Abstract
Background
Abnormal calcium (Ca2+) release from the sarcoplasmic reticulum (SR) contributes to the pathogenesis of atrial fibrillation (AF). Increased phosphorylation of two proteins essential for normal SR-Ca2+ cycling, the type-2 ryanodine receptor (RyR2) and phospholamban (PLN), enhances the susceptibility to AF, but the underlying mechanisms remain unclear. Protein phosphatase 1 (PP1) limits steady-state phosphorylation of both RyR2 and PLN. Proteomic analysis uncovered a novel PP1-regulatory subunit (PPP1R3A) in the RyR2 macromolecular channel complex which has been previously shown to mediate PP1 targeting to PLN. We tested the hypothesis that reduced PPP1R3A levels contribute to AF pathogenesis by reducing PP1 binding to both RyR2 and PLN.
Methods
Immunoprecipitation, mass spectrometry and complexome profiling were performed from AF patient atrial tissue and from cardiac lysates of WT and Pln-KO mice. Ppp1r3a-KO mice were generated by CRISPR-mediated deletion of exons 2–3. Ppp1r3a-KO mice and WT littermates were subjected to in vivo programmed electrical stimulation to determine AF susceptibility. Isolated atrial cardiomyocytes were used for STimulated Emission Depletion (STED) superresolution microscopy and confocal Ca2+ imaging.
Results
Proteomics identified the PP1-regulatory subunit PPP1R3A as a novel RyR2-binding partner, and co-immunoprecipitation confirmed PPP1R3A binding to RyR2 and PLN. Complexome profiling and STED imaging revealed PLN is present in the PPP1R3A-RyR2 interaction, suggesting the existence of a previously unknown SR nanodomain composed of both RyR2 and PLN/SERCA2a macromolecular complexes. This novel RyR2/PLN/SERCA2a complex was also identified in human atria. Genetic ablation of Ppp1r3a in mice impaired binding of PP1 to both RyR2 and PLN. Reduced PP1 targeting was associated with increased phosphorylation of RyR2 and PLN, aberrant SR-Ca2+ release in atrial cardiomyocytes and enhanced susceptibility to pacing-induced AF. Finally, PPP1R3A was progressively downregulated in atria of patients with paroxysmal and persistent (chronic) AF.
Conclusions
PPP1R3A is a novel PP1-regulatory subunit within the RyR2 channel complex. Reduced PPP1R3A levels impair PP1 targeting and increase phosphorylation of both RyR2 and PLN. PPP1R3A deficiency promotes abnormal SR-Ca2+ release and increases AF susceptibility in mice. Given that PPP1R3A is downregulated in AF patients, this regulatory subunit may represent a new target for AF therapeutic strategies.
Keywords: Atrial fibrillation, calcium release, protein phosphatase 1, PPP1R3A, GM, RGL, ryanodine receptor, RyR2
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with an increased risk of stroke and mortality.1 Current AF therapy with drugs is of moderate efficacy because AF is increasingly considered as a symptom or marker of an atrial cardiomyopathy resulting from a variety of risk factors, including genetic predisposition, sex, advanced age, and cardiovascular and non-cardiovascular diseases.2 The limitations of current AF therapies have at least in part been attributed to a one-size-fits-most approach that ignores the diversity of underlying cardiomyopathy and the related mechanisms that predispose to AF.3,4 Altered calcium (Ca2+) handling within atrial cardiomyocytes is widely regarded as a hallmark of AF.5 Emerging evidence has revealed abnormal Ca2+ release from the sarcoplasmic reticulum (SR) as a key mechanism promoting atrial arrhythmogenesis and AF development.6–9
Normal excitation-contraction coupling depends on the balanced influx and efflux of cytosolic Ca2+.10 During an action potential, Ca2+ enters the cell through L-type Ca2+ channels, activating the type-2 ryanodine receptor (RyR2), which in turn releases a much larger amount of Ca2+ from the SR into the cytosol to trigger myocyte contraction. Relaxation occurs when cytosolic Ca2+ is re-sequestered into the SR via the SR-Ca2+-ATPase (SERCA2a), or extruded from the myocyte via the Na+/Ca2+-exchanger (NCX).
In patients with early stage (paroxysmal) or long-standing persistent (chronic) AF (pAF and cAF, respectively), enhanced activities of both RyR2 and SERCA2a are associated with increased SR-Ca2+ leak, increased SR-Ca2+ load, and delayed afterdepolarizations that promote cellular arrhythmogenic events.9,11,12 Studies using human atrial tissue revealed that in cAF, hyperphosphorylation of RyR2 promotes spontaneous SR-Ca2+ leak, while hyperphosphorylation of the SERCA2a-regulator phospholamban (PLN) promotes increased SR-Ca2+ re-uptake through reduced inhibition of SERCA2a.13 This enhanced re-uptake likely compensates for the reduction in SR-Ca2+ load during AF and may have a permissive effect on SR-Ca2+ leak. The mechanisms underlying enhanced phosphorylation of SR-Ca2+-handling proteins in AF, especially that of RyR2, have remained subject of substantial controversy.14–16 Until recently, a majority of work has focused on understanding the role of kinases such as protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) in promoting RyR2 and PLN dysfunction.17,18 However, several studies including ours revealed that cardiac diseases lead to extensive changes in subcellular targeting of protein phosphatases as well.13,19,20
Protein phosphatase type-1 (PP1) is one of the most abundant serine-threonine phosphatases in the heart, and dephosphorylates both RyR2 and PLN.21 Proper targeting of the PP1-catalytic (PP1c) subunit depends on a variety of regulatory subunits (R-subunits) that confer substrate specificity, and we have recently shown that altered binding between PP1c and its R-subunits may contribute to impaired phosphatase targeting in AF.19,22 For example, PP1c is targeted to RyR2 by the regulatory subunit PPP1R9B (spinophilin). Genetic ablation of spinophilin in mice reduced the amount of RyR2-bound PP1c by ~60%, leading to increased phosphorylation of RyR2 at S2814 and enhanced AF susceptibility.22 Interestingly, the amount of residual PP1c bound to RyR2 in spinophilin-deficient mice suggests additional R-subunits may also mediate the interaction between PP1c and RyR2. On the other hand, targeting of PP1c to PLN is mediated by PPP1R3A (aka GM/RGL), however little is known about the role of this R-subunit in cardiac function.23
Here, we report that PPP1R3A is a novel RyR2-binding partner, and demonstrate that loss of PPP1R3A impairs targeting of PP1c to both PLN and RyR2. This leads to increased SR-Ca2+ leak and enhanced AF susceptibility in Ppp1r3A-KO mice. Complexome profiling24,25 suggests that PPP1R3A itself plays an integral part of a never-before-identified extended protein complex within the SR that is comprised among others of RyR2, PLN and SERCA2a, revealing that Ca2+ release/re-uptake complexes physically interact. Further, PPP1R3A is progressively downregulated and its extended SR protein complex disrupted in pAF and cAF patients, implicating this R-subunit as a potential contributor to AF progression to more persistent forms, and, hence, a novel therapeutic target for AF treatment.
