Abstract
Following the onset of ischemia/reperfusion (I/R), CD38 activation occurs and is associated with depletion of NAD(P)(H) in the heart as well as myocardial injury and endothelial dysfunction. Studies with pharmacological inhibitors suggest that the NADP+-hydrolyzing ability of CD38 can deplete the NAD(P)(H) pools. However, there is a need for more specific studies on the importance of CD38 and its role in the process of endothelial dysfunction and myocardial injury in the post-ischemic heart. Therefore, experiments were performed in hearts of mice with global gene knockout of CD38. Isolated perfused CD38−/− and wild type (WT) mouse hearts were studied to determine the link between CD38 activation, the levels of NADP(H), endothelial dysfunction, and myocardial injury after I/R. Genetic deletion of CD38 preserves the myocardial and endothelial NADP(H) pools compared to WT. Whole heart BH4 levels in CD38−/− hearts were also preserved. Post-ischemic levels of cGMP were greatly depleted in WT hearts, but preserved to near baseline levels in CD38−/− hearts. The preservation of these metabolite pools in CD38−/− hearts was accompanied by near full recovery of NOS-dependent coronary flow, while in WT hearts, severe impairment of endothelial function and NOS uncoupling occurred with decreased NO and enhanced superoxide generation. CD38−/− hearts also exhibited marked protection against I/R with preserved glutathione levels, increased recovery of left ventricular contractile function, decreased myocyte enzyme release, and decreased infarct size. Thus, CD38 activation causes post-ischemic depletion of NADP(H) within the heart, with severe depletion from the endothelium, resulting in endothelial dysfunction and myocardial injury.
Keywords: Coronary circulation, Endothelium, Nitric Oxide, Ischemia, Oxidant Stress
1. Introduction
Myocardial ischemia/reperfusion (I/R) injury leads to myocyte cell death caused, in large part, by increases in oxidative stress and inflammation through the formation of reactive oxygen species (ROS) [1–4]. ROS effect important changes in the cellular environment which contribute to cell death, including changes in the levels of important metabolites. Of those affected, NADPH and tetrahydrobiopterin (BH4) are both essential for the function of endothelial nitric oxide (NO) synthase (eNOS) [5]. eNOS is the primary vascular source of NO, catalyzing the conversion of L-arginine to NO and L-citrulline at the expense of NADPH using BH4, flavin adenine dinucleotide, flavin mononucleotide, and Ca2+/calmodulin as cofactors [5]. With BH4 and/or NADPH depletion after I/R [6, 7], eNOS-dependent production of NO decreases, and eNOS uncoupling may occur, leading to increased production of superoxide [6–9].
While BH4 depletion in I/R was found to result from direct oxidation [6, 10], this was not true for NADPH since the levels of both NADPH and its oxidized form NADP+ were decreased in I/R with NADP+ depletion even more marked than that of NADPH [7]. This suggested that enzymatic mechanisms of depletion were likely, as occurs with poly ADP-ribose polymerase (PARP)-mediated NAD+ depletion [11]. Furthermore, depletion of the NADP(H) pool was also more severe than the NAD(H) pool [7]. In targeting enzymes with efficient NADP+-degrading activity, CD38 was found to have a roughly 6-fold lower Km and higher Vmax with NADP+ as a substrate compared to NAD+ [12].
Recent studies in isolated rat hearts identified CD38 expression by western blotting and immunohistochemistry [7]. With I/R injury, levels of the CD38 signaling molecule, 2’-phospho-ADP-ribose (2’-P-ADPR), increased 5-fold compared to control levels. The CD38 inhibitor α-NAD blocked this increase and concurrently protected the levels of NADP(H) in the coronary endothelium [7]. Later studies with another more potent CD38 inhibitor, luteolinidin, indicated that CD38 inhibition protects post-ischemic NADP(H) levels and increases the recovery of left ventricular developed pressure and decreases infarct size after 30 minutes of global ischemia [13].
However, there is a need to more specifically demonstrate the role of CD38 and address key questions regarding how CD38 contributes to I/R injury within the heart. Prior studies implicating a role of CD38 in post-ischemic injury have been based solely on the use of pharmacological inhibitors [7, 13], the possible off target effects of which can have confounding effects. Therefore experiments utilizing specific genetic knockout of CD38 would be of great value. Experiments in this mouse knockout model can unambiguously delineate the role of CD38 activation in NAD(P)H depletion and in triggering endothelial dysfunction as well as in the overall process of post-ischemic cardioprotection.
In this study, we perform a series of experiments in isolated CD38−/− and WT mouse hearts. We observe that with CD38 gene knockout, the levels of myocardial and endothelial NADP(H) were markedly protected compared to levels in WT control hearts after I/R. Levels of BH4 were also higher in CD38−/− hearts after I/R compared to WT controls. Preservation of these metabolite pools in CD38−/− hearts led to protection of NOS function, as levels of NOS-dependent cGMP and NOS-dependent coronary flow (CF) were also higher, and eNOS uncoupling was prevented, unlike in WT hearts which had depressed NOS-dependent NO and enhanced NOS-dependent superoxide production. We also observed protection of total CF and left ventricle contractile function in CD38−/− hearts, along with preserved glutathione levels and greatly decreased myocyte protein release and infarct size. In summary, global knockout of CD38 is shown to be highly protective against I/R injury in the isolated heart with NADPH-mediated preservation of endothelial function and left ventricular contractile function, with decreased myocardial infarction.
2. Methods
2.1. Materials
All chemicals and reagents were purchased from Sigma except for BH4 and pterin (Schircks Laboratories), CD38 antibody ab90 (AbdSerotec), CD31 antibody (R&D Systems), GAPDH antibody (Cell Signaling Technologies), myoglobin antibody (Abcam), polypyrimidine tract binding protein 1 (PTBP1) antibody (Abcam), HRP-linked secondary antibodies (Abcam), ECL immunoblotting detection reagents (Amersham Biosciences), creatine phosphokinase kit (BioVision), and recombinant human CD38, which was provided by Hon-Cheung Lee and Y.J. Zhao [14].
2.2. Animals
All mice used for experiments were in the age range of 4–6 months. The CD38−/− mice breeding pairs were obtained from Dr. Eduardo Chini of the Mayo Institute [15]. As described, the targeting vector containing neomycin resistance and HSV-TK genes was used to disrupt CD38 [16]. Homologous recombination resulted in replacement of a 1.6-kb CD38 genomic region that includes the putative NAD(P)+ase enzyme catalytic site. The construct was transfected into 129P2/OlaHsd-derived E14–1 embryonic stem cells and selected cells were injected into C57Bl/6J blastocysts. Thus, C57Bl/6J mice were used as controls, and are termed wild type (WT). The CD38−/− mice have a normal life span and breed normally. The C57Bl/6J mice were obtained from Jackson Laboratories. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Ohio State University and conformed to the Guide for the Care and Use of Laboratory Animals published by the NIH.
2.3. Isolated heart perfusion
Isolated heart experiments were performed as described previously [7]. Male, C57Bl/6 mice (control) or CD38−/− mice weighing 25–30 g were heparinized and injected with 100 mg/kg ketamine and 15 mg/kg xylazine intraperitoneally. Hearts were excised, cleaned of non-myocardial tissue, cannulated via the aorta and perfused retrogradely in Langendorff mode with Krebs-Henseleit buffer (KHB) (119 mM NaCl, 17 mM glucose, 2 mM sodium pyruvate, 25 mM NaHCO3, 5.9 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 0.5 mM NaEDTA). A polyvinylchloride balloon connected to a pressure transducer (ADInstruments, Colorado Springs, CO) was placed in the left ventricle to measure left ventricular developed pressure (LVDP), systolic pressure (ESP), left ventricle end diastolic pressure (LVEDP), and heart rate (HR). An inline flow probe (Transonic, Ithaca, NY) measured CF.
2.4. CD38 activity assay
CD38 has been reported to function primarily as an NAD(P)+ase through its hydrolysis of NAD(P)+ to 2’-P-ADPR. To measure this enzyme activity specifically, a substrate analog of NAD+, nicotinamide 1,N6-ethenoadenine dinucleotide (ε-NAD), was used [13, 17]. WT and CD38−/− hearts subjected to control perfusion, 30 min ischemia, or 30 min ischemia/30 min reperfusion were ground in liquid nitrogen and homogenized in buffer containing 150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1% Triton X-100, and freshly added protease inhibitors. Homogenate totaling 100 μg of protein was added to a 200 μl reaction mixture containing 200 μM ε-NAD. Reactions were monitored for the conversion of ε-NAD to strongly fluorescent product etheno-ADP-ribose (ε-ADPR). Fluorescence was measured at an excitation wavelength of 300 nm and an emission wavelength of 410 nm on a Molecular Devices SpectraMax plate reader.
2.5. Western blotting
In experiments utilizing heart tissue, hearts were homogenized in 5 volumes of RIPA buffer (150 mM NaCl, 10 mM Tris, 1 mM EDTA, 0.1% SDS, 0.1% sodium deoxycholate, and 1% Triton X-100) with freshly added protease inhibitors. Homogenates were allowed to incubate on ice for 30 minutes before centrifugation to pellet cell debris. Supernatants were assayed for protein concentration by the detergent-compatible protein assay.
For western blotting of myoglobin and PTBP1 from coronary effluents, effluent was initially collected from hearts in 1 mL fractions and was immediately frozen in liquid nitrogen. We also performed experiments to test the presence of PTBP1 in coronary effluent after permeabilization of the coronary endothelium. In these experiments, coronary effluent was collected after a 25 μL bolus injection of 0.25% Triton X-100 delivered through a septum-sealed sidearm located directly above the heart with a Hamilton syringe. In some cases, frozen coronary effluent samples were completely lyophilized and reconstituted in 200 μL 1X sample loading buffer with 10 mM DTT. In other cases, protein in coronary effluent samples was concentrated using Corning Spin-X 5000 molecular weight cut off filters. For experiments assessing the monomer:dimer ratio of eNOS in hearts from WT and CD38−/− hearts, low temperature PAGE (LT-PAGE) with non-reducing conditions was used. Specifically, reducing agents were omitted from the protein samples prior to electrophoresis, and gels were run at 125V at 4°C with a sample SDS concentration of 2%. Protein samples were separated either on 4–20% gradient or 12% Tris-glycine polyacrylamide gels. Protein from gels was transferred to PVDF membranes and blocked for 1 hour at room temperature (RT) with 5% milk in Tris-buffered saline with 0.1% Tween-20 (TBST). Membranes were incubated overnight at 4°C with anti-CD38 antibody diluted at 1:2000, anti-eNOS antibody diluted at 1:1000, anti-GAPDH antibody diluted at 1:10,000, anti-myoglobin antibody diluted at 1:2000, or anti-PTBP1 antibody diluted at 1:2000. Membranes were then washed in TBST and incubated for 1 hour with HRP-conjugated secondary antibodies in TBST with 5% milk at RT. Imaging was performed with ECL immunoblotting detection reagents. The intensity of blotting was quantified using ImageJ from the NIH.
2.6. Immunohistochemistry of CD38
WT and CD38−/− hearts were perfused with Krebs buffer for 20 minutes and then embedded in OCT compound. Heart sections were then taken and used for immunohistochemistry (IHC) of CD38. Sections were first washed with PBS with 0.1% Tween-20 (PBST) to dissolve residual OCT. Then, sections were fixed with 4% paraformaldehyde, washed briefly with PBS, and blocked with 5% BSA in PBST containing 0.3 M glycine. After 1 hour of blocking at RT, CD38 antibody (ab90) and CD31 antibodies were added at a dilution of 1:100 in PBST overnight at 4°C. After overnight incubation, sections were washed 3×5 minutes with PBST, and secondary antibodies (Alexa Fluor 594 for CD38, Alexa Fluor 488 for CD31) were added at a final dilution of 1:200. Images were taken on an Olympus FV 1000 spectral confocal microscope with a 60X objective. For nuclei staining, DAPI was used at a concentration of ~1 μM.
