Abstract
Acetate and the related metabolism of acetyl-CoA confers numerous metabolic functions, including energy production, lipid synthesis and protein acetylation. Despite its importance as a nutrient for cellular metabolism, its source has been unclear. Recent studies have provided evidence to support the existence of a de novo pathway for acetate production derived from pyruvate, the end product of glycolysis. This mechanism of pyruvate-derived acetate generation could have far-reaching implications for the regulation of central carbon metabolism. In this opinion, we discuss our current understanding of acetate metabolism in the context of cell autonomous metabolic regulation, cell-cell interactions, and systemic physiology. Applications relevant to health and disease, particularly cancer will be emphasized.
Keywords: Lipogenesis, Alcoholism, Cancer, Nutrition, Acetate Metabolism
Acetyl-Coenzyme A’s central role in metabolism
Acetyl-Coenzyme A (CoA) is a two-carbon reactive unit that participates in central carbon metabolism (see Glossary)[1]. Catabolism or further oxidation of the acetyl moiety in the tricarboxylic acid cycle (TCA) generates ATP. Anabolic metabolism or reductive biosynthesis from acetyl-CoA results in the generation of lipids referred to as de novo lipogenesis. Possibly due to its central location within metabolism and its usage in both anabolic and catabolic processes, cellular acetyl-CoA concentration also confers signal transduction functions by mediating protein acetylation [2]. These functions include direct interactions with chromatin that may modify epigenetic status - a concept which has been developed and extensively reviewed elsewhere [3–5].
Acetyl-CoA is generated in the mitochondria from pyruvate by pyruvate dehydrogenase (PDH) and in the cytosol from citrate by ATP citrate lyase (ACLY). Both processes are coupled to the TCA cycle. In addition, acetyl-CoA is synthesized from acetate by acetyl CoA synthetase in both the mitochondria (ACSS1) and cytosol (ACSS2)[6, 7]. Recent work has also shown that acetate is generated directly from pyruvate both by nonenzymatic chemistry involving hydrogen peroxide metal catalysis and by alternative or neomorphic activities of ketoacid dehydrogenases[8, 9]. Together these multiple pathways for acetyl-CoA production provide a host of possibilities for biochemical and physiological regulation (Figure 1). This opinion article aims to bridge current knowledge about central carbon metabolism with recent advances in our understanding of acetate and acetyl-CoA in cellular metabolism, physiology and disease, most notably cancer.
Figure 1. Summary of Uptake Pathways and Potential Cellular Fates for Acetate in Mammalian Cells.
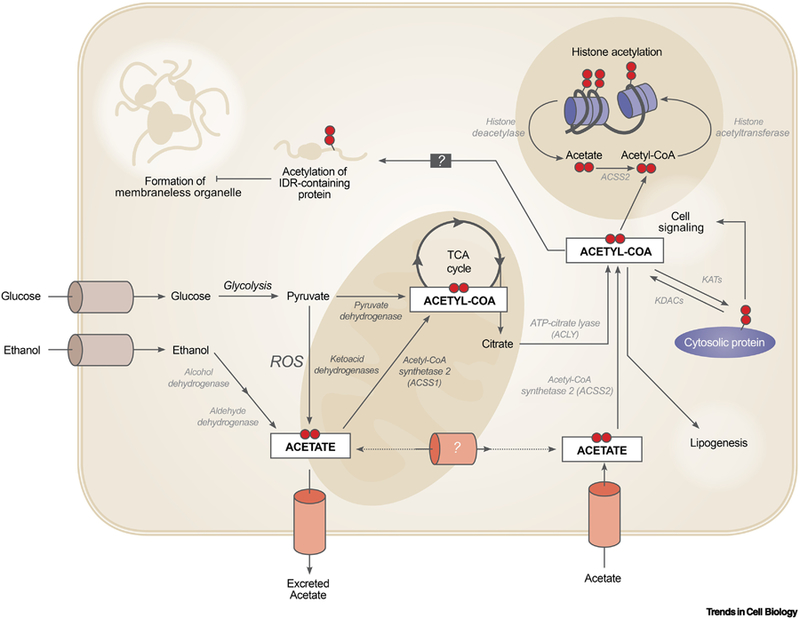
Acetate and related acetyl-CoA metabolism is important for cellular homeostasis and functions through a number of different pathways throughout the cell, including in the mitochondria, the cytosol, and the nucleus. Abbreviations: ROS, reactive oxygen species; IDR, intrinsically disordered regions; KATs, lysine acetyltransferase; KDACs, lysine deacetylase; TCA, tricarboxylic acid cycle
Sources of Acetate and Acetyl-CoA
Considering the central role of acetyl-CoA in a myriad of biological processes, the availability of precursor acetate is of considerable interest. [10]. Saccharolytic fermentation performed by the intestinal microbiota is thought to be the primary source of exogenous acetate through uptake in the colon [11]. Following ingestion of indigestible carbohydrates, enteric bacteria generate acetate, propionate, and butyrate in a 3:1:1 stoichiometry during fermentation processes [12]. The uptake of these short-chain fatty acids (SCFAs), the greatest in quantity of which is acetate, has been by some very rough estimates accountable for approximately 10% of total human energy expenditure after being absorbed from the colonic lumen[13, 14]. The largest proportion of acetate is absorbed in the proximal colon, and the absorptive capacity of the colonic epithelium decreases throughout the tract, with the lowest uptake occurring in the rectum[15]. Acetogenic bacteria like B. hydrogenotrophica are thought to be responsible for a smaller contribution of acetate, processing formate to acetyl CoA, H2, and CO2 via the Wood-Ljungdhal pathway [16].
