Summary
In this prospective trial evaluating the false negative rate in sentinel nodes for patients undergoing neoadjuvant chemotherapy post surgical radiation therapy was employed at the discretion of the treating physicians. Herein we report outcome of patients in the trial as a function of clinical-pathologic features and radiation therapy administered.
Introduction
Neoadjuvant chemotherapy (NAC) is commonly utilized in the management of breast cancer1–3. Increased utilization is based on a number of trials demonstrating similar overall survival rates with the use of NAC as opposed to postoperative adjuvant therapy, but also increased capacity to perform breast conservation surgery (BCS) as well as the prognostic value of pathologic response4–6. In addition to facilitating the surgical approach in patients with locally advanced breast cancer and converting selected patients to become eligible for breast conservation, response to NAC is a powerful prognostic factor that can aid clinicians in guiding future systemic approaches and multimodality treatment recommendations7. Increasingly, NAC can be used to assess novel drugs and targeted therapies in clinical trials8.
With respect to local-regional management issues, the utilization of NAC introduces some uncertainties and significant variabilities in practice regarding appropriate approaches to surgery and radiation, given that the majority of data concerning local-regional decision-making has traditionally been based on clinical and pathologic factors after upfront surgery3,9,10. Variability in practice includes not only the optimal surgical approach of the primary tumor, but also the reliability of sentinel lymph node (SLN) surgery after NAC, reconstruction options and timing, imaging, and the appropriate use of regional nodal or post-mastectomy radiation (PMRT). Currently NRG 9353 (NSABP B51/RTOG1304) is addressing the issue of omission of regional nodal radiation and PMRT in patients converting from biopsy-proven node-positive disease at presentation to pathologic node-negative following NAC. Alliance for Clinical Trials in Oncology A011202 is addressing whether axillary radiation alone is equivalent to ALND in SLN positive patients after NAC11–13.
The ACOSOG Z1071 (Now part of the ALLIANCE) Phase II trial, which is the subject of this report, investigated the false negative rate of SLN surgery following NAC for patients presenting with initial biopsy proven node-positive disease14 Patients with biopsy-proven node-positive breast cancer at diagnosis received NAC followed by breast surgery with SLN surgery and completion ALND. Beyond the requirement of a full axillary lymph node dissection to assess the false negative rate, which was the primary endpoint, local-regional management approaches were generally left to the discretion of the treating physicians, with only some general protocol guidelines regarding contemporary practice standards. This resulted in significant variability in regional nodal radiation which we recently reported with respect to the use of PMRT and regional nodal irradiation in patients treated with NAC, SLN surgery and ALND in ACOSOG Z107115. The selective discretionary use of post-mastectomy and regional nodal irradiation in ACOSOGZ1071 offers a unique opportunity to determine if variability in the patterns of practice in adjuvant RT impacts on local-regional outcomes. Although this analysis is a retrospective review of the outcomes, all data including radiation therapy details, local-regional and other outcomes were prospectively collected in accordance with the protocol guidelines for up to 10 years post surgery. In this study we evaluate the impact of RT and pathologic response on outcomes, focusing on local-regional recurrence (LRR) in women presenting with node-positive breast cancer treated with NAC on ACOSOG Z1071.
Methods and Materials
ACOSOG Z1071 was approved by the institutional review boards of participating institutions and all study participants provided written informed consent. Patients with biopsy proven breast cancer, T0–T4, N1–N2, M0 who were also Eastern Cooperative Oncology Group Performance status of 0 or 1, received NAC according to standard practice during this time interval. There was a requirement for confirmation of nodal metastasis before NAC by fine needle aspirate or core biopsy. There was also a requirement that surgery be performed within 84 days of the completion of NAC. Following NAC patients routinely underwent primary breast surgery by breast-conserving surgery (BCS), mastectomy (MAST), or mastectomy with reconstruction. These local-regional treatment approaches were determined at the discretion of the treating physicians based on patient and physician preference, as well as clinical indications. SLN surgery was routinely followed by completion ALND for all patients on the trial. The primary outcome of the trial including the false negative rate of SLN surgery were published previously14. Although there were general post-operative radiation therapy guidelines consistent with contemporary practice standards, administration of radiation and radiation field design was left primarily to the discretion of the treating physicians. Patients were treated in accordance with standard practice at 1.8–2.0 Gy Daily to a total dose of 45–5040 Gy with a boost as indicated to a total dose of 60–66 Gy. Regional nodal radiation, and in particular internal mammary radiation was not standardized and left to the discretion of the treating physicians. Radiation of the fully dissected axilla was discouraged.