2.7. Endothelial permeabilization for NAD(P)(H) measurements
A method to permeabilize the endothelium of perfused hearts, as previously performed in rat hearts [7, 18], was adapted for mouse hearts. For WT and CD38−/− hearts subjected to either control perfusion or 30 min I/R, a 7.5 μl bolus of 0.25% Triton X-100 in PBS was infused through a septum-capped sidearm directly above the perfusion cannula with a Hamilton syringe. Coronary effluent (1 ml) was immediately collected and frozen in liquid nitrogen. Only effluent samples from hearts with normal post-Triton X-100 contractile and smooth muscle function, as well as completely eliminated endothelial function, were studied. Normal contractile function was defined as hearts displaying greater than 80% of pre-Triton X-100 rate-pressure product (RPP), and normal smooth muscle function was defined as a maintained response to NO-donor sodium nitroprusside (SNP) (greater than 90% of pre-Triton X-100 response). Completely eliminated endothelial function was considered to have occurred if hearts had a less than 5% increase in CF in response to bolus injections of endothelium-dependent vasodilators acetylcholine (50 pmol) or bradykinin (1 nmol) [19]. Frozen effluents were then purified using anion-exchange solid phase extraction (SPE) with Strata-X-AW (SXAW) cartridges (Phenomenex). SXAW cartridges were conditioned with 1 ml methanol, 1 ml 2% acetic acid in 25% methanol, and 1 ml water. Prior to loading samples to the SXAW cartridges, sample pH was adjusted to ~4–5 with acetic acid. Samples were then applied to the SPE cartridges twice under light vacuum. After washing with 1 ml water and 1 ml 40% methanol, metabolites were eluted from the SPE cartridges with 10% ammonium hydroxide (NH4OH) in water. These samples were then frozen and lyophilized. Dried samples were then resuspended in 100 μl 200 mM potassium cyanide (KCN), 60 mM potassium hydroxide (KOH) and 1 mM diethylenetriaminepentaacetic acid (DTPA) for HPLC analysis of NAD(P)(H).
2.8. High performance liquid chromatography (HPLC) analysis of pyridine nucleotides
Pyridine nucleotides were measured by HPLC with fluorescence detection as detailed previously [7, 13]. In this method, cyanide ion from potassium cyanide (KCN) in basic solution is used to derivatize NAD+ and NADP+ to stable, fluorescent analytes, allowing for measurements of both the oxidized and reduced nucleotides in one chromatographic run [20]. Heart tissue from isolated mouse heart experiments was ground in liquid nitrogen and homogenized in a buffer consisting of 200 mM KCN, 60 mM KOH and 1 mM DTPA. The resulting homogenate was centrifuged for 10 minutes at 15,000 × g to pellet insoluble material. The supernatant was then filtered using a 3,000 MWCO regenerated cellulose filter (Millipore). Filtrates were then injected onto a Supelcosil LC-18-T column (25 cm × 4.6 mm × 5 μm) with mobile phase A (MPA) of 200 mM ammonium acetate (pH 5.8) and mobile phase B (MPB) of 200 mM ammonium acetate (pH 5.8) in 50% methanol. Separation was achieved with an initial flow rate of 1.0 mL/min consisting of 8% MPB and a linear methanol gradient (0.4% per minute for 25 minutes). Analytes were detected via fluorescence spectroscopy (excitation wavelength of 330 nm; emission wavelength of 460 nm). Peaks were assigned by co-elution with analytical standards, and quantitation was performed with use of standard curves prepared from analytical standards.
2.9. Solid phase extraction for BH4 measurements
Heart tissue was ground in liquid nitrogen and homogenized in 3 volumes of ice-cold, oxygen-free 0.1 N HCl degassed by argon. Resulting homogenate was added 1:1 with a 2% KI/3% iodine and 0.2 N KOH solution and allowed to react for 1 hour at ambient temperature in the dark. Reaction mixtures were then centrifuged at 15,000 × g for 10 min, and the resulting supernatant was filtered through a 3,000 MWCO filter for further processing using SPE. SPE was performed using Waters Oasis MCX cation-exchange cartridges. Cartridges were first conditioned with 1 ml of methanol and 1 ml of water. Filtrate samples (~300 μl) were acidified by 1:1 addition with 0.2 N HCl and loaded to the MCX cartridges by vacuum. Cartridges were washed with 1 ml 2% acetic acid and 1 ml methanol to remove acids and proteins. To elute the bound pteridines, 1 ml of 5% NH4OH in methanol was loaded to the cartridges. The resulting eluate was then frozen and lyophilized. Dried samples were resuspended in 20% of the original volume using HPLC buffer, resulting in a 5-fold concentrated sample allowing for accurate peak identification and quantitation.
2.10. HPLC analysis of BH4
The HPLC analysis of BH4 was performed as previously reported using fluorescence detection with an excitation wavelength of 348 nm and emission wavelength of 444 nm [7, 10]. This method was chosen for its sensitivity and selectivity in the detection of BH4 after controlled oxidation to pterin. HPLC analysis was carried out using a Waters Atlantis T3 reversed-phase column (150 × 4.6 mm). Isocratic elution was performed at a flow rate of 1.2 ml/min using a buffer consisting of 100 mM KH2PO4, 6 mM citric acid, 2.5 mM sodium octyl sulfate and 2% methanol, pH 2.5. Peaks were assigned by co-elution with analytical standards, and quantitation was performed with use of standard curves prepared from analytical standards.
2.11. cGMP measurements
WT and CD38−/− hearts subjected to control treatment or I/R were assayed for cGMP content [13]. To delineate NOS-dependent cGMP that is derived from sGC, matched experiments were performed in hearts pretreated with 1 mM L-NAME for 10 minutes. These values were subtracted from the total measured cGMP levels to obtain the NOS-dependent cGMP levels. Heart tissue was homogenized in five volumes of 0.1 N HCl and centrifuged at 10,000 × g. The supernatant was collected and used for ELISA detection of cGMP (Enzo Life Sciences, Farmingdale, NY). The acetylated assay protocol was used in order to improve sensitivity. Acetylation was performed by 1:20 addition of the acetylating reagent (1:2 acetyl anhydride:triethylamine) to the samples, which were then subjected to the ELISA assay according to the manufacturer’s instructions, with endpoint measurements performed on a Molecular Devices SpectraMax M5 at a wavelength of 405 nm. Myocardial cGMP levels were expressed as picomoles per mg of protein in homogenates.
2.12. NOS-dependent coronary flow measurements
NOS-dependent coronary flow (CF) was determined by the continuous infusion of NOS inhibitor L-NAME (1 mM final concentration) for 10 minutes either pre-ischemia or after 30 min reperfusion. NOS-dependent CF was defined as the amount of CF lost with L-NAME infusion.
2.13. Liposomal NADPH delivery
NADPH was delivered at a final concentration of 100 μM in liposomal formulation as prepared previously [7, 13]. Briefly, 100 mg of phosphatidylcholine (PPC) (Sigma) was dissolved in 1 ml chloroform in a glass jar. The chloroform was evaporated while rotating the jar to create a thin film of PPC around the jar. NADPH dissolved in KHB was added and rolled on a jar roller for one hour. Resulting liposomes were extruded with a 1 ml extruder using 200 nm filters (Avestin), and then diluted to a final NADPH concentration of 1 mM for infusion at 1/10 of CF, resulting in a final NADPH concentration of 100 μM.
2.14. HPLC analysis of GSH/GSSG
HPLC coupled to fluorescence spectroscopy was used to analyze both the reduced (GSH) and oxidized (GSSG) forms of glutathione from WT and CD38−/− hearts subjected to 20 min control perfusion or 30 min ischemia/30 min reperfusion (I/R). GSH/GSSG was also measured from heart coronary effluents with and without endothelial permeabilization from a parallel group of hearts of WT and CD38−/− mice, with endothelial permeabilization performed as described in section 2.7 for NAD(P)(H). For whole hearts, tissue was frozen and powdered in liquid nitrogen using a mortar and pestle. Powdered whole hearts were then homogenized in 5 volumes of 0.01 N HCl and centrifuged at 20,000 × G for 5 minutes to pellet insoluble material. Resulting supernatant was centrifuge-filtered through regenerated cellulose, 3,000 molecular weight cutoff filters for 15 minutes. Resulting filtrate was used to measure GSH and GSSG as follows. For GSH, 5 μl of filtrate was reacted in the dark at room temperature with 95 μl of 5 mg/ml O-Phthaldialdehyde (OPA) in 5% methanol in 100 mM sodium borate (pH 9.90) for 15 minutes [21]. This reaction mixture was diluted 1:5 in 100 mM sodium borate (pH 9.90) and this final diluted filtrate was used for HPLC analysis of GSH. For GSSG, filtrate was reacted first with 20 mM N-ethylmaleimide (NEM) for 5 minutes to block sample GSH. Reduction of sample GSSG was performed with addition of 10 mM DTT for 10 minutes. Resulting GSH was then similarly reacted with 5 mg/ml OPA in 100 mM sodium borate (pH 9.90) for 15 minutes. After reaction with OPA, the reaction mixture was used for HPLC analysis without further dilution. In the coronary effluent studies, effluents from hearts were reacted 1:1 directly with the 5 mg/mL OPA solution in 100 mM sodium borate (pH 9.90) for 15 minutes and this mix was used for HPLC analysis. HPLC was performed isocratically with a mobile phase of 150 mM sodium acetate (pH 7.0) in 5% methanol. GSH-OPA was detected using fluorescence spectroscopy with an excitation wavelength of 338 and emission wavelength of 458. GSH-OPA retention time was confirmed using analytical standards reacted with OPA. Peak intensities derived from analytical standards reacted with OPA were also used for quantitation of tissue GSH/GSSG levels.
2.15. Creatine kinase Activity
Creatine kinase (CK) activity was measured in coronary effluents using a commercially available kit (BioVision). Briefly, 1 mL fractions of coronary effluent collected from WT and CD38−/− hearts pre-ischemia (PI) and after 2, 5, 15, 30, 60, and 120 min reperfusion were dried to completion by lyophilization, and reconstituted in 300 μL of water. Samples were then mixed with kit contents per the manufacturers’ instructions and CK activity was measured by monitoring absorbance at 450 nm in a Molecular Devices SpectraMax plate reader.
2.16. Myocardial infarct size measurement
Ex vivo myocardial infarction was measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining of heart sections, as reported previously [7]. The heart was immediately removed after I/R and frozen for 20 min for hardening. The heart was then serially sectioned into transverse slices (1 mm) by a heart slicer (Zivic Laboratories) and incubated in 1.5% TTC in PBS for 15 min at 37°C to demarcate the viable (brick red) and infarcted (pale) myocardium. Heart slices were then fixed overnight in 10% neutral-buffered formaldehyde for improved color contrast and were digitally imaged. Computerized planimetry (with image-analysis software MetaVue, v. 6.0) of each section was used to determine percent infarction from the total cross-sectional area of the left ventricle.
2.17. EPR spin trapping of NO
Spin trapping measurements of NO formation from the heart were performed using the Fe2+-N-methyl-D-glucamine (Fe-MGD) complex. Fresh stock solutions of Fe-MGD (1:5) were prepared by adding ferrous sulfate (1 mM) to aqueous solutions of MGD (5 mM) in perfusion buffer with 10 mg/ml bovine serum albumin and 1 μM acetylcholine (Ach) to maximally active eNOS. After a 1 nmol bolus injection of Ach through a septum-capped sidearm to observe the effect on CF, the normal perfusate line was closed. Immediately after closing the perfusate line, Fe-MGD spin trapping solution was infused at a flow of 0.5 ml/min directly to the heart. Spin-trapping solution was then collected in 0.5 mL fractions for EPR analysis. In a separate set of experiments, L-NAME (1 mM) was administered to the heart for 10 minutes prior to acetylcholine stimulation and Fe-MGD infusion. EPR spectra of NO trapped by Fe-MGD were recorded at room temperature with a Bruker EMX spectrometer operating at X-band, ~9.77 GHz, with 100-kHz modulation frequency and a HS resonator, with a microwave power of 40 mW and a modulation amplitude 3.0 G.
2.18. EPR spin trapping of Superoxide
Spin trapping measurements of superoxide and superoxide-derived radicals were performed using 50 mM DMPO with 1 μM acetylcholine in perfusate immediately prepared and infused by a Harvard pump at 0.5 mL/min. The DMPO-infused samples were collected into tubes containing methyl-β-cyclodextrin (final concentration of 100 mM) to enhance spin adduct stability. EPR measurements were performed in a quartz flat cell and spectra obtained at room temperature at a microwave frequency of 9.77 GHz using 20 mW of microwave power and 1.0 G modulation amplitude. L-NAME was administered for 10 minutes prior to DMPO infusion at a concentration of 1 mM to block the production of NOS-dependent superoxide.
