Abstract
Objective.
This study examined factors associated with prescription opioid analgesic use in the US population using data from a nationally representative sample. It focused on factors previously shown to be associated with opioid use disorder or overdose. Variations in the use of different strength opioid analgesics by demographic subgroup were also examined.
Methods.
Data came from respondents aged 16 years and older who participated in the National Health and Nutrition Examination Survey (2011–2014). Respondents were classified as opioid users if they reported using one or more prescription opioid analgesics in the past 30 days.
Results.
Opioid users reported poorer self-perceived health than those not currently using opioids. Compared with those not using opioids, opioid users were more likely to rate their health as being “fair” or “poor” (40.4% [95% confidence interval {CI} = 34.9%–46.2%] compared with 15.6% [95% CI = 14.3%–17.1%]), experienced more days of pain during the past 30 days (mean = 14.3 [95% CI = 12.9–15.8] days compared with 2.3 [95% CI = 2.0–2.7] days), and had depression (22.5% [95% CI = 17.3%–28.7%] compared with 7.1% [95% CI = 6.2%–8.0%]). Among those who reported using opioids during the past 30 days, 18.8% (95% CI = 14.4%–24.1%) reported using benzodiazepine medication during the same period and 5.2% (95% CI = 3.5%–7.7%) reported using an illicit drug during the past six months. When opioid strength was examined, a smaller percentage of adults aged 60 years and older used stronger-than-morphine opioids compared with adults aged 20–39 and 40–59 years.
Conclusions.
Higher percentages of current opioid users than nonusers reported having many of the factors associated with opioid use disorder and overdose.
Keywords: Antidepressants, Benzodiazepines, CDC, Opioid Analgesics, NHANES
Introduction
The use of prescription opioid analgesics has increased during the past two decades [1–3]. As of 2011–2012, 6.9% of adults aged 20 years and older reported using a prescription opioid analgesic in the past 30 days [4]. This increase in opioid use has corresponded with increases in the rates of opioid use disorder and opioid-related deaths [5–7]. Significant resources have been mobilized to develop prescription guidelines to decrease these adverse outcomes [8–10].
As part of the effort to reduce opioid-related adverse outcomes, researchers have examined the characteristics of opioid users to understand factors associated with opioid use disorder and overdose. These associated factors include poor mental health, illicit drug use, alcohol abuse, and concurrent use of benzodiazepine and antidepressant medications [11–15]. In addition, variation in opioid analgesic strength is also a concern due to greater risk of opioid use disorder from using stronger-than-morphine opioids [8]. Most of this research, however, has focused on opioid users within specific cohorts or subgroups, for example, Medicaid users, opioid users within a specific geographic location, or people who report having chronic pain [13,16,17].
The study’s objective was to examine factors associated with the use of prescription opioid analgesics using the most current data available from a nationally representative sample of the noninstitutionalized US population. It focused on factors shown in prior research to be associated with opioid use disorder or overdose. Variations in the use of different strength opioid analgesics by demographic subgroups were also examined.
Methods
Data
The National Health and Nutrition Examination Survey (NHANES) is a continuous, cross-sectional survey conducted by the National Center for Health Statistics (NCHS). A complex, multistage probability sampling design is used to generate a representative sample of the civilian, noninstitutionalized US population [18,19]. Participants receive a detailed in-home interview followed by a physical examination at a mobile examination center (MEC). As part of the MEC exam, participants are asked to complete a health interview including questions on alcohol and drug use, pain experiences, and depression in a private setting. Study protocols were approved by the NCHS Research Ethics Review Board, and informed consent was obtained from all participants. Data are collected continuously but are released in two-year cycles. Data from two cycles of two years were included in the analysis: 2011–2012 and 2013–2014. The examination response rates for each cycle were 69.5% and 68.5%, respectively [20]. The analytic sample included all NHANES respondents aged 16 years and over who completed the household interview and physical examination and had no missing data on questions regarding their use of prescription medication (N = 12,114).
Variables
Prescription Opioid Use and Strength
Data on prescription medications used in the past 30 days were collected during the household interview. Respondents aged 16 years and older were asked: “In the past 30 days, have you used or taken medication for which a prescription is needed?” Those who answered affirmatively were asked to show their prescription medication containers to the interviewer and report details related to their use. The interviewer examined the containers and recorded the exact product names from their labels. If containers were not available, the participant verbally reported this information. In 2013–2014, interviewers could indicate that they had recorded the medication’s name from the documentation (printout) respondents received from the pharmacy when they filled their prescription (if the medication’s container was not available). In 2011–2014, 85.8% of the drug product names were recorded by direct observation of the container, 2.9% were recorded based on the pharmacy printout, and the rest (11.3%) were collected via respondents’ recall. Except for the minor change noted above, the collection methodology was similar for both NHANES cycles [21]. NCHS classified the prescription medications based on the therapeutic classification scheme of Cerner Multum’s Lexicon Plus propriety database [22].
