Abstract
Novel substituted phenoxyalkyl pyridinium oximes, previously shown to reactivate brain cholinesterase in rats treated with high sublethal dosages of surrogates of sarin and VX, were tested for their ability to prevent mortality from lethal doses of these two surrogates. Rats were treated subcutaneously with 0.6 mg/kg nitrophenyl isopropyl methylphosphonate (NIMP; sarin surrogate) or 0.65 mg/kg nitrophenyl ethyl methylphosphonate (NEMP; VX surrogate), dosages that were lethal within 24 hr to all tested rats when they received only 0.65 mg/kg atropine at the time of initiation of seizure-like behavior (about 30 min). If 146 mmol/kg 2-PAM (human equivalent dosage) was also administered, 40% and 33% survival was obtained with NIMP and NEMP, respectively, while the novel Oximes 1 and 20 provided 65% and 55% survival for NIMP and 75 and 65% for NEMP, respectively. In addition, both novel oximes resulted in a highly significant decrease in time to cessation of seizure-like behavior compared to 2-PAM during the first 8 hours of observation. Brain cholinesterase inhibition was slightly less in novel oxime treated rats compared to 2-PAM in the 24 hour survivors. The lethality data indicate that 24 hour survival is improved by two of the novel oximes compared to 2-PAM. The cessation of seizure-like behavior data strongly suggest that these novel oximes are able to penetrate the blood-brain barrier and can combat the hypercholinergic activity that results in seizures. Therefore this oxime platform has exceptional promise as therapy that could both prevent nerve agent-induced lethality and attenuate nerve agent-induced seizures.
Keywords: organophosphate, acetylcholinesterase reactivators, nerve agent surrogates, prevention of lethality, anticholinesterase
1.1 Introduction
Chemical warfare agents (CWAs) have long been a concern in both warfare and civilian terrorist attacks. One of the most toxic groups of CWAs is the class of organophosphate (OP) nerve agents whose mechanism of toxic action is the inhibition of acetylcholinesterase (AChE) resulting in the accumulation of the neurotransmitter acetylcholine and subsequent overstimulation of cholinergic pathways in both the peripheral and central nervous systems. High levels of AChE inhibition in the peripheral nervous system can result in respiratory failure and subsequent death. In addition, hyperstimulation of the cholinergic system in the central nervous system (CNS) can result in excitotoxicity, seizures, and cognitive dysfunction (Ecobichon et al., 2001).
Two of the most toxic OP nerve agents, sarin and VX, were synthesized during and immediately after World War II, and are still of concern today (Johnson et al., 2015). These compounds were synthesized and stockpiled in the United States, Germany, Russia, Great Britain, and other countries (Tucker, 2007). Despite a large amount of these CWAs being destroyed following the Chemical Weapons Convention in 1993 (Tucker, 2007), other countries including Iraq and Syria produced and used nerve agents in warfare and on civilians during the 1980s and 2000s, respectively (Smart, 1997; Johnson et al., 2015). Sarin was also synthesized and deployed by a terrorist group on a Japanese subway in 1994 resulting in several deaths as well as many victims suffering long term neurological deficits (Nishiwaki et al., 2001; Miyaki et al., 2005). The attacks with sarin on civilians in Syria in 2013 also emphasize the concern that unsuspecting persons remain at risk of nerve agent toxicity (Johnson et al., 2015). Therefore nerve agents are not only a military hazard, they are a threat to civilians from potential terrorism.
Treatment for OP exposure traditionally involves the administration of a muscarinic receptor antagonist, atropine, and an oxime AChE reactivator (e.g., pralidoxime or 2-PAM approved by the Food and Drug Administration in the USA). Benzodiazepines (e.g., diazepam) may be administered to mitigate OP induced seizures. A major limitation of the current drug regimen is the very limited ability of the approved oximes to cross the blood-brain barrier (BBB), and, therefore, their inability to reactivate AChE in the CNS (Clement, 1979; Kuca et al., 2005; Skovira et al., 2010).
