Abstract
Background
Gliomas are brain tumours arising from glial cells with an annual incidence of 4 to 11 people per 100,000. In this review we focus on gliomas with low aggressive potential in the short term, i.e. low‐grade gliomas. Most people with low‐grade gliomas are treated with surgery and may receive radiotherapy thereafter. However, there is concern about the possible long‐term effects of radiotherapy, especially on neurocognitive functioning.
Objectives
To evaluate the long‐term neurocognitive and other side effects of radiotherapy (with or without chemotherapy) compared with no radiotherapy, or different types of radiotherapy, among people with glioma (where 'long‐term' is defined as at least two years after diagnosis); and to write a brief economic commentary.
Search methods
We searched the following databases on 16 February 2018 and updated the search on 14 November 2018: Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 11) in the Cochrane Library; MEDLINE via Ovid; and Embase via Ovid. We also searched clinical trial registries and relevant conference proceedings from 2014 to 2018 to identify ongoing and unpublished studies.
Selection criteria
Randomised and non‐randomised trials, and controlled before‐and‐after studies (CBAS). Participants were aged 16 years and older with cerebral glioma other than glioblastoma. We included studies where patients in at least one treatment arm received radiotherapy, with or without chemotherapy, and where neurocognitive outcomes were assessed two or more years after treatment.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias. We assessed the certainty of findings using the GRADE approach.
Main results
The review includes nine studies: seven studies were of low‐grade glioma and two were of grade 3 glioma. Altogether 2406 participants were involved but there was high sample attrition and outcome data were available for a minority of people at final study assessments. In seven of the nine studies, participants were recruited to randomised controlled trials (RCTs) in which longer‐term follow‐up was undertaken in a subset of people that had survived without disease progression. There was moderate to high risk of bias in studies due to lack of blinding and high attrition, and in two observational studies there was high risk of selection bias. Paucity of data and risk of bias meant that evidence was of low to very low certainty. We were unable to combine results in meta‐analysis due to diversity in interventions and outcomes.
The studies examined the following five comparisons.
Radiotherapy versus no adjuvant treatment Two observational studies contributed data. At the 12‐year follow‐up in one study, the risk of cognitive impairment (defined as cognitive disability deficits in at least five of 18 neuropsychological tests) was greater in the radiotherapy group (risk ratio (RR) 1.95, 95% confidence interval (CI) 1.02 to 3.71; n = 65); at five to six years the difference between groups did not reach statistical significance (RR 1.38, 95% CI 0.92 to 2.06; n = 195). In the other study, one subject in the radiotherapy group had cognitive impairment (defined as significant deterioration in eight of 12 neuropsychological tests) at two years compared with none in the control group (very low certainty evidence).
With regard to neurocognitive scores, in one study the radiotherapy group was reported to have had significantly worse mean scores on some tests compared with no radiotherapy; however, the raw data were only given for significant findings. In the second study, there were no clear differences in any of the various cognitive outcomes at two years (n = 31) and four years (n = 15) (very low certainty evidence).
Radiotherapy versus chemotherapy One RCT contributed data on cognitive impairment at up to three years with no clear difference between arms (RR 1.43, 95% CI 0.36 to 5.70, n = 117) (low‐certainty evidence).
High‐dose radiotherapy versus low‐dose radiotherapy Only one of two studies reporting this comparison contributed data, and at two and five years there were no clear differences between high‐ and low‐dose radiotherapy arms (very low certainty evidence).
Conventional radiotherapy versus stereotactic conformal radiotherapy One study involving younger people contributed limited data from the subgroup aged 16 to 25 years. The numbers of participants with neurocognitive impairment at five years after treatment were two out of 12 in the conventional arm versus none out of 11 in the stereotactic conformal radiotherapy arm (RR 4.62, 95% CI 0.25 to 86.72; n = 23; low‐certainty evidence).
Chemoradiotherapy versus radiotherapy Two RCTs tested for cognitive impairment. One defined cognitive impairment as a decline of more than 3 points in MMSE score compared with baseline and reported data from 2‐year (110 participants), 3‐year (91 participants), and 5‐year (57 participants) follow‐up with no clear difference between the two arms at any time point. A second study did not report raw data but measured MMSE scores over five years in 126 participants at two years, 110 at three years, 69 at four years and 53 at five years. Authors concluded that there was no difference in MMSE scores between the two study arms (P = 0.4752) (low‐certainty evidence).
Two RCTs reported quality of life (QoL) outcomes for this comparison. One reported no differences in Brain‐QoL scores between study arms over a 5‐year follow‐up period (P = 0.2767; no raw data were given and denominators were not stated). The other trial reported that the long‐term results of health‐related QoL showed no difference between the arms but did not give the raw data for overall HRQoL scores (low‐certainty evidence).
We found no comparative data on endocrine dysfunction; we planned to develop a brief economic commentary but found no relevant economic studies for inclusion.
Authors' conclusions
Radiotherapy for gliomas with a good prognosis may increase the risk of neurocognitive side effects in the long term; however the magnitude of the risk is uncertain. Evidence on long‐term neurocognitive side effects associated with chemoradiotherapy is also uncertain. Neurocognitive assessment should be an integral part of long‐term follow‐up in trials involving radiotherapy for lower‐grade gliomas to improve the certainty of evidence regarding long‐term neurocognitive effects. Such trials should also assess other potential long‐term effects, including endocrine dysfunction, and evaluate costs and cost effectiveness.
Keywords: Humans, Antineoplastic Agents, Antineoplastic Agents/adverse effects, Antineoplastic Agents/therapeutic use, Cognition Disorders, Cognition Disorders/chemically induced, Cognition Disorders/epidemiology, Glioma, Glioma/therapy, Radiation Injuries, Radiation Injuries/complications, Radiosurgery, Radiotherapy, Radiotherapy/adverse effects, Radiotherapy/methods, Randomized Controlled Trials as Topic
Long‐term effects of radiotherapy for glioma treatment on brain functioning
Background Gliomas are brain tumours that can be very aggressive and result in death within months; however, people with less aggressive gliomas (low‐grade gliomas) can survive for a number of years. Most people are treated with surgery and may also receive radiotherapy with or without chemotherapy. However, radiotherapy can damage healthy brain tissue, and we do not know enough about the possible long‐term effects of radiotherapy on brain functioning, such as memory, communication, concentration and speed of thinking (called neurocognition). Progression of the tumour can also cause deterioration in brain functioning. In this review we looked at the possible long‐term effects of radiotherapy on the brain in adults with less aggressive gliomas who had survived for at least two years after receiving treatment.
Methods and results We searched for relevant research studies up to 14 November 2018. We only included studies with a control group (i.e. studies that included groups of people that had or had not received radiotherapy or had received different types or doses of radiotherapy). The review includes nine research studies that collected information on long‐term neurocognitive or quality of life outcomes, mostly among people with low‐grade gliomas. Altogether 2406 participants were involved in these studies. The studies looked at five different comparisons including radiotherapy versus no radiotherapy, radiotherapy versus chemotherapy, high‐ versus low‐dose radiotherapy, different types of radiotherapy, and radiotherapy versus chemoradiotherapy. Some evidence suggested that radiotherapy might increase the risk of cognitive impairment compared with no radiotherapy after surgery; however, this and evidence for the other comparisons was not convincing. This was partly because many of the people were not followed up, either because they had died or their disease had progressed, and so the resulting evidence was weak.
No studies compared effects of radiotherapy on relevant hormone functioning; we planned to develop a brief economic commentary to summarise information on whether the interventions represented a good use of health services but found no relevant studies.
Conclusions The risk of long‐term deterioration in brain functioning associated with radiotherapy for the treatment of less aggressive gliomas remains uncertain. Further research on glioma treatment options should assess potential long‐term cognitive and hormonal side effects, costs and value for money.
Summary of findings
Summary of findings for the main comparison.
| Long‐term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma | ||||
|
Patient or population: people with glioma surviving at least two years Settings: tertiary care | ||||
| Comparison and Outcomes | Relative effect (95% CI) | No of participants andstudies | Quality of the evidence (GRADE) | Comments |
|
Intervention: radiotherapy Comparison: no adjuvant treatment Outcome: neurocognitive impairment at 5‐ to 6‐year follow‐up |
RR 1.38 (0.92 to 2.06) | 1 study with data for 195 participants | ⊕⊝⊝⊝ very low1,2 | Outcome defined as cognitive disability deficits in at least 5 of 18 neuropsychological tests |
|
Intervention: radiotherapy Comparison: no adjuvant treatment Outcome: neurocognitive impairment at 12 year follow‐up |
RR 1.95 (1.02 to 3.71) | 1 study with data for 65 participants | ⊕⊝⊝⊝ very low1,3 | Outcome defined as cognitive disability deficits in at least 5 of 18 neuropsychological tests |
|
Intervention: radiotherapy Comparison: no adjuvant treatment Outcome: neurocognitive impairment at 2 year follow‐up |
RR 2.50 (0.11 to 56.98) | 1 study with data for 31 participants | ⊕⊝⊝⊝ very low1,2,3 | There was a single event for this outcome in this observational study. The outcome was defined as a significant deterioration (≥ 1 SD) in 8 out of 12 neuropsychological tests |
|
Intervention: radiotherapy Comparison: chemotherapy Outcome: neurocognitive impairment at 3 year follow‐up |
RR 1.43 (0.36 to 5.70) | 1 study with data for 117 participants | ⊕⊕⊝⊝ low2,3 | Outcome defined as a MMSE score of 26 or less |
|
Intervention: high‐dose radiotherapy Comparison: low‐dose radiotherapy Outcome: neurocognitive impairment at 2 years after treatment |
RR 0.53 (0.06, 4.85) | 1 study with data for 65 participants | ⊕⊝⊝⊝ very low2,3,4 | Outcome defined as decrease in MMSE score from baseline (more than 3 points).There was serious and uneven attrition between groups in this study. |
|
Intervention: high‐dose radiotherapy Comparison: low‐dose radiotherapy Outcome: neurocognitive impairment at 5 years after treatment |
RR 0.16 (0.01 to3.20) | 1 study with data for 38 participants | ⊕⊝⊝⊝ very low2,3,4 | Outcome defined as decrease in MMSE score from baseline (more than 3 points). There was serious and uneven attrition between groups in this study. |
|
Intervention: chemoradiotherapy Comparison: radiotherapy Outcome: neurocognitive impairment at 3 years after treatment |
RR 0.37 (0.02 to 8.88) | 1 study with data for 91 participants | ⊕⊕⊝⊝ low2,3 | Outcome defined as a decline (of more than 3 points in MMSE score) in cognitive state compared with baseline |
|
Intervention: stereotactic conformal radiotherapy Comparison: radiotherapy Outcome: neurocognitive impairment at 5 years after treatment |
RR 4.62 (95% CI 0.25 to 86.72) | 1 study with data for 23 participants | ⊕⊕⊝⊝ low2,3 | Outcome defined as a decline (of more than 3 points in MMSE score) in cognitive state compared with baseline. There was serious sample attrition at 5 years. |
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
Abbreviations: SD = standard deviation; MMSE = Mini Mental State Exam
1. Single study contributing data had very serious study design limitations (−2) 2. Uncertain findings; wide 95% CI crossing the line of no effect (−1) 3. Effect estimate based on small sample size (−1) 4. Single study contributing data had study design limitations (−1)
Background
Description of the condition
Primary brain and other central nervous system (CNS) tumours are less common than many other cancers, accounting for around 1.9% of new cancer diagnoses annually; however, they are associated with a relatively higher proportion of cancer deaths annually (2.3%), amounting to approximately 189,382 deaths worldwide in 2012 (GLOBOCAN 2012). Gliomas are brain tumours that arise from glial cells, usually oligodendrocytes and astrocytes. They occur at an annual incidence of four to 11 people per 100,000 and are more frequent in high‐income, industrialised countries (Ohgaki 2009). Gliomas are graded 1 to 4 by the World Health Organization (WHO) according to their aggressive potential in the short term. The 2007 WHO classification system (Louis 2007), used in completed clinical trials since 2007, graded gliomas based on histological characteristics only. However, in the 2016 WHO classification system, to be used in future trials, grading depends on both histological and molecular features, e.g. isocitrate dehydrogenase (IDH) status, chromosome 1p 19q, and other genetic parameters (Louis 2016). Using the 2007 WHO classification, gliomas graded 1 and 2 have low aggressive potential and are referred to as low‐grade gliomas; these include pilocytic astrocytomas (grade 1), diffuse astrocytomas, oligodendrogliomas and mixed oligoastrocytomas (grade 2). High‐grade gliomas have faster local growth rates and include anaplastic astrocytomas, anaplastic oligodendrogliomas (grade 3) and glioblastomas (grade 4). Grades correspond with prognosis: grade 1 has a good prognosis and can often be cured with surgery alone, whereas grade 4 has a poor prognosis, and can be rapidly fatal (Louis 2007). Thus, tumour grade is a key factor in deciding how to treat gliomas, particularly the need for additional treatment in the form of radiotherapy or chemotherapy or both (chemoradiotherapy) after surgery.
Description of the intervention
Most people with glioma first undergo surgery to resect (cut out) or biopsy the tumour. The latter is usually performed when resection is not possible, either due to the diffuse, infiltrative nature of the tumour, or its location near important structures. Additional radiotherapy targeting the tumour area (focal radiotherapy) is usually given immediately after surgery for high‐grade gliomas, whereas for grade 2 gliomas it can either be given immediately, or postponed if the tumour has been resected until the development of new symptoms or tumour progression (Sarmiento 2015). Fifty per cent of people with grade 2 and grade 3 gliomas survive at least seven years and four and a half years, respectively, after treatment (Buckner 2016; Cairncross 2013). However, for certain grade 2 and 3 gliomas with particular molecular features, median survival can be extended by a further seven years by the addition of adjuvant chemotherapy to radiotherapy (Buckner 2016; Cairncross 2013). Among people with grade 4 gliomas that are treated with chemoradiotherapy, only approximately 25% are alive two years after diagnosis (Stupp 2005).
Potential side effects
The treatment of glioma can be complicated by long‐term side effects that present months or years after treatment. This is due to the exposure of healthy brain tissue to radiation, which adversely affects brain plasticity (the ability of the brain to modify its connections and rewire) and repair processes (Dhermain 2016). As the frequency of side effects increases with time, these tend to be problematic for people with less aggressive tumours who survive long term, and are especially common among survivors of childhood brain tumours (Grill 1999; Seaver 1994; Spiegler 2004; Williams 2018). Certain parts of the brain such as the hippocampus, fornix and corpus callosum are more sensitive to irradiation (Connor 2017; Gondi 2012; Gondi 2018; Peiffer 2013); impairment of memory, communication, concentration and problem‐solving (neurocognition) can result. Studies among adults with low‐grade glioma show that the risk of neurocognitive impairment is increased when radiotherapy is administered to the whole brain (Gregor 1996; Surma‐aho 2001), but is less likely when radiotherapy is administered to the tumour area only (Brown 2003; Laack 2005; Taphoorn 1994; Vigliani 1996). Factors that are important to the risk of long‐term side effects in glioma treatment are the site of the tumour, the volume of brain tissue irradiated, the radiotherapy fraction size and the total radiotherapy dose. The use of chemotherapy with radiotherapy might plausibly add to the risk.
Endocrine (hormonal) dysfunction affecting adrenal (stress response) hormones, gonadal (sex) hormones, and thyroid hormones can also occur due to radiotherapy damage to the hypothalamic‐pituitary axis (Taphoorn 1995), the system that communicates with hormone‐producing glands in the body. Pituitary dysfunction is commonly diagnosed amongst children who have undergone radiotherapy for glioma, which in children frequently leads to hypothyroidism, growth hormone deficiency, and precocious puberty (Terashima 2013). In adults, recent studies suggest that pituitary dysfunction following radiotherapy for brain tumours is very underdiagnosed and that regular endocrine surveillance should be performed above a dose threshold of 30 Gy (Kyriakakis 2016; Kyriakakis 2019). In addition, fatigue, disturbed sleep and depression are also commonly reported side effects of treatment (Armstrong 2017). Such side effects can seriously interfere with a person's ability to work, maintain relationships, perform daily activities, and enjoy life (Armstrong 2016).
Why it is important to do this review
Long‐term cognitive side effects of radiotherapy were identified among the top 10 priority research questions in neuro‐oncology by the James Lind Alliance and the National Cancer Research Institute (NCRI) (JLA 2015). This is because uncertainty exists about the long‐term side effects of radiotherapy for brain tumours, especially among people with a good prognosis. Evaluating the long‐term consequences of treatment is important to understand what the real impact of this condition and its treatment are for individuals and health systems. We undertook this review to help inform clinical decision making in the context of a trend towards more aggressive early treatments for low‐grade gliomas.
The costs of care can be 'direct costs' due to health care resources used to treat the condition, or 'indirect costs' that are borne by the patient and their families. Radiotherapy is one of the highest direct costs of glioma management (Blomqvist 2000; Raizer 2015). The cost of malignant gliomas has been estimated to range between USD 50,600 and USD 92,700 (2015) per patient per year (Raizer 2015). It is, therefore, also important to understand the long‐term consequences of different glioma management strategies so that the costs and consequences of such strategies can be fully evaluated.
Objectives
To evaluate the long‐term neurocognitive and other side effects of radiotherapy (with or without chemotherapy) compared with no radiotherapy, or different types of radiotherapy, among people with glioma (where 'long‐term' is defined as at least two years after diagnosis); and to write a brief economic commentary.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and non‐randomised trials, and controlled before‐and‐after studies (CBAS). We considered non‐randomised trials and CBAS for inclusion if there were no primary outcome data from randomised trials for a particular treatment comparison. We excluded cross‐over designs, case‐control studies, and studies that did not have a control group.
Types of participants
People aged 16 years of age and older with a histopathologically confirmed diagnosis of cerebral glioma who are alive at least two years after diagnosis.
In this review, as we considered late effects to be those that are present at two years or more after diagnosis among people who have a good long‐term prognosis, rather than in those that have a short‐term prognosis, we excluded studies only involving people with glioblastoma. In studies with mixed high‐grade glioma participants (grade 3 and grade 4 gliomas) we planned to extract data for the participants with grade 3 glioma only where possible.
Types of interventions
Treatment interventions after surgery (biopsy or resection of the tumour) could include the following.
-
Radiotherapy compared with no radiotherapy, which includes the following comparison subgroups.
Radiotherapy versus no adjuvant treatment.
Chemoradiotherapy versus no adjuvant treatment.
Radiotherapy versus chemotherapy.
Chemoradiotherapy versus chemotherapy.
High‐dose radiotherapy versus low‐dose radiotherapy.
Conventional radiotherapy versus conformal radiotherapy
Chemoradiotherapy versus radiotherapy.
Types of outcome measures
Studies had to report at least one of the primary outcomes in both the intervention and control groups at least two years after receiving the intervention.
Primary outcomes
Cognitive impairment (objective or subjective), as measured by an overall cognitive function score, a change over time score, or as a categorical outcome. This includes evaluation of cognitive impairment as individual cognitive function domains, e.g. verbal fluency, processing speed, memory, attention, and executive functioning, using a standardised measurement tool, e.g. Mini Mental State Exam (MMSE), Cognitive Failures Questionnaire (CFQ).
Quality of life (QoL), as measured using a standardised questionnaire, e.g. the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 or QLQ‐BN20 (specific for brain cancer), or the Functional Assessment of Cancer Therapy scale (FACT‐G (general) or FACT‐Br (specific for brain cancer)).