Two Multum ingredient categories (narcotic analgesics and narcotic analgesic combinations) were used to identify opioid analgesic medications, similar to prior research [4]. Opioids that were identified were then categorized based on their strength relative to morphine using a classification scheme used in prior research [4,23]: weaker than morphine (codeine, dihydrocodeine, meperidine, pentazocine, propoxyphene, and tramadol), morphine equivalent (hydrocodone, morphine, and tapentadol), or stronger than morphine (fentanyl, hydromorphone, methadone, oxycodone, and oxymorphone). Respondents who reported using two or more opioid analgesics in the past 30 days (about 12% of opioid users) were categorized based on the strongest opioid they reported.
Other Prescription Medication Variables
Three additional variables were derived from the prescription medication data. Two were binary variables: One indicated whether respondents used prescription benzodiazepines during the past 30 days (Multum categories benzodiazepine anticonvulsants and benzodiaze-pines), and the other indicated whether respondents used prescription antidepressants during the past 30 days (Multum category antidepressants). The third was a variable assessing total number of nonopioid prescription medications used in the past 30 days (0, 1–3, 4–6, 7+).
Demographic Variables
Demographic variables included respondents’ age (16–19, 20–39, 40–59, 60+ years), sex (male, female), race/Hispanic origin (non-Hispanic white, non-Hispanic black, non-Hispanic Asian, Hispanic), and health insurance status (have health insurance, do not have health insurance).
Health Measures
Factors associated with opioid use included a dichotomous measure of self-rated health (excellent/very good/good, fair/poor) [24]; the number of days (out of 30) pain made it difficult to complete usual activities (0, 1–13, 14–29, 30 days); the number of days (out of 30) respondents felt worried, tense, or anxious (0, 1–13, 14–29, 30 days); and whether respondents had depression during the past two weeks, indicated by having a score of 10 or higher on the Patient Health Questionnaire (PHQ-9), a depression screening instrument [25]. These variables come from an interview conducted during the MEC examination.
Health Risk Behaviors
Two health risk behaviors were included. A measure of current alcohol consumption was included: nondrinker (0 drinks in past year, former drinkers, and lifetime abstainers), light drinker (average of 3 or fewer drinks per week), moderate drinker (average of 4–14 drinks per week for men; average of 4–7 drinks per week for women), and heavy drinker (average of 15 or more drinks per week for men; average of 8 or more drinks per week for women) [26]. A dichotomous variable (yes/no) indicating whether respondents used one or more of the following illicit drugs (cocaine, heroin, methamphetamine, and other injected drugs not prescribed by a doctor) during the past six months was also created. Like the health measures, information on these behaviors came from an interview conducted during the MEC examination.
Data Analysis
The analysis was conducted in three parts. First, estimates of demographics, health measures, and high-risk behaviors among those who used prescription opioids during the past 30 days were generated and then compared with estimates from those who did not use opioids during the past 30 days. Differences in the mean number of days experiencing pain, the mean number of days experiencing anxiety, and the mean number of nonopioid prescriptions were also compared. Adjusted Wald tests were used to test for significant differences in characteristics between opioid users and nonusers in the first part of the analysis.
Next, odds ratios of using a prescription opioid in the past 30 days were calculated by associated covariates identified in the first part of the study. Two types of logistic regression models were used: an unadjusted model and a model with adjustment for demographic characteristics (age, sex, race/Hispanic origin) and health insurance status to account for physiological differences, social context, and access to medical services [27].
Finally, variations in the strength of opioids used by age, sex, and race/Hispanic origin were assessed using chi-square tests. If significant differences were found, adjusted Wald tests were run to test for differences among age, sex, and race/Hispanic origin groups. For all tests performed, a significance level of 0.05 was utilized.
Statistical analyses were conducted applying the complex sampling parameters using the SVY commands in Stata 15 to adjust for differential probabilities of selection and the complex sampling design [28]. Exam sample weights were used to obtain estimates representative of the civilian, noninstitutionalized US population aged 16 years and older. Variance estimates were computed using the Taylor series linearization approximation method.
Results
Characteristics of Opioid Users
Table 1 presents the prevalence of factors associated with opioid use for current opioid analgesic users and those not currently using opioids. In 2011–2014, 6.7% (95% CI = 5.8–7.8) of respondents aged 16 years and older reported using at least one prescription opioid analgesic in the past 30 days.
Table 1.