Independent of the bacterial metabolism of dietary fiber, other dietary sources of acetate are prevalent in particular foods such as cheese and processed meats [17]. While ingested foods directly contribute to circulating acetate, their contribution is likely small. Intriguingly, alcohol consumption and hepatic metabolism of ethanol may be a considerable source, since one serving of alcohol contributes to approximately 100 calories of acetate with kinetics likely to be considerably faster than the digestion of other macronutrients. Following NAD+-dependent catabolism to acetaldehyde in the liver, aldehyde dehydrogenases catalyze the conversion to acetate[18]. As such, while baseline plasma concentrations of acetate in humans ranges between 50 μM to 200 μM[19–21], alcohol ingestion can induce sustained increases of plasma acetate to concentrations greater than 0.5 mM[22]. These increases in plasma acetate are more marked during chronic alcohol consumption, possibly due to an enhanced oxidative capacity to metabolize ethanol. Fluorodeoxyglucose (FDG) - positron emission tomography (PET)-based imaging of patients following acute intoxication has demonstrated increased uptake and utilization of [11-C] acetate as well with greater uptake noted in chronic alcoholics[22].
Since acetyl-CoA is the focus of central carbon metabolism, the ability to generate acetyl-CoA from endogenous sources could be useful for maintaining metabolic fitness, especially during times of limited nutrient availability. The metabolic flux through ACLY, which is responsible for cleaving citrate exported from the mitochondria to acetyl-CoA and oxaloacetate, is a key factor in this regulation [5]. Acetate can also be generated endogenously by the deacetylation of proteins such as histones [23]. In turn, acetate can generate acetyl-CoA via flux through ACSS2, which may be regulated in response to cellular stresses like hypoxia or serum starvation [24–26].
While under nutritionally replete conditions, acetyl-CoA is largely derived from glucose, hypoxia and other nutritional challenges lead to acetate utilization as a major metabolic source – an adaptation which has been well studied particularly in the context of the tumor microenvironment[4, 24, 25, 27]. Protein deacetylation and acetyl-CoA hydrolase activity also produce acetate but these contributions to the overall pool are likely small. New evidence supports a pathway of de novo acetate production from pyruvate. This pathway was observed to be especially pronounced during periods of overflow metabolism, when the metabolic supply outpaces the demand that results in the accumulation and excretion of intermediate products [28]. Hyperactive metabolism such as what occurs during the Warburg Effect in tumors leads to increased glucose uptake, incomplete metabolism, and the release of metabolic intermediates, like acetate, into the extracellular space [29]. As such, a model for metabolism in the tumor would include a symbiotic relationship between proximal cells – with well vascularized portions of the neoplasm providing metabolic intermediates as a resource for neighboring nutrient-limited cells (Figure 2). Several studies have provided evidence supporting the existence of this phenomenon for other downstream products of glucose metabolism such as lactate and alanine [30–33]. This pyruvate-derived acetate overflow pathway is proposed to occur via two mechanisms in parallel: (1) via the oxidative decarboxylation of pyruvate, facilitated by reactive oxygen species (ROS), and (2) via incomplete oxidation by ketoacid dehydrogenases (kDH) in a thiamine and glutathione dependent manner. Given the number of inputs to these reactions and the relations to mitochondrial function, this de novo acetate generation is likely an adaptive mechanism with interesting potential implications for pathophysiology linked to altered redox metabolism and other aspects of mitochondrial function that we will further discuss.
