Abstract
Objectives:
In patients with acute recurrent pancreatitis (ARP), pancreas divisum, and no other etiologic factors, endoscopic retrograde cholangiopancreatography (ERCP) with minor papilla endoscopic sphincterotomy (miES) is often performed to enlarge the minor papillary orifice, based on limited data. The aims of this study are to describe the rationale and methodology of a sham-controlled clinical trial designed to test the hypothesis that miES reduces the risk of acute pancreatitis.
Methods:
The SpHincterotomy for Acute Recurrent Pancreatitis (SHARP) trial is a multicenter, international, sham-controlled, randomized trial comparing endoscopic ultrasound + ERCP with miES vs. endoscopic ultrasound + sham for the management of ARP. A total of 234 consented patients having two or more discrete episodes of acute pancreatitis, pancreas divisum confirmed by magnetic resonance cholangiopancreatography, and no other clear etiology for acute pancreatitis will be randomized. Both cohorts will be followed for a minimum of 6 months and maximum of 48 months.
Results:
The trial is powered to detect a 33% risk reduction of acute pancreatitis frequency.
Conclusions:
The SHARP trial will determine whether ERCP with miES benefits patients with idiopathic ARP and pancreas divisum. Trial planning has informed the importance of blinded outcome assessors and long-term follow-up.
Keywords: acute pancreatitis, acute recurrent pancreatitis, chronic pancreatitis, endoscopic retrograde cholangiopancreatography, pancreas divisum
Introduction
Unlike patients with chronic pancreatitis, patients with acute recurrent pancreatitis (ARP) are unique in that most do not have end organ morphological and functional changes at the time of their clinical presentation. However, patients with ARP have a substantial (10–40%) risk of progressing to chronic pancreatitis and its sequelae of chronic pain, malabsorption, diabetes mellitus, poor quality of life, and pancreatic ductal adenocarcinoma.1–3 Treatments to attenuate ARP are needed.
The concept was promulgated in the 1970s that in pancreas divisum there is an anatomic impediment to the drainage of pancreatic exocrine secretions, resulting in an obstructive pancreatopathy.4 Treatment of pancreas divisum involves enlargement of the minor papillary orifice via minor papilla sphincterotomy, serial dilation and stent placement, or surgical sphincteroplasty. Endoscopic minor papilla sphincterotomy is favored over surgery and serial stenting because of its lower short-term morbidity and reduced need for repeated procedures to exchange or remove therapeutic stents, respectively; repeated stent exchanges confer some risk of stent-induced injury to the pancreatic duct.5
There are limited preliminary data supporting the use of endoscopic retrograde cholangiopancreatography (ERCP) with minor papilla endoscopic sphincterotomy (miES). Although the technique of miES has been performed for >30 years, there has been only one pilot, open-label, randomized trial of 19 patients with idiopathic ARP published over 20 years ago.6 This study compared serial dilation of the minor papillary orifice via pancreatic stents – a surrogate for miES – vs diagnostic ERCP. After mean follow-up of 29–32 months, 6/9 (67%) patients who underwent sham ERCP developed at least one bout of acute pancreatitis as compared to 1/10 (10%, P < 0.05) that underwent serial pancreatic duct stent placement.
Several retrospective cohort studies also support the practice of miES for ARP in the setting of pancreas divisum, with approximately 70% of patients in most studies reporting a significant improvement in their disease course.7 While supporting the role of miES, these studies chose a subjective endpoint (self-perceived improvement) despite their open-label design and absence of a sham comparison group. The controversy is a recurrent topic at national meetings, and opposite positions were nicely summarized after a debate at the 2006 meeting of the American Pancreatic Association.8 Both sides acknowledged the need for randomized trials, yet there has been little progress in clarifying the benefit of miES on idiopathic ARP with pancreas divisum over the past decade.9 Given the longstanding controversy, the SpHincterotomy for Acute Recurrent Pancreatitis (SHARP) randomized trial is being conducted to definitely answer the question of whether miES can reduce the risk of acute pancreatitis by relieving the purported obstruction of pancreatic juice outflow caused by divisum anatomy.
