Abstract
Background:
Preclinical and human positron emission tomography (PET) studies have produced inconsistent results regarding the effects of opioids on mesolimbic dopamine (DA). Here we quantify striatal DA release (measured by [11C]-raclopride displacement) in response to an intravenous infusion of morphine, and its relation with morphine-induced subjective effects, in healthy, non-dependent opioid-experienced participants.
Methods:
Fifteen healthy male participants were initially included. Sessions were on separate days. On Session 1, participants received intravenous morphine (10 mg / 70 kg) in the clinic to ensure tolerability. Participants without adverse reactions (n=10) then received intravenous morphine and placebo (saline) sessions, in counter-balanced order, while undergoing [11C]-raclopride PET scans. Subjective and physiological responses were assessed. Region-of-interest and voxelwise image analyses were used to assess changes in [11C]-raclopride non-displaceable binding potential (ΔBPnd).
Results:
Morphine produced marked subjective and physiological effects, and induced a significant decrease in [11C]-raclopride BPnd, particularly in the nucleus accumbens and globus pallidus, where ΔBPnd was approximately 9%. However, the subjective effects of morphine did not show a simple pattern of correlation with DA release.
Conclusions:
This is to our knowledge the first study providing in vivo human evidence that DA transmission in ventral striatum is affected by morphine. Further studies are required to fully delineate the DA contribution to the reinforcing effects of opioids.
Keywords: Opioids, Morphine, [11C]-raclopride, Dopamine, PET, Ventral Striatum
Introduction
Opioids have been used by humans for their analgesic and euphorigenic effects for thousands of years. In the last century, natural and synthetic opioids, including morphine, oxycodone, and fentanyl, have become mainstays of treatment for acute and chronic pain. The drastic increase in rates of prescription opioid use, particularly since the 1990s, posed the challenge of balancing the analgesic properties of opioids against their well-documented abuse potential due to their potent rewarding properties in vulnerable individuals.
Currently, the nonmedical use of prescription as well as illicit opioids represents a major public health crisis in the United States (1). Because the potent reinforcing properties of opioid agonists are combined with respiratory depressant effects, the increased prevalence of nonmedical opioid use has resulted in dramatically increased mortality, reaching 60000 deaths annually (2). The opioid epidemic requires broad implementation of available treatments for opioid use disorders (OUD), but also prompts a need for mechanistically novel interventions for pain.
Major advances have been made in the neuroscience of opioid actions and OUD, but the mechanisms underlying the effects of morphine and other opioids, particularly with regard to their reinforcing properties, are still highly debated (3–5). Preclinical studies indicate that opioids act in the ventral tegmental area (VTA) via Gi/o-coupled μ-receptors to disinhibit dopaminergic (DA) neurons by attenuating inhibitory GABAergic tone. This promotes burst firing of DA neurons and enhances DA release in their terminal fields (6, 7). In vivo microdialysis data also demonstrate that opioid administration increases DA release in the nucleus accumbens (NAc) (8).
While this dopaminergic response has been evidenced in animal investigations, modulation of mesolimbic DA transmission by opioids remains controversial in humans. Two previous [11C]-raclopride positron emission tomography (PET) studies have evaluated changes in extracellular DA release following the administration of opioids. These studies failed to detect increased striatal DA release in response to opioids, an effect reported for other addictive agents [for a review see (23, 24)]. Specifically, an intravenous dose of 50 mg heroin did not result in a measurable effect on striatal DA levels in heroin-dependent patients, despite producing marked euphoria (9). Similarly, heroin administration, as well as the expectation of a heroin reward, were not associated with DA release in patients with OUD receiving methadone or buprenorphine (10).
However, assessing the magnitude of DA release in individuals with a prolonged exposure to opioids may be misleading given that chronic drug exposure induces long-term adaptations within the DA system. These neuroadaptive processes result in a hypodopaminergic state, indexed experimentally by a blunted DA response. This phenomenon has been observed in opioid-dependent individuals (11), as well as in individuals with other substance use disorders (12–15), and is also supported by extensive preclinical data (for a review see (16)). Moreover, studies in rodents suggest that the differential DA response observed in opioid-naïve vs. dependent animals may underlie a different contribution of DA to opioid reinforcement, depending on opioid exposure state (17, 18). To date, the role of the DA response in opioid reinforcement remains the subject of debate ((19–27) but see also (28–31)). Thus, a better understanding of the dopaminergic response to opioids in the human brain is needed.
To begin addressing this set of questions, we hypothesized that in non-dependent opioid-experienced participants, morphine administration would increase striatal DA release compared to placebo, although to a lesser degree than elicited by substances acting directly on the DA system, as also indicated by preclinical studies (6). We used PET and the D2/3 receptor-preferring radioligand [11C]-raclopride to test this hypothesis. Together with measures of DA release, we evaluated the subjective effects induced by intravenous infusion of morphine, with the aim of exploring the relationship between those and DA release.
Methods
Participants
Fifteen healthy male participants who had previously received oral prescription opioid analgesics were recruited from the community via flyers, newspapers and internet advertisements [see supplementary table 1 for demographics]. Participants provided written informed consent, and underwent a thorough medical examination prior to enrollment in the study. Further details are provided in Supplemental Information.