Figure 2. Cell-Cell Interactions in the Tumor Microenvironment Confer Survival Advantages for Nutrient Poor Cells.
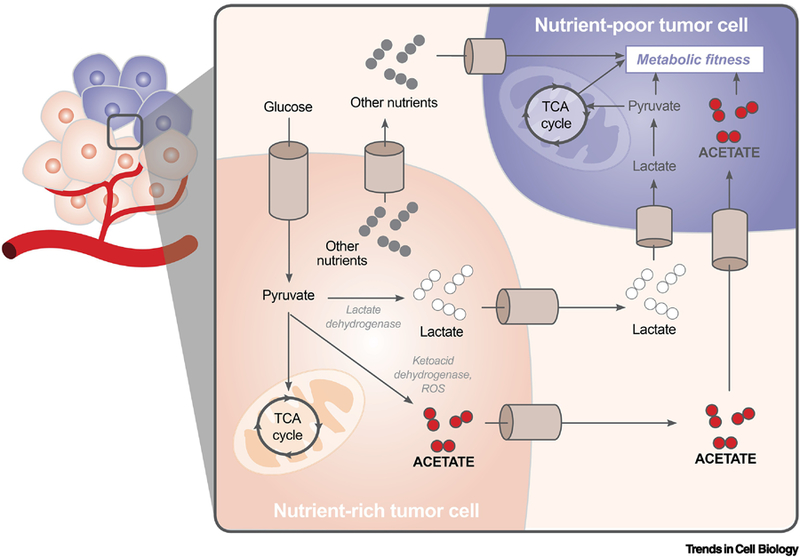
Metabolites generated during overflow metabolism can be released from nutrient rich tumor cells into the extracellular space, where they can be taken up by nutrient poor tumor cells. This metabolic coupling allows for the proliferation of tumor cells in poorly vascularized environments.
Regulation and Function of Acetate and Acetyl-CoA Metabolism Pathways
Cell autonomous
Acetate plays a critical role in maintaining intracellular pools of acetyl-CoA, and dysregulation of acetate metabolism has been linked to several human diseases [17, 34, 35]. While the precise mechanisms of extracellular uptake remain largely uncharacterized, certain monocarboxylate transporters (MCTs) have been implicated in the active transport of acetate coupled to the co-transport of sodium, proton, or bicarbonate molecules; however, the function and regulation of these transporters are still debated[17]. In light of the existence of pyruvate-derived, de novo acetate production, numerous scenarios exist in which this pathway is coupled to mitochondrial functions and other aspects of acetyl-CoA metabolism. For example, the reaction is catalyzed by ROS and thus defects in mitochondrial TCA and corresponding electron transport chain activity that increase ROS will lead to upregulation of de novo acetate metabolism. In some instances, this could be a compensatory pathway to maintain acetyl-CoA pools. Other interactions with mitochondrial metabolism are likely present in some situations. During limitations of PDH activity, acetate would be produced that could maintain acetyl-CoA in both the mitochondria and cytosol. In each of these cases, acetyl-CoA may be used to maintain or possibly enhance lipogenesis as is shown to require acetate through ACSS2 [36]. This could lead to intriguing possibilities say, for example, in liver or adipose tissue where de novo lipogenesis could be enhanced by these adaptive pathways. Thus, these connections imply interesting, albeit highly speculative, molecular links. For example, oxidative stress and lowered mitochondrial activity by promoting acetate-dependent lipogenesis could contribute to obesity. Also, the effects of oxidative-stress inducing exercise on reducing adiposity could be limited by this compensatory mechanism.
This de novo pathway allows for regulation through its coupling to other sources of acetate. When environmental acetate is limited, de novo acetate can compensate and vice versa. Additionally, excess acetate can be stored on histones for possible later uses [23]. This de novo pathway provides additional regulatory networks to couple to ACLY activity. When citrate is limited, pyruvate can compensate. Finally, considering that ROS contributes to pyruvate-derived acetate production, this pathway may also serve as a sink for endogenous or exogenous sources of ROS. The depletion of ROS as a result, might function to minimize oxidative stress in nutrient limiting conditions. Additionally, the conversion of acetate and resulting overflow metabolism results in the net cytosolic generation of NADH in contrast to the reduction of pyruvate to lactate which is redox neutral. These excess electrons may be of relevance in numerous contexts such as during increased ATP demands when they can be shuttled into the mitochondria. Thus the function of acetate in maintaining the intracellular redox balance through NADPH and NADH could also be crucial for some aspects of metabolic fitness [37].
