1. Introduction
The proliferation of prescription opioids for the treatment of chronic pain has been associated with significant increases in the non-medical use of prescription opioids, opioid use disorder, and opioid overdose deaths [5, 13, 58]. The prescription of high dose opioids, typically defined as morphine equivalent daily doses (MEDD) of 90 or more milligrams, is specifically associated with adverse drug events [9, 19, 21, 37, 52], morbidity and mortality [23]. Long-term opioid therapy (LTOT) has also been associated with the development of opioid use disorder (OUD); [23] the risk of incident OUD is greater with higher dose and longer duration of LTOT [9, 21, 47]. Despite the substantial body of evidence linking an increased risk of OUD with specific prescription opioid use patterns [41], the association between receipt of prescription opioids and subsequent heroin use is less understood. The use of heroin increases the risk of opioid-attributable overdose, the exposure to potential contaminants such as illicitly manufactured fentanyl, and an increased likelihood of acquisition and transmission of blood borne diseases such as HIV and hepatitis C virus [8, 34, 46].
Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important. Clinicians may prescribe opioids to men and women during military service or soon after discharge to address injuries sustained during combat, chronic pain, and/or psychological and physical ailments that can arise while readjusting to civilian life [27]. Prior studies conducted by our research team have shown that hydrocodone, codeine, and oxycodone are the three most common opioids prescribed to US veterans engaged in care [20]. A recent report from the Department of Veterans’ Affairs (VA) notes that more than one million US veterans engaged in care in the VA health system receive prescription opioids for pain, and nearly half of them continue beyond 90 days [22, 53]. Appropriate prescribing of opioids to this population, with a higher underlying prevalence both of substance use disorders and of pain conditions than the general population, is particularly nuanced and challenging [3, 5, 28].
The impact of prescription opioid receipt on subsequent heroin use is a phenomenon that has been described in the US mainstream media and among particular subpopulations [10, 33, 38, 49, 55], but the scope of this issue among veterans is still largely unexplored. In the current era, regular heroin use among the treatment-seeking general population is often preceded by non-medical use of prescription opioids [14]. We recently demonstrated that non-medical use of prescription opioids (NMUPO) was a strong and independent predictor of heroin initiation among veterans receiving care in the VA [4]. However, it is less clear how receipt of prescription opioids from a VA provider is associated with later heroin use. A better understanding of the relationship between specific opioid prescribing practices and the subsequent use of heroin could assist in creating strategies that mitigate such risk in veterans.
2. Material and methods
2.1. Data
This study used longitudinal behavioral and pharmacy fill/refill data from the Veterans Aging Cohort Study (VACS); detailed data collection and survey methodology for VACS are previously described [35, 36]. In brief, the VACS is a prospective cohort study of military veterans living with HIV and uninfected veterans receiving medical care at eight US VA sites. Since June of 2002, VACS has enrolled more than 7,000 patients from the Infectious Disease or General Medical clinics in Atlanta, Baltimore, Houston, Los Angeles, Pittsburgh, Washington DC and multiple sites in New York City. This longitudinal study contains variables from six follow-up behavioral surveys, completed approximately every 18 months, from 2002 to 2012. The survey data are linked to robust VA electronic medical records (EMR) containing data on prescribed medications based on pharmacy dispensing, medical diagnoses, and laboratory results for each patient. The VACS was approved by the institutional review boards at each participating VA Medical Center and affiliated academic institutions.
2.2. Participant eligibility
Of the 7,502 VACS participants who were potentially eligible for inclusion in our study because they had completed at least one survey, we excluded 689 (9.2%) of participants who reported any illicit opioid use (including heroin and non-medical use of prescription opioids) in the year prior to the first survey the patient completed (baseline survey). We also excluded participants who were never prescribed opioids from the VA during the 10 years of follow-up, n=2980 (39.7%), as well as participants who did not complete at least one additional follow-up survey, n=263 (3.5%). The final analytic sample consisted of 3,570 veterans. In all analyses, dropout was defined as a failure to complete any additional surveys after the baseline survey, while having been enrolled in the VACS study for greater than or equal to 2 years.
2.3. Primary exposures
Consistent with the methods outlined in previous VACS analyses using prescription opioid data from pharmacy records [20, 24, 58], we determined receipt of all outpatient opioids, including codeine, hydrocodone, oxycodone, oxycodone, morphine, fentanyl, hydromorphone, methadone for the treatment of pain, tramadol and propoxyphene. We then determined: 1) the average MEDD supplied in the year prior to each follow-up survey; and 2) number of days supplied in the year prior to each follow-up survey, starting with the baseline survey. Average MEDD was dichotomized into high dose (≥90 mg) and low dose (<90mg). This definition of high-dose prescription opioid receipt was based on VA and CDC opioid prescribing guidelines [2, 18]. The number of days supplied in the year prior was used to create a dichotomous indicator variable for long-term opioid therapy, defined as ≥90 days of continuous use, allowing for a 30 day gap between fill/refill in a given year [44]. We also categorized opioids into short-acting (i.e., with a duration of action generally less than 12 hours) versus long-acting medications (including sustained- and extended-release products), according to Drug Enforcement Administration classifications [56].
2.4. Primary outcome
Self-reported heroin use during the follow-up surveys was ascertained starting with a participant’s first follow-up survey and was based on the time-updated survey item: “How often in the past year you used each drug – Heroin?” Past year heroin use was defined as any change in respondent’s answer to the previous survey question from “Never” or “No use in the last year”, to indicating any frequency of heroin use in the previous year. We used the date of the corresponding survey to approximate the date of past year heroin use.
2.5. Confounders and other covariates
Selection of independent variables and potential confounders that were included in the analysis was based on a review of prior literature [17, 32, 40, 43, 45]. Baseline VACS survey demographics included age, sex, race/ethnicity, marital status, employment status, and gross annual income. HIV status was identified using VA Immunology Case Registry and Hepatitis C virus status was determined using International Classification of Disease (ICD)-9 codes and laboratory data. Level of social isolation was ascertained from responses to the following baseline survey items: “How often do you see or hear from relatives or close friends? Would you say less than once a month, about once a month, a few times a month, a few times a week, every day?” and “How many close friends or family do you have with whom you feel at ease, can talk about private matters, or can call on for help?” We determined whether the participant had ever been homeless or stayed at a homeless shelter from the following questions on the baseline survey: “Have you ever been without a permanent address that you call home?” and “Have you ever stayed one or more nights in a shelter, on the street, in a park or an abandoned building?” Additionally, we included time-updated survey variables for: other drug use (including marijuana, cocaine, and methamphetamines); unhealthy alcohol use; and diagnoses of post-traumatic stress disorder (PTSD) and major depression. Any past-year use of marijuana, cocaine and methamphetamines was dichotomized as yes/no. Unhealthy alcohol use was characterized using the Alcohol Use Disorders Identification Test (AUDIT)-C; a score of 4 and above indicated unhealthy alcohol use for men, and a threshold of 3 for women [16, 42]. Diagnoses of PTSD, major depression, and opioid use disorder were ascertained from ICD-9 codes and updated at every survey wave. Pain interference in daily life was ascertained from responses to the following time-updated survey item: “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” We dichotomized participants’ responses into “some amount of pain interference in daily life” vs. “no pain interference in daily life”.
