Abstract
INTRODUCTION:
Little is known about the longitudinal association between napping and cognitive impairment in older adults.
METHODS:
We used wrist actigraphy to measure naps in 2751 community-dwelling older men. Cognition was assessed repeatedly over 12 years, and clinically significant cognitive impairment was determined by physician diagnosis, Alzheimer’s medication use or a significant cognitive decline.
RESULTS:
After adjustment for all covariates, men with longer napping duration had greater cognitive decline and higher risk of cognitive impairment. Men who napped for ≥120 min/day (vs. <30 min/day) were 66% more likely to develop cognitive impairment (OR=1.66, 95%CI: 1.09–2.54) in 12 years. Further adjustment for nighttime sleep quality did not appreciably alter the results. The association between napping and cognitive impairment was more pronounced among those with higher sleep efficiency and average sleep duration.
DISCUSSION:
Napping might be useful as an early marker of cognitive impairment in the elderly, and its cognitive effects may differ by nighttime sleep.
Keywords: Sleep, Napping, Daytime sleepiness, Dementia, Cognitive impairment, Cognitive decline, Longitudinal study, Epidemiology
Introduction
Napping, both intentional and not, is very common in the elderly (1). In recent years, growing evidence has suggested a link between napping and a number of adverse health outcomes in older adults, including cardiovascular diseases (CVD), diabetes, Parkinson’s disease (PD) and mortality (2–6). However, little is known about the effects of napping on cognitive health in those without dementia.
While sleep is considered to enhance memory retention and consolidation, especially among healthy younger adults (7, 8), it remains controversial whether napping could benefit cognition by compensating for poor nighttime sleep, or if napping might be a prodromal or risk factor of cognitive impairment in the elderly. A few population-based studies have suggested cross-sectional associations between napping and worse cognitive function (9, 10), or showed increased risk of dementia or cognitive decline associated with reported excessive daytime sleepiness (EDS) or daily sleep (11–15). However, self-reported EDS only reflects tendency to fall asleep rather than the actual napping/sleep periods. Napping measured by self-reported questionnaires is also less reliable and valid than objective measures, particularly for older adults (16–18). Subjective reports often fail to provide an accurate estimate of napping duration, an attribute that may be strongly linked to cognitive function (8). Therefore, it is critical to use objective measures of napping and longitudinal study designs in order to fully understand the relationship of napping with health and cognitive outcomes, and to clarify whether increased napping is an antecedent risk factor for development of cognitive impairment.
No study to date has examined the longitudinal association between objective estimates of napping behaviors and cognitive decline or risk of cognitive impairment. Moreover, it is unclear if the effects of napping on cognition could differ among those who sleep well versus those who sleep poorly at night. Understanding these associations is critical for informing sleep recommendations across population groups.
Our goal was to prospectively study the association between actigraphy-assessed napping, cognitive decline and risk of developing clinically significant cognitive impairment in a large cohort of older men and to explore whether this differs by nighttime sleep quality and duration.
Methods
We examined participants enrolled in the Osteoporotic Fractures in Men Study (MrOS) (http://mrosdata.sfcc-cpmc.net) (19, 20), a prospective study of community-dwelling men aged 65 years or older, enrolled from 2000 to 2002 at six clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement.
The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, recruited 3,135 participants for a comprehensive sleep assessment between 2003 and 2005. To be included in the current analysis, men had to have undergone wrist actigraphy for multiple days at baseline (2003–2005) and to have cognitive function measured at baseline and at one or more follow-up visits (2005–2006, 2007–2009 or 2014–16) over the subsequent 12 years. We excluded 176 men with “significant cognitive impairment” at baseline (Modified Mini-Mental State Examination (3MS) score <80 or use of medication for dementia). Of the 2959 men without significant cognitive impairment and with baseline napping and cognition data, 2751 had at least one additional measure of cognition during follow-up and comprise our analytic cohort. All men provided written informed consent and the study was approved by the Institutional Review Board at each site.
Measure of napping
Napping was objectively assessed using an actigraph (SleepWatch-O, Ambulatory Monitoring, Inc., Ardsley, NY), worn continuously on the nondominant wrist for a minimum of 5 consecutive 24-hour periods (mean 5.2±0.9 days). Actigraphy is a reliable non-invasive tool for estimating sleep-wake activities (21, 22). It measures movement using a piezoelectric bimorph-ceramic cantilevered beam, and generates a voltage each time the actigraph is moved; these voltages are gathered continuously and stored in 1-minute epochs. The University of California, San Diego (UCSD) scoring algorithm in the ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to analyze the actigraphy data and differentiate sleep from wake times (23, 24). Participants also completed sleep logs for the period they wore the actigraph, and reported information regarding times they got into and out of bed, when the actigraph was removed and times they napped; these data were used to edit the actigraph records. This standardized scoring protocol has been found to have high inter-scorer reliability (25).