MATERIALS AND METHODS
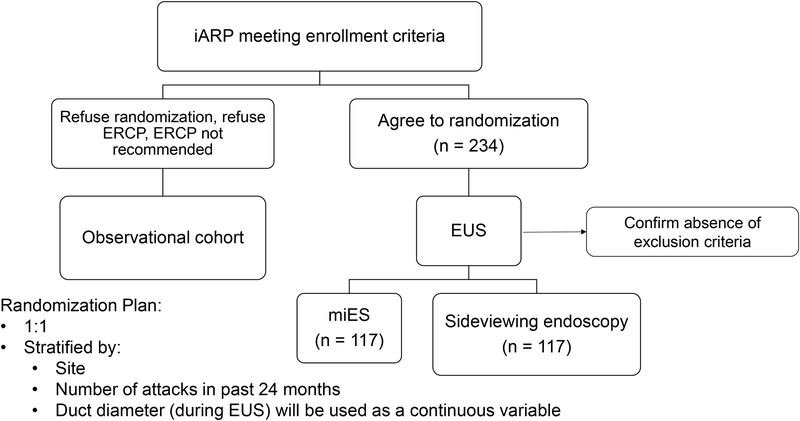
The SHARP trial is a sham-controlled, blinded outcome assessment, intention to treat, multi-center, randomized clinical trial of ERCP with miES for the treatment of ARP with pancreas divisum. The trial requires 234 randomized participants during a planned enrollment period of approximately 3.5 years at 17 or more medical centers, with total planned study duration of 5 years (Fig. 1).
FIGURE 1.
Enrollment schema. ARP indicates acute recurrent pancreatitis; ERCP, endoscopic retrograde cholangiopancreatography; miES; minor papilla endoscopic sphincterotomy; EUS, endoscopic ultrasound.
Trial Organization
The SHARP trial is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and registered in http://clinicaltrials.gov (). Recognizing the effort and skill required to execute the various aspects of a large clinical trial, the SHARP trial has three principal investigators, each having a specified focus. The administrative PI (G.A.C.) oversees the work of the Clinical Coordinating Center and chairs the Steering, Publication and Ancillary Study Committees. The protocol PI (D.Y.) chairs the Executive and Biorepository Committees and oversees the scientific aspects related to the trial protocol. The Statistical and Data Coordinating Center PI (V.L.D-M.) is responsible for all aspects related to statistical design and analysis, study database development, and management and data reporting and sharing. A study-appointed independent Medical Safety Monitor and an NIH-NIDDK appointed Data and Safety Monitoring Board (DSMB) oversee safety in the SHARP trial. The DSMB meets every six months to review study progress and accumulated data. Their main responsibilities are to ensure that study participants are not exposed to unnecessary or unreasonable risks, and that the study is conducted with high scientific and ethical standards. The DSMB is assisted by the independent safety monitor who reviews and adjudicates all serious adverse events throughout the study.
Study Hypothesis and Aims
The primary aim of the SHARP trial is to test the hypothesis that ERCP with miES will reduce the risk of a subsequent acute pancreatitis episode by 33%. This hypothesis will be tested by comparing the incidence of acute pancreatitis episodes that occur >30 days after the randomization procedure. The SHARP Steering Committee agreed that a 33% relative risk reduction is the minimally acceptable threshold above which the benefit of ERCP with miES would offset its known short- and long-term risks, including post-ERCP pancreatitis and post-sphincterotomy re-stenosis, respectively. Acute pancreatitis will be defined by consensus criteria during the baseline assessment and follow-up (Table 1).
TABLE 1.