We previously reported on the patterns of local-regional management with attention to surgical and radiation details as a function of clinical presentation, nodal status following NAC, and response to chemotherapy in which significant variability in the use of adjuvant RT was documented15. For the current study, outcomes including local-regional recurrence (LRR), distant metastasis (DM), disease-free (DFS) Breast Cancer Specific Survival (BCSS) and overall survival (OS) were recorded and analyzed as a function of response to NAC and use of RT.
Statistical analysis
Categorical variables were summarized as frequencies and percentages. Comparisons of categorical variables between groups were made with a chi-square test or Fisher’s exact test if expected cell sizes were too small for the chi-square test16. All tests were two-sided, and p-values less than 0.05 were considered to be statistically significant. Outcomes were assessed from the time of surgery to the date of local, regional, distant relapse or death and estimated with a Kaplan-Meier estimator. LRR events were defined as recurrences in the ipsilateral breast or axilla. Patients were censored for death, distant recurrence, or being alive at the time of analysis with no recurrence. Distant recurrence events were defined as any recurrence outside the ipsilateral breast/axilla. Patients were censored for death, local-regional recurrence, or being alive at the time of analysis with no recurrence. BCSS events were defined as deaths due to breast cancer. Patients were censored if they died from another cause or if they were alive at the time of analysis. OS events were defined as death due to any cause and patients who were alive at the time of analysis were censored. Univariable and multivariable Cox models were used to generate hazard ratios with 95% confidence intervals.17 The multivariable model was adjusted for known prognostic variables. Data collection and statistical analyses were conducted by the ALLIANCE Statistics and Data Center. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) on a dataset frozen on April 10, 2018.
Results
Of 756 women enrolled in ACOSOG Z1071, 701 patients were eligible and evaluable. Mastectomy was performed on 423 (59.6%) and BCS was performed on 277 (40.4%) (unknown in 1). Of the 701 eligible and evaluable patients, 506 (72.2%) had residual disease in the breast and/or lymph nodes after NAC and 195 (27.8%) achieved a pathologic complete response (pCR). Of the 701 patients 591 (85.3%) patients received RT and 102 (14.7%) did not (8 unknown). Characteristics of the patient population broken down by radiation approach are summarized in Table 1. Radiation following mastectomy included the chest wall and regional nodes, with a recommendation to exclude the fully dissected axilla. Internal mammary nodal irradiation was not separately recorded or analyzed. As expected, radiation following mastectomy was more likely to be omitted in patients with pathologically negative nodes and pathologic complete response in the breast. Regional nodal radiation in breast conservation was also omitted more frequently in those with pathologically response in the nodes and/or breast.
Table 1.
Clinical characteristics of the patient population (N = 686) by primary surgical approach
| Breast conserving surgery with Regional RT | conserving surgery without Regional RT | Mastectomy with RT | Mastectomy without RT | p-value | ||
|---|---|---|---|---|---|---|
| Number of patients | 153(22.1%) | 118(17.1%) | 347 (50.2%) | 73(10.6%) | ||
| Clinical tumor category at presentation | <0.0001 | |||||
| cT0/Tis | 2 (1.3%) | 1 (0.8%) | 4 (1.2%) | 1 (1.4%) | ||
| cT1 | 19(12.4%) | 17(14.4%) | 39 (11.3%) | 15(20.6%) | ||
