Abstract
Background
Pharmacological treatments for tobacco dependence, such as nicotine replacement therapy (NRT), have been shown to be safe and effective interventions for smoking cessation. Higher levels of adherence to these medications increase the likelihood of sustained smoking cessation, but many smokers use them at a lower dose and for less time than is optimal. It is important to determine the effectiveness of interventions designed specifically to increase medication adherence. Such interventions may address motivation to use medication, such as influencing beliefs about the value of taking medications, or provide support to overcome problems with maintaining adherence.
Objectives
To assess the effectiveness of interventions aiming to increase adherence to medications for smoking cessation on medication adherence and smoking abstinence compared with a control group typically receiving standard care.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register, and clinical trial registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform) to the 3 September 2018. We also conducted forward and backward citation searches.
Selection criteria
Randomised, cluster‐randomised or quasi‐randomised studies in which adults using active pharmacological treatment for smoking cessation were allocated to an intervention arm where there was a principal focus on increasing adherence to medications for tobacco dependence, or a control arm providing standard care. Dependent on setting, standard care may have comprised minimal support or varying degrees of behavioural support. Included studies used a measure that allowed assessment of the degree of medication adherence.
Data collection and analysis
Two authors independently screened studies for eligibility, extracted data for included studies and assessed risk of bias. For continuous outcome measures, we calculated effect sizes as standardised mean differences (SMDs). For dichotomous outcome measures, we calculated effect sizes as risk ratios (RRs). In meta‐analyses for adherence outcomes, we combined dichotomous and continuous data using the generic inverse variance method and reported pooled effect sizes as SMDs; for abstinence outcomes, we reported and pooled dichotomous outcomes. We obtained pooled effect sizes with 95% confidence intervals (CIs) using random‐effects models. We conducted subgroup analyses to assess whether the primary focus of the adherence treatment ('practicalities' versus 'perceptions' versus both), the delivery approach (participant versus clinician‐centred) or the medication type were associated with effectiveness.
Main results
We identified two new studies, giving a total of 10 studies, involving 3655 participants. The medication adherence interventions studied were all provided in addition to standard behavioural support.They typically provided further information on the rationale for, and emphasised the importance of, adherence to medication or supported the development of strategies to overcome problems with maintaining adherence (or both). Seven studies targeted adherence to NRT, two to bupropion and one to varenicline. Most studies were judged to be at high or unclear risk of bias, with four of these studies judged at high risk of attrition or detection bias. Only one study was judged to be at low risk of bias.
Meta‐analysis of all 10 included studies (12 comparisons) provided moderate‐certainty evidence that adherence interventions led to small improvements in adherence (i.e. the mean amount of medication consumed; SMD 0.10, 95% CI 0.03 to 0.18; I² = 6%; n = 3655), limited by risk of bias. Subgroup analyses for the primary outcome identified no significant subgroup effects, with effect sizes for subgroups imprecisely estimated. However, there was a very weak indication that interventions focused on the 'practicalities' of adhering to treatment (i.e. capabilities, resources, levels of support or skills) may be effective (SMD 0.21, 95% CI 0.03 to 0.38; I² = 39%; n = 1752), whereas interventions focused on treatment 'perceptions' (i.e. beliefs, cognitions, concerns and preferences; SMD 0.10, 95% CI –0.03 to 0.24; I² = 0%; n = 839) or on both (SMD 0.04, 95% CI –0.08 to 0.16; I² = 0%; n = 1064), may not be effective. Participant‐centred interventions may be effective (SMD 0.12, 95% CI 0.02 to 0.23; I² = 20%; n = 2791), whereas those that are clinician‐centred may not (SMD 0.09, 95% CI –0.05 to 0.23; I² = 0%; n = 864).
Five studies assessed short‐term smoking abstinence (five comparisons), while an overlapping set of five studies (seven comparisons) assessed long‐term smoking abstinence of six months or more. Meta‐analyses resulted in low‐certainty evidence that adherence interventions may slightly increase short‐term smoking cessation rates (RR 1.08, 95% CI 0.96 to 1.21; I² = 0%; n = 1795) and long‐term smoking cessation rates (RR 1.16, 95% CI 0.96 to 1.40; I² = 48%; n = 3593). In both cases, the evidence was limited by risk of bias and imprecision, with CIs encompassing minimal harm as well as moderate benefit, and a high likelihood that further evidence will change the estimate of the effect. There was no evidence that interventions to increase adherence to medication led to any adverse events. Studies did not report on factors plausibly associated with increases in adherence, such as self‐efficacy, understanding of and attitudes toward treatment, and motivation and intentions to quit.
Authors' conclusions
In people who are stopping smoking and receiving behavioural support, there is moderate‐certainty evidence that enhanced behavioural support focusing on adherence to smoking cessation medications can modestly improve adherence. There is only low‐certainty evidence that this may slightly improve the likelihood of cessation in the shorter or longer‐term. Interventions to increase adherence can aim to address the practicalities of taking medication, change perceptions about medication, such as reasons to take it or concerns about doing so, or both. However, there is currently insufficient evidence to confirm which approach is more effective. There is no evidence on whether such interventions are effective for people who are stopping smoking without standard behavioural support.
Plain language summary
Can we help smokers to increase their use of stop‐smoking medicines?
Background
Medicines designed to make it easier for people to stop smoking, such as nicotine replacement therapy (NRT), bupropion and varenicline, are safe and successfully help people to quit. However, people often do not follow the instructions that come with the medicines properly, which may mean that the medicines do not work as well as they could. This probably reduces a person's chances of giving up smoking for good. In this review, we looked at whether there are ways to help people to use stop‐smoking medicines correctly, and whether this makes people more likely to quit smoking.
Study characteristics
We searched for studies up to September 2018, and we found 10 studies, including 3655 people. All of these people were smokers, over 18 years of age. Studies tested different ways of helping people use their stop‐smoking medicines properly. Typically this meant providing additional information about the medicine or helping people to overcome problems they had with taking the medicine. One study delivered support by telephone, and the rest provided at least some face‐to‐face support. All included studies measured the amount that people used their medicines and all but one measured how many people quit smoking.
Key results
People who received help to improve their use of medicines to stop smoking used their medicines slightly more than people who did not receive this help. There was some evidence that this also led to slightly more people quitting smoking.
Quality of the evidence
The evidence that helping people improve their use of stop‐smoking medicines can successfully boost the use of these medicines is of moderate quality, meaning that more evidence could make us feel more certain of this effect. This is because there were problems with the methods of some of the included studies. The evidence suggesting that approaches to improve the use of stop‐smoking medicines can help more people to quit smoking is of low quality, which means that we are not confident that they do actually help more people to quit and further evidence may or may not strengthen our confidence in this effect. This is because there were problems with some of the study methods and because it is unclear whether providing extra support to encourage people to use their medicines leads to more or fewer people successfully quitting smoking.
Summary of findings
Summary of findings for the main comparison. Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking.
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: adult smokers Settings: typically in‐person clinical settings (China, UK, USA) Intervention: interventions to increase adherence through providing information and facilitating problem‐solving Comparison: behavioural support for smoking cessation | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of participants (studies; comparisons) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Intervention to increase adherence | ||||
| Adherence to medications for tobacco dependence | SMD 0.10 (0.03 to 0.18) | Mean proportion of prescribed medication consumed over 28 days was 63.6% | Mean proportion of prescribed medication consumed over 28 days was 3.9% higher (95% CI 1.2% to 7.0% higher) | 3655 (10 RCTs; 12 comparisons) | ⊕⊕⊕⊝ Moderatea,b |
| Short‐term abstinence from smoking (< 6 months) | RR 1.08 (0.96 to 1.21) | 357 people per 1000 achieve abstinence) | 386 people per 1000 achieve abstinence (95% CI 343 to 432) |
1795 (5 RCTs; 5 comparisons) | ⊕⊕⊝⊝ Lowa,b,c |
| Long‐term abstinence from smoking (≥ 6 months) | RR 1.16 (0.96 to 1.40) | 203 people per 1000 achieve abstinence | 236 per 1000 achieve abstinence (95% CI 195 to 284) |
3593 (5 RCTs; 7 comparisons) | ⊕⊕⊝⊝ Lowa,b,c |
| The basis for the illustrative comparative risks is provided in Footnotesd. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
|
GRADE Working Group grades of evidence High certainty: current evidence provides a very good indication of the likely effect, and the likelihood that the actual effect will be substantially different is low. Moderate certainty: current evidence provides a good indication of the likely effect, and the likelihood that the actual effect of the treatment will not be substantially different is moderate. Low certainty: current evidence provides some indication of the likely effect, but the likelihood that the actual effect will be substantially different is high. Very low certainty: current evidence does not provide a reliable indication of the likely effect, and the likelihood that the actual effect will be substantially different is very high. | |||||
aMost studies were at high or unclear risk of bias which lowers confidence in estimate of effect (risk of bias). bWe did not downgrade due to indirectness as we judged the evidence specifically relating to the general population receiving an adherence intervention in addition to behavioural support for smoking cessation, compared to behavioural support alone, is moderate. However, our conclusions cannot be generalised to populations not receiving behavioural support or that are unlikely to adhere, or both. cIncluded sufficient sample size for single adequately powered trial but 95% CI overlapped no effect and ranged from minimal harm to moderate benefit (imprecision). dConcerning adherence outcomes for the comparison group, as the basis for an illustration of potential effect size on a more familiar metric, we used data from the largest included study that reported adherence as assessed by tablet counts (Marteau 2012). In this study, mean proportion of prescribed nicotine replacement therapy that was consumed at 28 days by the 'standard care' arm was 63.6% (SD 39.0%). Further explanation is provided in Data synthesis. Concerning abstinence outcomes for the comparison group, the percentage of observed events seen in the review's data (which derives from studies with characteristics as specified by the 'Summary of findings' table) was applied to 1000 total events.
Background
Description of the condition
Smoking is one of the largest preventable causes of disease and premature death worldwide, being a key causal factor in heart disease, stroke, chronic lung disease and cancers (GBD 2018). Pharmacological treatments for tobacco dependence, such as nicotine replacement therapy (NRT), are widely considered to be safe and effective interventions for smoking cessation. One Cochrane systematic review found that participants using NRT were over 1.5 times more likely to achieve abstinence than those who did not (Hartmann‐Boyce 2018). Participants using bupropion, nortriptyline and varenicline are also more likely to stop smoking than those using placebo (Cahill 2016; Hughes 2014). However, studies have shown that many smokers who use medications for tobacco dependence do so at a lower dose and for less time than the evidence suggests is optimal (Cheong 2010; Hays 2010; Shiffman 2008; Swan 2010). For example, Burns and Levinson reported that users of NRT, on average, continue medication for less than half the time for which it was prescribed (Burns 2008). Observational evidence controlling for reverse causation (whereby people whose quit attempt was faltering choose not to adhere to their medication) showed that prior adherence to medication promoted later abstinence (Hollands 2013; Shiffman 2007; Shiffman 2008). One review of this relationship, although highlighting the lack of high‐quality studies, suggested that the degree of adherence predicted subsequent abstinence (Raupach 2014). Therefore, it is important to know whether interventions aiming to increase adherence are effective and whether this in turn improves abstinence, the evidence for which we reviewed here.
Description of the intervention
Interventions that specifically aim to increase adherence to prescribed medications vary widely in their content and characteristics (Nieuwlaat 2014). Examples may include, but are not limited to, improved or increased information provision, monitoring and feedback concerning performance, reminders, and psychological therapy or counselling. In the specific context of medications for tobacco dependence, general behavioural support for smoking cessation may include components that target increasing medication adherence. Interventions that are additional to standard behavioural support and that devote special attention to improving adherence may also be delivered, such as addressing individuals' beliefs about the value of taking medications or providing additional support to overcome barriers to adherence.
More‐specific intervention types can be characterised by reference to two key factors informed by the Perceptions and Practicalities Approach (PAPA) (Horne 2013). This approach proposes that non‐adherence can be both intentional and unintentional depending on a person's motivations and capabilities. Perceptual factors ('perceptions'), that is, beliefs, cognitions, concerns and preferences, as well as practical factors ('practicalities'), that is, capabilities, resources, levels of support or skills, can explain non‐adherence and be addressed by interventions to increase adherence. Current guidance in England on medicines adherence emphasises both perceptions and practicalities for improving medication adherence (NICE 2009). PAPA emphasises the importance of tailoring intervention content by eliciting and appreciating the needs, cognitions or behaviours of the patient or participant, and can, therefore, be considered 'participant‐centred'. By contrast, adherence focused interventions that are primarily 'clinician‐centred' tend to be standardised, directive or didactic in nature. We used this approach to categorise interventions in this review.
Why it is important to do this review
To our knowledge, no other published systematic review addresses this question. Reviews of studies of behavioural support interventions (e.g. Hartmann‐Boyce 2019; Lancaster 2017), which may include elements that target medication adherence, are not designed to disentangle the specific effects of those components that focus on increasing adherence. Previous reviews of interventions designed to increase adherence have focused on specific patient groups or treatment contexts, or have not covered smoking cessation treatments (Nieuwlaat 2014). A specific review of the topic is valuable because we cannot be certain that findings relating to adherence to other medications are generalisable to smoking cessation medications, as these provide a unique treatment context with specific issues for adherence. For example, many people see stopping smoking without medication as the best way to stop smoking (Morphett 2015). Additionally, the drawbacks of failing to adhere are less significant than they may be in the treatment of illness. For example, individuals may successfully quit smoking without adhering to therapy, or if they fail to adhere and continue to smoke, they may not feel that they have lost anything or experienced any adverse effects. There is evidence to suggest that it may be more difficult to persuade individuals of the benefits of using smoking cessation medications compared with other health conditions. Hammond 2004 found that over a third of smokers reported that use of pharmacotherapies (NRT or bupropion) would either make no difference or actually reduce the likelihood of quitting smoking. Smokers who perceived cessation assistance methods to be beneficial were more likely to use medication in the future. Finally, some users may perceive risks of harm to their health from the medication that outweigh the potential benefits.
Objectives
To assess the effectiveness of interventions aiming to increase adherence to medications for smoking cessation on medication adherence and smoking abstinence compared with a control group typically receiving standard care.
To assess which intervention approaches are most effective; and determine the impact of interventions on potential precursors of adherence, such as understanding of the treatment and efficacy perceptions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, cluster‐randomised or quasi‐randomised studies.
Types of participants
Adults (aged 18 years and over) smoking at point of entry into a study.
Types of interventions
All participants across relevant intervention and comparator study arms must have been offered effective pharmacological treatment for smoking cessation. Pharmacological treatments comprised those prescribed to increase cessation rates (e.g. NRT, bupropion, nortriptyline, varenicline and combination regimens).
Interventions to increase adherence may vary widely in their nature (Nieuwlaat 2014), and so the nature of the interventions considered for inclusion in this review were not specified beyond reference to exclusion criteria. Eligible interventions included any intervention that differed from standard care administered to smokers, and where the differing intervention content had a clear principal focus on increasing adherence to medications for tobacco dependence, reflected in described content and stated aims. We did not include interventions that systematically altered the active pharmacological characteristics of a given medication, such as dose strength, length of treatment or means of delivery. Interventions that included the use of financial incentives were not eligible.
Acceptable comparison groups were those that provided standard or usual care. Depending on setting, this could comprise of minimal support or varying degrees of behavioural support.
Types of outcome measures
Primary outcomes
Adherence to medication for tobacco dependence.
Studies must have used a quantitative measure of adherence. This could be defined as a continuous measure, such as the amount of medication consumed over a given treatment period, or as a dichotomous outcome, indicating whether the treatment was used to a specific quantified degree (e.g. adherence for x number of days, or x amount of medication consumed). This is in contrast to a binary (i.e. any amount of medication at any time versus non‐use) or categorical checklist measure, which we did not consider an appropriate measure. Adherence could have been measured by electronic measure, tablet counts by a third party or through self‐report (or combinations thereof).
Where studies reported multiple measures of adherence, we used the most stringent available. Where studies assessed treatment periods at multiple time points, we used the longest time point. Where available, we used primary outcome data for only those participants who continued a quit attempt and remained engaged for the duration of a treatment programme rather than dropping out, as opposed to using outcome data from all those randomised to receive a given intervention (i.e. intention‐to‐treat analysis (ITT)) (see Dealing with missing data for further details).
Secondary outcomes
Abstinence from smoking measured near or at a time point relevant to the measure of adherence (less than six months, i.e. short‐term abstinence).
Where there were data from multiple time points, we reported data measured near or at a time point closest to the measure of adherence, expected to be less than six months. Where studies reported multiple definitions of abstinence, we used the most stringent.
Abstinence at six months or longer (i.e. long‐term abstinence)
We reported abstinence at the longest available time point of six months or longer, in order to assess the long‐term benefit of the intervention on cessation rates. For both abstinence outcomes, we used data as randomised (ITT), assuming people not followed up to be smoking.
Other outcomes
Factors plausibly associated with increases in adherence, such as, but not limited to:
intention or motivation to quit smoking (as measured by the studies, likely using a self‐reported questionnaire measure);
attitudes towards treatment, or understanding of the treatment (as measured by the studies, likely using a self‐reported questionnaire measure);
self‐efficacy (as measured by the studies, likely using a self‐reported questionnaire measure).
Adverse events.
Any adverse events or harms reported in included trials, including clinical levels of depression or anxiety.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Tobacco Addiction Group Specialized Register on the 3 September 2018, and two trial registries (ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/)).
The most recent issues of the databases included in the Register, as searched for the current update of this review, were:
Cochrane Central Register of Controlled trials (CENTRAL), issue 8, 2018;
MEDLINE (via Ovid) to update 28 August 2018;
Embase (via Ovid) to week 36 2018;
PsycINFO (via Ovid) to update 20 August 2018.