SHARP Enrollment Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patient must consent to be in the study and must have signed and dated an approved consent form. | Prior minor papilla therapy (endoscopic or surgical) |
| ≥18 years | Calcific chronic pancreatitis, defined as parenchymal or ductal calcifications identified on computed tomography or magnetic resonance imaging scan that is reviewed by an expert radiologist at the recruiting site. |
Two or more episodes of acute pancreatitis, with each episode meeting at least two of the following three criteria:
|
Main pancreatic duct stricture |
| At least one episode of acute pancreatitis within 24 months of enrollment | Presence of a structural etiology for acute pancreatitis, such as anomalous pancreatobiliary union, periampullary mass, or pancreatic mass lesion on imaging |
| Pancreas divisum confirmed by prior MRCP that is reviewed by an abdominal radiologist at the recruiting site. | Presence of a local complication from acute pancreatitis which requires pancreatogram |
| By physician assessment, there is no certain explanation for recurrent acute pancreatitis. | Daily use of opioid medication for abdominal pain for the past three months |
| Subjects must be able to fully understand and participate in all aspects of the study, including completion of questionnaires and telephone interviews, in the opinion of the clinical investigator. | Medication as the etiology for acute pancreatitis by physician assessment |
| TWEAK score ≥4* | |
| Hypertriglyceridemia, defined as a serum triglyceride level >500mg/dL during a prior episode of acute pancreatitis | |
| Hypercalcemia, defined as a corrected serum calcium level >10.5mg/dL associated with a prior episode of acute pancreatitis | |
| Clinical presentation consistent with type I or type II autoimmune pancreatitis | |
| Pregnancy (urine test) | |
| Low probability of follow-up on a regular basis to achieve study objectives | |
| Life expectancy < 6 months based on the opinion of the physician investigator | |
| Incarceration |
TWEAK = Tolerance, Worry, Eye-opener, Amnesia, and “K”-ut down used to describe alcohol use. CECT indicates contrast-enhanced computed tomography scan; MRI, magnetic resonance imaging.
There are several potential secondary benefits of reducing ARP episodes, which will be explored through secondary aims of the trial. The frequency of acute pancreatitis, defined as the incidence rate ratio (episodes/time pre- and post-randomization), the change in patient-centered outcomes and progression to chronic pancreatitis and its sequelae will all be compared between the two treatment arms. A biological and imaging repository will be established for future exploratory analyses of genetic, laboratory and radiological associations with outcomes; the Standard Operating Procedures for the SHARP biological repository were derived from the ongoing Consortium for the study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer.10
Enrollment Criteria
Inclusion/exclusion criteria are summarized in Table 1. The possible presence of a pancreatic duct stricture or structural etiology for acute pancreatitis will be assessed for all patients during review of prior cross-sectional imaging, including magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP). If no evidence of either exclusion is identified, and all other eligibility criteria are met, patients who consent to randomization will be scheduled for the pre-randomization EUS.
The threshold at which alcohol causes acute pancreatitis is poorly understood and patient-dependent. Alcohol use will also be quantified using the TWEAK (Tolerance, Worry, Eye-opener, Amnesia, and “K”-ut down) during the baseline assessment.11,12 The SHARP trial excludes patients with a high probability of having alcoholic pancreatitis.
Sites and Recruitment
The SHARP trial will be completed at 17 centers in the U.S., Canada, Netherlands, and U.K. Each center represents a regional referral center for pancreatic disorders and ERCP. The recruitment process will not involve any procedures that may be construed as coercive, and there will be no restriction to recruitment based on sociodemographic factors including age, gender, or ethnic characteristics. Participants will be recruited from within clinical practices. It is expected that the informed consent process will occur in ambulatory clinics. If a patient is agreeable to participate, randomization procedures will commence thereafter. In an effort to stimulate recruitment and raise awareness, referring physician practices will be reminded of the SHARP trial through regular communications. The trial began enrollment in September, 2018 with a planned primary analysis to be completed in September, 2023. All authors had access to the study data and reviewed and approved the final manuscript.
Baseline Assessments, Randomization, and Study Procedures
In addition to assessments related to eligibility, all participants will be administered questionnaires and medical records will be reviewed to collect information relevant to study objectives (Table 2). If a participant has not been formally diagnosed, assessment for diabetes mellitus and exocrine insufficiency will be performed. Study participants will be asked to provide blood and urine samples for a study biorepository which will be processed within 4 hours of collection and stored at −80°C until testing.
TABLE 2.
