Abstract
Background:
Less than half of patients with major depressive disorder (MDD) respond to their first antidepressant trial. Our understanding of the underlying mechanisms of selective serotonin reuptake inhibitors (SSRIs) remains poor, and there is no reliable method of predicting treatment response.
Methods:
Thirty-seven MDD subjects and 41 healthy controls, somatically healthy and medication-free for at least six weeks, were recruited, and plasma serotonin (5-HT) levels were assessed at baseline. Twenty-six of the MDD subjects were then treated in an open-label manner with clinically appropriate doses of sertraline for 8 weeks, after which plasma 5-HT levels were again assessed. Response to treatment was defined as an improvement of 50 % or more on the Hamilton Depression Rating Scale.
Results:
Non-responders to sertraline treatment had significantly lower pre-treatment 5-HT levels compared to both healthy controls and responders (F=4.4, p=0.004 and p=0.036 respectively). There was a significant decrease in 5-HT levels over treatment in all MDD subjects (t=6.2, p=0.000003). The decrease was significantly more prominent in responders compared to non-responders (t=2.1, p=0.047). There was no significant difference in post-treatment 5-HT levels between responders and non-responders.
Limitations:
The study had a modest sample size. 5-HT levels in plasma may not reflect 5-HT levels in the brain.
Conclusions:
The results indicate that SSRI response may be facilitated by adequate baseline plasma 5-HT content and that successful SSRI treatment is associated with greater decreases in circulating 5-HT. Plasma 5-HT content may be a predictor of SSRI treatment outcome. Potential underlying mechanisms are discussed.
Keywords: Major Depressive Disorder, serotonin, antidepressant, treatment response, selective serotonin reuptake inhibitor (SSRI)
Introduction
Major Depressive Disorder (MDD) is one of the main contributors to worldwide morbidity. Over 300 million people are estimated to suffer from MDD, representing almost 5 % of the world population, and depression has been ranked as the single largest contributor to global disability by the World Health Organization (WHO, 2017).
Antidepressants are the first-line treatment for moderate to severe MDD. Currently used antidepressants do not substantially differ in their efficacy and are associated with response rates of less than 50 % after the first trial (Trivedi et al., 2006). The most frequently prescribed medications for MDD are the selective serotonin reuptake inhibitors (SSRIs), although there are currently no established predictors of SSRI treatment response or guidelines for treatment selection. Biomarkers for treatment prediction would save time and resources, clarify targets for antidepressant action, possibly delineate biologically distinct subgroups of depressed patients and mitigate patient burden.
The exact pharmacological mechanisms of action of SSRIs have remained unclear despite extensive research. The most widely accepted hypothesis is that SSRIs compensate for an underlying serotonin deficiency in the synaptic cleft (Morrissette and Stahl, 2014). Serotonin, or 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter that is present in, among other organs, the brain, the gut, and platelets. 5-HT is involved in a variety of behaviours and symptoms, including mood, aggression, sleep and higher cognitive processes (Fidalgo et al., 2013). In the peripheral blood circulation, the majority of 5-HT is found in platelets but smaller amounts can be found in cell-free plasma (Da Prada and Picotti, 1979).
Given the proposed mechanisms of action for SSRIs, plasma 5-HT levels have been investigated as biomarkers for treatment response, even though the relationship of plasma 5-HT to brain 5-HT is uncertain (Audhya et al., 2012; Sarrias et al., 1990). Results from these studies have, however, been conflicting. In a large-scale study, Gupta et al. found that, among MDD subjects, plasma 5-HT levels decreased after 8 weeks of SSRI treatment, and higher pre-treatment 5-HT levels and a greater decrease over the treatment course were associated with better clinical outcome (Gupta et al., 2016). This led them to hypothesize that baseline and treatment-associated changes in plasma 5-HT levels could be part of an “SSRI response phenotype”. However, other studies, many of them with shorter treatment duration, did not report associations between baseline plasma 5-HT levels and treatment response (Blardi et al., 2002; Blardi et al., 2005; Castrogiovanni et al., 2003; Kotzailias et al., 2004). Previous inconsistencies in the literature might be due to factors such as lack of a control group, varying follow-up time, and differences in diagnostic methods and medication status.
In the present study we investigated plasma 5-HT levels pre- and post-SSRI treatment in a rigorously characterized sample of MDD subjects who were medication-free for a minimum of 6 weeks before study enrolment. We also compared pre- and post-treatment plasma 5-HT levels between SSRI responders, non-responders and healthy controls. The aim of this study was to better understand the underlying mechanisms of SSRI treatment response and to investigate plasma 5-HT as a marker of treatment prediction.
Materials and methods
Ethics statement
This research was approved by the Committee on Human Research of the University of California, San Francisco (UCSF).