The search strategy for the Register is given in Appendix 1. For details of the searches used to create the Specialized Register see the Cochrane Tobacco Addiction Group's website.
Searching other resources
We conducted forwards and backwards citation searches from included studies.
Data collection and analysis
Selection of studies
Two review authors independently screened all search results (titles and abstracts) for possible inclusion, and those selected by either or both review authors were subjected to full‐text assessment. Two review authors independently assessed the selected full‐text articles for inclusion. Any discrepancies were resolved by consensus, overseen by a third review author acting as arbiter as necessary. We listed excluded studies after full‐text assessment and gave reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
We developed a data extraction form, which was piloted and amended as necessary. We extracted the following main sets of data from each included study:
lead author;
date;
study participant inclusion criteria;
participants (participant condition(s) and demographics: race/ethnicity, gender, religion/culture, socioeconomic status, age);
study design and timetable; randomisation; allocation concealment;
interventions (content and format of interventions, including details of information provided; intervention setting and delivery provider; delivery of any cointerventions, theoretical basis of intervention if stated; intervention type coded by reference to two factors: 1. focus on perceptions, practicalities, or both; 2. participant‐centred or clinician‐centred);
numbers of participants in each trial arm;
outcome measures; time(s) at which outcomes assessed;
results;
balance of baseline characteristics;
analysis;
additional comments;
study funding and authors' declarations of interest
Two review authors independently extracted data. A third review author checked data extraction and resolved any errors or inconsistencies. The first review author entered the data into Review Manager 5, with another review author checking the accuracy of the data entry (Review Manager 2014).
Assessment of risk of bias in included studies
We assessed and reported the risk of bias of included studies by outcome, in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We reported on the following individual domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of outcome assessment (detection bias) (assessed for each main outcome or class of outcome). We did not assess risk of performance bias pertaining to blinding of participants and personnel due to the difficulty of achieving that in this context, in line with the guidance of the Cochrane Tobacco Addiction Group. It would be impractical to blind those delivering the intervention and attempts to do so could introduce additional limitations, such as reducing potency of the intervention by impairing its delivery and introducing further systematic differences between the intervention exposures by group;
incomplete outcome data (attrition bias) (assessed for each main outcome or class of outcome);
selective reporting (reporting bias);
other sources of bias (consistency in intervention delivery, i.e. was the information standardised/structured; was fidelity to protocol monitored).
Two review authors independently assessed risk of bias of included studies, with any disagreements resolved by discussion and consensus, and with a third review author acting as arbiter as necessary. We present our assessment in Risk of Bias tables for each included study.
A summary risk of bias judgement was derived for each study by applying an algorithm suggested in Section 8.7 (Table 8.7a) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Specifically, if the judgement in at least one of these domains was 'high risk of bias' then summary risk of bias was determined to be high. If there were no judgements of 'high' risk, but the judgement in at least one domain was 'unclear risk of bias', then the summary risk of bias was determined to be unclear. Summary risk of bias was only judged 'low' if judgements in all domains were 'low risk of bias'.
Measures of treatment effect
For continuous outcomes where the precise nature of the measures used differed but the outcomes were regarded as comparable, they were integrated and standardised to have common effect sizes, defined as the standardised mean difference (SMD). The effect measure for comparable dichotomous outcomes was risk ratio (RR). When different studies reported either dichotomous or continuous data for the same outcome, we combined these data using the generic inverse variance method and reported summary effect sizes as SMDs. This followed methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Sections 7.7.7 and 9.4.6; Higgins 2011), whereby standard errors were computed for each study by converting CIs for log odds ratios and SMDs. Log odds ratios were converted to SMDs by multiplying each by the required constant. Where studies provided both dichotomous and continuous measures for the same outcome, a continuous outcome measure was selected. Finally, we accounted for studies that contributed multiple comparisons to the meta‐analysis by reducing their sample sizes in direct relation to how often corresponding data were used.
We obtained a pooled effect size with 95% confidence intervals (CI) using a random‐effects model.
Unit of analysis issues
We included no cluster‐randomised trials and observed no unit of analysis errors. Should we have identified any cluster‐randomised trials, where an analysis was reported accounting for the clustered study design, we would have estimated the effect on this basis. If that had not been possible and the information was not available from authors, then an 'approximately correct' analysis would have been carried out according to current guidelines (Higgins 2011). We would have imputed estimates of the intracluster correlation (ICC) using estimates derived from similar studies or by using general recommendations from empirical research. If it was not possible to implement these procedures, we would have given the effect estimate as presented but reported the unit of analysis error.
Dealing with missing data
In the context of smoking cessation medications, it would be informative for measures of adherence to include only those participants who continue a quit attempt and not all those allocated to receive a given intervention (Hollands 2013). Including those people who abandon a quit attempt is less appropriate because first, treatment such as NRT is not indicated when a person has ceased trying to quit smoking, and second, it potentially confounds adherence with initial uptake (which may be influenced by different factors). As such, we are most interested in adherence to medication in those individuals who continue to engage with a treatment programme and do not dropout from the intervention, and hence remain in the study. Therefore, we intended to analyse data for our primary outcome in this way where available. In practice, primary outcomes for included studies were often presented as ITT, with five instances where it was clear that adherence was assessed and reported only for those who remained engaged with treatment or at least with study follow‐up (Mooney 2005; Nollen 2011; Schlam 2018; Smith 2013; Tucker 2017). For secondary smoking cessation outcomes, we assumed that people not followed up had resumed smoking following Cochrane Tobacco Addiction Group guidance. For such abstinence outcomes, ITT data were reported in all cases.
Assessment of heterogeneity
We tested for heterogeneity by inspecting the overlap of CIs and quantified this using the I² statistic (which describes the percentage of the variability in effect estimates due to heterogeneity rather than sampling error). We considered a value greater than 50% to represent substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We assessed likelihood of publication bias using funnel plots for the primary adherence outcome as there were at least ten studies within that analysis (Sterne 2011).
Data synthesis
We conducted a narrative synthesis of the included studies, presenting studies' major characteristics and results. As studies were sufficiently similar in terms of setting, population, interventions and outcomes (including the time(s) at which these are assessed), we pooled the data statistically. We used a random‐effects model for meta‐analysis to obtain a pooled effect size with 95% CIs, due to observed clinical heterogeneity in study characteristics, such as differences in the treatment contexts and outcome measures used.
Certainty of the evidence
We used the GRADE framework to rate the certainty of each body of evidence relating to an outcome that was incorporated into a meta‐analysis, to indicate the confidence that may be placed in summary estimates of effect (Guyatt 2011). This is an assessment of the likelihood that the true effect will not be substantially different from what the research found. Within the GRADE approach, the certainty of a body of evidence for intervention effects is assessed based on the design of the underlying studies, with randomised controlled trials (RCTs) initially considered high certainty, and on a number of factors that can decrease or increase certainty. GRADE criteria for downgrading certainty of evidence encompass risk of bias, inconsistency, imprecision, indirectness, publication bias and other considerations. If such a criterion is identified, it is classified either as serious (leading to downgrading by one level) or very serious (downgrading by two levels). The four possible certainty ratings that can be applied are:
high certainty (meaning that current evidence provides a very good indication of the likely effect, and the likelihood that the actual effect will be substantially different is low);
moderate certainty (current evidence provides a good indication of the likely effect, and the likelihood that the actual effect of the treatment will not be substantially different is moderate);
low certainty (current evidence provides some indication of the likely effect, but the likelihood that the actual effect will be substantially different is high); and
very low certainty (current evidence does not provide a reliable indication of the likely effect, and the likelihood that the actual effect will be substantially different is very high).
'Summary of findings' tables
The 'Summary of findings' table comprises summaries of the estimated intervention effect and the number of participants and studies for each main outcome, and includes justifications underpinning GRADE assessments. In this case, we completed a 'Summary of findings' table for the primary adherence outcome and the secondary abstinence outcomes: short‐term abstinence and long‐term abstinence. Results of meta‐analyses are presented as SMDs and RRs, with 95% CIs. To facilitate interpretation of effect sizes for the primary outcome that were expressed as SMDs, we re‐expressed these in a more familiar metric (similar to the approach used in other Cochrane Reviews (e.g. Crockett 2018; Hollands 2015a). Because, to our knowledge, there is no larger more definitive survey that uses objective measurement of levels of adherence within standard care, for this translation we used outcome data from Marteau 2012. This was the largest study included within the current review that, first, reported adherence at least partly assessed by tablet counts, and second, used a general population sample in primary care (meaning that its data on adherence is likely to be relatively generalisable). Specifically, we used the standard deviation of the adherence outcome (here assessed as proportion of prescribed NRT that was consumed at 28 days) within the control group (here being the phenotype arm) as this best reflects typical adherence to medication in the absence of an intervention (i.e. within standard care). Such translations have important limitations and are only intended to be broadly illustrative to guide interpretation of the pooled result from the meta‐analysis. For example, what is considered 'standard care' inevitably differs, and in this study involved communicating to smokers that they were being prescribed a higher or lower dose based on their level of nicotine dependence. In addition, NRT may not be representative of all medications used to treat tobacco dependence. More generally, re‐expressed values relate directly to data derived from only one sample with its own context and measurement characteristics and so applying them more widely inevitably extrapolates beyond this.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses for the primary outcome to examine the specific characteristics or components of adherence interventions that may explain their effectiveness, an understanding of which could inform the design of maximally effective interventions. We coded more specific intervention types using the PAPA approach (Horne 2013). First, we coded whether interventions focused on perceptual factors ('Perceptions'; i.e. beliefs, cognitions, concerns and preferences) or practical factors ('Practicalities'; i.e. capabilities, resources, levels of support or skills), or both. Second, we coded whether the intervention content was shaped by eliciting and appreciating the needs, cognitions or behaviours of the patient or participant ('Participant‐centred') or was primarily standardised, directive or didactic in nature ('Clinician‐centred'). We also looked at these two factors in combination. Finally, we conducted a subgroup analysis looking at differential effects on adherence by the type of prescribed medication, although seven of the 10 studies focused on NRT medication.
Sensitivity analysis
We conducted a sensitivity analysis for the primary and secondary outcome analyses, removing the studies at high risk of bias.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; and Characteristics of studies awaiting classification tables for additional details of studies. Table 3 provides a brief overview of the nature of adherence interventions used in the included studies.
1. Brief descriptions of adherence interventions.
| Study | Brief description of specific intervention components intended to increase adherencea | Additional contact time relative to standard care? | Medication for which adherence was targeted | Intervention focused on perceptions, practicalities or both | Participant‐ or clinician‐centred intervention |
| Chan 2010 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant |
| Chan 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant |
| Marteau 2012 | Tailored and communicated about NRT dosage using a more potent rationale (genotype vs phenotype) | No | NRT | Perceptions | Clinician |
| Mooney 2005 | Personalised feedback of questionnaire responses regarding medication | No | NRT | Perceptions | Participant |
| Mooney 2007 | Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant |
| Nollen 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline | Both | Clinician |
| Schlam 2018 | Added contact time to standard behavioural support with: 1. medication adherence counselling; 2. automated reminder calls; 3. electronic monitoring counselling | Yes | NRT | 1. Perceptions 2. Both 3. Practicalities |
1. Participant 2. Clinician 3. Participant |
| Schmitz 2005 | Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant |
| Smith 2013 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Both | Participant |
| Tucker 2017 | Added contact time to standard behavioural support with module focused on improving adherence to nicotine patch | Yes | NRT | Both | Participant |
aFor further details see Characteristics of included studies table.
NRT: nicotine replacement therapy.
Results of the search
The searches for this update retrieved 294 unique records. 10 articles were identified as potentially eligible for inclusion after title and abstract screening. Of these, six articles were excluded at the full‐text screening stage. Of the remaining four records, one was classified as an ongoing study (NCT02635919), and three contained information on two new studies eligible for inclusion in the review (Schlam 2018; Tucker 2017). The flow of studies through the systematic review process for this update is shown in Figure 1.
1.
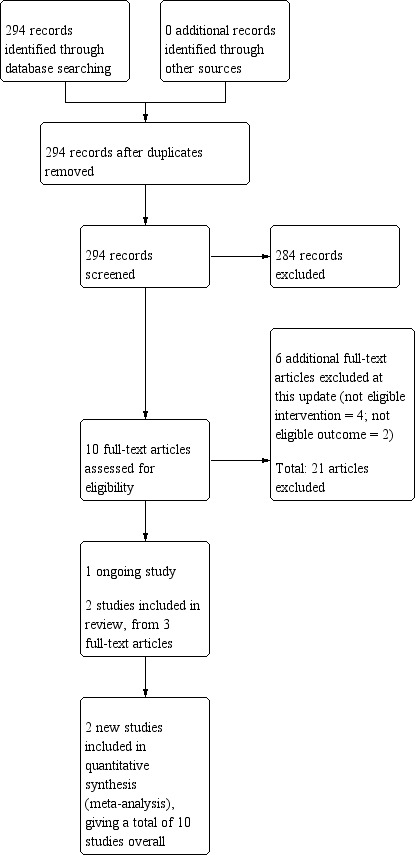
Study flow diagram for the current review update (eight studies were included in the previous version of the review).
Included studies
The review included 10 studies (eight previously included in Hollands 2015b), and two new at this update. These 10 studies included 3655 randomised participants (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017).
Types of studies
All trials were individually randomised controlled trials. Five trials involved randomisation into two groups which were both included in our analysis (Marteau 2012; Mooney 2007; Nollen 2011; Schmitz 2005; Tucker 2017), and three trials involved randomisation into three groups, where only two of these groups were eligible for this review (Chan 2010; Chan 2011; Mooney 2005). One trial involved a 2 × 2 × 2 factorial design with eight randomised groups, but these groups were collapsed into a two‐group comparison, relevant to this review, by the study authors (Smith 2013). One trial involved a 2 × 2 × 2 × 2 × 2 factorial design with 32 randomised groups, with these groups collapsed into three two‐group comparisons relevant to this review (Schlam 2018).
Types of participants and settings
Eight studies included a general population of smokers. Two studies included only participants with a specific clinical condition, namely erectile dysfunction (Chan 2010) and HIV/AIDS (Tucker 2017). The mean ages of participants in trials ranged from 34.6 years (Mooney 2005) to 49 years (Schmitz 2005). In two trials, all participants were women (Mooney 2007; Schmitz 2005). In one trial, all participants were men (Chan 2010). In the remaining trials, percentage women ranged from 7.5% (Tucker 2017) to 62.5% (Nollen 2011). Seven trials took place in the USA (Mooney 2005; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017), two in Hong Kong, China (Chan 2010; Chan 2011), and one in the UK (Marteau 2012). Regarding setting, all but one of the included studies featured interventions that were at least in part delivered in‐person, with the other delivering the intervention by telephone (Smith 2013). The interventions were delivered in clinic (e.g. smoking cessation or outpatient clinics) or social service settings, apart from one that was delivered by telephone (Smith 2013), one where one of the three adherence interventions was delivered by automated telephone call (Schlam 2018), and two where the setting was unclear (Chan 2010; Chan 2011). Those delivering the intervention were trained counsellors or project staff (Chan 2010; Chan 2011; Mooney 2005; Nollen 2011; Schlam 2018; Smith 2013; Tucker 2017), nurses (Marteau 2012; Schmitz 2005), or cognitive behavioural therapy (CBT) practitioners (Mooney 2007).
Types of interventions
The trials all offered pharmacological treatment and some behavioural support, comprising a form of smoking cessation counselling with no particular emphasis on adherence (e.g. providing dosing instructions and weekly checks of adverse effects; Schmitz 2005), to participants in the control arm. Support for the control arm varied from a single support session of 16 minutes (Tucker 2017) or 20 minutes (Mooney 2005) to seven weekly sessions (Marteau 2012; Mooney 2007; Schmitz 2005). In the main, the intervention consisted of an additional component to the standard behavioural support, with eight studies providing additional contact time for those in the intervention arm (Chan 2010; Chan 2011; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017). In the other two studies, the nature of the contact changed but its duration did not significantly differ (Marteau 2012; Mooney 2005). The interventions typically provided information on the rationale for, and emphasised the importance of, adherence to medication, and aided participants in developing strategies to overcome problems and barriers to maintaining adherence. As such, they included a combination of two intervention strategies outlined within a taxonomy of interventions to increase adherence (Haynes 2008), that is included in Appendix 2, namely 1. instruction for participants on medication use or 2. counselling about smoking, and the value of medication in overcoming addiction. Two studies included interventions that involved personalised feedback of medication taking, monitored electronically (Mooney 2007; Schmitz 2005); one study elicited participants' beliefs about medication taking and then provided personalised counselling relating to those beliefs (Mooney 2005); one study tailored medication dose to either genotype or degree of tobacco dependence and explained the rationale for this to participants (Marteau 2012); and five studies added additional counselling contact time to standard behavioural support, with content focusing on medication adherence, including the use of motivational interviewing techniques and the 4/5R approach to increasing motivation (counselling addressing risks, rewards, roadblocks, and repetition, and relevance in the case of the 5Rs; Chan 2010; Chan 2011), a focus on motivation to use the medication and behavioural skills for achieving this (Nollen 2011; Tucker 2017), and targeting medication beliefs with monitoring and feedback on adherence (Smith 2013). One study examined multiple adherence interventions concerning personalised feedback of adherence behaviour, automated medication reminder calls and additional behavioural support content focused on adherence (Schlam 2018).
Seven studies prescribed NRT (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Schlam 2018; Smith 2013; Tucker 2017), two studies prescribed bupropion (Mooney 2007; Schmitz 2005), and one study prescribed varenicline (Nollen 2011).