2.6. Analysis
We analyzed patterns of opioid prescribing over time for each of the six follow-up waves using the Cochran-Armitage test for trend. We used χ2 or Fisher’s exact tests to examine the baseline correlates of receipt of high dose prescription opioids, as well as past year heroin use at any time over the follow-up period. Unadjusted Kaplan–Meier analysis and the log-rank test were then used to calculate the incidence of past year heroin use and compare time to first report of heroin use during follow-up, stratifying by the past year receipt of high-dose opioid prescription at baseline and past year receipt of long-term opioid therapy, respectively. Next, we used Cox proportional hazards regression to estimate crude hazard ratios (CHRs) and 95% confidence intervals (CIs) related to factors associated with heroin use. We then constructed a separate multivariable Cox model, including all variables assessed in bivariable analyses, for the two main exposure variables: prior receipt of high-dose opioid prescription and prior receipt of long-term opioid therapy. All variables were found to meet the proportional hazards assumption for the Cox regression models (i.e. none exhibited significant deviance from this assumption at p < 0.05) [12]. Since employment status was highly correlated with education (p<0.001) and income (p<0.001), we did not include it in the fully adjusted final model.
We created inverse probability of censoring weights (IPCW) to account for potential biases arising from differential dropout. Weights were obtained through fitting a weighted pooled logistic regression model for dropout, including baseline and time-varying predictors of dropping out, defined as not completing all six follow-up surveys. We included a robust sandwich estimator in all Cox regression models to account for potential clustering heterogeneity by study site, and the estimation required to create the weights [29, 31]. Analyses were conducted using SAS version 9.4.
2.7. Sensitivity Analyses
To address the potential for confounding and selection bias due to the inclusion of time-updated covariates affected by prior exposure using a standard regression approach, we conducted a sensitivity analysis using inverse probability of treatment and censoring weights [15]. In brief, we used weighting to create a pseudo-population in which exposure and dropout were independent of measured confounders. We used pooled logistic models to estimate the inverse of the probability of each participant’s probability of receiving a high-dose prescription opioid and receiving long-term opioid therapy, respectively. The numerator of the stabilized weights included baseline covariates that predicted the probability of the exposure, and the denominator included baseline and time-updated covariates that were included in the main analysis. We examined the weights for means, standard deviations, and extreme minimum and maximum values. Finally, we conducted a sensitivity analysis in which opioid dose was categorized as a three-level variable: low (0–49 MME), medium (50–89 MME), and high (≥90 MME).
3. Results
3.1. Sample Composition
The mean age of VACS members in the final analytic sample was 49.8 (SD = 9.0 years); 2302 (65.1%) participants were black, 324 (9.2%) were Hispanic, and 764 (21.6%) were white. The majority of participants were male (94.5%); approximately half of them were living with HIV and 32.9% were positive for hepatitis C virus infection. At baseline, 235 (6.6%) participants reported ever having engaged in injection drug use, and 38.6% reported having ever spending the night in a homeless shelter, street, or abandoned building. At baseline, 28.0% of participants reported that they were currently working for wages, 9.9% reported that were currently seeking employment and unemployed for less than one year, 33.4% reported that they were currently unable to work, 7.6% reported being self-employed, and 16.2% reported being retired. Of the 3,570 participants who were in the analytic sample, 1160 (32.5%) were lost to follow-up over the study period.
3.2. Trends in prescription opioid receipt from the VA
Of the 3,570 eligible participants, the proportion of study participants who were receiving high-dose prescription opioids (≥ 90 mg MEDD) did not change over the follow-up period and ranged from 4.4% to 5.9% (Cochran-Armitage test for trend p = 0.222). The proportion receiving long-term opioid therapy (≥90 consecutive days) steadily increased from 23.3%, in 2002 to 39.9%, in 2012 (Cochran-Armitage test for trend p<0.001). A vast majority of participants received only short-acting opioids from the VA (see Table 1).
Table 1.
Characteristics of prescription opioid dose and duration dispensed to VACS participants, 2002–2012
| Survey | BL | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Days of Consecutive Opioid Rx Use, Previous Year | |||||||
| Short-Term Use (≥1 day & <90 days) | 1188 (76.7%) | 511 (67.1%) | 754 (67.4%) | 767 (66.0%) | 807 (61.1%) | 789 (62.0%) | 745 (60.1%) |
| Long-Term Use (≥90 days) | 361 (23.3%) | 251 (32.9%) | 365 (32.6%) | 395 (34.0%) | 514 (38.9%) | 483 (38.0%) | 494 (39.9%) |
| Opioid Rx MEDD, Previous Year | |||||||
| 1–50mg | 1353 (88.7%) | 665 (88.0%) | 977 (88.5%) | 1011 (87.7%) | 1156 (88.6%) | 1123 (88.8%) | 1108 (89.6%) |
| 51–90mg | 104 (6.8%) | 49 (6.5%) | 69 (6.2%) | 74 (6.4%) | 86 (6.6%) | 84 (6.6%) | 74 (6.0%) |
| ≥90mg | 68 (4.5%) | 42 (5.5%) | 58 (5.2%) | 68 (5.9%) | 62 (4.7%) | 58 (4.6%) | 54 (4.4%) |
| Median MEDD in mg (IQR) | 31.1 (13.4–48.8) | 32.0 (13.2–50.7) | 34.5 (16.0–53.0) | 39.1 (21.4–56.8) | 40.6 (23.5–57.7) | 40.3 (24.6–56.0) | 37.1 (21.2–53.0) |
| Opioid Formulation | |||||||
| Short-Acting Only | 1113 (81.7%) | 386 (78.1%) | 626 (79.6%) | 602 (77.6%) | 752 (81.2%) | 775 (82.3%) | 807 (84.7%) |
| Long-Acting Only | 54 (4.0%) | 33 (6.7%) | 41 (5.2%) | 67 (8.6%) | 59 (6.4%) | 45 (4.8%) | 45 (4.7%) |
| Both | 195 (14.3%) | 75 (15.2%) | 119 (15.1%) | 107 (13.8%) | 115 (12.4%) | 122 (13.0%) | 101 (10.6%) |
Note: mg=milligrams; Rx=prescription; MEDD=morphine-equivalent daily dose; IQR=Interquartile Range
3.3. Risk factors for past year heroin use
Over the 10-year follow-up period, 174 (4.9%) participants reported past year heroin use. At baseline, study participants who reported past year heroin use were more likely to be Black, report a lower gross annual income, report past year cocaine and methamphetamine use, and have experienced homelessness and social isolation. (Table 2)
Table 2.