| cT2 | 106(69.3%) | 78(66.1%) | 159(46.0%) | 36 (49.3%) | ||
| cT3 | 24(15.7%) | 20(17.0%) | 123(35.6%) | 14(19.2%) | ||
| cT4 | 2 (1.3%) | 2 (1.7%) | 21 (6.1%) | 7 (9.6%) | ||
| Unknown | 0 | 0 | 1 | 0 | ||
| Clinical nodal category at presentation | 0.56 | |||||
| cN1 | 147(96.1%) | 113(95.8%) | 319(93.3%) | 69 (94.5%) | ||
| cN2 | 6 (3.9%) | 5 (4.2%) | 23 (6.7%) | 4 (5.5%) | ||
| Unknown | 0 | 0 | 5 | 0 | ||
| Clinical stage at presentation | <0.0001 | |||||
| II | 123(80.4%) | 91 (77.1%) | 188(55.1%) | 51 (69.9%) | ||
| III | 30(19.6%) | 27 (22.9%) | 153(44.9%) | 22(30.1%) | ||
| Unknown | 0 | 0 | 6 | 0 | ||
| <0.0001 | ||||||
| pT0/is | 55 (36.0%) | 59 (50.0%) | 87 (25.4%) | 27 (37.5%) | ||
| Pathologic tumor | pT1 | 60 (39.2%) | 38 (32.2%) | 124(36.2%) | 27 (37.5%) | |
| category from | pT2 | 36 (23.5%) | 18(15.2%) | 82 (23.9%) | 12(16.7%) | |
| surgical specimen | pT3 | 2 (1.3%) | 3 (2.5%) | 50(14.6%) | 3 (4.2%) | |
| pT4 | 0 | 0 | 0 | 3 (4.2%) | ||
| Unknown | 0 | 0 | 4 | 1 | ||
| Pathologic nodal category from surgical specimen | 0.0004 | |||||
| pN0 | 62 (40.5%) | 65(55.1%) | 121 (34.9%) | 36 (50.0%) | ||
| pN1 | 58 (37.9%) | 41 (34.8%) | 120(34.6%) | 18(25.0%) | ||
| pN2 | 27(17.6%) | 11 (9.3%) | 78 (22.5%) | 13(18.1%) | ||
| pN3 | 6 (3.9%) | 1 (0.8%) | 28 (8.1%) | 5 (6.9%) | ||
| Unknown | 0 | 0 | 0 | 1 | ||
| Approximated tumor subtype | 0.062 | |||||
| Triple negative | 44 (28.8%) | 33 (28.0%) | 77 (22.2%) | 16(21.3%) | ||
| HER2 positive | 42(38.1%) | 45(38.1%) | 95 (27.4%) | 21 (28.8%) | ||
| HR positive, HER2 | 67 (43.8%) | 40 (33.9%) | 175(50.4%) | 36 (49.3%) | ||
| negative Unknown | 0 | 0 | 0 | 0 |
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; N, nodal; T, tumor
As of April 2018, median follow-up is 5.9 years (range 0.4–8.1 years). A LRR was observed in 43 patients (6.1%). Distant relapse developed in 145 patients (20.7%), 142 patients (20.4%) died, of whom 119 died of breast cancer.
As noted in Table 2, patients with a pCR (n=195) had the best LRR-free survival (HR=0.32 (0.12 – 0.81), p=0.016), DM-free survival (HR=0.31 (0.19 –0.52), p<0.0001), BCSS (HR=0.34 (0.19 – 0.59), p=0.0001) and OS (HR=0.39 (0.24 – 0.63), p=0.001) compared to patients with residual disease, as summarized in Table 2. Notably patients with a pCR had a 5-year LRR rate of 96.8 (92.4 – 98.7) compared to 91.7% (88.5 – 94.0) for those without a pCR and an overall survival of 93.8% (89.2 – 96.5) compared to 80.5% (76.6 – 83.8) without a pCR (data not shown).
Table 2.
Local-regional relapse by clinical and pathologic variables
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% Cl) | P-value | HR (95% Cl) | p-value | |
| Clinical tumor category | 0.14 | 1.00 (ref) | 0.024 | |
| T0-T2 | 1.00 (ref) | 2.16 (1.10–4.21) | ||
| T3-T4 | 1.60 (0.86–2.93) | |||
| Approximated tumor subtype | 1.00 (ref) | 0 | 1.00 (ref) | <0.0001 |
| Hormone receptor-positive/HER2-negative | 1.50 (0.67–3.33) | 2.29 (1.00–5.26) | ||
| HER2-positive | 3.46 (1.68–7.13) | 5.91 (2.80–12.49) | ||
| Triple negative | ||||
| Pathologic CR breast | 1.00 (ref) | 0.01 | 1.00 (ref) | 0.008 |
| No | 0.30 (0.13–0.72) | 0.25 (0.09 – 0.70) | ||
| Yes | ||||
| Pathologic tumor category | 1.00 (ref) | 0.01 | ||
| T0/Tis | 2.47 (0.96 – 6.37) | |||
| T1 | 3.87 (1.49–10.06) | |||
| T2 | 5.41 (1.82–16.10) | |||
| T3 | 12.70 (1.51 –106.85) | |||
| T4 | ||||
| Pathologic CR axilla | 1.00 (ref) | 0.02 | 1.00 (ref) | 0.18 |
| No | 0.42 (0.21 – 0.86) | 0.56 (0.24–1.30) | ||
| Yes | ||||
| Pathologic nodal category | 0.11 | |||
| N0 | 1.00 (ref) | |||
| N1 | 2.31 (1.07–4.97) | |||
| N2 | 2.57(1.09–6.06) | |||
| N3 | 2.44 (0.67–8.86) | |||
| Pathologic CR breast & axilla | 1.00 (ref) | 0.02 | ||
| No | 0.32 (0.12–0.81) | |||
| Yes | ||||
| Pathologic CR | 0.04 | |||