Secondary outcomes
Functional impairment or disability, as measured by an overall ability score, or as a change of ability over time score, or both, using a standardised measurement tool, e.g. Karnofsky Performance Status Scale, Neurological Functions Score; or as a categorical outcome, as defined by investigators.
Endocrine dysfunction, as determined by use of hormonal treatment, or as defined by study investigators, or both.
Depression, as measured by a standardised scale, e.g. Hospital Anxiety and Depression Scale (HADS).
Anxiety, as measured by a standardised scale, e.g. HADS.
Fatigue, according to Common Terminology Criteria for Adverse Events (CTCAE), or as defined by investigators.
Sleep disturbances, as defined by investigators.
Imaging evidence of physical deficit, e.g. general brain atrophy, white matter changes, radionecrosis, stroke.
Social outcomes (e.g. carer strain, relationship status, employment status).
Second cancers.
We present evidence regarding cost of care as a brief economic commentary.
Search methods for identification of studies
Electronic searches
We searched the following databases on the 16 February 2018 and updated the search on 14 November 2018.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 11), in the Cochrane Library;
MEDLINE via Ovid (1946 to October week 5 2018);
Embase via Ovid (1980 to 2018 week 46).
Please refer to Appendix 1 for CENTRAL, MEDLINE and Embase search strategies.
We did not apply language restrictions to any of the searches.
Searching other resources
We searched the following for ongoing trials.
ClinicalTrials.gov;
International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch).
Where we identified through these searches ongoing trials that had not been published, we approached the principal investigators to ask for an update on the trial status and relevant data. We used the related articles feature of PubMed and handsearched the reference lists of included studies to identify newly published articles and additional studies of relevance. We also handsearched conference proceedings from 2014 to 2018 (5 years) of conferences of the British Neuro‐Oncology Society, the Society for Neuro‐Oncology, the European Association of Neuro‐Oncology and the World Federation of Neuro‐Oncology Societies for relevant ongoing or unpublished studies.
Data collection and analysis
Selection of studies
The Information Specialist at the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group (GNOC) downloaded all titles and abstracts retrieved by electronic searching to Endnote and removed duplicates and those studies that clearly did not meet the inclusion criteria. Review authors in teams of two (TL and RG; JE and DG) independently screened the remaining records and excluded studies that clearly did not meet the inclusion criteria. We obtained copies of the full texts of potentially eligible references and at least two review authors independently assessed these for eligibility (TL and RG, JE or DG). The two review authors concerned resolved disagreements by discussion and, if necessary, consulted the other review authors. We used Covidence to facilitate this study selection process (Covidence 2018), and document reasons for exclusion in Characteristics of excluded studies.
Data extraction and management
Two review authors (TL, TD, RG, JE or DG) independently extracted the following data from included studies to a pre‐designed data extraction form.
Author contact details
Country
Setting
Dates of participant accrual
Trial registration number/identification
Funding source
Participant inclusion and exclusion criteria
Study design and methodology
-
Study population and baseline characteristics
Number of participants enrolled/analysed
Age
Gender
Tumour grade/type
Type of surgery (biopsy or resection)
Other medication, e.g. anti‐epileptics and anti‐depressants (selective serotonin reuptake inhibitors (SSRIs))
-
Intervention details
Type of intervention
Type of comparator
Duration of follow‐up
Primary outcome/s of the study
-
Review outcomes
For dichotomous outcomes, we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed
For continuous outcomes, we extracted the value and standard deviation of the outcome of interest and the number of participants assessed at the relevant time point in each group. We also extracted change‐from‐baseline score data where reported and noted the type of scale used
We extracted adjusted statistics where reported
Where possible, all data we extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in the groups to which they were assigned
We resolved differences between reviewers by discussion or by appeal to the other review authors when necessary
Risk of study bias (see below)
Assessment of risk of bias in included studies
For randomised trials, we assessed the risk of bias using Cochrane's tool and the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This includes assessment of:
random sequence generation;
allocation concealment;
blinding of participants and healthcare providers;
blinding of outcome assessors;
incomplete outcome data (more than 20% missing data considered high risk);
selective reporting of outcomes;
other possible sources of bias, e.g. lack of a power calculation, baseline differences in group characteristics.
For non‐randomised studies (non‐randomised trials and CBAS), we assessed the risk of bias in accordance with four criteria concerning sample selection comparability of treatment groups, namely:
relevant details of criteria for assignment of people with the condition to treatments;
representative group of people with the condition who received the experimental intervention;
representative group of people with the condition who received the comparison intervention;
baseline differences between groups controlled for, in particular with reference to age, gender, type and grade of glioma and surgical treatment.
At least two review authors (TL and at least one other) assessed risk of bias independently and resolved differences by discussion or by appeal to a third review author. We summarised judgements in 'Risk of bias' tables along with the characteristics of the included studies. We interpreted results in light of the 'Risk of bias' assessment. For more details about the assessment of risk of bias, see Appendix 2.
Measures of treatment effect
For dichotomous outcomes, we calculated the effect size as a risk ratio (RR) with its 95% confidence interval (CI).
For continuous outcomes (e.g. QoL scores) in which different measurement scales had been used, we did not pool data because time points, scales and measurement scales were too dissimilar to produce clinically meaningful estimates of effect.
Unit of analysis issues
At least two review authors (TL, TD) reviewed unit‐of‐analysis issues, as described in Higgins 2011, for each included study. These included reports where there were multiple observations for the same outcome, e.g. repeated measurements with different scales, or outcomes measured at different time points to those stipulated in the review protocol. Because data were sparse, after discussion amongst the authors we agreed to include data from different scales and time points and report the findings narratively.
Dealing with missing data
We did not impute missing data. In the event of missing data, we wrote to study authors to request the data and described in the Characteristics of included studies tables how we obtained any missing data. Where substantial volumes of data were missing, we took this into consideration in our grading of the evidence (see Data synthesis).
Assessment of heterogeneity
We did not pool data and assessed heterogeneity between studies by visual inspection of forest plots, where this was meaningful (Higgins 2003). As no data were pooled, we did not use a formal statistical test of the significance of the heterogeneity (Deeks 2001). Where there was evidence of substantial heterogeneity on visual inspection of the forest plots, we investigated and reported the possible reasons for this.
Assessment of reporting biases
Due to few included studies and limited data, it was not possible to use funnel plots to investigate reporting biases.
Data synthesis
We did not conduct meta‐analyses because data were sparse and comparisons and time points and measurements were too dissimilar for pooled estimates to be clinically meaningful. However, to help visualise the data and facilitate narrative syntheses, we created forest plots for the primary outcomes using Review Manager 5 (RevMan 5) (Review Manager 2014). For future meta‐analyses, we will use the random‐effects model with inverse variance weighting. If any trials contributing to a meta‐analysis have multiple intervention groups, we will divide the 'shared' comparison group into the number of treatment groups and comparisons between each treatment group and treat the split comparison group as independent comparisons. We will perform a meta‐analysis of the results assuming that we find at least two included studies that are sufficiently similar for the findings to be clinically meaningful.
'Summary of findings' table and reporting of results
Based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we prepared Table 1 to present the results of the primary outcomes, namely:
cognitive impairment at ≥ 2 years.
quality of life (QoL) score at ≥ 2 years.
We used the GRADE system to rank the quality of the evidence (Schünemann 2011). Two review authors independently graded the evidence. We resolved differences by discussion and, if necessary, by involving a third review author. Where the evidence was based on single studies, or where there was no evidence on a specific outcome, we included the outcome in the 'Summary of findings' table and graded or explained accordingly. In addition, we provided a rationale for each judgement of assumed risk in the table footnotes. In the absence of a single estimate of effect (when meta‐analysis was not possible), we rated the certainty of the effect using the GRADE approach (Murad 2017). We interpreted the results of the graded evidence based on Cochrane Effective Practice and Organisation of Care guidance (EPOC 2017).
Brief economic commentary
A brief economic commentary was planned to summarise the availability and principal findings of the economic evaluations relevant to this review. This included evaluations alongside trials and model based evaluations. The work was performed in line with current guidelines, including a supplementary search to identify economic studies (Shemilt 2018).
Subgroup analysis and investigation of heterogeneity
For the comparison 'radiotherapy versus no radiotherapy', we subgrouped studies according to the type of control group. However, as we did not pool the data we were unable to use formal tests for subgroup differences to determine whether the effect of interventions differ according to these subgroups.
Sensitivity analysis
In this version of the review, we have not performed sensitivity analysis because data were sparse. In future versions of this review, when more data are available, we plan to perform sensitivity analyses to investigate substantial heterogeneity identified in meta‐analyses of the primary outcomes, and also to estimate the effect after excluding studies at high risk of bias, to investigate how study quality affects the certainty of findings.
Results
Description of studies
Results of the search
Initial database searches conducted on the 16 February 2018 yielded the following results:
CENTRAL Issue 2 2018 ‒ 621 references
Medline: 1946 to February week 2 2018 ‒ 2302 references
Embase: 1980 to 2018 week 07 ‒ 2547 references
After de‐duplication and filtering out clearly irrelevant papers (e.g. studies of other cancers), we screened a total of 3197 references (including 10 references identified using the PubMed related‐articles feature) and short‐listed 57 references for full‐text screening. After full‐text screening, we classified 19 references (related to 9 studies) as included, 37 as excluded, and one as ongoing (Figure 1).
Figure 1.
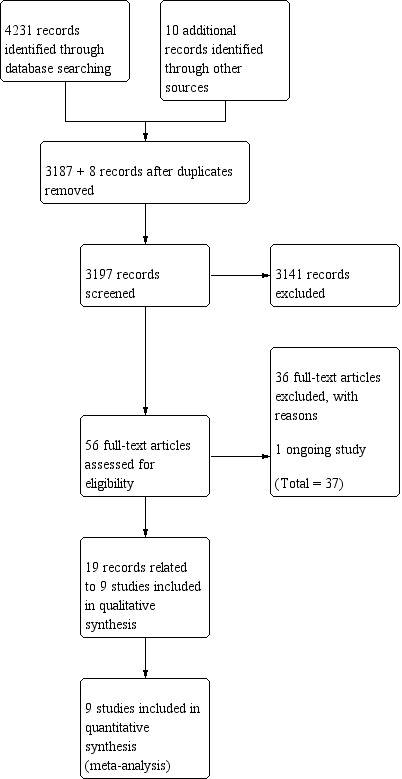
Study flow diagram (date of search 16/02/18).
The top‐up search in November 2018 yielded the following:
CENTRAL Issue 11, 2018 ‒ 95 additional references
Medline: February 2018 to October week 5 2018 ‒ 95 references
Embase: February 2018 to 2018 week 46 ‒ 126 references
We identified one additional study by searching the abstracts from conference proceedings. After de‐duplication, we screened 283 additional records plus the one conference abstract on title and abstract. This led to our assessment of eight full texts, seven of which we excluded and one (the conference abstract) we added to 'Characteristics of ongoing studies' (Figure 2). We identified four other potentially eligible ongoing studies by searching the clinical trials registries (NCT00457210; NCT02544178; NCT03055364; NCT03180502); and we identified one in the initial database search (CATNON 2017). We subsequently identified two related publications and added them to the related previously included studies.
Figure 2.
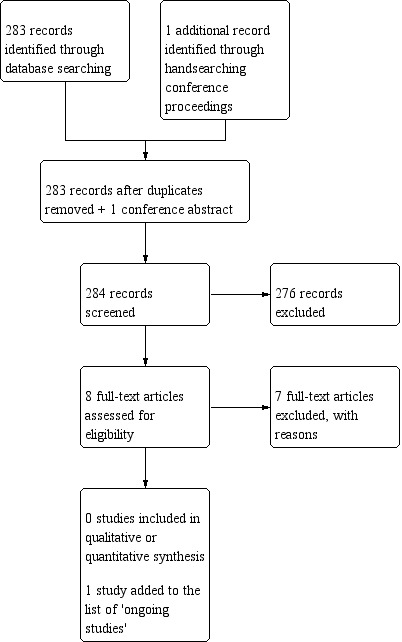
Study flow diagram (date of search 9/10/18).
Included studies
The review includes nine studies that collected data on long‐term neurocognitive or quality of life outcomes: seven were conducted among people with low‐grade gliomas (Brown 2003; Jalali 2017; Kiebert 1998 ‐ EORTC 22844; Klein 2002/Douw 2009; Prabhu 2014 ‐ RTOG 9802; Reijneveld 2016 ‐ EORTC 22033‐26033; Vigliani 1996); and two among people with grade 3 gliomas (Taphoorn 2007 ‐ EORTC 26951; Wang 2010 ‐ RTOG 9402). Of these nine studies, seven were randomised controlled trials (RCTs), which is to say that patients were randomly allocated to alternative treatments at recruitment. As the focus of this review is on long‐term outcomes, such outcome data derived from RCTs was from those subgroups of participants that survived and were able to complete long‐term assessments. Therefore, because participants with disease progression or who died were not followed up, the long‐term data from these trials are unlikely to be representative of the original randomised samples.
Two of the studies were observational (Klein 2002/Douw 2009; Vigliani 1996), with no attempt to randomly allocate participants to different treatments — patients receiving different treatments (physician or institution allocated) were simply followed up over time. Both of these studies reported outcomes in patients that had or had not received radiotherapy as part of their treatment for glioma.
Two studies (Klein 2002/Douw 2009; Taphoorn 2007) reported further long‐term data among survivors as a whole rather than by treatment group (Boele 2015; Habets 2014, respectively). We discuss these in more detail in the Agreements and disagreements with other studies or reviews section of the Discussion).
Numbers recruited and analysed
Altogether 2406 participants were recruited to the nine included studies. However, in all studies the number included in the analysis at various time points during follow‐up was generally considerably less than the total recruited or that had undergone baseline assessments. There was serious sample attrition due to death or disease progression, and there were missing data due to failure to carry out assessments or low participant response rates. In the Kiebert 1998 study the number followed up beyond two years was not clear; for the remaining studies, data were available for 503 participants (i.e. approximately a quarter of those recruited) at the final reported assessments, the timing of which varied between studies. In the Brown 2003 study, of 211 recruited there were follow‐up data for 97 at one year, 65 at two years and for only 38 at five years. Of 195 recruited to the Klein 2002/Douw 2009 study, data were available for 65 at the study end point with follow‐up times varying considerably for individual participants. Of 254 randomised in Prabhu 2014, MMSE data were available for 131 participants at one year and for 126 at two years; while for the 477 participants in Reijneveld 2016, data were reported for 253 at one year, 172 at two years and for 117 at three years. In Taphoorn 2007, of 268 randomised 149 were alive and progression‐free at 2.5 years and data were available for 94 of these patients. At four years there were data for 11 out of 31 participants in the Vigliani 1996 study; and at five years, of the 291 randomised in the Wang 2010 trial only 29 neurocognitive assessments were available. Finally, we included one study that recruited children, adolescents and young adults (Jalali 2017). This study included some participants with other types of brain tumour including craniopharyngioma, although the majority had glioma. We were only able to include data for a relatively small proportion of the sample; while 200 were recruited, only 66 were aged over 16 years and are included in the review, and at five years (the time point reported) only 23 provided outcome data.
Location of studies
Three studies were international and recruited patients from institutions in several countries (Kiebert 1998; Reijneveld 2016; Taphoorn 2007). The study by Wang 2010 was conducted in hospitals in the USA and Canada. The trials by Brown 2003 and Prabhu 2014 were carried out in the USA, and the remaining studies were carried out in the Netherlands (Klein 2002/Douw 2009), India (Jalali 2017), and France (Vigliani 1996).
Dates of recruitment
Participants were recruited to the various studies between 1985 and 2012, and follow‐up in some of the later studies continues. Three studies began recruitment in the 1980s (Brown 2003 1986 to 1996; Kiebert 1998 1985 to 1991; Vigliani 1996 1989 to 1993); four in the 1990s (Klein 2002/Douw 2009 1997 to 2000; Prabhu 2014 1998 to 2002; Taphoorn 2007 1996 to 2002; and Wang 2010 1994 to 2002); and two studies started recruitment after 2000 (Jalali 2017 2001 to 2012; Reijneveld 2016 2005 to 2012). In some studies recruitment was over a long period and it is possible that screening and diagnosis techniques, research personnel, aspects of care and adjuvant therapies changed over the course of the study.
Funding and conflict of interest
In the Kiebert 1998 study sources of funding were not reported. For the rest, all studies reported being financially supported by government, cancer charities or higher education research grants (Brown 2003; Jalali 2017; Klein 2002/Douw 2009; Prabhu 2014; Reijneveld 2016; Taphoorn 2007; Vigliani 1996; Wang 2010). In addition, three trials reported that they had received some support from commercial or private institutes (Klein 2002/Douw 2009; Reijneveld 2016; Taphoorn 2007). While in the Prabhu 2014 study it was reported that there was no commercial funding, several of the investigators reported receiving compensation from commercial organisations, although it was not clear whether this funding related to the reported work. Where conflict of interest was mentioned, no study authors reported conflict of interest other than Prabhu 2014 as stated above.
Characteristics of study participants
Age
All but one of the studies recruited only adult participants (> 18 years, although Kiebert 1998 recruited adults > 16 years). One study recruited children, adolescents and young adults up to the age of 25 years (Jalali 2017); approximately a third of the sample in this study were over 16 years and we have only included these young adults in our data and analysis. Vigliani 1996 had an upper age limit of 60 years for participants, whereas the remaining studies included older adults. For the eight studies recruiting adults, the median age of participants was between 40 and 49 years.
Gender
In most studies there was a larger proportion of male to female participants (approximately 60:40); In Kiebert 1998 and Vigliani 1996 there were similar numbers of men and women recruited.
Type and grade of glioma
Most studies recruited patients with low‐grade glioma and had criteria that excluded patients with other serious disease (e.g. other cancers or serious heart, liver or renal problems).
Five studies recruited participants affected by grades 1 or 2 supratentorial glioma including astrocytoma, oligodendroglioma or mixed disease (Brown 2003; Kiebert 1998; Klein 2002/Douw 2009; Prabhu 2014; Reijneveld 2016). Vigliani 1996 reported recruiting patients with grade 2 or 3 glioma (and in this non‐randomised study there was disparity between treatment groups in the type and grade of disease); and in the Wang 2010 and Taphoorn 2007 trials, participants had grade 3 disease and this was reflected in the poorer prognosis for patients in these studies compared with others. Finally, in the study recruiting children and young adults the sample included low‐grade glioma but also other types of brain tumours (Jalali 2017).
Surgical interventions
In all studies, most of the included patients had undergone surgical intervention prior to radio or chemo‐therapy although the proportions undergoing biopsy, partial or total resection varied. In the Jalali 2017 study the number of participants having surgery, and the type of surgical intervention, was not clear. In the remaining studies the proportions in treatment groups undergoing the different interventions was similar, except for the non‐randomised studies by Klein 2002/Douw 2009 and Vigliani 1996. In these observational studies, there was disparity between treatment groups in the numbers undergoing different surgical interventions and in the light of these differences in patient characteristics, between‐groups findings should be interpreted with particular caution. We have provided more information of the numbers undergoing surgery in the Characteristics of included studies tables.
Treatment with anti‐epileptic drugs
Only one of the included studies reported on the number of participants receiving anti‐epileptic drugs. In the Klein 2002/Douw 2009 study, 71% of patients in each of the treatment and control groups, respectively, received medication to prevent seizures.