We categorised the content of each intervention by reference to PAPA. Of 12 comparisons included in the review, three comparisons assessed the impact of changing perceptions (Marteau 2012; Mooney 2005; Schlam 2018 (medication adherence counselling intervention)), and five comparisons assessed the impact of interventions aiming to improve the practicalities of medication‐taking (Chan 2010; Chan 2011; Mooney 2007; Schlam 2018 (electronic monitoring feedback intervention); Schmitz 2005). Four comparisons assessed Interventions of perceptions and practicalities (Nollen 2011; Schlam 2018 (automated calls intervention); Smith 2013; Tucker 2017).
We also assessed whether interventions aimed at changing perceptions or practicalities assessed participants' particular beliefs or difficulties (patient‐centred) or provided a standardised intervention (clinician‐centred). Nine comparisons were patient‐centred (Chan 2010; Chan 2011; Mooney 2005; Mooney 2007; Schlam 2018 (medication adherence counselling intervention); Schlam 2018 (electronic monitoring feedback intervention); Schmitz 2005; Smith 2013; Tucker 2017), and three comparisons were clinician‐centred (Marteau 2012; Nollen 2011; Schlam 2018 (automated calls intervention)).
Types of outcome measures
Measures of adherence varied across studies. Five studies reported at least one continuous outcome, measured as the percentage or amount of prescribed medication that was consumed (Marteau 2012; Mooney 2005; Nollen 2011), number of days on which it was used (Smith 2013), or percentage of days on which a person was adherent (Schlam 2018). Five studies used a dichotomous outcome, meaning people were classified as either achieving or not achieving a specified degree of adherence that was deemed adequate (Chan 2010; Chan 2011; Mooney 2007; Schmitz 2005; Tucker 2017). The definitions of adequate adherence naturally varied by medication type and because there may not be agreed standards for what constitutes desirable levels of adherence. Furthermore, the operationalisation of this was not always clear. In assessing adherence, seven studies at least partly used tablet counts (Marteau 2012; Mooney 2005; Nollen 2011; Tucker 2017), or electronic monitoring systems (Mooney 2007; Schlam 2018; Schmitz 2005). One study used self‐report (Smith 2013), while the means of assessing adherence was unclear in two studies (Chan 2010; Chan 2011). The period for which the primary adherence outcome was being assessed ranged from approximately two weeks (Mooney 2005; Smith 2013), to three months (Nollen 2011).
To assess abstinence seven studies used biochemically validated outcomes (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Mooney 2007; Nollen 2011; Tucker 2017), but only six of these provided useable data in study reports (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Nollen 2011; Tucker 2017). Two studies provided self‐reported abstinence data (Schlam 2018; Smith 2013), and one study did not report abstinence (Schmitz 2005). Time of abstinence outcome measurement ranged from two weeks (Mooney 2005), to six months (Chan 2010; Chan 2011; Marteau 2012; Schlam 2018; Smith 2013), to one year (Schlam 2018).
Excluded studies
We excluded six additional studies at this update. Two did not include an eligible adherence outcome (ISRCTN33423896; McClure 2013), and four did not include an eligible intervention (Cropsey 2017; Gong 2016; McClure 2016; Tseng 2017). Tseng 2017 was previously included in this review as an ongoing study; however, based on information in the published report it was deemed ineligible for inclusion at this update. The detailed description of the intervention made it clear that the content was equally split between standard smoking cessation support and content focused specifically on increasing medication adherence ("Each day participants in the two TM [text message] arms received one adherence‐focused message and one IMB [information‐motivation‐behavioural skills model] smoking cessation‐themed message"). As one of the inclusion criteria for this review stated that differing intervention content should have a clear principal focus on increasing adherence to medications for tobacco dependence, reflected in both described content and stated aims, we decided that this study did not meet the eligibility criteria and would not allow us to assess the effect of the adherence intervention independently.
We excluded 21 studies in the previous version of this review (Hollands 2015b). Our previous searches also identified two studies awaiting classification, which we were still unable to fully assess and include due to a lack of information (Applegate 2007; Yuhongxia 2011). See Characteristics of studies awaiting classification table.
Risk of bias in included studies
It is clear from the risk of bias summary that the included studies were often difficult to assess for bias on our criteria because there was insufficient information in published reports (Figure 2). For summary risk of bias judgements, as described in Assessment of risk of bias in included studies, we were able to judge that these conferred a low summary risk of bias for one study (Marteau 2012). Four studies were assessed at high risk of bias (Mooney 2005; Mooney 2007; Schmitz 2005; Smith 2013), with the remaining studies assessed at unclear risk of bias. Few judgements were made suggesting a high risk of bias for any domain, with the only four examples being risk of bias due to blinding of outcome assessment for Smith 2013 and due to incomplete outcome data for Mooney 2005, Mooney 2007, and Schmitz 2005.
2.
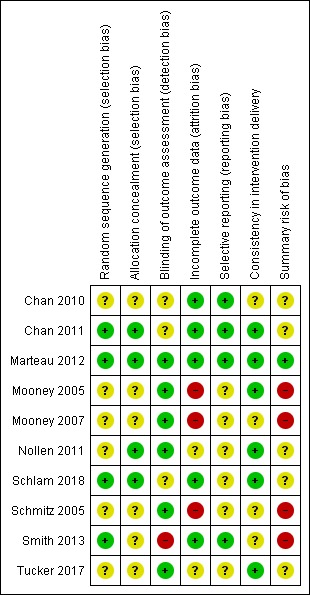
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies were at low risk of selection bias with details being provided of an adequate sequence generation process and steps to ensure allocation concealment (Chan 2011; Marteau 2012; Schlam 2018). One study provided details of adequate allocation concealment but not sequence generation (Nollen 2011), while one study provided details of adequate sequence generation but not allocation concealment (Smith 2013). In the other studies, there was insufficient detail to judge the risk of selection bias (Chan 2010; Mooney 2005; Mooney 2007; Schmitz 2005; Tucker 2017).
Blinding
We did not assess performance bias, as described in the Assessment of risk of bias in included studies section. We did assess whether outcomes were assessed blind to allocation (detection bias). Six studies were judged to be at low risk of detection bias (Marteau 2012; Mooney 2005; Mooney 2007; Nollen 2011; Schmitz 2005; Tucker 2017), one was judged at high risk of detection bias, as it used self‐report to assess all components of the primary adherence outcome (Smith 2013), and three were judged at unclear risk of bias (Chan 2010; Chan 2011; Schlam 2018). Some studies clearly attempted to blind outcome assessors to the secondary abstinence outcome (Chan 2010; Marteau 2012), although only in one study to the primary adherence outcome (Chan 2011). Elsewhere, attempts to blind outcome assessors were unclear (Mooney 2005; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017). However, the use of objective outcome measures of adherence and biochemical validation of abstinence (for all other than Schlam 2018 and Smith 2013), was evidence that these outcomes were unlikely to be affected by detection bias.
Incomplete outcome data
We deemed five studies at low risk of bias because they had low levels of attrition, or addressed substantial or differential (or both) attrition (Chan 2010; Chan 2011; Marteau 2012; Schlam 2018; Smith 2013). Two studies were judged to be at unclear risk of bias (Nollen 2011; Tucker 2017). Three studies were judged to be at high risk of bias because participant numbers were not fully reported, the overall number of participants lost was 50% or greater, the difference in percentage followed up between groups was 20% or more, or a combination of these (Mooney 2005; Mooney 2007; Schmitz 2005).
Selective reporting
Four trials were preregistered on a clinical trials register enabling us to corroborate that specified outcomes remained consistent and so we assessed risk of bias as low (Chan 2010; Chan 2011; Marteau 2012; Smith 2013). One of these also published a protocol (Marteau 2012). A further study was preregistered on a clinical trials register but the adherence outcomes were not specified (Schlam 2018) and so we assessed risk of bias as unclear. We were unable to find registrations for the other five studies so selective reporting within the final reports could not reasonably be ruled out and risk of bias was considered unclear (Mooney 2005; Mooney 2007; Nollen 2011; Schmitz 2005; Tucker 2017).
Other potential sources of bias
We regarded another potential source of bias that was relevant to this review to be consistency in intervention delivery, judging this by whether it was clear that the information given to participants was standardised or structured to some degree, and fidelity to protocol was systematically monitored. Six studies were judged to be at low risk of other bias (Chan 2011; Marteau 2012; Mooney 2005; Nollen 2011; Schlam 2018; Tucker 2017), with the remaining four assessed at unclear risk of bias (Chan 2010; Mooney 2007; Schmitz 2005; Smith 2013).
Effects of interventions
See: Table 1
Primary outcome
Adherence to medication for tobacco dependence
Five studies reported dichotomous adherence measures (Chan 2010; Chan 2011; Mooney 2007; Schmitz 2005; Tucker 2017). Chan 2010 and Chan 2011 assessed whether or not there had been continuous use of NRT, for four weeks (Chan 2010) and eight weeks (Chan 2011). Mooney 2007 and Schmitz 2005 both assessed whether or not participants had taken two daily doses of bupropion as prescribed over the seven‐week treatment period. Tucker 2017 assessed whether participants had used six or more nicotine patches per week, for those participants who provided complete data at baseline and follow‐up. Five studies used continuous adherence measures (Marteau 2012; Mooney 2005; Nollen 2011; Schlam 2018; Smith 2013). Marteau 2012 assessed the proportion of prescribed NRT consumed over the four‐week treatment period and reported the group mean. Mooney 2005 reported the mean pieces of nicotine gum used during the first 15 days of a quit attempt in those who completed the treatment period only. Nollen 2011 assessed the proportion of prescribed varenicline doses taken over three months, for those who remained engaged. Schlam 2018 measured the percentage of days in the first six weeks of the quit attempt where participants were adherent (i.e. where participants used both a nicotine patch and four or more pieces of gum), in those participants who completed the treatment phase. Smith 2013 assessed self‐reported number of days of nicotine patch use in the first two weeks, for those remaining engaged.
Pooled analysis of these data, comprising 12 comparisons from 10 studies, showed that adherence interventions produced a small improvement in adherence, with no significant statistical heterogeneity being observed (SMD 0.10, 95% CI 0.03 to 0.18; I² = 6%; n = 3655; Figure 3). Re‐expressing this effect size produced by the primary random‐effects meta‐analysis in a more familiar metric (see Data synthesis) suggested that interventions to increase adherence could have an effect equivalent to a 3.9% increase (95% CI 1.2% to 7.0%) in the mean proportion of prescribed medications consumed over 28 days.
3.
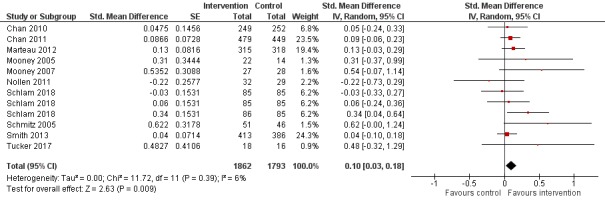
Forest plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).
GRADE assessment indicated that the evidence for this outcome was of moderate certainty, meaning that the true effect is probably close to the estimated effect. This judgement was reached through consideration of the following criteria. The current evidence was downgraded once due to risk of bias, because the majority of studies were judged to be at high or unclear risk of bias. We did not downgrade the evidence further based on other GRADE considerations. For imprecision, the number of participants (sample size) incorporated into this meta‐analysis exceeded the optimal information size (i.e. a sufficient sample size for a single adequately powered trial), and the 95% CI ranged from a very small to a small benefit. For inconsistency, there was minimal heterogeneity and considerable overlapping of CIs. There was no clear reason to downgrade certainty of evidence for indirectness (providing it was emphasised that moderate evidence related only to those receiving an adherence intervention in addition to behavioural support for smoking cessation, compared to behavioural support alone). Finally, for other considerations, including publication bias, the certainty of the evidence was not downgraded. Although a funnel plot of the primary outcome data suggested possible asymmetry (Figure 4), only one of 12 included comparisons was statistically significant, and there was not a clearly consistent pattern of smaller studies resulting in greater intervention effect estimates than larger studies. This limited the plausibility of publication bias as an explanation for asymmetry (Sterne 2011).
4.
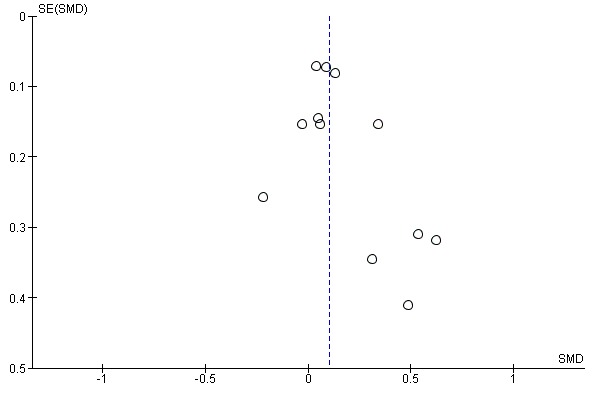
Funnel plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).
Subgroup analyses
We conducted three subgroup analyses of the primary analysis in order to examine the relative impact of specific intervention types in terms of their focus on 'perceptions'; 'practicalities'; or 'both' (Analysis 1.2); and whether the intervention was participant‐centred or clinician‐centred (Analysis 1.3). The third analysis considered these two factors in combination (Analysis 1.4).
1.2. Analysis.
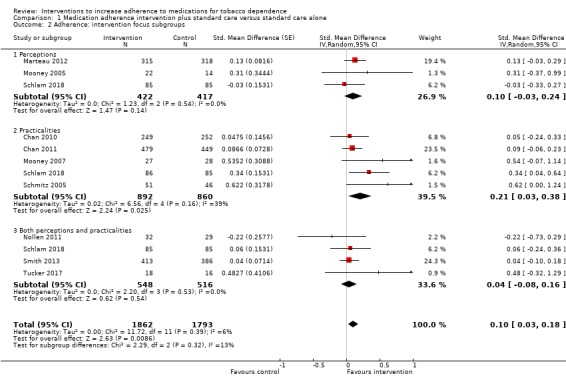
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 2 Adherence: intervention focus subgroups.
1.3. Analysis.
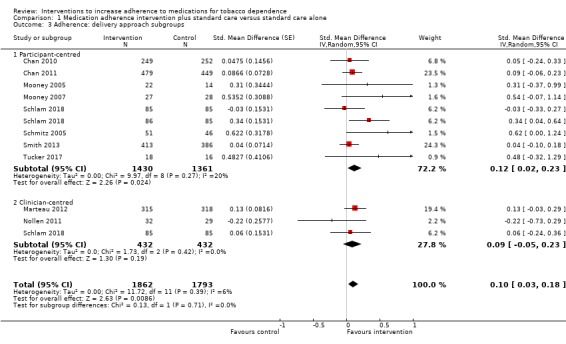
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 3 Adherence: delivery approach subgroups.
1.4. Analysis.
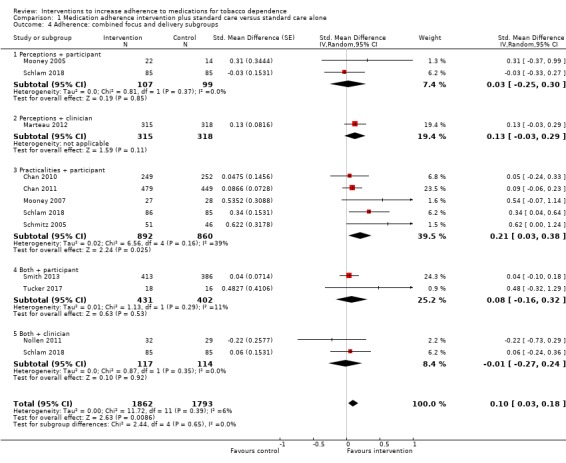
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 4 Adherence: combined focus and delivery subgroups.
There was no strong evidence that the effect of interventions that focused on perceptions, practicalities, or on both differed in their effect on adherence (I² = 13%, P = 0.32; Analysis 1.2). That said, the effect of interventions focused on practicalities appeared slightly larger than the other two groups, with an SMD of 0.21 (95% CI 0.03 to 0.38; I² = 39%; n = 1752), compared with perceptions (SMD 0.10, 95% CI –0.03 to 0.24; I² = 0%; n = 839), or a combination of both perceptions and practicalities (SMD 0.04, 95% CI –0.08 to 0.16; I² = 0%; n = 1064).
There was no clear evidence that participant‐centred interventions differed in effectiveness from clinician‐centred interventions (I² = 0%, P = 0.71; Analysis 1.3). The SMD for participant‐centred interventions was 0.12 (95% CI 0.02 to 0.23; I² = 20%; n = 2791) and for clinician‐centred interventions was 0.09 (95% CI –0.05 to 0.23; I² = 0%; n = 864).
There was also no strong evidence that combining these two classification systems led to subgroup differences in the effect of interventions on medication adherence (I² = 0%, P = 0.65; Analysis 1.4).
We conducted a further subgroup analysis to examine whether there were differential effects of the intervention depending on which medication was prescribed (Analysis 1.5). In this analysis, there was stronger evidence of subgroup differences (I² = 68%, P = 0.04). The effect of interventions to increase adherence to bupropion (SMD 0.58, 95% CI 0.14 to 1.01; I² = 0%; n = 152) was much larger than that for NRT (SMD 0.09, 95% CI 0.02 to 0.17; I² = 0%; n = 3442) or varenicline (SMD from only one study was –0.22, 95% CI –0.73 to 0.29; n = 61).
1.5. Analysis.
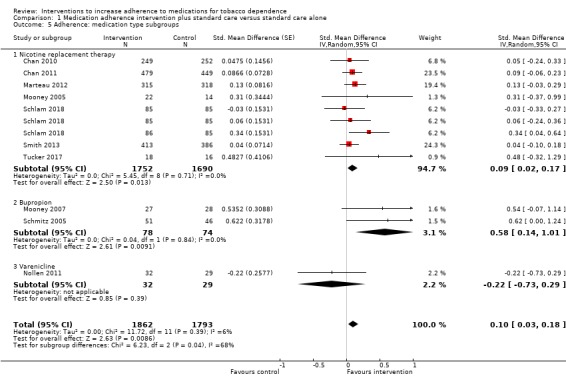
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 5 Adherence: medication type subgroups.