Factors associated with opioid use by prescription opioid use status, NHANES 2011–2014
| Current Opioid User | Nonopioid User | |||
|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | |
| Overall | 780 | 6.7 (5.8–7.8) | ||
| Age, y | ||||
| 16–19 | 24 | 2.1 (1.2–3.6) | 1,251 | 7.1 (6.5–7.8)* |
| 20–39 | 148 | 21.3 (16.5–27.0) | 3,592 | 34.7 (32.1–37.3)* |
| 40–59 | 288 | 44.6 (38.8–50.6) | 3,368 | 34.2 (32.6–35.9)* |
| 60+ | 320 | 32.0 (27.7–36.7) | 3,123 | 24.0 (22.5–25.5)* |
| Mean | 780 | 51.8 (49.8–53.7) | 11,334 | 44.9 (44.0–45.9)* |
| Sex | ||||
| Men | 331 | 43.5 (39.0–48.2) | 5,554 | 48.6 (47.6–50.0)* |
| Women | 449 | 56.5 (51.8–61.0) | 5,780 | 51.4 (50.4–52.4)* |
| Race/Hispanic origin† | ||||
| Non-Hispanic white | 392 | 72.9 (65.8–79.0) | 4,245 | 64.9 (59.6–69.8)* |
| Non-Hispanic black | 210 | 12.3 (8.8–17.0) | 2,676 | 11.7 (9.1–14.9) |
| Non-Hispanic Asian | 25 | 1.3 (0.8–2.1) | 1,521 | 5.5 (4.5–6.8)* |
| Hispanic | 118 | 9.3 (6.2–13.6) | 2,549 | 15.3 (12.1–19.2)* |
| Health insurance status | ||||
| Does not have insurance | 97 | 11.2 (8.2–15.1) | 2,557 | 19.2 (17.5–21.1) |
| Has insurance | 682 | 88.8 (84.9–91.8) | 8,760 | 80.8 (78.9–82.5) |
| Self-rated health | ||||
| Excellent/very good/good | 399 | 59.6 (53.8–65.1) | 8,162 | 84.4 (82.9–85.7)* |
| Fair/poor | 333 | 40.4 (34.9–46.2) | 2,111 | 15.6 (14.3–17.1)* |
| Pain (past 30 d)‡ | ||||
| 0 d | 93 | 27.3 (21.8–33.6) | 3,703 | 75.4 (73.2–77.5)* |
| 1–13 d | 80 | 20.8 (16.8–25.4) | 858 | 17.6 (15.9–19.4) |
| 14–29 d | 55 | 17.1 (11.0–25.6) | 143 | 3.3 (2.8–4.0)* |
| 30 d | 115 | 34.7 (27.2–43.1) | 199 | 3.6 (2.8–4.7)* |
| Mean | 343 | 14.3 (12.9–15.8) | 4,903 | 2.3 (2.0–2.7)* |
| Anxiety (past 30 d)‡ | ||||
| 0 d | 112 | 29.6 (23.9–36.0) | 2,358 | 45.5 (41.6–49.5)* |
| 1–13 d | 118 | 38.5 (32.9–44.5) | 1,824 | 38.4 (34.9–42.1) |
| 14–29 d | 53 | 15.9 (10.9–22.7) | 342 | 7.8 (6.9–8.9)* |
| 30 d | 59 | 16.0 (11.2–22.2) | 376 | 7.8 (6.8–9.0)* |
| Mean | 342 | 9.5 (7.7–11.2) | 4,900 | 5.3 (4.9–5.7)* |
| Depression (past 2 wk) | ||||
| 0–9 (not depressed) | 543 | 77.5 (71.3–82.7) | 9,346 | 92.9 (92.0–93.8)* |
| 10+ (depressed) | 174 | 22.5 (17.3–28.7) | 802 | 7.1 (6.2–8.0)* |
| No. of nonopioid prescription medications (past 30 d)§ | ||||
| 0 | 86 | 12.5 (9.1–16.9) | 5,672 | 46.4 (44.6–48.3)* |
| 1–3 | 276 | 36.9 (31.5–42.6) | 3,546 | 35.5 (34.0–37.1) |
| 4–6 | 198 | 24.4 (21.0–28.1) | 1,397 | 12.1 (11.3–13.1)* |
| 7+ | 220 | 26.2 (21.1–32.0) | 719 | 5.9 (5.2–6.6)* |
| Mean | 780 | 4.5 (4.0–4.9) | 11,334 | 1.7 (1.6—1.8)* |
| Used at least 1 benzodiazepine (past 30 d) | 136 | 18.8 (14.4–24.1) | 324 | 3.4 (2.9–4.0)* |
| Used at least 1 antidepressant (past 30 d) | 245 | 35.1 (30.6–39.8) | 997 | 11.8 (10.6–13.2)* |
| Current drinking status¶ | ||||
| Nondrinker | 292 | 34.7 (29.1–40.8) | 3,143 | 26.1 (23.2–29.3)* |
| Light drinker | 302 | 45.5 (40.1–51.0) | 4,329 | 46.3 (44.1–48.5) |
| Moderate drinker | 70 | 12.1 (8.9–16.1) | 1,557 | 20.1 (18.4–21.8)* |
| Heavy drinker | 46 | 7.8 (4.9–12.1) | 575 | 7.5 (6.7–8.5) |
| Illicit drug use (past 6 mo)‖ | 26 | 5.2 (3.5–7.7) | 224 | 2.8 (2.2–3.5)* |
Exam sample weights were utilized to adjust for differential probabilities of selection and the complex sampling design. Percentages do not sum to 100 due to rounding. Respondents were asked, “In the past 30 days, have you used or taken medication for which a prescription is needed?”
