Abstract
A wide spectrum of genetic causes may lead to non-immune hydrops fetalis (NIHF), and a thorough phenotypic and genetic evaluation are essential to determine the underlying etiology, optimally manage these pregnancies, and inform discussions about anticipated prognosis. In this review, we outline the known genetic etiologies of NIHF by fetal organ system affected, and provide a systematic approach to the evaluation of NIHF. Some of the underlying genetic disorders are associated with characteristic phenotypic features that may be seen on prenatal ultrasound, such as hepatomegaly with lysosomal storage disorders, hyperechoic kidneys with congenital nephrosis, or pulmonary valve stenosis with RASopathies. However, this is not always the case, and the approach to evaluation must include prenatal ultrasound findings as well as genetic testing and many other factors. Genetic testing that has been utilized for NIHF ranges from standard chromosomal microarray or karyotype to gene panels and broad approaches such as whole exome sequencing. Family and obstetric history, as well as pathology examination, can yield additional clues that are helpful in establishing a diagnosis. A systematic approach to evaluation can guide a more targeted approach to genetic evaluation, diagnosis, and management of NIHF.
INTRODUCTION
Hydrops fetalis occurs in approximately 1 in 1700 to 1 in 3000 pregnancies, and is diagnosed by prenatal ultrasound when at least two abnormal fetal fluid collections are present, including ascites, pleural effusion, pericardial effusion, or skin edema.1 The majority of cases are non-immune in nature when Rh(D) immune globulin is appropriately administered, and a wide variety of genetic causes are known to lead to non-immune hydrops fetalis (NIHF).1 Significant perinatal risks accompany NIHF, ranging from stillbirth to preterm birth and neonatal death, and risks of these adverse outcomes vary by the underlying cause of hydrops.1–6
An algorithm for the evaluation of NIHF has been published by the Society for Maternal-Fetal Medicine, which includes obstetric ultrasound with Doppler interrogation, fetal echocardiogram, fetal karyotype and/or chromosomal microarray analysis (CMA), and consideration of more targeted testing such as lysosomal enzyme assays.1 However, nearly half of NIHF cases remain of unknown etiology following standard evaluation.7 Prenatal phenotyping, or characterization of the unique features associated with each case, can be helpful to developing an approach to the diagnosis of the chromosomal abnormalities and single gene disorders leading to the fetal structural anomalies, genetic syndromes, and inborn errors of metabolism seen with NIHF.1,7,8
Understanding the etiology of NIHF is imperative in order to effectively manage these pregnancies, anticipate neonatal care requirements, implement time-sensitive treatments, and counsel families about prognosis and recurrence risk. While a number of non-genetic etiologies such as viruses may also lead to NIHF, we focus here on the genetic etiologies. This review provides a systematic approach to the genetic evaluation of NIHF, focusing on the etiologies by organ system affected. This framework allows providers to approach each NIHF case by considering the unique constellation of phenotypic features, and guides a more targeted approach to genetic evaluation, diagnosis, and management.
GENETIC CAUSES OF NIHF BY ORGAN SYSTEM
The presence of structural anomalies in the fetus, in addition to NIHF, may provide clues about the underlying genetic etiology. Both the constellation of findings as well as any unusual or uncommon features may be helpful in narrowing the differential diagnosis. While it can be difficult to detect subtle phenotypic features such as facial dysmorphism, defining the phenotype prenatally as much as possible can be helpful in the evaluation process.
Many genetic diseases and syndromes are difficult to classify into one primarily affected organ system, which highlights the importance of understanding the overall phenotypic picture for each case. The following sections take a practical approach by considering one organ system at a time, and reviewing considerations for underlying genetic etiologies when specific features are seen in addition to NIHF. Table 1 outlines genetic disorders and syndromes associated with NIHF by category, along with the major manifestations, associated genes, and prevalence.
Table 1.
Genetic disorders by organ system.
| Genetic disease or syndrome | Gene or Location | Prenatal Findings (in addition to NIHF) | Prevalence | References | |
|---|---|---|---|---|---|
| Aneuploidies | Monosomy X (Turner syndrome) | Chromosome X | Cystic hygroma (often septated), increased NT, cardiac anomalies (aortic coarctation, HLHS), horseshoe kidney, short femur | 1 in 2,000–2,500 female live births 10–20% of SABs | Papp 200653 |
| Trisomy 21 (Down syndrome) | Chromosome 21 | Cardiac anomalies (especially endocardial cushion defects, septal defects), duodenal atresia, increased NT or NF, absent/short nasal bone, ventriculomegaly | 1 in 800 live births | Smith Bindman 20019 Nyberg 199051 | |
| Trisomy 18 (Edwards syndrome) | Chromosome 18 | Cardiac defects (especially septal and valvular defects), skeletal anomalies (strawberry-shaped calvarium, clenched hands, rocker bottom feet), increased NT or cystic hygroma, CNS anomalies (abnormal cisterna magna, absent CC, NTDs, ventriculomegaly), facial anomalies (clefts, micrognathia), GI anomalies (omphalocele, CDH), GU (horseshoe kidney, hydronephrosis) | 1 in 5500 live births | Bronsteen 200410 | |
| Trisomy 13 (Patau syndrome) | Chromosome 13 | CNS anomalies (increased NT, holoprosencephaly, posterior fossa abnormalities, absent CC, ventriculomegaly), midline defects (cyclopia, midline clefts, anophthalmia), cardiac defects (septal defects, TOF, HLHS), GI anomalies (omphalocele, CDH), polycystic kidneys, postaxial polydactyly, FGR | 1 in 5,000–20,000 live births (but >75% die in utero) | Szigeti 200611 | |
| Triploidy (partial mole) | 69,XXX 69,XXY 69,XYY | CNS anomalies (ventriculomegaly, holoprosencephaly, Dandy Walker malformation, NTD), absent gallbladder, renal anomalies, syndactyly of 3rd and 4th digits, clubbed feet, clenched hands, 2 vessel umbilical cord Paternal (diandric, 75%): moderate, symmetric FGR; large, cystic placenta; increased NT Maternal (dyginic, 25%): asymmetric FGR; small, calcified placenta, oligohydramnios | 1–3% of clinically recognized pregnancies; 20% of spontaneous abortions | Massalska 201712 | |
| Central nervous system | Meckel Gruber syndrome | B9D1, B9D2, CC2D2A, CEP290, MKS1, RPGRIP1L, TMEM67, TMEM216 | Occipital encephalocele, bilateral large multicystic kidneys, fibrotic changes of the liver, polydactyly | 1 in 13,250–140,000 | Abrams 200722Barisic 201523 |
| Proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome | FLVCR2 | Hydranencephaly, cystic hygroma, arthrogrypsosis | Unknown | Petrovski 2019112 | |
| Smith Lemli Opitz | DHCR7 | Microcephaly, ventriculomegaly, FGR, CP, cryptorchidism, renal dysplasia, elevated NT, postaxial polydactyly, 2,3-syndactyly, cardiac defects | 1 in 20,000 to 60,000 (higher in Caucasians) | Maymon 199924 | |
| Zellweger syndrome | PEX1 (70%), PEX2, PEX3, PEX5, PEX6, PEX10, PEX11B, PEX12, PEX13, PEX14, PEX16, PEX19, PEX26 | Subependymal or periventricular pseudocysts, ventriculomegaly, increased NT, echogenic bowel, FGR, hypokinesia, hepatosplenomegaly, renal microcysts | 1 in 50,000 | Shen 2016127 Dursun 2009128 | |
| Cardiovascular | 22q11 microdeletion syndrome | 22q11.2 | Conotruncal heart defects (truncus arteriosus, aortic arch abnormalities, TOF, left ventricular noncompaction), CP, micrognathia, hypertelorism, thymic hypoplasia, increased NT or NF | 1 in 4,000 | Dupont 2014129 Machlitt 200232 |
| 9q duplication | 9q | Congenital heart defects, microcephaly, contractures, urogenital malformations, FGR | Unknown | Amarillo 2015130 | |
| ALPK3 related disorders | ALPK3 | Cardiomegaly, severe biventricular hypertrophy and dilation | Unknown | Almomani 201631 | |
| Barth syndrome | TAZ | Congenital heart disease (dilated cardiomyopathy, left ventricular noncompaction, endocardial fibroelastosis), X-linked | 1 in 300,000–400,000 | Steward 201028 | |
| Congenital long QT syndrome | KCNQ1, KCNH2, SCN5A | Arrhythmia | 1 in 2,000 live births | Komarlu 201136 | |
| GATA-5 related disorders | GATA5 | Congenital heart defects, genital anomalies | Unknown | Hempel 201730 | |
| Kabuki syndrome | KMT2D, KDM6A | Cardiac anomalies (HLHS, coarctation of the aorta, bicuspid aortic valve, septal defects), increased NT, echogenic bowel, micrognathia, FGR, polyhydramnios | 1 in 32,000 | Long 2016131 | |
| Klippel-Trenaunay-Weber syndorme | PIK3CA | Cardiomegaly, anemia, unilateral lower extremity hypertrophy, hemangiomas (placental, hepatic), polyhydramnios, cutaneous or subcutaneous cystic or multicystic lesions | 1 in 100,000 | Tanaka 201575 | |
| McKusick-Kaufman syndrome | MKKS | Cardiac anomalies (atrioventricular canal, HLHS, single atria), hydrometrocolpos, polydactyly | 1 in 10,000 of Old Order Amish population | Gaucherand 2002132 Tsai 2014133 | |
| Opitz G/BBB syndrome, X-linked | MID1 | Cardiac anomalies (VSD, ASD, aortic coarctation, persistent left SVC, PDA), hypertelorism, enlarged cisterna magna, polyhydramnios | 1 in 50,000–100,000 males | Patton 1986134 Cheng 2014135 | |
| PIK3CA-associated segmental overgrowth (including CLOVES and MCAP syndromes) | PIK3CA | CLOVES: lipomatous truncal masses, limb asymmetry, vascular or lymphatic malformations, polyhydramnios MCAP: macrocephaly, pleural effusion, ventriculomegaly, limb asymmetry, frontal bossing, polyhydramnios | Unknown | Nyberg 2005136 Mirzaa 2005137 Emrick 201413 | |
| Capillary malformation-arterioveous malformation syndrome (RASA1-related disorders) | RASA1 | Polyhydramnios, asymmetric MCA Dopplers, hyperechoic interventricular septum, arteriovenous malformations | 1 in 100,000 people of northern European descent | Overcash 201538 | |
| Tuberous sclerosis | TSC1, TSC2 | Cardiac rhabdomyoma, arrhythmias, cerebral cortical tubers on MRI | 1 in 6,000 | Pruksanusak 201226 | |
| Williams syndrome | 7q11.23 | Supravalvular aortic stenosis | 1 in 7,500–10,000 | Von Dadelszen 2000138 | |
| Wolff-Parkinson-White syndrome | Most unknown; PRKAG2 | Tachyarrhythmia | 1–3 in 1,000 | Abrams 200722 Hoeffler 201637 | |
| Pulmonary and thoracic | Cornelia de Lange Syndrome | NIPBL, SMC1A, HDAC8, RAD21, SMC3 | CDH, sacrococcygeal teratoma, polyhydramnios, FGR | 1 in 10,000–30,000 | Toomayan 200883Banait 201582 Lord 2019113 |
| Fraser syndrome | FRAS1 (>50%), FREM2, GRIP1 | Microphthalmia, cleft lip, renal agenesis or dysplastic kidneys, obstructive uropathy, CHAOS | 1 in 200,000 live births; 1 in 10,000 fetal losses/spontaneous abortions | Tessier 201643 | |
| Fryns syndrome | Unknown | CDH, distal limb hypoplasia, hypertelorism, broad nasal bridge, cleft lip and/or palate, micrognathia, polyhydramnios | Unknown; 1.3–10% of CDH cases | Ramsing 200141 | |
| Pallister-Killian syndrome | Tetrasomy 12p (usually with isochromosome 12p) | Cardiac anomalies, CDH, rhizomelic micromelia, ventriculomegaly, flat nasal bridge | 1 in 20,000 live births | Doray 200240 | |
