Abstract
Background
Motivational Interviewing (MI) is a directive patient‐centred style of counselling, designed to help people to explore and resolve ambivalence about behaviour change. It was developed as a treatment for alcohol abuse, but may help people to a make a successful attempt to stop smoking.
Objectives
To evaluate the efficacy of MI for smoking cessation compared with no treatment, in addition to another form of smoking cessation treatment, and compared with other types of smoking cessation treatment. We also investigated whether more intensive MI is more effective than less intensive MI for smoking cessation.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register for studies using the term motivat* NEAR2 (interview* OR enhanc* OR session* OR counsel* OR practi* OR behav*) in the title or abstract, or motivation* as a keyword. We also searched trial registries to identify unpublished studies. Date of the most recent search: August 2018.
Selection criteria
Randomised controlled trials in which MI or its variants were offered to smokers to assist smoking cessation. We excluded trials that did not assess cessation as an outcome, with follow‐up less than six months, and with additional non‐MI intervention components not matched between arms. We excluded trials in pregnant women as these are covered elsewhere.
Data collection and analysis
We followed standard Cochrane methods. Smoking cessation was measured after at least six months, using the most rigorous definition available, on an intention‐to‐treat basis. We calculated risk ratios (RR) and 95% confidence intervals (CI) for smoking cessation for each study, where possible. We grouped eligible studies according to the type of comparison. We carried out meta‐analyses where appropriate, using Mantel‐Haenszel random‐effects models. We extracted data on mental health outcomes and quality of life and summarised these narratively.
Main results
We identified 37 eligible studies involving over 15,000 participants who smoked tobacco. The majority of studies recruited participants with particular characteristics, often from groups of people who are less likely to seek support to stop smoking than the general population. Although a few studies recruited participants who intended to stop smoking soon or had no intentions to quit, most recruited a population without regard to their intention to quit. MI was conducted in one to 12 sessions, with the total duration of MI ranging from five to 315 minutes across studies. We judged four of the 37 studies to be at low risk of bias, and 11 to be at high risk, but restricting the analysis only to those studies at low or unclear risk did not significantly alter results, apart from in one case ‐ our analysis comparing higher to lower intensity MI.
We found low‐certainty evidence, limited by risk of bias and imprecision, comparing the effect of MI to no treatment for smoking cessation (RR = 0.84, 95% CI 0.63 to 1.12; I2 = 0%; adjusted N = 684). One study was excluded from this analysis as the participants recruited (incarcerated men) were not comparable to the other participants included in the analysis, resulting in substantial statistical heterogeneity when all studies were pooled (I2 = 87%). Enhancing existing smoking cessation support with additional MI, compared with existing support alone, gave an RR of 1.07 (95% CI 0.85 to 1.36; adjusted N = 4167; I2 = 47%), and MI compared with other forms of smoking cessation support gave an RR of 1.24 (95% CI 0.91 to 1.69; I2 = 54%; N = 5192). We judged both of these estimates to be of low certainty due to heterogeneity and imprecision. Low‐certainty evidence detected a benefit of higher intensity MI when compared with lower intensity MI (RR 1.23, 95% CI 1.11 to 1.37; adjusted N = 5620; I2 = 0%). The evidence was limited because three of the five studies in this comparison were at risk of bias. Excluding them gave an RR of 1.00 (95% CI 0.65 to 1.54; I2 = n/a; N = 482), changing the interpretation of the results.
Mental health and quality of life outcomes were reported in only one study, providing little evidence on whether MI improves mental well‐being.
Authors' conclusions
There is insufficient evidence to show whether or not MI helps people to stop smoking compared with no intervention, as an addition to other types of behavioural support for smoking cessation, or compared with other types of behavioural support for smoking cessation. It is also unclear whether more intensive MI is more effective than less intensive MI. All estimates of treatment effect were of low certainty because of concerns about bias in the trials, imprecision and inconsistency. Consequently, future trials are likely to change these conclusions. There is almost no evidence on whether MI for smoking cessation improves mental well‐being.
Plain language summary
Does motivational interviewing help people to quit smoking?
Background
Motivational interviewing is a type of counselling that can be used to help people to stop smoking. It aims to help people explore the reasons that they may feel unsure about quitting and find ways to make them feel more willing and able to stop smoking. Rather than telling the person why and how they should change their behaviour, counsellors try to help people to choose to change their own behaviour, increasing their confidence that they can succeed. This review explores whether motivational interviewing helps more people to stop smoking than no treatment, or other types of stop smoking treatment. It also looks at whether longer motivational interviewing, with more counselling sessions, helps more people to quit than shorter motivational interviewing with fewer sessions.
Study characteristics
This review included 37 trials covering over 15,000 people who smoked tobacco. Studies were conducted in a lot of different types of people, including people with health problems or drug use problems, young people, homeless people, and people who had been arrested or were in prison. Some people felt ready to quit smoking and others did not. Motivational interviewing was provided in one to 12 sessions and took from as little as five minutes, to as much as eight hours, to deliver. Studies lasted for at least six months. The evidence is up to date to August 2018.
Key results
There was not enough information available to decide whether motivational interviewing helped more people to stop smoking than no stop smoking treatment. People were slightly more likely to stop smoking if they were provided with motivational interviewing rather than another type of treatment to stop smoking, but our findings suggest that there is still a chance that motivational interviewing could also reduce a person's chances of quitting compared with other stop smoking treatments. This means more research is needed to decide whether motivational interviewing can help more people to quit than other types of treatment. Using longer motivational interviewing with more treatment sessions may help more people to give up smoking than shorter motivational interviewing with fewer sessions, however more research is needed to be sure that this is the case.
We also looked at whether being provided with motivational interviewing to quit smoking increased people's well‐being. Most studies did not provide any information about this, and so more studies are needed to answer this question.
Quality of the evidence
There is low‐quality evidence looking at whether motivational interviewing helps more people to quit smoking than no treatment. This means it is difficult to know whether motivational interviewing helps people to quit smoking or not, and more studies are needed. The quality of the evidence was also low for all of the other questions we asked about quitting smoking, which means that our findings may change when new research is carried out. The quality of the research is rated as low because there were problems with the design of studies, findings of studies were very different to one another, and there were not enough data, making it difficult to determine whether motivational interviewing or more intense motivational interviewing helped people to quit smoking or not.
Summary of findings
Background
Description of the condition
Tobacco use is one of the leading causes of preventable illness and death worldwide, accounting for over seven million deaths annually (GBD 2015 Risk Factors Collaborators 2016). Extrapolation based on current smoking trends suggests that, without widespread quitting, approximately 400 million tobacco‐related deaths will occur between 2010 and 2050, mostly among current smokers (Jha 2011). Most smokers would like to stop (CDC 2017); however, quitting is difficult.
Description of the intervention
The concept of motivational interviewing (MI) evolved from experiences in treating alcohol abuse, and was first described by Miller in 1983. It is defined as "a directive, client‐centred counselling style for eliciting behaviour change by helping clients to explore and resolve ambivalence" (Miller 1983). The four guiding principles: (a) expressing empathy, (b) developing discrepancy, (c) rolling with resistance, (d) supporting self efficacy, have been detailed elsewhere (Miller 2002).
The MI process is a brief psychotherapeutic intervention intended to increase the likelihood that a person will make an attempt to change their harmful behaviour. Adaptations of MI have ranged from brief 20‐minute office interventions (motivational consulting) to Motivation Enhancement Therapy (MET), a multi‐session course of treatment, including a lengthy assessment, personalised feedback and follow‐up interviews (Lawendowski 1998; Rollnick 1992). MI has also been provided by telephone consultations and in a group format. MI and its various forms have been applied both as a stand‐alone intervention or with other treatments, and in a range of settings. These include health settings such as general hospital wards, emergency departments, and general medical practice (Britt 2002).
How the intervention might work
Miller 1994 suggests that motivation may fluctuate over time or from one situation to another, and can be influenced to change in a particular direction. Thus, lack of motivation (or resistance to change) is seen as something fluid, that is open to change. Therefore, the main focus of MI is facilitating behaviour change using a directive approach, by helping people to explore and resolve any ambivalence they may have toward this change (Rollnick 1995), and in turn making them more likely to choose to change their behaviour in the desired direction. In this case, that behaviour is smoking and so the goal of MI is to increase motivation to quit, making smoking cessation more likely. Rollnick 1995 also suggests that adopting an aggressive or confrontational style is likely to produce negative responses from people (such as arguing), which may be interpreted by the practitioner as denial or resistance. MI guides people to explore and confront their behaviour, instead of telling them what to do.
Why it is important to do this review
MI has been used primarily for the management of health behaviours in those with behavioural disorders, such as alcohol abuse, drug addiction, weight loss, and treatment compliance, as well as for smoking cessation. Systematic reviews have shown some beneficial effects of MI on these behaviours (Cheng 2015; Cowlishaw 2012; Foxcroft 2016; Gates 2016; Heckman 2010; Hettema 2010; Klimas 2018; Mbuagbaw 2012; Morton 2015; Smedslund 2011). However, these effects are minimal or non‐existent at long‐term follow‐up and included studies are generally deemed to be of limited quality, making it difficult to draw clear conclusions. For example, Morton 2015 concluded that the design of many studies ‐ incorporating multi‐component interventions ‐ made it very difficult to isolate the effects of MI. The previous version of this review (Lindson‐Hawley 2015) resulted in a modest but significant increase in quitting smoking when MI was used in comparison to brief advice or usual care. However, this review encountered the same challenges described by Morton 2015 above, pooled studies with a range of different comparator types, and only included studies that reported providing a form of MI fidelity monitoring. This may have biased the inclusion of studies and thus the results. Therefore, inclusion criteria for this version of the review have been revised to reduce bias (although still control for fidelity monitoring), attempt to isolate the effects of MI, and to be mindful of the comparator group when pooling studies, to allow a range of useful comparisons.
Objectives
To evaluate the efficacy of MI for smoking cessation compared with no treatment, in addition to another form of smoking cessation treatment, and compared with other types of smoking cessation treatment. We also investigated whether more intensive MI is more effective than less intensive MI for smoking cessation.
We explored whether motivational interviewing for smoking cessation could enhance well‐being.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cluster RCTs.
Types of participants
Tobacco smokers, excluding pregnant women. We excluded trials that only recruited pregnant women, as their particular needs and circumstances warrant them being treated as a separate population. Studies in pregnant women are covered in a separate Cochrane Review (Chamberlain 2017).
Types of interventions
Interventions labelled as either MI or MET, targeted at tobacco smoking cessation. Eligible interventions were based on the principles and practices of MI (e.g. engaging, focussing, evoking, planning, exploring ambivalence, assessment of motivation and confidence to quit, eliciting 'change talk' and supporting self‐efficacy) as described in Miller 2013, and, in the opinion of the review authors, complied with these principles and practices beyond simply referring to the concepts. We included studies testing interventions that claimed to be based on both MI and another theoretical approach to counselling, such as cognitive behavioural therapy (CBT). However, we tested the effect of including these studies using sensitivity analysis.
MI is a specific motivational intervention, which has been incorrectly linked to other interventions or theories, such as the transtheoretical model of change, the decisional balance technique, and client‐centred counselling (Miller 2009). MI is conceptually and practically distinct from these interventions and principles. Therefore, we did not include trials that primarily tested these distinct approaches. Stage‐based interventions, such as the transtheoretical model for smoking cessation, are covered in a separate Cochrane Review (Cahill 2010).
We included studies where the intervention arm included MI as part of a multi‐component intervention (that may or may not have included pharmacotherapy), provided that the additional elements were also included in the control arm, and thus were not being tested. No exclusions were made based on the modality of the intervention.
Eligible studies included a comparison (control) intervention of either 1) no smoking cessation treatment, 2) another smoking cessation intervention, of any length or intensity (including usual care), or 3) another type of MI intervention (e.g. MI of a lower intensity).
Types of outcome measures
Primary outcomes
Our primary outcome was smoking cessation. We preferred continuous/prolonged cessation over point prevalence cessation, and biochemically validated over self‐reported cessation, where multiple measures were available in included studies. We reported cessation at the longest follow‐up, and excluded trials that did not include data on smoking cessation rates at least six months after baseline.
Secondary outcomes
MI has been linked to self‐determination theory. Markland 2005 proposed that MI can provide the circumstances under which people can initiate and action their own behaviour through 'self‐determination'. Self‐determination theory hypothesises that this self‐determination can lead to positive consequences, such as enhanced well‐being (Ryan 2000). This suggests that MI may increase well‐being as well as promote behaviour change. Therefore, we attempted to collect data on the following secondary outcomes:
Mental health and well‐being. Any measure of mental health and well‐being as defined by included studies
Quality of life (QOL). Any validated QOL scale reported in included studies. For example, the Quality of Life Scale (QOLS) (Burckhardt 2003); the Euro–Quality of Life Questionnaire (EQ‐5D) (EuroQol Group 1990)
We considered including adverse events as an outcome but decided against this. MI and comparator interventions comprise talk about smoking, which rarely gives rise to strong emotions and attendance for counselling is voluntary. Thus, it is unlikely that people who find such talk distressing will attend MI. As a result, we believe that few or no trials will have assessed adverse events, making assessment impossible.
Search methods for identification of studies
Electronic searches
We conducted a search of the Cochrane Tobacco Addiction Group's Specialised Register in August 2018. The search strategy is available in Appendix 1. The Register has been developed from electronic searching of the Cochrane Central Register of Controlled trials (CENTRAL), MEDLINE, Embase and PsycINFO, together with handsearching of specialist journals, conference proceedings and reference lists of previous trials and overviews. See the Tobacco Addiction Group's website for full details of how the Register is compiled. At the time of the Register search, results from the following databases were included:
Cochrane Central Register of Controlled trials (CENTRAL), issue 1, 2018;
MEDLINE (via OVID) to update 20180726;
Embase (via OVID) to week 201831;
PsycINFO (via OVID) to update 20180723.
We also searched the following online trial registries to identify unpublished studies: ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP).
Although this review is an update of a previous review, we carried out full searches of the literature, from database inception. This was because inclusion criteria were updated for this version and we wanted to ensure we identified relevant studies that may have been excluded in previous versions.
Data collection and analysis
Selection of studies
Two authors (of AF, JL, NL, TT) independently screened the title and abstract of each record returned for eligibility. Where there was uncertainty, the record was put forward to the next round of screening. We then acquired the full‐text reports of any trials deemed potentially relevant. Two authors (of AF, JL, NL, TT) independently assessed the full texts for inclusion, and any disagreements were referred to a third author.
Data extraction and management
Two authors (of AF, JL, NL, TT) independently extracted the following information about each eligible trial, where available:
Details of study design, including methods of randomisation and recruitment
Location and setting of the trial, e.g. hospital‐based, clinic‐based, community‐based
Participant characteristics, e.g. level of motivation, pre‐existing conditions, demographic descriptors
Intervention provider characteristics: e.g. type of provider and MI training provision
Description of the intervention(s), including the nature, frequency and duration of MI, and any co‐interventions used
Description of comparator(s), including the nature, frequency and duration of MI, and any co‐interventions used
Any procedures followed to ensure MI fidelity, and the results of any monitoring
Primary outcome measures: definition of smoking cessation used for primary outcome, timing of longest follow‐up, any biochemical validation
Secondary outcome measures: whether mental health and QoL were measured, definitions of outcomes (where measured), outcome data (where measured)
Loss to follow‐up
Funding source
Declarations of interest
Extraction was then compared and amalgamated for each study, with disagreements referred to a third author.
Assessment of risk of bias in included studies
We evaluated studies on the basis of randomisation procedure, allocation concealment, incomplete outcome data, and any other bias using standard Cochrane methods (Higgins 2011). We also assessed detection bias based on the outcome measure, according to standard methods of the Cochrane Tobacco Addiction Group. If the outcome was objective (i.e. biochemically validated) and/or if contact was matched between arms, we judged the studies as being at low risk of bias, but if the outcome was self‐reported and the intervention arm received more support than the control arm, we judged differential misreport to be possible and rated these studies as being at high risk of bias. For trials of behavioural interventions (such as those included here), it is deemed inappropriate to assess performance bias, as blinding of participants and personnel is not feasible due to the nature of the intervention.
Two authors (of AF, JL, NL, TT) independently rated each domain as being at high, low or unclear risk of bias, for each study. We resolved any disagreement between authors through discussion with a third author.
Measures of treatment effect
For our primary outcome, we extracted the most stringent definition of smoking cessation for each study (i.e. longest follow‐up, continuous/prolonged versus point prevalence, and biochemically validated versus self‐report). Where appropriate, we expressed trial effects as a risk ratio (RR), calculated as: (quitters in treatment group/total randomised to treatment group)/(quitters in control group/total randomised to control group), alongside 95% confidence intervals (CI). A risk ratio greater than 1 indicates a potentially better outcome in the intervention group than in the control group.
Secondary outcomes (mental health and QoL) were discussed narratively.
Unit of analysis issues
We included both individually and cluster‐randomised trials. For cluster RCTs, we considered whether authors had accounted for clustering in their reported analyses. Where possible and appropriate, we adjusted for clustering using the trial's reported intra‐class correlation (ICC), calculated an ICC from the information provided, or applied the reported ICC from a similar trial.
Dealing with missing data
We conducted our analyses on an intention‐to‐treat basis, i.e. using all participants randomised to their original groups as denominators where data were available, and assuming that those lost to follow‐up were continuing to smoke. We extracted numbers lost to follow‐up from study reports and used these to assess the risk of attrition bias. Where any required primary outcome data were not available in study reports, we contacted the authors in an attempt to obtain these.
Assessment of heterogeneity
Before pooling studies, we considered both methodological and clinical variance between studies. Where pooling was deemed appropriate, we investigated statistical heterogeneity using the I² statistic (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance).
Assessment of reporting biases
We used funnel plots to assess small‐study effects and investigate the possibility of publication bias for the 'MI as an adjunct' and 'MI versus other smoking cessation treatment' comparisons. There were not enough studies (fewer than ten) included in the other analyses to create funnel plots.
Data synthesis
For the primary outcome ‐ smoking cessation ‐ we synthesised groups of studies using Mantel‐Haenszel random‐effects models to estimate separate pooled treatment effects (as RRs and 95% CIs), for four types of comparison:
MI versus no smoking cessation intervention (comparison 1)
MI in addition to another smoking cessation treatment versus that smoking cessation treatment alone (comparison 2)
MI alone versus another smoking cessation intervention (comparison 3)
Higher intensity MI versus lower intensity MI (comparison 4)
Secondary outcomes ‐ mental health and QoL ‐ were reported sparsely and so were summarised narratively.
Subgroup analysis and investigation of heterogeneity
In view of possible heterogeneity between studies, where relevant and there were sufficient studies, we analysed the trials in the following subgroups:
Stratified by whether intensity of smoking cessation support was matched between trial arms, or differed between the MI and comparison group. Intensity was defined as a combination of the number of treatment sessions provided and the overall intervention/comparator contact time.
Stratified by age of participant: adult versus adolescent
Stratified by intervention provider: GP, nurse, counsellor/psychologist, lay healthcare worker
Stratified by counselling modality: face‐to‐face contact (including interventions delivered completely face‐to‐face or partially face‐to‐face) versus no face‐to‐face contact (i.e. via telephone, text messages, virtual reality setting)
Stratified by whether MI fidelity monitoring was reported or not
Stratified by the participants' motivation to quit at baseline, i.e. whether those recruited were motivated to quit, were not motivated to quit, or had not been selected based on their motivation to quit
Sensitivity analysis
We carried out the following sensitivity analyses to see if the pooled results of analyses were sensitive to the removal of:
Studies judged to be at high risk of bias
Studies that measured the fidelity of MI and found that the requirements of MI were not met (fidelity subgroup analyses only)
Studies where the MI intervention was also based on another theoretical approach, such as CBT
'Summary of Findings' table
Following standard Cochrane methodology (Higgins 2011), we created 'Summary of findings' tables for all comparisons:
MI versus no smoking cessation intervention
MI in addition to another smoking cessation treatment versus that smoking cessation treatment alone
MI versus another smoking cessation intervention
Higher intensity MI versus lower intensity MI
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for the smoking cessation outcome, and to draw conclusions about the certainty of the evidence within the text of the review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification; .
Results of the search
Our searches resulted in 1325 records. After duplicates were removed, 1299 records remained for title and abstract screening. We ruled out 1139 records at this stage, leaving 160 for full‐text screening. We identified 37 completed studies, five ongoing studies, one study awaiting classification, and excluded 117 studies at the full‐text screening stage. See Figure 1 for study flow information relating to the most recent search.
1.
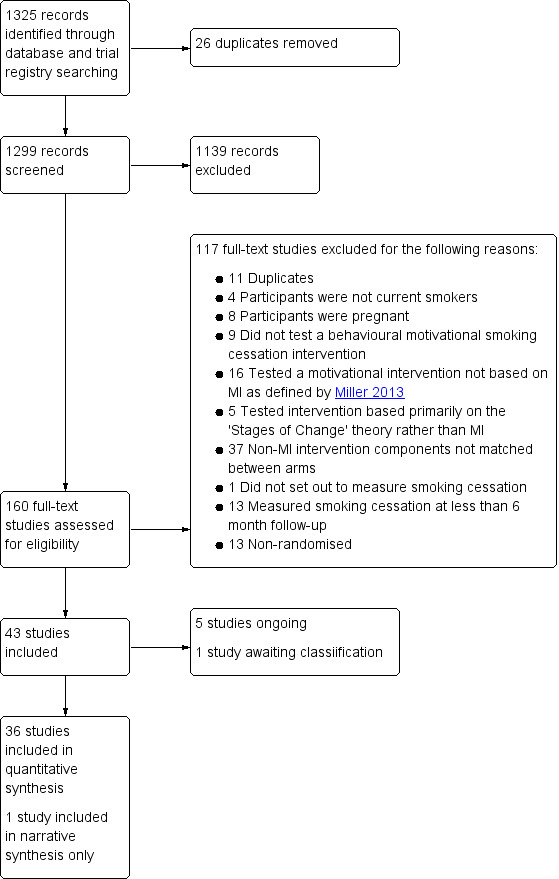
Study flow diagram for this update
Included studies
Included studies
This review includes 37 RCTs, including over 15,000 participants. Trials were conducted in Australia (two studies), Brazil (two studies), the USA (28 studies), China, India, South Africa, Spain and the UK (one study each).
Participants
All participants were tobacco smokers. Eleven of the 37 included studies (Butler 1999; Catley 2016; Cook 2016; Davis 2011; Demétrio Faustino‐Silva 2018; Ellerbeck 2009; Hollis 2007; NCT02645838; Soria 2006; Vidrine 2019; Wu 2009) recruited from the general population, through advertisements, attendance at primary care or other community venues, or through calling a smoking quit‐line. However, the majority of studies in this review recruited from specialist populations:
Adolescents or young people (eight studies; Audrain‐McGovern 2011; Colby 2005; Colby 2012; Harris 2010; Helstrom 2007; Kelly 2006; Tevyaw 2009; Woodruff 2007). One of these studies specifically recruited adolescent offenders (Helstrom 2007). Participants had been arrested or given notice to appear in court for a variety of offences and had been given the option for a diversionary program, but were not incarcerated.
People with substance abuse problems (three studies): Rohsenow 2015 recruited people with a range of substance abuse issues, whereas Rohsenow 2014 specifically recruited people with alcohol dependency and Stein 2006 recruited opoid dependent people receiving methadone treatment.
People attending, or who had attended screening, for smoking‐related cancers (two studies): Marshall 2016 recruited people who were being screened for lung cancer, and McClure 2005 recruited women who had attended for cervical screening, and had been told that they had an elevated risk of cervical cancer.
Patients with a variety of acute health problems (eight studies): In four studies, participants were being treated as hospital inpatients, for unspecified, varied health issues (De Azevedo 2010; Lewis 1998; Sherman 2016) or operative fractures (Matuszewski 2018). In the remaining four studies, patients were attending the emergency department for chest pain (Bock 2008), or receiving outpatient treatment for post traumatic stress disorder (PTSD) (Battaglia 2016), HIV (Lloyd‐Richardson 2009), or tuberculosis (Louwagie 2014).
African‐American/black light smokers: defined as smoking ten or fewer cigarettes per day (Ahluwalia 2006)
Incarcerated men in a prison in India (Naik 2014)
Homeless adults recruited from homeless shelters (Okuyemi 2013)
Friends and family of people who had been diagnosed with lung cancer (Bastian 2013)
People with a low income: defined as primary care patients who were uninsured or receiving healthcare benefits (Bock 2014)
The majority of the included studies (29 of 37) did not recruit participants specifically based on their motivation to quit at baseline, i.e. there was not an eligibility criterion that specified that participants needed to be motivated to quit or not; however five studies only recruited participants motivated to quit (Ahluwalia 2006; Demétrio Faustino‐Silva 2018; Hollis 2007; Lewis 1998; Vidrine 2019) and three studies specifically recruited participants who were not motivated to quit (Catley 2016; Cook 2016; Davis 2011). The studies that recruited people motivated to quit had an eligibility criteria specifying that participants had to be willing to quit smoking within a specific time period (e.g. the next two weeks, within a month); recruited people based on their willingness to receive smoking cessation treatment; or recruited people because they had expressed an interest in quitting. The studies that recruited people not motivated to quit advertised for participants who were not ready to quit smoking; had an eligibility criterion specifying that participants should have no interest in quitting over the next month; or participants were not told that the aim of the study was smoking cessation and, when asked about their quitting plans, were excluded if they said they were ready to quit.
Intervention
Motivational Interviewing (MI)
All of the studies included in this review made explicit reference to using MI principles defined by Miller and Rollnick (as described in Miller 2013). Most studies merely specified that the intervention was carried out according to established MI techniques, rather than providing a more detailed description of counselling content. Three studies reported that the counselling in the intervention arm was based on another theoretical approach in addition to MI: Bastian 2013 combined the principles of MI with adaptive coping skills, and both Lewis 1998 and Vidrine 2019 combined MI with principles of cognitive behavioural therapy (CBT). Another study combined the adolescent participant MI intervention with a parent MI intervention in the intervention arm only (Colby 2012). Researchers discussed participants' quit attempts and supporting it with their parents, using MI principles.This study was borderline for inclusion as one of our eligibility criteria was to exclude studies where extra non‐MI components were not matched between study arms. However, we decided to include this study, as the extra component complied with the principles of MI, and we went on to test whether its exclusion impacted upon the results of meta‐analysis using sensitivity analysis.
MI fidelity monitoring
Twenty‐one of the 37 studies reported that they carried out MI fidelity monitoring during the study to assess whether the principles of MI were adhered to, to improve adherence to the principles, or both (Ahluwalia 2006; Audrain‐McGovern 2011; Bastian 2013; Battaglia 2016; Bock 2008; Bock 2014; Catley 2016; Colby 2005; Colby 2012; Davis 2011; De Azevedo 2010; Ellerbeck 2009; Harris 2010; Hollis 2007; Kelly 2006; Lloyd‐Richardson 2009; Okuyemi 2013; Rohsenow 2014; Rohsenow 2015; Sherman 2016; Tevyaw 2009). This usually comprised one or a range of the following methods: the observation of all or a subset of sessions by clinicians or the study lead; rating sessions on their adherence to MI using fidelity scales, such as the Motivational Interviewing Treatment Integrity (MITI) code (Pierson 2007) or study specific scales; supervision meetings with counselling providers to reflect on practice and learn and improve based on these experiences. Only ten of these 21 studies then went on to report on the results of this fidelity monitoring (Audrain‐McGovern 2011; Catley 2016; Colby 2005; Colby 2012; Davis 2011; Harris 2010; Lloyd‐Richardson 2009; Rohsenow 2014; Rohsenow 2015; Tevyaw 2009). Only one of these studies reported that some of the benchmarks for competency were not widely met (Audrain‐McGovern 2011), and we accounted for this using sensitivity analysis; however, criteria were very close to being met. As fidelity monitoring and benchmarks for fidelity differed across studies, it is plausible that studies that met their own adherence standards may not have met the standards of other studies and vice versa. For further details of fidelity monitoring (where this occurred) see Table 5.
1. Details of MI fidelity monitoring (studies that reported monitoring only).
| Study ID | Details of monitoring | Monitoring results | Fidelity achieved? (defined by individual study parameters) |
| Ahluwalia 2006 | Weekly supervision; subset of sessions rated using MISC | Not reported | n/a |
| Audrain‐McGovern 2011 | Weekly supervision, subset of sessions rated using MITI code | Benchmarks for MI competency (>= 6) approached/achieved for 2 ratings of empathy (mean: 5.2; SD: 0.87) & spirit (mean: 5.9; SD: 0.81) using 7‐point Likert scale. Behavioural counts met benchmarks for proficiency, including ratio of reflections to questions (1.8), percentage of open questions (61%), and MI adherence (96%). 28% of complex reflections approached benchmark for beginning proficiency (40%). | No, in some cases marker did not quite meet the benchmarks set; however were close |
| Bastian 2013 | Each counsellors first 3 sessions monitored & feedback provided; weekly supervision; random sessions rated using MITI code | Not reported | n/a |
| Battaglia 2016 | Random calls observed; nurse participated in ongoing MI training | Not reported | n/a |
| Bock 2008 | Subset of sessions audited using a decisional balance review tool and intervention component checklists | Not reported | n/a |
| Bock 2014 | Subset of sessions reviewed; weekly supervision | Not reported | n/a |
| Catley 2016 | Training continued until counsellors met fidelity criteria for 3 consecutive sessions; subset of sessions rated using MITI code | Mean (SD) global ratings (1–5): Empathy MI = 4.5 (0.6) 95% above criterion, HE = 2.3 (1.2) 24% above criterion, MD = 2.3 (95% CI = 1.8, 2.8). Direction MI = 4.9 (0.4) 97% above criterion, HE = 4.7 (0.8) 95% above criterion, MD = 0.3 (–0.2, 0.8), P = 0.17. Collaboration MI = 4.2 (0.9) 79% above criterion, HE = 2.1 (1.2) 14% above criterion, MD = 2.1 (1.6, 2.5). Evocation MI = 4.4 (0.7) 92% above criterion, HE = 2.3 (1.1) 19% above criterion, MD = 2.2 (1.8, 2.7). Autonomy support MI = 4.3 (0.8) 87% above criterion, HE 2.8 (1.2) 27% above criterion, MD = 1.5 (1.1, 2.0). Giving information (counts): MI = 3.9 (4.8) n/a % above criterion, HE = 12.8 (9.5) n/a % above criterion, MD = 1.2 (95% CI 0.7, 1.7). Reflections: questions (ratio of counts): MI = 3.1 (2.4) 92% above criterion, HE = 0.2 (0.3) 5% above criterion, MD = 1.7 (95% CI 1.2, 2.1). Open‐ended questions (%): MI = 66.0 (27.6) 76% above criterion, HE = 10.5 (11.0) 3% above criterion, MD = 2.6 (95% CI 2.2, 3.1). Complex reflections (%) MI = 53.9 (16.3) 82% above criterion, HE = 19.6 (27.6) 24% above criterion, MD = 1.5 (95% 1.1, 2.0). MI adherent (%) MI = 79.4 (37.9) 71% above criterion, HE = 30.3 (42.6) 22% above criterion, MD = 1.2 (95% CI 0.8, 1.7). MI adherent behaviour counts MI = 2.3 (1.7) n/a % above criterion, HE = 0.8 (1.3) n/a % above criterion, MD = 1.0 (95% 0.5, 1.5). MI non‐adherent behaviour counts MI = 0.2 (0.7) n/a % above criterion, HE = 1.5 (3.0) n/a % above criterion, MD = 0.6 (95% CI 0.1, 1.1) | Yes |
| Colby 2005 | Weekly group supervision; each session rated on scales from 1 (strongly disagree) to 4 (strongly agree) on rapport, counsellor empathy & self‐efficacy enhancement; delivery of 15 essential elements of the protocol were also rated as 0 (topic not introduced), 1 (not at all useful), 2 (somewhat useful), or 3 (very useful) | Participant ratings high for counsellor rapport (M = 3.8, SD = 0.6), empathy (M = 3.5, SD = 0.8), and self‐efficacy enhancement (M = 3.7, SD = 0.7). Participants recalled 94% of essential elements; interventionists reported discussing 98% of the elements. Utility judged high by participants (M = 2.4, SD = 0.5) and interventionists (M = 2.4, SD = 0.3) | Yes |
| Colby 2012 | Weekly supervision; interventionists & adolescent participants rated sessions | Interventionists & adolescents indicated that nearly all 16 MI session components were delivered (M = 15.6, SD = 0.80 and M = 15.4, SD = 1.39 respectively). Interventionists indicated that they provided 10.7 of 12 (SD = 1.78) parent MI components. | Yes |
| Davis 2011 | Sessions reviewed for protocol adherence and discussed at weekly meetings | Two cases did not meet treatment standard. Cases not reaching criterion were removed from the analyses. | Yes |
| De Azevedo 2010 | Fortnightly supervision | n/a | n/a |
| Ellerbeck 2009 | Each session rated on key concepts of MI and specific content of MI protocol; counsellors rated themselves and were rated during supervision using MI markers. | Not reported | n/a |
| Harris 2010 | Counsellors had to demonstrate proficiency in MI; weekly supervision; supervisors rated counsellors' in‐session proficiency on 18 items, including reflective listening, asking permission, and MI spirit; where fidelity scores dropped, additional supervision was provided until they increased or the counsellor was dismissed | Fidelity scores remained high throughout (mean rating of 6.12 (0.87 SD) on the MI‐spirit item) | Yes |
| Hollis 2007 | Calls monitored and rated on adherence | Not reported | n/a |
| Kelly 2006 | Supervision, including a review of each session to reduce content drift/contamination | Not reported | n/a |
| Lloyd‐Richardson 2009 | Supervision on sample of sessions; participant exit interviews; documentation of time spent in intervention; subset of sessions rated on degree of adherence | Content delivered was appropriate, and exceeded SC arm | Yes |
| Okuyemi 2013 | Sessions reviewed during weekly supervision | Not reported | n/a |
| Rohsenow 2014 | Subset of sessions reviewed in weekly supervision, rated for MI style & adherence with feedback given. Rated from 1 (not at all) to 5 (extensively) scale for 5 motivational style measures, and on adequacy of six MI adherence items | Therapist style ratings did not differ across conditions for arguing (on average not at all), but MI therapists showed more empathy, used more reflective listening, supported self‐efficacy more, and emphasised personal responsibility more. MI therapists more likely to discuss topics to increase ambivalence (100% of MI, 4% of BA sessions), provide assessment feedback (100% of MI, 0% of BA sessions), explore barriers (82.1% of MI, 0% of BA sessions), provide summaries (100% of MI, 0% of BA sessions), and discuss possible goals (100% of MI, 14.8% of BA sessions) | Yes |
| Rohsenow 2015 | Subset of sessions reviewed in weekly supervision, rated for MI style & adherence & feedback given. Rated from 1 (not at all) to 5 (extensively) scale for 5 motivational style measures, and on adequacy of six MI adherence items | MI more likely to discuss: ambivalence about smoking (93% of MI, 4% of BA sessions), assessment feedback (100% of MI, 0% of BA sessions), barriers to quitting smoking (100% of MI, 17% of BA sessions), provide summaries (100% of MI, 0% of BA sessions), methods of quitting or preparing to quit (100% of MI, 58% of BA sessions), and possible goals (100% of MI, 14.8% of BA sessions). Therapist style ratings did not differ between conditions for arguing, empathy, or reflective listening, but MI therapists more likely to support self‐efficacy & emphasise personal responsibility. | Yes |
| Sherman 2016 | Subset of calls reviewed & feedback given on MI techniques; weekly supervision | Not reported | n/a |
| Tevyaw 2009 | Participants & counsellors rated which of 15 MET elements and 4 REL elements were completed | Therapists reported covering 14.9 (SD = 0.4) of 15 MET components and 0 (SD = 0.1) of 4 REL components during MET; and all 4 (SD = 0) of the REL components and 0.7 (SD = 0.5) of the MET components during REL. Student ratings reported that therapists covered 14.3 (SD = 1.3) of 15 MET components & 1.6 (SD = 1.5) of 4 REL components during MET sessions. They reported therapists covered 3.3 ( SD = 1.1) of the 4 REL components and 6.3 (SD = 5.3) of the 15 MET components during REL sessions. | Yes |
BA:brief advice HE: Health Education M: mean MD: mean difference MET: Motivational Enhancement Therapy MISC: Motivational Interviewing Skills Code MI: Motivational Interviewing MITI: Motivational Interviewing Treatment Integrity n/a: not applicable REL: muscle relaxation training SC: smoking cessation SD: standard deviation
Pharmacotherapy
Twenty of the 37 studies offered or recommended the use of pharmacotherapy for smoking cessation to all, or a subset of participants, in the study groups of interest for this review. This was typically nicotine replacement therapy (NRT) only (Ahluwalia 2006; Bastian 2013; Bock 2008; Bock 2014; Cook 2016; Hollis 2007; Lloyd‐Richardson 2009; Okuyemi 2013; Rohsenow 2014; Rohsenow 2015; Sherman 2016; Stein 2006; Vidrine 2019; Wu 2009), however one study offered bupropion only (Soria 2006), and some studies provided a choice of pharmacotherapies from two or all three of the following: NRT, varenicline or bupropion (Battaglia 2016; Catley 2016; Ellerbeck 2009; Harris 2010; McClure 2005). In all cases, pharmacotherapy was offered or recommended in all relevant trial arms, and so the use and type of pharmacotherapy was not being tested. Where included studies did have trial arms testing additional components to MI, these study arms were not included in analyses (Ellerbeck 2009; Lewis 1998).