Comparisons
The nine included studies examined a range of five different comparisons, as follows.
Radiotherapy versus no adjuvant treatment
Radiotherapy versus chemotherapy
High‐dose versus low‐dose radiotherapy
Standard versus stereotactic conformal radiotherapy
Chemoradiotherapy versus radiotherapy alone
1. Radiotherapy versus no adjuvant treatment
Two studies are included in this comparison and both used observational study designs (Klein 2002/Douw 2009; Vigliani 1996). In Vigliani 1996 allocation was by physician choice and in the retrospective study by Klein 2002/Douw 2009 there was no information on how allocation was made. The latter study involved a simple comparison between those participants that had or had not been treated with radiotherapy during the study period.
In the Klein 2002/Douw 2009 study the total mean radiotherapy dose was 55.6 Gy (standard deviation (SD) 6.1) with a fractional dose of 1.8 Gy to 2 Gy in 86 of the 104 participants. However, in 18 participants the fractional dose was greater than 2 Gy. The control group were patients with glioma who did not receive radiotherapy.
In the Vigliani 1996 study the radiotherapy dose was 54 Gy to 55.8 Gy in 1.8 Gy fractions over 6 weeks.
2. Radiotherapy versus chemotherapy
Reijneveld 2016 examined outcomes in participants randomised to either receiving radiotherapy (50.4 Gy in 28 fractions of 1.8 Gy up to 6.5 weeks) versus oral temozolomide daily for 21 out of 28 days repeated for up to 12 cycles (hence the duration of treatment was quite different in the two experimental groups).
3. High‐dose versus low‐dose radiotherapy
Two randomised studies examined higher versus lower total doses of radiotherapy; in both studies, although the fractional doses in the two arms were the same, the treatment period was longer in the higher dose groups (Brown 2003; Kiebert 1998). In Brown 2003 the total dose in the higher dose group was 64.8 Gy in 36 fractions over seven weeks compared with 50.4 Gy in 28 fractions over five and a half weeks weeks in the lower dose group. In Kiebert 1998 the higher dose was 59.4 Gy over six weeks compared with a lower dose of 45 Gy over five weeks.
4. Standard versus stereotactic conformal radiotherapy
This comparison included only one study that mainly recruited children under 16 years of age, but included a subgroup of participants between 16 and 25 years old (Jalali 2017). The radiotherapy dose in both arms was 54 Gy in 30 fractions over six weeks.
5. Chemoradiotherapy versus radiotherapy alone
Three studies examined the effects of chemoradiotherapy versus radiotherapy alone. In all studies the chemotherapy regimen comprised procarbazine, lomustine and vincristine. Radiotherapy was the same in both arms of each trial, although the dose used in the two studies was different. In the Prabhu 2014 trial, the radiotherapy dose was a total of 54 Gy in 30 fractions of 1.8 Gy over six weeks; while in the Taphoorn 2007 and Wang 2010 studies, the total dose was 59.4 Gy in fractions of 1.8 Gy. As in the Reijneveld 2016 study above, the duration of chemotherapy meant that the treatment period was more protracted in the chemoradiotherapy arms.
Outcomes and follow‐up
In this review, we aimed to include studies that reported longer term (two years or longer) neurocognitive or quality of life outcomes (or both). Several of the studies reported cognitive changes or impairment using the MMSE (Mini Mental State Examination) (Brown 2003; Prabhu 2014; Reijneveld 2016; Wang 2010). An MMSE score of 26 or lower out of 30 was the threshold applied as indicative of neurocognitive impairment in most of these studies. For the rest, Jalali 2017 collected data on intelligence quotients (for participants < 16 years), memory (Wechsler Memory Scale for participants > 16 years), and anxiety and depression; Vigliani 1996 used a battery of 12 neuropsychological tests and patients were considered globally deteriorated or improved when at least eight of 12 items were significantly modified by more than one standard deviation; and Klein 2002/Douw 2009 reported cognitive disability defined as deficits in at least five of 18 applied neuropsychological tests. Kiebert 1998, Reijneveld 2016, Taphoorn 2007 and Wang 2010 reported quality of life (QoL) outcomes.
Periods of follow‐up varied in these studies; while all studies followed up participants beyond two years, the number of participants at each progressive follow‐up point was reduced due to death or disease progression. For example, Brown 2003 followed up participants for a mean of 7.4 years but by this time more than half of the original sample had died (101/203 alive). In the Wang 2010 trial, median follow‐up time was 6.9 years in the surviving participants but 64% of participants had died, and in Taphoorn 2007 data from 2.5 years after radiotherapy were reported, by which point 59% of the original sample had died, and data on 32 of the long‐term survivors were reported in 2014. In Klein 2002/Douw 2009 the median follow‐up period was 12 years but the treatment groups were assessed at different time points and, in the intervention group, (radiotherapy) participants had received radiotherapy up to 20 years previously making results difficult to interpret. Vigliani 1996 reported outcomes up to four years after treatment. Kiebert 1998 followed up participants annually from two years; however, the published report contained QoL outcome data for participants between seven and 15 months only, which could not be used for our review purposes.
Excluded studies
After initial screening and full assessment of study reports we excluded 43 studies from the review. Fifteen studies were excluded as they did not assess or report neurocognitive or quality of life outcomes (Buglione 2014; Cairncross 2006; Combs 2008; Dai 2011; Ding 2017; Ediebah 2015; Eyre 1993; Goda 2017; Karim 2002; Malmstrom 2017; MRC 2001; Satoer 2014; Thomas 2001; van den Bent 2006; Wick 2009). Frequently in these studies the outcomes of interest were survival and disease progression. In eight studies all participants or a large proportion had high‐grade glioma such as glioblastoma, and in those studies where some participants had lower grade glioma separate results were not reported for these patients (Ali 2018; Chung 2018; NCT02655601; Repka 2018; Sichez 1996; Wheeler 2016; Wirsching 2018; Zhu 2017). In the study by Williamson 2017, participants had recurrent glioma and were undergoing re‐irradiation after initial treatment; this study also included participants with glioblastoma. Packer 2002 looked at a paediatric population which is outside the remit of this review.
Other important reasons for exclusion related to study design or the way results were reported. There were five observational studies with no comparator arm (Anand 2012; Armstrong 2002; Gregor 1996; Shaw 2006; Taylor 1998); and in a further five studies the control groups were not relevant to the aims of the review (e.g. the comparator group were healthy controls or had other types of disease or malignancy (Archibald 1994; Corn 2009; Costello 2004; Johannesen 2003; Sherman 2016)). In the study by Correa 2008 that included participants that had received radiotherapy, results were not analysed or reported separately for the radiotherapy arm which made results difficult to interpret. Issues relating to study design and sample selection also meant that results in Surma‐aho 2001 were difficult to interpret and likely to be at high risk of bias.
Finally six studies were excluded as they did not report original study data but were either reviews, commentary or letters to journal editors (Behrend 2014; Brown 2003b; Brown 2009; Klein 2004; Lunsford 2001; Taphoorn 1994); these reports may have included reference to studies already included or excluded from the review.
Risk of bias in included studies
See Figure 3 for the risk of bias summary table.
Figure 3.
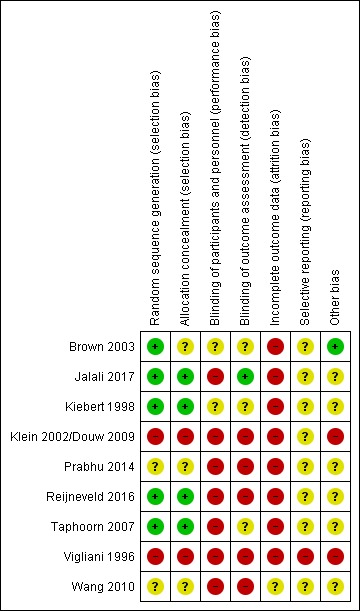
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the seven RCTs, the methods used to randomise participants to experimental groups were mainly low risk or not clearly described. In four studies randomisation was carried out centrally and in these studies we assessed sequence generation and allocation concealment as low risk of bias (Jalali 2017; Kiebert 1998; Reijneveld 2016; Taphoorn 2007). In Brown 2003, there was probably centralised randomisation but this was not entirely clear. In the studies by Prabhu 2014 and Wang 2010, methods of sequence generation and allocation concealment were not well described (assessed as unclear risk of bias for both domains).
In the two non‐randomised studies there was likely to have been high risk of selection bias. In Vigliani 1996 allocation was down to physician choice and there were disparities between groups in terms of patient characteristics. For Klein 2002/Douw 2009, again without random allocation there was a likelihood of bias although methods were not described.
Blinding
In the randomised trials, blinding staff and participants was generally not feasible as treatment regimens in groups were different. Blinding of outcome assessment was also likely to have been at high risk of bias due to lack of blinding, as in this review we focus on subjective outcomes. Four studies were assessed as high risk of bias for both performance and detection bias (Prabhu 2014; Reijneveld 2016; Taphoorn 2007; Wang 2010). Brown 2003 and Kiebert 1998 did not mention blinding and it was unclear whether there was any attempt to blind those collecting outcomes to treatment allocation. Jalali 2017 had no treatment masking but reported that investigators collecting outcome data were unaware of treatment group.
In the non‐randomised studies there was no blinding (Klein 2002/Douw 2009; Vigliani 1996). In Klein 2002/Douw 2009, participants may have been unaware that their data were being used in a study as data were collected as part of clinical assessment. In Vigliani 1996, physicians chose treatment and recorded outcomes.
Incomplete outcome data
All of these studies were assessed as being at high risk of bias for sample attrition (defined by attrition of more than 20%; see Appendix 2). In Wang 2010 there was serious sample attrition but the investigators attempted to take sample loss into account in their analysis. For the rest, by two to three years following treatment there was a significant loss to follow‐up (with a half or more of the sample suffering disease progression or death). In Prabhu 2014 and Reijneveld 2016 there was considerable sample loss and at some assessment points there were different response rates in the two arms of these trials.
Selective reporting
Selective reporting bias is not easy to assess and this is reflected in our judgements, with all of the randomised studies being assessed as unclear risk of bias for this domain.
For the non‐randomised studies, we assessed Klein 2002/Douw 2009 as unclear risk of bias and Vigliani 1996 as high risk of bias.
Other potential sources of bias
Risk of other bias was generally not clear. In the Taphoorn 2007 trial progression‐free survival was better in one of the treatment arms and this may have affected some outcomes. In the non‐randomised studies there were baseline differences between groups (Klein 2002/Douw 2009; Vigliani 1996).
Effects of interventions
See: Table 1
We were not able to combine results in meta‐analysis due to differences in treatment comparisons, time points of follow‐up, and the different outcomes reported. However, we have entered data applicable to primary outcomes on forest plots, without totals, for narrative synthesis purposes. Due to the paucity of data available we have produced a single 'Summary of findings' table covering several different comparisons (Table 1). The table includes dichotomous data for our primary outcome (neurocognitive impairment) for all but one of our comparisons (the Jalali 2017 study mainly recruited children and the limited data we summarise in the text below is for a subgroup aged over 16 years). We did not include estimates of absolute risk as part of our 'Summary of findings' table; this was because we considered that such estimates could be misleading. Findings reported in the review were based on subsets (progression‐free survivors) of samples originally recruited. As sample sizes at follow‐up tended to be small and event rates for outcomes low, there was considerable uncertainly in effect estimates. Absolute risks would reflect these serious uncertainties in the relative effect of interventions and were unlikely to be helpful in the interpretation of findings.
We had intended producing a 'Summary of findings' table for outcomes relating to quality of life but there were insufficient data to create a meaningful summary.
Primary outcomes
Cognitive impairment at 2 years or more after diagnosis/treatment
A. Radiotherapy versus no radiotherapy
A.1. Radiotherapy versus no adjuvant treatment
Two observational studies contributed data (Klein 2002/Douw 2009; Vigliani 1996), with the Klein 2002/Douw 2009 study authors reporting two time points up to 12 years after diagnosis/treatment, and Vigliani 1996 reporting the results of a battery of cognitive functioning tests from follow‐up up to four years after diagnosis/treatment. In the Klein 2002/Douw 2009 cohort, at the 12‐year follow‐up, the risk of cognitive impairment (defined as cognitive disability deficits in at least five of 18 neuropsychological tests) was greater in the radiotherapy group; at five to six years the difference between groups did not reach statistical significance (at five to six years, RR 1.38, 95% CI 0.92 to 2.06; n = 195; at 12 years, RR 1.95, 95% CI 1.02 to 3.71; n = 65) (Figure 4). In the Vigliani 1996 study, one study subject in the radiotherapy group had cognitive impairment at two years compared with none in the control group. We judged the evidence from these observational studies suggesting a possible negative relative effect of radiotherapy on long‐term cognitive impairment to be of very low certainty.
Figure 4.
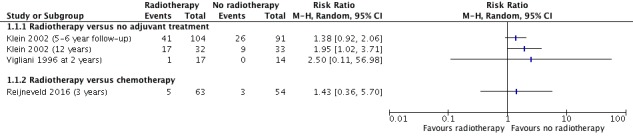
Forest plot of comparison A. Radiotherapy versus no radiotherapy, outcome: Neurocognitive impairment at 2 or more years after treatment. (dichotomous data)
With regard to neurocognitive scores, in the later Klein 2002/Douw 2009 study report (Douw 2009), the radiotherapy group had significantly worse mean executive functioning, attentional functioning and processing speed than the group that received no radiotherapy (Figure 5) and psychomotor functioning, verbal memory and working memory were reported as not significantly different; however, the raw data of the non‐significant findings were not given. In Vigliani 1996, there were no clear differences in any of the various cognitive outcomes measured at two years (n = 31) and four years (n = 15) after diagnosis. We judged this evidence as very low certainty due to inconsistency between these studies.
Figure 5.
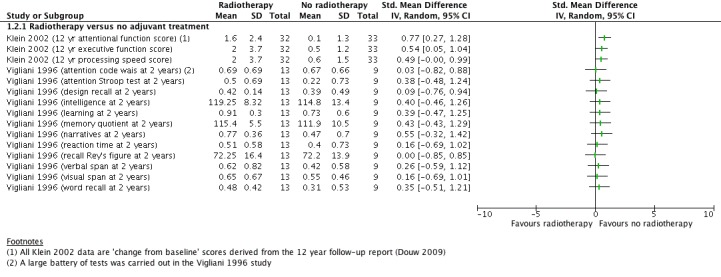
comparison A. Radiotherapy versus no radiotherapy, outcome: Neurocognitive impairment at 2 or more years after treatment. (continuous data)
A.2. Radiotherapy versus chemotherapy
One RCT contributed data on cognitive impairment, assessed at three years after randomisation (Reijneveld 2016). There was no clear difference in the proportion of participants with cognitive impairment between the trial arms at this time point (RR 1.43, 95% CI 0.36 to 5.70, n = 117) (Figure 4). MMSE scores were also measured at different time points and changes from baseline in MMSE scores up to 36 months were presented in a graph, with authors reporting that "no significant difference was recorded between the groups for the change in MMSE scores during the 36 month follow up” (p1533). Sparse data due to attrition and the wide 95% CIs for findings (imprecision) led us to judge the certainty of this evidence as low.
B. High‐dose radiotherapy versus low‐dose radiotherapy
Only one of the two studies — Brown 2003 and Kiebert 1998 — reporting this comparison contributed data. In the observational Brown 2003 study, only a small proportion of study subjects experienced a clinically significant decrease in MMSE score from baseline (more than 3 points) at the 2‐ and 5‐year follow‐ups and there were no clear differences between high‐ and low‐dose radiotherapy arms at either time point (Figure 6). Only 38 subjects of the original cohort of 203 contributed data at the 5‐year follow‐up. We judged the certainty of this evidence of no difference to be very low.
Figure 6.

Forest plot of comparison B: High dose versus low dose radiotherapy, outcome: 2.1 Neurocognitive impairment at 2 years or more after treatment.
C. Conventional radiotherapy versus stereotactic conformal radiotherapy
One study involving younger people with low‐grade glioma contributed limited data from the subgroup aged 16 to 25 years (Jalali 2017). The numbers of participants with neurocognitive impairment at five years after treatment, assessed by the Wechsler Memory Scale, were two out of 12 versus none out of 11 participants in the conventional radiotherapy and conformal radiotherapy arms, respectively (Figure 7). These findings are inconclusive because the study was not powered to detect a difference in this subgroup of its participants (RR 4.62, 95% CI 0.25 to 86.72; n = 23; low‐certainty evidence).
Figure 7.

Forest plot of comparison C: Conventional versus stereotactic conformal radiotherapy, outcome: Neurocognitive impairment at 2 years or more after treatment.
D. Chemoradiotherapy versus radiotherapy
Two RCTs reported cognitive impairment based on MMSE measurements for this comparison.
Prabhu 2014 defined it as a decline (of more than 3 points in MMSE score) in cognitive state compared with baseline and reported comparative data for 2‐year (110 participants), 3‐year (91 participants), and 5‐year (57 participants) time points (Figure 8); these dichotomous data showed no clear difference between the two study arms at any time point.
Figure 8.
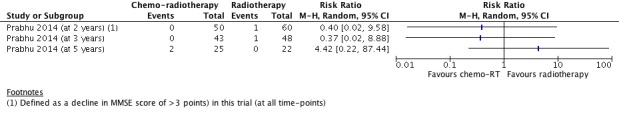
Forest plot of comparison D: Chemoradiotherapy versus radiotherapy, outcome: Neurocognitive impairment at 2 years or more after treatment.
Wang 2010 reported little raw data. Graphic representation of mean MMSE scores up to five years suggested that there was little difference between groups at any time point and authors reported that there was no difference between MMSE scores between the two study arms (P = 0.4752). Those assessed at two, three, four, and five years numbered 126, 110, 69 and 53 survivors respectively in this study. Only 29 out of 191 had completed all assessments at five years for the assessment of cognitive function (MMSE). Authors also reported that the group that received chemoradiotherapy had improving MMSE scores after two years, whereas in the radiotherapy‐only group mean MMSE scores among survivors remained constant over time.
We judged the findings (of no difference) to be low‐certainty evidence due to risk of (attrition) bias and imprecision.
A 2014 Taphoorn 2007 publication reported no difference in cognitive impairments between surviving patients treated initially with radiotherapy and chemoradiotherapy (7 and 20 patients, respectively) at a median survival of 147 months; however data were not reported separately.
Quality of life
We found no data on this outcome for comparisons A to C.
D. Chemoradiotherapy versus radiotherapy
Two RCTs involving people with grade 3 gliomas reported quality of life outcomes for this comparison. One reported no differences in Brain‐QoL scores between its two study arms over a 5‐year follow‐up period ( P = 0.2767; no raw data were given and denominators are not stated) (Wang 2010).
The other trial reported that the long‐term results of health‐related quality of life (HRQoL) "showed no difference between the arms" but did not give the raw data for overall HRQoL scores (Taphoorn 2007). However, authors reported appetite loss, fatigue, nausea and vomiting, physical functioning and drowsiness QoL mean scores; and at 2.5 years after radiotherapy there was no difference between the groups for any of these HRQoL components among participants with data at this time point (55 in the chemoradiotherapy arm and 39 in the radiotherapy arm).