| VACTERL association | Unknown | Vertebral defects, anal atresia, cardiac defects, TEF, renal anomalies, limb abnormalities | 1 in 10,000 to 40,000 newborns | Sanford 2012139 | |
| Gastrointestinal | Beckwith-Wiedemann syndrome | 11p15.5 | Omphalocele, placental cysts, macroglossia, enlarged kidneys, adrenal cysts, hepatosplenomegaly, polyhydramnios | 1 in 13,700 live births | Hillstrom 199552 |
| Lysosomal Storage Disorders | |||||
| Galactosialidosis | CTSA | Hepatosplenomegaly, echogenic bowel | Unknown (100 reported cases) | Patel 1999140 Caciotti 2013141 | |
| Gaucher disease | GBA | Arthrogryposis, ventriculomegaly, microcephaly, ichthyosis, hepatosplenomegaly | 1:855 in Ashkenazi Jewish, 1:57,000 to 1.16:100,000 in general | Beaujot 201377 | |
| Mucolipidosis I (Sialidosis) | NEU1 | Hepatosplenomegaly | Unknown (1–4:200,000 to 1:4.2 million) | Gillan 198446 | |
| Mucolipidosis II (I-cell disease) | GNPTAB | Skeletal dysplasia, FGR | 1:640,000 | Capobres 2015142 Heo 2012143 | |
| Mucopolysaccharidosis IVa (Morquio syndrome) | GALNS | Isolated hydrops | 1:250,000 | Applegarth 1987144 | |
| Mucopolysaccharidosis type IVB (GM1- gangliosidosis) | GLB1 | Isolated or with hepatosplenomegaly | 1:100,000–1:200,000 | Sinelli 2005145 Gillan 198446 | |
| Mucopolysaccharidosis VII (Slysyndrome) | GUSB | Short femurs, ventriculomegaly, increased nuchal translucency/nuchal fold, hydrothorax, echogenic bowel, myocardial hypertrophy, placentomegaly | 1:250,000–1:<1,000,000 | Cheng 2003 Hollander 2000 Geipel 2002146–148 | |
| Niemann-Pick A | SMPD1 | Placental thickening | 1:40,000 in Ashkenazi Jewish, 1:250,000 in general populations | Schoenfeld 1985149 | |
| Niemann-Pick C | NPC1, NPC2 | Cystic hygroma, hepatosplenomegaly, echogenic bowel, placentomegaly | 1:150,000 | Ples 2018150 | |
| Salla Disease | SLC17A5 | Hepatosplenomegaly, clubbed feet, cardiomegaly, FGR, ventriculomegaly, brachycephaly, micromelia | Unknown, <1:1,000,00 | Aula 2006151 | |
| Mevalonate kinase deficiency | MVK | Hepatosplenomegaly, hypotonia | Unknown (200 reported worldwide) | Peciuliene 2016152 | |
| Wolman Disease | LIPA | Hepatosplenomegaly, enlarged, calcified adrenal glands | 1:350,000 | Özmen 1992153 Blitz 2017154 | |
| Genitourinary and Renal | Bartter syndrome | SLC12A1, KCNJ1, CLCNKB, BSND, CLCNKA, CLCNKB | Large renal cysts | Unknown | Çetinkaya 201354 |
| Autosomal recessive polycystic kidney disease | PKHD1 | Enlarged, echogenic kidneys with multiple cysts | 1 in 20,000 to 40,000 | Van Maldergam 199255 | |
| Congenital nephrosis | |||||
| Pierson syndrome | LAMB2 | Renal hyperechogenicity, pyelectasis, placentomegaly, oligohydramnios, anencephaly | Unknown | Mark 2006155 | |
| Finnish nephrosis | NPHS1, NPHS2 | Hyperechoic, enlarged kidneys, placentomegaly | 1–3 in 100,000 worldwide 1 in 10,000 in Finland | Zenker 2004156 | |
| Musculoskeletal | Skeletal dysplasias | ||||
| Achondrogenesis | TRIP11, SLC26A2, COL2A1 | Short, bent long bones, poor spine ossification, hydrocephalus, polyhydramnios | Unknown | Pretorius 198619 Heinrich 201556 | |
| Achondroplasia | FGFR3 | Short long bones with relative macrocephaly | 1 in 15–40,000 | Etches 1979157 Hatzaki 2011158 | |
| Asphyxiating thoracic dystrophy (Jeune syndrome) | CEP120, CSPP1, DYNC2H1, IFT80, IFT140, IFT172, TTC21B, WDR19, WDR34, WDR35, WDR60 | Bell-shaped thorax, short horizontal ribs, mesomelia, renal cysts | 1–5 in 500,000 live births | Nakamura 1987159 Tonni 2013160 | |
| Congenital disorders of glycosylation type I | PMM2, ALG1, ALG9 | Short long bones, clubfeet, polyhydramnios, cardiomyopathy | 800 cases reported | Kranz 200761 | |
| Hydrops-ectopic calcification moth eaten (HEM) skeletal dysplasia (Greenberg dysplasia) | LBR | Severe shortening of all long bones, moth-eaten radiographic appearance, FGR, placentomegaly, rib fractures, narrow thorax, hepatomegaly | Unknown | Konstantinidou 200849 | |
| Osteogenesis Imperfecta | COL1A1, COL1A2, CRTAP, P3H1 | Reduced echogenicity of fetal bone; bowed, crumpled femurs; beaded ribs; evidence of fractures; markedly diminished calvarial mineralization | 6–7 in 100,000 | Nakamura 1987159 Steiner 2013161 | |
| Osteopetrosis congenita | CLCN7 (75%), CA2, IKBKG, ITGB3, OSTM1, PLEKHM1, TCIRG1, TNFRSF11A, TNFSF11 | Dense bones, macrocephaly, hydrocephaly, hepatosplenomegaly | 1 in 20,000 | Mathur 198448 | |
| Roberts syndrome | ESCO2 | Mesomelia, cleft lip/palate, oligodactyly | 150 cases reported | Dalal 2006162 | |
| Schneckenbecken dysplasia | SLC35D1 | Hypoplastic iliac bones, oval-shaped vertebral bodies, short long bones | Unknown | Nikkels 2001163 | |
| Short rib polydactyly, type I (Saldino-Noonan) | DYNC2H1 | Short, horizontal ribs, small thorax, polydactyly, micromelia, pointed metaphyses, absent fibulae, delayed ossification, natal teeth; cardiac, pulmonary, GU, and GI anomalies; cleft palate; females more likely | 1 in 200,000 live births | Badiner 2017 Montemarano 1995 Kumru 2005 Silveira 2017164–167 | |
| Short rib polydactyly, type II (Majewski) | NEK1 | Enlarged kidneys, oligohydramnios, micromelia | |||
| Short rib polydactyly, type III (Verma-Naumoff) | DYNC2H1 | Micromelia, narrow thorax (less severe than type 1), widened metaphyses, absent fibulae, arrhythmia, postaxial polydactyly, aortic coarctation, increased NT | |||
| Short rib polydactyly, type IV (Beemer-Langer) | IFT122 | Increased NF, micromelia, small thorax, postaxial polydactyly, hypoplastic clavicles | |||
| Thanatophoric dysplasia | FGFR3 | Hydrocephalus, absent CC, cloverleaf skull, bent long bones, normal spine ossification | 1 in 20,000 to 50,000 newborns | Pretorius 198619 | |
| Yunis-Varon syndrome | FIG4 | Absent clavicles, ventriculomegaly, Dandy-Walker malformation, microcephaly, hypoplastic phalanges of fingers and toes | Unknown | Basel-Vanagaite 2008168 | |
| Oral-facial-digital syndrome | OFD1 | Polydactyly, short and low implanted thumbs, bifid tongue, CP, agenesis of the CC | 1 in 50,000 to 250,000 newborns | Van Maldergam 199255 Alby 2018169 | |
| Fetal akinesia | |||||
| Arthrogryposis multiplex congenita (Pena-Shokeir syndrome) | DOK7, MUSK, RAPSN | Multiple contractures, pulmonary hypoplasia, polyhydramnios, FGR, micrognathia, camptodactyly, reduced fetal movements | 1 in 12,000 | Gupta 201059 | |
| Lethal multiple pterygium syndrome | CHRNG, CHRNA1, CHRND, RAPSN | Multiple contractures, pulmonary hypoplasia, cystic masses in neck, skin webs, reduced fetal movements, cystic hygroma or increased NT, pterygia | Unknown | Jauniaux 199057 Chen 201258 | |
| Myotonic dystrophy | DMPK | Decreased fetal movements, bradycardia, tented mouth facies | 1 in 8000 | Stratton 199360 | |
| Neu-Laxova syndrome | PHGDH, PSATI1, PSPH | FGR, contractures, distal hypoplasia, clubbed feet, microcephaly, microganthia, ichthyosis, scoliosis | Unknown | Jauniaux 199057 Mattos 201476 | |
| IPEX syndrome | FOXP3 | Echogenic bowel, skin desquamation, FGR, akinesia, echogenic kidneys, clubfeet, X-linked | n/a | Reichert 2015170 Shehab 2017171 | |
| Hematologic | Alpha thalassemia (HbH and Hb Barts) | HBA1, HBA2 | Hydrocephaly, microcephaly, increased NT, placentomegaly, anemia, hepatomegaly | 1 in 200–2,000 births in SE Asia | Chui 199862 |
| Congenital dyserythropoietic anemia | CDAN1, SEC23B | Anemia | Unknown | Tamary 199365 | |
| Congenital erythropoietic porphyria | CEP, UROS | Cystic hygroma, hydropic placenta, FGR, hypoplasia of cerebellar vermis, echogenic dysplastic kidneys, echogenic bowel and kidneys; dark brown amniotic fluid | Unknown | Pannier 2002172 | |
| Diamond Blackfan anemia | GATA1, RPL5, RPL11, RPL15, RPL26, RPL27, RPL31, RPL35A, RPL36, RPS7, RPS10, RPS15, RPS17, RPS19, RPS24, RPS26, RPS27, RPS28, RPS29, TSR2 | Anemia, FGR, oligohydramnios | 5–7 per 1 million | Dunbar 200364 | |
| εγδβ-thalassemia | HBB, locus control region of beta-globin locus on 11p | Anemia, echogenic bowel | Unknown | Goel 2017173 | |
| Glucose-6-phosphate dehydrogenase deficiency | G6PD | Anemia | 400 million people worldwide | Arcasoy 199550 | |
| Glucose phosphate isomerase deficiency | GPI | Anemia, hepatosplenomegaly | Unknown (~50 cases reported) | Arcasoy 199550 | |
| Pyruvate kinase deficiency | PKLR | Anemia, multicystic encephalomalacia, hepatomegaly | 1 in 20,000 people of European descent, especially Old Order Amish of Pennsylvania | Arcasoy 199550 | |
| Lymphatic | RASopathies | ||||
| Noonan syndrome | PTPN11, SOS1, RAF1, RIT1 | Cardiac anomalies (hypertrophic cardiomyopathy, HLHS, valvular anomalies), hydrothorax, chylothorax, pyelectasis, increased NT, cystic hygroma | 1:1,000–1:2,500 | Croonen 201333 | |
| Costello syndrome | HRAS | Increased NT, short long bones, abnormal hand posture, ventriculomegaly, large size, macrocephaly, atrial tachycardia | 1:300,000–1:1,230,000 | Lin 2002, 200914,35 | |
| Cardiofaciocutaneous syndrome | MAP2K2 | Increased NT, polyhydramnios | Unknown (200–300 worldwide) | Gos 2017174 | |
| Casitas B-cell lymphoma syndrome | CBL | Pleural effusion, chylothorax | Unknown | Bülow 201434 | |
| Noonan-like syndrome with loose anagen hair | SHOC2 | Elevated nuchal fold, hypertelorism, low-set ears | Unknown | Gargano 2013178 | |
| Generalized lymphedema | |||||
| ITGA9-associated fetal chylothorax | ITGA9 | Chylothorax, polyhydramnios | 1 in 12,000 | Yang 201268 | |
| Generalized lymphatic dysplasia | PIEZO1 | Cystic hygroma | Unknown | Fotiou 201566 | |
| Hennekam lymphangiectasia-lymphedema syndrome | CCBE1, FAT4 | Polyhydramnios | <1:1,000,000 | Bellini 200374 | |
| Hereditary lymphedema (Nonne-Milroy disease) | VEGFR3 | Leg edema, hydrothorax, lung hypoplasia | 1 in 6,000 | Gordon 201272 Lev-Sagie 2003175 | |
| Hypotrichosis-lymphedema-telangiectasia syndrome | SOX18 | Chylothorax | Unknown | Irrthum 200369 | |
| Lymphedema-cholestasis syndrome (Aagenaes syndrome) | 15q, gene unknown | Placentomegaly | <1:1,000,000 | Shah 2013176 | |
| Lymphedema-distichiasis | FOXC2 | Cardiac anomalies, cleft palate, spinal extradural cysts, distichiasis, lymphedema, large NT | Unknown | Gulati 2018177 | |
| Primary lymphedema (Emberger syndrome) | GATA2 | Bilateral lower extremity and genitalia edema | <1:1,000,000 | Ostergaard 201173 | |
| Yellow Nail Syndrome | Unknown | Chylothorax, polyhydramnios | Unknown | Nanda 201067 | |
| Skin | Sphingosine-1-phosphate lyase deficiency | SGPL1 | Ichthyosis, adrenal calcifications, gonadal defects, microcephaly, cerebellar hypoplasia | Unknown | Choi 201878 |
CC- corpus callosum, CDH- congenital diaphragmatic hernia, CNS- central nervous system, CP- cleft palate, FGR- fetal growth restriction, GI- gastrointestinal, GU- genitourinary, HLHS- hypoplastic left heart syndrome, NF- nuchal fold, NT- nuchal translucency, NTD- neural tube defect, TEF- tracheoesophageal fistula, TOF- tetralogy of Fallot
Central Nervous System
Many genetic etiologies of NIHF are associated with central nervous system (CNS) anomalies. The majority of these anomalies are not thought to be causative of NIHF; rather, they are more often a manifestation of the underlying etiology leading to hydrops. The majority of associated CNS anomalies are non-specific and are not pathognomonic for a particular genetic disorder. However, each anomaly may be part of an overall pattern of anomalies that is useful for identifying the underlying diagnosis.