We defined napping as periods of inactivity of at least 5 consecutive minutes scored as sleep (inactivity) outside of the main sleep interval (26, 27). Daily napping duration was calculated by summing up the duration of napping periods throughout the day and averaging across all days of recording. Subjective daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS), a self-administered questionnaire with scores ranging from 0–24. EDS was defined as having a ESS score of >10 (28, 29).
Cognitive Assessment
Two tests of cognitive function were administered at the clinic visits by trained staff: Trails B and the Modified Mini-Mental State examination (3MS).
Trails B is a timed test of processing speed that measures attention, sequencing, visual scanning and executive function. Trails B test requires the participant to continuously scan a page to identify numbers and letters in a specified sequence while shifting from number to letter sets (30). The participant is given 300 seconds to complete the test. A lower time for completion (in seconds) represents better executive functioning.
The 3MS is a global measurement of cognitive function, with components for orientation, concentration, language, praxis, and immediate and delayed memory. The 3MS test is a broad sampling of cognitive domains. Scores range from 0 to 100, with higher scores representing better cognitive functioning (31). In total, there were three follow-ups of cognitive function after the baseline visit: between 2005–2006 (visit 2), 2007–2009 (visit 3) and 2014–16 (visit 4). We defined the development of clinically significant cognitive impairment during follow-up as follows: report of physician-diagnosed dementia, use of dementia medication (verified by clinic staff based on examination of pill bottles), or having a change in 3MS scores ≥ 1.5 standard deviation worse than the mean change from baseline to any follow-up visit (equal to a decline of 7.32, 9.43 and 13.62 points on 3MS from baseline to visit 2, visit 3 and visit 4, respectively). Once an individual met the criteria for cognitive impairment at any visit, this was considered as an endpoint in the analysis. For the study of change in cognitive function, we used cognitive data collected from all follow-ups.
Other measures
Participants completed exams and questionnaires, which included information on demographics, smoking, physical activity, depressive symptoms and medical history. Level of physical activity was examined using the Physical Activity Scale for the Elderly (PASE) (32), and depressive symptoms were assessed using the Geriatric Depression Scale (GDS) (33). Body mass index (BMI; weight in kilograms divided by height in meters squared) was also measured. During the clinic visit, participants were also asked to bring in all prescription and nonprescription medications used over the past month. Medication use was entered into an electronic database, with each matched to its ingredients based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (34). Sleep medication use includes the use of nonbenzodiazepines and nonbarbiturate sedative hypnotics.
We used average nighttime sleep duration and efficiency (SE; the average percentage of time asleep while in bed) recorded by actigraphy to represent nighttime duration and sleep quality, respectively. In addition, the apnea-hypopnea index (AHI, total number of obstructive and central apneas and hypopneas associated with ≥3% oxygen desaturation), measured by unattended in-home polysomnography (PSG, Safiro ambulatory EEG system; Compumedics, Abbotsford, Australia) following a standardized protocol as described previously (35), was used to account for the effects of sleep-disordered breathing (SDB).
Statistical analysis
Participants were first categorized according to their mean daily napping durations: <30min, 30 to <60min, 60 to <120min, and ≥120min. We compared baseline characteristics of participants with ANOVA for normally distributed continuous variables, Kruskal Wallis test for skewed variables, and chi-square test for categorical variables. We used mixed-effects linear regression cubic splines to examine the association between napping duration and change in cognitive function, carefully evaluating linear assumptions. We compared the overall changes in adjusted mean scores of 3MS and Trails B tests by different napping durations. We used multivariable logistic regression to study the association between napping duration and risk of developing cognitive impairment, adjusting for age, education, BMI, smoking, physical activity, depressive symptoms, medical comorbidities, sleep medication use and baseline global cognition. In order to minimize effects of “reverse causality” (i.e., modelling napping as an antecedent risk factor for cognitive impairment), we introduced a lag time of 2 years, only including cases identified after two years following the measurement of napping. To explore the effects of nighttime sleep, we further adjusted for nighttime sleep duration, sleep efficiency and SDB (AHI≥15), and stratified the analysis according to different categories of these nighttime sleep parameters. Given that this is a sample of very old men with relatively short nighttime sleep duration and low sleep efficiency, we categorized nighttime sleep duration into <6h, 6–8h and >8h, and divided sleep efficiency into ≤70% and >70%, as with previous publications in the same cohort (36, 37). To explore if the association between napping and subsequent cognitive trajectory differs by baseline cognitive status, we further compared the association between participants with possible mild cognitive impairment (MCI) (3MS ≤88) and with normal cognition at baseline (3MS>88) (38, 39). Finally, to address the limitation of actigraphy in differentiating naps from quiet wakefulness, we repeated the analysis using ≥10 instead of ≥5 consecutive minutes of inactivity to define naps. Results are presented as odds ratios (OR) with 95% confidence intervals. All statistical tests were two-sided, and a p-value of less than .05 was considered significant. Analyses were performed using Stata, version 14.1 (Stata Corp LP, College Station, TX).