METHODS
Please see the Online Data Supplements for detailed materials and methods. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Human samples
Right atrial appendages of patients undergoing open-heart surgery were collected with patients’ written informed consent. All experimental protocols were approved by the ethics committee of the Medical Faculty at the University of Duisburg-Essen (AZ:12–5268-BO). Patient characteristics are listed in Supplemental Tables 1 and 2.
Animal studies
Studies involving animals were performed according to protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine, and conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996). Ppp1r3a-KO mice were generated by CRISPR-Cas9 targeted deletion of exons 2 and 3 and were maintained on a C57Bl6/J background. Male and female mice were used for all experiments, with WT littermates as controls. Mice were anaesthetized with 2% isoflurane in 0.5L/min 100% 02 prior to surgery or tissue collection. Mice that appeared unhealthy or runted (body-weight < 2 SD below average) were excluded. Analyses were performed in blinded manner.
Co-immunoprecipitation and mass spectrometry
Co-immunoprecipitation and mass spectrometry were performed as described.19
Quantitative real-time PCR
Quantitative real-time (qRT)-PCR was performed as described.26 Primers are listed in Supplemental Table 3.
Intracardiac electrophysiology studies
In vivo electrophysiology studies were performed in 4–5 month old Ppp1r3a-KO mice and WT littermates.27
STED and confocal microscopy
Atrial cardiomyocytes were isolated via retrograde Langendorff perfusion using a modified collagenase protocol.6,12 Cells were either fixed and stained with PPP1R3A, RyR2, and PLN antibodies for STimulated Emission Depletion (STED) superresolution imaging, or loaded with Fluo-4 AM for live-cell confocal Ca2+-imaging.8,28
Complexome profiling
Total membrane pellets (100,000xg) from isolated mouse ventricular cardiomyocytes or human right-atrial tissue were solubilized with digitonin. Higher molecular complexes including mitochondrial OXPHOS supercomplexes were separated by blue native gel electrophoresis, and protein composition and migration profiling analyzed by mass spectrometry as described.29,30
Statistical analysis
Results are expressed as mean±standard error of the mean (SEM). Fisher’s exact test, chi-square test or unpaired two-tailed Student’s t-test was used for comparisons between two groups, as specified in figure legends. Generalized Estimating Equation was used for analysis of hierarchical/non-independent data (see Online Data Supplements). A P-value of less than 0.05 was considered statistically significant.
RESULTS
Identification of PPP1R3A as a novel regulator of RyR2
To identify novel regulators of RyR2 phosphorylation, RyR2 was co-immunoprecipitated from WT mouse hearts followed by mass spectrometry analysis. As a negative control, each sample was also immunoprecipitated with murine non-specific IgG. Of the proteins identified, six were either serine/threonine kinases, phosphatases or phosphatase regulatory subunits (Supplemental Table 4). When compared with the mass spectrometry signal from the control immunoprecipitation (IP) using non-specific IgG, 2 of these proteins were found exclusively in the RyR2 IP (top 2 rows with ratio of “Infinity”). The protein with the highest abundance in the IP as estimated by the label-free quantification (LFQ) signal normalized to molecular weight was the PP1 catalytic subunit PP1c (right most column). The protein with the second highest abundance was PPP1R3A, a known regulatory subunit of PP1c.31 Since PP1c is known to regulate RyR2 function, but not through PPP1R3A, we conducted in-depth studies to investigate potentially important roles of PPP1R3A in regulating RyR2.
PPP1R3A mediates targeting of PP1c to both RyR2 and PLN in mouse heart
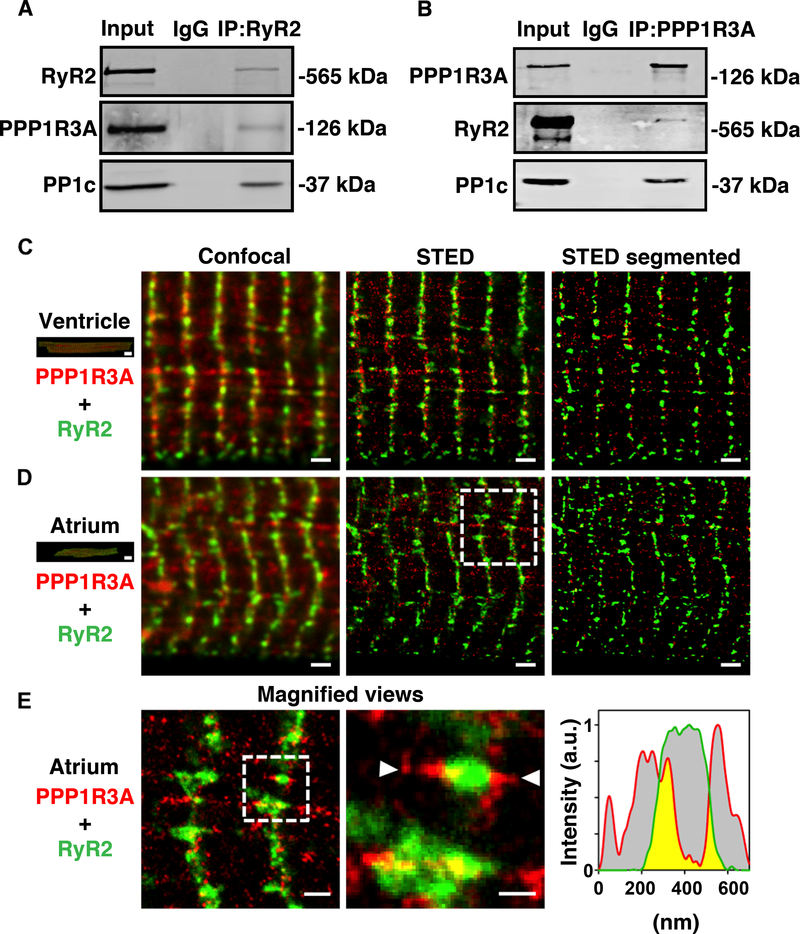
PPP1R3A is a 126-kDa protein reported to target PP1c to glycogen and PLN.23,32,33 However, there were no prior reports suggesting that PPP1R3A mediates the interaction between PP1c and RyR2. We therefore sought to validate the mass spectrometry results implicating PPP1R3A as a novel RyR2 interactor. Reciprocal co-immunoprecipitation from mouse heart lysates using either RyR2 or PPP1R3A antibodies confirmed that PPP1R3A binds to both RyR2 and PP1c in mouse heart (Figure 1A–B). Interestingly, super-resolution microscopy using STimulated Emission Depletion (STED) revealed that the interaction of PPP1R3A with RyR2 in ventricular or atrial cardiomyocytes occurs in a highly localized fashion in SR nanodomains, often near Z-line striations (Figure 1C–D). Magnified views of atrial myocytes depict larger, axially extended RyR2 clusters, apparently containing distinct PPP1R3A co-localization domains (Figure 1E). In addition, triple-color STED imaging further confirmed PPP1R3A nanodomains typically aligned along the main cell axis and co-localized with PLN right next to RyR2 clusters (Supplemental Figure 1). Validation of the signal specificity of the PPP1R3A antibody used for STED experiments can be found in Supplemental Figure 2. Taken together, these findings confirm PPP1R3A as a novel RyR2-binding partner and suggest this PP1 regulatory (R) subunit may play a dual or integrative role in regulating both PLN and RyR2.