We graded this evidence as low certainty because participants with progressive disease were excluded from assessment in these studies. Survivors in the chemoradiotherapy arms outnumbered the survivors in the radiotherapy arms and those with disease progression would be expected to experience a worse HRQoL; therefore the findings are biased towards no difference when there might be one.
Secondary outcomes
None of the review's secondary outcomes were reported.
Discussion
Summary of main results
We included nine studies altogether: these compared radiotherapy versus no adjuvant treatment (2 observational studies), radiotherapy versus chemotherapy (1 RCT), high‐dose radiotherapy versus low‐dose radiotherapy (subgroup analysis of patients without disease progression from 2 RCTs), conventional radiotherapy versus stereotactic conformal radiotherapy (1 RCT) and chemoradiotherapy versus radiotherapy (3 RCTs). All studies except for those of chemoradiotherapy versus radiotherapy involved people with low‐grade gliomas; whereas two of the chemoradiotherapy trials involved people with grade 3 glioma. As review outcomes are long‐term outcomes (2 or more years after treatment), attrition was high in most studies and, even in the RCTs, long‐term data were observational because the benefits of randomisation were lost through attrition. We did not perform meta‐analysis because the studies reported different time points and outcomes; however, where possible we entered data into forest plots to facilitate narrative synthesis, evidence grading and discussion.
Radiotherapy versus no radiotherapy
For cognitive impairment at two or more years after treatment (measured as a categorical variable in 3 studies), limited evidence suggested that radiotherapy may increase the risk of long‐term cognitive impairment; however the magnitude of this effect was not estimable and we graded the evidence as 'very low certainty'. Evidence on the associated continuous variables that comprised different components of cognitive functioning were also very low certainty. We found no comparative data on quality of life.
High‐dose versus low‐dose radiotherapy
Only one study contributed data on cognitive impairment at two and five years after treatment and its findings showed no difference between these radiotherapy options (very low certainty evidence).
Conventional radiotherapy versus stereotactic conformal radiotherapy
Low‐certainty evidence suggested there may be little or no difference in cognitive impairment at five years after randomisation between these options.
Chemoradiotherapy versus radiotherapy alone
Low‐certainty evidence suggested there may be little or no difference between these options in cognitive impairment among survivors at two and five years' follow‐up. The evidence also suggested that there may also be little or no difference in quality of life at two years or more among glioma survivors who receive either treatment option (low‐certainty evidence).
We identified no relevant data on the review's secondary outcomes or for the brief economic commentary.
Overall completeness and applicability of evidence
With regard to radiotherapy versus no radiotherapy, the included studies were fairly old so this very low to low‐certainty evidence of an increased risk of cognitive impairment might not be applicable to modern radiotherapy techniques, such as image‐guided and conformal radiotherapy, which aim to reduce radiation exposure to normal tissue.
Findings on the cognitive effects of high‐dose versus low‐dose radiotherapy were inconclusive; however, in these studies, death rates and toxicity rates were slightly but consistently higher in the high‐dose arms. As high‐dose radiotherapy in low‐grade glioma is not advocated, further studies on this are unlikely.
Evidence from studies of radiotherapy versus chemotherapy or chemoradiotherapy suffered from high attrition and insensitive measurement tests. Data from various studies employing better measurement tests are not yet mature (Klein 2017).
With regard to conventional radiotherapy versus stereotactic conformal radiotherapy, we derived evidence relating to the review's primary outcomes from a group of young participants (aged 16 to 25 years) in only one trial and, unfortunately, the findings were underpowered to be conclusive. Further research among adult populations with low‐grade glioma would be of interest. Whilst neuroendocrine dysfunction was measured in this study, we were unsuccessful in obtaining separate data from the authors for the subgroup of patients older than 16 years with glioma.
We were unable to synthesise evidence on secondary review outcomes due to a lack of data.
Quality of the evidence
The main review results suggesting that radiotherapy may have a negative effect on cognitive functioning in the long term should be interpreted with caution because the quality of the evidence is low.
Evidence on cognitive function was most commonly derived from study data collected using the MMSE, which lacks sensitivity to mild changes in cognitive impairment and changes due to focal lesions. This is an important limitation of the evidence, as neurocognitive problems related to brain tumours can be subtle or restricted to certain neurocognitive domains only (as suggested by the Klein 2002/Douw 2009 data), depending on their location (Day 2016).
Brief economic commentary
To supplement the main systematic review of the long‐term complications of radiotherapy in those with glioma we sought to identify economic evaluations which included the long‐term effects of radiotherapy as part of the evaluation. No economic studies were identified that analysed the long‐term consequences of radiotherapy. The apparent shortage of relevant economic evaluations indicates that economic evidence regarding the long‐term effects of radiotherapy on long‐term glioma survivors is needed.
Potential biases in the review process
Whilst we did not pool data, it might have been reasonable to do so for the primary dichotomous outcome 'Cognitive impairment at 2 years or more after treatment' of the 'Radiotherapy versus no radiotherapy' comparison. We included three studies in this forest plot, two comparing radiotherapy with no adjuvant treatment and one comparing radiotherapy with chemotherapy. We chose not to pool these data because of the clinical heterogeneity (different measurement time points and different control interventions). Had we done so (using the 5‐ to 6‐year data from Klein 2002/Douw 2009, not the 12‐year data; see Figure 9) the effect estimate in favour of no radiotherapy would have been an RR of 1.40 (95% CI 0.95 to 2.05). With downgrading for imprecision and risk of bias, we would most likely have graded this evidence as low certainty. Whilst our narrative synthesis does not provide an overall effect estimate, the grading and interpretation of the evidence is reasonably consistent with the latter.
Figure 9.
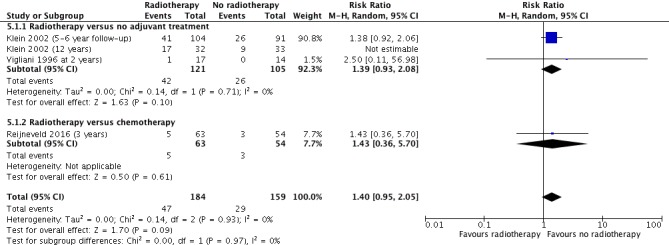
Forest plot of comparison A (exploratory with totals): Radiotherapy versus no radiotherapy, outcome: Neurocognitive impairment at 2 or more years after treatment.
Other potential biases are as follows.
Neurocognitive impairment was variously measured across included studies. We extracted and analysed both dichotomous and continuous data where available. As it is possible for differences in mean scores between treatment groups to be statistically significant but not clinically meaningful, evidence on changes in continuous data (mean scores) should be — and were — interpreted with caution.
In the Klein 2002/Douw 2009 study, which reported 12‐year follow‐up data (Douw 2009), only the significant results were reported as raw data in the text. Psychomotor functioning, verbal memory, and working memory were not significant (data shown in graphs) but the results tended to be in the same direction (favouring the 'no radiotherapy' group). We did not attempt to obtain these numerical data from the authors as we considered that any data obtained would be of a very low quality and did not warrant the (investigator's) efforts required to retrieve it, given that the findings were at high risk of bias anyway.
We included Kiebert 1998, which compared high‐dose radiotherapy with low‐dose radiotherapy; however, the study ended up contributing no usable data to the review. Whilst the study methods stated that participants were followed up annually after 24 months, only data from participants between 7 and 15 months after diagnosis were reported in the published paper and we were unable to obtain any subsequent follow up data. Findings from the 7‐ to 15‐month assessment showed no significant difference in neurological impairment and no significant difference in the proportion of patients with the worst neurological scores (data were not shown in the paper). There was no significant difference in QoL scores overall but some QoL items were worse with high dose, namely emotional functioning and leisure time activities (P = 0.009 and P = 0.017, respectively). By not using these data, we might have missed an opportunity to estimate the effects of high‐dose versus low‐dose radiotherapy on quality of life outcomes.
Prabhu 2014, which compared chemoradiotherapy with radiotherapy, in addition to reporting neurocognitive decline, reported the numbers of participants in each group that experienced an improvement in cognitive functioning (based on a 3‐point increase in MMSE score) over a 5‐year period. Similarly, Vigliani 1996 reported cognitive improvement according to author‐specified criteria in two patients in this study following radiotherapy. As cognitive improvement was not a pre‐specified outcome, we did not present or analyse these data.
We included a trial of conventional radiotherapy versus stereotactic conformal radiotherapy, a more recent radiotherapy technique that aims to reduce the radiation exposure of normal tissue; however this trial was conducted mainly in younger people (Jalali 2017). The sample mainly comprised children and young people with glioma but also included other brain tumours including craniopharyngioma. Most results were not broken down by diagnosis or age group and we were unable to obtain additional data for the subgroup of interest from study authors. In this study, most participants were under 16 years but one‐third were aged 16 to 25 years, and we limited our data extraction to the older age group. Overall, however, neurocognitive (intelligence quotient or memory scores) of patients in the stereotactic conformal radiotherapy arm were either stable or showed an improvement over five years compared with patients in the conventional radiotherapy arm (difference in slope = 1.48; P = 0.04), which was the same direction of the neurocognitive effect reported for the older subgroup only, but for which the data were sparse. This trial also reported the incidence of new endocrine dysfunctions, which were significantly fewer in the stereotactic conformal arm compared with the conventional arm (52% versus 29%; P = 0.02); we did not use these data in the review, however, because they were derived mainly from patients under 16 years old and we were unsuccessful in obtaining subgroup data from the authors.
We excluded studies of glioblastoma because of the poor rates of survival at two years and more. However, with improved survival rates for IDH‐mutated gliomas, useful long‐term data might become available from such studies in the future and we might need to reconsider our study inclusion/exclusion criteria.
Agreements and disagreements with other studies or reviews
Two cohort studies (Boele 2015 and Habets 2014) reported additional long‐term data related to included studies (Klein 2002/Douw 2009 and Taphoorn 2007, respectively) for survivors as a group, rather than separately by treatment group. Both of these longer term studies compared HRQoL in glioma patients (low‐grade glioma (LGG) and grade 3 patients, respectively) with healthy controls and previous assessments. Habets 2014 also assessed cognitive functioning. In Boele 2015, the assessments were at around six and 12 years after diagnosis and initial treatment, respectively, and in Habets 2014 assessment was at a median of 147 months after diagnosis. Neither of these studies had comparative data that could be included in our meta‐analyses. Their main findings are as follows.
Boele 2015 found that LGG patients had lower physical role functioning (P = 0.004) and general health perceptions (P = 0.004), but no other statistically significant differences were observed compared with healthy controls. In the majority of patients both physical (87.7%) and mental (80%) HRQoL remained stable; however, the mean physical HRQoL score was reported to be significantly worse at 12 years than at six years (49.5 versus. 46.9, P < 0.01). Authors concluded that "although HRQoL remains mostly preserved in the majority of LGG patients, a subset of patients experience detectable decline on one or more HRQoL scales despite long‐term stable disease."
Habets 2014 reported findings of 32 out of 37 long‐term survivors of grade 3 gliomas who had participated in the Taphoorn 2007 trial comparing radiotherapy with chemoradiotherapy. The number of survivors was less than 10% of the original sample and about a third of those assessed at 2.5 years. Ten out of 32 patients evaluated had received radiotherapy initially, the rest had received chemoradiotherapy. Compared with healthy controls, survivors who had never progressed had lower working memory capacity, information processing speed, psychomotor functioning, attention, and executive functioning. Investigators reported that initial treatment did not correlate with HRQoL or cognitive functioning findings and that HRQoL in the long term for this cohort was similar to the findings at 2.5 years.
With such studies, it is important to bear in mind that differences between glioma patients and healthy controls could be related to the glioma itself, as well as to treatment. Also, long‐term data on cognitive effects and quality of life are inherently biased by the effect of a given treatment on survival. This is particularly relevant to studies that show substantial differences in survival, as quality of life would plausibly be worse among patients with a shorter survival time at a particular time point distant from treatment, and cognitive data would be influenced by the greater attrition of participants in the study arm with the shorter survival. Such a positive correlation between survival and quality of life is evident in the Wang 2010 study, which evaluated the effect of chemoradiotherapy versus radiotherapy alone among people with anaplastic oligodendrogliomas, and also in CATNON 2017 (see below).
Ongoing studies
The review process identified six ongoing studies that hopefully will contribute data to a future version of this review (Characteristics of ongoing studies). Ongoing randomised trials include long‐term neurocognitive outcomes of the EORTC study 22033‐26033 (radiotherapy versus temozolomide; Klein 2017); CATNON 2017 (radiotherapy versus radiotherapy plus adjuvant temozolomide and other comparisons ‒ 4 study arms); and NCT03180502 (proton beam versus intensity‐modulated radiotherapy). Ongoing observational studies include NCT00457210, NCT02544178 and NCT03055364; one of these was registered in 2007 and forthcoming data would seem unlikely at this stage. All the other studies are likely to report results from 2020 onwards.
Authors' conclusions
Low‐certainty evidence suggests that in good‐prognosis patients with lower grade glioma, radiotherapy may increase the risk of neurocognitive side effects in the long term; however the magnitude of the risk is uncertain. The long‐term neurocognitive effects of adding chemotherapy to radiotherapy are also uncertain. In general, there were insufficient data to detect possible differences between groups, and a lack of evidence of effect does not provide evidence of no effect. Doctors should make patients aware that radiotherapy may increase the risk of neurocognitive problems, bearing in mind that cognitive deterioration can also occur with tumour progression. This review found no evidence on endocrine dysfunction following radiotherapy and more research on this potential treatment‐related effect is needed.
To improve the certainty of evidence around long‐term cognitive effects, neurocognitive assessment should be an integral part of long‐term follow‐up in trials of lower grade glioma. Such evaluation should not exclude patients with disease progression, otherwise long‐term findings might under‐estimate the positive effects on these outcomes for treatments that improve survival. Ongoing studies, such as CATNON 2017, that help to distinguish which types of glioma respond to more or less aggressive therapies will hopefully lead to improvements in the management of this condition and reduce any undesirable side effects associated with overtreatment. Trials should also include systematic long‐term evaluation of endocrine function, particularly in light of a recent report suggesting a high prevalence (Kyriakakis 2019). High‐quality comparative studies should include economic evaluations that reflect the long‐term treatment side effects.
In terms of the types of neurocognitive data that are most useful, more comprehensive neuropsychological tests, or tests selected to examine cognitive domains considered likely to be most vulnerable, are preferable to the brief MMSE because they are likely to be more sensitive to subtle neurocognitive changes and the selective neurocognitive impairment that occurs due to focal lesions. Day 2016 provides a helpful discussion about test choices.
Finally, a qualitative review on patients' and carers' views and experiences of treatment for low‐grade glioma would be of value to improve our understanding of what is important to the individuals affected by this condition.
Acknowledgements
We would like to thank the Information Specialist, Jo Platt, and the Gynaecological, Neuro‐oncology and Orphan Cancer Group (GNOC) Managing Editors, Gail Quinn and Clare Jess, for their advice and support in the preparation of this review. In addition, we would like to thank the library staff at the Royal United Hospital, Bath, UK for sourcing many of the articles cited.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Programme Grant funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
The authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers, are grateful to the following peer reviewers for their time and comments: Helen Bulbeck ‒ brainstrust, UK; Julia Day ‒ Edinburgh Centre for Neuro‐Oncology (ECNO), UK; Michael Hart ‒ Addenbrooke's Hospital, UK; Riccardo Soffietti ‒ Italy.