CI = confidence interval.
Statistically significant difference between current opioid users and nonopioid users (P < 0.05).
Estimates for persons of other race/Hispanic origin groups are not provided separately, but members of this group are included in total estimates.
Question only asked in 2011–2012.
Benzodiazepine and antidepressant prescription medications were included in this count.
Nondrinker (0 drinks in past year, includes former drinkers and abstainers); light drinker (average of 3 or fewer drinks per week); moderate drinker (average of 4–14 drinks per week for men; average of 4–7 drinks per week for women); heavy drinker (average of 15 or more drinks per week for men; average of 8 or more drinks per week for women). These questions were only asked of respondents aged 18 years and older.
Using cocaine, heroin, methamphetamine, and/or other injected drugs not prescribed by a doctor in the past six months. Questions about illicit drug use were only asked of respondents aged 16–59 years.
Across the demographic variables examined, significant differences between adult opioid users and adults who had not used opioids in the past 30 days were identified. The mean age of opioid users (mean = 51.8 [95% CI = 49.8–53.7] years) was higher than the mean age of adults not using opioids (mean = 44.9 [95% CI = 44.0–45.9] years) due to the larger proportion of opioid users in the 40–59 and 60+ age groups. Sex differences between current opioid users and nonopioid users were also identified (for men, 43.5% [95% CI = 39.0%–48.2%] vs 48.6% [95% CI = 47.6%–50.0%]; for women 56.5% [95% CI = 51.8%–61.0%] vs 51.4% [95% CI = 50.4%–52.4%]). Compared with adults not currently using opioids, opioid users were more likely to be non-Hispanic white (72.9% [95% CI = 65.8%–79.0%] vs 64.9% [95% CI = 59.6%–69.8%]) and less likely to be non-Hispanic Asian (1.3% [95% CI = 0.8%–2.1%] vs 5.5% [95% CI = 4.5%–6.8%) or Hispanic (9.3% [95% CI = 6.2%–13.6%] vs 15.3% [95% CI = 12.1%–19.2%]).
Compared with those not currently using opioids, opioid users reported worse health outcomes across all examined health measures. Compared with those not using opioids, opioid users were more likely to report their overall health as “fair” or “poor” (40.4% [95% CI = 34.9%–46.2%] vs 15.6% [95% CI = 14.3%–17.1%]), more days of pain (during the past 30 days) that made it difficult to complete their usual activities (mean = 14.3 [95% CI = 12.9–15.8] days vs mean = 2.3 [95% CI = 2.0–2.7] days), experiencing more days of anxiety (during the past 30 days) that impacted their usual activities (mean = 9.5 [95% CI = 7.7–11.2] days vs mean = 5.3 [95% CI = 4.9–5.7] days), and having depression during the past two weeks (22.5% [95% CI = 17.3%–28.7%] vs 7.1% [95% CI = 6.2%–8.0%]).
Opioid users reported using a higher number of nonopioid prescription medications in the past 30 days than those not currently using opioids. A larger proportion of opioid users used benzodiazepines in the past 30 days (18.8% [95% CI = 14.4%–24.1%] vs 3.4% [95% CI = 2.9%–4.0%]) or used antidepressants in the past 30 days (35.1% [95% CI = 30.6%–39.8%] vs 11.8% [95% CI = 10.6%–13.2%]) compared with those not currently using opioids.
Opioid users were more likely to be nondrinkers compared with those not currently taking opioids (34.7% [95% CI = 29.1%–40.8%] vs 26.1% [95% CI = 23.2%–29.3%]) and were no more likely to be heavy drinkers compared with that group (7.8% [95% CI = 4.9%–12.1%] vs 7.5% [95% CI = 6.7%–8.5%]). Despite being more likely to be nondrinkers, 19.9% of opioid users reported that they were “moderate” or “heavy” drinkers. Opioid users were more likely to have used illicit drugs during the past six months than those not currently taking opioids (5.2% [95% CI = 3.5%–7.7%] vs 2.8% [95% CI = 2.2%–3.5%]).
Table 2 presents the odds ratios and adjusted odds ratios between the covariates and using at least one opioid analgesic. All covariates were associated with opioid use in the past 30 days, although non-Hispanic black race and being a heavy alcohol drinker did not reach statistical significance. These associations persisted after adjusting for demographic variables and health insurance status, except for respondent’s sex, which was no longer significant. The largest adjusted odds ratios observed were for experiencing pain on 14–29 days and 30 days out of the past 30 days (adjusted odds ratio [AOR] = 14.2 [95% CI = 8.3–24.4]; AOR = 26.6 [95% CI = 17.9–39.5]), using seven or more prescription medications (AOR = 20.8 [95% CI = 13.8–31.5]), and using benzodiazepines concurrently with opioids (AOR = 5.6 [95% CI = 4.1–7.7]).