2.19. Statistical Analysis
All values were expressed as mean ± SE. Comparisons between two groups were statistically evaluated by Student’s t-test. To compare data with 2 factors, statistical analysis was performed by two-way repeated measures ANOVA. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Measurement of CD38 expression in WT and CD38−/− hearts and NAD(P)+ase activity
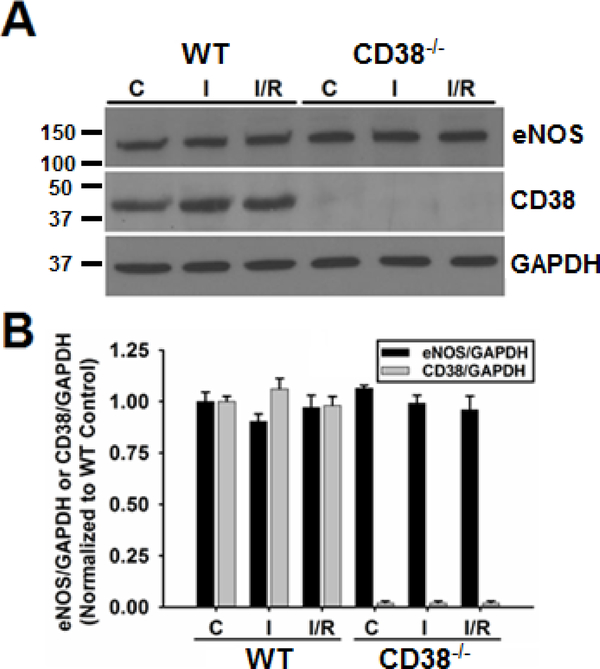
The CD38−/− mouse was initially generated by Cockayne et al in order to study the role of CD38 in humoral immune responses [16]. In that study, and others, it was shown using the CD38−/− mouse that CD38 is the principle NAD(P)+ase found in mammalian tissues [15, 16]. In the study by Aksoy et al, NAD(P)+ase activity was only found in the kidneys and testes of CD38−/− mice, with no detectable activity found in the heart or other tissues tested. Consistent with this, we found that CD38 expression was undetectable in CD38−/− hearts by western blotting, while in WT hearts we found CD38 expression that was unchanged by ischemia or I/R (Fig. 1). We also tested whether eNOS expression was different in WT and CD38−/− hearts under normal control conditions and after ischemia or I/R. In these experiments, similar expression of eNOS was present in both groups and not significantly changed following ischemia or I/R (Fig. 1).
Figure 1. Expression of eNOS and CD38 in WT and CD38−/− hearts.
Western blotting of heart homogenates showed that the expression of eNOS and CD38 were unchanged in WT hearts undergoing control perfusion (C), 30 min ischemia (I), or 30 min ischemia/30 min reperfusion (I/R). In CD38−/− hearts, the expression of eNOS was also unchanged with the different treatments, while CD38 expression was absent (n=3).
3.2. Measurement of the effect of ischemia and I/R on NAD(P)+ase activity in WT and CD38−/− hearts
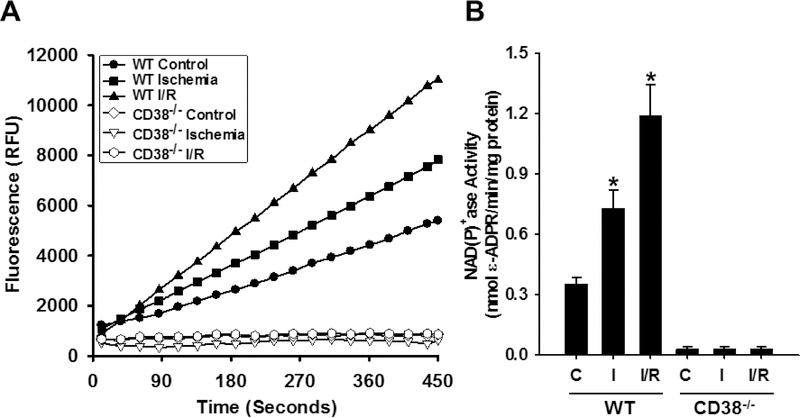
Measurements of CD38 NAD(P)+ase activity were performed in hearts from WT and CD38−/− hearts undergoing control perfusion, ischemia, or I/R. While NAD(P)+ase activity was absent from CD38−/− hearts, a basal, non-ischemic level of 0.35 ± 0.03 nmol/min/mg protein was measured in WT hearts. With ischemia and with I/R, NAD(P)+ase activity increased to 0.73 ± 0.09 and 1.20 ± 0.16 nmol/min/mg protein, respectively (Fig. 2). Thus, the NAD(P)+ase activity of CD38 is activated in mouse hearts subjected to ischemia and I/R.
Figure 2. CD38 is the main NAD(P)+ase in the mouse heart.
A) Representative data tracing from a CD38 activity assay with heart homogenates from WT and CD38−/− hearts undergoing control perfusion (Control), 30 min ischemia (Ischemia), or 30 min ischemia followed by 30 min reperfusion (I/R). B) Summarized data demonstrating increased CD38 activity with ischemia and I/R in WT hearts (*P<0.05, mean ± SEM, n=4).
3.3. Visualization and cellular localization of CD38 expression in the heart
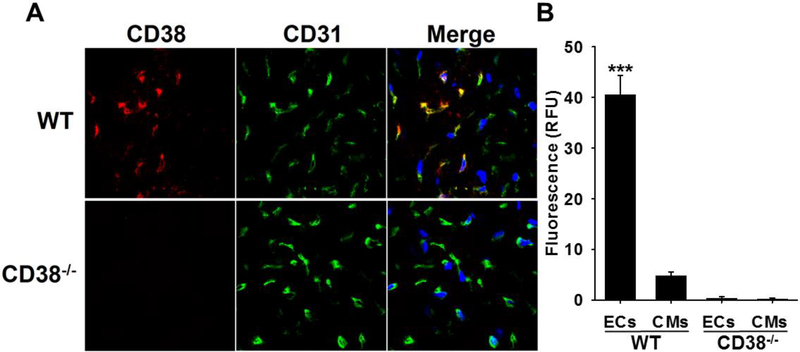
Immunohistology was performed to map CD38 expression and cellular localization in WT hearts. Initially, lack of CD38 staining in CD38−/− hearts was confirmed with immunohistochemistry (Fig. 3). However, in WT hearts, there was a distinctive pattern of staining. In WT heart sections, CD38 primarily co-localized with the endothelial cell-specific marker CD31 [22], indicating that CD38 expression occurs primarily in the endothelium of the heart, with approximately 10-fold more intense staining in endothelial cells compared to surrounding myocytes (Fig. 3B). This was consistent with our previous studies finding that CD38 primarily expressed in the endothelium of the heart [7, 23].
Figure 3. Immunohistochemistry of CD38.
A, WT and CD38−/− hearts were sectioned and stained for CD38 (red) and CD31 (green). CD38 (red) stained prominently in heart sections of WT mice but not in those of CD38−/− mice. In WT heart sections, CD38 staining co-localized with staining of endothelial marker CD31 (merged in yellow), indicating endothelial CD38 expression. In the merged images, nuclei are stained blue with DAPI. B, CD38 staining intensity was compared in ECs (defined by co-localization of CD38 and CD31) and CMs by phase contrast. CD38 staining intensity was approximately 10 times higher in ECs than in CMs in WT hearts. In CD38−/− hearts, there was no detectable CD38 staining in either ECs or CMs. (***P<0.005 vs. WT CMs, mean ± SEM; n=4).
3.4. Effect of I/R on myocardial content of NAD(H) and NADP(H)
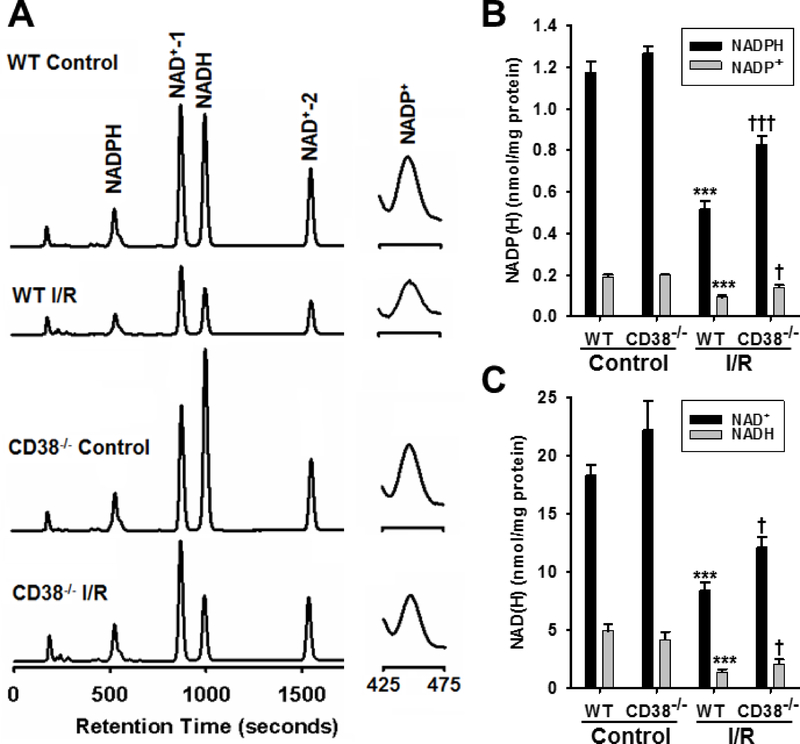
We recently showed that pre-ischemic delivery of CD38 inhibitors led to increased recovery of myocardial NADP(H) after I/R [13]. Thus, we hypothesized that genetic deletion of CD38 would result in similarly protected NADP(H) levels after I/R in mouse hearts. In non-ischemic WT hearts, levels of NADP(H) were 1.17 ± 0.05 nmol/mg protein for NADPH, and 0.19 ± 0.01 nmol/mg protein for NADP+, while in non-ischemic CD38−/− hearts, these levels were not significantly different with values of 1.26 ± 0.03 nmol/mg protein and 0.20 ± 0.002 nmol/mg protein, respectively (Fig. 4A, B). After I/R, however, the whole heart levels of NADP(H) in WT hearts fell by ~60% to 0.51 ± 0.05 nmol/mg protein for NADPH and 0.08 ± 0.01 nmol/mg protein for NADP+. In CD38−/− hearts, post-ischemic levels were higher with values of 0.83 ± 0.01 and 0.14 ± 0.01 nmol/mg protein, respectively, for NADPH and NADP+, corresponding to 63% and 75% higher levels than in WT (Fig. 4A, B).
Figure 4. NAD(P)(H) levels in hearts.
A, Fluorescence chromatograms of all pyridine nucleotides from WT and CD38−/− hearts subjected to Control and I/R treatments. Peaks for NADP+ are shown in the right panel, and are zoomed ~20X. B & C, To determine the effect of CD38 activation on the NAD(P)(H) content of hearts subjected to I/R injury, both WT and CD38−/− hearts were subjected to either 20 minute control perfusion (Control), or 30 min ischemia/30 min reperfusion (I/R) and tissue levels of NADP(H) and NAD(H) were measured by HPLC. These experiments demonstrated that despite similar baseline levels, CD38−/− hearts had higher levels of all of these compounds compared to WT after I/R injury (***P<0.005 vs. WT Control, †††P<0.005, †P<0.05 vs. WT I/R. Mean ± SEM; n=5–7).
The preservation of the NAD(H) pool was observed to be slightly less in magnitude, but still significantly higher in CD38−/− hearts. In non-ischemic WT hearts, the levels of NAD+ and NADH were 18.34 ± 0.88 and 4.97 ± 0.54 nmol/mg protein, with these levels falling to 8.46 ± 0.78 and 1.42 ± 0.07 nmol/mg protein in hearts subjected to I/R. Baseline values of the NAD(H) pool for CD38−/− hearts were 22.26 ± 2.45 and 4.20 ± 0.65 nmol/mg protein for NAD and NADH, respectively. After I/R, these levels fell to 12.14 ± 1.01 and 2.10 ± 0.62 nmol/mg protein for NAD+ and NADH (Fig. 4A, C). From these results, the I/R-associated CD38 activation leads to decreases in both the NADP(H) pool and the NAD(H) pool.
