 answers are "Yes". High risk: at least 1 answer is "No". Unclear risk: there is no "No" and at least 1 is "Unclear". |
|
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 PCR | 15 | 1884 |
| 2 PCR 1‐9 DPO | 1 | 180 |
| 3 PCR 8‐9 DPO | 1 | 62 |
| 4 qPCR | 16 | 3210 |
| 5 qPCR Serum/plasma | 2 | 255 |
| 6 qPCR rrs/lipl32 (Woods 2017) Buffy coat | 1 | 750 |
| 7 qPCR rrs (Slack 2007) | 1 | 266 |
| 8 qPCR rrs/lipl32 (Woods 2017) Urine | 1 | 626 |
| 9 qPCR rrs (Smythe 2002) | 2 | 83 |
| 10 qPCR lipL32 (Villumsen 2012) | 2 | 83 |
| 11 qPCR rrs (Backstedt 2015) | 1 | 25 |
| 12 qPCR rrs (Slack 2007) Serum | 1 | 766 |
| 13 qPCR rrs (Slack 2007) Buffy coat | 1 | 750 |
| 14 qPCR rrs (Slack 2007) Urine | 1 | 626 |
| 15 qPCR 1‐4 DPO | 1 | 75 |
| 16 qPCR 5‐10 DPO | 1 | 62 |
| 17 qPCR Multiplex | 1 | 55 |
| 18 PCR Urine | 5 | 459 |
| 19 PCR 2x | 2 | 233 |
| 20 nPCR | 4 | 726 |
| 21 nPCR lipL32 (Bomfim 2008) | 1 | 47 |
| 22 LAMP lipL41 (Lin 2009) | 1 | 266 |
| 23 LAMP rrs (Sonthayanon 2011) | 1 | 266 |
| 24 LAMP Plasma | 1 | 287 |
| 25 LAMP Urine | 1 | 184 |
| 26 ICG‐based LFA | 1 | 44 |
| 27 Dipstick | 1 | 44 |
| 28 MAb‐based dot‐ELISA | 1 | 43 |
| 29 ELISA lipl32 | 1 | 29 |
| 30 ELISA fla1 | 1 | 29 |
| 31 ELISA lipl41 | 1 | 29 |
| 32 ELISA hbpA | 1 | 29 |
| 33 ELISA sphCD210 | 1 | 29 |
| 34 ELISA sph2 | 1 | 29 |
| 35 ELISA sph4 | 1 | 29 |
1. Test.
PCR.
2. Test.
PCR 1‐9 DPO.
3. Test.
PCR 8‐9 DPO.
4. Test.
qPCR.
5. Test.
qPCR Serum/plasma.
6. Test.
qPCR rrs/lipl32 (Woods 2017) Buffy coat.
7. Test.
qPCR rrs (Slack 2007).
8. Test.
qPCR rrs/lipl32 (Woods 2017) Urine.
9. Test.
qPCR rrs (Smythe 2002).
10. Test.
qPCR lipL32 (Villumsen 2012).
11. Test.
qPCR rrs (Backstedt 2015).
12. Test.
qPCR rrs (Slack 2007) Serum.
13. Test.
qPCR rrs (Slack 2007) Buffy coat.
14. Test.
qPCR rrs (Slack 2007) Urine.
15. Test.
qPCR 1‐4 DPO.
16. Test.
qPCR 5‐10 DPO.
17. Test.
qPCR Multiplex.
18. Test.
PCR Urine.
19. Test.
PCR 2x.
20. Test.
nPCR.
21. Test.
nPCR lipL32 (Bomfim 2008).
22. Test.
LAMP lipL41 (Lin 2009).
23. Test.
LAMP rrs (Sonthayanon 2011).
24. Test.
LAMP Plasma.
25. Test.
LAMP Urine.
26. Test.
ICG‐based LFA.
27. Test.
Dipstick.
28. Test.
MAb‐based dot‐ELISA.
29. Test.
ELISA lipl32.
30. Test.
ELISA fla1.
31. Test.
ELISA lipl41.
32. Test.
ELISA hbpA.
33. Test.
ELISA sphCD210.
34. Test.
ELISA sph2.
35. Test.
ELISA sph4.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agampodi 2012.
| Study characteristics | |||
| Patient sampling | Single‐gate case‐control study with non‐consecutive/random enrolment of people suspected of having leptospirosis. The selection of cases and controls was non‐consecutive (only people with paired samples). However, the enrolment of the original cohort study was consecutive. We found the risk of bias of this selection procedure to be minimal. Instead, we rated the concerns regarding applicability as high, because people with only a single sample could differ in disease spectrum. |
||
| Patient characteristics and setting | Sample size: 49 cases, 56 non‐cases (total 105) Clinical presentation: acute fever (< 15 days), headache OR myalgia OR prostration, conjunctival suffusion/haemorrhage OR meningeal irritation OR an/olig/protein/haematuria Risk factors: NR Region: Sri Lanka, Kegalle, Kandy, and Matale districts Clinical setting: 3 major hospitals, outbreak setting Prevalence of leptospirosis: 21.7% DPO at moment of enrolment: ≤ 10 Only patients with paired samples |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) on whole blood Target gene: rrs (Smythe 2002) Timing of sample collection at # DPO: 1–10 DPO Cut‐off value: NR Patient material: whole blood Company/brand: inhouse Frozen samples: no Index test 2: real‐time PCR (TaqMan) on serum Target gene: rrs (Smythe 2002) Timing of sample collection at # DPO: 1–10 DPO Cut‐off value: NR Patient material: serum Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO (MAT): acute – see index test; convalescent: 14 DPO (minimum 7 days after acute sample) Single or paired sera (MAT): paired Cut‐off value (MAT): seroconversion or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Agampodi 2016.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with consecutive/random enrolment of people suspected of leptospirosis. | ||
| Patient characteristics and setting | Sample size: 22 cases, 74 non‐cases (total 96) Clinical presentation: acute fever with headache with or without myalgia, and ≥ 1 of: oliguria, polyuria, conjunctival haemorrhage/suffusion, dyspnoea, chest pain. Region: Sri Lanka, Anuradhapura district Clinical setting: outbreak setting Prevalence of leptospirosis: 43.8% DPO of disease at moment of enrolment: median 4 (IQR 3–7) |
||
| Index tests |
Index test: real‐time PCR (TaqMan) Target gene: rrs (Smythe 2002) Timing of sample collection at # DPO of disease: median 4 (IQR 3–7) Cut‐off value: NR Patient material: whole blood or serum Company/brand: inhouse Frozen sample: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: 14 DPO Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400, seroconversion or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ahmed 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with consecutive/random enrolment of people suspected of leptospirosis. | ||
| Patient characteristics and setting | Sample size: 1–4 DPO: 12 cases, 63 non‐cases (total 75); 5–10 DPO: 16 cases, 46 non‐cases (total 62); 1–10 DPO: 26 cases, 107 non‐cases (total 133) Clinical presentation: suspected for leptospirosis, 92% hospitalised, 45.8% attended ICU. Risk factors: NR Region: the Netherlands, countrywide Clinical setting: NR Prevalence of leptospirosis: 19.5% DPO of disease at moment of enrolment: < 10 |
||
| Index tests |
Index test: real‐time PCR (SYBR green) Target gene: SecY (Ahmed 2009) Timing of sample collection at # DPO of disease: 1–10 DPO Cut‐off value: 35 Ct Patient material: blood or serum Company/brand: inhouse Frozen sample: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or IgM ELISA positive or culture positive, or a combination of these (all people underwent all 3 tests) Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:160, seroconversion or a 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (IgM ELISA): see MAT Single or paired sera (IgM ELISA): single and paired Cut‐off value (IgM ELISA): ≥ 1:80, seroconversion or a 4‐fold titre rise Patient material (IgM ELISA): serum Timing of sample collection at # DPO of disease (culture): 1–10 DPO Cut‐off value (culture): 4 months Patient material (culture): whole blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ananyina 2000.
| Study characteristics | |||
| Patient sampling | 2‐gate case‐control design including people with leptospirosis and people without leptospirosis | ||
| Patient characteristics and setting | Sample size: 103 cases, 55 non‐cases (total 158) Clinical presentation: NR for cases; non‐cases had diseases such as acute respiratory infections, hepatitis, lues, Lyme, etc. Risk factors: NR Region: Russia and China, various regions Clinical setting: NR Prevalence of leptospirosis: NA DPO of disease at moment of enrolment: only reported for cases: range 1–5 weeks |
||
| Index tests | Index test: PCR Target gene: G and B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: only reported for cases: range 1–5 weeks Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): NR Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| Low | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Backstedt 2015.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 18 cases, 7 non‐cases (total 25) Clinical presentation: NR ("clinically suspected for severe leptospirosis") Risk factors: NR Region: Brazil, Salvador Clinical setting: hospital Prevalence of leptospirosis: 72.0% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: EDTA blood Company/brand: inhouse Frozen samples: yes Index test 2: real‐time PCR (SYBR green) Target gene: rrs (Backstedt 2015) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: EDTA blood Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both (all people underwent both tests) Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800, seroconversion or a 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): NR Cut‐off value (culture): NR Patient material (culture): NR |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Biscornet 2017.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with consecutive sampling method. | ||
| Patient characteristics and setting | Sample size: 31 cases, 192 non‐cases (total 223) Clinical presentation: acute fever of unknown origin > 3 days, with or without any of the following: headaches, myalgia, haemorrhagic manifestations in the absence of any definite diagnosis. Risk factors: NR Region: Seychelles, nationwide Clinical setting: population‐based national survey Prevalence of leptospirosis: 13.9% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: real‐time PCR (TaqMan) Target gene: rrs (Smythe 2002) Timing of sample collection at # DPO of disease: NR Cut‐off value: 35 Ct Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT or IgM ELISA positive, or both Timing of sample collection at # DPO of disease (MAT): acute: NR; convalescent: ≥ 4 weeks DPO Single or paired sera (MAT): single and paired Cut‐off value (MAT): 1:400 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (IgM ELISA): acute: NR; convalescent: ≥ 4 weeks DPO Single or paired sera (IgM ELISA): NR Cut‐off value (IgM ELISA): NR Patient material (IgM ELISA): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Blanco 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 28 cases, 493 non‐cases (total 521) Clinical presentation: NR ("suspected leptospirosis") Risk factors: NR Region: Brazil Clinical setting: NR Prevalence of leptospirosis: 5.4% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: PCR Target gene: rrs (Merien 1992) Timing of sample collection at # DPO of disease: only reported for cases: 1–10 DPO Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: unclear Index test 2: nested PCR Target gene: rrs (Merien 1992) Timing of sample collection at # DPO of disease: only reported for cases: 1–10 DPO Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): only reported for cases – acute: see index test; convalescent: 11–17 DPO. Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800, seroconversion, or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Nested PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Cardona 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 20 cases, 53 non‐cases (total 73) Clinical presentation: fever, headache, myalgia, vomiting, diarrhoea, fatigue, weakness, jaundice Risk factors: contact with stagnant water contaminated with animal urine. Region: Venezuela, several regions Clinical setting: NR Prevalence of leptospirosis: 27.4% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: PCR on serum Target gene: G and B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen sample: no Index test 2: PCR on urine Target gene: G and B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen sample: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800, seroconversion and a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Chandrasiri 2010.