| None | 1.00 (ref) | |||
| Axilla only | 0.28 (0.11–0.72) | |||
| Breast only | 0.66 (0.26–1.69) | |||
| Both | 0.29 (0.04–2.12) | |||
| Breast Surgery Procedure | 0.05 | 0.008 | ||
| BCS | 1.00 (ref) | 1.00 (ref) | ||
| Mastectomy | 0.55 (0.30–1.01) | 0.41 (0.21–0.79) | ||
| Breast Surgery Procedure | 0.16 | |||
| BCS | 1.00 (ref) | |||
| Mastectomy & reconstruction | 0.64 (0.32–1.27) | |||
| Mastectomy no reconstruction | 0.46(0.19–1.13) | |||
| Radiation therapy | 0.1 | 0.018 | ||
| Yes | 1.00 (ref) | 1.00 (ref) | ||
| No | 1.80 (0.89–3.65) | 2.35 (1.15–4.79) | ||
| Age (continuous) | 1.01 (0.98–1.04) | 0.58 | ||
| Age | 0.48 | |||
| <50 | 1.00 (ref) | |||
| ≥50 | 1.24 (0.68–2.27) | |||
| Residual Cancer Burden | 1.00 (ref) | 0.02 | ||
| 0 | 1.27 (0.15–10.85) | |||
| 1 | 2135 (0.70–6.52) | |||
| 2 | 4.52 (1.66–12.35) | |||
| 3 | ||||
BCS, breast conserving surgery; CR, complete response; HER2, human epidermal growth factor receptor 2; HR, hazard ratio
Throughout the entire cohort of mastectomy and breast conserving therapy patients, although patients receiving PMRT or regional nodal RT were of a higher risk group, LRR was lower in patients who received RT compared to those who did not. This was significant on multivariable analysis (HR = 2.35, p = .018). However there was no statistically significant impact of RT in the entire cohort on OS, DFS, or BCSS in both univariate and multivariate analysis.
Table 3, 3a and 4 and Supplemental Figures 1–3 summarize the outcomes in mastectomy and breast-conserving patients as a function of the use of post-mastectomy RT or regional nodal RT in the patients receiving breast-conserving surgery. Notably, in mastectomy patients, omission of PMRT in multivariate analysis was associated with a higher risk of local-regional relapse (HR: 4.84 (1.50 – 15.61) p = 0.008). As noted in Table 3a, further subset analysis by pathologic nodal status after NAC revealed that the lower rate of LRR in mastectomy patients was significant on multivariable analysis in those patients with residual node positive disease ( HR 4.14 (1.15 – 14.92), p = .030), but was not significant in those patients with a pathologically negative axilla.
Table 3.
Outcome in Mastectomy Patients by Radiation Administered
| RT Received | NoRT | |||||
|---|---|---|---|---|---|---|
| HR(P) | Events/ # Patients | 5 year | HR(P) | Events/ # Patients | 5 year | |
| All Mastectomy Patients | ||||||
| LRR | 1.00 (ref) | 11/347 | 96.1% (93.0–97.8) | 1.83(0.65–5.14) (0.25) | 5/73 | 90.7% (78.9–96.1) |
| DFS | 1.00 (ref) | 71/347 | 72.3% (66.2–77.5) | 1.10(0.67–1.82) (0.71) | 18/73 | 68.5% (54.5–79.0) |
| OS | 1.00 (ref) | 83.2% (78.7–86.9) | 1.08(0.64–1.83) 0.78 | 13/73 | 81.3% (70.0–88.7) | |
| BCSS | 1.00 (ref) | 46/347 | 85.6% (81.3–89.0) | 1.04(0.58–1.85) (0.91) | 10/73 | 85.5% (74.6–91.9) |
| Pathologic status of axillary nodes | ||||||
| Axillary pathologic complete response (pCR) | ||||||
| LRR | 1.00 (ref) | 0/119 | 100% | --- | 1/36 | 96.2% (75.7–99.4) |
| DS | 1.00 (ref) | 10/119 | 88.8% (80.1–93.8) | 1.34 (0.47–3.83) (0.58) | 5/36 | 82.1% (62.1–92.2) |
| OS | 1.00 (ref) | 6/119 | 94.6% (88.4–97.5) | 1.44 ((0.50–4.07) 0.50 | 5/36 | 85.3% (68.1–93.7) |
| BCSS | 1.00 (ref) | 4/119 | 96.4% (90.6–98.6) | 1.73 (0.52–5.76) (0.37) | 4/36 | 88.0% (70.8–95.4) |
| No Axillary pCR | ||||||
| LRR | 1.00 (ref) | 11/228 | 94.1% (89.5–96.7) | 1.83 (0.60–5.61) (0.29) | 4/37 | 86.6% (67.8–94.8) |
| DFS | 1.00 (ref) | 62/228 | 64.7% (56.8–71.5) | 1.25 (0.70–2.23) (0.44) | 58.2% (38.8–73.4) | |
| OS | 1.00 (ref) | 48/228 | 77.3% (71.1–82.4) | 1.24(0.67–2.30) 0.50 | 8/37 | 77.4% (59.7–88.0) |
| BCSS | 1.00 (ref) | 43/228 | 80.0% (74.0–84.8) | 1.16 (0.59–2.29 (0.66) | 6/37 | 83.0% (65.9–92.0) |
Table 3 a.