Appendices
Appendix 1. Search strategies
Medline Search Strategy
1. exp Glioma/ 2. (glioma* or astrocytoma* or medulloblastoma* or ependymoma* or craniophyrangioma* or oligodendroglioma* or glioblastoma* or GBM*).ti,ab. 3. 1 or 2 4. exp Radiotherapy/ 5. radiotherapy.fs. 6. (radiotherap* or radiat* or irradiat*).ti,ab. 7. exp Antineoplastic Agents/ 8. Antineoplastic Combined Chemotherapy Protocols/ 9. chemotherap*.mp. 10. exp Chemoradiotherapy/ 11. (radiochemo* or chemoradio*).mp. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. 3 and 12 14. Radiation Effects/ 15. exp Radiation Injuries/ 16. adverse effects.fs. 17. ((late or adverse* or long term or side or long‐term or chronic* or residual* or delay* or undesirable or unexpected) adj5 (effect* or event* or outcome* or reaction* or complication* or harm* or injur* or toxic* or cognit*)).ti,ab. 18. (adrs or tolerab*).ti,ab. 19. (radiation induced* or radiation‐induced).ti,ab. 20. 14 or 15 or 16 or 17 or 18 or 19 21. randomized controlled trial.pt. 22. controlled clinical trial.pt. 23. randomized.ab. 24. placebo.ab. 25. clinical trials as topic.sh. 26. randomly.ab. 27. trial.ti. 28. exp Cohort Studies/ 29. cohort*.tw. 30. longitudinal*.tw. 31. prospective*.tw. 32. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. 13 and 20 and 32 34. exp animals/ not humans.sh. 35. 33 not 34
Key
mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab = abstract sh = subject heading ti = title pt = publication type
Medline Search with economic filter:
1. exp Glioma/ 2. (glioma* or astrocytoma* or medulloblastoma* or ependymoma* or craniophyrangioma* or oligodendroglioma* or glioblastoma* or GBM*).ti,ab. 3. 1 or 2 4. exp Radiotherapy/ 5. radiotherapy.fs. 6. (radiotherap* or radiat* or irradiat*).ti,ab. 7. exp Antineoplastic Agents/ 8. Antineoplastic Combined Chemotherapy Protocols/ 9. chemotherap*.mp. 10. exp Chemoradiotherapy/ 11. (radiochemo* or chemoradio*).mp. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. 3 and 12 14. Radiation Effects/ 15. exp Radiation Injuries/ 16. adverse effects.fs. 17. ((late or adverse* or long term or side or long‐term or chronic* or residual* or delay* or undesirable or unexpected) adj5 (effect* or event* or outcome* or reaction* or complication* or harm* or injur* or toxic* or cognit*)).ti,ab. 18. (adrs or tolerab*).ti,ab. 19. (radiation induced* or radiation‐induced).ti,ab. 20. 14 or 15 or 16 or 17 or 18 or 19 21. 13 and 20 22. Economics/ 23. exp "costs and cost analysis"/ 24. Economics, Dental/ 25. exp economics, hospital/ 26. Economics, Medical/ 27. Economics, Nursing/ 28. Economics, Pharmaceutical/ 29. (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. 30. (expenditure$ not energy).ti,ab. 31. value for money.ti,ab. 32. budget$.ti,ab. 33. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 34. ((energy or oxygen) adj cost).ti,ab. 35. (metabolic adj cost).ti,ab. 36. ((energy or oxygen) adj expenditure).ti,ab. 37. 34 or 35 or 36 38. 33 not 37 39. letter.pt. 40. editorial.pt. 41. historical article.pt. 42. 39 or 40 or 41 43. 38 not 42 44. 21 and 43
Key
mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab = abstract sh = subject heading ti = title pt = publication type
Embase Search Strategy
1. exp Glioma/ 2. (glioma* or astrocytoma* or medulloblastoma* or ependymoma* or craniophyrangioma* or oligodendroglioma* or glioblastoma* or GBM*).ti,ab. 3. 1 or 2 4. exp radiotherapy/ 5. radiotherapy.fs. 6. (radiotherap* or radiat* or irradiat*).ti,ab. 7. exp chemotherapy/ 8. exp antineoplastic agent/ 9. chemotherap*.mp. 10. exp chemoradiotherapy/ 11. (radiochemo* or chemoradio*).mp. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. 3 and 12 14. radiation response/ 15. exp radiation injury/ 16. ae.fs. 17. ((late or adverse* or long term or side or long‐term or chronic* or residual* or delay* or undesirable or unexpected) adj5 (effect* or event* or outcome* or reaction* or complication* or harm* or injur* or toxic* or cognit*)).ti,ab. 18. (adrs or tolerab*).ti,ab. 19. (radiation induced* or radiation‐induced).ti,ab. 20. 14 or 15 or 16 or 17 or 18 or 19 21. crossover procedure/ 22. randomized controlled trial/ 23. single blind procedure/ 24. random*.mp. 25. factorial*.mp. 26. (crossover* or cross over* or cross‐over).mp. 27. placebo*.mp. 28. (doubl* adj blind*).mp. 29. (singl* adj blind*).mp. 30. assign*.mp. 31. allocat*.mp. 32. volunteer*.mp. 33. exp cohort analysis/ 34. cohort*.tw. 35. longitudinal*.tw. 36. prospective*.tw. 37. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38. 13 and 20 and 37
Key
mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab = abstract sh = subject heading ti = title pt = publication type
Embase Search with economic filter:
1. exp Glioma/ 2. (glioma* or astrocytoma* or medulloblastoma* or ependymoma* or craniophyrangioma* or oligodendroglioma* or glioblastoma* or GBM*).ti,ab. 3. 1 or 2 4. exp radiotherapy/ 5. radiotherapy.fs. 6. (radiotherap* or radiat* or irradiat*).ti,ab. 7. exp chemotherapy/ 8. exp antineoplastic agent/ 9. chemotherap*.mp. 10. exp chemoradiotherapy/ 11. (radiochemo* or chemoradio*).mp. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. 3 and 12 14. radiation response/ 15. exp radiation injury/ 16. ae.fs. 17. ((late or adverse* or long term or side or long‐term or chronic* or residual* or delay* or undesirable or unexpected) adj5 (effect* or event* or outcome* or reaction* or complication* or harm* or injur* or toxic* or cognit*)).ti,ab. 18. (adrs or tolerab*).ti,ab. 19. (radiation induced* or radiation‐induced).ti,ab. 20. 14 or 15 or 16 or 17 or 18 or 19 21. 13 and 20 22. Health Economics/ 23. exp Economic Evaluation/ 24. exp Health Care Cost/ 25. pharmacoeconomics/ 26. 22 or 23 or 24 or 25 27. (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. 28. (expenditure$ not energy).ti,ab. 29. (value adj2 money).ti,ab. 30. budget$.ti,ab. 31. 27 or 28 or 29 or 30 32. 26 or 31 33. letter.pt. 34. editorial.pt. 35. note.pt. 36. 33 or 34 or 35 37. 32 not 36 38. (metabolic adj cost).ti,ab. 39. ((energy or oxygen) adj cost).ti,ab. 40. ((energy or oxygen) adj expenditure).ti,ab. 41. 38 or 39 or 40 42. 37 not 41 43. 21 and 42 44. (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 45. 43 not 44
Key
mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab = abstract sh = subject heading ti = title pt = publication type
CENTRAL search strategy
#1. MeSH descriptor: [Glioma] explode all trees #2. glioma* or astrocytoma* or medulloblastoma* or ependymoma* or craniophyrangioma* or oligodendroglioma* or glioblastoma* or GBM* #3. #1 or #2 #4. MeSH descriptor: [Radiotherapy] explode all trees #5. radiotherap* or radiat* or irradiat* #6. MeSH descriptor: [Antineoplastic Agents] explode all trees #7. MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] this term only #8. Any MeSH descriptor with qualifier(s): [Radiotherapy ‐ RT] #9. Any MeSH descriptor with qualifier(s): [Drug therapy ‐ DT] #10. Chemotherap* #11. MeSH descriptor: [Chemoradiotherapy] explode all trees #12. radiochemo* or chemoradio* #13. #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14. #3 AND #13 #15. MeSH descriptor: [Radiation Effects] this term only #16. MeSH descriptor: [Radiation Injuries] explode all trees #17. Any MeSH descriptor with qualifier(s): [Adverse effects ‐ AE] #18. ((late or adverse* or long term or side or long‐term or chronic* or residual* or delay* or undesirable or unexpected) near/5 (effect* or event* or outcome* or reaction* or complication* or harm* or injur* or toxic* or cognit*)) #19. adrs or tolerab* #20. radiation induced* or radiation‐induced #21. #15 or #16 or #17 or #18 or #19 or #20 #22. #14 AND #21
Appendix 2. Assessment of risk of bias
For randomised trials
(1) Random sequence generation
Low risk of bias, e.g. participants assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers
High risk of bias, e.g. participants assigned to treatments on basis of date of birth, clinic id‐number or surname, or no attempt to randomise participants
Unclear risk of bias, e.g. not reported, information not available
(2) Allocation concealment
Low risk of bias, e.g. where the allocation sequence could not be foretold
High risk of bias, e.g. allocation sequence could be foretold by patients, investigators or treatment providers
Unclear risk of bias, e.g. not reported
(3) Blinding of participants and personnel
Low risk of bias if participants and personnel were adequately blinded
High risk of bias if participants and/or personnel were not blinded to the intervention that the participant received
Unclear risk of bias if this was not reported or unclear
(4) Blinding of outcomes assessors
Low risk of bias if outcome assessors were adequately blinded to the intervention that the participant received
High risk of bias if outcome assessors were not blinded to the intervention that the participant received
Unclear risk of bias if this was not reported or unclear
(5) Incomplete outcome data
We will record the proportion of participants whose outcomes were not reported at the end of the study. We will code a satisfactory level of loss to follow‐up for each outcome as follows.
Low risk of bias, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
High risk of bias, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear risk of bias, if loss to follow‐up was not reported
(6) Selective reporting of outcomes
Low risk of bias, e.g. review reports all outcomes specified in the protocol
High risk of bias, e.g. it is suspected that outcomes have been selectively reported
Unclear risk of bias, e.g. it is unclear whether outcomes had been selectively reported
(7) Other bias
Low risk of bias, i.e. no other source of bias suspected and the trial appears to be methodologically sound
High risk of bias, i.e. we suspect that the trial was prone to an additional bias
Unclear risk of bias, i.e. we are uncertain whether an additional bias may have been present
For non‐randomised trials
We will assess the risk of bias in accordance with four criteria concerning sample selection comparability of treatment groups.
(1) Relevant details of criteria for assignment of participants to treatments
Low risk of bias, e.g. yes, details provided
High risk of bias, e.g. no details provided
Unclear risk of bias, e.g. details unclear
(2) Representative group of people who received the experimental intervention
Low risk of bias, if representative of patients with gliomas who receive treatment for their condition
High risk of bias, if groups of patients were selected (non‐consecutive)
Unclear, if selection of the group was not described
(3) Representative group of people who received the comparison intervention
Low risk of bias, if drawn from the same population as the experimental group
High risk of bias, if drawn from a different source
Unclear risk of bias, if selection of group not described
(4) Baseline differences between groups controlled for, in particular with reference to age, gender, grade/type of glioma, type of surgery
Low risk of bias, if all of these characteristics were reported
High risk of bias, if the groups differed in these baseline characteristics and differences were not controlled for
Unclear risk of bias, if fewer than three of these characteristics were reported even if there were no other differences between the groups, and other characteristics were controlled for
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2019 | Amended | Edits made to text. |
Differences between protocol and review
We moved the Health‐Related Quality of Life (HRQoL) outcome from a secondary outcome in the protocol to a primary outcome in the review. This facilitated the inclusion of Taphoorn 2007, which reported HRQoL but not neurocognitive outcomes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: primary study was an RCT for which outcomes were previously reported (Shaw 2002). Cognitive function data from the subgroup of participants without tumour progression were analysed in the Brown 2003 substudy Country: USA Accrual dates: 1986 to 1994 Trial reg: NCCTG 86‐72‐51 Funding: Public Health Service grant nos. CA‐25224, CA‐37404, CA‐15083, and CA‐35415, and the Linse Bock Foundation, Rochester, MN. |
|
| Participants | No. randomised: 211 No. analysed: 203 Inclusion/exclusion criteria: to be eligible, patients had to be 18 years of age or older and have histologic proof of a supratentorial Kernohan grade 1 or 2 astrocytoma, oligodendroglioma, or mixed oligoastrocytoma within 3 months of study entry (pilocytic astrocytomas and other LGG variants were excluded) Age: approx. 50% ≥ 40 years Gender: 42% female Glioma type: LGGs (astrocytoma, oligodendroglioma or mixed) Glioma grade: grade 1 (10), grade 2 (193) Resection/biopsy: gross total resection (29), subtotal resection (71), biopsy (103) Anti‐epileptics/SSRIs: NR Duration of FU: median follow‐up for the cognitive function study was 7.4 years in 101 patients still alive. At the time of the Shaw 2002 analysis, 83 patients (41%) were dead, and median follow‐up was 6.43 years in the 120 participants who were still alive. |
|
| Interventions | Arm 1: (n = 101) low‐dose RT 50.4 Gy in 28 fractions over 5.5 weeks Arm 2: (n = 102) high‐dose RT 64.8 Gy in 36 fractions over 7 weeks After progression, patients could receive chemotherapy off protocol |
|
| Outcomes | Reported review outcomes: neurocognitive effects as measured by an MMSE score, change from baseline, change of more than 3 points considered significant Other reported study outcomes: Neurologic Function Score (0 to 4) Evaluations were completed at study entry and then every 4 months for 2 years, every 6 months for 3 years, and yearly until year 15 |
|
| Notes | Neurologic Function Scores were not reported according to randomisation group Authors conclusions: "In this population, most low‐grade glioma patients maintained a stable neurocognitive status after focal radiotherapy as measured by the MMSE. Patients with an abnormal baseline MMSE were more likely to have an improvement in cognitive abilities than deterioration after receiving radiotherapy. Only a small percentage of patients had cognitive deterioration after radiotherapy. However, more discriminating neurocognitive assessment tools may identify cognitive decline not apparent with the use of the MMSE." Death rates and toxicity rates were slightly but consistently higher in the high‐dose arm than the low‐dose arm, but the differences were not statistically significant Of the 5 MMSE domains (orientation, short‐term memory retention, attention, short‐term memory recall, language), the most frequently affected (over time in the study population) were those of language and orientation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The main paper states "an adaptive stratified randomisation method was used" and central randomization likely, but this is not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described in the study reports |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel is unlikely but this is not described in the study reports |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the study reports |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 97/145 patients without tumour progression had data for the year 1 assessment, 65/95 patients without tumour progression had data for year 2, and 38/76 patients without tumour progression had data for year 5. The number of participants with data differed between the groups, particularly for the year 2 assessment when 62% of data were from the low‐dose arm and 38% from the high‐dose arm. |
| Selective reporting (reporting bias) | Unclear risk | Neurologic Function Scores were assessed but not reported according to randomisation group |
| Other bias | Low risk | None noted |
| Methods | Design: RCT Country: single centre study in Mumbai, India Accrual dates: April 2001 to March 2012 Trial reg: NCT00517959 Funding: reported no conflict of interest. Funded by Tate Memorial Centre and Terry Fox India and the Brain Tumor Foundation of India. It was stated that the funders had no influence on the design or conduct of the study |
|
| Participants | No. randomised: 200 children were randomised. Only 66 were more than 16 years and results for the over‐16 age group are very limited No. analysed: results for 23 patients over 16 for IQ at 5 years (overall there were 142 measured with neurological outcomes and 181 with endocrine outcomes) Inclusion/exclusion criteria: children and young adults (up to age 25) with low‐grade and benign residual and/or progressive brain tumours (< 7 cm and require RT, NPS 0 to 3) Age: median age 13 (3 to 25 years). Sample included 66 young adults over 16 years (45 aged 16 to 20 years and 21 aged 21 to 25 years) Gender: gender distribution in the sample aged over 16 years not clear, over all age groups approximately 65% (133) male and 35% (67) female Glioma type: glioma type for those over 16 years not clear. Overall the sample included patients with craniopharyngioma, astrocytoma, optic pathway gliomas, ependymoma, and other tumours Glioma grade: low grade (grade 2) and benign Resection/biopsy: not clear Anti‐epileptics/SSRIs: not clear Duration of FU: up to 5 years (at 6 months, 2, 4, and 5 years) |
|
| Interventions | Arm 1: Conventional radiotherapy at a dose 54 Gy in 30 fractions over 6 weeks (total 104, with n = 31 participants over 16, but results were available for only 12 at 5 years) Arm 2: Conformal radiotherapy at a dose 54 Gy in 30 fractions over 6 weeks (total 96 with n = 35 participants over 16 but results were available for only 11 at 5 years) |
|
| Outcomes | Review outcomes: IQ, memory, depression and anxiety, endocrine function (but results available for over‐16s for findings of the Wechsler Memory Scale only) Other reported study outcomes: survival |
|
| Notes | We extracted data only for the 66 young adults over 16 years (45 aged 16 to 20 years and 21 21 to 25 years); these data were scant and we were unable to obtain any additional data from the authors by email request. Authors reported that in the conformal stereotactic radiotherapy group IQ scores were either stable or showed some improvement over 5 years compared with the conventional radiotherapy arm; in the latter arm scores improved in the first 6 months and gradually declined, reaching pre‐radiotherapy baseline scores by the second year and remaining stable thereafter. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. Stratified by tumour location, pre or post puberty, neurological performance and presence of hydrocephalus |
| Allocation concealment (selection bias) | Low risk | Allocation and enrolment was carried out by an external organisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff performing intervention would be aware of allocation. It was not clear whether patients were aware |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was stated that outcome assessment was performed by staff unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was considerable loss to follow‐up that was not explained |
| Selective reporting (reporting bias) | Unclear risk | This is a registered trial and expected outcomes are reported although means and median scores over the broad age range may not be meaningful in the context of this review |
| Other bias | Unclear risk | For the group of interest to the review most results are not reported (and subgroup results may not represent overall findings – the sample was not stratified by age) |
| Methods | Design: sub‐study of a RCT Country: multinational sites ‒ 14 out of 27 sites contributed to the QoL substudy Accrual dates: April 1985 to September 1991 Trial reg: EORTC 22844 (Karim 1996) Funding: NR |
|
| Participants | No. randomised: 379 No. analysed: 109 of 113 that complete a baseline QoL questionnaire Inclusion/exclusion criteria: all adult patients (age 16 to 65 years) having a definite histopathologic diagnosis of low‐grade astrocytomas (G1 and G2), oligodendroglioma, and mixed oligoastrocytomas of the supratentorial areas. Grade 1 (pilocytic) astrocytoma, if totally excised, was excluded, while grade 2 astrocytoma, even if totally excised, was included. Oligodendrogliomas and mixed oligoastrocytomas were included. The patients had to have been in reasonable good general condition as indicated by performance score after surgery: Karnofsky index ≥ 60 and WHO score ≤ 2. Neurologic deficit status was also recorded and defined: 1 = no deficit; 2 = some deficit but with adequate functioning for useful work; 3 = moderate functional impairment with movement difficulties, moderate dysparesis, paresis, and visual or memory impairment; 4 = major functional impairment; and 5 = lack of conscious response. The patients in categories 4 and 5 were excluded from this trial. Patients with pregnancy or gross hepatic, renal, or cardiovascular diseases of malignancy other than curable skin cancers were excluded. However, patients thought to be cured of cancer for at least 5 years before inclusion in the protocol were eligible. Age: < 35 years (40), 35 to 44 years (35), ≥ 45 years (38) Gender: low dose: 24 female, 33 male; high dose: 28 female, 28 male. Glioma type: low dose: astrocytoma (37), oligodendoglioma (15), mixed (5); high‐dose: astrocytoma (35), oligodendoglioma (17), mixed (4) Glioma grade: low dose: 0 or 1 (6), > 1 (51); high dose: 0 or 1 (6), > 1 (50) Resection/biopsy: low dose: < 50% tumour excised (23), ≥ 50% tumour excised (34); high dose: < 50% tumour excised (30), ≥ 50% tumour excised (26) Anti‐epileptics/SSRIs: NR Duration of FU: 2+ years |