Experimental Design
Participants underwent three single-blind infusion sessions on separate days, separated by approximately 1 week. At the start of each session, a urine drug screen [iScreen One Step 10 Panel Dip Card, Alere Toxicology®, testing for amphetamine, phencyclidine, buprenorphine, methadone, morphine, oxycodone, THC, cocaine, methamphetamine, MDMA], a breathalyzer test and CO monitoring were performed to exclude the use of psychoactive drugs, alcohol and recent tobacco smoking (CO ≥15 ppm disqualified the subject from the study). Cotinine levels were assessed for participants reporting e-cigarettes use (cotinine ≥1 ng/mL disqualified the subject from the study). As a safety measure, food and beverages (except for water) were discontinued at least 4 h prior to imaging studies.
A baseline infusion session (first session) was performed in the clinic to ensure that participants tolerated the morphine infusion without experiencing nausea or marked sedation. During this session, which lasted approximately 5 hours, participants received, in a fixed order, a placebo infusion (normal saline) followed by an IV challenge of morphine (10 mg/70 kg over 1 minute; morphine concentration 2 mg/ml). This is at the high end of doses used in clinical practice to achieve analgesia. Pre- and post-injection, the following measures were acquired: 1) subjective responses as measured by the Drug Effects Questionnaire [DEQ] (32), which assessed ‘drug-liking’, ‘drug-wanting’, ‘high’, and ‘feel drug’; 2) physiological response, including respiratory rate, oxygen saturation and pupil response to light. The DEQ was administered at baseline and at 5, 15, 30, 45, 60, 75 and 90 min after each injection. The pupillary constriction test was modified from a previously reported method (33). In brief, participants were instructed to focus on a fixation point and to avoid blinking while the pupilometer (PLR-200™ Pupillometer, NeurOptics®) was positioned over the eye by the operator. The resting pupil diameter of each eye was measured for 2 s every 20 s for a total of one minute. The test was performed at baseline and at 10, 20 and 30 min post-injection. Participants were also monitored throughout the experimental session with pulse oximetry for safety. Following the first session, five participants were excluded from the study since they experienced adverse effects (mostly nausea) in response to morphine.
PET and MRI Scans
In the second and third sessions, which lasted approximately 3.5 hours, the remaining 10 participants received, in counter-balanced, randomized order, an IV infusion of morphine (10 mg/70 kg) or an equivalent volume of normal saline over 20–30 s, while undergoing a PET scan with [11C]-raclopride. Participants were scanned supine with their head held in place using a custom-made thermoplastic facemask fixation system, to minimize head movements during scanning. Subjective effects were assessed using the DEQ at baseline and every 10 min during the scan, while vital signs and electrocardiograms (ECG) were collected at baseline and every 5 min during the scan. Details on the PET scan protocol are provided in Supplemental Information.
For structural reference, 3D T1-weighted magnetic resonance imaging (MRI) scans were obtained prior to the PET sessions using a 3T General Electric MRI scanner (General Electric, Milwaukee, WI).
Data Processing
The raw PET data were acquired in 3D mode, reconstructed into dynamic timeframes of variable duration (0.5–5 min), and then co-registered with standard T1 weighted (MPRAGE) MRI scans using PMOD (version 2.8.5; PMOD Technologies Ltd., Zürich, Switzerland). All PET images were then resliced using the Statistical Parametric Mapping package (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). Parametric Binding Potential (BPnd) images were obtained using the Simple Reference Tissue Model 2 (SRTM2) (34), and the cerebellum was used as the reference region to derive a quantitative estimate of binding potential relative to the non-displaceable compartment. The PET data from the placebo session were used as a measure of baseline raclopride BPnd. Morphine-induced changes in binding potential in each ROI were computed relative to baseline as %ΔBPnd = 100 × ΔBPnd (placebo − morphine) / BPnd (placebo). Reduction in raclopride binding is attributed to competition with endogenous dopamine, and the percent change in binding potential has been shown to be proportional to the magnitude of DA release (35). Further details on PET data processing are provided in Supplemental Information.
Voxel-wise analysis
Voxelwise parameter estimates of [11C]-raclopride were generated using SRTM2, with the cerebellar cortex as reference region. PET images were first analyzed on a pixel by pixel basis with PMOD. Next, BPND changes from placebo to morphine condition were investigated statistically using a paired t-test in SPM8. Analysis was performed on voxels with BPND values greater than 1 across participants (which primarily included the striatum). A stringent statistical significance threshold was set by p(FWE) < 0.05, corrected for multiple comparisons at the cluster level using the family-wise error (FWE) correction, a cluster-defining threshold p < 0.005 (uncorrected) and a minimum cluster size of 450 voxels (1-mm isotropic), consistent with current statistical standards (36).
Region-of-Interest Analysis
As a complementary approach to voxel-wise analysis, we also performed an additional ROI-level analysis. ROI-based average values are considered more robust than single voxel measures, for they are less prone to imaging noise. In addition, our ROI analysis was performed in the subject space with ROI defined for each individual, which is more robust to individual differences in regional morphometry. Specifically, subcortical ROIs were defined using an automated segmentation approach (‘run_first_all’ command) implemented in the University of Oxford’s Center for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL version 5.0; http://www.fmrib.ox.ac.uk/fsl) (37). Four a priori regions were used: bilateral caudate, putamen, NAc, and pallidum, which included the dorsal pallidum or globus pallidus (internal and external segments). To remove the effect of voxels with low BPND for each bilateral ROI the average of the top 10% voxels with the highest BPND values was estimated. This approach was also motivated by the fact that ROIs are not functionally homogeneous and that the mean of all voxels within the ROI may not optimally represent activity when activated voxels are grouped with inactive or de-activated voxels (38–42). For example, the average map of top 10% voxels indicated that the voxels with the highest BPND values in the pallidum ROI were located primarily within the external globus pallidus (GPe), thus we refer to this particular sub-region (GPe) when referring to the pallidum ROI in the rest of the paper [Figure S1].