| Study characteristics | |||
| Patient sampling | Study assessment based only on the abstract. Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 7 cases, 52 non‐cases (total 59) Clinical presentation: NR ("suspected of leptospirosis") Risk factors: NR Region: Sri Lanka Clinical setting: teaching hospital Prevalence of leptospirosis: 11.8% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: G1 and G2 primers (original reference unknown) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: blood product (not specified which) Company/brand: inhouse Frozen samples: NR |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:800 Patient material (MAT): blood product, most likely serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | This study was reported as a meeting abstract. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Unclear | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Chaurasia 2018.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 23 cases, 6 non‐cases (total 29) Clinical presentation: most patients presented with fever and myalgia. Other symptoms included jaundice, low blood pressure, diarrhoea, bronchial asthma, pancreatitis, and anaemia. Involvement of the liver, lungs, and kidneys was occurred in some of patients. Risk factors: NR Region: India (Kolenchery, Kerala) Clinical setting: hospital Prevalence of leptospirosis: 79.3 DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: ELISA (lipl32) Target antigen: lipl32 Timing of sample collection at # DPO of disease: NR Cut‐off value: not reported Patient material: urine Company/brand: inhouse Frozen samples: no Index test 2: ELISA (fla1) Target antigen: fla1 Timing of sample collection at # DPO of disease: NR Cut‐off value: not reported Patient material: urine Company/brand: inhouse Frozen samples: no Index test 3: ELISA (lipl41) Target antigen: lipl41 Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: no Index test 4: ELISA (hbpA) Target antigen: hbpA Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: no Index test 5: ELISA (sphCD210) Target antigen: sphCD210 Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: no Index test 6: ELISA (sph2) Target antigen: sph2 Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: no Index test 7: ELISA (sph4) Target antigen: sph2 Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:100 Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Céspedes 2007.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 26 cases, 92 non‐cases (total 118) Clinical presentation: temperature > 38 °C; malaise; headache; ≥ 1 of chills, dizziness, myalgia, arthralgia, cough, dyspnoea, diarrhoea, nausea or vomiting, conjunctival haemorrhage, haemorrhagic phenomena, jaundice and oliguria Risk factors: some patients from an outbreak Region: Peru Clinical setting: hospital and health centres, outbreak setting Prevalence of leptospirosis: 22.0% DPO of disease at moment of enrolment: < 10 DPO |
||
| Index tests |
Index test: PCR Target gene: rrs (Merien 1992) Timing of sample collection at # DPO of disease: 1–7 DPO Cut‐off value: not applicable Patient material: EDTA blood Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or IgM ELISA positive or culture positive, or a combination of these (all people underwent all 3 tests) Timing of sample collection at # DPO of disease (MAT): acute: 1–9 DPO; convalescent: 7–21 DPO Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400, seroconversion or a 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (IgM ELISA): see MAT Single or paired sera (IgM ELISA): see MAT Cut‐off value (IgM ELISA): NR Patient material (IgM ELISA): serum Timing of sample collection at # DPO of disease (culture): see MAT Patient material (culture): EDTA blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
de Abreu Fonseca 2006.
| Study characteristics | |||
| Patient sampling | 2‐gate case‐control designs including people with leptospirosis and people without leptospirosis. | ||
| Patient characteristics and setting | Sample size: 60 cases, 20 non‐cases (total 80) Clinical presentation: cases: NR. Non‐cases: patients with other febrile diseases diagnosed through microbiological or serological testing. Risk factors: NR Region: Brazil, Sao Paolo Clinical setting: infirmary and ICU of a university hospital Prevalence of leptospirosis: NR DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: PCR on whole blood Target gene: G1/G2 primers (Gravekamp 1993), and LP1/LP2 primers (Kee 1994) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: whole blood Company/brand: inhouse Frozen samples: unclear Index test 2: PCR on urine Target gene: G1/G2 primers (Gravekamp 1993), and LP1/LP2 primers (Kee 1994) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both (all people underwent both tests) Timing of sample collection at # DPO of disease (MAT): acute: NR; convalescent: 10–15 days later Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800, seroconversion or a 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): NR Cut‐off value (culture): NR Patient material (culture): NR |
||
| Flow and timing | 6 urine samples from people without leptospirosis were excluded from the analysis because "they presented inhibitor in positive controls samples spiked with Leptospira". | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Unclear | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Denipitiya 2016.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 65 cases, 46 non‐cases (total 111) Clinical presentation: all patients had fever, influenza‐like illness, headache, chills, and malaise. Some patients had vomiting, jaundice, and conjunctivitis. Risk factors: NR Region: Sri Lanka, Gampaha district Clinical setting: hospital Prevalence of leptospirosis: 58.6% DPO of disease at moment of enrolment: 1–5 DPO |
||
| Index tests |
Index test: real‐time PCR (SYBR Green) Target gene: SecY (Ahmed 2009) Timing of sample collection at # DPO of disease: 1–5 DPO Cut‐off value: 35 Ct Patient material: whole blood Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: 7–14 days after acute sample. Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400, seroconversion to ≥ 1:100, or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Fan 1999.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 5 cases, 10 non‐cases (total 15) Clinical presentation: NR ("suspected leptospirosis") Risk factors: NR Region: China, Guangdong Province, Qingyuan City Clinical setting: hospital Prevalence of leptospirosis: 33.3% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: rrs (Fan 1999) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): NR Cut‐off value (MAT): NR Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Unclear | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Gokmen 2016.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 21 cases, 26 non‐cases (total 47) Clinical presentation: fever, jaundice, hepatosplenomegaly, haematuria, and kidney failure Risk factors: NR Region: Turkey, Cukurova University Hospital and the Adana State Hospital Clinical setting: hospital Prevalence of leptospirosis: 44.7% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: nested PCR targeting lipl32 Target gene: lipL32 (Bomfim 2008) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: yes Index test: nested PCR targeting 16S Target gene: rrs (Merien 1992) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:400 Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Nested PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Gonzalez 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 85 cases, 98 non‐cases (total 183) Clinical presentation: people suspected of leptospirosis. Symptoms reported for cases: 90% had fever, fatigue, myalgia, and headaches (conjunctival hyperaemia in 30%). Risk factors: cases were mainly rural workers Region: Uruguay Clinical setting: NR Prevalence of leptospirosis: 46.4% DPO of disease at moment of enrolment: NR Only people with paired samples were included. |
||
| Index tests |
Index test: real‐time PCR (SYBR green) Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute: NR; convalescent: 10–15 days after acute samples Single or paired sera (MAT): paired Cut‐off value (MAT): NR Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Gravekamp 1993.
| Study characteristics | |||
| Patient sampling | Unclear study design and sampling method. | ||
| Patient characteristics and setting | Sample size: 79 cases, 40 non‐cases (total 119) Clinical presentation: NR Risk factors: NR Region: the Netherlands and Barbados Clinical setting: NR Prevalence of leptospirosis: NR DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: G and B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or IgM ELISA positive, or both (all people underwent both tests) Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): NR Cut‐off value (MAT): NR Patient material (MAT): serum Timing of sample collection at # DPO of disease (IgM ELISA): NR Single or paired sera (IgM ELISA): NR Cut‐off value (IgM ELISA): NR Patient material (IgM ELISA): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kitashoji 2015.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 132 cases, 155 non‐cases (total 287) Clinical presentation: 1, fever and ≥ 2 other signs and symptoms of leptospirosis: headache, myalgia, eye pain, nausea, vomiting, abdominal pain, diarrhoea, conjunctival suffusion, jaundice, tea‐coloured urine, oliguria, anuria, or unusual bleeding and 2. history of exposure to floodwater or animals Risk factors: slum residents, outside occupation Region: Philippines, Manila Clinical setting: hospital, 3 outbreak seasons Prevalence of leptospirosis: 46.0% DPO of disease at moment of enrolment: (for plasma only) 1st sample collection (at admission): 6.5 DPO (IQR 2–19), 2nd sample (at discharge): 11.2 DPO (IQR 4–27) |
||
| Index tests |
Index test 1: LAMP on plasma Target gene: rrs (Koizumi 2012) Timing of sample collection at # DPO of disease: median 6.5 (IQR 2–19) Cut‐off value: not applicable Patient material: plasma Company/brand: inhouse Frozen samples: yes Index test 2: LAMP on urine Target gene: rrs (Koizumi 2012) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: median 11.2 (IQR 4–27) Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400, seroconversion, or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All patients were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test LAMP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Koizumi 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 26 cases, 81 non‐cases (total 107) Clinical presentation: acute febrile illness with headache, myalgia and prostration, with any of the following: conjunctival suffusion/haemorrhage, meningeal irritation, an/olig/protein/haematuria, jaundice, haemorrhages, skin rash, cardiac symptoms AND a history of exposure to infected animals or an environment contaminated with animal urine (clinical case definition of Sri Lanka's surveillance book). Risk factors: NR Region: Sri Lanka, Central Province, Kandy, Teaching Hospital Peradeniya Clinical setting: hospital Prevalence of leptospirosis: 24.3% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: nested PCR Target gene: flaB (Kawabata 2001 / mod: Koizumi 2008) Timing of sample collection at # DPO of disease: 7 DPO Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): 7 DPO Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:400 Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Nested PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Merien 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 17 cases, 34 non‐cases (total 51) Clinical presentation: possible systemic leptospirosis 'presented with various symptoms compatible with this endemic disease'. Risk factors: NR Region: Pacific Island Countries and Territories (Oceania) Clinical setting: NR Prevalence of leptospirosis: 33.3% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: nested PCR Target gene: rrs (Merien 1992) Timing of sample collection at # DPO of disease: median 5 DPO (IQR 3–8; range 1–30) Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: no Index test 2: real‐time PCR Target gene: LFB1 (Merien 2005) Timing of sample collection at # DPO of disease: Median 5 DPO (IQR 3–8; range 1–30) Cut‐off value: NR Patient material: serum Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: median 15 DPO (IQR 10–20) Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:100 or seroconversion Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Nested PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ooteman 2006.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 47 cases,78 non‐cases (total 125) Clinical presentation: fever, myalgia, headache, jaundice, nausea and vomiting, kidney disturbances, conjunctival suffusion, diarrhoea, etc. Risk factors: rural workers (10.3%), garbage workers (3.9%), other occupations Region: Brazil, Minas Gerais State, Fundacao Ezequiel Dias Clinical setting: NR Prevalence of leptospirosis: 37.6% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: G1/G2 primers (Gravekamp 1992) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800 or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Pakoa 2018.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 15 cases, 115 non‐cases (total 130) Clinical presentation: 'Feeling unwell', headache, fever or chills, myalgia or arthralgia, prostration, cough, jaundice, and oliguria or anuria Risk factors: NR Region: Port Vila, Vanuatu (island of Efate) Clinical setting: outpatient clinic at a hospital Prevalence of leptospirosis: 11.5% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: real‐time PCR (TaqMan) Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: serum Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:800 Patient material (MAT): serum |
||
| Flow and timing | 31 people not included because the amount of serum was not enough for MAT. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Does the index test, its conduct and interpretation, match the review question? | Unclear | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Riediger 2007.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 15 cases, 51 non‐cases (total 66) Clinical presentation: NR (clinical suspicion) Risk factors: NR Region: NR Clinical setting: NR Prevalence of leptospirosis: 22.7% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: PCR on whole blood Target gene: G1/G2 and G/B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: whole blood Company/brand: inhouse Frozen samples: no Index test 2: PCR on urine Target gene: G1/G2 and G and B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800 or seroconversion Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Riediger 2017.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with consecutive sampling method. | ||
| Patient characteristics and setting | Sample size: 127 cases, 23 non‐cases (total 150) Clinical presentation: fever, jaundice, conjunctival suffusion, and oliguria Risk factors: NR Region: Brazil (Salvador and Curitiba) Clinical setting: hospital and municipal health unit Prevalence of leptospirosis: 84.7% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) on whole blood Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: NR Cut‐off value: 40 Ct Patient material: whole blood Company/brand: inhouse Frozen samples: yes Index test 2: real‐time PCR (TaqMan) on serum Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: NR Cut‐off value: 40 Ct Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT or culture positive, or both Timing of sample collection at # DPO of disease (MAT): acute: NR; convalescent: approximately 2 weeks after acute sample Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:800 or seroconversion or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): NR Cut‐off value (culture): 3 months Patient material (culture): whole blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Saengjaruk 2002.