|
| Interventions | Arm 1: (n = 57) low dose RT (45 Gy in 5 weeks) Arm 2: (n = 56) high dose RT (59.4 Gy in 6 weeks) |
|
| Outcomes | Reported review outcomes: QoL (self‐reported scale) including physical, social, psychological, and symptom domains. Signs and symptoms were rated using a Likert scale 1 to 4 (4 = severe); Rand HIS‐Physical capacities scale. Other reported outcomes: Survival (OS, PFS) Timing of follow up: 3, 6, 12, 18, 24 months and then annually. Data were analysed in 2 time points ‒ immediately post RT and at 7 to 15 months. |
|
| Notes | Longer term (2+ year) follow‐up data have not been reported because "compliance with further follow‐up was so poor that analysis of these latter data were considered inappropriate". At the 7 to 15 month follow‐up, there was no significant difference in neurological impairment observed, and no significant difference was found in the proportion of patients with the worst neurological scores (data were not shown). Emotional functioning and leisure time activities were significantly worse with high dose (P = 0.009 and P = 0.017, respectively). Authors found no major difference in QoL overall but some individual QoL items were worse with high‐dose RT. In the primary study, high‐dose RT did not lead to better survival than low‐dose RT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization with stratification according to institution and grade Baseline characteristics of patients that completed the QoL questionnaires were not significantly different to those of the whole sample |
| Allocation concealment (selection bias) | Low risk | "Central randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Long‐term (2+ year) follow‐up data have not been reported because "compliance with further follow‐up was so poor that analysis of these latter data were considered inappropriate". |
| Selective reporting (reporting bias) | Unclear risk | Not possible to make a judgement |
| Other bias | Unclear risk | None noted |
| Methods | Design: retrospective observational study with controls and long‐term follow‐up Country: the Netherlands. Multicentre study. Accrual dates: February 1997 and January 2000 Trial reg: not an RCT Funding: reported no conflict of interest and funders had no influence on study design or conduct. Later follow‐up funded by Kaptein Fonds and Schering Plough. Grant from Dutch Cancer Society (˜VU96‐1155). |
|
| Participants | No. randomised: n/a No. analysed: 195 patients with low‐grade glioma for initial assessments, 65 for longer‐term follow‐up at a mean of 12 years Inclusion/exclusion criteria: adult patients with low‐grade glioma with no clinical signs of tumour recurrence at 1 year after diagnosis and primary treatment and no radiological signs of recurrence 3 months before testing. Radiotherapy patients were only included if RT had been given within 8 weeks of histological diagnosis. Patients were excluded if they were treated with corticosteroids or if they were not proficient in the Dutch language. Age: mean age 42.6 (SD12.2) in the group treated with radiotherapy (n = 104) and 38.7 (SD 11.5) in the 'no radiotherapy' group (n = 91) (this age difference was statistically significant) Gender: male sex 62/104 (60%) in the radiotherapy group and 58/91 (64%) in the non‐treated group Glioma type: astrocytoma 139/195, oligodendroglioma 43/195, oligoastrocytoma 13/195 Glioma grade: grades not reported, all described as low‐grade glioma Resection/biopsy: biopsy 55/104 (53%) in the radiotherapy group, 29/91 (32%) in the non‐treated group. Resection 49/104 (47%) versus 62/ 91 (68%) (significant difference between groups for surgical interventions) Anti‐epileptics/SSRIs: anti‐epileptics 74/104 (71%) versus 65/91 (71%) Duration of FU: followed up for a median of 12 years. Patients were assessed at different time points following RT |
|
| Interventions | Arm 1: 104 had received RT ‒ mostly focal. Mean total dose was 55.6 (6.1). Fractional dose 1.8 Gy to 2 Gy in 86/104 patients, 18/104 received a fractional dose above 2 Gy. Arm 2: (n = 91); patients with low‐grade glioma with no early radiotherapy. The study also included adults with haematological malignancies and healthy controls; these patients are not included in this review. |
|
| Outcomes | Reported review outcomes: Cognitive test data across different cognitive domains (intellectual functioning, perception and psychomotor speed, memory, attention and executive function). Other reported study outcomes: Brain tissue atrophy (Postma 2002). Correlation between brain tissue atrophy and cognitive functioning. |
|
| Notes | In the intervention group, RT was received from 1 to 20 years previously, with a mean of 6 years after diagnosis. Authors concluded that low‐grade glioma patients do have cognitive problems when compared to healthy controls and to patients with non‐CNS cancers, and those who received radiotherapy had lower functioning than those not in receipt of RT, but cognitive disability was only found in patients receiving high fraction doses (exceeding 2 Gy). HRQoL was reported in a subsequent publication (Boele 2015). In this paper, HRQoL in 65 LGG patients (irrespective of treatment arm) was compared to that of healthy controls at around 6 and 12 years after diagnosis and initial treatment, respectively, and change of time was also assessed. Compared with healthy controls, LGG patients had lower physical role functioning (P = 0.004) and general health perceptions (P = 0.004), but no other statistically significant differences were observed. The majority of patients maintained a stable level of both physical (87.7%) and mental (80%) HRQoL. However, the mean physical HRQoL score was reported to be significantly worse at 12 years than at 6 years (49.5 versus. 46.9, P < 0.01). Authors concluded that "although HRQOL remains mostly preserved in the majority of LGG patients, a subset of patients experience detectable decline on one or more HRQOL scales despite long‐term stable disease." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study; non‐randomised. Patients were not allocated to a treatment randomly. |
| Allocation concealment (selection bias) | High risk | Observational study; non‐randomised. No allocation concealment. Patients not allocated to a treatment by research team. Treatment depended on the recruiting centre; therapeutic policies differed as regards the use of early physiotherapy and irradiated patients were recruited from centres where early radiotherapy was favoured. There may have been other important differences in the characteristics of centres and treatment choices. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Observational study; non‐randomised. No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observational study; non‐randomised. No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Observational study; non‐randomised. There was considerable loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to make a judgement |
| Other bias | High risk | Representativeness and comparability of study groups: Radiotherapy treated patients were older (the number of years since diagnosis was also slightly greater (mean difference 1 year)). Neurosurgical interventions were also significantly different between groups with a higher proportion of biopsies in the radiotherapy treated group (52.9% versus 31.9% in the non‐treated group) and a lower proportion of resections (47.1% versus 68.1%). The patients in the study with haematological cancers may not be representative of all such patients – more of this group than the glioma group declined to participate, which might have introduced a selection bias (18% versus 28%). Baseline differences: There were also other differences in baseline characteristics of the two groups which may indicate that intervention or comparison participants were not representative. The participants appeared representative in terms of clinical characteristics. There was an attempt to match groups by premorbid IQ. Other bias: For longer‐term follow‐up there was considerable attrition due to death, disease progression and other reasons. 65/195 were followed up at mean 12 years (data presented in this review). There was a very broad range of follow‐up times. This makes results more difficult to interpret. |
| Methods | Design: RCT (with observational arm – non‐randomised) Country: USA. Multicentred Accrual dates: 31 October 1998 to 27 June 2002, with long‐term follow‐up (results up to 5 years reported in the published paper). Trial reg: NCT00003375 Funding: it was reported that there was no commercial sponsorship, but in 'Conflicts of interest' it appeared that several investigators had received compensation from commercial organisations: Pharmacyclics, Merck Serono, Genentech, Bristol‐Myers Squibb, Merck, Novartis, Elekta, GlaxoSmithKline. It was not clear whether the compensation was outside this study. NIH funding. |
|
| Participants | No. randomised: (254 originally randomised) 251 eligible for evaluation. No. analysed: 230 included in analyses of cognitive function. Inclusion/exclusion criteria: WHO grade 2 glioma age 40 or more with any extent of resection or less than 40 with subtotal resection/biopsy. (Histologically confirmed grade 2 astrocytoma, oligodendroglioma or mixed oligoastrocytoma). Karnofsky performance status 60% or greater, neurological functioning score 3 or less and supratentorial location. Age: median age in RT arm 40 and 41 in RT + chemotherapy. Range overall 18 to 82 (ages 18 to 39 with subtotal resection or 40 or more with total resection and KPS > 60 and neuro ≤ 3) Gender: male 77/126, female 49/126 in RT arm, male 65/125 and female 60/125 in RT + Chemo + RT (lower proportion of females (39% ) in RT arm versus 48% in RT + chemo arm (NS)) Glioma type: confirmed grade 2 astrocytoma (65) , oligodendroglioma (107) or mixed oligoastrocytoma (79) Glioma grade: grade 2 (low grade) Resection/biopsy: biopsy 119/251, partial resection 107/251, total resection 25/251 Anti‐epileptics/SSRIs: not stated. Duration of follow‐up: results up to year 5 reported for cognitive outcomes (survival follow‐up ongoing). At 4, 8,12, 18 and 24 months and annually thereafter. MMSE evaluated at each follow‐up point but discontinued with tumour progression. |
|
| Interventions | Arm 1: (n = 128 randomised, 122 analysed) radiotherapy alone (54 Gy in 30 fractions of 1.8 Gy) over 6 weeks Arm 2: (n = 125 randomised, 116 analysed) radiotherapy plus chemotherapy. Following radiotherapy (as arm 1) patients received 6 cycles of procarbazine (60 mg/m² orally per day on days 8 and 21 of each cycle), lomustine (110 mg/m² on day 1 of each cycle) and vincristine (1.4 mg/m² (maximum 2 g) IV on days 8 and 29 of each cycle. The cycle length was 8 weeks. |
|
| Outcomes | Reported review outcomes: cognitive function assessed by MMSE at 1, 2, 3 and 5 years from randomisation. Significant MMSE decline was defined as a decrease of more than 3 points and gain as an increase in score of more than 3 points compared with baseline. Other reported study outcomes: survival reported in main trial report |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Published papers and trial registration do not state how randomisation was carried out |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised trial with parallel assignment and randomisation stratified by tumour type but methods of allocation concealment were not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was described as an open label trial with no masking in the trial registration |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For some outcomes (survival), lack of masking may not have been important, but for assessment of cognitive function lack of blinding may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was considerable loss to follow‐up due to death and a large proportion of patients (approximately 1/3) had no MMSE assessment at 1 year. There seems to have been fewer responses at all time points in the radiotherapy plus chemotherapy arm. |
| Selective reporting (reporting bias) | Unclear risk | The trial was registered but there was very little information about methods |
| Other bias | Unclear risk | It was not explained why large numbers of patients were not assessed using the MMSE |
| Methods | Design: Results of follow‐up of RCT Country: 19 countries (Australia, Austria, Belgium, France, Germany, Hungary, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland, Australia, New Zealand, Singapore, Canada, Egypt, Israel, UK) Accrual dates: December 2005 to December 2012 Trial reg: EudraCT. Number 2004‐002714‐11 and Clinical/Trials.gov, number NCT00182819 Funding: Merck Sharp & Dohme, Merck & Co (study chemotherapy drugs and grant), National Cancer Institute, Swiss Cancer League, National Centre for Health Research, Cancer Research UK, Canadian Cancer Research Institute, National Health and Medical Research Council, European Organisation for Research and Treatment of Cancer, Cancer Research Fund. It was stated that the funders of the research had no role in study design, data collection, data analysis, data interpretation or writing the report. Conflict of interest: two investigators report personal fees from Hoffmann La Roche outside the submitted work. One author reports grants and non financial support from Roche, Ipsen, and Astra‐Zeneca outside the submitted work. One author reports grants from Celgene, Novartis and Pharmamar and personal fees from Celgene, Boehringer, Genentec, Lilly and Merck‐Serono outside the submitted work. Grants from funders as above. |
|
| Participants | No. randomised: 477 assigned No. analysed: reported that 477 in ITT analyses (but considerable amounts of missing data for outcomes relevant to this review) Inclusion/exclusion criteria: Age: adults aged 18 years or more (median, 43 (36 to 52 interquartile range) in the radiotherapy group and 45 (37 to 53) in the chemotherapy group; 38% (92/240) less than 40 in the radiotherapy group and 36% (85/237) in the chemotherapy group) Gender: 58% men and 42% women in both groups. Glioma type: Astrocytoma WHO grade II 37% in the radiotherapy group and 33% in the chemotherapy group. Oligoastrocytoma WHO grade II 24% and 25%, Oligodendroglioma WHO grade II 39% and 41%. Glioma grade: low‐grade glioma confirmed 95% and 89%. Resection/biopsy: radiotherapy: biopsy 40%, partial removal 44%, total removal 15%; chemotherapy: biopsy 39%, partial removal 42%, total removal 19% Anti‐epileptics/SSRIs: reason for treatment: refractory seizures 12% and 14%. Duration of FU: time between biopsy or surgery to study treatment median 4.8 months in both groups but considerable variation (2.9 to 18.3 IQR months in the radiotherapy group and 2.6 and 26.4 months in the chemotherapy group). Time from initial diagnosis and study treatment medians 5.1 and 6.0 months. |
|
| Interventions | Arm 1: (n = 240) radiotherapy. Total dose of 50.4 Gy in 28 fractions of 1.8 Gy once daily for 5 days per week up to a maximum treatment period of 6.5 weeks. Reasons for treatment discontinuation included major worsening of neurological or mental status or other medical condition that would preclude continuation. Dose adjustments were not recommended. Arm 2: (n = 237) chemotherapy. 75 mg/m² oral temozolomide daily for 21 of 28 days (1 cycle) repeated for a maximum of 12 cycles until disease progression or unacceptable toxicity. The treatment was withheld if low neutrophil and platelet counts and resumed on recovery. Patients with severe recurrent toxicity despite dose reduction discontinued treatment. |
|
| Outcomes | Reported review outcomes: adverse events, health‐related quality of life (HRQoL scales including global health or quality of life status, role and functioning, social functioning, communication deficit, visual disorder, motor dysfunction, drowsiness) and cognitive functioning (MMSE). Outcomes reported up to 36 months. Other reported study outcomes: Primary outcome of trial was progression‐free survival. |
|
| Notes | Authors emailed 6 February 2019. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation using a minimisation technique (stratified for WHO performance status, age, presence or absence of contrast enhancement on MRI, 1p status, and treatment centre |
| Allocation concealment (selection bias) | Low risk | Probably low risk as randomisation was centralised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no attempt to blind participants and staff as treatments were different. The different types of treatment may have affected patient compliance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For self‐assessed outcomes relevant to this review the different treatments may have affected response rates and those experiencing worse outcomes may have been less likely to respond. It was reported that response was lowest in patients with poor performance status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For long‐term outcomes relevant to this review response rates were less than 70% at 2 years; for early assessments response rates were greater in the chemotherapy group although this disparity between groups decreased over time. Denominators for some outcomes were not clear. There was variation between treatment centres in response rates. |
| Selective reporting (reporting bias) | Unclear risk | This was a registered trial with specified outcomes using standard measures. The frequency of testing may have introduced risk of multiple testing. |
| Other bias | Unclear risk | Treatment modalities differed significantly in duration and intensity and early differences detected in QoL may have reflected these differences in treatment modalities. |
| Methods | Design: RCT Country: Multicentre study in 40 hospitals in Europe (the Netherlands, France, Austria, Hungary, Italy, Belgium) Accrual dates: August 1996 to March 2002 Trial reg: European Organisation for Research and Treatment of Cancer (EORTC)26951 Funding: authors report no conflict of interest. Supported by EORTC Grant , Astra Zeneca, and Dutch Cancer Society |
|
| Participants | No. randomised: 368 patients randomised. No. analysed: QoL data available for 288 patients at baseline and 94 patients at follow‐up at 2.5 years Inclusion/exclusion criteria: patients aged 16 to 70 years diagnosed with anaplastic oligodendroglioma or mixed oligoastrocytoma with at least 25% oligodendroglial elements and at least 3 or 5 anaplastic characteristics (high cellularity, mitosis, nuclear abnormalities, endothelial proliferation and necrosis); patients had performance status 0 to 2 and had not undergone previous chemotherapy or radiotherapy to the skull, had no other diseases interfering with follow‐up and had adequate haematologic, renal and hepatic function. Age: median in radiotherapy plus chemotherapy group 48.6 (range 18.6 to 68.7), Median in radiotherapy only group 49.8 (range 19.2 to 68.7) Gender: male/female sex, 102/83 in the radiotherapy plus chemotherapy group and 110/73 in the radiotherapy only group. Overall 58% male. Glioma type: oligodendroglioma (265/ 368) or mixed oligoastrocytoma (100/368) (pathology missing for 3 patients) Glioma grade: not clear Resection/biopsy: all patients had surgery. biopsy 52/368; partial resection 183, total resection 133. Anti‐epileptics/SSRIs: not clear Duration of FU: 2.5 years for quality of life outcomes. (59% patients died by this time) |
|
| Interventions | Arm 1: (n = 185) radiotherapy plus chemotherapy. Radiotherapy within 6 weeks of surgery dose 45 Gy to the planning target volume in 25 daily fractions of 1.8 Gy 5 fractions a week. After that a boost of 14.4 Gy up to a cumulative dose of 59.4 Gy in 8 fractions of 1.8 Gy. Chemotherapy was 6 cycles of standard procarbazine, lomustine and vincristine to start within 4 weeks after the end of radiotherapy. Cycle consisted of lomustine 110 mg/m² orally on day 1 with antiemetics, procarbazine 60 mg/m² orally on days 8 to 21 and vincristine 1.4 mg/m² IV on days 8 to 29 (maximum dose 2 mg). Cycles were repeated every 6 weeks with dose reductions. Arm 2: (n = 183) radiotherapy as above without adjuvant chemotherapy. Disease progression led to 54 patients in the RT/PCV group and 85 in the RT group having other chemotherapy. |