Statistical Analysis
All statistical comparisons, apart from voxel-wise analyses, were performed using SAS (version 9.3; SAS Institute, Cary, NC).
To determine whether %ΔBPnd differed significantly between the placebo and the morphine condition at p < 0.05 (i.e. whether a significant displacement occurred following morphine administration), a one–sample t-test was performed separately for each ROI. In addition, the absolute BPND values in each ROI measured were compared between the placebo and morphine conditions using analysis of covariance, with the order of infusion, age, and BMI as covariates.
Scores on the self-reported DEQ measures were analyzed in two contexts: 1) on the first session (baseline session), where participants received both placebo and morphine during the same visit outside of the scanner; and 2) between the two following visits, each conducted in the scanner, where participants received either placebo or morphine during each visit. Data were only analyzed for the ten subjects that also underwent the PET scan. One included subject had missing DEQ data at several timepoints during the morphine infusion in the PET scanner. For each context, data were analyzed using a two-way repeated measures ANOVA (rmANOVA) with 2 within-subject factors: condition (placebo, morphine) and time (7 time points from 0 to 60 minutes). Similarly, the pupillometry data were analyzed with a two-way rmANOVA with 2 within-subject factors: condition (placebo, morphine) and time (4 time points from 0 to 30 minutes), with separate analyses run for the left and right eyes. In all analyses, age and BMI were included as covariates. All ANOVA analyses were conducted using the PROC MIXED procedure in SAS.
Correlation analyses between regional changes in [11C]-raclopride BPND and subjective responses were analyzed using the Pearson product moment correlation coefficient.
Results
Physiological measures
Pupil diameter: Injection of morphine produced the expected decrease in pupil diameter, with a significant condition × time interaction for both the right (F[335] = 16.18, p<0.0001) and the left eye (F[3,35] = 17.98, p<0.0001) [Figure S2], thus indirectly confirming the acute central effects of morphine.
Subjective measures
On the first visit, participants showed significant increases in the subjective DEQ measures of ‘feel high’ (condition x time interaction: F[6,56] = 11.52, p<0.0001), and ‘feel drug’ (condition x time interaction: F[6,51] = 15.19, p<0.0001), following morphine compared to placebo. The subjective experience of ‘like drug’ also showed a significant condition × time interaction (F[6,55] = 2.67, p = 0.02), however there were no significant differences between morphine and placebo at any of the individual timepoints (all Tukey post hoc tests p > 0.05). Finally, there was no effect of morphine on the ‘want more’ measure (condition x time interaction: F[6,59] = 0.18, p = 0.98) [Figure 1]. Subjective responses to morphine were similar when participants were in the scanner environment: significant condition x time interactions for ‘feel high’ (F[6,51] = 31.19, p<0.0001) and ‘feel drug’ (F[6,47] = 72.32, p<0.0001) was observed , while no significant effects of condition were found for ‘want more’ ratings (condition x time interaction: F[6,51] = 0.51, p = 0.80). ‘Like drug’ measures showed a trend-level condition × time interaction (F[6,61] = 2.18, p = 0.06), but no significant differences between conditions at any of the time points were found [Figure 2].
Figure 1.
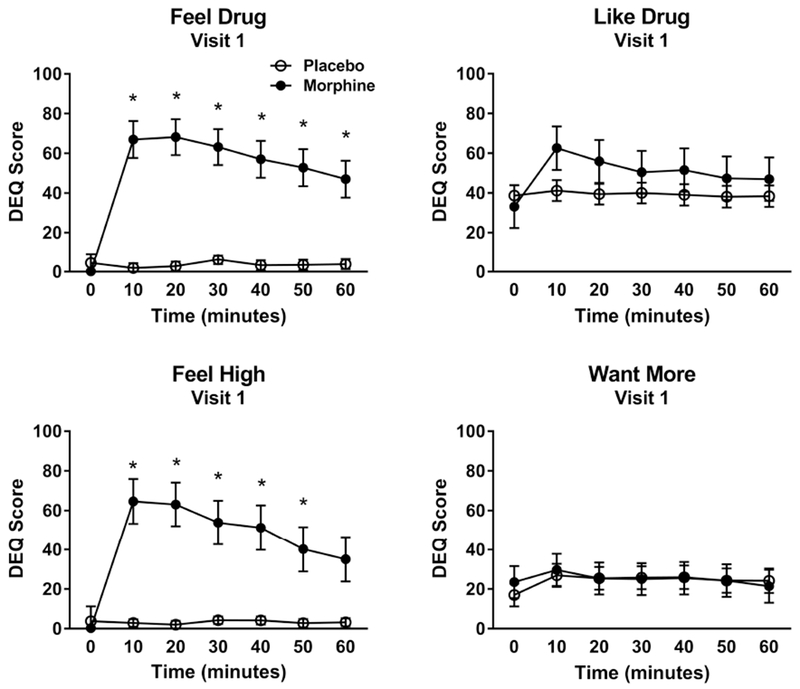
Subjective responses to morphine and placebo outside the scanner environment (Visit 1) on the DEQ. Injection of morphine produced significant condition x time interactions on ‘feel high’ (F[6,56] = 11.52, p<0.0001), ‘feel drug’ (F[6,51] = 15.19, p<0.0001), and ‘like drug’ (F[6,55] = 2.67, p = 0.02), with no effect on ‘want more’ (F[6,59] = 0.18, p = 0.98). Sample size: 10 healthy, non-smoking men.