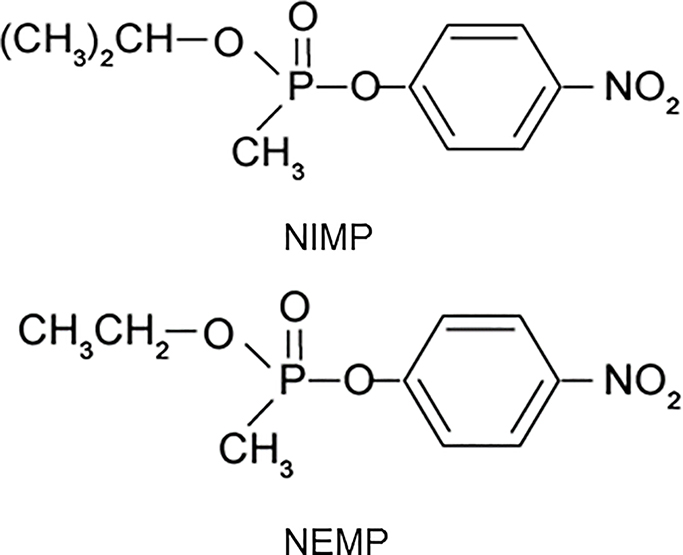
A series of novel pyridinium oximes, designed and synthesized in our laboratories, incorporate moieties to increase their lipophilicity, thus enhancing the likelihood of crossing the BBB and reactivating OP-inhibited brain cholinesterase (ChE) (Chambers et al., 2013). Additionally, experimental OPs were synthesized as highly relevant surrogates for the nerve agents sarin and VX to investigate the ChE reactivation potential of the novel oximes both in vitro and in vivo (Meek et al., 2012). These surrogates, nitrophenyl isopropyl methylphosphonate (NIMP, sarin surrogate) and nitrophenyl ethyl methylphosphonate (NEMP, VX surrogate) are non-volatile and phosphylate ChE with the same moiety as their respective nerve agents, making them highly relevant for testing of reactivator efficacy (Fig. 1). The novel oximes were screened in vitro for efficacy as reactivators of NIMP- and NEMP-inhibited rat brain ChE (Chambers et al., 2013) A subset of oximes that demonstrated good reactivation potential in vitro were subsequently tested in vivo in rats using the treatment paradigm of a high sub-lethal dosage of the surrogates, which induced about 80% peak brain ChE inhibition and yielded seizure-like behavior, but did not require atropine or other drugs for survival. Several of the novel oximes displayed substantial efficacy in vivo at reactivating rat brain ChE activity and attenuating seizure-like behavior in this sub-lethal paradigm.
Figure 1.
Structures of nitrophenyl isopropyl methylphosphonate (NIMP; sarin surrogate) and nitrophenyl ethyl methylphosphonate (NEMP; VX surrogate).
Several groups have developed and/or tested new oxime structures for better efficacy and a broader spectrum of activity (Kalisiak et al., 2012; Kuca et al., 2005; Worek et al., 2004). Some of the most efficacious of the new reactivators are bis-pyridinium oximes that possess two positive charges and would have even greater difficulty penetrating the BBB than the mono-pyridinium oximes (Musilek et al., 2011; Worek and Thiermann, 2013). There have also been efforts at developing reactivators that can penetrate the BBB (Kalisiak et al., 2011; Mercey et al., 2012; Radic et al., 2012). However, these are still in the developmental stages and their efficacy has not been proven. Additionally some older oximes have been revisited to determine their ability to cross the BBB with limited success, including the non-charged tertiary oxime MINA (Skovira et al., 2010), and pro-PAM, which is more lipophilic and can be converted to 2-PAM in the CNS (DeMar et al., 2010).
The most urgent goal following an OP nerve agent exposure is saving the lives of those experiencing lethal levels. However restoring full nervous system function is also critical and the current oxime antidote, 2-PAM, cannot because it does not penetrate the BBB appreciably and, therefore, it cannot prevent or attenuate OP-induced seizures and subsequent brain damage. Thus an improved therapeutic strategy would not only save lives but would also rescue the brain from damage by preventing or attenuating seizures. The goal of the present study was to expand on the earlier studies, that demonstrated novel oxime efficacy in reactivating brain ChE and attenuating seizure-like behavior in a sub-lethal paradigm (Chambers et al., 2013), by testing the efficacy of the novel oximes to prevent lethality, reactivate inhibited brain ChE, and attenuate OP induced seizure-like behavior at lethal levels of the surrogates in a rat model.
1.2 Materials and methods
1.2.1 Materials
1.2.1.1 Nerve agent surrogates
Two highly relevant nerve agent surrogates were studied for efficacy testing. Nitrophenyl isopropyl methyl phosphonate (NIMP; sarin surrogate) and nitrophenyl ethyl methyl phosphonate (NEMP; VX surrogate) were synthesized in our laboratories as described earlier (Meek et al., 2012; Fig. 1).
1.2.1.2 Novel oxime reactivators
The novel oximes (patent pending) were first described in Chambers et al. (2013) and the structures of the specific oximes tested in the present study are shown in Fig. 2.
Figure 2.
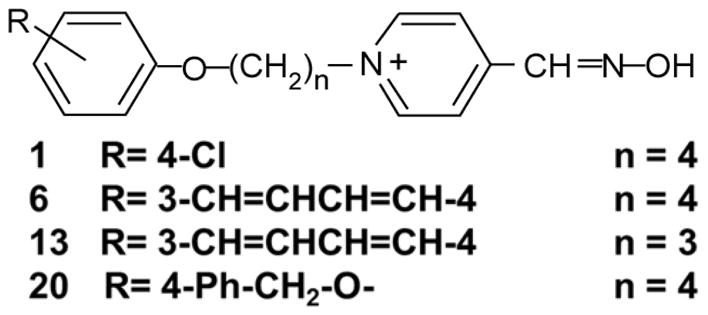
Structures of novel substituted phenoxy alkyl pyridinium oximes tested for their ability to provide survival from lethal level exposures of NIMP and NEMP. R indicates the substitution on the phenoxy group and n indicates the number of methylene groups in the linker chain.