Baseline characteristics associated with recent heroin use among veterans participating in VACS, 2002–2012
| Characteristic | Total (%) (N = 3,570) | Reported Recent Heroin Use | p – value* | |
|---|---|---|---|---|
| Yes (%) (N = 174) | No (%) (N =3,396) | |||
| Sex | 0.025 | |||
| Male | 3373 (94.5) | 171 (98.3) | 3202 (94.3) | |
| Age | 0.005 | |||
| ≤42 years | 708 (19.8) | 32 (18.4) | 676 (19.9) | |
| 43–49 years | 1043 (29.2) | 61 (35.1) | 982 (28.9) | |
| 50–56 years | 1146 (32.1) | 65 (37.4) | 1081 (31.8) | |
| ≥57 years | 673 (18.8) | 16 (9.2) | 657 (19.3) | |
| HIV Infection | <0.001 | |||
| Yes | 1830 (51.3) | 116 (66.7) | 1714 (50.5) | |
| HCV Infection | <0.001 | |||
| Yes | 1117 (32.9) | 126 (74.1) | 991 (30.8) | |
| Race | <0.001 | |||
| White | 764 (21.6) | 14 (8.2) | 750 (22.3) | |
| Black | 2302 (65.1) | 133 (77.8) | 2169 (64.5) | |
| Hispanic | 324 (9.2) | 14 (8.2) | 310 (9.2) | |
| Other | 144 (4.1) | 10 (5.8) | 134 (4.0) | |
| Education | <0.001 | |||
| High School or Less | 1409 (39.9) | 93 (54.7) | 1316 (39.2) | |
| Some College or Greater | 2119 (60.1) | 77 (45.3) | 2042 (60.8) | |
| Currently Employed | <0.018 | |||
| Yes | 997 (28.0) | 35 (20.1) | 962 (28.4) | |
| Gross Annual Income | <0.001 | |||
| <$6,000 | 713 (20.7) | 59 (35.3) | 654 (20.0) | |
| $6,000 - $11,999 | 896 (26.1) | 54 (32.3) | 842 (25.7) | |
| $12,000 - $24,999 | 880 (25.6) | 28 (16.8) | 852 (26.0) | |
| $25,000 - $49,999 | 684 (19.9) | 25 (14.9) | 659 (20.1) | |
| ≥$50,000 | 265 (7.7) | 1 (0.6) | 264 (8.1) | |
| Marital Status | 0.024 | |||
| Married/Living with Partner | 1064 (30.1) | 38 (22.2) | 1026 (30.5) | |
| Divorced/Separated/Widowed | 1491 (42.2) | 88 (51.5) | 1403 (41.8) | |
| Never Married | 975 (27.6) | 45 (26.3) | 930 (27.7) | |
| Smoke Cigarettes | <0.001 | |||
| Never | 860 (24.4) | 21 (12.4) | 839 (25.0) | |
| Current | 1734 (49.1) | 122 (72.2) | 1612 (48.0) | |
| Former | 936 (26.5) | 26 (15.4) | 910 (27.1) | |
| Marijuana use, Past Year | 0.497 | |||
| Yes | 793 (23.0) | 42 (25.1) | 751 (22.9) | |
| Stimulant use, Past Year | 0.001 | |||
| Yes | 92 (2.7) | 12 (7.3) | 80 (2.5) | |
| Cocaine use, Past Year | <0.001 | |||
| Yes | 599 (17.4) | 54 (32.5) | 545 (16.6) | |
| Any Injection Drug Use, Lifetime | <0.001 | |||
| Yes | 235 (6.6) | 46 (26.4) | 189 (5.6) | |
| Diagnosed Opioid Use Disorder, Past Year | <0.001 | |||
| Yes | 205 (5.7) | 54 (31.0) | 151 (4.4) | |
| Unhealthy alcohol use, Past Year (AUDIT-C score ≥ 3 or 4) | 0.196 | |||
| Yes | 1393 (39.0) | 76 (43.7) | 1317 (38.8) | |
| Ever without a permanent address to call home | <0.001 | |||
| Yes | 1496 (42.1) | 101 (58.0) | 1395 (41.2) | |
| Ever stayed one or more nights in homeless shelter | <0.001 | |||
| Yes | 1373 (38.6) | 101 (58.0) | 1272 (37.7) | |
| Diagnosed PTSD, Past Year | 0.135 | |||
| Yes | 408 (11.4) | 26 (14.9) | 382 (11.2) | |
| Diagnosed Major Depressive Disorder, Past Year | 0.010 | |||
| Yes | 467 (13.1) | 34 (19.5) | 433 (12.7) | |
| Pain Interference in Daily Life, Past Year | 0.342 | |||
| Yes | 1122 (69.4) | 33 (63.5) | 1089 (69.6) | |
| # Close Friends/Family | <0.001 | |||
| None | 400 (11.3) | 34 (19.6) | 366 (10.8) | |
| 1–4 | 2297 (64.7) | 110 (63.6) | 2187 (64.8) | |
| ≥5 | 853 (24.0) | 29 (16.8) | 824 (24.4) | |
| How often you see friends/family | 0.039 | |||
| < once a month | 687 (19.6) | 45 (26.2) | 642 (19.2) | |
| Monthly | 1008 (28.7) | 52 (30.2) | 956 (28.6) | |
| Weekly/Daily | 1816 (51.7) | 75 (43.6) | 1741 (52.1) | |
| Receipt of High-Dose Opioid Rx from the VA | 0.754 | |||
| Yes | 68 (4.5) | 3 (4.8) | 65 (4.4) | |
| Receipt of Long-term Opioid Rx from the VA | 0.878 | |||
| Yes | 361 (23.3) | 15 (24.2) | 346 (23.3) | |
Note:
From Chi-square tests or Fisher’s exact tests
HIV=Human Immunodeficiency Virus; HCV=Hepatitis C Virus; PTSD=Post Traumatic Stress Disorder
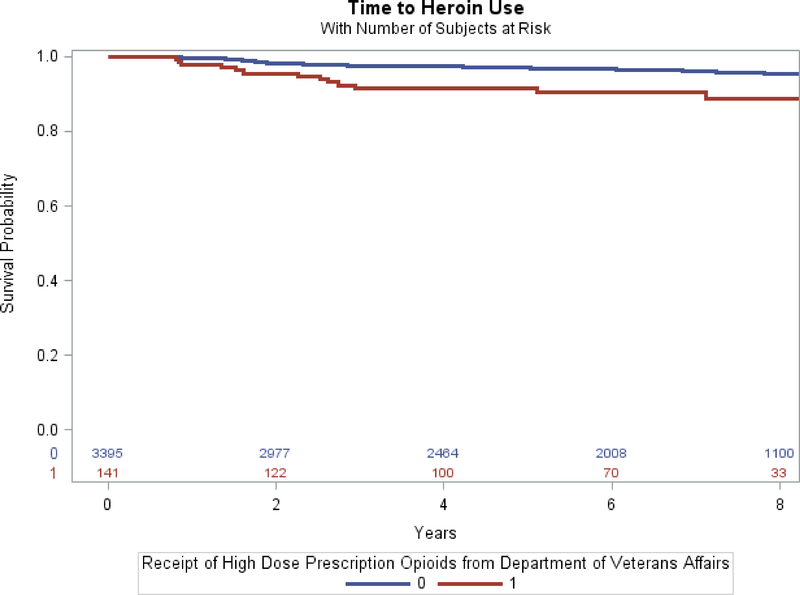
The ten-year unadjusted incidence rate of past year heroin use was 8.15 events per 1,000 person-years. Time to past year heroin use differed significantly between participants who had prior receipt of a high-dose prescription opioid at baseline and participants who had no prior receipt of high-dose opioids; the log-rank test was significant at p<0.001. (Figure 1)
Figure 1.