Table 2.
Odds ratios and adjusted odds ratios of factors associated with opioid use, NHANES 2011–2014
| Unadjusted Comparison of Those Who Used vs Those Who Did Not Use | Adjusted Comparison of Those Who Used vs Those Who Did Not Use | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | AOR | 95% CI | P | |
| Age, y | ||||||
| 16–19 | 1.0 | Ref. | - | 1.0 | Ref. | - |
| 20–39 | 2.1 | 1.2–3.6 | ≤0.05 | 2.2 | 1.2–3.8 | ≤0.01 |
| 40–59 | 4.4 | 2.5–7.5 | ≤0.001 | 4.4 | 2.5–7.5 | ≤0.001 |
| 60+ | 4.5 | 2.6–7.7 | ≤0.001 | 4.1 | 2.4–7.2 | ≤0.001 |
| Sex | ||||||
| Men | 1.0 | Ref. | - | 1.0 | Ref. | - |
| Women | 1.2 | 1.0–1.5 | ≤0.05 | 1.2 | 1.0–1.4 | >0.05 |
| Race/Hispanic origin* | ||||||
| Non-Hispanic white | 1.0 | Ref. | - | 1.0 | Ref. | - |
| Non-Hispanic black | 0.9 | 0.8–1.1 | >0.05 | 1.1 | 0.8–1.4 | >0.05 |
| Non-Hispanic Asian | 0.2 | 0.1–0.3 | ≤0.001 | 0.2 | 0.1–0.4 | ≤0.001 |
| Hispanic | 0.5 | 0.4–0.7 | ≤0.001 | 0.7 | 0.5–1.0 | ≤0.05 |
| Health insurance status | ||||||
| Does not have insurance | 1.0 | Ref. | - | 1.0 | Ref. | - |
| Has insurance | 1.9 | 1.4–2.6 | ≤0.001 | 1.5 | 1.1–2.0 | ≤0.05 |
| Self-rated health | ||||||
| Excellent/very good/good | 1.0 | Ref. | - | 1.0 | Ref. | - |
| Fair/poor | 3.7 | 2.9–4.6 | ≤0.001 | 3.7 | 2.9–4.7 | ≤0.001 |
| Pain (past 30 d)† | ||||||
| 0 d | 1.0 | Ref. | - | 1.0 | Ref. | - |
| 1–13 d | 3.2 | 2.3–4.5 | ≤0.001 | 3.2 | 2.3–4.6 | ≤0.001 |
| 14–29 d | 14.3 | 8.2–24.9 | ≤0.001 | 14.2 | 8.3–24.4 | ≤0.001 |
| 30 d | 26.3 | 18.1–38.2 | ≤0.001 | 26.6 | 17.9–39.5 | ≤0.001 |
| Anxiety (past 30 d)† | ||||||
| 0 d | 1.0 | Ref. | - | 1.0 | Ref. | - |
| 1–13 d | 1.5 | 1.2–2.0 | ≤0.01 | 1.6 | 1.2–2.1 | ≤0.01 |
| 14–29 d | 3.2 | 2.0–5.0 | ≤0.001 | 3.3 | 2.1–5.2 | ≤0.001 |
| 30 d | 3.1 | 2.0–4.9 | ≤0.001 | 3.3 | 2.1–5.2 | ≤0.001 |
| Depression (past 2 wk) | ||||||
| 0–9 (not depressed) | 1.0 | Ref. | - | 1.0 | Ref. | - |
| 10+ (depressed) | 3.8 | 2.7–5.5 | ≤0.001 | 4.0 | 2.8–5.7 | ≤0.001 |
| No. of nonopioid prescription medications (past 30 d)‡ | ||||||
| 0 | 1.0 | Ref. | - | 1.0 | Ref. | - |
| 1–3 | 3.8 | 2.6–5.6 | ≤0.001 | 4.2 | 2.9–6.1 | ≤0.001 |
| 4–6 | 7.5 | 5.1–10.9 | ≤0.001 | 9.1 | 6.3–13.1 | ≤0.001 |
| 7+ | 16.5 | 11.0–24.7 | ≤0.001 | 20.8 | 13.8–31.5 | ≤0.001 |
| Used at least 1 benzodiazepine (past 30 d) | 6.5 | 4.8–8.8 | ≤0.001 | 5.6 | 4.1–7.7 | ≤0.001 |
| Used at least 1 antidepressant (past 30 d) | 4.0 | 3.4–4.8 | ≤0.001 | 3.5 | 2.9–4.1 | ≤0.001 |
| Current drinking status§ | ||||||
| Nondrinker | 1.0 | Ref. | - | 1.0 | Ref. | - |
| Light drinker | 0.7 | 0.6–0.9 | ≤0.01 | 0.8 | 0.7–1.0 | ≤0.05 |
| Moderate drinker | 0.5 | 0.3–0.6 | ≤0.001 | 0.5 | 0.3–0.7 | ≤0.01 |
| Heavy drinker | 0.8 | 0.4–1.4 | >0.05 | 0.9 | 0.5–1.5 | >0.05 |
| Illicit drug use (past 6 mo)¶ | 1.9 | 1.3–2.9 | ≤0.01 | 2.4 | 1.6–3.7 | ≤0.001 |
Exam sample weights were utilized to adjust for differential probabilities of selection and the complex sampling design. Percentages do not sum to 100 due to rounding. Respondents were asked, “In the past 30 days, have you used or taken medication for which a prescription is needed?” Adjusted models include respondents’ age (continuous), sex (dichotomous), race/Hispanic origin (categorical), and health insurance status (dichotomous).