1.2.1.3 Animals
Adult male Sprague Dawley-derived rats (Harlan Laboratories) were housed in an AAALAC-accredited facility in a temperature-controlled environment (22±2°C) with a 12-h dark-light cycle with lights on between 0700 and 1900 hours. LabDiet rodent chow and tap water were provided ad libitum. All animal protocols received prior approval from the Mississippi State University Animal Care and Use Committee.
1.2.2 Methods
1.2.2.1 Treatment paradigm
Initial testing occurred with a variety of dosages and testing paradigms. Briefly, the times of atropine and NIMP or NEMP administration were varied to identify the best testing paradigm. Atropine was administered 1, 5, 15, or 30 min post-surrogate exposure with the oxime administration at 15 min or at seizure onset (about 30 min). Atropine administration at early time points (1, 5, or 15 min) required higher dosages of surrogate to achieve LD99 results and yielded varied responses in lethality and seizure-like behavior onset, perhaps due to rapid absorption and clearance of atropine and absorption of the surrogates. Administration of oxime prior to seizure onset also resulted in varied lethality and seizure-like behavior development.
A treatment paradigm was selected for the OP’s that provided a lethal dose by 24 hr when only atropine was provided and which provided only a moderate level of survival with atropine plus 2-PAM so that any improved survival that was afforded by the novel oximes could be detected. Consistent with the time delay that would be likely in the event of a terrorist attack or accident, the antidotes were delivered at the time of initiation of signs of seizure-like behavior, about 30 min post OP administration. An LD99 dosage (SC) of each of the OP’s (dissolved in a biocompatible solvent, Multisol) was established with the following criteria: 1) the dosage was lethal within 24 hr to all rats treated together with 0.65 mg/kg atropine (free base) in saline IM (dosage within the range of atropine dosages typically used in experiments testing antidote efficacy for lethal dosages of nerve agents) and 2) the dosage was lethal within 24 hr to a moderate number of rats treated IM with atropine plus 2-PAM in Multisol at 0.146 mmol/kg (human equivalent dosage for 3 autoinjectors). Multisol is composed of 48.5% water, 40% propylene glycol, 10% ethanol, and 1.5% benzyl alcohol. Dosages of the OP’s are 0.6 mg/kg for NIMP and 0.65 mg/kg for NEMP. Novel oximes in Multisol were administered at 0.146 mmol/kg (2-PAM molar equivalent dosage). Antidote (atropine ± oxime) was administered at time of seizure onset: NIMP, 25 min; NEMP, 30 min. Animals were observed for signs of toxicity including seizure-like behavior during the first 8 hr following treatment. Rats surviving to 24 hr were humanely euthanized. At the time of death, brain, blood (separated into plasma and erythrocytes), and skeletal muscle were frozen for subsequent ChE analysis. Twenty replications were conducted for vehicle controls, 2-PAM, Oxime 1 and Oxime 20 with both surrogates, and 15 replications were conducted for Oxime 1 + 2-PAM and Oxime 20 + 2-PAM (same dosages of each oxime as done in the single oxime trials) with both surrogates. Because of poorer efficacy with Oximes 6 and 13, only 11 replications were conducted.
1.2.2.2 Documentation of seizure-like behavior
Animals were monitored post OP exposure using the following cholinergic toxicity scale, which closely follows the Racine scale for scoring of seizures (Luttjohann et al., 2009). Scores were as follows: 0) normal behavior; 1) oro-alimentary movements and head nodding; 2) anterior limb clonus and rearing; 3) loss of balance (falling), jumping and running; 4) tonic convulsions; 5) death. Animals were monitored constantly for the first 4 hours following OP exposure and every 30 min thereafter during the first 8 hours. All surviving animals were sacrificed at 24 hours post OP exposure.