Results
Overall, the 2751 men (mean age 76.0 years) had a mean napping duration of 40.2 minutes (0–411 min). 1409 (51.2%) of the men napped for <30min per day, 736 (26.8%) napped for 30–59min, 642 (16.6%) napped for 60–119min, and 253 (5.4%) napped for >120 min per day. Table 1 shows participants’ baseline characteristics according to their napping duration. Men with longer napping duration had higher BMI and higher depressive symptoms, and were more likely to have a history of stroke, coronary heart disease, hypertension or diabetes, and had worse (but normal) Trails B scores at baseline. Those who napped for longer duration also had greater EDS, were more likely to have either short or long sleep duration at night but had similar sleep efficiency compared to those with less napping.
Table 1.
Baseline characteristics by napping duration in 2751 older men
| Characteristics | Napping duration | P value | |||
|---|---|---|---|---|---|
| mean±SD or N (%) | <30min | 30~59min | 60~119min | ≥120min | |
| (N=1061) | (N=795) | (N=642) | (N=253) | ||
| Age | 75.3±5.1 | 76.1±5.1 | 76.6±5.6 | 77.5±5.7 | 0.01 |
| Body Mass Index | 26.8±3.5 | 27.3±3.9 | 27.4±3.9 | 28.3±4.2 | <0.01 |
| Physical Activity Scale for the Elderly score | 156.4±69.3 | 149.0±69.9 | 144.1±72.2 | 123.6±69.5 | 0.70 |
| Geriatric Depression Scale score | 1.3±1.7 | 1.8±2.2 | 1.8±2.1 | 2.3±2.4 | <0.01 |
| Current smoking | 13 (1.2) | 19 (2.4) | 17 (2.7) | 7 (2.8) | 0.12 |
| Stroke history | 27 (2.5) | 27 (3.4) | 26 (4.1) | 20 (7.9) | <0.01 |
| Coronary heart disease | 137 (12.9) | 148 (18.6) | 120 (18.7) | 53 (21.0) | <0.01 |
| Hypertension | 475 (44.8) | 393 (49.4) | 328 (51.1) | 159 (62.9) | <0.01 |
| Diabetes | 112 (10.6) | 105 (13.2) | 91 (14.2) | 51 (20.2) | <0.01 |
| Sleep medication use | 133 (12.5) | 94 (11.8) | 61 (9.5) | 37 (14.7) | 0.12 |
| Modified Mini-Mental State score | 94.1±4.3 | 93.7±4.3 | 93.3±4.6 | 92.9±4.8 | 0.05 |
| Trails B score | 110.0±46.0 | 118.3±51.1 | 121.7±51.7 | 128.6±57.4 | <0.01 |
| Excessive daytime sleepiness | 85 (8.0) | 119 (15.0) | 91 (14.2) | 42 (16.6) | <0.01 |
| Nighttime sleep duration | <0.01 | ||||
| <6h | 296 (27.9) | 249 (31.3) | 211 (32.9) | 104 (41.1) | |
| 6–8h | 701 (66.1) | 500 (62.9) | 376 (58.6) | 128 (50.6) | |
| >8h | 64 (6.0) | 46 (5.8) | 55 (8.6) | 21 (8.3) | |
| Lower sleep efficiency (≤70%) | 193 (18.2) | 153 (19.3) | 102 (15.9) | 44 (17.4) | 0.42 |
Over 12 years of follow-up, the 3MS scores of those who napped for <30 min, 30–59 min, 60–119 min and ≥120 min declined by 4.2, 5.9, 4.3 and 5.6 points, respectively (p<0.001). Trails B scores increased by 59, 71, 74 and 79 seconds for those who napped for <30min, 30–59min, 60–119min and ≥120min (p=0.02).
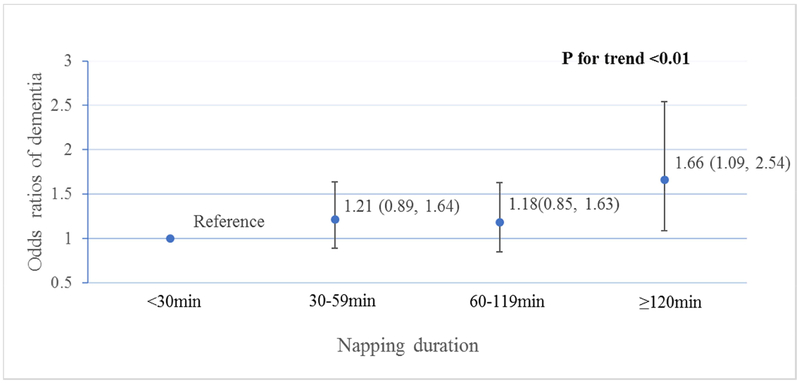
After excluding 121 cases developed within two years after baseline, we identified 320 (12%) cases of clinically significant cognitive impairment at follow up, of which 106 (11%) napped for <30min, 95 (13%) napped for 30–59min, 78 (13%) napped for 60–119min, and 41 (18%) napped for ≥120min. Figure 1 shows the multivariable adjusted ORs (95%CI) of developing cognitive impairment for different napping groups. After adjustment for age, education, BMI, smoking, physical activity, depressive symptoms, history of stroke, coronary heart disease, hypertension, diabetes, sleep medication use and baseline 3MS score, longer napping duration was associated with greater risk of developing cognitive impairment (p for trend<0.01). Men with a napping duration of ≥120min at baseline were 66% more likely to develop cognitive impairment compared to those who napped for <30min (OR=1.66, 95%CI: 1.09–2.54). Further adjustment for nighttime sleep duration, efficiency and hypoxemia did not appreciably alter the results, and the association remained statistically significant (p=0.01). EDS was not associated with risk of cognitive impairment (OR=0.82, 95% CI: 0.56–1.21).