Summary and Schedule for Data Collection for the SHARP Trial
| SHARP Data Collection | Study Timepoint |
|---|---|
| Assessment of two qualifying attacks of Acute pancreatitis, Inclusion and exclusion criteria, TWEAK questionnaire, Informed consent | Screening |
| Demographics, socioeconomic status, prior acute pancreatitis episodes (number, timing, severity), genetic test results (if performed) | Baseline visit |
| Tobacco and alcohol use | Baseline visit, 6-month, 18-month, 36-month, 48-month visits |
| Pain and disability | Baseline visit, every 6-months until 48-month visit |
| Quality of life and pain type (PROMIS Global health, PROMIS-29, PROMIS Nociceptive and Neuropathic pain) | Baseline visit, 6-month, 18-month, 36-month, 48-month visits |
| Medical History | Baseline visit, 18-month visit |
| Evaluation for Exocrine and Endocrine insufficiency | Baseline or Randomization visit; 18-month visit |
| Concomitant medication | Randomization; 30-day visit; every 6 months until 48-month visit |
| Biological sample collection (blood, urine) | Baseline or randomization visit |
| EUS + sham or EUS + ERCP with miES | Randomization visit |
| Abdominal x-ray | Day 30-visit |
| Best Guess (Subject, Study Coordinator, Blinded Physician) | Day 30-visit; 18-month visit; 36-month visit |
| Patients Global Impression of Change | 6-month, 12-month, 24-month, 36-month visits |
| MRI and MRCP | 18-visit visit |
| Any adverse event | Until Day-30 visit |
| Severe adverse event (Acute pancreatitis event, pancreatitis-related pain event, any other severe event) | Throughout study until month 48-visit |
| ERCP after randomization | Any time during follow-up |
| Interval development of calcific chronic pancreatitis, exocrine and endocrine insufficiency | 6-month, 18-month, 24-month, 36-month, 48-month visits |
PROMIS indicates Patient-Reported Outcomes Measurement Information System.
A linear echoendoscope will be used for EUS exam. In addition to confirming the absence of exclusion criteria, the EUS exam will assess for pancreatic duct diameter and document parenchymal and ductal changes in the pancreas. Prior to randomization, the amount and type of intravenous fluids to be administered during the periprocedural period (irrespective of treatment allocation) will be documented.
Unless a definitive obstructive lesion or any other exclusion criterion is identified during EUS, the participant will be randomized 1:1 to either EUS + sham or EUS + ERCP with miES. Clinical center, pancreatic duct diameter and a dichotomized variable for number of acute pancreatitis episodes (1–2 vs ≥3) in the two years prior to randomization will be included in the randomization algorithm to ensure a balanced distribution between treatment arms. The SHARP trial utilizes a web-based central randomization system housed at the SHARP Statistical and Data Coordinating Center at the Medical University of South Carolina.
Participants randomized to treatment (ERCP + miES), will undergo the procedure following the EUS, under the same anesthetic. Indomethacin or diclofenac (100mg per rectum) will be administered for post-ERCP pancreatitis prophylaxis at the onset of the ERCP procedure in patients with no known allergy. Techniques for minor papilla cannulation will be left to the discretion of the treating endoscopist. Variables of interest pertaining to ERCP include the use of enhanced maneuvers to facilitate cannulation (e.g., intravenous secretin and mucosal contrast agents such as methylene blue), performance of precut sphincterotomy, extent of pancreatography, presence of complete vs. incomplete pancreas divisum, miES technique, and type of prophylactic pancreatic duct stent. This technical approach is intended to mimic real-life practice and maximize the study’s external validity. These variables will be tracked in order to determine their impact on study outcomes (Table 2). If a mucosal contrast agent was not used to assist in cannulation, 3 ml of dilute dye will be injected into the duodenum before the end of the procedure; and, a stent will be left in the duodenum if one cannot be placed into the pancreatic duct.
For participants assigned to the sham group, a duodenoscope will be passed to the second portion of the duodenum. The minor papilla will be photo documented, 3 ml of dilute dye such as methylene blue in US sites, or similar dye in non-US sites, will be injected into the duodenum, and a plastic stent will be left in the duodenum.