Recruitment procedures and study participants
Thirty-seven subjects with MDD and 41 healthy controls were recruited by flyers, bulletin board notices, Craigslist postings, newspaper ads, and clinical referrals. All MDD subjects were diagnosed with MDD without psychotic symptoms according to Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID)(First, 1997), which was the diagnostic manual employed at the time of this study, and the diagnosis was verified by clinical interview with a board-certified psychiatrist. Depressive symptomatology was evaluated with the 17-item Hamilton Score for Depression Rating Scale (HDRS) (Hamilton, 1960), with a current score of ≥ 17 being an inclusion criterion. Depressed subjects were excluded for presence of the following: bipolar disorder, psychotic symptoms during their current major depressive disorder, history of psychosis outside of a mood disorder episode, any eating disorder or post-traumatic stress disorder (PTSD) within one month of entering the study, and substance abuse or dependence (including alcohol) within six months of entering the study. Comorbid anxiety disorders (except post-traumatic stress disorder) were allowed if MDD was considered the primary diagnosis. The study participants had no acute illnesses or infections, chronic inflammatory disorders, neurological disorders, or any other major medical conditions considered to be potentially confounding (e.g. cancer, HIV, diabetes, history of cardiovascular disease or stroke, etc.), as assessed by history, physical examinations, and routine blood screening. All subjects were free of psychotropic medications (including antidepressants), hormone supplements, and steroid-containing birth control or other potentially interfering medications, and had not had any vaccinations, for at least 6 weeks prior to enrolment in the study, and none were taking vitamin supplements above the US recommended daily allowances (e.g. 90 mg/day for vitamin C). Short-acting sedative-hypnotics were allowed in the MDD subjects as needed up to a maximum of 3 times per week, but none within 1 week prior to blood draws. Prior to each study visit, all subjects had to pass a urine toxicology screen for drugs of abuse (marijuana, cocaine, amphetamines, phencyclidine, opiates, methamphetamine, tricyclic antidepressants, and barbiturates) and a urine test for pregnancy for women of child-bearing potential.
SSRI treatment
Twenty-six of the 37 MDD subjects underwent eight weeks of open-label outpatient treatment with the SSRI antidepressant sertraline. The decision to offer treatment to this subgroup was based solely on funding; there was no systematic bias in offering treatment or not to subjects. Outpatient compliance with the medication regimen, as well as clinical evaluations and assessments of drug tolerability, were assessed by a telephone check-in at the end of week 1 and an in-person check-in at the end of week 4 and week 8, at which times pill counts were performed. To assess treatment outcome, in-person HDRS ratings were repeated at the end of treatment, at week 8. All 26 treated MDD subjects were treated with sertraline to minimize variability in biological or behavioral responses secondary to different antidepressant medications. In all cases, sertraline was judged to be a clinically appropriate choice for the subjects, and alternative treatment options were discussed with the subjects prior to their consent for treatment. Medication dosages were increased over the course of treatment as tolerated and as warranted by clinical response. Sertraline dosing began with 25 mg per day and increased to a maximum of 200 mg per day based on the clinician’s judgment of efficacy and side effects. MDD subjects were rated with the HDRS at baseline and after eight weeks of SSRI treatment. HDRS ratings were done blind to the knowledge of 5-HT levels. “Responders” were defined as subjects with greater than or equal to 50% improvement on HDRS ratings at week 8 compared to baseline; “non-responders” had less than 50% improvement on the HDRS. Those with post-treatment HDRS ratings ≤7 were classified as “remitters.”
Blood sampling and assays
Subjects were admitted as outpatients to the UCSF Clinical and Translational Science Institute between the hours of 0800 and 1100, having fasted (except water) since 2200 h the night before. Subjects were instructed to sit quietly and relax for 25–45 min before blood samples were obtained.
In addition to 5-HT, we also quantified plasma tryptophan levels in order to estimate whether differences in plasma 5-HT were due to dietary intake, as a majority of the body’s 5-HT is produced from dietary tryptophan in the enterochromaffin cells in the digestive tract (Sarrias et al., 1990), and it has previously been shown that diet influences plasma tryptophan (Wolfe et al., 1997).