Figure 1. Validation of binding between RyR2, PPP1R3A and PP1c in mouse heart.
Representative Western blots confirming the interaction between RyR2, PPP1R3A, and PP1c in WT mouse heart lysates immunoprecipitated with (A) RyR2 antibody or (B) PPP1R3A antibody. Confocal and STED imaging of co-immunostained ventricular (C) and atrial (D) mouse cardiomyocytes. STED, but not confocal imaging, resolves the PPP1R3A (red) and RyR2 (green) signals, which enables image segmentation for regional nanodomain visualization (right). Of note, RyR2 and PPP1R3A clusters show considerable differences in their subcellular distribution between ventricular and atrial myocytes. Scale bars 10 μm (left, cell overview) or 1 μm (image panels). (E) Left: Magnified view (as indicated in D). Center: zoom-in showing the local association of PPP1R3A with RyR2 clusters at nanometric scale. The white triangles indicate the nanodomain orientation used for signal intensity profiling. Right: The PPP1R3A (red) and RyR2 (green) signal distribution confirms sub-cluster areas exhibiting co-localized signals (yellow). Scale bars 500 nm (left) or 200 nm (center). Dashed boxes indicate magnified views in (D) and (E).
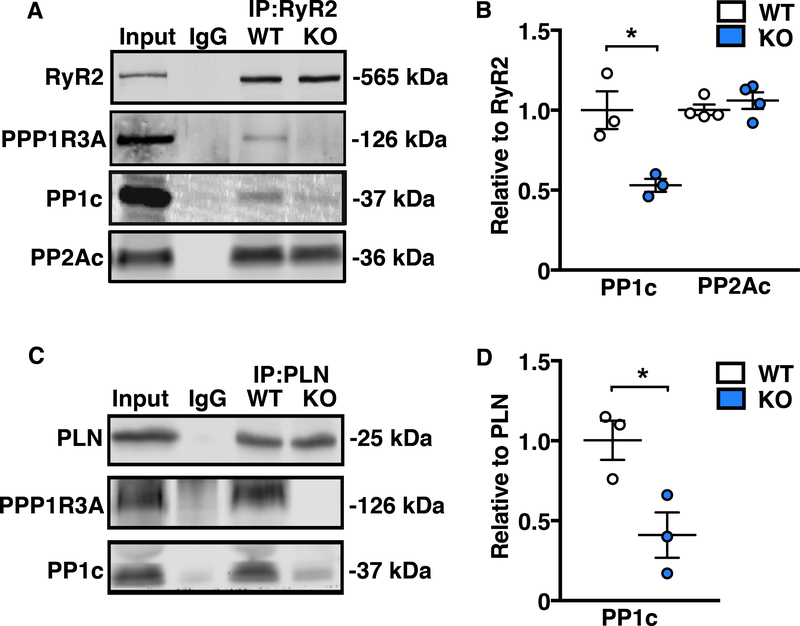
To explore the functional importance of PPP1R3A in the heart, we generated Ppp1r3a knockout (KO) mice by CRISPR-Cas9 mediated deletion of exons 2 and 3 (Supplemental Figure 3). Co-immunoprecipitation experiments revealed a 47% reduction in the amount of PP1c bound to RyR2 in Ppp1r3a-KO mouse hearts (p<0.05 vs. WT; Figure 2A–B) confirming that PPP1R3A is necessary for mediating (at least in part) the interaction between PP1c and RyR2. Importantly, loss of PPP1R3A did not alter PP2Ac binding to RyR2. Similarly, we found a 58% reduction in the amount of PP1c bound to PLN (p<0.05 vs. WT; Figure 2C–D), in agreement with prior reports that PPP1R3A mediates targeting of PP1c to PLN.23,32 These findings suggest that some PP1c might bind to PLN through another yet-to-be-identified PP1 R-subunit.
Figure 2. Genetic ablation of PPP1R3A impairs binding of PP1c to both RyR2 and PLN.
Representative Western blots and corresponding dot plots showing that with PPP1R3A ablation, (A and B) the association between RyR2 and PP1c is significantly reduced with no change in the association between RyR2 and PP2A, and (C and D) the association between PLN and PP1c is significantly reduced. Data represent mean±SEM and were analyzed using unpaired 2-tailed Student’s t-test. (*P<0.05 vs. WT).
Impaired local PP1 regulation enhances RyR2 and PLN phosphorylation
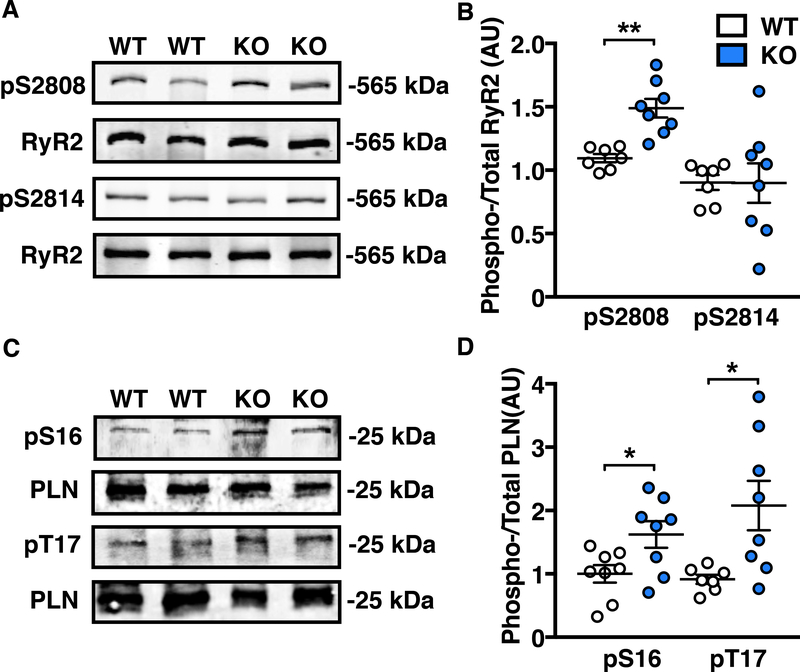
Enhanced phosphorylation of RyR2 at both S2808 and S2814 promotes SR-Ca2+ release, while phosphorylation of PLN at S16 and T17 promotes SR-Ca2+ re-uptake through SERCA2a by reducing PLN’s inhibition of the channel. Because we observed impaired targeting of PP1c to both RyR2 and PLN in Ppp1r3a-KO hearts, we expected an increase in the phosphorylation levels of both proteins. Consistently, relative phosphorylation of RyR2 was increased at the primary PKA site S2808 by 50% (p<0.01 vs WT), with no change at the CaMKII site S2814 (Figure 3A–B). Moreover, phosphorylation of PLN was increased at both the PKA site S16 by 62% and the CaMKII site T17 by 100% in Ppp1r3a-KO atria (p<0.05 vs WT for both; Figure 3C–D).