3.5. Effect of I/R on post-ischemic endothelial NADP(H) levels
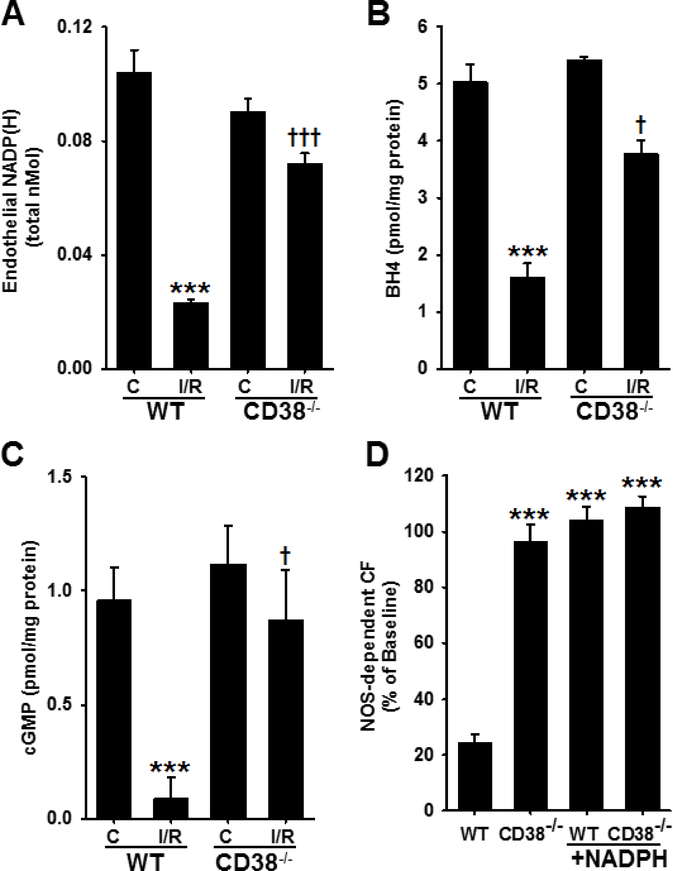
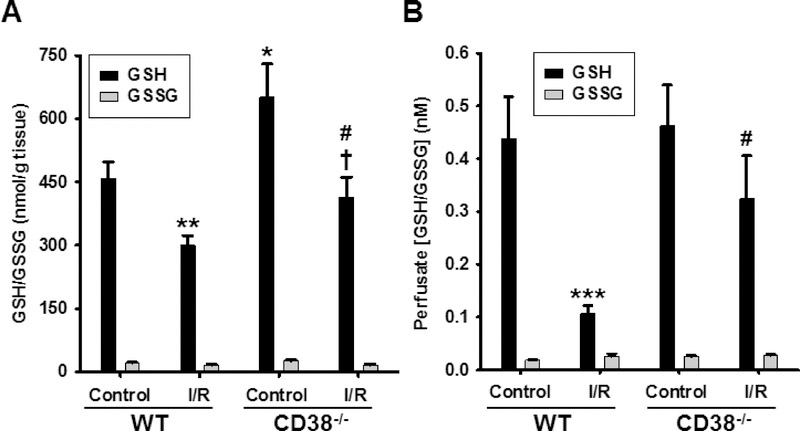
To determine the specific effect of I/R on the endothelial pools of NADP(H) in WT and CD38−/− hearts, endothelial permeabilization and measurement of the released NADP(H) in the coronary effluent was performed with HPLC [7, 18]. Previously, and as confirmed above in Figure 3, we found that CD38 was highly expressed in endothelial cells compared to cardiac myocytes [7, 23]. Thus, we hypothesized that there would be a greater effect of CD38 activation on NAD(P)(H) levels in the endothelium following I/R than that observed in whole hearts. Baseline levels of endothelial NADP(H) detected were 0.10 ± 0.008 and 0.09 ± 0.005 total nmol in WT and CD38−/− hearts, respectively. After I/R, WT levels fell to 0.02 ± 0.001 (~20% of baseline), while in CD38−/− hearts, I/R levels were 0.072 ± 0.004 (~80% of baseline) (Fig. 5A). These results indicate that CD38 depletes the NADP(H) pool more in the endothelium than in the whole heart.
Figure 5. Endothelial NADP(H), BH4, cGMP, and NOS-dependent CF in WT and CD38−/− hearts.
A, Endothelial metabolite pools were measured from endothelial-permeabilized hearts and analyzed for NADP(H) by HPLC. Levels were similar in control groups, with ~80% NADP(H) depletion in WT hearts, but slight loss in CD38−/− hearts (***P<0.005 vs. WT C, †††P<0.005 vs. WT I/R, n=5–11). B, Control levels of BH4 were similar between groups; however, after 30 min ischemia/30 min reperfusion, BH4 levels were much higher in CD38−/− hearts compared to WT hearts (***P<0.005 vs WT C, †††P<0.005 vs. CD38−/− C, †P<0.05 vs. WT I/R, n=3–6). C, WT and CD38−/− hearts were subjected to control (C) or 30 min ischemia/reperfusion (I/R) and the levels of cGMP were measured. After I/R, tissue levels of cGMP fell by ~90% in the WT group, but only ~20% in the CD38−/− group (***P<0.005 vs. WT C, †P<0.05 vs. WT I/R, n=4–6). D, At 30 min reperfusion in WT and CD38−/− hearts, NOS-dependent CF was largely decreased in WT hearts, while there was near total preservation of NOS-dependent CF in CD38−/− hearts. Acute NADPH restoration after 30 min reperfusion recovered NOS-dependent CF to pre-ischemic levels in WT hearts with minimal effect on CD38−/− hearts (***P<0.005 compared to WT. Mean ± SEM; n=3–5).
3.6. Effect of I/R on the myocardial content of BH4 in WT and CD38−/− hearts
Previous work has demonstrated marked depletion of myocardial BH4 with myocardial ischemia [6]. However, the process by which BH4 recovers in the acute time window after ischemia remains unanswered. Interestingly, both the recycling pathway through dihydrofolate reductase (DHFR) and the final step in the de novo synthesis pathway through sepiapterin reductase (SPR) are NADPH-dependent reactions [24, 25]. With preservation of the NADP(H) pool after I/R in CD38−/− hearts, we questioned whether this would lead to preserved BH4 levels as well. To address this, WT and CD38−/− hearts were subjected to non-ischemic perfusion (control) or 30 min ischemia followed by 30 min reperfusion, and BH4 was measured by HPLC. Levels of BH4 were not significantly different in non-ischemic hearts with values of 5.04 ± 0.30 pmol/mg protein and 5.42 ± 0.05 pmol/mg protein for WT and CD38−/−, respectively. However, after 30 min reperfusion, levels of BH4 were ~2.4-fold higher in CD38−/− compared to WT hearts, with values of 1.60 ± 0.24 pmol/mg protein for WT, and 3.77 ± 0.24 pmol/mg protein for CD38−/− hearts (Fig. 5B).
3.7. Effect of I/R on eNOS signaling via cGMP
As a way to assess eNOS-derived NO signaling, cGMP levels were measured after control perfusion or I/R in WT and CD38−/− hearts with and without NOS-inhibition. Levels of NOS-dependent cGMP in control-perfused hearts were not significantly different for WT and CD38−/− hearts with levels of 0.96 ± 0.14 and 1.12 ± 0.17 pmol/mg protein, respectively. After I/R, cGMP levels decreased to 0.09 ± 0.09 pmol/mg protein in WT hearts, with only a minor decrease to 0.87 ± 0.22 pmol/mg protein occurring in CD38−/− hearts (Fig. 5C). Thus, a >90% decrease was seen in WT hearts, but only a ~20% decrease in CD38−/− hearts, indicating preservation of NO signaling in post-ischemic hearts lacking CD38.
3.8. Effect of I/R on vascular function in WT and CD38−/− hearts
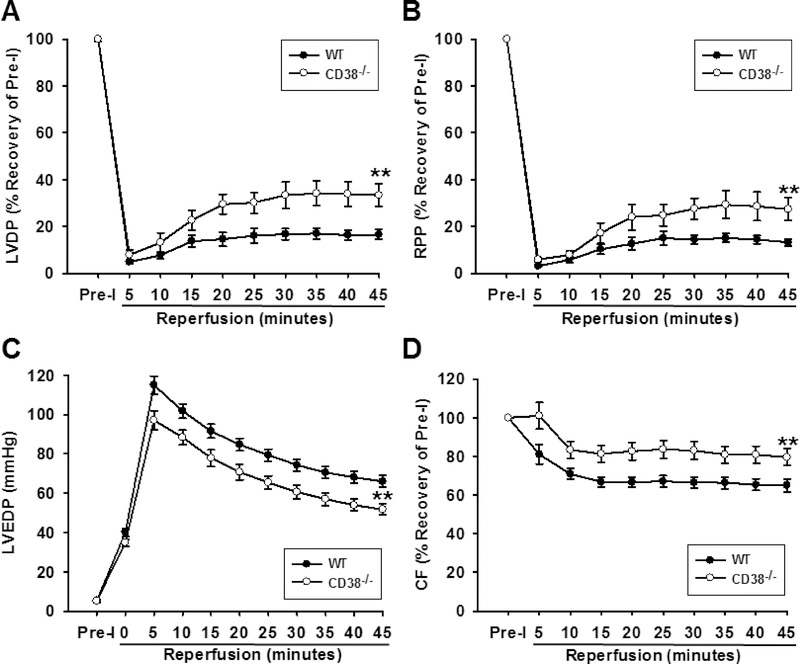
With higher post-ischemic levels of NADPH and BH4, as well as cGMP, we hypothesized that there would also be higher recovery of endothelium-dependent vascular function in CD38−/− hearts. To test this, we measured NOS-dependent CF after 30 min reperfusion by the infusion of NOS inhibitor L-NAME (1 mM) for 10 min. The recovery of NOS-dependent CF in WT hearts was only 24.3 ± 2.8% of baseline levels, confirming marked impairment of eNOS function after I/R. In CD38−/− hearts, however, the recovery of eNOS function was nearly complete, with a 96.5 ± 5.9% recovery. Similar measurements made following acute liposomal NADPH (100 μM) infusions showed that NADPH supplementation resulted in the full recovery of NOS-dependent CF in WT hearts, with only a small additive effect in CD38−/− hearts (Fig. 5D). Thus, in WT hearts, post-ischemic vascular function is severely impaired due to dysfunctional eNOS, but this is preserved in CD38−/− hearts which have preserved NADPH, BH4, and cGMP levels. We also see that replenishment of NADPH post-ischemia restores NOS-dependent CF to pre-ischemic levels in WT hearts. Further supporting this, we found that total CF in CD38−/− hearts undergoing I/R was significantly higher than in WT hearts. In WT hearts, recovery of total CF at 30 min reperfusion was 66.5 ± 2.9% of baseline, while in CD38−/− hearts, recovery was 83.0 ± 4.6% of baseline (Fig. 9D).
Figure 9. Effect of CD38 deletion on the recovery of contractile function and coronary flow after I/R.
Studies were performed on isolated hearts of WT and CD38−/− subjected to 30 min ischemia followed by 45 min reperfusion. Results show the time course of reperfusion for LVDP, RPP, CF, and LVEDP. After I/R, LVDP, RPP, and CF were all higher in CD38−/− hearts compared to WT controls, while LVEDP was lower in CD38−/− hearts compared to WT controls. In sum, these data show that CD38−/− hearts are strongly protected against I/R injury. Statistical significance was determined by ANOVA with two-way repeated measures (**P<0.01 compared to WT. Mean ± SEM; n=11 for WT, 15 for CD38−/−).