Outcome in Mastectomy Patients by Radiation Administered—Multivariable models
| RT Received | NoRT | |||
|---|---|---|---|---|
| HR(p) | 5 year | HR(p) | 5 year | |
| All Mastectomy Patients | ||||
| LRR* | 1.00 (ref) | 96.1% (93.0–97.8) | 4.84(1.50–15.61) (0.008) | 90.7% (78.9–96.1) |
| DFS* | 1.00 (ref) | 72.3% (66.2–77.5) | 1.48(0.88–2.46) (0.078) | 68.5% (54.5–79.0) |
| OS* | 1.00 (ref) | 83.2% (78.7–86.9) | 1.48(0.86–2.55) 0.16 | 81.3% (70.0–88.7) |
| BCSS* | 1.00 (ref) | 85.6% (81.3–89.0) | 1.49(0.82–2.71) (0.19) | 85.5% (74.6–91.9) |
| Pathologic status of axillary nodes | ||||
| Axillary pathologic complete response (pCR) | ||||
| LRR** | 1.00 (ref) | 100% | --- | 96.2% (75.7–99.4) |
| DS** | 1.00 (ref) | 88.8% (80.1–93.8) | 1.46 (0.50–4.34) (0.49) | 82.1% (62.1–92.2) |
| OS** | 1.00 (ref) | 94.6% (88.4–97.5) | 1.50 (0.52–4.37) (0.45) | 85.3% (68.1–93.7) |
| BCSS** | 1.00 (ref) | 96.4% (90.6–98.6) | 1.73 (0.51 −5.87) (0.38) | 88.0% (70.8–95.4) |
| No Axillary pCR | ||||
| LRR** | 1.00 (ref) | 94.1% (89.5–96.7) | 4.14 (1.15–14.92) (0.030) | 86.6% (67.8–94.8) |
| DFS** | 1.00 (ref) | 64.7% (56.8–71.5) | 1.42 (0.78–2.56) (0.25) | 58.2% (38.8–73.4) |
| OS** | 1.00 (ref) | 77.3% (71.1–82.4) | 1.49 (0.78–2.84) 0.23 | 77.4% (59.7–88.0) |
| BCSS** | 1.00 (ref) | 80.0% (74.0–84.8) | 1.43 (0.71–2.88) (0.32) | 83.0% (65.9–92.0) |
Multivariable models include: RT, Clinical T-stage, path CR breast, path CR axilla, and tumor biology
Multivariable models include: RT, Clinical T-stage, path CR breast, and tumor biology
Table 4.