Relationship between receipt of high-dose prescription opioids and past year heroin use among VACS participants 2002–2012
Note: 0=No prior receipt of high dose prescription opioids; 1=Prior receipt of high dose prescription opioids
In contrast, the rate of past year heroin use did not differ significantly between participants who had long-term prior receipt of prescription opioids and participants who had short-term prior receipt of prescription opioids (p=0.469, figure not shown). The rate of past year heroin use did not vary significantly between participants receiving short-acting opioids, long-acting opioids, or both (data not shown).
Prior receipt of a high-dose opioid prescription opioid, having HCV infection, fewer years of education, diagnosis with PTSD, diagnosis with depression, and self-reported pain interference were all significantly associated with an increased risk of past year heroin use in unadjusted Cox regression analyses (Table 3). In the first fully adjusted and weighted multivariable Cox regression analysis, prior receipt of high-dose prescription opioids from the VA was positively and significantly associated with past year heroin use (adjusted hazard ratio [AHR] = 2.54, 95% CI = 1.26–5.10, see Table 4). In the second fully adjusted Cox regression model, prior receipt of long-term opioid therapy from the VA was not significantly associated with past year heroin use (AHR =1.09, 95% CI = 0.75–1.57). Being age 50–56 years, compared to participants younger than 42 years of age, having HCV infection, past year cocaine use, and past year stimulant use all significantly increased the hazard of past year heroin use in both models. In addition, participants reporting pain interference in daily life had an increased risk of heroin use.
Table 3.
Inverse probability of censoring weighted unadjusted Cox proportional hazard model of factors associated with time to self-reported past year heroin use among veterans receiving prescription opioids from the VA, participating in VACS, 2002–2012
| Characteristic | CHR*† (95% CI) | P – value |
|---|---|---|
| Receipt of High-Dose Opioid Rx from the VA (ref: low dose) | 2.52 (1.69–3.76) | <0.001 |
| Receipt of Long-term Opioid Rx From the VA (ref: short-term) | 0.87 (0.56–1.35) | 0.531 |
| MEDD of Opioid Prescription (ref: low dose (1–50mg)) | ||
| Medium Dose (50–90mg) | 1.40 (0.83–2.35) | 0.211 |
| High Dose (≥90mg) | 2.58 (1.73–3.85) | <0.001 |
| Sex (ref: Female) | 2.22 (0.80–6.15) | 0.126 |
| Age (ref: ≤42 years) | ||
| 43 – 49 years | 1.16 (0.59–2.30) | 0.659 |
| 50 – 56 years | 1.25 (0.84–1.88) | 0.273 |
| ≥57 years | 0.54 (0.25–1.16) | 0.113 |
| HIV (infected versus uninfected) | 1.62 (1.12–2.35) | 0.010 |
| HCV (infected versus uninfected) | 7.81 (6.17–9.88) | <0.001 |
| Race (ref: White) | ||
| Black | 3.13 (1.96–4.99) | <0.001 |
| Hispanic | 2.38 (0.92–6.18) | 0.074 |
| Other | 4.54 (2.65–7.78) | <0.001 |
| Education (ref: High School or Less) | ||
| Some College or Greater | 0.56 (0.42–0.76) | <0.001 |
| Currently Employed (yes versus no) | 0.65 (0.53–0.79) | <0.001 |
| Gross Annual Income (ref: <$6,000) | ||
| $6,000 - $11,999 | 0.72 (0.57–0.91) | 0.006 |
| $12,000 - $24,999 | 0.41 (0.26–0.65) | <0.001 |
| ≥$25,000 | 0.30 (0.21–0.43) | <0.001 |
| Marital Status (ref: Married/Living w/Partner) | ||
| Divorced/Separated/Widowed | 1.73 (1.35–2.21) | <0.001 |
| Never Married | 1.40 (0.90–2.18) | 0.130 |
| PTSDᶲ (ever diagnosis vs. none) | 1.62 (1.22–2.16) | <0.001 |
| Depressionᶲ (ever diagnosis vs. none) | 1.77 (1.11–2.82) | 0.017 |
| Pain Interference in Daily Lifeᶲ (any vs. none) | 1.57 (1.13–2.17) | 0.007 |
| Unhealthy alcohol useᶲ, Past Year (AUDIT-C score ≥ 3 or 4) | 2.17 (1.48–3.20) | <0.001 |
| Smoker (ref: Never) | ||
| Current | 4.04 (1.71–9.54) | 0.001 |
| Former | 1.32 (0.60–2.89) | 0.494 |
| Marijuana Useᶲ, Past Year (ref: none) | 5.55 (3.58–8.61) | <0.001 |
| Cocaine Useᶲ, Past Year (ref: none) | 31.48 (19.35–51.21) | <0.001 |
| Stimulant Useᶲ, Past Year (ref: none) | 18.82 (10.73–33.00) | <0.001 |
| Any Injection Drug Use, Lifetime (ref: none) | 5.75 (4.08–8.11) | <0.001 |
| Opioid Use Disorder (diagnosis vs. none) | 9.99 (7.03–14.19) | <0.001 |
| Ever stayed in homeless shelter (ref: no) | 2.19 (1.58–3.05) | <0.001 |
| Number of close friends/family (ref: none) | ||
| 1–4 | 0.55 (0.34–0.87) | 0.012 |
| ≥ 5 | 0.38 (0.20–0.72) | 0.003 |
Note:
Crude Hazard Ratio
Using robust sandwich estimator
HIV = human immunodeficiency virus; HCV = hepatitis C virus; PTSD = post-traumatic stress disorder
Time-updated variable; Rx=prescription
Table 4.