Secondary outcomes
We reported assessments measured at the time point that most closely accorded with the assessment of adherence. If this selected abstinence measure assessed short‐term abstinence (less than six months), we additionally report abstinence at the longest available time point of six months or longer in order to assess the long‐term benefit of the intervention on cessation rates.
Short‐term abstinence (less than six months)
Five studies contributed data to the analysis of short‐term abstinence (Marteau 2012; Mooney 2005; Nollen 2011; Smith 2013; Tucker 2017). Marteau 2012 assessed biochemically validated prolonged abstinence at 28 days, Mooney 2005 assessed biochemically validated point‐prevalent abstinence at two weeks and Nollen 2011 assessed biochemically validated point‐prevalent abstinence at three months. Smith 2013 measured self‐reported 30‐day point‐prevalent abstinence at six weeks, while Tucker 2017 assessed biochemically validated continuous abstinence over 90 days.
Random‐effects meta‐analysis pooling these data produced an RR of 1.08 (95% CI 0.96 to 1.21; I² = 0%; n = 1795; Analysis 1.8). This suggested a potential small effect of adherence interventions on short‐term abstinence from smoking but with considerable uncertainty due to CIs overlapping no effect and including the possibility of a very small negative effect on abstinence.
1.8. Analysis.
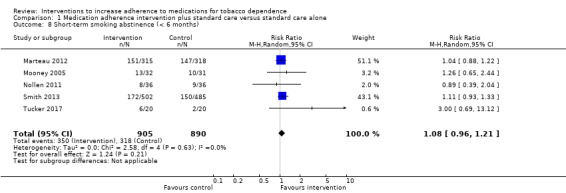
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 8 Short‐term smoking abstinence (< 6 months).
GRADE assessment indicated that the evidence for this outcome was of low certainty, meaning that the true effect might be markedly different from the estimated effect. This judgement was reached through consideration of the following criteria. The current evidence was downgraded by one level due to risk of bias, because the majority of studies were judged to be at high or unclear risk of bias. It was also downgraded by one level due to imprecision, because while the number of participants (sample size) incorporated into this meta‐analysis exceeded the optimal information size (i.e. a sufficient sample size for a single adequately powered trial), the 95% CI overlapped no effect and ranged from a very small harm to a small benefit. We did not downgrade further due to other considerations, namely inconsistency (because there was negligible heterogeneity), indirectness or publication bias (with insufficient studies for formal assessment).
Long‐term abstinence (six months or longer)
Five studies (seven comparisons) contributed data to long‐term abstinence (Chan 2010; Chan 2011; Marteau 2012; Schlam 2018; Smith 2013). All five studies assessed abstinence at six months, which was biochemically validated in three studies (Chan 2010; Chan 2011; Marteau 2012), and based on self‐report in two studies (Schlam 2018; Smith 2013). Random‐effects meta‐analysis pooling these data produced an RR of 1.16 (95% CI 0.96 to 1.40; I² = 48%; n = 3593; Analysis 1.9). This suggested a potential small effect of adherence interventions on long‐term abstinence; however, considerable uncertainty arose due to the lower CI including the possibility of a very small negative effect on abstinence. Participants subject to interventions to improve adherence were between 4% less likely and 16% more likely to be abstinent at six months than those given standard behavioural support.
1.9. Analysis.
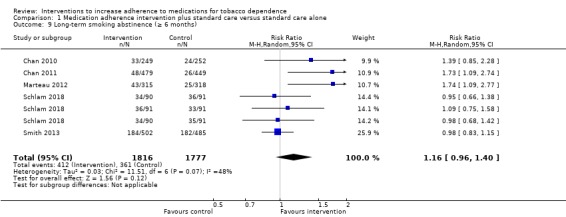
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 9 Long‐term smoking abstinence (≥ 6 months).
GRADE assessment indicated that the evidence for this outcome was of low certainty, meaning that the true effect might be markedly different from the estimated effect. This judgement was reached through consideration of the following criteria. The current evidence was downgraded by one level due to risk of bias because the majority of studies were at high or unclear risk of bias. It was also downgraded by one level for imprecision, because while the number of participants (sample size) incorporated into this meta‐analysis exceeded the optimal information size (i.e. a sufficient sample size for a single adequately powered trial), the 95% CI overlapped no effect and ranged from a very small harm to a small benefit. We did not downgrade further for other considerations, namely inconsistency (because heterogeneity was not classed as substantial), indirectness or publication bias (there were insufficient studies for formal assessment).
Sensitivity analyses
In sensitivity analyses, we excluded those studies at high risk of bias to determine if the primary and secondary outcome analyses were affected. Removing the two studies at high risk of bias (Mooney 2005; Smith 2013) did not affect results and interpretation for either the primary outcome (adherence to medication for tobacco dependence: SMD 0.12, 95% CI 0.02 to 0.22) or secondary outcomes (short‐term abstinence: RR 1.05, 95% CI 0.83 to 1.33; long‐term abstinence: RR 1.23, 95% CI 0.99 to 1.54).
Other outcomes
Factors plausibly associated with increases in adherence
No studies reported any relevant outcomes (i.e. factors plausibly associated with increases in adherence, such as intention or motivation, or attitudes towards treatment).
Adverse events
Four studies explicitly reported adverse events (Marteau 2012; Mooney 2005; Schlam 2018; Smith 2013). In Marteau 2012, there were no adverse events that were plausibly related to the intervention or its effect on participants' exposure to medication. There were also no differences between groups in levels of anxiety at either one‐week or six‐month assessment times. In Mooney 2005, there was no difference in adverse events between groups and in Schlam 2018 and Smith 2013 there were no serious adverse events during the study.
Discussion
Summary of main results
There is evidence of moderate certainty that interventions that devote special attention to improving adherence to smoking cessation medications can improve this to a small degree, when added to behavioural support for smoking cessation. Such interventions involve addressing the practicalities of taking medication, including facilitating problem solving, or providing information to address perceptions about the value of taking medication or concerns about doing so. There is low‐certainty evidence that such interventions may slightly improve the likelihood of achieving abstinence. The evidence for these findings was limited in both quality and quantity – characterised by a small number of studies, clinical heterogeneity, impaired study quality and imprecise estimates of effect, incorporating both potential benefit and harm.
Concerning the small improvement seen in adherence, translating the small statistical effect size into a more familiar metric suggests a potential effect equivalent to a 4% increase in the mean proportion of prescribed medication consumed (although see Data synthesis for limitations of such translations). One estimate is that each additional milligram per day of NRT consumed could increase the odds of abstinence by 5% (Hollands 2013), so this would represent a small but appreciable increase, equivalent to consuming one extra milligram of NRT with a prescription for 25 mg. Given evidence that greater adherence improves cessation outcomes for people using NRT, and evidence that higher doses of varenicline are more effective than lower doses (Cahill 2016), it stands to reason that this would apply to other medications too, because medication cannot work if it is not consumed. Characteristics of the treatment could also be shaped to attempt to increase the overall background levels of adherence. For example, characteristics of the medication (Hollands 2013), and its delivery (Hajek 1999), have been shown to impact on adherence. Even if adherence interventions demonstrate effect sizes of the small magnitudes seen here, the potential for aggregate impact is substantial given the extent to which medications for tobacco dependence are currently used, at least in high‐income countries. The degree to which this ultimately applies globally is dependent on increasing the uptake of effective pharmacotherapies, in part via increasing their availability and reducing their cost (van den Brand 2017).
Given that these interventions typically involve relatively minor additions to standard behavioural support, much of the content of the included interventions appeared relatively homogeneous. A detailed assessment of the content specifically concerning adherence did, however, reveal some potential avenues for further investigation. Those interventions that focus on addressing the practical barriers to adherence (as opposed to perceptions of treatment) and respond to participants' needs (as opposed to being governed by a set clinical agenda), and those that combine both of these foci, may be the most effective. However, the evidence suggesting this is very weak, particularly as there was no strong evidence of subgroup differences, and hence should be treated with caution. These tentative findings accord with English guidance on medication adherence (NICE 2009), which itself is based on a review of literature about effective interventions in adherence. This guidance emphasises that practical factors and barriers need to be considered key to explaining non‐adherence, not solely participants' beliefs and preferences about treatment. Furthermore, they reflect evidence suggesting that simply providing information to target cognitions and motivate changes in behaviour is often insufficient without also addressing factors, such as practical actions to overcome structural barriers, that prevent good intentions being realised (Hollands 2012; Webb 2006). It is also possible that a more detailed examination of intervention content would provide clearer insight into effective and ineffective mechanisms. Deriving a more precise understanding of the composition and processes of effective interventions will require a greater depth of evidence, including interventions that assess mediators, and improvements in the science and reporting of behavioural interventions (Sumner 2018).
Overall completeness and applicability of evidence
The review included only 10 trials, which were variable in terms of their context, the components of the intervention and the measures of the primary outcome, medication adherence, which makes summarising the data more difficult and reduces the certainty of the estimates produced. All these studies featured participants who were motivated to quit or reduce smoking, had sought and were receiving some degree of behavioural support – either face‐to‐face or by telephone – to take medication and were not paying for that medication. Furthermore, no studies targeted participants who were more likely to be non‐adherent, such as those who had not adhered to medication previously. Consequently, perhaps, medication adherence was overall reasonably high. For example, Nollen 2011 and Marteau 2012 reported the mean percentage of prescribed doses taken in the intervention arm was over 82% and control arm was over 63%, even though in the latter study, participants who had given up their quit attempt and ceased follow‐up were counted as non‐adherent. In studies using dichotomous measures of adequate adherence, three studies reported over 50% of participants achieving satisfactory levels of adherence (Chan 2011; Mooney 2007; Schmitz 2005). Perhaps in the context of the general population of people seeking support to quit, medication use is relatively high – contrary to perceptions that adherence is commonly suboptimal – and interventions have only limited potential to enhance adherence further. However, most people who stop smoking with the aid of medication do so without behavioural support (Fidler 2011), and typically any medication must be purchased at considerable cost. It is likely that adherence in this context is much lower and that interventions to improve adherence may be particularly helpful, but also that delivering these interventions will be especially challenging. There is currently no evidence on what may be effective in such unsupported contexts, though it seems likely that targeting perceptions or practicalities or both are likely to be relevant. A final point is that there is moderate‐certainty evidence that reimbursing the costs of medication where it is not freely provided improves adherence (van den Brand 2017).
Quality of the evidence
Most studies were judged at unclear risk of bias due to poor reporting of randomisation, even though all of them were published since first publication of the CONSORT statement (Moher 2010). Only three studies reported procedures clearly enough to be classified as having a low risk of bias (Chan 2011; Marteau 2012; Schlam 2018). It is possible, but relatively unlikely, that this led to bias. In the context of smoking cessation clinics, trial participants are usually unknown to the therapists, and this likely decreases, but does not eliminate, the likelihood of therapists assigning particular participants to particular arms and subverting the randomisation. Nonetheless, this should be addressed in reports from future trials. One potential source of bias is that practitioners who provided the adherence intervention also collected data on the degree to which people were adhering. As such they were unblinded, which may also motivate participants to report better adherence. This concern was mitigated substantially by the use of 'tablet counts', common to most of these trials. It is encouraging that the use of more objective measures appears commonplace in these types of trials, meaning that measurement issues were for the majority of studies not considered to confer particular risk of bias. This contrasts with another Cochrane Review focusing on adherence to prescription medications, where most studies used self‐report measures (Nieuwlaat 2014). Furthermore, while in the past, electronic monitoring approaches have been applied primarily to the opening and closing of tablet bottles, making them suitable for certain types of medications only, technology has been developed that will enable this to be used for other types of medication storage.
We assessed the certainty of the evidence for each outcome using the GRADE system. For the primary adherence outcome, GRADE assessment indicated that the evidence for this outcome was of moderate certainty, meaning that the true effect is probably close to the estimated effect. The current evidence was downgraded only once, due to risk of bias, because the majority of studies were judged to be at high or unclear risk of bias. We did not downgrade the evidence further based on other GRADE considerations. Evidence was of low certainty for both secondary outcomes of short‐ and long‐term abstinence, meaning that the true effect might be markedly different from the estimated effect. The current evidence was downgraded twice for each of these outcomes, in both cases being first, for risk of bias, because the majority of studies were judged to be at high or unclear risk of bias, and second, for imprecision, because the 95% CI overlapped no effect and ranged from a very small harm to a small benefit. This suggests that further research on abstinence outcomes will be valuable in increasing the reliability and precision of effect estimates and the certainty we can place in them.
Potential biases in the review process
Key possible limitations of the review are that first, we may have failed to identify all relevant research for inclusion in the review. We did take steps to minimise this possibility, including searching the Tobacco Addiction Group's specialised register in addition to electronic database searches, but this remains possible. Second, it is possible that there was publication bias, given there was asymmetry in the funnel plot, but we did not consider publication bias a likely explanation (see Effects of interventions). Unfortunately, two studies were classified as 'awaiting classification' as there was insufficient available information to confirm inclusion, and we were unable to contact the authors (Applegate 2007; Yuhongxia 2011).
Agreements and disagreements with other studies or reviews
We are not aware of other reviews addressing this topic. Cochrane Reviews show that behavioural support increases smoking cessation and, typically, included studies included people using medication, and receiving adherence advice as part of standard smoking cessation support (Hartmann‐Boyce 2019; Lancaster 2017). However, the studies did not randomise people to receive or not receive a medication adherence component so they do not provide specific evidence on its effect. Nieuwlaat 2014 examined the effect of interventions to improve adherence to a wider range of medication in a general setting. They found that information and counselling approaches improved adherence and patient outcomes but were unable to identify key components of the interventions. Nieuwlaat 2014 excluded tobacco dependence medications, hence the necessity of this current review.
Authors' conclusions
Implications for practice.
In people who are stopping smoking and receiving behavioural support, there is moderate evidence that enhanced behavioural support focusing on adherence to smoking cessation medication can modestly improve medication adherence, but less certainty that this may slightly increase abstinence in the short or longer term. The additional support could be brief and focus on reasons to take medication or concerns about doing so or the practicalities of taking it, or both, but there is currently insufficient evidence to confirm which approach is more effective.
There is no evidence on whether interventions to increase medication adherence are effective for people who are stopping smoking without behavioural support, and these findings may not generalise to populations and settings where low adherence is likely.
Implications for research.
More high‐quality randomised controlled trials investigating the effects of smoking cessation medication adherence interventions on both adherence and smoking cessation are needed to allow us to reliably estimate and interpret effects. The specific active components of interventions that may increase medication use also remain to be delineated, requiring more systematic research but also more detailed and consistent reporting by researchers.
Trials should be conducted in settings and populations where medication adherence is likely to be low because it appears that, in the context of people quitting with medication in a behavioural support programme, medication adherence is relatively good. As such, future studies might investigate interventions to increase medication adherence in population subgroups who exhibit lower adherence and who may benefit more from an adherence intervention, such as people who are quitting without behavioural support, who purchase their own cessation medication, who have failed to adhere in the past or women who smoke during pregnancy (Coleman 2012; Fish 2009).
Investigations of remotely delivered interventions could capitalise on technological solutions for prompting, monitoring and feeding back adherence behaviours in real‐time, incorporating sensor technology and digital communications and apps, as with behavioural support for cessation (Naughton 2017).
Research should consider supplementing interventions with changes to proximal environmental cues that shape our behaviour (Hollands 2017), such as how medication is stored, presented, packaged or labelled.
Future syntheses would benefit if researchers were able to define a common outcome metric for measuring adherence. Following recommendations elsewhere (Hollands 2013), first, a distinction should be made between overall consumption and adherence to a prescribed regimen and, because a prescribed regimen may not be optimal, the former should be privileged (with both reported preferably). Second, the actual degree of adherence is usually more meaningfully assessed by continuous outcome measures – such as milligrams of medication consumed – and we would encourage future use where possible. This is because there is unlikely to be clear guidance as to what should be regarded as an adequate or effective level of adherence to a given medication, meaning dichotomous measures concerning a specified level of adherence may be subject to greater variation and arbitrariness, and may be less directly comparable and interpretable.
Outcomes should be objectively assessed as far as is possible, and as well as reporting intention‐to‐treat data for the entire randomised sample, data should also be reported for the subset of participants who are continuing a quit attempt at the time of assessment, because cessation medication is not indicated when a person has given up a quit attempt.
What's new
| Date | Event | Description |
|---|---|---|
| 18 July 2019 | New search has been performed | Updated searches: two new studies |
| 18 July 2019 | New citation required but conclusions have not changed | Conclusions unchanged |
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health. PA is also part‐funded by the NIHR Oxford Biomedical Research Centre (BRC).
The authors would like to thank Jonathan Livingstone‐Banks who ran searches of the Tobacco Addiction Group Specialized Register, Jamie Hartmann‐Boyce and all at the Cochrane Tobacco Addiction Group. We also greatly appreciate the input of the peer reviewers who have commented on this review throughout its development.