Outcome in patients treated with breast conservation by use of Regional Nodal Radiation
| Pathologic status of axillary nodes | Regional Nodal RT | No Regional Nodal RT | ||||
|---|---|---|---|---|---|---|
| HR(p) | Events/ # Patients | 5yr | HR(p) | Events/ # Patients | 5yr | |
| Axillary pCR (ypN0) | ||||||
| LRR | 1.00 (ref) | 91.5% (78.8–96.7) | 1.66 (0.37–7.40) (0.51) | 94.6% (84.1–98.2) | ||
| DS | 1.00 (ref) | 90.9% (77.4–96.5) | 0.98 (0.26–3.65) (0.98) | 93.3% (83.0–97.4) | ||
| OS | 1.00 (ref) | 95.5% (83.2–98.9) | 0.88 (0.28–2.77) (0.83) | 91.5% (80.8–96.4) | ||
| BCSS | 1.00 (ref) | 95.5% (83.2–98.9) | 0.97 (0.26–3.61) (0.96) | 93.2% (82.8–97.4) | ||
| No Axillary pCR (ypN+) | ||||||
| LRR | 1.00 (ref) | 90.4% (72.7 – 96.8) | 1.55 (0.51–4.75) (0.44) | 90.7% (82.2–95.3) | ||
| DS | 1.00 (ref) | 72.9% (52.5 – 85.6) | 0.88 (0.39–1.93) (0.72) | 74.5% (63.4–82.7) | ||
| OS | 1.00 (ref) | 82.2% (64.6–91.6) | 0.65(0.26–1.60) (0.35) | 79.8% (69.9–86.8) | ||
| BCSS | 1.00 (ref) | 84.6% (66.9–93.3) | 0.63 (0.24–1.68) (0.35) | 80.9% (71.0–87.7) | ||
BCSS, breast cancer-specific survival; DS, disease-free survival; LRR, local-regional recurrence; OS, overall survival
Subset analysis of patients with a pCR in the axilla was focused on as these patients would be eligible for the ongoing NRG 9353 (NSABP B51) study, which randomizes (1) post-mastectomy patients who had an axillary CR after NAC to receive RT or no RT, and (2) post-lumpectomy patients who had an axillary CR after NAC to receive whole breast RT plus or minus regional nodal RT.
Notably, in this subset, omission of radiation in mastectomy patients was not associated with a significant compromise in LRR (100% with PMRT and 96.2% (75.7 – 99.4%) without PMRT), and omission of regional nodal irradiation in lumpectomy patients in those patients with a pCR in the axilla was not associated with a significant compromise in LRR 90.1% (78.8 – 96.7%) with regional nodal RT vs. 94.6% (84.1 – 98.2%) without regional nodal RT (HR=1.66 (0.37 – 7.40), p=0.51). There was also no impact of RT in this subset on OS, DFS or BCSS on univariate or multivariate analysis. These observations suggest equipoise in administration of RT in patients with pathologically negative axilla after NAC and support the ongoing NRG 9353 randomized trial to address this clinical issue.
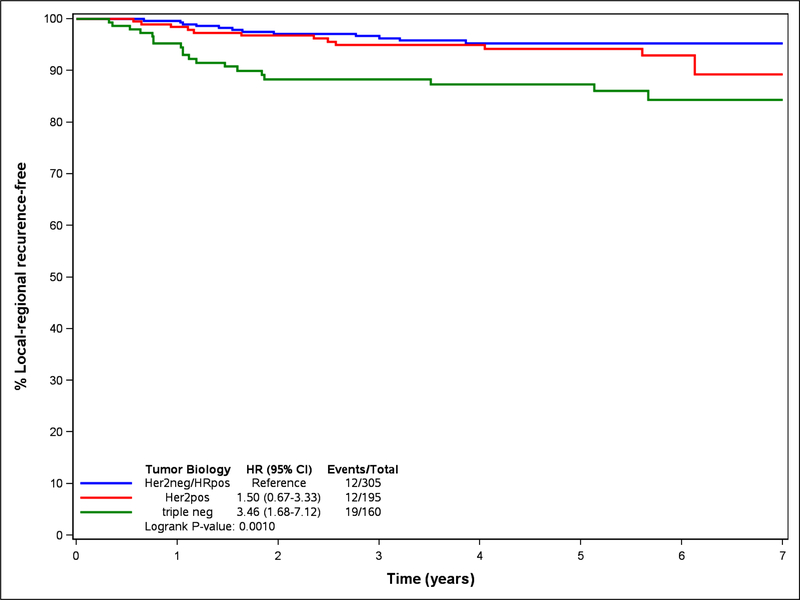
Tumor biology had a significant impact on local-regional outcomes (Table 2) with HER2-positive and triple negative patients having higher LRR rates compared to ER+/HER2-negative patients. Patients with triple negative disease had a particularly high hazard ratio (HR) of 3.46 (1.68–7.13) (Table 5, Figure 1). Although not statistically significant due to relatively small numbers in this subset analysis of triple negative patients, LRR-free rates were higher in both the mastectomy patients 91.0% (79.6 – 96.2%) with PMRT vs. 77.9% (35.4 – 94.2%) without PMRT, p = 0.44) (Table 5) and lumpectomy patients 90.2% (65.9 – 94.5%) with regional nodal RT vs. 85.8% (71.1 – 93.4%) without regional nodal RT, p=.49, (Table 5). Although numbers were small and not statistically significant, in triple negative patients with a pCR who did undergo PMRT or regional nodal RT, the LRR at 5 years was 100% (no events) compared to 90.4% (66.8 – 97.5) in those triple negative patients with a pCR who did not receive radiation.