| Study characteristics | |||
| Patient sampling | 2‐gate case‐control design including people with leptospirosis and people without leptospirosis. | ||
| Patient characteristics and setting | Sample size: 25 cases, 18 non‐cases (total 43) Clinical presentation: cases: NR. Non‐cases consisted of 10 patients with liver‐fluke (Opisthorchis viverrini) associated cholangiocarcinoma, 3 patients with falciparum malaria, 1 patient with scrub typhus and 4 patients with liver fluke infection (O viverrini). Risk factors: NR Region: Thailand, Nakhon Ratchaseema Province, Muang District Clinical setting: hospital Prevalence of leptospirosis: NA DPO of disease at moment of enrolment: mean 5 days (range 1–14) (reported only for cases) |
||
| Index tests |
Index test: dot‐ELISA Target gene: not applicable Timing of sample collection at # DPO of disease: mean 5 days (range 1–14) (reported only for cases) Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: yes for 1 group, unclear for 2nd group. |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: culture positive Timing of sample collection at # DPO of disease (culture): NR Patient material (culture): blood or urine or cerebrospinal fluid |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Dot‐ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Unclear | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | |||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Samsonova 1997.
| Study characteristics | |||
| Patient sampling | 2‐gate case‐control design including people with leptospirosis and people without leptospirosis. | ||
| Patient characteristics and setting | Sample size: 47 cases. 28 non‐cases (total 75) Clinical presentation: NR for cases, controls had diseases such as serous meningitis, viral hepatitis, and influenza. Risk factors: NR (cases from outbreak setting) Region: Russia and China (Hunan province) Clinical setting: NR Prevalence of leptospirosis: NA DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: G and B primers (Gravekamp 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: none applicable Patient material: serum Company/brand: inhouse Frozen samples: yes (probably) |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): NR Cut‐off value (MAT): NR Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Seng 2007.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with consecutive sampling of participants. | ||
| Patient characteristics and setting | Sample size: 4 cases, 117 non‐cases (total 121) Clinical presentation: ≥ 1 of the following signs or symptoms: fever, headache, myalgia, temperature > 38.3 °C, conjunctival suffusion or muscle tenderness Risk factors: walking through water, throwing garbage on the ground, pulling out sprouts in the wet field for < 6 hours, keeping animals, fertilising the wet fields for < 6 hours. Region: Cambodia, Takeo Pronvincial Hospital Clinical setting: hospital Prevalence of leptospirosis: 3.3% DPO of disease at moment of enrolment: 14 DPO (median) |
||
| Index tests |
Index test: PCR done twice Target gene: 23S rDNA (original reference unknown) Timing of sample collection at # DPO of disease: 1. Median 14, 2. Median 35 Cut‐off value: none applicable Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): acute – median 14 DPO; convalescent – median 35 DPO Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:100 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): median 14 DPO Cut‐off value (culture): 4 months Patient material (culture): whole blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PCR 2x | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sonthayanon 2013.
| Study characteristics | |||
| Patient sampling | Single‐gate case‐control study with with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 100 cases, 150 controls (total 250) Clinical presentation: NR, except for fever (> 37.8 °C) of unknown cause Risk factors: NR Region: Thailand (northeast) Clinical setting: hospital Prevalence of leptospirosis: 31.8% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: real‐time PCR (TaqMan) Target gene: rrs (Slack 2007) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: EDTA blood Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): acute: NR; convalescent: 2 weeks after acute sample Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): NR Cut‐off value (culture): NR Patient material (culture): heparin blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sukmark 2018.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 86 cases, 116 controls (total 202) Clinical presentation: body temperature > 38 °C, myalgia, jaundice, nausea or vomiting, headache, malaise, fatigue, dyspnoea, and abdominal pain Risk factors: history of exposure to flood water, farmers, reservoir animals Region: Thailand (8 different provinces) Clinical setting: NR Prevalence of leptospirosis: 42.6% DPO of disease at moment of enrolment: cases: median 4 DPO, non‐cases: median 3 DPO |
||
| Index tests |
Index test: PCR Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: cases: median 4 (IQR 3–5), non‐cases: 3 (IQR 2–4.3) Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): acute: cases: median 4 (IQR 3–5), non‐cases: median 3 (IQR 2–4.3); convalescent: 7 days after acute sample. Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): cases: median 4 (IQR 3–5), non‐cases: median 3 (IQR 2–4.3) Cut‐off value (culture): 2 weeks Patient material (culture): whole blood |
||
| Flow and timing | 10 people were excluded because of unavailable samples. Additionally, 5 people were excluded because there was no EDTA blood available and 4 people were excluded because there was no clinical information. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Thaipadungpanit 2011.
| Study characteristics | |||
| Patient sampling | Single‐gate case‐control study with consecutive/random enrolment of people suspected of leptospirosis. | ||
| Patient characteristics and setting | Sample size: 133 cases, 133 non‐cases (total 266) Symptoms: NR expect for "Fever (>37.8C) of unknown origin" Risk factors: NR Region: Thailand, northeast, Udon Thani hospital Clinical setting: hospital Prevalence of leptospirosis: 31.8% DPO of disease at moment of enrolment: cases: median 4 (IQR 2–5), controls: median 6 (IQR 3–9) Only patients with paired samples (high concern regarding applicability) |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: cases: median 4 (IQR 2–5), controls: median 6 (IQR 3–9). Cut‐off value: NR Patient material: EDTA blood Company/brand: inhouse Frozen samples: yes Index test 2: real‐time PCR (TaqMan) Target gene: rrs (Slack 2007) Timing of sample collection at # DPO of disease: cases: median 4 (IQR 2–5), controls: median 6 (IQR 3–9). Cut‐off value: NR Patient material: EDTA blood Company/brand: inhouse Frozen samples: yes Index test 3: LAMP Target gene: lipL41 (Lin 2009) Timing of sample collection at # DPO of disease: cases: median 4 (IQR 2–5), controls: median 6 (IQR 3–9) Cut‐off value: not applicable Patient material: EDTA blood Company/brand: inhouse Frozen samples: yes Index test 4: LAMP Target gene: rrs (Sonthayanon 2007) Timing of sample collection at # DPO of disease: cases: median 4 (IQR 2–5), controls: median 6 (IQR 3–9). Cut‐off value: not applicable Patient material: EDTA blood Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): acute – see index test; convalescent: median 17 (IQR 13–21) Single or paired sera (MAT): paired Cut‐off value (MAT): ≥ 1:400 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): see index test. Patient material (culture): heparin blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test LAMP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Vanasco 2016.