CI = confidence interval; OR = odds ratio.
Estimates for persons of other race/Hispanic origin groups are not provided separately, but members of this group are included in total estimates.
Question only asked in 2011–2012.
Benzodiazepine and antidepressant prescription medications were included in this count.
Nondrinker (0 drinks in past year, includes former drinkers and abstainers); light drinker (average of 3 or fewer drinks per week); moderate drinker (average of 4–14 drinks per week for men; average of 4–7 drinks per week for women); heavy drinker (average of 15 or more drinks per week for men; average of 8 or more drinks per week for women). These questions were only asked of respondents aged 18 years and older.
Using cocaine, heroin, methamphetamine, and/or other injected drugs not prescribed by a doctor in the past six months. Questions about illicit drug use were only asked of respondents aged 16–59 years.
Strength of Opioids by Demographic
Characteristics
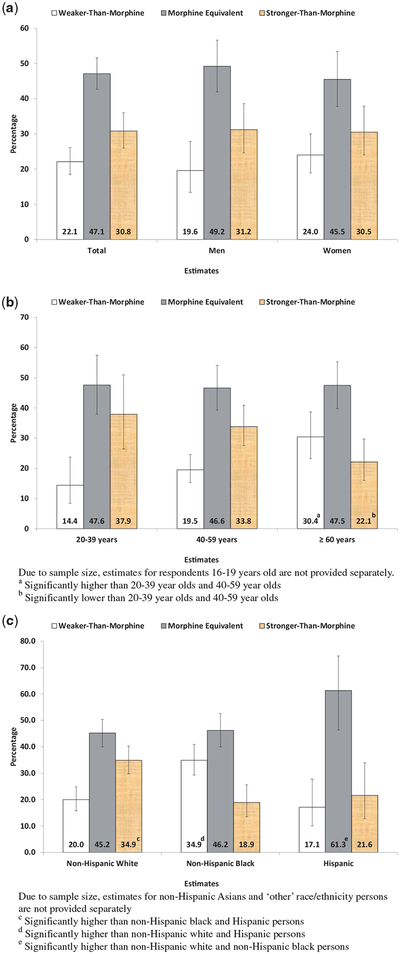
Figure 1 presents the estimates of strength of opioid used overall, by age group, sex, and race/Hispanic origin. During 2011–2014, 22.1% (95% CI = 18.5%–26.1%) of current opioid users used weaker-than-morphine opioids, 47.1% (95% CI = 42.7%–51.6%) used morphine equivalent opioids, and 30.8% (95% CI = 26.0%–36.0%) used stronger-than-morphine opioids. There was no significant difference in strength of opioid use between men and women. A larger percentage of opioid users aged 60 years and older used weaker-than-morphine opioids compared with adults aged 20–39 and 40–59 years (30.4% [95% CI = 23.2%–38.7%] vs 14.4% [95% CI = 8.4%–23.7%] and 19.5% [15.3%–24.6%], respectively). Conversely, a smaller percentage of opioid users aged 60 years and older used stronger-than-morphine opioids compared with adults aged 20–39 and 40–59 years (21.1% [95% CI = 16.0%–29.7%] vs 37.9% [95% CI = 26.4%–51.0%] and 33.8% [27.5%–40.9%], respectively). A larger percentage of non-Hispanic white opioid users used a stronger-than-morphine opioid compared with non-Hispanic black and Hispanic opioid users (34.9% [95% CI = 29.8%–40.3%] vs 18.9% [95% CI = 13.5%–25.6%] and 21.6% [95% CI = 12.8%–34.0%], respectively). A larger percentage of Hispanic users used morphine equivalent opioids than non-Hispanic white and non-Hispanic black users (61.3% [95% CI = 46.4%–74.4%] vs 45.2% [95% CI = 40.0%–50.4%] and 46.2% [95% CI = 40.0%–52.6%]). A larger percentage of non-Hispanic black opioid users used weaker-than-morphine opioids compared with non-Hispanic white and Hispanic opioid users (34.9% [95% CI = 29.3%–40.9%] vs 20.0% [95% CI = 15.8%–24.8%] and 17.1% [95% CI = 10.0%–27.7%]).