Figure 2.
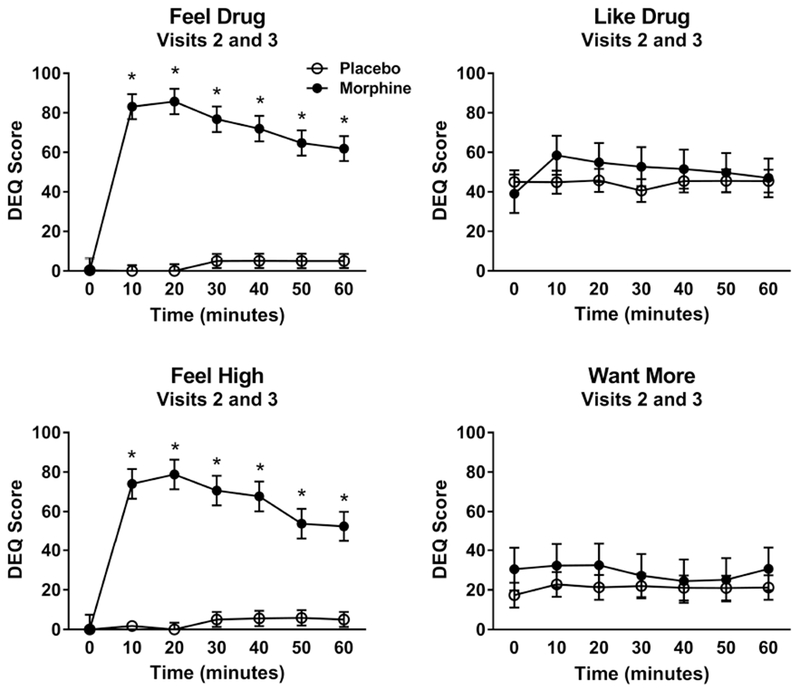
Subjective responses to morphine and placebo inside the scanner environment (Visit 2 and 3) on the DEQ. Injection of morphine produced significant condition x time interactions on ‘feel high’ (F[6,51] = 31.19, p<0.0001) and ‘feel drug’ (F[6,47] = 72.32, p<0.0001), a trend-level condition x time interaction for ‘like drug’ (F[6,61] = 2.17, p = 0.06), and no significant effects on ‘want more’ ratings (F[6,51] = 0.51, p = 0.80). Sample size: 10 healthy, non-smoking men.
[11C]-raclopride PET
The voxel-wise analysis showed a significant decrease in [11C]-raclopride BPND in a large cluster encompassing the putamen, caudate and pallidum in response to morphine compared to placebo infusion (p(FWE) < 0.05) as shown by the averaged statistical maps [Figure 3, Table 1].
Figure 3.
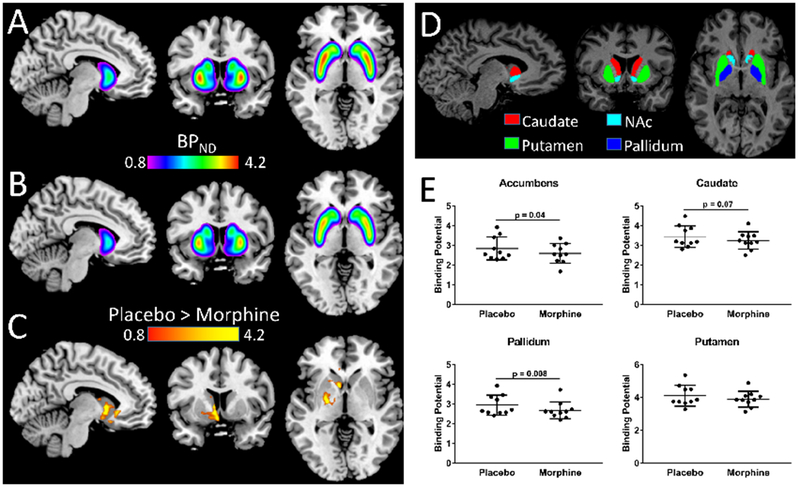
Changes in [11C]-raclopride binding potential (BPND) between the morphine and placebo sessions. (A) BPND maps averaged across all participants, coregistered to a common template for the morphine and (B) placebo infusion. (C) Statistical significance (t-score) map show a significant decrease in [11C]-raclopride BPnd in a large cluster encompassing the Putamen, Caudate and GP in response to morphine compared to placebo infusion (t = 3.5; p(FEW) = 0.005). (D) Four striatal regions of interest. (E) ΔBPnd for [11C]-raclopride between placebo and morphine sessions in four striatal regions of interest. Data are least square means (±s.e.m.). Sample size: 10 healthy, non-smoking, right-handed men. FWE, family-wise error. Error bars are s.e.m.
Table 1.
Statistics and spatial coordinates of clusters that demonstrated significant differences in [11C]-raclopride BPND between the morphine and placebo condition.
| Brain Region | MNI coordinates [mm] | Cluster | PL > MOR | ||
|---|---|---|---|---|---|
| x | y | z | k | T | |
| Caudate (left) | −7 | 9 | 3 | 1489 | 7.0 |
| Caudate Body | |||||
| Caudate Head | |||||
| Putamen | |||||
| Pallidum | |||||
Abbreviations: FWE, family-wise error; PL, placebo; MOR, morphine.
The locations of the clusters are based on the coordinates from the stereotactic space of the Montreal Neurological Institute (MNI) in (x, y, z). The values correspond to the T-scores and significance was set at PFWE<0.005, k⩾450.
