Abstract
Background
Larviciding refers to the regular application of chemical or microbial insecticides to water bodies or water containers to kill the aquatic immature forms of the mosquito (the larvae and pupae).
Objectives
To summarize research evidence evaluating whether larviciding with chemical or microbial insecticides prevents malaria transmission.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE; Embase; CAB Abstracts; LILACS; the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP); ClinicalTrials.gov; and the ISRCTN registry up to 6 June 2019.
Selection criteria
We included cluster‐randomized controlled trials (cRCTs), interrupted time series (ITS), randomized cross‐over studies, non‐randomized cross‐over studies, and controlled before‐and‐after studies (CBAs) that compared larviciding with no larviciding.
Data collection and analysis
We independently assessed trials for eligibility and risk of bias, and extracted data. We assessed the certainty of evidence using the GRADE approach.
Main results
Four studies (one cRCT, two CBAs, and one non‐randomized cross‐over design) met the inclusion criteria. All used ground application of larvicides (people hand‐delivering larvicides); one evaluated chemical and three evaluated microbial agents. Studies were carried out in The Gambia, Tanzania, Kenya, and Sri Lanka. Three studies were conducted in areas where mosquito aquatic habitats were less extensive (< 1 km²), and one where habitats were more extensive (> 1 km²; a cross‐over study from The Gambia).
For aquatic habitats of less than 1 km², one cRCT randomized eight villages in Sri Lanka to evaluate chemical larviciding using insect growth regulator; and two CBA studies undertaken in Kenya and Tanzania evaluated microbial larvicides. In the cRCT, larviciding across all villages was associated with lower malaria incidence (rate ratio 0.24, 4649 participants, low‐certainty evidence) and parasite prevalence (risk ratio (RR) 0.26, 5897 participants, low‐certainty evidence) compared to no larviciding. The two CBA studies reported lower malaria prevalence during the intervention period (parasite prevalence RR 0.79, 95% confidence interval (CI) 0.71 to 0.89; 70,902 participants; low‐certainty evidence). The Kenyan study also reported a reduction in the incidence of new malaria cases (RR 0.62, 95% CI 0.38 to 1.01; 720 participants; very low‐certainty evidence).
For aquatic habitats of more than 1 km², the non‐randomized cross‐over trial using microbial larvicides did not detect an effect for malaria incidence (RR 1.58, 95% CI 0.94 to 2.65; 4226 participants), or parasite prevalence (RR 1.15, 95% CI 0.41 to 3.20; 3547 participants); both were very low‐certainty evidence. The Gambia trial also reported the mean haemoglobin level, and there was no difference across the four comparisons (mean difference –0.13, 95% CI –0.40 to 0.13; 3586 participants).
We were unable to summarize or pool entomological outcomes due to unreported and missing data.
Authors' conclusions
Most controlled studies on larviciding have been performed with microbial agents. Ground larviciding for non‐extensive larval habitats may have an effect on malaria transmission, and we do not know if there is an effect in large‐scale aquatic habitats. We found no studies using larviciding application techniques that could cover large aquatic habitats, such as aerial spraying using aircraft.
16 September 2019
Up to date
All studies incorporated from most recent search
All published trials found in the last search (6 Jun, 2019) were included, and we did not identify any ongoing trials.
Plain language summary
Larviciding to control malaria
What was the aim of this review?
Larviciding is the regular application of microbial or chemical insecticides to water bodies or water containers. The aim of larviciding is to reduce the adult population of mosquitoes by killing the aquatic immature forms, so that fewer will develop into adults. This should reduce the number of mosquitoes that bite and infect humans with malaria.
Key messages
All four studies included in this review distributed larvicides manually. Hand larviciding of small mosquito habitats may be effective in preventing malaria. Only one study was conducted in an area where larval habitats spanned a large area and this study found no effect of larviciding.
What was studied in the review?
We searched for trials that evaluated the impact of larviciding, using a microbial agent or chemical insecticide on malaria transmission. We considered effects on both human health outcomes and on mosquito populations.
What were the main results of the review?
Evidence from three studies shows that larviciding may decrease at least one malaria disease outcome in some studies, and this was in areas where the mosquito aquatic habitats were less than 1 km2 (low‐certainty evidence). We do not know if larviciding in large water bodies shows an impact on malaria based on results from one study in The Gambia (very low‐certainty evidence).
How up to date is the review?
We searched for relevant trials up to 6 June 2019.
Summary of findings
Background
Description of the condition
Malaria is caused by the Plasmodium parasite, which is transmitted by female Anopheles mosquitoes. There are five Plasmodium species that cause disease in humans; however, the most important species in terms of disease burden are Plasmodium falciparum, which is prevalent in sub‐Saharan Africa, and Plasmodium vivax, which is more common in Asia and South America. There were an estimated 219 million malaria cases and 435,000 deaths worldwide due to malaria in 2017 (WHO 2018). Sub‐Saharan Africa carries a disproportionately high share of the malaria burden, with 92% of cases and 93% of malaria deaths in 2017 (WHO 2018). As well as direct effects on health, malaria is a major cause of poverty and underdevelopment in many countries, due to household and health system costs, absenteeism from school or work, reduced productivity, and premature death (Chima 2008). Malaria‐endemic countries are, on average, poorer by more than five‐fold and have lower rates of economic growth than non‐malaria endemic countries, with a mean growth of per‐capita gross domestic product (GDP) of 0.4% per year versus 2.3% between 1965 and 1990 (Sachs 2002).
Vector control tools, such as long‐lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) of insecticides, play a major role in malaria control, alongside diagnosis and effective treatment of malaria cases, and chemoprevention in some population groups. Scale‐up of vector control, diagnosis, and treatment averted 663 million clinical cases of malaria between 2000 and 2015 (Bhatt 2015). However, progress against malaria is stalling and a high burden of morbidity and mortality still remains (WHO 2017; WHO 2018). The World Health Organization (WHO) set out ambitious targets in the Global Technical Strategy to eliminate malaria in at least 35 countries by 2030 (WHO 2015a).
Description of the intervention
Larviciding refers to the regular application of microbial or chemical insecticides to water bodies or water containers to kill the aquatic immature forms of the mosquito (the larvae and pupae) (Tusting 2013).
Malaria vectors lay their eggs in standing water and the eggs develop through a series of life stages (larvae and pupae) into adults. The type of standing water selected by ovipositing females depends on the species in question and can be natural or man‐made, temporary or permanent (Bruce‐Chwatt 1985). For example, Anopheles stephensi prefers containers such as water tanks, some species prefer brackish habitats (Anopheles aquasalis in Latin America), while others prefer riceland habitats (Anopheles arabiensis).
There are several different types of larvicide, including chemical larvicides (using conventional insecticides, such as temephos, or insect growth regulators, such as pyriproxyfen, methoprene, and diflubenzuron), microbial larvicides (such as Bacillus thuringiensis israeliensis (Bti) and Bacillus sphaericus (Bs)) and oils. Larvivorous fish have also been used as a form of malaria control. Larvicides have varying modes of action. For example surface films, such as mineral oils and alcohol‐based surface products, suffocate the mosquito larvae and pupae by covering the surface of a water body. This is different from synthetic organic chemicals, such as organophosphates, which inhibit cholinesterase and affect the central nervous system of the mosquito. Insect growth regulators interfere with insect metamorphosis and prevent adult emergence from the pupal stage. Microbial larvicides function by bacterial proteins binding to the larval gut, which cause the larvae to stop eating and die (WHO 2013).
How the intervention might work
Larviciding aims to reduce malaria transmission by targeting the immature stages (larvae and pupae) of the anopheline mosquito, to reduce the number of mosquitoes that reach adulthood. By reducing adult vector populations in this way, larviciding is expected to reduce the transmission of Plasmodium species by anopheline mosquitoes, and reduce morbidity and mortality from malaria (Figure 1).
1.
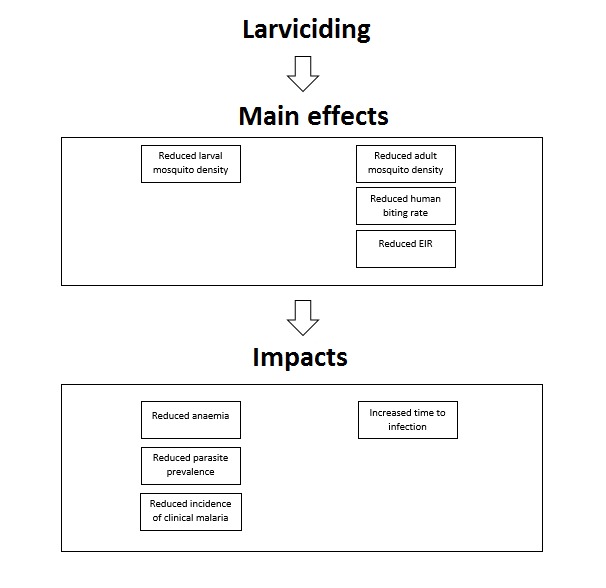
Logic model of the proposed effect of larviciding on various entomological and epidemiological outcomes. EIR: entomological inoculation rate.
Many of the principles behind vector control come from the theory of vectorial capacity developed by George Macdonald in the 1950s (Macdonald 1957). Vectorial capacity describes the total number of potentially infectious bites that would eventually arise from all the mosquitoes biting a single perfectly infectious (i.e. all mosquito bites result in infection) human on a single day. Vectorial capacity can be linked to the basic reproduction ratio of a disease which is the estimated number of secondary infections potentially transmitted by a single infected individual in a totally susceptible population (Black 1968). The basic reproduction number represents the theoretical estimate of the intensity of transmission. The George‐Macdonald model shows that vectorial capacity is most sensitive to changes in adult mosquito survival, which led to the prioritization of IRS and LLINs as vector control tools in the 1950s. However, the vectorial capacity model does not adequately consider the aquatic stages of the vector and so the potential of larviciding is likely to have been underestimated (Brady 2016). Models show that larval source management (LSM) reduces mosquito population density linearly with coverage if adult mosquitoes avoid laying eggs in treated habitats, but quadratically if eggs are laid in treated habitats and the effort is therefore wasted (Smith 2013). This would mean that if the most productive habitats are targeted, larviciding could be highly effective even without extensive coverage. Larviciding may also operate against both indoor and outdoor (e.g. An arabiensis) biting and resting mosquitoes, unlike LLINs and IRS. This is beneficial, since in some settings anthropophillic vectors are able to sustain transmission even with high coverage of LLINs or IRS, or both (Bayoh 2010; Russell 2010; Lwetoijera 2014), and several studies have also shown evidence of behavioural adaptation of vectors towards early evening biting which may reduce the effectiveness of indoor interventions (Gatton 2013). Thus larviciding may be effective against ‘residual malaria transmission', which is generally defined as transmission that exists despite universal coverage of LLINs or IRS to which vector populations are fully susceptible (Durnez 2013; Killeen 2014).
Why it is important to do this review
There is a need for new tools in malaria vector control if the goals set by the WHO Global Technical Strategy are to be achieved (WHO 2018). Malaria vector control currently relies largely on LLINs and IRS. Although the WHO recommends the use of LSM (including larviciding) as a supplementary control measure (WHO 2013), larviciding is not widely used by malaria control programmes. This is despite historical and contemporary successes with the use of larviciding for vector control. Programmatic application of Paris Green, an arsenic‐based compound toxic to larvae, contributed to the elimination of species belonging to the Anopheles gambiae complex in Egypt and Brazil (Soper 1943; Shousha 1948). Larviciding is routinely practiced by mosquito control programmes in the USA and Europe (Becker 1997; Floore 2006). Larviciding has also been hugely successful against other vector‐borne diseases; for example, Bti and temephos were used to control species of the Simulium damnosum complex – vectors of onchocerciasis – in Brazil and the continent of Africa as a supplement to mass drug administration (MDA) (Sékétéli 2002; Gustavsen 2011).
Larviciding has the potential to overcome several challenges currently facing malaria vector control. First, larviciding is able to target outdoor resting and biting mosquitoes that are less affected by LLINs and IRS. Second, it could be used to tackle residual foci of malaria where high coverage of LLINs and IRS is not sufficient to eliminate malaria. Last, larviciding could be used together with other interventions as part of an insecticide resistance management strategy. Insecticide resistance has been reported in all major malaria vectors and involves all classes of insecticide (but particularly pyrethroids) and may threaten the effectiveness of insecticide‐based vector control (WHO 2012a). The distribution and intensity of insecticide resistance has been increasing over time. Of 80 malaria‐endemic countries reporting insecticide resistance monitoring data since 2010, 68 reported resistance to at least one insecticide class and 57 reported resistance to two or more insecticide classes (WHO 2018). The WHO Global Plan for Insecticide Resistance Management recommends the use of insecticide‐based and non‐insecticide‐based interventions targeting both immature and adult mosquitoes as an insecticide resistance management strategy (WHO 2012a). This is also aligned with Integrated Vector Management (IVM), an adaptive, evidence‐based, and multi‐sectorial approach to vector control, which is recommended by the WHO for more effective, sustainable, and ecologically sound vector control (WHO 2008).
A Cochrane Review of LSM for controlling malaria was published in 2013 (Tusting 2013). This contributed to WHO deliberations that led to the recommendation of LSM as a supplementary malaria vector control intervention, and a WHO operational manual on LSM (WHO 2012b; WHO 2013). Although all LSM interventions have the aim of reducing mosquito larvae, the ways they are carried out are very different and effectiveness is likely to differ. For example, habitat modification (a permanent alteration to the environment such as drainage of aquatic habitats) is different to regular application of chemical or microbial larvicides to a water body. Due to the diversity of forms of LSM, a new assessment of larviciding alone is justified, thus splitting the original Cochrane Review on LSM (Tusting 2013).
Objectives
To summarize and appraise experimental and quasi‐experimental studies evaluating the effect of larviciding with chemical or microbial insecticides on malaria transmission.
Methods
Criteria for considering studies for this review
Types of studies
Cluster‐randomized controlled trials (cRCTs) with: the unit of randomization being a cluster, and at least two clusters per arm. As larvicides are distributed at a community level, we did not expect to find trials with individual randomization.
Randomized and non‐randomized cross‐over trials with: the unit of randomization being a cluster, at least two clusters per arm, and a suitable washout period during which malaria or entomological indices have returned to baseline levels. As larvicides are distributed at a community level, we did not expect to find trials with individual randomization.
Controlled before‐and‐after studies (CBAs) with: a contemporaneous control group, and at least two sites per arm.
Interrupted time series (ITS) studies with: a clearly defined point in time when the intervention occurred, and at least three data points before and three during or after cessation of larviciding.
We excluded studies if:
the intervention was applied for less than one year in sites with perennial (year‐round) transmission (as reported by the study authors); or less than one transmission season (defined as the period from the onset of rains until one month afterwards) in sites with seasonal transmission (as reported by the study authors);
the follow‐up periods for the intervention and control periods were not identical.
Types of participants
All people living in a rural or urban malarious area that is at any level of endemicity, including both stable and unstable transmission.
We planned to include and analyse studies specific to special groups, such as refugees and soldiers, separately from other studies but none were identified.
Types of interventions
Intervention
Larviciding using chemicals (insecticides and insect growth regulators), microbial agents, or oils. We excluded plant products, because formulations have not been standardized and studies are thus not comparable. We also excluded biological larviciding using larvivorous fish, covered in a separate Cochrane Review (Walshe 2017).
Control
Not receiving larviciding interventions as described above. Any co‐interventions such as LLINs, IRS, topical repellents, spatial repellents, environmental manipulation, environmental modification, MDA, and case management must have been received in both control and intervention arms.
Types of outcome measures
Studies must have reported at least one primary outcome for inclusion.
Primary outcomes
Clinical malaria incidence: we used site‐specific definitions, provided they include: demonstration of malaria parasites by blood smear or a rapid diagnostic test (RDT), or both; and clinical symptoms including fever or history of fever, detected passively or actively.
Malaria parasitaemia incidence: measured as a count per person unit time of infections or new infections, both defined as parasitaemia confirmed by blood smear microscopy or RDT. New infections were defined as either infection in participants who were negative for parasites at an earlier survey or infection in participants who were cleared of parasites using drug treatment at an earlier survey.
Malaria parasite prevalence: proportion of surveyed people with confirmed parasitaemia.
Secondary outcomes
Entomological
Adult mosquito density measured by a technique previously shown to be appropriate for the vector (measured using human baits, light traps, knock‐down catches, baited huts, or other methods). Adult mosquito density would most likely have been reported as bites/person/night for human landing catches and mosquitoes/traps/night for trap catches or pyrethrum spray catches.
Sporozoite rate measured as the number of caught adult mosquitoes positive for malaria sporozoites. Sporozoites can be detected through molecular or immunological methods.
Entomological inoculation rate (EIR): the estimated number of bites by infectious mosquitoes per person per unit time. This was measured using the human biting rate (the number of mosquitoes biting a person over a stated time period measured directly using human baits or indirectly using light traps, knock‐down catches, baited huts, or other methods of biting rate determination) multiplied by the sporozoite rate.
Epidemiological
-
Incidence of severe malaria: we used site‐specific definitions, provided they include (a) and either (b) or (c):
(a) demonstration of parasitaemia by blood smear;
(b) symptoms of cerebral malaria including coma or prostration or multiple seizures, or both;
(c) severe life‐threatening anaemia (WHO 2015b).
Malaria‐related deaths.
Mean haemoglobin levels (g/dL).
Anaemia prevalence defined using WHO cut‐offs (WHO 2011).
Hospital admissions for malaria.
Adverse events
Any indicators of adverse events of the intervention, including the following.
Non‐target effects such as the larvicide killing other animals in the water body.
Reports of poisoning in humans due to exposure to larviciding chemicals.