Figure 1.
Strength of opioid analgesic among opioid users aged 16 years and older by demographic characteristics, NHANES 2011–2014.
Discussion
The Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain [8], published in 2016, addressed several factors associated with opioid use disorder and overdose. These factors included poor mental health, alcohol consumption, illicit drug use, and concurrent use of opioids and benzodiaze-pines [8–10,14,29]. This study examined the prevalence of these factors in a recent nationally representative sample of the noninstitutionalized US population. Using prior research as a guide, it examined these factors among those aged 16 years and older using a wide array of self-reported health and health risk behavior measures [3,12,17]. In many instances, these factors were more prevalent among current opioid users than among those not currently using opioids. Larger percentages of opioid users reported poorer health including mental health problems (i.e., frequent anxiety and depression), concurrent use of benzodiazepines and antidepressants, and illicit drug use. Although opioid users were less likely to report moderate alcohol consumption and as likely to report heavy drinking as nonopioid users, approximately 20% of opioid users reported currently being moderate or heavy drinkers, which is not recommended when taking opioids [9].
Current opioid users reported experiencing more frequent pain that made it difficult to complete usual activities than those not currently taking opioids. In the adjusted regression analysis, the adjusted odds ratios for pain were some of the largest observed in this study. As opioid analgesics are used to reduce pain, this association may not be unexpected. However, approximately one-quarter of current opioid users reported experiencing zero days of pain that limited current activities. The findings presented here could be due to the effectiveness of opioids and their ability to relieve pain to the point where it no longer limits users’ activities. However, it is not possible to confirm this because the data come from a cross-sectional survey and data on opioid use and some of the factors examined were collected at different times (i.e., during the household exam and the physical exam at the MEC). These findings might indicate adverse selection, a phenomenon in which high-risk patients, including those with more severe and treatment-resistant pain conditions, tend to be prescribed high-risk opioid regimes rather than nonopioid medications, such as nonsteroidal anti-inflammatory drugs [30–32].
The examination of opioid strength by demographic characteristics expands the understanding of the variation in strength-of-opioid use. As shown in prior research, morphine equivalent opioids were the most frequently used, followed by stronger-than-morphine opioids, and then weaker-than-morphine opioids [4]. Although women were more likely to be using opioids than men in this study, the strength of the opioids used did not differ. Opioid users aged 60 years and older were more likely to use weaker-than-morphine opioids and less likely to use stronger-than-morphine opioids than opioid users aged 20–39 and 40–59 years. This finding is in line with general clinical guidelines to avoid giving high-strength opioids to elderly adults due to their greater pharmacodynamic sensitivity to them [33]. The findings also identified differences in strength of opioid use among race/Hispanic origin groups. Compared with non-Hispanic white opioid users, a larger percentage of non-Hispanic black opioid users used a weaker-than-morphine opioid. In contrast, a larger percentage of non-Hispanic white opioid users used stronger-than-morphine opioids than non-Hispanic black and Hispanic opioid users.
Limitations
The NHANES is a cross-sectional survey, so it is not possible to determine the causal link between current opioid use and the factors examined. The survey does not collect medication dosage of reported medications or discern how often the medication was taken during the past 30 days. Thus, it was not possible to calculate the total daily dosage of opioids (morphine milligram equivalent per day [MME/d]), which would provide a more nuanced assessment of opioid use and could avoid potentially misclassifying opioid strength based on the classification system used for this analysis. Finally, NHANES does not have data on misuse of opioids.
Strengths
Data for this analysis come from a nationally representative sample of the US noninstitutionalized population, which allowed for the generation of estimates that were representative of the US noninstitutionalized population aged 16 years and older. Prescription medication data were primarily collected by direct observation of medicine containers and categorized based on procedures used in prior pharmacoepidemiological studies on opioid analgesic use [4]. A wide of array of self-reported health measures and measures of health risk behaviors were incorporated into the analysis. Finally, the study examined variations in the strength of opioids across several demographic subgroups.
Conclusion
Current opioid users in the United States reported poor health status and engaging in health risk behaviors that have been shown in prior research to be associated with opioid use disorder or overdose. These behaviors included illicit drug use and concurrent use of benzodiazepines. The use of different strengths of opioids may vary by age and race/Hispanic origin. These findings may inform other research to further the understanding of opioid use in the US population.
Acknowledgment
The authors would like to thank Dr. Brian Kit, MD, MPH, for his assistance with conceptualizing the project and revising the manuscript.
Funding sources: None.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, Centers for Disease Control and Prevention, or any other entity of the US Government.
Conflicts of interest: The authors have no personal, commercial, or academic conflicts of interest to report and no financial relationships relevant to this article to disclose.
Prior presentation: Findings related to this project were presented in a poster at the 2017 Society for Epidemiological Research annual meeting.