Modality
MI was delivered in face‐to‐face sessions in 17 of the 37 studies; in another 12 studies, the counselling was delivered in a combination of face‐to‐face and telephone sessions, usually with an initial session or sessions conducted face‐to‐face, followed by follow‐up counselling over the phone. Six studies provided counselling over the phone only (Bastian 2013; Battaglia 2016; Ellerbeck 2009; Hollis 2007; McClure 2005; Sherman 2016); a further study had an MI intervention group that received calls and text messages based on CBT and MI and another MI group that received text messages only (Vidrine 2019), and a final study provided MI counselling for adolescents in an online virtual environment (Woodruff 2007). Participants were represented by an avatar in the online world and received MI group counselling with other participants and a counsellor within a virtual shopping mall.
Intensity
Nine studies provided a single session of MI in at least one of the MI intervention groups (Butler 1999; Davis 2011; Helstrom 2007; Kelly 2006; Louwagie 2014; Marshall 2016; Matuszewski 2018; Rohsenow 2014; Vidrine 2019); the number of sessions offered ranged from one to 12 across studies. Some studies had more than one MI intervention group of different intensities (Ellerbeck 2009; Hollis 2007; Matuszewski 2018; Rohsenow 2014; Sherman 2016; Vidrine 2019). These studies compared a lower intensity MI intervention comprised of one to two sessions to a higher intensity MI intervention which ranged from two to 11 sessions. The total duration of MI interventions varied greatly across studies, from five minutes to 315 minutes; however length of sessions was not reported in a minority of cases. For further detail on the content and intensity of interventions, see Table 6.
2. Details of intervention & comparator content & intensity.
| Study ID | MI intervention description | Intervention intensity (no. of sessions; total duration) | Non‐MI comparator description | Comparator intensity | Intensity matched? | Pharmacotherapy used? | Other common intervention components |
| Ahluwalia 2006 | MI counselling using semi‐structured script | 6 sessions; 2 h | SC counselling providing information & advice to develop a quit plan | 6 sessions; 2 h | Yes | NRT in half each condition; placebo NRT in other half | Tailored smoking cessation booklet |
| Audrain‐McGovern 2011 | MET counselling | 5 sessions; 3 h 15 min | Structured brief advice, using '5As' or '5Rs' | 5 sessions; 1 h 15 min | No, comparator lower | n/a | n/a |
| Bastian 2013 | MI & adaptive coping skills counselling + self‐directed materials. Skills training informed by Transactional Model of Stress & Coping | 6 sessions; 3 h | Self‐help materials ‐ letter from oncologist encouraging quitting, quit kit (including SC guide), individually tailored booklet | n/a | No, comparator lower | NRT | Self‐help materials |
| Battaglia 2016 | MI counselling + written SC information | 12 sessions; 3 h 20 min | PTSD home telehealth programme + electronic (Health Buddy) device | n/a | No, comparator lower | NRT, bupropion or varenicline | PTSD home telehealth programme + electronic (Health Buddy) device |
| Bock 2008 | MI counselling + self help resources | 5 sessions; 1 h 20 min | Non‐MI counselling calls + self‐help resources | 2 sessions; 20 min | No, comparator lower | NRT | 2 brief non‐MI counselling calls, review of NRT use instructions |
| Bock 2014 | MI counselling + 5As intervention | 3 sessions; approx 1 h | 5As intervention | 1 session; 5 min | No, comparator lower | NRT | 5As intervention |
| Butler 1999 | Brief MI session | 1 session; 10 min | Brief SC advice | 1 session; 2 min | No, comparator lower | n/a | n/a |
| Catley 2016 | MI counselling | 4 sessions; length unclear | 1. brief advice 2. SC counselling based on clinical guidelines |
1. 1 session 2. 4 sessions (lengths unclear) | Yes (health education intensity matched) & No (brief advice ‐ lower) | NRT or varenicline | Self‐help guide |
| Colby 2005 | MI counselling | 2 sessions; 50 min | Brief recommendation to quit + follow‐up call | 2 sessions; 10 min | No, comparator lower | n/a | SC pamphlet + treatment referrals information |
| Colby 2012 | MI counselling. Participants' parents also discussed child's quit attempt and supporting it with researchers, using MI principles. | 2 sessions for adolescents + 1 for parents; 1 h adolescents; 15 min parents | Brief SC advice with follow‐up session | 2 sessions for adolescents; 10 min (none for parents) | No, comparator lower | n/a | SC pamphlet + treatment referrals information |
| Cook 2016 | MI counselling | 4 sessions; 50 min | 1. Behavioural smoking reduction guidance 2. No treatment |
1. 7 sessions; 1 h 20 min. 2. No sessions |
1. No, higher 2. No, lower |
NRT dependent on trial arm (balanced across arms of interest) | n/a |
| Davis 2011 | Mi counselling | 1 session; 15 min | Prescriptive interview regarding smoking ‐ firm & authoritative | 1 session; 15 min | Yes | n/a | n/a |
| De Azevedo 2010 | MI counselling | 8 sessions; 1 h 40 min | Brief SC advice | 1 session; 15 min | No, comparator lower | n/a | n/a |
| Demétrio Faustino‐Silva 2018 | MI taught to SC advisors as additional resource to standard CBT approach used | Average 3 sessions; length unclear | Standard CBT approach advocated by Brazilian Ministry of Health's smoking programme | Average 3 sessions, length unclear | Yes | n/a | CBT counselling approach |
| Ellerbeck 2009 | 1. High intensity MI 2. Moderate intensity MI |
1. 6 sessions every 6 min; length unclear 2. 2 sessions every 6 min; length unclear |
n/a | n/a | n/a | NRT or bupropion | Welcome letter, information about medication, smoking cessation pamphlets, 6‐monthly personalised newsletter, periodic progress reports with counselling suggestions faxed to participants' physicians |
| Harris 2010 | MI counselling | 4 sessions; average 1 h 40 min | MI counselling focused on increasing fruit & vegetable consumption | 4 sessions; average 1 h 40 min | n/a | Pharmacotherapy for highly dependent smokers | n/a |
| Helstrom 2007 | MET counselling | 1 session; length unclear | Information session on tobacco use based on American Cancer Society pamphlet | 1 session; length unclear | Yes | n/a | n/a |
| Hollis 2007 | 1. high intensity MI 2. moderate intensity MI 3. low intensity MI |
1. 5 sessions; at least 1 h 2. 2 sessions; at least 45 min 3. 1 session; 15 min |
n/a | n/a | n/a | NRT for half of participants in each group | “Quit kit” including SC booklet |
| Kelly 2006 | MI counselling | 1 session; 1 h | SC counselling based on psychoeducation model | 1 session; 1 h | Yes | n/a | Written materials |
| Lewis 1998 | Brief motivational message to quit smoking, with follow‐up counselling incorporating CBT & MI | 5 sessions; approx 1 h | Brief motivational message to quit smoking + SC pamphlet | 1 session; 3 min | No, comparator lower | Placebo NRT | Brief motivational message to quit + SC pamphlet |
| Lloyd‐Richardson 2009 | MET counselling | 5 sessions; at least 2 h | Brief assessment of quitting plans with brief in‐person follow‐up | 2 sessions; approx 10 m | No, comparator lower | NRT | n/a |
| Louwagie 2014 | Brief MI session + short standardised SC message + SC booklet | 1 session; 15‐20 min | Short standardised SC message + SC booklet | 1 session; 1 min | No, comparator lower | n/a | Short SC message + SC booklet |
| Marshall 2016 | MI counselling session + audio quit material + printed materials + quit‐line details | 1 session; approx 25 min | Non‐tailored printed materials + quit‐line details | n/a | No, comparator lower | n/a | Written materials + quit‐line details |
| Matuszewski 2018 | 1. MI counselling + control intervention 2. MI counselling intervention + additional brief follow‐up |
1. 1 session; average 10 min 2. 2 sessions; average 15 min |
Referral to patient resource centre + quit‐line brochure | n/a | No, comparator lower | n/a | Referral to patient resource centre + quit‐line brochure |
| McClure 2005 | MET counselling + control intervention | 4 sessions; 1 h | Letter explaining association between cervical cancer & smoking + SC booklet + quit‐line details | n/a | No, comparator lower | NRT or bupropion | Letter explaining association between cervical cancer & smoking, SC booklet, quit‐line details |
| Naik 2014 | MI counselling | not reported | No intervention (waiting list control) | n/a | n/a | n/a | n/a |
| NCT02645838 | MI counselling | 7 sessions; unclear‐ over 20 min | Brief SC advice | 1 session; 5 min | No, comparator lower | n/a | n/a |
| Okuyemi 2013 | MI SC and NRT adherence counselling | 6 sessions; average 1h 45 min | Brief smoking cessation advice + SC guide | 1 session; 10‐15 min | No, control lower | NRT | SC guide |
| Rohsenow 2014 | 1. MI counselling session 2. MI counselling session + booster sessions |
1. 1 session; 45 min 2. 3 sessions 1h 5min |
1. Brief advice using US AHRQ method 2. Brief advice + 2 booster sessions |
1. 1 session; 15 min 2. 3 sessions; 35 min |
No, comparator lower | NRT | SC pamphlets, information on SC skills groups & hard candy |
| Rohsenow 2015 | MI session + booster sessions (half received non contingent payments & half contingent payments) | 4 sessions; approx 2 h | Brief advice using US AHRQ methods (half received non contingent payments & half contingent payments) | 4 sessions; approx 55 min | No, comparator lower | NRT | SC pamphlets & hard candy |
| Sherman 2016 | 1. MI & Problem Solving Therapy counselling 2. Referral to state Quitline (usually New York state) ‐ counsellors trained in MI |
1. 7 sessions; approx 1 h 30 min 2. 2 sessions; approx 30 min |
n/a | n/a | n/a | NRT | n/a |
| Soria 2006 | MI counselling | 3 sessions; 60 min | Brief anti‐smoking advice | 1 session; 3 min | No, comparator lower | Bupropion for highly dependent smokers | n/a |
| Stein 2006 | MI counselling | 3 sessions; approx 1h | Brief advice using 4As + self‐help materials | 2 sessions; approx 5 min | No, comparator lower | NRT | n/a |
| Tevyaw 2009 | MET (half received non contingent payments & half contingent payments) | 3 sessions; 2 h | Progressive muscle relaxation training (half received non contingent payments & half contingent payments) | 3 sessions; 2 h | n/a | n/a | n/a |
| Vidrine 2019 | 1. Brief advice + text messages based on CBT & MI 2. Brief advice + texts + counselling calls based on CBT & MI |
1. 1 session; approx 5 min. 2. 11 sessions; approx 2 h |
Brief SC advice | 1 session; approx 5 min | No, comparator lower | NRT | Brief quitting advice, written materials, quit‐line details |
| Woodruff 2007 | Online virtual world (The Breathing Room) ‐ participants' avatars had MI group counselling with other participants & the counsellor within a virtual shopping mall setting | 7 sessions; 5 h 15 min | No treatment | n/a | n/a | n/a | n/a |
| Wu 2009 | MI counselling + SC self‐help materials | 4 sessions; 4 h | Health education counselling + self‐help materials covering nutrition, exercise, and tobacco use | 4 sessions; 4 h | Yes | NRT | n/a |
5As: 'Ask, Advise, Assess, Assist, and Arrange'
5Rs: 'Relevance, Risks, Rewards, Roadblocks, Repetition' AHRQ: Agency for Healthcare Research and Quality approx: approximately CBT: Cognitive Behavioural Therapy h: hour(s)
m: month(s) min: minute(s) MI: Motivational interviewing MET: Motivational Enhancement Therapy n/a: not applicable NRT:nicotine replacement therapy PTSD: post traumatic stress disorder SC: smoking cessation
Provider
MI was delivered by physicians (Butler 1999; Marshall 2016; NCT02645838; Soria 2006), nurses (Battaglia 2016; Davis 2011; Lewis 1998), counsellors/psychologists (Ahluwalia 2006; Audrain‐McGovern 2011; Bastian 2013; Bock 2008; Bock 2014; Catley 2016; Colby 2005; Colby 2012; Cook 2016; Ellerbeck 2009; Harris 2010; Kelly 2006; Lloyd‐Richardson 2009; McClure 2005; Okuyemi 2013; Rohsenow 2014; Rohsenow 2015; Sherman 2016; Stein 2006; Tevyaw 2009; Vidrine 2019; Woodruff 2007; Wu 2009), some of whom were described as specialist smoking cessation advisors (De Azevedo 2010; Demétrio Faustino‐Silva 2018; Hollis 2007; Matuszewski 2018), and lay healthcare workers (Louwagie 2014). Helstrom 2007 and Naik 2014 did not specify the type of provider delivering support.
Comparator
We grouped studies dependent on the nature of the relevant comparator. Comparators either consisted of no smoking cessation interventions (Cook 2016; Harris 2010; Naik 2014; Tevyaw 2009; Woodruff 2007), a non‐MI smoking cessation intervention (Ahluwalia 2006; Audrain‐McGovern 2011; Bastian 2013; Battaglia 2016; Bock 2008; Bock 2014; Butler 1999; Catley 2016; Colby 2005; Colby 2012; Cook 2016; Davis 2011; De Azevedo 2010; Demétrio Faustino‐Silva 2018; Helstrom 2007; Kelly 2006; Lewis 1998; Lloyd‐Richardson 2009; Louwagie 2014; Marshall 2016; Matuszewski 2018; McClure 2005; NCT02645838; Okuyemi 2013; Rohsenow 2014; Rohsenow 2015; Soria 2006; Stein 2006; Tevyaw 2009; Vidrine 2019; Wu 2009), or another MI intervention of lower intensity (Ellerbeck 2009; Hollis 2007; Matuszewski 2018; Rohsenow 2014; Sherman 2016; Vidrine 2019). Some studies had multiple study arms and so fell into more than one category. We further split the studies with a non‐MI smoking cessation intervention into two groups ‐ those where the MI interventions stood alone and were directly compared with the other cessation interventions, and those where the intervention groups received the MI interventions in addition to the non‐MI smoking cessation interventions, which were also offered in the comparator groups.
No smoking cessation treatment comparator
Three of the five studies that compared MI to no smoking cessation treatment provided no intervention (Cook 2016; Naik 2014; Woodruff 2007). Participants were simply followed up to assess the outcome. Naik 2014 offered participants in the comparator the opportunity to receive the MI intervention following the initial treatment period. Two of the studies provided participants with a 'dummy' intervention designed to match the intensity of the MI smoking cessation intervention. In Harris 2010, this was MI counselling focussed on increasing participants' fruit and vegetable consumption; both study arms received counselling over four sessions for an average duration of 100 minutes. In Tevyaw 2009, participants in the comparison group received 'progressive muscle relation training' over three sessions for an overall duration of 120 minutes.
Non‐MI smoking cessation intervention comparator
In the minority of cases (7 of 31; Ahluwalia 2006; Catley 2016; Davis 2011; Demétrio Faustino‐Silva 2018; Helstrom 2007; Kelly 2006; Wu 2009), the comparator group (or one of the comparator groups in the study) received smoking cessation counselling that was matched in intensity to the MI counselling in the intervention group. This was either described simply as smoking cessation counselling with information giving or advice, or as a specific approach, i.e. prescriptive interviewing (Davis 2011), CBT (Demétrio Faustino‐Silva 2018), or the psychoeducation model (Kelly 2006). Wu 2009 provided participants in the comparator group with general health education counselling, which covered smoking cessation, as well as nutrition and exercise.
In most cases, the support provided in the comparator group was of lower intensity than the MI intervention arm and consisted of brief advice on cessation, self‐help materials (such as printed materials and contact details for smoking cessation services or quit‐lines), or both. In one study, one of the comparator interventions was more intensive than the MI intervention (Cook 2016). Cook 2016 was a 16‐arm factorial trial where some study arms were provided with a behavioural smoking reduction intervention. This reduction intervention was delivered over seven sessions with a total duration of 80 minutes, whereas the MI intervention was delivered over four sessions with a total duration of 50 minutes.
Two studies offered half of their participants payments contingent on them being abstinent from smoking (Rohsenow 2015; Tevyaw 2009). In both cases, these contingency payments were matched in the intervention arm.
Outcomes
The majority of studies measured cessation at six months follow‐up (25 of 37); however, nine studies measured cessation at 12 months follow‐up (Bastian 2013; Bock 2014; Hollis 2007; Marshall 2016; McClure 2005; Rohsenow 2014; Rohsenow 2015; Soria 2006; Woodruff 2007), and one study each measured cessation at nine months (Battaglia 2016), 11 months (Demétrio Faustino‐Silva 2018) and 24 months (Ellerbeck 2009) follow‐up. It was possible to use biochemically validated (using expired carbon monoxide or urinary/salivary cotinine) cessation rates for 22 of the 37 studies. We were unsure whether the rates reported in Naik 2014 and Demétrio Faustino‐Silva 2018 were biochemically verified. The Naik 2014 study report stated that carbon monoxide was measured; however, it was unclear whether this was used to motivate participants, verify cessation rates, or both.
Only one study measured one of our secondary outcomes ‐ mental health. Battaglia 2016 recruited veterans with PTSD attending Veterans Health Administration (VHA) healthcare clinics and investigated the effect of integrating MI smoking cessation counselling into the standard telehealth programme already provided (which included access to pharmacological and behavioural smoking cessation treatments). Throughout the study, PTSD symptoms were assessed using the PTSD Checklist (range of 17 to 85 with a score > 50, indicating PTSD diagnosis), depression was monitored using the 15‐item Geriatric Depression Scale‐Short Form (GDS‐SF), where a score greater than 6 indicated probable depression, and suicidal thoughts were assessed every 30 sessions via a single question. Some of the other studies measured markers of mental health or well‐being at baseline or reported mental health at follow‐up overall; however, only Battaglia 2016 measured mental health at follow‐up and presented the results by study group.
Excluded studies
We listed 117 studies that were potentially relevant but excluded, with reasons, in the Characteristics of excluded studies table. Reasons that studies were excluded at full‐text stage are also summarised in Figure 1. The reason why most studies were excluded at full‐text screening stage was because the intervention group received non‐MI intervention components that were not included in the comparator arm, such as pharmacotherapy, a text messaging intervention, or incentives.
We also classified five studies as ongoing (Lloyd‐Richardson 2003; NCT01387516; NCT02905656; NCT03002883; Salgado Garcia 2018), which are likely to be relevant for inclusion once completed and/or reported. We classified Zhou 2014 as 'awaiting classification' as only a conference abstract was available and it was impossible to determine from this whether smoking cessation was definitely measured (reduction in cigarette consumption was reported) and at what time points. Attempts to contact the authors were unsuccessful.
Risk of bias in included studies
Full details of 'Risk of bias' assessments are given for each trial within the Characteristics of included studies tables. Overall, we judged four studies to be at low risk of bias (low risk of bias across all domains), 11 at high risk of bias (high risk of bias in at least one domain), and the remaining 22 at unclear risk of bias. A summary illustration of the 'Risk of bias' profile across trials is shown in Figure 2.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We assessed selection bias through investigating methods of random sequence generation and allocation concealment for each study. We rated 15 studies as having low risk for random sequence generation, and the remaining 22 as having unclear risk. We judged nine studies to be at low risk for allocation concealment, 27 at unclear risk, and one study at high risk (Woodruff 2007). Woodruff 2007 was judged as having high risk as clusters were randomised to treatments, and study personnel knew which condition a cluster was in before participant recruitment began. Recruitment was then tailored to this, using different recruitment materials dependent on assigned condition. This meant that participants may not have been equivalent across groups. We judged studies as having unclear risk of bias when authors provided insufficient information about methods used.
Outcome assessment (detection bias)
We did not formally assign a risk of performance bias for each trial. It is almost always impossible to blind providers of behavioural support to treatment allocation. Moreover, nonspecific effects of being in treatment are part of the intervention effect that studies were aiming to assess.
We judged detection bias on the basis of biochemical validation and, where biochemical validation was not provided, on the basis of differential levels of contact between participants and the study team across relevant study groups. We judged ten studies to be at high risk of detection bias as outcomes were defined as self‐report only and the intervention and control arms received different levels of support, making differential misreporting possible (Bastian 2013; Bock 2008; Cook 2016; De Azevedo 2010; Hollis 2007; Kelly 2006; Marshall 2016; Sherman 2016; Vidrine 2019; Woodruff 2007). We judged two studies to be at unclear risk of detection bias (Demétrio Faustino‐Silva 2018; Naik 2014) as we were unsure whether the rates reported were biochemically verified. We judged the remaining 25 studies to be at low risk of detection bias.
Incomplete outcome data
We judged studies to be at a low risk of attrition bias where the numbers of participants lost to follow‐up were clearly reported, the overall number lost to follow‐up was not more than 50%, and the difference in loss to follow‐up between groups was no greater than 20%. This is in accordance with 'Risk of bias' guidance produced by the Cochrane Tobacco Addiction Group for assessing smoking cessation studies. We judged 29 of the studies to be at low risk of bias, six at unclear risk (Lewis 1998; Matuszewski 2018; McClure 2005; Naik 2014; Stein 2006; Woodruff 2007) and two at high risk (Bastian 2013; Bock 2014). These two studies were judged to be at high risk because overall loss to follow‐up was more than 50%. Judgements of unclear risk were made either because information on follow‐up was not reported in the sources available to us (Lewis 1998; Matuszewski 2018; McClure 2005; Naik 2014), or because loss to follow‐up was reported for the relevant time point overall, but not split by study group (Stein 2006; Woodruff 2007).
Other potential sources of bias
Two sources of other bias were identified for two of the included studies (Cook 2016; Naik 2014). The comparator intervention in Naik 2014 was a 'waiting list' to receive the MI intervention treatment following the intervention group (verified through contact with author); however, it was unclear whether participants knew that they were on a waiting list. We contacted the authors a second time to verify whether the intervention was delivered to the comparator group after the six‐month assessment time point and received no further reply. However, the quit rates were much higher in the intervention group than in the comparator group (48/300 and 6/300, respectively), suggesting that this was the case. Due to this uncertainty, we have assigned this study a rating of unclear risk for 'other potential sources of bias'. Cook 2016 was a factorial trial with four factors: 1) MI/no MI; behavioural reduction counselling/no behavioural reduction counselling; nicotine gum/no nicotine gum; and nicotine patch/no nicotine patch. The authors reported an unexpected interaction between MI and nicotine gum, where the combination of the two resulted in lower quit rates than any other interventions or combinations. As a result, we have assigned Cook 2016 a rating of high risk of other bias. For details of how data from Cook 2016 have been entered into meta‐analyses, see the Characteristics of included studies table.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Motivational interviewing compared with no treatment for smoking cessation.
| Motivational interviewing compared with no treatment for smoking cessation | ||||||
| Patient or population: tobacco smokers (adolescents, university students, adult primary care patients) Setting: high schools, university & primary care (USA) Intervention: motivational interviewing Comparison: no smoking cessation treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with MI | |||||
| Smoking cessation at ≥ 6 months follow‐up | Study population | RR 0.84 (0.63 to 1.12) | adjusted N = 684 (4 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | One eligible study (Naik 2014) has been excluded from this pooled analysis as it recruited a substantially different population (incarcerated men) compared with the other studies, which recruited adults and adolescents from the general population. When included in the analysis, it resulted in substantial heterogeneity ‐ removal of Naik 2014 decreased statistical heterogeneity to zero. | |
| 22 per 100 | 19 per 100 (14 to 25) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level as all studies were at high or unclear risk of bias; removing the studies at high risk changed the direction of the effect estimate so that it favoured MI, however the CIs still spanned one and suffered substantial imprecision
2 Downgraded one level due to imprecision: the upper and lower limits of the confidence intervals included both meaningful benefit and harm, and the overall number of events was low (n = 144)
Summary of findings 2. Motivational interviewing in addition to other smoking cessation treatment for smoking cessation.
| Motivational interviewing in addition to other smoking cessation treatment for smoking cessation | ||||||
| Patient or population: tobacco smokers (general population, low income, inpatients and outpatients with mixed diagnoses) Setting: community, hospital, healthcare clinics (Australia, Brazil, South Africa, USA) Intervention: motivational interviewing in addition to other smoking cessation (SC) treatment Comparison: other smoking cessation treatment alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other SC treatment only | Risk with MI in addition to other SC treatment | |||||
| Smoking cessation at ≥ 6 months follow‐up | Study population | RR 1.07 (0.85 to 1.36) | adjusted N = 4167 (12 RCTs) | ⊕⊕⊝⊝ LOW 1, 2, 3 | ||
| 15 per 100 | 16 per 100 (13 to 20) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Five studies judged to be at high risk of bias, however sensitivity analysis suggested this is unlikely to impact on the result ‐ not downgraded
2 Downgraded one level due to inconsistency: study effects differed across studies, demonstrated by moderate unexplained statistical heterogeneity (I2 = 47%)
3 Downgraded one level due to imprecision: the upper and lower limits of the confidence intervals included both meaningful benefit and harm
Summary of findings 3. Motivational interviewing compared with another smoking cessation intervention for smoking cessation.
| Motivational interviewing compared with another smoking cessation intervention for smoking cessation | ||||||
| Patient or population: tobacco smokers (general population, adolescents, offenders, homeless, substance users, hospital inpatients, HIV‐positive) Setting: community, universities, homeless shelters, inpatient and outpatient healthcare clinics, primary care (Australia, Brazil, China, Spain, UK, USA) Intervention: motivational interviewing Comparison: another SC intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other SC intervention | Risk with MI | |||||
| Smoking cessation at ≥ 6 months follow‐up | Study population | RR 1.24 (0.91 to 1.69) | 5192 (19 RCTs) | ⊕⊕⊝⊝ LOW 1, 2, 3 | ||
| 9 per 100 | 11 per 100 (8 to 15) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Three studies judged at high risk of bias, however sensitivity analysis suggested this was unlikely to impact on the result ‐ not downgraded
2 Downgraded one level due to inconsistency: study effects differ across studies, demonstrated by moderate unexplained statistical heterogeneity (I2 = 54%)
3 Downgraded one level due to imprecision: the upper and lower limits of the confidence intervals included both meaningful benefit and harm
Summary of findings 4. Higher compared with lower intensity motivational interviewing for smoking cessation.
| Higher compared with lower intensity motivational interviewing for smoking cessation | ||||||
| Patient or population: tobacco smokers (general population, hospital inpatients with mixed diagnoses) Setting: community‐based telephone quit‐line, primary care, hospital, inpatient substance abuse treatment centre (USA) Intervention: higher intensity motivational interviewing Comparison: lower intensity motivational interviewing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lower intensity MI | Risk with higher intensity MI | |||||
| Smoking cessation at ≥ 6 months follow‐up | Study population | RR 1.23 (1.11 to 1.37) | adjusted N = 5620 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 17 per 100 | 21 per 100 (19 to 23) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to risk of bias: three of the five studies were judged to be at high risk of bias and removing these studies in a sensitivity analysis changed the interpretation of the effect, so that the confidence intervals encompassed both appreciable benefit and harm of higher intensity motivational interviewing for smoking cessation
MI versus no smoking cessation treatment (comparison 1)
Smoking cessation outcome
We pooled five studies, including an adjusted N of 1284 (adjusted for clustering in one study ‐ Harris 2010), comparing an MI smoking cessation intervention with no smoking cessation treatment. However, heterogeneity was substantial (I2 = 87%; Analysis 1.1), and so we did not deem it appropriate to present the pooled result of this analysis. Examining the forest plots, individual RRs and 95% CIs provided evidence that this heterogeneity was due to the large positive effect of MI in Naik 2014 (RR 8.00; 95% CI 3.48 to 18.41; N = 600). This was confirmed by a sensitivity analysis removing Naik 2014 (Analysis 1.2; I2 = 0%). None of the four remaining studies (Cook 2016; Harris 2010; Tevyaw 2009; Woodruff 2007) demonstrated a clear benefit of MI, as the confidence intervals spanned both clinical benefit and harm (pooled RR 0.84, 95% CI 0.63 to 1.12; I2 = 0%; adjusted N = 684). The heterogeneity introduced by Naik 2014 can potentially be explained by the nature of the population recruited, which differs substantially to the populations studied in Cook 2016, Harris 2010, Tevyaw 2009 and Woodruff 2007. Naik 2014 recruited incarcerated male smokers and, as a result, took place in a prison setting where participants were potentially unable to drop out, and also very unlikely to try to quit smoking in the no treatment group; whereas Cook 2016 recruited adults in a primary care setting, Harris 2010 and Tevyaw 2009 recruited young college and university students (aged 18 to 24 years) and Woodruff 2007 recruited adolescents (aged 14 to 19 years).
1.1. Analysis.
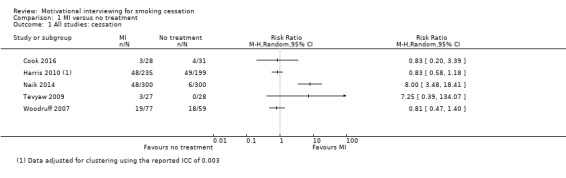
Comparison 1 MI versus no treatment, Outcome 1 All studies: cessation.
1.2. Analysis.
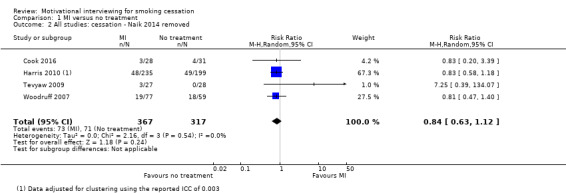
Comparison 1 MI versus no treatment, Outcome 2 All studies: cessation ‐ Naik 2014 removed.
Harris 2010 cluster‐randomised 30 university fraternities and sororities rather than individuals. They reported an ICC of 0.003, allowing us to adjust for this in our analysis. Cook 2016 was a four factor, 16‐arm, factorial RCT included in this comparison, as well as comparisons 2 and 3. Please refer to the Characteristics of included studies table for full details of how this study was included in all analyses.
Removing the two studies judged to be at high risk of bias in the pooled analysis (Cook 2016; Woodruff 2007) changed the direction of the pooled estimate so that it was in favour of motivational interviewing; however, confidence intervals still incorporated evidence of both benefit and harm, and so this did not change our interpretation of the result. The estimate resulting from this sensitivity analysis should be treated with caution as it was based on only two studies (Harris 2010; Tevyaw 2009) and there was substantial imprecision due to a paucity of participants and events (RR 1.50, 95% CI 0.22 to 10.14; I2 = n/a; adjusted N = 434).
We did not carry out any subgroup analyses on the presented pooled analysis (Analysis 1.2) as there were insufficient data to draw meaningful conclusions.
Mental health and QoL outcomes
None of the studies relevant to this comparison measured mental health or QoL at any follow‐up, by study group.
MI in addition to another smoking cessation treatment versus that smoking cessation treatment alone (comparison 2)
Smoking cessation outcome
We pooled twelve studies comparing a smoking cessation intervention supplemented by MI with the same smoking cessation intervention without the MI component (Analysis 2.1). This resulted in a pooled RR of 1.07 (95% CI 0.85 to 1.36; adjusted N = 4167). The point estimate suggests a small potential benefit of MI when offered in addition to other smoking cessation treatment; however, CIs spanned one and moderate heterogeneity was detected (I2 = 47%).
2.1. Analysis.
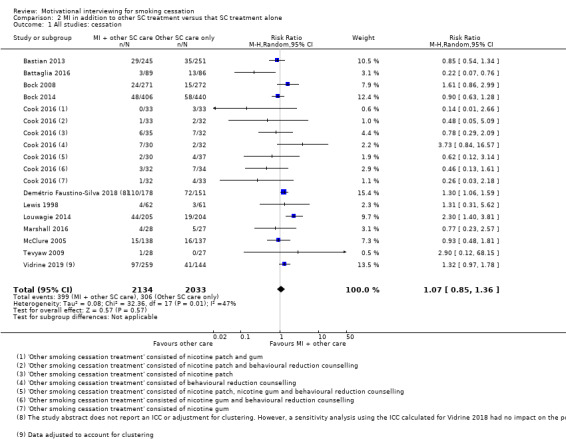
Comparison 2 MI in addition to other SC treatment versus that SC treatment alone, Outcome 1 All studies: cessation.
Two cluster RCTs were included in the analysis; Vidrine 2019 randomised neighbourhood sites and conducted adjusted analyses, accounting for the type of site (church, housing complex or community centre) and the individual site (46 sites). This allowed us to calculate an ICC of 0.06 and adjust for this in our analysis. We were unsure whether Demétrio Faustino‐Silva 2018 carried out adjustment for clustering and an ICC was not reported. As a result, we entered the data from the abstract into our main analysis and performed a sensitivity analysis replacing this data with data adjusted for the ICC calculated for Vidrine 2019 (0.06). This sensitivity analysis had no effect on the interpretation of the result (RR 1.06, 95% CI 0.83 to 1.36; I2 = 46%; adjusted N = 3965).
We also carried out sensitivity analyses removing three studies with interventions based on other theoretical approaches alongside MI (Bastian 2013 ‐ MI + Adaptive coping skills; Lewis 1998 ‐ MI + CBT; Vidrine 2019 ‐ MI + CBT), and removing six studies judged to be at high risk of bias (Bastian 2013; Bock 2008; Bock 2014; Cook 2016; Marshall 2016; Vidrine 2019). In neither case did this significantly affect the interpretation of the result, as confidence intervals continued to encompass both harm and benefit (RR 1.02; 95% CI 0.75 to 1.40; I2 = 53%; N = 3145; and RR 1.17; 95% CI 0.71 to 1.93; I2 = 65%; N = 1366, respectively).
Intensity of the comparator
We split the twelve included studies into two groups dependent on whether the intensities of the interventions provided to the MI groups and the comparator groups were matched, or whether the intensity of the intervention received by the MI group exceeded that in the comparator group (Analysis 2.2). One study matched the intensity of the intervention and comparator treatments (Demétrio Faustino‐Silva 2018), and provided evidence of an effect of MI. Both groups received the standard CBT‐based smoking cessation support advocated by the Brazilian Ministry of Health, but the intervention providers in the MI arm were also taught MI as an additional resource to use in their treatment sessions. However, in most studies the intervention provided in the comparator group was of a lower intensity, as the MI intervention was being offered as an additional element to a standard smoking cessation intervention. These eleven studies resulted in a pooled estimate of 1.01, with CIs encompassing both harm and benefit of MI in addition to other smoking cessation treatment. There was no evidence of statistically significant subgroup differences (I2 = 47.3%, P = 0.17).
2.2. Analysis.
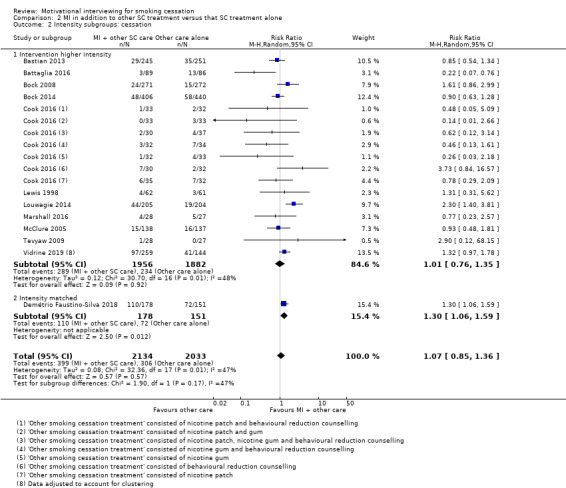
Comparison 2 MI in addition to other SC treatment versus that SC treatment alone, Outcome 2 Intensity subgroups: cessation.