Follow-up Procedures and Guidelines for Intervention
Enrolled participants will be followed for a maximum of 48 months depending on their time of enrollment. Scheduled in-person follow-up will occur at day 30 and month 18 (Table 2). These in-person visits will be directed by blinded study personnel at each site. During the day 30 follow-up, the participant will be assessed for adverse events and complete an abdominal X-ray to confirm spontaneous passage of the pancreatic duct stent. During the month 18 follow-up visit, participants will undergo an MRI with MRCP to assess for morphologic changes in the pancreas along with functional pancreas testing. All other follow-up visits will be completed by telephone every 6 months (scheduled) until the end of the study or ad hoc (if symptoms suggestive of acute pancreatitis develop). The date of diagnosis of acute pancreatitis as reported by the enrolling site will be used for the primary outcome. Investigators will make every effort to avoid ERCPs during the follow-up period until this outcome has been reached. The SHARP protocol provides guidance (Table 3) on repeat ERCPs to assist a blinded investigator who is evaluating participants during the follow-up period. Ultimately the decision to recommend ERCP during the follow-up period is based on the best clinical judgement of a blinded physician investigator evaluating the participant.
TABLE 3.
Guidelines for ERCP During the Follow-up Period
| 1. Two or more episodes of acute pancreatitis |
| 2. One episode of acute pancreatitis with local complication as defined by Atlanta criteria, that warrants pancreatogram |
| 3. One episode of acute pancreatitis plus at least one independent pancreatitis-related pain event. |
| 4. Interval development of symptomatic pancreatic duct obstruction (main duct stricture or stone) on cross sectional imaging |
| 5. Two or more pancreatitis-related pain events and minimum follow-up of 12 months |
Maintenance of the Blind
All sites will have a minimum of two physician investigators, of which one will serve as blinded investigator and one as unblinded investigator for each participant. In order to maintain blinding of participants, healthcare providers making clinical decisions that may directly impact the primary endpoint, and study coordinators who will obtain outcome data, the endoscopy report will include language indicating the participation in a blinded research study in which he/she may or may not have undergone the ERCP with miES procedure. All facility and professional charges associated with the randomization procedure will be billed to the research study.
To test the effectiveness of blinding procedures, participants and blinded study personnel will be asked at the 30-day follow-up assessment to which group they believe the participant was assigned. This question will be repeated at the Month 18 visit. If the participant and/or assessor becomes knowledgeable of treatment assignment at any point during study participation, this will be documented in the study database. The participant will remain in the study and be part of the analysis population (intention to treat analysis).
Observational Cohort
Approximately 100 patients who meet all eligibility criteria but who decline randomization, decline ERCP or those in whom ERCP is not recommended by the unblinded physician will be invited to participate in an observational cohort study (Fig. 1). Patients who provide consent will be followed for subsequent acute pancreatitis episodes, systematic, semiannual assessments for patient-centered outcomes and clinical (observational) assessments for the interval development of chronic pancreatitis and exocrine or endocrine insufficiency. These participants will also provide biological samples at baseline for the SHARP biorepository.
Statistical Analysis Plan and Sample Size Estimation
Natural history studies specific to pancreas divisum are lacking, but retrospective cohort studies and one surgical series suggest the recurrence rate following minor papilla stenting, miES, or surgical sphincteroplasty is 15–50%.13–15 Given the short-term risk of post-ERCP pancreatitis (~10%), costs of ERCP, and potential for post-sphincterotomy re-stenosis, we believe a minimum effect size of 33% relative risk reduction is of clinical relevance. Based on the limited information in the literature, we anticipate that the risk of recurrence within 12 months of randomization is 60% in the EUS + sham arm. A 33% relative risk reduction would demonstrate an event rate of 40% in the miES arm. Assuming an exponential hazard (constant hazard), these proportions translate into median times to recurrence of 9.1 and 16.3 months, respectively. Setting the subject accrual period to 42 months, a maximum follow-up period of 48 months, a 2-sided type I error of 5%, power of 85%, and non-adherence rate of 20%, the trial requires a total sample size of 234 randomized participants (164 observed events). The non-adherence factor is primarily to account for the potential dilution of effect size due to technical failures in the miES arm, where a patient is assigned to the miES arm but the treating endoscopist cannot access the duct as well as the potential competing risks.