1.2.2.3 Cholinesterase assay
Tissues were analyzed spectrophotometrically for ChE activity using our modification (Chambers et al., 1988) of the Ellman technique (Ellman et al., 1961), and percent inhibition was calculated compared to vehicle controls. All incubations were performed at 37°C in a shaking water bath. Brain and skeletal muscle samples were homogenized and assayed at a concentration of 1 mg/ml or 5 mg/ml, respectively, in Tris-HCl (0.05 M, pH 7.4 at 25°C). Blanks had all ChE activity inhibited by adding 0.01mM (final concentration) eserine sulfate and incubating for 15 minutes. Next, 1mM acetylthiocholine iodide (final concentration) was added as the substrate to all samples and blanks, with the reaction terminated after 15 minutes by adding a mixture of 0.05% sodium dodecyl sulfate and 2.6 mM 5,5′-dithio-bis(nitrobenzoic acid). The absorbance was measured at 412nm. Blood was collected with EDTA as an anticoagulant and was centrifuged at 1,000 g for 10 min at 4°C to separate plasma and erythrocytes, which were then diluted in sodium phosphate buffer (0.1M, pH 8.0 at 25°C) to 0.001 μl/ml and 0.0005 μl/ml, respectively. Diluted plasma and erythrocytes were assayed continuously with a mixture of 1 mM (final concentration) acetylthiocholine and 0.33 mM 5,5′-dithio-bis(nitrobenzoic acid) for 60 minutes at 37°C in sodium phosphate buffer (0.1 M, pH 8.0 at 25°C). Additionally, protein concentrations were quantified for all samples using the Folin phenol reagent (Lowry et al., 1951).
1.2.3 Statistical Methods
The effect of treatment on time to death and time to seizure cessation was analyzed using survival analysis. Separate models were developed for each outcome when rats were exposed to each of the nerve agent surrogates. The Kaplan-Meier estimator was used to estimate the survivor functions using PROC LIFETEST, SAS for Windows 9.4 (SAS Institute, Inc., Cary, NC, USA). The data for both outcomes were right censored at 8 hours post exposure. The log-rank test statistic was used to assess the effect of treatment. When treatment was found to have a significant effect, Dunnett’s adjustment for multiple comparisons was used to compare each of the novel oximes to 2-PAM. The survivor functions were graphically displayed as Kaplan-Meier plots using PROC SGPLOT.
The effect of treatment on 24 hour survival was assessed using logistic regression with PROC LOGISTIC, SAS for Windows 9.4. 2-PAM was used as the referent for the other treatments. Separate models were fit for each of the nerve agent surrogates. An alpha level of 0.10 was selected to determine statistical significance rather than the more conventional 0.05 in recognition that the objective of the study was to screen novel oximes to find the best candidates for future, more intensive evaluation but to also use as few rats as possible for humaneness.
Differences in inhibition of ChE in brain and in peripheral tissues was assessed by ANOVA followed by Tukey’s mean separation test, with an alpha level of 0.05.
1.3 Results
Four novel oximes together with atropine were tested with lethal level exposures to NIMP and NEMP; structures are shown in Fig. 2. With NIMP all animals which received only atropine and no oxime died with an average time of 1.5 hr (Table 1). Animals receiving 2-PAM showed 40% survival at 24 hr with an average time to death of those not surviving of 2 hr. Poorer efficacy than with 2-PAM was observed with Oxime 6 (27%) and Oxime 13 (0%), so fewer replications (11) were conducted with these two oximes, and they were not pursued further. Oximes 1 and 20 showed better efficacy alone (65% and 55%, respectively) and in combination with 2-PAM (73 and 80%, respectively) than 2-PAM alone. Average time to death of animals which died with these therapies was 1.5–3 hr. There were no differences in time to death among any of the oxime treatments.
Table 1.
Percent survival (% S) at 24 hr of rats treated with nitrophenyl isopropyl methylphosphonate (NIMP; sarin surrogate; 0.6 mg/kg SC) or nitrophenyl ethyl methylphosphonate (NEMP; VX surrogate; 0.65 mg/kg SC) followed by atropine (0.65 mg/kg IM) ± oxime (2-PAM or novel oximes; 0.146 mmol/kg IM) at 25 (NIMP) or 30 min (NEMP). S/T=number surviving to 24 hr/number treated. Odds ratios are for survival from novel oximes alone or in combination with 2-PAM to survival from 2-PAM alone; number greater than 1 indicates improved survival of novel oxime compared to 2-PAM; probability of statistical significance was set at 0.1.
| NIMP | NEMP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds | P | Odds | P | |||||||
| Oxime | Time, h | S/T | % S | Ratio | Value | Time, h | S/T | % S | Ratio | Value |
| None | 1.5 | 0/20 | 0 | 2 | 0/20 | 0 | ||||
| 2-PAM | 2 | 8/20 | 40 | 2.5 | 6/20 | 33 | ||||
| Oxime 1 | 1.5 | 13/20 | 65 | 2.785 | 0.117 | 2 | 15/20 | 75 | 6.993 | 0.006 |
| Oxime 20 | 1.5 | 11/20 | 55 | 1.834 | 0.344 | 4 | 13/20 | 65 | 4.926 | 0.030 |
| Ox1+2-PAM | 2.5 | 11/15 | 73 | 4.132 | 0.056 | 4 | 8/15 | 53 | 2.667 | 0.168 |
| Ox20+2-PAM | 3 | 13/15 | 80 | 9.708 | 0.010 | 4 | 8/15 | 53 | 2.667 | 0.168 |
With NEMP, all animals receiving only atropine died with an average time to death of 2 hr (Table 1). Animals receiving 2-PAM showed 33% survival with an average time to death of 2.5 hr. Similar to NIMP, Oximes 6 and 13 showed poorer efficacy than 2-PAM, 27% and 18%, respectively, and fewer replications were conducted. Oximes 1 and 20 showed considerably better efficacy alone (75% and 65%, respectively) and in combination with 2-PAM (53% for both oximes) than 2-PAM alone. Average time to death of animals which died with these therapies was 2–4 hr. There were no differences in time to death among any of the oxime treatments.