Another speculative role for acetate metabolism is in the compartmentalization of biochemical reactions through protein acetylation by forming membrane-less organelles through liquid-liquid phase separation. These are domains of proteins and nucleic acids that interact through non-covalent interactions and exhibit properties of liquid droplets in that they are flexible, reversible and in dynamic equilibrium with the surrounding cytoplasm or nucleoplasm. Their formation is dictated by the physical properties (e.g. concentration or valency) of their component macromolecules, making them tightly controlled cellular events. Various intracellular functions for LLPS include the formation of stress granules, Cajal bodies, nucleoli and potentially transcriptional regulation through super-enhancers [38, 39]. Acetylation has been shown to allow or inhibit the formation of phase separated domains in different cellular contexts by neutralizing positively charged lysines - enabling or disabling non-covalent cross-linking of component molecules. It has been shown to inhibit LLPS-dependent maturation of stress granules - implicated in the activation of the stress response [40]; and to inhibit LLPS-dependent nucleation of the amyloid protein Tau, although its role in related pathophysiologies remains controversial [41, 42]. The spatiotemporal control of these events by acetate and acetyl-CoA metabolism remains poorly understood and points to new, unexplored roles for the metabolic regulation of cellular physiology [43].
Notably, these mechanisms likely have specific effects in different contexts within tissues. For example, in neuronal development, ACSS2-dependent histone acetylation has been shown to activate neuronal genes and is implicated in memory consolidation. In a hippocampal-ACSS2 knockout mouse model, altered histone acetylation was shown to inhibit the assimilation of long-term spatial memory [44]. In the context of immunity, acetate has also been shown to be transiently released into circulation in response to systemic bacterial infection, as an adaptive host resistance mechanism. Acetate can be taken up by CD8+ memory T-cells where it upregulates glycolysis and subsequently enhances immune function [45]. Cells infected by human cytomegalovirus (HCMV) have also been shown to upregulate pyruvate-derived acetate production, and generate cytosolic acetyl-CoA through ACSS2 to support lipogenesis, a process which is critical for successful viral infection [9].
Non-Cell Autonomous and/or Cell-cell Interactions
Symbiosis of neighboring cells during periods of metabolic stress is responsible for ensuring metabolic fitness. Metabolite generation and shuttling between proximal cells has been largely studied in the context of tumors, where hypoxic, nutrient poor and well-vascularized areas are relatively delineated. Nutritionally replete areas of the tumor exhibit aberrant metabolism, with rapid glucose uptake outpacing the oxidative capacity of the cell in a metabolic rewiring known as the Warburg Effect. Overflow metabolism from these cells leads to the production of metabolic intermediates, like acetate and lactate, and previous work has suggested that these metabolites can be taken up from the tumor microenvironment by cells with limited nutrient availability [31]. These nutrient-limited cells are dependent on these alternative nutrient sources from the intratumoral space, and adapt as such to preferentially metabolize them [46]. Recent studies have also underscored the importance of acetate utilization in tumor cell interactions [25, 47, 48]. Nutrient-limited tumor cells have been shown to capture acetate as a carbon source to support catabolic and anabolic demands [6, 49]. In this context, the intracellular mechanisms of generating and shuttling acetate between cells suggests metabolic vulnerabilities which can be targeted by pharmacologic therapies.
While cancers provide an intriguing model for studying metabolic substrate shuttling, metabolic symbiosis between proximal cells exists in healthy tissues as well and the function of acetate in these cooperative pathways has not been fully elucidated. Some of these pathways, including lactate shuttling, have been observed in the brain [50–52]. While the preferential uptake of acetate by astrocytes remains controversial [53], the upregulation of particular acetate importers in glial cells has supported the notion of acetate uptake as a marker for astrocytic metabolism . Isotope tracing and in vivo imaging has shown that the combination of acetate with an anaplerotic substrate facilitates the conversion of α-ketoglutarate into glutamate and glutamine, which can then be shuttled to neurons [54]. This metabolic coupling between astrocytes and neurons potentially confers an advantage for neurons during periods of glucose deprivation such as during hypoglycemia or chronic alcoholic consumption [55].