It is possible that participants will experience a pancreatitis-related event such as severe pain, without reaching the actual event of primary interest (acute pancreatitis). In some of these cases, participants may be treated with an ERCP. An ERCP for pain alone will be treated as a competing risk in the primary analysis. We recognize that sample size estimation is based on assumptions and if the assumed event rate is lower than expected, then we may begin to see a decrease in power. For this reason, the SHARP design includes a blinded sample size re-estimation plan.16,17 This information will be shared with the Data and Safety Monitoring Board during closed session and it will be the DSMB’s decision to recommend an increase in the total sample size.
The SHARP trial does not incorporate any interim analyses to stop the trial early for overwhelming efficacy or for futility of the primary outcome. To stop the trial early would lead to potentially biased estimates of the treatment effect as well as reduced data for interpretation of the secondary outcomes that require long term follow up. The study team does have in place a detailed safety monitoring plan that will allow the DSMB to examine safety data and provide recommendations to the NIDDK if the trial should indicate stopping early due to safety issues. The primary efficacy analysis will be conducted when all participants have completed follow up. The primary hypothesis will be tested using the intention-to-treat analysis population (all randomized) and a two-sided 0.05 level of significance with the method of Fine and Gray for modeling the hazard of the sub distribution in the presence of competing risks. Two variables included in the randomization scheme, duct diameter and number of attacks within 24 months prior to enrollment, will be included as covariates in the primary analysis. As an exploratory analysis of the primary, the model will be run with additional potential covariates/prognostic variables such as the presence of chronic pain at baseline, number of EUS features (parenchymal and ductal), age, gender, race, and presence of endocrine and exocrine insufficiency. All adverse events will be summarized in terms of frequency of the event, number of participants having the event, severity, expectedness (anticipated/unanticipated) and relatedness to the study treatment.
DISCUSSION
Rationale
The practice of ERCP with miES for patients with idiopathic ARP and pancreas divisum is widespread yet decades of clinical practice have failed to refute or establish its utility, observational research suggests a benefit, and its practice in the West is widespread. It is biologically plausible that pancreas divisum represents an obstructive pancreatopathy, and thus treating the minor papillary orifice could ameliorate acute pancreatitis episodes. Pancreatic duct obstruction and transient increases in intraductal pressure is a well-recognized mechanism for acute pancreatitis.18–25 However, pancreas divisum, a congenital variant that is observed in roughly 10% of the population, is not unequivocally overrepresented in ARP patient populations. Moreover, there seems to be a clear association between pancreas divisum and several genetic susceptibility mutations, although the reason for this phenomenon is poorly understood.26,27 For example, it is suspected that mutations in the Cystic Fibrosis Transmembrane Receptor gene, which impairs the amount and composition of pancreatic fluid, will relate to the probability of ARP following miES. The relationship between susceptibility mutations and response to miES will be explored using the SHARP Biological Repository; testing for known susceptibility mutations will be completed as part of the post hoc primary analysis.
Justification for Primary Outcome and Importance of Blinding
A reduction in the risk of acute pancreatitis was selected as the primary outcome since this is the principal objective of clinicians who offer ERCP with miES in clinical practice. In addition, it is implausible that an intervention which fails to reduce the risk of subsequent acute pancreatitis will reduce the risk of other sequelae such as interval development of chronic pancreatitis or its associated complications. While episodes of severe acute pancreatitis are typically self-evident, powering a study to reduce the risk of severe acute pancreatitis is impractical since these episodes represent the minority of attacks experienced by individuals with idiopathic ARP. There also was consideration in using incidence rate ratio for the primary outcome. While reducing episode frequency is important, this requires careful adherence to the original treatment allocation for a long follow-up period. As participants develop episodes of acute pancreatitis during follow-up, it will be very difficult for clinicians to withhold interventions (including ERCP with miES) and preserve the blind.