Figure 3. Loss of local PP1c regulation in the absence of PPP1R3A increases RyR2 and PLN phosphorylation.
Representative Western blots and corresponding dot plots showing (A and B) increased phosphorylation of RyR2 at S2808 but not at S2814 in Ppp1r3a-KO (KO) mouse atria compared with wild-type (WT) atria. (C and D) increased phosphorylation of PLN at both S16 and T17 in Ppp1r3a-KO mouse atria. Data represent mean±SEM and were analyzed using unpaired 2-tailed Student’s t-test (*P<0.05, **P<0.01 vs. WT).
To demonstrate that the observed changes in RyR2 and PLN phosphorylation were due exclusively to decreased targeting of PP1c, we investigated global changes in protein expression of the phosphatases and kinases known to regulate the two proteins. We did not observe compensatory changes in expression levels of PP1 and PP2A catalytic subunits, or of the PP1c R-subunit spinophilin in Ppp1r3a-KO atria (Supplemental Figure 4A–B). Atrial protein levels of kinases PKA and CaMKII were also unchanged between Ppp1r3a-KO and WT (Supplemental Figure 4C–D). Priming phosphorylation sites of PKA (T197) and CaMKII (T286), which are essential for their catalytic activity, were also unchanged, indicating the changes in RyR2 and PLN phosphorylation are specifically due to reduced phosphatase targeting, and not enhanced kinase activities (Supplemental Figure 4 E–F).
Impaired local PP1c regulation by Ppp1r3a knockout increases AF susceptibility
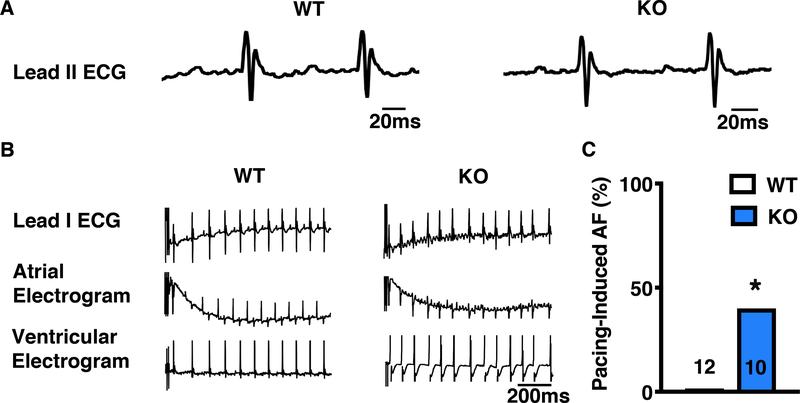
To understand how locally reduced PP1c targeting to RyR2 and PLN affects cardiac function, we performed in vivo programmed electrical stimulation (PES) studies in 4-month-old Ppp1r3a-KO mice and WT littermates. Heart rate and baseline ECG parameters were similar between groups, (Figure 4A; Supplemental Table 5). Furthermore, we found no indication of baseline ventricular dysfunction in Ppp1r3a-KO mice (Supplemental Table 6). However, following atrial burst pacing 40% of the Ppp1r3a-KO mice had reproducible episodes of AF, compared to none of the WT mice (p<0.05; Figure 4 B–C). Ventricular PES did not induce VT in any Ppp1r3a-KO mice (data not shown). Interestingly, PPP1R3A protein levels were 2.9 fold higher in mouse atria compared to ventricle (Supplemental Figure 5). These data indicate that genetic ablation of PPP1R3A results in an atrial-specific phenotype, and position PPP1R3A as a novel atrial-selective therapeutic target.
Figure 4. Ppp1r3a-KO mice exhibit increased susceptibility to pacing-induced AF.
A, Representative recordings of surface ECG lead II showing no change in baseline ECG parameters comparing 4-month old Ppp1r3a-KO mice and WT littermates. B, Simultaneous recordings from surface and intracardiac (atrial and ventricular) leads demonstrating AF in Ppp1r3a-KO mice (right) and normal sinus rhythm in WT littermate (left) following atrial burst pacing. C, Bar graph summarizing the incidence of inducible AF in Ppp1r3a-KO mice. Data were analyzed using Fisher’s exact test (*P<0.05 vs. WT).
To determine the underlying cause of AF in Ppp1r3a-KO mice, we looked for signs of structural and electrical remodeling. We observed no changes in heart morphology, atrial weight, or baseline ECG parameters (Supplemental Figure 6A; Supplemental Table 5). Quantitative real-time PCR showed unchanged expression of fibrotic gene markers (collagen-I, collagen-III and TGFβ) in Ppp1r3a-KO atria (Supplemental Figure 6B), and Masson’s trichrome staining confirmed the absence of fibrosis in Ppp1r3a-KO atria (Supplemental Figure 6C–D). Similarly, we found no changes in atrial expression of the ion-channel genes Cacna1c, Kcna5, Kcnj3, or Kcnj5 (Supplemental Figure 7). Although Cacna1c mRNA levels showed a non-significant increase in KO mice, no change was found at the protein level. Overall, these results indicate that the AF phenotype observed in Ppp1r3a-KO mice is unlikely to be due to structural or electrical remodeling.