Intervention provider
Interventions were provided by physicians (one study; N = 55), nurses (two studies; N = 298), counsellors (including those specifically trained as smoking cessation advisors; eight studies; N = 3405), or lay healthcare workers (one study; N = 409). When studies were split into these groups, there was evidence of moderate subgroup differences (I2 = 66.2%, P = 0.03); with some evidence of a benefit of MI when delivered by lay healthcare workers (Analysis 2.3). However, the number of participants and events in most subgroups were low, resulting in imprecise effects, which should be treated with caution.
2.3. Analysis.

Comparison 2 MI in addition to other SC treatment versus that SC treatment alone, Outcome 3 Provider subgroups: cessation.
Counselling modality
We grouped studies into those where the intervention was delivered either wholly or partially face‐to‐face and those where none of the intervention was delivered face‐to‐face (Analysis 2.4). Eight studies involved face‐to‐face contact and four no face‐to‐face contact (interventions were delivered either solely by telephone or via telephone and text message). There was very little heterogeneity between subgroup effects (I2 = 4.1%, P = 0.31).
2.4. Analysis.

Comparison 2 MI in addition to other SC treatment versus that SC treatment alone, Outcome 4 Counselling modality subgroups: cessation.
MI fidelity monitoring
Some included studies reported study mechanisms to monitor or ensure the fidelity of the MI intervention, or both; therefore, we split those that did and did not into separate subgroups (Analysis 2.5). For this comparison, five of the 12 studies reported that they had used a form of fidelity monitoring. There was no evidence of statistically significant heterogeneity between subgroups (I2 = 16.9%, P = 0.27). The point estimate favoured the comparator in the studies that included fidelity monitoring and favoured MI in those that did not include fidelity monitoring; however, in both cases, CIs incorporated both potential benefit and harm of the intervention. We planned a sensitivity analysis to test the effect of removing studies that monitored fidelity and discovered fidelity to the principles of MI was low; however, only one of the five studies that reported they carried out monitoring reported on the results, and fidelity was deemed to be high (Table 5).
2.5. Analysis.
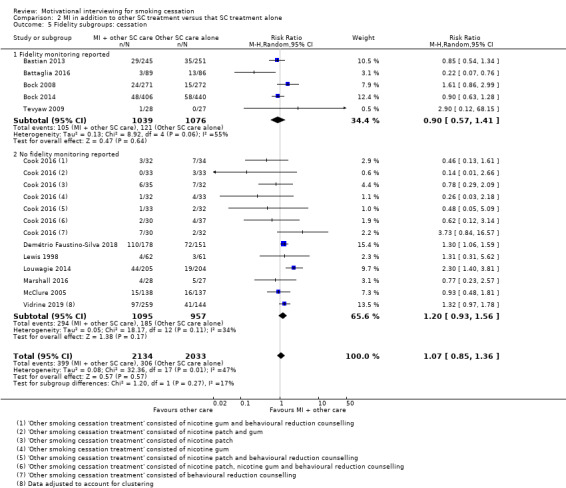
Comparison 2 MI in addition to other SC treatment versus that SC treatment alone, Outcome 5 Fidelity subgroups: cessation.
Baseline participant motivation
Three studies in this comparison recruited participants who were already motivated to quit smoking at baseline, one recruited participants who were not motivated to quit, and the remaining eight studies recruited people regardless of motivation to quit. Although there was some heterogeneity between subgroups this did not reach statistical significance (I2 = 47.6%, P = 0.15) (Analysis 2.6).
2.6. Analysis.
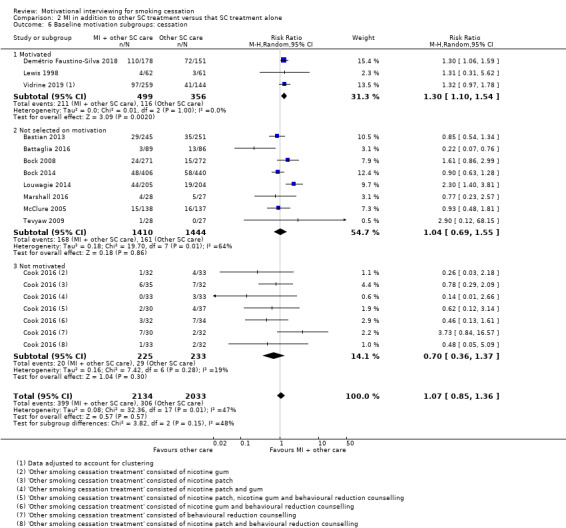
Comparison 2 MI in addition to other SC treatment versus that SC treatment alone, Outcome 6 Baseline motivation subgroups: cessation.
Age of participants
We also planned to carry out subgroup analyses investigating the effects of MI in addition to another form of smoking cessation support in adolescent participants versus adult participants, however, all of the studies included in the analysis for this comparison recruited adults.
Mental health and QoL outcomes
One study relevant to this comparison, which recruited military veterans with PTSD, measured and reported on mental health outcomes (Battaglia 2016). At end of treatment, the MI intervention group had a mean score of 54.5 (SD = 13.2, N = 62) on the PTSD Checklist (range 17 to 85) and the comparator group had an average score of 55.9 (SD = 13.5, N = 59). However, at final follow‐up, six months after the end of treatment (nine months post‐baseline), the MI intervention group had a statistically significantly (P < 0.05 for analysis adjusting for covariates) lower PTSD symptom score (58.4; SD = 11.4, N = 61) compared with the comparator group (62.4; SD = 10.9, N = 59). For both groups, there had been an increase in PTSD scores between the end of treatment and final follow‐up, however, this increase was smaller in the intervention group. At both the end of treatment and final follow‐up, the average depression score was significantly higher in the comparator group than the MI group (P < 0.05 for analyses adjusting for covariates). In both groups, all scores indicated probable depression. There was no significant difference in the frequency of reported suicidal thoughts between groups during the intervention period (3.4% (n = 3) of the MI group and 10.5% (n = 9) of the comparator group reported thoughts of self‐harm) and no participants died by suicide during the study.
MI versus another smoking cessation intervention (comparison 3)
Smoking cessation outcome
We pooled 19 studies comparing MI to another type of smoking cessation intervention (Analysis 3.1). The point estimate was in favour of MI; however the confidence intervals were compatible with potential harm as well as substantial benefit (RR 1.24, 95% CI 0.91 to 1.69; I2 = 54%; N = 5192). In sensitivity analyses, we 1) removed Colby 2012, as parents also received an MI intervention to motivate them to support their child's quit attempt, making the intervention different to the other interventions included (RR 1.24, 95% CI 0.90 to 1.70; I2 = 56%; N = 5030); and 2) removed Cook 2016, De Azevedo 2010 and Kelly 2006 as we judged these studies to be at high risk of bias (RR 1.32, 95% CI 0.89 to 1.98; I2 = 63%; N = 4602). Neither of these analyses meaningfully changed the summary estimates.
3.1. Analysis.
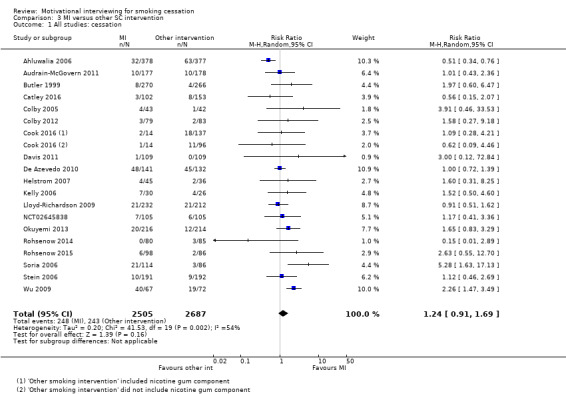
Comparison 3 MI versus other SC intervention, Outcome 1 All studies: cessation.
Intensity of the comparator
In 14 studies, the treatment provided in the comparator group (or in some of the comparator groups) was of a lower intensity than in the MI intervention group. In one study, the comparator group in some of the study arms received a higher intensity treatment than the intervention group (Cook 2016 ‐ behavioural reduction counselling); and in six studies the intensity of the treatments was similar in the intervention and comparator groups. In both Catley 2016 and Cook 2016, there was more than one comparator arm and the intensity of the comparators varied. Therefore, both studies were included in more than one subgroup and the total participants and number of events in the intervention group were split across subgroups. There was no evidence of heterogeneity between subgroups (I2 = 0%, P = 0.93; Analysis 3.2).
3.2. Analysis.
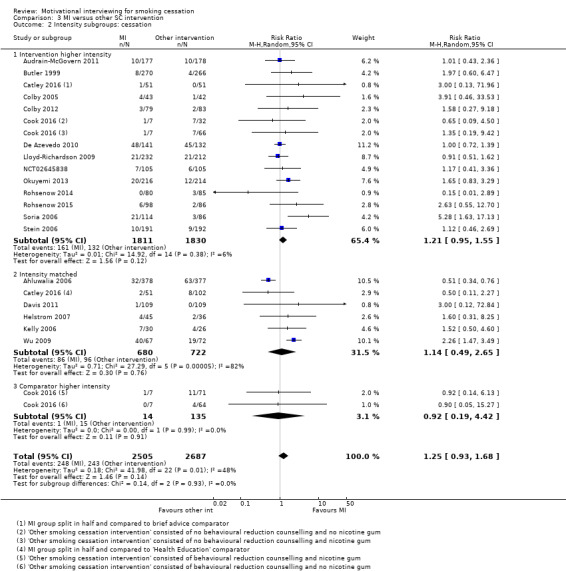
Comparison 3 MI versus other SC intervention, Outcome 2 Intensity subgroups: cessation.
Age of participants
Of the 19 studies comparing MI to another type of smoking cessation intervention, five recruited adolescents only. There was no evidence of a subgroup difference for the effect of MI in adolescents compared with adult participants (I2 = 0%, P = 0.73; Analysis 3.3).
3.3. Analysis.
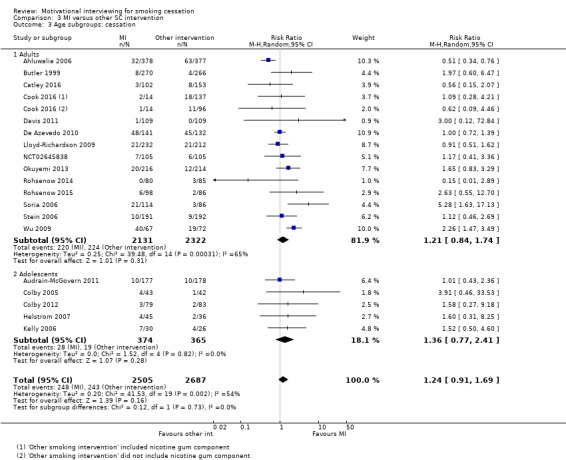
Comparison 3 MI versus other SC intervention, Outcome 3 Age subgroups: cessation.
Intervention provider
Across the studies in this comparison, interventions were provided by physicians (three studies), nurses (one study), or counsellors/psychologists (including those specialised in smoking cessation; 14 studies). Helstrom 2007 was not included in this subgroup analysis because they did not report the treatment providers' main role. There was no evidence that the effect size differed by these subgroups (I2 = 17.2%, P = 0.30; Analysis 3.4).
3.4. Analysis.

Comparison 3 MI versus other SC intervention, Outcome 4 Provider subgroups: cessation.
MI fidelity monitoring
For this comparison, 12 of the 19 studies reported that they had monitored the fidelity of MI. There was evidence that this modified the effectiveness of MI relative to comparators in this subgroup (I2 = 84.0%, P = 0.01; Analysis 3.5). There was no evidence that MI outperformed the comparator in studies where fidelity monitoring had taken place (RR 0.98, 95% CI 0.71 to 1.37; I2 = 41%; N = 3382), with stronger evidence of a benefit of MI in studies where fidelity was not assessed (RR 1.83, 95% CI 1.28 to 2.60; I2 = 9%; N = 1810). However, this latter effect appears to be partly driven by only two of the six studies in the group (Soria 2006; Wu 2009), which suggest a stronger benefit than the other studies in the subgroup. Eight of the 12 studies that reported they had carried out fidelity monitoring provided results of fidelity assessment (Audrain‐McGovern 2011; Catley 2016; Colby 2005; Colby 2012; Davis 2011; Lloyd‐Richardson 2009; Rohsenow 2014; Rohsenow 2015); all but one of these appeared to meet the study defined thresholds for good fidelity. The one study that did not meet prespecified thresholds only narrowly missed these (Audrain‐McGovern 2011); removing this single study did not meaningfully change the subgroup estimate nor the difference between subgroups.
3.5. Analysis.
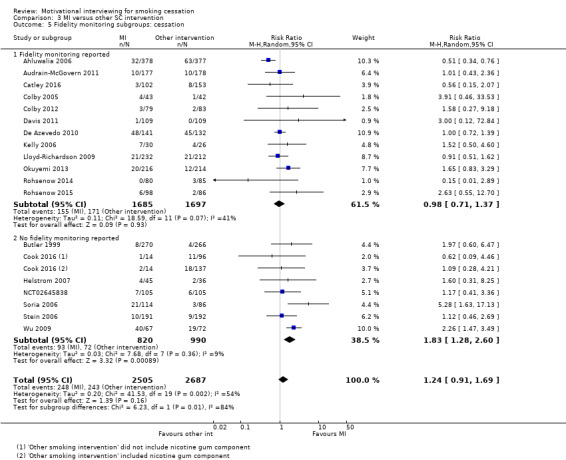
Comparison 3 MI versus other SC intervention, Outcome 5 Fidelity monitoring subgroups: cessation.
Baseline participant motivation
One study in this comparison recruited only participants motivated to quit smoking at baseline, three recruited participants not motivated to quit, and the remaining 15 recruited participants regardless of their motivation. There was substantial evidence of effect modification (I2 = 88.8%, P = 0.0001; Analysis 3.6). In the subgroup that recruited participants regardless of motivation, there was evidence that MI interventions resulted in superior quit rates to other smoking cessation interventions (RR 1.44, 95% CI 1.09 to 1.90; I2 = 33%; N = 3703), whereas the one study that recruited participants motivated to quit (Ahluwalia 2006) found substantial evidence that MI worsened outcomes (RR 0.51, 95% CI 0.34 to 0.76; I2 = n/a; N = 755). The comparator in Ahluwalia 2006 was termed 'health education', and was described as the current best smoking cessation support, focussed on providing information and advice by reviewing the addictive nature of nicotine, the health consequences of smoking, the benefits of quitting, and providing strategies to develop a quit plan and identify an alternative to smoking when meeting triggers to smoke. In trials where participants were recruited who were not motivated to quit smoking (Catley 2016; Cook 2016; Davis 2011) the confidence intervals provided evidence of substantial imprecision, and for potential harm and benefit of MI (RR 0.81, 95% CI 0.36 to 1.85; I2 = 0%; N = 734).
3.6. Analysis.

Comparison 3 MI versus other SC intervention, Outcome 6 Baseline motivation subgroups: cessation.
Counselling modality
We also planned to assess whether the effect of MI might depend on whether face‐to‐face sessions or remote sessions were provided but all interventions incorporated at least one face‐to‐face session.
Mental health and QoL outcomes
No studies relevant to this comparison measured mental health or QoL at any follow‐up, by study group.
Intensity of the MI intervention (comparison 4)
Smoking cessation outcome
Five included studies examined whether the intensity of MI affected smoking cessation rates. When pooled, these studies resulted in an RR of 1.23 (95% CI 1.11 to 1.37; adjusted N = 5620; I2 = 0%; Analysis 4.1) favouring more intensive over less intensive intervention. As described above, Vidrine 2019 randomised neighbourhood sites rather than individual participants and conducted adjusted analyses. This allowed us to calculate an ICC of 0.06 and adjust for this in our analysis. We carried out three sensitivity analyses to test the robustness of the effect of high versus low intensity MI: 1) we removed Sherman 2016 as the two MI interventions differed not only in intensity but also provider (RR 1.22, 95% CI 1.07 to 1.40; I2 = 0%; adjusted N = 4002) (in the higher intensity arm, participants were treated by the study team, whereas in the lower intensity arm they were referred to a state quit‐line); 2) we removed Vidrine 2019 as both relevant intervention groups were based on CBT as well as MI (RR 1.23, 95% CI 1.09 to 1.37; I2 = 0%; adjusted N = 5361); and 3) we removed Hollis 2007; Sherman 2016 and Vidrine 2019 together, as they were all judged to be at high risk of bias. The former two analyses did not meaningfully change the estimate of higher versus lower intensity; however, removing the three studies at higher risk of bias reduced the estimate to 1.00 (95% CI 0.65 to 1.54; I2 = n/a; N = 482), with CIs incorporating both a substantial benefit and harm of increased intensity MI interventions. Although there were two studies included in this analysis that were judged to be at low or unclear risk of bias (Ellerbeck 2009; Rohsenow 2014), the latter RR and CIs were calculated from Ellerbeck 2009 only as no participants quit in Rohsenow 2014, making it impossible to calculate a point estimate for that individual study.
4.1. Analysis.
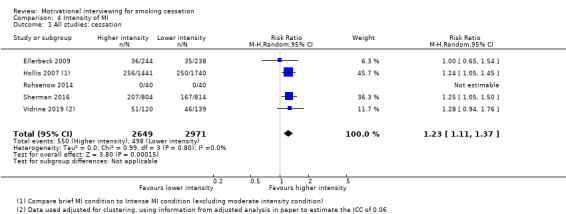
Comparison 4 Intensity of MI, Outcome 1 All studies: cessation.
Counselling modality
We grouped studies into those where the intervention was delivered either wholly or partially face‐to‐face and those where none of the intervention was delivered face‐to‐face (Analysis 4.2). One study involved face‐to‐face contact (Rohsenow 2014) and four studies involved no face‐to‐face contact (interventions were delivered either solely by telephone or via telephone and text message). However, this subgroup analysis had no effect (I2 = 0%, P = 0.80), as no participants quit in either group in Rohsenow 2014.
4.2. Analysis.
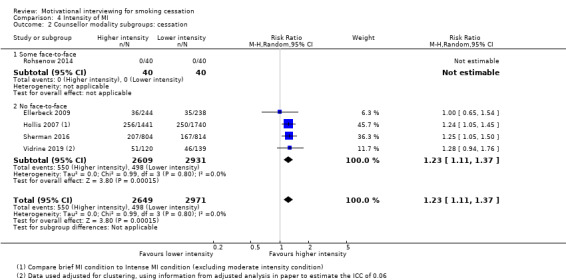
Comparison 4 Intensity of MI, Outcome 2 Counsellor modality subgroups: cessation.
MI fidelity monitoring
Four of the five studies included in this comparison reported MI fidelity monitoring. There was no evidence of a difference between the four that did report monitoring and the single study that did not (I2 = 0%, P = 0.78; Analysis 4.3). We had planned to assess the sensitivity of the results to studies where fidelity was poor, but only one of the four studies that monitored fidelity reported the results, and that study found adequate fidelity.
4.3. Analysis.

Comparison 4 Intensity of MI, Outcome 3 Fidelity monitoring subgroups: cessation.
Baseline participant motivation
Two studies in this comparison recruited participants who were already motivated to quit smoking at baseline, and the remaining three recruited participants regardless of their motivation to quit. Again, there was no evidence of a difference between these subgroups and both groups showed evidence of a benefit of higher intensity MI versus lower intensity MI for smoking cessation (I2 = 0%, P = 0.81; Analysis 4.4).
4.4. Analysis.
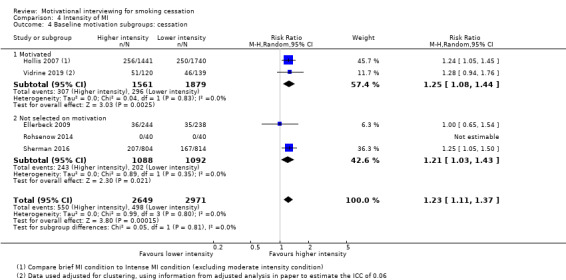
Comparison 4 Intensity of MI, Outcome 4 Baseline motivation subgroups: cessation.
Age of participants
We also planned to carry out subgroup analyses investigating whether providing higher intensity MI in adolescents versus adults had any affect on smoking cessation; however, this was not possible, as all of the studies recruited adults.
Intervention provider
We also planned to carry out subgroup analyses investigating whether the effect varied dependent on intervention provider. However, this was not possible as all of the studies were delivered by counsellors.
Mental health and QoL outcomes
None of the studies in this comparison measured mental health or QoL at any follow‐up, by study group.
Additional study
We included one additional study, which was relevant to more than one comparison, that we were unable to include in any meta‐analyses (Matuszewski 2018). Matuszewski 2018 (N = 237) investigated two MI interventions delivered in addition to brief smoking cessation support (referral to a patient resource centre that provided details of a smoking quit‐line and a quit‐line brochure). The MI interventions both consisted of a single session of MI counselling (10 minutes); however the second MI group also received a brief follow‐up session (5 minutes). Thus, this study investigated the intensity of MI counselling as well as MI in addition to another type of smoking cessation intervention. Analysis of study data was completed at the end of 2018 and has not yet been published. However, results were presented at a conference in 2018. The conference abstract stated that at six months, 35% of the comparison group had quit, and 21% and 30% of the lower intensity and higher intensity MI groups had quit respectively, with no evidence of differences between these groups. The abstract did not state whether analysis was carried out on an intention‐to‐treat or complete case basis; however, the data presented suggests that this was probably not an intention‐to‐treat analysis and it was not possible to conduct one using data provided in the abstract alone.
Discussion
Summary of main results
This review included 37 trials. Five of these trials compared MI to no smoking cessation intervention, 12 provided MI in addition to another smoking cessation intervention and compared this to the same intervention without MI, and 19 compared MI to another smoking cessation intervention. Five studies compared a more intensive MI intervention to a lower intensity one. One study could not be included in meta‐analyses.
Pooling all available studies comparing MI to no smoking cessation treatment resulted in substantial heterogeneity between studies caused by one study carried out in incarcerated men. As this study differed considerably from the other included studies, we excluded this study from the analysis and pooled the remaining studies carried out in adults and adolescents representing the general population. This resulted in a pooled RR of 0.84 (95% CI 0.63 to 1.12; I2 = 0%; adjusted N = 684). The pooled estimate was in favour of no treatment; however the CIs incorporated the possibility of both benefit and harm. This estimate was judged to be of low certainty as it was imprecise, and all studies were judged to be at high or unclear risk of bias. When studies at high risk of bias were removed, the point estimate changed to be in favour of MI; however, the CIs were still imprecise and spanned one; suggesting the possibility of both harm and benefit. The comparison between MI plus another smoking cessation intervention and that smoking cessation intervention alone (RR 1.07, 95% CI 0.85 to 1.36; I2 = 47%; adjusted N = 4167), and the comparison between MI and another smoking cessation intervention (RR 1.24, 95% CI 0.91 to 1.69; I2 = 54%; N = 5192) also produced CIs incorporating both benefit and harm. Concerns about unexplained inconsistency and imprecision resulted in low certainty relating to both of these estimates. We investigated the impact of studies at high risk of bias, the impact of face‐to‐face contact, fidelity monitoring, and participant motivation to quit. Across these sensitivity and subgroup analyses, there were some differences splitting by subgroup, but no consistent pattern emerged across the body of evidence.
Five trials examined the effectiveness of more intensive compared with less intensive MI smoking cessation interventions and produced an RR of 1.23 (95% CI 1.11 to 1.37; adjusted N = 5620; I2 = 0%) in favour of more intensive MI. However, this analysis included three studies at high risk of bias and removing these produced an RR of 1.00 (95% CI 0.65 to 1.54; I2 = n/a; N = 482). We judged the overall summary estimate as having low certainty because of this.
Only one study investigated the effect of an MI smoking cessation intervention on the well‐being of participants (Battaglia 2016). Battaglia 2016 studied participants with PTSD, and found modest benefits of MI on PTSD and depression scores at final follow‐up, compared with a usual smoking cessation care group. However, due to the paucity of evidence, no conclusions can be drawn on whether MI smoking cessation interventions can improve the well‐being of people attempting to quit smoking.
Overall completeness and applicability of evidence
The studies identified for this review were mainly conducted in the USA; most others took place in other high‐income countries, though some were conducted in middle‐income countries. In addition, the majority of studies were carried out in specific populations rather than recruitment being carried out in the general population. The diversity of populations studied may have contributed to the moderate to substantial heterogeneity observed across all comparisons. We did not plan to, and therefore did not look for evidence that population characteristics (other than participants' baseline motivation to quit) modified the effectiveness of MI. Typically, the specialist populations studied represented what may be referred to as 'hard to reach' groups (people with substance use disorders, adolescents, offenders, hospital inpatients and outpatients) and this should be considered when interpreting results; however, where the characteristics of a population have led to low quit rates in the intervention arm, this is also likely to have affected quit rates in the comparator arm, and this may not have affected the relative effectiveness of MI.
The significant heterogeneity detected within analyses is likely to also have been influenced by considerable variation across the characteristics of both the intervention and comparator arms. Despite dividing studies into separate comparisons for this update and conducting preplanned subgroup analyses in an attempt to reduce and explain some of this variation, key differences remained in the components of the MI and more general smoking cessation support provided. Studies typically provided only limited explanation of the content of the MI interventions; most specifying simply that MI techniques were adhered to, but giving sparse description of the counselling overall. This made it hard to differentiate between studies based on the nature of the MI support provided, which could have given further insight into why heterogeneity existed between studies. There was substantial variation in the intensity of the support in both intervention and comparator groups, which we did attempt to control for. However, it would have been impossible to control for all possible sources of variation across studies and this means that although we can hypothesise about the causes of this heterogeneity, this remains largely unexplained.
MI can be a difficult technique to learn and enact and therefore monitoring the fidelity of the intervention can give assurance that MI was delivered as intended and was consonant with Miller and Rollnick's key principles (Miller 2002; Miller 2013). Jelsma 2015 gave guidance on why this is important and how trialists can ensure that they satisfy this suggested requirement. Scales have been developed, such as the motivational interviewing treatment integrity (MITI) code (Pierson 2007), in order to measure adherence to MI consistently. However, most of the studies in this review did not attempt to monitor and/or improve the fidelity of MI interventions; and when monitoring was reported, they often did not report the results of that monitoring. The inconsistency of monitoring techniques and standards to define acceptable fidelity across studies means it was impossible to report overall fidelity of implementation. This, in turn, makes it difficult to determine whether the minimal beneficial effects detected in this review were due to a genuine lack of MI efficacy, or whether the included studies were not delivering MI as intended.
Finally, MI is an intervention designed to help people change their behaviour by increasing motivation to quit; however, only a minority of the included studies specifically recruited smokers who were not motivated to quit (Catley 2016; Cook 2016; Davis 2011). Five studies specifically recruited smokers motivated to quit and the remainder did not specify that they had recruited based on motivation at baseline and, thus, we assume these were populations of mixed motivation. That said, it is likely that only people somewhat motivated to quit would join a study about smoking cessation and it is likely that most studies in this review presented themselves to potential participants in this manner. It is plausible that, if MI is effective, it would be more helpful for people with low motivation. However, there was no evidence of this in this review as too few studies have recruited this population.
Certainty of the evidence
Of the 37 studies included in this review, we judged four to be at low risk of bias for all domains, and 11 to be at high risk in one or more domains. In many cases, we had to rate studies at an unclear risk, because they did not report key information. In these cases, it is impossible to know whether these studies were at any risk of bias or whether the information was simply not reported. To investigate the potential impact of studies that we judged to be at high risk of bias on results, we removed studies judged to be at high risk of bias in sensitivity analyses. In most cases, this did not materially change the estimates of effect. However, removing the three studies judged to be at high risk of bias from the analysis of higher versus lower intensity MI did affect the results, changing the summary estimate from clear evidence of modest benefit to no evidence of benefit.
We assessed the certainty of the evidence by creating 'Summary of findings' tables for all four comparisons and carrying out GRADE ratings for the smoking cessation outcome for each (Table 1; Table 2; Table 3; Table 4). For the MI versus no treatment comparison, we judged the certainty of the cessation evidence to be low. We downgraded the evidence as all of the included studies were at high or unclear risk of bias and the pooled estimate was imprecise, as the upper and lower limits of the CIs included both meaningful benefit and harm. We also judged the cessation evidence to be of low certainty for all of the remaining comparisons. When investigating MI in addition to another type of smoking cessation treatment or versus another type of smoking cessation intervention, this was due to imprecision, but also unexplained variation in effect size between studies. We judged the evidence contributing to the 'intensity of MI' comparison as low certainty because of the 'Risk of bias' assessment, where three of the five studies were at high risk of bias. As previously discussed, removing these studies in a sensitivity analysis changed the interpretation of the effect, so that the confidence intervals encompassed both appreciable benefit and harm of higher intensity motivational interviewing for smoking cessation.
We generated funnel plots for the two comparisons that included over ten studies ('MI in addition to another form of smoking cessation treatment' or 'MI versus another non‐MI smoking cessation intervention') in an attempt to identify any differential reporting of studies finding negative effects of MI. In neither case did these plots provide evidence of publication bias (Figure 3; Figure 4).
3.
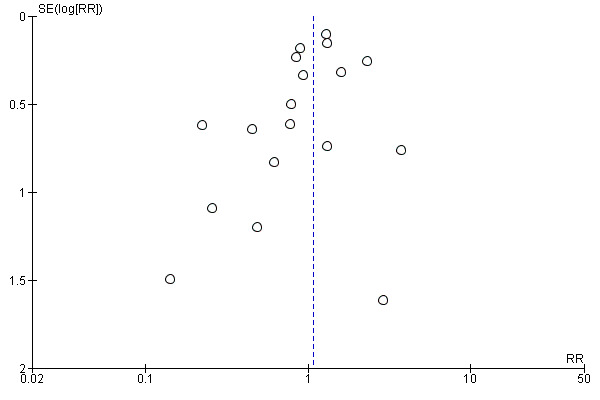
Funnel plot of comparison: 2 MI in addition to other smoking cessation treatment, outcome: 2.1 cessation.
4.
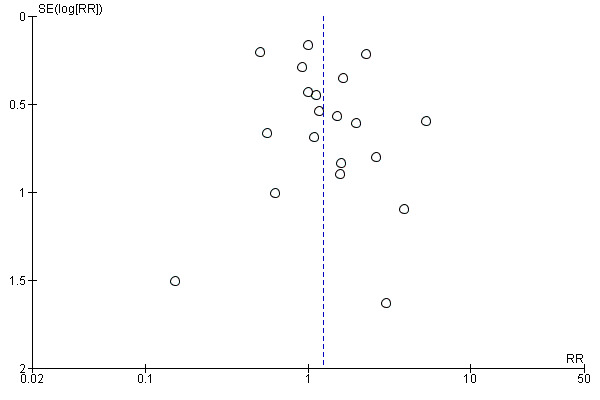
Funnel plot of comparison: 3 MI versus other SC intervention, outcome: 3.1 cessation.
Potential biases in the review process
We consider the review process used to be robust, and are unaware of any introduced bias. For outcome assessment, we followed the standard methods used for Cochrane Tobacco Addiction Review Group cessation reviews. Our search strategy included the Cochrane Tobacco Addiction Group Specialised Register and we also searched trial registries in an attempt to capture unpublished and ongoing studies. There may be unpublished data that our searches did not uncover; however funnel plots suggested that this is unlikely to bias results for the two relevant comparisons. For this update of the review, we modified the inclusion criteria and therefore conducted a full search of the literature (rather than just updating the searches run previously). As a result, we included 16 new studies and excluded ten previously included studies and two ongoing studies. We introduced an exclusion criterion to exclude studies that incorporated additional non‐MI components in the MI intervention arm but not the comparison arm (nine previously included/ongoing studies have been now excluded for this reason). It is plausible that the apparent effect of MI seen in the previous review may have been partly because the interventions incorporated these other active elements. We excluded quasi‐randomised studies at this update as non‐randomised studies are of lower quality and the larger body of randomised trials allowed us to draw conclusions on the best quality evidence (we excluded one previously included study for this reason). We also excluded one previously included study that tested an MI intervention to encourage people to participate in the trial rather than to aid them to quit smoking, and another study that was based primarily on the stages of change theory. We believe that these changes have reduced biases that previously existed in the review.
Agreements and disagreements with other studies or reviews
Two previous reviews of MI for smoking cessation (Heckman 2010; Hettema 2010) provided evidence of a very modest effect of MI at long‐term follow‐up (six months or more). Our own effect estimates are compatible when comparing MI (on its own or in addition to other smoking cessation care) to another form of smoking cessation treatment, with point estimates suggesting very modest benefit but, unlike the previous reviews, our summary estimate CIs incorporated potential harm of MI as well as benefit. A key difference is that these other reviews pooled together all comparisons whereas we separated ours into four based on the type and intensity of the comparator groups. Our findings reflect the findings of the MI literature more generally, across a variety of health behaviours (Cheng 2015; Cowlishaw 2012; Foxcroft 2016; Gates 2016; Klimas 2018; Mbuagbaw 2012; Morton 2015; Smedslund 2011). These systematic reviews typically found modest effects of MI that were not sustained at long‐term follow‐up. As in this review, these reviews often detected moderate unexplained heterogeneity, possibly relating to substantial differences between the intervention and comparator, other than the presence or absence of MI across the included studies.
Hettema 2010 found evidence that the baseline motivation of participants recruited moderated the effect of MI. The studies included in their meta‐analysis that recruited participants with low motivation to quit found a significant moderate effect of MI on quit rates at both short‐ and long‐term follow‐up, whereas those which recruited highly‐motivated participants resulted in a very small, non‐significant overall effect of MI on smoking cessation.
Authors' conclusions
Implications for practice.
There is insufficient evidence to assess whether MI to promote smoking cessation increases cessation compared with no intervention, and further evidence may change our estimate of the effect.
MI may modestly increase the likelihood of long‐term smoking cessation when used in addition to other smoking cessation intervention components or when compared with non‐MI smoking cessation interventions; however, there is also the possibility that MI may reduce quit rates relative to other smoking cessation interventions. Further evidence is likely to strengthen or weaken this effect.
There is no clear evidence to suggest that the effect of MI is moderated by the intervention provider, age of participant, participants' motivation to quit at baseline, whether MI is delivered face‐to‐face or whether MI fidelity monitoring takes place.
Higher intensity MI may increase smoking cessation rates relative to lower intensity MI, however, due to risks of bias in the existing studies, further research could strengthen or weaken this effect.
Implications for research.
Greater clarity and consistency of study methods, components and counselling techniques would improve comparability between trials.
Trials should aim to reduce confounding by minimising the number of co‐interventions when testing MI, and where co‐interventions are used, match these in the comparator arm.
Future studies of MI should aim to maximise the fidelity to MI, consider independent monitoring of the fidelity of intervention delivery, and report these data. Standardising methods used to monitor fidelity would allow easier comparisons across studies.
Future research should attempt to identify which core components of the motivational interviewing approach successfully help people to quit smoking, and whether modifying them enhances or reduces the likelihood of quitting.
Future studies should monitor the well‐being of participants throughout the study and at follow‐up, reporting results by trial arm, to investigate whether MI for smoking cessation improves the well‐being of smokers attempting to quit.
Trialists should consider testing the effects of baseline motivation to quit when investigating MI interventions and recruiting participants where motivation could benefit from improvement.
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2019 | New search has been performed | Updated with 16 new studies and with ten previously included studies excluded due to changes in eligibility criteria |
| 23 May 2019 | New citation required and conclusions have changed | New authors added. Conclusions have changed ‐ evidence for benefit of MI for smoking cessation is inconclusive ‐ confidence intervals now incorporate both benefit and harm. The certainty of the evidence has changed from moderate to low, indicating that new evidence is likely to have an impact on the effect estimates and their 95% confidence intervals. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 5 January 2015 | New citation required but conclusions have not changed | Authors have changed. Main conclusions remain stable, with only minor changes in subgroup findings |
| 5 January 2015 | New search has been performed | Updated with 14 new included studies |
| 5 September 2011 | Amended | Reference to companion review updated |
| 10 February 2010 | Amended | Spelling correction in tables and change in |
| 21 October 2008 | Amended | Converted to new review format |
Acknowledgements
We thank Douglas Lai, Kate Cahill, Ying Qin, Jin‐Ling Tang and Rachna Begh for their authorship of previous versions of this review. We are thankful for statistical support from Dr Thomas Fanshawe, and the many authors who have provided us with additional information about their studies. We gratefully acknowledge peer review by Dr Claire Garnett (University College London) and Assistant Professor Peter Kelly (Illawarra Health and Medical Research Institute and School of Psychology, University of Wollongong), consumer review by Lee Bromhead and editorial support from Dr Jonathan Livingstone‐Banks and Professor Robert West.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care. Professor Paul Aveyard is an NIHR senior investigator and funded by NIHR Oxford Biomedical Research Centre and CLAHRC.