The independent ROI analyses corroborated that [11C]-raclopride BPnd was significantly lower following morphine infusion than placebo infusion the NAc (t(10) = 2.27, p=0.04, uncorrected), and in the GPe (t(10) = 3.52, p=0.006). Differences in [11C]-raclopride BPnd in the GPe remained significant (p=0.007) after partial volume effects correction (PVEc) to account for spillover effects resulting from the limited spatial resolution of the PET camera (see Supplement Information for PVEc methodology). There was a trend level effect in the caudate (t(10)=1.96; p=0.08) but no significant differences in [11C]-raclopride BPnd in the putamen (t(10) = 1.6, p=0.14), [Figure 3E]. ANCOVA showed similar results after controlling for age, BMI, and order of infusion. Specifically, morphine induced a significantly lower [11C]-raclopride BPnd compared with placebo in the GPe (F[1,9]=11.5, p=0.008; Cohen’s d=0.67; percent reduction=9.0%) and in the NAc (F[1,9]=5.43; p=0.04; Cohen’s d=0.52; percent reduction=8.8%). As already observed, a trend level condition effect was found in the caudate (F[1,9]=4.27; p=0.07; Cohen’s d =0.56), but no effect of condition in the putamen (F[1,9] 3.1, p=0.11).
Regional [11C]-raclopride BPnd in the morphine and placebo conditions are reported in Table 2.
Table 2.
Regional [11C]-raclopride BPND in the morphine and placebo conditions (Mean ±SD)
| Caudate | Putamen | Nucleus Accumbens | Globus Pallidus (external) | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Morphine | Placebo | Morphine | Placebo | Morphine | Placebo | Morphine | |
| BPND | 3.45±0.5 | 3.25±0.4 | 4.11±0.6 | 3.9±0.4 | 2.84±0.4 | 2.60±0.5 | 2.94±0.5 | 2.68±0.4 |
Abbreviations: BPND, nondisplaceable binding potential
Correlations between PET data and subjective responses to morphine
[11C]-raclopride %ΔBPnd was negatively correlated with self-reported drug wanting in caudate (r2 = 0.51; p= 0.03) and putamen (r2 = 0.43; p= 0.05), while only a trend toward significance was observed in GPe (r2 = 0.39; p= 0.07) and NAc (r2 = 0.43; p= 0.09) [Supplementary Figure 3a]. %ΔBPnd within the GPe was also negatively correlated with subjective ratings of ‘high’ (r2 = 0.62; p= 0.01), and marginally negatively correlated with self-reported ‘feel drug’ measures (r2 = 0.44; p= 0.05) [Supplementary Figure 3b and 3c]. Finally, we did not observe any correlation between drug-liking ratings and [11C]-raclopride displacement in any region of interest (data not shown).
Discussion
To our knowledge, this is the first study investigating morphine-induced DA release in mesolimbic areas in non-dependent, opioid-experienced healthy individuals. Using both ROI and voxelwise analyses, we found that morphine produced a decrease in striatal [11C]-raclopride binding potential, an observation typically interpreted as reflecting an increased release of endogenous DA.
We detected a significant decrease in [11C]-raclopride BPND in response to morphine in the GPe (−9.0% in BPND) and NAc (−8.8% in BPND), i.e. in the ventral striatum, which is thought to be most directly linked to reinforcing properties of drugs. Dorsal striatal regions only showed a trend for morphine-induced DA-release. The changes in BPND in the ventral striatum are of somewhat lower magnitude than commonly reported in similar studies investigating psychostimulants effects in healthy participants (43–45). A contributing factor to the more robust effect of stimulants could be their direct action on the DA transporter (46), but comparison between drug classes are challenging, and would require an assessment of the dose-response relationship for the respective class. Increases in DA release similar in magnitude to what we detected, as measured by reduced [11C]-raclopride binding, have been reported following acute exposure to alcohol in social drinkers (47–49). Similar findings have also been reported in response to nicotine in smokers (50–52), although these have been less consistent across studies and seem to also be influenced by the sensitivity of the radiotracer used and genetic variation within the DA system (53).
To interpret the magnitude of the BPND change observed in our study, it is of interest to compare our findings with data obtained from preclinical studies. In their seminal study, Di Chiara and Imperato (6) used in vivo microdialysis to demonstrate that opioids induce DA release but do so to a lesser extent than psychostimulants (<300% vs 400 - 1000%). They also observed that DA release was particularly pronounced in the shell of the NAc, at the terminal site of opioid sensitive neurons in the VTA. Subsequently, in a key bridging study in non-human primates, Breier and colleagues (54) found that a fivefold increase of extracellular DA in the striatum was required to produce a 10% decrease in [11C] raclopride binding. The magnitude of BPND change observed in our study, therefore, seems consistent with the animal literature.
Two previous [11C]-raclopride PET studies in opioid-dependent individuals failed to detect increased striatal DA release following acute opioid administration (9, 10). Direct comparison with our study is difficult as the protocols and sample characteristics were substantially different. Sensitivity estimates of [11C]-raclopride to changes in extracellular DA concentration are variable, ranging from 8:1 to 44:1 (% increase DA: % decrease [11C]-raclopride) (9). Furthermore, the magnitude of DA release is affected by several factors, including the nature of the challenge (54–56), and the drug exposure state (12, 13, 57, 58).