Ca2+ handling is altered in Ppp1r3a-KO atrial cardiomyocytes due to increased activities of RyR2 and SERCA2a
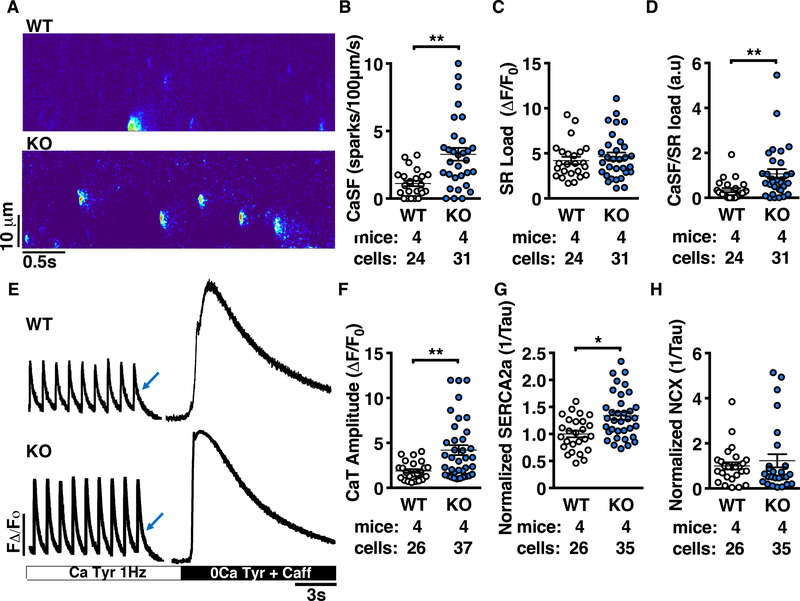
Since RyR2 and PLN are both critical mediators of SR-Ca2+ release and re-uptake, we expected their dysregulation to lead to altered SR-Ca2+ handling in Ppp1r3a-KO atrial cardiomyocytes. While total protein expression of SR-Ca2+-handling proteins was unaltered (Supplemental Figure 8), linescan confocal Ca2+ imaging revealed an increase in the frequency of RyR2-mediated Ca2+ sparks (CaSF) in Ppp1r3a-KO atrial cardiomyocytes (3.3±0.5 a.u.) compared to WT (1.1±0.2 a.u.; P<0.01; Figure 5 A–B). Total SR-Ca2+ content (SR load) was not significantly different between groups (4.8±0.5 a.u. for KO vs. 4.2±0.4 a.u. for WT; Figure 5C), and the increase in SR-Ca2+ leak was maintained after normalizing CaSF to SR load (1.1±0.2 a.u. for KO vs. 0.34±0.1 a.u. for WT; P<0.01; Figure 5D). Sparks characteristics are listed in Supplemental Table 7. The amplitude of the Ca2+ transient (CaT) was also increased in cardiomyocytes from Ppp1r3a-KO mice (4.2±0.6 a.u. for KO vs. 1.9±0.2 a.u. for WT; P<0.01; Figure 5E–F). In addition to enhanced RyR2-mediated Ca2+ leak, Ppp1r3a-KO atrial cardiomyocytes exhibited faster re-uptake of cytosolic Ca2+ into the SR proportional to a 30% increase in SERCA2a activity (P<0.05 vs. WT; Figure 5E, 5G). These results reveal aberrant SR-Ca2+ release and re-uptake in atrial cardiomyocytes from Ppp1r3a-KO mice consistent with enhanced activities of both RyR2 and SERCA2a. Additionally, challenging atrial cardiomyocytes with 100 nM isoprenaline further exacerbated RyR2-mediated Ca2+ leak in Ppp1r3a-KO cells compared to WT, with no difference in SERCA2a activity between groups (Supplemental Figure 9).
Figure 5. Ca2+ handling is altered in atrial cardiomyocytes from Ppp1r3a-KO mice due to increased activities of RyR2 and SERCA2a.
A, Representative confocal line-scan images of atrial myocytes from Ppp1r3a-KO mice and WT littermates. B, Dot plots summarizing spontaneous Ca2+ spark frequency (CaSF). C, Summary of SR-Ca2+ load and (D) CaSF normalized to caffeine-induced SR-Ca2+ load. E, Representative tracings of Ca2+ transient recordings during 1Hz pacing and after exposure to 10mM caffeine. F, Dot plot summarizing Ca2+ transient amplitude. G, SERCA2a activity calculated as the difference between the decay of the pacing-induced transient and the caffeine-induced transient. H, NCX activity calculated from the decay of the caffeine-induced transient. Abbreviations: CaSF, Ca2+ spark frequency; CaT, Ca2+ transient; Tyr, Tyrode’s buffer; Caff, caffeine. Data represent mean±SEM and were analyzed using the Generalized Estimating Equation function in SPSS (*P<0.05; **P<0.01 vs. WT).
Although RyR2 phosphorylation at S2808 was increased in Ppp1r3a-KO ventricle (Supplemental Figure 10A–B), RyR2-mediated Ca2+ leak was comparable between WT and Ppp1r3a-KO ventricular cardiomyocytes (Supplemental Figure 11A–C). Nevertheless, like in atria, CaT amplitude and SERCA2a activity were significantly higher in Ppp1r3a-KO ventricular cardiomyocytes (Supplemental Figure 11D–E), and PLN phosphorylation was increased at S16, but not T17 (Supplemental Figure 10C–D). Similar to atria, no change in ventricular NCX activity was observed (Supplemental Figure 11F). Thus, unlike in atrial cardiomyocytes, PPP1R3A appears not to play a critical role in regulating ventricular E-C coupling.
Identification of PPP1R3A within an extended RyR2/PLN/SERCA2a complex
Our findings reveal PPP1R3A as a novel “integrator” of SR-Ca2+ cycling in cardiomyocytes. This is of great interest since little is known about the interplay between RyR2 and PLN/SERCA2a and how they work together to precisely coordinate SR-Ca2+ release and re-uptake locally. To better understand how PPP1R3A interacts with both RyR2 and the PLN/SERCA2a complex, we performed Blue Native (BN)-PAGE/mass spectrometry-based complexome profiling from total membrane fractions of mouse ventricular cardiomyocytes. In order to identify large (10 kDa to 5 MDa) multiprotein complexes, samples were fractionated by BN gel electrophoresis, and 60 gel slices subjected to in-gel tryptic digestion for analysis by mass spectrometry. Apparent molecular weight calibration for soluble and integral membrane proteins including larger complexes from the gel slices can be found in Supplemental Figure 12.34 Notably, mitochondrial OXPHOS complexes of known molecular weight were preserved in PLN KO membranes, confirming proteomic quality and quantitative profiling as a viable approach for ventricular cardiomyocyte membranes (Supplemental Figure 13).29
Strikingly, complexome profiling identified PPP1R3A as part of previously unknown higher molecular weight complexes that include PLN, SERCA2a and RyR2, as indicated by their overlapping migration pattern following BN gel electrophoresis (Figure 6A–C). Interestingly, the abundance of both the SERCA2a and RyR2 complexes was disrupted in PLN deficient (PLN-KO) cardiomyocytes (Figure 6D–F). This unexpected finding positions PLN as a necessary mediator of PPP1R3A binding to RyR2, strongly supporting the existence of a novel extended complex within SR nanodomains that includes PPP1R3A, RyR2, PLN and SERCA2a. In contrast to prevailing models of functionally discrete core complexes, these results together with our STED imaging data indicate that RyR2 and PLN/SERCA2a may in fact physically interact and function as joint Ca2+ release/re-uptake regulatome complexes.