Plasma 5-HT and tryptophan levels were measured in all subjects at baseline, and after 8 weeks of treatment with sertraline in the treated group. For 3 of the 26 subjects treated with an SSRI, week 8 blood samples were not available. Relative concentrations of plasma 5-HT and tryptophan were determined as part of metabolomic profiling performed at Metabolon, Inc. (Durham, NC). The metabolomic profiling used ultrahigh performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS2). Samples were processed as described (Evans et al., 2009; Ohta et al., 2009). For each sample, 100 μL of plasma was used for analyses. Using an automated liquid handler (Hamilton LabStar, Salt Lake City, UT), protein was precipitated from the plasma with methanol that contained four standards, used to determine extraction efficiency. The resulting supernatant was split into equal aliquots for analysis on the three platforms. Aliquots, dried under nitrogen and vacuum-desiccated, were subsequently either reconstituted in 50 μL 0.1% formic acid in water (acidic conditions) or in 50 μL 6.5 mM ammonium bicarbonate in water, pH 8 (basic conditions) for the two UHPLC/MS/MS2 analyses. In addition, three types of controls were analyzed in concert with the experimental samples: aliquots of a well-characterized human plasma pool served as technical replicates throughout the data set, extracted water samples served as process blanks, and a cocktail of standards spiked into every analyzed sample allowed monitoring of instrument performance. For UHPLC/MS/MS2 analysis, aliquots were separated using a Waters Acquity UPLC (Waters, Millford, MA) and analyzed using an LTQ mass spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA), which consisted of an electrospray ionization source and linear ion-trap mass analyzer. The MS instrument scanned 99-1000 m/z and alternated between MS and MS2 scans using dynamic exclusion with approximately 6 scans per second. Metabolites were identified by automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, in-source fragments, and associated MS spectra, and were curated by visual inspection for quality control using software developed at Metabolon (Dehaven et al., 2010).
Instrument variability was determined by calculating the median relative standard deviation for the internal standards that were added to each sample prior to injection into the mass spectrometers. Overall process variability was determined by calculating the median relative standard deviation for all endogenous metabolites (i.e., non-instrument standards) present in 100% of the matrix samples, which are technical replicates of pooled samples. Values for instrument and process variability were 6% and 12%, respectively.
Statistics
All statistical analyses were performed using IBM SPSS Statistics (version 24). Independent-samples T-tests and one-way ANOVAs were used for between-group comparisons. Skewed data were log transformed before analyses. Binary logistic regression was additionally used to test the relationship between baseline plasma 5-HT and responder/non-responder status. Changes in 5-HT levels in responders and non-responders were analyzed using paired-samples T-test. Pearson’s chi-squared test was used to compare proportions. Correlations were investigated using Spearman’s test. Yates correction for continuity was used to compare proportions in responders and non-responders due to the small number of individuals in each group. A p-value of <0.05 was considered statistically significant.
Results
Demographics
Demographic characteristics are summarized in Table 1. MDD subjects and controls were well-matched regarding age, sex distribution and BMI, but MDD subjects were more likely to be smokers. Responders and non-responders were well-matched regarding age, sex and HDRS score at baseline, but non-responders had a higher average BMI and were less likely to be smokers. There was no significant difference in baseline 5-HT between smokers and non-smokers (t=0.35, p=0.73), and 5-HT did not correlate significantly with BMI (r=−0.17, p=0.14). There were also no significant differences in platelet counts between controls, responders and non-responders at baseline (F=0.71, p=0.50). Platelet counts were available in 37 of the 41 healthy controls. Six MDD subjects had a current co-morbid anxiety disorder. Frequency of anxiety disorder co-morbidity did not differ between responders and non-responders (X2=0.22, p=0.64).
Table 1.
Demographic characteristics of all subjects and divided by responder/non-responder status in those 26 MDD subjects treated with open label sertraline for 8 weeks.
| All MDD patients (n=37) | Controls (n=41) | Statistical value | P-value | Non-responders (n=9) | Responders (n=17) | Statistical value | P-value | |
|---|---|---|---|---|---|---|---|---|
| Age (±SD) | 38.1±13.5 | 36.2±13.0 | 0.65 | 0.52 | 40.3±15.8 | 37.8±10.7 | 0.48 | 0.64 |
| Sex % women (n) | 64.9 (24) | 61.0 (25) | 0.13 | 0.72 | 77.8 (7) | 58.8 (10) | 0.28 | 0.59 |
| BMI baseline (±SD) | 25.0±4.5 | 24.9±4.9 | 0.13 | 0.89 | 28.4±5.1 | 24.0±3.7 | 2.53 | 0.02 |
| Smoker % (n) | 29.7 (11) | 7.3 (3) | 6.6 | 0.01 | 11.1 (1) | 47.1 (8) | 1.96 | 0.16 |
| HDRS at baseline | 19.4±2.6 | N/A | N/A | N/A | 19.1±1.8 | 18.9±2.4 | 0.18 | 0.86 |
P-values represent independent T-tests (continuous variables) or Pearson chi-square with continuity correction for comparisons between responders vs non-responders (categorical variables).
Baseline 5-HT measurements were available in 26 MDD subjects treated with open-label sertraline for 8 weeks. These subjects were characterized as responders/non-responders. Three of these subjects did not have post-treatment 5-HT levels.