Figure 1. Multivariable*-adjusted ORs (95%CI) of cognitive impairment by napping duration.
*Adjusted for age, education, BMI, smoking, physical activity, depressive symptoms, history of stroke, coronary heart disease, hypertension, diabetes, sleep medication use and baseline 3MS score. N for each napping duration group of <30min, 30–59min, 60–119min and ≥120min was 1061, 795, 642 and 253, respectively.
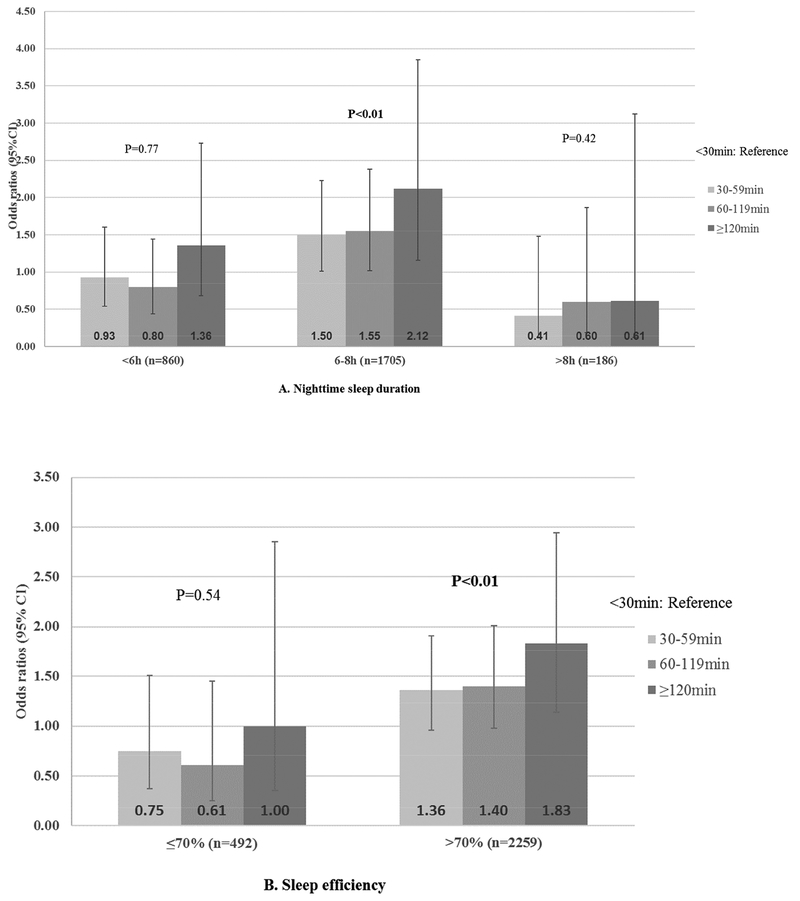
Figure 2 shows the ORs of cognitive impairment associated with categories of napping duration and stratified by nighttime sleep duration and efficiency. There was statistically significant interaction by nighttime sleep duration (p=0.04) but not sleep efficiency (p=0.14). Napping was only associated with increased risk of cognitive impairment among those with higher sleep efficiency (>70%) and average sleep duration (6–8h). Among those who slept for 6–8h at night, those who napped for ≥120min were more than twice as likely to develop cognitive impairment as compared to those napping for <30min (OR=2.12, 1.16–3.85); among those who had higher sleep efficiency, those who napped for ≥120min were almost twice as likely to develop cognitive impairment as compared to those napping for <30min (OR= 1.83, 1.14–2.94). The association between napping and risk of cognitive impairment did not differ significantly by SDB or baseline cognition: ORs (95%CI) associated with napping for at least 120min were 1.90 (1.11–3.25) and 1.68 (0.88–3.23) for those with an AHI of <15 and ≥15, respectively (p for interaction=0.22); ORs (95%CI) were 2.26 (0.85–6.00) and 1.61 (1.00–2.61) for those with a baseline 3MS score of ≤88 and >88, respectively (p for interaction=0.84). We found similar results when defining a nap as ≥10 consecutive minutes of inactivity (data not shown).