Logistic regression analysis of the 24 hr survival data in which novel oxime treatments were compared to 2-PAM treatments indicated statistically significant odds ratios greater than 1 for both novel oximes alone and in combination with 2-PAM with both NIMP and NEMP (P < 0.1) (Table 1). Odds ratios were lower for Oximes 1 and 20 alone (2.8 and 1.8, respectively) than in combination with 2-PAM (4.1 and 9.7, respectively) for NIMP, while odds ratios were higher for Oximes 1 and 20 alone (7.0 and 4.9, respectively) than in combination with 2-PAM (2.7 for both oximes) for NEMP.
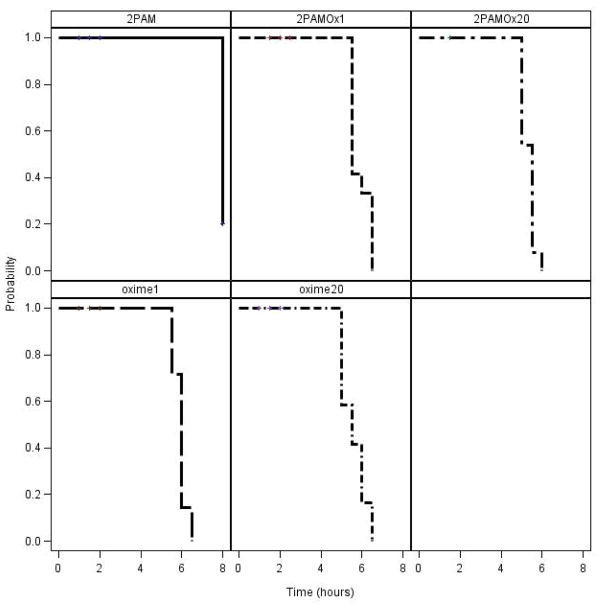
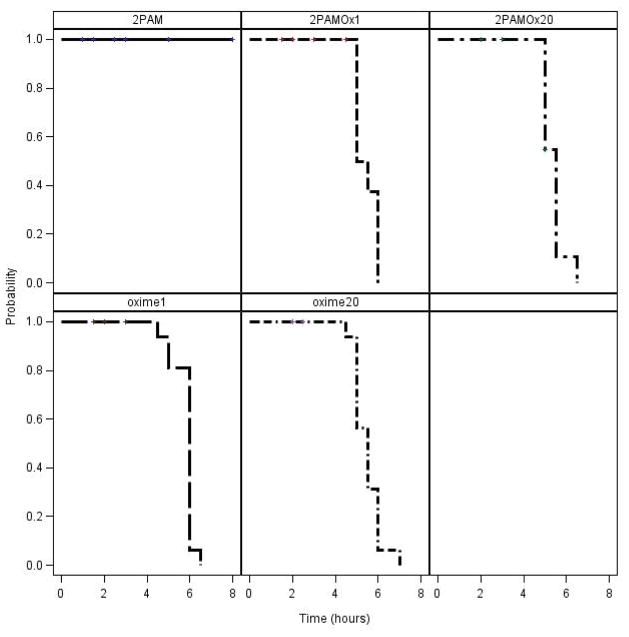
Assessment of time of cessation of seizure-like behavior in surviving rats indicated that all rats surviving NIMP to 8 hrs with Oximes 1 or 20, alone or in combination with 2-PAM, had ceased displaying seizure-like behavior by about 6 hrs whereas the 2-PAM alone surviving rats were still showing seizure-like behavior at 8 hrs (Fig. 3) and some as far out as 24 hrs. Similar results were obtained with NEMP (Fig. 4). These differences with both surrogates, either alone or in combination with 2-PAM, were highly significant (P at least < 0.0006) compared to 2-PAM alone (Fig. 3 and 4). Additionally, rats receiving 2-PAM alone often demonstrated more severe seizure-like behavior characteristic of brain stem seizures, such as rapid running in the cage, than was seen with any of the novel oxime treatments.
Figure 3.
Kaplan-Meier analysis of time to cessation of seizure-like behavior during the first 8 hours following NIMP administration in rats treated with novel oximes ± 2-PAM. Statistical analysis results of novel oximes ± 2-PAM compared to 2-PAM alone: Oxime 1, P = 0.0006; Oxime 20, P < 0.0001; Oxime 1 + 2-PAM, P = 0.0002; Oxime 20 + 2-PAM, P < 0.0001.
Figure 4.