Table 5.
Triple negative Subset by Radiation Administered
| Post Mastectomy RT Received | NoRT | |||||
|---|---|---|---|---|---|---|
| Triple Negative Mastectomy patients | HR(p) | 5yr | HR(p) | 5 yr | ||
| LRR | 1.00 (ref) | 91.0% (79.6–96.2) | 1.86 (0.39 0 8.97) (0.44) | 77.9% (35.4–94.2) | ||
| Regional Nodal RT Received | No Regional Nodal RT | |||||
| Triple negative BCS patients who had whole breast RT | HR(p) | 5yr | HR(p) | 5 yr | ||
| LRR | 1.00 (ref) | 90.2% (65.9–94.5) | 0.57 (0.12–2.82) (0.49) | 85.8% (71.1–93.4) | ||
HR, hazard ratio; LRR, local-regional recurrence; RT, radiation therapy
Figure 1.
Local-regional recurrence by tumor biology
Discussion
The increased utilization of NAC in the management of stage II and III breast cancer, while providing significant clinical advantages, including the ability to assess response to chemotherapy, increase utilization of breast conservation and improve surgical management of advanced disease, has introduced significant variability in practice with respect to local-regional management of disease, particularly in radiation oncology15,18. Traditionally, with upfront surgery, radiation management decisions are based on initial tumor size, nodal status and other clinical-pathologic factors. There are available data, randomized trials and additional literature that provide high level evidence to help guide and shape decision-making based on clinical, surgical and pathologic factors at the time of initial diagnosis11,19. When patients are treated with NAC followed by surgery, decisions regarding radiation management are more complex, with less robust or evidence-based data18. Specifically, in patients with initial biopsy-documented node-positive disease who respond to NAC, it remains unclear whether radiation decisions should be based on the initial presentation or on disease status after NAC18. In patients with sentinel node-positive disease after NAC, the issue of axillary radiation compared to completion dissection remains unclear. These and other local-regional management issues are currently being addressed in prospective, randomized trials such as NRG 9353 and Alliance A01120211,18.
ACOSOG Z1071 was designed to determine the false negative rate of sentinel node biopsy in patients with pathologically confirmed node-positive breast cancer treated with NAC, with all patients undergoing completion axillary dissection after sentinel lymph node surgery14. While the ACOSOG Z1071 protocol had some general radiation therapy guidelines, there were no specific protocol mandated radiation requirements and radiation field design and decisions were mainly left to the discretion of the treating physicians.
As previously reported there was significant variability in practice with respect radiation therapy administration11. Since administration of radiation was left to the discretion of the treating physicians, any conclusions regarding impact of radiation on outcomes is subject to bias and should be interpreted with caution. The use of regional nodal and PMRT was typically tailored to the pathologic findings and more frequently used with more aggressive pathology after NAC.
As has been reported in multiple other series, this study confirms that local-regional, distant, disease-free and overall survival rates are impacted by the response to NAC, with those patients achieving a pathologic complete response having more favorable outcomes compared with those with residual disease20,21. It is important to note that the acceptable outcomes experienced in this study, with elimination of PMRT and regional nodal RT in patients converting to node-negative disease, is reassuring regarding the ongoing NRG 9353 clinical trial, and is consistent with a number of other reports. These findings suggest equipoise in patients who have complete pathologic responses in the breast and/or axilla whether PMRT or regional nodal RT is administered or not, and support the ongoing trials addressing this issue. Clearly, however, the prospective randomized NRG 9353 trial (NSABP B51) will more definitively address the role of RT after complete axillary response and we encourage enrollment which ultimately will help to decrease practice variability. Our results, which demonstrate a statistically significant higher LRR on multivariable analysis with omission of RT in those with residual positive nodes after NAC, do confirm that these patients should be considered for PMRT or regional nodal RT after BCS.
Since all patients in ACOSOG Z1 underwent completion axillary dissection, the role of axillary radiation compared to axillary dissection could not be assessed in this study. AMAROS and other studies have demonstrated that axillary radiation results in similar local-regional control with lower morbidity compared to axillary dissection in patients presenting with low volume sentinel node- positive disease at initial presentation in the setting of upfront surgery22. For patients with sentinel node-positive disease after NAC, the ongoing Alliance A011202 trial is comparing axillary dissection to axillary radiation. All patients in this Alliance trial will undergo post-mastectomy chest wall and regional nodal radiation or post-lumpectomy breast and regional nodal radiation. The higher rate of LRR with omission of post-mastectomy radiation supports the use of radiation for those patients with residual node-positive disease following mastectomy.