Ventriculomegaly may be seen with NIHF in the setting of aneuploidy9–12 as well as with genetic disorders such as Costello syndrome and PIK3CA-associated segmental overgrowth syndromes.13,14 The presence of ventriculomegaly should also prompt the investigation of viral etiologies, which may also present with this feature.1 Neural tube defects and strawberry-shaped calvarium may be seen with trisomy 18,15,16 and posterior fossa abnormalities with trisomy 13,11 trisomy 18,10 and triploidy.17 More rare CNS anomalies that have been reported in association with NIHF include hydrocephalus with Turner syndrome,18 achondrogenesis,19 and mucopolysaccharidosis type VII;20 holoprosencephaly with trisomy 1311 and short-rib thoracic dysplasia;21 occipital encephalocele with Meckel Gruber syndrome;22,23 and microcephaly with Smith Lemli Opitz syndrome.24 Additional features of genetic syndromes reported in association with NIHF are outlined in further detail in Table 1.
Cardiovascular
Cardiac anomalies are the most common type of structural anomaly seen with NIHF, present in about 17–35% of cases.1,25 Structural cardiac abnormalities may lead to impaired venous return or other dysfunction, leading to NIHF. Associated cardiac anomalies may also be mild, and not necessarily the primary cause of the hydrops. It is particularly important to investigate for an underlying genetic abnormality that explains the presence of both the cardiac anomaly and the NIHF.
Cardiac rhabdomyomas are classically associated with tuberous sclerosis, and may present with NIHF.26 Cardiomyopathy in association with NIHF has been reported with arthrogryposis,27 mitochondrial disorders such as Barth syndrome,28 RASopathies such as Noonan and Costello syndrome,29 lysosomal storage disorders such as mucopolysaccharidosis type VII,20 and other single gene disorders.30,31 Truncus arteriosus and interrupted aortic arch can be seen with NIHF in the setting of 22q11 microdeletion syndrome.32 Pulmonary valve or supravalvular pulmonary stenosis have been well described with RASopathies, such as Noonan and Casitas B-cell lymphoma (CBL) syndrome, which are among the more common genetic syndromes underlying NIHF.33,34 Cardiac arrhythmias may also lead to NIHF, with genetic etiologies including Costello, Barth, long QT, and Wolff-Parkinson White syndromes.22,28,35–37 More rarely reported vascular disorders associated with NIHF include: Klippel-Trenaunay-Weber syndrome,93 RASA1-related disorders,38 and PIK3CA-associated segmental overgrowth syndromes.13 Further details as well as other more rare genetic etiologies of cardiovascular anomalies and NIHF are listed in Table 1.
Pulmonary and Thoracic
Pulmonary and thoracic anomalies are seen in approximately 6% of NIHF cases.1 Anomalies such as congenital pulmonary airway malformation (CPAM), congenital diaphragmatic hernia (CDH) or congenital highway airway obstruction syndrome (CHAOS), can obstruct blood flow or fetal swallowing of amniotic fluid. CPAMs have not been reported as part of specific genetic disorders, but the genes HOXB-5, FGF-7 and PDGFB have been implicated in their development.39 CDH is associated with NIHF in a number of genetic disorders, including Pallister-Killian syndrome, Fryns syndrome, and deletions such as 1p21.1p12.40–42 CHAOS has been reported with NIHF and an underlying diagnosis of VACTERL association (Vertebral defects, Anal atresia, Cardiac defects, Tracheo-Esophageal fistula, Renal anomalies, and Limb abnormalities), as well as with several rare genetic disorders.43,44 Pleural effusions are seen in association with a number of genetic etiologies, and are discussed further under the Lymphatic section below.
Gastrointestinal
Gastrointestinal (GI) anomalies are seen infrequently with NIHF, comprising 0.5–4% of cases.1 Mechanisms suggested to lead to NIHF with GI anomalies include venous congestion, decreased hepatic function, hypoalbuminemia, and intrahepatic proliferation of metabolites.1 Lysosomal storage disorders (LSD) are an increasingly recognized underlying etiology of NIHF, occurring in up to 29.6% of otherwise unexplained NIHF cases when a comprehensive work up is performed for these metabolic disorders.45 LSDs may present with fetal hepatosplenomegaly due to accumulation of metabolites, and have been reported to present with isolated ascites as well.46 Hepatomegaly has been also reported in association with NIHF and trisomy 21,47 possibly due to transient myeloproliferative disorder, and has also been reported in association with skeletal and hematologic disorders (Table 1).48–50 Duodenal atresia may be seen in trisomy 21,51 and fetal omphalocele with Beckwith Wiedemann syndrome52 or aneuploidy. Other more rarely reported GI anomalies in association with NIHF are listed in Table 1.
Genitourinary and Renal
Genitourinary (GU) and renal anomalies are seen infrequently with NIHF, reported in approximately 2–3% of cases.1 Congenital nephrosis presents with enlarged, hyperechoic kidneys and an elevated maternal serum alpha feto-protein (MSAFP), and can lead to fetal hypoproteinemia, decreased oncotic pressure, and ultimately NIHF. Fraser syndrome may present with renal agenesis and NIHF.10,43,53 NIHF has been described in association with Bartter syndrome,54 in which renal cysts may be seen prenatally. Finally, enlarged, multicystic kidneys have been reported with NIHF in the setting of both Meckel Gruber syndrome22,23 and infantile polycystic kidney disease.55 Additional details are listed in Table 1.
Musculoskeletal
Musculoskeletal anomalies are present in approximately 3–4% of NIHF cases.1 A variety of skeletal dysplasias and fetal movement disorders have been reported in association with NIHF, through hypothesized mechanisms including a small chest with increased intra-thoracic pressure and obstructed venous return, or intrahepatic proliferation of blood cell precursors due to small bone marrow volume leading to volume overload and heart failure.1
A wide variety of skeletal dysplasias have been reported in the setting of NIHF, such as thanatophoric dysplasia and achondrogenesis.19,56 Fetal movement disorders have also been associated with NIHF, including lethal multiple pterygium syndrome, fetal akinesia/hypokinesia sequence (Pena-Shokeir type I), and myotonic dystrophy.57–60 Examples of specific abnormalities of the musculoskeletal system seen in disorders associated with NIHF are: rocker bottom feet and clenched hands with trisomy 18,10 undermineralization of bone and fractures with osteogenesis imperfecta,48 shortened long bones with congenital disorders of glycosylation type 1,61 contractures with arthrogryposis,59 and limb asymmetry with PIK3CA-associated segmental overgrowth syndromes.13 Table 1 lists further details of typical features associated with skeletal dysplasias and fetal akinesia sequences associated with NIHF.
Hematologic
Hematologic disorders are a more common cause of NIHF, implicated in approximately 4–12% of cases. Causes of anemia vary, and include genetic disorders, infections, vascular malformations, and fetomaternal hemorrhage. Anemia is suggested when the middle cerebral artery peak systolic velocity is elevated, reaching 1.5 multiples of the median or higher for the gestational age.
Alpha thalassemia is common worldwide, particularly in Southeast Asian populations. Hemoglobin Bart syndrome occurs with the loss of all 4 α-globin genes, and hemoglobin H disease occurs with the loss of 3 α-globin genes, leading to a greater proportion of ineffective hemoglobin in Bart form. NIHF may be seen with both hemoglobin Bart and hemoglobin H disease, although is more common with hemoglobin Bart.62 Transient abnormal myelopoeisis may present with fetal anemia and NIHF in the setting of trisomy 21.63 Other genetic disorders that may lead to anemia and NIHF are listed in Table 1, such as Diamond Blackfan anemia,64 pyruvate kinase deficiency,50 and congenital dyserythropoietic anemias.65
Lymphatic
Lymphatic vessel dysplasia or obstruction leads to NIHF in approximately 5–6% of cases.1 Ultrasound findings may include increased nuchal translucency or nuchal fold, cystic hygroma, chylothorax, or NIHF. Cystic hygromas are particularly common in the setting of Turner syndrome,53 but are also seen with other genetic syndromes including multiple pterygium syndrome,58 generalized lymphatic dysplasia,66 and RASopathies such as Noonan syndrome.33
Another feature that is common among RASopathies is hydrothorax, chylothorax, or pleural effusion. Fetal chylothorax has also been observed with yellow nail syndrome,67 ITGA9 variants,68 and hypotrichosis-lymphedema-telangiectasia syndrome.69 Recent studies have demonstrated PIEZO1 variants to be associated with generalized lymphatic dysplasia, likely resulting from abnormal electrical currents through ion channels on the plasma membrane.66,70,71 Additional rare lymphedema syndromes reported to present with NIHF are listed in Table 1, such as hereditary lymphedema IA (Milroy disease),72 primary lymphedema (Emberger syndrome),73 and Hennekam lymphangiectasia-lymphedema syndrome.74
Skin
Although skin manifestations beyond edema are quite rare in cases of NIHF, there are several that should be mentioned. Enlargement of soft tissues in the limb as well as lipomatous truncal masses can be seen in PIK3CA-associated mutations,13 and cutaneous cystic lesions with multiple pterygium and Klippel-Trenaunay-Weber syndromes.75 Severe cases of ichthyosis have been reported in the setting of NIHF with Neu-Laxova,57,76 Gaucher,77 and sphingosine-1-phosphate lyase deficiency syndromes.78 Echogenic debris representing large sheets of sloughed skin cells may be observed on ultrasound in the amniotic fluid in these cases.
Fetal tumors
Fetal and placental tumors constitute approximately 2–7% of all NIHF cases.1,79 These tumors are highly vascular, and can lead to shunting and sequestering of blood followed by high-output cardiac failure and NIHF. Fetal teratomas are the most commonly reported, occurring in the sacrococcygeal, mediastinal, cardiac, and intracranial regions. While many teratomas result from postzygotic somatic mutations, germline mutations have also been reported.
Sacrococcygeal teratomas are an especially common cause of NIHF, and reported genetic etiologies include MNX1 variants,80 partial monosomy 7q/trisomy 2p,81 and Cornelia de Lange syndrome.82,83 Other tumors occurring with NIHF include neuroblastomas, hemangiomas, fibrosarcomas, and rhabdomyosarcomas.79 Examples of chromosome imbalances reported in association with fetal tumors and NIHF include a translocation between chromosomes 2q35 and 8q21.2 in a fetus with a cervical rhabdomyosarcoma,84 a mosaic ring X chromosome in a fetus with an epignathus teratoma,85 and a 22q11.2 deletion in a fetus with a rhabdoid tumor.86
Placenta
Many cases of NIHF are associated with placentomegaly, likely resulting from intravillous edema. On the other hand, severe polyhydramnios may compress the placenta and cause it to appear thinned or compressed.
Fetal triploidy can be associated with placentomegaly when the extra chromosomal material is diandric, or paternally inherited.12 Beckwith Wiedemann syndrome can present with NIHF and placental cysts, in addition to other structural anomalies.52 Klippel-Trenaunay-Weber syndrome may present with placental hemangiomas.75 Other infrequent placental and umbilical cord lesions that have been associated with NIHF include chorioangioma, angiomyxoma of the umbilical cord, and aneurysm of the umbilical artery.1
GENETIC EVALUATION OF NIHF
The morbidity, mortality, and recurrence risk for NIHF varies widely by underlying etiology.1,3,6 Determining the specific cause is essential in order to counsel families about prognosis and recurrence risk, guide decision-making, provide targeted antenatal care, and anticipate neonatal needs. The genetic evaluation of NIHF includes a detailed family and obstetric history, imaging to identify additional phenotypic features, and step-wise laboratory evaluations to address the spectrum of genetic and structural etiologies that contribute to NIHF. The Society for Maternal-Fetal Medicine has published guidelines for the evaluation of NIHF cases to address the heterogeneous group of potential etiologies.1 The approach to evaluation in this review is focused specifically on the range of genetic factors leading to NIHF.
Family and Obstetric History
A detailed family history is an essential component of the NIHF evaluation, and can yield important information to narrow the differential diagnosis. In particular, attention should be focused on a family history of recurrent pregnancy losses, stillbirths, hydrops, specific genetic diagnoses, developmental delay or regression, dysmorphic features, and early deaths. Detailed collection of information about prior pregnancies is also essential, with attention paid to similar details as well as imaging results and laboratory data. For living children that were affected by hydrops in utero, phenotypic data such as dysmorphic features, developmental progress, and comorbidities may also be informative. Finally, a pedigree is integral to understanding inheritance patterns of disease.