Kaplan-Meier analysis of time to cessation of seizure-like behavior during the first 8 hours following NEMP administration in rats treated with novel oximes ± 2-PAM. Statistical analysis results of novel oximes ± 2-PAM compared to 2-PAM alone: all had P < 0.0001.
Brain ChE inhibition in animals which died during the first 8 hrs was about 95% or greater. Brain ChE was still substantially inhibited in the 24 hr survivors (Table 2). Brain ChE showed 85% inhibition in animals receiving NIMP and 2-PAM, whereas animals treated with Oximes 1 or 20, alone or in combination with 2-PAM, displayed 74–78% inhibition. Brain ChE showed 66% inhibition in animals receiving NEMP and 2-PAM, whereas animals treated with Oxime 1 or 20 alone had 59 and 63% inhibition, respectively. Animals treated with NEMP and Oxime 1 or 20 in combination with 2-PAM displayed more inhibition than 2-PAM alone, 70 and 73%, respectively. Even though Oximes 6 and 13 were not effective enough for pursuing the full complement of replications, the 11 replications conducted yielded brain ChE inhibition similar to the other values seen in Table 2: Oxime 6, 78% with NIMP and 68% with NEMP; and Oxime 13, 63% with NEMP and there were no 24 hr survivors with NIMP. There were no statistical differences among any of the oxime treated groups within each surrogate.
Table 2.
Brain cholinesterase percent inhibition (% I; mean ± SD) in rats surviving 24 hours following treatment with 0.6 mg/kg NIMP (sarin surrogate) or 0.65 mg/kg NEMP (VX surrogate) followed by 0.65 mg/kg atropine ± 0.146 mmol/kg oxime (2-PAM or novel oximes). Numbers of 24-hr survivors contributing to these data are indicated in Table 1.
| Oxime | NIMP | NEMP |
|---|---|---|
| % I | % I | |
| None | NA | NA |
| 2-PAM | 85±3.5 | 66±3.9 |
| Oxime 1 | 78±4.1 | 59±5.2 |
| Oxime 20 | 76±5.3 | 63±9.9 |
| Ox1+2-PAM | 74±4.1 | 70±8.1 |
| Ox20+2-PAM | 75±3.0 | 73±4.7 |
NA, not applicable because no survivors.
Peripheral (skeletal muscle, erythrocyte, and plasma) ChE inhibition in animals which died during the first 8 hrs was about 80% or greater (data not shown). Peripheral tissue ChE did not show any significant differences in inhibition among any of the oximes with ChE Inhibition for the 24 hour survivors ranging from 50–60% for skeletal muscle, erythrocytes and plasma.
An initial test of toxicity was conducted on Oximes 1 and 20. Two weeks after oxime administration, there was no difference in body weight compared to vehicle controls, nor was there evidence of any gross pathology other than injection site irritation.
Discussion
The novel oximes in the present study were a subset of the substituted phenoxyalkyl pyridinium oximes that were shown earlier to reactivate brain ChE when rats were treated in vivo with the sarin and VX surrogates, NIMP and NEMP, respectively (Chambers et al., 2013). In the earlier studies with high sublethal dosages, 20 of the novel oximes were tested with NIMP and 12 showed moderate ability to increase ChE activity in the brain; 7 of these were tested with NEMP and all showed efficacy. The novel oximes have incorporated lipophilic moieties with the expectation that the charge of the quaternary ammonium will be, in part, counterbalanced by the lipophilic moiety, theoretically allowing the oxime to cross the BBB. A substantial amount of reactivating ability of the pyridinium moiety was expected to be retained, and a number of these novel oximes displayed reactivation efficacies approaching that of 2-PAM (Chambers et al., 2013). The sublethal paradigm administered the oxime at the time of peak brain ChE inhibition (1 hr post surrogate treatment), so that the surrogate was in the process of being excreted. These experiments provided convincing evidence that a reduction of brain ChE inhibition was the result of brain penetration by the novel oximes and not just peripheral reactivation; with this paradigm, 2-PAM did not result in any decrease in brain ChE inhibition. While the perspective of these original studies was to test sublethal dosages of the OP’s, the present study was designed to determine whether these same novel oximes could also combat lethality similar to or better than 2-PAM; 4 novel oximes that showed among the best efficacies in the sublethal studies were tested in the lethal level paradigm.
Since the goal of this study was to reduce lethality and seizure activity, we selected the paradigm of the administration of atropine IM and the novel oxime (alone or in combination with 2-PAM) IM at the time of initiation of seizure-like behavior (25–30 min) following SC administration of the surrogate. (Intramuscular administration is the most likely route of therapy administration and SC is the typical route employed in nerve agent research.) Our paradigm incorporated the delay that would be expected for the administration of antidotes by first responders in the event of a terrorist attack or an accident with the nerve agents. While the results on survival with Oximes 6 and 13 were very disappointing despite their positive results in the earlier sublethal studies, the results with Oximes 1 and 20 were positive, with both showing enhanced survival compared to 2-PAM when used alone and in combination with 2-PAM. The combination treatments were even more effective with NIMP, but the combination treatments were slightly worse with NEMP compared to Oximes 1 or 20 alone. The reason for the lesser efficacy with NEMP with combinations of novel oximes and 2-PAM is not known. Oximes reactivate through transphosphorylation producing an oxime-phosphate that can be a ChE inhibitor (Maxwell et al., 2013), and it is possible that there was sufficient oxime-phosphate formed quickly enough with both oximes to present an increased reinhibition of ChE.