3.9. NO and superoxide production in WT and CD38−/− hearts
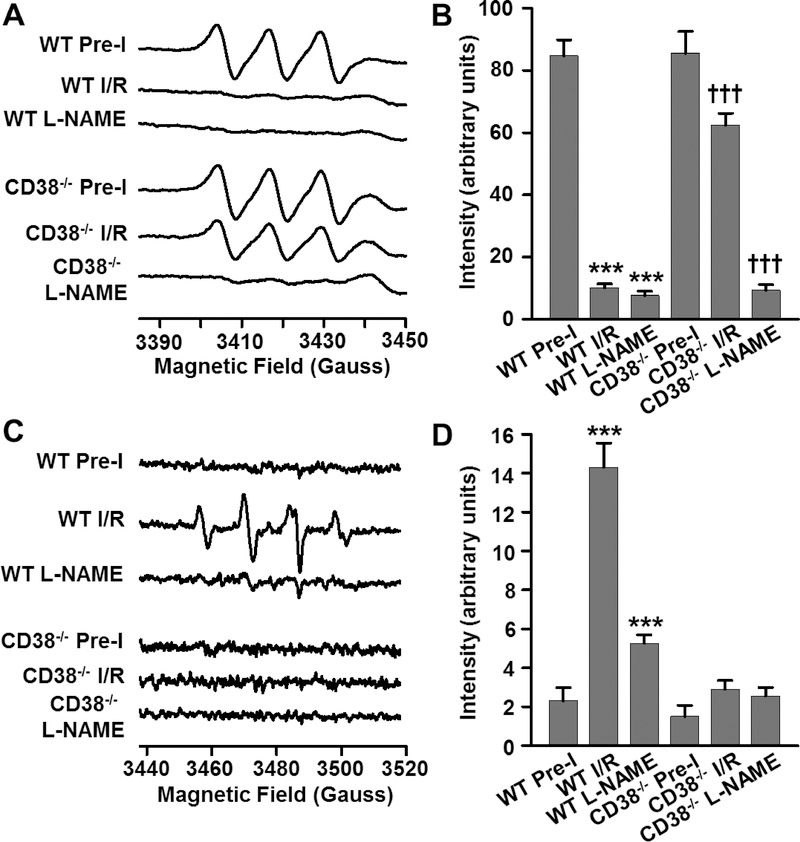
After measuring NADPH, BH4, and cGMP levels, as well as NOS-dependent vasodilatory function, we sought to understand how total and NOS-dependent NO and superoxide production were affected by I/R and if this is altered by CD38 knockout. In control WT and CD38−/− hearts using the NO spin trap Fe-MGD, we measured similar baseline levels of NO production following acetylcholine-induced activation of the endothelium. This NO production was largely blocked by pre-infusion of L-NAME (1 mM) for 10 minutes prior to NO spin trapping, demonstrating the NOS-dependence of the measured signal. Following I/R, we observed that the high basal levels of NO production elicited by agonist-induced endothelial activation was lost in WT hearts, ~90% decrease; however, this was largely preserved in CD38−/− hearts, only ~25% decrease (Fig. 6A). This was consistent with the higher levels of BH4, endothelial NADPH, and cGMP, as well as NOS-dependent CF, measured in CD38−/− compared to WT hearts (Fig. 5).
Figure 6. NO and superoxide production in WT and CD38−/− hearts.
Spin-trapping experiments of NO and superoxide were performed with agonist-induced NOS activation using 1 μM acetylcholine with and without pre-infusion with L-NAME (1 mM) to determine NOS coupling state. A, B, Pre-ischemic levels of NO production, which were inhibited by L-NAME, were largely lost with I/R in WT, but preserved in CD38−/− hearts. C, D, Superoxide, which was nearly undetectable in pre-ischemic hearts, increased sharply with I/R in WT hearts, but not in CD38−/− hearts. L-NAME blocked the majority of this superoxide generation, indicating that it was largely NOS-derived. Together these results demonstrate NOS uncoupling with loss of NOS-derived NO and increase in NOS derived superoxide (***P<0.005 compared to WT pre-I, †††P<0.005 compared to CD38−/− pre-I. Mean ± SEM; n=6).
Next, we performed spin trapping of superoxide and superoxide derived radical generation using the spin trap DMPO to ascertain the extent of eNOS uncoupling induced by I/R and the effect of CD38 knockout on this process. Post-ischemic superoxide production was measured following acetylcholine-induced endothelial activation with and without NOS inhibition using L-NAME. As shown previously, the observed spin trap signals can be quenched by SOD indicating that they are superoxide-derived [1, 7, 26]. We initially measured only trace pre-ischemic levels of superoxide in both WT and CD38−/− hearts. With agonist-induced activation of the endothelium after 30 minutes ischemia/30 minutes reperfusion, superoxide levels increased, primarily in WT hearts with little if any effect in CD38−/− hearts. Interestingly, infusion of the NOS inhibitor L-NAME (1 mM), prior to endothelial activation and superoxide spin trapping, largely blocked this signal increase, suggesting that post-ischemic NOS uncoupling occurred in WT but not CD38−/− hearts (Fig. 6B).
3.10. eNOS monomer/dimer ratio in WT and CD38−/− hearts
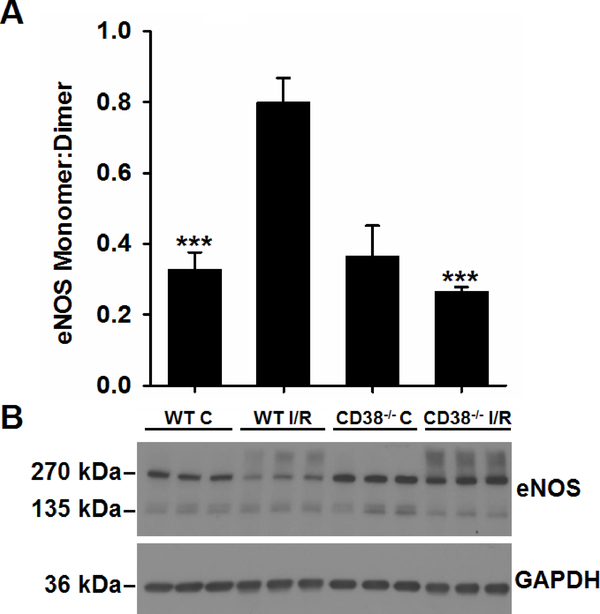
While depletion of cellular BH4 levels have been shown to cause NOS uncoupling, the monomer:dimer ratio of eNOS has also been reported to be an important measure of NOS function and coupling. Oxidant stress as occurs following I/R can induce a loss of NOS dimer [ref, ref]. Thus, we measured the ratio of eNOS monomer to dimer in WT and CD38−/− hearts prior to and after I/R. With cold, non-reducing PAGE and western blotting, we found that the eNOS monomer:dimer ratio increased ~2.5-fold following I/R of WT hearts, but not in CD38−/− hearts (Fig. 7). The monomer:dimer ratio in CD38−/− hearts was found to be ~3-fold lower than in WT hearts. As it has been shown that BH4 levels can regulate the eNOS monomer:dimer ratio through stabilization of the dimer [27] [28], the higher post-ischemic levels of BH4 seen in CD38−/− hearts (Fig. 5B) may serve to preserve the eNOS dimer and limit the formation of the monomer so as to preserve the monomer:dimer ratio after I/R [27, 29]. Interestingly, the eNOS antibody also recognized a relatively small broad high molecular weight band at about twice the molecular weight of the dimer in both WT and CD38−/− hearts following I/R, with this band not seen in either set of control-perfused heart samples. This band at the molecular weight of the tetramer was not seen with normal reducing PAGE in the presence of DTT, Figure 1. Thus, this band suggests the presence of higher order oligomers of eNOS arising due to thiol-crosslinking, likely triggered by the increased oxidative stress that accompanies I/R.
Figure 7. eNOS monomer and dimer levels in hearts.
WT and CD38−/− hearts undergoing control perfusion (C) or I/R were assessed by low temperature non-reducing gel electrophoresis and western blotting for levels of eNOS monomer and dimer. With I/R, the ratio of eNOS monomer (inactive) to dimer (active) increased in WT hearts, but not in CD38−/− hearts (***P<0.005 compared to WT I/R. Mean ± SEM, n=3).
3.11. Effect of I/R on myocardial glutathione levels
As the reduction of GSSG to GSH requires NADPH [30], we sought to measure both the myocardial and endothelial GSH/GSSG pools in both WT and CD38−/− hearts prior to and after I/R. Initial measurements in control-perfused hearts showed that CD38−/− hearts actually contained significantly higher levels of GSH in baseline conditions, with around 25% higher levels compared to WT. Accompanying I/R there is reported to be a loss of GSH/GSSG pool secondary to GSSG formation and wash out [31]. With I/R, we found that the levels of GSH in both groups fell significantly (approximately 35%), as previously reported to occur in hearts undergoing I/R [32, 33]. Interestingly, this brought the GSH levels in the CD38−/− hearts to levels near the pre-ischemic baseline levels in WT hearts (Fig. 8A). Using endothelial permeabilization, we also measured the endothelial pools of GSH from perfused hearts as we did with NAD(P)(H) (Fig. 5A). Similar to the whole heart, endothelial GSH levels fell with I/R; however, the loss of GSH was much more severe in the WT hearts which showed ~75% depletion while the CD38−/− hearts showed only ~30% depletion (Fig. 8B). Thus, marked endothelial depletion of GSH occurs in WT hearts following I/R, but only mild depletion was seen in CD38−/− hearts.
Figure 8. Myocardial and endothelial glutathione levels in WT and CD38−/− hearts undergoing control perfusion or I/R.
HPLC with fluorescence was used to measure GSH-OPA adducts before or after NEM labeling with subsequent reduction by DTT to assess GSH and GSSG, respectively in A) heart tissue, and B) coronary effluent of endothelium-permeabilized hearts. In heart tissue, I/R resulted in significant reduction in GSH levels in both groups with about 35% reduction seen for each. However, baseline GSH levels were significantly higher in CD38−/− compared to WT hearts. Analysis of coronary effluent after endothelial pemeabilization showed marked GSH depletion from the endothelium of WT hearts. In CD38−/− hearts, this depletion was much less severe and did not reach statistical significance, indicating protection of post-ischemic endothelial GSH pools in CD38−/− hearts. *P<0.05, **P<0.01, ***P<0.005 compared to WT Control, †P<0.05 compared to CD38−/− control, #P<0.05 compared to WT I/R. Mean ± SEM; n= 5–8).
3.12. Effect of I/R on contractile function in WT and CD38−/− hearts
Cardiac function was also assessed in isolated hearts from WT and CD38−/− mice after 30 min ischemia. Pre-ischemic hemodynamic function was similar between groups (Table 1). In WT hearts, baseline pre-ischemic LVDP values were 80 ± 4 mmHg and recovered to 16 ± 2 % of baseline by 45 min of reperfusion, while in CD38−/− hearts, pre-ischemic LVDP values were 79 ± 4 mmHg, with LVDP recovering to 31 ± 4 % of baseline. Baseline values of rate pressure product (RPP) were 30.7 ± 2.1 × 103 mmHg/min in WT and 29.7 ± 1.8 × 103 mmHg/min in CD38−/− hearts. The recovery of RPP was also much higher in the CD38−/− hearts with a value of 26 ± 4% compared to 13 ± 1.5% in WT hearts after 45 min reperfusion. Consistent with these findings, LVEDP, a measure of left ventricle relaxation, was found to be lower in CD38−/− hearts (50.2 ± 2.7 mmHg) after 45 min reperfusion compared to WT hearts (66.0 ± 3.0 mmHg) (Fig. 9A, B, C). Thus in hearts lacking CD38, post-ischemic myocardial contractile function is greatly preserved.
Table 1.
Baseline hemodynamic properties in WT and CD38−/− mouse hearts after 20 minutes of perfusion. CF is expressed as ml/min/g of heart weight. Values are mean ± SEM (N=15/grp).
| Parameter | WT | CD38−/− | P value |
|---|---|---|---|
| LVDP (mmHg) | 80 ± 4 | 79 ± 4 | 0.79 |
| RPP (mmHg/min) | 30.7 ± 2.1 × 103 | 29.7 ± 1.8 × 103 | 0.73 |
| CF (ml·min−1·g−1) | 10.3 ± 0.6 | 9.6 ± 0.5 | 0.34 |
| HR (BPM) | 381 ± 13 | 383 ± 15 | 0.92 |
3.13. Myocyte and endothelial cell protein/enzyme release from WT and CD38−/− hearts
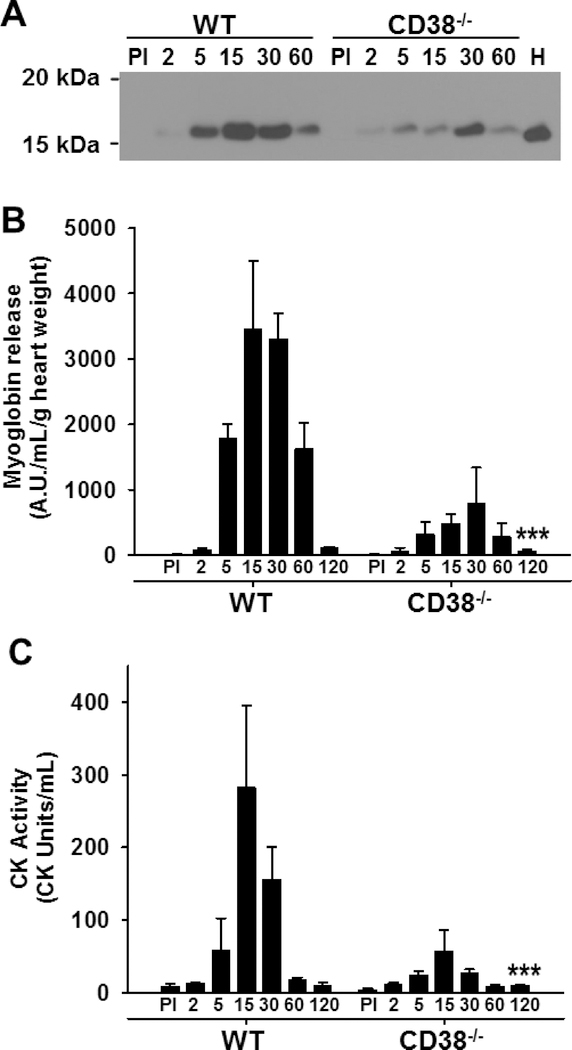
Myoglobin levels and CK activity were measured in coronary effluents in order to determine the effect of I/R on cardiac myocyte enzyme release during reperfusion of WT and CD38−/− hearts. As myoglobin and CK in the heart are almost exclusively expressed in cardiac myocytes, these experiments directly assay the level of myocyte injury occurring in the isolated hearts undergoing I/R. We found that while myoglobin was undetectable in coronary effluent prior to ischemia in both groups, there was much higher myoglobin release from WT hearts compared to CD38−/− hearts after reperfusion (Fig. 10A, B). Prominent myoglobin release occurred after 5 minutes of reperfusion and continued through 60 minutes of reperfusion, with myoglobin release tapering off by 120 minutes of reperfusion in both groups. We then performed calculations of total myoglobin release in each interval of reperfusion and summed the total release over the entire reperfusion period studied (0–120 min). We then compared this to the myoglobin content of heart homogenate of a pre-ischemic control heart. This analysis suggested that ~40% of the total heart’s content of myoglobin content was lost over the 120 minute reperfusion period in WT hearts, with only ~10% loss in CD38−/− hearts.