Finally, our results confirm the more aggressive local-regional failure patterns in patients with triple negative breast cancer, where elimination of radiation post-mastectomy and elimination of regional nodal radiation were associated with a trend toward higher LRR rates. Outside of a clinical trial, these results suggest that patients presenting with node-positive triple negative disease, despite response to NAC, should be treated aggressively.23 NRG 9353 (NSABP B51) and Alliance A011202 will help to identify if biological subtype should further influence radiation decision-making after administration of NAC in initial node-positive patients.
Supplementary Material
Supplemental Figure 1 Local-regional recurrence-free survival, comparing patients who received adjuvant radiation with those who did not.
Supplemental Figure 2 Pathologic N0 patients by use of radiation.
Supplemental Figure 3 Pathologic N1 patients by use of radiation.
Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180790, and U10CA180858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Amoroso V, Generali D, Buchholz T, et al. International Expert Consensus on Primary Systemic Therapy in the Management of Early Breast Cancer: Highlights of the Fifth Symposium on Primary Systemic Therapy in the Management of Operable Breast Cancer, Cremona, Italy: (2013). J Natl Cancer Inst Monogr 2015;2015:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 2008;26:814–9. [DOI] [PubMed] [Google Scholar]
- 3.Teshome M, Hunt KK. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin N Am 2014;23:505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr 2001:96–102. [DOI] [PubMed] [Google Scholar]
- 5.Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol 2009;27:2474–81. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- 7.Colleoni M, Viale G, Goldhirsch A. Lessons on responsiveness to adjuvant systemic therapies learned from the neoadjuvant setting. Breast 2009;18 Suppl 3:S137–40. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883–91. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz TA, Lehman CD, Harris JR, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol 2008;26:791–7. [DOI] [PubMed] [Google Scholar]
- 10.Fowble BL, Einck JP, Kim DN, et al. Role of postmastectomy radiation after neoadjuvant chemotherapy in stage II-III breast cancer. Int J Radiat Oncol Biol Phys 2012;83:494–503. [DOI] [PubMed] [Google Scholar]
- 11.Haffty BG, Mahmoud O. The evolution of regional nodal irradiation in breast cancer. Breast J 2015;21:32–41. [DOI] [PubMed] [Google Scholar]
- 12.Smith BD. Using chemotherapy response to personalize choices regarding locoregional therapy: a new era in breast cancer treatment? J Clin Oncol 2012;30:3913–5. [DOI] [PubMed] [Google Scholar]
- 13.Mak KS, Harris JR. Radiotherapy Issues After Neoadjuvant Chemotherapy. J Natl Cancer Inst Monogr 2015;2015:87–9. [DOI] [PubMed] [Google Scholar]
- 14.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haffty BG, McCall LM, Ballman KV, et al. Patterns of Local-Regional Management Following Neoadjuvant Chemotherapy in Breast Cancer: Results From ACOSOG Z1071 (Alliance). Int J Radiat Oncol Biol Phys 2016;94:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantel N Maximum likelihood vs. minimum chi-square. Biometrics 1985;41:777–83. [PubMed] [Google Scholar]
- 17.Gellar JE, Colantuoni E, Needham DM, Crainiceanu CM. Cox Regression Models with Functional Covariates for Survival Data. Stat Modelling 2015;15:256–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krug D, Baumann R, Budach W, et al. Individualization of post-mastectomy radiotherapy and regional nodal irradiation based on treatment response after neoadjuvant chemotherapy for breast cancer : A systematic review . Strahlenther Onkol 2018. [DOI] [PubMed] [Google Scholar]
- 19.Harris J, Lippman M, Morrow M, Osborne C. Diseases of the Breast 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 20.Symmans WF, Wei C, Gould R, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype . J Clin Oncol 2017;35:1049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. [DOI] [PubMed] [Google Scholar]
- 22.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton JK, Jagsi R, Woodward WA, Ho A. Breast Cancer Biology: Clinical Implications for Breast Radiation Therapy. Int J Radiat Oncol Biol Phys 2018;100:23–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Local-regional recurrence-free survival, comparing patients who received adjuvant radiation with those who did not.
Supplemental Figure 2 Pathologic N0 patients by use of radiation.
Supplemental Figure 3 Pathologic N1 patients by use of radiation.