A recognized risk of miES is the development of symptomatic post-sphincterotomy stenosis. While large-scale, prospective studies with discrete criteria are lacking, rates of post-sphincterotomy stenosis are estimated to be 20% or higher.28–32 There are no objective definitions for post-sphincterotomy stenosis. However, symptomatic post-sphincterotomy stenosis is expected to result in either acute pancreatitis (primary outcome) or pancreatitis-related pain event(s) as defined previously. Differences in these outcomes between participants randomized to EUS + ERCP with miES and EUS + sham will clarify whether the risk of post-sphincterotomy stenosis is outweighed by its benefit.
Blinding adds substantial complexity to the SHARP protocol but is critically important in the assessment of outcomes. For SHARP participants who contact their providers with pain reminiscent of their prior pancreatitis episodes, knowledge of treatment allocation is likely to impact recommendations. In such a scenario, participants who knew they underwent miES may be less likely to seek medical attention since they received “maximal treatment” previously. Similarly, clinicians with knowledge of treatment allocation may have a lower threshold to recommend hospital evaluation or admission (eg, laboratory or radiology testing, emergency room evaluation) to patients assigned to the sham group. In addition to clinical care, patient-reported outcomes are most susceptible to bias in an unblinded study, and yet are of paramount importance in SHARP. Quality of life is lower among patients with chronic pancreatitis and ARP, yet difficult to quantify in day-to-day clinical practice. Abdominal pain and pain-related disability may be increasingly burdensome for some patients with idiopathic ARP, especially if the disease is evolving to chronic pancreatitis. Masking subjects and those assessing outcomes will reduce bias in the measurement of these important patient-reported outcomes.
Impact and Future Directions
The absence of viable medical therapies, the plausibility that improving pancreatic flow may improve the disease course, the available (but weak) data in support of miES, and patients’ “desperation” have created the perfect storm for more than three decades of endoscopic intervention for idiopathic ARP. The SHARP trial will prove whether or not ERCP should be performed for idiopathic ARP with pancreas divisum.
Disclosures:
Grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK):DK112673 (for planning phase) and DK116743 (for trial phase).
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
Clinical trials registration: (http://ClinicalTrials.gov number, )
REFERENCES
- 1.Bang UC, Benfield T, Hyldstrup L, et al. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146:989–994. [DOI] [PubMed] [Google Scholar]
- 2.Sankaran SJ, Xiao AY, Wu LM, et al. Frequency of Progression From Acute to Chronic Pancreatitis and Risk Factors: A Meta-analysis. Gastroenterology. 2015;149:1490–1500 e1491. [DOI] [PubMed] [Google Scholar]
- 3.Cote GA, Yadav D, Abberbock JA, et al. Recurrent Acute Pancreatitis Significantly Reduces Quality of Life Even in the Absence of Overt Chronic Pancreatitis. Am J Gastroenterol. 2018;113:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980;21:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakman YG, Safdar K, Freeman ML. Significant clinical implications of prophylactic pancreatic stent placement in previously normal pancreatic ducts. Endoscopy. 2009;41:1095–1098. [DOI] [PubMed] [Google Scholar]
- 6.Lans JI, Geenen JE, Johanson JF, et al. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc. 1992;38:430–434. [DOI] [PubMed] [Google Scholar]
- 7.Michailidis L, Aslam B, Grigorian A, et al. The efficacy of endoscopic therapy for pancreas divisum: a meta-analysis. Ann Gastroenterol. 2017;30:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogel EL, Toth TG, Lehman GA, et al. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas. 2007;34:21–45. [DOI] [PubMed] [Google Scholar]
- 9.Guda NM, Muddana V, Whitcomb DC, et al. Recurrent Acute Pancreatitis: International State-of-the-Science Conference With Recommendations. Pancreas. 2018;47:653–666. [DOI] [PubMed] [Google Scholar]