Environmental impacts such as changes to the biodiversity and ecosystem due to the use of larvicides.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases up to 6 June 2019, using the search terms and strategy described in Appendix 1:
Cochrane Infectious Diseases Group Specialized Register;
Cochrane Central Register of Controlled Trials (CENTRAL) published in the Cochrane Library (Issue 6, 2019)
MEDLINE (Pubmed, from 1966);
Embase (OVID, from 1974);
CAB Abstracts, from 1973 (Web of Science);
Latin American Caribbean Health Sciences Literature (LILACS) (BIREME, from 1982).
We also searched ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/trialsearch), and the International Standard Randomized Controlled Trials Number (ISRCTN) registry (www.isrctn.com/) for trials in progress, using "malaria", "mosquito", and "larvicid*" as search terms.
Searching other resources
Tusting 2013 handsearched the US Armed Forces Pest Management Board Defense Pest Management Literature Retrieval System and the Tropical Diseases Bulletin using the terms: malaria or mosquito and larvicides up to the end of 2010 and incorporated the results into the Cochrane Review ‘Mosquito larval source management for controlling malaria'. We had planned to update this search but decided it was unlikely any new studies that would fit the review's inclusion criteria would be found.
We contacted researchers in the field to identify unpublished data, and checked the reference lists of studies identified using electronic searches.
Data collection and analysis
Selection of studies
Two review authors (LC and AW) independently assessed the titles and abstracts of trials identified by the literature searches. We obtained the full‐text articles of any potentially relevant articles. The same two review authors assessed the full‐text articles of potentially relevant studies for inclusion using an eligibility form based on predetermined inclusion criteria. We resolved any disagreements by discussion and consensus, with arbitration by a third review author (SM), when necessary. We ensured that multiple publications of the same trial were included only once. We listed studies excluded after full‐text assessment, together with their reasons for exclusion, in the Characteristics of excluded studies table. We illustrated the study selection process in a PRISMA flow chart (Moher 2009).
Data extraction and management
Two review authors (LC and AW) independently extracted information from the trials using pre‐piloted electronic data extraction forms. SM was a primary investigator and author of one included study. He was not involved in the screening, data extraction or risk of bias assessment, and analysis for this particular study. When differences in extracted data arose, the two review authors discussed these differences to reach consensus and involved a third review author (SM), where necessary. For missing data, we contacted the original study author(s) for clarification.
We extracted the following data.
Trial design: type of trial; method of participant selection; adjustment for clustering (for cRCTs); sample size; method of blinding of participants and personnel.
Participants: trial settings and population characteristics; recruitment rates; withdrawal and loss to follow‐up.
Intervention: description of intervention (active ingredient, dose, formulation, method, frequency and timing of application, buffer zone between clusters); quality control of the larvicide (e.g. WHO Pesticide Evaluation Scheme (WHOPES) approved); quality assurance of implementation of larviciding; co‐interventions; description of control; duration of follow‐up; passive or active case detection; coverage of larvicide (as reported by the study authors) and co‐interventions (e.g. vector control, vaccines, chemoprophylaxis, diagnosis, and treatment); duration of the activity of the larvicide; compliance (with application of larvicide and co‐interventions).
Outcomes: definition of outcome; diagnostic method or surveillance method; number of events; number of participants or unit time; time point at which outcome was assessed in relation to larviciding implementation, statistical power; unit of analysis; incomplete outcomes or missing data.
-
Other:
primary and secondary vector(s) species; vector(s) behaviour (nature, stability, and extent (number and size) of aquatic habitats, proximity of aquatic habitats to human habitation, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic); method of mosquito collection(s); phenotypic insecticide resistance (based on WHO definitions if WHO cylinder assays, Centers for Disease Control and Prevention (CDC) bottle bioassays, intensity assays or synergist assays were performed while the trial was running); genotypic insecticide resistance profile (either performed during the trial or if the trial references data from previous studies done on the same local vector population within the previous five years); insecticide and larvicide resistance detected in the larvae (as reported by study authors);
malaria endemicity; eco‐epidemiological setting; population proximity and density; Plasmodium species.
For dichotomous outcomes, we extracted the number of participants experiencing each outcome and the number of participants in each treatment group. For count data outcomes, we extracted the number of outcomes in the treatment and control groups, the total person time at risk in each group or the rate ratio, and a measure of variance (e.g. standard error). For continuous outcomes, we extracted the mean and a measure of variance (e.g. standard deviation).
For cRCTs we recorded the number of clusters randomized; number of clusters analyzed; measure of effect (such as risk ratio (RR), odds ratio (OR), rate ratio, or mean difference (MD)) with confidence intervals (CI) or standard deviations; number of participants; and the intracluster correlation coefficient (ICC) value.
For non‐randomized studies (NRS), we extracted adjusted measures of intervention effects that attempt to control for confounding.
Assessment of risk of bias in included studies
Two review authors (LC and AW) independently assessed the risk of bias for each cRCT using the Cochrane ‘Risk of bias' tool and the five additional criteria listed in Section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions that relate specifically to cRCTs (Higgins 2011a; Higgins 2011b). For assessing the risk of bias for randomized cross‐over trials, we used the Cochrane ‘Risk of bias' tool also and the additional criteria listed in Section 16.4.3 of the Cochrane Handbook for Systematic Reviews of Interventions that relate specifically to randomized and non‐randomized cross‐over trials (Higgins 2011a). We planned to assess non‐randomized controlled studies and ITS for risk of bias using the Cochrane Effective Practice and Organisation of Care (EPOC) ‘Risk of bias' tool. We resolved any discrepancies through discussion or by consulting a third review author (SM). We judged studies at low, high, or unclear risk of bias, and used summary graphs (‘Risk of bias' summary and ‘Risk of bias' graph) to display results.
Measures of treatment effect
We compared intervention and control data using RRs if the outcome was dichotomous. Where effect sizes from studies were presented as an OR, we converted these to RRs following the methodology stated in Section 12.5.4.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented rate data as rate ratios. We calculated the MD for continuous measures. We used adjusted measures of effect to summarize treatment effect from NRS. We presented all results with their associated 95% CIs.
We aimed to report any accounts of possible adverse effects. We appreciated that the specified inclusion criteria were not designed to detect effects on animals in the water, people exposed to the larvicides, and the ecosystem overall, and we intended to note this in the discussion, but there were no adverse events.
Unit of analysis issues
For cRCTs, we planned to extract adjusted measures of effect where possible. If the study authors did not perform any adjustment for clustering, we planned to adjust the raw data using an ICC value. If the study did not report an ICC value, we contacted the study authors, obtained this from similar studies, or estimated the ICC. When we estimated the ICC, we performed sensitivity analyses to investigate the robustness of our analyses (Richardson 2016).
For cross‐over trials, we applied the principles stated in Sections 16.4.4 and 16.4.5 of the Cochrane Handbook for Systematic Reviews of Interventions that relate specifically to randomized and non‐randomized cross‐over trials (Higgins 2011a).
Dealing with missing data
In case of missing data, we intended to apply available‐case analysis and to only include data on the known results. The denominator would have been the total number of participants who had data recorded for the specific outcome. For outcomes with no missing data, we planned to perform analyses on an intention‐to‐treat basis. We intended to include all participants randomized to each group in the analyses and analyse participants in the group to which they were randomized.
Assessment of heterogeneity
We inspected forest plots for overlapping CIs and assessed statistical heterogeneity in each meta‐analysis using the I² statistic and Chi² test. We regarded heterogeneity as moderate if I² statistic values were between 30% and 60%; substantial if they were between 50% and 90%; and considerable if they were between 75% and 100%. We regarded a Chi² test statistic with a P value ≤ 0.10 as indicative of statistically significant heterogeneity. We explored clinical and methodological heterogeneity through consideration of the trial populations, methods, and interventions, and by visualization of trial results.
Assessment of reporting biases
If there were 10 or more trials included in each meta‐analysis, we intended to investigate reporting biases (such as publication bias) using funnel plots. We planned to assess funnel plot asymmetry both visually and using formal tests (Harbord 2006), and explore possible reasons for asymmetry.
Data synthesis
We analyzed data using Review Manager 5 (Review Manager 2014). We used a fixed‐effect meta‐analysis to combine data if heterogeneity was absent. If there was considerable heterogeneity, we combined data using a random‐effects meta‐analysis and reported a mean treatment effect (RRs and ORs for dichotomous outcomes and rate ratio for count data). We decided whether to use a fixed‐ or random‐effects model based on the consideration of clinical and methodological heterogeneity between trials.
We combined data across follow‐up time points for each included study.
Certainty of the evidence
We assessed the certainty of evidence using the GRADE approach (Guyatt 2011). We rated each primary epidemiological outcome (malaria incidence and prevalence) as described by Balshem 2011.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect.
Low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
RCTs started as high‐certainty evidence but we downgraded the certainty of the evidence if there were valid reasons within the following five categories: risk of bias, imprecision, inconsistency, indirectness, and publication bias. We upgraded the certainty of the evidence for studies where there was a large effect, a dose–response effect, and if all plausible residual confounding would reduce a demonstrated effect or would suggest a spurious effect if the was no effect (Balshem 2011). We presented the GRADE assessments in a ‘Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity
We initially analyzed all types of larvicide (e.g. surface films, synthetic organic chemicals, insect growth regulators, and microbial larvicides) together. If there was a sufficient number of studies then we grouped these and analyzed them separately.
We explored reasons for substantial heterogeneity using subgroup analysis. We intended to perform the following subgroup analyses.
-
Seasonality of malaria:
perennial, defined as year‐round transmission;
seasonal as reported by study authors in the manuscript or defined as 75% or more of all malaria episodes occurring in six or fewer months of the year (Roca‐Feltrer 2009);
epidemic, defined as a sharp rise in malaria incidence, higher than typical levels.
-
Extent of aquatic habitat:
container habitat;
habitats smaller than 1 km² (excluding containers);
habitats larger than 1 km².
-
Continent:
Africa;
non‐Africa.
We only performed a subgroup analysis based on the extent of aquatic habitat as there were insufficient studies to perform the other subgroup analyses.
Sensitivity analysis
We performed sensitivity analysis on the primary outcome to determine the effect of exclusion of trials at high risk of bias (for allocation concealment and incomplete outcome data) on the overall results. If the ICC value was estimated, we undertook sensitivity analyses to investigate the impact of varying the ICC value on meta‐analysis results.
Results
Description of studies
Results of the search
We identified 2510 reports using electronic searches. We removed one duplicate and screened all remaining 2509 abstracts against the review's inclusion criteria. Abstract screening resulted in 98 unique reports for full‐text screening (Figure 2).
2.
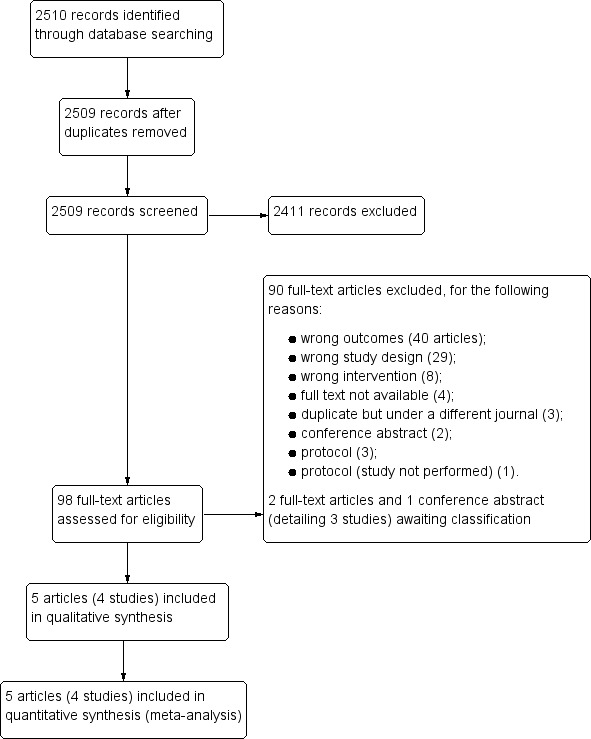
Study flow diagram.
Included studies
Design
Five reports detailing four separate studies met the inclusion criteria and these are described in the Characteristics of included studies table. One study was a cRCT (Yapabandara 2001), one was a non‐randomized cross‐over trial (Majambere 2010), and two were CBAs (Fillinger 2009; Maheu‐Giroux 2013a).
Transmission
Three studies were each conducted in sub‐Saharan Africa with one in Kenya where P falciparum was present and malaria transmission was moderate (Fillinger 2009), one in Gambia where P falciparum was present and malaria transmission was seasonal (Majambere 2010), and one in Tanzania where P falciparum accounted for more than 90% of cases and malaria transmission was perennial with peaks in incidence after the two rainy seasons a year (Maheu‐Giroux 2013a). The remaining study was conducted in Sri Lanka where both P falciparum and P vivax were present (Yapabandara 2001). Yapabandara 2001 did not report on the level of malaria transmission.
Habitat and vectors
The four studies targeted a range of aquatic habitats and vector species and all applied larvicides by hand. In the Elahera gem‐mining area situated in Matale District, Sri Lanka, Yapabandara 2001 targeted shallow pits dug by gem miners that harbour An culicifacies and An subpictus. In The Gambia, investigators larvicided large flooded areas of the floodplain of the lower reaches of the Gambia River which are ideal larval habitats for An gambiae s.s., An melas, and An arabiensis (Majambere 2010). Fillinger 2009 targeted aquatic habitats in the valley bottoms in the Kenyan highlands. These habitats harbour predominantly An gambiae s.s. and An funestus and are becoming more important as papyrus swamps are deforested to create agricultural land. Maheu‐Giroux 2013a evaluated the effect of the Urban Malaria Control Programme (UMCP) in Dar es Salaam, Tanzania. Here there were numerous aquatic habitats including natural habitats (swamps, river beds, springs), agricultural habitats (rice paddies, ridge and furrow agriculture) and artificial non‐agricultural habitats (drains, construction pits, etc.). Habitats harboured predominantly An gambiae s.l. but An funestus and An coustani were also present, along with large numbers of culicines.
Interventions
A summary of the interventions implemented is given in Table 4. There are two main microbial larvicides: Bs and Bti, and these exist in two formulations – water dispersible and corn cob granule. Two studies used both water‐dispersible and corn cob granule formulations of Bti (Majambere 2010; Maheu‐Giroux 2013a). Fillinger 2009 used a water dispersible formulation of Bs for six months but switched to corn cob Bti after six months due to a lack of residual effect. Maheu‐Giroux 2013a also used a corn cob Bs formulation to treat closed aquatic habitats that predominantly harboured culicines. All studies that used Bti or Bs used commercial formulations manufactured by Valent BioSciences LLC. Yapabandara 2001 used the insect growth regulator pyriproxyfen as a larvicide in Sri Lanka.
1. Characteristics of larviciding.
| Study | Active ingredient, formulation, dose, and manufacturer | Frequency of application | Targeted aquatic habitats | Who carried out the larviciding | Vector species |
| Fillinger 2009 | Commercial strains of Bs (water‐dispersible, Valent BioSciences LLC) | Weekly intervals for first 6 months of the study | All water bodies | Project staff | An gambiae s.l. and An funestus s.l. |
| Commercial strains of Bti (water‐dispersible, Valent BioSciences LLC) | Weekly intervals for remainder of the study | ||||
| Maheu‐Giroux 2013a | Commercial strains of Bs (0.04 g/m², water‐dispersible, Valent BioSciences LLC) | Weekly intervals | All open light‐exposed water bodies | Community‐owned resource person | An gambiae s.s., An funestus s.l., and An coustani |
| Commercial strains of Bti (0.1 g/m², corn cob Valent BioSciences LLC) | Once every 3 months | All closed, covered, often highly polluted water bodies | |||
| Majambere 2010 | Commercial strains of Bti (0.2 kg/hectare, water‐dispersible, Valent BioSciences LLC) | Weekly intervals | Areas of low vegetation across the Gambia river | Project staff using knapsack compression sprayers | An gambiae s.s., An melas, and An arabiensis |
| Commercial strains of Bti (0.5 kg/hectare, corn cob, Valent BioSciences LLC) | Areas of high vegetation across the Gambia river | Project staff by hand | |||
| Yapabandara 2001 | Pyriproxyfen, S31183 (Adeal 0.5% G) applied at a rate of 0.01 mg active ingredient/L (2 g of granules/m³) | 3 applications: December 1994, June–July 1995, end of November 1995 | Gem mining pits | Project staff | An culicifacies, An subpictus, and An aruna |
Abbreviations: An: Anopheles; Bs:Bacillus sphaericus;Bti:Bacillus thuringiensis israeliensis.
The frequency of application varied across the studies that used a microbial larvicide. Two studies applied the larvicide at weekly intervals (Fillinger 2009; Majambere 2010). In the Tanzanian study, open habitats were treated every week with Bti whereas closed habitats were treated every three months with Bs (Maheu‐Giroux 2013a). Yapabandara 2001 applied pyriproxyfen on three occasions during the study, one in December 1994, the second between June and July 1995 and the last application at the end of November 1995. Only one study reported on the duration of the activity of the larvicide (Fillinger 2009).
In Sri Lanka, local volunteers helped field staff to access villages and locate gem pits and assisted with administering the intervention (Yapabandara 2001). In The Gambia, field applicators were recruited from communities within each zone to make use of their local knowledge of the environment (Majambere 2010). They were supervised by one field supervisor in each zone and trained for one month before larviciding. In the Kenyan study, larviciding was implemented by project staff (Fillinger 2009). The UMCP in Dar es Salaam utilized community‐owned resource people, each assigned to a particular neighbourhood, to deliver the larvicides (Maheu‐Giroux 2013a).