Weighted, multivariable, Cox proportional hazard model of factors associated with time to self-reported past year heroin use among recipients of prescription opioids from the participating in the VACS, 2002–2012
| Model 1 Main exposure: Receipt of High-Dose Opioid Rx† | Model 2 Main exposure: Receipt of Long-term Opioid Rx† | |||
|---|---|---|---|---|
| Variables | HR* (95% CI) | P-value | HR (95% CI) | P-value |
| High Dose Opioid Rx (ref: low dose) | 2.54 (1.26–5.10) | 0.009 | N/A | N/A |
| Long-Term Opioid Rx ( ref: short-term) | N/A | N/A | 1.09 (0.75–1.57) | 0.645 |
| Sex (ref: Female) | 1.07 (0.27–4.22) | 0.918 | 1.07 (0.24–4.89) | 0.926 |
| Age (ref: ≤42 years) | -- | -- | -- | -- |
| 43 – 49 years | 0.66 (0.41–1.06) | 0.083 | 0.69 (0.42–1.14) | 0.152 |
| 50 – 56 years | 0.56 (0.35–0.90) | 0.016 | 0.62 (0.42–0.92) | 0.018 |
| ≥57 years | 0.61 (0.29–1.28) | 0.190 | 0.74 (0.38–1.45) | 0.384 |
| HIV (infected versus uninfected) | 0.88 (0.63–1.23) | 0.466 | 0.80 (0.54–1.17) | 0.250 |
| HCV (infected versus uninfected) | 2.70 (1.83–3.97) | <0.001 | 3.46 (2.44–4.91) | <0.001 |
| Race (ref: White) | -- | -- | -- | -- |
| Black | 1.55 (0.72–3.34) | 0.334 | 1.50 (0.79–2.87) | 0.218 |
| Hispanic | 0.96 (0.28–3.30) | 0.949 | 1.34 (0.41–4.41) | 0.625 |
| Other | 1.91 (0.55–6.57) | 0.305 | 1.86 (0.72–4.77) | 0.198 |
| Education (ref: High School or Less) | -- | -- | -- | -- |
| Some College or Greater | 0.62 (0.40–0.97) | 0.036 | 0.52 (0.35–0.78) | 0.001 |
| Gross Annual Income (ref: <$6,000) | -- | -- | -- | -- |
| $6,000 - $11,999 | 1.04 (0.70–1.55) | 0.842 | 0.93 (0.64–1.34) | 0.694 |
| $12,000 - $24,999 | 1.03 (0.72–1.47) | 0.863 | 1.07 (0.71–1.59) | 0.753 |
| ≥$25,000 | 0.80 (0.38–1.70) | 0.567 | 0.75 (0.41–1.37) | 0.350 |
| Marital Status (ref: Married/Living w/Partner) | -- | -- | -- | -- |
| Divorced/Separated/Widowed | 1.40 (1.08–1.80) | 0.011 | 1.18 (0.78–1.78) | 0.444 |
| Never Married | 0.93 (0.59–1.45) | 0.931 | 0.84 (0.51–1.40) | 0.842 |
| PTSDᶲ (ever diagnosis vs. none) | 0.84 (0.50–1.39) | 0.501 | 0.82 (0.48–1.40) | 0.475 |
| Depressionᶲ (ever diagnosis vs. none) | 0.69 (0.41–1.15) | 0.155 | 0.63 (0.30–1.29) | 0.206 |
| Pain Interference in Daily Lifeᶲ (any vs. none) | 1.41 (1.17–1.69) | <0.001 | 1.18 (0.89–1.57) | 0.241 |
| Unhealthy alcohol useᶲ, Past Year | 0.96 (0.61–1.49) | 0.844 | 0.79 (0.50–1.24) | 0.306 |
| Smoker (ref: Never) | -- | -- | -- | -- |
| Current | 1.61 (0.61–4.27) | 0.338 | 1.35 (0.40–4..52) | 0.624 |
| Former | 1.01 (0.41–2.47) | 0.986 | 0.67 (0.22–2.08) | 0.491 |
| Any Injection Drug Use with Heroin, lifetime (yes vs no) | 1.78 (1.11–2.86) | 0.017 | 2.66 (1.60–4.41) | <0.001 |
| Opioid Use Disorder (diagnosis vs. none) | 4.47 (2.65–7.55) | <0.001 | 4.67 (2.77–7.88) | <0.001 |
| Marijuana Useᶲ, Past Year (ref: none) | 1.54 (0.83–2.89) | 0.183 | 1.58 (0.89–2.80) | 0.113 |
| Cocaine Useᶲ, Past Year (ref: none) | 13.21 (7.11–24.52) | <0.001 | 15.94 (8.15–31.15) | <0.001 |
| Stimulant Useᶲ, Past Year (ref: none) | 4.63 (2.07–10.39) | <0.001 | 4.06 (1.85–8.92) | <0.001 |
| Ever stayed in homeless shelter (ref: no) | 0.92 (0.64–1.34) | 0.681 | 0.88 (0.60–1.30) | 0.528 |
| Number of close friends/family (ref: none) | -- | -- | -- | -- |
| 1–4 | 0.70 (0.49–0.99) | 0.044 | 0.75 (0.47–1.20) | 0.230 |
| ≥ 5 | 0.37 (0.20–0.69) | 0.002 | 0.48 (0.27–0.87) | 0.016 |
Note:
Hazard ratio
Time-updated values
Using robust sandwich estimator
HIV = human immunodeficiency virus; HCV = hepatitis C virus; PTSD = post-traumatic stress disorder; Rx=prescription
3.4. Sensitivity Analyses
The Cox regression with prescription opioid dose categorized into low, medium, and high revealed that participants receiving a medium dose versus a low dose did not have a significantly increased CHR of past year heroin use. In the fully adjusted model, high dose compared to low dose was positively and significantly associated with past year heroin use (AHR = 3.16, 95% CI = 1.75–5.68).
The stabilized inverse probability of exposure weights were statistically well-behaved for both primary exposures of interest. The mean of the weights for receipt of high-dose prescription opioids was 0.99 (SD= 0.23) and they ranged from 0.25 to 4.51. The mean of the weights for receipt of a long-term prescription opioid was 1.00 (SD= 0.30) and they ranged from 0.18 to 3.61. In the sensitivity analysis with treatment and censoring weights, participants who received high-dose prescription opioids from the VA had 2.11 the hazard of past year heroin use compared to participants who received prescription opioids in doses less than 90 mg MEDD (95%CI: 1.30–3.43). The receipt of long-term opioid therapy from the VA was not significantly associated with past year heroin use in the sensitivity analysis.