|
| Outcomes | Reported review outcomes: quality of life (EORTC quality of life questionnaire C30 with specific brain cancer module with 20 topics relevant to brain tumour). Cognitive function (Mini mental status examination – but results not reported in published papers). Fatigue, nausea, physical functioning, appetite loss, drowsiness. Reported at baseline and 3‐monthly. For this review, outcomes at 1 year, 2 years and 2.5 years reported. Other reported study outcomes: survival, progression‐free survival. |
|
| Notes | SDs were calculated for HRQoL scores where possible. Long‐term HRQoL and cognitive functioning in a cohort of survivors was reported in a subsequent paper (Habets 2014). Results of 32 patients were compared to healthy controls and to their earlier findings at 2.5 years. Findings showed that 31% were severely cognitively impaired; HRQoL was worse compared with controls but similar to their HRQoL at the 2.5 year assessment. Authors concluded that "In progression‐free patients, HRQOL is relatively stable during the disease course." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | External randomisation service (EORTC) data centre (computer randomisation via the internet or phone). Stratified by age, extent of resection, performance score and previous surgery |
| Allocation concealment (selection bias) | Low risk | External randomisation service (EORTC) data centre (computer randomisation via the internet) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not feasible and it is not clear whether lack of blinding affected other treatment decisions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Some outcomes may not have been affected by lack of blinding but it is possible quality of life outcomes were affected |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Due to death and disease progression there was considerable loss to follow‐up at the later assessments. Less than half of those randomised had recurrent disease or had died by 30 months. (149/368 still alive and progression free; of these 94/149 returned QoL assessment forms) |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes were not reported in published papers |
| Other bias | Unclear risk | Progression‐free survival was increased in the radiotherapy plus chemotherapy (but not overall survival): this may have had an impact on findings if 1 group remained healthier for slightly longer. (This was stated in the paper as a possible bias in HRQoL assessments.) |
| Methods | Design: cohort of patients either treated with radiotherapy or not Country: single hospital in France Accrual dates: February 1989 to December 1993 Trial reg: not a trial Funding: Associazione Italiana per la Ricerca sul Cancro (AIRC) Milan, and EEC Radioprotection Program Grant. Conflict of interest not stated |
|
| Participants | No. randomised: not randomised. Total 31. Irradiated group 17, no radiotherapy 14 No. analysed: decreasing numbers over time; at 1 year 29, 2 years 21, 3 years 15 and 4 years 11 Inclusion/exclusion criteria: patients attending a Paris hospital with low‐grade glioma or anaplastic astrocytoma with good prognostic factors (total or subtotal resection, age < 60 years, Karnofski index > 70). Age: radiotherapy group mean 35.3 (range 24 to 49), no radiotherapy 37.7 years (16 to 56 years) Gender: M/F radiotherapy group 12/5, no radiotherapy 12/2 Glioma type: in radiotherapy group 11 had astrocytoma, 1 mixed glioma, 1 oligodendroglioma, 1 anaplastic oligodendroglioma and 3 anaplastic astrocytoma. In the non‐radiotherapy group it was stated all had low‐grade astrocytoma (in 8 cases diagnosis was available – 4 astrocytomas, 2 mixed gliomas and 2 oligodendrogliomas) Glioma grade: grade II and III described as low grade with good prognosis Resection/biopsy: in the radiotherapy group 2 had total resection, 8 partial resection and 7 biopsy. In the 'no radiotherapy' group 4 had total resection, 3 partial resection, 1 biopsy and 6 patients had no biopsy. Anti‐epileptics/SSRIs: not mentioned Duration of FU: annually up to 4 years |
|
| Interventions | The decision whether or not to use radiotherapy was made by the physician caring for the patient Arm 1: (n = 17) radiotherapy. Limited field radiotherapy (tumour bed and 2 cm to 3 cm margin) dose 54 Gy to 55.8 Gy in 1.8 Gy fractions (30 to 31) over 6 weeks. (In addition 4 patients had chemotherapy.) Arm 2: (n = 14) no radiotherapy (and none had chemotherapy) |
|
| Outcomes | Reported review outcomes: neuropsychological tests – battery of tests administered by a neuropsychologist (Stroop color word test, WAIS subtest code, reaction time, verbal span, visual span, Raven progressive matrices (PM38), Wechsler memory scale, recall of word series, recall of design, recall of Rey‐Osterrieth complex figure). Testing 120 to 150 minutes at yearly intervals for 4 years (radiotherapy group also tested at 6 months). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The decision to administer radiotherapy was made by the attending physician. Those receiving radiotherapy may have different (worse diagnoses) or groups may not have been treated by the same doctors with some doctors being more or less likely to opt for radiotherapy. 6 of the 'no radiotherapy' group had no histologically confirmed diagnosis |
| Allocation concealment (selection bias) | High risk | The decision to administer radiotherapy was made by the attending physician. Those receiving radiotherapy may have different (worse diagnoses) or groups may not have been treated by the same doctors with some doctors being more or less likely to opt for radiotherapy. 6 of the 'no radiotherapy' group had no histologically confirmed diagnosis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 6/14 had no clear diagnosis. No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was considerable attrition over time with 11/31 assessed at 4 years. (15/31 at 3 years.) |
| Selective reporting (reporting bias) | High risk | Reporting by doctors who selected treatment |
| Other bias | High risk | There were no significant differences reported although groups were too small to identify possible differences. Age and sex were similar. It was not clear if type of glioma were the same in the two groups and there were differences in rates of neurological resection/biopsy. There were some differences in neuropsychological scores at baseline (IQ and reaction time). The changes were assessed at the individual level (ANOVA) but group means were reported and with serious sample attrition these are not simple to interpret. |
| Methods | Design: randomised controlled trial Country: 76 institutions in USA and Canada Accrual dates: 1994 to 2002 with long‐term follow‐up Trial reg: Radiation Therapy Oncology Group Trial 9402. NCT 00002569 Funding: Radiotherapy oncology group grants, North Central Cancer Treatment Group Grant, Eastern Cooperative Oncology Group Grant, Southwest Oncology Group Grant, Community Clinical Oncology Program Grant, National Cancer Institute, National Cancer Institute of Canada. Authors state no conflict of interest |
|
| Participants | No. randomised: 291 No. analysed: 291. No patients were lost to follow‐up but due to death and other factors the number analysed at different time points decreased over time. Only 29/191 had completed all assessments at 5 years for the assessment of cognitive function (MMSE) Inclusion/exclusion criteria: patients 18 years or more with confirmed diagnoses of anaplastic oligodendroglioma and anaplastic oligoastrocytoma with Karnofsky performance status 60 or more after surgery, adequate marrow and organ function, not pregnant and with no other serious illness. Age: Median age in 'chemotherapy plus radiotherapy' group 43 (range 18 to 75) and in the radiotherapy group median 43 (range 19 to 76). Gender: MF; radiotherapy plus chemotherapy group 90/58; radiotherapy alone 84/59 (approximately 60% male in both groups) Glioma type: anaplastic oligodendroglioma 150/291; anaplastic oligoastrocytoma 141/291. Glioma grade: grade III. 161 had moderately anaplastic disease and 130 highly anaplastic. Resection/biopsy: total resection in the CRT and RT alone groups 40/148 and 53/143; partial resection 85 and 75, biopsy only 21 and 14. Anti‐epileptics/SSRIs: not stated (corticosteroids at baseline CRT group and 79 in the RT group Duration of FU: median survival for surviving patients was 6.9 years (64% had died) |
|
| Interventions | Arm 1: (n = 148) chemotherapy and radiotherapy. Chemotherapy within 1 week of randomisation. 4 cycles every 6 weeks before radiotherapy: lomustine 130 mg/m² orally on day 1, procarbazine 75 mg/m² orally daily days 8 to 21 and vincristine 1.4 mg/m² IV on days 8 and 29. There was no 2 mg limit on vincristine. Radiotherapy 59.4 Gy in 33 fractions of 1.8 Gy each 5 days a week. Arm 2: (n = 143) radiotherapy alone as above |
|
| Outcomes | Reported review outcomes: Cognitive function MMSE and Quality of life (B‐QOL, Brain Quality of Life,) baseline, 9 and 12 months, 4 monthly in year 2, 6 monthly years 3 to 5 then annually Other reported study outcomes: survival; toxicity and symptoms were assessed but not reported in detail or by randomisation group |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not fully described but was a RTOG trial and randomisation was stratified |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not feasible and staff and patients would have been aware of randomisation group. It was not clear whether lack of blinding would have affected those outcomes assessed. (It is not clear if those with radiotherapy alone completed treatment before those receiving chemotherapy.) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Detection of survival outcomes are unlikely to have been affected by assignment. Patients completed forms themselves for cognitive function |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was considerable loss to follow‐up due to death and disease progression. There was an attempt by the authors to take account of these factors in analysis and analysis was mainly relating to within‐subject factors |
| Selective reporting (reporting bias) | Unclear risk | Outcomes that may be important to patients were not reported in full (toxicity, nausea, etc). Also much of the analysis was not by randomisation group |
| Other bias | Unclear risk | None noted |
CNS = central nervous system; EORTC = European Organisation for Research and Treatment of Cancer; Gy = Grays; LGG = low grade glioma; HGG = high grade glioma; NR = not reported; HRQoL = Health‐related quality of life; IQ = intelligence quotient; ITT = intention to treat; KPS = Karnofsky Performance Score; MMSE = Mini Mental State Examination; NAT = no adjuvant treatment; NS = not statistically significant; QoL = quality of life; PFS = progression free survival; OS = overall survival; RCT = randomised controlled trial; RT= radiotherapy; RTOG = Radiation Therapy Oncology Group; SD = standard deviation; WHO = World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2018 | This trial compared hyperfractionated versus standard radiotherapy for patients with glioma; more than 70% of participants had glioblastoma |
| Anand 2012 | No comparator arm |
| Archibald 1994 | Wrong comparator arm (healthy controls) |
| Armstrong 2002 | No comparator arm, mixed population |
| Behrend 2014 | Not a study report |
| Brown 2003b | Review article |
| Brown 2009 | Commentary |
| Buglione 2014 | Study of early versus late RT for LGG but only 6 patients had neurocognitive function assessed and neurocognitive scores were not compared between study groups |
| Cairncross 2006 | No neurocognitive outcomes reported |
| Chung 2018 | Paricipants all had glioblastoma |
| Combs 2008 | No neurocognitive outcomes |
| Corn 2009 | Before and after study of different doses of RT with no comparator arm |
| Correa 2008 | Treated patients compared with NAT group included patients who had RT (n = 5), RT plus CT (n = 1) but also CT only (n = 3). Data on participants who had RT are not reported separately from those who only had CT |
| Costello 2004 | Wrong comparator (non‐malignant tumour group) |
| Dai 2011 | No neurocognitive outcomes reported |
| Ding 2017 | No neurocognitive outcomes reported |
| Ediebah 2015 | No neurocognitive outcomes reported |
| Eyre 1993 | No neurocognitive outcomes reported |
| Goda 2017 | No neurocognitive data reported |
| Gregor 1996 | No comparator arm |
| Johannesen 2003 | No neurocognitive outcomes reported; no relevant comparison |
| Karim 2002 | No neurocognitive outcomes reported |
| Klein 2004 | Not a study but a letter to the editor |
| Laack 2005 | A cohort study of 20 adults who received radiotherapy (50.4Gy or 64.8Gy) for LGG. Cognitive function was reported as stable at 3‐year follow up, but findings were not reported separately by treatment group. |
| Lunsford 2001 | Not a study but a letter to the editor |
| Malmstrom 2017 | All received RT; no late effects data |
| MRC 2001 | All received RT; no late effects neurocognitive outcome data reported |
| NCT02655601 | Study examining participants with high‐grade glioma |
| Packer 2002 | Commentary on a paediatric study |
| Repka 2018 | Recruited patients with high‐grade glioma and glioblastoma |
| Satoer 2014 | Wrong intervention and no late neurocognitive outcomes reported |
| Shaw 2006 | No comparator arm |
| Sherman 2016 | Wrong comparator (published normals) |
| Sichez 1996 | Wrong population ‒ mixed HGG population |
| Surma‐aho 2001 | Retrospective study of effects of RT versus no RT but the study groups were highly selected at baseline and so the findings are impossible to interpret with any certainty |
| Taphoorn 1994 | Not a study but a letter to the editor |
| Taylor 1998 | No comparator arm |
| Thomas 2001 | All received RT; no late effects neurocognitive outcomes data reported |
| van den Bent 2006 | No neurocognitive outcome data reported |
| Wheeler 2016 | Mixed HGG population with low numbers with grade 3; all had RT (question was the effect of immunotherapy) |
| Wick 2009 | No long‐term (> 2 years) neurocognitive outcome data reported |
| Williamson 2017 | This study looked at re‐irradiation of patients with recurrent glioma; some had glioblastoma and no neurocognitive outcomes were reported |
| Wirsching 2018 | This trial examined chemoradiotherapy versus radiotherapy alone in elderly patients (ARTE trial). All participants had glioblastoma |
| Zhu 2017 | All participants in this trial had glioblastoma |
Gy = Grays; LGG = low grade glioma; HGG = high grade glioma;nNAT = no adjuvant treatment; RCT = randomised controlled trial; RT= radiotherapy;
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | CATNON trial (EORTC study 26053‐22054) |
| Methods | Phase 3 randomised, open‐label study with 2x2 factorial design. Web‐based randomisation (1:1:1:1) |
| Participants | 784 randomised Patients were 18 years or older with newly diagnosed non‐co‐deleted anaplastic glioma with WHO performance scores of 0 to 2 |
| Interventions | RT RT + adjuvant TMZ RT + concurrent TMZ RT + concurrent TMZ + adjuvant TMZ |
| Outcomes | 5‐year PFS and OS, adverse events, HRQoL and cognitive effects |
| Starting date | Dec 2007 ‒ ongoing |
| Contact information | Martin van den Bent; m.vandenbent@erasmusmc.nl |
| Notes | Interim results were published in 2017, which showed that adjuvant TMZ was associated with significant 5‐year survival benefit in this population. (One might expect that cognitive data will therefore be biased by the greater attrition in the study arms with adjuvant TMZ). Neurocognitive data will be presented in 2020/21 (personal communication) |
| Trial name or title | Memory functioning in low‐grade glioma patients treated with either RT or TMZ ‒ EORTC study 22033‐26033 |
| Methods | Memory functioning using the Rey Auditory Verbal Learning Test applied at baseline and every 6 months subsequently, and memory functions compared between treatment arms over time. Minimal compliance was set at 60%. |
| Participants | 98 patients with low‐grade glioma were assessed at baseline |
| Interventions | RT ‒ 52 participants TMZ ‒ 46 participants |
| Outcomes | Memory functioning (free recall and delayed recall) |
| Starting date | Date is not clear from the conference abstract, in which 12‐month data on memory functioning are reported (no clear difference in memory functioning between RT and TMZ groups at 12 months). |
| Contact information | Martin Klein |
| Notes | Mature data from this study are expected to contribute to the review in due course.Reijneveld 2016 Reijneveld 2016 reports the QoL data for EORTC 22033‐26033 and survival data are reported in New Reference. |
| Trial name or title | The influence of radiotherapy on cognitive function |
| Methods | Prospective observational cross‐sectional study |
| Participants | 150 20‐ to 80‐year‐old oncologic patients, of both genders, that are referred to brain radiotherapy or stereotactic radiosurgery due to primary brain tumour/brain metastases or increased risk for brain metastases |
| Interventions | (observational) Radiotherapy or sterotactic radiosurgery |
| Outcomes | Cognitive function questionnaire |
| Starting date | April 2007 |
| Contact information | bencorn@tasmc.health.gov.il |
| Notes | Study status is unknown and, given the date of registration, publication seems unlikely. No response to email query. |
| Trial name or title | Study of neurological complication after radiotherapy for high‐grade glioblastoma (EPIBRAINRAD) |
| Methods | Prospective observational cohort |
| Participants | 200 consecutive adult patients treated by radiotherapy and chemotherapy for a glioma stage 3 or 4 in the Pitié‐Salpêtrière Hospital or in the Paul Strauss hospital from April 2015 to April 2017 will be included. |
| Interventions | (observational) Treatment by radiotherapy and chemotherapy |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2015 |
| Contact information | marie‐odile.bernier@irsn.fr |
| Notes | Estimated completion date: 2020 |
| Trial name or title | Cognitive function after treatment of primary CNS malignancy |
| Methods | Prospective observational cohort study |
| Participants | 160 people with primary CNS malignancy receiving brain radiotherapy |
| Interventions | (observational) Brain radiotherapy |
| Outcomes | Rate and magnitude of change in cognitive performance within 12 months of completion of therapy in patients with intracranial malignancies receiving photon‐ or proton‐based cranial irradiation with curative intent (time frame: baseline, completion of treatment, 3 months, 6 months, 1 year and 2 years after radiotherapy) |
| Starting date | May 2017 |
| Contact information | tranby.brianna@mayo.edu |
| Notes | Estimated completion date: May 2021 |
| Trial name or title | Proton beam or intensity‐modulated radiation therapy in preserving brain function in patients with IDH mutant grade II or III glioma. |
| Methods | Phase 2, randomized, parallel arm, open‐label trial |
| Participants | Patients with IDH mutant, low to intermediate grade gliomas |
| Interventions | Active Comparator: Arm I (IMRT, temozolomide) Patients undergo IMRT daily, 5 days a week for 6 weeks for a total of 30 fractions. Beginning 4 weeks after completion of radiation therapy, patients receive standard of care temozolomide for 5 days. Treatment repeats every 28 days for up to 12 courses in the absence of disease progression of unacceptable toxicity. Experimental: Arm II (proton beam radiation therapy, temozolomide) Patients undergo proton beam radiation therapy daily, 5 days a week for 6 weeks for a total of 30 fractions. Beginning 4 weeks after completion of radiation therapy, patients receive standard of care temozolomide for 5 days. Treatment repeats every 28 days for up to 12 courses in the absence of disease progression of unacceptable toxicity. |
| Outcomes | Primary:
Secondary:
|
| Starting date | 2 August 2017 |
| Contact information | Claudine.Gamster@CadenceHealth.org |
| Notes | Estimated completion date: May 2022 |
CNS = central nervous system; EORTC = European Organisation for Research and Treatment of Cancer; Gy = Grays; IMRT = intensity modulated radiotherapy; LGG = low grade glioma; HGG = high grade glioma; HRQoL = Health‐related quality of life; PFS = progression free survival; OS = overall survival; RT= radiotherapy; TMZ = temozolomide
Contributions of authors
Theresa Lawrie and Therese Dowswell wrote the first draft of the review. All authors contributed to study screening and data extraction. All authors advised on and approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR 16/144 Cochrane Programme Grant Scheme, UK.