Appendices
Appendix 1. CRS Search Strategy 2019
#1 (motivat* NEAR2 interview*):TI,AB,MH,EMT,KY,XKY,KW,XRT
#2 (motivat* NEAR2 enhanc*):TI,AB,MH,EMT,KY,XKY,KW
#3 (motivat* NEAR2 (session* OR counsel* OR practi* OR behav*)):TI,AB
#4 #1 OR #2 OR #3 AND (INREGISTER) [SET 1]
#5 motivation*:MH,EMT,XKY,KY,KW AND (INREGISTER) [SET 2]
Notes: Set 1 identifies the most relevant records. Set 2 identifies records with the keyword 'motivation' not otherwise identified in set 1, and is over sensitive. Studies in both sets were screened for inclusion.
In lines 4 and 5 'motivat*' captures the variants of 'motivational' used in the original search strategy.
Data and analyses
Comparison 1. MI versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies: cessation | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 All studies: cessation ‐ Naik 2014 removed | 4 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.12] |
Comparison 2. MI in addition to other SC treatment versus that SC treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies: cessation | 12 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.36] |
| 2 Intensity subgroups: cessation | 12 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.36] |
| 2.1 Intervention higher intensity | 11 | 3838 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.35] |
| 2.2 Intensity matched | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.06, 1.59] |
| 3 Provider subgroups: cessation | 12 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.36] |
| 3.1 Physician | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.23, 2.57] |
| 3.2 Nurse | 2 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.09, 2.95] |
| 3.3 Counsellor | 8 | 3405 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.32] |
| 3.4 Lay healthcare worker | 1 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.40, 3.81] |
| 4 Counselling modality subgroups: cessation | 12 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.36] |
| 4.1 Some face‐to‐face | 8 | 2818 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.87, 1.56] |
| 4.2 No face‐to‐face | 4 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.42] |
| 5 Fidelity subgroups: cessation | 12 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.36] |
| 5.1 Fidelity monitoring reported | 5 | 2115 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.57, 1.41] |
| 5.2 No fidelity monitoring reported | 7 | 2052 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.93, 1.56] |
| 6 Baseline motivation subgroups: cessation | 12 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.36] |
| 6.1 Motivated | 3 | 855 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.10, 1.54] |
| 6.2 Not selected on motivation | 8 | 2854 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.69, 1.55] |
| 6.3 Not motivated | 1 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.36, 1.37] |
Comparison 3. MI versus other SC intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies: cessation | 19 | 5192 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.69] |
| 2 Intensity subgroups: cessation | 19 | 5192 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.68] |
| 2.1 Intervention higher intensity | 14 | 3641 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.95, 1.55] |
| 2.2 Intensity matched | 6 | 1402 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.49, 2.65] |
| 2.3 Comparator higher intensity | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.19, 4.42] |
| 3 Age subgroups: cessation | 19 | 5192 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.69] |
| 3.1 Adults | 14 | 4453 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.84, 1.74] |
| 3.2 Adolescents | 5 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.77, 2.41] |
| 4 Provider subgroups: cessation | 18 | 5111 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.90, 1.70] |
| 4.1 Physician | 3 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.92, 5.45] |
| 4.2 Nurse | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.84] |
| 4.3 Counsellor/psychologist | 14 | 3947 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.79, 1.55] |
| 5 Fidelity monitoring subgroups: cessation | 19 | 5192 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.69] |
| 5.1 Fidelity monitoring reported | 12 | 3382 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.37] |
| 5.2 No fidelity monitoring reported | 7 | 1810 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.28, 2.60] |
| 6 Baseline motivation subgroups: cessation | 19 | 5192 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.69] |
| 6.1 Motivated | 1 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 6.2 Not selected on motivation | 15 | 3703 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.09, 1.90] |
| 6.3 Not motivated | 3 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.85] |
Comparison 4. Intensity of MI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies: cessation | 5 | 5620 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.11, 1.37] |
| 2 Counsellor modality subgroups: cessation | 5 | 5620 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.11, 1.37] |
| 2.1 Some face‐to‐face | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 No face‐to‐face | 4 | 5540 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.11, 1.37] |
| 3 Fidelity monitoring subgroups: cessation | 5 | 5620 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.11, 1.37] |
| 3.1 Fidelity monitoring reported | 4 | 5361 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.09, 1.37] |
| 3.2 No fidelity monitoring reported | 1 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.94, 1.76] |
| 4 Baseline motivation subgroups: cessation | 5 | 5620 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.11, 1.37] |
| 4.1 Motivated | 2 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.08, 1.44] |
| 4.2 Not selected on motivation | 3 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.03, 1.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahluwalia 2006.
| Methods | Study design: factorial RCT (2 x 2) Location: USA Setting: urban community‐based clinic Recruitment: through clinic, media and community outreach efforts, including radio, television, gas pump, billboard advertising, community health fairs, posting signs in minority‐owned businesses and mailing of referral letters from physicians |
|
| Participants | Defining eligibility criteria?: African‐American or black adults who smoked 10 or fewer cigarettes a day for at least 6 months prior to enrolment (light smokers) Participant characteristics: 755 adult smokers; 505/755 (66.9%) female; mean age: 45; mean cpd: 7.5; nicotine dependence: mean Fagerstrom test for nicotine dependence (FTND) = 4.3 Motivation to quit?: motivated |
|
| Interventions | Control 1: Health Education plus 2 mg nicotine gum (HE + NG): HE was a standard counselling approach, based on the current US Department of Health & Human Services treatment guidelines that focused on providing information and advice. Participants received the counselling over six 20‐minute sessions (three in‐person visits and three telephone calls). During HE sessions, trained counsellors used the ‘KIS II Quit Smoking Guide’ (a 36‐page booklet developed for African‐American light smokers) and semi‐structured scripts to review the addictive nature of nicotine, health consequences of smoking and benefits of quitting, and provided concrete strategies on developing a quit plan and identifying alternatives against triggers to smoke. Participants were provided with an eight‐week supply of 2 mg nicotine gum. Control 2: Health Education plus placebo gum (HE + PG): As control 1, however participants received placebo gum rather than 2 mg nicotine gum. Intervention 1: Motivational Interviewing plus 2 mg nicotine gum (MI + NG): MI counselling was provided by trained counsellors over six 20‐minute sessions (three in‐person visits and three telephone calls). Counsellors followed semi‐structured scripts that explored the pros and cons of smoking/quitting, and motivation and confidence to quit. A values clarification strategy based on the work of Miller & Rollnick was used. Participants also received the 36‐page ‘KIS II Quit Smoking Guide’. Intervention 2: Motivational Interviewing plus placebo gum (MI + PG): As intervention 1, however participants received placebo gum rather than 2 mg nicotine gum. Provider: trained counsellors (counsellors participated in two days of in‐service training). All counsellors participated in weekly group supervision to ensure the integrity of the respective counselling protocols. Intensity: counselling took place during six 20‐minute sessions (3 face‐to‐face and 3 telephone) over 16 weeks in all study arms. Was MI fidelity monitored?: Yes. Each session was tape‐recorded to maintain fidelity and consistency throughout the study. A subset of audiotapes were rated by investigators for adherence to MI principles using a modified version of the Motivational Interviewing Skills Code (results not reported). MI counsellors and supervisors reviewed audiotapes and discussed current issues at their weekly meetings. |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 26 weeks Validation: saliva cotinine‐verified. A salivary cotinine cut‐off of ≤ 20 ng/mL was used. Was mental health and/or well‐being measured at follow‐up?: No Was quality of life measured at follow‐up?: No |
|
| Funding source | National Cancer Institute at the National Institutes of Health (R01CA091912). Glaxo‐SmithKline provided study medication but played no role in the design, conduct of the study or interpretation and analysis of the data. | |
| Author conflicts of interest | None | |
| Notes | For purposes of analysis, the two HE groups and the two MI groups were merged to create one HE group and one MI group. This was acceptable as there was no interaction detected between study factors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated random‐numbers table was used to randomize patients". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "a sealed envelope with pre‐assigned randomization numbers was drawn to determine which form of counseling the participant would receive". Did not state that envelope was opaque. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking outcome was biochemically verified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18.8% lost to follow‐up in MI groups; 12.5% lost to follow‐up in HE groups. Therefore, less than 50% overall and similar loss to follow‐up between intervention groups of interest. |
Audrain‐McGovern 2011.
| Methods | Study design: RCT Location: USA Setting: children's hospitals Recruitment: through flyers and brochures advertising the study, available at the participating medical sites. Participants were also referred by their physicians. |
|
| Participants | Defining eligibility criteria?: adolescents aged 14 to 18 years Participant characteristics: 355 adolescent smokers; 195/355 (54.9%) female; mean age: 17.02; mean cpd: 9.8; nicotine dependence: mean modified Fagerstrom Tolerance Questionnaire (MFTQ)= 4.26 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: structured brief advice (SBA): based on clinical practice guidelines for treating nicotine dependence ‐ the “5 A’s” for those interested in quitting, and the “5 R’s” for participants not interested in quitting smoking. In each session, the 5 A’s/R’s were followed by a review of self‐help materials (smoking cessation print materials, list of resources), and a brief check‐in to see if the adolescent needed help in gaining access to services (e.g. appointment with their physician for pharmacotherapy). Intervention: motivational interviewing (MI), based on motivational enhancement therapy (MET), an adaptation of motivational interviewing. MET adds personalised feedback about assessment results (e.g. adolescent's tobacco use at baseline and during treatment) and collaborative development of a formal change plan to the standard principles and techniques of MI. Provider: counsellor Intensity: the MI intervention consisted of three 45‐minute office sessions and two 30‐minute office or telephone sessions over 12 weeks. Was MI fidelity monitored?: "To promote treatment integrity, all treatment sessions were audio recorded and reviewed weekly by the treatment supervisor, who used an adherence checklist. MI and SBA counselors received extensive training on the treatment protocol and received weekly individual or group supervision." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 24 weeks Validation: Saliva cotinine (<= 15 ng/mL classified as abstinent) Was mental health and/or well‐being measured at follow‐up?: yes, however results were not reported by group at follow‐up. Was quality of life measured at follow‐up?: no |
|
| Funding source | "This study was supported by grant SAP 4100027295 from the Commonwealth of Pennsylvania, Pennsylvania Department of Health". | |
| Author conflicts of interest | "The authors have indicated they have no financial relationships relevant to this article to disclose." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | At this initial assessment, participants were randomly assigned (stratified by precontemplation stage of quitting smoking). |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of smoking outcome was blinded and cessation was biochemically verified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up low and similar across study arms 14/177 (8%) in MI group; 4/178 (2%) in control group. |
Bastian 2013.
| Methods | Study design: RCT Location: USA Setting: telephone support Recruitment: lung cancer patients identified relatives and friends who smoked through four clinical sites. A letter was written to the friend/relative explaining the study and asking them to call a toll‐free number if they wanted to decline participation. Those who did not decline were called by the study team seven days later to assess eligibility. |
|
| Participants | Defining eligibility criteria?: in social network/family of someone diagnosed with lung cancer Participant characteristics: 496 adult smokers, randomised to intervention (245) control (251). 58% female. Mean age 47, mean cpd 19.5. Motivation to quit?: not selected on motivation |
|
| Interventions | Control: self‐directed materials: letter from an oncologist encouraging participants to give up smoking, quit kit (including an ALA cessation guide, straws, candy, cards, and a notepad), and an individually‐tailored information booklet. Mailing of 2‐week nicotine patch starter kit and advised to call for a further 2‐week supply as needed Intervention: As control, plus 6 weekly telephone calls over the 12‐week intervention period ‐ standard smoking cessation counselling using MI techniques and adaptive coping skills training Provider: counsellors Intensity: 1 x 30‐minute session a week for 6 weeks Was MI fidelity monitored?: not reported |
|
| Outcomes | Definition of cessation used: 7‐day PPA Length of longest follow‐up: 12 months Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?:no |
|
| Funding source | Supported by the National Cancer Institute grant 5U01‐CA‐92622, also in part by the Intramural Program of the National Human Genome Research Institute, National Institutes of Health | |
| Author conflicts of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was blocked by patient, with entire social network units stratified by site and size of social network enrolled (one vs. two or more) assigned to the same condition." No further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was blocked by patient, with entire social network units stratified by site and size of social network enrolled (one vs. two or more) assigned to the same condition." No further information given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "We attempted to verify self‐report cessation at 2 weeks, 6 months and 12 months postintervention with saliva cotinine analysis, but were unable to do so because return rates (via mail) for saliva samples were low". Therefore, quit rates were not validated and the amount of support differed between arms. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 136/245 lost to follow‐up in intervention arm; 132/251 lost to follow‐up in control arm. Therefore, loss to follow‐up was high (more than 50%). |
Battaglia 2016.
| Methods | Study design: RCT Location: USA Setting: over the telephone within the VHA healthcare system Recruitment: recruited using informational flyers, provider referrals, and outreach letters |
|
| Participants | Defining eligibility criteria?: veteran smokers with post traumatic stress disorder (PTSD) ‐ Diagnostic and Statistical Manual of Mental Disorders IV criteria for diagnosis code 309.81 PTSD documented in their medical record Participant characteristics: 175 adult smokers; 24/175 (13.7%) female; mean age: 55.6; mean cpd: not provided; nicotine dependence: FTND: intervention arm: mean 5.4 (SD = 2.0); control arm: mean 5.1 (SD = 2.3) Motivation to quit?: not selected on motivation |
|
| Interventions | Control: usual PTSD Health Buddy Care: PTSD home telehealth care management program (Health Buddy) and nurse care management alongside usually offered smoking cessation treatments. The Health Buddy (BoschHealthcare, Palo Alto,CA) is designed to help individuals with PTSD self‐monitor and self‐manage. This computer‐like device is 12 × 8 × 4 inches with an LCD screen, on which participants read information, and four large buttons for responding to questions. A typical session is completed in approximately 2 minutes. Participants given access to nicotine replacement therapy (patch, lozenge, gum, and inhaler), bupropion or varenicline Intervention: enhanced PTSD Health Buddy and Motivational Interviewing: as control, plus MI‐based written smoking cessation curricula on home telehealth and weekly telephone MI smoking cessation counselling with a nurse Provider: nurses Intensity: weekly (average duration 16.7 minutes) Was MI fidelity monitored?: "To ensure fidelity, random calls made by the intervention research nurse are observed throughout the study. Additionally, the research nurse will participate in ongoing MI training and workshops throughout the study to diminish “drift” away from the principles of MI." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 9 months post‐baseline Validation: exhaled CO ≤ 10 ppm Was mental health and/or well‐being measured at follow‐up?: PTSD symptoms assessed using the PTSD Checklist. The 15‐item Geriatric Depression Scale‐Short Form was used to monitor depression. Suicidal thoughts were assessed every 30 sessions during the intervention period with a question on the Health Buddy. Was quality of life measured at follow‐up?: no |
|
| Funding source | "Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development" | |
| Author conflicts of interest | "The authors declare that they have no competing interests". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A research pharmacist who was unaffiliated with the study performed randomisation using a blocked randomisation process. Did not specify how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Study personnel were blinded to the randomisation process. Was not clear how this took place |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking/quitting was reported by participants via a handheld computer and not directly to study personnel. Quitting was validated at end of treatment and final follow‐up using exhaled CO verification (CO verified rates obtained through communication with author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall loss to follow‐up 31% and was evenly matched between trial arms. |
Bock 2008.
| Methods | Study design: RCT Location: USA Setting: observation unit of a hospital emergency department Recruitment: admission records used to identify participants |
|
| Participants | Defining eligibility criteria?: admitted to emergency department of hospital with chest pain Participant characteristics: 543 adult smokers, randomised to intervention (271) usual care (272). 69.1% female; mean age 47.7. Mean cpd 18.9 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: referral sheet to local SC resources (usual care) Intervention: Single 30‐min MI session, including use of decision‐balance tool, summation of reasons to quit versus continuing to smoke etc. If trying to quit, given ALA manual, 2 brief (< 15 min) follow‐up telephone calls at 2 and 4 weeks after counselling session All participants offered NRT if decided to quit, and received brief call on quit day and a week later Provider: counsellors Intensity: 1 x 30‐minute session followed by 2 further 15‐minute sessions Was MI fidelity monitored?: "A subsample of 10% of all counseling sessions was audio‐taped. Tapes were audited by the study investigators for quality control and treatment fidelity. Counselors used a decisional balance review tool and intervention component checklists to enhance treatment fidelity and to document delivery of the intervention components and the amount of time spent on each component." |
|
| Outcomes | Definition of cessation used: continuous Length of longest follow‐up: 6 months Validation: continuous abstinence defined as self‐reported abstinent at all time points Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A National Institutes of Health, National Heart, Lung and Blood Institute grant (1 R01HL60986) | |
| Author conflicts of interest | "The authors report no competing interests". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "After providing informed consent, participants were randomly assigned…" No further information given |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "After providing informed consent, participants were randomly assigned…" No further information given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Continous cessation rates were not biochemically validated and contact differed between trial arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 133/271 (49%) of the intervention group and 118/272 (43%) of the control group were lost to follow‐up. Therefore overall there was less than 50% loss to follow‐up and rates were similar between groups. |
Bock 2014.
| Methods | Study design: RCT Location: USA Setting: 3 hospital‐based primary care clinics located in separate inner‐city hospitals Recruitment: during routine healthcare visits at primary care clinics. Patients invited to participate in a study of smoking patterns and cessation |
|
| Participants | Defining eligibility criteria?: all attending routine primary care appointments for variety of reasons Participant characteristics: 846 adult smokers randomised to intervention (406) and control (440), 68.8% female; mean age 39.6. cpd of at least 10 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: smoking cessation assistance following guidelines for best practice, using the 5As. Participants asked about smoking status, assessed for nicotine dependence, advised to quit smoking and offered assistance with quitting (nicotine patches, self‐help pamphlets and/or referral to the state quit‐line) Intervention: As control, plus 45‐min individual counselling session with Health Educators, using MI techniques. Participants ready to quit received behavioural skills training. Those who decided to quit during this baseline visit were given 2 follow‐up telephone counselling calls (on quit day and 2 weeks later). Those choosing not to quit were called 2 and 4 weeks later. All participants received 8 weeks of nicotine patches. Provider: counsellor Intensity: 1 x 45‐minute face‐to‐face session followed by 2 telephone calls at 2 and 4 weeks Was MI fidelity monitored?: "ME interventionists were trained and supervised by licensed clinical psychologists. Ongoing fidelity was monitored through selected session observation and weekly clinical supervision. All counselling sessions were tape recorded, and 20% of tapes were selected at random for review by the study intervention coordinator, a PhD psychologist who was certified in MI. Regular, weekly meetings were conducted to review the intervention procedures and results of counselling tape audits to enhance treatment fidelity." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 12 months Validation: Exhaled CO ≤ 5 ppm Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A National Institutes of Health,National Institute on Drug Abuse grant (R01DA010860) | |
| Author conflicts of interest | "Authors declare no conflicts". | |
| Notes | Outcome data not clearly provided for ITT unadjusted analysis in the paper; therefore we obtained data directly from the author for meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "the computer used a random number program to assign participants at random to one of two treatment conditions". |
| Allocation concealment (selection bias) | Low risk | QUOTE: "the computer used a random number program to assign participants at random to one of two treatment conditions". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: " reports of tobacco abstinence were confirmed using expired carbon monoxide testing with a Bedfont MicroSmokerlyzer™ machine with ≥ 5 ppm as the cutoff indicating a positive smoking result". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 238/406 (59%) in the intervention arm and 232/440 (53%) in the control arm lost to follow‐up. Therefore, more than 50% dropout overall. |
Butler 1999.
| Methods | Study design: RCT Location: Wales, UK Setting: general practices Recruitment: GPs asked to recruit 1st smoker coming to each surgery |
|
| Participants | Defining eligibility criteria?: all participants were consulting within primary care for various reasons. Participant characteristics: 536 adult smokers, randomised to MI (270) or brief advice (266). 29% M. Mean age 41. Mean cpd 25.5 Motivation to quit?: not selected on motivation |
|
| Interventions | 1. Control: standardised brief advice (2 mins)
2. Intervention: structural motivational counselling for 1 session (mean 10 mins)
Provider: physicians Intensity: 1 x 10‐minute session Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: point prevalence Length of longest follow‐up: 6 months Validation: attempted, but abandoned Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | The Welsh Office of Research and Development for Health and Social Care | |
| Author conflicts of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Clinicians then opened sealed envelopes assigning patients to an intervention group. These numbered envelopes were filed in a study pack and clinicians were instructed to open them in order. Sequential blocks of six envelopes contained three allocations to each group, but the order varied." No further information given. Therefore, the methods for generating the allocation sequence are unclear. |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Clinicians then opened sealed envelopes assigning patients to an intervention group. These numbered envelopes were filed in a study pack and clinicians were instructed to open them in order. Sequential blocks of six envelopes contained three allocations to each group, but the order varied." Unclear if envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Biochemical validation of quitting was attempted, but uptake was low and results did not alter conclusions from self‐report data". Cessation was measured by self‐report only, however the amount of contact was similar between arms. This means the risk of misreporting was likely to be similar across study arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64/270 (24%) in the motivational arm and 54/266 (20%) in the brief advice arm lost to follow‐up at 6 months |
Catley 2016.
| Methods | Study design: RCT Location: USA Setting: university Recruitment: through word of mouth, newspaper ads, flyers, billboards, Internet advertising, and physician referral using printed cards |
|
| Participants | Defining eligibility criteria?: adult community resident smokers reporting low motivation and readiness to quit Participant characteristics: 255 adult smokers; 110/255 (43%) female; mean age: MI = 45.0, HE = 46.7, BA = 45.5; mean cpd: MI = 16.2, HE = 16.9, BA = 18.0; nicotine dependence: Severity of Dependence Scale (five‐item dependence scale, with a score ranging from 0 to 15): MI = 6.6, HE = 6.9, BA = 5.7 Motivation to quit?: not motivated |
|
| Interventions | Control 1: brief smoking cessation advice Control 2: Health Education (HE) (intensity‐matched comparison): the four‐session HE intervention was based on the relevant risks of smoking, rewards of quitting and roadblocks to cessation of the US Clinical Practice Guideline but excluded elements characteristic of MI. To ensure HE was distinct from MI, counsellors followed a script and presented information via a computer during in‐person visits. Intervention 1: Motivational Interviewing (MI): "The MI sessions were unscripted and counselors used the style (e.g. empathic, collaborative, and autonomy‐supportive) and methods (e.g. open‐ended questions, affirmations, and reflections) of MI. Counselors encouraged patient engagement in the conversation by exploring patient ambivalence regarding smoking cessation; developing discrepancy between the client’s goals/values (e.g. health) and current behaviors (i.e. smoking); and increasing “change talk” while avoiding arguing or disputing “sustain talk.” Provision of information was minimized and offered only when judged necessary. For participants who expressed an interest in quitting, the MI counselor worked to strengthen the commitment for change and used an MI style to complete the guideline‐based quit plan and follow‐up sessions as described above." All participants who expressed any interest in quitting were offered a self‐help guide and, for those who set a quit date, free pharmacotherapy (varenicline and NRT offered) Provider: counsellors Intensity: four sessions over a 6‐month period Was MI fidelity monitored?: "Each counselor delivered all three treatments. This avoided confounding counselor and treatment effects. To prevent treatment contamination, the HE and BA arms were scripted and stringent measures were implemented to ensure fidelity. Training, practice, and supervision for each of the interventions continued until counselors met fidelity criteria for three consecutive sessions (training hours per counselor were 96 for MI and 28.5 for HE). Counselors then began counseling enrolled participants and received regular group supervision of a randomly selected recent audio recording from separate expert clinicians for each of the interventions (weekly for MI, every other week for HE, and monthly for BA). Study‐specific rating scales were completed to verify fidelity. To verify treatment integrity, the duration of sessions was assessed and randomly selected 10% of regular sessions (i.e. excluding quit plans and follow‐ups) for evaluation (38 MI and 37 HE), using the MI Treatment Integrity Code by an independent expert coding group blind to group assignment. The Code yields ratings of counselor adherence to MI, including overall ratings of the session (e.g. expression of empathy) and behavior counts (e.g. frequency of open‐ended questions)." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: saliva cotinine Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This study was supported by a grant (R01 CA133068) from NIH, National Cancer Institute. Pfizer provided varenicline (Chantix) through Investigator‐ Initiated Research Support (No. WS759405)". | |
| Author conflicts of interest | "Delwyn Catley reports grants from NIH, Patient‐Centered Outcomes Research Institute (PCORI), and the National Multiple Sclerosis (MS) Foundation and non‐financial support from Pfizer during the conduct of the study; Delwyn Catley occasionally received fees for providing Motivational Interviewing training. Kathy Goggin reports grants from NIH, PCORI, and the National MS Foundation and consultant fees for providing Motivational Interviewing training. Karen Williams reports personal fees from Proctor and Gamble (P&G) and from P&G Global Advisory Committee, during the conduct of the study but outside of the submitted work. Ken Resnicow occasionally conducts Motivational Interviewing training and reports personal fees from University of Missouri, Kansas City during the conduct of the study. Edward Ellerbeck reports grants from NIH. James Grobe reports research consulting fees from the University of Texas, Southwestern & Texas Women’s University unrelated to the study. No other financial disclosures were reported by the authors of this paper." | |
| Notes | Control groups merged for all analyses apart from the intensity subgroup analysis, where the intervention group was split and compared with the two separate control groups in the appropriate subgroups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "A predetermined computer‐generated randomization sequence was prepared by the study statistician". |
| Allocation concealment (selection bias) | Low risk | QUOTE: "A predetermined computer‐generated randomization sequence was prepared by the study statistician and provided in sealed opaque envelopes. After research assistants enrolled participants and baseline measures were collected, research assistants opened envelopes to allocate participants to treatment group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence was biochemically verified. QUOTE: "self‐report 7‐day point‐prevalence smoking abstinence was collected at 3 and 6 months, and verified biochemically at 6 months using saliva cotinine." "Although participants can differentiate whether they are in the BA versus MI or HE because of the different number of sessions, they are not informed in any way regarding the names, the nature, or distinctions between HE and MI and therefore will be blind to which of these two treatments they receive." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | QUOTE: "Logistic regression analyses revealed no significant differences in attrition rates between the groups (Figure 1), with overall completion rates of 89.4% (n = 228) at Month 6. 12/102 of MI group, 7/51 of BA group and 8/102 of the HE group were lost to follow‐up, therefore attrition did not differ greatly across groups". |
Colby 2005.
| Methods | Study design: RCT Location: USA Setting: hospital outpatient clinic or Emergency Department (ED), then over the phone Recruitment: in an eating disorder and an adolescent outpatient clinic at an urban hospital in the Northeast |
|
| Participants | Defining eligibility criteria?: adolescents (12 to 19 years of age) Participant characteristics: 85 adolescents; 60/85 (71%) female; mean age: 16.3; mean cpd: 10.5; nicotine dependence: mean FTND = 5.9 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: Brief Advice; pamphlet on quitting smoking and list of local treatment referrals Intervention: Motivational interview (MI). As control, plus feedback sheet, goal sheet, and information about strategies for quitting and coping with withdrawal. Interventionists contacted participants by telephone 1 week after baseline. Provider: counsellors Intensity: one 15 to 20‐minute face‐to‐face session, plus one telephone call the following week Was MI fidelity monitored?: "Interventionists participated in weekly group supervision. Patients and interventionists rated each session as a compliance check. Rapport, counselor empathy, and self‐efficacy enhancement were rated on scales from 1 (strongly disagree) to 4 (strongly agree). The delivery of 15 essential elements of the protocol were rated as either 0 (topic not introduced), 1 (not at all useful), 2 (somewhat useful), or 3 (very useful)." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: CO validated (> 8 ppm) or cotinine validated (>= 15 ng/mL) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This study was supported by grant number 030330 from the Robert Wood Johnson Foundation. Additional support was provided by grant #DA11204 from the National Institute on Drug Abuse, and by two Department of Veterans Affairs Career Research Scientist Awards to Dr. Monti and Dr. Rohsenow." | |
| Author conflicts of interest | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified ‐ only specified that allocation was random |
| Allocation concealment (selection bias) | Unclear risk | Not specified ‐ only specified that allocation was random |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants were blind to treatment condition and assured confidentiality at each assessment. Biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rates were 80% at 1 and 6 months and 86% at 3 months, and did not differ by group. |
Colby 2012.
| Methods | Study design: RCT Location: USA Setting: "in a private setting", conducted face‐to‐face and via telephone Recruitment: from various sites: emergency department, hospital‐based adolescent outpatient clinic, paediatrician's office, high schools. In medical settings, flyers advertising the study were posted and research staff proactively screened and recruited patients waiting for appointments/treatment. In schools, classroom presentations were made and table displays in school cafeterias provided study information during lunch. Adolescents in the general community who heard about the study through flyers, radio ads, and word of mouth called the research office and were screened for eligibility. |
|
| Participants | Defining eligibility criteria?: adolescent smokers aged 14 to 18 years Participant characteristics: 162 adolescents; 77/162 (47.5%) female; mean age: 16.2; mean cpd: intervention: 11.3; control: 9.2; nicotine dependence: mean Stanford Dependence Index: intervention:14.1; control: 13.5 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: brief advice (BA) followed by a 5‐minute telephone booster. Participants who reported quit attempts were praised; those who reported continued smoking were strongly encouraged to try to quit as soon as possible. Baseline parent assessment by telephone. Handouts on quitting smoking mailed to adolescents and parents. Intervention: motivational interviewing: "Interventionists' therapeutic style followed MI principles (Miller 2002). The MI manual included the following sections: 1) establishing rapport; 2) exploring pros and cons of smoking and quitting; 3) delivery of computer‐generated personalized assessment feedback; 4) imagining the future with and without smoking; 5) reviewing a menu of change options and developing a change plan; and 6) enhancing self‐efficacy for change." MI participants were provided with the same handouts as in BA, and also received an assessment feedback sheet and change plan. The length of the baseline session was 45 min, with a 15 to 20‐min telephone booster one week later, designed to reinforce progress toward goals. The interventionist assisted in problem‐solving, discussed coping skills, promoted self‐efficacy for change, and updated change plans if appropriate. Revised change plans were mailed to participants afterwards. This group also received a parent intervention: parents of MI participants took part in a 15 to 20‐min discussion. This intervention was also designed to be consistent with MI principles, emphasised the adolescent's responsibility for making decisions/changes related to smoking, and focussed on increasing parent support for the adolescent's goals for changing smoking, increasing clear communication, and establishing home smoking rules. Interventionists used open‐ended questions to elicit information about the parent's attitudes and behaviour relevant to these topics and, based on parent interest, introduced strategies for enhancing communication, enforcing household smoking restrictions, and reinforcing adolescent efforts toward change goals. Provider: counsellors Intensity: 1 x 45‐minute session and 1 x 15 to 20‐minute booster call Was MI fidelity monitored?: "Interventionists participated in weekly group supervision. Post‐MI and BA, interventionists and adolescent participants completed session ratings." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: expired air CO (< 9 ppm); saliva cotinine (< 14 ng/mL) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "Funded by NIDA grant # 1R01 DA11204. Preparation of the manuscript was also supported by NIAAA grant # 1R01 AA016000 and NIDA grant # 1T32 DA016184. NIDA and NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication." | |
| Author conflicts of interest | "All authors declare that they have no conflicts of interest". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "A computer‐generated random number sequence allocated participants to treatment groups prior to enrollment". |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "assignments were sealed in envelopes which were filed in a series of sequentially numbered folders. Interventionists used folders in order and completed baseline assessment before opening the envelope." However, it was not stated whether envelopes were opaque, hence unclear rating. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Two biochemical markers were used to validate self‐reported abstinence at follow up"; QUOTE: "all interviewers were blind to condition assignment during assessments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | QUOTE: "There were no significant group differences on booster or follow‐up completion rates". Follow‐up rates at 6 months: MI (n = 61; 77.2%); BA (n = 71; 85.5%) |
Cook 2016.
| Methods | Study design: factorial RCT (4 factors, 16 trial arms) Location: USA Setting: primary care clinics Recruitment: smokers were invited during primary care clinic visits to participate in a research program to help them to reduce their smoking. Those interested were referred electronically to the research office. |
|
| Participants | Defining eligibility criteria?: adult smokers with no interest in quitting in the next 30 days but willing to cut down Participant characteristics: 517 adult smokers; 328/517 (63.4%) female; mean age: 47; mean cpd: 17.5; nicotine dependence: mean FTND = 4.8 Motivation to quit?: not motivated to quit |
|
| Interventions | Intervention factors: 1. Motivational interviewing: initial 20‐minute in‐person counselling session followed by three biweekly, 10‐minute counselling calls over the 6‐week intervention period. Based on Miller & Rollnick (Miller 2002), the counselling sessions included motivation‐building exercises to reinforce intrinsic motivation and to help participants overcome ambivalence about quitting. Case managers engaged participants in a series of motivation building exercises such as reviewing feelings and thoughts about the pros and cons of quitting and smoking, reinforcing the positives of quitting, helping to dispel myths and concerns about the negatives of quitting, and posing questions about the "good" aspects of smoking. 2. Behavioural smoking reduction counselling: initial 20‐minute in‐person counselling session followed by six weekly 10‐minute counselling calls. During these sessions, participants set smoking reduction goals and developed reduction strategies (e.g. delaying smoking, eliminating smoking in specific situations). Participants were also instructed to record daily smoking, which case managers used to identify successes and challenges. 3. Nicotine gum: participants were instructed to use 2 mg gum for the 6‐week intervention period (≥ nine per day, one piece per 1–2 hours) in place of smoking. 4. Nicotine patch: participants were instructed to use 14 mg patches daily for the 6‐week intervention period. Where all intervention factors were OFF, this resulted in a 'no treatment' condition. Provider: counsellors Intensity: 1 x 20 minutes face‐to‐face session, followed by fortnightly 10‐minute phone calls Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 26 weeks Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This research was supported by grants 9P50CA143188 and 1K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. This work was carried out in part while T.R.S. was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. W.‐Y.L. is also supported by NSF grant DMS‐1305725. L.M.C. is also supported by NIH grants P50DA10075 and R01DK097364. J.W.C. is supported by Merit Review Award 101CX00056 from the US Department of Veterans Affairs." | |
| Author conflicts of interest | "The authors have received no direct or indirect funding from, nor do they have a connection with, the tobacco, alcohol, pharmaceutical or gaming industries or anybody funded substantially by one of these organizations. W.‐Y.L. is supported partially by a grant from Eli Lilly and Company for research that is unrelated to smoking or tobacco dependence treatment." | |
| Notes | In‐line with guidance in the Cochrane Handbook, we looked for potential interactions between the factors in this factorial trial. An interaction between the MI and nicotine gum factor was reported by the authors. Rather than exclude data from this trial from analyses, which we believe would introduce bias we accounted for the risk of bias potentially introduced by this interaction in our 'Risk of bias assessment' below and carried out sensitivity analyses removing it from analyses alongside other studies judged to be at high risk of bias. For the MI versus no treatment analyses, we compared the one study arm with MI and no other smoking cessation treatment (behavioural reduction, gum and patch) to the one study arm with no MI and no other smoking cessation treatment. For the analyses comparing MI plus other SC treatment to other treatment alone, we compared any study arms receiving MI plus reduction and/or nicotine gum, and/or nicotine patch to study arms receiving reduction, and/or nicotine gum, and/or nicotine patch with no MI. For the analyses comparing MI to another form of smoking cessation treatment, we compared the one study arm receiving MI and no other smoking cessation treatment (behavioural reduction, gum and patch) to all study arms receiving reduction and/or nicotine gum, and/or nicotine patch. Where relevant (analyses 2 and 3), we have ensured that study arms that received nicotine gum have been entered into analyses separately to study arms that did not receive nicotine gum. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to treatment conditions using stratified permuted, computer‐generated block randomisation; stratified by gender and clinic with a fixed block size of 16 based on the 16 unique possible combinations of intervention components (in random order within each block). |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report (no biochemical validation). The lack of MI meant that participants had less face‐to‐face contact and less intensive support in some of the comparison trial arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no significant differences in missing data across the contrasting levels of each intervention factor. 46/253 (18%) were lost to follow‐up in the MI groups and 37/264 (14%) in the non‐MI groups. |
| Other bias | High risk | This is a factorial trial and an interaction was found between motivational interviewing and nicotine gum. This was not an a priori hypothesised interaction, and challenged the assumption that the factors studied were independent. |
Davis 2011.