Appendices
Appendix 1. Cochrane Tobacco Addiction Groups Specialized Register search strategy
1. (adhere* or complian* or concord* or discontinu*):TI,AB,MH,EMT,KY,KW,XKY
2. Medication Adherence:MH,EMT,KY,KW,XKY
3. ((NRT or nicotine replacement therap* or bupropion or wellbutrin or zyban or voxra or budeprion or aplenzin or amfebutamone or varenicline or chantix or champix) OR (nicotine adj7 (patch* or gum* or inhaler* or inhalator* or lozenge* or microtab* or tablet* or spray*))):TI,AB,MH,EMT,KY,KW,XKY
4. #1 OR #2
5. #3 AND #4
Appendix 2. Taxonomy of possible interventions (adapted from Haynes 2008)
More instruction for patients, e.g. verbal, written or visual material; programmed learning and formal education sessions;
counselling about the patients' target condition, the importance of therapy and compliance with therapy, the possible adverse effects, patient empowerment, couple‐focused therapy to increase social support;
automated telephone, computer‐assisted patient monitoring and counselling;
manual telephone follow‐up;
family intervention;
various ways to increase the convenience of care, e.g. provision at the work site or at home;
simplified dosing;
involving patients more in their care through self‐monitoring;
reminders, e.g. programmed devices, and tailoring the regimen to daily habits;
special 'reminder' medication packaging;
dose‐dispensing units of medication and medication charts;
appointment and prescription refill reminders;
reinforcement or rewards for both improved adherence and treatment response, e.g. reduced frequency of visits;
different medication formulations, such as tablet versus syrup;
crisis intervention conducted when necessary;
direct observation of treatments (DOTS) by health workers or family members;
lay health mentoring;
augmented pharmacy services;
psychological therapy, e.g. cognitive behaviour therapy, multisystemic therapy;
mailed communications;
group meetings.
Data and analyses
Comparison 1. Medication adherence intervention plus standard care versus standard care alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence (combined dichotomous and continuous) | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 2 Adherence: intervention focus subgroups | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 2.1 Perceptions | 3 | 839 | Std. Mean Difference (Random, 95% CI) | 0.10 [‐0.03, 0.24] |
| 2.2 Practicalities | 5 | 1752 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.38] |
| 2.3 Both perceptions and practicalities | 4 | 1064 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.08, 0.16] |
| 3 Adherence: delivery approach subgroups | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 3.1 Participant‐centred | 8 | 2791 | Std. Mean Difference (Random, 95% CI) | 0.12 [0.02, 0.23] |
| 3.2 Clinician‐centred | 3 | 864 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.05, 0.23] |
| 4 Adherence: combined focus and delivery subgroups | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 4.1 Perceptions + participant | 2 | 206 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.25, 0.30] |
| 4.2 Perceptions + clinician | 1 | 633 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.03, 0.29] |
| 4.3 Practicalities + participant | 5 | 1752 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.38] |
| 4.4 Both + participant | 2 | 833 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.16, 0.32] |
| 4.5 Both + clinician | 2 | 231 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.27, 0.24] |
| 5 Adherence: medication type subgroups | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 5.1 Nicotine replacement therapy | 7 | 3442 | Std. Mean Difference (Random, 95% CI) | 0.09 [0.02, 0.17] |
| 5.2 Bupropion | 2 | 152 | Std. Mean Difference (Random, 95% CI) | 0.58 [0.14, 1.01] |
| 5.3 Varenicline | 1 | 61 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.73, 0.29] |
| 6 Dichotomous adherence data (for calculation purposes) | 6 | 1664 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.05, 2.21] |
| 7 Continuous adherence data (for calculation purposes) | 5 | 4604 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.03, 0.19] |
| 8 Short‐term smoking abstinence (< 6 months) | 5 | 1795 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.21] |
| 9 Long‐term smoking abstinence (≥ 6 months) | 5 | 3593 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.96, 1.40] |
1.1. Analysis.
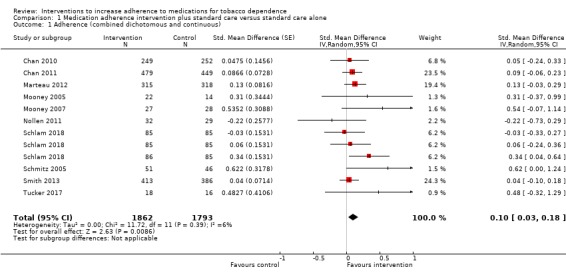
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 1 Adherence (combined dichotomous and continuous).
1.6. Analysis.
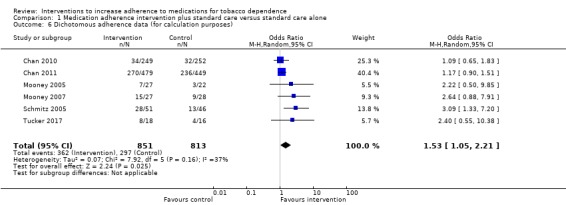
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 6 Dichotomous adherence data (for calculation purposes).
1.7. Analysis.
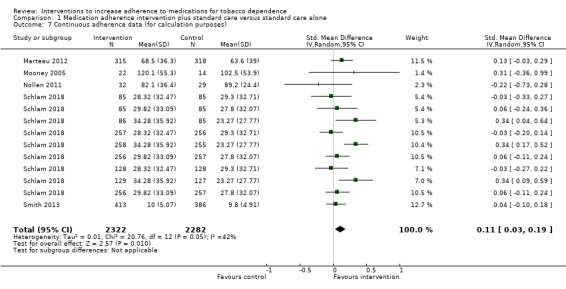
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 7 Continuous adherence data (for calculation purposes).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 2010.
| Methods | Design: RCT Country: Hong Kong, China Recruitment methods: mass media publicity and referrals from hospitals/clinics and physicians Setting: no information other than a non‐clinical setting |
|
| Participants | Inclusion criteria: Chinese men; aged ≥ 18 years; self‐reported erectile dysfunction; smoked ≥ 1 cigarette per day; intended to quit smoking within 7 days of first contact; willing to use NRT; not following any other smoking cessation regimen Exclusion criteria: psychologically or physically unable to communicate; taking regular psychotropic medications; serious health problems preventing use of NRT Participants randomised: 501 participants in eligible groups (mean age 48.8 years (SD 11.5); 100% Chinese) |
|
| Interventions | Aim of intervention: to increase adherence to NRT and smoking cessation Intervention: additional counselling component focused on medication adherence, delivered by trained male counsellor. Patient‐centred approach, utilising motivational interviewing techniques and the 4R approach. The NRT adherence intervention was developed from WHO guidelines on adherence interventions which emphasise the importance of adhering to the prescribed dosage, assessed and discussed ways to overcome barriers, and delivered problem‐oriented interventions to improve adherence. Participants received 15 minutes of face‐to‐face smoking cessation counselling and 3 minutes of NRT adherence counselling, plus 1 week of free NRT (gum or patch) at first contact. They were tested for CO and given a self‐help quitting pamphlet. They also received a telephone hotline number of a counsellor. There was further counselling and CO testing at 1 week and 4 weeks, plus 1 week of NRT at 1 week. At 1 week, NRT usage was checked and additional adherence counselling was given. At 4 weeks NRT usage was checked and additional counselling given as needed. Control: same content apart from the NRT adherence counselling at baseline and the NRT checking and adherence counselling at week 1. |
|
| Outcomes | Primary adherence outcome (dichotomous data): continuous use of NRT for 4 weeks, assessed at 3 months (ITT data). Checked by self‐report via telephone contact and possibly tablet counts of medication also used, although procedure unclear. Other adherence outcomes: 8‐week NRT adherence rate at 3 months. Checked by telephone call at 3 months. This outcome related to adherence beyond the treatment period with no NRT being supplied. Secondary outcomes: self‐reported 7‐day point prevalent abstinence, assessed at 6 months; Biochemically validated quit rate, assessed at 6 months (selected as abstinence outcome by review authors); self‐reported reduction (≥ 50%) in cigarette consumption, assessed at 6 months. |
|
| Notes | An additional 218 participants were randomised to a third arm (B) which was ineligible for this review: "Group B received a 10‐minute face‐to‐face counseling session, with simple advice to quit smoking". Abstinence outcome not reported by arm and so not useable data in report. Study authors were contacted and supplied data in April 2014, confirming that the biochemically validated quit rate for the intervention group (A1) was 13.3% (33/249) and for the control group (A2) was 9.5% (24/252). The study was funded by the Research Grants Council, Hong Kong. Nicotine patches/gums were provided free of charge from Pfızer. No conflicts of interest were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement beyond stating that it was randomised (p. 252, paragraph 7). No evidence of systematic differences between arms, with no reported differences in baseline demographic and smoking characteristics (p. 253, paragraph 7). |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details of procedure to enable judgement although longer‐term follow‐up by telephone was conducted by staff blinded to assignment (p. 253, paragraph 2). Adherence outcomes may have included tablet counts of medication used as well as self‐report by telephone but procedure unclear (p. 253, paragraph 1). Abstinence outcome was biochemically validated (p. 253, paragraph 3). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used and reported (p. 253, paragraph 7). No differences in attrition between arms (p. 253, paragraph 8) with numbers being reported, the overall number of participants lost being < 50% and the difference in percentage followed up between groups being < 20%. |
| Selective reporting (reporting bias) | Low risk | Trial was preregistered ISRCTN13070778 with specified outcomes remaining consistent for the study report. |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement. |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
Chan 2011.
| Methods | Design: RCT Country: Hong Kong, China Recruitment methods: local media publicity and by contacting previous cohorts of smokers who had cessation counselling but failed to quit. Setting: no information, but appeared to be smoking cessation clinic. |
|
| Participants | Inclusion criteria: aged ≥ 18 years; Chinese; smoked ≥ 2 cigarettes per day; no intention to quit in the next 4 weeks but interested in reducing smoking; not following any other smoking cessation regimen; no contraindication to NRT Exclusion criteria: psychologically or physically unable to communicate; taking regular psychotropic medications; serious health problems preventing use of NRT; pregnant/intending to become pregnant in next 6 months Participants randomised: 928 participants in eligible groups (mean age 41.9 years (SD 10.3); 19.4% women; 100% Chinese) |
|
| Interventions | Aim of intervention: to increase adherence to NRT, and smoking reduction and cessation. Intervention: additional counselling component focused on medication adherence, delivered by trained smoking cessation counsellor. Patient‐centred approach, utilising motivational interviewing techniques and the 5R approach. The NRT adherence intervention was developed from WHO guidelines on adherence interventions which emphasise the importance of adhering to the prescribed dosage, assessed and discussed ways to overcome barriers, and delivered problem‐oriented interventions to improve adherence. Participants received 15 minutes of face‐to‐face smoking reduction intervention, including information on the health consequences of smoking and counselling emphasising achieving the goal of cessation by focusing on reduction before quitting, highlighting how reduction is effective when quitting is difficult and how to reduce their smoking. They also received 3 minutes of NRT adherence counselling plus 1 week of free NRT (gum or patch) at first contact. They were tested for CO and given a self‐help quitting pamphlet. There was further smoking reduction and adherence counselling and CO testing at 1 week, plus administration of a further 3 weeks of NRT. NRT usage was also checked. At 4 weeks, participants received a similar intervention as at 1 week. Control: same content apart from the NRT adherence counselling at baseline, week 1 and week 4. |
|
| Outcomes | Primary adherence outcome (dichotomous data): continuous use of NRT over 8 weeks, assessed at 3 months (ITT data). Checked by self‐report via telephone contact but possibly also by tablet counts and procedure not clear. Other adherence outcomes: continuous use of NRT over 4 weeks, assessed at 3 months. Secondary outcomes: self‐reported 7‐day point prevalent abstinence, assessed at 6 months; biochemically validated quit rate, assessed at 6 months (selected as abstinence outcome by review authors); self‐reported 7‐day point prevalent abstinence, assessed at 3 months; Self‐reported reduction (≥ 50%) in cigarette consumption, assessed at 6 months. |
|
| Notes | An additional 226 participants were randomised to a third arm (B) which was ineligible for this review: "Subjects in control group B received 10 min simple advice on the health hazards of smoking and the importance of smoking cessation". The study was funded by the Health and Health Services Research Fund, Hong Kong SAR. Nicotine patches/gums were provided free of charge from McNeil AB (Helsingborg, Sweden), which had no other role in this trial. There were no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random numbers generated by a computer prior to participant recruitment (p. 1156, paragraph 6). No evidence of systematic differences between arms, although reported difference between arms in baseline CO level and not mentioned if this is adjusted for in the analysis (p. 253, paragraph 7). |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was determined by a research assistant not conducting the intervention. Assignment was by opening sealed, opaque envelopes and followed informed consent (p. 1156, paragraph 6). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistants contacting participants at follow‐up were blinded to arm allocation (p. 1157, paragraph 2), but it was not clear that this was the only means by which the primary outcome was assessed. Adherence outcome was seemingly checked by self‐report but combination of tablet counts of medication used and self‐report may have been used and procedure not clear (p. 1156, paragraph 6; p. 1157, paragraph 2). Abstinence outcome was biochemically validated (p. 1157, paragraph 5). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used and reported with non‐respondents at follow‐up treated conservatively as non‐adherent and continuing smokers (p. 1158, paragraph 1). There were no differences in attrition between arms (p. 1158, paragraph 2) with numbers being reported, the overall number of participants lost < 50% and the difference in percentage followed up between groups < 20%. |
| Selective reporting (reporting bias) | Low risk | Trial was preregistered ISRCTN05172176 with specified outcomes remaining consistent for the study report. |
| Consistency in intervention delivery | Low risk | Some sessions conducted by each of the counsellors were recorded and validated by an experienced nurse supervisor. |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
Marteau 2012.
| Methods | Design: RCT Country: UK Recruitment methods: participants were recruited through NHS primary care practices. Smokers were identified through practice registers and sent a letter offering assistance to quit and an invitation to participate in the trial. Setting: smoking cessation clinics in primary care |
|
| Participants | Inclusion criteria: smoking ≥ 10 cigarettes per day; wanting to quit smoking; aged ≥ 18 years Exclusion criteria: none stated Participants randomised: 633 participants (mean age 47.3 years (SD 13.3); 54.3% women; 90.2% white |
|
| Interventions | Aim of intervention: to increase adherence to NRT by informing participants that their oral dose is tailored based on an analysis of their genotype, rather than their phenotype (FTND score). Intervention: communicating different means of tailoring prescribed medication, delivered by trained research nurses. Behavioural support (based on withdrawal‐orientated therapy) and nicotine patches were provided (with the patch dose tailored in relation to cigarettes per day) to all participants. Participants were also prescribed an oral NRT product of their choice. The dose of oral NRT in the intervention arm was tailored based on gene variant. Participants were given both forms of NRT 1‐day prequit and told the basis for their dosage. They were also provided with a personalised booklet and an appointment card documenting the dose of NRT to use daily and giving the reason for the dose. The rationale for the dose was reiterated at each subsequent clinic. Behavioural support was offered twice prior to quit day, weekly afterwards for 4 weeks and then at 8 weeks. Quit day was set 2 weeks and 1 day after baseline. Support sessions lasted 10–30 minutes, depending on progress and stage of quit attempt. Control: same content apart from the dose of oral NRT and the corresponding communication of the rationale was tailored based on FTND score. |
|
| Outcomes | Primary adherence outcome (continuous data): proportion of all prescribed NRT taken over 28 days, assessed at 28 days of treatment period (ITT data). Checked by tablet counts of medication used. Other adherence outcomes: proportion of all prescribed NRT taken over 7 days; proportion of participants showing no use of NRT; proportion of participants showing use of NRT beyond 28 days. Secondary outcomes: biochemically validated prolonged abstinence at 28 days; biochemically validated prolonged abstinence at 6 months; anxiety assessed using the short‐form Spielberger State‐Trait Anxiety Inventory‐6. |
|
| Notes | Phenotype arm was regarded as control arm as it is more similar to standard care. Funded by Medical Research Council, UK. 1 author reported having completed consultancy and research on smoking cessation for pharmaceutical companies. The remaining authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer generated (p. 4, paragraph 2). No evidence of systematic differences between arms, with no reported differences in baseline demographic and smoking characteristics (p. 6, Table 1). |
| Allocation concealment (selection bias) | Low risk | Allocation was conducted from a central isolated location, separate from trial co‐ordination and participant recruitment (p. 4, paragraph 2). The randomisation sequence was revealed sequentially and concealed from the trial team, nurses and participants (p. 4, paragraph 3). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors for primary outcome were not blinded, but because tablet counts were used it was unlikely that this constituted a clear risk of bias. Outcome assessors for longer‐term follow‐up were blinded to allocation (p. 4, paragraph 3). Primary adherence outcome was checked by tablet counts of medication used (p. 3, paragraph 5). Abstinence outcomes were biochemically validated (p. 3, paragraph 13). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was used and reported with non‐respondents at follow‐up treated conservatively as non‐adherent and continuing smokers (p. 4, paragraph 11). There were no differences in attrition between arms (p. 7, paragraph 3) with numbers being reported, the overall number of participants lost being < 50% and the difference in percentage followed up between groups being < 20%. |
| Selective reporting (reporting bias) | Low risk | Study was preregistered including specified outcomes and these were unchanged in study report (ISRCTN14352545). This is also clear in a published protocol. |
| Consistency in intervention delivery | Low risk | Standardised script used, detailed in the published protocol (p. 3, paragraph 3). |
| Summary risk of bias | Low risk | Summary risk of bias assessed as low. |
Mooney 2005.