Figure 10. Enzyme release as a measure of myocardial cell death following I/R in hearts from WT and CD38−/− mice. A & B, Representative western blot and densitometry of myoglobin release in the coronary effluent that was used as a measure of myocyte injury in WT and CD38−/− hearts.
Levels of myoglobin in coronary effluents were much higher from WT compared to CD38−/− hearts. Myoglobin was also blotted in a heart lysate (H). C, CK release as measured by CK activity assay. CK release upon reperfusion was much greater in WT than CD38−/− hearts. Statistical significance was determined by ANOVA with two-way repeated measures (***P<0.005 compared to WT, n=3).
We also measured CK activity in coronary effluents of both groups. While baseline release of CK was negligible, release of CK started to occur in reperfusion in hearts beginning after 5 minutes of reperfusion. Release was considerably higher in WT compared to CD38−/− hearts (Fig. 10C). Overall, CK release followed a similar pattern to that of myoglobin from WT and CD38−/− hearts, both in relative abundance, and in the pattern of release over time. These experiments demonstrate that WT hearts were much susceptible to I/R induced cardiomyocyte injury than CD38−/− hearts.
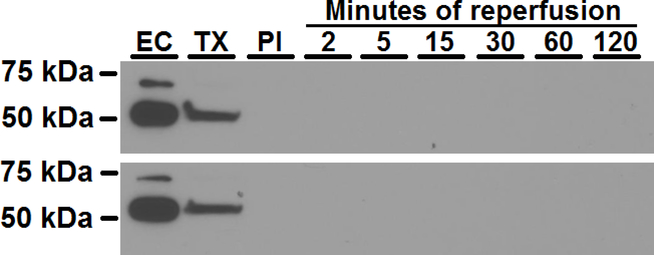
We further measured the release of cytosolic endothelial protein polypyrimidine tract binding protein 1 (PTBP1) [34, 35] in coronary effluent as a means to assess endothelial cell injury or death. We were able to detect strong PTBP1 bands both in purified aortic endothelial cell lysates and in coronary effluent collected after permeabilization of the coronary endothelium with bolus infusions of 0.25% Triton X-100 to the heart. However, in the same coronary effluent samples containing high levels of myoglobin and CK (Fig. 10A, B, C), PTBP1 was not detected, even in samples which were 25-fold concentrated (Fig. 11). This suggests that less than 1% endothelial cell death occurred following I/R.
Figure 11. Representative western blot of PTBP1 as a marker of endothelial cell death.
PTBP1 was measured in pure endothelial cells (EC), coronary effluent from an endothelial-permeabilized heart using Triton X-100 (TX), and coronary effluent collected pre-ischemia (PI) and after 2, 5, 15, 30, 60, and 120 min reperfusion. While PTBP1 was highly expressed in ECs and present in coronary effluent after endothelial permeabilization, it was undetectable in coronary effluent of hearts undergoing 30 minutes ischemia and 2–120 min reperfusion indicating the lack of endothelial cell death.
3.14. Infarct size in WT and CD38−/− hearts
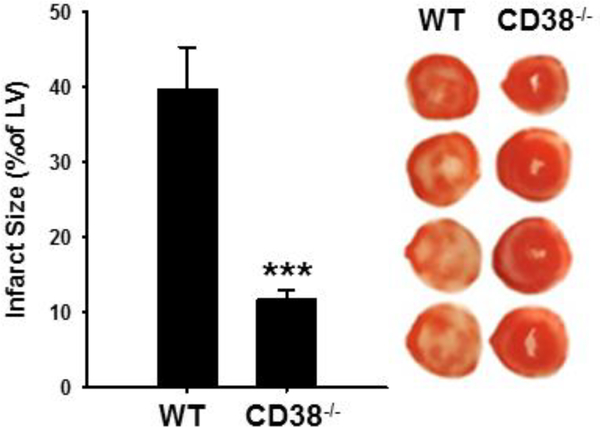
In order to further compare functional recovery with irreversible myocardial damage, TTC staining was performed to delineate between viable and non-viable tissue of WT and CD38−/− hearts. After 30 min ischemia and 120 min reperfusion, WT hearts displayed significant infarction, with an average of 39.8 ± 5.5% of the LV. However, CD38−/− hearts displayed much less infarction, with an average LV infarction of 11.7 ± 1.3% (Fig. 12). These data were consistent with the protein/enzyme release measurements above and demonstrate that CD38 activation is an important mechanism contributing to myocardial infarction in the isolated post-ischemic heart.
Figure 12. Myocardial infarction after I/R in hearts from WT and CD38−/− mice.
After 30 min ischemia and 120 min reperfusion, hearts were TTC-stained and imaged for viable and non-viable tissue. Myocardial infarction was significantly reduced in CD38−/− hearts (***P<0.005 vs WT, n=10–12).
4. Discussion
The use of genetically modified mice has provided the opportunity to directly and definitively test hypotheses regarding the roles of genes and their protein products. In this study, we utilize the CD38−/− mouse to more specifically test the role of CD38 and its activation in the process of I/R-induced injury with NAD(P)(H) pool depletion, resultant endothelial dysfunction and myocardial injury that is associated with loss of eNOS function [7, 13].
We initially tested CD38 and eNOS expression in WT and CD38−/− hearts undergoing non-ischemic control perfusion, global ischemia, and global I/R. These experiments showed that CD38 expression, which was absent in CD38−/− hearts, was unchanged in WT hearts undergoing these treatments. We also found that the expression of eNOS was similar across groups at baseline and with I/R (Fig. 1). In heart sections of WT and CD38−/− mice, CD38 staining was found in WT, but not CD38−/− hearts, with CD38 primarily co-staining with the endothelium-specific protein CD31 (PECAM-1) (Fig. 3A). Thus, CD38 was shown to be primarily expressed at high levels in the endothelial cells of the heart. Of note, in these sections, CD38 staining was approximately 10-fold weaker in surrounding cardiac myocytes than in endothelial cells (Fig. 3B).
Consistent with prior reports [15, 16], we found that CD38 is the sole NAD(P)+ase in the heart. After confirming that CD38−/− hearts lack NAD(P)+ase activity, we tested the changes in CD38 activity in WT hearts as a result of ischemia and I/R. While CD38−/− hearts lacked NAD(P)+ase activity in all conditions tested, NAD(P)+ase activity increased in WT hearts ~100% with ischemia alone, and ~200% with I/R (Fig. 2), consistent with prior measurements in the rat heart [7, 13].
Despite an absence of NAD(P)+ase activity in CD38−/− hearts, baseline levels of NADP(H) and NAD(H) were similar in CD38−/− and WT hearts (Fig. 4). However, after I/R there was much less depletion of both nucleotide pools in CD38−/− hearts compared to WT, approximately 30% versus 60%. These results suggest that I/R functions as a stimulus to trigger CD38 activation. This phenomenon may also be true in aging, where, in WT mice, NAD(H) levels were shown to decrease and CD38 activity was shown to increase, with age [36]. As oxidative stress has been implicated in I/R injury and aging [3, 37], CD38 activation in each condition may occur through a mechanism involving oxidative stress [7]. Though levels of NADP(H) and NAD(H) were largely preserved in CD38−/− hearts, modest depletion still occurred after I/R and this may be due to other NAD(P)(H)-dependent enzymes such as poly-ADP-ribose polymerase (PARP), sirtuin deacetylases (SIRTs), mono-ADP ribosyltransferases, or CD157 [38–41]. Non-enzymatic depletion also would be expected to occur through the process of cell death that accompanies I/R injury with release of pyridine nucleotides into the extracellular space [42]; however, the observed 60% depletion well exceeded the measured infarct size.
After establishing similar expression of eNOS in each group, we explored the levels of its substrates/cofactors with I/R injury. Using endothelial permeabilization, we measured the endothelial NADP(H) pool [7, 18]. While baseline levels were similar in both groups, with I/R greater than 80% depletion of NADP(H) occurred in WT coronary endothelium, with only ~ 20% depletion in CD38−/− hearts (Fig. 5A). Thus, NADP(H) depletion in WT hearts is more severe in the endothelium relative to the whole heart, likely due to the much higher expression of CD38 in endothelial cells as noted above [7]. We also showed that while BH4 levels were similar at baseline, BH4 levels recovered over 2-fold higher in CD38−/− hearts compared to WT after 30 minutes of reperfusion (Fig. 5B). With depletion of whole heart and endothelial NADP(H) levels, we reasoned that the activity of NADPH-dependent enzymes in the BH4 recycling and de novo synthesis pathways may also be affected. These are DHFR, which catalyzes the conversion of BH2 to BH4 in the recycling pathway, and SPR which catalyzes the final step in BH4 synthesis by converting 6-pyruvoyl tetrahydrobiopterin to BH4 in the de novo synthesis pathway [43, 44]. Thus, post-ischemic BH4 recycling from BH2, or BH4 synthesis from GTP, could be impaired in WT hearts but protected in CD38−/− hearts due to preserved myocardial and endothelial NADPH levels.
With increased recovery of NADPH and BH4 in CD38−/− hearts, both of which are essential for NO production by eNOS, we hypothesized that increased production of cGMP would occur due to NO binding to and activation of soluble guanylate cyclase (sGC). cGMP levels were similar between groups at baseline, demonstrating normal NOS-dependent function pre-ischemia. Following I/R, however, WT hearts exhibited very low levels ~10–20% of baseline pre-ischemic levels of myocardial cGMP, while levels in CD38−/− hearts were largely preserved (~80% of baseline) (Fig. 5C). Thus, NO signaling through sGC is largely lost in WT hearts but preserved with CD38−/−.
The physiological significance of higher post-ischemic cGMP levels in CD38−/− hearts was evaluated by measuring the change in CF with L-NAME (1 mM) with and without prior liposomal NADPH infusions. We found that there was significantly higher NOS-dependent CF in CD38−/− compared to WT hearts after I/R. Recovery of NOS-dependent CF was approximately 95% in CD38−/− hearts, but only 25% in WT hearts (Fig 5D). However, NADPH supplementation fully restored NOS-dependent CF in WT hearts, with little additional effect in CD38−/− hearts which had intrinsically preserved NADP(H) levels. This is consistent with our previous studies where replenishment of NADPH after I/R led to near complete recovery of NOS-dependent CF, with tandem replenishment of NADPH and BH4 restoring it completely [7].