| Methods | Study design: RCT Location: USA Setting: laboratory Recruitment: precontemplative and contemplative smokers were recruited from the community through advertisements and direct recruitment (no further explanation). Participants were offered USD 25 for participation. |
|
| Participants | Defining eligibility criteria?: unmotivated adult smokers Participant characteristics: 218 adult smokers randomised to intervention (109) and control (109), 45% female; mean age 37.6. cpd: 25.4 Motivation to quit?: not motivated |
|
| Interventions | Control: Prescriptive 15‐min interview regarding smoking. Described as the current dominant approach (i.e. usual care), which maintains a firm and authoritative approach Intervention: 15‐min motivational interview regarding smoking. Motivational interviewing described as seeking to establish supportive and empathic alliance Provider: nurses Intensity: 1 x 15‐minute session Was MI fidelity monitored?: "All tapes were reviewed for adherence to the protocol and weekly meetings were held with the study nurses. Sessions not reaching criterion were removed from the analyses". |
|
| Outcomes | Definition of cessation used: point prevalence Length of longest follow‐up: 6 months Validation: urinary cotinine Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from The Arizona Disease Control Research Commission | |
| Author conflicts of interest | Not reported | |
| Notes | The outcome used for meta‐analysis was point prevalence reported at both 1 m and 6 m (i.e. cross between point prevalence and prolonged abstinence). This outcome was used as for all others, the manner of reporting made it impossible to tell which time point numbers referred to (i.e. abstinent at 1 m or 6 m). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "participants completed informed consent, baseline assessments, and were randomized to receive either a 15‐min motivational or prescriptive interview". No further information given |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "participants completed informed consent, baseline assessments, and were randomized to receive either a 15‐min motivational or prescriptive interview". No further information given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was verified through urinary cotinine levels. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47/116 (41%) in the motivational arm and 61/114 (54%) in the prescriptive arm were lost to follow‐up at 6 months. Overall less than 50% loss to follow‐up ‐ similar rates between arms |
De Azevedo 2010.
| Methods | Study design: RCT Location: Brazil Setting: public university hospital Recruitment: patients admitted to a public university hospital approached by research team to take part ‐ screening interview took place at patients’ bedside within 72 hours of admission |
|
| Participants | Defining eligibility criteria?: hospital inpatients for variety of reasons Participant characteristics: 273 adult smokers randomised to intervention (141) and control (132), 63.6% M; mean age 47; cpd (range = 11 to 20) Motivation to quit?: not selected on motivation |
|
| Interventions | 1. Control: 15‐min session of individual counselling where participants were advised to stop smoking. Counsellor reviewed the dangers of smoking and benefits of quitting. The counsellor suggested that, after discharge, the participant should seek help to stop smoking. 2. Intervention: 30‐min session of individual counselling consisting of a motivational interview, after hospital discharge. Participants were given 7 follow‐up telephone calls over 6 m (at 1, 2 and 3 weeks, and at 1, 2, 3 and 4 m). Each call lasted 10 mins. It was an opportunity to reinforce motivation for stopping smoking (or maintaining abstinence). Style of interview was in line with MI performed during hospitalisation. Intervention provider: smoking cessation advisor Intensity: 1 x 30‐minute session with 7 x 15‐minute follow‐up calls over 4 months Was MI fidelity monitored?: "Counselors and main researchers met fortnightly along the study period for clarification of any doubt that might have arisen." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the Research Foundation of the State of São Paulo (grant no. 06/61885‐6) | |
| Author conflicts of interest | Not reported | |
| Notes | There were 3 arms in the study; however, the usual‐care arm was not randomised, so was not eligible for the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "An allocation sequence based on a random‐number table was used to randomly assign all enrolled subjects to either LII or HII." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "The allocation was maintained in a serially numbered, opaque envelope, which was opened at the Phase 2 interview to prevent counselor bias." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Abstinence was obtained via self‐report and the amount of researcher‐participant contact varied between trial arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34/141 (24%) participants in the MI intervention group and 24/132 (18%) in the control group were lost to follow‐up. Less than 50% lost overall and similar between groups |
Demétrio Faustino‐Silva 2018.
| Methods | Study design: cluster RCT Location: Brazil Setting: primary care smoking cessation clinics Recruitment: from smoking groups performed by the primary care teams of the Conceição Hospitalar Group, Porto Alegre, Brasil |
|
| Participants | Defining eligibility criteria?: adult smokers motivated to quit Participant characteristics: 329 adult smokers; gender not provided; mean age: not provided; mean cpd: not provided; nicotine dependence: not provided Motivation to quit?: motivated (already attending smoking group) |
|
| Interventions | Control: traditional CBT, as advocated by the Brazilian Ministry of Health's smoking programme Intervention: motivational interviewing: "The professionals who coordinated the smoking groups were trained to use Motivational Interviewing as an additional resource to the motivation and cognitive‐behavioral approach usually performed in the groups." Provider: Smoking cessation advisors Intensity: not reported Was MI fidelity monitored?: none reported |
|
| Outcomes | Definition of cessation used: 30‐day point prevalence Length of longest follow‐up: 11 months Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Not reported | |
| Author conflicts of interest | Not reported | |
| Notes | Information from conference abstract. Limited information available on how clustering dealt with. We entered data as presented in abstract and carried out sensitivity analysis applying the ICC of another similar study (Vidrine 2019). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further information given |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not look as though any biochemical validation took place and it was unclear whether the amount of contact with investigators was the same across study arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers lost to follow‐up were less than 50% overall and similar between groups (34.8% in MI group; 42.4% in control group). |
Ellerbeck 2009.
| Methods | Study design: randomised controlled trial Location: Kansas, USA Setting: rural primary care practices Recruitment: from 50 rural primary care practices in the Kansas Physicians Engaged in Prevention Research network. Trained medical students systematically screened patients, identified smokers, and recruited them for the study, obtaining consent. Participants’ contact information was forwarded to research staff who contacted them via telephone, verified eligibility, and conducted the baseline survey. |
|
| Participants | Defining eligibility criteria?: primary care patients Participant characteristics: 726 adult smokers randomised to intervention (482) and control (244), 58.5% female; mean age 47.2; cpd 23.7 Motivation to quit?: general population (not selected on motivation) |
|
| Interventions | Intervention: high intensity: educational support, telephone counselling, periodic progress reports with counselling suggestions faxed to their physician, and a 6‐monthly personalised KanQuit newsletter with tips on quitting smoking. Offered up to 6 counselling calls every 6 months to either promote quitting or prevent relapse. Counsellors used MI techniques and followed a semi‐structured protocol. Control: moderate‐intensity MI: as intervention, however were only offered up to 2 telephone‐based counselling sessions every 6 months (1 session to promote a quit attempt and 1 additional follow‐up session for those who made a quit attempt). Provider: counsellors Intensity: twice every 6 months in moderate intensity arm; 6 times every 6 months in high intensity arm Was MI fidelity monitored?: "After each session, counsellors were asked to complete a checklist asking about several of the key concepts of MI that served as process markers of appropriate MI delivery (e.g. rolling with resistance, making appropriate reflective statements, encouraging change talk) and a checklist asking about specific content that should have been covered per the MI protocol guiding the sessions (e.g. pros/cons of quitting, developing behavioural action plan). Counsellors rated themselves and during supervision they were also rated using motivational interview markers." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 24 months Validation: salivary cotinine level < 15 ng/mL in a mailed saliva sample. Because of resistance by participants to providing salivary samples at month 12, validation by proxy report from a significant other at month 24 was used for quitters who did not return a salivary sample. The validated quit rate at 24 months was a mixture of the 2 approaches. Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Cancer Institute (R01‐101963). Study medication provided by GlaxoSmithKline | |
| Author conflicts of interest | Authors reported no conflicts. | |
| Notes | We compared the high intensity MI group and the moderate intensity MI group in this review and in our analyses investigating the intensity of MI counselling support. The study also included a 'pharmacotherapy alone' condition; however this was not relevant to this review as it included non‐MI intervention components that were not received by the two MI intervention groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "A computer‐generated random‐number table was used to generate allocation cards in blocks of 24, with allocation equally distributed across treatment groups." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "To conceal allocation, we placed these [allocation] cards in sequentially numbered, opaque, sealed envelopes. After research assistants verified participant eligibility and completed the baseline assessment, the project director opened the next sequential sealed envelope and determined the participant’s treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Abstinence validated by salivary cotinine measurement (15 ng/mL) or a significant other." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50/249 (20%) in the moderate intensity disease management group and 47/251 (19%) in the high intensity disease management were lost to follow‐up at the final follow‐up (month 24). |
Harris 2010.
| Methods | Study design: cluster RCT Location: USA Setting: university Recruitment: proactive recruitment at fraternity and sorority chapter meetings at 1 large Midwestern university at the start of 3 academic years (2006 ‐ 2008) |
|
| Participants | Defining eligibility criteria?: university students (sorority or fraternity members) Participant characteristics: 452 adult smokers (college students) randomised to intervention (245) and control (207), 54.4% M; mean age 19.5; cpd 3.5 Motivation to quit?: general population (not selected on motivation) |
|
| Interventions | 1. Control: no smoking cessation treatment ‐ up to 4 sessions of MI focussed on increasing consumption of fruits and vegetables to at least 5 servings a day. The first 3 sessions occurred approximately every other week following baseline assessment and the fourth session occurred approximately 4 weeks after session 3. Sessions were typically 20 to 30 mins. A self‐help guide on the benefits and methods for eating fruit and vegetables was given to participants. 2. Intervention: up to 4 sessions of MI focussed on motivating and assisting participants to quit cigarette smoking. The first 3 sessions occurred approximately every other week following baseline and the fourth approximately 4 weeks after session 3. Sessions were typically 20 to 30 mins. For students who became motivated to change during the sessions, counsellors used a MI style to follow the outline of a 'plan module' in which cognitive–behavioural principles were used to develop a change plan. A self‐help guide on quitting was also given to participants. All students who smoked at a high level were encouraged to use pharmacotherapy obtainable through the university and other resources. Provider: clinical or counselling psychology students (counsellors) Intensity: 4 x 20 to 30‐min sessions over 7 weeks Was MI fidelity monitored?: "We assessed fidelity to MI using supervisors' rating of counselors' in‐session proficiency on 18 items, including reflective listening, asking permission, and MI spirit, used in prior studies". |
|
| Outcomes | Definition of cessation used: 30‐day point prevalence Length of longest follow‐up: 6 months Validation: saliva cotinine ≤ 15 ng/mL Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Cancer Institute (R01CA107191) | |
| Author conflicts of interest | "The authors declare that there are no conflicts of interest". | |
| Notes | An ICC of 0.003 is reported, which implied (30 clusters, n = 452) a design effect of 1 + (452/30 − 1) × 0.003 = 1.0422. We applied this design effect to account for clustering in our analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "For each cohort, after all students completed the baseline survey, fraternities and sororities were randomized to either treatment (smoking) or comparison (fruits/vegetables) conditions without blocking". No further information given |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "For each cohort, after all students completed the baseline survey, fraternities and sororities were randomized to either treatment (smoking) or comparison (fruits/vegetables) conditions without blocking". No further information given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was verified using cotinine and amount of contact matched between trial arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25/245 (10%) in the MI for smoking cessation group and 23/207 (11%) in the MI for fruits and vegetables group were lost to follow‐up. Therefore, loss to follow‐up was low and similar between groups. |
Helstrom 2007.
| Methods | Study design: RCT Location: USA Setting: community Recruitment: "Participants identified from larger longitudinal study of problem drinking in adolescents. Eligible adolescents were located through telephone calls to randomly generated telephone numbers." |
|
| Participants | Defining eligibility criteria?: adolescent offenders who had been arrested or given notice to appear in court Participant characteristics: 81 adolescent smokers; 34/81 (42%) female; mean age: 16; mean cpd: intervention: 11.22; control: 9.56; nicotine dependence: not reported Motivation to quit?: not selected on motivation |
|
| Interventions | Control: tobacco education: an information session based on a pamphlet about tobacco use by the American Cancer Society Intervention: motivational enhancement therapy (MET): MET sessions began with individualised feedback about participants' smoking based on information from the baseline assessment. Then, participants' likes/dislikes, beliefs, and pattern of tobacco use were discussed and participants were assisted in identifying goals for behaviour change and addressing their ambivalence about their smoking. For participants ready to make changes, cessation strategies were provided, goals were defined, and a behaviour change plan was developed. Provider: not specified Intensity: one session ‐ duration not specified Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 28‐day point prevalence Length of longest follow‐up: 6 months Validation: salivary cotinine (<= 15 ng/mL) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Research supported by "a grant from the National Institute on Drug Abuse awarded to the first author (DA13182‐02)" | |
| Author conflicts of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No methods specified beyond reporting that participants were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | No methods specified beyond reporting that participants were randomly allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was verified using salivary cotinine. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fewer than 50% of participants dropped out overall; there was a difference in dropout rates between groups (6.7% in the MET arm and 25% in the education control arm), however this did not meet the threshold advised by the Cochrane Tobacco Addiction Group (difference of 20% or more in follow‐up between arms) to signal a 'high' risk of bias. |
Hollis 2007.
| Methods | Study design: factorial RCT (3 x 2) Location: Oregon USA Setting: community‐based telephone quit‐line programme Recruitment: callers to quit‐line invited to participate |
|
| Participants | Defining eligibility criteria?: quit‐line callers Participant characteristics: 4614 smokers randomised to: brief counselling (872, no NRT; 868, with NRT), moderate counselling (718, no NRT; 715, with NRT), or intensive counselling (720, no NRT; 721, with NRT). 60% female, mean age 41. Mean cpd 21. Motivation to quit?: as participants were callers to a telephone quit‐line, they were assumed to be fully or partly motivated to quit. |
|
| Interventions | Two factors: intensity of MI counselling and NRT versus no NRT. The three levels of the intensity factor were as follows: 1. Single brief (15‐min) negotiation based on MI (usual care), 15‐min call + referral material + tailored self‐help materials 2. Moderate counselling (40‐min) based on MI + 1 brief call to encourage use of community services, tailored self‐help materials 3. Intensive counselling (as moderate counselling, plus offer of ≤ 4 additional telephone calls). Each call incorporated MI techniques, stage assessment and relapse prevention as needed. NRT offered free to the 'with NRT' groups Provider: smoking cessation advisors Intensity: brief: one off; moderate: 1‐2 weekly; intensive: 4 calls over 3 months Was MI fidelity monitored?: "We taped calls for quality assurance monitoring and rated counsellors regularly on adherence to key elements of each protocol." |
|
| Outcomes | Definition of cessation used: 30‐day point prevalence Length of longest follow‐up: 12 months Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Cancer Institute (R01 CA86242). Nicotine patches supplied by GlaxoSmithKline | |
| Author conflicts of interest | "JFH, JLF and KR have no competing interests. TAMcA and SMZ are with Free & Clear, Inc, which is a for‐profit company providing telephone counselling services." | |
| Notes | We compared the brief intensity MI to the high intensity MI in our analyses. NRT versus no NRT groups were combined as no interaction effects were detected. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "a computer algorithm randomly assigned participants". |
| Allocation concealment (selection bias) | Low risk | QUOTE: "a computer algorithm randomly assigned participants". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation of cessation and amount of contact varied between arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 550/1740 (32%) of the brief groups; 448/1433 (31%) of the moderate groups and 497/1441 (35%) of the intensive groups were lost to follow‐up at the 12‐month follow‐up. Therefore, loss to follow‐up overall was less than 50% and similar between arms. |
Kelly 2006.
| Methods | Study design: RCT Location: Australia Setting: community Recruitment: high school students caught smoking were recruited. "Participants were included if the drug of concern was tobacco and if parent/guardian active informed consent was obtained." |
|
| Participants | Defining eligibility criteria?: high school student smokers aged 14 to 16 years Participant characteristics: 56 adolescent smokers; 19/56 (34%) female; mean age: 15; mean cpd: 7.4; nicotine dependence: mean nicotine dependence (MTFQ): 3.6 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: standard care: included an education about the broad effects of smoking regardless of the participant's experience. Built on a widely used psychoeducation model, where knowledge dissemination/attainment was assumed to result in change. This involved reviewing reading materials on the effects of smoking (and other drugs) and a 'Quit kit' on smoking. Intervention: MI: explored the meaning of smoking in participants' lives, the positives and negatives of smoking/quitting, the impact of smoking on self‐concept, health goals, and identification of obstacles to goal attainment. The intervention included information only where relevant to the participant's direct experiences (e.g. effects of smoking on respiration if breathlessness in sport was reported). All participants provided with reading materials Provider: "The two interventions were delivered by the second author (KL), a PhD candidate and registered psychologist, with 4 years experience in adolescent psychotherapy." (counsellor/psychologist) Intensity: single 1‐hour session Was MI fidelity monitored?: "The therapy manual documented a number of behavioral indices that would normally characterize close adherence to the two interventions. Relative to the SC condition, the MI intervention would normally be characterized by: more talking by the participant than the therapist, open probes, summary statements aimed to develop discrepancy, asking permission of the student to extend/expand the content focus, and reflexive delivery of intervention components. The therapist regularly completed a behavioral checklist for each session to reduce content drift/contamination and promote discussion during supervision." |
|
| Outcomes | Definition of cessation used: 30‐day point prevalence abstinence Length of longest follow‐up: 6 months Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "The manuscript was completed during an NHMRC Career Development Award to the first author. The study was funded by NHMRC Project 189414 awarded to the first author." | |
| Author conflicts of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Students were randomly assigned to either the MI or SC conditions." No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Students were randomly assigned to either the MI or SC conditions." No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status measured by self‐report; MI was one hour but length of SC not stated. SC appeared to include less contact with therapist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was less than 50% overall and similar between groups according to the standard practice of the Cochrane Tobacco Addiction Group (6/30; 20% in MI group; 8/26; 31% in standard care group). |
Lewis 1998.
| Methods | Study design: RCT Location: USA Setting: hospital and over telephone Recruitment: from inpatients admitted to the University of Wisconsin Hospital and clinics who expressed an interest in quitting smoking |
|
| Participants | Defining eligibility criteria?: hospital inpatient smokers interested in quitting Participant characteristics: 185 adult smokers; 85/185 (46.0%) female; mean age: control: 43, intervention: 44.7; mean cpd: control: 22.5, intervention: 24.9; nicotine dependence: mean FTND = control: 6.6, intervention: 6.9 Motivation to quit?: motivated |
|
| Interventions | Control: minimal care: brief (2–3 min) motivational message to quit smoking and a copy of the National Cancer Institute’s Clearing the Air self‐help smoking cessation pamphlet Intervention: counselling and placebo patch: as control, plus a placebo nicotine replacement patch, and a study nurse provided brief (10 to 15 minute) phone counselling at 1, 3, 6, and 24 weeks after the initiation of patch treatment. Phone counselling incorporated basic techniques of cognitive‐behavioural therapy and motivational interviewing. The nurse also frequently reminded participants of the Clearing the Air pamphlets and encouraged them to look over the pamphlet between sessions. Provider: nurse Intensity: five 10 to 15‐minute sessions over 24 weeks Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 24 weeks Validation: expired carbon monoxide <= 10 ppm Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This research was supported by a research grant provided by the Elan Pharmaceutical Research Corporation, Gainsville, Georgia, and Athlone, Ireland." | |
| Author conflicts of interest | Not specified | |
| Notes | This study also included an additional intervention arm, which was the same as the intervention arm reported above but included active rather than placebo nicotine patch. This study arm was not eligible for this review and was not included in analyses as the use of pharmacotherapy was not matched to the control arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patient was randomized to either the MC condition or a patch condition using a predetermined computer‐generated randomization code." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patient was randomized to either the MC condition or a patch condition using a predetermined computer‐generated randomization code." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was biochemically verified. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates not reported |
Lloyd‐Richardson 2009.
| Methods | Study design: RCT Location: USA Setting: immunology clinics (6 outpatient HIV clinics and 2 primary care medical offices) Recruitment: patients who smoked, were deemed eligible to participate by their physician, and were willing to speak with a health educator (HE) were referred to the study. |
|
| Participants | Defining eligibility criteria?: HIV‐positive Participant characteristics: 444 HIV‐positive, adult smokers, randomised to intervention (232) and control (212); 56.7% female; mean age 42.0; cpd 18.3 Motivation to quit?: not selected on motivation |
|
| Interventions | 1. Control: NRT + brief standard care intervention (SC). 2 brief sessions, including brief assessment of quitting plans. Participants returned to the clinic biweekly for distribution of additional patches, allowing the counsellors to briefly (5 mins) reinforce quit efforts, check on patch side effects and answer questions. HEs were instructed to provide praise of participant’s efforts and answer any questions asked, but not to initiate additional discussion of the quit effort. Participants unwilling to set a quit date were instructed to contact the counsellor when ready. This reflects the minimum standard of care recommended by the Agency for Health Research and Quality (AHRQ). 2. Intervention: NRT + intensive motivationally enhanced counselling intervention (ME). Participants received 4 30‐min intervention sessions, as well as a quit‐day counselling call. Quit dates determined by individual participants in consultation with counsellors. MI elements delivered throughout all contacts. Participants not willing to set a quit date were engaged in discussion of ‘quitting as a process’ and barriers to quitting. Provider: counsellors Intensity: 4 biweekly medication contacts plus 5 counselling contacts Was MI fidelity monitored?: "We monitored the delivery of both intervention conditions by: (i) audio‐taped supervision on a random subsample of counseling sessions; (ii) patient exit interviews (conducted by the intervention‐blinded research assistant); and (iii) documentation of time spent in each intervention. To examine fidelity to each intervention protocol, two independent raters reviewed audio tapes of 20% of all sessions and rated (i) the degree to which intervention providers of the ME intervention adhered faithfully to the spirit of motivational interviewing (i.e. establish rapport, express empathy, reflective listening, explore ambivalence); and (ii) the degree to which there was contamination across conditions." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: exhaled CO (< 10 ppm) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Grants from the National Institute of Drug Abuse (R01‐DA12344‐06), the National Heart, Lung and Blood Institute (K23‐HL069987), the National Cancer Institute (K07‐CA95623), the NIH‐funded Transdisciplinary Tobacco Use Research Center (P50 CA084719), NIH‐funded Lifespan/Tufts/Brown Center for AIDS Research (P30 AI42853), and by the Robert Wood Johnson Foundation | |
| Author conflicts of interest | Paper stated that authors had no declarations of conflicts of interests. | |
| Notes | Different Ns and different loss to follow‐up allocated to intervention and control arms in the results section in comparison to the participant flow chart. Table 1 seemed consistent with text. Data inferred based on this assumption as there was no response to a data request from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Patients were then randomized (using block randomization) to ensure stratification by gender and level of motivation to quit smoking". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Patients were then randomized (using block randomization) to ensure stratification by gender and level of motivation to quit smoking". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation validated using exhaled carbon monoxide measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60/212 (28%) in the intervention (ME) group, and 66/232 (28%) in the control (SC) group were lost to follow‐up at 6 months. Therefore, rates were similar between groups. |
Louwagie 2014.
| Methods | Study design: RCT Location: South Africa Setting: tuberculosis (TB) clinics Recruitment: newly diagnosed adult patients initiating TB treatment were approached to participate. |
|
| Participants | Defining eligibility criteria?: TB patients initiating TB treatment Participant characteristics: 409 adult smokers newly diagnosed with TB randomised to intervention (205) and control (204); 10% female; mean age 41.3; cpd 10.0 Motivation to quit?: not selected on motivation |
|
| Interventions | Control (brief smoking cessation advice): the following short standardised smoking cessation message from the TB nurse: "Tobacco use is extremely harmful for your health. If you stop smoking now, your TB will heal better and you will have a lower risk of getting TB again in the future. You will also reduce your risk of heart disease and cancer and protect your children against TB. As a professional nurse, I advise you to stop using tobacco in the interests of your health", plus a smoking cessation booklet supplied by the National Council against Smoking of South Africa 2. Intervention (brief motivational interviewing): as control, plus a brief motivational interviewing session (15 to 20 mins) consisting of a quick assessment, the participant identifying problems and solutions and the setting of targets. Participants already highly motivated to quit were helped to design a quit plan. Provider: lay healthcare workers Intensity: single 15 to 20‐minute session Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: only occurred in small subset of participants and so has not been used; however outcomes were the same with validation. As participants did not know whether the monitor was allocated to their clinics at specific time points, this approach introduced a 'bogus pipeline' procedure, thus increasing the likelihood of truthful answers. Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Grants from the KNCV Tuberculosis Foundation (12.402.2/MvdW/U.10.0696/cal), and the National Research Foundation of South Africa (80843), and by the Global Bridges Health Care Alliance for Tobacco Dependence Treatment | |
| Author conflicts of interest | "K.O. received Pfizer funding for an FDA‐approved research project (unrelated to this project) involving the use of nicotine patch, bupropion and varenicline. O.A.A.‐Y. is a sub‐awardee of an unrestricted Pfizer Education grant to Mayo Clinic for the Global Bridges Health Alliance project and received an honorarium as a speaker at the 2012 congress of the South African Dental Association for a session on treatment funded by Pfizer". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "The randomization sequence was generated by an independent epidemiologist who was not otherwise involved in the research project, with a 1:1 allocation and random block sizes of 2, 4, 6, 8 and 10." No further information given therefore method of sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Current smokers were then allocated by the LHCWs to either the intervention or the control arm by means of sequentially numbered sealed opaque envelopes, thus ensuring allocation concealment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Validation occurred in a small subset of participants and has not been applied to data; however, outcomes were the same with validation. As participants did not know whether the monitor was allocated to their clinics at specific time points, this approach introduced a 'bogus pipeline' procedure, thus increasing the likelihood of truthful answer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 53/205 (26%) in the intervention arm and 43/204 (21%) in the control arm were lost to follow‐up at 6 months. Therefore rates were similar between arms. |
Marshall 2016.
| Methods | Study design: RCT Location: Australia Setting: outpatient clinic Recruitment: "Smokers in the Queensland Lung Cancer Screening Study were invited to enrol in the sub‐study by letter prior to each scheduled CT screening scan". |
|
| Participants | Defining eligibility criteria?: attending lung cancer screening (age 60 to 74 years; ≥ 30 pack year smoking) Participant characteristics: 55 older smokers; 20/55 (36.4%) female; mean age: control = 63.0, intervention = 63.0; mean cpd: control = 25.0, intervention = 25.0; nicotine dependence: mean FTND = control = 6.0, intervention = 6.0 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: non‐tailored printed materials, quit‐line details Intervention: MI counselling: single face‐to‐face counselling session on the day of attendance for lung cancer screening plus audio cessation advice (on mp3 player), plus written quit materials Provider: physician Intensity: one‐off session lasting an average of 26.5 minutes Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 12 months Validation: exhaled carbon monoxide >= 10 parts per million "However CO not always obtainable at the 12‐month time point because the CT protocol allowed scans to take place between 11 and 15 months after the previous scan (one intervention group and three control group quitters had exhaled CO < 10 ppm, the remainder were not tested)." Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "Queensland Health, Smart State Research Grant (388600); National Health and Medical Research Council (NHMRC) National Research Centre for Asbestos‐Related Diseases (NRCARD) (440812); The Prince Charles Hospital Foundation (FRC0207‐24)." | |
| Author conflicts of interest | "None to declare" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocation sequence was generated at randomisation using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Group allocation was concealed at randomization". No further detail given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although biochemical verification was planned, the investigators could not attempt it in all participants due to the timing of hospital appointments. Therefore, we have not been able to use verified rates. Amount and intensity of contact differed between study groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/28 (10.7%) intervention group participants and 2/27 (7.4%) control group participants did not return 12‐month questionnaires and were assumed to be smokers. |
Matuszewski 2018.
| Methods | Study design: RCT Location: USA Setting: hospital Recruitment: inpatients with an operative fracture were enrolled from hospital |
|
| Participants | Defining eligibility criteria?: hospital inpatients with an operative fracture Participant characteristics: 237 smokers; sex: not reported; mean age: not reported; mean cpd: not reported; nicotine dependence: not reported Motivation to quit?: not selected on motivation |
|
| Interventions | Control: standard care: informational materials about smoking cessation, referred to the patient resource centre and provided with a quit‐line brochure Intervention 1: standard care + brief counselling + extended follow‐up: as control, plus 10 to 30 minutes of guided discussion about the risks and benefits of smoking for the healing of their traumatic injuries, plus smoking educator checked in with participants' progress for approximately 5 minutes at follow‐up appointments Intervention 2: standard care + brief counselling: as control, plus 10 to 30 minutes of guided discussion about the risks and benefits of smoking for the healing of their traumatic injuries. No additional 'check‐in' at follow‐up Provider: smoking cessation advisors Intensity: single 20 to 30‐minute face‐to‐face session. Intervention 1 also received 5‐minute check‐ins at 2 weeks, 6 weeks, 3 months and 6 months. Was MI fidelity monitored?: unclear |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: exhaled carbon monoxide (8 ppm) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Not reported | |
| Author conflicts of interest | Not reported | |
| Notes | Completed trial, not published yet. Has been presented at 2018 Annual Meeting of the Orthopaedic Trauma Association in Kissimmee (Orlando), Florida, October 17‐20 2018. Unable to calculate numbers quit from abstract, contacted authors for quit rates, however did not receive all the information needed. Study discussed narratively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation: randomised. No further information |
| Allocation concealment (selection bias) | Unclear risk | Allocation: randomised. No further information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was CO validated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout at 6 months not reported in conference abstract. Paper not yet published |
McClure 2005.
| Methods | Study design: RCT Location: USA Setting: Group Health Co‐operative, a staff‐model integrated health care organisation Recruitment: women smokers with an abnormal pap smear or colposcopy were invited to participate. |
|
| Participants | Defining eligibility criteria?: women with abnormal pap smear or a colposcopy within the preceding 2 months (i.e. elevated risk for cervical cancer) Participant characteristics: 275 women, randomised to intervention (138) or control (137). Mean age 33, Mean cpd 14 Motivation to quit?: not selected on motivation |
|
| Interventions | 1. Control: usual care: a letter explaining the association between cervical cancer and smoking, self‐help booklet, contact information for a phone‐based smoking cessation treatment programme. Encouraged to use NRT or bupropion
2. Intervention: as control, plus ME telephone counselling (4 x 15‐min proactive calls), focussed on motivation building and strengthening, action plans for quitting or relapse prevention strategies, depending on readiness to quit Provider: counsellors Intensity: one off mailing plus four 15‐minute calls over 6 months Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 12 months Validation: CO < 10 ppm or salivary cotinine, at 12 months only Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Grants from the National Cancer Institute (CA84603; CA74517), and the National Institute on Drug Abuse (DA11194) | |
| Author conflicts of interest | Not reported | |
| Notes | For this update, cessation rates were changed to 12‐month verified rates (taking into account data in table 2). The ones previously used were 6 m and 12 m combined self‐report (from table 3). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Participants were randomly assigned to usual care (UC) or motivationally enhanced counseling (MEC)." No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Participants were randomly assigned to usual care (UC) or motivationally enhanced counseling (MEC)." No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "biochemical confirmation of abstinence was obtained only at the 12‐month follow‐up from nonsmokers. Women were given the option of providing a breath sample in person or returning a salivary cotinine test strip by mail". 12‐month validated rates were used in our analyses as the amount of contact differed between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported |
Naik 2014.
| Methods | Study design: RCT Location: India Setting: prison Recruitment: random sampling of male prisoners at Central jail in Bangalore City |
|
| Participants | Defining eligibility criteria?: incarcerated males Participant characteristics: 600 adult smokers; 0/600 (0%) female; mode age: 21 to 30 years; mean cpd: 21 to 30 in intervention group, control not reported; nicotine dependence: not reported Motivation to quit?: not selected on motivation |
|
| Interventions | Control: waiting‐list control Intervention: motivational interviewing: the topics for the intervention included: introduction to tobacco, prevalence of tobacco use, effects of tobacco use on general health and dental health, psychosocial factors influencing tobacco use, healthy diet and behavioural intervention for prevention of tobacco use. Provider: not specified Intensity: not specified Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: unclear ‐ "stopped smoking" Length of longest follow‐up: 6 months Validation: Unclear ‐ CO was measured but didn't specify if used for validation. Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no, quality of life was measured in the intervention group only, not allowing comparison to the control group. |
|
| Funding source | "Nil" | |
| Author conflicts of interest | "None declared" | |
| Notes | Contacted authors to confirm that the control group received no smoking cessation treatment as this was unclear in the study report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Among 600 prisoners, 300 were selected for each group (study and control) by simple random sampling". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | "Among 600 prisoners, 300 were selected for each group (study and control) by simple random sampling". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether investigators were blind to treatment condition. Although carbon monoxide was measured, it was unclear whether these were used to validate cessation rates. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts provided |
| Other bias | Unclear risk | As reported in the publication, the content of the control group was unclear, therefore we contacted the authors. The authors replied as follows: "Control group received 3 month smoking cessation motivational intervention after finishing the study group intervention", suggesting that the control group were a waiting‐list control. Based on the extra information provided, it was unclear whether the control group received the intervention immediately after the study group, within the 6‐month follow‐up. We sought additional clarification on this from the authors but did not receive a response; however, the much higher result in the MI group suggested the control group did not receive the intervention before follow‐up. |
NCT02645838.
| Methods | Study design: RCT Location: China Setting: primary care Recruitment: via general practice |
|
| Participants | Defining eligibility criteria?: adult smokers Participant characteristics: 210 adult smokers; 12/210 (5.7%) female; mean age: 45.3; mean cpd: not reported; nicotine dependence: mean FTND = 4 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: brief smoking cessation advice Intervention: motivational interviewing: 20‐minute discussion with physician, determining stage of change in smoking cessation and using motivational interviewing skills Provider: physicians Intensity: single 20‐minute face‐to‐face session, plus up to six follow‐up calls Was MI fidelity monitored?: not stated |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: exhaled CO (< 10 ppm) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Not reported | |
| Author conflicts of interest | Not reported | |
| Notes | Contacted authors and received extensive additional data and information, beyond what was reported in trial registry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SAS 9.4 was used to generate random numbers, random grouping was used to perform random grouping, and random grouping schemes were sequentially saved into opaque sealed envelopes with sequential numbers. |
| Allocation concealment (selection bias) | Low risk | After the patient consented to take part in the study, the researcher opened the sealed opaque envelopes according to the envelope number sequence, and assigned the included smoking patients to the intervention group or the control according to the assignment in the envelope. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking cessation was biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/105 participants in the MI group were lost to follow‐up and 18/105 in the BA group. Therefore, loss to follow‐up was less than 20% and was similar between arms. |
Okuyemi 2013.
| Methods | Study design: RCT Location: USA Setting: emergency homeless shelters and transitional housing units Recruitment: through health fairs, staff informational sessions, fliers at homeless shelters and word of mouth |
|
| Participants | Defining eligibility criteria?: homeless people Participant characteristics: 430 homeless adult smokers randomised to intervention (216) and control (214); 74.7% M; mean age 44.4; cpd 19.3 Motivation to quit?: not selected on motivation |
|
| Interventions | 1. Control: single session of brief advice to quit smoking lasting approximately 10 to 15 mins. Included topics of smoking history, current smoking, direct advice about the health risks of smoking and the health benefits of quitting, affirmation of the participant’s decision to quit, an assessment of preparedness to quit and addressing strategies for coping with smoking cues 2. Intervention: six individual MI counselling sessions, each lasting 15 to 20 minutes, which occurred at baseline and follow‐up at weeks 1, 2, 4, 6 and 8. The focus of sessions was to encourage cessation and NRT adherence. Pharmacotherapy: At baseline, participants in both groups received a 2‐week supply of 21‐mg nicotine patches, and every 2 weeks they received an additional 2‐week supply of 21 mg nicotine patches, over the 8 week treatment period. All participants received health educational resource called The Power to Quit: A Quit Smoking Guide, developed by the project investigators, and a 2‐week supply of 21‐mg nicotine patches. Every 2 weeks, they received an additional 2‐week supply of 21 mg nicotine patches, over the 8‐week treatment period. Provider: counsellors Intensity: six 15 to 20‐minute sessions over 8 weeks Was MI fidelity monitored?: "Sessions were audio recorded and reviewed during weekly supervision". |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: expired carbon monoxide (≤ 10 ppm). Salivary cotinine testing was performed if the expired CO was greater than 10 ppm. for those who self‐reported cessation. A cut‐off of ≤ 20 ng/mL for salivary cotinine was used to verify abstinence. Was mental health and/or well‐being measured at follow‐up?: no, change in depression was measured but not reported by intervention group. Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Heart Lung and Blood Institute (R01HL081522) | |
| Author conflicts of interest | Paper stated that there were no conflicts. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "pre‐assigned randomization numbers prepared by the study statistician determined into which study arm the participant would be enrolled". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "pre‐assigned randomization numbers prepared by the study statistician determined into which study arm the participant would be enrolled". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A cessation was biochemically verified using exhaled CO and cotinine. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47/216 (22%) in MI intervention group and 59/214 (28%) in control group lost to follow‐up. Therefore, rates were similar between groups. |
Rohsenow 2014.