4. Discussion
In this study, we examined the relationship between specific opioid prescribing patterns and subsequent heroin use in a population of US military veterans receiving medical care in the VA system. Our results indicate that, among veterans who reported no past year illicit opioid use at baseline and who received prescription opioids from the VA, prior receipt of high-dose prescription opioids increased the risk of subsequent heroin use.
Our findings are broadly consistent with the literature, which shows that veterans who use heroin are also more likely to engage in polysubstance use, including use of non-opioid illicit drugs [50, 51]. The proportion of participants who reported past year heroin use at each year of follow-up was slightly higher than the percent of individuals who reported past year heroin use in the 2013 National Survey of Drug Use and Health [38]. However, we suggest caution when comparing our study results to those of a national survey of the general population, since by design, all study participants received opioid analgesics at least once during the study period, and approximately half were persons living with HIV. Both of these eligibility criteria likely elevated the probability of heroin use over the study period. However, by identifying a population with a higher prevalence of risk factors for future heroin use, clinicians can more appropriately direct these patients to overdose education and naloxone distribution [48] in hopes of reducing the risk of potential heroin attributable overdoses.
At baseline, study participants who reported past year heroin use were more likely to be Black. Additionally, Black participants were at an increased risk of past year heroin use in the unadjusted Cox regression analyses. However, in multivariable models, the adjusted rate of past year heroin use did not differ significantly between Black participants and participants of other races. It is possible that variables serving as proxies for structural inequalities and included in the final model (e.g. education, income), confounded the association between race and past year heroin use. As greater levels of education and a higher number of close friends/family were protective against past year heroin use in our study, involvement with support groups and improved access to opioid safety and overdose prevention programs may mitigate the risk of future heroin use. Additionally, peer engagement and outreach programs have proven to be an effective intervention in encouraging treatment-seeking for opioid use disorders, as well as for connecting veterans to evidence-based approaches to addiction treatment (e.g., opioid agonist treatment with methadone or buprenorphine, non-pharmacologic treatments) [7].
Our findings suggest that diagnosis and treatment of psychiatric disorders are important considerations when prescribing opioid therapy, though existing guidelines concerning the use of opioids vary widely in their recommendations regarding patients with co-morbid depression and other psychiatric disorders [54]. Lower household wealth is associated with chronic pain that substantially limits participation in daily activities [30]. Therefore, the fact that work-related pain interference in daily life remained associated with an increased risk of past year heroin use in the fully adjusted model suggests that patients may be engaging in heroin use to alleviate pain that has not been adequately addressed by prescription opioid therapy and that efforts to reduce the burden of disabling chronic pain should prioritize socioeconomically vulnerable groups
More than half of VA primary care patients report pain, with many reporting chronic pain; however, current VA/Department of Defense opioid therapy guidelines recommend use of long-acting opioids only for persistent pain, and prescription of opioid therapy for chronic pain only after other non-opioid analgesic pharmacotherapies and non-pharmacologic pain treatments have insufficiently improved pain-related function [39]. Though our results further substantiate current VA and CDC opioid prescribing guidelines, which recommended prescribing opioids for short durations and recommend against prescribing opioids in doses greater than 90 mg MEDD [1], Gaither and colleagues found that, from 1998 to 2010, the majority of VA patients initiating long-term opioid therapy did not receive opioid therapy guideline-concordant care (although older guidelines were in effect when this study was published.) [24] Future research is needed to determine the extent to which opioid prescribing is consistent with current guidelines.
We posit that prior receipt of long-term opioid prescriptions was not associated with past year heroin use because our data were collected prior to the enactment of recent CDC and VA/DOD guidelines regarding the tapering of prescription opioids, and it is unlikely that opioid tapering and/or abrupt discontinuation of opioid therapy drove patients to engage in heroin use during this time period [18]. Nonetheless, future studies should examine whether abrupt discontinuation or inappropriate tapering of opioid therapy is associated with an increased risk of transitioning to heroin use among veterans and high-risk populations. This is particularly important in the context of the VA’s Opioid Safety Initiative, which strongly encourages opioid tapering, if not discontinuation, without explicit examination of unintended harms of this initiative [60]. It is essential to continue addressing unmet pain management needs of veterans, as is also recommended by guidelines from the American College of Physicians, which specifically recommends increased use of non-opioid pharmacological and non-pharmacological approaches [11].
Our study had a number of limitations. First, there is a potential for confounding by indication, whereby patients at higher risk of heroin use were also more likely to receive high-dose prescription opioids. We addressed this by using inverse probability weighting methods, and creating weights based on patients’ probability of receiving high dose opioids, conditioned on a comprehensive list of potential, time-updated confounders [59]. Second, our exposure data was based on pharmacy fill/refill records from the VA, and we were not able to account for opioid prescriptions filled outside of the VA [25, 26]. One recent study demonstrated that 18% of veterans receive opioids both inside and outside of the VA [6]. In that vein, receipt of a prescription opioid prescription may not have accurately captured the true amount of opioids actually being taken by VACS participants. There is also some likelihood of measurement error and bias in our outcome variable: past year heroin use. While this variable captures self-report of any heroin use in the ten-year follow-up period, we were not able to discern whether this is truly lifetime incident heroin use or represents re-initiation of heroin use. We only used a single domain (e.g. work-related interference) to measure pain interference in daily life. The complexity of chronic pain and its interference in daily activities is likely not fully captured by this question. Additionally, almost 50% of participants reported being retired or unable to work at baseline, which may have affected the validity and measurement of this item. Moreover, as we did not measure employment status over time, we are unable to examine whether daily pain interfered in employment status, or loss of employment related to chronic pain increased the risk of heroin use. Despite these limitations, our study has important implications for clinical care, policy and future research. The finding that prior receipt of a high-dose prescription opioid may increase the risk of past year heroin use among veterans receiving care at the VA supports the current CDC and VA guidelines that recommend prescribing opioid at lower doses. Our results indicate that further efforts are needed to develop effective screening strategies to identify heroin use among those prescribed high dose opioids.
Supplementary Material
5. Acknowledgements
This work was funded by a NIDA NIH Dissertation Grant, R36DA042877, U01 AA020790, U24 AA020794, and RO1 DA040471.
Footnotes
Conflict of Interest
None
7. References
- 1.US Department of Veterans Affairs/Department of Defense. Clinical Practice Guidelines for Management of Opioid Therapy for Chronic Pain. 2017; Available from: https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf.