Consistent with the above, we found that NO production was severely decreased following I/R in WT hearts but much less so in CD38−/− hearts. This was found along with increased NOS-dependent superoxide production in WT but not in CD38−/− hearts, suggesting that CD38 knockout prevents post-ischemic NOS uncoupling (Fig 7). Aside from measuring BH4 as a potential mechanism of NOS uncoupling, we also assessed the eNOS monomer:dimer ratio which has been considered as a mechanism of NOS dysfunction and uncoupling, possibly regulated by BH4 availability [27, 29, 45, 46]. Consistently, we found increased monomer:dimer ratio following I/R in WT hearts, perhaps due to the lower BH4 levels found in these hearts after I/R. Further supporting this, we found that the increased monomer:dimer occurring with I/R was largely prevented in the CD38−/− hearts which have higher post-ischemic BH4 levels (Fig 6).
Another mechanism of NOS uncoupling is due to the S-glutathionylation of eNOS, a post-translational modification (PTM) of cysteine residues determined in large part by the glutathione redox state [47, 48]. Interestingly, we found higher myocardial levels of GSH at baseline in CD38−/− compared to WT hearts. While I/R had a similar effect in each with around 30% depletion, the resulting GSH levels were higher in CD38−/− hearts. In the endothelium, where NADP(H) is more severely depleted, GSH recovery was higher in CD38−/− compared to WT hearts (Fig 8B). As the reduction of oxidized glutathione (GSSG) to GSH by glutathione reductase is NADPH-dependent [30], the higher concentration of NADPH throughout I/R could help maintain GSH levels minimizing the formation and loss of GSSG that is accompanied by wash out from the heart. This could further lead to the preservation of the cellular glutathione pool that was observed in the CD38−/− hearts. Further studies on post-ischemic PTMs of eNOS will be needed to determine how they may be modulated by CD38 activation and how this further contributes to NOS dysfunction and/or uncoupling.
With regard to the effect of CD38 knockout on physiological function, we observed that the recovery of total CF, similar to NOS-dependent CF, was higher in CD38−/− hearts (Fig. 9D). This accompanied the increased recovery of LV contractile function, with higher LVDP and RPP and significantly reduced post-ischemic LVEDP in CD38−/− hearts (Fig. 9A, B, C). Strong protection against I/R injury was also evidenced by the dramatic reduction in enzyme release (both myoglobin and CK), and LV infarct size seen in CD38−/− hearts, where only small infarction occurred compared to much larger infarction in WT hearts (Figs. 10 and 12). Measurement of the release of PTBP1, a cytosolic endothelial protein, demonstrated negligible endothelial cell death following I/R (Fig. 11). Therefore, while some depletion of NAD(P)(H) in the whole heart could be attributed to cell washout, as observed with myoglobin and CK, cellular washout of NAD(P)(H) from the endothelium would be negligible. Thus, endothelial cell depletion of NAD(P)(H) is due primarily to CD38-mediated depletion.
In determining the mechanism(s) of cardioprotection observed in CD38−/− hearts, the multifunctionality of CD38 has to be considered. From the present studies in the isolated heart model, we observe that the preserved vascular function found in CD38−/− hearts due to enhanced recovery of NADPH and BH4 is an important mechanism conferring cardioprotection against I/R injury. Previously, we studied eNOS−/− hearts to determine whether the protective effects of CD38 inhibition found in WT hearts were lost in hearts lacking eNOS [7]. In that study, we showed that treatment with CD38 inhibitor α-NAD elicited cardioprotection against I/R in WT mouse hearts, with the protective effect largely lost in eNOS−/− mouse hearts [7]. While this demonstrated that normal eNOS expression was key to the effects of CD38 inhibitors in I/R, the incomplete loss of protection suggested that CD38 inhibition may also confer some protection through eNOS-independent mechanisms.
The CD38−/− brain has previously been shown to be protected against in vivo I/R injury [49]. The authors suggested that ectocellular CD38 expressed on circulating leukocytes was responsible for leukocyte migration to the vascular injury site, causing an increased production of chemokines which exacerbated I/R injury [49]. However, in our model of ex vivo I/R, the systemic immune response is eliminated as a factor, and thus, the protective effect of CD38 deletion in our model was not due to a tempered immune response. However, this raises the possibility that there could be even more profound protection with this additive effect in vivo, where the immune response is intact.
Aside from these mechanisms, other distinct cardioprotective mechanisms of CD38 knockout may also exist. These include sparing of the NAD(H) pool which CD38 regulates [15, 50], eliminating the production of CD38-derived Ca2+-mobilizing second messengers, and, as shown in this paper for the first time, higher myocardial glutathione levels (Fig. 8A). While maintaining the NAD(H) pool may be beneficial to cellular ATP production, inhibiting the production of Ca2+ mobilizers such as (2’P-)ADPR, (2’-P-)cADPR and NAADP [12, 51, 52] could lessen myocardial Ca2+ overload, a well-documented cause of I/R injury of myocytes [7, 53–55].
While most of the effects of CD38 inhibition or knockout in mediating NAD(P)(H) salvage would be expected to be protective including preservation of eNOS function and coupling, maintenance of reduced glutathione levels, prevention of excessive Ca2+ mobilization and overload, one can question whether preservation of NADPH levels might also enhance Nox2 NADPH oxidase function and secondary superoxide generation. Our measurements of superoxide generation in post-ischemic hearts indicate that the observed superoxide generation in WT hearts subjected to I/R was primarily NOS-derived with inhibition by L-NAME. Furthermore, no significant increase in superoxide generation was seen in the CD38−/− hearts after I/R with less superoxide detected than in WT (Fig. 6C and D). Thus, there was no evidence of any Nox-mediated or any other increase in superoxide production in this model. This may be due to the relatively low levels of Nox expression in the isolated heart, or the lack of its activation and complex assembly. Of note, our recent study evaluating the sources of radical generation in endothelial cells subjected to hypoxia and reoxygenation and the effects of CD38 inhibition or siRNA-mediated knockdown, demonstrated that oxygen radical generation was derived from xanthine oxidase and uncoupled eNOS, with no measurable effect of Nox inhibition [23]. This is consistent with prior studies that have shown the importance of these two pathways in post-ischemic hearts or hypoxic/reoxygenated cells [10, 56–60].
The cardioprotected-phenotype of CD38−/− hearts provides a strong rational to search for effective and specific CD38 inhibitors. Recently, there have been several publications demonstrating novel inhibitors of CD38. Using NAD+ analogues, Wang et al demonstrated strong inhibition against the NAD(P)+ase function of CD38 [61]. Another group has shown the potential for two different families of chemicals for CD38 inhibition: the thiazoloquin(az)olinones and 4-amino-8-quinoline carboxamides [62, 63]. Perhaps most intriguing, however, is the potential for natural products to inhibit CD38. Flavonoids, which are rich in plant foods, have recently been shown to inhibit CD38 in vitro and in vivo [13, 64, 65]. We have recently found that the 3-deoxyanthocyanin flavonoid luteolinidin is highly cardioprotective in the rat heart [13]. Another group of natural products, the anthranoids, have also been shown to effectively inhibit CD38 [66]. Further studies on the bioavailability, biocompatibility, and potency of such naturally-occurring compounds are needed to clarify their potential use as CD38 inhibitors.
In summary, the use of a genetic model of CD38 deletion enabled the demonstration that CD38 is the major enzyme in the heart with NAD(P)+ase enzymatic function, conferring it with a critical role in the pathogenesis of I/R injury. While baseline levels of NADP(H) and NAD(H) pools are similar between WT and CD38−/− hearts, the post-ischemic levels of each are strongly preserved in CD38−/− hearts and coronary endothelium. Preservation of the NADP(H) pool led to increased recovery of BH4 and eNOS function, which resulted in greater cGMP production, preserved eNOS coupling, and increased recovery of NOS-dependent and total CF in CD38−/− hearts. Increased recovery of LV contractile function in the CD38−/− hearts was also found, with higher myocardial and endothelial GSH levels, and lessened post-ischemic enzyme release and enhanced myocardial survival in CD38−/− hearts. Further studies investigating the mechanisms of CD38 activation, as well as its inhibition, with future extension of the current observations to in vivo models will be important for advancement of CD38-related therapeutics for the treatment of cardiovascular disease.
Highlights.
Knockout of CD38 decreases myocardial/endothelial NAD(P)(H) depletion in I/R.
Post-ischemic eNOS-dependent vasodilation is protected in CD38 knockout hearts.
CD38 knockout hearts have higher recovery of contractile function after I/R.
Myocardial infarction is greatly decreased with knockout of CD38.
Overall, CD38 activation has a critical role in the pathogenesis of I/R injury.
Acknowledgements
We would like to acknowledge the help of Craig Hemann for technical assistance in experiments and manuscript preparation. We would also like to acknowledge the Ohio State Campus Microscopy and Imaging Facility (CMIF) for microscopy assistance.
Funding
This work was supported by National Institutes of Health grants HL131941 and HL135648.
Abbreviations
- 2’-P-(c)ADPR
2’-phospho (cyclic) adenosine diphosphate ribose
- BH2
Dihydrobiopterin
- BH4
Tetrahydrobiopterin
- BSA
Bovine serum albumin
- CF
Coronary flow
- cGMP
Cyclic guanosine monophosphate
- DHFR
Dihydrofolate reductase
- DTPA
Diethylenetriaminepentaacetic acid
- ε-ADPR
1,N6-etheno adenosine diphosphate ribose
- ε-NAD
1,N6-ethenoadenine dinucleotide
- EDTA
Ethylenediaminetetraacetic acid
- eNOS
Endothelial nitric oxide synthase
- GSH
Reduced glutathione
- GSSG
oxidized glutathione
- HR
Heart rate
- HRP
Horseradish peroxidase
- IHC
Immunohistochemistry
- I/R
Ischemia/reperfusion
- KCN
Potassium cyanide
- KH2PO4
Potassium phosphate
- KI
Potassium iodide
- KOH
Potassium hydroxide
- L-NAME
Nω-Nitro-L-arginine methyl ester
- LV
Left ventricle
- LVDP
Left ventricle developed pressure
- LVEDP
Left ventricle end diastolic pressure
- NAADP
Nicotinic acid adenine dinucleotide phosphate
- NH4OH
Ammonium hydroxide
- NO
Nitric oxide
- PPC
Phosphatidylcholine
- PTBP1
Polypyrimidine tract binding protein
- ROS
Reactive oxygen species
- RPP
Rate pressure product
- SDS
Sodium dodecyl sulfate
- SGC
Soluble guanylate cyclase
- SIRT
Sirtuin deacetylase
- SNP
Sodium nitroprusside
- SPE
Solid phase extraction
- SPR
Sepiapterin reductase
- TBST
Tris buffer saline with 0.1% Tween-20
- Tris
Tris(hydroxymethyl)aminomethane
- TTC
2,3,5-triphenyltetrazolium chloride
Footnotes
Conflict of Interest
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT, Measurement and characterization of postischemic free radical generation in the isolated perfused heart, J Biol Chem 264(32) (1989) 18890–5. [PubMed] [Google Scholar]
- [2].Ferrari R, Alfieri O, Curello S, Ceconi C, Cargnoni A, Marzollo P, Pardini A, Caradonna E, Visioli O, Occurrence of oxidative stress during reperfusion of the human heart, Circulation 81(1) (1990) 201–11. [DOI] [PubMed] [Google Scholar]
- [3].Zweier JL, Talukder MA, The role of oxidants and free radicals in reperfusion injury, Cardiovasc Res 70(2) (2006) 181–90. [DOI] [PubMed] [Google Scholar]
- [4].Bolli R, Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research, J Mol Cell Cardiol 33(11) (2001) 1897–918. [DOI] [PubMed] [Google Scholar]
- [5].Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S, Update on mechanism and catalytic regulation in the NO synthases, J Biol Chem 279(35) (2004) 36167–70. [DOI] [PubMed] [Google Scholar]
- [6].Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL, Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4, Proc Natl Acad Sci U S A 104(38) (2007) 15081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reyes LA, Boslett J, Varadharaj S, De Pascali F, Hemann C, Druhan LJ, Ambrosio G, El-Mahdy M, Zweier JL, Depletion of NADP(H) due to CD38 activation triggers endothelial dysfunction in the postischemic heart, Proc Natl Acad Sci U S A 112(37) (2015) 11648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xia Y, Tsai AL, Berka V, Zweier JL, Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process, J Biol Chem 273(40) (1998) 25804–8. [DOI] [PubMed] [Google Scholar]