Plasma 5-HT at baseline
While plasma 5HT levels were lower in MDD subjects compared to controls (1.18±0.58, vs. 1.42±0.67, respectively), this difference missed statistical significance t=1.7, p=0.095). There was a significant difference in 5-HT levels at baseline between MDD responders, MDD nonresponders and healthy controls (F=4.4, p=0.016), where responders (p=0.036), as well as healthy controls (p=0.004) had significantly higher baseline plasma 5-HT levels than nonresponders. Plasma 5-HT levels in the responder group did not significantly differ from those in the control group (p=0.47). The relationship between plasma 5-HT and responder/non-responder status was also significant using logistic regression (B=2.6, OR=14.1, p<0.05).
Baseline plasma 5-HT levels did not correlate significantly with baseline HDRS scores in all MDD subjects or when responders and non-responders were analysed separately (all rs<0.23 and all p>0.55). Baseline 5-HT levels were nominally higher in remitters (n=10) than in nonremitters (n=16), but the difference did not reach statistical significance (t=1.8, p=0.09).
Pre-to-post-treatment changes in plasma 5-HT
There was a highly significant pre- to post SSRI treatment-associated decrease in 5-HT in all treated MDD subjects (t=6.2, p=0.000003) (Figure 1). This effect was seen in both responders (t=5.9, p=0.00003) and non-responders (t=3.0, p=0.025), but the decrease in 5-HT was significantly greater in responders than in non-responders (t=2.1, p=0.047). There was no significant difference in post-treatment 5-HT levels between responders and non-responders (t=0.63, p=0.53) (Table 2). There was no significant difference in the decrease in 5-HT in remitters vs non-remitters (t=1.2, p=0.23).
Figure 1.
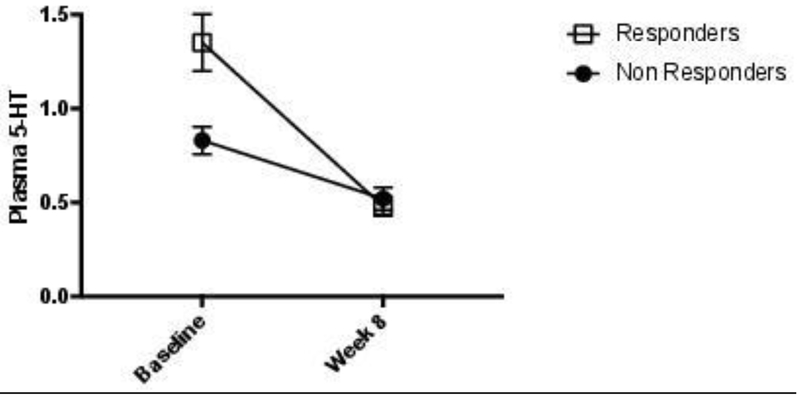
Mean (SEM) plasma 5-HT levels in responders and non-responders at baseline and at week 8. Baseline 5-HT levels were significantly different between responders and non-responders (p<0.05), and the decrease in 5-HT was significantly greater in responders than in non-responders (p<0.05).
Table 2.
Relative concentrations of plasma 5-HT at baseline and after 8 weeks SSRI treatment and delta 5-HT (measured as 5-HT levels at week 8 minus 5-HT levels at baseline) in MDD subjects treated with SSRI, divided by responder/non-responder status. Twenty-six subjects (9 non-responders and 17 responders) had 5-HT baseline data but only 23 of these (7 non-responders and 16 responders) had additional 5-HT data at week 8.
| Non-responders | Responders | P-value | |
|---|---|---|---|
| 5-HT at baseline (mean±SD) | 0.78±0.19 | 1.30±0.62 | 0.036 |
| 5-HT at 8 weeks (mean±SD) | 0.52±0.16 | 0.48±0.21 | 0.533 |
| Delta 5-HT | −0.31±0.26 | −0.88±0.68 | 0.047 |
Across all MDD subjects, a larger decrease in 5-HT during treatment tended to be associated with a continuous measure of better SSRI response, although these results did not reach statistical significance (rS=0.39, p=0.07). There were no significant differences in sertraline doses between responders and non-responders or between remitters and non-remitters (all p>0.34), and doses were not significantly correlated with change in 5-HT levels (rS=0.08, p=0.75) or with change in HDRS ratings (rS=0.34, p=0.14).
Tryptophan levels
Plasma 5-HT levels were not significantly correlated with plasma tryptophan levels at baseline (rS=0.14, p=0.21) or at week 8 (rS=−0.043, p=0.85). Plasma tryptophan levels at baseline did not differ significantly between MDD subjects and controls (t=−0.22, p=0.83), or between responders and non-responders (t=−0.65, p=0.53). There was no significant change in tryptophan levels over the course of treatment (t=x−0.51, p=0.62).