Figure 6. Complexome profiling reveals PPP1R3A as a protein within a novel high molecular weight RyR2/PLN/SERCA2a complex.
A, Heat map and B-C, migration profiles of PLN, SERCA2a, RyR2 and PPP1R3A in cardiomyocytes from PLN wildtype (WT) mice. Arrows indicate high-molecular weight complexes. D, Heat map and E-F, migration profiles of SERCA2a, RyR2, and PPP1R3A in cardiomyocytes from PLN-KO mice.
PPP1R3A expression is downregulated with progression of AF in patients
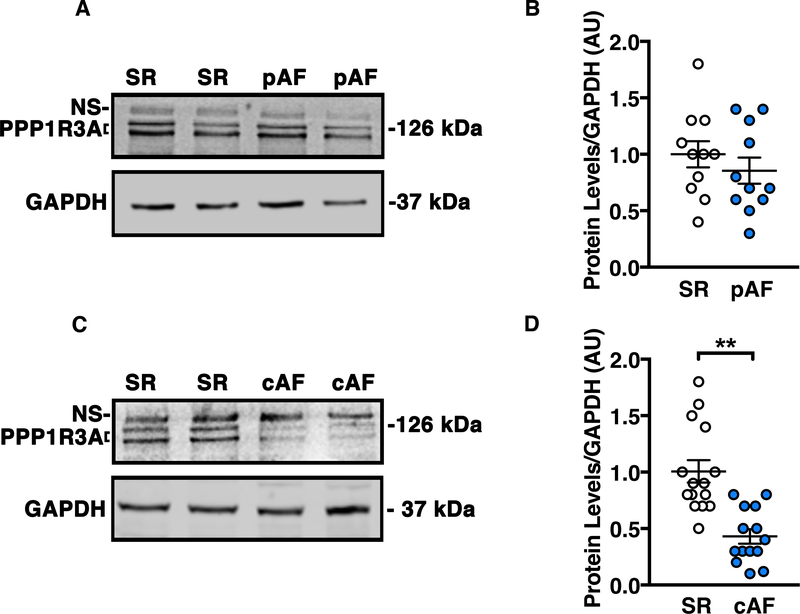
Since PPP1R3A deficiency enhances the susceptibility to inducible AF in mice, we assessed PPP1R3A protein levels in atrial tissues from patients with pAF and cAF. Patient characteristics are listed in Supplemental Table 1, and human PPP1R3A antibody validation can be found in Supplemental Figure 14. PPP1R3A expression was not significantly decreased in pAF patients (Figure 7 A–B), but was 60% lower in cAF patients (P<0.01 vs. control patients in sinus rhythm; Figure 7 C–D). These findings suggest PPP1R3A is a clinically relevant PP1 R-subunit whose downregulation may contribute to proarrhythmic alterations in atrial cardiomyocyte Ca2+ homeostasis in AF patients.
Figure 7. PPP1R3A protein expression is reduced in atria of cAF patients.
Representative Western blots and corresponding dot plots from human atrial biopsy samples showing (A and B) slight but non-significant decrease in PPP1R3A expression levels (bottom two bands) in early stage (paroxysmal) AF patients and (C and D) significant decrease in PPP1R3A expression levels in late stage (chronic) AF patients. Abbreviations: pAF, paroxysmal AF; cAF, chronic AF; SR, sinus rhythm. Data represent mean±SEM and were analyzed using unpaired 2-tailed Student’s t-test (**P<0.01 vs. SR control).
PPP1R3A deficiency in mice prevented PP1c from targeting to the SR, resulting in increased phosphorylation of RyR2 and PLN. Since previous work showed that phosphorylation of RyR2 and PLN is increased in cAF patients, we correlated PPP1R3A protein levels with those of P-RyR2, P-PLN, and PP1c in a second cAF cohort. Consistent with other groups, we found significant increases in phosphorylation of RyR2 (at S2814) and of PLN (at S16) in cAF patients, and confirmed a significant reduction in PPP1R3A protein expression (Supplemental Figure 15). Our correlation analyses revealed that PPP1R3A expression negatively correlates with phosphorylation levels of RyR2 and PLN. Similarly, PP1c protein expression also showed a negative correlation with the degree of RyR2 and PLN phosphorylation (Supplemental Figure 16). These patient data are consistent with our mouse studies, where we found a reduction in local PP1c targeting to the SR-Can cause hyper-phosphorylation of key SR-Ca2+ handling proteins, thereby increasing diastolic SR-Ca2+ leak and susceptibility to AF.
Disruption of the extended RyR2/PLN/SERCA2a complex in human AF
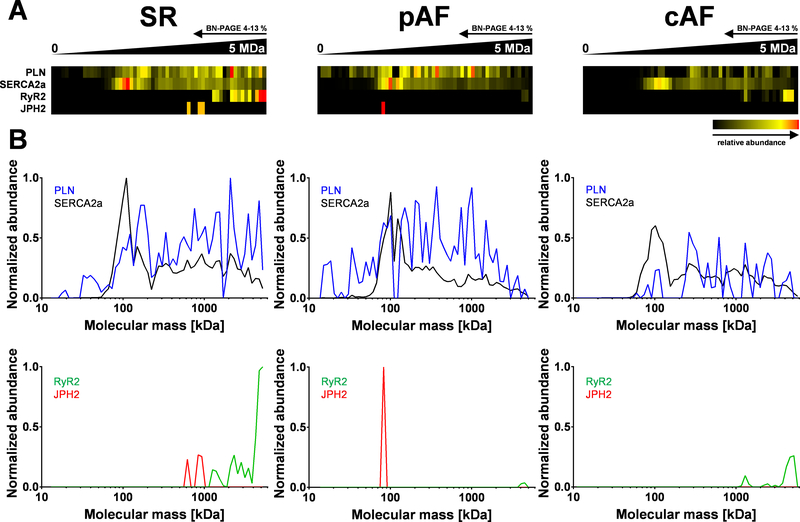
Our complexome study identified a novel, extended SR-Ca2+ cycling complex in the mouse heart that included RyR2 and PLN/SERCA2a. To determine the relevance of this complex in human atria, we performed complexome profiling on right-atrial samples from 1 patient each in sinus rhythm (pseudo-control, due to coronary artery disease), pAF and cAF (Supplemental Table 2). Samples were size-fractionated by BN gel electrophoresis and 60 gel slices subjected to digestion for mass spectrometry profiling (Supplemental Figure 17A). Soluble and integral membrane proteins/complexes showed a robust linear calibration throughout apparent molecular weights (Supplemental Figure 17B).34 Moreover, human mitochondrial OXPHOS complexes appeared intact in pAF and cAF samples (Supplemental Figure 18), confirming a similar quality of complexome profiling as in mouse cardiomyocytes (Supplemental Figure 13).