In the Sri Lankan study, the control arm received no interventions (Yapabandara 2001). Both CBA studies had two arms: the control arm received standard practice vector control (insecticide‐treated nets (ITNs) in Fillinger 2009 and predominantly untreated bednets in Maheu‐Giroux 2013a), while the intervention arm received larviciding plus standard practice vector control. The cross‐over trial had two intervention arms; standard practice vector control (ITNs), and standard practice vector control (ITNs) plus larviciding (Majambere 2010). There were two units per arm, called zones. Zones 1 and 3 had the larviciding in the first year and served as control in the second year. Zones 2 and 4 received the interventions in the reverse order.
Fillinger 2009 reported an increase in ITN use from 4.8% (95% CI 3.0% to 6.6%; range in control valleys 1% to 9% and in intervention valleys 2% to 6%) at baseline to 40.8% (95% CI 36.7% to 45.0%; range in control valleys 24% to 51% and in intervention valleys 25% to 51%) during the intervention year. Majambere 2010 also reported an increase in net use during the study period, from between 6.1% to 38.3% in 2006 to between 37.2% to 81.4% in 2007.
Outcomes
Two studies measured clinical outcomes in children aged six months to 10 years only (Fillinger 2009; Majambere 2010). The other two studies measured outcomes in participants of all ages. Two studies measured the incidence of clinical malaria (Yapabandara 2001; Majambere 2010). Four studies measured the prevalence of Plasmodium infection (Yapabandara 2001; Fillinger 2009; Majambere 2010; Maheu‐Giroux 2013a). Fillinger and colleagues also reported the incidence of new Plasmodium infections (Fillinger 2009). They used children with no parasites at the first cross‐sectional survey of the season who had become infected two months later to calculate the incidence rate of new parasite infections over the two‐month follow‐up. One study measured mean haemoglobin concentration (Majambere 2010).
Three studies reported EIR (Fillinger 2009; Majambere 2010; Maheu‐Giroux 2013a). Three studies presented adult mosquito density as a biting rate (Yapabandara 2001; Fillinger 2009; Maheu‐Giroux 2013a). One study presented this outcome as totals caught in traps (Majambere 2010). One study measured sporozoite rate (Majambere 2010).
Excluded studies
We excluded 90 full‐text articles for the following reasons (see Characteristics of excluded studies table).
No relevant outcomes (40 articles).
Study design did not match inclusion criteria (29 articles).
Intervention did not match inclusion criteria (eight articles).
Full text not available (four articles).
Duplicate but under a different journal (three articles).
Conference abstract (two articles).
Protocol (three articles).
Protocol of a study not performed (one article).
We found three studies awaiting classification (see Characteristics of studies awaiting classification table). We contacted the authors of Fuseini 2017, Javadian 1974, and Zhou 2013 for additional data to determine whether the studies would meet the review's inclusion criteria but we did not receive the necessary information.
Risk of bias in included studies
Judgement of the risk of bias in the included studies is summarized in Figure 3. We listed individual risk of bias assessments in the Characteristics of included studies table.
3.
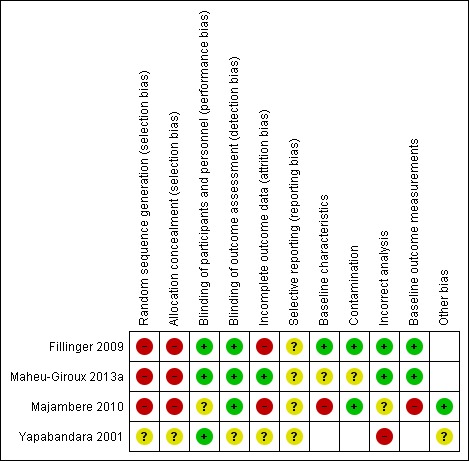
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies were not randomized trials, and, therefore. we judged them at high risk of selection bias (Fillinger 2009; Majambere 2010; Maheu‐Giroux 2013a). We judged the cRCT to have an unclear risk of selection bias as the trial authors did not explicitly state whether they conducted random sequence generation or allocation concealment (Yapabandara 2001).
Blinding
We judged studies to have a low (Yapabandara 2001; Fillinger 2009; Maheu‐Giroux 2013a), or unclear (Majambere 2010) risk of bias for performance bias and low (Fillinger 2009; Majambere 2010; Maheu‐Giroux 2013a) or unclear (Yapabandara 2001) detection bias.
Incomplete outcome data
Two studies were at high risk of attrition bias. Fillinger 2009 reported absences from cross‐sectional surveys and the magnitude of these absences differed by study arm. There was also over 10% loss to follow‐up in study groups in Majambere 2010. Maheu‐Giroux 2013a had low risk of attrition bias and Yapabandara 2001 was at unclear risk.
Selective reporting
All studies had unclear risk of reporting bias.
Other potential sources of bias
We considered the risk of additional types of bias in the studies (baseline characteristics, contamination, incorrect analysis, and baseline outcome). We judged Majambere 2010 at high risk of bias due to significant differences in baseline characteristics between the zones. For example, the prevalence of P falciparum infections was much higher in zone 1 (38.4%) compared to the others (range 9.5% to 16.8%).
Although not a form of bias, Yapabandara 2001 used an inappropriate analysis technique which did not adjust for the clustered nature of the data in their analysis. Unadjusted estimates from cRCTs contribute disproportionately to the pooled result in meta‐analysis since they receive too much weight.
Effects of interventions
Summary of findings for the main comparison. Summary of findings table 1.
| Larviciding versus no larviciding where mosquito aquatic habitats are < 1 km² | ||||||
|
Patient or population: people at risk of malaria Setting: areas where mosquito aquatic habitats are < 1 km² (one RCT carried out in Sri Lanka, and two CBA studies carried out in Kenya and Tanzania (Yapabandara 2001; Fillinger 2009; Maheu‐Giroux 2013a)). Intervention: larviciding Comparison: no larviciding | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Rate or risk with no larviciding | Rate or risk with larviciding | |||||
| Malaria incidence | 23 episodes per 100 person‐years | 5 episodes per 100 person‐years | Rate ratio 0.24 | 4649 person‐years (1 cluster‐RCT) |
⊕⊕⊝⊝
Lowa,b due to imprecision |
Larviciding may decrease malaria incidence |
| Parasite prevalence | 4 per 100 | 1 per 100 | RR 0.26 | 5868 (1 cluster‐RCT) |
⊕⊕⊝⊝
Lowc due to imprecision |
Larviciding may decrease parasite prevalence |
| 12 per 100 | 9 per 100 (9 to 11) | RR 0.79 (0.71 to 0.89) | 70,902 (2 controlled before‐and‐after studies) |
⊕⊕⊝⊝
Lowd due to non‐randomized design |
||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; ICC: intracluster correlation coefficient; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision: the rate ratio and CIs reported in the study were not adjusted for clustering. Sensitivity analysis with a mean cluster population of 675 showed the most conservative estimate of an ICC of 0.1 gave a rate ratio of 0.24 (95% CI 0.05 to 1.08) whereas the least conservative estimate of an ICC of 0.01 gave a rate ratio of 0.24 (95% CI 0.14 to 0.4). This created uncertainty around the point estimate. bAn additional study measured incidence of new infections. As this study was not a RCT, it was not combinable. However, the study showed a large effect consistent with the results of the RCT (RR 0.62, 95% CI 0.38 to 1.01) (Fillinger 2009). On GRADE assessment, the point estimate of 0.62 was very low‐certainty evidence. This was due to the study being a non‐randomized controlled trial, therefore baseline GRADE assessment started at ‘low'. Further downgraded one level for imprecision due to wide CIs. cDowngraded two levels for imprecision: the odds ratio and CIs reported in the study were not adjusted for clustering. Sensitivity analysis with a mean cluster population of 675 showed the most conservative estimate of an intracluster coefficient of 0.1 gave a RR of 0.26 (95% CI 0.03 to 2.42) whereas the least conservative estimate of an ICC of 0.01 gave an odds ratio of 0.08 (95% CI 0.02 to 0.46). The wide range in CIs generated very serious uncertainty around the point estimate. dNon‐RCTs, so baseline GRADE assessment started at ‘low', therefore no further downgrading required for risk of bias.
Summary of findings 2. Summary of findings table 2.
| Larviciding versus no larviciding where mosquito aquatic habitats are > 1 km² | ||||||
|
Patient or population: people at risk of malaria Setting: areas where the extent of mosquito aquatic habitats are > 1 km² (one non‐randomized cross‐over study in The Gambia (Majambere 2010)). Intervention: larviciding Comparison: no larviciding | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Rate or risk with no larviciding | Rate or risk with larviciding | |||||
| Malaria incidence | 23 episodes per 100 child‐years | 36 episodes per 100 child‐years (22 to 61) | RR 1.58 (0.94 to 2.65) | 1793 child‐years (1 non‐randomized cross‐over trial) | ⊕⊝⊝⊝
Very lowa,b due to inconsistency and imprecision |
We are uncertain of the effects on malaria incidence. |
| Parasite prevalence | 14 per 100 | 16 per 100 (6 to 45) | RR 1.15 (0.41 to 3.20) | 3574 (1 non‐randomized cross‐over trial) | ⊕⊝⊝⊝
Very lowa,b due to inconsistency and imprecision |
We are uncertain of the effects on parasite prevalence. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk of the comparison group is calculated from the total number of events/total number of participants in the control arms of the trials contributing to the meta‐analysis. Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for inconsistency: both comparisons indicated an effect favouring no larviciding, but there was considerable quantitative heterogeneity (I² = 77%). bDowngraded two levels for imprecision: very wide CIs.
Primary epidemiological outcomes
Clinical malaria incidence
Cluster‐randomized controlled trial
Yapabandara 2001 found a reduction of 76% in the incidence of clinical malaria when communities in four villages received larviciding compared to those in four villages that did not receive larviciding. The study authors did not adjust for clustering. Using an ICC value of 0.1 (a conservative estimate), gave wide CIs ranging from a 95% reduction to an 8% increase (Analysis 1.1). Using an ICC value of 0.01 resulted in a smaller range (rate ratio 0.24, 95% CI 0.14 to 0.40).
1.1. Analysis.
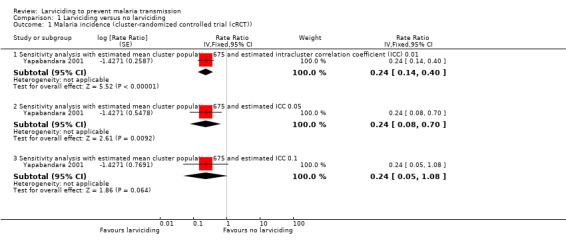
Comparison 1 Larviciding versus no larviciding, Outcome 1 Malaria incidence (cluster‐randomized controlled trial (cRCT)).
Non‐randomized studies
Two NRS investigated the impact of larviciding on malaria incidence. Majambere 2010 measured clinical malaria incidence and Fillinger 2009 measuring malaria parasitaemia incidence. They were subgrouped by the extent of aquatic habitats due to considerable heterogeneity (Analysis 1.2; I² statistic = 89%). Majambere 2010 reported two time points. The first time point in 2006 showed an increase in the risk of clinical malaria in the study group receiving larviciding by 101% compared to the control arm which did not receive larviciding (95% CI 51% to 168% increase). The second time point showed no effect of larviciding on the incidence of clinical malaria (RR 1.18, 95% CI 0.79 to 1.78).
1.2. Analysis.
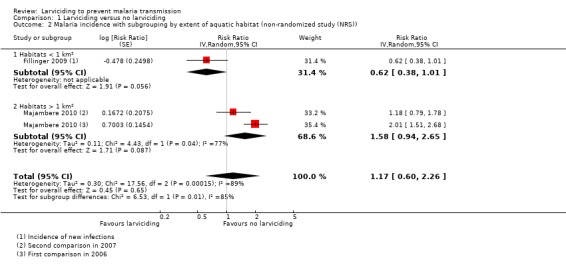
Comparison 1 Larviciding versus no larviciding, Outcome 2 Malaria incidence with subgrouping by extent of aquatic habitat (non‐randomized study (NRS)).
Malaria parasitaemia incidence
Non‐randomized studies
Fillinger 2009 found a reduction in the risk of new infections in the study group receiving larviciding of 38% compared to the control arm which did not receive larviciding (95% CI 62% decrease to 1% increase).
Parasite prevalence
Cluster‐randomized controlled trial
Yapabandara 2001 reported two separate time periods postintervention for parasite prevalence. Similar to the malaria incidence outcome measured in this study, sensitivity analysis to take account of clustering showed some uncertainty around the precision. The most conservative ICC gave a very imprecise result, a RR of 0.25 (95% CI 0.03 to 2.42), while the least conservative gave a RR of 0.08 (95% CI 0.02 to 0.46), somewhat more precise but CIs were still wide.
Non‐randomized studies
All three NRSs reported the effect of larviciding on parasite prevalence (Analysis 1.5). Fillinger 2009 and Maheu‐Giroux 2013a reported an adjusted OR. When converted to a RR and pooled, the effect size showed a reduction of 21% in parasite prevalence in areas receiving larviciding compared to areas not receiving larviciding (95% CI 11% to 29% reduction).
1.5. Analysis.
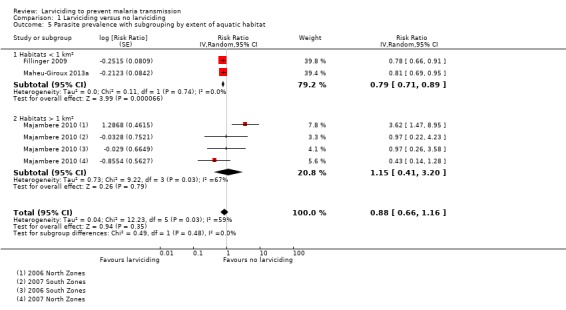
Comparison 1 Larviciding versus no larviciding, Outcome 5 Parasite prevalence with subgrouping by extent of aquatic habitat.
Majambere 2010 reported an unadjusted OR. We took the two northern zones as one comparison and the two southern zones as another. We also took each follow‐up year as separate comparisons. This led to four separate comparisons for Majambere 2010. The pooled analysis across the three NRS taking the least conservative estimate for the ICC in Majambere 2010 gave a RR of 0.88 with CIs crossing 1 (95% CI 0.66 to 1.16). However, when we took the most conservative estimate for the ICC for Majambere 2010, the pooled RR showed a reduction in parasite prevalence associated with larviciding (RR 0.80, 95% CI 0.71 to 0.89). This was because less weighting was given in the meta‐analysis for the Majambere 2010 comparisons as the ICC increased.
Due to concerns of a high risk of bias for baseline imbalance, we also conducted a sensitivity analysis excluding two comparisons from Majambere 2010 (Analysis 1.4). Excluding the northern zones of Majambere 2010 and taking an estimated ICC of 0.01, the pooled result suggested a lower 21% in parasite prevalence in the area that received larviciding compared to the area that did not receive larviciding (95% CI 11% to 29% lower).
1.4. Analysis.
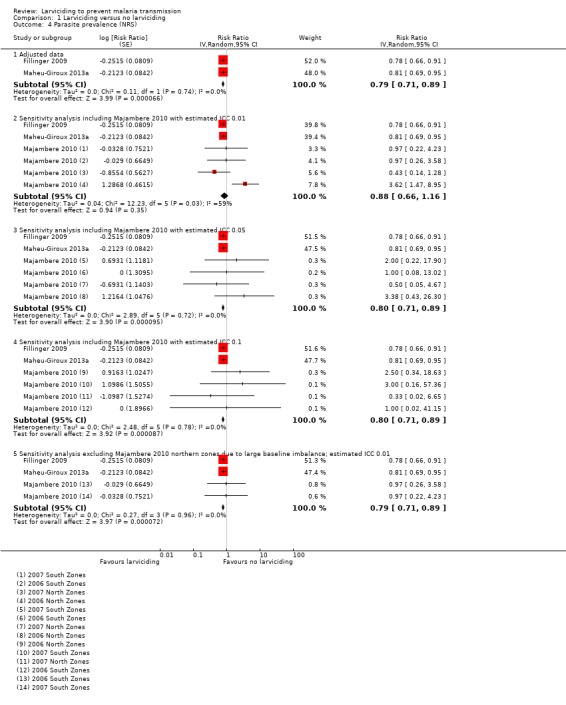
Comparison 1 Larviciding versus no larviciding, Outcome 4 Parasite prevalence (NRS).
Subgroup analyses
There was moderate heterogeneity in Analysis 1.4 when the comparisons from Majambere 2010 with an estimated ICC of 0.01 were pooled with the other two NRS (Fillinger 2009; Maheu‐Giroux 2013a) (I² = 59%; P = 0.003). This was explained when we subgrouped the studies by extent of the aquatic habitat in Analysis 1.5. The subgroup analysis showed there was a reduction in parasite prevalence when ground application of larvicides was conducted in areas where the extent of aquatic habitats were smaller than 1 km² (RR 0.79, 95% CI 0.71 to 0.89). The analysis did not show an effect of larviciding on parasite prevalence when larvicides were administered by ground application in areas where the extent of aquatic habitats exceeded 1 km² (RR 1.15, 95% CI 0.41 to 3.20). We did not conduct the other subgroup analyses planned due to the small number of studies identified.
Secondary outcomes
Entomological
Several studies reported EIR in the intervention and control arms (summarized in Table 5). Entomological outcomes could not be pooled due to issues with the way the figures were reported in the manuscripts. All studies reported a mean number with 95% CIs except for Majambere 2010. Both Fillinger 2009 and Maheu‐Giroux 2013a analyzed the data by using a model to adjust for confounders, whereas Majambere 2010 reported the raw data with no analysis. Fillinger 2009 and Maheu‐Giroux 2013a reported a statistically significant lower EIR in areas receiving larviciding compared to control areas. For the first year in Majambere 2010, there was no difference in EIR in the northern zones (each EIR 0) and an increase in EIR in the southern intervention zone (EIR 5.82) compared to the southern control zone (EIR 3.13). For the second year of the study, there was barely any difference in EIR between the northern intervention zone (EIR 2.32) and the northern control zone (EIR 2.24). The southern zones for 2007 showed a large difference between arms with an EIR of 17 in the southern control zone compared to 3.91 in the southern intervention zone.