Imaging and Prenatal Phenotyping
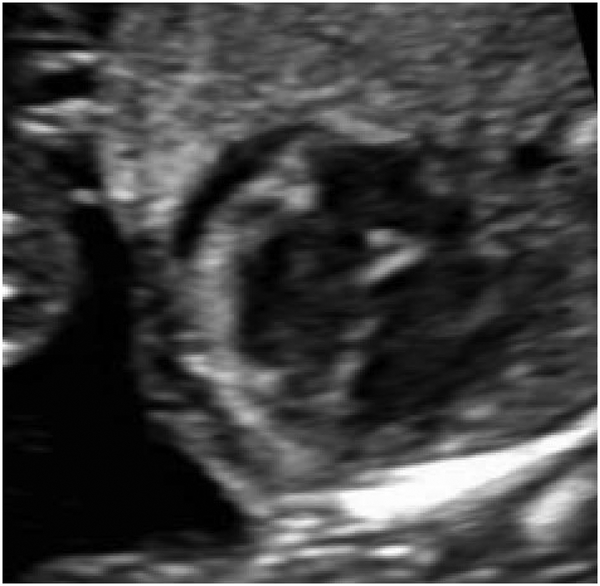
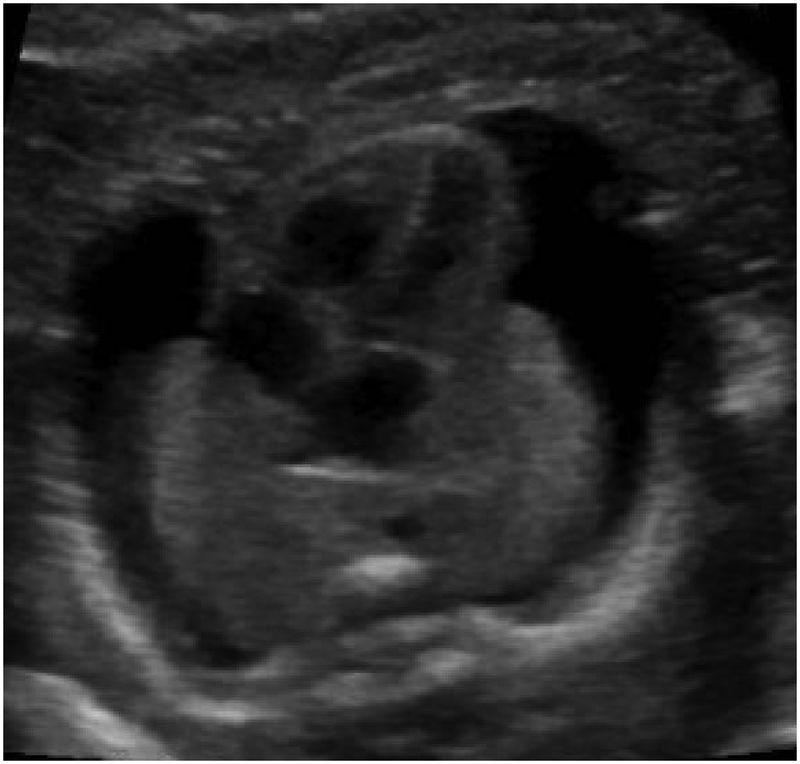
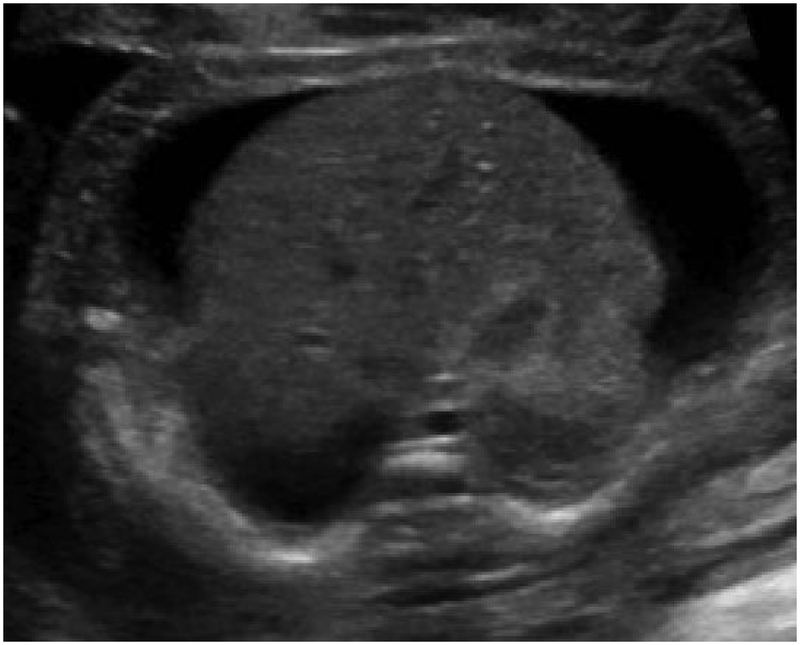
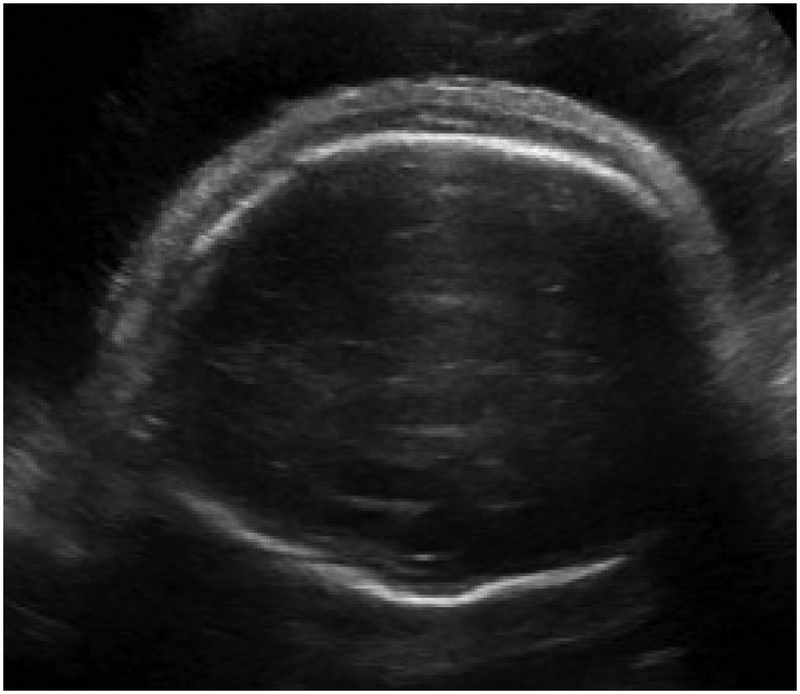
Hydrops is formally diagnosed when at least two abnormal fetal fluid collections are present, including ascites, pleural effusion, pericardial effusion, or skin edema (skin thickness > 5mm) (Figure 1).1 While polyhydramnios and placentomegaly (placental thickness ≥ 4cm in the second trimester, or ≥ 6cm in the third trimester) are often seen in conjunction with these abnormal fluid collections, they are not strictly part of the diagnostic criteria. NIHF can present at any point during pregnancy. In a recent review of 65 NIHF cases, the median gestational age at diagnosis was 22 weeks, ranging from 11 to 37 weeks.7 Early findings of NIHF may include cystic hygroma, which is commonly seen with aneuploidy. However, NIHF may occur at any point during pregnancy with a multitude of genetic disorders.
Figure 1.
Abnormal fetal fluid collections in hydrops.
(a) Pericardial effusion. (b) Pleural effusions. (c) Ascites. (d) Skin edema.
Hydrops is formally diagnosed when at least two abnormal fetal fluid collections are present, including ascites, pleural effusion, pericardial effusion, or skin edema (skin thickness > 5mm).
Obstetric ultrasound evaluation of the fetus with NIHF should be detailed and systematic, addressing each organ system, in order to detect any concurrent structural anomalies as discussed above. Because of the frequency with which cardiac anomalies are seen in conjunction with NIHF, fetal echocardiogram is additionally recommended.1 In addition to identifying structural anomalies of the heart, fetal echocardiogram is useful for detecting cardiomyopathy, high output failure, arrhythmias, and other abnormalities that may lead to NIHF. Middle cerebral artery (MCA) Doppler assessment is a critical component of the ultrasound evaluation, as elevation suggests the presence of anemia and a different subset of etiologies.1 The presence of appropriate fetal movements should be noted, to assure a fetal akinesia syndrome is not present.
As discussed above, NIHF is often not diagnosed until the second trimester of pregnancy or later.7 As a result, ultrasonographic evaluations are often performed during the second and third trimesters. However, hydrops can also be detected in the first trimester and early ultrasound can be helpful in situations where a prior pregnancy was affected with NIHF, when early evidence of hydrops is present such as with a cystic hygroma, or if structural anomalies are suspected.87–89 Additional imaging modalities such as three- and four-dimensional ultrasound, as well as magnetic resonance imagining (MRI), have been investigated as potential ways to improve prenatal diagnosis of concurrent structural anomalies.23,90,91 While the situations in which these modalities are most useful have not been defined, fetal MRI does appear promising in cases where intracranial anomalies are suspected, and may provide additional information about cortical sulcation, gyration, leukomalacia, vascular defects, and other differences.90,91
Prenatal phenotyping is key in the evaluation, meaning the identification of prenatal features unique to each case that allows providers to narrow the differential diagnosis and target further testing. In addition to information gained from imaging, prenatal phenotyping may include maternal serum screening results that suggest a specific disorder or range of disorders, such as elevated MSAFP with congenital nephrosis or ichthyoses, or low maternal serum unconjugated estriol with metabolic disorders.92,93 Accurate phenotyping can be more challenging in the prenatal setting though, and is sometimes limited to those features detectable through prenatal imaging. Subtle dysmorphic features and more minor fetal structural anomalies may be missed with standard prenatal ultrasound. Some anomalies become evident in later gestational ages, genetic disorders with variable expressivity or incomplete penetrance may present differently and thus be missed, and prenatal imaging cannot predict certain features such as developmental delay or intellectual disability.8,94,95 Finally, an important component of phenotyping is fetal autopsy in cases for which a live birth is not the outcome. Fetal autopsy can supplement other diagnostic tests by confirming the presence of structural anomalies and other features, as well as by identifying additional anomalies that were not previously recognized on prenatal imaging.96–98 Autopsy is further discussed in the Pathology section below.
Importantly, structural anomalies of the fetus are often cited as the underlying cause of the NIHF. However, in many cases where fetal structural anomalies are observed in conjunction with NIHF, it is unclear whether the structural anomaly itself is truly causative of the NIHF. Further, the genetic or environmental etiology of the structural anomaly itself remains unexplained in many of these cases.7 As a result, genetic testing is an essential component of the NIHF evaluation.
Biochemical, Cytogenetic and Molecular genetic testing
A thorough approach to genetic testing is integral to establishing a diagnosis in cases of NIHF. The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend that chromosomal microarray (CMA) is offered in place of karyotype as the primary genetic test for patients undergoing prenatal diagnosis for a fetal structural abnormality seen on ultrasound examination.99,100 CMA has greater resolution than karyotype, and is able to detect copy number variants (CNVs), submicroscopic deletions and duplications (as small as 50 – 100 kb), that are much smaller than seen with karyotype.99 A large cohort study demonstrated an incremental yield of 6% when CMA was used for establishing a genetic diagnosis in cases of fetal structural anomalies.101
The yield of CMA for cases of NIHF, however, has not specifically been investigated, but may be in the range of 6 to 14% based on cohort studies of all anomalies.7,107 Also unclear is how much CMA adds to the diagnostic yield of karyotype for NIHF cases beyond detection of aneuploidy.7 This likely reflects the heterogeneous group of genetic etiologies that underlie NIHF, including single gene disorders that CMA is not able to detect. For example, 19% of all NIHF cases have been attributed to rare genetic syndromes and nearly one-third of idiopathic NIHF cases to LSDs,45,103 the vast majority of which are not detected by CMA. Despite these limitations, karyotype and CMA are still useful first-line tests for evaluation, given the 7–17% of NIHF cases that result from chromosomal abnormalities or copy number variants that are detectable with these tests.1,7
As discussed above, the constellation of affected organ systems and other phenotypic data may suggest a more specific genetic disorder or category. In such situations, testing strategies may include single gene sequencing or genotyping, multiple gene sequencing or genotyping through panels, or testing for specific deletions or duplications as appropriate for the disorders under consideration. For example, RASopathies such as Noonan syndrome and Costello syndrome may present with NIHF and other lymphatic disturbances.104 When features such as increased nuchal translucency, NIHF, polyhydramnios, and cardiac defects are seen, genetic sequencing for genes such as PTPN11, KRAS, and RIT1 associated with the RASopathies should be considered.33,104,105 Occasionally, the family or obstetric history will include prior identification of a specific variant, in which case targeted genotyping for the familial variant may be performed if consistent with the presentation in the currently affected pregnancy.