The negative studies enrolled opioid-dependent patients in maintenance therapy with methadone [15mg/day (9) or 30 mg/day (10)] or buprenorphine [8mg/day (10)]. These medications were discontinued 24 hours prior to the PET scan, but their long half-life and prolonged repeated dosing could have blunted DA release after the opioid challenge. In addition, patients in the previous, negative studies had a history of opioid dependence. Chronic exposure to opioids may lead to profound changes within the DA system. Several PET studies in opioid-dependent individuals have shown that striatal D2/3 receptor binding potential is reduced compared to controls [(11, 58, 59) but see also (10)]. Studies in rodents have also shown that opioid exposure is associated with a decrease in D2/3 receptor binding in the striatum (11, 60, 61). A similar phenomenon has also been observed in cocaine and methamphetamine users, during both early and protracted withdrawal (62–66), as well as in detoxified alcoholics (14).
Taken together, our data suggest that findings from studies conducted in patients with long-term exposure to opioids may not generalize to non-dependent opioid users. An important implication of this observation is that reinforcing properties of opioid-induced DA release may be an important mechanism in the initiation of opioid use. With prolonged opioid exposure and progression into dependence, other effects of opioids, including effects that are negatively reinforcing, may take on an increasingly dominant role in maintaining use (67).
Methodological factors could also contribute to the divergence of results between studies. First, there are substantial differences in the extent of dopaminergic projections to striatal subdivisions (68). As a consequence, differences in ROI definition could contribute to the divergent findings (69). Secondly, our study excluded individuals who experienced nausea or marked sedation in response to morphine, which may have enriched for individuals with a high level of opioid reinforcement. Finally, differences in the study protocol, specifically the choice of opioid and PET tracer, may have influenced the results. For instance, Spreckelmeyer and colleagues (70) reported that a remifentanil infusion increased striatal DA release measured with [18F]-fallypride PET in both abstinent alcohol-dependent individuals and healthy volunteers, with greater magnitudes of the effect than what was observed in this study. Notably, opioids differ in terms of receptor affinity and efficacy (71, 72) and abuse liability (73), as well as in their ability to provoke DA release (74, 75).
In the current study, we also detected a significant decrease in [11C]-raclopride BPND in response to morphine in the GPe (−9% in BPND). [11C]-Raclopride is commonly used in PET studies to assess D2/3 receptor availability and to infer DA levels in the striatum, In extrastriatal areas, [11C]-raclopride has been considered to be less adequate for measuring DA activity given the lower density of D2/3 receptors in these areas (76). However, the GPe is part of the indirect pathway, wherein neurons express D2 receptors, and there is evidence that decreases in [11C]-raclopride binding potential (BPND) can also be observed in this region, in a range similar to the NAc (77, 78). Furthermore, our finding in the GPe remained significant after correction for multiple comparisons and PVEc, which was performed to account for the limited spatial resolution of the PET camera which may cause spillover effects in the basal ganglia (79).
In addition to inducing DA release in mesolimbic areas, the dose of morphine administered in our study was sufficient to produce marked objective opioid effects such as miosis and a pronounced subjective response to morphine, including ‘feel high’ and ‘feel drug’. We explored the possible relationship between subjective effects and changes in [11C]-raclopride displacement in response to morphine and noted negative correlations between self-report measures of ‘high’ and ‘feel drug’ and ΔBPND in GPe, and between drug-wanting ratings and ΔBPND in caudate and putamen. These negative correlations may suggest that the involvement of DA release as measured by [11C]-raclopride displacement in modulating opioid-induced reinforcing effects is complex and variable, as previously indicated for several addictive agents (43, 80–82). They also support the need to investigate the causal relationship between DA release and opioid reward, for example by examining whether dopamine receptor blockade would reduce opioid self-administration in humans.
Our study should be interpreted in light of some limitations. First, our sample size was modest and consisted only of males, although it was in line with previous PET studies investigating the effects of different drugs of abuse (9–11, 83, 84). However, the sample size did not allow us to investigate whether the OPRM1 118G allele carrier status would have conferred a more vigorous DA response to morphine in the ventral striatum, as previously described for alcohol (34). Second, in the ROI analysis, we detected a significant difference in percent change in [11C]-raclopride BPND in the voxels showing the top 10% BPND values within each ROI. Additionally, after a conservative Bonferroni correction, the ROI-based finding of decreased in [11C]-raclopride BPND in response to morphine in the NAc, did not reach significance. Nevertheless, morphine-induced raclopride displacement was identified by two methods, ROI and voxel-wise analysis.
In summary, our findings indicate that DA transmission in subcompartments of the human striatum is promoted by morphine. The behavioral significance of this increased DA remains to be established. Future studies, in a larger sample of healthy volunteers as well as in individuals at high risk for OUD, are required to fully delineate the DA contribution to opioid reward and addiction.
Supplementary Material
Acknowledgments
This work was supported by NIDA intramural research program and the NIAAA Division of Intramural Clinical and Biological Research (Z1A-AA000466).