| Methods | Design: RCT Country: USA Recruitment methods: recruited from community via radio, newspaper and handbill advertisements Setting: research clinic at tobacco research centre |
|
| Participants | Inclusion criteria: aged 18–65 years; physically healthy; smoking 15–50 cigarettes per day for ≥ 1 year; no untreated major mental illness; no contraindications for nicotine gum use; no concurrent use of other nicotine or tobacco products; experienced past nicotine withdrawal syndrome according to DSM Exclusion criteria: pregnancy Participants randomised: 63 participants (mean age 34.6 years (SD 10.9); 55.6% women; 87.3% white) |
|
| Interventions | Aim of intervention: investigate if a brief low‐cost intervention increased adherence to NRT. Intervention: additional personalised feedback component focused on medication use/adherence, delivered by smoking cessation counsellors. Participants initially received a presentation on the benefits of quitting, a review of coping skills, and support and encouragement. Personalised feedback was then delivered that addressed the effectiveness, safety and necessity of nicotine replacement. First, facts were presented about NRT followed by personalised feedback based on responses to 3 questionnaires completed at visit 1 (the BMQ, the ANRT‐12 and the PRNR). Tailored scripts were used to reinforce correct knowledge and promedication beliefs. In contrast, incorrect knowledge, negative or ambivalent positions were raised using non‐confrontational language that allowed for engagement, reflection and clarification. A clarifying statement would then be offered. The broader goal was to define the pros and cons of treatment and shift the decisional balance toward adequate use of gum. The intervention was a single session of approximately 20 minutes. Control: participants received a presentation on the benefits of quitting, a review of coping skills, and support and encouragement. A smoking history section reviewed general smoking experiences. This section was intended as a 'placebo' topic with some face relevance, but little probable influence on gum use. |
|
| Outcomes | Primary adherence outcomes: (dichotomous data) rates of gum adherence of 12 pieces per day (for those who received medication and started the treatment phase, not ITT); (continuous data) total gum use (in participants completing the treatment phase, not ITT). Total gum use selected as primary outcome as most stringent continuous measure that is more meaningful and informative than potentially arbitrary dichotomised variable. Checked by tablet counts of medication used. Assessed for days 1–15. Other adherence outcomes: daily gum use Secondary outcomes: biochemically validated point‐prevalent abstinence at 1 week; biochemically validated point‐prevalent abstinence at 2 weeks (selected by the review authors as most stringent and consistent with adherence outcome time point); self‐reported point‐prevalent abstinence at 4, 5, 6 and 7 weeks; NHLBI defined abstinence at 3 and 6 weeks. Additional secondary outcome measures for which the data are not reported were as follows: 3 measures of attitudes and knowledge about NRT at weeks 1, 6 and 7 (BMQ, ANRT‐12, PRNR); The Minnesota Nicotine Withdrawal Scale. Adverse events relating to nicotine toxicity and nicotine gum were also assessed. |
|
| Notes | An additional 34 participants were randomised to an additional "contingency management" arm not eligible for inclusion in this review, in which participants received payment for using nicotine gum. Funded by National Institute on Drug Abuse. GlaxoSmithKline provided the nicotine gum. No information on conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement, although no evidence of systematic differences between arms, with no differences observed at baseline (p. 571, paragraph 3). |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear that outcome assessors were blinded, but because tablet counts were used it is unlikely that this constitutes a clear risk of bias. Primary adherence outcome was checked by tablet counts of medication used (p. 569, paragraph 5). Abstinence outcomes were biochemically validated (p. 570, paragraph 3). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no significant differences in attrition over time across all 3 arms (p. 571, paragraph 4), but the 2 arms of interest had substantial and differing (by > 20%) attrition levels over the treatment period of 31% (intervention) and 55% (control). Data reported for the primary outcome dId not refer to all randomised participants and reasons for dropout were not detailed. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol. |
| Consistency in intervention delivery | Low risk | A standardised script and checklist used (p. 568 paragraph 7). |
| Summary risk of bias | High risk | Summary risk of bias assessed as high. |
Mooney 2007.
| Methods | Design: RCT Country: USA Recruitment methods: not reported Setting: outpatient research clinic, located at a university medical centre |
|
| Participants | Inclusion criteria: women; aged 20–65 years; physically healthy; smoking ≥ 10 cigarettes per day; no current DSM‐IV Axis 1 disorder Exclusion criteria: pregnancy/breastfeeding; current treatment with bupropion or other smoking cessation medication Participants randomised: 55 participants (mean age 42.1 years (SD 10); 100% women; 61.8% white) |
|
| Interventions | Aim of intervention: to provide feedback on medication use (using electronic MEMS to increase bupropion adherence. Intervention: provision of additional feedback on adherence levels, given by a CBT therapist. Following baseline assessment all participants began 7 weeks of open‐label treatment with bupropion SR 300 mg dispensed in MEMS bottles (containing a computer chip that recorded the times when bottle opening occurred). In addition, all participants received individual weekly CBT sessions for smoking cessation, focusing on identification of high‐risk situation for smoking, coping skills training and lapse recovery strategies. In the intervention condition the weekly CBT was increased in duration by 10 minutes a session, during which time the MEMS feedback was given in graphical form and the treatment regimen was clarified. Problem‐solving techniques were used to help the participant to tailor the regimen to their schedule by associating medication taking with regular activities or routines. Potential barriers to adherence identified and strategies for removing barriers discussed. Participants were encouraged to self‐monitor tablet consumption on daily diaries reviewed at the next therapy session. Control: as above but without the extra 10 minutes added to each session for enhanced therapy. |
|
| Outcomes | Primary adherence outcome (dichotomous data): rates of full adherence, i.e. 2 doses taken per day in an optimal schedule (ITT data). Assessed daily over 7‐week treatment period, objectively using MEMS bottles. Other adherence outcomes: rates of dose adherence, i.e. 2 doses taken per day over 7‐week treatment period. Secondary outcomes: biochemically validated abstinence at week 6 (selected as abstinence outcome by review authors. as most consistent with adherence outcome time point but there is no useable data in the report); biochemically validated abstinence at week 3. |
|
| Notes | Authors contacted in 2015 to request data for secondary abstinence outcome but no response received. Funded by National Institute on Drug Abuse. GlaxoSmithKline provided the bupropion SR. No information on conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement. |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear that outcome assessors were blinded, but because MEMS monitoring data used it was unlikely that this constituted a clear risk of bias. Primary adherence outcome was only measured objectively using MEMS monitoring data. Abstinence biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no differences in attrition between arms (p. 878, paragraph 2), but more than 50% of participants did not complete the study. Data reported for the primary outcome appeared to refer to all randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol. |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement. |
| Summary risk of bias | High risk | Summary risk of bias assessed as high. |
Nollen 2011.
| Methods | Design: RCT Country: USA Recruitment methods: not detailed Setting: community‐based clinic serving a predominantly black population |
|
| Participants | Inclusion criteria: black; aged ≥ 18 years; smoking > 10 cigarettes per day; wanting to quit; willing to take varenicline. Exclusion criteria: planning to move from the area within 3 months; contraindications to the use of varenicline, including a cardiovascular event in the month prior to enrolment, renal impairment, taking insulin for diabetes but unwilling to closely monitor blood sugar or history of clinically significant allergic reactions to varenicline; a major depressive disorder in the past year requiring treatment; history of alcohol or drug dependency in the past year; history of psychosis, panic disorder, bipolar disorder or any eating disorders; current breastfeeding, pregnancy, or plans to get pregnant in the next 3 months. Participants randomised: 72 participants (mean age 46.8 years (SD 11.3); 62.5% women; 100% black) |
|
| Interventions | Aim of intervention: to improve varenicline use. Intervention: standard components plus additional adherence support counselling. These were delivered by study counsellors although their disciplinary backgrounds/training were not detailed. The standard components comprised: 1. culturally targeted quit smoking guide addressing the health consequences of smoking, benefits of quitting and strategies to promote abstinence; 2. a 1‐month supply of varenicline in a monthly tablet box. Participants were verbally instructed on how to take the medication. Participants were encouraged to initiate varenicline on day 1, set a quit date on day 8 and to not smoke cigarettes during the 3‐month treatment phase. Participants returned to the clinic at the end of months 1 and 2 for medication refills; 3. standard counselling: all participants met with a study counsellor during the randomisation visit to develop a plan for quitting on day 8. Counsellors followed semi‐structured scripts to provide information about the risks of continued smoking, benefits of quitting, discuss strategies for coping with withdrawal and assist participants in developing a quit plan. The additional adherence support counselling comprised 5 additional counselling sessions on days 8, 12, 20, 30 and 60 of the treatment period. Using the Information‐Motivation‐Behavioural skills model of adherence behaviour change, counsellors provided information to enhance participants' motivation in their ability to take the medication as prescribed (e.g. consequences of adherence/non‐adherence) and behavioural skills for managing adverse effects (e.g. nausea) and remembering to take their medication (e.g. timing doses with daily activities). Control: 3 standard components only. |
|
| Outcomes | Primary adherence outcome (continuous data): percentage of prescribed varenicline doses taken at 3 months (for those remaining engaged to provide data). Assessed during monthly medication refill clinic visits by research staff with tablet counts. Other adherence outcomes: percentage of prescribed varenicline doses taken at 1 month; percentage of prescribed varenicline doses at 2 months. Secondary outcomes: biochemically validated 7‐day PPA at 3 months, verified by salivary cotinine (selected as abstinence outcome by review authors as most consistent with adherence outcome time point); biochemically validated 7‐day PPA at 1 month, verified by CO; biochemically validated 7‐day PPA at 2 months, verified by CO. Reduction in self‐reported cigarettes per day from baseline, assessed at 3 months. Adverse events assessed. |
|
| Notes | Participant numbers per arm not given for primary outcome in published paper. We contacted the authors for clarification and they confirmed that 29 participants for control arm and 32 for intervention arm (August 2014). Funded by University of Kansas Cancer Center and Pfizer Global Pharmaceuticals. Pfizer Global Pharmaceuticals provided study medication. Reported that 1 author serves as a paid consultant to Pfizer Global Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of allocation sequence not detailed, although no significant differences between arms at baseline reported. |
| Allocation concealment (selection bias) | Low risk | Allocation determined by drawing a sealed envelope with preassigned randomisation numbers, at the randomisation visit (p. 869, paragraph 4). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear that outcome assessors were blinded, but because tablet counts were used it was unlikely that this constituted a clear risk of bias. Primary adherence outcome was by tablet counts. Abstinence was biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The overall level of attrition was moderate across the treatment period (15–21%), but reasons for dropout not detailed. No reported differences in attrition by arm (p. 870, Results paragraph 1) with numbers being reported, the overall number of participants lost being < 50% and the difference in percentage followed up between groups being < 20%. Data reported for the primary outcome did not appear to refer to all randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol. |
| Consistency in intervention delivery | Low risk | Standard counselling was delivered according to semi‐structured scripts. Adherence counselling was delivered based on a model of adherence behaviour change. All counselling sessions were audiotaped and integrity of protocols was checked by weekly supervision of audiotaped sessions. |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
Schlam 2018.
| Methods | Design: RCT Country: USA Recruitment methods: participants recruited from primary care clinics. Clinical staff invited smokers interested in quitting to participate. Interested participants were called by researchers to assess eligibility. Setting: primary care clinics in 2 healthcare systems in southern Wisconsin |
|
| Participants | Inclusion criteria: aged ≥ 18 years; smoking ≥ 5 cigarettes per day for previous 6 months; motivated to quit; read, write and speak English; agree to complete assessments; planning to remain in area for ≥ 12 months; not currently taking bupropion or varenicline; agree to use only study cessation medication during treatment; no medical contraindications to NRT; agreeing to use approved contraception. Exclusion criteria: none stated Participants randomised: 544 participants (mean age 46.2 years (SD 12.8); 59.0% women; 87.4% white; 56.9% at least some college education). |
|
| Interventions | Aim of intervention: to examine the effect of various interventions on smokers' adherence to combined nicotine patch and nicotine gum during a quit attempt. Study was a 2 × 2 × 2 × 2 × 2 randomised factorial experiment (i.e. 32 treatment conditions) evaluating 5 intervention components: 1. MAC vs none; 2. automated medication adherence calls vs none; 3. electronic medication monitoring with feedback and counselling vs e‐monitoring alone; 4. 26 vs 8 weeks of nicotine patch plus nicotine gum and 5. maintenance counselling vs none. Interventions 1–3 were eligible interventions for the purposes of this review. Intervention: all participants received a base cessation treatment (8 weeks of nicotine patch plus nicotine gum, and a total of 50 minutes of counselling. Randomised intervention components were as follows: 1. MAC vs none: comprised 2 × 10‐minute in‐person counselling sessions delivered by case managers that provided information tailored to correct misconceptions regarding cessation medication; 2. automated medication adherence calls vs none: comprised 7–11 brief automated medication reminder calls that offered information and encouragement for using medication as recommended; 3. electronic medication monitoring with feedback and counselling vs e‐monitoring alone: comprised being given printouts showing daily gum use (as electronically recorded by the dispenser that all participants instructed to use) along with 5–9 10‐minute counselling sessions delivered by case managers that focused on printouts and problem solving to increase adherence. Control: all participants received a base cessation treatment (8 weeks of nicotine patch plus nicotine gum, and a total of 50 minutes of counselling. Each of the 3 eligible interventions described above were compared to the absence of that intervention. |
|
| Outcomes | Primary adherence outcome (continuous data): combined patch and gum use (percentage of days in first 6 weeks of the quit attempt adherent to both nicotine patch and gum, meaning where participants used both a patch and ≥ 4 pieces of gum) for those remaining engaged to provide data (i.e. in participants completing the treatment phase, not ITT). Assessed during monthly medication refill clinic visits by research staff with nicotine patch use assessed by timeline follow‐back and gum use assessed via electronic medication dispenser given to participants. Other adherence outcomes: patch use (percentage of days participants used the patch in the first 6 weeks of the quit attempt); gum use (mean pieces of gum per day used in the first 6 weeks of the quit attempt). Secondary outcomes: self‐reported 7‐day PPA at 26 weeks in those randomised (selected as abstinence outcome by review authors as most consistent with adherence outcome time point), assessed by participants reporting smoking since last contact in a timeline follow‐back interview; self‐reported 7‐day PPA at 52 weeks. |
|
| Notes | Authors contacted 7 November 2018 to obtain adherence and abstinence data in form needed. Outcomes included in the review related to sample on which adherence analysis conducted (513 participants). Multiple comparisons from this study were entered into meta‐analyses. To account for this and ensure these data were not overweighted in the analyses, sample sizes were reduced in proportion to how many times the study data were used for any given analysis. Comparisons used from this study for primary and secondary analysis were as follows (in order from top to bottom of the forest plot): first = 1. MAC vs none; second = 2. automated medication adherence calls vs none; third = 3. electronic medication monitoring with feedback and counselling vs e‐monitoring alone. Data used were as follows for primary analysis: MAC vs none (28.32 (SD 32.47) vs 29.30 (SD 32.71). Number reduced by one third and rounded down (so 85 participants used), as study data entered 3 times into analysis; automated adherence calls vs none (29.82 (SD 33.09) vs 27.80 (SD 32.07). Number reduced by one third and rounded down (so 85 participants used); electronic medication monitoring counselling vs e‐monitoring alone (34.28 (SD 35.92) vs 23.27 (SD 27.77). Number reduced by one third and rounded down (so 86 and 85 participants used respectively). Funded by grants from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. No conflicts of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via a database that used stratified, computer‐generated, permuted block randomisation. No evidence reported of systematic differences between arms. |
| Allocation concealment (selection bias) | Low risk | Staff could not view the allocation sequence and the database did not reveal participants' treatment condition to staff until participants' eligibility was confirmed. Participants were blinded to treatment condition until they provided consent. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear that outcome assessors (case managers) were blinded. Medication counts were partly used, meaning it was unlikely that this constituted a clear risk of bias, but also unclear that risk of bias could be reasonably excluded. Primary adherence outcome was checked in part by tablet counts of medication used assessed via electronic medication dispenser (gum use) given to participants (p. 2067, paragraph 2), but also part assessed via self‐report via timeline follow‐back (patch use). Abstinence outcomes were self‐report (p. 2067, paragraph 1) via timeline follow‐back. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants were included in analyses if they had ≥ 14 days of both nicotine gum and patch use data in the first 6 weeks post‐target quit day (513; 94% of the 544 randomised participants) (p. 2067, final paragraph). Attrition therefore very low for the primary outcome and did not clearly differ by intervention arm. |
| Selective reporting (reporting bias) | Unclear risk | Study was preregistered (NCT01120704) though adherence outcomes not specified. |
| Consistency in intervention delivery | Low risk | Standard protocol used with example scripts for all counselling sessions (see supplementary tables to Schlam 2016). |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
Schmitz 2005.
| Methods | Design: RCT Country: USA Recruitment methods: advertisements in local papers and radio announcements Setting: outpatient research clinic, located at a university medical centre |
|
| Participants | Inclusion criteria: English‐speaking; women; aged 30–70 years; physically healthy; smoking ≥ 10 cigarettes per day Exclusion criteria: dependence on other substances; evidence of psychotic, depressive or anxiety disorders; pregnancy/breastfeeding; serious medical problems Participants randomised: 97 participants (mean age 49 years (SD 9.9); 100% women; 72% white) |
|
| Interventions | Aim of intervention: to determine whether tablet taking instructions and personalised feedback using MEMS enhanced bupropion adherence. Intervention: provision of additional feedback on adherence levels, given by a clinic nurse. Participants received written and verbal instructions on proper administration of bupropion. All doses were administered in MEMS bottles (containing a computer chip that recorded the times when bottle opening occurred) in the morning and 1 in the evening with at least 8 hours (but not more than 12 hour) between. Participants in the intervention group were told about the recording device in the bottle cap, specifically that the cap would record the time and date that they took the medication. MEMS feedback was given in graphical form weekly with repeated instructions to increase adherence and a check of adverse effects. Feedback sessions lasted approximately 5–10 minutes. Treatment regimen 7 weeks in duration with weekly counselling visits. Control: no particular information, direction or feedback beyond the standard dosing instructions. Participants met briefly with nurse for a weekly check of adverse effects. The control arm was designed to typify usual care in a medical setting. |
|
| Outcomes | Primary adherence outcome (dichotomous data): rates of full adherence i.e. 2 doses taken per day in an optimal schedule (ITT data). Assessed daily over 7‐week treatment period, objectively using MEMS bottles. Other adherence outcomes: rates of dose adherence i.e. 2 doses taken per day over 7‐week treatment period. Secondary outcomes: none reported |
|
| Notes | Funded by National Institute on Drug Abuse and the Department of Psychiatry at the University of Texas – Houston. No information on conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement. No reported systematic differences between arms in baseline demographic and smoking characteristics (p. 142, paragraph 5). |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear that outcome assessors were blinded, but because MEMS monitoring data used it was unlikely that this constituted a clear risk of bias. Primary adherence outcome was measured objectively using MEMS monitoring data. Abstinence biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no differences in attrition between arms (p. 142, paragraph 7), but > 50% of participants did not complete the study. We assumed that data reported refers to all randomised participants (given wording used and consistent with reported degrees of freedom for F‐tests). |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol. |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement. |
| Summary risk of bias | High risk | Summary risk of bias assessed as high. |
Smith 2013.