Some commercial laboratories offer gene panels to analyze a group of genes implicated in NIHF development, although the yield of these panels has not been well-studied. In one retrospective review, next-generation sequencing was performed for 41 genes associated with inborn errors of metabolism and NIHF, which led to a diagnosis in 12.5% of cases sequenced.106 Genes included on commercially available panels vary from one to the next, and additional research is needed to determine which genes should be included.
Enzyme assays and other biochemical tests to assess for LSDs are also available, although limitations include availability on a commercial basis for prenatal samples, standardized protocols for analysis, handling of isoenzymes, and prenatal reference ranges for interpretation.107,108 A systematic review of NIHF publications from 1979 to 2014 found that LSDs were reported to underlie NIHF in 5.2% cases, but that when a comprehensive work up was completed for this category of disorders, 29.6% of idiopathic cases were attributable to a LSD.45 LSDs are thus an important consideration in the differential diagnosis of NIHF, although the clinical availability of prenatal enzyme assays has decreased in lieu of molecular testing given the limitations noted above. However, it is important to note that enzyme assays may still provide important information to establish a diagnosis in situations such as when a variant of uncertain significance is identified in a gene of interest.
Whole exome sequencing (WES) has become more widely used in the prenatal setting in recent years, and has the potential to yield clinically useful information about underlying genetic variants. WES sequences the exome, or the protein-coding regions of the genome. Prenatal phenotyping is an essential component of WES interpretation, and availability of detailed phenotypic data significantly improves the diagnostic yield.70,109,110 WES has not been applied specifically to a cohort of NIHF cases, although it has been used in studies considering a range of fetal anomalies including NIHF. In cohorts of all prenatal anomalies, WES has demonstrated a clear or likely diagnostic yield in 9 to 47% of cases when microarray and/or karyotype are normal.70,109,111–113 A recently published large prospective cohort study demonstrated a yield of 8.5% for diagnostic genetic variants in fetuses with a variety of structural anomalies, and the diagnostic yield specifically for the NIHF cases within this cohort was 9%.113 Finally, among a cohort of deceased fetuses with anomalies undergoing WES, 41% of cases with pathogenic variants and 31% of cases with variants in candidate genes had hydrops.114
For cases in which clinically relevant variants are not found, re-analysis is cost-effective at an interval of 12 months in postnatal settings,115 although the optimal interval for re-analysis based primarily on prenatal phenotypic data remains unclear. Limitations of WES include that it is not designed to detect CNVs or sequence intronic regions, and it relies on adequate probe density as well as accurate phenotypic data.116 While WES has not yet been studied specifically in a cohort of NIHF cases to determine its diagnostic utility or cost effectiveness, it should be considered for cases in which a genetic etiology is suspected but standard testing is unrevealing.117
Pathology
Evaluation of the placenta as well as fetal autopsy through detailed pathology examination may yield further important data for establishing a diagnosis in cases of NIHF. One study found that fetal autopsy identified a definitive cause in 58% of NIHF cases.96 Chromosome abnormalities were the most frequent etiology, followed by single structural anomalies, multiple structural anomalies, and lysosomal storage disorders. Specialized techniques such as immunohistochemical staining to detect lymphatic dysplasia can significantly improve the ability of autopsy to establish a diagnosis.118 Detailed examination of the placenta may also identify changes that suggest a cause of the NIHF, such as placental chorioangioma, vacuolization of placental cells as seen with LSDs, or lymphedema of the villi associated with genetic abnormalities or congenital anomalies.119–121
MANAGEMENT
Central to developing a management plan for pregnancies with NIHF is determining the underlying cause. For some genetic disorders, interventions to improve outcomes are not available either before or after birth. However, for others, antenatal or postnatal interventions are available, sometimes with greater effects on outcomes the earlier an intervention is initiated.
A discussion of the options for in utero intervention for the range of fetal anomalies associated with NIHF is beyond the scope of this paper. However, examples of situations in which in utero interventions may be applied include maternal antiarrhythmic medications for fetal arrhythmias, intrauterine transfusions for genetic disorders causing fetal anemia, and drainage or shunt placement for fetal pleural effusion or chylothorax. Further, in utero enzyme replacement therapy (ERT), as well as in utero stem cell transplantation and gene therapy, are active areas of research that have the potential to improve the in utero and neonatal course in cases of NIHF.122–125
When determining an overall management plan for pregnancies with NIHF, it is essential to factor in the underlying cause and anticipated prognosis, so the options presented and decisions made are consistent with a realistic assessment of the outcome. Cases with a diagnosis of a serious or potentially lethal genetic syndrome should be offered termination, or a discussion of comfort care if termination is not pursued. For continuing pregnancies, cases in which in utero intervention is available and desired should be referred to an institution with appropriate expertise. More general recommendations for timing and mode of delivery with NIHF, as well as antenatal testing and other considerations, have been outlined in guidelines published by the Society for Maternal-Fetal Medicine.1 Discussions for continuing pregnancies with NIHF should further include plans for monitoring during labor, location of delivery, mode of delivery, specialists needed at delivery, and neonatal resuscitation.
In some cases with identified genetic disorders, prompt initiation of an established treatment protocol, or enrollment in a research study protocol, may be beneficial for the outcomes of the affected neonate. For example, neonates with NIHF resulting from an arrhythmia can be managed medically and surveilled for cardiac events that impose risk. Protocols to screen for malignancies can be implemented early on for cases of NIHF resulting from PIK3CA-associated segmental overgrowth syndromes or other syndromes associated with greater risk of malignant transformation. Enzyme replacement therapy can be efficiently prepared and administered in neonates with NIHF resulting from a LSD such as mucopolysaccharidosis type VII, when a postnatal treatment is available. Individuals with LSDs in particular are known to benefit from early diagnosis and initiation of treatment after birth, in order to mitigate some of the more severe manifestations of disease.126
CONCLUSION
It is well known that a heterogeneous group of underlying genetic disorders may lead to NIHF. A systematic approach is warranted for the evaluation of each case, considering personal and family history, concurrent structural anomalies, additional phenotypic features, and genetic testing as well as pathology examinations. Particularly important to consider are the unique phenotypic features of each case, as these may guide the approach to genetic testing and the interpretation of testing in many scenarios. Establishing a genetic diagnosis allows providers to improve the care for these fetuses and neonates by more accurately characterizing prognosis, anticipating neonatal needs, and implementing available treatments earlier. Moving forward, research to improve prenatal phenotyping, identify the underlying genetic cause in idiopathic cases, and revise the algorithm for genetic evaluation will be instrumental for better understanding and managing NIHF.
What is already known about this topic?
A wide spectrum of genetic etiologies may lead to non-immune hydrops fetalis. A thorough genetic evaluation is essential to determine the underlying etiology, optimally manage these pregnancies, and inform discussions about prognosis. Considering the unique phenotypic features in each case can aid in establishing the underlying genetic diagnosis.
What does this study add?
This review provides a systematic approach to the genetic evaluation of NIHF, focusing on structural anomalies by system and the genetic underpinnings of disease. This allows providers to approach each NIHF case in a way that considers the unique constellation of phenotypic features by organ system, and guides a targeted approach to genetic evaluation, diagnosis, and management.
Acknowledgments
Funding and disclosures: T.N.S. is supported by grant 5K12HD001262–18 from the National Institutes of Health (NIH). The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. T.N.S. is also supported by a grant from the Fetal Health Foundation. Ultragenyx has provided financial support for studies conducted through the UCSF Center for Maternal–Fetal Precision Medicine. M.E.N. is a consultant to Invitae and has received research funding from Natera, but this funding was not applied to this study. The other authors declare no conflicts of interest.
REFERENCES
- 1.Norton ME, Chauhan SP, Dashe JS. Society for maternal-fetal medicine (SMFM) clinical guideline #7: Nonimmune hydrops fetalis. Am J Obstet Gynecol 2015;212(2):127–39. [DOI] [PubMed] [Google Scholar]
- 2.Gedikbasi A, Oztarhan K, Gunenc Z, et al. Preeclampsia due to fetal non-immune hydrops: Mirror syndrome and review of literature. Hypertens. Pregnancy. 2011;30(3):322–30. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima K, Morokuma S, Fujita Y, et al. Short-term and long-term outcomes of 214 cases of non-immune hydrops fetalis. Early Hum Dev 2011;87(8):571–5. [DOI] [PubMed] [Google Scholar]
- 4.Derderian SC, Jeanty C, Fleck SR, et al. The many faces of hydrops. J Pediatr Surg 2015;50(1) 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nassr AA, Ness A, Hosseinzadeh P, et al. Outcome and Treatment of Antenatally Diagnosed Nonimmune Hydrops Fetalis. Fetal Diagn Ther 2018;43(2) 123–128. [DOI] [PubMed] [Google Scholar]
- 6.Steurer MA, Peyvandi S, Baer RJ, et al. Epidemiology of Live Born Infants with Nonimmune Hydrops Fetalis—Insights from a Population-Based Dataset. J Pediatr 2017;(187)182–188.e3. [DOI] [PubMed] [Google Scholar]
- 7.Sparks TN, Thao K, Lianoglou BR, et al. Nonimmune hydrops fetalis: identifying the underlying genetic etiology. Genet Med 2018;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best S, Wou K, Vora N, Van der Veyver IB, Wapner R, Chitty LS. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat Diagn 2018;38(1) 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith-Bindman R, Hosmer W. Second-trimester ultrasound to detect fetuses with Down syndrome. JAMA J … 2001;285(8):1044–55. [DOI] [PubMed] [Google Scholar]
- 10.Bronsteen R, Lee W, Vettraino IM, Huang R, Comstock CH. Second-Trimester Sonography and Trisomy 18. J Ultrasound Med 2004;23(2):233–40. [DOI] [PubMed] [Google Scholar]
- 11.Szigeti Z, Csapó Z, Joó JG, Pete B, Papp Z, Papp C. Correlation of prenatal ultrasound diagnosis and pathologic findings in fetuses with trisomy 13. Prenat Diagn 2006;26(13):1262–6. [DOI] [PubMed] [Google Scholar]