In the sinus rhythm patient, complexome profiling confirmed higher molecular weight complexes composed of PLN, SERCA2a, RyR2, and Junctophilin-2 (JPH2) by their overlapping migration profiles (Figure 8A–B, sinus rhythm). In contrast, in pAF RyR2 channels interacting with JPH2 proteins were nearly abolished in higher complexes, with only monomeric JPH2 detected. (Figure 6A and 6B, pAF). Similarly, in cAF the abundance of higher molecular complexes was clearly reduced and again JPH2 was not detected (Figure 8A and 8B, cAF). While PPP1R3A was not detected for technical reasons in these samples, co-IP confirmed the presence of PPP1R3A in the complex in human atria (Supplemental Figure 19). These data identify a previously unknown qualitative and quantitative disruption of higher molecular weight RyR2/SERCA2a/PLN complexes, which confirms and extends our data from mouse ventricle to human atria, and shows a potentially relevant loss of JPH2 in AF.
Figure 8. Higher molecular complexes in human atrial tissue are disrupted in atrial fibrillation.
A, Heat map representation of the migration profile of PLN, SERCA2a, RyR2, and JPH2 in human atrial tissue (total membrane fraction). Atrial samples were obtained from patients with sinus rhythm (SR), paroxysmal AF (pAF), or chronic AF (cAF). B, In SR (pseudo-control; see Supplemental Table 2), profiling shows comigration of PLN/SERCA2a and RyR2 in higher molecular complexes (>1 MDa) and additionally JPH2 (≤1 MDa). In contrast, in paroxysmal AF the RyR2 channel was nearly abolished in higher complexes and only monomeric JPH2 exists. In contrast, in cAF the abundance of higher molecular complexes was decreased and JPH2 was not detected any longer.
DISCUSSION
Dysregulation of steady-state protein phosphorylation plays an important role in atrial arrhythmogenesis, but the mechanisms remain poorly understood due to a lack of understanding of global vs. local phosphatase regulation. In the heart, PP1c associates with nearly 200 known R-subunits that govern its local regulation by modulating subcellular localization, substrate specificity, and catalytic activity. We demonstrate for the first time that the PP1c R-subunit PPP1R3A mediates PP1c regulation of both RyR2 and PLN, and is an essential integrator of cardiac SR-Ca2+ cycling. Our IP/MS data identified PPP1R3A as a novel RyR2 binding partner, and further studies confirmed PPP1R3A mediates binding of the PP1-catalytic subunit PP1c to RyR2. Similarly, we confirmed PPP1R3A also mediates binding of PP1c to PLN. Using complexome profiling, we identified PPP1R3A within an extended SR macromolecular complex that included RyR2, PLN and SERCA2a. We discovered that cardiac PPP1R3A deficiency in mice is associated with increased atrial RyR2 and PLN phosphorylation, arrhythmogenic atrial SR-Ca2+ leak, and susceptibility to AF. Finally, we found PPP1R3A is reduced in cAF patients, revealing PPP1R3A downregulation as a potentially novel mechanism underlying aberrant phosphatase targeting in AF pathogenesis.
RyR2 regulation by PP1 in AF
The impact of altered RyR2 phosphorylation in cardiac diseases remains controversial.14 Nevertheless, the majority of studies exploring dysregulation of RyR2 in AF have shown that hyperphosphorylation of RyR2 at residues S2814 and S2808 promotes SR-Ca2+ leak, spontaneous Ca2+-release events, and DADs that may underlie arrhythmogenic triggered activity in atrial cardiomyocytes.35–37 Phosphorylation of RyR2 is mediated by PKA and CaMKII, while dephosphorylation is mediated by PP1 and PP2A. However, it is still incompletely understood which RyR2 phosphorylation sites are dephosphorylated by PP1 and/or PP2A.21 Chiang et al.22 demonstrated that PP1-regulatory subunit PPP1R9B (spinophilin) targets PP1c to RyR2. However, genetic ablation of spinophilin in mice reduced PP1c binding to RyR2 by only ~60%, suggesting that PP1c may bind to the channel through alternative targeting mechanism(s). Consistent with this observation, our findings from Ppp1r3a-KO mice revealed that PPP1R3A also mediates PP1c targeting to RyR2, accounting for approximately 47% of total PP1c binding. Interestingly, while ablation of spinophilin increased RyR2 phosphorylation exclusively at S2814, ablation of PPP1R3A increased RyR2 phosphorylation exclusively at S2808. These findings suggest differential roles for spinophilin vs. PPP1R3A in site-specific regulation of RyR2, the significance of which remains to be elucidated.
One unexpected finding was that Ppp1r3a ablation resulted in an atrial-specific phenotype in 4–5 month-old mice. Although RyR2-S2808 phosphorylation was increased in both atria and ventricle of Ppp1r3a-KO mice, only atrial cardiomyocytes exhibited excessive RyR2-mediated Ca2+ leak, and only atrial arrhythmias were detected with PES in Ppp1r3a-KO mice. This may be due to 3-fold higher PPP1R3A protein levels in atria, but also suggests a differential role for RyR2-S2808 phosphorylation in atrial vs. ventricular E-C coupling. Indeed, recently we identified a conserved, differential subcellular phosphorylation mechanism specific for atrial cardiomyocytes, where only junctional RyR2 clusters are constitutively phosphorylated by PKA and CaMKII, in contrast to non-junctional clusters.6,38 The Ca2+-handling alterations observed in ventricular cardiomyocytes of Ppp1r3a−/− mice (~30% increase in SERCA2a activity without diastolic SR-Ca2+ leak) may even be beneficial in the setting of chronic cytosolic Ca2+ overload, however further studies are needed to fully understand the importance of PPP1R3A in the ventricle.
PLN regulation by PP1
PLN is a critical mediator of SR-Ca2+ re-uptake through regulation of SERCA2a. In its de-phosphorylated form, PLN inhibits SERCA2a activity. Phosphorylation by PKA at PLN-S16 and by CaMKII at PLN-T17 relieves this inhibition, increasing the rate of SR-Ca2+ transport and SERCA2a activity. Early work established that dephosphorylation of PLN is mediated primarily by PP1.39 However, recently Akaike et al.40 reported that the newly identified PP2Ce is also able to dephosphorylate cardiac PLN, specifically at T17, but not at S16. Our findings support the notion that PP1 dephosphorylates both S16 and T17 of PLN, since both sites were hyperphosphorylated in the absence of PPP1R3A. Nevertheless, it is possible that PPP1R3A also mediates targeting of PP2Ce to PLN, which may explain why we observed a larger increase in T17 compared to S16 phosphorylation in Ppp1r3a-KO mice. This possibility may be the subject of future investigation, as currently no antibodies sensitive enough to detect cardiac PP2Ce are available.