| Study characteristics | |||
| Patient sampling | Single‐gate case‐control design with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 67 cases, 121 controls (total 188) Clinical presentation: NR Risk factors: NR Region: Argentina (all regions – reference laboratory) Clinical setting: NA (reference laboratory) Prevalence of leptospirosis: 35.3% DPO of disease at moment of enrolment: 4 (median) |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: median 5 (IQR 4–7) Cut‐off value: 40 Ct Patient material: serum/whole blood Company/brand: inhouse Frozen samples: yes Index test 2: PCR Target gene: lipL32 (Stoddard 2009) Timing of sample collection at # DPO of disease: median 5 (IQR 4–7) Cut‐off value: not applicable Patient material: serum/whole blood Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or IgM ELISA positive, or both Timing of sample collection at # DPO of disease (MAT): > 9 DPO for convalescent sample Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (IgM ELISA): > 9 DPO for convalescent sample Single or paired sera (IgM ELISA): single and paired Cut‐off value (IgM ELISA): NR Patient material (IgM ELISA): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Villumsen 2012 BC.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 7 cases, 22 non‐cases (total 29) Clinical presentation: NR Risk factors: NR Region: Denmark Clinical setting: NR Prevalence of leptospirosis: 24.1% DPO of disease at moment of enrolment: NR Note: people using antibiotics were excluded |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) Target gene: lipL32 (Villumsen 2012) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: blood culture Company/brand: inhouse Frozen samples: unclear Index test 2: real‐time PCR (TaqMan) Target gene: rrs (Smythe 2002) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: blood culture Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:1000 or seroconversion or 2‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | Of 36 patients suspected of leptospirosis, only 29 were tested by MAT (reason unstated in paper). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Villumsen 2012 U.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 3 cases, 51 non‐cases (total 54) Clinical presentation: NR Risk factors: NR Region: Denmark Clinical setting: NR Prevalence of leptospirosis: 5.6% DPO of disease at moment of enrolment: NR Note: people using antibiotics were excluded. |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) Target gene: lipL32 (Villumsen 2012) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: unclear Index test 2: real‐time PCR (TaqMan) Target gene: rrs (Smythe 2002) Timing of sample collection at # DPO of disease: NR Cut‐off value: NR Patient material: urine Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:1000 or seroconversion or 2‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | Of 60 people suspected of leptospirosis, only 54 were tested by MAT (reason unstated in paper). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Waggoner 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 6 cases, 49 non‐cases (total 55) Clinical presentation: NR Risk factors: NR Region: Brazil Clinical setting: NR Prevalence of leptospirosis: 10.9% DPO of disease at moment of enrolment: 8 DPO (range 1–19 DPO) |
||
| Index tests |
Index test 1: real‐time PCR (UFI Assay or Lepto‐MD Assay) (TaqMan) Target gene: rrs (Waggoner 2014) Timing of sample collection at # DPO of disease: acute; range 1–19 DPO Cut‐off value: 45 Ct Patient material: plasma/serum Company/brand: inhouse Frozen samples: yes Note: multiplex real‐time PCR for dengue, plasmodium, and leptospirosis Index test 2: real‐time PCR (TaqMan) Target gene: rrs (Waggoner 2014) Timing of sample collection at # DPO of disease: 8.0 (5–12 IQR) Cut‐off value: 45 Ct Patient material: plasma/serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): see index tests. Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:400 Patient material (MAT): plasma/serum |
||
| Flow and timing | Of 65 people suspected of leptospirosis, only 55 people were tested by MAT (reason unstated in paper). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Waggoner 2015.
| Study characteristics | |||
| Patient sampling | Single‐gate case‐control study with non‐random sampling of participants: up to 55 MAT‐tested samples were selected per month, but if the number exceeded 55, only MAT‐negatives were selected for that month. | ||
| Patient characteristics and setting | Sample size: 33 cases, 445 non‐cases (total 478) Clinical presentation: NR Risk factors: NR Region: Brazil, Rio de Janeiro state Clinical setting: NR Prevalence of leptospirosis: NA DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: real‐time PCR (UFI Assay or Lepto‐MD Assay) (TaqMan) Target gene: rrs (Waggoner 2014) Timing of sample collection at # DPO of disease: NR Cut‐off value: 45 Ct Patient material: serum Company/brand: inhouse Frozen samples: yes Note: multiplex real‐time PCR for dengue, plasmodium, and leptospirosis |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:800 Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wangroongsarb 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 15 cases, 78 non‐cases (total 93) Clinical presentation: NR Risk factors: NR Region: Thailand, Buriram region Clinical setting: hospital Prevalence of leptospirosis: 16.1% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: rrs (no reference) and flaB (Kawabata 2001) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: EDTA blood Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): NR (at admission, 2nd sample 5–14 days later) Single or paired sera (MAT): paired Cut‐off value (MAT): seroconversion or a 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): NR (at admission) Patient material (culture): whole blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Widiyanti 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 28 cases, 16 non‐cases (total 44) Clinical presentation: NR Risk factors: NR Region: Philippines Clinical setting: NR Prevalence of leptospirosis: 63.6% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test 1: PCR Target gene: flaB (Kawabata 2001) Timing of sample collection at # DPO of disease: median 5.5 (IQR 3–9) Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: yes Index test 2: ICG‐based LFA Target gene: not applicable Timing of sample collection at # DPO of disease: median 5.5 (IQR 3–9) Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: yes Index test 3: dipstick assay Target gene: not applicable Timing of sample collection at # DPO of disease: median 5.5 (IQR 3–9) Cut‐off value: not applicable Patient material: urine Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): NR (some samples were collected in the acute phase) Single or paired sera (MAT): single Cut‐off value (MAT): ≥ 1:400 Patient material (MAT): serum |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test ICG‐based LFA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dipstick | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Woods 2018.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with consecutive sample collection. | ||
| Patient characteristics and setting | Sample size: 32 cases, 734 non‐cases (total 766) Clinical presentation: NR (all people had febrile illness) Risk factors: NR Region: Laos (Vientiane city) Clinical setting: hospital Prevalence of leptospirosis: 4.4% DPO of disease at moment of enrolment: median 5 (IQR 3–7, range 1–30) (samples were collected at presentation). |
||
| Index tests |
Index test 1: real‐time PCR (TaqMan) Target gene: rrs (Slack 2007) Timing of sample collection at # DPO of disease: median 5 (IQR 3–7, range 1–30) Cut‐off value: 40 Ct Patient material: serum Company/brand: inhouse Frozen samples: no Index test 2: real‐time PCR (TaqMan) Target gene: rrs (Slack 2007) Timing of sample collection at # DPO of disease: median 5 (IQR 3–7, range 1–30) Cut‐off value: 40 Ct Patient material: buffy coat Company/brand: inhouse Frozen samples: no Index test 3: real‐time PCR (TaqMan) Target gene: rrs (Slack 2007) Timing of sample collection at # DPO of disease: median 5 (IQR 3–7, range 1–30) Cut‐off value: 40 Ct Patient material: urine Company/brand: inhouse Frozen samples: no Index test 4: real‐time PCR (TaqMan) Target gene: rrs/lipL32 (Woods 2018) Timing of sample collection at # DPO of disease: median 5 (IQR 3–7, range 1–30) Cut‐off value: 45 Ct Patient material: serum Company/brand: inhouse Frozen samples: no Index test 5: real‐time PCR (TaqMan) Target gene: rrs/lipL32 (Woods 2018) Timing of sample collection at # DPO of disease: median 5 (IQR 3–7, range 1–30) Cut‐off value: 45 Ct Patient material: buffy coat Company/brand: inhouse Frozen samples: no Index test 6: real‐time PCR (TaqMan) Target gene: rrs/lipL32 (Woods 2018) Timing of sample collection at # DPO of disease: median 5 (IQR 3–7, range 1–30) Cut‐off value: 45 Ct Patient material: urine Company/brand: inhouse Frozen samples: no |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): acute: median 5 (IQR 3–7, range 1–30); convalescent: median 10 (IQR 7–14) Single or paired sera (MAT): single and paired Cut‐off value (MAT): ≥ 1:400 or 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): median 5 (IQR 3–7, range 1–30) Cut‐off value (culture): NR Patient material (culture): blood clot |
||
| Flow and timing | From 811 consecutively sampled people, only 785 serum samples, 774 buffy coats and 644 urine samples were tested by PCR. No reason was provided in the paper. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Real‐time PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Wu 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study with unclear sampling method. | ||
| Patient characteristics and setting | Sample size: 9 cases, 10 non‐cases (total 19) Clinical presentation: typical clinical manifestations ("classic disease triad") Risk factors: NR Region: Xichang city, China Clinical setting: NR Prevalence of leptospirosis: 47.4% DPO of disease at moment of enrolment: NR |
||
| Index tests |
Index test: PCR Target gene: rrs (Wu 1993) Timing of sample collection at # DPO of disease: NR Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: no, but salt buffer added to blood |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): NR Single or paired sera (MAT): NR Cut‐off value (MAT): NR Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): NR Patient material (culture): NR |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Yersin 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study. Although the words 'consecutive' and 'random' were not used, the objective of the survey was to include everyone with suspicion of leptospirosis in the Seychelles. So it is reasonable to conclude that the investigators enrolled everyone meeting the inclusion criteria. | ||
| Patient characteristics and setting | Sample size: 60 cases, 52 non‐cases (total 112) Clinical presentation: any of the following: fever, myalgia, tender liver, jaundice, acute renal failure, bleeding tendency, radiological lung infiltrates, and meningism (inclusion criteria) Risk factors: NR Region: Seychelles, various regions Clinical setting: hospital Prevalence of leptospirosis: 53.6% DPO of disease at moment of enrolment: mean cases: 3.9 (SD 2.1); controls: 4.5 (SD 3.6) |
||
| Index tests |
Index test: PCR done twice Target gene: rrs (Merien 1992) Timing of sample collection at # DPO of disease: PCR #1: mean cases: 3.9 (SD 2.1); controls: 4.5 (SD 3.6) DPO. PCR #2: ≥ 14 DPO Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: yes |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive Timing of sample collection at # DPO of disease (MAT): acute: mean cases: 3.9 (SD 2.1), controls: 4.5 (SD 3.6) DPO; convalescent: ≥ 14 DPO Single or paired sera (MAT): paired Cut‐off value (MAT): seroconversion or a 4‐fold titre rise Patient material (MAT): serum |
||
| Flow and timing | 17 patients who had a single sample were excluded; this decision appeared to have been made post‐hoc. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PCR 2x | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | No | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | No | ||
| Did the execution of the MAT include paired samples? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Zhang 1992.