Discussion
Our results suggest that, while alterations in plasma 5-HT levels are not associated with MDD per se, relatively higher pre-treatment 5-HT levels predict a better response to an SSRI. Moreover, a greater decrease in plasma 5-HT over the course of treatment is associated with a superior antidepressant response.
These findings are in line with a previous study by Gupta et al. (Gupta et al., 2016), in which plasma 5-HT was analyzed in 306 MDD subjects before and after 8 weeks of SSRI treatment. They similarly found that plasma 5-HT levels decreased after SSRI treatment, and that higher baseline 5-HT levels and greater decreases in plasma 5-HT levels after 8 weeks of SSRI treatment were associated with better clinical outcome. Our study replicates and extends those findings in a small but rigorously screened sample of unmedicated MDD subjects and healthy controls. In line with Gupta et al. (Gupta et al., 2016), we found that higher baseline plasma 5-HT levels and a greater decrease in 5-HT over the treatment course were associated with better SSRI antidepressant responses. Figueras et al. (Figueras et al., 1999) did not find an association between pre-treatment levels of plasma 5-HT and clinical response to paroxetine; however, follow up-time in that study was only 4 weeks, which might be too short to detect an antidepressant response in all subjects (Taylor et al., 2006). Moreover, subjects in that study were not required to be medication free, and the SSRI dose was fixed and not adjusted as warranted by clinical response (Figueras et al., 1999). Alvarez et al. (Alvarez et al., 1999) also did not find any significant correlations between pre-treatment levels of plasma 5-HT and clinical outcome in a small sample with short follow-up (8 subjects, 28 days). Discrepancies between these two studies and the present one, as well as Gupta et al. (Gupta et al., 2016), may be due to the differences in follow-up times, as both we and Gupta et al., evaluated clinical response after 8 weeks of SSRI treatment.
Although we found that plasma 5-HT levels were nominally lower in MDD subjects compared to controls, this did not reach statistical significance. Results from earlier studies comparing peripheral 5-HT levels between MDD subjects and healthy individuals have been inconsistent (Franke et al., 2000; Stahl, 1977; Urbina et al., 1999), which may be due to differences in assay methods across studies and failure to take into account concurrent antidepressant treatment (Li et al., 2015). Moreover, some previous studies measured 5-HT in platelets, which may limit comparability, since the relationship of platelet 5-HT to plasma 5-HT is not well known. Importantly, our data raise the possibility that there are MDD subgroups that can be distinguished based on plasma 5-HT levels and SSRI responsivity, which previous case-control studies lacking a treatment component have not been able to identify.
We found a robust decrease in plasma 5-HT after treatment with sertraline across all treated MDD subjects, in both responders and non-responders This is in line with several previous studies (Alvarez et al., 1999; Celada et al., 1992; Gupta et al., 2016; Urbina et al., 1999), although other studies have found increased plasma 5-HT following SSRI treatment (Blardi et al., 2002; Blardi et al., 2005; Castrogiovanni et al., 2003; Kotzailias et al., 2004; Saldanha et al., 2009). Again, these conflicting results may be due to variations in follow-up time. Most (Blardi et al., 2002; Blardi et al., 2005; Castrogiovanni et al., 2003; Kotzailias et al., 2004), but not all (Saldanha et al., 2009), studies reporting an increase in plasma 5-HT used shorter periods of treatment (24 hours to 40 days), while the majority of studies reporting a decrease in 5-HT (Celada et al., 1992; Gupta et al., 2016; Urbina et al., 1999) had longer treatment durations (ranging from 6 to 12 weeks). This suggests that SSRI treatment duration of 6 weeks or more may be necessary in order to observe a decrease in plasma 5-HT. This observation is also mainly in line with animal literature showing that there is an initial increase in central 5-HT levels in rodents during SSRI treatment, but a return to baseline or even lower levels with prolonged treatment (Caccia et al., 1993; Popa et al., 2010).