2. Entomological inoculation rate from included studies.
| Study | Study arm | Mean numbers (95% CI) |
| Fillinger 2009a | No larviciding | 1.68 (1.16 to 2.43) |
| Larviciding | 0.39 (0.16 to 0.79) | |
| Maheu‐Giroux 2013aa | No larviciding | 1.28 |
| Larviciding | 0.683 (0.491 to 0.952) | |
| Majambere 2010 (2006 data) | No larviciding North Zone | 0 |
| Larviciding North Zone | 0 | |
| No larviciding South Zone | 3.13 | |
| Larviciding South Zone | 5.82 | |
| Majambere 2010 (2007 data) | No larviciding North Zone | 2.24 |
| Larviciding North Zone | 2.32 | |
| No larviciding South Zone | 17.00 | |
| Larviciding South Zone | 3.91 |
Abbreviations: CI: confidence interval. aThere is a statistically significant difference between the study arms (P < 0.05).
Epidemiological
The mean haemoglobin level reported by study arm by Majambere 2010 was converted into an MD. The pooled result from the four comparisons showed no statistically significant difference in the mean haemoglobin level of children living in areas that received larviciding compared to areas that received no larviciding (MD –0.13, 95% CI –0.40 to 0.13). We adjusted using an ICC of 0.01 for this analysis as the CIs were already very wide.
Discussion
Summary of main results
See Table 1 and Table 2. We included four studies: one cRCT in Sri Lanka, and three NRSs in sub‐Saharan Africa.
Primary outcomes
Malaria incidence
The cRCT reported a protective efficacy against malaria of 76% and adjusting using a conservative ICC of 0.1 gave the same effect estimate but wide CIs (95% CI 0.05 to 1.08) (Yapabandara 2001).
The pooled estimate of the two comparisons from the cross‐over trial showed a statistically non‐significant 58% increase (95% CI 6% decrease to 165% increase) in the incidence of malaria in children in the group that received larviciding compared to those who did not receive larviciding (Majambere 2010). However, the trial authors found that the year of study was a potential effect modifier which generated uncertainty around this pooled analysis.
Fillinger 2009 reported a protective efficacy of 38% against incidence of new parasite infections in the study group receiving larviciding compared to the control arm (95% CI 62% reduction to 1% increase).
Parasite prevalence
Yapabandara 2001 reported on the effect of larviciding on parasite prevalence. Utilizing the most conservative ICC value gave a statistically non‐significant protective efficacy of 74% (95% CI 97% reduction to 142% increase). Pooled estimates from two NRS showed a significant protective efficacy of larviciding against parasite prevalence of 21% (95% CI 11% to 29% reduction). The extent of aquatic habitat explained the moderate heterogeneity present when all NRS studies were pooled for this outcome.
Secondary outcomes
Majambere 2010 was the only study to measure mean haemoglobin level. The pooled analysis showed there was no statistically significant difference between the two study arms (MD –0.13, 95% CI –0.40 to 0.13).
For entomological outcomes, three studies reported on the impact of larviciding on EIR. Due to missing data and differences in how EIR was measured in each study, it was not possible to pool across studies. Two studies reported a statistically significant reduction in EIR when an area received larviciding compared to an area that did not receive larviciding (Fillinger 2009; Maheu‐Giroux 2013a). The entomological results from Majambere 2010 were mixed.
Certainty of the evidence
We appraised the certainty of evidence using the GRADE approach. The GRADE assessments are presented in Table 1 and Table 2.
Three studies (one cRCT, two NRS) evaluated the efficacy of ground application of larvicides where aquatic habitats were less than 1 km². One cRCT provided low‐certainty evidence that ground application of larvicides could have had a large impact on malaria incidence. The analysis of this study was not adjusted for clustering and so the CIs may have been misleadingly narrow, and thus we downgraded by two levels due to imprecision. The same trial also reported a large beneficial effect of ground larviciding on parasite prevalence but again this was not adjusted for clustering. We downgraded by two levels to low‐certainty evidence. We judged the certainty of the evidence for the pooled estimate for the prevalence of malaria from NRS to be low.
One study evaluated the efficacy of ground application of larvicides where the extent of aquatic habitats was more than 1 km². We judged the certainty of the evidence for the pooled OR for the incidence of malaria as very low, as we downgraded by one level due to unexplained heterogeneity and by two levels due to imprecision. The level of certainty for the pooled estimate for parasite prevalence was also very low, as we downgraded by two levels for imprecision and by one level for inconsistency.
Overall completeness and applicability of evidence
Larviciding is a context‐dependent intervention which requires knowledge of malaria transmission dynamics, vector ecology, behaviour, and the extent of water bodies and population density in proposed target areas. It is currently listed as a supplementary intervention for malaria control (WHO 2012b), compared to LLINs and IRS which are potentially more broadly applicable to different settings.
All studies included in this review looked at the efficacy of ground application of larvicides. Our review provided low‐certainty evidence of the efficacy of larviciding where habitats could be feasibly treated by hand, such as drainage channels, irrigation channels, ponds, and pools. The certainty of evidence was downgraded due to imprecision; however, point estimates of effect from the three studies consistently showed a high reduction in malaria incidence and prevalence.
There was very low‐certainty evidence on the efficacy against malaria of ground larvicide application where aquatic habitats were extensive, such as flood plains. The larval habitats treated in the Majambere study were huge as marshland areas stretched for several kilometres along the River Gambia and some larval habitats were up to 2 km wide (Bogh 2003; Majambere 2008). Not all aquatic habitats were treated in this study due to deep water which prevented access by ground staff, especially during high tides. Another reason for the lack of effect in this study could have been that mosquitoes can fly long distances in this part of The Gambia (Bøgh 2007), potentially leading to spillover of mosquitoes from non‐intervention areas into study zones treated with larvicide. Because there was only one study with very low‐certainty evidence conducted in such a setting using ground application of larvicides, it was difficult to draw any conclusions.
LSM, primarily larviciding, is the basis of contemporary mosquito control in large parts of the USA (Mosquito Abatement Districts) and Europe (e.g. Rhine valley, Germany) (Becker 1997; Floore 2006). Programmes in the USA and Europe routinely use aerial application technologies such as planes and helicopters which enable higher coverage of extensive larval habitats compared to ground larviciding, and also target cryptic aquatic habitats. There is also a vast body of historical literature on the programmatic use of LSM including larviciding. For example, LSM was the primary intervention responsible for the eradication of An gambiae from Brazil (Soper 1943) and in Wadi Haifa, Egypt (Najera 2001). Unfortunately, this evidence could not be included in this review due to the study designs employed and extensive literature searches only identified four studies. Many large‐scale field trials were excluded from this review as they only measured entomological outcomes, while epidemiological outcomes are typically required to demonstrate the public health benefit of an intervention (Wilson 2015). Larviciding, as with other wide area vector control interventions like environmental management, does not lend itself to cRCTs since the cost of studies with sufficient numbers of large clusters would be prohibitive.
Potential biases in the review process
We identified no potential biases in the review process. SM is a trial author of one of the included studies but was not involved in the screening or data extraction of this study.
Agreements and disagreements with other studies or reviews
There is an existing Cochrane Review that included all LSM interventions (Tusting 2013). Aside from that review, we are not aware of any other systematic reviews on larviciding for malaria control.
Authors' conclusions
Implications for practice.
The WHO currently recommends larviciding and other larval source management (LSM) interventions as a supplementary malaria control intervention. Unlike insecticide‐treated nets (ITNs) and indoor residual spraying (IRS) which target indoor vectors, LSM could potentially target outdoor as well as indoor transmission. As a result, many programmes in the elimination phase are now considering LSM including larviciding to tackle the remaining foci of malaria transmission. This review supports the use of ground larviciding for non‐extensive larval habitats. We do not know if larviciding by hand in extensive habitats, largely inaccessible on foot or where water is tidal has any effect on malaria based on the results of one study of very low‐certainty evidence. Operational research could strengthen the evidence base in these particular settings, with an aim of identifying effective methods for distributing larvicides over large areas
Most countries do not have the capacity or capability to conduct larviciding. If malaria control programmes are to implement larviciding, then support will be required to assess feasibility, and implement, and monitor and evaluate the intervention.
Implications for research.
The findings of this review indicate low‐certainty evidence of benefit from controlled studies; however, the reality is that few, if any, studies will be conducted in the coming years. Further evidence on the effects of larviciding should be generated through monitoring and evaluation of programmatic implementation using concurrent control areas, perhaps in pragmatic stepped wedge designs.
Although not evaluated or discussed in this review, evaluation of new technologies for identifying aquatic habitats (such as high‐resolution imaging) and aerial application of larvicides in malaria‐endemic areas may well be relevant to further refine larviciding strategies.
What's new
| Date | Event | Description |
|---|---|---|
| 6 September 2019 | Amended | Corrected link to Appendix 1 in review text |
Acknowledgements
The Academic Editor is Dr Jimee Hwang.
We are grateful to Vittoria Lutje, Information Specialist with the Cochrane Infectious Diseases Group (CIDG), for help with the literature search strategy.
Leslie Choi is supported by the Research, Evidence and Development Initiative (READ‐It) project. Anne Wilson was supported for this work by a subcontract from READ‐It. The CIDG editorial base and READ‐It are funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the official policies of the UK government.
Leslie Choi is supported by PIIVeC, the Partnership for Increasing the Impact of Vector Control. PIIVeC is funded by the Medical Research Council of the UK (grant number MR/P027873/1) through the Global Challenges Research Fund.
This work was partly supported through a grant from the Global Malaria Programme, WHO (WHO Global Malaria Programme Agreement for Performance of Work (APW) Grant 2017 (number 709319)). The views expressed in this review have not been influenced by, or necessarily reflect, WHO policy.
Appendices
Appendix 1. Detailed search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINE | Embase | LILACS | CABS Abstracts |
| 1 | Mosquito* | Malaria [ti, ab, Mesh] | Malaria [ti, ab, Mesh] | Malaria [ti, ab, Emtree] | Mosquito$ | Mosquito* |
| 2 | Anopheles | Anopheles [Mesh] | Anopheles [ti, ab, Mesh] | Anopheles ti, ab, Emtree | Anopheles | Anopheles |
| 3 | malaria | Mosquito* ti, ab | Mosquito* ti, ab | Mosquito* ti, ab | malaria | malaria |
| 4 | 1 or 2 or 3 | Mosquito control [Mesh] | Mosquito control [Mesh] | Mosquito control ti, ab | 1 or 2 or 3 | 1 or 2 or 3 |
| 5 | Larvicid* | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | Larvicid$ or larval or larva or larvae ti, ab | Larvicid* or larval or larva or larvae ti, ab |
| 6 | 4 and 5 | Larvicid* or larval or larva or larvae ti, ab | Larvicid* or larval or larva or larvae ti, ab | Larvicid* or larval or larva or larvae ti, ab | 4 and 5 | Bacillus thuringiensis |
| 7 | — | "Larval control" ti, ab | "Larval control" ti, ab | "Larval control" ti, ab | — | Bacillus sphericus |
| 8 | — | 6 or 7 | Bacillus thuringiensis [ti, ab, Mesh] | Bacillus thuringiensis ti, ab | — | Paris green |
| 9 | — | 5 and 8 | Bacillus sphericus ti, ab | Bacillus sphericus ti, ab | — | Temefos |
| 10 | — | — | Paris green ti, ab, sn | Paris green ti, ab | — | Pyriproxyfen or methoprene OR fenthion OR abate OR "surface oils" OR "surface films" OR chlorpyrifos OR pirimiphos‐methyl OR diflubenzuron OR novaluron OR spinosad |
| 11 | — | — | Temefos ti, ab, sn | Temefos ti, ab | — | Insect growth regulator* |
| 12 | — | — | (Pyriproxyfen or methoprene OR fenthion OR abate OR "surface oils" OR "surface films" OR chlorpyrifos OR pirimiphos‐methyl OR diflubenzuron OR novaluron OR spinosad) ti, ab | (Pyriproxyfen or methoprene OR fenthion OR abate OR "surface oils" OR "surface films" OR chlorpyrifos OR pirimiphos‐methyl OR diflubenzuron OR novaluron OR spinosad) ti, ab | — | Biological pest control |
| 13 | — | — | Juvenile hormones [Mesh] | Insect growth regulator* ti, ab | — | 5‐12/OR |
| 14 | — | — | Insect growth regulator* ti, ab | Biological pest control [Emtree] | — | 4 AND 13 |
| 15 | — | — | Pest Control, Biological [Mesh] | Larvicidal agent [Emtree] | — | — |
| 16 | — | — | 6‐15/OR | 6‐15/OR | — | — |
| 17 | — | — | 5 AND 16 | 5 AND 16 | — | — |
| 18 | — | — | — | — | — | — |
aCochrane Infectious Diseases Group Specialized Register.
Data and analyses
Comparison 1. Larviciding versus no larviciding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Malaria incidence (cluster‐randomized controlled trial (cRCT)) | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Sensitivity analysis with estimated mean cluster population 675 and estimated intracluster correlation coefficient (ICC) 0.01 | 1 | Rate Ratio (Fixed, 95% CI) | 0.24 [0.14, 0.40] | |
| 1.2 Sensitivity analysis with estimated mean cluster population 675 and estimated ICC 0.05 | 1 | Rate Ratio (Fixed, 95% CI) | 0.24 [0.08, 0.70] | |
| 1.3 Sensitivity analysis with estimated mean cluster population 675 and estimated ICC 0.1 | 1 | Rate Ratio (Fixed, 95% CI) | 0.24 [0.05, 1.08] | |
| 2 Malaria incidence with subgrouping by extent of aquatic habitat (non‐randomized study (NRS)) | 2 | Risk Ratio (Random, 95% CI) | 1.17 [0.60, 2.26] | |
| 2.1 Habitats < 1 km² | 1 | Risk Ratio (Random, 95% CI) | 0.62 [0.38, 1.01] | |
| 2.2 Habitats > 1 km² | 1 | Risk Ratio (Random, 95% CI) | 1.58 [0.94, 2.65] | |
| 3 Parasite prevalence (cRCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sensitivity analysis with estimated mean cluster population 675 and estimated ICC 0.01 | 1 | 763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.46] |
| 3.2 Sensitivity analysis with estimated mean cluster population 675 and estimated ICC 0.05 | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.68] |
| 3.3 Sensitivity analysis with estimated mean cluster population 675 and estimated ICC 0.1 | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.42] |
| 4 Parasite prevalence (NRS) | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Adjusted data | 2 | Risk Ratio (Random, 95% CI) | 0.79 [0.71, 0.89] | |
| 4.2 Sensitivity analysis including Majambere 2010 with estimated ICC 0.01 | 3 | Risk Ratio (Random, 95% CI) | 0.88 [0.66, 1.16] | |
| 4.3 Sensitivity analysis including Majambere 2010 with estimated ICC 0.05 | 3 | Risk Ratio (Random, 95% CI) | 0.80 [0.71, 0.89] | |
| 4.4 Sensitivity analysis including Majambere 2010 with estimated ICC 0.1 | 3 | Risk Ratio (Random, 95% CI) | 0.80 [0.71, 0.89] | |
| 4.5 Sensitivity analysis excluding Majambere 2010 northern zones due to large baseline imbalance; estimated ICC 0.01 | 3 | Risk Ratio (Random, 95% CI) | 0.79 [0.71, 0.89] | |
| 5 Parasite prevalence with subgrouping by extent of aquatic habitat | 3 | Risk Ratio (Random, 95% CI) | 0.88 [0.66, 1.16] | |
| 5.1 Habitats < 1 km² | 2 | Risk Ratio (Random, 95% CI) | 0.79 [0.71, 0.89] | |
| 5.2 Habitats > 1 km² | 1 | Risk Ratio (Random, 95% CI) | 1.15 [0.41, 3.20] | |
| 6 Mean haemoglobin level | 1 | 3586 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.40, 0.13] |
| 6.1 Sensitivity analysis with estimated ICC 0.01 | 1 | 3586 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.40, 0.13] |
1.3. Analysis.
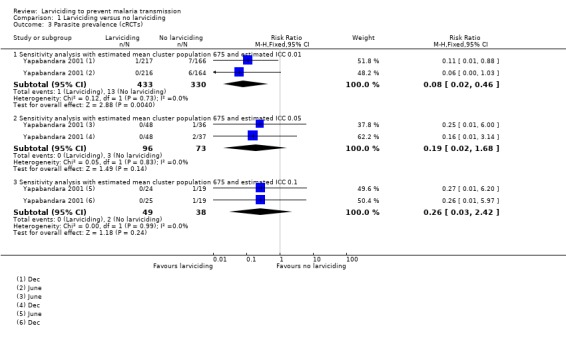
Comparison 1 Larviciding versus no larviciding, Outcome 3 Parasite prevalence (cRCTs).