| Methods | Study design: RCT Location: USA Setting: state‐funded inner‐city residential substance abuse treatment programme with state‐wide catchment Recruitment: residents of the abstinence‐oriented programme were told the study would provide informational sessions about smoking without requiring cessation, and asked if they would like to take part. |
|
| Participants | Defining eligibility criteria?: alcohol‐dependent smokers Participant characteristics: 165 adult smokers meeting current alcohol dependence criteria, randomised to intervention (80) and control (85); 32.4% female; mean age 33.8; cpd 21.2 Motivation to quit?: not selected on motivation |
|
| Interventions | 1. Control: brief advice used AHRQ‐recommended methods. At the Initial session (15 mins), therapists assessed smoking rate and interest in quitting, directly advised participants to stop smoking now during substance use treatment for their health, and given advice about useful methods. 43 participants were randomised to receive booster sessions (5 to 15 mins each), 7 and 30 days after the initial session. The remaining 42 participants did not receive boosters. 2. Intervention: used motivational therapist style with assessment feedback, based on motivational interviewing. Initial session (45 mins) involved discussing pros and cons of smoking, interpreting health risks, costs of smoking, smoking rate, relationship of smoking to ongoing alcohol use, and barriers to change, with corrective information. 40 participants were randomised to booster sessions (5 to 15 mins each), 7 and 30 days after the initial session. The remaining 40 participants did not receive boosters. All participants informed of free access to smoking cessation pamphlets, smoking cessation skills groups, hard candy, and free access to NRT (transdermal nicotine or nicotine gum) if medically eligible and willing to cease smoking while using it. Provider: counsellors Intensity: single 45‐minute initial session, with two 5‐ to 15‐minute booster sessions (in booster group only) Was MI fidelity monitored?: "Treatment session audiotapes (24% of initial sessions, 19% of booster sessions) were reviewed in weekly group supervision with Dr. Rohsenow and a treatment coordinator, and rated for MI style and adherence to the manual (see 2.4.4), with immediate feedback to therapists to prevent drift. Treatment sessions were rated by the treatment coordinator (primary rater) or the first author on 1 (not at all) to 5 (extensively) scales for five motivational style measures (arguing, demonstrating empathy, reflective listening, supporting self‐efficacy, emphasizing personal responsibility for change), and supervisors endorsed adequacy of six MI adherence items (discuss ambivalence (pros and cons, goal discrepancies), discuss feedback about smoking effects, explore barriers to change, provide summaries, discuss various goals, discuss methods)." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 12 months Validation: exhaled CO ≤ 10 ppm Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Institute of Alcohol Abuse and Alcoholism (1 RO1 AA11318) and two Senior Research Career Scientist Awards from the United States Department of Veterans Affairs | |
| Author conflicts of interest | Not reported | |
| Notes | The study was made up of 4 trial arms: MI with and without booster sessions and brief advice with and without booster sessions. For the purpose of MI versus other smoking cessation support analyses, we combined these into 2 groups: 1. MI and 2. brief advice. However, we also compared the two MI groups in our intensity analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization to MI or BA and to booster sessions versus no boosters within each gender occurred in the first week of the program using a random numbers table". |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Assignment was placed in a sealed envelope opened just before the first treatment session." Did not state whether envelopes were opaque. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence was validated by exhaled carbon monoxide. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27/80 (34%) in the MI intervention group and 25/85 (29%) in the BA control group were lost to 12‐month follow‐up. Therefore, dropout rate was similar between arms. |
Rohsenow 2015.
| Methods | Study design: factorial RCT (2 x 2) Location: USA Setting: residential substance abuse treatment programme Recruitment: from the substance abuse treatment programme (no further information given) |
|
| Participants | Defining eligibility criteria?: drug use disorder and in residential substance abuse treatment Participant characteristics: 184 adult smokers; 102/184 (55.4%) female; mean age: 34.5; mean cpd: 22.3; nicotine dependence: mean FTND = 5.28 Motivation to quit?: not selected on motivation |
|
| Interventions | Control 1: brief advice with non‐contingent vouchers: participants received a session (15 minutes) of brief advice to promote motivation to quit used AHRQ‐recommended methods, adapted for substance use disorder recovery issues. Counsellors assessed smoking rate and interest in quitting, directly advised participants to stop smoking now for their health, assisted by giving advice about useful methods, and asked them to set a quit‐date within the next 2 weeks. If participants expressed concern about effects on sobriety, they were given corrective information. Additional sessions were provided at 7, 14 and 19 days after the first session (10 to 15 minutes each) where progress toward smoking cessation was checked, participants were engaged in problem‐solving around barriers to quitting, successes in accomplishing goals were noted, repeated direct advice to quit was given, and reminders of methods available were given. Participants could earn payments per day for 19 days, simply for providing breath samples as scheduled (not contingent on abstinence). In the last session there was discussion addressing the transition away from the contingency payments provided in the study. Control 2: brief advice with contingency vouchers: as control 1, however the payments participants could earn were awarded for providing reduced CO breath samples rather than just for providing samples. Intervention 1: motivational interviewing with non contingent vouchers: As control 1, however participants received motivational interviewing rather than the brief advice intervention. “The initial session (45 minutes) involved discussing pros and cons of smoking, the health risks associated with their carbon monoxide (CO) level, the costs of smoking relative to their income, their smoking rate compared to state and national norms, the relationship of smoking to alcohol use and to sobriety, and their barriers to change with corrective information (since more barriers are associated with lower motivation). Patients chose goals and methods from a menu of suggestions, and were provided with their choice of a variety of smoking cessation pamphlets. At additional sessions at 7, 14 and 19 days after the first session (15–30 minutes each), patients were asked about progress toward their own stated goals, barriers and ways to overcome barriers, successes (focussing on self‐efficacy), and revised goal preferences.” Intervention 2: motivational interviewing with contingency vouchers: as control 2, however participants received motivational interviewing rather than the brief advice intervention. All participants received free access to NRT (patch or gum), smoking cessation pamphlets and hard candy. Provider: counsellors Intensity: single 45‐minute face‐to‐face session plus three 15‐ to 30‐minute sessions Was MI fidelity monitored?: "Treatment session audiotapes (15% of initial sessions, 10% of additional sessions) were reviewed in weekly group supervision with the treatment coordinator and a psychologist trained in MI, and rated for MI style and adherence to the manual, with immediate feedback to therapists to prevent drift." |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 12 months Validation: CO level ≤ 4 ppm and salivary cotinine level ≤ 15 ng/mL Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "Supported by 1 RO1 DA13616 from the National Institute on Drug Abuse; two Senior Career Research Scientist Awards from the Department of Veterans Affairs (DJR and PMM); and K05AA019681 from the National Institute on Alcohol Abuse and Alcoholism." | |
| Author conflicts of interest | "No authors declare conflicts". | |
| Notes | Two MI groups and 2 brief advice (BA) groups merged into one MI and one BA group to contribute to 'MI versus other smoking cessation treatment' comparison as no interaction effects were detected. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used stratified random assignment, using urn randomisation. No further detail given |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research interviewers blind to treatment condition conducted all assessments. Cessation was biochemically verified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45/184 (24.5%) lost to follow‐up, with no significant differences by condition |
Sherman 2016.
| Methods | Study design: RCT Location: USA Setting: hospital Recruitment: "Using the electronic medical record, a daily list was generated of inpatients documented as current smokers on admission screening. Research assistants (RAs) reviewed the list twice daily and went to the bedside of every patient on the list. In addition to the inpatient units, RAs approached admitted patients who remained in the emergency department and patients in the intensive care units." |
|
| Participants | Defining eligibility criteria?: admitted to hospital for various healthcare problems Participant characteristics: 1619 adult smokers; 346/1619 (21.4%) female; mean age: control: 48, intervention: 49; mean cpd: control: 12.5, intervention: 12.3; nicotine dependence: level of nicotine addiction, first cigarette within: 5 minutes: 685/1619 (42.3%), 6 to 30 minutes: 321/1619 (19.8%), 31 to 60 minutes: 180/1619 (11.1%), > 60 minutes: 422/1619 (26.1%). Motivation to quit?: not selected on motivation |
|
| Interventions | Control: referral to quit‐line based on MI: participants referred to a quit‐line and offered NRT. Participants received one 15 to 20‐minute counselling session with a follow‐up call to assess quit status and assure any requested NRT was received. Most participants were referred to the New York state quit‐line where the counsellors are trained in MI. Intervention: MI intensive counselling plus 8 weeks of NRT if they had not received an NRT prescription at discharge. "The structured counselling protocol was based on Motivational Interviewing and Problem Solving Therapy". Provider: counsellors Intensity: single 15 to 20‐minute initial call, followed by six 10 to 15‐minute calls over 42 weeks Was MI fidelity monitored?: "The program staff members use a structured protocol to maintain a record of each of the counselling calls for internal quality assurance. To ensure intervention standardization and fidelity after study implementation, a random sample of the counsellors' phone calls will be audio taped and reviewed by a clinical psychologist and the study’s counsellor supervisor". |
|
| Outcomes | Definition of cessation used: 30‐day point prevalence Length of longest follow‐up: 6 months Validation: "Where possible salivary cotinine was used: several sites (including New York) made exhaustive efforts to obtain saliva from a consecutive subsample of participants reporting abstinence and no NRT or e‐cigarette use in the past 7 days at 6‐month follow‐up." Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This work was supported by a grant from the National Heart, Lung and Blood Institute (NHLBI) of NIH (#1U01HL105229) and a Hurricane Sandy Supplement (#3U01HL105229‐04S1), and also in part by the New York University CTSA grant UL1TR000038 from the National Center for Advancing Translational Sciences, NIH." | |
| Author conflicts of interest | "None of the authors have any conflicts of interest to report". | |
| Notes | Both intervention groups received counselling based on MI albeit from different providers. This study was included in our comparison investigating the intensity of MI; however, it was removed in a sensitivity analysis due to the difference in providers between arms, which made it different to other studies included in the same comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study database generated random assignments. QUOTE: "The randomisation scheme, designed by the biostatistician, employed a computerized random number generator and stratified participants on hospital site." |
| Allocation concealment (selection bias) | Low risk | The study database generated random assignments. QUOTE: "The randomisation scheme, designed by the biostatistician, employed a computerized random number generator and stratified participants on hospital site." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Biochemical verification did not occur in full sample and intervention contact was not matched across study arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 247/804 (30.7%) lost to follow‐up in the hospital intervention arm, and 278/814 (34.2%) lost to follow‐up in the quit‐line arm. Less than 50% overall and similar rates between groups |
Soria 2006.
| Methods | Study design: RCT Location: Spain Setting: family health centres Recruitment: smokers making routine GP visits |
|
| Participants | Defining eligibility criteria?: patients attending primary care for variety of reasons Participant characteristics: 200 smokers, randomised to intervention (114) or control (86). 53% female, mean age 38. Mean cpd 18 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: brief (3 mins) anti‐smoking advice
Intervention: three 20‐min MI‐based interviews, at intervals to suit doctor and participant
Pharmacotherapy: bupropion offered to highly nicotine‐dependent members of both groups Provider: physicians Intensity: three 20‐minute sessions Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: point prevalence Length of longest follow‐up: 12 months Validation: expired CO < 6 ppm Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | Grant from the Department of Health, Health Science Institute of the Government of the Autonomous Communities of Castille ‐ La Mancha (Spain) | |
| Author conflicts of interest | "The authors have stated that there are none". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "The patients were randomly assigned to either one of the actions groups by means of a non‐block table of random numbers", "patient randomisation was achieved by applying a non‐block table of random numbers as opposed to a block table, resulting in unbalanced group sizes". |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Two hundred non‐transparent sealed envelopes containing the interventions (either brief advice or MI) were prepared. Before the start of daily consultations, the GPs conducting the interventions would extract one of the envelopes, not knowing the type of action it contained. The first smoker patient who attended the consultation would be offered the possibility of taking part in the study. If they accepted and signed the informed consent form, the envelope would be opened, upon which the GP would learn of the patient’s group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was validated using exhaled carbon monoxide measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17/114 (14.9%) of the MI intervention group and 9/86 (10.5%) of the brief advice control group were lost to follow‐up at the 12‐month follow‐up. |
Stein 2006.
| Methods | Study design: RCT Location: USA Setting: methadone maintenance treatment programme centres Recruitment: offered to smokers routinely attending maintenance clinic |
|
| Participants | Defining eligibility criteria?: opiate‐dependent people on methadone maintenance treatment for 3 months or more Participant characteristics: 383 methadone‐maintained adult smokers, randomised to maximal (191) or minimal (192) SC programmes. 48% female, mean age 40, mean cpd 27 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: up to 2 visits, i.e. baseline and quit‐date (if set). Brief advice using National Cancer Institute's 4As model (< 3 mins), plus self‐help materials
Intervention: up to 3 visits from study counsellor, i.e. one 30‐min MI‐based tailored interview, plus 15 to 30‐min quit‐date session + follow‐up relapse prevention session. Those not ready to quit only received 2 sessions. All participants willing to make quit attempt offered NRT patches. Provider: counsellors Intensity: single 30‐minute session, followed by two follow‐up sessions within 30 days Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: Expired CO < 8 ppm Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Cancer Institute (R01CA84392). Transdermal nicotine therapy provided by GlaxoSmithKline | |
| Author conflicts of interest | "The authors have no conflicts of interest, financial or otherwise, to report for this article or this research." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "At this point, randomization and group assignment occurred." No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "At this point, randomization and group assignment occurred." No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was validated using exhaled carbon monoxide measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | QUOTE: "A total of 383 participants were assessed at baseline; 312 (81.5%) were successfully located and assessed at 6 months". However, rates of follow‐up were not reported by study arm. |
Tevyaw 2009.
| Methods | Study design: RCT Location: USA Setting: colleges and universities Recruitment: advertisements posted in campuses, in campus newspapers, and on the Internet (e.g. Craigslist) |
|
| Participants | Defining eligibility criteria?: young people 18‐24 years Participant characteristics: 110 adult (18 to 24 years), student, daily smokers verified by a CO > 10 ppm, randomised to intervention (55) and control (55); 0.1% female; mean age 19.8; cpd 12.3 Motivation to quit?: not selected on motivation |
|
| Interventions | Control 1: progressive muscle relaxation (REL) with non‐contingent payments: i.e. non‐smoking cessation support, matched to the intervention for contact time. Therapists followed a standardised manual for implementation. In session 1, therapists guided the participant through progressive muscle REL exercises. Muscle REL techniques were then practiced during sessions 2 and 3. Participants received payments for providing breath samples, regardless of CO level across 3 weeks. Payments were provided to promote session attendance and to minimise differences in attendance between groups. Control 2: progressive muscle relaxation (REL) with contingency payments: as control 1 however, as well as receiving the non‐contingent payments participants received contingent reinforcement for CO reductions of 25% or greater from their baseline levels. Intervention 1: MET with non‐contingent payments: as control 1, however rather than REL therapy, participants received three sessions of motivational enhancement therapy (MET), incorporating central principles of MI. The first session (60 mins) focussed on enhancing motivation to cut down and quit smoking. Students received information about smoking effects, coping with withdrawal symptoms, and strategies for quitting.The therapist and student developed an action plan for behaviour change. Sessions 2 and 3 (each 30 mins) used MET principles, focussed on progress made and planning for the future. Intervention 2: MET with contingency payments: as control 2, however, rather than REL therapy, participants received three sessions of motivational enhancement therapy (MET), incorporating central principles of MI. The first session (60 mins) focussed on enhancing motivation to cut down and quit smoking. Students received information about smoking effects, coping with withdrawal symptoms, and strategies for quitting.The therapist and student developed an action plan for behaviour change. Sessions 2 and 3 (each 30 mins) used MET principles, focussed on progress made and planning for the future. Intervention provider: counsellors Intensity: single 60‐minute session, followed by two 30‐minute sessions over the following two weeks Was MI fidelity monitored?: "Students and therapists separately rated which of 19 possible session elements (15 MET elements and 4 REL elements) had been completed at posttreatment". |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: salivary cotinine < 15 ng/mL or CO ≤ 8 ppm Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Institute on Drug Abuse (DA011204), and a Senior Career Research Scientist Award from the Department of Veterans Affairs | |
| Author conflicts of interest | "None declared" | |
| Notes | The study was made up of 4 trial arms: MET with and without contingency reinforcement, and REL with and without contingency reinforcement. We compared the two non‐contingent groups for our MI versus no smoking cessation treatment comparison, and compared the two contingent groups for our MI as an adjunct comparison. The authors kindly provided the quit data for individual study arms in response to an information request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Participants were randomly assigned to one of four conditions". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Participants were randomly assigned to one of four conditions". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was validated using saliva cotinine and exhaled carbon monoxide. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/55 (6%) in the MET intervention group and 3/55 (6%) in the relaxation control group were lost to follow‐up at the 6‐month follow‐up. Therefore, dropout rate was low and the same across study arms. |
Vidrine 2019.
| Methods | Study design: cluster RCT Location: USA Setting: community (over telephone) Recruitment: took place at churches, public housing sites, and community centres (no further information given) |
|
| Participants | Defining eligibility criteria?: adult smokers Participant characteristics: 624 adult smokers; 316/624 (50.6%) female; mean age: 45.8; cpd: 1 to 10: 188/624 (30.1%), 11 to 20: 285/624 (45.7 %), >= 21: 151/624 (24.2%); nicotine dependence: mean FTND = 5.59 Motivation to quit?: motivated |
|
| Interventions | Control: brief advice to quit smoking, self‐help written materials, a quit‐line referral, and a 10‐week supply of NRT patches Intervention 1: as control, plus tailored text messaging. "Message delivery began several days before a scheduled quit date and continued for a 12‐week period. Frequency of messages was highest (i.e. 5 per day) near the time of the quit date, but gradually reduced to 1 per day. Message content was informed by cognitive behavioral and motivational enhancement principles and was designed to increase health knowledge, quit motivation, use of coping skills, support, and self‐efficacy. Messages were tailored based on participants’ first name and current smoking status (proactively assessed weekly by mobile phone), and on disease history, future disease concerns, and preferred coping skills (each assessed at the baseline audio computer assisted self‐interview)." Intervention 2: as intervention 1, plus proactive telephone counselling. As with text messaging, counselling session content was primarily drawn from cognitive‐behavioural and MI techniques. Provider: counsellors Intensity: 11 10‐ to 12‐minute sessions over a 12‐week period Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 30‐day point prevalence Length of longest follow‐up: 6 months Validation: saliva cotinine (< 20 ng/mL) via postal swab but only introduced in second year of recruitment (N = 377; 60.4%) Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This study was supported by grant R01 CA141628 from the National Cancer Institute (principal investigators [PIs]: Drs D. J. Vidrine and Prokhorov); grant P30 CA225520 from the Stephenson Cancer Center (PI: Dr Robert S. Mannel, MD); grant P30 CA016672 from The University of Texas MD Anderson Cancer Center (PI: Louis L. Pisters, MD); grant 092‐016‐0002l from the Oklahoma Tobacco Settlement Endowment Trust (PI: Dr J. I. Vidrine); and grant U54GM104938 from the National Institute of General Medical Sciences (PI: Judith A. James, MD, PhD)." | |
| Author conflicts of interest | "Authors reported no conflicts of interest." | |
| Notes | Intervention groups merged and compared with control for MI as an adjunct comparison. Intervention groups compared with one another for intensity of MI comparison. The study randomised neighbourhood sites and conducted adjusted analyses, accounting for the type of site (church, housing complex or community centre) and the individual site (46 sites). This allowed us to calculate an ICC of 0.06 and adjust for this in our analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Neighborhood sites were stratified based on type (i.e. church, community center, or public housing complex) and racial/ethnic composition, then randomized to a treatment group using a random number list generated by a staff statistician". |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Research staff who recruited, consented, and administered the assessments were blinded to the treatment group assignment." No further information |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research staff who recruited, consented, and administered the assessments were blinded to the treatment group assignment. However, validation of abstinence only began at year 2 of recruitment so we have not used validated rates, and the amount of contact with counsellors differed between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | QUOTE: "The overall 6‐month follow‐up rate was 73.6%, and no significant group differences (P > .57 for all) were observed". |
Woodruff 2007.
| Methods | Study design: cluster RCT Location: USA Setting: high schools (via virtual environment) Recruitment: from 14 local high school using classroom presentations, lunch‐hour sign‐up tables, flyers, posters, school newspaper ads and articles, school‐wide announcements, and school liaison referrals |
|
| Participants | Defining eligibility criteria?: high school students (adolescents) Participant characteristics: 136 adolescent smokers; 63/136 (46%) female; mean age: 16; mean cpd: 2 to 5; nicotine dependence: latency to first cigarette of the day assessed on a scale ranging from 1 (immediately after waking) to 6 (more than 2 hours after waking), mean: intervention; 4.44; control; 4.78 Motivation to quit?: not selected on motivation |
|
| Interventions | Control: no treatment (measurement only) Intervention: "The Breathing Room" virtual world incorporating motivational interviewing: participants were represented by an avatar in the virtual world and received counselling in a group, delivered by a counsellor, in a shopping mall setting. "The Breathing Room virtual world", used proprietary interactive software known as ActiveWorlds that created a virtual mall environment with chat box communication. Provider: counsellor Intensity: single 45‐minute session per week for 7 weeks Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 12 months Validation: none Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | "This research was funded by California's Tobacco‐related Disease Research Program (TRDRP), grant number 11HT‐3301". | |
| Author conflicts of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization to condition was done by school to avoid contamination between intervention and control groups". No further information given |
| Allocation concealment (selection bias) | High risk | Clusters knew which condition they were in, and recruitment was tailored to this. Participants recruited were different at each site due to recruitment materials associated with condition ‐ this resulted in non‐equivalent groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status measured by self‐report and control group had no interaction with study counsellor (compared with seven sessions of MI/chat room). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall loss to follow‐up was 27% for the 12‐month follow‐up survey. QUOTE: "There was tendency for survey non‐response to be higher among intervention participants than among controls. For example, at the post‐intervention assessment, 15% of controls did not respond compared to 33% of intervention participants." Exact numbers per group lost to follow‐up not reported and 18% difference between controls and intervention participants at post‐intervention assessment (no report of difference at 12‐month follow‐up). |
Wu 2009.
| Methods | Study design: RCT Location: USA Setting: Asian community health coalition’s member organisations. Community setting in New York City Recruitment: participants were recruited through the Asian Community Health Coalition’s Chinese member organisations by bilingual staff from Temple University’s Center for Asian Health in cooperation with trained community volunteers. |
|
| Participants | Defining eligibility criteria?: self‐identification as ethnic Chinese Participant characteristics: 139 adult ethnic Chinese smokers, randomised to intervention (67) and control (72); 12.3% female; mean age 44.4; cpd not stated Motivation to quit?: not selected on motivation |
|
| Interventions | Control: four 60‐min 'health education' sessions and general self‐help health information, covering nutrition, exercise, and harmful effects of tobacco. Quitting strategies were provided. Intervention: four in‐person 60‐min sessions of MI counselling for smoking cessation and self‐help smoking cessation materials. The effects of tobacco use, secondhand smoke, and participants’ experiences with smoking were discussed. Participants were counselled about the addictive nature of nicotine, encouraged to examine the pros and cons of smoking, and contemplate quitting behaviour. All participants were provided with nicotine patches. Provider: counsellors Intensity: four in‐person 60‐minute sessions. Frequency unclear Was MI fidelity monitored?: no |
|
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: 6 months Validation: expired CO was measured; however results by arm not reported and so non‐validated data used. Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
|
| Funding source | A grant from the National Cancer Institute Community Network Program (U01CA114582‐02S2) | |
| Author conflicts of interest | "None declared" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Eligible participants/smokers aged 18 years and older were randomly assigned to MI or to the general health – counseling program". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Eligible participants/smokers aged 18 years and older were randomly assigned to MI or to the general health – counseling program". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "All participants were measured by breath CO as cross validation of their smoking status at two timepoints: baseline and 6‐month follow‐up". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/67 (11%) in the MI intervention group, and 10/72 (14%) of the general health control group were lost to follow‐up at 6 months. |
5As: 'Ask, Advise, Assess, Assist, and Arrange'
5Rs: 'Relevance, Risks, Rewards, Roadblocks, Repetition' AHRQ: Agency for Health care Research and Quality ALA: American Lung Association AMI: adapted motivational interviewing BA: brief advice CA: continuous abstinence CBT: Cognitive Behavioural Therapy CO: carbon monoxide cpd: cigarettes per day CT: computerised tomography FTND: Fagerstrom Test for Nicotine Dependence HE: health education HII: high intensity intervention HIV: Human Immunodeficiency Virus ICC: intraclass correlation ITT: intention to treat LCD: liquid‐crystal display LHCW: lay health care workers LII: low intensity intervention M: male m: month MA: meta‐analysis ME: motivational enhancement MEC: motivationally enhanced counselling MET: motivational enhancement therapy MFTQ: modified Fagerstrom Tolerance Questionnaire MI: motivational interviewing MISC: Motivational Interviewing Skill Code mp3: Moving Picture Experts Group Layer‐3 Audio (audio file format/extension) N: number of participants NG:nicotine gum NRT: Nicotine replacement therapy PA: prolonged abstinence PG: placebo gum PPA: point prevalence abstinence ppm: parts per million PTSD: post‐traumatic stress disorder RCT: randomised controlled trial REL: progressive muscle relaxation RP: relapse prevention SBA: structured brief advice SC: smoking cessation S‐H: self help SUD: substance use disorder TB: tuberculosis TQD: target quit date UC: usual care USD: United States dollars VHA: Veterans Health Administration
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12609000627257 | Non‐MI intervention components not matched between arms |
| ACTRN12609001039279 | Non‐MI intervention components not matched between arms |
| ACTRN12612000016831 | Was non‐randomised or quasi‐randomised |
| ACTRN12614000876695 | Non‐MI intervention components not matched between arms |
| ACTRN12614001147673 | Was non‐randomised or quasi‐randomised |
| ACTRN12616000314426 | Participants not current smokers |
| Aertsen Van Der Kuip 2006 | Measured cessation at less than 6 m follow‐up |
| Ahluwalia 1998 | Did not test a behavioural motivational smoking cessation intervention |
| Auer 2016 | Was non‐randomised or quasi‐randomised |
| Baker 2006 | Non‐MI intervention components not matched between arms |
| Bernstein 2018 | Measured cessation at less than 6 m follow‐up |
| Boccio 2017 | Was non‐randomised or quasi‐randomised |
| Bolger 2010 | Measured cessation at less than 6 m follow‐up |
| Bonevski 2018 | Non‐MI intervention components not matched between arms |
| Borrelli 2002 | Non‐MI intervention components not matched between arms |
| Borrelli 2005 | Non‐MI intervention components not matched between arms |
| Borrelli 2016 | Non‐MI intervention components not matched between arms |
| Borrelli 2017 | Non‐MI intervention components not matched between arms |
| Boyle 2007 | Tested a motivational intervention not based on M & R's MI |
| Breland 2014 | Measured cessation at less than 6 m follow‐up |
| Bronson 1989 | Tested a motivational intervention not based on M & R's MI |
| Brooks 2017 | Non‐MI intervention components not matched between arms |
| Brown 2003 | Non‐MI intervention components not matched between arms |
| Caponnetto 2017 | Intervention based on stages of change theory |
| Carpenter 2004 | Tested a motivational intervention not based on M & R's MI |
| Cigrang 2002 | Did not test a behavioural motivational smoking cessation intervention |
| Collicott 2001 | Measured cessation at less than 6 m follow‐up |
| Cornuz 2002 | Tested a motivational intervention not based on M & R's MI |
| Curry 2003 | Non‐MI intervention components not matched between arms |
| Dornelas 2000 | Intervention based on stages of change theory; only participants in precontemplative and contemplative stages received MI counselling, the rest received relapse prevention counselling only |
| Eakin 2014 | Did not test a behavioural motivational smoking cessation intervention |
| Emmons 2001 | Non‐MI intervention components not matched between arms |
| Ershoff 1999 | Participants were pregnant smokers |
| Gariti 2002 | Non‐MI intervention components not matched between arms |
| George 2000 | Non‐MI intervention components not matched between arms |
| Glasgow 2000 | Non‐MI intervention components not matched between arms |
| Ha 2012 | Was non‐randomised or quasi‐randomised |
| Ha 2015 | Was non‐randomised or quasi‐randomised |
| Haas 2015 | Non‐MI intervention components not matched between arms |
| Hennrikus 2002 | Did not test a behavioural motivational smoking cessation intervention |
| Hennrikus 2005 | Non‐MI intervention components not matched between arms |
| Hokanson 2006 | Non‐MI intervention components not matched between arms |
| Horn 2007 | Non‐MI intervention components not matched between arms |
| Huang 2015 | Non‐MI intervention components not matched between arms |
| Hughes 2017 | Participants not current smokers |
| Hutchinson 2017 | Participants not current smokers |
| Hyman 2007 | Did not test a behavioural motivational smoking cessation intervention |
| Idrisov 2013 | Non‐MI intervention components not matched between arms |
| Ingersoll 2005 | Measured cessation at less than 6 m follow‐up |
| IRCT2017080435257N1 | Tested a motivational intervention not based on M & R's MI |
| ISRCTN11353250 | Non‐MI intervention components not matched between arms |
| ISRCTN50627997 | Tested a motivational intervention not based on M & R's MI |
| Klemperer 2017 | Tested a motivational intervention not based on M & R's MI |
| Krigel 2011 | Measured cessation at less than 6 m follow‐up |
| Lasser 2012 | Did not test a behavioural motivational smoking cessation intervention |
| Lennox 1998 | Intervention based on stages of change theory |
| Lindqvist 2013 | Was non‐randomised or quasi‐randomised |
| Luna 2005 | Measured cessation at less than 6 m follow‐up |
| Ma 2005a | Was non‐randomised or quasi‐randomised |
| Ma 2005b | Was non‐randomised or quasi‐randomised |
| Mahajan 2017 | Was non‐randomised or quasi‐randomised |
| Manfredi 1999 | Measured cessation at less than 6 m follow‐up |
| Manfredi 2004 | Did not test a behavioural motivational smoking cessation intervention |
| Martin‐Lujan 2011 | Non‐MI intervention components not matched between arms |
| Mazas 2007 | Didn't measure smoking cessation |
| McCambridge 2005 | Participants not current smokers |
| Menzie 2018 | Measured cessation at less than 6 m follow‐up |
| Metse 2017 | Non‐MI intervention components not matched between arms |
| Metz 2006a | Non‐MI intervention components not matched between arms. NRT only recommended to participants in one trial arm |
| Metz 2006b | Tested a motivational intervention not based on M & R's MI |
| Meyer 2003 | Tested a motivational intervention not based on M & R's MI |
| Mujcic 2018 | Non‐MI intervention components not matched between arms |
| NCT00169260 | Tested a motivational intervention not based on M & R's MI |
| NCT00701896 | Tested a motivational intervention not based on M & R's MI |
| NCT00907309 | Did not test a behavioural motivational smoking cessation intervention |
| NCT01098955 | Tested a motivational intervention not based on M & R's MI |
| NCT01846910 | Non‐MI intervention components not matched between arms |
| NCT01982617 | Measured cessation at less than 6 m follow‐up |
| NCT02086162 | Tested a motivational intervention not based on M & R's MI |
| Nichter 2018 | Tested a motivational intervention not based on M & R's MI |
| Pardavila‐Belio 2015 | Non‐MI intervention components not matched between arms |
| Parker 2007 | Participants were pregnant smokers |
| Persson 2006 | Non‐MI intervention components not matched between arms |
| Pineiro 2014 | Was non‐randomised or quasi‐randomised |
| Polosa 2011 | Intervention based on stages of change theory |
| Reitzel 2010 | Participants were pregnant smokers |
| Rigotti 1997 | Non‐MI intervention components not matched between arms |
| Rigotti 2006 | Participants were pregnant smokers |
| Rogers 2016 | Was non‐randomised or quasi‐randomised |
| Schuck 2014 | Non‐MI intervention components not matched between arms |
| Severson 2009 | Non‐MI intervention components not matched between arms |
| Sherbot 2005 | Intervention based on stages of change theory |
| Sims 2013 | Tested a motivational intervention not based on M & R's MI |
| Skov‐Ettrup 2016 | Intervention based on stages of change theory |
| Smith 2001 | Did not test a behavioural motivational smoking cessation intervention |
| Sobell 2017 | Non‐MI intervention components not matched between arms |
| Steinberg 2016 | Measured cessation at less than 6 m follow‐up |
| Stotts 2002 | Participants were pregnant smokers |
| Stotts 2009 | Participants were pregnant smokers |
| Tappin 2000 | Participants were pregnant smokers |
| Tappin 2005 | Participants were pregnant smokers |
| Thomsen 2010 | Non‐MI intervention components not matched between arms |
| Van Rossem 2015 | Tested a motivational intervention not based on M & R's MI |
| Wakefield 2004 | Non‐MI intervention components not matched between arms |
| Weaver 2015 | Measured cessation at less than 6 m follow‐up |
m: month M & R: Miller & Rollnick MI: Motivational Interviewing NRT: Nicotine replacement therapy
Characteristics of studies awaiting assessment [ordered by study ID]
Zhou 2014.
| Methods | Study design: unclear Location: China Setting: not specified Recruitment: unclear |
| Participants | N: 139 Defining eligibility criteria?: smokers with coronary heart disease Motivation to quit?: unknown |
| Interventions | Control: usual clinic care and health education Intervention: motivational interviewing: "MI participants received six MI sessions over 3 months. Interviews include: (1) To help patients recognize that smoking and coronary heart disease are closely related. (2) To help patients realize the dangers of smoking on cardiovascular health. (3) To help patients recognize the potential benefits of quitting smoking. (4) Encourage the patients to face obstacles and setbacks in the process of smoking cessation bravely, and provide a solution available for the patients, enhancing patient confidence and motivation. (5) Encountering with the patients do not want to try to change, repeat the above explanation optionally." Provider: not specified Intensity: 6 sessions over 3 months Was MI fidelity monitored?: unclear |
| Outcomes | Definition of cessation used: unclear (abstinence not reported in abstract) Length of longest follow‐up: unclear Validation: unclear Was mental health and/or well‐being measured at follow‐up?: unclear Was quality of life measured at follow‐up?: unclear |
| Funding source | Not reported |
| Authors' declarations of interest | Not reported |
| Notes | Unclear whether smoking cessation was measured (although seems likely) and at what follow‐up points, making it impossible to ascertain whether the study met eligibility criteria. No cessation rates were reported in the abstract. Tried to contact authors however was unable to identify any contact details and an email to a generic university email address did not receive a response. |
Characteristics of ongoing studies [ordered by study ID]
Lloyd‐Richardson 2003.
| Trial name or title | Informed development of smoking cessation interventions for college students |
| Methods | Study design: RCT Location: not specified Setting: not specified Recruitment: not specified |
| Participants | N: 136 Defining eligibility criteria?: college students Motivation to quit?: not specified |
| Interventions | Control: brief individualised smoking cessation treatment plus 8 weeks of NRT Intervention: motivationally‐enhanced group treatment plus NRT Provider: not specified Intensity: not specified Was MI fidelity monitored?: not specified |
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: six months Validation: yes ‐ details not stated Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
| Starting date | Not reported |
| Contact information | EE Lloyd‐Richardson |
| Funding source | Not reported |
| Authors' declarations of interest | Not reported |
| Notes | Whole sample had not been recruited when abstract was submitted, therefore six‐month cessation rates were not reported. Attempt made to contact the author with no response |
NCT01387516.