In the absence of direct assessment of 5-HT in the brain, clinical studies have typically measured 5-HT levels in plasma, serum or in platelets, and more rarely, its metabolite 5- hydroxyindoleacetic acid (5-HIAA) in cerebrospinal fluid (CSF). Several animal studies, however, have measured 5-HT in the brain during SSRI treatment. After administration of fluoxetine to monkeys, Smith et al. (Smith et al., 2000) found that 5-HT levels initially increased in the hippocampus and caudate, but after 7 days, they had declined to pre-drug baseline values. Related findings have been reported in rodents (Caccia et al., 1993; Honig et al., 2009; Popa et al., 2010). Thus our findings, together with these other studies, raise the possibility that the mechanisms of action for SSRIs (and the time latency to antidepressant response) may not mainly depend on increased 5-HT levels, but rather on long-term adaptive responses of the 5-HT system to the administration of SSRIs, which result in (or are reflected by) decreases in 5-HT. The exact nature of such adaptive changes are currently not known but may involve alterations in the expression and efficiency of the 5-HT transporter, as well as density or sensitivity of presynaptic 5-HT receptor subtypes, 5-HT1A/1B, which in turn regulate 5-HT release (Smith et al., 2000). Consistent with this hypothesis, Popa et al found that central 5-HT levels in rodents initially increase but then decrease after 3-4 weeks of SSRI treatment, and that these changes may relate to a desensitization of the 5-HT1A receptor (Popa et al., 2010). Another possible mechanism explaining our findings that superior SSRI response is associated with higher pre-treatment plasma 5-HT levels is 5-HT1A autoreceptor hypofunctioning. Although speculative, this hypothesis is supported by previous data linking a relative deficiency in 5-HT1A autoreceptor functioning and levels to superior SSRI response (Lemonde et al., 2004; Richardson-Jones et al., 2010), and a hypofunction of the 5-HT1A autoreceptor could theoretically lead to higher baseline 5-HT levels due to less feedback-induced inhibition of 5-HT release. Other mechanisms, including a potential SSRI-induced blockade of platelet uptake of 5-HT from cells in the gut, could underlie the observed association between superior treatment response and a greater 5-HT decrease, but more studies are needed to test these hypotheses.
Alternatively, our results of worse antidepressant response in subjects with lower baseline 5- HT could be due to a “floor effect”. Individuals who are less likely to respond to an SSRI may have a lower serotonergic tone, either caused by a disease process or by a compensatory mechanism, to the point that an SSRI cannot exert a therapeutic effect through further lowering of 5-HT. One potential mechanism that could underlie this type of floor effect is a shift in tryptophan metabolism from the serotonin pathway towards the kynurenine pathway (Lapin and Oxenkrug, 1969). This shift is promoted by inflammatory stimuli and could theoretically lead to lower basal 5-HT levels (Chen and Guillemin, 2009). Interestingly, increased inflammation has also been associated with worse antidepressant treatment (Strawbridge et al., 2015). This theory is mainly in line with our findings of no differences in tryptophan, the precursor to 5-HT, and future studies should investigate interactions between the 5-HT system, inflammation and the kynurenine pathway and how they relate to antidepressant treatment response. Yet another hypothesis that could explain our findings is that the effect of SSRIs is mediated by 5-HT, to the point where these drugs are not efficacious unless there is sufficient 5-HT present. Although speculative, this hypothesis is in line with previous studies showing that tryptophan depletion causes a decrease of mood and relapse into depression even in remitted MDD patients who are currently medicated with SSRIs (Ruhe et al., 2007). However, it is important to note that this study was not designed to be able to explain the underlying mechanisms of these results, and further studies are needed to shed light on the underlying mechanisms of our findings.
Our findings raise the question whether the demonstrated difference in 5-HT levels and SSRI responsivity is a result of different MDD subgroups with distinct underlying pathophysiologies. It has long been discussed that the diagnostic category of MDD according the DSM is very heterogeneous, and has the potential to include subjects with highly variable symptomatology (Zimmerman et al., 2015). This might in turn be a result of heretofore unknown different pathological mechanisms. Future studies should also investigate if the responder and non-responder groups differ in, for example, depression symptom profiles or in other biological indices that impinge on 5-HT activity, e.g. inflammation. In the current sample, there was no significant between-group difference (responders vs non-responders) in anxiety disorder co-morbidity; however, this should be further investigated in a larger sample.
Strengths and limitations
This study has several strengths. The sample was well-defined; the subjects were not taking any interfering medications (including antidepressants for at least six weeks), and they did not suffer from any potentially confounding somatic illnesses. All patients were prescribed the same SSRI, sertraline, in doses that were adjusted using real-world clinical criteria of response and tolerability, and compliance was monitored by pill counts. Further, a healthy control group was included to determine normative values of plasma 5-HT.
There are, however, also limitations to this study. 5-HT levels in plasma do not fully mirror 5- HT levels in the CNS, as the 5-HT molecule is charged and does not cross the blood brain barrier (BBB) (Kaufman et al., 2016), and since the majority of plasma 5-HT comes from platelets and the gut, as opposed to the brain. Previous studies have, however, shown a correlation between plasma 5-HT and CSF 5-HT (Audhya et al., 2012; Sarrias et al., 1990). Despite this, we cannot be certain that our peripheral findings mirror serotonergic function in the CNS, limiting our ability to draw mechanistic conclusions. A potential confounding factor could also be differences in dietary factors among responders and non-responders, as certain foods can temporarily raise plasma 5-HT levels. We attempted to minimize this limitation by restricting the participants’ dietary intake for 12 hours before sample collection. Moreover, we did not find any differences in tryptophan levels between response groups, which speaks against dietary differences as a confounding factor, as plasma 5-HT is largely derived from dietary tryptophan in the enterochromaffin cells in the digestive tract (Sarrias et al., 1990). Also, as the SSRI treatment in the study was open-label, a placebo effect on the 5-HT levels or on behavioral ratings cannot be completely ruled out. Furthermore, while we have argued that the association between a greater decrease in 5-HT levels over treatment and better SSRI response has a biological foundation, we cannot fully rule that this effect, or part of it, is due to a regression to the mean. Finally, this was a relatively small study, and our results will need to be replicated in larger samples.