1.6. Analysis.
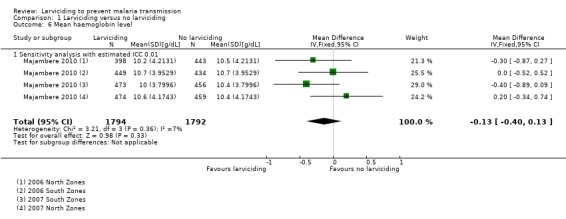
Comparison 1 Larviciding versus no larviciding, Outcome 6 Mean haemoglobin level.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fillinger 2009.
| Methods |
Study design: controlled before‐and‐after study Unit of allocation: clusters (valleys) Number of units: 3 valleys per arm Outcome assessment/surveillance type: 6 paired cross‐sectional surveys in cohort of children conducted during the long rains (April–June) and short rains (November–January) each year. Blood smears were collected from all children in each cohort to be assessed by microscopy for parasite identification and density. On each occasion, each pair of surveys were carried out 2 months apart. Thus, parasite infection status was assessed during 6 consecutive rainy seasons (the first 6 surveys at baseline, the following 6 during the intervention) from April 2004 to January 2007. Length of follow‐up: April 2004 to January 2007 (survey 1–3 at baseline, survey 4–6 during the intervention) Adjustment for clustering: yes |
|
| Participants |
Number of participants: 120 children in each valley (360:360) Population characteristics: children aged 6 months to 10 years. Approximately equal numbers of boys and girls. Withdrawal and loss to follow‐up: some absences from cross‐sectional surveys. Children said to be due to travelling in all cases. Figure 1 in the study paper reported numbers of each survey. Numbers present appeared to be consistently higher by above 10% in the control group compared to the intervention group. |
|
| Interventions |
Larvicide: Active ingredient and dosage: commercial strains of Bs orBti Formulation: water‐dispersible (Bs) and granule formulations (Bti) Manufacturer: Valent BioSciences LLC, USA Quality control of the larvicide: not reported Duration of the activity of the larvicide: Bs was used for the first 6 months of the intervention but, due to a lack of a residual effect, it was replaced with Bti, which is cheaper and forestalls the development of resistance due to its more complex mode of action. Method of application: not reported Frequency of application: applied to 3/6 valleys beginning in July 2005. Larvicide was applied to all water bodies at weekly intervals. Coverage: not reported Buffer size between clusters: valleys at least 1 km apart Cointerventions: ITNs, mainly long‐lasting insecticidal nets Types of nets used: PermaNet, Vestergaard Frandsen, Lausanne, Switzerland; Olyset, Sumitomo Chemical, Tokyo, Japan Delivery method: not delivered as part of study – from 2005 onwards, ITNs were intensively promoted and supplied by government health facilities and non‐governmental organizations. In addition, in July and August 2006, joint measles–malaria campaigns combining the distribution of ITNs with measles vaccinations were conducted. Coverage: not reported Net use: ITN use in sentinel households increased from 4.8% (95% CI 3.0% to 6.6%; range in control valleys 1–9% and in intervention valleys 2–6%) at baseline to 40.8% (95% CI 36.7% to 45.0%; range in control valleys 24–51% and in intervention valleys 25–51%) during the intervention year. The probability that ITNs were used was the same in the control and intervention group (OR 1.06, 95% CI 0.56 to 1.99) in both years but increased in both groups during the intervention (OR 13.58, 95% CI 7.30 to 25.28). |
|
| Outcomes |
Primary: Incidence of new Plasmodium infections in children aged 0.5–10 years, measured as children who were negative at the first paired survey but positive at the second Prevalence of Plasmodium infections in children aged 0.5–10 years Secondary: Annual EIR Mean human biting rate Anopheline late instar larval abundance Anopheline adult abundance |
|
| Location profile |
Study location: Kakamega and Vihiga highlands, western Kenya. Hilly areas characterized by steep‐sided valleys with flat bottoms and plateaus, where most homes were built. Small streams ran along the valley bottoms and papyrus swamps were common. Altitude of the highland valley communities ranged from 1453 to 1632 m. Malaria endemicity: moderately endemic EIR: annual EIR of An gambiae s.l. and An funestus s.l. combined was 10–12 infectious bites per person in both groups at baseline Population proximity/density: densely populated districts Plasmodiumspecies:Plasmodium falciparum |
|
| Vector profile |
Primary (and secondary) vector species:An gambiae s.s. (An funestus, An arabiensis, An rufipes) Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): not reported Phenotypic resistance profile: not reported Genotypic resistance profile: not reported Method of mosquito collection: Larval surveys done weekly in all valleys. The presence or absence of anopheline and culicine larvae was recorded in all aquatic habitats. Purposive dipping was used to sample larvae (10 dips per site). Larvae were categorized as early stage (first and second instars) and late stage (third and fourth instars). In 10 randomly selected sentinel sites per valley, weekly larval densities (mean number of larvae per dip per habitat) were recorded and the proportion of late instar larvae was calculated as an indicator of larval survival and emergence. Indoor‐resting mosquitoes collected monthly using pyrethrum spray catches from 10 sentinel houses in each valley that were randomly selected from households within 500 m of the valley bottom. The type of household, number of occupants during the night before, and mosquito control methods used were recorded routinely. An gambiae s.l. were identified to the species level using PCR, and presence of sporozoites was determined by ELISA of pooled samples of 10 mosquitoes per test. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized. |
| Allocation concealment (selection bias) | High risk | Not randomized. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded; however, the cointervention (ITNs) that were used in study arms had a similar usage in both. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Microscopists were blinded. RDTs and ELISA are objective measurements, but microscopy is objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some absences from cross‐sectional surveys. Children said to be due to travelling in all cases. Figure 1 in the study paper reported numbers of each survey. Numbers presented appeared to be consistently higher by > 10% in the control group compared to the intervention group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published beforehand. All expected outcomes from a trial such as this were reported. |
| Baseline characteristics | Low risk | Baseline characteristics appeared similar between the control and intervention arms. Key characteristics such as age and sex were the same. |
| Contamination | Low risk | Valleys were at least 1 km apart so assumed no contamination risk. |
| Incorrect analysis | Low risk | Cluster adjustment was carried out. Valleys were treated as the unit of geographic location and included as confounders in their models for analysis. |
| Baseline outcome measurements All outcomes | Low risk | Baseline outcome measurements appeared similar between the control and intervention arms. |
Maheu‐Giroux 2013a.
| Methods |
Study design: CBA of a staged programmatic implementation with randomized, cluster sampled household surveys Unit of allocation: clusters as wards Number of units: initially 15 control wards, period 1 (3 intervention, 12 control), period 2 (9 intervention, 6 control), period 3 (15 intervention) Outcome assessment/surveillance type: 6 rounds of cross‐sectional household surveys). A list of TCUs (small administrative units) was assembled for each ward before March 2004 and was regularly updated throughout the study duration. During the first round of the survey, 10 TCUs were randomly sampled from each of the 15 wards. All households located in the sampled TCUs were invited to participate in the survey. From the second round onwards, the TCUs sampled in the first round were followed up longitudinally, and another 10 TCUs per ward were selected for cross‐section surveys. Household survey administered and blood films taken. Length of follow‐up: from May 2004 to Dec 2008 Adjustment for clustering: yes |
|
| Participants |
Number of participants: > 610,000 residents Population characteristics: used total population Withdrawal and loss to follow‐up: not reported |
|
| Interventions |
Larvicide: Active ingredient and dosage: Bti (VectoBacH) for open (light‐exposed) habitats and Bs (VectoLexH) for closed (covered, often highly polluted) habitats. Dosages of 0.04 g/m² for water‐dispersible granule formulations and 1 g/m² for corn cob granule formulations of Bti. 1 g/m² of Bs. The targeting of closed habitats was for Culex mosquitoes. Formulation: water‐dispersible granule and corn cob formulations for Bti and corn cob formulations for Bs Manufacturer: Valent BioSciences LLC, USA Quality control of the larvicide: not reported Duration of the activity of the larvicide: not reported Method of application: community based but vertically managed intervention implementation. Open habitats (potential to produce Anopheles larvae), were treated by the Mosquito Control CORPs, each of whom was assigned to a specific mtaa (administrative subunits) or portions of an mtaa. Closed habitat were treated by an additional team of CORPs. Frequency of application: open habitats were treated every week with Bti. Closed habitats treated every 3 months. Buffer size between clusters: not reported. Cointerventions: all existing interventions such as ITNs, house screening, ceiling boards, repellents, coils, and spray. |
|
| Outcomes | Infection prevalence in all ages EIR Adult mosquito density |
|
| Location profile |
Study location: Dar es Salaam, Tanzania Malaria endemicity: climate was tropical humid with 2 rainy seasons – the long rains during the months of April and May and the short rains of October and November. Malaria transmission was year‐round with peaks in incidence after the 2 rainy seasons. EIR: 1.28 (all Anopheles) Population proximity/density: not reported Plasmodium species:Plasmodium falciparum accounted for > 90% of cases |
|
| Vector profile |
Primary (and secondary) vector species:An gambiae s.l. and An funestus s.l. An coustani has a low contribution to transmission. Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): exophagic Phenotypic resistance profile: not reported Genotypic resistance profile: not reported Method of mosquito collection: HLC performed in all clusters. In each of the 67 mtaa, 4 different, well‐distributed sampling locations were chosen non‐randomly to maximize coverage of surveillance, resulting in a total of 268 routinely maintained surveillance sites. HLC was conducted once every 4 weeks, overnight. In order to estimate the total true exposure experienced both indoors and outdoors by residents, directly measured outdoor mosquito densities were multiplied by the coefficient of the estimated total true human exposure divided by the estimated total outdoor biting rate obtained from detailed studies of mosquito–human interactions. These coefficients were derived from an in‐depth behavioural survey of both mosquitoes and humans which was conducted during the main rainy season of April to June 2006. Captured mosquitoes were Identified to genus morphologically and to subspecies by PCR. ELISA was used to detect infection of sporozoites. |
|
| Notes | The entomological outcomes were extracted from an earlier published paper, before all clusters received the intervention (3/15), related to the same study (Geissbühler 2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention was not randomly allocated; however, participants selected for outcome assessment were randomly selected. |
| Allocation concealment (selection bias) | High risk | Intervention was not randomly allocated;, however, participants selected for outcome assessment were randomly selected. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded; however, cointerventions used in study arms had a similar usage in both. Also analysis was adjusted, taking into account as possible covariates such as cointervention usage. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but outcome data collected independently of those implementing control. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors stated loss to follow‐up was minimal; however, no numbers were reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published beforehand. All expected outcomes from a trial such were reported. |
| Baseline characteristics | Unclear risk | Baseline characteristics appeared similar between the control and intervention arms which we took as their third survey round. Key characteristics such as age and sex were the same. |
| Contamination | Unclear risk | No mention of a buffer zone or population migration (both in terms of participants and mosquitoes). Potential for bias but unclear from what the study authors report. |
| Incorrect analysis | Low risk | Cluster adjustment was carried out. TCU and household was taken into account with their multivariate model. |
| Baseline outcome measurements All outcomes | Low risk | Baseline outcome measurements appeared similar between the control and intervention arms. |
Majambere 2010.
| Methods |
Study design: non‐randomized cross‐over trial Unit of allocation: zones Number of units: 4 zones approximately 12 × 8 km in area and divided into 3 parallel 4‐km wide bands (subzones) perpendicular to the river. Outcome assessment/surveillance type: cross‐sectional surveys (start and end of transmission season in 2 years (June and December 2006, then June and December 2007) and passive case detection. Census of residents, including children aged 6 months to 10 years, was carried out in 50 study villages during the dry season in 2006. Children were selected from random lists, with the total in each village proportional to village size. Length of follow‐up: baseline entomological data, but not clinical data, were collected during July–November 2005. In 2006 and 2007, entomological and clinical data collection started in May and ended in November. A cross‐over design was used for the application of larvicide. From June to November 2006, larvicide was applied to all accessible aquatic habitats in zones 1 and 3 at weekly intervals and zones 2 and 4 served as controls. From May to November 2007, larvicide intervention was applied to zones 2 and 4 and zones 1 and 3 served as controls. Adjustment for clustering: no |
|
| Participants |
Number of participants: 2039 total children at the first cross‐sectional survey. 1862 in the final survey. Population characteristics: children aged 0.5–10 years old Withdrawal and loss to follow‐up: from enrolment and first survey to the second survey, there was a high number of participants who were absent (98 in zone 1, 65 in zone 2, 76 in zone 3, and 76 in zone 4). A new cohort of children was used from survey 3 onwards which included previous children that were still under 10 years if age and a selection of new children which replaced either children over 10 years of age or any that had left the study site. From this new cohort, a total of 184 were absent across all zones (33 in zone 1, 50 in zone 2, 47 in zone 3, and 54 in zone 4). |
|
| Interventions |
Larvicide: Active ingredient and dosage: commercial strains of Bti at 0.2 kg/hectare for water‐dispersible granules and at 5 kg/hectare for corn granules Formulation: water‐dispersible granule and corn granules Manufacturer: Valent BioSciences LLC, USA Quality control of the larvicide: field applicators were recruited from communities supervised by 1 field supervisor in each zone and trained for 1 month before larviciding. Larval surveys were carried out continuously by the zone supervisor. In 2005, during the baseline period, all aquatic habitats in each zone were visited and the presence or absence of anopheline and culicine larvae recorded as described elsewhere. Each habitat was visited monthly. During the intervention years (2006 and 2007) random larval spot checks were implemented throughout the season to estimate the proportion of habitats containing early and late instar larvae to determine the effectiveness of larvicide application. Of the total number of habitats identified in each zone during baseline (1076), 40 habitats were randomly (computer‐ generated) selected every day for each zone respectively by the programme manager (S.M.) and the habitat identification number, including global positioning system co‐ordinates, forwarded to the field supervisor for habitat inspection as described above. Selection of sites was stratified according to subzone and the timetable of larvicide application to ensure that inspection of sites took place 1–2 days after the habitat was treated with larvicide and that an equal number of sites were visited weekly in all three subzones in each zone. In addition, 10 sentinel habitats per zone were randomly selected after the first round of complete habitat surveys in 2005 and larval densities measured weekly in these. At each site visit, purposive dipping was used to sample larvae (10 dips per site), which were categorized as early (first and second instars) stages and late (third and fourth) stages. Duration of the activity of the larvicide: not reported. Method of application: the water‐dispersible granules were applied as liquid with knapsack compression sprayers (15‐L capacity diaphragm knapsack sprayers, Solo 475; Solo Kleinmotoren GmbH, Sindelfingen, Germany) in areas with low vegetation coverage. The corn granules were applied by hand from buckets held with a strap around the waist or neck or motorized knapsack granuleblowers (13‐L capacity motorized sprayers; MD 150DX‐13; Maruyama, Tokyo, Japan) when aquatic habitats were covered by vegetation and difficult to access. Frequency of application: weekly Buffer size between clusters: study villages were recruited from the central band of each zone. The study authors assumed that when larvicide was applied to an entire study zone, the 2 × 4‐km bands, either side of the central band, would be sufficiently wide to minimize mosquito movement from untreated sites outside the study zone into the central band, where the study villages were located. Cointerventions: existing ITNs Compliance: use increased during study range in 2006 was 6.1% to 38.3%, range in 2007 37.2% to 81.4%. |
|
| Outcomes | Incidence of malaria cases per 100 child‐years defined as a history of fever within the last 48 hours or axillary temperature ≥ 37.5 °C later confirmed with the presence of Plasmodium falciparum identified microscopically. Prevalence of P falciparum infection Mean haemoglobin level Prevalence of splenomegaly Prevalence of gametocytaemia Seasonal EIR Number of female adult An gambiae s.l. Sporozoite rate |
|
| Location profile |
Study location: floodplains of river Gambia, east of Farafenni, The Gambia. Study was carried out in 4 separate areas (referred to as zones 1–4), 2 on the north banks and 2 on the south banks of the Gambia River. Flat open Sudan savannah broadly consisting of farmlands, sparse woodland, and the extensive alluvial floodplains of the river. Malaria endemicity: seasonal transmission EIR: not reported Population proximity/density: % of villages in each zone < 1 km from the floodplain was reported. In zones 1 and 2, this was < 20% on average. In zones 3 and 4, this was > 80%. Plasmodiumspecies:Plasmodium falciparum |
|
| Vector profile |
Primary (and secondary) vector species:An gambiae s.s., An melas, and An arabiensis Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): not reported Aquatic habitats (type, stability and extent (number and size), proximity of aquatic habitats to human habitation): flood plains of River Gambia Phenotypic resistance profile: not reported Genotypic resistance profile: not reported Method of mosquito collection: adult vector surveys were implemented in 39 villages (10 in zone 1, 11 in zone 2, 9 in zone 3, and 9 in zone 4) at 2‐week intervals from July to November in 2005 and for the duration of larviciding in the intervention years. Each zone had 15 traps divided between the villages with 1–3 sentinel houses per village proportional to village size. Within randomly selected compounds, all houses with open eaves, a thatched roof, no ceiling, and where a single man slept were numbered and 1 was selected randomly. Mosquitoes were sampled using miniature CDC light traps (Model 512; John W. Hock Company, Gainesville, FL) positioned 1 m above the floor at the foot end of the bed where a person slept under an untreated bed net. Traps were set at 7:00 p.m. and collected at 7:00 a.m. the following morning. If the occupant moved house, the trap was moved to the nearest similar house in the same village. If the occupant did not spend the night in the selected room or the trap was faulty, the data were excluded from the analysis. Mosquitoes were identified to the level of species by microscopy and the numbers of An gambiae s.l. females recorded. The presence of sporozoites was identified using ELIZA. In 2005 and 2006, a 1% random sample of the An gambiae s.l. females, stratified by zone and sampling period, was typed to the species by PCR. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention was not randomly allocated; however, participants selected for outcome assessment were randomly selected. |
| Allocation concealment (selection bias) | High risk | Intervention was not randomly allocated; however, participants selected for outcome assessment were randomly selected. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Residents were aware of ongoing interventions but this was unlikely to have impacted results. There was a large difference in net use in zone 1 compared to the other zones; however, this was measured at baseline and net use increased at a similar rate throughout all zones after the intervention was introduced. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reading of blood films was blinded and RDTs are objective assessments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 10% loss in study groups. See above under ‘Withdrawal and loss to follow‐up' |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published beforehand. All expected outcomes from a trial such were reported. |
| Baseline characteristics | High risk | Large differences in key baseline characteristics (sex ratio, ethnicity, net use) |
| Contamination | Low risk | Study authors do not specifically mention contamination. However, the intervention is expected to have a very short‐lasting effect so would not carry over to the following year when the cross‐over of intervention happened. |
| Incorrect analysis | Unclear risk | Suggested no paired analysis was done, therefore, it should have an inappropriate weighting in a meta‐analysis. However, this is not so much a risk of bias issue and this study was not meta‐analyzed. |
| Baseline outcome measurements All outcomes | High risk | Taking the prevalence of Plasmodium falciparum infections, zone 1 had a much higher prevalence (38.4%) compared to the others (9.5–16.8%) |
| Other bias | Low risk | Suitability of a cross‐over design: low risk. Malaria can be highly seasonal but study authors reported rainfall to be consistent throughout the years of the trial period. Whether only first‐period data are available: low risk. Multiple periods of data reported. Comparability of results with those from parallel‐group trials: low risk. |
Yapabandara 2001.