| Study characteristics | |||
| Patient sampling | Unclear study design, selection procedures not reported. | ||
| Patient characteristics and setting | Sample size: 132 cases, 43 non‐cases (total 175) Clinical presentation: NR Risk factors: NR Region: China Clinical setting: NR Prevalence of leptospirosis: 75.4% (assuming cross‐sectional design) DPO of disease at moment of enrolment: NR ("early") |
||
| Index tests |
Index test: PCR Target gene: 23S rRNA (Zhang 1992) Timing of sample collection at # DPO of disease: 1–5 DPO Cut‐off value: not applicable Patient material: serum Company/brand: inhouse Frozen samples: unclear |
||
| Target condition and reference standard(s) | Target condition: leptospirosis Case definition: MAT positive or culture positive, or both Timing of sample collection at # DPO of disease (MAT): 1–5 DPO and 14–21 DPO Single or paired sera (MAT): paired Cut‐off value (MAT): seroconversion or a 4‐fold titre rise Patient material (MAT): serum Timing of sample collection at # DPO of disease (culture): 1–5 DPO Patient material (culture): whole blood |
||
| Flow and timing | All people were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a 2‐gate case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PCR | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Does the index test, its conduct and interpretation, match the review question? | Unclear | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is this the type of test that is likely to correctly classify the target condition? | Yes | ||
| Did the execution of the MAT include paired samples? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Does the case definition match the review question? | Yes | ||
| Is the execution of the test reported in such detail that reproduction is possible? | No | ||
| Low | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ct: threshold cycle; DPO: days postonset; EDTA: ethylenediaminetetraacetic acid; ICG‐LFA: immunochromatography‐based lateral flow assay; ICU: intensive care unit; IgM ELISA: immunoglobulin M enzyme‐linked immunosorbent assay; IQR: interquartile range; LAMP: loop‐mediated isothermal amplification; MAT: microscopic agglutination test; NR: not reported; PCR: polymerase chain reaction; rDNA: ribosomal DNA; rRNA: ribosomal ribonucleic acid.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ananyina 1999 | Based on the title, the study might have been eligible. However, the abstract and the full‐text article were not retrievable. |
| Bal 1994 | Controls were partly healthy people. |
| Barreto 2000 | This seemed like a relevant DTA study, but no data were reported for the construction of 2 × 2 tables (meeting abstract). |
| Brown 1995 | Sample types (blood, urine) were not separately analysed among controls. |
| Calderaro 2002 | Based on the title, the study might have been eligible. However, the abstract and the full‐text article were not retrievable. |
| Capriles 2017a | Based on the abstract, the study might have been eligible. However, the full‐text article was not retrievable. No data were reported for the construction of 2×2 tables. |
| Capriles 2017b | Based on the abstract, the study might have been eligible. However, the full‐text article was not retrievable. No data were reported for the construction of 2×2 tables. |
| Cermakova 2013 | Sample types (urine, EDTA blood, CSF) are not separately analysed. Authors contacted, but no reply received. |
| Chanket 2003 | Based on the title, the study might have been eligible. However, the abstract and the full‐text article were not retrievable. |
| Chu 1998 | The accuracy of MAT in the diagnosis of leptospiral uveitis was unclear. |
| Destura 2007 | Data in table 4 and table 5 disagree. Authors contacted for clarification, but no reply received. |
| Dittrich 2016 | MAT was not the reference test, but it was done for all patients. Authors contacted to request the 2 × 2 table for qPCR vs MAT, but no reply received. |
| Esteves 2018 | Only nested PCR‐positive patients are included in the 2 × 2 tables provided by the authors. |
| Fhogartaigh 2014 | No data were reported for the construction of 2 × 2 tables (meeting abstract). |
| Gosi 2012 | No data were reported for the construction of 2 × 2 tables (meeting abstract). |
| Hochedez 2013 | Sample size was 8. |
| Hodge 1996 | The target condition seemed to be leptospirosis uveitis. The abstract was not retrievable (meeting abstract). |
| Iwasaki 2016 | Index tests LAMP and flab PCR on urine were not separately analysed, Authors contacted to request the 2 × 2 table for both index tests separately, but no reply received. |
| Jie 2017 | Not enough data for 2 × 2 tables. Authors contacted, but no reply received. |
| Kucerova 2013 | Sample types (plasma, urine, CSF, bronchoalveolar lavage, sputum) were not separately analysed. Authors contact for separately analysed data, but no reply received. |
| Kuntawunginn 2013 | No data were reported for the construction of 2 × 2 tables (meeting abstract). |
| Macak 1971 | Based on the title, the study might have been eligible. However, the abstract and the full‐text article were not retrievable. |
| Merien 1995 | Sample types (serum, CSF) were not separately analysed. Authors contacted for separately analysed data, but no reply received. |
| Mullan 2016 | Not enough data for 2 × 2 tables. Authors contacted, but no reply received. |
| Narayanan 2016 | Sensitivity and specificity numbers were available, but not enough data for 2 × 2 tables. Authors contacted for 2 × 2 tables, but no reply received. |
| Natarajaseenivasan 2012 | Sample types (blood, urine) are not separately analysed. Authors contacted for separately analysed data, but no reply received. |
| Ooteman 2004 | Probably the same study as Ooteman 2006. |
| Patil 2016 | Unclear whether this truly met inclusion criteria. No data were reported for the construction of 2 × 2 tables (meeting abstract). |
| Romero 1998 | The accuracy of MAT on CSF in the diagnosis of leptospiral meningitis was unclear. |
| Romero 2000 | The accuracy of MAT on CSF in the diagnosis of leptospiral meningitis was unclear (meeting abstract). |
| Romero 2010 | The accuracy of MAT on CSF in the diagnosis of leptospiral meningitis was unclear. |
| Samsonova 2004 | Based on the title, the study might have been eligible. However, the abstract and the full‐text article were not retrievable. |
| Saravanan 2014 | Not enough data for 2 × 2 tables. Authors contacted, but no reply received. |
| Shekatkar 2010 | Data in table and text disagree. Authors contacted for clarification, but no reply received. |
| Tagoe 2011 | Contacted authors for more data, but no reply received. No data were reported for the construction of 2 × 2 tables (meeting abstract). |
| Taurustiati 2013 | No data were reported for the construction of 2 × 2 tables. |
| Teamkrim 2005 | Based on the title, the study might have been eligible. However, the abstract and the full‐text article were not retrievable. |
| Toma 2018 | PCR was part of the reference standard. Authors contacted for 2 × 2 tables without PCR, but no reply received. |
CSF: cerebrospinal fluid; DTA: diagnostic test accuracy; EDTA: ethylenediaminetetraacetic acid; LAMP: loop‐mediated isothermal amplification; MAT: microscopic agglutination test; PCR: polymerase chain reaction; qPCR: real‐time polymerase chain reaction.
Differences between protocol and review
Author affiliations
At the initiation of the review, the Leptospirosis Reference Center was part of the Department of Biomedical Research (KIT‐BR) within the Royal Tropical Institute (KIT). From 1 July 2016, it became an integral part of the Department of Medical Microbiology at the Academic Medical Center in Amsterdam, the Netherlands. Therefore, the affiliations of MGA Goris and RA Hartskeerl have changed.
Regarding in/exclusion criteria
We decided to exclude studies with fewer than 10 participants, as they would have added little value to our analyses while possibly introducing more sources of heterogeneity.
We decided to exclude studies of ocular and neurological manifestations of leptospirosis, as it was unclear whether MAT was a valid reference standard for these conditions.
We did not prespecify whether or not to include abstracts without full‐text articles. We decided to include abstract‐only studies that reported data for two‐by‐two tables. Additionally, we decided to conduct a sensitivity analysis excluding abstract‐only studies.
In order to prevent incorporation bias, we excluded studies which contained a nucleic acid or antigen detection test in the reference standard.
Regarding investigations of heterogeneity
The covariate 'blood sample type' was added later to the assessment based on the study designs of newly identified studies.
The covariates 'target gene/primer' and 'real‐time PCR visualisation method' was added later to the assessment based on expert recommendation.
The covariate 'endemicity (endemic versus non‐endemic)' was changed to 'prevalence', as it was difficult to classify studies into 'endemic' or 'non‐endemic' settings.
The covariate 'study design' was not assessed because of overlap with 'risk of bias for patients domain' in the sensitivity analysis.
The covariates 'choice of reference standard' and 'timing of MAT testing' were not assessed because of overlap with 'risk of bias for reference standard domain' in the sensitivity analysis.
The covariate 'unit of analysis (participant versus sample)' was not assessed because 'participant' was the unit of analysis in all but one study (Chandrasiri 2010).
Contributions of authors
| Contribution | Authors |
| Title registration | BY, SdV, MG, ML |
| Draft the protocol | BY, SdV, BJV, MG, ML, MPG, RH |
| Develop a search strategy | BY, SdV, BJV, MG, ML, IN |
| Search for studies | IN, RS |
| Obtain copies of studies | BY, BJV, MG |
| Screening titles and abstracts | BY, MG |
| Full‐text assessments | BY, SdV, MG |
| Data extraction | BY, SdV, MG |
| Data cleaning | BY |
| Data analysis | ML |
| Data interpretation | BY, SdV, MG, AA, ML |
| First draft of manuscript | BY |
| Revision of manuscript | BY, SdV, MG, AA, BJV, MPG, RH, ML |
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Conflict of interest
The Leptospirosis Reference Center (AMC), formerly part of the Royal Tropical Institute (KIT), has done multiple diagnostic accuracy studies. Three such studies are included in this review (Gravekamp 1993; Ahmed 2009; Denipitiya 2016).
Financial conflicts
BY: none. SGV: none. AA: none. BJV: none. IN: none. RS: none. MPG: none. RH: none. MG: none. ML: none.