Conclusion
We found that MDD subjects who responded to SSRI treatment had baseline plasma 5-HT levels similar to those seen in healthy controls, and significantly higher than in those who did not respond. SSRI responders also had a more prominent decrease in plasma 5-HT over the treatment course. We hypothesize that SSRI response is facilitated by adequate baseline 5-HT content, and that successful SSRI treatment is associated with a decrease in plasma 5-HT content. Future studies should simultaneously assess brain (CSF), plasma and platelet 5-HT content, as well as levels of 5-HIAA and kynurenine metabolites, to better elucidate the relationship of SSRI response to tissue-specific 5-HT content and to 5-HT metabolic pathways. This may help predict treatment response and further our knowledge of necessary and sufficient bases of SSRI response.
Highlights.
Plasma serotonin levels were associated with the outcome of SSRI treatment
Higher baseline serotonin was associated with better treatment outcome
SSRI responders had serotonin levels similar to healthy controls
Greater decrease in serotonin during treatment was linked to better response
Acknowledgements
We acknowledge research coordinator Rebecca Rosser.
This study was funded by grants from the National Institute of Mental Health (NIMH) (Grant Number R01-MH083784), the O’Shaughnessy Foundation, the Tinberg family, and grants from the UCSF Academic Senate, the UCSF Research Evaluation and Allocation Committee (REAC). This project was also supported by National Institutes of Health/National Center for Research Resources (NIH/NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Daniel Lindqvist was supported by the Swedish Research Council (registration number 2015-00387), Marie Sklodowska Curie Actions, Cofund (Project INCA 600398), the Swedish Society of Medicine, the Söderström König Foundation, the Sjöbring Foundation, OM Persson Foundation and the province of Scania (Sweden) state grants (ALF).
Amanda Holck was supported by the province of Scania (Sweden) state grants (ALF).
Footnotes
Declaration of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Declarations of interest: none.
Role of the Funding Source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez JC, Gluck N, Fallet A, Gregoire A, Chevalier JF, Advenier C, Spreux-Varoquaux O, 1999. Plasma serotonin level after 1 day of fluoxetine treatment: a biological predictor for antidepressant response? Psychopharmacology (Berl) 143, 97–101. [DOI] [PubMed] [Google Scholar]
- Audhya T, Adams JB, Johansen L, 2012. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim Biophys Acta 1820, 1496–1501. [DOI] [PubMed] [Google Scholar]
- Blardi P, De Lalla A, Leo A, Auteri A, Iapichino S, Di Muro A, Dell'Erba A, Castrogiovanni P, 2002. Serotonin and fluoxetine levels in plasma and platelets after fluoxetine treatment in depressive patients. J Clin Psychopharmacol 22, 131–136. [DOI] [PubMed] [Google Scholar]
- Blardi P, de Lalla A, Urso R, Auteri A, Dell'Erba A, Bossini L, Castrogiovanni P, 2005. Activity of citalopram on adenosine and serotonin circulating levels in depressed patients. J Clin Psychopharmacol 25, 262–266. [DOI] [PubMed] [Google Scholar]
- Caccia S, Anelli M, Codegoni AM, Fracasso C, Garattini S, 1993. The effects of single and repeated anorectic doses of 5-hydroxytryptamine uptake inhibitors on indole levels in rat brain. Br J Pharmacol 110, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrogiovanni P, Blardi P, De Lalla A, Dell'Erba A, Auteri A, 2003. Can serotonin and fluoxetine levels in plasma and platelets predict clinical response in depression? Psychopharmacol Bull 37, 102–108. [PubMed] [Google Scholar]
- Celada P, Dolera M, Alvarez E, Artigas F, 1992. Effects of acute and chronic treatment with fluvoxamine on extracellular and platelet serotonin in the blood of major depressive patients. Relationship to clinical improvement. J Affect Disord 25, 243–249. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guillemin GJ, 2009. Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res 2, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Prada M, Picotti GB, 1979. Content and subcellular localization of catecholamines and 5-hydroxytryptamine in human and animal blood platelets: monoamine distribution between platelets and plasma. Br J Pharmacol 65, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaven CD, Evans AM, Dai H, Lawton KA, 2010. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E, 2009. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81, 6656–6667. [DOI] [PubMed] [Google Scholar]
- Fidalgo S, Ivanov DK, Wood SH, 2013. Serotonin: from top to bottom. Biogerontology 14, 21–45. [DOI] [PubMed] [Google Scholar]