- 12.Massalska D, Bijok J, Lnicka A, Jakiel G, Roszkowski T. Triploidy – variability of sonographic phenotypes. Prenat Diagn 2017;37(8):774–80. [DOI] [PubMed] [Google Scholar]
- 13.Emrick LT, Murphy L, Shamshirsaz AA, et al. Prenatal diagnosis of CLOVES syndrome confirmed by detection of a mosaic PIK3CA mutation in cultured amniocytes. Am J Med Genet Part A 2014;164(10):2633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin AE, O’Brien B, Demmer LA, et al. Prenatal features of Costello syndrome: ultrasonographic findings and atrial tachycardia. Prenat Diagn 2009;29(7):682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolaides KH, Salvesen DR, Snijders RJ, Gosden CM. Strawberry-shaped skull in fetal trisomy 18. Fetal Diagn Ther 1992;7(2):132–7. [DOI] [PubMed] [Google Scholar]
- 16.Rosa RFM, Trevisan P, Rosa RCM, et al. Trisomy 18 and neural tube defects. Pediatr Neurol 2013;49(3):203–4. [DOI] [PubMed] [Google Scholar]
- 17.Zalel Y, Shapiro I, Weissmann-Brenner A, Berkenstadt M, Leibovitz Z, Bronshtein M. Prenatal sonographic features of triploidy at 12–16 weeks. Prenat Diagn 2016;36(7):650–5. [DOI] [PubMed] [Google Scholar]
- 18.Kalpatthi R, Lieber E, Rajegowda B, Sharma J. Hydrocephalus in a hydropic fetus with Turner syndrome: a rare association. J Matern Fetal Neonatal Med 2003;14(2):136–8. [DOI] [PubMed] [Google Scholar]
- 19.Pretorius DH, Rumack CM, Manco-Johnson ML, et al. Specific skeletal dysplasias in utero: sonographic diagnosis. Radiology 1986;159(1):237–42. [DOI] [PubMed] [Google Scholar]
- 20.Walter-Nicolet E, Rakza T, Storme L, et al. A new case of mucopolysaccharidosis VII presenting as non immune hydrops fetalis. Eur J Pediatr 2003;162(7–8):520–1. [DOI] [PubMed] [Google Scholar]
- 21.Passarge E Familial occurrence of a short rib syndrome with hydrops fetalis but without polydactyly. Am J Med Genet 1983;14(2):403–5. [DOI] [PubMed] [Google Scholar]
- 22.Abrams ME, Meredith KS, Kinnard P, Clark RH. Hydrops fetalis: a retrospective review of cases reported to a large national database and identification of risk factors associated with death. Pediatrics 2007;120(1):84–9. [DOI] [PubMed] [Google Scholar]
- 23.Barišić LS, Stanojević M, Kurjak A, Porović S, Gaber G. Diagnosis of fetal syndromes by three- and four-dimensional ultrasound: is there any improvement? J. Perinat. Med. 2017;45(6):651–665. [DOI] [PubMed] [Google Scholar]
- 24.Maymon R, Ogle RF, Chitty LS. Smith-Lemli-Opitz syndrome presenting with persisting nuchal oedema and non-immune hydrops. Prenat Diagn 1999;19(2):105–7. [DOI] [PubMed] [Google Scholar]
- 25.Bellini C, Donarini G, Paladini D, et al. Etiology of non-immune hydrops fetalis: An update. Am J Med Genet Part A 2015;167(5):1082–8. [DOI] [PubMed] [Google Scholar]
- 26.Pruksanusak N, Suntharasaj T, Suwanrath C, Phukaoloun M, Kanjanapradit K. Fetal cardiac rhabdomyoma with hydrops fetalis: report of 2 cases and literature review. J Ultrasound Med 2012;31(11):1821–4. [DOI] [PubMed] [Google Scholar]
- 27.Stassou S, Nadroo A, Schubert R, Chin S, Gudavalli M. A new syndrome of myopathy with muscle spindle excess. J Perinat Med 2005;33(2):179–82. [DOI] [PubMed] [Google Scholar]
- 28.Steward CG, Newbury-Ecob RA, Hastings R, et al. Barth syndrome: an X-linked cause of fetal cardiomyopathy and stillbirth. Prenat Diagn 2010;30(10):970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz S, Petersen C, Kordaß U, Seidel H, Zenker M, Kutsche K. Two cases with severe lethal course of Costello syndrome associated with HRAS p.G12C and p.G12D. Eur J Med Genet 2012;55(11):615–9. [DOI] [PubMed] [Google Scholar]
- 30.Hempel M, Casar Tena T, Diehl T, et al. Compound heterozygous GATA5 mutations in a girl with hydrops fetalis, congenital heart defects and genital anomalies. Hum Genet 2017;136(3):339–46. [DOI] [PubMed] [Google Scholar]
- 31.Almomani R, Verhagen JMA, Herkert JC, et al. Biallelic Truncating Mutations in ALPK3 Cause Severe Pediatric Cardiomyopathy. J Am Coll Cardiol 2016;67(5):515–25. [DOI] [PubMed] [Google Scholar]
- 32.Machlitt A, Tennstedt C, Korner H, Bommer C, Chaoui R. Prenatal diagnosis of 22q11 microdeletion in an early second-trimester fetus with conotruncal anomaly presenting with increased nuchal translucency and bilateral intracardiac echogenic foci. Ultrasound Obstet Gynecol 2002;19(5):510–3. [DOI] [PubMed] [Google Scholar]
- 33.Croonen EA, Nillesen WM, Stuurman KE, et al. Prenatal diagnostic testing of the Noonan syndrome genes in fetuses with abnormal ultrasound findings. Eur J Hum Genet 2013;21(9):936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bülow L, Lissewski C, Bressel R, et al. Hydrops, fetal pleural effusions and chylothorax in three patients with CBL mutations. Am J Med Genet A 2015;167A(2):394–9. [DOI] [PubMed] [Google Scholar]
- 35.Lin AE, Grossfeld PD, Hamilton RM, et al. Further delineation of cardiac abnormalities in Costello syndrome. Am J Med Genet 2002;111(2):115–29. [DOI] [PubMed] [Google Scholar]
- 36.Komarlu R, Beerman L, Freeman D, Arora G. Fetal and neonatal presentation of long QT syndrome. Pacing Clin Electrophysiol 2012;35(4). [DOI] [PubMed] [Google Scholar]
- 37.Hoeffler CD, Krenek ME, Brand MC. Wolff-Parkinson-White Syndrome in a Term Infant Presenting With Cardiopulmonary Arrest. Adv Neonatal Care 2016;16(1):44–51. [DOI] [PubMed] [Google Scholar]
- 38.Overcash RT, Gibu CK, Jones MC, Ramos GA, Andreasen TS. Maternal and fetal capillary malformation-arteriovenous malformation (CM-AVM) due to a novel RASA1 mutation presenting with prenatal non-immune hydrops fetalis. Am J Med Genet Part A 2015;167(10):2440–3. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RD, Hedrick HL, Liechty KW, et al. Cystic adenomatoid malformation of the lung: review of genetics, prenatal diagnosis, and in utero treatment. Am J Med Genet A 2006;140(2):151–5. [DOI] [PubMed] [Google Scholar]
- 40.Doray B, Girard-Lemaire F, Gasser B, et al. Pallister-Killian syndrome: Difficulties of prenatal diagnosis. Prenat Diagn 2002;22(6):470–7. [DOI] [PubMed] [Google Scholar]
- 41.Ramsing M, Gillessen-Kaesbach G, Holzgreve W, Fritz B, Rehder H. Variability in the phenotypic expression of Fryns syndrome: A report of two sibships. Am J Med Genet 2000;95(5):415–24. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim M, Hunter M, Gugasyan L, et al. Interstitial deletion of chromosome 1 (1p21.1p12) in an infant with congenital diaphragmatic hernia, hydrops fetalis, and interrupted aortic arch. Clin Case Reports 2017;5(2):164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tessier A, Sarreau M, Pelluard F, et al. Fraser syndrome: features suggestive of prenatal diagnosis in a review of 38 cases. Prenat Diagn 2016;36(13):1270–5. [DOI] [PubMed] [Google Scholar]
- 44.Sanford E, Saadai P, Lee H, Slavotinek A. Congenital high airway obstruction sequence (CHAOS): A new case and a review of phenotypic features. Am J Med Genet Part A 2012;158 A(12):3126–36. [DOI] [PubMed] [Google Scholar]
- 45.Gimovsky AC, Luzi P, Berghella V. Lysosomal storage disease as an etiology of nonimmune hydrops. Am J Obstet Gynecol 2015;212(3):281–90. [DOI] [PubMed] [Google Scholar]
- 46.Gillan JE, Lowden JA, Gaskin K, Cutz E. Congenital ascites as a presenting sign of lysosomal storage disease. J Pediatr 1984;104(2):225–31. [DOI] [PubMed] [Google Scholar]
- 47.Hojo S, Tsukimori K, Kitade S, et al. Prenatal sonographic findings and hematological abnormalities in fetuses with transient abnormal myelopoiesis with Down syndrome. Prenat Diagn 2007;27(6):507–11. [DOI] [PubMed] [Google Scholar]
- 48.Mathur BP, Karan S. Non-immune hydrops fetalis due to osteopetrosis congenita. Indian Pediatr 1984;21(8):651–3. [PubMed] [Google Scholar]
- 49.Konstantinidou A, Karadimas C, Waterham HR, et al. Pathologic, radiographic and molecular findings in three fetuses diagnosed with HEM/Greenberg skeletal dysplasia. Prenat Diagn 2008;28(4):309–12. [DOI] [PubMed] [Google Scholar]
- 50.Arcasoy MO, Gallagher PG. Hematologic disorders and nonimmune hydrops fetalis. Semin Perinatol 1995;19(6):502–15. [DOI] [PubMed] [Google Scholar]
- 51.Nyberg DA, Resta RG, Luthy DA, Hickok DE, Mahony BS, Hirsch JH. Prenatal sonographic findings of Down syndrome: review of 94 cases. Obstet Gynecol 1990;76(3 Pt 1):370–7. [PubMed] [Google Scholar]
- 52.Hillstrom MM, Brown DL, Wilkins-Haug L, Genest DR. Sonographic appearance of placental villous hydrops associated with Beckwith-Wiedemann syndrome. J Ultrasound Med 1995;14(1):61–4. [DOI] [PubMed] [Google Scholar]
- 53.Papp C, Beke A, Mezei G, Szigeti Z, Bán Z, Papp Z. Prenatal diagnosis of Turner syndrome: report on 69 cases. J Ultrasound Med 2006;25(6):711–7; quiz 718–20. [DOI] [PubMed] [Google Scholar]
- 54.Çetinkaya M, Durmaz O, Büyükkale G, et al. Neonatal Bartter syndrome and unilateral ectopic renal cyst as new renal causes of hydrops fetalis: two case reports and review of the literature. J Matern Fetal Neonatal Med 2013;26(11):1147–50. [DOI] [PubMed] [Google Scholar]
- 55.Van Maldergem L, Jauniaux E, Fourneau C, Gillerot Y. Genetic causes of hydrops fetalis. Pediatrics 1992;89(1):81–6. [PubMed] [Google Scholar]
- 56.Heinrich T, Nanda I, Rehn M, et al. Co-Occurence of Reciprocal Translocation and COL2A1 Mutation in a Fetus with Severe Skeletal Dysplasia: Implications for Genetic Counseling. Cytogenet Genome Res 2015;145(1):25–8. [DOI] [PubMed] [Google Scholar]
- 57.Jauniaux E, Van Maldergem L, De Munter C, Moscoso G, Gillerot Y. Nonimmune hydrops fetalis associated with genetic abnormalities. Obstet Gynecol 1990;75(3 Pt 2):568–72. [PubMed] [Google Scholar]
- 58.Chen CP. Prenatal diagnosis and genetic analysis of fetal akinesia deformation sequence and multiple pterygium syndrome associated with neuromuscular junction disorders: A review. Taiwan J Obstet Gynecol 2012;51(1):12–7. [DOI] [PubMed] [Google Scholar]
- 59.Gupta P, Sharma JB, Sharma R, Gadodia A, Kumar S, Roy KK. Antenatal ultrasound and MRI findings of Pena-Shokeir syndrome. Arch Gynecol Obstet 2011;283(SUPPL. 1):27–9. [DOI] [PubMed] [Google Scholar]
- 60.Stratton RF, Patterson RM. DNA confirmation of congenital myotonic dystrophy in non‐immune hydrops fetalis. Prenat Diagn 1993;13(11):1027–30. [DOI] [PubMed] [Google Scholar]
- 61.Kranz C, Basinger AA, Güçsavaş-Calikoğlu M, et al. Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am J Med Genet A 2007;143A(12):1371–8. [DOI] [PubMed] [Google Scholar]
- 62.Chui DH, Waye JS. Hydrops fetalis caused by alpha-thalassemia: an emerging health care problem. Blood 1998;91(7):2213–22. [PubMed] [Google Scholar]
- 63.Hendricks SK, Sorensen TK, Baker ER. Trisomy 21, fetal hydrops, and anemia: prenatal diagnosis of transient myeloproliferative disorder? Obstet Gynecol 1993;82(4 Pt 2 Suppl):703–5. [PubMed] [Google Scholar]
- 64.Dunbar AE, Moore SL, Hinson RM. Fetal Diamond-Blackfan Anemia Associated with Hydrops Fetalis. Am J Perinatol 2003;20(7):391–4. [DOI] [PubMed] [Google Scholar]
- 65.Tamary H, Dgany O. Congenital Dyserythropoietic Anemia Type I. 2009 Apr 21 [updated 2016 Aug 25] In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A editors. [Internet]. GeneReviews®. 2009;Available from: http://www.ncbi.nlm.nih.gov/pubmed/20301759 [Google Scholar]
- 66.Fotiou E, Martin-Almedina S, Simpson MA, et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat Commun 2015;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanda A, Al-Essa FH, El-Shafei WM, Alsaleh QA. Congenital yellow nail syndrome: a case report and its relationship to nonimmune fetal hydrops. Pediatr Dermatol 27(5):533–4. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y-S, Ma G-C, Shih J-C, et al. Experimental treatment of bilateral fetal chylothorax using in-utero pleurodesis. Ultrasound Obstet Gynecol 2012;39(1):56–62. [DOI] [PubMed] [Google Scholar]