Systemic Physiology
In addition to cell-cell transport of acetate within tissues, the systemic physiology associated with the release and utilization of acetate in different tissues throughout an organism is also of relevance. Since acetyl-CoA is the focus of central carbon metabolism, the ability to produce and consume acetate is likely a critical adaptive response at the systemic level (Figure 3). During periods of nutritional stress, acetyl-CoA thioesterase (ACOT12), an acetyl-CoA hydrolase found in the liver, is activated and generates free acetate from acetyl-CoA[56]. This free acetate subsequently enters into circulation where it can be taken up by peripheral tissues [57–59]. There is ongoing speculation about a more extensive role for acetate as a signaling molecule including as a ligand for G-protein coupled receptors [17]. Nevertheless, molecular specificity for acetate as a ligand would be hard to establish given its low chemical complexity, and modest concentration dynamics in plasma under most conditions. So, its physiological role as a nutrient source and metabolite is more plausible.
Figure 3. Mechanisms of Systemic Metabolic Crosstalk Via Acetate.
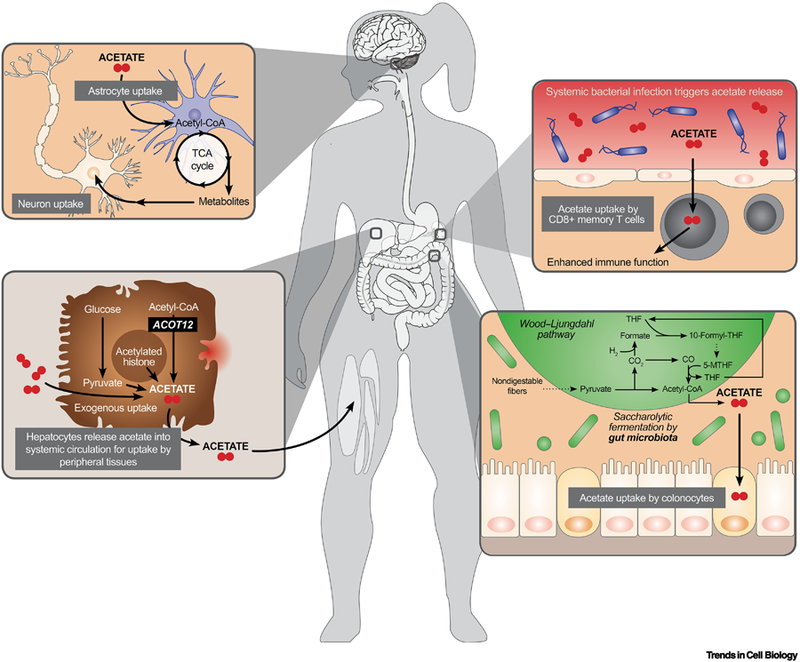
Acetate can be released into systemic circulation and taken up by different tissues in diverse contexts during health and disease. Here we illustrate the processing and release of acetate with particular emphasis on its role in neuron-astrocyte interactions, hepatic compensation during nutrient stress, immune response, and gut microbiome commensalism.
The impact of acetyl-CoA and acetate on autonomic nervous system activation and related systemic physiology has been an emerging area of interest. The gut microbiota and host interact extensively through immune, humoral, endocrine and neural pathways, and this brain-gut-microbiota axis has been recently highlighted as a key factor for insulin resistance and metabolic syndrome [60]. Evidence to support the role of microbial metabolites as a modulating influence on brain function and behavior suggests that microbial acetate production may exert extensive autonomic effect. In fact, in mice and rats with increased circulating acetate, either due to altered gut microbiota or intraarterial infusion of exogenous acetate, parasympathetic nervous system activation was noted, associated with increased pancreatic β-cell activity, glucose stimulated insulin secretion (GSIS), hyperphagia, and obesity [34]. These effects were abrogated by vagotomy to prevent efferent parasympathetic communication, thus precluding direct effects of acetate on the pancreatic β-cell activity as the key driver. As such, brain-gut-microbiota interactions through acetate were concluded to be the initial point of control.
Concluding Remarks
Overall, we have discussed emerging roles for acetate metabolism in health and disease. These concepts include at the cellular level the recent quantitative biochemical elucidation of a de novo acetate generating pathway that occurs through direct conversion of pyruvate to acetate. The cellular contexts and possible regulatory mechanisms are largely unexplored and thus these ideas are largely speculative. More work is undoubtedly needed (see Outstanding questions). Similarly, the role of acetate in mediating cell-cell interactions and the extent to which acetate functions in mediating crosstalk in the tumor microenvironment as well as in other situations is also largely unknown.