- Figueras G, Perez V, San Martino O, Alvarez E, Artigas F, 1999. Pretreatment platelet 5-HT concentration predicts the short-term response to paroxetine in major depression. Grupo de Trastornos Afectivos. Biol Psychiatry 46, 518–524. [DOI] [PubMed] [Google Scholar]
- First MB, 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. America Psychiatric Press, Washington, DC. [Google Scholar]
- Franke L, Schewe HJ, Muller B, Campman V, Kitzrow W, Uebelhack R, Berghofer A, Muller-Oerlinghausen B, 2000. Serotonergic platelet variables in unmedicated patients suffering from major depression and healthy subjects: relationship between 5HT content and 5HT uptake. Life Sci 67, 301–315. [DOI] [PubMed] [Google Scholar]
- Gupta M, Neavin D, Liu D, Biernacka J, Hall-Flavin D, Bobo WV, Frye MA, Skime M, Jenkins GD, Batzler A, Kalari K, Matson W, Bhasin SS, Zhu H, Mushiroda T, Nakamura Y, Kubo M, Wang L, Kaddurah-Daouk R, Weinshilboum RM, 2016. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: pharmacometabolomics-informed pharmacogenomics. Mol Psychiatry 21, 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig G, Jongsma ME, van der Hart MC, Tecott LH, 2009. Chronic citalopram administration causes a sustained suppression of serotonin synthesis in the mouse forebrain. PLoS One 4, e6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, DeLorenzo C, Choudhury S, Parsey RV, 2016. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol 26, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzailias N, Marker M, Jilma B, 2004. Early effects of paroxetine on serotonin storage, plasma levels, and urinary excretion: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 24, 536–539. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF, 1969. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1, 132–136. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR, 2004. Association of the C(−1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 7, 501–506. [DOI] [PubMed] [Google Scholar]
- Li X, Fan Y, Xiao S, Peng S, Dong X, Zheng X, Liu CC, Li H, Xiao Z, 2015. Decreased platelet 5-hydroxytryptamin (5-HT) levels: a response to antidepressants. J Affect Disord 187, 84–90. [DOI] [PubMed] [Google Scholar]
- Morrissette DA, Stahl SM, 2014. Modulating the serotonin system in the treatment of major depressive disorder. CNS Spectr 19 Suppl 1, 57–67; quiz 54-57, 68. [DOI] [PubMed] [Google Scholar]
- Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L, 2009. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol 37, 521–535. [DOI] [PubMed] [Google Scholar]
- Popa D, Cerdan J, Reperant C, Guiard BP, Guilloux JP, David DJ, Gardier AM, 2010. A longitudinal study of 5-HT outflow during chronic fluoxetine treatment using a new technique of chronic microdialysis in a highly emotional mouse strain. Eur J Pharmacol 628, 83–90. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED, 2010. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH, 2007. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry 12, 331–359. [DOI] [PubMed] [Google Scholar]
- Saldanha D, Kumar N, Ryali V, Srivastava K, Pawar AA, 2009. Serum Serotonin Abnormality in Depression. Med J Armed Forces India 65, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrias MJ, Cabre P, Martinez E, Artigas F, 1990. Relationship between serotoninergic measures in blood and cerebrospinal fluid simultaneously obtained in humans. J Neurochem 54, 783–786. [DOI] [PubMed] [Google Scholar]
- Smith TD, Kuczenski R, George-Friedman K, Malley JD, Foote SL, 2000. In vivo microdialysis assessment of extracellular serotonin and dopamine levels in awake monkeys during sustained fluoxetine administration. Synapse 38, 460–470. [DOI] [PubMed] [Google Scholar]
- Stahl SM, 1977. The human platelet. A diagnostic and research tool for the study of biogenic amines in psychiatric and neurologic disorders. Arch Gen Psychiatry 34, 509–516. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z, 2006. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry 63, 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team SDS, 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Urbina M, Pineda S, Pinango L, Carreira I, Lima L, 1999. [3H]Paroxetine binding to human peripheral lymphocyte membranes of patients with major depression before and after treatment with fluoxetine. Int J Immunopharmacol 21, 631–646. [DOI] [PubMed] [Google Scholar]
- WHO, 2017. Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization, Geneva. [Google Scholar]
- Wolfe BE, Metzger ED, Stollar C, 1997. The effects of dieting on plasma tryptophan concentration and food intake in healthy women. Physiol Behav 61, 537–541. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Ellison W, Young D, Chelminski I, Dalrymple K, 2015. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr Psychiatry 56, 29–34. [DOI] [PubMed] [Google Scholar]