- [9].Cardounel AJ, Xia Y, Zweier JL, Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin, J Biol Chem 280(9) (2005) 7540–9. [DOI] [PubMed] [Google Scholar]
- [10].De Pascali F, Hemann C, Samons K, Chen CA, Zweier JL, Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation, Biochemistry 53(22) (2014) 3679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pieper AA, Walles T, Wei G, Clements EE, Verma A, Snyder SH, Zweier JL, Myocardial postischemic injury is reduced by polyADPripose polymerase-1 gene disruption, Mol Med 6(4) (2000) 271–82. [PMC free article] [PubMed] [Google Scholar]
- [12].Vu CQ, Lu PJ, Chen CS, Jacobson MK, 2’-Phospho-cyclic ADP-ribose, a calcium-mobilizing agent derived from NADP, J Biol Chem 271(9) (1996) 4747–54. [PubMed] [Google Scholar]
- [13].Boslett J, Hemann C, Zhao YJ, Lee HC, Zweier JL, Luteolinidin Protects the Postischemic Heart through CD38 Inhibition with Preservation of NAD(P)(H), J Pharmacol Exp Ther 361(1) (2017) 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Munshi CB, Fryxell KB, Lee HC, Branton WD, Large-scale production of human CD38 in yeast by fermentation, Methods Enzymol 280 (1997) 318–30. [DOI] [PubMed] [Google Scholar]
- [15].Aksoy P, White TA, Thompson M, Chini EN, Regulation of intracellular levels of NAD: a novel role for CD38, Biochem Biophys Res Commun 345(4) (2006) 1386–92. [DOI] [PubMed] [Google Scholar]
- [16].Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, Murray R, Schuber F, Howard MC, Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses, Blood 92(4) (1998) 1324–33. [PubMed] [Google Scholar]
- [17].Graeff RM, Lee HC, Determination of ADP-ribosyl cyclase activity, cyclic ADP-ribose, and nicotinic acid adenine dinucleotide phosphate in tissue extracts, Methods Mol Biol 1016 (2013) 39–56. [DOI] [PubMed] [Google Scholar]
- [18].Giraldez RR, Panda A, Zweier JL, Endothelial dysfunction does not require loss of endothelial nitric oxide synthase, Am J Physiol Heart Circ Physiol 278(6) (2000) H2020–7. [DOI] [PubMed] [Google Scholar]
- [19].Gwozdz P, Drelicharz L, Kozlovski VI, Chlopicki S, Prostacyclin, but not nitric oxide, is the major mediator of acetylcholine-induced vasodilatation in the isolated mouse heart, Pharmacol Rep 59(5) (2007) 545–52. [PubMed] [Google Scholar]
- [20].Klaidman LK, Leung AC, Adams JD Jr., High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions, Anal Biochem 228(2) (1995) 312–7. [DOI] [PubMed] [Google Scholar]
- [21].Michaelsen JT, Dehnert S, Giustarini D, Beckmann B, Tsikas D, HPLC analysis of human erythrocytic glutathione forms using OPA and N-acetyl-cysteine ethyl ester: evidence for nitrite-induced GSH oxidation to GSSG, J Chromatogr B Analyt Technol Biomed Life Sci 877(28) (2009) 3405–17. [DOI] [PubMed] [Google Scholar]
- [22].Liu L, Shi GP, CD31: beyond a marker for endothelial cells, Cardiovasc Res 94(1) (2012) 3–5. [DOI] [PubMed] [Google Scholar]
- [23].Boslett J, Hemann C, Christofi FL, Zweier JL, Characterization of CD38 in the Major Cell Types of the Heart: Endothelial Cells Highly Express CD38 with Activation by Hypoxia/Reoxygenation Depleting NAD(P)H, Am J Physiol Cell Physiol (2017) ajpcell001392017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bailey SW, Ayling JE, The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake, Proc Natl Acad Sci U S A 106(36) (2009) 15424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao L, Pung YF, Zhang J, Chen P, Wang T, Li M, Meza M, Toro L, Cai H, Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability, Am J Physiol Heart Circ Physiol 297(1) (2009) H331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zweier JL, Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury, J Biol Chem 263(3) (1988) 1353–7. [PubMed] [Google Scholar]
- [27].Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, Tsai AL, Zweier JL, Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition, Biochemistry 49(14) (2010) 3129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen PF, Tsai AL, Wu KK, Cysteine 99 of endothelial nitric oxide synthase (NOS-III) is critical for tetrahydrobiopterin-dependent NOS-III stability and activity, Biochem Biophys Res Commun 215(3) (1995) 1119–29. [DOI] [PubMed] [Google Scholar]
- [29].Cai S, Khoo J, Channon KM, Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells, Cardiovasc Res 65(4) (2005) 823–31. [DOI] [PubMed] [Google Scholar]
- [30].Vanoni MA, Wong KK, Ballou DP, Blanchard JS, Glutathione reductase: comparison of steady-state and rapid reaction primary kinetic isotope effects exhibited by the yeast, spinach, and Escherichia coli enzymes, Biochemistry 29(24) (1990) 5790–6. [DOI] [PubMed] [Google Scholar]
- [31].Ambrosio G, Santoro G, Tritto I, Elia PP, Duilio C, Basso A, Scognamiglio A, Chiariello M, Effects of ischemia and reperfusion on cardiac tolerance to oxidative stress, Am J Physiol 262(1 Pt 2) (1992) H23–30. [DOI] [PubMed] [Google Scholar]
- [32].Cheung PY, Wang W, Schulz R, Glutathione protects against myocardial ischemia-reperfusion injury by detoxifying peroxynitrite, J Mol Cell Cardiol 32(9) (2000) 1669–78. [DOI] [PubMed] [Google Scholar]
- [33].Singh A, Lee KJ, Lee CY, Goldfarb RD, Tsan MF, Relation between myocardial glutathione content and extent of ischemia-reperfusion injury, Circulation 80(6) (1989) 1795–804. [DOI] [PubMed] [Google Scholar]
- [34].Alonso J, Sanchez de Miguel L, Monton M, Casado S, Lopez-Farre A, Endothelial cytosolic proteins bind to the 3’ untranslated region of endothelial nitric oxide synthase mRNA: regulation by tumor necrosis factor alpha, Mol Cell Biol 17(10) (1997) 5719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yi B, Ozerova M, Zhang GX, Yan G, Huang S, Sun J, Post-Transcriptional Regulation of Endothelial Nitric Oxide Synthase Expression by Polypyrimidine Tract-Binding Protein 1, Arterioscler Thromb Vasc Biol 35(10) (2015) 2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Camacho-Pereira J, Tarrago MG, Chini CC, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN, CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism, Cell Metab 23(6) (2016) 1127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS, Mitochondrial oxidative stress in aging and healthspan, Longev Healthspan 3 (2014) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Halmosi R, Berente Z, Osz E, Toth K, Literati-Nagy P, Sumegi B, Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system, Mol Pharmacol 59(6) (2001) 1497–505. [DOI] [PubMed] [Google Scholar]
- [39].Abdellatif M, Sirtuins and pyridine nucleotides, Circ Res 111(5) (2012) 642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schwab CJ, Colville MJ, Fullerton AT, McMahon KK, Evidence of endogenous mono-ADP-ribosylation of cardiac proteins via anti-ADP-ribosylarginine immunoreactivity, Proc Soc Exp Biol Med 223(4) (2000) 389–96. [DOI] [PubMed] [Google Scholar]
- [41].Malavasi F, Deaglio S, Ferrero E, Funaro A, Sancho J, Ausiello CM, Ortolan E, Vaisitti T, Zubiaur M, Fedele G, Aydin S, Tibaldi EV, Durelli I, Lusso R, Cozno F, Horenstein AL, CD38 and CD157 as receptors of the immune system: a bridge between innate and adaptive immunity, Mol Med 12(11–12) (2006) 334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P, Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart, J Biol Chem 276(4) (2001) 2571–5. [DOI] [PubMed] [Google Scholar]
- [43].Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM, Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways, J Biol Chem 284(41) (2009) 28128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schoedon G, Blau N, Schneemann M, Flury G, Schaffner A, Nitric oxide production depends on preceding tetrahydrobiopterin synthesis by endothelial cells: selective suppression of induced nitric oxide production by sepiapterin reductase inhibitors, Biochem Biophys Res Commun 199(2) (1994) 504–10. [DOI] [PubMed] [Google Scholar]
- [45].Stuehr D, Pou S, Rosen GM, Oxygen reduction by nitric-oxide synthases, J Biol Chem 276(18) (2001) 14533–6. [DOI] [PubMed] [Google Scholar]
- [46].Zou MH, Shi C, Cohen RA, Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite, J Clin Invest 109(6) (2002) 817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL, S-glutathionylation uncouples eNOS and regulates its cellular and vascular function, Nature 468(7327) (2010) 1115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL, Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling, J Biol Chem 286(33) (2011) 29098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Choe CU, Lardong K, Gelderblom M, Ludewig P, Leypoldt F, Koch-Nolte F, Gerloff C, Magnus T, CD38 exacerbates focal cytokine production, postischemic inflammation and brain injury after focal cerebral ischemia, PLoS One 6(5) (2011) e19046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chini EN, CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions, Curr Pharm Des 15(1) (2009) 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gul R, Park DR, Shawl AI, Im SY, Nam TS, Lee SH, Ko JK, Jang KY, Kim D, Kim UH, Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) and Cyclic ADP-Ribose (cADPR) Mediate Ca2+ Signaling in Cardiac Hypertrophy Induced by beta-Adrenergic Stimulation, PLoS One 11(3) (2016) e0149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kuhn FJ, Heiner I, Luckhoff A, TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose, Pflugers Arch 451(1) (2005) 212–9. [DOI] [PubMed] [Google Scholar]
- [53].Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E, Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts, J Clin Invest 79(3) (1987) 950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cheung JY, Bonventre JV, Malis CD, Leaf A, Calcium and ischemic injury, N Engl J Med 314(26) (1986) 1670–6. [DOI] [PubMed] [Google Scholar]
- [55].Braunwald E, Kloner RA, Myocardial reperfusion: a double-edged sword?, J Clin Invest 76(5) (1985) 1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Thompson-Gorman SL, Zweier JL, Evaluation of the role of xanthine oxidase in myocardial reperfusion injury, J Biol Chem 265(12) (1990) 6656–63. [PubMed] [Google Scholar]
- [57].Xia Y, Zweier JL, Substrate control of free radical generation from xanthine oxidase in the postischemic heart, J Biol Chem 270(32) (1995) 18797–803. [DOI] [PubMed] [Google Scholar]
- [58].Zweier JL, Kuppusamy P, Lutty GA, Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues, Proc Natl Acad Sci U S A 85(11) (1988) 4046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zweier JL, Broderick R, Kuppusamy P, Thompson-Gorman S, Lutty GA, Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation, J Biol Chem 269(39) (1994) 24156–62. [PubMed] [Google Scholar]
- [60].Zweier JL, Kuppusamy P, Thompson-Gorman S, Klunk D, Lutty GA, Measurement and characterization of free radical generation in reoxygenated human endothelial cells, Am J Physiol 266(3 Pt 1) (1994) C700–8. [DOI] [PubMed] [Google Scholar]
- [61].Wang S, Zhu W, Wang X, Li J, Zhang K, Zhang L, Zhao YJ, Lee HC, Zhang L, Design, synthesis and SAR studies of NAD analogues as potent inhibitors towards CD38 NADase, Molecules 19(10) (2014) 15754–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR, Jeune MR, Kaldor I, Milliken N, Petrov KG, Preugschat F, Schulte C, Shearer BG, Shearer T, Smalley TL Jr., Stewart EL, Stuart JD, Ulrich JC, Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors, J Med Chem 58(8) (2015) 3548–71. [DOI] [PubMed] [Google Scholar]
- [63].Becherer JD, Boros EE, Carpenter TY, Cowan DJ, Deaton DN, Haffner CD, Jeune MR, Kaldor IW, Poole JC, Preugschat F, Rheault TR, Schulte CA, Shearer BG, Shearer TW, Shewchuk LM, Smalley TL Jr., Stewart EL, Stuart JD, Ulrich JC, Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38, J Med Chem 58(17) (2015) 7021–56. [DOI] [PubMed] [Google Scholar]
- [64].Kellenberger E, Kuhn I, Schuber F, Muller-Steffner H, Flavonoids as inhibitors of human CD38, Bioorg Med Chem Lett 21(13) (2011) 3939–42. [DOI] [PubMed] [Google Scholar]
- [65].Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN, Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome, Diabetes 62(4) (2013) 1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Blacher E, Ben Baruch B, Levy A, Geva N, Green KD, Garneau-Tsodikova S, Fridman M, Stein R, Inhibition of glioma progression by a newly discovered CD38 inhibitor, Int J Cancer 136(6) (2015) 1422–33. [DOI] [PubMed] [Google Scholar]