- 2.VA/DoD Clinical Practice Guideline: management of opioid therapy for chronic pain. Department of Veterans Affairs, Department of Defense. 2010; Available from: https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_fulltext.pdf.
- 3.Back SE, Killeen TK, Teer AP, Hartwell EE, Federline A, Beylotte F, and Cox E, Substance use disorders and PTSD: an exploratory study of treatment preferences among military veterans. Addict Behav, 2014. 39(2): p. 369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee G, Edelman EJ, Barry DT, Becker WC, Cerda M, Crystal S, Gaither JR, Gordon AJ, Gordon KS, Kerns RD, Martins SS, Fiellin DA, and Marshall BD, Non-medical use of prescription opioids is associated with heroin initiation among US veterans: a prospective cohort study. Addiction, 2016. 111(11): p. 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker WC, Sullivan LE, Tetrault JM, Desai RA, and Fiellin DA, Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend, 2008. 94(1–3): p. 38–47. [DOI] [PubMed] [Google Scholar]
- 6.Becker WC, Fenton CT, Brandt CA, Doyle EL, Francis J, Goulet J, Moore BA, Torrise V, Kerns RD, and Kreiner PW, Multiple Sources of Prescription Payment and Risky Opioid Therapy Among Veterans. Med Care, 2017. 7(1): p. S33–S36. [DOI] [PubMed] [Google Scholar]
- 7.Bennett AS, Elliott L, Golub A, Wolfson-Stofko B, and Guarino H, Opioid-Involved Overdose Among Male Afghanistan/Iraq-Era U.S. Military Veterans: A Multidimensional Perspective. Subst Use Misuse, 2017: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode AD, Singh M, Andrews J, Kapur G, and Baez AA, Fentanyl laced heroin and its contribution to a spike in heroin overdose in Miami-Dade County. The American Journal of Emergency Medicine, 2017. 35(9): p. 1364–1365. [DOI] [PubMed] [Google Scholar]
- 9.Bohnert ASB, Marcia Valenstein, Bair Matthew J, Ganoczy D, McCarthy John F., Ilgen MA, and Blow FC, Association Between Opioid Prescribing Patterns and Opioid Overdose-Related Deaths. JAMA, 2011. 305(13): p. 1315–1321. [DOI] [PubMed] [Google Scholar]
- 10.Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, and Stewart WF, Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction, 2010. 105(10): p. 1776–82. [DOI] [PubMed] [Google Scholar]
- 11.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, and Brodt ED, Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med, 2017. 166(7): p. 493–505. [DOI] [PubMed] [Google Scholar]
- 12.Christensen E, Multivariate survival analysis using Cox’s regression model. Hepatology, 1987. 7(6): p. 1346–1358. [DOI] [PubMed] [Google Scholar]
- 13.Cicero TJ, Surratt H, Inciardi JA, and Munoz A, Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiol Drug Saf, 2007. 16(8): p. 827–40. [DOI] [PubMed] [Google Scholar]
- 14.Cicero TJ, Ellis MS, Surratt HL, and Kurtz SP, The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry, 2014. 71(7): p. 821–6. [DOI] [PubMed] [Google Scholar]
- 15.Cole SR and Hernan MA, Constructing Inverse Probability Weights for Marginal Structural Models. American Journal of Epidemiology, 2008. 168(6): p. 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson DA, Smith SM, Saha TD, Rubinsky AD, and Grant BF, Comparative performance of the AUDIT-C in screening for DSM-IV and DSM-5 alcohol use disorders. Drug Alcohol Depend, 2012. 126(3): p. 384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobscha SK, Morasco BJ, Duckart JP, Macey T, and Deyo RA, Correlates of prescription opioid initiation and long-term opioid use in veterans with persistent pain. Clin J Pain, 2013. 29(2): p. 102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowell D, Haegerich TM, and Chou R, CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. JAMA, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan M, Welsner CM, Silverberg MJ, Campbell CI, Psaty BM, and Von Korff M, Opioid Prescriptions for Chronic Pain and Overdose. Ann Intern Med, 2010. 152: p. 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman EJ, Gordon K, Becker WC, Goulet JL, Skanderson M, Gaither JR, Brennan Braden J, Gordon AJ, Kerns RD, Justice AC, and Fiellin DA, Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med, 2013. 28(1): p. 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, and Sullivan MD, The Role of Opioid Prescription in the Incident Opioid Abuse and Dependence Among Individuals with Chronic Noncancer Pain. Clin J Pain, 2014. 30(7): p. 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlund MJ, Austen MA, Sullivan MD, Martin BC, Williams JS, Fortney JC, and Hudson TJ, Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain, 2014. 155(11): p. 2337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin GM, Fulton-Kehoe D, Turner JA, Sullivan MD, and Wickizer TM, Changes in opioid prescribing for chronic pain in Washington State. J Am Board Fam Med, 2013. 26(4): p. 394–400. [DOI] [PubMed] [Google Scholar]
- 24.Gaither JR, Goulet JL, Becker WC, Crystal S, Edelman EJ, Gordon K, Kerns RD, Rimland D, Skanderson M, Weisberg DF, Justice AC, and Fiellin DA, Guideline-concordant management of opioid therapy among human immunodeficiency virus (HIV)-infected and uninfected veterans. J Pain, 2014. 15(11): p. 1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gellad WF, Zhao X, Thorpe CT, Thorpe JM, Sileanu FE, Cashy JP, Mor M, Hale JA, Radaomski T, Hausmann LR, Fine MJ, and Good CB, Overlapping buprenorphine, opioid, and benzodiazepine prescriptions among veterans dually enrolled in Department of Veterans Affairs and Medicare Part D. Subst Abus, 2017. 38(1): p. 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gellad WF, Thorpe JM, Zhao X, Thorpe CT, Sileanu FE, Cashy JP, and Hale JA, Impact of Dual Use of Department of Veterans Affairs and Medicare Part D Drug Benefits on Potentially Unsafe Opioid Use. American Journal of Public Health, 2018. 108(1): p. 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golub A and Bennett AS, Prescription opioid initiation, correlates, and consequences among a sample of OEF/OIF military personnel. Subst Use Misuse, 2013. 48(10): p. 811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourlay Douglas L., Heit Howard A., and Almahrezi Abdulaziz, Universal Precautions in Pain Medicine: A Rational Approach to the Treatment of Chronic Pain. Pain Medicine, 2005. 6(2): p. 107–112. [DOI] [PubMed] [Google Scholar]
- 29.Graubard BI and Korn EL, Regression Analysis with Clustered Data. Statistics in Medicine, 1994. 13: p. 509–522. [DOI] [PubMed] [Google Scholar]