New
References
References to studies included in this review
Agampodi 2012 {published data only}
- Agampodi SB, Matthias MA, Moreno AC, Vinetz JM. Utility of quantitative polymerase chain reaction in leptospirosis diagnosis: association of level of leptospiremia and clinical manifestations in Sri Lanka. Clinical Infectious Diseases 2012;54(9):1249‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agampodi 2016 {published data only}
- Agampodi SB, Dahanayaka NJ, Nöckler K, Anne MS, Vinetz JM. Redefining gold standard testing for diagnosing leptospirosis: further evidence from a well‐characterized, flood‐related outbreak in Sri Lanka. American Journal of Tropical Medicine and Hygiene 2016;95:531‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2009 {published data only}
- Ahmed A, Engelberts MF, Boer KR, Ahmed N, Hartskeerl RA. Development and validation of a real‐time PCR for detection of pathogenic Leptospira species in clinical materials. PloS One 2009;4(9):e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananyina 2000 {published data only}
- Ananyina YV, Samsonova AP, Lebedev VV, Petrov EM, Esipov EN. Gene diagnostics of acute persistent Leptospira infection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2000;4(Suppl):23‐6. [PubMed] [Google Scholar]
Backstedt 2015 {published data only}
- Backstedt BT, Buyuktanir O, Lindow J, Wunder EA, Mitermayer GR, Usmani‐Brown S, et al. Efficient detection of pathogenic leptospires using 16S ribosomal RNA. PloS One 2015;10:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biscornet 2017 {published data only}
- Biscornet L, Dellagi K, Pages F, Bibi J, Comarmond J, Melade J, et al. Human leptospirosis in Seychelles: a prospective study confirms the heavy burden of the disease but suggests that rats are not the main reservoir. PLoS Neglected Tropical Diseases 2017;11(8):e0005831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanco 2014 {published data only}
- Blanco RM, Romero EC. Evaluation of nested polymerase chain reaction for the early detection of Leptospira spp. DNA in serum samples from patients with leptospirosis. Diagnostic Microbiology & Infectious Disease 2014;78(4):343‐6. [DOI] [PubMed] [Google Scholar]
Cardona 2008 {published data only}
- Cardona MN, Moros RM, López EA. Diagnosis of leptospirosis by PCR in patients with febrile icterohemorrhagic syndrome [Diagnóstico de leptospirosis mediante la PCR en pacientes con síndrome febril icterohemorrágico]. Revista de la Sociedad Venezolana de Microbiología 2008;28(1):24‐30. [Google Scholar]
Céspedes 2007 {published data only}
- Céspedes M, Tapia R, Balda L, Gonzalez D, Peralta C, Condori P. Standardization and validation of a PCR test for early human leptospirosis [Estandarización y validación de una prueba de PCR para el diagnóstico precoz de Leptospirosis humana]. Revista Peruana de Medicina Experimental y Salud Pública 2007;24(1):20‐6. [Google Scholar]
Chandrasiri 2010 {unpublished data only}
- Chandrasiri P, Wahala P, Ramesh R, Kulathunge K. Detection of Leptospira by PCR and comparison of different methods for early diagnosis of leptospirosis. Clinical Microbiology and Infection 2010;16:S394. [Google Scholar]
Chaurasia 2018 {published data only}
- Chaurasia R, Thresiamma KC, Eapen CK, Zachariah BJ, Paul R, Sritharan M. Pathogen‐specific leptospiral proteins in urine of patients with febrile illness aids in differential diagnosis of leptospirosis from dengue. European Journal of Clinical Microbiology & Infectious Diseases 2018;37(3):423‐33. [DOI] [PubMed] [Google Scholar]
de Abreu Fonseca 2006 {published data only}
- Abreu Fonseca C, Teixeira de Freitas VL, Calo Romero E, Spinosa C, Arroyo Sanches MC, Silva MV, et al. Polymerase chain reaction in comparison with serological tests for early diagnosis of human leptospirosis. Tropical Medicine & International Health 2006;11(11):1699‐707. [DOI] [PubMed] [Google Scholar]
Denipitiya 2016 {published data only}
- Denipitiya DT, Chandrasekharan NV, Abeyewickreme W, Hartskeerl CM, Hartskeerl RA, Jiffrey AM, et al. Application of a real time polymerase chain reaction (PCR) assay for the early diagnosis of human leptospirosis in Sri Lanka. Biologicals 2016;44(6):497‐502. [DOI] [PubMed] [Google Scholar]
Fan 1999 {published data only}
- Fan Q, Cao D, Liu J. Rapid detection of Leptospira DNA by polymerase chain reaction. en.cnki.com.cn/Article_en/CJFDTOTAL‐ZRSZ902.025.htm 1999 (accessed 3 August 2017).
Gokmen 2016 {published data only}
- Gokmen T, Soyal A, Kalayci Y, Onlen C, Koksal F. Comparison of 16S rRNA‐PCR‐RFLP, LipL32‐PCR and OmpL1‐PCR methods in the diagnosis of leptospirosis. Revista do Instituto de Medicina Tropical de São Paulo 2016;58:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2013 {published data only}
- Gonzalez S, Geymonat JP, Hernandez E, Marques JM, Schelotto F, Varela G. Usefulness of real‐time PCR assay targeting lipL32 gene for diagnosis of human leptospirosis in Uruguay. Journal of Infection in Developing Countries 2013;7(12):941‐5. [DOI] [PubMed] [Google Scholar]
Gravekamp 1993 {published data only}
- Gravekamp C, Kemp H, Franzen M, Carrington D, Schoone GJ, Eys GJ, et al. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. Journal of General Microbiology 1993;139(8):1691‐700. [DOI] [PubMed] [Google Scholar]
Kitashoji 2015 {published data only}
- Kitashoji E, Koizumi N, Lacuesta TL, Usuda D, Ribo MR, Tria ES, et al. Diagnostic accuracy of recombinant immunoglobulin‐like protein A‐based IgM ELISA for the early diagnosis of leptospirosis in the Philippines. PLoS Neglected Tropical Diseases 2015;9:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koizumi 2009 {published data only}
- Koizumi N, Gamage CD, Muto M, Kularatne SA, Budagoda BD, Rajapakse RP, et al. Serological and genetic analysis of leptospirosis in patients with acute febrile illness in Kandy, Sri Lanka. Japanese Journal of Infectious Diseases 2009;62(6):474‐5. [PubMed] [Google Scholar]
Merien 2005 {published data only}
- Merien F, Portnoi D, Bourhy P, Charavay F, Berlioz‐Arthaud A, Baranton G. A rapid and quantitative method for the detection of Leptospira species in human leptospirosis. FEMS Microbiology Letters 2005;249(1):139‐47. [DOI] [PubMed] [Google Scholar]
Ooteman 2006 {published data only}
- Ooteman MC, Vago AR, Koury MC. Evaluation of MAT, IgM ELISA and PCR methods for the diagnosis of human leptospirosis. Journal of Microbiological Methods 2006;65(2):247‐57. [DOI] [PubMed] [Google Scholar]
Pakoa 2018 {published data only}
- Pakoa JG, Soupe‐Gilbert ME, Girault D, Takau D, Gaviga J, Gourinat AC, et al. High incidence of leptospirosis in an observational study of hospital outpatients in Vanuatu highlights the need for improved awareness and diagnostic capacities. PLoS Neglected Tropical Diseases 2018;12(6):e0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riediger 2007 {published data only}
- Riediger IN, Moreirab SD, Skrabaa I, Leônidas J, Hoffmannc LU, Nakatania SM, et al. Use of Urine and Blood Samples for PCR Diagnosis of Human Leptospirosis [Dissertation]. Paraná (Brazil): Federal University of Paraná, 2007. [Google Scholar]
Riediger 2017 {published data only}
- Riediger IN, Stoddard RA, Ribeiro GS, Nakatani SM, Moreira SD, Skraba I, et al. Rapid, actionable diagnosis of urban epidemic leptospirosis using a pathogenic Leptospira lipL32‐based real‐time PCR assay. PLoS Neglected Tropical Diseases 2017;11(9):e0005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saengjaruk 2002 {published data only}
- Saengjaruk P, Chaicumpa W, Watt G, Bunyaraksyotin G, Wuthiekanun V, Tapchaisri P, et al. Diagnosis of human leptospirosis by monoclonal antibody‐based antigen detection in urine. Journal of Clinical Microbiology 2002;40(2):480‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samsonova 1997 {published data only}
- Samsonova AP, Chzhun‐Fu L, Petrov EM, Aliapkina IS, Epon L, Gintsburg AL, et al. Development of polymerase chain reaction‐based test systems for detecting leptospira in polytypical leptospirosis foci. Molekuliarnaia Genetika, Mikrobiologiia i Virusologiia 1997;4:15‐9. [PubMed] [Google Scholar]
Seng 2007 {published data only}
- Seng H, Sok T, Tangkanakul W, Petkanchanapong W, Kositanont U, Sareth H, et al. Leptospirosis in Takeo Province, Kingdom of Cambodia, 2003. Journal of the Medical Association of Thailand 2007;90(3):546‐51. [PubMed] [Google Scholar]
Sonthayanon 2013 {published data only}
- Sonthayanon P, Chierakul W, Wuthiekanun V, Limmathurotsakul D, Amornchai P, Smythe LD, et al. Molecular confirmation of co‐infection by pathogenic Leptospira spp. and Orientia tsutsugamushi in patients with acute febrile illness in Thailand. American Journal of Tropical Medicine & Hygiene 2013;89(4):797‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sukmark 2018 {published data only}
- Sukmark T, Lumlertgul N, Peerapornratana S, Khositrangsikun K, Tungsanga K, Sitprija V, et al. Thai‐Lepto‐on‐admission probability (THAI‐LEPTO) score as an early tool for initial diagnosis of leptospirosis: result from Thai‐Lepto AKI study group. PLoS Neglected Tropical Diseases 2018;12(3):e0006319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thaipadungpanit 2011 {published data only}
- Sonthayanon P, Chierakul W, Wuthiekanun V, Thaipadungpanit J, Kalambaheti T, Boonsilp S, et al. Accuracy of loop‐mediated isothermal amplification for diagnosis of human leptospirosis in Thailand. American Journal of Tropical Medicine & Hygiene 2011;84(4):614‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaipadungpanit J, Chierakul W, Wuthiekanun V, Limmathurotsakul D, Amornchai P, Boonslip S, et al. Diagnostic accuracy of real‐time PCR assays targeting 16S rRNA and lipL32 genes for human leptospirosis in Thailand: a case‐control study. PloS One 2011;6(1):e16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanasco 2016 {published data only}
- Vanasco NB, Jacob P, Landolt N, Chiani Y, Schmeling MF, Cudos C, et al. Diagnostic accuracy of an IgM enzyme‐linked immunosorbent assay and comparison with 2 polymerase chain reactions for early diagnosis of human leptospirosis. Diagnostic Microbiology and Infectious Disease 2016;84:292‐7. [DOI] [PubMed] [Google Scholar]
Villumsen 2012 BC {published data only}
- Villumsen S, Pedersen R, Borre MB, Ahrens P, Jensen JS, Krogfelt KA. Novel TaqMan PCR for detection of Leptospira species in urine and blood: pit‐falls of in silico validation. Journal of Microbiological Methods 2012;91(1):184‐90. [DOI] [PubMed] [Google Scholar]
Villumsen 2012 U {published data only}
- Villumsen S, Pedersen R, Borre MB, Ahrens P, Jensen JS, Krogfelt KA. Novel TaqMan PCR for detection of Leptospira species in urine and blood: pit‐falls of in silico validation. Journal of Microbiological Methods 2012;91(1):184‐90. [DOI] [PubMed] [Google Scholar]
Waggoner 2014 {published data only}
- Waggoner JJ, Abeynayake J, Balassiano I, Lefterova M, Sahoo MK, Liu Y, et al. A multiplex nucleic acid amplification test for the diagnosis of dengue, malaria, and leptospirosis. Journal of Clinical Microbiology 2014;52(5):2011‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner JJ, Balassiano I, Abeynayake J, Sahoo MK, Mohamed‐Hadley A, Liu Y, et al. Sensitive real‐time PCR detection of pathogenic Leptospira spp. and a comparison of nucleic acid amplification methods for the diagnosis of leptospirosis. PloS One 2014;9:e112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waggoner 2015 {published data only}
- Waggoner JJ, Balassiano I, Mohamed‐Hadley A, Vital‐Brazil JM, Sahoo MK, Pinsky BA. Reverse‐transcriptase PCR detection of Leptospira: absence of agreement with single‐specimen microscopic agglutination testing. PloS One 2015;10:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wangroongsarb 2005 {published data only}
- Wangroongsarb P, Yasaeng S, Petkanchanapong W, Naigowit P, Hagiwara T, Kawabata H, et al. Applicability of polymerase chain reaction to diagnosis of leptospirosis. Journal of Tropical Medicine and Parasitology 2005;28(2):43‐7. [Google Scholar]
Widiyanti 2013 {published data only}
- Widiyanti D, Koizumi N, Fukui T, Muslich LT, Segawa T, Villanueva SY, et al. Development of immunochromatography‐based methods for detection of leptospiral lipopolysaccharide antigen in urine. Clinical and Vaccine Immunology 2013;20(5):683‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woods 2018 {published data only}
- Woods K, Nic‐Fhogartaigh C, Arnold C, Boutthasavong L, Phuklia W, Lim C, et al. A comparison of two molecular methods for diagnosing leptospirosis from three different sample types in patients presenting with fever in Laos. Clinical Microbiology & Infection 2018;24(9):1017.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 1996 {published data only}
- Weihua W, Shengfu L, Baomin D. Rapid treatment and PCR detection of serum samples from patients with leptospirosis. www.rsghb.cn/CN/abstract/article_19932.shtml 1996 (accessed 3 August 2017).