- 69.Irrthum A, Devriendt K, Chitayat D, et al. Mutations in the Transcription Factor Gene SOX18 Underlie Recessive and Dominant Forms of Hypotrichosis-Lymphedema-Telangiectasia. Am J Hum Genet 2003;72(6):1470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vora NL, Powell B, Brandt A, et al. Prenatal exome sequencing in anomalous fetuses: New opportunities and challenges. Genet Med 2017;19(11):1207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Datkhaeva I, Arboleda VA, Senaratne TN, et al. Identification of novel PIEZO1 variants using prenatal exome sequencing and correlation to ultrasound and autopsy findings of recurrent hydrops fetalis. Am J Med Genet A 2018;176(12):2829–34. [DOI] [PubMed] [Google Scholar]
- 72.Gordon K, Spiden SL, Connell FC, et al. FLT4/VEGFR3 and Milroy Disease: Novel Mutations, a Review of Published Variants and Database Update. Hum Mutat 2013;34(1):23–31. [DOI] [PubMed] [Google Scholar]
- 73.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 2011;43(10):929–31. [DOI] [PubMed] [Google Scholar]
- 74.Bellini C, Mazzella M, Arioni C, et al. Hennekam syndrome presenting as nonimmune hydrops fetalis, congenital chylothorax, and congenital pulmonary lymphangiectasia. Am J Med Genet 2003;120 A(1):92–6. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka K, Miyazaki N, Matsushima M, et al. Prenatal diagnosis of Klippel-Trenaunay-Weber syndrome with Kasabach-Merritt syndrome in utero. J Med Ultrason (2001) 2015;42(1):109–12. [DOI] [PubMed] [Google Scholar]
- 76.Mattos EP, da Silva AA, Magalhães JAA, et al. Identification of a premature stop codon mutation in the PHGDH gene in severe Neu-Laxova syndrome-evidence for phenotypic variability. Am J Med Genet Part A 2015;167(6):1323–9. [DOI] [PubMed] [Google Scholar]
- 77.Beaujot J, Joriot S, Dieux A, et al. Phenotypic variability of prenatally presenting Gaucher’s disease. Prenat Diagn 2013;33(10):1004–6. [DOI] [PubMed] [Google Scholar]
- 78.Choi Y-J, Saba JD. Sphingosine phosphate lyase insufficiency syndrome (SPLIS): A novel inborn error of sphingolipid metabolism. Adv Biol Regul 2019;71:128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isaacs H Fetal hydrops associated with tumors. Am J Perinatol 2008;25(1):43–68. [DOI] [PubMed] [Google Scholar]
- 80.Lin Y-H, Huang R-L, Lai H-C. Presacral teratoma in a Curarrino syndrome woman with an unreported insertion in MNX1 gene. Taiwan J Obstet Gynecol 2011;50(4):512–4. [DOI] [PubMed] [Google Scholar]
- 81.Le Caignec C, Winer N, Boceno M, et al. Prenatal diagnosis of sacrococcygeal teratoma with constitutional partial monosomy 7q/trisomy 2p. Prenat Diagn 2003;23(12):981–4. [DOI] [PubMed] [Google Scholar]
- 82.Banait N, Fenton A, Splitt M. Cornelia de Lange syndrome due to mosaic NIPBL mutation: antenatal presentation with sacrococcygeal teratoma. BMJ Case Rep 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toomayan GA, Gaca AM. Aberrant course of the umbilical vein in a newborn with Cornelia de Lange syndrome. Pediatr Radiol 2009;39(4):406–8. [DOI] [PubMed] [Google Scholar]
- 84.Yoshino K, Takeuchi M, Nakayama M, Suehara N. Congenital cervical rhabdomyosarcoma arising in one fetus of a twin pregnancy. Fetal Diagn Ther 2005;20(4):291–5. [DOI] [PubMed] [Google Scholar]
- 85.Witters I, Moerman P, Louwagie D, Van Assche FA, Migeon BR, Fryns JP. Second trimester prenatal diagnosis of epignathus teratoma in ring X chromosome mosaicism with inactive ring X chromosome. Ann Genet 2001;44(4):179–82. [DOI] [PubMed] [Google Scholar]
- 86.White FV, Dehner LP, Belchis DA, et al. Congenital disseminated malignant rhabdoid tumor: a distinct clinicopathologic entity demonstrating abnormalities of chromosome 22q11. Am J Surg Pathol 1999;23(3):249–56. [DOI] [PubMed] [Google Scholar]
- 87.Edwards L, Hui L. First and second trimester screening for fetal structural anomalies. Semin. Fetal Neonatal Med. 2018; [DOI] [PubMed] [Google Scholar]
- 88.Karim JN, Roberts NW, Salomon LJ, Papageorghiou AT. Systematic review of first-trimester ultrasound screening for detection of fetal structural anomalies and factors that affect screening performance. Ultrasound Obstet Gynecol 2017;50(4):429–41. [DOI] [PubMed] [Google Scholar]
- 89.Kenkhuis MJA, Bakker M, Bardi F, et al. Effectiveness of 12–13-week scan for early diagnosis of fetal congenital anomalies in the cell-free DNA era. Ultrasound Obstet Gynecol 2018;51(4):463–9. [DOI] [PubMed] [Google Scholar]
- 90.Gonçalves LF, Lee W, Mody S, Shetty A, Sangi-Haghpeykar H, Romero R. Diagnostic accuracy of ultrasonography and magnetic resonance imaging for the detection of fetal anomalies: a blinded case-control study. Ultrasound Obstet Gynecol 2016;48(2):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossi AC, Prefumo F. Additional value of fetal magnetic resonance imaging in the prenatal diagnosis of central nervous system anomalies: A systematic review of the literature. Ultrasound Obstet Gynecol 2014;44(4):388–93. [DOI] [PubMed] [Google Scholar]
- 92.Kallinen J, Heinonen S, Ryynänen M, Pulkkinen L, Mannermaa A. Antenatal genetic screening for congenital nephrosis. Prenat Diagn 2001;21(2):81–4. [DOI] [PubMed] [Google Scholar]
- 93.Jari SD, Fraer LM, Hogge WA. Association of undetectable unconjugated estriol on multiple marker screening with steroid sulfatase deficiency. Fetal Diagn Ther 19(1):43–8. [DOI] [PubMed] [Google Scholar]
- 94.Rossi AC, Prefumo F. Correlation between fetal autopsy and prenatal diagnosis by ultrasound: A systematic review. Eur J Obstet Gynecol Reprod Biol 2017;210:201–6. [DOI] [PubMed] [Google Scholar]
- 95.Kaasen A, Tuveng J, Heiberg A, Scott H, Haugen G. Correlation between prenatal ultrasound and autopsy findings: A study of second-trimester abortions. Ultrasound Obstet Gynecol 2006;28(7):925–33. [DOI] [PubMed] [Google Scholar]
- 96.Puri RD, Kotecha U, Lall M, Dash P, Bijarnia-Mahay S, Verma IC. Is the diagnostic yield influenced by the indication for fetal autopsy? Am J Med Genet Part A 2016;170(8):2119–26. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez MM, Chaves F, Romaguera RL, Ferrer PL, de la Guardia C, Bruce JH. Value of autopsy in nonimmune hydrops fetalis: series of 51 stillborn fetuses. Pediatr Dev Pathol 5(4):365–74. [DOI] [PubMed] [Google Scholar]
- 98.Bellini C, Hennekam RCM, Fulcheri E, et al. Etiology of nonimmune hydrops fetalis: A systematic review. Am J Med Genet Part A 2009;149(5):844–51. [DOI] [PubMed] [Google Scholar]
- 99.Dugoff L, Norton ME, Kuller JA. The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol 2016;215(4):B2–9. [DOI] [PubMed] [Google Scholar]
- 100.Practice Bulletin No. 162: Prenatal Diagnostic Testing for Genetic Disorders. Obstet Gynecol 2016;127(5)e:108–e122. [DOI] [PubMed] [Google Scholar]
- 101.Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. Obstet Gynecol Surv 2013;68(4):276–8. [Google Scholar]
- 102.Parchem JG, Sparks TN, Gosnell K, Norton ME. Utility of chromosomal microarray in anomalous fetuses. Prenat Diagn 2018;38(2):140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moreno CA, Kanazawa T, Barini R, et al. Non-immune hydrops fetalis: A prospective study of 53 cases. Am J Med Genet A 2013;161A(12):3078–86. [DOI] [PubMed] [Google Scholar]
- 104.Myers A, Bernstein Ja Fau - Brennan M-L, Brennan Ml Fau - Curry C, et al. Perinatal features of the RASopathies: Noonan syndrome, Cardiofaciocutaneous syndrome and Costello syndrome. LID - 10.1002/ajmg.a.36737 [doi]. Am J Med Genet A 2014;164A(11):2814–21. [DOI] [PubMed] [Google Scholar]
- 105.Bakker M, Pajkrt E, Mathijssen IB, Bilardo CM. Targeted ultrasound examination and DNA testing for Noonan syndrome, in fetuses with increased nuchal translucency and normal karyotype. Prenat Diagn 2011;31(9):833–40. [DOI] [PubMed] [Google Scholar]
- 106.Sudrié-Arnaud B, Marguet F, Patrier S, et al. Metabolic causes of nonimmune hydrops fetalis: A next-generation sequencing panel as a first-line investigation. Clin Chim Acta 2018;481:1–8. [DOI] [PubMed] [Google Scholar]
- 107.Verma J, Thomas DC, Sharma S, et al. Inherited metabolic disorders: Prenatal diagnosis of lysosomal storage disorders. Prenat Diagn 2015;35(11):1137–47. [DOI] [PubMed] [Google Scholar]
- 108.Burin MG, Scholz AP, Gus R, et al. Investigation of lysosomal storage diseases in nonimmune hydrops fetalis. Prenat Diagn 2004;24(8):653–7. [DOI] [PubMed] [Google Scholar]
- 109.Fu F, Li R, Li Y, et al. Whole exome sequencing as a diagnostic adjunct to clinical testing in fetuses with structural abnormalities. Ultrasound Obstet Gynecol 2018;51(4):493–502. [DOI] [PubMed] [Google Scholar]
- 110.Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet 2018;137(2):175–81. [DOI] [PubMed] [Google Scholar]
- 111.Drury S, Williams H, Trump N, et al. Exome sequencing for prenatal diagnosis of fetuses with sonographic abnormalities. Prenat Diagn 2015;35(10):1010–7. [DOI] [PubMed] [Google Scholar]
- 112.Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet 2019;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 113.Lord J, McMullan DJ, Eberhardt RY, et al. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet (London, England) 2019;393(10173):747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yates CL, Monaghan KG, Copenheaver D, et al. Whole-exome sequencing on deceased fetuses with ultrasound anomalies: Expanding our knowledge of genetic disease during fetal development. Genet Med 2017;19(10):1171–8. [DOI] [PubMed] [Google Scholar]
- 115.Ewans LJ, Schofield D, Shrestha R, et al. Whole-exome sequencing reanalysis at 12 months boosts diagnosis and is cost-effective when applied early in Mendelian disorders. Genet Med 2018;20:1564–74. [DOI] [PubMed] [Google Scholar]
- 116.Pena LDM, Jiang YH, Schoch K, et al. Looking beyond the exome: A phenotype-first approach to molecular diagnostic resolution in rare and undiagnosed diseases. Genet Med 2018;20(4):464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn 2018;38(1):6–9. [DOI] [PubMed] [Google Scholar]
- 118.Bellini C, Fulcheri E, Rutigliani M, et al. Immunohistochemistry in non-immune hydrops fetalis: A single center experience in 79 fetuses. Am J Med Genet Part A 2010;152(5):1189–96. [DOI] [PubMed] [Google Scholar]
- 119.Suri V, Aggarwal N, Deo ND, Malhotra S, Vasishta K. Placental chorioangioma with hydrops foetalis: A case report. Acta Obstet Gynecol Scand 2005;84(6):603–4. [DOI] [PubMed] [Google Scholar]
- 120.Soma H, Yamada K, Osawa H, Hata T, Oguro T, Kudo M. Identification of Gaucher cells in the chorionic villi associated with recurrent hydrops fetalis. Placenta 2000;21(4):412–6. [DOI] [PubMed] [Google Scholar]
- 121.Roncati L, Barbolini G, Pusiol T, Piscioli F. New advances on placental hydrops and related villous lymphatics. Lymphology 2015;48(1):28–37. [PubMed] [Google Scholar]
- 122.Grubb JH, Vogler C, Sly WS. New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res 13(2–3):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rastall DP, Amalfitano A. Recent advances in gene therapy for lysosomal storage disorders. Appl Clin Genet 2015;8:157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacKenzie TC, David AL, Flake AW, Almeida-Porada G. Consensus statement from the first international conference for in utero stem cell transplantation and gene therapy. Front Pharmacol 2015;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pearson EG, Flake AW. Stem cell and genetic therapies for the fetus. Semin Pediatr Surg 2013;22(1):56–61. [DOI] [PubMed] [Google Scholar]