Figure 2. >Multivariable*-adjusted ORs (95%CI) of cognitive impairment associated with napping duration, stratified by A) nighttime sleep duration and B) sleep efficiency.
*Adjusted for age, education, BMI, smoking, physical activity, depressive symptoms, history of stroke, coronary heart disease, hypertension, diabetes, sleep medication use and baseline 3MS score.
Discussion
In this study of 2751 non-demented older men, men with long napping duration had greater cognitive decline and higher risk of developing clinically significant cognitive impairment. Those who napped for ≥120min had 66% higher risk of developing cognitive impairment in 12 years, compared to those who napped for less than 30min per day. This association was moderated by nighttime sleep characteristics with those having higher sleep efficiency (>70%) and intermediate sleep duration (6–8h) having worse outcomes with napping, though the interaction effect was not statistically significant for sleep efficiency. Among men with low sleep efficiency and short or long sleep duration, napping was not associated with increased risk of cognitive impairment.
There have been heated debates over whether napping as a restorative form of sleep could help improve cognition, or if it might be associated with cognitive decline or impairment. Some of the key considerations include the age and health of the population, as well as the length and timing of the naps (8). One recent experimental study showed that a 90-min afternoon nap benefits episodic memory retention in young adults but not in older adults (40). Several epidemiologic studies have shown that older adults who reported EDS had 20% to 3 times increase in the risk of cognitive decline or dementia over a follow-up of 18 months to 10 years (11–13, 41). Findings from the MRC Cognitive Function and Ageing Study (CFAS) suggested that reported EDS was associated with more than double the risk of incident cognitive impairment in 10 years, whereas reported napping was protective against cognitive decline, with those napping for at least 1h per day linked to a 70% reduction in the risk of cognitive impairment (42). Such divergent findings could be due to the subjective measurement of napping/ EDS. On one hand, it is challenging for older adults and especially those who already undergo cognitive difficulties to accurately report the onset or length of naps; on the other hand, report of EDS only reflects tendency to fall asleep and could be a very different measure to the actual napping behavior. This study found that longer objective napping was indeed associated with greater EDS, but the prevalence of EDS did not differ by napping duration for those who had at least 30min of objective naps. In addition, we did not find an association between EDS and risk of cognitive impairment. Therefore, it is critical for epidemiologic studies to employ objective assessments of napping to help understand the nature of this behavior.
Prior cross-sectional studies have shown that older women who had long objective napping were more likely to have poorer cognitive function or to have had a diagnosis of dementia (18, 43). To our knowledge, this is the first longitudinal study that demonstrates higher long-term risk of cognitive impairment and greater cognitive decline associated with longer napping durations, measured objectively by 24-h actigraphy, among non-demented older men. In order to help assess the direction of the relationship, we excluded cases developed within two years following the measurement of napping. After adjustment for demographics, comorbidities and baseline cognition, we found a significant increase in the risk of cognitive impairment and greater decline in both global cognition and executive function among those who had a daily napping duration of at least 2h, which makes up 10% of this population. This suggests that excessive napping could be considered as a robust independent early marker of future cognitive decline and impairment in elderly persons who do not have signs of significant cognitive impairment.
The association between napping and risk of cognitive impairment remained after controlling for nighttime sleep duration and disturbances, suggesting an important role of napping itself, independent of nighttime sleep. Interestingly, napping was not associated with increased risk of cognitive impairment among those with low sleep efficiency or with extreme sleep duration, especially short sleep duration. This indicates that the effects of napping on cognition are different among those who sleep poorly and those who sleep well at night. Napping might help compensate for poor nighttime sleep and thus provide extra benefits on cognition among those who sleep poorly at night. Meanwhile, it is unclear why napping might be associated with increased risk of cognitive impairment among those who sleep well at night. The “long nappers” might be genetically phenotyped as a “hypersomnolent” group who are more likely to fall asleep thus having higher sleep efficiency and relatively long sleep duration. This group might share common pathways to neuropsychiatric conditions. Alternatively, long naps outside the main sleep period might have detrimental cognitive effects related to sleep inertia, which is represented by cognitive and sensory-motor impairment that occurs immediately after awakening especially from deep stages of sleep. Additional studies with information on the frequency of naps and duration of each individual nap are needed before conclusions can be made regarding these speculations. At the very least, our findings suggest a need for future studies to carefully consider the joint effects of napping and nighttime sleep and their potential interaction in order to fully understand the association between sleep and cognitive aging.