References
- 1.Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician 2014;17(2):E119–28. [PubMed] [Google Scholar]
- 2.CDC. Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011;60:1487–92. [PubMed] [Google Scholar]
- 3.Mojtabai R National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf 2018;27(5):526–34. [DOI] [PubMed] [Google Scholar]
- 4.Frenk SM, Porter KS, Paulozzi LJ. Prescription Opioid Analgesic Use Among Adults: United States, 1999–2012 NCHS Data Brief, No 189. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 5.Dart RC, Surratt HL, Cicero TJ. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372(3):241–8. [DOI] [PubMed] [Google Scholar]
- 6.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016;65:1445–52. [DOI] [PubMed] [Google Scholar]
- 7.Warner M, Hedegaard H, Chen LH. Trends in drug-poisoning deaths involving opioid analgesics and heroin: United States, 1999–2012. NCHS health e-stats. 2014. Available at: http://www.cdc.gov/nchs/data/hestat/drug_poisoning/drug_poisoning_deaths_1999-2012.pdf (accessed December 2016). [PubMed]
- 8.Dowell D, Haegerich TM, Chou R. CDC guidelines for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. 2016. FDA warns about serious risk and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Drug Safety Communications. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM518672.pdf (accessed December 2016).
- 10.Weiner SG, Raja AS, Bittner JC, et al. Opioid-related policies in New England emergency departments. Acad Emerg Med 2016;23(9):1086–90. [DOI] [PubMed] [Google Scholar]
- 11.Jann M, Kennedy WK, Lopez G. Benzodiazepines: A major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract 2014;27(1):5–16. [DOI] [PubMed] [Google Scholar]
- 12.Larochelle MR, Zhang F, Ross-Degnan D, et al. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001–2010. Pharmacoepidemiol Drug Saf 2015;24(8):885–92. [DOI] [PubMed] [Google Scholar]
- 13.Mack KA, Zhang K, Paulozzi L, et al. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved 2015;26(1):182–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulozzi LJ. Prescription drug overdoses: A review. J Safety Res 2012;43(4):283–9. [DOI] [PubMed] [Google Scholar]
- 15.West NA, Dart RC. Prescription opioid exposures and adverse outcomes among older adults. Pharmacoepidemiol Drug Saf 2016;25(5):539–44. [DOI] [PubMed] [Google Scholar]
- 16.Baumblatt JAG, Wiedeman C, Dunn J, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med 2014;174(5):796–801. [DOI] [PubMed] [Google Scholar]
- 17.Edlund MJ, Martin BC, Fan M- Y, et al. Risk for opioid abuse and dependence among recipients of chronic opioid therapy: Results from the TROUP study. Drug Alcohol Depend 2010;112(1–2):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zipf G, Chiappa M, Porter KS, et al. National health and nutrition examination survey: Plan and operations, 1999–2010. National Center for Health Statistics. Vital Health Stat 2013;1:1–37. [PubMed] [Google Scholar]
- 19.National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed December 2016). [Google Scholar]
- 20.Division of Health and Nutrition Examination Surveys. NHANES response rates and population totals. 2015. Available at: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx (accessed December 2016).
- 21.National Center for Health Statistics. 2011. Dietary supplements and prescription medication—DSQ. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/questionnaires/dsq.pdf (accessed December 2016).
- 22.NCHS. National Health and Nutrition Examination Survey, 1988–2014 data documentation, codebook, and frequencies: Prescription medications—drug information. 2016. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm (accessed December 2016).
- 23.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24(6):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manor O, Matthews S, Power C. Dichotomous or categorical response? Analysing self-rated health and lifetime social class. Int J Epidemiol 2000;29(1):149–57. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fryar CD, Hirsch R, Porter KS, et al. Smoking and Alcohol Behaviors Reported by Adults, United States 1999–2002 Advance Data from Vital and Health Statistics; No 378. Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 27.Zedler B, Xie L, Wang L, Joyce A, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration Patients. Pain Med 2014;15(11):1911–29. [DOI] [PubMed] [Google Scholar]
- 28.StataCorp. Stata Statistical Software: Release 15 [computer program]. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 29.Webster LR, Dasgupta N. An analysis of the root cause for opioid-related overdose deaths in the United States. Pain Med 2011;12(suppl 2):S26–35. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: Promises and perils. Pain 2013;154:S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell G, Nielsen S, Larance B, et al. Pharmaceutical opioid use and dependence among people living with chronic pain: Associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med 2015;16(9):1745–58. [DOI] [PubMed] [Google Scholar]
- 32.Henry SG, Wilsey BL, Melnikow J, Iosif AM. Dose elscalation during the first year of long-term opioid therapy for chronic pain. Pain Med 2015;16(4):733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naples JG, Gellad WF, Hanlon JT. Managing pain in older adults: The role of opioid analgesics. Clin Geriatr Med 2016;32(4):725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]