| Methods |
Study design: cRCT Unit of allocation: clusters (villages) Number of units: 8 villages divided equally into 2 arms. On the basis of 1 year's preintervention data the villages were stratified into 4 with high levels of malaria transmission and 4 with lower transmission. Within each strata 2 villages randomly selected for intervention. Outcome assessment/surveillance type: passive case detection. Also 2 mass blood surveys were carried out in July and December during the pre‐ and postintervention years. Blood films were taken, regardless of the presence/ absence of fever, from all the residents of the 8 villages. Length of follow‐up: January 1994 to December 1995 Adjustment for clustering: yes |
|
| Participants |
Number of participants: 4/8 villages had populations < 500 while the other 4 had populations of 600–1100. Population characteristics: not reported. Withdrawal and loss to follow‐up: not reported. |
|
| Interventions |
Larvicide: Active ingredient and dosage: pyriproxyfen, S31183 (Adeal 0.5% G) applied at a rate of 0.01 mg active ingredient/L (2 g granules/m³) Formulation: not reported. Manufacturer: not reported. Quality control of the larvicide: community engagement to encourage community to inform about new gem pits so that they could be rapidly treated. Duration of the activity of the larvicide: not reported but assays were conducted to determine if residual activity was present. Method of application: not reported. Frequency of application: 3 applications – 1 in December 1994, 1 between June and July 1995 in the postmonsoon season when river bed pools were formed, and 1 at end of November 1995. Buffer size between clusters: not reported. |
|
| Outcomes | Malaria incidence defined as fever/history of fever and parasites detected by blood film. Infection prevalence (slide positivity rates) Number of anophelines |
|
| Location profile |
Study location: This study was carried out in Kaluganga, which is part of Elahera gem‐mining area situated in Matale District (7°40N, 80°50E) in the dry central zone of Sri Lanka. A cluster of 8 villages with a total area of 23 km² was selected for this study. The numbers of gem pits per village ranged from 311 to 3622. The villages were surrounded by thick jungle. The area was a settlement scheme, which was established about 30 years before the trial was conducted around the rivers, Aban ganga and Kalu ganga. Malaria endemicity: not reported. EIR: not reported. Population proximity/density: treated gem pits and pools up to 1.5 km from villages. Plasmodiumspecies:Plasmodium falciparum and Plasmodium vivax |
|
| Vector profile |
Primary (and secondary) vector species:An culicifacies (An subpictus and An varuna) Vector behaviour (nature, stability, adult habitat, peak biting times, exophilic/endophilic, exophagic/endophagic, anthropophilic/zoophilic): not reported Aquatic habitats (type, stability and extent (number and size), proximity of aquatic habitats to human habitation): Shallow pits dug by gem miners that filled with water. Breeding of An culicifacies was almost entirely in gem pits but some breeding of An subpictus and most of An varuna was in other sites such as river bed pools and slow‐moving river margins Phenotypic resistance profile: not reported Genotypic resistance profile: not reported Method of mosquito collection: Anopheline populations in the study area were estimated by 7 sampling methods: window exit trap collection; pyrethrum spray sheet collection; indoor HCs; all night or for the first part of the night up to midnight; cattle‐baited hut collection and cattle‐baited net trap collection; and light trap collection. The locations chosen for applying these methods were near the centres of each village to try to avoid interference by immigration of mosquitoes from neighbouring villages. Data reported were only from cattle‐baited huts, partial night landing catches, and all night landing catches. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that they randomized, but unclear how. |
| Allocation concealment (selection bias) | Unclear risk | Stated that they randomized, but unclear how. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not possible for the intervention; however, unlikely to affect the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported whether blinding was used. Unclear whether slide readers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No cohort established. Movement in and out of study area not documented. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published beforehand. Not all the stated entomological outcomes described in the methods were reported. |
| Incorrect analysis | High risk | Inappropriate analysis, no adjustment for clustering |
| Other bias | Unclear risk | Recruitment bias: low risk – randomized study where they had selected clusters based on malaria cases before the intervention was introduced to ensure this was equal in both arms. Mass blood surveys and census attempted to include the entire population. Baseline imbalance: low risk – baseline characteristics appeared similar Loss of clusters: low risk – no clusters were lost Comparability with RCTs randomizing participants: low risk – larviciding is expected to have a community wide effect and should be implemented at a community level. |
Abbreviations: An: Anopheles; Bti: Bacillus thuringiensis israeliensis; Bs: Bacillus sphaericus; EIR: entomological inoculation rate; ELISA: enzyme‐linked immunosorbent assay; HLC: human landing catches; ITN: insecticide‐treated nets; OR: odds ratio; PCR: polymerase chain reaction; RCT: randomized controlled trial; RDT: rapid diagnostic test; TCU: Ten‐Cell Unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdalmagid 2012 | No relevant outcomes |
| Afrane 2016 | No relevant outcomes |
| Ansari 2005 | No relevant outcomes |
| Balaraman 1983 | No relevant outcomes |
| Balaraman 1987 | No relevant outcomes |
| Bertram 1950 | No relevant outcomes |
| Bhalwar 1995 | No relevant outcomes |
| Biswas 1997 | No relevant outcomes |
| Bond 2004 | No relevant outcomes |
| Brescia 1947 | Study design did not match inclusion criteria |
| Castro 2002 | Study design did not match inclusion criteria |
| Castro 2009 | Intervention did not match inclusion criteria |
| Chaki 2011 | Study design did not match inclusion criteria |
| Chen 1985 | Study design did not match inclusion criteria |
| Chen 1988 | Study design did not match inclusion criteria |
| Claborn 2002 | No relevant outcomes |
| Derua 2017 | No relevant outcomes |
| Djènontin 2014 | No relevant outcomes |
| El Safi 1986 | No relevant outcomes |
| Farashiani 2000 | Full text not available |
| Fillinger 2003 | No relevant outcomes |
| Galardo 2013 | No relevant outcomes |
| Giurcă 1978 | Duplicate but in a different journal |
| Haq 2004 | No relevant outcomes |
| Houten 1980 | Full text not available |
| Imbahale 2012 | Intervention did not match inclusion criteria |
| Johnson 1947 | No relevant outcomes |
| Julvez 1987a | Study design did not match inclusion criteria |
| Julvez 1987b | Duplicate but in a different journal |
| Kanda 1995 | Study design did not match inclusion criteria |
| Karanja 1994 | No relevant outcomes |
| Konradsen 1999 | Study design did not match inclusion criteria |
| Kramer 2014 | Protocol |
| Kumar 1994 | Intervention did not match inclusion criteria |
| Kumar 1998 | Intervention did not match inclusion criteria |
| Kumar 2013 | Intervention did not match inclusion criteria |
| Kusumawathie 2008 | No relevant outcomes |
| Ladoni 1986 | Full text not available |
| Lee 1990 | No relevant outcomes |
| Liu 2009 | Study design did not match inclusion criteria |
| Lunin 1979 | No relevant outcomes |
| Mahdi 1967 | No relevant outcomes |
| Maheu‐Giroux 2013b | No relevant outcomes |
| Maheu‐Giroux 2013c | Duplicate but in a different journal |
| Maheu‐Giroux 2014 | No relevant outcomes |
| Marina 2014 | No relevant outcomes |
| McCann 2017a | Protocol |
| McCann 2017b | Intervention did not match inclusion criteria |
| Meng 1996 | Study design did not match inclusion criteria |
| Minakawa 2007 | Protocol (study not performed) |
| Mossadegh 1973 | Study design did not match inclusion criteria |
| Msellemu 2016 | Study design did not match inclusion criteria |
| Müller 1984 | Study design did not match inclusion criteria |
| Obopile 2018 | Study design did not match inclusion criteria |
| Ouedraogo 2017 | Study design did not match inclusion criteria |
| Parvez 2003 | No relevant outcomes |
| Perich 1990 | No relevant outcomes |
| Prabhu 2011 | No relevant outcomes |
| Pridantseva 1980 | No relevant outcomes |
| Ranjbar 2012 | Study design did not match inclusion criteria |
| Rettich 1973 | Conference abstract |
| Rifaat 1974 | Full text not available |
| Sharma 1983 | No relevant outcomes |
| Sharma 1989 | Intervention did not match inclusion criteria |
| Sharma 2003 | No relevant outcomes |
| Shililu 2003 | No relevant outcomes |
| Shimada 2007 | Study design did not match inclusion criteria |
| Skovmand 1997 | No relevant outcomes |
| Skovmand 1999 | No relevant outcomes |
| Skovmand 2011 | Intervention did not match inclusion criteria |
| Some 1994 | Study design did not match inclusion criteria |
| Song 2013 | Study design did not match inclusion criteria |
| Srivastava 1996 | No relevant outcomes |
| Tchicaya 2009 | No relevant outcomes |
| Tchicaya 2010 | Conference abstract |
| Teng 2005 | No relevant outcomes |
| Tâcu 1977 | Study design did not match inclusion criteria |
| Usenbaev 2006 | Study design did not match inclusion criteria |
| Vasuki 1992 | Study design did not match inclusion criteria |
| Wang 1983 | Study design did not match inclusion criteria |
| Xu 1980 | Study design did not match inclusion criteria |
| Xu 1983 | Study design did not match inclusion criteria |
| Xu 2004 | Study design did not match inclusion criteria |
| Yapabandara 2002 | No relevant outcomes |
| Yapabandara 2004 | Intervention did not match inclusion criteria |
| Yapabandara 2005 | No relevant outcomes |
| Zhou 2010 | Study design did not match inclusion criteria |
| Zhou 2016 | Protocol |
| Zohdy 1982 | Study design did not match inclusion criteria |
Characteristics of studies awaiting assessment [ordered by study ID]
Fuseini 2017.
| Methods | Unavailable |
| Participants | Unavailable |
| Interventions | Unavailable |
| Outcomes | Unavailable |
| Notes |
Javadian 1974.
| Methods |
Study design: CBA Unit of allocation: clusters (dehestans which are administrative units above villages) Number of units: 4 dehestans per arm Outcome assessment/surveillance type: active case detection Length of follow‐up: 1 year preintervention and 3 years postintervention. Overall period of April 1965 to the end of 1968 Adjustment for clustering: unclear as not reported |
| Participants |
Number of participants: total population varied throughout study period. 1965: 40,794 in control, 28,999 in intervention 1966: 41,514 in control, 27,446 in intervention 1967: 46,226 in control, 43,663 in intervention 1968: 46,757 in control, 32,649 in intervention Population characteristics: total population was used. Population denominator not stable, swelled by migration during date palm harvest season which coincides with peak transmission in Aug/Sept. Withdrawal and loss to follow‐up: not reported |
| Interventions |
Larvicide: Active ingredient and dosage: the text suggested it is petroleum oil Formulation: not reported Manufacturer: not reported Quality control of the larvicide: not reported Duration of the activity of the larvicide: not reported Method of application: not reported Frequency of application: not reported Buffer size between clusters: not reported Cointerventions: IRS Active ingredient and dosage: DDT 2 g/m² and malathion 2 g/m² Formulation: not reported Frequency of spraying: 2 rounds per year of DDT until 1967. After that, DDT was used for the first round and malathion was used for the second each year. Coverage: not reported |
| Outcomes | Malaria incidence Indoor resting density of An stephensi |
| Notes |
Zhou 2013.
| Methods |
Study design: cRCT assessing a combination of vector control interventions. The study spanned 2 years (2010 to 2011), but for this review, only the data from 2011 were included as this was when larviciding was used. Unit of allocation: clusters Number of units: 3 study sites which each had 3 paired clusters, making a total of 9 paired clusters. Each of these pair would then be randomly assigned the control (ITNs) or the intervention (ITNs + larviciding). Furthermore, within each cluster, an area was targeted with IRS. Outcome assessment/surveillance type: active case detection in cohort of 350 participants per cluster in 2010 and 450 per cluster in 2011. Cross‐sectional surveys were done during February and March 2010 before the IRS application and 2011 before the IRS and larvicide application. Another survey was conducted post intervention in May 2010, May 2011, and July 2011. Blood smears were taken from randomly selected participants of different ages within each cluster: approximately 150 in 2010 and 250 in 2011. Only 12/18 clusters were monitored in 2011. Length of follow‐up: around 2 years: February 2010 to July 2011 Adjustment for clustering: yes |
| Participants |
Number of participants: in 2010, numbers ranged from 2884 to 2906 for the cross‐sectional survey. In 2011, numbers ranged from 4323 to 5139. For the cohort, there were 6248 participants in 2010 and 5574 in 2011. Population characteristics: not reported but no particular age group was targeted. Withdrawal and loss to follow‐up: not reported. |
| Interventions |
Larvicide: Active ingredient and dosage: commercial strains of Bti (VectoMax) Formulation: corn granules Manufacturer: Valent BioSciences LLC, USA Quality control of the larvicide: aquatic habitats were searched thoroughly by a team of technicians accompanied by field assistants from local villages. Duration of the activity of the larvicide: not reported. Method of application: not reported. Frequency of application: first round of application was completed in February/March 2011 and the second in March/April 2011, 4 weeks after the first round. Buffer size between clusters: 250 m buffer zone between control and intervention clusters; however, there was no buffer between the IRS targeted zones and the non‐targeted zones. Cointervention: ITNs Active ingredient and dosage: not reported. Method of distribution: Global Fund supported mass distribution in June to September 2006 (targeting < 5 s and pregnant women) in all study areas. A second round was accomplished during May to July 2011 (targeting everyone at risk). Coverage: increased from 40.7% (range 34.3 to 47.8%) in 2010 to 93.0% (range 81.6 to 100%) in 2011. Compliance: not reported |
| Outcomes | Malaria incidence defined as fever/history and plasmodium parasites detected by smear Parasite prevalence Indoor resting density of mosquitoes |
| Notes |
Abbreviations: An: Anopheles; Bti: Bacillus thuringiensis israeliensis; CBA: controlled before‐and‐after; cRCT: cluster‐randomized controlled trial; DDT: dichlorodiphenyltrichloroethane; IRS: indoor residual spraying.
Differences between protocol and review
We changed the title from ‘Larviciding to control malaria' in the protocol (Choi 2017), to ‘Larviciding to prevent malaria transmission'.
We amended the study inclusion criteria to allow inclusion of non‐randomized cross‐over trials.
In the published protocol, Choi 2017, we stated that we would not present results from cluster‐randomized controlled trials (cRCTs) that were not adjusted for clustering. In the review we presented unadjusted measures of effect and we have taken this into account in the GRADE assessments.
In the protocol we stated, "If there was considerable heterogeneity i.e. an I² statistic value of 75% to 100% or inconsistency in the direction of the effect, or both, then we did not perform a meta‐analysis". We performed a meta‐analysis in Analysis 1.2 where heterogeneity was I² = 82%. We accounted for the heterogeneity in the GRADE assessments, and this is reflected in the ‘Summary of findings' tables.
We added Silas Majambere as an author.
Contributions of authors
All authors contributed to the protocol design, wrote the protocol, and approved the final version.
LC and AW screened articles, extracted data from the included studies, and conducted the analysis.
SM acted as arbitrator.
All authors sought to interpret the data, and prepared and interpreted the GRADE summaries.
LC and AW wrote the full text and SM edit modified this. All authors read and approved the final manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Project number 300342‐104
-
World Health Organization (WHO), Switzerland.
WHO Global Malaria Programme Agreement for Performance of Work (APW) Grant 2017 (number 709319)
-
Partnership for Increasing the Impact of Vector Control (PIIVeC), UK.
Provided support to LC. PIIVeC is funded by the Medical Research Council of the UK (grant number MR/P027873/1) through the Global Challenges Research Fund.
Declarations of interest
LC has no known conflicts of interest.
SM is an independent consultant, and occasionally advises governments on malaria control. His main sources of consultancy work are from a research project funded by the Innovative Vector Control Consortium (IVCC). The title of the review is related to the use of insecticide applications for malaria vector control. The IVCC as an organization has a programme of working with industry on the development of novel insecticides and other vector control tools. He is a co‐chair of the Larval Source Management workstream of the Roll Back Malaria's Vector Control Working Group. SM was a primary investigator and author of one study reviewed. He was not involved in the screening, data extraction, risk of bias assessment, and analysis for this particular study.
AW sits on the Innovative Vector Control Consortium (IVCC) External Scientific Advisory Committee 1 (ESAC1), which provides the IVCC management team with independent scientific advice on all projects proposed to the IVCC relating to the development of new products for malaria vector control.