The exact biological mechanisms linking napping and cognitive impairment remain to be elucidated. Growing evidence suggested an elevated dementia risk associated with circadian rhythm disruption, which is often presented as disrupted 24-h sleep wake cycle including excessive daytime naps (44). Self-reported napping has been associated with increased risk of CVD, diabetes and chronic inflammation (5, 45, 46), all of which could contribute to increased risk of dementia via microvascular disease (47–49). While the association between reported EDS and cognitive impairment remains controversial, emerging evidence showed pathophysiological changes related to self-reported EDS. For example, recent findings from the Baltimore Longitudinal Study of Aging suggested that baseline EDS rather than self-reported napping was associated with greater β-Amyloid deposition 15.7 years later (50). The Mayo Clinical Study of Aging found both a longitudinal association between EDS and β-Amyloid accumulation (51) and a cross-sectional association between EDS and global cortical thinning (52), which is known to be key brain structural changes that predicts future cognitive decline and impairment (53, 54). Another study found a cross-sectional correlation between frequent actigraphy measured napping and β-Amyloid deposition in cognitively normal adults, suggesting the presence of napping in the preclinical stage of Alzheimer disease (55). Future longitudinal studies that examine the association between objectively measured napping and subsequent pathophysiological change in healthy older adults are required to help clarify the nature of the relationship between objective napping and dementia risk. Strengths of this study include a relatively large sample with long follow-up, objective measures of napping and sleep. Unlike previous studies that included only self-reported sleepiness or naps, the current study design has given us a unique opportunity to report for the first time that it is the objective daily napping duration rather than the perceived daytime sleepiness that precedes cognitive decline or impairment in the future. There are also a few limitations. First, this study involves older men who were mostly white, and thus our findings may not be generalizable to women, younger populations or other ethnicities. We defined daily napping duration as the accumulated time of extreme inactivity (≥5 consecutive minutes of inactivity by actigraphy) throughout the day and did not use neurophysiological measurement of sleep. Given the sedentary lifestyle of many older adults and especially parkinsonism in some individuals, some of the periods we refer to as naps may represent quiet wakefulness. Since there is no gold standard definition of objective naps in the field, we used the same approach as in our previous similar studies in both older men and older women (6, 18) to address this problem. While this approach does not eliminate the problem of misclassification, it is more accurate than subjective measures and is also more feasible than PSG as a measure of 24-h sleep-wake patterns. To account for the possibility that some of the naps are quiet wakefulness, especially for those with parkinsonism, we performed sensitivity analysis using a stricter definition of naps and found similar results. The current study focused on cumulative daily napping duration as the primary exposure variable. Future studies are needed to determine whether different frequency of naps or individual nap duration might influence cognitive trajectory differently, i.e. if “frequent dozing-off” might have different effects compared to “a prolonged extended nap”. Second, as with most large population-based cohort studies, we did not have clinical dementia adjudication or information on subtypes of dementia. In order to establish a clinically relevant outcome measure, we defined clinically significant cognitive impairment using a prespecified algorithm incorporating hospitalization records, medication use and neurocognitive test scores, which is similar to the approach used by previous studies (56–58). As such, we did not have the exact dates when cognitive impairment was developed. . As with previous study (59), we excluded participants with significant cognitive impairment at baseline based on a 3MS score of less than 80 or use of dementia medication. It is possible that some patients with MCI are still included in the study sample and might have different napping patterns and cognitive trajectory compared to those with normal cognition. To address this problem, we further stratified the sample by presence of possible MCI based on baseline 3MS scores and found similar association between groups. Finally, in secondary analysis we used nighttime sleep duration of 6–8h and sleep efficiency of >70% to indicate average sleep duration and higher sleep efficiency. These cut-offs reflect the distribution of sleep quality in this population but might not be applied to a younger population who generally sleep well. Besides, the number of people who had long nighttime sleep in our cohort is small. Larger studies are needed to further explore the interaction effects of napping and nighttime sleep on future risk of dementia.
Conclusions
Among community-dwelling older men without significant cognitive impairment, objectively measured long napping was associated with higher risk of developing clinically significant cognitive impairment and greater decline in both global cognition and executive function over 12 years. Excessive napping could be useful as an early sign or risk factor of cognitive decline or clinically significant cognitive impairment in the elderly. Our study also showed for the first time that the cognitive effects of napping in older adults might differ according to their nighttime sleep. Excessive napping among those who have higher sleep efficiency and intermediate sleep duration could contribute to, or could be an early indicator of significant cognitive impairment. Clinicians should pay close attention to the 24-h sleep-wake cycles in the elderly. Future studies are needed to examine the underlying mechanisms of these associations. This might provide insights into the early detection of dementia, and open up new opportunities for prevention of dementia through better management of 24-h sleep-wake cycles.
Highlights.
Older men with long daily naps had greater risk of cognitive impairment in 12 years.
Napping is more relevant to cognitive impairment in those with better nocturnal sleep.
Objective naps might be useful as an early marker of clinically significant cognitive impairment in the elderly.
Research in context.