Declarations of interest
Theresa Lawrie: none known Therese Dowswell: none known David Gillespie: none known Jonathan Evans: none known Sara Erridge: none known Luke Vale: Member of NIHR Health Technology Assessment Clinical Evaluation and Trials Panel until March 2018 Ashleigh Kernohan: none known Robin Grant: none known
New
References
References to studies included in this review
- Brown PD, Buckner JC, O'Fallon JR, Iturria NL, Brown CA, O'Neill BP, et al. Effects of radiotherapy on cognitive function in patients with low‐grade glioma measured by the folstein mini‐mental state examination. Journal of Clinical Oncology 2003;21(13):2519‐24. [DOI] [PubMed] [Google Scholar]; Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, Dinapoli R, et al. Prospective randomized trial of low‐ versus high‐dose radiation therapy in adults with supratentorial low‐grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 2002;20(9):2267‐76 XIR. [DOI] [PubMed] [Google Scholar]
- Jalali R, Gupta T, Goda JS, Goswami S, Shah N, Dutta D, et al. Efficacy of Stereotactic Conformal Radiotherapy vs Conventional Radiotherapy on Benign and Low‐Grade Brain Tumors: A Randomized Clinical Trial. JAMA Oncology 2017;3(10):1368‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, et al. A randomized trial on dose‐response in radiation therapy of low‐grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. International Journal of Radiation Oncology, Biology, Physics 1996;36(3):549‐56. [DOI] [PubMed] [Google Scholar]; Kiebert GM, Curran D, Aaronson NK, Bolla M, Menten J, Rutten EH, et al. Quality of life after radiation therapy of cerebral low‐grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co‐operative Group. European Journal of Cancer 1998;34(12):1902‐9. [DOI] [PubMed] [Google Scholar]
- Boele FW, Douw L, Reijneveld JC, Robben R, Taphoorn MJ, Aaronson NK, et al. Health‐related quality of life in stable, long‐term survivors of low‐grade glioma. Journal of Clinical Oncology 2015;33(9):1023‐9. [DOI] [PubMed] [Google Scholar]; Douw L, Klein M, Fagel SS, Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low‐grade glioma: long‐term follow‐up. Lancet Neurology 2009;8(9):810‐8. [DOI] [PubMed] [Google Scholar]; Klein M, Heimans JJ, Aaronson NK, Ploeg HM, Grit J, Muller M, et al. Effect of radiotherapy and other treatment‐related factors on mid‐term to long‐term cognitive sequelae in low‐grade gliomas: a comparative study. Lancet 2002;360(9343):1361‐8. [DOI] [PubMed] [Google Scholar]; Postma TJ, Klein M, Verstappen CC, Bromberg JE, Swennen M, Langendijk JA, et al. Radiotherapy‐induced cerebral abnormalities in patients with low‐grade glioma. Neurology 2002;59(1):121‐3. [DOI] [PubMed] [Google Scholar]
- Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus procarbazine, CCNU, and vincristine in low‐grade glioma. New England Journal of Medicine 2016;374(14):1344‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prabhu RS, Won M, Shaw EG, Hu C, Brachman DG, Buckner JC, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low‐grade glioma: secondary analysis of RTOG 98‐02. Journal of Clinical Oncology 2014;32(6):535‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low‐grade glioma: initial results of RTOG 9802. Journal of Clinical Oncology 2012;30(25):3065‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumert BG, Hegi ME, Bent MJ, Deimling A, Gorlia T, Hoang‐Xuan K, et al. Temozolomide chemotherapy versus radiotherapy in high‐risk low‐grade glioma (EORTC 22033‐26033): a randomised, open‐label, phase 3 intergroup study. Lancet Oncology 2016;17(11):1521‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reijneveld JC, Taphoorn MJB, Coens C, Bromberg JEC, Mason WP, Hoang‐Xuan K, et al. Health‐related quality of life in patients with high‐risk low‐grade glioma (EORTC 22033‐26033): a randomised, open‐label, phase 3 intergroup study. Lancet Oncology 2016;17(11):1533‐42. [DOI] [PubMed] [Google Scholar]
- Habets EJ, Taphoorn MJ, Nederend S, Klein M, Delgadillo D, Hoang‐Xuan K, Bottomly A, et al. Health‐related quality of life and cognitive functioning in long‐term anaplastic oligodendroglioma and oligoastrocytoma survivors. Journal of Neuro‐oncology 2014;116(1):161‐8. [DOI] [PubMed] [Google Scholar]; Taphoorn MJ, Bent MJ, Mauer ME, Coens C, Delattre JY, Brandes AA, et al. Health‐related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: results of a European Organisation for Research and Treatment of Cancer randomized clinical trial. Journal of Clinical Oncology 2007;25(36):5723‐30. [DOI] [PubMed] [Google Scholar]
- Vigliani MC, Sichez N, Poisson M, Delattre JY. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4‐year results. International Journal of Radiation Oncology, Biology, Physics 1996;35(3):527‐33. [DOI] [PubMed] [Google Scholar]
- Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long‐term results of RTOG 9402. Journal of Clinical Oncology 2013;31(3):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang M, Cairncross G, Shaw E, Jenkins R, Scheithauer B, Brachman D, et al. Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: radiation therapy oncology group trial 9402. International Journal of Radiation Oncology, Biology, Physics 2010;77(3):662‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ali AN, Zhang P, Yung WK, Chen Y, Movsas B, Urtasun RC, et al. NRG oncology RTOG 9006: a phase III randomized trial of hyperfractionated radiotherapy (RT) and BCNU versus standard RT and BCNU for malignant glioma patients. Journal of Neuro‐oncology 2018;137(1):39‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand AK, Chaudhory AR, Aggarwal HN, Sachdeva PK, Negi PS, Sinha SN, et al. Survival outcome and neurotoxicity in patients of high‐grade gliomas treated with conformal radiation and temozolamide. Journal of Cancer Research and Therapeutics 2012;8(1):50‐6. [DOI] [PubMed] [Google Scholar]
- Archibald YM, Lunn D, Ruttan LA, Macdonald DR, Maestro RF, Barr HWK, et al. Cognitive functioning in long‐term survivors of high‐grade glioma. Journal of Neurosurgery 1994;80(2):247‐53. [DOI] [PubMed] [Google Scholar]
- Armstrong CL, Hunter JV, Ledakis GE, Cohen B, Tallent EM, Goldstein BH, et al. Late cognitive and radiographic changes related to radiotherapy: initial prospective findings. Neurology 2002;59(1):40‐8. [DOI] [PubMed] [Google Scholar]
- Behrend SW. Patients with primary brain tumors. Oncology Nursing Forum 2014;41(3):335‐6. [DOI] [PubMed] [Google Scholar]
- Brown PD, Buckner JC, Uhm JH, Shaw EG. The neurocognitive effects of radiation in adult low‐grade glioma patients. Neuro‐oncology 2003;5(3):161‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Cerhan JH. Same, better or worse? Neurocognitive effects of radiotherapy for low‐grade gliomas remain unknown. Lancet Neurology 2009;8(9):779‐81. [DOI] [PubMed] [Google Scholar]
- Buglione M, Pedretti S, Gipponi S, Todeschini A, Pegurri L, Costa L, et al. Radiotherapy in low‐grade glioma adult patients: a retrospective survival and neurocognitive toxicity analysis. Radiologia Medica 2014;119(6):432‐9. [DOI] [PubMed] [Google Scholar]
- Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman DXS‐G, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. Journal of Clinical Oncology 2006;24(18):2707‐14. [DOI] [PubMed] [Google Scholar]
- Chung C, Brown P, Liu D, Grosshans D, Dibaj S, et al. Ph II randomized trial comparing cognitive outcomes of proton vs. photon radiation for glioblastoma. Radiotherapy and Oncology ‐ 2018 Annual Conference of the European Society for Radiotherapy and Oncology, ESTRO 37. Spain. 127. 2018:S686. [Google Scholar]
- Combs SE, Nagy M, Edler L, Rausch R, Bischof M, Welzel T, et al. Comparative evaluation of radiochemotherapy with temozolomide versus standard‐of‐care postoperative radiation alone in patients with WHO grade III astrocytic tumors. Radiotherapy and Oncology 2008;88(2):177‐82. [DOI] [PubMed] [Google Scholar]
- Corn BW, Wang M, Fox S, Michalski J, Purdy J, Simpson J, et al. Health related quality of life and cognitive status in patients with glioblastoma multiforme receiving escalating doses of conformal three dimensional radiation on RTOG 98‐03. Journal of Neuro‐oncology 2009;95(2):247‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Shi W, Thaler HT, Cheung AM, DeAngelis LM, Abrey LE. Longitudinal cognitive follow‐up in low grade gliomas. Journal of Neuro‐oncology 2008;86(3):321‐7. [DOI] [PubMed] [Google Scholar]
- Costello A, Shallice T, Gullan R, Beaney R. The early effects of radiotherapy on intellectual and cognitive functioning in patients with frontal brain tumours: the use of a new neuropsychological methodology. Journal of Neuro‐oncology 2004;67(3):351‐9. [DOI] [PubMed] [Google Scholar]
- Dai PY, Wang SW, Zhang CR, Chen FQ. Three‐dimensional conformal radiotherapy combined with temozolomide for the treatment of brain glioma [Chinese]. Chinese Journal of Cancer Prevention and Treatment 2011;18(2):128‐30. [Google Scholar]
- Ding Z, Ding M, Ning X, Pang J, Zhao C. Clinical safety study of temozolomide combined with radiotherapy after glioma resection. Biomedical Research (India) 2017;28(13):5987‐91. [Google Scholar]
- Ediebah DE, Galindo‐Garre F, Uitdehaag BM, Ringash J, Reijneveld JC, Dirven L, et al. Joint modeling of longitudinal health‐related quality of life data and survival. Quality of Life Research 2015;24(4):795‐804. [DOI] [PubMed] [Google Scholar]
- Eyre HJ, Crowley JJ, Townsend JJ, Eltringham JR, Morantz RA, Schulman SF, et al. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low‐grade gliomas: a Southwest Oncology Group study. Journal of Neurosurgery 1993;78(6):909‐14. [DOI] [PubMed] [Google Scholar]
- Goda JS, Gupta T, Jalali R. Long term outcomes of stereotactic conformal radiation therapy in the treatment of benign and low‐grade brain tumors. Translational Cancer Research 2017;6(Supplement9):S1489‐S90. [Google Scholar]
- Gregor A, Cull A, Traynor E, Stewart M, Lander F, Love S. Neuropsychometric evaluation of long‐term survivors of adult brain tumours: relationship with tumour and treatment parameters. Radiotherapy and Oncology 1996;41(1):55‐9. [DOI] [PubMed] [Google Scholar]
- Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long‐term brain tumour survivors after radiotherapy. Radiotherapy and Oncology 2003;69(2):169‐76. [DOI] [PubMed] [Google Scholar]
- Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, Witte O, et al. Randomized trial on the efficacy of radiotherapy for cerebral low‐grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. International Journal of Radiation Oncology, Biology, Physics 2002;52(2):316‐24. [DOI] [PubMed] [Google Scholar]
- Klein M, Heimans JJ. The measurement of cognitive functioning in low‐grade glioma patients after radiotherapy. Journal of Clinical Oncology 2004;22(5):966‐7; author reply 7. [DOI] [PubMed] [Google Scholar]
- Laack NN, Brown PD, Ivnik RJ, Furth AF, Ballman KV, Hammack JE, et al. Cognitive function after radiotherapy for supratentorial low‐grade glioma: a North Central Cancer Treatment Group prospective study. International Journal of Radiation Oncology, Biology, Physics 2005;63(4):1175‐83. [DOI] [PubMed] [Google Scholar]
- Lunsford LD, Kondziolka D. Adverse long‐term effects of brain radiotherapy in adult low‐grade glioma patients. Neurology 2001;57(11):2150‐1. [DOI] [PubMed] [Google Scholar]
- Malmstrom A, Poulsen HS, Gronberg BH, Stragliotto G, Hansen S, Asklund T, et al. Postoperative neoadjuvant temozolomide before radiotherapy versus standard radiotherapy in patients 60 years or younger with anaplastic astrocytoma or glioblastoma: a randomized trial. Acta Oncologica 2017;56(12):1776‐85. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Brain Tumour Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high‐grade astrocytoma: a Medical Research Council trial. Journal of Clinical Oncology 2001;19(2):509‐18. [DOI] [PubMed] [Google Scholar]
- NCT. Trial of Newly Diagnosed High Grade Glioma Treated With Concurrent Radiation Therapy, Temozolomide and BMX‐001. clinicaltrials.gov/show/nct026556012016.
- Packer RJ. Radiation‐induced neurocognitive decline: the risks and benefits of reducing the amount of whole‐brain irradiation. Current Neurology and Neuroscience Reports 2002;2(2):131‐3. [DOI] [PubMed] [Google Scholar]
- Repka M, Lei S, Campbell L, Suy S, Voyadzis J, Kalhorn C, et al. Long‐term outcomes following conventionally fractionated stereotactic boost for high‐grade glioma. Radiotherapy and Oncology ‐ Conference: 2018 Annual Conference of the European Society for Radiotherapy and Oncology, ESTRO 37. Spain. 127. 2018, issue 1:S673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoer D, Visch‐Brink E, Smits M, Kloet A, Looman C, Dirven C, et al. Long‐term evaluation of cognition after glioma surgery in eloquent areas. Journal of Neuro‐oncology 2014;116(1):153‐60. [DOI] [PubMed] [Google Scholar]
- Shaw EG, Rosdhal R, D'Agostino, RB, Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. Journal of Clinical Oncology 2006;24(9):1415‐20. [DOI] [PubMed] [Google Scholar]
- Sherman JC, Colvin MK, Mancuso SM, Batchelor TT, Oh KS, Loeffler JS, et al. Neurocognitive effects of proton radiation therapy in adults with low‐grade glioma. Journal of Neuro‐oncology 2016;126(1):157‐64. [DOI] [PubMed] [Google Scholar]
- Sichez N, Chatellier G, Poisson M, Delattre JY. Supratentorial gliomas: neuropsychological study of long‐term survivors. Revue Neurologique 1996;152(4):261‐6. [PubMed] [Google Scholar]
- Surma‐aho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, et al. Adverse long‐term effects of brain radiotherapy in adult low‐grade glioma patients. Neurology 2001;56(10):1285‐90. [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ, Schiphorst AK, Snoek FJ, Lindeboom J, Wolbers JG, Karim AB, et al. Cognitive functions and quality of life in patients with low‐grade gliomas: the impact of radiotherapy. Annals of Neurology 1994;36(1):48‐54. [DOI] [PubMed] [Google Scholar]
- Taylor BV, Buckner JC, Cascino TL, O'Fallon JR, Schaefer PL, Dinapoli RP, et al. Effects of radiation and chemotherapy on cognitive function in patients with high‐grade glioma. Journal of Clinical Oncology 1998;16(6):2195‐201. [DOI] [PubMed] [Google Scholar]
- Thomas D, Stenning S, Lantos P, Ironside J, Moss T, Whaley J, et al. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high‐grade astrocytoma: a Medical Research Council Trial. Journal of Clinical Oncology 2001;19(2):509‐18. [DOI] [PubMed] [Google Scholar]
- Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression‐free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. Journal of Clinical Oncology 2006;24(18):2715‐22. [DOI] [PubMed] [Google Scholar]
- Wheeler LA, Manzanera AG, Bell SD, Cavaliere R, McGregor JM, Grecula JC, et al. Phase II multicenter study of gene‐mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro‐oncology 2016;18(8):1137‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, et al. NOA‐04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. Journal of Clinical Oncology 2009;27(35):5874‐80 XIR. [DOI] [PubMed] [Google Scholar]
- Williamson A, Mackinnon M, James A, Chalmers A, Nowicki S. Reirradiation of recurrent glioma. Neuro‐Oncology ‐ Conference: 5th Quadrennial Meeting of the World Federation of Neuro‐Oncology Societies, WFNOS. Switzerland. 19. 2017, issue Supplement 3:iii120. [Google Scholar]
- Wirsching H. G, Tabatabai G, Hottinger A, Plasswilm L, Roelcke U, Conen K, et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the arte trial. Neuro‐Oncology. Conference: 5th quadrennial meeting of the world federation of neuro‐oncology societies, WFNOS. Switzerland. 2017. [Google Scholar]; Wirsching H. G, Tabatabai G, Roelcke U, Hottinger A. F, Jorger F, Schmid A, et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the randomized, open‐label, phase II ARTE trial. Annals of Oncology 2018;29(6):1423‐1430. [DOI] [PubMed] [Google Scholar]
- Zhu J. J, Demireva P, Kanner A. A, Pannullo S, Mehdorn M, Avgeropoulos N, et al. Health‐related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF‐14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. Journal of Neuro‐oncology 2017;135(3):545‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053‐22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non‐co‐deleted anaplastic glioma: a phase 3, randomised, open‐label intergroup study. Lancet 2017;390(10103):1645‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Drijver J, Bent M, Hoang‐Xuan K, Taphoorn MJB, Reijneveld JC, et al. Memory functioning in low‐grade glioma patients treated with either radiotherapy (RT) or temozolomide (TMXZ) chemotherapy. A correlative analysis of European organization for research and treatment (EORTC) study 22033‐26033. Neuro‐oncology. 2017; Vol. 19 Suppl 6:Society for Neuro‐Oncology 2017 conference proceedings. [Google Scholar]
- Corn B. The influence of radiotherapy on cognitive function. www.clinicaltrials.gov2007.
- Jacob S. Study of neurological complication after radiotherapy for high grade glioblastoma (EPIBRAINRAD). www.clinicaltrials.gov2015.
- Laack N. Cognitive function after treatment of primary CNS malignancy. www.clinicaltrials.gov2017.
- NRG Oncology. Proton beam or intensity‐modulated radiation therapy in preserving brain function in patients with IDH mutant grade II or III glioma. www.clinicaltrials.gov2017.
Additional references
- Armstrong T, Vera‐Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T. The symptom burden of primary brain tumours: evidence for a core set of tumor‐ and treatment‐related symptoms. Neuro‐oncology 2016;18(2):252‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TS, Shade MY, Breton G, Gilbert MR, Mahajan A, Scheurer ME, et al. Sleep‐wake disturbance in patients with brain tumors. Neuro‐oncology 2017;19(3):323‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist P, Lycke J, Strang P, Törnqvist H, Ekbom A. Brain tumours in Sweden 1996: care and costs. Journal of Neurology, Neurosurgery, and Psychiatry 2000;69:792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele FW, Douw L, Reijneveld JC, Robben R, Taphoorn MJ, Aaronson NK, et al. Health‐related quality of life in stable, long‐term survivors of low‐grade glioma. Journal of Clinical Oncology 2015;33(9):1023‐9. [DOI] [PubMed] [Google Scholar]
- Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus procarbazine, CCNU, and vincristine in low‐grade glioma. New England Journal of Medicine 2016;374(14):1344‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long‐term results of RTOG 9402. Journal of Clinical Oncology 2013;31(3):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Karunamuni R, McDonald C, Seibert T, White N, Moiseenko V, et al. Regional susceptibility to dose‐dependent white matter damage after radiotherapy. Radiotherapy and Oncology 2017;123:209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veritas Health Innovation. Covidence. Version accessed 6 June 2018. Melbourne, Australia: Veritas Health Innovation, 2018.
- Day J, Gillespie DC, Rooney AG, Bulbeck HJ, Zienius K, Boele F, et al. Neurocognitive deficits and neurocognitive rehabilitation in adult brain tumors. Current Treatment Options in Neurology 2016;18(5):22. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
- Dhermain F, Barani IJ. Complications from radiotherapy. In: Berger MS, Weller M editor(s). Handbook of Clinical Neurology. 3rd Edition. Vol. 134, Elsevier, 2016:219‐34. [DOI] [PubMed] [Google Scholar]
- Douw L, Klein M, Fagel SS, Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low‐grade glioma: long‐term follow‐up. Lancet Neurology 2009;8(9):810‐8. [DOI] [PubMed] [Google Scholar]
- Effective Practice, Organisation of Care (EPOC). EPOC Resources for review authors, 2017. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed 14 July 2016).
- Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low‐grade adult brain tumours. International Journal of Radiation Oncology, Biology, Physics 2012;83:e487‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondi V, Pugh S, Brown PD, Wefel J, Gilbert M, Bovi J, et al. Preservation of neurocognitive function with hippocampal avoidance during whole‐brain radiotherapy for brain metastases: preliminary results of phase III trial NRG oncology CC001. Neuro‐oncology. 2018; Vol. 20 Suppl 6:Society of Neuro‐Oncology 2018 conference proceedings. [Google Scholar]
- Grill J, Renaux VK, Bulteau C, Viguier D, Levy‐Piebois C, Sainte‐Rose C, et al. Long‐term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. International Journal of Radiation Oncology, Biology, Physics 1999;45(1):137‐45. [DOI] [PubMed] [Google Scholar]
- Habets EJ, Taphoorn MJ, Nederend S, Klein M, Delgadillo D, Hoang‐Xuan K, Bottomly A, et al. Health‐related quality of life and cognitive functioning in long‐term anaplastic oligodendroglioma and oligoastrocytoma survivors. Journal of Neuro‐oncology 2014;116(1):161‐8. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- MacDonald L, Neuro‐Oncology Group. Top 10 priorities for clinical research in primary brain and spinal cord tumours. www.jla.nihr.ac.uk/priority‐setting‐partnerships/neuro‐oncology/top‐10‐priorities (accessed 1 March 2018).
- Kyriakakis N, Orme SM, Gerrard G, Hatfield P, Loughrey C, et al. Pituitary dysfunction following cranial radiotherapy for adult‐onset non‐pituitary brain tumours. Clinical Endocrinology 2016;84:372‐97. [DOI] [PubMed] [Google Scholar]
- Kyriakakis N, Lynch J, Orme SM, Gerrard G, Hatfield P, Short SC, et al. Hypothalamic‐pituitary axis irradiation dose thresholds for the development of hypopituitarism in adult‐onset gliomas. Clinical Endocrinology (accessed prior to 10 July 2019). [DOI: 10.1111/cen.13971] [DOI] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathology 2007;114(2):97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, Deimling A, Figarella‐Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathology 2016;131(6):803‐20. [DOI] [PubMed] [Google Scholar]
- Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty of the evidence in the absence of a single estimate of effect. Evidence‐based Medicine 2017;22(3):85‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H. Epidemiology of brain tumours. In: Verma, M editor(s). Methods of Molecular Biology, Cancer Epidemiology. Vol. 472, Totowa (NJ): Humana Press, 2009:323‐42. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Leyrer CM, Greene‐Schloesser DM, Shing E, Kearns WT, Hinson WH, et al. Neuroanatomical target theory as a predictive model for radiation‐induced cognitive decline. Neurology 2013;80:747‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma TJ, Klein M, Verstappen CC, Bromberg JE, Swennen M, Langendijk JA, et al. Radiotherapy‐induced cerebral abnormalities in patients with low‐grade glioma. Neurology 2002;59(1):121‐3. [DOI] [PubMed] [Google Scholar]
- Raizer J, Fitzner K, Jacobs D, Bennett C, Liebling D, Luu T, et al. Economics of Malignant Gliomas: A Critical Review. Journal of Oncology Practice / American Society of Clinical Oncology 2015;11:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Sarmiento JM, Venteicher AS, Patil CG. Early versus delayed postoperative radiotherapy for treatment of low‐grade gliomas. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD009229.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Seaver E, Geyer R, Sulzbacher S, Warner M, Batzel L, Milstein J, et al. Psychosocial adjustment in long‐term survivors of childhood medulloblastoma and ependymoma treated with craniospinal irradiation. Pediatric Neurosurgery 1994;20(4):248‐53. [DOI] [PubMed] [Google Scholar]
- Shemilt I, Aluko P, Graybill E, Craig D, Henderson C, Drummond M, et al. Chapter 20: Economics evidence. Draft version (13 September 2018). In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch V editor(s). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane, 2018:1‐21. [Google Scholar]
- Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. Journal of Clinical Oncology 2004;22(4):706‐13. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine 2005;10:987‐96. [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ, Heimans JJ, Veen EA, Karim AB. Endocrine functions in long‐term survivors of low‐grade supratentorial glioma treated with radiation therapy. Journal of Neuro‐oncology 1995;2:97‐102. [DOI] [PubMed] [Google Scholar]
- Terashima K, Chow K, Jones J, Ahern C, Jo E, Ellezam B, et al. Long‐term outcome of centrally located low‐grade glioma in children. Cancer 2013;119(14):2630‐8. [DOI] [PubMed] [Google Scholar]
- Williams NL, Rotondo RL, Bradley JA, Pincus DW, Fort JA, Wynn T, et al. Late effects after radiotherapy for childhood low‐grade glioma. American Journal of Clinical Oncology 2018;41(3):307‐12. [DOI] [PubMed] [Google Scholar]