| Trial name or title | Motivation and skills for detained teen smokers |
| Methods | Study design: factorial RCT (2 x 2) Location: USA Setting: adolescent detention centre Recruitment: adolescents who had been detained at the Rhode Island Training School (no further details) |
| Participants | N: 314 Defining eligibility criteria?: adolescents detained at the Rhode Island Training School Motivation to quit?: not selected on motivation |
| Interventions | Control 1: relaxation intervention (no smoking cessation treatment): "The Relaxation Therapy intervention is a 60‐90 minute individual session. The session encompasses several techniques, including Progressive Muscle Relaxation and Visualization‐Imagination, and as a whole is really a meditation protocol. The Relaxation Therapy intervention encompasses several techniques, including Progressive Muscle Relaxation and Visualization‐Imagination, and meditation to reduce stress." Control 2: self‐help programming: "Self Help intervention is administered during two 90 minute group sessions. The intervention modules are based on the principles of Nicotine Anonymous (NicA), to provide those who use nicotine but want a nicotine‐free life, with a community of people that have also experienced nicotine addiction and strive to be nicotine free. Elements incorporated in this intervention include the 12 Steps and the NicA "tools" (i.e. meetings, phone list, literature, sponsorship, and service) to facilitate and maintain abstinence from nicotine." Control 3: cognitive behavioural therapy: "The Cognitive Behavioral Therapy (CBT) Intervention is administered during two 90 minute group sessions. The focus is on the interrelationship between thoughts, feelings, and behaviors. It is used to address specific deficits, such as improving problem solving skills and developing social supports, and behaviors such as substance abuse and smoking." Intervention 1: motivational intervention: "Motivational Interviewing (MI) will be a 60‐90 minutes individual session. The focus is on establishing rapport and building motivation. The counselor explores youth's reasons for entering treatment, prior treatment experience, previous attempts to change use, possible goals for treatment, substance effect expectancy, and perceptions of self‐efficacy. A personalized feedback report outlines assessment results, highlights any problems or concerns related to cigarette use expressed by teen, and compares tobacco use levels with national norms for same age and gender peers." Provider: counsellor Intensity: MI/RT: 60 to 90 minutes over one session; CBT/SHP: 60 to 75 minutes over two sessions Was MI fidelity monitored?: not specified |
| Outcomes | Definition of cessation used: 7‐day point prevalence Length of longest follow‐up: six months (post‐release) Validation: CO levels & saliva cotinine tests Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
| Starting date | July 2007 |
| Contact information | Lynda Stein, University of Rhode Island |
| Funding source | Not reported |
| Authors' declarations of interest | Not reported |
| Notes | Contacted author to ask about study status ‐ author replied that the study was complete and that they were about to start study write‐up. No data were supplied. |
NCT02905656.
| Trial name or title | Strategies to promote cessation in smokers who are not ready to quit (PACE) |
| Methods | Study design: factorial RCT Location: USA Setting: quit‐line Recruitment: not specified |
| Participants | N: 828 Defining eligibility criteria?: not specified Motivation to quit?: not motivated to quit |
| Interventions | Control: brief advice: "Participants will receive brief advice to quit smoking, and be provided psycho‐education citing health consequences and the positive impact on mortality and morbidity". Intervention 1: motivational Interviewing (MI): "Motivational interviewing (MI) is a collaborative conversation style for strengthening a person's own motivation and commitment to change. MI attempts to avoid a confrontational style and, instead, guides participants toward choosing to make a change in their behavior." Intervention 2: rate reduction (RR): "Participants will be informed of the strong medical evidence of systematic reductions in smoking behavior can lead to long‐term smoking cessation." This condition will receive Nicotine Replacement Therapy in the form of gum. Intervention 3: motivation interviewing + rate reduction: participants will receive both intervention 1 and intervention 2 combined. Provider: not specified Intensity: 3 to 6 30‐minute sessions + 3 booster sessions Was MI fidelity monitored?: not specified |
| Outcomes | Definition of cessation used: prolonged abstinence (defined as continuous abstinence with a two‐week grace period) Length of longest follow‐up: 12 months Validation: not specified Was mental health and/or well‐being measured at follow‐up?: not specified Was quality of life measured at follow‐up?: not specified |
| Starting date | September 2016 |
| Contact information | Karen Derefinko: kderefin@uthsc.edu Sarah Hand: sarkbill@uthsc.edu |
| Funding source | "Funded by the US NIH". |
| Authors' declarations of interest | Not reported |
| Notes | Trial registry record stated that the study is due to complete in 2020. |
NCT03002883.
| Trial name or title | STAND community college tobacco cessation trial |
| Methods | Study design: RCT Location: USA Setting: community college Recruitment: from Sacremento Community Colleges (no further information) |
| Participants | N: 113 Defining eligibility criteria?: community college students Motivation to quit?: motivated to quit |
| Interventions | Control 1: usual care: "Students educated about and referred to student health for tobacco cessation resources and provided with campus "Quit Kits" ("Quit Kit" water bottle with small cessation aids (e.g. sunflower seeds))" Control 2: direct referral to quit‐line: as control 1 plus "Students were directly referred to the state quitline for follow‐up counseling. Peer educator educates about state quitline services and gets verbal consent to use quitline's direct referral web portal for quitline to contact participant in 1‐2 business days about free counseling services to make a quit plan". Intervention: brief motivational interviewing: as control 1, plus "students received brief motivational interviewing by a student peer educator about tobacco cessation and participant goals" Provider: student peer educator Intensity: one session Was MI fidelity monitored?: not specified |
| Outcomes | Definition of cessation used: point prevalence (period not defined) Length of longest follow‐up: 6 months Validation: saliva cotinine level Was mental health and/or well‐being measured at follow‐up?: not specified Was quality of life measured at follow‐up?: not specified |
| Starting date | September 2014 |
| Contact information | Elisa Tong, University of California, Davis |
| Funding source | Not reported |
| Authors' declarations of interest | Not reported |
| Notes | Contacted author to ask about study status ‐ author replied that the study was complete and that they were currently writing up results. No data was supplied. |
Salgado Garcia 2018.
| Trial name or title | Planning a change easily (PACE): a randomized controlled trial for smokers who are not ready to quit |
| Methods | Study design: RCT Location: USA Setting: nationwide quit‐line Recruitment: "Recruitment is multi‐faceted, including local and regional strategies. Including traditional strategies, such as flyers, business cards, and medical referrals, and electronic strategies, such as Facebook, Pandora Radio. A “refer‐a‐friend” program is also used, where participants receive an extra $20 gift card for referring a person who is eligible and enrolls in the study." |
| Participants | N: not specified Defining eligibility criteria?: not specified Motivation to quit?: not motivated to quit |
| Interventions | Control 1: brief advice (BA): Control 2: rate reduction (RR): participants encouraged to reduce the amount they smoke, and instructed on the benefits this will have to their health. 26 weeks' worth of 4 mg nicotine gum provided Intervention 1: motivational interviewing (MI): "Basic MI principles will be used for each call with the intention of eliciting language that indicates behavioral change (i.e. “change talk”) using open‐ended questions, affirmations, reflections, and summaries. First, each telephone call will include an initial period of engagement. Then, motivation and confidence to change smoking behavior will be assessed separately using scales from 1 (not at all motivated/confident) to 10 (extremely motivated/confident). Next, the counsellor will focus the discussion using the “5Rs” to increase the participants' motivation for change and eventual odds of cessation. The 5Rs will provide opportunities to elicit information from participants. At the end of each session, a summary of the discussion will be provided, and motivation and confidence to change smoking behavior will, again, be assessed. Should the participant wish to quit at any point during the sessions, the interventionist may assist in creating a participant‐centred cessation plan, but no specific skills will be provided in this condition (e.g. “it seems like distraction and exercise could help you quit”). At the end of each session, the interventionist will ask the participant about their willingness to set a quit date using the elicit‐provide‐elicit approach, where the interventionist will elicit permission to provide information, will provide the information (e.g. higher likelihood of quitting if setting a quit date), and will elicit the participant's thoughts about setting a quit date." Intervention 2: motivational interviewing and rate reduction (MI + RR): control 2 and intervention 1 combined Provider: "The interventionists will be recruited based on educational background and past experience with counselling or delivering behavioral interventions. All interventionists will be required to have at least master's degrees in diverse areas of study (e.g. social work, public health, counselling, and psychology)." Intensity: 3 sessions provided over 3 to 6 weeks (weekly or biweekly); 3 booster sessions provided bimonthly; 30 minutes overall Was MI fidelity monitored?: "Audio from all study sessions will be recorded, and approximately one out of every ten sessions will be reviewed and scored for fidelity and MI adherence (when applicable) by doctoral‐level clinical supervisors. Interventionists will receive weekly clinical supervision from doctoral level supervisors for feedback on scored sessions and further training when needed. MI training by local and national experts will be provided periodically throughout the study period." |
| Outcomes | Definition of cessation used: prolonged abstinence (length of time since the quit‐date with a two‐week grace period) Length of longest follow‐up: 12 months Validation: saliva cotinine Was mental health and/or well‐being measured at follow‐up?: no Was quality of life measured at follow‐up?: no |
| Starting date | Not specified |
| Contact information | F.I. Salgado García: fsalgado@uthsc.edu |
| Funding source | "National Institutes of Health [grant number 1R01CA193245‐01A1]" |
| Authors' declarations of interest | "All authors confirm that there are no known conflicts of interest". |
| Notes | Contacted author to ask about study status ‐ author replied that the study was still recruiting. Recruitment was planned to end in summer 2019, with results expected autumn 2020. |
BA: brief advice CBT: Cognitive Behavioural Therapy CO: carbon monoxide MI: Motivational Interviewing NicA: Nicotine Anonymous NRT: nicotine replacement therapy PACE: Planning a Change Easily RR: rate reduction RT: relaxation therapy SHP:self‐help programming STAND: Sacramento Taking Action Against Tobacco Dependence
Differences between protocol and review
The cost‐effectiveness objective has been removed.
We excluded pregnant women, as their particular needs and circumstances warrant them being treated as separate populations. They are covered in another Cochrane Review (Chamberlain 2017).
We introduced a new eligibility criterion excluding studies incorporating additional non‐MI components into the intervention group that were not matched in the comparator group, in order to reduce bias.
We have now included comparators of all intensities; however, we controlled for this using subgroup analyses.
We have now included studies regardless of whether they carried out fidelity monitoring; however, we controlled for this using subgroup analyses.
We excluded non‐randomised controlled trials, to keep the quality of the evidence as high as possible.
We introduced secondary outcomes (mental health and quality of life) to assess whether MI for smoking cessation has any impact on the well‐being of participants.
We grouped the included studies into four separate comparisons rather than incorporating all studies into one meta‐analysis.
We added additional prespecified subgroup analyses, splitting studies by 1) the relative intensity of the intervention and comparator conditions; 2) participant motivation to quit; 3) and whether fidelity monitoring took place.
Contributions of authors
AF, JL, NL, PA and TT all reviewed the previous version of the review and amended eligibility criteria. AF, JL, NL, and TT assessed study eligibility and extracted data from eligible studies.
NL authored a draft of the review and AF, JL, PA and TT all provided comments that were incorporated into this final version.
Sources of support
Internal sources
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
Clinical Trials & Health Research ‐ Institute of Translational & Stratified Medicine; University of Plymouth, UK.
College of Medicine & Health, University of Exeter, UK.
External sources
-
NIHR Infrastructure funding, UK.
Funding for the infrastructure of the Cochrane Tobacco Addiction Group
NIHR Cochrane Programme Grant funding, UK.
Declarations of interest
AF is employed by the University of Oxford to work as a Senior Researcher in the Health Behaviours group on projects related to nicotine consumption. Anne has been the recipient of a grant funded by Cancer Research UK to explore clinician attitudes towards electronic cigarettes. None of this is deemed a conflict of interest.
JL has no known conflicts of interest.
NL is employed by the University of Oxford to work as Managing Editor for the Cochrane Tobacco Addiction Group (TAG). TAG's infrastructure is funded by the NIHR. Nicola has received payment for lectures on systematic review methodology, and has been an applicant on project funding to carry out priority setting and systematic reviews in the area of tobacco control (NIHR funded). None of this is deemed a conflict of interest.
PA has no known conflicts of interest.
TT has no known conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahluwalia 2006 {published data only}
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction 2006;101(6):883‐91. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JS, Okuyemi KS, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers (PA7‐6). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18; Orlando (FL). 2006.
- Ahluwalia JS, Richter K, Mayo MS, Ahluwalia HK, Choi WS, Schmelzle KH, et al. African American smokers interested and eligible for a smoking cessation clinical trial: predictors of not returning for randomization. Annals of Epidemiology 2002;12(3):206‐12. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Thomas JL, An LC, Guo H, Collins T, Okuyemi KS, et al. Change in smoking, diet, and walking for exercise in Blacks. Health Education & Behavior 2012;39(2):191‐7. [DOI: 10.1177/1090198111432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen N, Ahluwalia JS, Okuyemi K, Choi W, Kaur H, Mayo M. Kick it at SWOPE II (KIS II): baseline data from a smoking cessation trial among African American (AA) light smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20‐23; Prague, Czech Republic. 2005.
- Nollen NL, Mayo MS, Sanderson Cox L, Okuyemi KS, Choi WS, Kaur H, et al. Predictors of quitting among African American light smokers enrolled in a randomized, placebo‐controlled trial. Journal of General Internal Medicine 2006;21(6):590‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 2007;102(12):1979‐86. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Sanderson Cox L, Nollen NL, Snow TM, Kaur H, Choi W, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African‐American light smokers. American Journal of Health Promotion 2007;21(3):183‐91. [DOI] [PubMed] [Google Scholar]
- Stahre MA, Toomey TL, Erickson DJ, Forster JL, Okuyemi KS, Ahluwalia JS. The effects of a tobacco intervention on binge drinking among African American light smokers. Journal of Addictive Diseases 2013;32(4):377‐86. [DOI] [PubMed] [Google Scholar]
Audrain‐McGovern 2011 {published data only}
- Audrain‐McGovern J, Stevens S, Murray PJ, Kinsman S, Zuckoff A, Pletcher J, et al. The efficacy of motivational interviewing versus brief advice for adolescent smoking behavior change. Pediatrics 2011;128(1):e101‐11. [DOI] [PubMed] [Google Scholar]
- Kalkhuis‐Beam S, Stevens SL, Baumritter A, Carlson EC, Pletcher JR, Rodriguez D, et al. Participant‐ and study‐related characteristics predicting treatment completion and study retention in an adolescent smoking cessation trial. Journal of Adolescent Health 2011;49(4):371‐8. [DOI] [PubMed] [Google Scholar]
- NCT00381329. Pennsylvania Adolescent Smoking Study (PASStudy). ClinicalTrials.gov/show/NCT00381329 (first received 27 September 2006).
Bastian 2013 {published data only}
- Bastian LA, Fish LJ, Peterson BL, Biddle AK, Garst J, Lyna P, et al. Assessment of the impact of adjunctive proactive telephone counseling to promote smoking cessation among lung cancer patients' social networks. American Journal of Health Promotion 2013;27(3):181‐90. [] [DOI] [PubMed] [Google Scholar]
Battaglia 2016 {published and unpublished data}
- Battaglia C, Peterson J, Whitfield E, Min S‐J, Benson SL, Maddox TM, et al. Integrating motivational interviewing into a home telehealth program for veterans with posttraumatic stress disorder who smoke: a randomized controlled trial. Journal of Clinical Psychology 2016;72(3):194‐206. [DOI] [PubMed] [Google Scholar]
- Peterson J, Prochazka AV, Battaglia C. Smoking cessation and care management for veterans with posttraumatic stress disorder: a study protocol for a randomized controlled trial. BMC Health Services Research 2015;15(1):46. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bock 2008 {published data only}
- Becker BM, Bock BC, Partridge R. Smoking cessation interventions in the emergency department for smokers with chest pain. Academic Emergency Medicine 2003;10:507. [Google Scholar]
- Bock BC, Becker BM, Niaura RS, Partridge R, Fava JL, Trask P. Smoking cessation among patients in an emergency chest pain observation unit: outcomes of the Chest Pain Smoking Study (CPSS). Nicotine & Tobacco Research 2008;10(10):1523‐31. [DOI] [PubMed] [Google Scholar]
Bock 2014 {published and unpublished data}
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low‐income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413‐22. [CENTRAL: 986341; CRS: 9400050000000050; EMBASE: 2014185069; PUBMED: 24174612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 1999 {published data only}
- Butler CC, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. British Journal of General Practice 1999;49(445):611–6. [Google Scholar]
Catley 2016 {published data only}
- Bani‐Yaghoub M, Elhomani A, Catley D. Effectiveness of motivational interviewing, health education and brief advice in a population of smokers who are not ready to quit. BMC Medical Research Methodology 2018;18(1):52. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, et al. A randomized trial of motivational interviewing: cessation induction among smokers with low desire to quit. American Journal of Preventive Medicine 2016;50(5):573‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Harris KJ, Goggin K, Richter K, Williams K, Patten C, et al. Motivational Interviewing for encouraging quit attempts among unmotivated smokers: study protocol of a randomized, controlled, efficacy trial. BMC Public Health 2012;12:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AT, Martin LE, Bruce J, Moreno JL, Staggs VS, Lee HS, et al. Executive function fails to predict smoking outcomes in a clinical trial to motivate smokers to quit. Drug and Alcohol Dependence 2017;175:227‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01188018. Testing counseling styles to motivate smokers to quit. ClinicalTrials.gov/show/NCT01188018 (first received 25 August 2010).
- Rasu R, Thelen J, Agbor Bawa W, Goggin K, Harris K, Richter K, et al. Resource utilized in a randomized clinical trial to recruit smokers with low motivation to quit. ISPOR 17th Annual European Congress; 2014 Nov 8‐12; Amsterdam, Netherlands. 2014. [DOI] [PubMed]
Colby 2005 {published data only}
- Colby SM, Monti PM, O'Leary‐Tevyaw T, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in medical settings. Addictive Behaviors 2005;30(5):865‐74. [DOI] [PubMed] [Google Scholar]
Colby 2012 {published data only}
- Colby SM, Nargiso J, Tevyaw TO, Barnett NP, Metrik J, Lewander W, et al. Enhanced motivational interviewing versus brief advice for adolescent smoking cessation: results from a randomized clinical trial. Addictive Behaviors 2012;37(7):817‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantini R, McGrath AC, Stein LAR, Barnett NP, Monti PM, Colby SM. Misreporting in a randomized clinical trial for smoking cessation in adolescents. Addictive Behaviors 2015;45:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2016 {published data only}
- Cook JW, Collins LM, Fiore MC, Smith SS, Fraser D, Bolt DM, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction 2016;111(1):117‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01122238. Identifying treatments to motivate smokers to quit. ClinicalTrials.gov/show/NCT01122238 (first received 17 March 2015).
Davis 2011 {published data only}
- Davis MF, Shapiro D, Windsor R, Whalen P, Rhode R, Miller HS, et al. Motivational interviewing versus prescriptive advice for smokers who are not ready to quit. Patient Education and Counseling 2011;83(1):129‐33. [DOI] [PubMed] [Google Scholar]
De Azevedo 2010 {published data only}
- Azevedo RC, Mauro ML, Lima DD, Gaspar KC, Silva VF, Botega NJ. General hospital admission as an opportunity for smoking‐cessation strategies: a clinical trial in Brazil. General Hospital Psychiatry 2010;32(6):599‐606. [DOI] [PubMed] [Google Scholar]
Demétrio Faustino‐Silva 2018 {published data only}
- Demétrio Faustino‐Silva D, Melnick R, Keller Celeste R. Efficacy of motivational interviewing in smoking groups in primary health care: a randomized clinical trial. International Journal of Behavioral Medicine 2018;25(Suppl 1):S68. [Google Scholar]
- NCT03221010. Use of the Motivational Interviewing in the Treatment of smokers in Groups in primary health care (MITG). clinicaltrials.gov/ct2/show/NCT03221010 (first received 18 July 2017).
Ellerbeck 2009 {published data only}
- Cox LS, Wick JA, Nazir N, Cupertino AP, Mussulman LM, Ahluwalia JS, et al. Predictors of early versus late smoking abstinence within a 24‐month disease management program. Nicotine & Tobacco Research 2011;13(3):215‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupertino AP, Berg C, Gajewski B, Hui SA, Richter K, Catley D, et al. Change in self‐efficacy, autonomous and controlled motivation predicting smoking cessation. Journal of Health Psychology 2012;17(5):640‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupertino AP, Wick JA, Richter KP, Mussulman L, Nazir N, Ellerbeck EF. The impact of repeated cycles of pharmacotherapy on smoking cessation: a longitudinal cohort study. Archives of Internal Medicine 2009;169:1928‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, Mussulman LM, et al. Effect of varying levels of disease management on smoking cessation. Annals of Internal Medicine 2009;150(7):437‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HW, Ellerbeck EF, Mahnken JD. Simultaneous evaluation of abstinence and relapse using a Markov chain model in smokers enrolled in a two‐year randomized trial. BMC Medical Research Methodology 2012;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2010 {published data only}
- Harris KJ, Catley D, Good GE, Cronk NJ, Harrar S, Williams KB. Motivational interviewing for smoking cessation in college students: a group randomized controlled trial. Preventive Medicine 2010;51(5):387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JD, Catley D, Lee HS, Harrar SW, Harris KJ. Health risk perceptions predict smoking‐related outcomes in Greek college students. Psychology of Addictive Behaviors 2014;28(3):743‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel S, Cronk NJ, Harris KJ, Scott AB. Adaptation of a lay health advisor model as a recruitment and retention strategy in a clinical trial of college student smokers. Health Promotion Practice 2010;11(5):751‐9. [DOI: 10.1177/1524839908325065] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SJ, Cronk NJ, Harris KJ, Scott AB. Adaptation of a lay health advisor model as a recruitment and retention strategy in a clinical trial of college student smokers. Health Promotion Practice 2010;11:751‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helstrom 2007 {published data only}
- Helstrom A, Hutchison K, Bryan A. Motivational enhancement therapy for high‐risk adolescent smokers. Addictive Behaviors 2007;32(10):2404‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helstrom AW. A Smoking Intervention for High‐Risk Adolescents [Dissertation]. Boulder (USA): University of Colorado, 2004. [Google Scholar]
Hollis 2007 {published data only}
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tobacco Control 2007;16(Suppl 1):i53‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2006 {published data only}
- Kelly AB, Lapworth K. The HYP program‐targeted motivational interviewing for adolescent violations of school tobacco policy. Preventive Medicine 2006;43(6):466‐71. [DOI] [PubMed] [Google Scholar]
Lewis 1998 {published data only}
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: a randomized clinical trial. Preventive Medicine 1998;27(2):296‐303. [DOI] [PubMed] [Google Scholar]
Lloyd‐Richardson 2009 {published data only (unpublished sought but not used)}
- Lloyd‐Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction 2009;104(11):1891‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Richardson EE, Stanton C, Carton‐Lopez S, Morrow K, Shadel W. Motivation and patch treatment for HIV‐positive smokers: psychosocial barriers to cessation (POS1‐057). Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 Feb 18‐21; Phoenix, Arizona. 2004.
- Stanton CA, Lloyd‐Richardson EE, Papandonatos GD, Dios MA, Niaura R. Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Education and Prevention 2009;21(3 Suppl):65‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louwagie 2014 {published data only}
- Louwagie GM, Ayo‐Yusuf OA. Tobacco use patterns in tuberculosis patients with high rates of human immunodeficiency virus co‐infection in South Africa. BMC Public Health 2013;13:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie GM, Okuyemi KS, Olalekan AA. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction 2014;109(11):1942‐52. [DOI] [PubMed] [Google Scholar]
- Louwagie GMC, Ayo‐Yusuf OA. Predictors of tobacco smoking abstinence among tuberculosis patients in South Africa CNO ‐ CN‐01165925. Journal of Behavioral Medicine 2015;38(3):472‐82. [DOI] [PubMed] [Google Scholar]
Marshall 2016 {published data only (unpublished sought but not used)}
- Marshall HM, Courtney DA, Passmore LH, McCaul EM, Yang IA, Bowman RV, et al. Brief tailored smoking cessation counseling in a lung cancer screening population is feasible: a pilot randomized controlled trial.. Nicotine & Tobacco Research 2016;18(7):1665‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Marshall HM, Yang IA, Passmore L, McCaul EM, Bowman R, Fong KM. A randomized controlled trial of brief counselling intervention and audio materials for smoking cessation in a low‐dose CT screening study. Journal of Thoracic Oncology 2013;8(Suppl 2):S703. [Google Scholar]
Matuszewski 2018 {published data only}
- Matuszewski P, Ordonio K, O’Hara N, O’Toole R. Prospective randomized trial on smoking cessation in orthopaedic trauma patients: could the 'Hawthorne Effect' of carbon monoxide monitoring be the best treatment?. ota.org/education/meetings‐and‐courses/abstracts/prospective‐randomized‐trial‐smoking‐cessation‐orthopaedic (Accessed 11 May 2019).
- NCT02428244. Let's STOP now trial: smoking in trauma orthopaedic patients. ClinicalTrials.gov/show/NCT02428244 (first received 4 July 2011).
McClure 2005 {published data only}
- McClure J, Westbrook E, Wetter DW, Curry SJ. Proactive cessation treatment in a managed care setting. Society for Behavioral Medicine 10th Annual Meeting; 2005 Feb 18‐21; Phoenix, Arizona. 2005.
- McClure J, Wetter D, Curry SJ. Acceptability of proactive contact and counseling among female smokers (POS3‐039). Nicotine & Tobacco Research 2004;6:722. [Google Scholar]
- McClure JB, Westbrook E, Curry SJ, Wetter DW. Proactive, motivationally enhanced smoking cessation counseling among women with elevated cervical cancer risk. Nicotine & Tobacco Research 2005;7(6):881‐9. [DOI] [PubMed] [Google Scholar]
Naik 2014 {published and unpublished data}
- Naik S, Khanagar S, Kumar A, Ramachandra S, Vadavadagi SV, Dhananjaya KM. Assessment of effectiveness of smoking cessation intervention among male prisoners in India: a randomized controlled trial. Journal of International Society of Preventive & Community Dentistry 2014;4(Suppl 2):S110‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02645838 {published data only}
- NCT02645838. Motivational interviewing for smoking cessation. clinicaltrials.gov/ct2/show/NCT02645838 (first received 5 January 2016).
Okuyemi 2013 {published data only}
- Goldade K, Jarlais D, Everson‐Rose SA, Guo H, Thomas J, Gelberg L, et al. Knowing quitters predicts smoking cessation in a homeless population. American Journal of Health Behavior 2013;37(4):517‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldade K, Whembolua G, Thomas J, Eischen S, Guo H, Connett J, et al. Designing a smoking cessation intervention for the unique needs of homeless persons: a community‐based randomized clinical trial. Clinical Trials 2011;8(6):744‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00786149. Improving varenicline adherence and outcomes in homeless smokers. ClinicalTrials.gov/show/NCT00786149 (first received 6 November 2008).
- Ojo‐Fati O, Thomas JL, Vogel RI, Ogedegbe O, Jean‐Louis G, Okuyemi KS. Predictors of adherence to nicotine replacement therapy (nicotine patch) among homeless persons enrolled in a randomized controlled trial targeting smoking cessation. Journal of Family Medicine 2016;3(7):1‐13. [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua G, Thomas JL, Eischen S, Guo H, et al. Smoking characteristics and comorbidities in the power to quit randomized clinical trial for homeless smokers. Nicotine & Tobacco Research 2013;15(1):22‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua G, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108(6):1136‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Guo H, et al. Effects of motivational interviewing and the nicotine patch for smoking cessation among homeless smokers (PA7‐2). Society for Research on Nicotine and Tobacco 18th Annual Meeting; 2012 Mar 13‐16; Houston Texas. 2012.
- Richards CM, Sharif F, Eischen S, Thomas J, Wang Q, Guo H, et al. Retention of homeless smokers in the power to quit study. Nicotine & Tobacco Research 2015;17(9):1104‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CD, Rogers CR, Okuyemi KS. Depression symptoms among homeless smokers: effect of motivational interviewing. Substance Use & Misuse 2016;51(10):1393‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C, Sewali B, Olayinka A, Eischen S, Wang Q, Guo H, et al. Smoking cessation in homeless populations: who participates and who does not. Nicotine & Tobacco Research 2014;16(3):369‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohsenow 2014 {published data only}
- Rohsenow DJ, Martin RA, Monti PM, Colby SM, Day AM, Abrams DB, et al. Motivational interviewing versus brief advice for cigarette smokers in residential alcohol treatment. Journal of Substance Abuse Treatment 2014;46(3):346‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Colby SM, Martin RA. Brief interventions for smoking cessation in alcoholic smokers. Alcoholism, Clinical and Experimental Research 2002;26(12):1950‐1. [DOI] [PubMed] [Google Scholar]
Rohsenow 2015 {published data only}
- Rohsenow D, Martin R, Tidey J, Monti P, Swift R. Motivational and contingency interventions for unmotivated smokers in substance abuse treatment (PA‐7). Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 Mar 20‐23; Prague, Czech Republic. 2005.
- Rohsenow DJ, Tidey JW, Kahler CW, Martin RA, Colby SM, Sirota AD. Intolerance for withdrawal discomfort and motivation predict voucher‐based smoking treatment outcomes for smokers with substance use disorders. Addictive Behaviors 2015;43:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Sirota AD, Swift RM, et al. Contingent vouchers and motivational interviewing for cigarette smokers in residential substance abuse treatment. Journal of Substance Abuse Treatment 2015;55:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2016 {published data only}
- Grossman E, Shelley D, Braithwaite RS, Lobach I, Goffin A, Rogers E, et al. Effectiveness of smoking‐cessation interventions for urban hospital patients: study protocol for a randomized controlled trial. Trials 2012;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01363245. Effectiveness of smoking‐cessation interventions for urban hospital patients. clinicaltrials.gov/ct2/show/NCT01363245 (first received 1 June 2011).
- Sherman SE, Link AR, Rogers ES, Krebs P, Ladapo JA, Shelley DR, et al. Smoking‐cessation interventions for urban hospital patients: a randomized comparative effectiveness trial. American Journal of Preventive Medicine 2016;51(4):566‐77. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soria 2006 {published data only}
- Soria R, Legido A, Escolano C, Lopez Yeste A, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. British Journal of General Practice 2006;56(531):768‐74. [PMC free article] [PubMed] [Google Scholar]
Stein 2006 {published data only}
- Stein MD, Anderson BJ, Niaura R. Smoking cessation patterns in methadone‐maintained smokers. Nicotine & Tobacco Research 2007;9(3):421‐8. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ. Respiratory symptom relief related to reduction in cigarette use. Journal of General Internal Medicine 2005;20(10):889‐94. [DOI: 10.1111/j.1525-1497.2005.0190.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone‐maintained. Addiction 2006;101(4):599‐607. [DOI] [PubMed] [Google Scholar]
Tevyaw 2009 {published data only}
- Tevyaw TO, Colby SM, Tidey JW, Kahler CW, Rohsenow DJ, Barnett NP, et al. Contingency management and motivational enhancement: a randomized clinical trial for college student smokers. Nicotine & Tobacco Research 2009;11(6):739‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidrine 2019 {published data only}
- Daly AT, Deshmukh AA, Vidrine DJ, Prokhorov AV, Frank SG, Tahay PD, et al. Cost‐effectiveness analysis of smoking cessation interventions using cell phones in a low‐income population. Tobacco Control 2019;28(1):88‐94. [DOI: 10.1136/tobaccocontrol-2017-054229] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00948129. Smoking cessation for low‐income adults. clinicaltrials.gov/ct2/show/NCT00948129 (first received 29 July 2009).
- Vidrine DJ, Fletcher FE, Danysh HE, Marani S, Vidrine JI, Cantor SB, et al. A randomized controlled trial to assess the efficacy of an interactive mobile messaging intervention for underserved smokers: project ACTION. BMC Public Health 2012;12:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Frank‐Pearce SG, Vidrine JI, Tahay PD, Marani SK, Chen S, et al. Efficacy of mobile phone–delivered smoking cessation interventions for socioeconomically disadvantaged individuals: a randomized clinical trial. JAMA Internal Medicine 2019;179(2):167‐74. [DOI: 10.1001/jamainternmed.2018.5713] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodruff 2007 {published data only}
- Woodruff SI, Conway TL, Edwards CC, Elliott SP, Crittenden J. Evaluation of an Internet virtual world chat room for adolescent smoking cessation. Addictive Behaviors 2007;32(9):1769‐86. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu D, Ma GX, Zhou K, Zhou D, Liu A, Poon AN. The effect of a culturally tailored smoking cessation for Chinese American smokers. Nicotine & Tobacco Research 2009;11(12):1448‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12609000627257 {published data only}
- ACTRN12609000627257. Using admissions to a "smokefree" hospital to promote cessation of smoking in mental health inpatients versus a representation of the general population [The effect of a quit smoking program on the intention of mental health inpatients versus orthopaedic, plastic surgery and neurosurgery inpatients (as representative of the general population) to adopt smoking cessation strategies]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308251 (first received 20 Jul 2009).
ACTRN12609001039279 {published data only}
- ACTRN12609001039279. Healthy lifestyle intervention for cardiovascular disease risk reduction among smokers with psychotic disorders [In smokers with psychotic disorders, is an intervention consisting of nicotine replacement therapy (NRT) and motivational interviewing and cognitive‐behaviour therapy (MICBT) and contingency management (CM) as or more effective as a one‐session intervention followed by NRT plus brief contact for reduction of cardiovascular disease (CVD) risk and smoking cessation?]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609001039279 (first received 17 Jul 2009).
ACTRN12612000016831 {published data only}
- ACTRN12612000016831. A secondary prevention patient centred model for smoking cessation [A pilot study of a nurse‐led evidence based smoking cessation program in smokers with cardiovascular disease to develop a secondary prevention model of care for smoking cessation]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000016831 (first received 21 Dec 2011).
ACTRN12614000876695 {published data only}
- ACTRN12614000876695. Improving radiotherapy outcomes with smoking cessation: feasibility trial in head and neck cancer patients [Improving radiotherapy outcomes in head and neck cancer patients: a preliminary comparison of smoking cessation intervention ‘Varenicline plus support’ with ‘treatment as usual’]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366605 (first received 7 Jul 2014).
ACTRN12614001147673 {published data only}
- ACTRN12614001147673. Smoke‐free recovery from trauma surgery: a pilot trial of an online smoking cessation programme for trauma surgery patients [A pilot trial of an online smoking cessation program investigating uptake and usage of an online smoking cessation programme]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366829 (first received 14 Oct 2014).
ACTRN12616000314426 {published data only}
- ACTRN12616000314426. Efficacy of a pre‐release group‐based abstinence program on extending prisoners' smoking abstinence post release from smoke‐free prisons in Queensland [Efficacy of a pre‐release group‐based abstinence program on extending prisoners' smoking abstinence post release from smoke‐free prisons in Queensland: a randomised controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370213 (first received 25 Feb 2016).
Aertsen Van Der Kuip 2006 {published data only}
- Aertsen‐Van Der Kuip MSA, Besselink RM, Vlugt MJ, Peters E, Fouwels AJ, Wollersheim H, et al. Successful motivational interviewing for smoking cessation in a secondary cardiovascular prevention clinic. European Heart Journal 2006;27(Suppl 1):165. [Google Scholar]
Ahluwalia 1998 {published data only}
- Ahluwalia JS, Resnicow K, Clark WS. Knowledge about smoking, reasons for smoking, and reasons for wishing to quit in inner‐city African Americans. Ethnicity & Disease 1998;8(3):385‐93. [PubMed] [Google Scholar]
Auer 2016 {published data only}
- Auer R, Gencer B, Tango R, Nanchen D, Matter CM, Luscher TF, et al. Uptake and efficacy of a systematic intensive smoking cessation intervention using motivational interviewing for smokers hospitalised for an acute coronary syndrome: a multicentre before‐after study with parallel group comparisons. BMJ Open 2016;6(9):e011520. [DOI: 10.1136/bmjopen-2016-011520] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2006 {published data only}
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. American Journal of Psychiatry 2006;163(11):1934‐42. [DOI: 10.1176/appi.ajp.163.11.1934] [DOI] [PubMed] [Google Scholar]
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. Characteristics of smokers with a psychotic disorder and implications for smoking interventions. Psychiatry Research 2007;150(2):141‐52. [DOI: 10.1016/j.psychres.2006.05.021] [DOI] [PubMed] [Google Scholar]
- Baker A, Richmond R, Kay‐Lambkin F, Lewin TJ, Carr VJ. Randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder: 3‐year follow‐up (PA2‐2). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007:19.