Systematic review:
The authors conducted a comprehensive search of studies on napping using PubMed. The effects of napping are controversial with some evidence suggesting of beneficial effects and other suggesting associations with indicators of ill health. No study has focused on the longitudinal association between objective napping and cognitive decline or impairment, and it is unclear if the cognitive effects of napping differ by nocturnal sleep.
Interpretation:
We showed for the first time that objectively measured long napping was associated with higher long-term risk of developing clinically significant cognitive impairment, and that the cognitive effects of napping differ by nighttime sleep quality. These novel findings contribute to the heated debate over the effects of napping on cognition in the elderly.
Future directions:
Excessive napping might be useful as an early sign of cognitive decline or cognitive impairment in the elderly. Future longitudinal studies with objective estimates should consider both nighttime and daytime sleep and their interactions in relation to cognitive impairment, and the underlying mechanisms need to be elucidated.
Acknowledgements:
Y.L. is supported by National Institute on Aging (NIA) 1K99AG056598–01. K.Y. is supported in part by NIA K24AG031155.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declared no conflict of interest.
References
- 1.Foley DJ, Vitiello MV, Bliwise DL, et al. : Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry 2007; 15:344–350 [DOI] [PubMed] [Google Scholar]
- 2.Gao J, Huang X, Park Y, et al. : Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol 2011; 173:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leng Y, Cappuccio FP, Surtees PG, et al. : Daytime napping, sleep duration and increased 8-year risk of type 2 diabetes in a British population. Nutr Metab Cardiovasc Dis 2016; 26:996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leng Y, Wainwright NW, Cappuccio FP, et al. : Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol 2014; 179:1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada T, Hara K, Shojima N, et al. : Daytime Napping and the Risk of Cardiovascular Disease and All-Cause Mortality: A Prospective Study and Dose-Response Meta-Analysis. Sleep 2015; 38:1945–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leng Y, Goldman SM, Cawthon PM, et al. : Excessive daytime sleepiness, objective napping and 11-year risk of Parkinson’s disease in older men. Int J Epidemiol 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasch B, Born J: About sleep’s role in memory. Physiol Rev 2013; 93:681–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milner CE,Cote KA: Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res 2009; 18:272–281 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Cacchione PZ, Hodgson N, et al. : Afternoon Napping and Cognition in Chinese Older Adults: Findings from the China Health and Retirement Longitudinal Study Baseline Assessment. J Am Geriatr Soc 2017; 65:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross N, Terpening Z, Rogers NL, et al. : Napping in older people ‘at risk’ of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res 2015; 24:494–502 [DOI] [PubMed] [Google Scholar]
- 11.Elwood PC, Bayer AJ, Fish M, et al. : Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health 2011; 65:820–824 [DOI] [PubMed] [Google Scholar]
- 12.Tsapanou A, Gu Y, Manly J, et al. : Daytime Sleepiness and Sleep Inadequacy as Risk Factors for Dementia. Dement Geriatr Cogn Dis Extra 2015; 5:286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley D, Monjan A, Masaki K, et al. : Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc 2001; 49:1628–1632 [DOI] [PubMed] [Google Scholar]
- 14.Benito-Leon J, Louis ED, Villarejo-Galende A, et al. : Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES). Neurology 2014; 83:1530–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benito-Leon J, Louis ED,Bermejo-Pareja F: Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES). J Psychiatr Res 2013; 47:1998–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean-Louis G, Kripke DF, Assmus JD, et al. : Sleep-wake patterns among postmenopausal women: a 24-hour unattended polysomnographic study. J Gerontol A Biol Sci Med Sci 2000; 55:M120–123 [DOI] [PubMed] [Google Scholar]
- 17.Vitiello MV: We have much more to learn about the relationships between napping and health in older adults. J Am Geriatr Soc 2008; 56:1753–1755 [DOI] [PubMed] [Google Scholar]
- 18.Leng Y, Stone K, Ancoli-Israel S, et al. : Who Take Naps? Self-Reported and Objectively Measured Napping in Very Old Women. J Gerontol A Biol Sci Med Sci 2018; 73:374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orwoll E, Blank JB, Barrett-Connor E, et al. : Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials 2005; 26:569–585 [DOI] [PubMed] [Google Scholar]
- 20.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. : Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 2005; 26:557–568 [DOI] [PubMed] [Google Scholar]
- 21.Kanady JC, Drummond SP, Mednick SC: Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res 2011; 20:214–222 [DOI] [PubMed] [Google Scholar]
- 22.Blackwell T, Redline S, Ancoli-Israel S, et al. : Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep 2008; 31:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole RJ, Kripke DF, Gruen W, et al. : Automatic sleep/wake identification from wrist activity. Sleep 1992; 15:461–469 [DOI] [PubMed] [Google Scholar]
- 24.Jean-Louis G, Kripke DF, Mason WJ, et al. : Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods 2001; 105:185–191 [DOI] [PubMed] [Google Scholar]
- 25.Blackwell T, Ancoli-Israel S, Gehrman PR, et al. : Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep 2005; 28:1599–1605 [DOI] [PubMed] [Google Scholar]