- 126.Staretz-Chacham O, Lang TC, LaMarca ME, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics 2009;123(4):1191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen O, Michaelson-Cohen R, Gross-Tsur V, et al. Prenatal observation of nystagmus, cataracts, and brain abnormalities in a case of Zellweger spectrum disorder syndrome. Prenat Diagn 2016;36(9):894–5. [DOI] [PubMed] [Google Scholar]
- 128.Dursun A, Gucer S, Ebberink MS, Yigit S, Wanders RJA, Waterham HR. Zellweger syndrome with unusual findings: non-immune hydrops fetalis, dermal erythropoiesis and hypoplastic toe nails. J Inherit Metab Dis 2009;32 Suppl 1:S345–8. [DOI] [PubMed] [Google Scholar]
- 129.Dupont C, Grati FR, Choy KW, et al. Prenatal diagnosis of 24 cases of microduplication 22q11.2: An investigation of phenotype-genotype correlations. Prenat Diagn 2015;35(1):35–43. [DOI] [PubMed] [Google Scholar]
- 130.Amarillo IE, O’Connor S, Lee CK, Willing M, Wambach JA. De novo 9q gain in an infant with tetralogy of Fallot with absent pulmonary valve: Patient report and review of congenital heart disease in 9q duplication syndrome. Am J Med Genet Part A 2015;167(12):2966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Long A, Sinkovskaya ES, Edmondson AC, Zackai E, Schrier Vergano SA. Kabuki syndrome as a cause of non-immune fetal hydrops/ascites. Am J Med Genet Part A 2016;170(12):3333–7. [DOI] [PubMed] [Google Scholar]
- 132.Gaucherand P, Vavasseur-Monot C, Ollagnon E, et al. McKusik-Kaufman syndrome: Prenatal diagnosis, genetics and follow up. Prenat Diagn 2002;22(11):1048–50. [DOI] [PubMed] [Google Scholar]
- 133.Tsai HF, Wu MH, Cheng YC, Chang CH, Chang FM. Prenatal ultrasonography and postnatal follow-up of a case of McKusick-Kaufman syndrome. Taiwan J Obstet Gynecol 2014;53(2):241–4. [DOI] [PubMed] [Google Scholar]
- 134.Patton MA, Baraitser M, Nickolaides K, Rodeck CH, Gamsu H. Prenatal treatment of fetal hydrops associated with the hypertelorism‐dysphagia syndrome (opitz‐g syndrome). Prenat Diagn 1986;6(2):109–15. [DOI] [PubMed] [Google Scholar]
- 135.Cheng YKY, Huang J, Law KM, Chan YM, Leung TY, Choy KW. Prenatal diagnosis of maternally inherited X-linked Opitz G/BBB syndrome by chromosomal microarray in a fetus with complex congenital heart disease. Clin Chim Acta 2014;436:140–2. [DOI] [PubMed] [Google Scholar]
- 136.Nyberg RH, Uotila J, Kirkinen P, Rosendahl H. Macrocephaly-cutis marmorata telangiectatica congenita syndrome--prenatal signs in ultrasonography. Prenat Diagn 2005;25(2):129–32. [DOI] [PubMed] [Google Scholar]
- 137.Mirzaa G, Conway R, Graham JM, Dobyns WB. PIK3CA-Related Segmental Overgrowth. 2013 Aug 15 In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2019. Available from http://www.ncbi. [Google Scholar]
- 138.von Dadelszen P, Chitayat D, Winsor EJ, et al. De novo 46,XX,t(6;7)(q27;q11;23) associated with severe cardiovascular manifestations characteristic of supravalvular aortic stenosis and Williams syndrome. Am J Med Genet 2000;90(4):270–5. [DOI] [PubMed] [Google Scholar]
- 139.Sanford E, Saadai P, Lee H, Slavotinek A. Congenital high airway obstruction sequence (CHAOS): a new case and a review of phenotypic features. Am J Med Genet A 2012;158A(12):3126–36. [DOI] [PubMed] [Google Scholar]
- 140.Patel MS, Callahan JW, Zhang S, et al. Early-infantile galactosialidosis: Prenatal presentation and postnatal follow-up. Am J Med Genet 1999;85(1):38–47. [PubMed] [Google Scholar]
- 141.C A, C S, T R, et al. Galactosialidosis: Review and analysis of CTSA gene mutations. Orphanet J Rare Dis 2013;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Capobres T, Sabharwal G, Griffith B. CASE REPORT A case of I-cell disease ( mucolipidosis II ) presenting with short femurs on prenatal ultrasound and profound diaphyseal cloaking. 2016;(February):0–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heo JS, Choi KY, Sohn SH, et al. A case of mucolipidosis II presenting with prenatal skeletal dysplasia and severe secondary hyperparathyroidism at birth. Korean J Pediatr 2012;55(11):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Applegarth DA, Toone JR, Wilson RD, Yong SL, Baldwin VJ. Morquio Disease Presenting as Hydrops Fetalis and Enzyme Analysis of Chorionic Villus Tissue in a Subsequent Pregnancy. Pediatr Pathol 1987;7(5–6):593–9. [DOI] [PubMed] [Google Scholar]
- 145.Sinelli MT, Motta M, Cattarelli D, Cardone ML, Chirico G. Fetal hydrops in GM1 gangliosidosis: A case report. Acta Paediatr Int J Paediatr 2005;94(12):1847–9. [DOI] [PubMed] [Google Scholar]
- 146.Cheng Y, Verp MS, Knutel T, Hibbard JU. Mucopolysaccharidosis type VII as a cause of recurrent non-immune hydrops fetalis. J Perinat Med 2003;31(6):535–7. [DOI] [PubMed] [Google Scholar]
- 147.Den Hollander NS, Kleijer WJ, Schoonderwaldt EM, Los FJ, Wladimiroff JW, Niermeijer MF. In-utero diagnosis of mucopolysaccharidosis type VII in a fetus with an enlarged nuchal translucency. Ultrasound Obstet Gynecol 2000;16(1):87–90. [DOI] [PubMed] [Google Scholar]
- 148.Geipel A, Berg C, Germer U, Krapp M, Kohl M, Gembruch U. Mucopolysaccharidosis VII (Sly disease) as a cause of increased nuchal translucency and non-immune fetal hydrops: Study of a family and technical approach to prenatal diagnosis in early and late pregnancy [5]. Prenat Diagn 2002;22(6):493–5. [DOI] [PubMed] [Google Scholar]
- 149.Schoenfeld A, Abramovici A, Klibanski C, Ovadia J. Placental ultrasonographic biochemical and histochemical studies in human fetuses affected with Niemann-Pick disease type A. Placenta 1985;6(1):33–43. [DOI] [PubMed] [Google Scholar]
- 150.Ples L, Sima RM, Nedelea F, Moga M. First prenatal diagnosis of a Niemann-Pick disease type C2 revealed by a cystic hygroma: A case report and review of the literature. Front Endocrinol (Lausanne) 2018;9(JUN):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aula N, Aula P. Prenatal diagnosis of free sialic acid storage disorders (SASD). Prenat Diagn 2006;26(8):655–8. [DOI] [PubMed] [Google Scholar]
- 152.Peciuliene S, Burnyte B, Gudaitiene R, et al. Perinatal manifestation of mevalonate kinase deficiency and efficacy of anakinra. Pediatr Rheumatol Online J 2016;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Radiol P Pediatric Radiology Wolman ‘ s disease : ultrasonographic and computed tomographic findings. 1992;541–2. [DOI] [PubMed] [Google Scholar]
- 154.Blitz MJ, Rochelson B, Sood M, Bialer MG, Vohra N. Prenatal sonographic findings in a case of Wolman’s disease. J Clin Ultrasound 2018;46(1):66–8. [DOI] [PubMed] [Google Scholar]
- 155.Mark K, Reis A, Zenker M. Prenatal findings in four consecutive pregnancies with fetal Pierson syndrome, a newly defined congenital nephrosis syndrome. Prenat Diagn 2006;26(3):262–6. [DOI] [PubMed] [Google Scholar]
- 156.Zenker M, Tralau T, Lennert T, et al. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A 2004;130A(2):138–45. [DOI] [PubMed] [Google Scholar]
- 157.Etches PC, Lemons JA. Nonimmune hydrops fetalis: report of 22 cases including three siblings. Pediatrics 1979;64(3):326–32. [PubMed] [Google Scholar]
- 158.Hatzaki A, Sifakis S, Apostolopoulou D, et al. FGFR3 related skeletal dysplasias diagnosed prenatally by ultrasonography and molecular analysis: presentation of 17 cases. Am J Med Genet A 2011;155A(10):2426–35. [DOI] [PubMed] [Google Scholar]
- 159.Nakamura Y, Komatsu Y, Yano H, et al. Nonimmunologic hydrops fetalis: a clinicopathological study of 50 autopsy cases. Pediatr Pathol 1987;7(1):19–30. [DOI] [PubMed] [Google Scholar]
- 160.Tonni G, Panteghini M, Bonasoni M, Pattacini P, Ventura A. Prenatal ultrasound and MRI Diagnosis of Jeune syndrome type I (asphyxiating thoracic dystrophy) with histology and post-mortem three-dimensional CT confirmation. Fetal Pediatr Pathol 2013;32(2):123–32. [DOI] [PubMed] [Google Scholar]
- 161.Steiner RD, Adsit J, Basel D. COL1A1/2-Related Osteogenesis Imperfecta 2005. January 28 [updated 2013 Feb 14]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews®. [Google Scholar]
- 162.Dalal AB, Phadke SR. Twin pregnancy with Roberts syndrome in one fetus and trisomy 18 in the other. J Clin Ultrasound 34(3):146–9. [DOI] [PubMed] [Google Scholar]
- 163.Nikkels PG, Stigter RH, Knol IE, van der Harten HJ. Schneckenbecken dysplasia, radiology, and histology. Pediatr Radiol 2001;31(1):27–30. [DOI] [PubMed] [Google Scholar]
- 164.Badiner N, Taylor SP, Forlenza K, et al. Mutations in DYNC2H1, the cytoplasmic dynein 2, heavy chain 1 motor protein gene, cause short-rib polydactyly type I, Saldino–Noonan type. Clin Genet 2017;92(2):158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Montemarano H, Bulas DI, Chandra R, Tifft C. Prenatal diagnosis of glomerulocystic kidney disease in short-rib polydactyly syndrome type II, Majewski type. Pediatr Radiol 1995;25(6):469–71. [DOI] [PubMed] [Google Scholar]
- 166.Kumru P, Aka N, Köse G, Vural ZT, Peker Ö, Kayserili H. Short rib polydactyly syndrome type 3 with absence of fibulae (Verma-Naumoff syndrome). Fetal Diagn Ther 2005;20(5):410–4. [DOI] [PubMed] [Google Scholar]
- 167.Silveira KC, Moreno CA, Cavalcanti DP. Beemer–Langer syndrome is a ciliopathy due to biallelic mutations in IFT122. Am J Med Genet Part A 2017;173(5):1186–9. [DOI] [PubMed] [Google Scholar]
- 168.Basel-Vanagaite L, Kornreich L, Schiller O, Yacobovich J, Merlob P. Yunis-Varon syndrome: further delineation of the phenotype. Am J Med Genet A 2008;146A(4):532–7. [DOI] [PubMed] [Google Scholar]
- 169.Alby C, Boutaud L, Bonnière M, et al. In utero ultrasound diagnosis of corpus callosum agenesis leading to the identification of orofaciodigital type 1 syndrome in female fetuses. Birth defects Res 2018;110(4):382–9. [DOI] [PubMed] [Google Scholar]
- 170.Reichert SL, Mckay EM, Moldenhauer JS. Identification of a novel nonsense mutation in the FOXP3 gene in a fetus with hydrops-Expanding the phenotype of IPEX syndrome. Am J Med Genet Part A 2016;170(1):226–32. [DOI] [PubMed] [Google Scholar]
- 171.Shehab O, Tester DJ, Ackerman NC, Cowchock FS, Ackerman MJ. Whole genome sequencing identifies etiology of recurrent male intrauterine fetal death. Prenat Diagn 2017;37(10):1040–5. [DOI] [PubMed] [Google Scholar]
- 172.Pannier E, Viot G, Aubry MC, et al. Congenital erythropoietic porphyria (Günther’s disease): Two cases with very early prenatal manifestation and cystic hygroma. Prenat Diagn 2003;23(1):25–30. [DOI] [PubMed] [Google Scholar]
- 173.Goel R, Snow J, Pri-Paz SM, Cushing M, Vasovic L V. Intrauterine transfusions for severe fetal anemia and hydrops due to de novo εγδβ-thalassemia. Transfusion 2017;57(4):876. [DOI] [PubMed] [Google Scholar]
- 174.Gos M, Smigiel R, Kaczan T, et al. MAP2K2 mutation as a cause of cardio-facio-cutaneous syndrome in an infant with a severe and fatal course of the disease. Am J Med Genet Part A 2018;176(7):1670–4. [DOI] [PubMed] [Google Scholar]
- 175.Lev-Sagie A, Hamani Y, Raas-Rothschild A, Yagel S, Anteby EY. Prenatal ultrasonographic diagnosis of atypical Nonne-Milroy lymphedema. Ultrasound Obstet Gynecol 2003;21(1):72–4. [DOI] [PubMed] [Google Scholar]
- 176.Shah S, Conlin LK, Gomez L, et al. CCBE1 Mutation in Two Siblings, One Manifesting Lymphedema-Cholestasis Syndrome, and the Other, Fetal Hydrops. PLoS One 2013;8(9):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gulati N, Morris RK, Williams D, Kilby MD. Prenatal thoraco-amniotic chest drain insertion to manage a case of fetal hydrops secondary to FOXC2. BMJ Case Rep 2018;2018:2017–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Gargano G, Guidotti I, Balestri E, et al. Hydrops fetalis in a preterm newborn heterozygous for the c.4A>G SHOC2 mutation. Am J Med Genet Part A 2014;164(4):1015–20. [DOI] [PubMed] [Google Scholar]