Kaplan-Meier analysis indicated that Oximes 1 and 20 (alone or in combination with 2-PAM) showed a shorter time for cessation of seizure-like behavior (about 6 hr in the 24-hr survivors) than survivors with 2-PAM which were still showing seizure-like behavior at 8 hrs. While these data on cessation of seizure-like behavior do not prove brain penetration by Oximes 1 and 20 compared to 2-PAM, they are consistent with a reactivator which has the ability to penetrate the BBB and it is unlikely that this attenuation of on-going seizure-like behavior could be the result of only reactivation of peripheral AChE. If these oximes can ultimately be used as replacements for 2-PAM, they would still probably need to be used along with an anticonvulsant.
Likewise the 24-hr brain AChE inhibition levels were slightly lower in the rats receiving Oximes 1 and 20 compared to those receiving 2-PAM. Although not statistically significant, these data are consistent with an oxime that has the ability to enter the brain. It is not surprising that there was relatively little difference in the brain ChE inhibition between the novel oximes and 2-PAM at 24 hr. There were high levels of these two OP’s circulating at these lethal dosages and oximes probably are bioavailable for only a relatively short period of time (Clement et al., 1995; Murray et al., 2012). It is expected that reactivation of brain ChE by the novel oximes would occur only shortly after the OP exposure. Our results showing a reduction of brain ChE inhibition from a high sublethal dose level of surrogates occurred at 90 min after OP exposure (30 min after oxime administration) (Chambers et al., 2013).
In order to conserve animals, this research has not calculated LD50 levels for the surrogates, so the level of protection cannot be compared directly to the protection afforded by new oximes in actual nerve agent tests (Shih et al., 2010). However, our range finding studies suggested that the LD50 for NIMP and NEMP under experimental conditions similar to those employed here (using atropine) were about 0.46 and 0.475 mg/kg, respectively. Therefore the paradigm used in the present experiments tested at about 1.3 and 1.4 times the LD50 of NIMP and NEMP, respectively.
In summary, two of the novel substituted phenoxyalkyl pyridinium oximes, that had previously demonstrated an ability to cross the BBB and reactivate ChE that was inhibited by surrogates of sarin and VX administered at high sublethal dosages, also showed the ability in the present study to provide appreciable protection from lethality and also the ability to reduce the time until cessation of seizure-like behavior from lethal levels of surrogates of sarin and VX. All data at this time are supportive of the ability of these two novel oximes to provide protection in the brain. While considerably more information about the pharmacokinetics, pharmacodynamics and toxicology of these novel oximes would be needed before concluding that they could be used as therapeutics in humans, at present this oxime platform displays the potential to be developed into nerve agent antidotes that could be more effective than 2-PAM and some other oximes.
Highlights.
Novel oxime reactivators that show ability to cross the blood-brain barrier at high sublethal dosages of surrogates for the nerve agents sarin and VX also can prevent lethality of these surrogates in rats.
The novel oximes also attenuate seizure-like behavior induced by lethal level sarin and VX surrogates.
This novel oxime platform has promise for development as antidotes that can both save lives and provide neuroprotection.
Acknowledgments
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number U01NS083430.
The authors wish to thank Erle Chenney and Royce Nichols for their assistance with technical procedure and to Dr. Jeffrey Eells for his advice on seizure behavior, all at Mississippi State University. We also wish to thank Dr. Tom Snider of Battelle for his helpful advice on experimental conditions.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Janice E. Chambers, Email: chambers@cvm.msstate.edu.
Edward C. Meek, Email: emeek@cvm.msstate.edu.
Joshua P. Bennett, Email: jpb280@msstate.edu.
W. Shane Bennett, Email: wsb33@cvm.msstate.edu.
Howard W. Chambers, Email: hwc2@msstate.edu.
C. Andrew Leach, Email: cal57@msstate.edu.
Ronald B. Pringle, Email: rpb62@msstate.edu.
Robert W. Wills, Email: wills@cvm.msstate.edu.