- 10.Fisher WE, Cruz-Monserrate Z, McElhany AL, et al. Standard Operating Procedures for Biospecimen Collection, Processing, and Storage: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47:1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley KA, Boyd-Wickizer J, Powell SH, et al. Alcohol screening questionnaires in women: a critical review. JAMA. 1998;280:166–171. [DOI] [PubMed] [Google Scholar]
- 12.Russell M, Martier SS, Sokol RJ, et al. Detecting risk drinking during pregnancy: a comparison of four screening questionnaires. Am J Public Health. 1996;86:1435–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attwell A, Borak G, Hawes R, et al. Endoscopic pancreatic sphincterotomy for pancreas divisum by using a needle-knife or standard pull-type technique: safety and reintervention rates. Gastrointest Endosc. 2006;64:705–711. [DOI] [PubMed] [Google Scholar]
- 14.Chacko LN, Chen YK, Shah RJ. Clinical outcomes and nonendoscopic interventions after minor papilla endotherapy in patients with symptomatic pancreas divisum. Gastrointest Endosc. 2008;68:667–673. [DOI] [PubMed] [Google Scholar]
- 15.Borak GD, Romagnuolo J, Alsolaiman M, et al. Long-term clinical outcomes after endoscopic minor papilla therapy in symptomatic patients with pancreas divisum. Pancreas. 2009;38:903–906. [DOI] [PubMed] [Google Scholar]
- 16.Todd S, Valdes-Marquez E, West J. A practical comparison of blinded methods for sample size reviews in survival data clinical trials. Pharm Stat. 2012;11:141–148. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead J Predicting the duration of sequential survival studies. Drug Information Journal. 2001;35:1387–1400. [Google Scholar]
- 18.Lerch MM, Saluja AK, Runzi M, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993;104:853–861. [DOI] [PubMed] [Google Scholar]
- 19.Runzi M, Saluja A, Lerch MM, et al. Early ductal decompression prevents the progression of biliary pancreatitis: an experimental study in the opossum. Gastroenterology. 1993;105:157–164. [DOI] [PubMed] [Google Scholar]
- 20.Chen JW, Thomas A, Woods CM, et al. Sphincter of Oddi dysfunction produces acute pancreatitis in the possum. Gut. 2000;47:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sofuni A, Maguchi H, Mukai T, et al. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastro Hepatol. 2011;9:851–858; quiz e110. [DOI] [PubMed] [Google Scholar]
- 22.Arendt T, Hansler M, Stoffregen C, et al. Does high pancreatic duct pressure compromise the duct mucosal barrier function to pancreatic exocrine proteins? APMIS. 1996;104:615–622. [DOI] [PubMed] [Google Scholar]
- 23.Haciahmetoglu T, Ertekin C, Dolay K, et al. The effects of contrast agent and intraductal pressure changes on the development of pancreatitis in an ERCP model in rats. Langenbecks Arch Surg. 2008;393:367–372. [DOI] [PubMed] [Google Scholar]
- 24.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. [DOI] [PubMed] [Google Scholar]
- 25.Munigala S, Kanwal F, Xian H, et al. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clin Gastro Hepatol. 2014;12:1143–1150 e1141. [DOI] [PubMed] [Google Scholar]
- 26.Ballard DD, Flueckiger JR, Fogel EL, et al. Evaluating Adults With Idiopathic Pancreatitis for Genetic Predisposition: Higher Prevalence of Abnormal Results With Use of Complete Gene Sequencing. Pancreas. 2015;44:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertin C, Pelletier AL, Vullierme MP, et al. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. Am J Gastroenterol. 2012;107:311–317. [DOI] [PubMed] [Google Scholar]
- 28.Clarke B, Slivka A, Tomizawa Y, et al. Endoscopic therapy is effective for patients with chronic pancreatitis. Clin Gastro Hepatol. 2012;10:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elton E, Howell DA, Parsons WG, et al. Endoscopic pancreatic sphincterotomy: indications, outcome, and a safe stentless technique. Gastrointest Endosc. 1998;47:240–249. [DOI] [PubMed] [Google Scholar]
- 30.Heyries L, Barthet M, Delvasto C, et al. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc. 2002;55:376–381. [DOI] [PubMed] [Google Scholar]
- 31.Joo YW, Yoon JH, Cho SC, et al. Endoscopic pancreatic sphincterotomy: indications and complications. Korean J Intern Med. 2009;24:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dufault DL, Elmunzer BJ, Cotton PB, et al. Adverse Events and Reinterventions Following Pancreatic Endoscopic Sphincterotomy. Pancreas. 2018;47:880–883. [DOI] [PubMed] [Google Scholar]