Outstanding Questions.
How does de novo acetate production impact our understanding of conditions of acetate limitation/addiction?
How does overflow metabolism of acetate and other metabolites contribute to intercellular metabolism?
Does diet or host metabolism play a role in the acetate metabolism within tumors or acetate dependent tissues?
Does acetate and acetyl-coA metabolism have a role for mediating the assembly of membraneless organelles through phase separation?
Finally, at the systemic level, while it is currently appreciated that interorgan metabolic communication occurs via acetate metabolism, whether this crosstalk may have a role in mediating tumorigenesis and cancer outcomes is unknown. A speculative role for acetate metabolism in supporting tumor growth through gut microbiota and liver interactions is intriguing, and warrants further investigation. Furthermore, as acetate uptake is involved in immunity, whether this crosstalk may be involved in the response to pathogenic challenges or the mediation of tumor immunity is mostly unexplored. Nevertheless, it is our hope that some of the speculations in this opinion may serve as frameworks for further investigation of the role of acetate in health and disease.
Highlights.
Our understanding of the origin of acetate in physiologic and disease states is currently evolving and is not limited to exogenous acetate uptake.
Regulation of the production of endogenous pyruvate-derived acetate remains largely unknown.
Pathways of metabolism during periods of nutritional excess and limitation allow for metabolic coupling to confer fitness advantages to proximal and systemic cellular partners.
Preferential uptake of acetate by certain tissues is driven not only by the availability of transporters, but by environmental pressures such as hypoxia and nutrient scarcity.
Glossary
- Central Carbon Metabolism
The routes of carbon metabolism involving the uptake of carbohydrates, amino acids and lipids. It involves oxidation to make ATP (catabolism) and reduction to undergo biosynthesis (anabolism)
- De Novo Lipogenesis
The endogenous synthesis of fatty acids from the two-carbon unit acetyl-CoA derived from several sources including glucose, amino acids, and acetate
- Ketoacid Dehydrogenases
Family of enzyme-complexes catalyzing the oxidative decarboxylation of alpha-ketoacids, aided by coenzymes and cofactors. An example is the Pyruvate Dehydrogenase (PDH) Complex which generates acetyl-CoA from pyruvate
- Metabolic Flux
The rate of flow of metabolites through a metabolic pathway. A measure of how active a metabolic pathway is in response to a stimulus
- Overflow metabolism
Condition where cells take in excessive amounts of nutrients and inefficiently catabolize them, leading to the production of metabolic intermediates that are released into extracellular space
- Warburg Effect
One type of overflow metabolism typically a feature of hyperproliferative cells, where cells rely on the increased uptake of glucose and generation of lactate to fuel their metabolic demands despite the presence of oxygen, instead of the more energy efficient oxidative phosphorylation
- Wood-Ljungdhal pathway
Process of acetyl-CoA generation which involves the reduction of CO2 to a methyl group, that reacts with CO and Coenzyme A through condensation
- Redox Balance
A type of cellular homeostasis involving maintaining a balance between the generation and removal of reactive oxygen species (ROS) to help regulate several cellular processes while preventing irreversible damage to cellular components
- Liquid-liquid phase separation
The process by which components of the cytosol, typically disordered proteins and nucleic acids interact non-covalently, and precipitate into liquid droplet like structures. This event can allow for the concentration of cellular materials into the liquid droplet and has been proposed to play a role in the regulation of biochemical reactions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh CT, Tu BP, & Tang Y (2017) Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chemical reviews 118 (4), 1460–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell SL, & Wellen KE (2018) Metabolic Signaling to the Nucleus in Cancer. Molecular cell 71 (3), 398–408. [DOI] [PubMed] [Google Scholar]
- 3.Cai L, & Tu BP (2011) On acetyl-CoA as a gauge of cellular metabolic state. . In Cold Spring Harbor symposia on quantitative biology, Cold Spring Harbor Laboratory Press; 76, 195–202. [DOI] [PubMed] [Google Scholar]