| Methods | Design: RCT. 2 × 2 × 2 factorial design examining 3 manipulations, only 1 of which was relevant to this review. Country: USA Recruitment methods: people who called the Wisconsin Tobacco Quit Line (WTQL) were invited to participate in the study. There was no additional advertising or targeted recruitment. Setting: counselling intervention conducted by telephone |
|
| Participants | Inclusion criteria: aged ≥ 18 years; English speaking; smoking ≥ 10 cigarettes/day; willing to set a quit date within the next 30 days Exclusion criteria: pregnant or lactating; medical contraindications for study medications (e.g. past 30 days, myocardial infarction or stroke; past 6 months, serious or worsening angina, very rapid or irregular heartbeat requiring medication); unwillingness to use study medications Participants randomised: 987 participants (mean age 41.9 years (SD 13.0); 57.6% women; 76.4% white) |
|
| Interventions | Aim of intervention: to address problematic beliefs or knowledge about NRT that might adversely affect appropriate use of the pharmacotherapies. Intervention: all participants received a standard quit guide in the mail, access to recorded medication information (via telephone), and access to an online cessation programme maintained by the quitline. They could make ad hoc calls to the quitline for additional assistance. They received standard cessation counselling. During call 1, quitline counsellors discussed smoking history, prior quit attempts, problem‐solving and coping strategies, social support, and appropriate use of cessation medications; also, a target quit date was set during this first call. Call 2 occurred on or close to the quit date and focused on management of withdrawal symptoms, appropriate use of medications, strategies to maintain abstinence in high‐risk situations and early relapse prevention. Calls 3 and 4 addressed relapse prevention but counselling was tailored to address concerns and questions raised by the participant. In addition, intervention participants received MAC during all standard counselling calls. The MAC protocol was developed by study investigators and involved the following: 1. prequit assessment of beliefs that might undermine NRT adherence, 2. ongoing medication adherence assessment by counsellors and 3. tailored coaching based on the ongoing assessments. Control: standard quit materials and standard counselling only. |
|
| Outcomes | Primary adherence outcome (continuous data): self‐reported number of days of nicotine patch use in the first 2 weeks in those remaining engaged at this time point (this was the most relevant outcome given the factorial design because all participants irrespective of randomised arm received nicotine patches for ≥ 2 weeks). Other adherence outcomes: self‐reported number of days of gum use in the first 2 weeks; self‐reported number of weeks of nicotine patch use in the first 6 weeks; self‐reported number of weeks of gum use in the first 6 weeks. Secondary outcomes: 30‐day PPA at 6 weeks postquit (selected as time point most relevant to adherence outcome), 30‐day PPA at 12 weeks postquit, 30‐day PPA at 26 weeks postquit (selected as longest time point). 7‐day PPA at 2 weeks postquit; 7‐day PPA at 6 weeks postquit; 7‐day PPA at 12 weeks postquit; 7‐day PPA at 26 weeks postquit. Abstinence outcomes were assessed by self‐report. |
|
| Notes | The study used a factorial design to examine the effect of 3 different enhancements to quitline treatment: 1. patch only vs combination (patch plus oral) NRT; 2. shorter vs longer duration of NRT; 3. standard counselling vs counselling to increase NRT adherence. We are only interested in the effect of the latter, with data for this comparison collapsing the other factor conditions. Study authors contacted and responded August 2014 in seeking exact number of participants by arm for primary outcome. Their response indicated that there were 386 participants in the standard counselling group and 413 participants in the adherence counselling group. Study funded by a National Cancer Institute grant. 3/10 authors reported no financial conflicts of interest with other authors declaring interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to the 8 treatment combinations via a list of randomised numbers generated by SAS Proc Plan (SAS Institute Inc., Cary, NC) (p. 719). No evidence of systematic differences between arms, with no reported differences in baseline demographic and smoking characteristics (p. 721, paragraph 4). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details to determine that allocation was adequately concealed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome data were collected by university‐based research staff not affiliated with the quitline, but it was unclear if they were blinded (p. 720, paragraph 5). Primary adherence outcome was only measured by self‐report via telephone, and abstinence measures were not biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The level of attrition was moderate (18–20%) and not different between arms (p. 721, paragraph 5) and reasons for dropout were given. Numbers were reported, the overall number of participants lost was < 50% and the difference in percentage followed up between groups was < 20%. Not ITT (p. 720, Analysis plan and statistical methods paragraph 1). |
| Selective reporting (reporting bias) | Low risk | Study was preregistered including specified primary outcomes and these were unchanged in study report (NCT01087905). |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement although seemed to (if not clearly stated) follow a basic protocol in terms of outlining the intended focus of each call. |
| Summary risk of bias | High risk | Summary risk of bias assessed as high. |
Tucker 2017.
| Methods | Design: RCT Country: USA Recruitment methods: study was advertised at health clinics and social service agencies serving people with HIV in the study area, magazines targeting the lesbian, homosexual, bisexual and transgender Latino community, and social networking sites geared toward homosexual and bisexual men. Setting: single in‐person session at a social service agency |
|
| Participants | Inclusion criteria: aged ≥ 18 years; self‐identified as both Latino and HIV‐positive; smoked ≥ 20 days and 5 cigarettes per day in last month; ready to set a quit date within next 30 days (score 7 on the Readiness to Quit Ladder); willing to use the nicotine patch Exclusion criteria: self‐reporting any medical condition preventing use of nicotine patch; currently using other tobacco products or e‐cigarettes; currently in another smoking cessation treatment; participated in the formative phase of the project Participants randomised: 40 participants (mean age 42.9 years (SD: standard care 8.1; adherence 9.4); 7.5% women; 0% white) |
|
| Interventions | Aim of intervention: brief adherence‐focused intervention module designed specifically to increase adherence with the nicotine patch. Intervention: adherence treatment followed the same basic structure as the standard treatment, but included an additional module on improving adherence to patch use. The module was designed to help smokers build motivation to use the patch (e.g. by weighing the pros and cons of patch use through a decisional balance exercise), establish realistic expectations about the patch (e.g. by understanding the extent to which withdrawal symptoms and urges will be reduced and how long it would take), develop personalised strategies to remember to use the patch (e.g. by linking patch application to daily routine like brushing teeth), and deal with temptations to not use the patch (e.g. by identifying personal triggers for not using the patch and devising a plan for avoiding or dealing with these triggers). Contact time was 27 minutes in the adherence group. Single in‐person session. Control: standard brief smoking cessation treatment (followed the 5 As protocol (Ask, Advise, Assess, Assist, Arrange and nicotine patch). All participants received an 8‐week supply of nicotine patches and a pamphlet on quitting smoking. Contact time in the standard group was 16 minutes. Single in‐person session. |
|
| Outcomes | Primary adherence outcome (dichotomous data): participants were classified as adherent with the nicotine patch if they used ≥ 6 patches per week. Assessed using self‐report of number of days of patch use between baseline and follow‐up (8 weeks) and by asking participants to return at follow‐up all of the patches they had been given and conducting a count of their used patches. Outcome was for complete case sample (i.e. participants who had complete data at baseline and follow‐up/participants completing the treatment phase, not ITT). Other adherence outcomes: none Secondary outcomes: 7‐day PPA and 90‐day continuous abstinence (i.e. time since quit day), verified via breath CO monitoring (≤ 5 ppm). 90‐day continuous abstinence (assessed at 90 days) was selected as the outcome most relevant to adherence outcome, using ITT data for all randomised. |
|
| Notes | 22/34 follow‐up participants returned their patches; the remaining 12 participants provided only self‐reports of number of patches used. Study funded by National Institute on Drug Abuse. No information on conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement. No evidence of systematic differences between arms, although participants in the adherence intervention condition reported significantly greater last year quit attempts (treated as a covariate in the analyses) (p. 151, paragraph 1). |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear that outcome assessors were blinded, but because used patch counts were used for majority of participants completing treatment, considered unlikely to constitute a clear risk of bias. Primary adherence outcome was assessed by objective patch counts of medication used where possible and this was the case for majority of participants (22/34) with self‐report data where tablet counts not available (1234) (p. 150, paragraph 5). Abstinence outcomes were biochemically validated (p. 150, paragraph 4). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition across both groups was 15% (calculated from information on p. 150), but numbers not reported by group and with no information as to whether there was differential dropout although study had small absolute numbers of participants (p. 151, Results). |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol. |
| Consistency in intervention delivery | Low risk | Intervention was a module with structure. Quote: "Sessions were audio‐recorded, with 30% of sessions reviewed by the second or third author to ensure intervention fidelity" (p. 150, paragraph 2). |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
4R: Risks, Rewards, Roadblocks, and Repetition; 5R: Relevance, Risks, Rewards, Roadblocks, and Repetition; ANRT‐12: Attitudes about Nicotine Replacement Therapy questionnaire; BMQ: Beliefs about Medicines Questionnaire; CBT: cognitive behavioural therapy; CO: carbon monoxide; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; FTND: Fagerstrom Test for Nicotine Dependence; NHLBI: National Heart, Lung, and Blood Institute; ITT: intention to treat; MAC: medication adherence counselling; MEMS: Medication Event Monitoring Systems; NHS: National Health Service; NRT: nicotine replacement therapy; PPA: point‐prevalence abstinence; PRNR: Perceived Risks of Nicotine Replacement questionnaire; RCT: randomised controlled trial; SD: standard deviation; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aveyard 2007 | Intervention not principally focused on increasing adherence to medications for tobacco dependence – the protocol for the behavioural support interventions "did not specify the nature of the support offered". Adherence outcome was of use/not of use for specific time periods – assessing "whether NRT was being used in general and not the degree of adherence". |
| Bansal‐Travers 2010 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control included a focus on medication use. |
| Berlin 2011 | Intervention not principally focused on increasing adherence to medications for tobacco dependence – it was not suggested that this was an aim for the study or that the intervention was being employed to encourage increased adherence in participants. |
| Bock 2014 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. |
| Brendryen 2008 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Participants in both the intervention and control arms "recommended the use of NRT and contained information about such products and their use". |
| Buchanan 2004 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control included a focus on medication use and for the intervention arm the component focused on medication use was 1 of multiple elements relating to smoking cessation. |
| Cropsey 2017 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. |
| Gariti 2009 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control included a focus on medication use. |
| Gong 2016 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. |
| ICRFGPRG 1993 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. |
| Ingersoll 2009 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Intervention and control conditions were 2 different formats both "designed to provide motivation for cessation and patch use through attention to the participants' own assessment of their reasons to quit, tools needed to quit, and goal‐setting around quitting or reducing smoking". |
| ISRCTN33423896 | No eligible adherence outcome assessed. |
| Lando 1988 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. For the intervention materials the adherence component was 1 of multiple elements – "emphasis was placed upon a range of behavioral coping mechanisms of which gum was simply one major strategy for combating urges to smoke". |
| Lifrak 1997 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control included a focus on medication use. |
| McClure 2013 | No eligible adherence outcome assessed. |
| McClure 2016 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. |
| Okuyemi 2006 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control included a focus on medication use. |
| Okuyemi 2013 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Intervention was seemingly focused on both smoking cessation and adherence components with smoking cessation being the primary outcome. |
| Raupach 2010 | Not an eligible study design – historical cohort study. |
| Rigotti 2013 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Intervention was focused on both smoking cessation and adherence components with smoking cessation being the focus of the stated aim and the stated primary outcome. |
| Shaughnessy 1987 | No eligible adherence outcome assessed. |
| Shiffman 2000 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. The stated aim of the intervention was to evaluate the efficacy of tailored and untailored materials as supplements to NRT. The specified primary outcome was rate of continuous abstinence. Prompts to comply with the medication were 1 of multiple reported elements of the intervention. |
| Strecher 2005 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control included a focus on medication use. |
| Swan 2010 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. All arms included a focus on medication use. |
| Tseng 2017 | Although study reports described the intervention as an adherence Intervention it was not principally focused on increasing adherence to medications for tobacco dependence. The intervention tested was equally split between providing standard smoking cessation advice and material designed to enhance adherence. |
| Tønnessen 2006 | Intervention not principally focused on increasing adherence to medications for tobacco dependence. The stated aim of the intervention was "to evaluate the efficacy of the nicotine sublingual tablet or placebo combined with either low or high behavioral support for smoking cessation in COPD [chronic obstructive pulmonary disease] patients after 6 months and 12 months" with specified primary and secondary outcomes being smoking cessation, smoking reduction and quality of life. The intervention was described as "counselling on smoking cessation… and subjects were also given take‐home material with tips on smoking cessation". Participants were "recommended to use study medication" as 1 of multiple reported elements of the counselling intervention but it was not reported that this was administered differentially to intervention and control arms. |
| Willemsen 2006 | No eligible adherence outcome assessed – included a measure of use vs no use of medication. |
NRT: nicotine replacement therapy.
Characteristics of studies awaiting assessment [ordered by study ID]
Applegate 2007.
| Methods | Quote (from abstract): "A secure web program was created to properly dose cigarette smokers to gum strength (2 vs. 4 mg) and dosing program (# of pieces/day [PPD]). The program then sends SMS text messaging to the user's cellular telephone to prompt medication use at regular intervals. We then conducted a randomised trial examining tailored text messaging (TTM) to support text messaging (STM) in 110 cigarette smokers attempting to quit smoking while using nicotine gum." |
| Participants | The sample was 53% men, 63% white, aged 43 years (SD 11) and smoked 19 (SD 7.6) CPD. There were no differences between groups at baseline for CPD, gum dosing and recommended pieces per day. |
| Interventions | Tailored text messaging vs support text messaging |
| Outcomes | Outcome variables included self‐reported 7‐day recalls of nicotine gum use and cigarette smoking at 7, 28 and 56 days post quit date. |
| Notes | Requires assessment of full text to confirm eligibility but only an abstract is seemingly available. Lead author unable to be contacted, although member of author team who was able to be contacted (May 2013) indicated that the study was conducted by a company and had not been written up for publication. Abstract presented results as follows: on an intent‐to‐treat basis, independent‐sample t‐tests revealed that subjects in the tailored text messaging group reported chewing more nicotine gum than participants in the support text messaging group, (6.5 pieces per day with tailored text messaging vs 4.5 pieces per day with support text messaging; P = 0.003). No significant differences at 4 or 8 weeks, or for cigarette use variables. |
Yuhongxia 2011.
| Methods | Design: randomised controlled trial Country: China |
| Participants | Smokers willing to make a quit attempt. |
| Interventions | Intervention group: varenicline combined with a mobile telephone text messaging smoking cessation programme. The programme comprised motivational messages, support for behavioural change and 'medicine attention'. Control group: varenicline only |
| Outcomes | Primary outcomes were varenicline usage for 12 weeks and self‐reported continuous smoking abstinence, biochemically verified by exhaled carbon monoxide test at 3 and 6 months. |
| Notes | Only an abstract available. Unclear from this whether the principal focus of the intervention was on increasing adherence, although this seems unlikely from the abstract content. We were unable to contact the authors to receive more information. |
CPD: cigarettes per day.
Characteristics of ongoing studies [ordered by study ID]
NCT02635919.
| Trial name or title | Stage Ib Trial of mSMART for Smoking Cessation Medication Adherence (mSMART‐Ib) |
| Methods | Parallel RCT. Primary aim to conduct a 60‐patient feasibility, acceptability and preliminary efficacy study of mSMART (Mobile App based Personalized Solutions and Tools for Medication Adherence of Rx tablet), a smartphone application ('app') for improving medication adherence among substance users. The investigators will compare 2 groups of cigarette smokers undergoing a quit attempt with varenicline (Chantix). |
| Participants | 60 participants; aged 18–65 years; recently prescribed varenicline (Chantix) with the intention to quit smoking in the next 3 months; has an Android smartphone (using v5.x.x or lollipop) or Apple smartphone (iPhone) Operating System (iOS) (using v6.0). |
| Interventions | Experimental group: using the mSMART app on their smartphone and a MEMS Cap, a smart tablet box that will a record a date and time‐stamped medication event whenever tablet box is opened and closed, and thus allow for primary measurement of medication adherence). Control group: using the MEMS Cap and mobile web‐based surveys on their smartphone but no mSMART application. |
| Outcomes | Primary outcomes
|
| Starting date | April 2016 |
| Contact information | F Joseph McClernon; joseph.mcclernon@duke.edu |
| Notes | Detailed in registration at ClinicalTrials.gov: NCT02635919 |
MEMS: medication event monitoring system; RCT: randomised controlled trial.
Differences between protocol and review
For the 2015 version of the review (Hollands 2015b), the criteria for eligible interventions was refined between the protocol and the review. The original primary intention of the review was to examine the effect of interventions to increase adherence where this was the clearly intended focus of those intervening. However, this primary intention was not adequately reflected in the original criteria. As such, a large number of studies of interventions that could in theory alter adherence but where this was not the researchers' intention would have been relevant for inclusion. Furthermore, this lack of clarity meant that most extant studies that featured any intervention in smokers would have to be examined at the full‐text screening stage because a clear focus on increasing adherence (which can typically be derived from the title and abstract screening process) was not necessary for consideration for inclusion.
For the current update, we made additional changes to the original protocol.