- 30.Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, and Piette JD, Racial and Socioeconomic Disparities in Disabling Chronic Pain: Findings From the Health and Retirement Study The Journal of Pain, 2017. 18(12): p. 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joffe MM, Have TRT, Feldman H, and Kimmel SE, Model Selection, Confounder Control, and Marginal Structural Models. The American Statistician, 2004. 58(4): p. 272–279. [Google Scholar]
- 32.Jones CM, Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug Alcohol Depend, 2013. 132(1–2): p. 95–100. [DOI] [PubMed] [Google Scholar]
- 33.Jones CM, Logan J, Gladden RM, and Bohm MK, Demographic and Substance Use Trends Among Heroin Users -- United States, 2002–2013. MMWR Morb Mortal Wkly Rep, 2015. 64(26): p. 719–725. [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan AE, Des Jarlais DC, and Hagan H, Prescription opioid misuse and its relation to injection drug use and hepatitis C virus infection: protocol for a systematic review and meta-analysis. Systematic Reviews, 2014. 3(95): p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, and Bryant K, Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care, 2006. 44(8): p. S52–S60. [DOI] [PubMed] [Google Scholar]
- 36.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, and Bryant K, Veterans aging cohort study (VACS): overview and description. Med Care, 2006. 44(8 Suppl 2): p. S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krebs Erin E., Ramsey Darin C., Miloshoff James M., and Matthew J Blair, Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med, 2011. 12: p. 740–746. [DOI] [PubMed] [Google Scholar]
- 38.Lipari RN and Hughes A, The NSDUH report: trends in heroin use in the United States: 2002 to 2013, in Center for Behavioral Health Statistics and Quality Data Review 2015, Substance Abuse and Mental Health Services Administration (SAMHSA): Rockville, MD. [PubMed] [Google Scholar]
- 39.Lovejoy TI, Dobscha SK, Turk DC, Weimer MB, and Morasco BJ, Correlates of prescription opioid therapy in Veterans with chronic pain and history of substance use disorder. J Rehabil Res Dev, 2016. 53(1): p. 25–36. [DOI] [PubMed] [Google Scholar]
- 40.Mars SG, Bourgois P, Karandinos G, Montero F, and Ciccarone D, “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy, 2014. 25(2): p. 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Krosten TR, and Fiellin DA, Systematic Review: Opioid Treatment for Chronic Back Pain: Prevalence, Efficacy, and Association with Addiction. Annals of Internal Medicine, 2007. 146(2): p. 116–127. [DOI] [PubMed] [Google Scholar]
- 42.McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, Kraemer KL, Maisto SA, Crystal S, Fiellin DA, and Justice AC, Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend, 2016. 168: p. 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, and Jamison RN, Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage, 2004. 28(3): p. 250–8. [DOI] [PubMed] [Google Scholar]
- 44.Miller M, Barber CW, Leatherman S, Fonda J, Hermos JA, Cho K, and Gagnon DR, Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med, 2015. 175(4): p. 608–15. [DOI] [PubMed] [Google Scholar]
- 45.Morasco BJ, Duckart JP, Carr TP, Deyo RA, and Dobscha SK, Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain, 2010. 151(3): p. 625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Friedman SR, and Des Jarlais DC, Transitions to Injecting Drug Use Among Noninjecting Heroin Users. J Acquir Immune Defic Syndr, 2006. 41(4): p. 493–503. [DOI] [PubMed] [Google Scholar]
- 47.Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, and Chou R, Opioid Prescribing: A Systematic Review & Critical Appraisal of Guidelines for Chronic Pain. Annals of Internal Medicine, 2014. 160(1): p. 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliva EM, Nevedal A, Lewis ET, McCaa MD, Cochran MF, Konicki PE, Davis CS, and Wilder C, Patient perspectives on an opioid overdose education and naloxone distribution program in the U.S. Department of Veterans Affairs. Subst Abus, 2016. 37(1): p. 118–26. [DOI] [PubMed] [Google Scholar]
- 49.Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, and Garfein RS, Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil, 2011. 2(1): p. 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robins LN, Helzer JE, Hesselbrock M, and Wish E, Vietnam veterans three years after Vietnam: how our study changed our view of heroin. Am J Addict, 2010. 19(3): p. 203–11. [DOI] [PubMed] [Google Scholar]
- 51.Robins Lee N. and Slobdyan Sergey, Post-Vietnam heroin use and injection by returning US veterans: clues to preventing injection today. Addiction, 2003. 98: p. 1053–1060. [DOI] [PubMed] [Google Scholar]
- 52.Saunders KW, Dunn KM, Merrill JO, Sullivan M, Weisner C, Branden JB, Psaty BM, and Von Korff M, Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med, 2010. 25(4): p. 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan M Half of veterans prescribed medical opioids continue to use them chronically. American Academy of Pain Medicine (AAPM) 2014. [cited 2016 2/8]; Available from: www.sciencedaily.com/releases/2014/03/140308094821.htm. [Google Scholar]
- 54.Sullivan MD, Edlund MJ, and Zhang L, Association Between Mental Health Disorders, Problem Drug Use, and Regular Prescription Opioid Use. JAMA Internal Med, 2006. 166(19): p. 2087–2093. [DOI] [PubMed] [Google Scholar]
- 55.Unick GJ, Rosenblum D, Mars S, and Ciccarone D, Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PLoS One, 2013. 8(2): p. e54496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.U.S. Drug Enforcement Administration.Controlled Substance Schedules. 2010; Available from: https://www.deadiversion.usdoj.gov/schedules/index.html.
- 57.Volkow ND and McLellan TA, Curtailing Diversion and Abuse of Opioid Analgesics Without Jeopardizing Pain Treatment. JAMA, 2011. 305(13): p. 1346–1347. [DOI] [PubMed] [Google Scholar]
- 58.Weisberg DF, Gordon KS, Barry DT, Becker WC, Crystal S, Edelman EJ, Gaither JR, Gordon AJ, Goulet JL, Kerns RD, Moore BA, Tate J, Justice AC, and Fiellin DA, Long-term prescription of opioids and/or benzodiazepines and mortality among HIV-infected and uninfected patients. J Acquir Immune Defic Syndr, 2015. 69(2): p. 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisberg DF, Gordon KS, Barry DT, Becker WC, Crystal S, Edelman EJ, Gaither J, Gordon AJ, Goulet J, Kerns RD, Moore BA, Tate J, Justice AC, and Fiellin DA, Long-term Prescription of Opioids and/or Benzodiazepines and Mortality Among HIV-Infected and Uninfected Patients. J Acquir Immune Defic Syndr, 2015. 69(2): p. 223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westanmo A, Marshall P, Jones E, Burns K, and Krebs EE, Opioid Dose Reduction in VA Health Care System - Implementation of Primary Care Population-Level Initiative. Pain Med, 2015. 16(5): p. 1019–1026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.