- 4.Shi L, & Tu BP (2015) Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Current opinion in cell biology 33, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, & Thompson CB (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324 (5930), 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshii Y, Furukawa T, Yoshii H, Mori T, Kiyono Y, Waki A, … & Yonekura Y (2009) Cytosolic acetyl‐CoA synthetase affected tumor cell survival under hypoxia: the possible function in tumor acetyl‐CoA/acetate metabolism. Cancer science 100 (5), 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, & Verdin E (2006) Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proceedings of the National Academy of Sciences 103 (27), 10224–10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeaman SJ (1989) The 2-oxo acid dehydrogenase complexes: recent advances. Biochemical Journal 257 (3), 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vysochan A, Sengupta A, Weljie AM, Alwine JC, & Yu Y (2017) ACSS2-mediated acetyl-CoA synthesis from acetate is necessary for human cytomegalovirus infection. Proceedings of the National Academy of Sciences 114 (8), E1528–E1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundquist F, & Wolthers H (1958) The kinetics of alcohol elimination in man. Acta pharmacologica et Toxicologica 14 (3), 265–289. [DOI] [PubMed] [Google Scholar]
- 11.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, & Gordon JI (2010) Dissecting the in vivo metabolic potential of two human gut acetogens. Journal of Biological Chemistry 285 (29), 22082–22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis P, Hold GL, & Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology 12 (10), 661. [DOI] [PubMed] [Google Scholar]
- 13.Chambers ES, Morrison DJ, & Frost G (2015) Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proceedings of the Nutrition Society 74 (3), 328–336. [DOI] [PubMed] [Google Scholar]
- 14.Royall D, Wolever T, & Jeejeebhoy KN (1990) Clinical significance of colonic fermentation. American Journal of Gastroenterology 85 (10). [PubMed] [Google Scholar]
- 15.Scheppach W, Pomare EW, Elia M, & Cummings JH (1991) The contribution of the large intestine to blood acetate in man. Clinical science (London, England: 1979) 80 (2), 177–182. [DOI] [PubMed] [Google Scholar]
- 16.Ragsdale SW (2008) Enzymology of the Wood–Ljungdahl pathway of acetogenesis. Annals of the New York Academy of Sciences 1125 (1), 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schug ZT, Voorde JV, & Gottlieb E (2016) The metabolic fate of acetate in cancer. Nature Reviews Cancer 16 (11), 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Israel Y, Orrego H, & Carmichael FJ (1994) Acetate‐mediated effects of ethanol. Alcoholism: Clinical and Experimental Research 18 (1), 144–148. [DOI] [PubMed] [Google Scholar]
- 19.Pomare EW, Branch WJ, & Cummings JH (1985) Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. The Journal of clinical investigation 75 (5), 1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tollinger CD, Vreman HJ, & Weiner MW (1979) Measurement of acetate in human blood by gas chromatography: effects of sample preparation, feeding, and various diseases. Clinical chemistry 25 (10), 1787–1790. [PubMed] [Google Scholar]
- 21.Richards RH, Dowling JA, Vreman HJ, Feldman C, & Weiner MW (1976) Acetate levels in human plasma. In Proceedings of the Clinical Dialysis and Transplant Forum 7, 73–79. [PubMed] [Google Scholar]
- 22.Nuutinen H, Lindros K, Hekali P, & Salaspuro M (1985) Elevated blood acetate as indicator of fast ethanol elimination in chronic alcoholics. Alcohol 2 (4), 623–626. [DOI] [PubMed] [Google Scholar]
- 23.Ye C, & Tu BP (2018) Sink into the epigenome: Histones as repositories that influence cellular metabolism. Trends in Endocrinology & Metabolism 29 (9), 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamphorst JJ, Chung MK, Fan J, & Rabinowitz JD (2014) Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer & metabolism 2 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, ... & McGarry L (2015) Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27 (1), 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S, Torres A, Henry RA, Trefely S, Wallace M, Lee JV, ... & Frey AJ (2016) ATP-citrate lyase controls a glucose-to-acetate metabolic switch. . Cell Reports 17 (4), 1037–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher EA, Marin‐Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, ... & Mathews D (2012) Metabolism of [U‐13C] glucose in human brain tumors in vivo. NMR in biomedicine 25 (11), 1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Cooper DE, Cluntun AA, Warmoes MO, Zhao S, Reid MA, ... & Wellen KE (2018) Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175 (2), 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberti MV, & Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends in biochemical sciences 41 (3), 211–218.26778478 [Google Scholar]