Yersin 1998 {published data only}
- Yersin C, Bovet P, Merien F, Wong T, Panowsky J, Perolat P. Human leptospirosis in the Seychelles (Indian Ocean): a population‐based study. American Journal of Tropical Medicine & Hygiene 1998;59(6):933‐40. [DOI] [PubMed] [Google Scholar]
Zhang 1992 {published data only}
- Zhang Y, Dai B. Detection of leptospiral DNA in the serum of 175 patients with early leptospirosis by polymerase chain reaction. Hua Xi Yi Ke da Xue Xue Bao [Journal of West China University of Medical Sciences] 1992;23:256‐60. [PubMed] [Google Scholar]
References to studies excluded from this review
Ananyina 1999 {published data only}
- Ananyina YV, Samsonova AP. Diagnostic efficacy of the polymerase chain reaction at different stages of leptospira infection. Klinicheskaia Laboratornaia Diagnostika 1999;11:10. [Google Scholar]
Bal 1994 {published data only}
- Bal AE, Gravekamp C, Hartskeerl RA, Meza‐Brewster J, Korver H, Terpstra WJ. Detection of leptospires in urine by PCR for early diagnosis of leptospirosis. Journal of Clinical Microbiology 1994;32(8):1894‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barreto 2000 {unpublished data only}
- Barreto IM, Bernardo CCM, Pregnolatto BP, Romero EC. Comparison of polymerase chain reaction with microscopic agglutination test for diagnosis of human leptospirosis. Abstracts of the General Meeting of the American Society for Microbiology 2000;100:171. [Google Scholar]
Brown 1995 {published data only}
- Brown PD, Gravekamp C, Carrington DG, Kemp H, Hartskeerl RA, Edwards CN, et al. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. Journal of Medical Microbiology 1995;43(2):110‐4. [DOI] [PubMed] [Google Scholar]
Calderaro 2002 {published data only}
- Calderaro A, Guegan R, Piccolo G, Ragni P, Conter M, Zuelli C, et al. Leptospira spp. research by double polymerase chain reaction (nested‐PCR) [Ricerca di Leptospira spp. mediante doppia reazione polimerasica a catena (nested‐PCR)]. Microbiologia Medica 2002;17(1):37‐42. [Google Scholar]
Capriles 2017a {published data only}
- Capriles S, Dos Santos C, Espino GC, Salas E, Crozzoli R, Flores E, et al. Leptospirosis: surveillance of cases of the icterohemorrhagic febrile syndrome program. Aragua, Venezuela [Leptospirosis: vigilancia de casos del programa sindrome febril icterohemorragico. Aragua, Venezuela]. Comunidad Salud 2017;15(1):9‐19. [Google Scholar]
Capriles 2017b {published data only}
- Capriles S, Dos Santos C, Garcia CE, Salas E, Crozzoli R, Flores E, et al. Leptospirosis: surveillance of cases in the Aragua Hemorrhagic Jaundice Febrile Syndrome Program. Comunidad Y Salud 2017;15(1):9‐19. [Google Scholar]
Cermakova 2013 {published data only (unpublished sought but not used)}
- Cermakova Z, Kucerova P, Valenta Z, Pliskova L, Bolehovska R, Prasil P, et al. Leptospirosis: possibilities of early laboratory and clinical diagnosis. Central European Journal of Medicine 2013;8(1):84‐9. [Google Scholar]
Chanket 2003 {published data only}
- Chanket T. PCR diagnosis of leptospirosis based on genes from the Rfb Locus [Masters thesis]. Mahidol University, 2003. [Google Scholar]
Chu 1998 {published data only}
- Chu KM, Rathinam R, Namperumalsamy P, Dean D. Identification of Leptospira species in the pathogenesis of uveitis and determination of clinical ocular characteristics in south India. Journal of Infectious Diseases 1998;177(5):1314‐21. [DOI] [PubMed] [Google Scholar]
Destura 2007 {published data only (unpublished sought but not used)}
- Destura RV, Alejandria MM, Mendoza MT, Cajucom MA, Concepcion FA. The clinical utility of monoclonal antibody based DOT‐ELISA urine antigen detection in the diagnosis of leptospirosis. Philippine Journal of Internal Medicine 2007;45:1‐6. [Google Scholar]
Dittrich 2016 {published data only (unpublished sought but not used)}
- Dittrich S, Rudgard WE, Woods KL, Silisouk J, Phuklia W, Davong V, et al. Utility of blood culture fluid for the molecular diagnosis of leptospira: a prospective evaluation. American Journal of Tropical Medicine & Hygiene 2016;94(4):736‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esteves 2018 {published data only}
- Esteves LM, Bulhões SM, Branco CC, Carreira T. Diagnosis of human leptospirosis in a clinical setting: real‐time PCR high resolution melting analysis for detection of leptospira at the onset of disease. Scientific Reports 2018;8(1):9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fhogartaigh 2014 {unpublished data only}
- Fhogartaigh CN, Dittrich S, Chanthongthip A, Dance DA, Newton PN, Shetty N, et al. Comparison of two molecular diagnostic approaches using serum and buffy coat for rapid diagnosis of acute leptospirosis. American Journal of Tropical Medicine and Hygiene 2014;91(5 Suppl):317. [Google Scholar]
Gosi 2012 {unpublished data only}
- Gosi P, Lanteri C, Fukuda M, Jongsakul K, Tyner S, Saunders D, et al. Development and validation of a quantitative PCR (TaqMan) assay for detection and early diagnosis of leptospirosis interrogans using the Joint Biological Agent Identification and Diagnostic System (JBAIDS). American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl 1):324. [Google Scholar]
Hochedez 2013 {published data only}
- Hochedez P, Escher M, Decoussy H, Pasgrimaud L, Martinez R, Rosine J, et al. Outbreak of leptospirosis among canyoning participants, Martinique, 2011. Euro Surveillance 2013;18(18):20472. [PubMed] [Google Scholar]
Hodge 1996 {unpublished data only}
- Hodge W, Sivakumar R, Dean D. Leptospirosis induced uveitis: ophthalmic characteristics and methods of diagnosis. Investigative Ophthalmology & Visual Science 1996;37(3):S361. [Google Scholar]
Iwasaki 2016 {published data only (unpublished sought but not used)}
- Iwasaki H, Chagan‐Yasutan H, Leano PS. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagnostic Microbiology and Infectious Disease 2016;84(4):287‐91. [DOI] [PubMed] [Google Scholar]
Jie 2017 {published data only}
- Jie WU, Dan W, Shao‐ling W, Li C, Hao‐ran YE, Zhong‐hua DU, et al. Leptospirosis detection among patients with fever in Hainan Province, China. Chinese Journal of Zoonoses 2017;33(3):276‐9. [Google Scholar]
Kucerova 2013 {published data only (unpublished sought but not used)}
- Kucerova P, Cermakova Z, Pliskova L, Pavlis O, Kubickova P, Kleprlikova H, et al. Our experience using real‐time PCR for the detection of the gene that encodes the superficial lipoprotein LipL32 of the pathogenic leptospires to confirm the acute form of human leptospirosis. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, Czech Republic 2013;157(4):387‐91. [DOI] [PubMed] [Google Scholar]
Kuntawunginn 2013 {unpublished data only}
- Kuntawunginn W, Gosi P, Jongsakul K, Tyner S, Arsanok M, Assawariyathipat T, et al. Development and validation of a quantitative PCR (TaqMan) assay for detection of acute early cases of pathogenic leptospira species in clinical samples from Thailand. American Journal of Tropical Medicine and Hygiene 2013;11:S89. [Google Scholar]
Macak 1971 {published data only}
- Macak J. Diagnosis of Weil's disease using immunofluorescence technic. Ceskoslovenska Patologie 1971;7(3):154‐6. [PubMed] [Google Scholar]
Merien 1995 {published data only (unpublished sought but not used)}
- Merien F, Baranton G, Perolat P. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. Journal of Infectious Diseases 1995;172(1):281‐5. [DOI] [PubMed] [Google Scholar]
Mullan 2016 {published data only}
- Mullan S, Panwala TH. Polymerase chain reaction: an important tool for early diagnosis of leptospirosis cases. Journal of Clinical and Diagnostic Research : JCDR 2016;10(12):DC08‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayanan 2016 {published data only (unpublished sought but not used)}
- Narayanan R, Sumathi G, Prabhakaran SG, Shanmughapriya S, Natarajaseenivasan K. Paediatric leptospirosis: a population based case‐control study from Chennai, India. Indian Journal of Medical Microbiology 2016;34(2):228‐32. [DOI] [PubMed] [Google Scholar]
Natarajaseenivasan 2012 {published data only (unpublished sought but not used)}
- Natarajaseenivasan K, Raja V, Narayanan R. Rapid diagnosis of leptospirosis in patients with different clinical manifestations by 16S rRNA gene based nested PCR. Saudi Journal of Biological Sciences 2012;19(2):151‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ooteman 2004 {published data only}
- Ooteman MC, Vago AR, Koury MC. Potential application of low‐stringency single specific primer‐PCR in the identification of Leptospira in the serum of patients with suspected leptospirosis. Canadian Journal of Microbiology 2004;50(12):1073‐9. [DOI] [PubMed] [Google Scholar]
Patil 2016 {unpublished data only}
- Patil DY, Dahake RV, Chowdhary AS, Deshmukh RA. Clinico‐epidemiological observations of human leptospirosis from Mumbai, India. Journal of Infection and Public Health 2016;10(2):247‐8. [DOI] [PubMed] [Google Scholar]
Romero 1998 {published data only}