References
- Chambers JE, Chambers HW, Meek EC, Pringle RB. Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates. Chem Biol Interact. 2013;203:135–138. doi: 10.1016/j.cbi.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, Chambers HW. Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci Res Comm. 1988;3:85–92. [Google Scholar]
- Clement JG, Bailey DG, Madill HD, Tran LT, Spence JD. The acetylcholinesterase oxime reactivator HI-6 in man: pharmacokinetics and tolerability in combination with atropine. Biopharm Drug Dispos. 1995;16:415–25. doi: 10.1002/bdd.2510160506. [DOI] [PubMed] [Google Scholar]
- Clement JG. Efficacy of pro-PAM (n-methyl-1,6-dihydropyridine-2-carbaldoxime hydrochloride) as a prophylaxis against organophosphate poisoning. Toxicol Appl Pharmacol. 1979;47:305–311. doi: 10.1016/0041-008x(79)90325-9. [DOI] [PubMed] [Google Scholar]
- Demar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schulz SM, Marek E, Medynets MA, Ku TC, Evans SA, Khan FA, Owens RR, Nambiar MP, Gordon RK. Pro-2-PAM therapy for central and peripheral cholinesterases. Chem Biol Interact. 2010;187:191–198. doi: 10.1016/j.cbi.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecobichon DJ. Toxic effects of pesticides. In: Klasseen CD, editor. Casarett and Doull’s Toxicology. McGraw-Hill; New York, NY: 2001. pp. 763–810. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetyl-cholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Johnson NH, Larson JC, Meek EC. Historical perspectives of chemical warfare agents. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. London, U.K: Elsevier; 2015. pp. 7–16. [Google Scholar]
- Kalisiak J, Ralph EC, Cashman JR. Nonquatermary reactivators for organophosphate-inhibited cholinesterases. J Med Chem. 2012;55:465–474. doi: 10.1021/jm201364d. [DOI] [PubMed] [Google Scholar]
- Kalisiak J, Ralph EC, Zhang J, Cashman JR. Amidine-oximes: reactivators for organophosphate exposure. J Med Chem. 2011;54:3319–3330. doi: 10.1021/jm200054r. [DOI] [PubMed] [Google Scholar]
- Kuca K, Cabal J, Jun D, Kassa J, Bartosovà L, Kunesovà G, Dohnal V. Strategy for the development of new acetylcholinesterase reactivators – antidotes used for treatment of nerve agent poisonings. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:429–431. doi: 10.5507/bp.2005.074. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lüttjohann A, Fabene PF, van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. 2009;98:579–586. doi: 10.1016/j.physbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Maxwell DM, Brecht KM, Sweeney RE. A common mechanism for resistance to oxime reactivation of acetylcholinesterase inhibited by organophosphorus compounds. Chem Biol Interact. 2013;203:72–76. doi: 10.1016/j.cbi.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Meek EC, Chambers HW, Coban A, Funck KE, Pringle RB, Ross MK, Chambers JE. Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol Sci. 2012;126:525–533. doi: 10.1093/toxsci/kfs013. [DOI] [PubMed] [Google Scholar]
- Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, Omae K. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- Murray DB, Eddleston M, Thomas S, Jefferson RD, Thompson A, Dunn M, Vidler DS, Clutton RE, Blain PG. Rapid and complete bioavailability of antidotes for organophosphorus nerve agent and cyanide poisoning in minipigs after intraosseous administration. Ann Emerg Med. 2012;60:424–30. doi: 10.1016/j.annemergmed.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Musilek K, Dolezal M, Gunn-Moore F, Kuca K. Design, evaluation and structure-activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med Res Rev. 2011;31:548–575. doi: 10.1002/med.20192. [DOI] [PubMed] [Google Scholar]
- Nishiwaki N, Maekawa K, Ogawa Y, Asukai N, Minami M, Omae K Sarin Health Effects Study Group. Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environ Health Perspect. 2001;109:1169–1173. doi: 10.1289/ehp.011091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem. 2012;287:19337. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Skovira JW, O’Donnell JC, McDonough JH. In vivo reactivation by oximes of inhibited blood, brain and peripheral tissue cholinesterase activity following exposure to nerve agents in guinea pigs. Chem Biol Interact. 2010;187:207–214. doi: 10.1016/j.cbi.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Skovira JW, O’Donnell JC, Koplovitz I, Kan RK, McDonough JH, Shih TM. Reactivation of brain acetylcholinesterase by monoisonitrosoacetone increases the therapeutic efficacy against nerve agents in guinea pigs. Chem Biol Interact. 2010;187:318–324. doi: 10.1016/j.cbi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Smart JK. History of chemical and biological warfare: an American perspective. Medical Aspects of Chemical and Biological Warfare, Textbook of Military Medicine. In: Sidell FR, Takafuji ET, Franz DR, editors. Office of the Surgeon General. Bordon Institute; Washington, DC: 1997. pp. 9–86. [Google Scholar]
- Tucker JB. War of Nerves: Chemical Warfare from World War 1 to Al-Queda. Pantheon Books; New York, NY: 2007. [Google Scholar]
- Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Worek F, Thiermann H. The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol Ther. 2013;139:249–259. doi: 10.1016/j.pharmthera.2013.04.009. [DOI] [PubMed] [Google Scholar]