- 30.McDonnell E, Crown SB, Fox DB, Kitir B, Ilkayeva OR, Olsen CA, ... & Hirschey MD (2016) Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell reports 17 (6), 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, ... & Kelley MJ (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. The Journal of clinical investigation 118 (12), 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, ... & Li H (2017) Lactate metabolism in human lung tumors. Cell 171 (2), 358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, ... & Asara JM (2016) Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536 (7617), 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, ... & Shulman GI (2016) Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 534 (7606), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendrick SF, O’boyle G, Mann J, Zeybel M, Palmer J, Jones DE, & Day CP (2010) Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 51 (6), 1988–1997. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Zhang M, Plec AA, Estill SJ, Cai L, Repa JJ, ... & Tu BP (2018) ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proceedings of the National Academy of Sciences 115 (40), E9499–E9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong H, & Chandel NS (2018) Regulation of redox balance in cancer and T cells. Journal of Biological Chemistry 293 (20), 7499–7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hnisz D, Shrinivas K, Young RA, Chakraborty AK, & Sharp PA (2017) A phase separation model for transcriptional control. Cell 169 (1), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holehouse AS, & Pappu RV (2018) Functional implications of intracellular phase transitions. Biochemistry 57 (17), 2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito M, Hess D, Eglinger J, Fritsch AW, Kreysing M, Weinert BT, ... & Matthias P (2019) Acetylation of intrinsically disordered regions regulates phase separation. Nature chemical biology 15 (1), 51. [DOI] [PubMed] [Google Scholar]
- 41.Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, & Lee VM (2011) The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nature communications 2, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreon J, Jain A, Choi KJ, Tsoi P, MacKenzie K, Jung S, & Ferreon A (2018) Acetylation Disfavors Tau Phase Separation. International journal of molecular sciences 19 (5), 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivanand S, Viney I, & Wellen KE (2018) Spatiotemporal control of acetyl-CoA metabolism in chromatin regulation. Trends in biochemical sciences 43 (1), 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mews P, Donahue G, Drake AM, Luczak V, Abel T, & Berger SL (2017) Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546 (7658), 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, ... & Krzyzaniak MA (2016) Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 44 (6), 1312–1324. [DOI] [PubMed] [Google Scholar]
- 46.Muir A, & Vander Heiden MG (2018) The nutrient environment affects therapy. Science 360 (6392), 962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, ... & Huang Z (2014) Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159 (7), 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, ... & Horton JD (2014) Acetate dependence of tumors. Cell 159 (7), 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bulusu V, Tumanov S, Michalopoulou E, van den Broek NJ, MacKay G, Nixon C, ... & Gottlieb E (2017) Acetate recapturing by nuclear acetyl-CoA synthetase 2 prevents loss of histone acetylation during oxygen and serum limitation. Cell reports 18 (3), 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barros LF, & Weber B (2018) CrossTalk proposal: an important astrocyte‐to‐neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. The Journal of physiology 596 (3), 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittar PG, Charnay Y, Pellerin L, Bouras C, & Magistretti PJ (1996) Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. Journal of Cerebral Blood Flow & Metabolism 16 (6), 1079–1089. [DOI] [PubMed] [Google Scholar]
- 52.Magistretti PJ, & Allaman I (2018) Lactate in the brain: from metabolic end-product to signalling molecule. Nature Reviews Neuroscience 19 (4), 235. [DOI] [PubMed] [Google Scholar]
- 53.Yellen G (2018) Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. . J Cell Biol 217 (7), 2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyss MT, Magistretti PJ, Buck A, & Weber B (2011) Labeled acetate as a marker of astrocytic metabolism. Journal of Cerebral Blood Flow & Metabolism 31 (8), 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, ... & Mason GF (2013) Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest 123 (4), 1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horibata Y, Ando H, Itoh M, & Sugimoto H (2013) Enzymatic and transcriptional regulation of the cytoplasmic acetyl-CoA hydrolase ACOT12. Journal of lipid research 54 (8), 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashita H, Kaneyuki T, & Tagawa K (2001) Production of acetate in the liver and its utilization in peripheral tissues. Biochim Biophys Acta 1532 (1–2), 79–87. [DOI] [PubMed] [Google Scholar]
- 58.Busch H, & Baltrush HA (1954) Rates of metabolism of acetate-1-C14 in tissues in vivo. Cancer research 14 (6), 448–455. [PubMed] [Google Scholar]
- 59.Mayfield ED, Bensadoun A, & Johnson BC (1966) Acetate metabolism in ruminant tissues. The Journal of nutrition 89 (2), 189–196. [DOI] [PubMed] [Google Scholar]
- 60.Dinan TG, & Cryan JF (2017) Gut–brain axis in 2016: Brain–gut–microbiota axis—mood, metabolism and behaviour. Nature Reviews Gastroenterology & Hepatology 14 (2), 69. [DOI] [PubMed] [Google Scholar]