- Romero EC, Billerbeck AE, Lando VS, Camargo ED, Souza CC, Yasuda PH. Detection of Leptospira DNA in patients with aseptic meningitis by PCR. Journal of Clinical Microbiology 1998;36(5):1453‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romero 2000 {unpublished data only}
- Romero EC, Barreto IM, Bernardo CC, Yasuda PH. Leptospiral meningitis diagnosed by PCR. Abstracts of the General Meeting of the American Society for Microbiology 2000;100:171‐2. [Google Scholar]
Romero 2010 {published data only}
- Romero EC, Blanco RM, Yasuda PH. Aseptic meningitis caused by Leptospira spp diagnosed by polymerase chain reaction. Memorias do Instituto Oswaldo Cruz 2010;105(8):988‐92. [DOI] [PubMed] [Google Scholar]
Samsonova 2004 {unpublished data only}
- Samsonova AP, Petrov EM, Lebedev VV, Lysenko IV, Alyapkina YS, Ananina YV. Genome polymorphism of pathogenic leptospires and PCR diagnosis of leptospirosis. Klinicheskaia Laboratornaia Diagnostika 2004;9:30. [Google Scholar]
Saravanan 2014 {published data only (unpublished sought but not used)}
- Saravanan R, Saradhai P, Rani E, Rajasekar V. A comparative study on microscopic agglutination test and counterimmunoelectrophoresis for early detection of human leptospirosis. Indian Journal of Medical Microbiology 2014;32(1):26‐30. [DOI] [PubMed] [Google Scholar]
Shekatkar 2010 {published data only (unpublished sought but not used)}
- Shekatkar S, Harish BN, Parija SC. Diagnosis of leptospirosis by polymerase chain reaction. International Journal of Pharma and Bio Sciences 2010;1(3):1‐6. [Google Scholar]
Tagoe 2011 {unpublished data only}
- Tagoe JA, Puplampu N, Nimo‐Paintsil SC, Kronmann KC, Clemens M, Nyarko E, et al. Leptospirosis in acute febrile patients in Ghana: diagnosis by culture, serology and polymerase chain reaction. American Journal of Tropical Medicine and Hygiene 2011;85(6 Suppl):328‐9. [Google Scholar]
Taurustiati 2013 {published data only (unpublished sought but not used)}
- Taurustiati D. Combination of antibody detection and antigen detection for the development of a diagnosis of leptospirosis [Kombinasi deteksi antibodi dan deteksi antigen untuk pengembangan diagnosis leptospirosis]. repository.unpad.ac.id/17506/1/pustaka_unpad_kombinasi_deteksi.pdf (accessed 3 August 2017).
Teamkrim 2005 {published data only}
- Teankrim S. Sensitivity and specificity of PCR for the diagnosis of leptospirosis [Dissertation]. Bangkok: Department of Medicine. Faculty of Medicine, Siriraj Hospital. Mahidol University, 2005. [Google Scholar]
Toma 2018 {published data only}
- Toma C, Koizumi N, Kakita T, Yamaguchi T, Hermawan I, Higa N, et al. Leptospiral 3‐hydroxyacyl‐CoA dehydrogenase as an early urinary biomarker of leptospirosis. Heliyon 2018;4(4):e00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bharti 2003
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infectious Diseases 2003;3:757‐71. [DOI] [PubMed] [Google Scholar]
Brett‐Major 2012
- Brett‐Major DM, Coldren R. Antibiotics for leptospirosis. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008264.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Budihal 2014
- Budihal SV, Perwez K. Leptospirosis diagnosis: competency of various laboratory tests. Journal of Clinical and Diagnostic Research 2014;8(1):199‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2003
- Costa E, Lopes AA, Sacramento E, Costa YA, Matos ED, Lopes MB, et al. Penicillin at the late stage of leptospirosis: a randomized controlled trial. Revista do Instituto de Medicina Tropical de São Paulo 2003;45:141‐5. [DOI] [PubMed] [Google Scholar]
Costa 2015
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez‐Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Neglected Tropical Diseases 2015;9(9):e0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duarte 2019
- Duarte JL, Giatti LL. Leptospirosis incidence in a state capital in the Western Brazilian Amazon and its relationship with climate and environmentalvariability, 2008‐2013.. Epidemiologia e Serviços de Saúde 2019;28(1):e2017224. [DOI] [PubMed] [Google Scholar]
Edwards 1988
- Edwards CN, Nicholson GD, Hassell TA, Everard CO, Callender J. Penicillin therapy in icteric leptospirosis. American Journal of Tropical Medicine and Hygiene 1988;39:388‐90. [DOI] [PubMed] [Google Scholar]
Farr 1995
- Farr RW. Leptospirosis. Clinical Infectious Diseases 1995;21:1‐6; quiz 7‐8. [DOI] [PubMed] [Google Scholar]
Goris 2011
- Goris MG, Boer KR, Bouman‐Strijker M, Hartskeerl R, Lucas C, Leeflang M. Serological laboratory tests for diagnosis of human leptospirosis in patients presenting with clinical symptoms. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009406] [DOI] [Google Scholar]
Goris 2012
- Goris MG, Leeflang MM, Boer KR, Goeijenbier M, Gorp EC, Wagenaar JF, et al. Establishment of valid laboratory case definition for human leptospirosis. Journal of Bacteriology and Parasitology 2012;3:2. [Google Scholar]
Goris 2013
- Goris MG, Boer KR, Kliffen SJ, Hartskeerl RA. Human leptospirosis trends, the Netherlands, 1925‐2008. Emerging Infectious Diseases 2013;19(3):371‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerra 2013
- Guerra MA. Leptospirosis: public health perspectives. Biologicals 2013;41:295‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2014
- Hall C, Lambourne J. The challenges of diagnosing leptospirosis. Journal of Travel Medicine 2014;21(2):139‐40. [DOI] [PubMed] [Google Scholar]
Jensenius 2013
- Jensenius M, Han PV, Schlagenhauf P, Schwartz E, Parola P, Castelli F, et al. GeoSentinel Surveillance Network. Acute and potentially life‐threatening tropical diseases in western travelers – a GeoSentinel multicenter study, 1996‐2011. American Journal of Tropical Medicine and Hygiene 2013;88(2):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2010
- Lau C, Smythe L, Weinstein P. Leptospirosis: an emerging disease in travellers. Travel Medicine and Infectious Disease 2010;8(1):33‐9. [DOI] [PubMed] [Google Scholar]
Levett 2001
- Levett PN. Leptospirosis. Clinical Microbiology Reviews 2001;14(2):296‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Limmathurotsakul 2012
- Limmathurotsakul D, Turner EL, Wuthiekanun V, Thaipadungpanit J, Suputtamongkol Y, Chierakul W, et al. Fool's gold: why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clinical Infectious Diseases 2012;55:322‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
McBride 2005
- McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Current Opinion in Infectious Diseases 2005;18:376‐86. [DOI] [PubMed] [Google Scholar]
McClain 1984
- McClain JB, Ballou WR, Harrison SM, Steinweg DL. Doxycycline therapy for leptospirosis. Annals of Internal Medicine 1984;100:696‐8. [DOI] [PubMed] [Google Scholar]
Mwachui 2015
- Mwachui MA, Crump L, Hartskeerl R, Zinsstag J, Hattendorf J. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Neglected Tropical Diseases 2015;9(9):e0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pappas 2008
- Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. International Journal of Infectious Diseases 2008;12:351‐7. [DOI] [PubMed] [Google Scholar]
Picardeau 2014
- Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, Durski K, Hartskeerl RA. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagnostic Microbiology and Infectious Disease 2014;78:1‐8. [DOI] [PubMed] [Google Scholar]
Pijnacker 2016
- Pijnacker R, Goris MG, Wierik MJ, Broens EM, Giessen JW, Rosa M, et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Eurosurveillance 2016;21(17):1‐7. [DOI] [PubMed] [Google Scholar]
Rutjes 2005
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case‐control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51:1335‐41. [DOI] [PubMed] [Google Scholar]
Rutjes 2007
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. Health Technology Assessment 2007;11(50):iii, ix‐51. [DOI] [PubMed] [Google Scholar]
Vijayachari 2008
- Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. Journal of Biosciences 2008;33:557‐69. [DOI] [PubMed] [Google Scholar]
Warnasekara 2019
- Warnasekara J, Koralegedara I, Agampodi S. Estimating the burden of leptospirosis in Sri Lanka; a systematic review. BMC Infectious Diseases 2019;19(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 1988
- Watt G, Padre LP, Tuazon ML, Calubaquib C, Santiago E, Ranoa CP, et al. Placebo‐controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 1988;1:433‐5. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies.. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Human leptospirosis: guidance for diagnosis, surveillance and control, 2003. whqlibdoc.who.int/hq/2003/WHO_CDS_CSR_EPH_2002.23.pdf (accessed 3 August 2017).
References to other published versions of this review
Yang 2015
- Yang B, Vries SG, Visser BJ, Nagel IM, Goris MG, Leeflang MM, et al. Molecular and antigen detection tests for leptospirosis. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011871] [DOI] [PMC free article] [PubMed] [Google Scholar]