These authors contributed equally to this work
These authors contributed equally to this work
Unchanged
References
References to studies included in this review
Fillinger 2009 {published data only}
- Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide‐treated nets in western Kenya: a controlled trial. Bulletin of the World Health Organization 2009;87(9):655‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maheu‐Giroux 2013a {published data only}
- Geissbühler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, et al. Microbial larvicide application by a large‐scale, community‐based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLOS One 2009;4(3):e5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu‐Giroux M, Castro MC. Impact of community‐based larviciding on the prevalence of malaria infection in Dar es Salaam, Tanzania. PLOS One 2013;8(8):e71638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Majambere 2010 {published data only}
- Majambere S, Pinder M, Fillinger U, Ameh D, Conway DJ, Green C, et al. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in The Gambia? A cross‐over intervention trial. American Journal of Tropical Medicine and Hygiene 2010;82(2):176‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yapabandara 2001 {published data only}
- Yapabandara AM, Curtis CF, Wickramasinghe MB, Fernando WP. Control of malaria vectors with the insect growth regulator pyriproxyfen in a gem‐mining area in Sri Lanka. Acta Tropica 2001;80(3):265‐76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdalmagid 2012 {published data only}
- Abdalmagid MA, Elkhalifa SM, Ismail AB, Ismail SM, Abdalrahman AH, Jamal AE. The mosquito dunk (Bacillus thuringensis israelienses) as a control agent against mosquito larvae in Khartoum State, Sudan. Sudanese Journal of Public Health 2012;7(2):51‐5. [Google Scholar]
Afrane 2016 {published data only}
- Afrane YA, Mweresa NG, Wanjala CL, Gilbreath TM III, Zhou G, Lee MC, et al. Evaluation of long‐lasting microbial larvicide for malaria vector control in Kenya. Malaria Journal 2016;15(1):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansari 2005 {published data only}
- Ansari MA, Razdan RK, Sreehari U. Laboratory and field evaluation of Hilmilin against mosquitoes. Journal of the American Mosquito Control Association 2005;21(4):432‐6. [DOI] [PubMed] [Google Scholar]
Balaraman 1983 {published data only}
- Balaraman K, Balasubramanian M, Jambulingam P. Field trial of Bacillus thuringiensis H‐14 (VCRC B‐17) against Culex and Anopheles larvae. Indian Journal of Medical Research 1983;77:38‐43. [PubMed] [Google Scholar]
Balaraman 1987 {published data only}
- Balaraman K, Gunasekaran K, Pillai PK, Manonmani AM. Field trial with different formulations of Bacillus sphaericus for mosquito control. Indian Journal of Medical Research 1987;85:620‐5. [PubMed] [Google Scholar]
Bertram 1950 {published data only}
- Bertram DM. Larvicidal treatments with DDT and ‘Gammexane' in Upper Assam, with particular reference to their effect on Anopheles minimus. Annals of Tropical Medicine & Parasitology 1950;44(3):255‐9. [DOI] [PubMed] [Google Scholar]
Bhalwar 1995 {published data only}
- Bhalwar R, Deshpande VR, Sandhu HS, Gokarn AG. Randomised, controlled, blinded field trial on the efficacy of biocide formulation (Bacillus spp.) in the control of mosquito vectors. Medical Journal Armed Forces India 1995;51(1):4‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biswas 1997 {published data only}
- Biswas D, Ghosh SK, Dutta RN, Mukhopadhyay AK. Field trial of bacticide on larval populations of two species of vector mosquitoes in Calcutta. Indian Journal of Malariology 1997;34(1):37. [PubMed] [Google Scholar]
Bond 2004 {published data only}
- Bond JG, Marina CF, Williams T. The naturally derived insecticide spinosad is highly toxic to Aedes and Anopheles mosquito larvae. Medical and Veterinary Entomology 2004;18(1):50‐6. [DOI] [PubMed] [Google Scholar]
Brescia 1947 {published data only}
- Brescia F. Effect of concentration of DDT in oil aerosols on toxicity to mosquito larvae. Journal of Economic Entomology 1947;40(3):441. [DOI] [PubMed] [Google Scholar]
Castro 2002 {published data only}
- Castro SD, Colombi E, Flores LN, Canales D. Biolarvicide Bacillus sphaericus‐2362 (GRISELESF) for the control of malaria in a health area of the Republic of Honduras [Aplicación del biolarvicida Bacillus sphaericus‐2362 (GRISELESF) para el control de la Malaria en un área de salud de la República de Honduras]. Revista Cubana de Medicina Tropical 2002;54(2):134‐41. [PubMed] [Google Scholar]
Castro 2009 {published data only}
- Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S. Community‐based environmental management for malaria control: evidence from a small‐scale intervention in Dar es Salaam, Tanzania. Malaria Journal 2009;8(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaki 2011 {published data only}
- Chaki PP, Dongus S, Fillinger U, Kelly A, Killeen GF. Community‐owned resource persons for malaria vector control: enabling factors and challenges in an operational programme in Dar es Salaam, United Republic of Tanzania. Human Resources for Health 2011;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1985 {published data only}
- Chen SF, Xiao YC, Lü JF. Open solid plate medium cultured Bacillus thuringiensis and its mosquitocidal effect in field application. Journal of Parasitology & Parasitic Diseases 1985;3(3):195. [PubMed] [Google Scholar]
Chen 1988 {published data only}
- Chen SF. Comparison of insecticidal effects against mosquito larvae between Bacillus sphaericus strain 1593 and Bacillus thuringiensis H‐14 B. t. israelensis. Chinese Journal of Parasitology & Parasitic Diseases 1988;6(4):312. [Google Scholar]
Claborn 2002 {published data only}
- Claborn DM, Masuoka PM, Klein TA, Hooper T, Lee A, Andre RG. A cost comparison of two malaria control methods in Kyunggi Province, Republic of Korea, using remote sensing and geographic information systems. American Journal of Tropical Medicine and Hygiene 2002;66(6):680‐5. [DOI] [PubMed] [Google Scholar]
Derua 2017 {published data only}
- Derua YA, Kahindi SC, Mosha FW, Kweka JE, Atieli HE, Zhou G, et al. Microbial larvicides for mosquito control: impact of long lasting formulation of Bacillus thuringiensis var. Israelensis and Bacillus sphaericus on non target organisms in western Kenya highlands. American Journal of Tropical Medicine and Hygiene 2017;49:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Djènontin 2014 {published data only}
- Djènontin A, Pennetier C, Zogo B, Soukou KB, Ole‐Sangba M, Akogbéto M, et al. Field efficacy of Vectobac GR as a mosquito larvicide for the control of Anopheline and Culicine mosquitoes in natural habitats in Benin, West Africa. PLOS One 2014;9(2):e87934. [DOI] [PMC free article] [PubMed] [Google Scholar]
El Safi 1986 {published data only}
- Safi SH, Haridi AM. Field trial of the insect growth regulator, Dimilin, for control of Anopheles pharoensis in Gezira, Sudan. Journal of the American Mosquito Control Association 1986;2(3):374. [PubMed] [Google Scholar]
Farashiani 2000 {published data only}
- Farashiani E, Ladonni H. Laboratory and field evaluation of triflumuron and a larvicide against Anopheline larvae on rice field in South of Iran. Journal of Entomological Society of Iran 2000;19(1/2):45‐55. [Google Scholar]
Fillinger 2003 {published data only}
- Fillinger U, Knols BG, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Tropical Medicine & International Health 2003;8(1):37‐47. [DOI] [PubMed] [Google Scholar]
Galardo 2013 {published data only}
- Galardo AK, Zimmerman R, Galardo CD. Larval control of Anopheles (Nyssorhinchus) darlingi using granular formulation of Bacillus sphaericus in abandoned gold‐miners excavation pools in the Brazilian Amazon Rainforest. Revista da Sociedade Brasileira de Medicina Tropical 2013;46(2):172‐7. [DOI] [PubMed] [Google Scholar]
Giurcă 1978 {published data only}
- Giurcă I, Tâcu V, Petrescu S. Action of some chemical products – insecticides and detergents on the larvae of mosquitoes of Anopheles labranchiae atroparvus and Culex pipiens autogenicus species. Revista de Igiena, Bacteriologie, Virusologie, Parazitologie, Epidemiologie, Pneumoftiziologie. Pneumoftiziologia 1978;23(4):237‐46. [PubMed] [Google Scholar]
Haq 2004 {published data only}
- Haq S, Bhatt RM, Vaishnav KG, Yadav RS. Field evaluation of biolarvicides in Surat city, India. Journal of Vector Borne Diseases 2004;41(3/4):61. [PubMed] [Google Scholar]
Houten 1980 {published data only}
- Houten AT, Aminah NS, Suroso T, Seregeg IG. Effects of diflubenzuron (OMS 1804) against malaria vectors Anopheles sundaicus breeding in lagoons in Bali, Indonesia. WHO/VBC/80 1980;795:1‐7. [Google Scholar]
Imbahale 2012 {published data only}
- Imbahale SS, Githeko A, Mukabana WR, Takken W. Integrated mosquito larval source management reduces larval numbers in two highland villages in western Kenya. BMC Public Health 2012;12(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1947 {published data only}
- Johnson CG, Walton WH. The effect of area dosage, solution concentration and drop size of sprayed solutions and emulsions of DDT against mosquito larvae. Bulletin of Entomological Research 1947;38(3):405‐30. [DOI] [PubMed] [Google Scholar]
Julvez 1987a {published data only}
- Julvez J, Galtier J, Halidi A, Henry M, Mouchet J. Epidemiology of malaria and the antimalarial campaign in Mayotte (Comoro archipelago, Indian Ocean). Development of the situation between 1976 and 1986. Outlook. Bulletin de la Societe de Pathologie Exotique et de ses Filiales 1987;90(3 Pt 2):505‐19. [PubMed] [Google Scholar]
Julvez 1987b {published data only}
- Julvez J, Galtier J, Halidi A, Henry M, Mouchet J. Epidemiology of malaria and the antimalarial campaign in Mayotte (Comoro archipelago, Indian Ocean). Development of the situation between 1976 and 1986. Outlook. Bulletin de la Societe de Pathologie Exotique et de ses Filiales 1987;80(3 bis):505‐19. [PubMed] [Google Scholar]
Kanda 1995 {published data only}
- Kanda TO, Bunnag DA, Deesin VA, Deesin TH, Leemingsawat SO, Komalamisra NA, et al. Integration of control measures for malaria vectors in endemic areas of Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1995;26(1):154‐63. [PubMed] [Google Scholar]
Karanja 1994 {published data only}
- Karanja DM, Githeko AK, Vulule JM. Small‐scale field evaluation of the monomolecular surface film ‘Arosurf MSF' against Anopheles arabiensis Patton. Acta Tropica 1994;56(4):365‐9. [DOI] [PubMed] [Google Scholar]
Konradsen 1999 {published data only}
- Konradsen F, Steele P, Perera D, Hoek W, Amerasinghe PH, Amerasinghe FP. Cost of malaria control in Sri Lanka. Bulletin of the World Health Organization 1999;77(4):301. [PMC free article] [PubMed] [Google Scholar]
Kramer 2014 {published data only}
- Kramer RA, Mboera LE, Senkoro K, Lesser A, Shayo EH, Paul CJ, et al. A randomized longitudinal factorial design to assess malaria vector control and disease management interventions in rural Tanzania. International Journal of Environmental Research and Public Health 2014;11(5):5317‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 1994 {published data only}
- Kumar A, Sharma VP, Sumodan PK, Thavaselvam D, Kamat RH. Malaria control utilizing Bacillus sphaericus against Anopheles stephensi in Panaji, Goa. Journal of the American Mosquito Control Association 1994;10(4):534‐9. [PubMed] [Google Scholar]
Kumar 1998 {published data only}
- Kumar A, Sharma VP, Sumodan PK, Thavaselvam D. Field trials of biolarvicide Bacillus thuringiensis var. israelensis strain 164 and the larvivorous fish Aplocheilus blocki against Anopheles stephensi for malaria control in Goa, India. Journal of the American Mosquito Control Association 1998;14(4):457‐62. [PubMed] [Google Scholar]
Kumar 2013 {published data only}
- Kumar AN, Murugan K, Shobana K, Abirami D. Isolation of Bacillus sphaericus screening larvicidal, fecundity and longevity effects on malaria vector Anopheles stephensi. Scientific Research and Essays 2013;8(11):425‐31. [Google Scholar]
Kusumawathie 2008 {published data only}
- Kusumawathie PH, Wickremasinghe AR, Karunaweera ND, Wijeyaratne MJ. Costs and effectiveness of application of Poecilia reticulata (guppy) and temephos in anopheline mosquito control in river basins below the major dams of Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(7):705‐11. [DOI] [PubMed] [Google Scholar]
Ladoni 1986 {published data only}
- Ladoni H, Zaim M, Motabar M. The effect of Bacillus thuringiensis on the anopheline mosquitoes in Southern Iran. Journal of Entomological Society of Iran 1986;8(1/2):45‐52. [Google Scholar]
Lee 1990 {published data only}
- Lee HL, Salleh H. Preliminary field trial of permethrin against Anopheles and Culex larvae in Malaysia. Mosquito‐Borne Diseases Bulletin 1990;7(4):127‐30. [Google Scholar]
Liu 2009 {published data only}
- Liu Z, Sun C, Wang W, Shen P. Efficacy of mosquito larvicides against mosquito larvae in field. Chinese Journal of Hygienic Insecticides & Equipments 2009;15(6):439‐41. [Google Scholar]
Lunin 1979 {published data only}
- Lunin SV. Effect of the preparation altozar having juvenile hormone activity on mosquito larvae during treatment of temporary bodies of water in the northern Baikal region USSR. Biologicheskie Nauki (Moscow) (8): 50‐53 1979;0:50. [Google Scholar]
Mahdi 1967 {published data only}
- Mahdi A, Taha AM, Ezz ME. Preliminary small scale field trial for evaluation of aerial spraying of Malathion LV and Abate against A. pharoensis in the Nile delta 1966. Journal of the Egyptian Public Health Association 1967;42(1):8‐13. [PubMed] [Google Scholar]
Maheu‐Giroux 2013b {published data only}
- Maheu‐Giroux M, Castro M. Do malaria vector control measures impact disease‐related behaviors and knowledge? Evidence from a large‐scale larviciding intervention in Tanzania. Tropical Medicine & International Health 2013;18:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maheu‐Giroux 2013c {published data only}
- Maheu‐Giroux M, Castro MC. Do malaria vector control measures impact disease‐related behaviour and knowledge? Evidence from a large‐scale larviciding intervention in Tanzania. Malaria Journal 2013;12(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maheu‐Giroux 2014 {published data only}
- Maheu‐Giroux M, Castro MC. Cost‐effectiveness of larviciding for urban malaria control in Tanzania. Malaria Journal 2014;13(1):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marina 2014 {published data only}
- Marina CF, Bond JG, Muñoz J, Valle J, Novelo‐Gutiérrez R, Williams T. Efficacy and non‐target impact of spinosad, Bti and temephos larvicides for control of Anopheles spp. in an endemic malaria region of southern Mexico. Parasites & Vectors 2014;7(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2017a {published data only}
- McCann RS, Berg H, Diggle PJ, Vugt M, Terlouw DJ, Phiri KS, et al. Assessment of the effect of larval source management and house improvement on malaria transmission when added to standard malaria control strategies in southern Malawi: study protocol for a cluster‐randomised controlled trial. BMC Infectious Diseases 2017;17(1):639. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2017b {published data only}
- McCann RS, Berg H, Vugt M, Terlouw DJ, Phiri KS, Diggle PJ, et al. Community‐led implementation of integrated malaria control in southern Malawi. American Journal of Tropical Medicine and Hygiene 2017;49:421‐2. [Google Scholar]
Meng 1996 {published data only}
- Meng XX. Observations on the mosquito‐killing effects of a mixed fermentative preparation of Bacillus sphaericus and Bacillus thuringiensis. Chinese Journal of Parasitic Disease Control 1996;9(3):204. [Google Scholar]
Minakawa 2007 {published data only}
- Minakawa N, Sonye G, Futami K, Kaneko S, Mushinzimana E, Fillinger U. A large‐scale field trial to evaluate the efficacy of bacillus larvicides for controlling malaria in western Kenya: study design and methods. Tropical Medicine and Health 2007;35(2):41‐5. [Google Scholar]
Mossadegh 1973 {published data only}
- Mossadegh A, Motabar M, Javadian E. Evaluation and investigation of the effect of oil spraying on the interruption of malaria disease. Bulletin de la Société de Pathologie Exotique 1973;66(1):92‐100. [PubMed] [Google Scholar]
Msellemu 2016 {published data only}
- Msellemu D, Namango HI, Mwakalinga VM, Ntamatungiro AJ, Mlacha Y, Mtema ZJ, et al. The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures. Malaria Journal 2016;15(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller 1984 {published data only}
- Müller P. Characteristics of Bacillus thuringiensis var. israelensis and its effect on mosquito larvae (Diptera: Culicidae). Angewandte Parasitologie 1984;25(3):157‐63. [PubMed] [Google Scholar]
Obopile 2018 {published data only}
- Obopile M, Segoea G, Waniwa K, Ntebela DS, Moakofhi K, Motlaleng M, et al. Did microbial larviciding contribute to a reduction in malaria cases in eastern Botswana in 2012–2013?. Public Health Action 2018;8(1):S50‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouedraogo 2017 {published data only}
- Ouedraogo A, Diarra A, Kargougou D, Barry A, Ouédraogo NI, Tiono A, et al. Impact of biolarvicides on the malaria morbidity in schoolchildren living in Ouagadougou, Burkina Faso. American Journal of Tropical Medicine and Hygiene 2017;49:307. [Google Scholar]
Parvez 2003 {published data only}
- Parvez SD, Al Wahaibi SS. Comparison of three larviciding options for malaria vector control. Eastern Mediterranean Health Journal 2003;9(4):627‐36. [PubMed] [Google Scholar]
Perich 1990 {published data only}
- Perich MJ, Boobar LR, Stivers JC, Rivera LA. Field evaluation of four biorational larvicide formulations against Anopheles albimanus in Honduras. Medical and Veterinary Entomology 1990;4(4):393‐6. [DOI] [PubMed] [Google Scholar]
Prabhu 2011 {published data only}
- Prabhu K, Murugan K, Nareshkumar A, Bragadeeswaran S. Larvicidal and pupicidal activity of spinosad against the malarial vector Anopheles stephensi. Asian Pacific Journal of Tropical Medicine 2011;4(8):610‐3. [DOI] [PubMed] [Google Scholar]
Pridantseva 1980 {published data only}
- Pridantseva EA, Popova NA, Myrzin AS, Lukiyanchikov VP. Dimilin for control of larvae of the mosquito Anopheles maculipennis messeae Fall. and the rice midge in the Krasnodar region. Meditsinskaya Parazitologiya i Parazitarnye Bolezni 1980;49(3):53‐7. [PubMed] [Google Scholar]
Ranjbar 2012 {published data only}
- Ranjbar M, Gorgij K, Mohammadi M, Haghdoost AA, Ansari‐Moghaddam A, Nikpour F, et al. Efficacy of applying self‐assessment of larviciding operation, Chabahar, Iran. Malaria Journal 2012;11(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rettich 1973 {published data only}
- Rettich F. Effect of fenitrothion on mosquito larvae under laboratory and field conditions. Conference on disinfection and disinsection, with international participation 1973:59.