Rather than including separate meta‐analyses combining each of continuous outcome data and dichotomous outcome data for a given outcome, we produced a single meta‐analysis that combined these using generic inverse variance methods. If a study reported both continuous and dichotomous outcomes that were similar in meeting other criteria, continuous data were used. In the previous version of the review, we had applied fixed‐effect models to our pooling of the data. This was in line with the protocol, in which we stated that we intended to group substantially similar studies. In practice, there was considerable methodological and clinical variance between studies, reflecting different characteristics of settings, participant groups, interventions and measures. As such, a random‐effects model was considered more appropriate.
We added formal coding of the focus of the content of the intervention, which while stated as an objective, had not been conducted for the previous version of this review. We coded studies by reference to two key factors: 1. focus on perceptions, practicalities, or both; 2. participant‐centred or clinician‐centred. This was then used as the basis for subgroup analyses to determine which types of interventions were most effective.
In the previous version of this review we proposed to assess the impact of using intention‐to‐treat data for adherence outcomes instead of using data for only those participants who remained engaged with treatment (the latter being our specified preferred approach). This was no longer considered necessary given justification for using the latter data, reflected in the two newly identified studies which both report adherence data for participants who remained engaged with treatment.
For risk of bias assessment, following guidance from the Cochrane Tobacco Addiction Group, we removed three domains (blinding of participants and personnel (performance bias); validity and reliability of outcome measures (other sources of bias); comparability of baseline characteristics (other sources of bias)). We followed guidance in Section 8.7 (Table 8.7a) of the Cochrane Handbook for Systematic Reviews of Interventions for deriving a summary risk of bias judgement from the domains that were assessed (Higgins 2011).
Contributions of authors
Draft the protocol: all authors.
Develop the search strategy: GJH, NL.
Search for trials: GJH, NL, FN.
Obtain copies of trials: GJH, FN.
Select which studies to include: GJH, FN, NL.
Extract data from studies: GJH, FN.
Enter data into Review Manager 5: GJH, FN.
Carry out the analysis: GJH, FN.
Interpret the analysis: all authors.
Draft the final review: all authors.
Update the review: GJH.
Sources of support
Internal sources
-
University of Cambridge, UK.
Computer use, database access
-
NIHR senior investigator, UK.
Paul Aveyard is an NIHR senior investigator.
-
NIHR Oxford Biomedical Research Centre, UK.
Paul Aveyard is supported by the NIHR Oxford Biomedical Research Centre.
-
NIHR Oxford CLAHRC, UK.
Paul Aveyard is supported by NIHR Oxford CLAHRC.
-
NIHR Infrastructure funding, UK.
The Cochrane Tobacco Addiction Group is funded by the NIHR. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
External sources
No sources of support supplied
Declarations of interest
GJH is an author of one of the studies included in the review (Marteau 2012).
FN declares no competing interests.
AF declares researcher‐led funding from Johnson and Johnson (Ethicon) being awarded to her institution.
NL is managing editor of the Cochrane Tobacco Addiction Group, although this is not deemed a conflict of interest.
PA is an author of one the studies included in the review (Marteau 2012). PA is also the co‐ordinating editor of the Cochrane Tobacco Addiction Group, although this is not deemed a conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chan 2010 {published data only}
- Chan SS, Leung DY, Abdullah AS, Lo SS, Yip AW, Kok W‐M, et al. Smoking‐cessation and adherence intervention among Chinese patients with erectile dysfunction. American Journal of Preventive Medicine 2010;39(3):251‐8. [DOI] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam T‐H. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction 2011;106:1155‐63. [DOI] [PubMed] [Google Scholar]
Marteau 2012 {published data only}
- Hollands GJ, Sutton S, McDermott M, Marteau TM, Aveyard P. Adherence to and consumption of nicotine replacement therapy and the relationship with abstinence within a smoking cessation trial in primary care. Nicotine & Tobacco Research 2013;15(9):1537‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Aveyard P, Munafò MR, Prevost AT, Hollands GJ, Armstrong D, et al. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PloS One 2012;7(4):e35249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Munafò MR, Aveyard P, Hill C, Whitwell S, Willis TA, et al. Trial protocol: using genotype to tailor prescribing of nicotine replacement therapy: a randomised controlled trial assessing impact of communication upon adherence. BMC Public Health 2010;10(1):680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mooney 2005 {published data only}
- Mooney M, Babb D, Jensen J, Hatsukami D. Interventions to increase use of nicotine gum: a randomized, controlled, single‐blind trial. Nicotine & Tobacco Research 2005;7(4):565‐79. [DOI] [PubMed] [Google Scholar]
- Mooney M, Green C, Hatsukami D. Nicotine self‐administration: cigarette versus nicotine gum diurnal topography. Human Psychopharmacology 2006;21(8):539‐48. [DOI] [PubMed] [Google Scholar]
Mooney 2007 {published data only}
- Mooney ME, Sayre SL, Hokanson PS, Stotts AL, Schmitz JM. Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: a replication and extension study. Addictive Behaviors 2007;32:875‐80. [DOI] [PubMed] [Google Scholar]
Nollen 2011 {published data only}
- Nollen NL, Sanderson Cox L, Nazir N, Ellerbeck EF, Owen A, Pankey S, et al. A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine & Tobacco Research 2011;13(9):868‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schlam 2018 {published data only}
- Schlam TR, Cook JW, Baker TB, Hayes‐Birchler T, Bolt DM, Smith SS, et al. Can we increase smokers' adherence to nicotine replacement therapy and does this help them quit?. Psychopharmacology 2018;235:2065‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Comparative effectiveness of intervention components for producing long‐term abstinence from smoking: a factorial screening experiment. Addiction 2016;111:142‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2005 {published data only}
- Schmitz JM, Sayre SL, Stotts AL, Rothfleisch J, Mooney ME. Medication compliance during a smoking cessation clinical trial: a brief intervention using MEMS feedback. Journal of Behavioral Medicine 2005;28(2):139‐47. [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith SS, Keller PA, Kobinsky KH, Baker TB, Fraser DL, Bush T, et al. Enhancing tobacco quitline effectiveness: identifying a superior pharmacotherapy adjuvant. Nicotine & Tobacco Research 2013;15(3):718‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2017 {published data only}
- Tucker JS, Shadel WG, Galvan FH, Naranjo D, Lopez C, Setodji C. Pilot evaluation of a brief intervention to improve nicotine patch adherence among smokers living with HIV/AIDS. Psychology of Addictive Behaviors 2017;31(2):148‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aveyard 2007 {published data only}
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafo MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62:898‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansal‐Travers 2010 {published data only}
- Bansal‐Travers M, Cummings KM, Hyland A, Brown A, Celestino P. Educating smokers about their cigarettes and nicotine medications. Health Education Research 2010;25(4):678‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlin 2011 {published data only}
- Berlin I, Jacob N, Coudert M, Perriot J, Schultz L, Rodon N. Adjustment of nicotine replacement therapies according to saliva cotinine concentration: the ADONIS* trial – a randomized study in smokers with medical comorbidities. Addiction 2011;106(4):833‐43. [DOI] [PubMed] [Google Scholar]
Bock 2014 {published data only}
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low‐income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brendryen 2008 {published data only}
- Brendryen H, Kraft P. Happy ending: a randomized controlled trial of a digital multi‐media smoking cessation intervention. Addiction 2008;103:478‐84. [DOI] [PubMed] [Google Scholar]
Buchanan 2004 {published data only}
- Buchanan LM, El‐Banna M, White A, Moses S, Siedlik C, Wood M. An exploratory study of multicomponent treatment intervention for tobacco dependency. Journal of Nursing Scholarship 2004;36(4):324‐30. [DOI] [PubMed] [Google Scholar]
Cropsey 2017 {published data only}
- Cropsey KL, Hendricks PS, Schiavon S, Sellers A, Froelich M, Shelton RC, et al. A pilot trial of In vivo NRT sampling to increase medication adherence in community corrections smokers. Addictive Behaviors 2017;67:92‐9. [DOI] [PubMed] [Google Scholar]
Gariti 2009 {published data only}
- Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment 2009;37(3):247‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gong 2016 {published data only}
- Gong J, Baker CL, Zou KH, Bruno M, Jumadilova Z, Lawrence D, et al. A pragmatic randomized trial comparing telephone‐based enhanced pharmacy care and usual care to support smoking cessation. Journal of Managed Care & Specialty Pharmacy 2016;22(12):1417‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICRFGPRG 1993 {published data only}
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice. BMJ 1993;306:1304‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Randomised trial of nicotine patches in general practice: results at one year. BMJ 1994;308:1476‐7. [PMC free article] [PubMed] [Google Scholar]
Ingersoll 2009 {published data only}
- Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS and Behavior 2009;13(3):545‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN33423896 {published data only}
- ISRCTN33423896. Study of effectiveness of a smartphone app for stopping smoking focused on use of nicotine replacement therapy. isrctn.com/ISRCTN33423896 (first received 16 March 2015).
Lando 1988 {published data only}
- Lando HA, Kalb EA, McGovern PG. Behavioral self‐help materials as an adjunct to nicotine gum. Addictive Behaviors 1988;13(2):181‐4. [DOI] [PubMed] [Google Scholar]
Lifrak 1997 {published data only}
- Lifrak P, Gariti P, Alterman A, McKay J, Volpicelli J, Sparkman T, et al. Results of two levels of adjunctive treatment used with the nicotine patch. American Journal on Addictions 1997;6(2):93‐8. [PubMed] [Google Scholar]
McClure 2013 {published data only}
- McClure JB, Swan GE, John J, Fauver R, Javitz HS, Bergen AW, et al. Pharmacogenetic smoking cessation intervention in a health care setting: a pilot feasibility study. Nicotine & Tobacco Research 2013;15(2):518‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2016 {published data only}
- McClure JB, Anderson ML, Bradley K, An LC, Catz SL. Evaluating an adaptive and interactive mHealth smoking cessation and medication adherence program: a randomized pilot feasibility study. JMIR mHealth and uHealth 2016;4(3):e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuyemi 2006 {published data only}
- Okuyemi KS, Thomas JL, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: a pilot clinical trial. Nicotine & Tobacco Research 2006;8(5):689‐99. [DOI] [PubMed] [Google Scholar]
Okuyemi 2013 {published data only}
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108:1136‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raupach 2010 {published data only}
- Raupach T, Shahab L, Eimer S, Puls M, Hasenfuss G, Andreas S. Increasing the use of nicotine replacement therapy by a simple intervention: an exploratory trial. Substance Use & Misuse 2010;45(3):403‐13. [DOI] [PubMed] [Google Scholar]
Rigotti 2013 {published data only}
- Rigotti NA, Japuntich S, Regan S, Kelley JH, Chang Y, Reyen M, et al. Promoting smoking cessation after hospital discharge: the helping hand randomized controlled comparative effectiveness trial. Journal of General Internal Medicine 2013;28:S160. [Google Scholar]
Shaughnessy 1987 {published data only}
- Shaughnessy AF, Davis RE, Reeder CE. Nicotine chewing gum: effectiveness and the influence of patient education in a family practice. Journal of Family Practice 1987;25(3):266‐9. [PubMed] [Google Scholar]
Shiffman 2000 {published data only}
- Shiffman S, Paty JA, Rohay JM, Marino ME, Gitchell J. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine polacrilex gum therapy. Archives of Internal Medicine 2000;160:1675‐81. [DOI] [PubMed] [Google Scholar]
Strecher 2005 {published data only}
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web‐based computer‐tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100(5):682‐8. [DOI] [PubMed] [Google Scholar]
Swan 2010 {published data only}
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine 2010;38(5):482‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tseng 2017 {published data only}
- NCT01898195. Improving adherence to smoking cessation medication among PLWHA (HIV). clinicaltrials.gov/ct2/show/NCT01898195 (first received 12 July 2013).
- Tseng TY, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, et al. Combining text messaging and telephone counseling to increase varenicline adherence and smoking abstinence among cigarette smokers living with HIV: a randomized controlled study. AIDS and Behavior 2017;21(7):1964‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tønnessen 2006 {published data only}
- Tønnessen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334‐42. [DOI] [PubMed] [Google Scholar]
Willemsen 2006 {published data only}
- Willemsen MC, Wiebing M, Emst A, Zeeman G. Helping smokers to decide on the use of efficacious smoking cessation methods: a randomized controlled trial of a decision aid. Addiction 2006;101(3):441‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Applegate 2007 {published data only}
- Applegate BW, Raymond C, Collado‐Rodriguez A, Riley WT, Schneider NG. Improving adherence to nicotine gum by SMS text messaging: a pilot study. Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 Feb 21‐24; Austin (TX). 2007.
Yuhongxia 2011 {published data only}
- Yuhongxia L. The compliance of varenicline usage and the smoking abstinence rate via mobile phone text messaging combine with varenicline: a single‐blind, randomised control trial. Respirology 2011;16:46‐7. [Google Scholar]
References to ongoing studies
NCT02635919 {published data only}
- NCT02635919. Stage Ib Trial of mSMART for Smoking Cessation Medication Adherence (mSMART‐Ib). clinicaltrials.gov/ct2/show/NCT02635919 (first received 21 December 2015).
Additional references
Burns 2008
- Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking‐cessation attempters. American Journal of Preventive Medicine 2008;34:212‐5. [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheong 2010
- Cheong YS, Ahn SH. Effect of multi‐modal interventions for smoking cessation in a university setting: a short course of varenicline, financial incentives, e‐mail and short message service. Korean Journal of Family Medicine 2010;31:355‐60. [Google Scholar]
Coleman 2012
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine‐replacement therapy patches in pregnancy. New England Journal of Medicine 2012;366(9):808‐18. [DOI] [PubMed] [Google Scholar]
Crockett 2018
- Crockett RA, King SE, Marteau TM, Prevost AT, Stubbs B, Bignardi G, et al. Nutritional labelling for healthier food or non‐alcoholic drink purchasing and consumption. Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD009315.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fidler 2011
- Fidler JA, Shahab L, West O, Jarvis MJ, McEwen A, Stapleton JA, et al. 'The smoking toolkit study': a national study of smoking and smoking cessation in England. BMC Public Health. 2011;11:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fish 2009
- Fish LJ, Peterson BL, Namenek Brouwer RJ, Lyna P, Oncken CA, Swamy GK, et al. Adherence to nicotine replacement therapy among pregnant smokers. Nicotine & Tobacco Research 2009;11(5):514‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2018
- GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hajek 1999
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 1999;159:2033‐8. [DOI] [PubMed] [Google Scholar]
Hammond 2004
- Hammond D, McDonald PW, Fong GT, Borland R. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction 2004;99:1042‐8. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2018
- Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2019
- Hartmann‐Boyce J, Hong B, Livingstone‐Banks J, Wheat H, Fanshawe TR. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 6. [DOI: 10.1002/14651858.CD009670.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haynes 2008
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Hays 2010
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010;12(6):574‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.10 (updated March 2011). Chichester (UK): John Wiley & Sons, 2011. [Google Scholar]
Hollands 2012
- Hollands GJ, Whitwell SC, Parker RA, Prescott NJ, Forbes A, Sanderson J, et al. Effect of communicating DNA based risk assessments for Crohn's disease on smoking cessation: randomised controlled trial. BMJ 2012;345:e4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollands 2013
- Hollands GJ, Sutton S, McDermott M, Marteau TM, Aveyard P. Adherence to and consumption of nicotine replacement therapy and the relationship with abstinence within a smoking cessation trial in primary care. Nicotine & Tobacco Research 2013;15(9):1537‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollands 2015a
- Hollands GJ, Shemilt I, Marteau TM, Jebb SA, Lewis HB, Wei Y, et al. Portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011045.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollands 2017
- Hollands GJ, Bignardi G, Johnston M, Kelly MP, Ogilvie D, Petticrew M, et al. The TIPPME intervention typology for changing environments to change behaviour. Nature Human Behaviour 2017;1:0140. [Google Scholar]
Horne 2013
- Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients' adherence‐related beliefs about medicines prescribed for long‐term conditions: a meta‐analytic review of the necessity‐concerns framework. PloS One 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical research ed) 2010;340:c332. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morphett 2015
- Morphett K, Partridge B, Gartner C, Carter A, Hall W. Why don't smokers want help to quit? A qualitative study of smokers' attitudes towards assisted vs. unassisted quitting. International Journal of Environmental Research and Public Health 2015;12(6):6591‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naughton 2017
- Naughton F. Delivering "just‐in‐time" smoking cessation support via mobile phones: current knowledge and future directions. Nicotine & Tobacco Research 2017;19(3):379‐83. [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Clinical guideline CG76. London: National Institute for Health and Clinical Excellence 2009.
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD000011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raupach 2014
- Raupach T, Brown J, Herbec A, Brose L, West R. A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction 2014;109(1):35‐43. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shiffman 2007
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102:809‐14. [DOI] [PubMed] [Google Scholar]
Shiffman 2008
- Shiffman S, Sweeney ST, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10 week randomized, double‐blind, placebo‐controlled clinical trial simulating over‐the‐counter use in adult smokers. Clinical Therapeutics 2008;30:1852‐8. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sumner 2018
- Sumner JA, Carey RN, Michie S, Johnston M, Edmondson D, Davidson KW. Using rigorous methods to advance behaviour change science. Nature Human Behaviour 2018;2:797‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Brand 2017
- Brand FA, Nagelhout GE, Reda AA, Winkens B, Evers SM, Kotz D, et al. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD004305.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb 2006
- Webb TL, Sheeran P. Does changing behavioral intentions engender behaviour change? A meta‐analysis of the experimental evidence. Psychological Bulletin 2006;132(2):249‐68. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hollands 2015b
- Hollands GJ, McDermott MS, Lindson‐Hawley N, Vogt F, Farley A, Aveyard P. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD009164.pub2] [DOI] [PubMed] [Google Scholar]