The authors would like to thank the staff of the NIDA outpatient clinic at the Bayview Research Center, 1SE inpatient unit and the PET Department of the NIH Clinical Center. The authors also acknowledge the support and effort of clinical research staff in the NIAAA and NIDA intramural program, including Kathy Lightfoot, Lisa Farinelli, John Etter, Monte Phillips, Sam Hall, Anuj Shah, Timothy Klepp and Tasha Cornish , as well as William Dieckmann in the PET Department.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier:
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.General’s USS (2017): Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. [PubMed] [Google Scholar]
- 2.Compton WM, Jones CM, Baldwin GT (2016): Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 374:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mello NK, Negus SS (1996): Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 14:375–424. [DOI] [PubMed] [Google Scholar]
- 4.Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011): Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 12:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR (2015): The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 16:305–312. [DOI] [PubMed] [Google Scholar]
- 6.Di Chiara G, Imperato A (1988): Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SW, North RA (1992): Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 12:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spanagel R, Herz A, Shippenberg TS (1990): The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 55:1734–1740. [DOI] [PubMed] [Google Scholar]
- 9.Daglish MR, Williams TM, Wilson SJ, Taylor LG, Eap CB, Augsburger M, et al. (2008): Brain dopamine response in human opioid addiction. Br J Psychiatry. 193:65–72. [DOI] [PubMed] [Google Scholar]
- 10.Watson BJ, Taylor LG, Reid AG, Wilson SJ, Stokes PR, Brooks DJ, et al. (2014): Investigating expectation and reward in human opioid addiction with [(11) C]raclopride PET. Addict Biol. 19:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, et al. (2012): Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 71:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. (2005): Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 58:779–786. [DOI] [PubMed] [Google Scholar]
- 13.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. (2007): Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 164:622–629. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. (2007): Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 27:12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, et al. (2008): Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 165:507–514. [DOI] [PubMed] [Google Scholar]
- 16.Fields HL, Margolis EB (2015): Understanding opioid reward. Trends Neurosci. 38:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nader K, van der Kooy D (1997): Deprivation state switches the neurobiological substrats mediating opiate reward in the ventral tegmental area. J Neurosci. 17:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D (2004): Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 7:160–169. [DOI] [PubMed] [Google Scholar]
- 19.Sellings LH, Clarke PB (2003): Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 23:6295–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olmstead MC, Franklin KB (1997): The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 111:1324–1334. [DOI] [PubMed] [Google Scholar]
- 21.Mackey WB, van der Kooy D (1985): Neuroleptics block the positive reinforcing effects of amphetamine but not of morphine as measured by place conditioning. Pharmacol Biochem Behav. 22:101–105. [DOI] [PubMed] [Google Scholar]
- 22.Nader K, Bechara A, Roberts DC, van der Kooy D (1994): Neuroleptics block high- but not low-dose heroin place preferences: further evidence for a two-system model of motivation. Behav Neurosci. 108:1128–1138. [DOI] [PubMed] [Google Scholar]
- 23.Van Ree JM, Ramsey N (1987): The dopamine hypothesis of opiate reward challenged. Eur J Pharmacol. 134:239–243. [DOI] [PubMed] [Google Scholar]
- 24.Gerrits MA, Ramsey NF, Wolterink G, van Ree JM (1994): Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology (Berl). 114:486–494. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin SI, Guerin GF, Co C, Goeders NE, Smith JE (1988): Lack of an effect of 6-hydroxydopamine lesions of the nucleus accumbens on intravenous morphine self-administration. Pharmacol Biochem Behav. 30:1051–1057. [DOI] [PubMed] [Google Scholar]
- 26.Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982): Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl). 78:204–209. [DOI] [PubMed] [Google Scholar]
- 27.Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984): Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl). 84:167–173. [DOI] [PubMed] [Google Scholar]
- 28.Tzschentke TM (1998): Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 56:613–672. [DOI] [PubMed] [Google Scholar]
- 29.Wise RA, Leone P, Rivest R, Leeb K (1995): Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 21:140–148. [DOI] [PubMed] [Google Scholar]
- 30.Shippenberg TS, Bals-Kubik R, Herz A (1993): Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 265:53–59. [PubMed] [Google Scholar]
- 31.Bechara A, Nader K, van der Kooy D (1998): A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol Biochem Behav. 59:1–17. [DOI] [PubMed] [Google Scholar]
- 32.Holdstock L, de Wit H (1999): Ethanol impairs saccadic and smooth pursuit eye movements without producing self-reports of sedation. Alcohol Clin Exp Res. 23:664–672. [PubMed] [Google Scholar]
- 33.Grace PM, Stanford T, Gentgall M, Rolan PE (2010): Utility of saccadic eye movement analysis as an objective biomarker to detect the sedative interaction between opioids and sleep deprivation in opioid-naive and opioid-tolerant populations. J Psychopharmacol. 24:1631–1640. [DOI] [PubMed] [Google Scholar]
- 34.Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies. Neuroimage. 4:153–158. [DOI] [PubMed] [Google Scholar]
- 35.Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. (2011): A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 16:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsis GD, Iannetti GD, Smart TS, Tracey I, Wise RG (2008): Regions of interest analysis in pharmacological fMRI: how do the definition criteria influence the inferred result? Neuroimage. 40:121–132. [DOI] [PubMed] [Google Scholar]
- 39.Salas-Gonzalez D, Gorriz JM, Ramirez J, Illan IA, Lopez M, Segovia F, et al. (2010): Feature selection using factor analysis for Alzheimer’s diagnosis using 18F-FDG PET images. Med Phys. 37:6084–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veronese M, Bertoldo A, Tomasi G, Smith CB, Schmidt KC (2018): Impact of tissue kinetic heterogeneity on PET quantification: case study with the L-[1-(11)C]leucine PET method for cerebral protein synthesis rates. Sci Rep. 8:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bentourkia M (2001): A flexible image segmentation prior to parametric estimation. Comput Med Imaging Graph. 25:501–506. [DOI] [PubMed] [Google Scholar]
- 42.Mohy-Ud-Din H, Lodge MA, Rahmim A (2015): Quantitative myocardial perfusion PET parametric imaging at the voxel-level. Phys Med Biol. 60:6013–6037. [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. (1999): Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 156:1440–1443. [DOI] [PubMed] [Google Scholar]