- Lan TH, Chiu HJ, Wu BJ, Hung TH, Hu TM. Readiness to quit and smoking reduction outcomes. American Journal of Psychiatry 2007;164:827‐8. [DOI] [PubMed] [Google Scholar]
Bernstein 2018 {published data only}
- Bernstein SL, Dziura J, Weiss J, Miller T, Vickerman KA, Grau LE, et al. Tobacco dependence treatment in the emergency department: a randomized trial using the Multiphase Optimization Strategy. Contemporary Clinical Trials 2018;66:1‐8. [DOI: 10.1016/j.cct.2017.12.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boccio 2017 {published data only}
- Boccio M, Sanna RS, Adams SR, Goler NC, Brown SD, Neugebauer RS, et al. Telephone‐based coaching. American Journal of Health Promotion 2017;31(2):136‐42. [DOI: 10.4278/ajhp.140821-QUAN-424] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolger 2010 {published data only}
- Bolger K, Carter K, Curtin L, Martz DM, Gagnon SG, Michael KD. Motivational interviewing for smoking cessation among college students. Journal of College Student Psychotherapy 2010;24:116‐29. [Google Scholar]
Bonevski 2018 {published data only}
- Bonevski B, Twyman L, Paul C, D'Este C, West R, Siahpush M, et al. Smoking cessation intervention delivered by social service organisations for a diverse population of Australian disadvantaged smokers: a pragmatic randomised controlled trial. Preventive Medicine 2018;112:38‐44. [DOI] [PubMed] [Google Scholar]
Borrelli 2002 {published data only}
- Borrelli B, McQuaid EL, Becker B, Hammond K, Papandonatos G, Fritz G, et al. Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Education Research 2002;17(5):659‐69. [DOI] [PubMed] [Google Scholar]
Borrelli 2005 {published data only}
- Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community‐nurse Assisted Research and Education on Smoking). Preventive Medicine 2005;41(5‐6):815‐21. [DOI: 10.1016/j.ypmed.2005.08.004] [DOI] [PubMed] [Google Scholar]
Borrelli 2016 {published data only}
- Borrelli B, McQuaid EL, Tooley EM, Busch AM, Hammond SK, Becker B, et al. Motivating parents of kids with asthma to quit smoking: the effect of the teachable moment and increasing intervention intensity using a longitudinal randomized trial design. Addiction 2016;111(9):1646‐55. [DOI: 10.1111/add.13389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borrelli 2017 {published data only}
- Borrelli B, Endrighi R, Hammond SK, Dunsiger S. Smokers who are unmotivated to quit and have a child with asthma are more likely to quit with intensive motivational interviewing and repeated biomarker feedback CNO ‐ CN‐01430706. Journal of Consulting and Clinical Psychology 2017;85(11):1019‐28. [DOI: 10.1037/ccp0000238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2007 {published data only}
- Boyle RG, Solberg LI, Asche SE, Maciosek MV, Boucher JL, Pronk NP. Proactive recruitment of health plan smokers into telephone counseling. Nicotine & Tobacco Research 2007;9(5):581‐9. [DOI: 10.1080/14622200701243201] [DOI] [PubMed] [Google Scholar]
Breland 2014 {published data only}
- Breland AB, Almond L, Kienzle J, Ondersma SJ, Hart AJr, Weaver M, et al. Targeting tobacco in a community‐based addiction recovery cohort: results from a computerized, brief, randomized intervention trial. Contemporary Clinical Trials 2014;38(1):113‐20. [DOI: 10.1016/j.cct.2014.03.008] [DOI] [PubMed] [Google Scholar]
Bronson 1989 {published data only}
- Bronson DL, Flynn BS, Solomon LJ, Vacek PM, Secker‐Walker RH. Smoking cessation counseling during periodic health examinations. Archives of Internal Medicine 1989;149(7):1653‐6. [PubMed] [Google Scholar]
Brooks 2017 {published data only}
- Brooks DR, Burtner JL, Borrelli B, Heeren TC, Evans T, Davine JA, et al. Twelve‐month outcomes of a group‐randomized community health advocate‐led smoking cessation intervention in public housing. Nicotine & Tobacco Research 2017;20(12):1434‐41. [DOI: 10.1093/ntr/ntx193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2003 {published data only}
- Brown RA, Ramsey SE, Strong DR, Myers MG, Kahler CW, Lejuez CW, et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tobacco Control 2003;12 Suppl 4:IV3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Strong DR, Abrantes AM, Myers MG, Ramsey SE, Kahler CW. Effects on substance use outcomes in adolescents receiving motivational interviewing for smoking cessation during psychiatric hospitalization. Addictive Behaviors 2009;34(10):887‐91. [DOI: 10.1016/j.addbeh.2009.03.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L, Strong DR, Kahler CW, Abrantes AM, Ramsey SE, Brown RA. Association of post‐treatment smoking change with future smoking and cessation efforts among adolescents with psychiatric comorbidity. Nicotine & Tobacco Research 2007;9:1297‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L, Strong DR, Palm KM, Abrantes AM, Brown RA. Post‐intervention lapse and relapse in adolescent smokers with psychiatric comorbidity (POS1‐84). Society for Research on Nicotine and Tobacco 13th Annual Meeting, 2007 February 21‐24, Austin, Texas. 2007:58.
- Tzilos GK, Strong DR, Abrantes AM, Ramsey SE, Brown RA. Quit intention as a predictor of quit attempts over time in adolescents with psychiatric disorders. American Journal on Addictions 2014;23:84‐9. [DOI: 10.1111/j.1521-0391.2013.12067.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caponnetto 2017 {published data only}
- Caponnetto P, DiPiazza J, Aiello MR, Polosa R. Training pharmacists in the stage‐of‐change model of smoking cessation and motivational interviewing: a randomized controlled trial. Health Psychology Open 2017;4(2):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2004 {published data only}
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology 2004;72(3):371‐81. [DOI: 10.1037/0022-006X.72.3.371] [DOI] [PubMed] [Google Scholar]
Cigrang 2002 {published data only}
- Cigrang JA, Severson HH, Peterson AL. Pilot evaluation of a population‐based health intervention for reducing use of smokeless tobacco. Nicotine & Tobacco Research 2002;4(1):127‐31. [DOI: 10.1080/14622200110101603] [DOI] [PubMed] [Google Scholar]
Collicott 2001 {published data only}
- Collicott SF. Increasing readiness to change among smokers in a primary care setting. Dissertation Abstracts International 2001;61:6699. [Google Scholar]
Cornuz 2002 {published data only}
- Cornuz J, Humair JP, Seematter L, Stoianov R, Melle G, Stalder H, et al. Efficacy of resident training in smoking cessation: a randomized, controlled trial of a program based on application of behavioral theory and practice with standardized patients. Annals of Internal Medicine 2002;136(6):429‐37. [DOI] [PubMed] [Google Scholar]
Curry 2003 {published data only}
- Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric‐based smoking cessation intervention for low‐income women: a randomized trial. Archives of Pediatrics & Adolescent Medicine 2003;157(3):295‐302. [DOI] [PubMed] [Google Scholar]
Dornelas 2000 {published data only}
- Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Preventive Medicine 2000;30(4):261‐8. [DOI] [PubMed] [Google Scholar]
Eakin 2014 {published data only}
- Bender BG. The challenge of reducing smoking in low‐income parents. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1457‐8. [DOI: 10.1164/rccm.201405-0893ED] [DOI] [PubMed] [Google Scholar]
- Eakin MN, Rand CS, Borrelli B, Bilderback A, Hovell M, Riekert KA. Effectiveness of motivational interviewing to reduce head start children's secondhand smoke exposure: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1530‐7. [DOI: 10.1164/rccm.201404-0618OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmons 2001 {published data only}
- Emmons KM, Hammond SK, Fava J, Velicer W, Evans J, Monroe A. The impact of motivational interviewing on passive smoke reduction in low income households with young children. 11th World Conference on Tobacco or Health; 2000 August 6‐11; Chicago. 2000:142.
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low‐income households with young children. Pediatrics 2001;108(1):18‐24. [DOI] [PubMed] [Google Scholar]
Ershoff 1999 {published data only}
- Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking‐cessation trial: when more isn't better, what is enough?. American Journal of Preventive Medicine 1999;17(3):161‐8. [DOI] [PubMed] [Google Scholar]
Gariti 2002 {published data only}
- Gariti P, Alterman A, Mulvaney F, Mechanic K, Dhopesh V, Yu E, et al. Nicotine intervention during detoxification and treatment for other substance use. American Journal of Drug and Alcohol Abuse 2002;28:671‐9. [DOI] [PubMed] [Google Scholar]
George 2000 {published data only}
- George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. American Journal of Psychiatry 2000;157(11):1835‐42. [DOI: 10.1176/appi.ajp.157.11.1835] [DOI] [PubMed] [Google Scholar]
Glasgow 2000 {published data only}
- Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low‐income planned parenthood clinics. American Journal of Public Health 2000;90(5):786‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ha 2012 {published data only}
- Ha YS, Choi YH. Effectiveness of a motivational interviewing smoking cessation program on cessation change in adolescents. Journal of Korean Academy of Nursing 2012;42(1):19‐27. [DOI: 10.4040/jkan.2012.42.1.19] [DOI] [PubMed] [Google Scholar]
Ha 2015 {published data only}
- Ha YS, Choi YH. Effectiveness of the self‐determination theory based a motivational interviewing YOU‐TURN program for smoking cessation among adolescents. Journal of Korean Academy of Nursing 2015;45(3):347‐56. [DOI: 10.4040/jkan.2015.45.3.347] [DOI] [PubMed] [Google Scholar]
Haas 2015 {published data only}
- Haas JS, Linder JA, Park ER, Gonzalez I, Rigotti NA, Klinger EV, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. JAMA Internal Medicine 2015;175(2):218‐26. [DOI: 10.1001/jamainternmed.2014.6674] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hennrikus 2002 {published data only}
- Hennrikus DJ, Jeffery RW, Lando HA, Murray DM, Brelje K, Davidann B, et al. The SUCCESS project: the effect of program format and incentives on participation and cessation in worksite smoking cessation programs. American Journal of Public Health 2002;92(2):274‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hennrikus 2005 {published data only}
- Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: the effectiveness of smoking cessation interventions with hospital patients. Preventive Medicine 2005;40(3):249‐58. [DOI: 10.1016/j.ypmed.2004.05.030] [DOI] [PubMed] [Google Scholar]
Hokanson 2006 {published data only}
- Hokanson JM, Anderson RL, Hennrikus DJ, Lando HA, Kendall DM. Integrated tobacco cessation counseling in a diabetes self‐management training program: a randomized trial of diabetes and reduction of tobacco. Diabetes Educator 2006;32(4):562‐70. [DOI: 10.1177/0145721706289914] [DOI] [PubMed] [Google Scholar]
Horn 2007 {published data only}
- Horn K, Dino G, Hamilton C, Noerachmanto N. Efficacy of an emergency department‐based motivational teenage smoking intervention. Preventing Chronic Disease 2007;4(1):A08. [PMC free article] [PubMed] [Google Scholar]
- Horn K, Dino G, Hamilton C, Noerachmanto N, Zhang J. Feasibility of a smoking cessation intervention for teens in the emergency department: reach, implementation fidelity, and acceptability. American Journal of Critical Care 2008;17(3):205‐16. [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang FF, Jiao NN, Zhang LY, Lei Y, Zhang JP. Effects of a family‐assisted smoking cessation intervention based on motivational interviewing among low‐motivated smokers in China. Patient Education and Counseling 2015;98(8):984‐90. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hughes 2017 {published data only}
- Hughes SC, Corcos I, Hovell M, Hofstetter CR. Feasibility pilot of a randomized faith‐based intervention to reduce secondhand smoke exposure among Korean Americans. Preventing Chronic Disease 2017;14:E19. [DOI: 10.5888/pcd14.160549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchinson 2017 {published data only}
- Hutchinson S, Breukelen G, Schayck O, Essers B, Muris J, Feron F, et al. A randomised trial to stop passive smoking in children with a high risk of asthma (abstract). European Respiratory Journal 2014;44:1404. [Google Scholar]
- Hutchinson SG, Breukelen G, Schayck CP, Essers B, Hammond SK, Muris JWM, et al. Motivational interviewing and urine cotinine feedback to stop passive smoke exposure in children predisposed to asthma: a randomised controlled trial. Scientific Reports 2017;7(1):15473. [DOI: 10.1038/s41598-017-15158-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyman 2007 {published data only}
- Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Archives of Internal Medicine 2007;167(11):1152‐8. [DOI: 10.1001/archinte.167.11.1152] [DOI] [PubMed] [Google Scholar]
Idrisov 2013 {published data only}
- Idrisov B, Sun P, Akhmadeeva L, Arpawong TE, Kukhareva P, Sussman S. Immediate and six‐month effects of Project EX Russia: a smoking cessation intervention pilot program. Addictive Behaviors 2013;38(8):2402‐8. [DOI: 10.1016/j.addbeh.2013.03.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingersoll 2005 {published data only}
- Ingersoll KS, Cropsey KL, Heckman CJ. Reducing smoking among people with HIV: outcomes and lessons from three pilot projects. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20‐23; Prague, Czech Republic. 2005.
IRCT2017080435257N1 {published data only}
- IRCT2017080435257N1. The effect of motivational counseling on smoking cessation. en.irct.ir/trial/26698 (first received September 11 2017).
ISRCTN11353250 {published data only}
- ISRCTN11353250. Efficacy of smoking cessation treatment interventions for individuals with severe mental illness: a pilot randomized controlled clinical trial. www.isrctn.com/ISRCTN11353250 (first received February 6 2015).
ISRCTN50627997 {published data only}
- ISRCTN50627997. Evaluation of the effectiveness of brief counseling for tobacco cessation during dental care in Sweden. www.isrctn.com/ISRCTN50627997 (first received July 23 2012).
Klemperer 2017 {published data only}
- Klemperer EM, Hughes JR, Callas PW, Solomon LJ. A mediation analysis of motivational, reduction, and usual care interventions for smokers who are not ready to quit. Nicotine & Tobacco Research 2017;19(8):916‐21. [DOI: 10.1093/ntr/ntx025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction 2017;112(1):146‐55. [DOI: 10.1111/add.13594] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krigel 2011 {published data only}
- Krigel SW. Motivational interviewing and the effect of the decisional balance exercise on college smokers. Dissertation Abstracts International 2011;71:7067. [Google Scholar]
Lasser 2012 {published data only}
- Lasser KE, Kenst KS, Quintiliani L, Wiener RS, Murillo J, Bowen DJ. Patient navigation to promote smoking cessation in primary care: baseline results from a pilot randomized controlled trial. Journal of General Internal Medicine 2012;27:S263. [Google Scholar]
Lennox 1998 {published data only}
- Lennox AS, Bain N, Taylor RJ, McKie L, Donnan PT, Groves J. Stages of change training for opportunistic smoking intervention by the primary health care team. Part I: randomised controlled trial of the effect of training on patient smoking outcomes and health professional behaviour as recalled by patients. Health Education Journal 1998;57:140‐9. [Google Scholar]
Lindqvist 2013 {published data only}
- Lindqvist H, Forsberg LG, Forsberg L, Rosendahl I, Enebrink P, Helgason AR. Motivational interviewing in an ordinary clinical setting: a controlled clinical trial at the Swedish National Tobacco Quitline. Addictive Behaviors 2013;38(7):2321‐4. [DOI: 10.1016/j.addbeh.2013.03.002] [DOI] [PubMed] [Google Scholar]
Luna 2005 {published data only}
- Luna L. The effectiveness of motivational enhancement therapy on smoking cessation in college students. Dissertation Abstracts International: Section B: the Sciences and Engineering 2005;65:5383. [Google Scholar]
Ma 2005a {published data only}
- Ma GX, Tan Y, Knauer CA, Toubbeh J, Choi J. A study of culturally appropriate smoking cessation for underserved Korean American smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 2005 March 20‐23; Prague, Czech Republic. 2005.
Ma 2005b {published data only}
- Ma GX. Study of culturally appropriate smoking cessation for underserved Korean American smokers. American Public Health Association 133rd Annual Meeting & Exposition; 2005 Dec 10; Philadelphia, MA. 2005.
Mahajan 2017 {published data only}
- Mahajan R, Solanki J, Kurdekar RS, Gupta S, Modh A, Yadav O. Educating the handicraft factory workers about tobacco cessation and to assess its effectiveness by motivational interviewing: an Intervention study. Journal of Experimental Therapeutics & Oncology 2017;12(1):43‐9. [PubMed] [Google Scholar]
Manfredi 1999 {published data only}
- Manfredi C, Crittenden KS, Warnecke R, Engler J, Cho YI, Shaligram C. Evaluation of a motivational smoking cessation intervention for women in public health clinics. Preventive Medicine 1999;28(1):51‐60. [DOI: 10.1006/pmed.1998.0377] [DOI] [PubMed] [Google Scholar]
Manfredi 2004 {published data only}
- Manfredi C, Crittenden KS, Cho YI, Gao S. Long‐term effects (up to 18 months) of a smoking cessation program among women smokers in public health clinics. Preventive Medicine 2004;38:10‐9. [DOI] [PubMed] [Google Scholar]
Martin‐Lujan 2011 {published data only}
- Martin‐Lujan F, Piñol‐Moreso JL, Martin‐Vergara N, Basora‐Gallisa J, Pascual‐Palacios I, Sagarra‐Alamo R, et al. Effectiveness of a structured motivational intervention including smoking cessation advice and spirometry information in the primary care setting: the ESPITAP study. BMC Public Health 2011;11(1):859‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazas 2007 {published data only}
- Mazas C, Li Y, Cofta‐Woerpel L, Wetter DW, Daza P, Nguyen L, et al. Disparities and smoking cessation among Latinos (SYM 2B). Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24, Austin, Texas. 2007:3.
McCambridge 2005 {published data only}
- McCambridge J, Strang J. Deterioration over time in effect of motivational Interviewing in reducing drug consumption and related risk among young people. Addiction 2005;100(4):470‐8. [DOI: 10.1111/j.1360-0443.2005.01013.x] [DOI] [PubMed] [Google Scholar]
Menzie 2018 {published data only}
- Menzie NS. A motivational interviewing intervention to increase utilization of smoking cessation services among veterans undergoing substance use treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2018;79:No pagination specified. [Google Scholar]
Metse 2017 {published data only}
- Metse AP, Bowman JA, Wye P, Stockings E, Adams M, Clancy R, et al. Evaluating the efficacy of an integrated smoking cessation intervention for mental health patients: study protocol for a randomised controlled trial. Trials 2014;15:266. [DOI: 10.1186/1745-6215-15-266] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metse AP, Wiggers J, Wye P, Wolfenden L, Freund M, Clancy R, et al. Efficacy of a universal smoking cessation intervention initiated in inpatient psychiatry and continued post‐discharge: a randomised controlled trial. Australian and New Zealand Journal of Psychiatry 2017;51(4):366‐81. [DOI: 10.1177/0004867417692424] [DOI] [PubMed] [Google Scholar]
Metz 2006a {published and unpublished data}
- Metz K, Kroeger C, Schuetz C, Floeter S, Donath C. Which smoking cessation intervention works for smokers with an alcohol addiction?. 68th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2006. 2006.
Metz 2006b {published data only}
- Metz K, Kroger C, Donath C, Floter S, Gradl S, Piontek D. Evaluation of a motivational intervention for smokers in rehabilitation centers. Verhaltenstherapie & Verhaltensmedizin 2006;27:445‐63. [Google Scholar]
Meyer 2003 {published data only}
- Meyer C, Ulbricht S, Schumann A, Hannover W, Hapke U, Rumpf HJ, et al. Interventions fostering the motivation to quit for smokers in general practice. Suchtmedizin in Forschung und Praxis 2003;5:134‐6. [Google Scholar]
- Ulrich JU, Ulbricht S, Goeze C, Meyer C. Brief intervention to motivate smokers to quit in primary medical care. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18:S40. [Google Scholar]
Mujcic 2018 {published data only}
- Mujcic A, Blankers M, Boon B, Engels R, Laar M. Internet‐based self‐help smoking cessation and alcohol moderation interventions for cancer survivors: a study protocol of two RCTs. BMC Cancer 2018;18(1):364. [DOI: 10.1186/s12885-018-4206-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00169260 {published data only}
- NCT00169260. Proactive health intervention for tobacco users (Get PHIT). clinicaltrials.gov/ct2/show/NCT00169260 (first received September 15 2005).
NCT00701896 {published data only}
- NCT00701896. Smoking cessation and the natural history of HIV‐associated emphysema. clinicaltrials.gov/show/nct00701896 (first received June 19 2008).
NCT00907309 {published data only}
- NCT00907309. Dental and medical office internet/intranet motivational enhancement therapy to reduce teen tobacco, alcohol, and drug use. clinicaltrials.gov/show/NCT00907309 (first received May 22 2009).
NCT01098955 {published data only}
- NCT01098955. Smoking cessation treatment for head & neck cancer patients: acceptance and commitment therapy. clinicaltrials.gov/show/nct01098955 (first received April 5 2010).
NCT01846910 {published data only}
- NCT01846910. Prepare to quit ‐ a randomized clinical trial to help people quit smoking in a community dental clinic setting. clinicaltrials.gov/show/NCT01846910 (first received May 6 2014).
NCT01982617 {published data only}
- NCT01982617. A randomized, controlled trial of motivational interviewing compared to psychoeducation for smoking precontemplators with severe mental illness. clinicaltrials.gov/show/nct01982617 (first received November 13 2013).
NCT02086162 {published data only}
- NCT02086162. Randomized controlled trial (RCT) of a motivational decision support system. clinicaltrials.gov/show/NCT02086162 (first received March 13 2014).
Nichter 2018 {published data only}
- Nichter M, Mini GK, Thankappan KR. Low‐level smoking among diabetes patients in India: a smoking cessation challenge. Clinical Epidemiology and Global Health 2018;6(4):176‐80. [DOI: 10.1016/j.cegh.2017.11.005] [DOI] [Google Scholar]
Pardavila‐Belio 2015 {published data only}
- Pardavila‐Belio MI, Garcia‐Vivar C, Pimenta AM, Canga‐Armayor A, Pueyo‐Garrigues S, Canga‐Armayor N. Intervention study for smoking cessation in Spanish college students: pragmatic randomized controlled trial. Addiction 2015;110(10):1676‐83. [DOI: 10.1111/add.13009] [DOI] [PubMed] [Google Scholar]
Parker 2007 {published data only}
- Parker DR, Windsor RA, Roberts MB, Hecht J, Hardy NV, Strolla LO, et al. Feasibility, cost, and cost‐effectiveness of a telephone‐based motivational intervention for underserved pregnant smokers. Nicotine & Tobacco Research 2007;9(10):1043‐51. [DOI: 10.1080/14622200701591617] [DOI] [PubMed] [Google Scholar]
Persson 2006 {published data only}
- Persson LG, Hjalmarson A. Smoking cessation in patients with diabetes mellitus: results from a controlled study of an intervention programme in primary healthcare in Sweden. Scandinavian Journal of Primary Health Care 2006;24:75‐80. [DOI] [PubMed] [Google Scholar]
Pineiro 2014 {published data only}
- Pineiro B, Rio Elena F, Lopez‐Duran A, Becona E. Does motivational interviewing improve the effectiveness of a psychological treatment to quit smoking?. Anales de Psicologia 2014;30:124‐33. [Google Scholar]
Polosa 2011 {published data only}
- Polosa R, Caponnetto P, Caruso M. Training pharmacists in the stage‐of‐change model of smoking cessation: a randomised controlled trial in Sicily (abstract). European Respiratory Society Annual Congress; 2011 Sept 24‐28; Amsterdam, the Netherlands. 2011; Vol. 38:780s [P4247].
Reitzel 2010 {published data only}
- Heppner WL, Ji L, Reitzel LR, Reitzel L, Castro Y, Correa‐Fernandez V, et al. The role of prepartum motivation in the maintenance of postpartum smoking abstinence. Health Psychology 2011;30(6):736‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel LR, Vidrine JI, Businelle MS, Kendzor DE, Costello TJ, Li Y, et al. Preventing postpartum smoking relapse among diverse low‐income women: a randomized clinical trial. Nicotine & Tobacco Research 2010;12(4):326‐35. [DOI: 10.1093/ntr/ntq001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 1997 {published data only}
- Rigotti NA, Arnsten JH, McKool KM, Wood‐Reid KM, Pasternak RC, Singer DE. Efficacy of a smoking cessation program for hospital patients. Archives of Internal Medicine 1997;157(22):2653‐60. [PubMed] [Google Scholar]
Rigotti 2006 {published data only}
- Rigotti NA, Park ER, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of telephone counseling for pregnant smokers: a randomized controlled trial. Obstetrics and Gynecology 2006;108(1):83‐92. [DOI: 10.1097/01.AOG.0000218100.05601.f8] [DOI] [PubMed] [Google Scholar]
Rogers 2016 {published data only}
- Rogers ES, Smelson DA, Gillespie CC, Elbel B, Poole S, Hagedorn HJ, et al. Telephone smoking‐cessation counseling for smokers in mental health clinics: a patient‐randomized controlled trial. American Journal of Preventive Medicine 2016;50(4):518‐27. [DOI: 10.1016/j.amepre.2015.10.004] [DOI] [PubMed] [Google Scholar]
Schuck 2014 {published data only}
- Schuck K, Bricker JB, Otten R, Kleinjan M, Brandon TH, Engels RC. Effectiveness of proactive quitline counselling for smoking parents recruited through primary schools: results of a randomized controlled trial. Addiction 2014;109(5):830‐41. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Effectiveness of proactive telephone counselling for smoking cessation in parents: study protocol of a randomized controlled trial. BMC Public Health 2011;11(1):732. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Predictors of cessation treatment outcome and treatment moderators among smoking parents receiving quitline counselling or self‐help material.. Preventive Medicine 2014;69:126‐31. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Promoting smoking cessation among parents: effects on smoking‐related cognitions and smoking initiation in children. Addictive Behaviors 2015;40:66‐72. [DOI: ] [DOI] [PubMed] [Google Scholar]
Severson 2009 {published data only}
- Severson HH, Peterson AL, Andrews JA, Gordon JS, Cigrang JA, Danaher BG, et al. Smokeless tobacco cessation in military personnel: a randomized controlled trial. Nicotine & Tobacco Research 2009;11(6):730‐8. [DOI: 10.1093/ntr/ntp057] [DOI] [PubMed] [Google Scholar]
Sherbot 2005 {published data only}
- Sherbot NA. The use of motivational enhancement therapy and the quit 4 life program as a means to facilitate adolescent smoking cessation. Dissertation Abstracts International: Section B: the Sciences and Engineering 2005;66:574. [Google Scholar]
Sims 2013 {published data only}
- Sims TH, McAfee T, Fraser DL, Baker TB, Fiore MC, Smith SS. Quitline cessation counseling for young adult smokers: a randomized clinical trial. Nicotine & Tobacco Research 2013;15(5):932‐41. [DOI: 10.1093/ntr/nts227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skov‐Ettrup 2016 {published data only}
- Skov‐Ettrup LS, Dalum P, Bech M, Tolstrup JS. The effectiveness of telephone counselling and internet‐ and text‐message‐based support for smoking cessation: results from a randomized controlled trial. Addiction 2016;111(7):1257‐66. [DOI: 10.1111/add.13302] [DOI] [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith SS, Jorenby DE, Fiore MC, Anderson JE, Mielke MM, Beach KE, et al. Strike while the iron is hot: can stepped‐care treatments resurrect relapsing smokers?. Journal of Consulting and Clinical Psychology 2001;69(3):429‐39. [DOI] [PubMed] [Google Scholar]
Sobell 2017 {published data only}
- Sobell MB, Peterson AL, Sobell LC, Brundige A, Hunter CM, Goodie JL, et al. Reducing alcohol consumption to minimize weight gain and facilitate smoking cessation among military beneficiaries. Addictive Behaviors 2017;75:145‐51. [DOI: 10.1016/j.addbeh.2017.06.018] [DOI] [PubMed] [Google Scholar]
Steinberg 2016 {published data only}
- Steinberg ML, Williams JM, Stahl NF, Budsock PD, Cooperman NA. An adaptation of motivational interviewing increases quit attempts in smokers with serious mental illness. Nicotine & Tobacco Research 2016;18(3):243‐50. [DOI: 10.1093/ntr/ntv043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stotts 2002 {published data only}
- Stotts AL, Diclemente CC, Dolan‐Mullen Patricia. One‐to‐one: a motivational intervention for resistant pregnant smokers. Addictive Behaviors 2002;27(2):275‐92. [DOI] [PubMed] [Google Scholar]
Stotts 2009 {published data only}
- Stotts AL, Groff JY, Velasquez MM, Benjamin‐Garner R, Green C, Carbonari JP, et al. Ultrasound feedback and motivational interviewing targeting smoking cessation in the second and third trimesters of pregnancy. Nicotine & Tobacco Research 2009;11(8):961‐8. [DOI: 10.1093/ntr/ntp095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tappin 2000 {published data only}
- Tappin DM, Lumsden MA, McIntyre D, Mckay C, Gilmour WH, Webber R, et al. A pilot study to establish a randomized trial methodology to test the efficacy of a behavioural intervention. Health Education Research 2000;15(4):491‐502. [DOI] [PubMed] [Google Scholar]
Tappin 2005 {published data only}
- Tappin DM, Lumsden MA, Gilmour WH, Crawford F, McIntyre D, Stone DH, et al. Randomised controlled trial of home based motivational interviewing by midwives to help pregnant smokers quit or cut down. BMJ (Clinical Research Edition) 2005;331(7513):373‐7. [DOI: 10.1136/bmj.331.7513.373] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomsen 2010 {published data only}
- Thomsen T, Tønnesen H, Okholm M, Kroman N, Maibom A, Sauerberg ML, et al. Brief smoking cessation intervention in relation to breast cancer surgery: a randomized controlled trial. Nicotine & Tobacco Research 2010;12(11):1118‐24. [DOI: 10.1093/ntr/ntq158] [DOI] [PubMed] [Google Scholar]
Van Rossem 2015 {published data only}
- Rossem C, Spigt M, Smit ES, Viechtbauer W, Mijnheer KK, Schayck CP, et al. Combining intensive practice nurse counselling or brief general practitioner advice with varenicline for smoking cessation in primary care: study protocol of a pragmatic randomized controlled trial. Contemporary Clinical Trials 2015;41:298‐312. [DOI: 10.1016/j.cct.2015.01.017] [DOI] [PubMed] [Google Scholar]
Wakefield 2004 {published data only}
- Wakefield M, Olver I, Whitford H, Rosenfeld E. Motivational interviewing as a smoking cessation intervention for patients with cancer: randomized controlled trial. Nursing Research 2004;53(6):396‐405. [DOI] [PubMed] [Google Scholar]
Weaver 2015 {published data only}
- Weaver KE, Kaplan S, Griffin L, Urbanic J, Zbikowski S, Danhauer SC. Satisfaction with a quitline‐based smoking cessation intervention among cancer survivors. Cancer Epidemiology, Biomarkers & Prevention 2015;24(4):759. [DOI: 10.1158/1055-9965.EPI-15-0100] [DOI] [Google Scholar]
References to studies awaiting assessment
Zhou 2014 {published data only}
- Zhou Y, Feng X, Yu L, Gu Y. Research on the effects of motivational interviewing in smoking cessation for patients with coronary heart diseases. Cardiology (Switzerland) 2014;129:135. [Google Scholar]
References to ongoing studies
Lloyd‐Richardson 2003 {published data only}
- Lloyd‐Richardson EE, Niaura R, Abrams DB. Informed development of smoking cessation interventions for college students (POS4‐25). Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 Feb 19‐22; New Orleans, Louisiana. 2003.
NCT01387516 {published data only}
- NCT01387516. Motivation and skills for detained teen smokers. ClinicalTrials.gov/show/NCT01387516 (first received 4 July 2011).
NCT02905656 {published data only}
- NCT02905656. Strategies to promote cessation in smokers who are not ready to quit. ClinicalTrials.gov/show/NCT02905656 (first received 5 January 2016).
NCT03002883 {published data only}
- NCT03002883. STAND community college tobacco cessation trial. ClinicalTrials.gov/show/NCT03002883 (first received 5 January 2016).
Salgado Garcia 2018 {published data only}
- Salgado Garcia FI, Derefinko KJ, Bursac Z, Hand S, Klesges RC. Planning A Change Easily (PACE): a randomized controlled trial for smokers who are not ready to quit. Contemporary Clinical Trials 2018;68:14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Britt 2002
- Britt E, Hudson SM, Blampied NM. Motivational Interviewing in health settings: a review. Patient Education and Counselling 2004;53(2):147‐55. [DOI] [PubMed] [Google Scholar]
Burckhardt 2003
- Burckhardt CS, Anderson KL. The Quality Of Life Scale (QOLS): reliability, validity, and utilization. Health and Quality of Life Outcomes 2003;1(1):60‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2010
- Cahill K, Lancaster T, Green N. Stage‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD004492.pub4] [DOI] [PubMed] [Google Scholar]
CDC 2017
- Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2000–2015. Morbidity and Mortality Weekly Report 2017;65(52):1457‐64. [DOI] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara‐Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2015
- Cheng D, Qu Z, Huang J, Xiao Y, Luo H, Wang J. Motivational interviewing for improving recovery after stroke. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011398.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cowlishaw 2012
- Cowlishaw S, Merkouris S, Dowling N, Anderson C, Jackson A, Thomas S. Psychological therapies for pathological and problem gambling.. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008937.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol Group 1990
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
Foxcroft 2016
- Foxcroft DR, Coombes L, Wood S, Allen D, Almeida Santimano NML, Moreira MT. Motivational interviewing for the prevention of alcohol misuse in young adults. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD007025.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2016
- Gates PJ, Sabioni P, Copeland J, Foll B, Gowing L. Psychosocial interventions for cannabis use disorder. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD005336.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2015 Risk Factors Collaborators 2016
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1659‐724. [DOI: 10.1016/S0140-6736(16)31679-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heckman 2010
- Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta‐analysis. Tobacco Control 2010;19(5):410‐6. [DOI: 10.1136/tc.2009.033175] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hettema 2010
- Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta‐analytic review. Journal of Consulting and Clinical Psychology 2010;78(6):868‐84. [PUBMED: 21114344] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;7414:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jelsma 2015
- Jelsma JGM, Mertens V‐C, Forsberg L, Forsberg L. How to measure motivational interviewing fidelity in randomized controlled trials: practical recommendations. Contemporary Clinical Trials 2015;43:93‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jha 2011
- Jha P. Avoidable deaths from smoking: a global perspective. Public Health Reviews 2011;33(2):569‐600. [Google Scholar]
Klimas 2018
- Klimas J, Fairgrieve C, Tobin H, Field CA, O'Gorman CSM, Glynn LG, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD009269.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawendowski 1998
- Lawendowski LA. A motivational intervention for adolescent smokers. Preventive Medicine 1998;5(3):A39‐46. [DOI] [PubMed] [Google Scholar]
Markland 2005
- Markland D, Ryan RM, Tobin VJ, Rollnick S. Motivational interviewing and self–determination theory. Journal of Social and Clinical Psychology 2005;24(6):811‐31. [Google Scholar]
Mbuagbaw 2012
- Mbuagbaw L, Ye C, Thabane L. Motivational interviewing for improving outcomes in youth living with HIV. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009748.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1983
- Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy 1983;11:147‐72. [Google Scholar]
Miller 1994
- Miller WR. Motivational interviewing. III. On the ethics of motivational interviewing. Behavioural and Cognitive Psychotherapy 1994;22(2):111‐23. [Google Scholar]
Miller 2002
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. Guilford Press, 2002. [Google Scholar]
Miller 2009
- Miller WR, Rollnick S. Ten things motivational interviewing is not. Behavioural and Cognitive Psychotherapy 2009;37:129‐140. [DOI] [PubMed] [Google Scholar]
Miller 2013
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd Edition. New York: Guildford Press, 2013. [Google Scholar]
Morton 2015
- Morton K, Beauchamp M, Prothero A, Joyce L, Saunders L, Spencer‐Bowdage S, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychology Review 2015;9(2):205‐23. [https://doi.org/10.1080/17437199.2014.882006] [DOI] [PubMed] [Google Scholar]
Pierson 2007
- Pierson HM, Hayes SC, Gifford EV, Roget N, Padilla M, Bissett R, et al. An examination of the motivational interviewing treatment integrity code. Journal of Substance Abuse Treatment 2007;32(1):11‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rollnick 1992
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. Journal of Mental Health 1992;1:25‐37. [Google Scholar]
Rollnick 1995
- Rollnick SR, Miller WR. What is motivational interviewing?. Behavioural and Cognitive Psychotherapy 1995;23(4):325‐34. [DOI] [PubMed] [Google Scholar]
Ryan 2000
- Ryan RM, Deci EL. Self‐determination theory and the facilitation of intrinsic motivation, social development, and well‐being. American Psychologist 2000;55(1):68‐78. [DOI] [PubMed] [Google Scholar]
Smedslund 2011
- Smedslund G, Berg RC, Hammerstrøm KT, Steiro A, Leiknes KA, Dahl HM, et al. Motivational interviewing for substance abuse. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008063.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lai 2008
- Lai DTC, Qin Y, Tang J‐L. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006936] [DOI] [PubMed] [Google Scholar]
Lai 2010
- Lai DTC, Cahill K, Qin Y, Tang J‐L. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006936.pub2; CD006936] [DOI] [PubMed] [Google Scholar]
Lindson‐Hawley 2015
- Lindson‐Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD006936.pub3] [DOI] [PubMed] [Google Scholar]