- 26.Patel SR, Hayes AL, Blackwell T, et al. : The association between sleep patterns and obesity in older adults. Int J Obes (Lond) 2014; 38:1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dautovich ND, McCrae CS,Rowe M: Subjective and objective napping and sleep in older adults: are evening naps “bad” for nighttime sleep? J Am Geriatr Soc 2008; 56:1681–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns MW: A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545 [DOI] [PubMed] [Google Scholar]
- 29.Johns MW: Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992; 15:376–381 [DOI] [PubMed] [Google Scholar]
- 30.Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276 [Google Scholar]
- 31.Teng EL,Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987; 48:314–318 [PubMed] [Google Scholar]
- 32.Washburn RA, Smith KW, Jette AM, et al. : The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993; 46:153–162 [DOI] [PubMed] [Google Scholar]
- 33.Brink TL: Clinical gerontology: a guide to assessment and intervention, New York, Howarth Press, 1986 [Google Scholar]
- 34.Pahor M, Chrischilles EA, Guralnik JM, et al. : Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 1994; 10:405–411 [DOI] [PubMed] [Google Scholar]
- 35.Claman DM, Ewing SK, Redline S, et al. : Periodic leg movements are associated with reduced sleep quality in older men: the MrOS Sleep Study. J Clin Sleep Med 2013; 9:1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackwell T, Yaffe K, Ancoli-Israel S, et al. : Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 2011; 34:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackwell T, Ancoli-Israel S, Redline S, et al. : Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med 2011; 7:357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K, Middleton LE, Lui LY, et al. : Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol 2011; 68:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espeland MA, Rapp SR, Robertson J, et al. : Benchmarks for designing two-stage studies using modified mini-mental state examinations: experience from the Women’s Health Initiative Memory Study. Clin Trials 2006; 3:99–106 [DOI] [PubMed] [Google Scholar]
- 40.Scullin MK, Fairley J, Decker MJ, et al. : The Effects of an Afternoon Nap on Episodic Memory in Young and Older Adults. Sleep 2017; 40: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabelle A, Gutierrez LA, Jaussent I, et al. : Excessive Sleepiness and Longer Nighttime in Bed Increase the Risk of Cognitive Decline in Frail Elderly Subjects: The MAPT-Sleep Study. Front Aging Neurosci 2017; 9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keage HA, Banks S, Yang KL, et al. : What sleep characteristics predict cognitive decline in the elderly? Sleep Med 2012; 13:886–892 [DOI] [PubMed] [Google Scholar]
- 43.Blackwell T, Yaffe K, Ancoli-Israel S, et al. : Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2006; 61:405–410 [DOI] [PubMed] [Google Scholar]
- 44.Leng Y, Musiek ES, Hu K, et al. : Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 2019; 18:307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leng Y, Ahmadi-Abhari S, Wainwright NW, et al. : Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open 2014; 4:e006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q, Song Y, Hollenbeck A, et al. : Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care 2010; 33:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt R, Schmidt H, Curb JD, et al. : Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 2002; 52:168–174 [DOI] [PubMed] [Google Scholar]
- 48.Mayeda ER, Haan MN, Kanaya AM, et al. : Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care 2013; 36:2600–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman AB, Fitzpatrick AL, Lopez O, et al. : Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 2005; 53:1101–1107 [DOI] [PubMed] [Google Scholar]
- 50.Spira AP, An Y, Wu MN, et al. : Excessive daytime sleepiness and napping in cognitively normal adults: associations with subsequent amyloid deposition measured by PiB PET. Sleep 2018; 41: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carvalho DZ, St Louis EK, Knopman DS, et al. : Association of Excessive Daytime Sleepiness With Longitudinal beta-Amyloid Accumulation in Elderly Persons Without Dementia. JAMA Neurol 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvalho DZ, St Louis EK, Boeve BF, et al. : Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med 2017; 32:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pacheco J, Goh JO, Kraut MA, et al. : Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiol Aging 2015; 36:903–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eskildsen SF, Coupe P, Garcia-Lorenzo D, et al. : Prediction of Alzheimer’s disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. Neuroimage 2013; 65:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju YE, McLeland JS, Toedebusch CD, et al. : Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013; 70:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deal JA, Betz J, Yaffe K, et al. : Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci 2017; 72:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong CH, Falvey C, Harris TB, et al. : Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology 2013; 81:528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujiyoshi A, Jacobs DR Jr, Fitzpatrick AL, et al. : Coronary Artery Calcium and Risk of Dementia in MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging 2017; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leng Y, Blackwell T, Stone KL, et al. : Periodic Limb Movements in Sleep are Associated with Greater Cognitive Decline in Older Men without Dementia. Sleep 2016; 39:1807–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]