- 44.Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, et al. (1999): PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 21:694–709. [DOI] [PubMed] [Google Scholar]
- 45.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. (2003): Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 23:285–300. [DOI] [PubMed] [Google Scholar]
- 46.Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N (1999): Is synaptic dopamine concentration the exclusive factor which alters the in vivo binding of [11C]raclopride?: PET studies combined with microdialysis in conscious monkeys. Brain Res. 841:160–169. [DOI] [PubMed] [Google Scholar]
- 47.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. (2003): Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 49:226–231. [DOI] [PubMed] [Google Scholar]
- 48.Aalto S, Ingman K, Alakurtti K, Kaasinen V, Virkkala J, Nagren K, et al. (2015): Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [(11)C]raclopride. J Cereb Blood Flow Metab. 35:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, et al. (2016): Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend. 160:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. (2004): Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 161:1211–1218. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery AJ, Asselin MC, Farde L, Grasby PM (2007): Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab. 27:369–377. [DOI] [PubMed] [Google Scholar]
- 52.Le Foll B, Guranda M, Wilson AA, Houle S, Rusjan PM, Wing VC, et al. (2014): Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology. 39:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, et al. (2006): Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 63:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. (1997): Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 94:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D’Souza DC, Krystal J, et al. (2000): Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry. 47:371–379. [DOI] [PubMed] [Google Scholar]
- 56.Schiffer WK, Volkow ND, Fowler JS, Alexoff DL, Logan J, Dewey SL (2006): Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse. 59:243–251. [DOI] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. (1997): Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 386:830–833. [DOI] [PubMed] [Google Scholar]
- 58.Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, et al. (1997): Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 16:174–182. [DOI] [PubMed] [Google Scholar]
- 59.Zijlstra F, Booij J, van den Brink W, Franken IH (2008): Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 18:262–270. [DOI] [PubMed] [Google Scholar]
- 60.Georges F, Stinus L, Bloch B, Le Moine C (1999): Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 11:481–490. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Cardoso P, Higuera-Matas A, Martin S, Miguens M, Del Olmo N, Garcia-Lecumberri C, et al. (2009): Strain differences between Lewis and Fischer 344 rats in the modulation of dopaminergic receptors after morphine self-administration and during extinction. Neuropharmacology. 57:8–17. [DOI] [PubMed] [Google Scholar]
- 62.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. (2011): Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 168:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narendran R, Martinez D (2008): Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 62:851–869. [DOI] [PubMed] [Google Scholar]
- 64.Schrantee A, Vaclavu L, Heijtel DF, Caan MW, Gsell W, Lucassen PJ, et al. (2015): Dopaminergic system dysfunction in recreational dexamphetamine users. Neuropsychopharmacology. 40:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trifilieff P, Martinez D (2014): Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 76 Pt B:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. (2012): Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 17:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF, Le Moal M (2005): Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 8:1442–1444. [DOI] [PubMed] [Google Scholar]
- 68.Lynd-Balta E, Haber SN (1994): The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 59:625–640. [DOI] [PubMed] [Google Scholar]
- 69.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- 70.Spreckelmeyer KN, Paulzen M, Raptis M, Baltus T, Schaffrath S, Van Waesberghe J, et al. (2011): Opiate-induced dopamine release is modulated by severity of alcohol dependence: an [(18)F]fallypride positron emission tomography study. Biol Psychiatry. 70:770–776. [DOI] [PubMed] [Google Scholar]
- 71.Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR (2001): mu Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem Pharmacol. 62:447–455. [DOI] [PubMed] [Google Scholar]
- 72.Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ (2008): Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 33:1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wightman R, Perrone J, Portelli I, Nelson L (2012): Likeability and abuse liability of commonly prescribed opioids. J Med Toxicol. 8:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, et al. (2014): Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci. 40:3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gottas A, Boix F, Oiestad EL, Vindenes V, Morland J (2014): Role of 6-monoacetylmorphine in the acute release of striatal dopamine induced by intravenous heroin. Int J Neuropsychopharmacol. 17:1357–1365. [DOI] [PubMed] [Google Scholar]
- 76.Farde L, Pauli S, Hall H, Eriksson L, Halldin C, Hogberg T, et al. (1988): Stereoselective binding of 11C-raclopride in living human brain--a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology (Berl). 94:471–478. [DOI] [PubMed] [Google Scholar]
- 77.Black KJ, Piccirillo ML, Koller JM, Hseih T, Wang L, Mintun MA (2015): Levodopa effects on [ (11)C]raclopride binding in the resting human brain. F1000Res. 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballanger B, Beaudoin-Gobert M, Neumane S, Epinat J, Metereau E, Duperrier S, et al. (2016): Imaging Dopamine and Serotonin Systems on MPTP Monkeys: A Longitudinal PET Investigation of Compensatory Mechanisms. J Neurosci. 36:1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frouin V, Comtat C, Reilhac A, Gregoire MC (2002): Correction of partial-volume effect for PET striatal imaging: fast implementation and study of robustness. J Nucl Med. 43:1715–1726. [PubMed] [Google Scholar]
- 80.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. (2002): Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 46:79–82. [DOI] [PubMed] [Google Scholar]
- 81.Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, et al. (2012): Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry. 71:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M (2014): Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry. 76:23–30. [DOI] [PubMed] [Google Scholar]
- 83.Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A (2002): Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 27:1027–1035. [DOI] [PubMed] [Google Scholar]
- 84.Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, et al. (1997): Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 154:50–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