Rifaat 1974 {published data only}
- Rifaat MA, Khalil HM, Gad AM, Sadek S. Effect of sublethal concentrations of the insecticides DDT, Abate and Sevin applied to 3rd stage larvae of Anopheles pharoensis on malaria cycle in the adult mosquito. Journal of the Egyptian Public Health Association 1974;49(6):329‐40. [PubMed] [Google Scholar]
Sharma 1983 {published data only}
- Sharma SK, Kalra NL, Bhargava YS. Small scale field trials with Bacillus thuringiensis variety israelensis H‐14 strain against larvae of anopheline and culicine mosquitoes. Journal of Communicable Diseases 1983;15(4):223. [PubMed] [Google Scholar]
Sharma 1989 {published data only}
- Sharma VP, Sharma RC. Community based bioenvironmental control of malaria in Kheda District, Gujarat, India. Journal of the American Mosquito Control Association 1989;5(4):514‐21. [PubMed] [Google Scholar]
Sharma 2003 {published data only}
- Sharma SN, Shukla RP, Mittal PK, Adak T, Kumar A. Efficacy of a new formulation of Bacillus thuringiensis var israelensis (Bti) in laboratory and field conditions of Kumaun foothills of Uttaranchal, India. Journal of Communicable Diseases 2003;35(4):290‐9. [PubMed] [Google Scholar]
Shililu 2003 {published data only}
- Shililu JI, Tewolde GM, Brantly E, Githure JI, Mbogo CM, Beier JC, et al. Efficacy of Bacillus thuringiensis israelensis, Bacillus sphaericus and temephos for managing Anopheles larvae in Eritrea. Journal of the American Mosquito Control Association 2003;19(3):251‐8. [PubMed] [Google Scholar]
Shimada 2007 {published data only}
- Shimada M, Ichinose I, Kaneko S, Minakawa N. Profile of the Nagasaki University Kenya Research Station and Activities. Tropical Medicine and Health 2007;35:29‐31. [Google Scholar]
Skovmand 1997 {published data only}
- Skovmand O, Bauduin S. Efficacy of a granular formulation of Bacillus sphaericus against Culex quinquefasciatus and Anopheles gambiae in West African countries. Journal of Vector Ecology 1997;22(1):43‐51. [PubMed] [Google Scholar]
Skovmand 1999 {published data only}
- Skovmand O, Sanogo E. Experimental formulations of Bacillus sphaericus and B. thuringiensis israelensis against Culex quinquefasciatus and Anopheles gambiae (Diptera: Culicidae) in Burkina Faso. Journal of Medical Entomology 1999;36(1):62‐7. [DOI] [PubMed] [Google Scholar]
Skovmand 2011 {published data only}
- Skovmand O, Ouedraogo TD, Sanogo E, Samuelsen H, Toé LP, Bosselmann R, et al. Cost of integrated vector control with improved sanitation and road infrastructure coupled with the use of slow‐release Bacillus sphaericus granules in a tropical urban setting. Journal of Medical Entomology 2011;48(4):813‐21. [DOI] [PubMed] [Google Scholar]
Some 1994 {published data only}
- Some ES. Effects and control of highland malaria epidemic in Uasin Gishu District, Kenya. East African Medical Journal 1994;71(1):2‐8. [PubMed] [Google Scholar]
Song 2013 {published data only}
- Song GZ, Ma MH, Qu J, Liu DH, Cao GS, Yuan QD, et al. A study on the technical measures and effect evaluation for the mosquito sustainable management in the demonstration area of Dongying city of Shandong Province. Chinese Journal of Hygienic Insecticides & Equipments 2013;19(6):498‐501. [Google Scholar]
Srivastava 1996 {published data only}
- Srivastava R, Tilak VW, Mukherjee S, Yadav JD. Field trial of Bacillus thuringiensis var israelensis pellet formulation in the control of mosquitoes. Medical Journal Armed Forces India 1996;52(4):233‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tâcu 1977 {published data only}
- Tâcu V, Giurcă I, Petrescu S. The larvicide effect of some local and foreign chemical products (insecticides and detergents) on the Anopheles labranchiae atroparvus and Culex pipiens autogenicus. Archives Roumaines de Pathologie Experimentales et de Microbiologie 1977;36(2):181. [PubMed] [Google Scholar]
Tchicaya 2009 {published data only}
- Tchicaya ES, Koudou BG, Keiser J, Adja AM, Cissé G, Tanner M, et al. Effect of repeated application of microbial larvicides on malaria transmission in central Côte d'Ivoire. Journal of the American Mosquito Control Association 2009;25(3):382‐5. [DOI] [PubMed] [Google Scholar]
Tchicaya 2010 {published data only}
- Tchicaya E, Koudou BG, Utzinger J. Effect of repeated application of microbial larvicides on malaria transmission in central Côte d'Ivoire. Malaria Journal 2010;9(S2):68. [DOI] [PubMed] [Google Scholar]
Teng 2005 {published data only}
- Teng H, Lu L, Wu Y, Fang J. Evaluation of various control agents against mosquito larvae in rice paddies in Taiwan. Journal of Vector Ecology 2005;30(1):126. [PubMed] [Google Scholar]
Usenbaev 2006 {published data only}
- Usenbaev NT, Ezhov MN, Zvantsov AB, Annarbaev A, Zhoroev AA, Almerekov K. An outbreak of Plasmodium vivax malaria in Kyrghyzstan. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2006;1:17‐20. [PubMed] [Google Scholar]
Vasuki 1992 {published data only}
- Vasuki V, Rajavel AR. Influence of short time exposure to an insect growth regulator, hexaflumuron, on mortality and adult emergence of vector mosquitoes. Memorias do Instituto Oswaldo Cruz 1992;87(2):275‐83. [DOI] [PubMed] [Google Scholar]
Wang 1983 {published data only}
- Wang MX. Experimental observation of urea derivatives for their disinfection effect on larva of mosquitoes. Zhonghua Liu Xing Bing Xue za Zhi 1983;4(6):354‐8. [PubMed] [Google Scholar]
Xu 1980 {published data only}
- Xu QF. Laboratory and field evaluation of the mosquito larvicidal effect of Bacillus thuringiensis var. israelensis (author's translation). Zhonghua Yu Fang Yi Xue za Zhi [Chinese Journal of Preventive Medicine] 1980;14(4):193‐6. [PubMed] [Google Scholar]
Xu 1983 {published data only}
- Xu QF. Studies on the prolongation of mosquito larvicidal effect of Bacillus thuringiensis var israelensis. Zhonghua Yu Fang Yi Xue za Zhi [Chinese Journal of Preventive Medicine] 1983;17(6):333‐5. [PubMed] [Google Scholar]
Xu 2004 {published data only}
- Xu Y, Liang T, He S. Study on the effect of abate granules against the mosquito larvae in rivers in Urban. Chinese Journal of Vector Biology and Control 2004;15(4):299‐300. [Google Scholar]
Yapabandara 2002 {published data only}
- Yapabandara AM, Curtis CF. Laboratory and field comparisons of pyriproxyfen, polystyrene beads and other larvicidal methods against malaria vectors in Sri Lanka. Acta Tropica 2002;81(3):211‐23. [DOI] [PubMed] [Google Scholar]
Yapabandara 2004 {published data only}
- Yapabandara AM, Curtis CF. Control of vectors and incidence of malaria in an irrigated settlement scheme in Sri Lanka when using the insect growth regulator pyriproxyfen. Journal of the American Mosquito Control Association 2004;20(4):395‐400. [PubMed] [Google Scholar]
Yapabandara 2005 {published data only}
- Yapabandara M. Review of the efficacy and persistence of pyriproxyfen 0.5% granules on Anopheles sp and Aedes sp breeding sites in Sri Lanka.. Proceedings of the Fifth International Conference on Urban Pests. Malaysia, 2005 Jul.
Zhou 2010 {published data only}
- Zhou G, Liu Q, Jiang J, Liu X, Li H, Zhang Y, et al. Effects of malaria vector mosquito control approaches in Yongcheng city, Henan province, 2008. Zhongguo Meijie Shengwuxue ji Kongzhi Zazhi [Chinese Journal of Vector Biology and Control] 2010;21(4):346‐9. [Google Scholar]
Zhou 2016 {published data only}
- Zhou G, Wiseman V, Atieli HE, Lee MC, Githeko AK, Yan G. The impact of long‐lasting microbial larvicides in reducing malaria transmission and clinical malaria incidence: study protocol for a cluster randomized controlled trial. Trials 2016;17(1):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zohdy 1982 {published data only}
- Zohdy NZ, Matter MM. Effect of Bacillus thuringiensis var. israelensis on some Egyptian mosquito larvae. Journal of the Egyptian Society of Parasitology 1982;12(2):349‐57. [PubMed] [Google Scholar]
References to studies awaiting assessment
Fuseini 2017 {published data only}
- Fuseini G, Phiri WP, Nlang JA, Efiri PB, Akum A, Smith J, et al. Effectiveness of community‐based larviciding program on malaria vector abundance on Bioko Island, Equatorial Guinea. American Journal of Tropical Medicine and Hygiene 2017;49:537. [Google Scholar]
Javadian 1974 {published data only}
- Javadian E, Acheson MA. An evaluation of the effect of larviciding operations in rural areas near Abadan, Iran. Iranian Journal of Public Health 1974;3(1):54‐61. [Google Scholar]
Zhou 2013 {published data only}
- Zhou G, Afrane YA, Dixit A, Atieli HE, Lee MC, Wanjala CL, et al. Modest additive effects of integrated vector control measures on malaria prevalence and transmission in western Kenya. Malaria Journal 2013;12(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;54(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bayoh 2010
- Bayoh NM, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide‐treated bed nets in western Nyanza Province, Kenya. Malaria Journal 2010;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 1997
- Becker N. Microbial control of mosquitoes: management of the upper Rhine mosquito population as a model programme. Parasitology Today 1997;13:485‐7. [DOI] [PubMed] [Google Scholar]
Bhatt 2015
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015;526(7572):207‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 1968
- Black RH. Manual of epidemiology and epidemiological services in malaria programmes, 1968. apps.who.int/iris/bitstream/10665/40401/1/924154015X.pdf (accessed 11 July 2017).
Bogh 2003
- Bogh C, Clarke S, Jawara M, Thomas CJ, Lindsay SW. Localized breeding of the Anopheles gambiae complex (Diptera: Culicidae) along the River Gambia, West Africa. Bulletin of Entomological Research 2003;93(4):279‐87. [DOI] [PubMed] [Google Scholar]
Brady 2016
- Brady OJ, Godfray HC, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(2):107‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruce‐Chwatt 1985
- Bruce‐Chwatt LJ. Essential Malariology. 2nd Edition. London (UK): William Heineman Medical Books Ltd, 1985. [Google Scholar]
Bøgh 2007
- Bøgh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, et al. High spatial resolution mapping of malaria transmission risk in the Gambia, west Africa, using LANDSAT TM satellite imagery. American Journal of Tropical Medicine and Hygiene 2007;76(5):875‐81. [PubMed] [Google Scholar]
Chima 2008
- Chima RI, Goodman CA, Mills A. The economic impact of malaria in Africa: a critical review of the evidence. Health Policy 2008;63(1):17‐36. [DOI] [PubMed] [Google Scholar]
Durnez 2013
- Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S editor(s). Anopheles Mosquitoes – New Insights into Malaria Vectors. Rijeka (Croatia): InTech, 2013. [Google Scholar]
Floore 2006
- Floore T. Mosquito Larval Control Practices: Past and Present. Journal of the American Mosquito Control Association 2006;22:527‐33. [DOI] [PubMed] [Google Scholar]
Gatton 2013
- Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution 2013;67(4):1218‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustavsen 2011
- Gustavsen K, Hopkins A, Sauerbrey M. Onchocerciasis in the Americas: from arrival to (near) elimination. Parasites & Vectors 2011;4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Killeen 2014
- Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malaria Journal 2014;13:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lwetoijera 2014
- Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malaria Journal 2014;13:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macdonald 1957
- Macdonald G. The Epidemiology and Control of Malaria. London (UK): Oxford University Press, 1957. [Google Scholar]
Majambere 2008
- Majambere S, Fillinger U, Sayer DR, Green C, Lindsay SW. Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. American Journal of Tropical Medicine and Hygiene 2008;79(1):19‐27. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Najera 2001
- Najera JA. Malaria control: achievements, problems and strategies. Parassitologia 2001;43(1‐2):1‐89. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2016
- Richardson M, Garner P, Donegan S. Cluster randomised trials in Cochrane reviews: evaluation of methodological and reporting practice. PLOS One 2016;11(3):e0151818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roca‐Feltrer 2009
- Roca‐Feltrer A, Schellenberg JR, Smith L, Carneiro I. A simple method for defining malaria seasonality. Malaria Journal 2009;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 2010
- Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer‐lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre‐existing high coverage of untreated nets. Malaria Journal 2010;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachs 2002
- Sachs JD. Macroeconomics and health: investing in health for economic development. Revista Panamericana de Salud Pública 2002;12(2):143‐4. [Google Scholar]
Shousha 1948
- Shousha AT. The eradication of Anopheles gambiae from Upper Egypt 1942–1945. Bulletin of the World Health Organization 1948;1(2):309‐42. [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith DL, Perkins TA, Tusting LS, Scott TW, Lindsay SW. Mosquito population regulation and larval source management in heterogeneous environments. PLOS One 2013;8(8):e71247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soper 1943
- Soper FL, Wilson DB. Anopheles gambiae in Brazil: 1930 – 1940. New York (NY): Rockefeller Foundation, 1943:1‐262. [Google Scholar]
Sékétéli 2002
- Sékétéli A, Adeoye G, Eyamba A, Nnoruka E, Drameh P, Amazigo UV, et al. The achievements and challenges of the African Programme for Onchocerciasis Control (APOC). Annals of Tropical Medicine & Parasitology 2002;96(Suppl 1):S15‐28. [DOI] [PubMed] [Google Scholar]
Tusting 2013
- Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walshe 2017
- Walshe DP, Garner P, Abdel‐Hameed Adeel AA, Pyke GH, Burkot T. Larvivorous fish for preventing malaria transmission. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD008090.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. WHO position statement on integrated vector management, 2008. apps.who.int/iris/bitstream/10665/69745/1/WHO_HTM_NTD_VEM_2008.2_eng.pdf (accessed 11 July 2017).
WHO 2011
- World Health Organization Vitamin and Mineral Nutrition Information System. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity, 2011. apps.who.int/iris/bitstream/10665/85839/3/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1. Geneva: World Health Organization, (accessed 11 July 2017).
WHO 2012a
- World Health Organization. Global plan for insecticide resistance management in malaria vectors, 2012. apps.who.int/iris/bitstream/10665/44846/1/9789241564472_eng.pdf?ua=1. Geneva: World Health Organization, (accessed 11 July 2017).
WHO 2012b
- World Health Organization. Interim Position Statement The role of larviciding for malaria control in sub‐Saharan Africa, April 2012. www.who.int/malaria/publications/atoz/interim_position_statement_larviciding_sub_saharan_africa.pdf?ua=1 (accessed prior to 5 June 2019).
WHO 2013
- World Health Organization. Larval source management. A supplementary measure for malaria vector control. An operational manual. apps.who.int/iris/bitstream/10665/85379/1/9789241505604_eng.pdf?ua=1. Geneva, Switzerland: WHO, (accessed 11 July 2017).
WHO 2015a
- World Health Organization. Global Technical Strategy for Malaria 2016‐2030. Geneva: World Health Organization, 2015. [Google Scholar]
WHO 2015b
- World Health Organization. Guidelines for the Treatment of Malaria. 3rd Edition. Geneva: World Health Organization, 2015. [Google Scholar]
WHO 2017
- World Health Organization. World Malaria Report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
WHO 2018
- World Health Organization. World Malaria Report 2018. Geneva: World Health Organization, 2018. [Google Scholar]
Wilson 2015
- Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, et al. Evidence‐based vector control? Improving the quality of vector control trials. Trends in Parasitology 2015;31:380‐90. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Choi 2017
- Choi L, Wilson A. Larviciding to control malaria. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012736] [DOI] [Google Scholar]