PLN is apparently less important in regulating atrial (vs. ventricular) SERCA2a function due to a lower PLN/SERCA2a ratio in murine atrial tissue.6 In our studies, Ppp1r3a ablation resulted in increased phosphorylation of atrial PLN at S16 and T17, which was associated with a 30% increase in SERCA2a activity in Ppp1r3a-KO atrial cardiomyocytes. While RyR2-mediated Ca2+ leak was exacerbated with 100nM isoprenaline in KO cardiomyocytes, the difference in SERCA2a activity disappeared, indicating that maximal SERCA2a activity was restored in Ppp1r3a-KO atrial cardiomyocytes, consistent with 2-fold higher expression of SERCA2a in atria vs. ventricle. 6,38
New insights into the SR-Ca2+-handling regulatome
Complexome profiling is a powerful, unbiased method for analysis of higher molecular weight complexes, previously established to identify protein constituents of mitochondrial OXPHOS supercomplexes (S0–Sn).25,30 Here, we used complexome profiling in combination with superresolution STED microscopy to study the dual role of PPP1R3A in regulating both RyR2 and SERCA2a/PLN complexes.24,41 Our data suggest that PPP1R3A forms an integral part of a previously unappreciated SR membrane complex comprised of both the RyR2 Ca2+ release channel and the SERCA2a/PLN Ca2+ re-uptake unit. In addition, we found that this higher molecular weight Ca2+ cycling complex is disrupted in AF patients. Recent work has revealed that membrane proteins including RyR2 form supramolecular clusters with inbuilt functional heterogeneity in order to enhance and coordinate the critical threshold to external stimuli.42 Our findings provide fundamental and potential clinical insight into the spatial and molecular architecture of these important SR-Ca2+ handling machines, and suggest that the RyR2 and SERCA2a complexes may physically interact to precisely coordinate SR-Ca2+ release and re-uptake in local SR nanodomains.
Global vs. local PP1 dysfunction in AF
We found that PPP1R3A targets PP1c to both RyR2 and PLN in mouse heart, and that PPP1R3A protein levels are reduced in AF patients. Interestingly, while global PP1c levels were decreased in our cAF tissue samples, earlier studies from AF patient tissues have reported paradoxically increased PP1c expression and activity.43,44 Nevertheless, this was associated with heterogeneous steady-state phosphorylation of contractile and Ca2+-handling proteins.13,45 For example, PLN and RyR2 were found to be hyperphosphorylated in cAF (consistent with our findings), while cardiac myosin binding protein C (cMyBP-C) was hypophosphorylated.21 The surprising finding that PP1c targeting to RyR2 seems to be locally reduced despite globally increased expression and activity may be explained by altered binding to one (or more) of the PP1 regulatory subunits. Recent IP/MS findings by Chiang and colleagues have revealed that the PP1-R-subunits interactome is altered in AF patients, underscoring the importance of investigating changes in local phosphatase regulation rather than changes in global phosphatase levels and activity.19 Atrial cardiomyocytes from Ppp1r3a-KO mice revealed increased diastolic SR-Ca2+ leak, which may promote arrhythmogenic DADs. This, together with our data from AF patients, suggest that reduced PPP1R3A levels contribute to aberrant atrial Ca2+ handling by altering local targeting of PP1c to both RyR2 and PLN.
POTENTIAL LIMITATIONS
For this work, we generated and validated a Ppp1r3a-KO mouse model. One limitation of this model is that ablation of PPP1R3A was not atrial-specific. Nevertheless, we did not observe ventricular abnormalities that may have confounded the AF phenotype, nor did we find gross morphological changes in Ppp1r3a-KO mice, in contrast to others who reported increased weight gain in Ppp1r3a-KO due to impaired glycogen metabolism in skeletal muscle.46 Another potential limitation is that Ppp1r3a-KO mice did not exhibit spontaneous AF at the age studied (4–5 months), however studies in older mice may reveal an aging phenotype with subsequent development of spontaneous AF. PPP1R3A mediates phosphatase regulation of two key Ca2+-handling proteins. Future studies crossing Ppp1r3a-KO mice to either PLN or RyR2 mutants should be performed to decipher the precise functional effects of PPP1R3A on each protein. We cannot exclude the possibility that PPP1R3A mediates PP1 targeting to proteins other than RyR2 and PLN; however, we believe this is unlikely since PPP1R3A binds to the SR membrane and is therefore not easily accessible to non SR-bound proteins.30 Finally, in the present AF patient cohort we did not observe an increase in RyR2 phosphorylation at Ser2808 as reported in some,8,11,37,47 but not all48–50 previous studies. Differences in type and evolution of the underlying atrial cardiomyopathy may potentially contribute to and explain these inconsistent results.
CONCLUSIONS
We have shown for the first time that the PP1-regulatory subunit PPP1R3A is an RyR2-binding protein responsible for targeting PP1c to the PKA site (S2808) of RyR2. We also discovered that PPP1R3A targets PP1c to both the PKA site (S16) and CaMKII site (T17) of PLN, ultimately forming higher molecular weight protein complexes that include RyR2, PLN and SERCA2a. To our knowledge, our study is the first to detect this native, extended SR-Ca2+ cycling complex in the heart. Furthermore, our finding that PPP1R3A modulates PP1 regulation of both RyR2 and PLN to promote SR-Ca2+ leak sheds light on the complex mechanisms by which altered phosphatase targeting may contribute to aberrant SR-Ca2+ cycling in AF pathophysiology.
Supplementary Material
CLINICAL PERSPECTIVE.
What Is New?
This study demonstrates for the first time that reduced levels of protein phosphatase 1 regulatory subunit R3A (PPP1R3A) in human atria are causally linked to abnormal calcium handling and atrial fibrillation (AF) pathogenesis.
In the absence of PPP1R3A, reduced binding of PP1 catalytic subunit increases phosphorylation levels of the ryanodine receptor (RyR2) calcium release channel and phospholamban (PLN).
Complexome profiling, a technique that combines native gel electrophoresis with mass spectrometry to obtain the composition of multiprotein assemblies, revealed that PPP1R3A is part of a macromolecular protein complex containing the RyR2 calcium release channel and the SERCA2a/PLN calcium reuptake transporter.
What Are the Clinical Implications?
Reduced levels of a PP1 regulatory subunit contribute to abnormal calcium release and reuptake in atrial myocytes thereby promoting AF pathogenesis.
Normalizing levels of the PPP1R3A phosphatase subunit represents a novel therapeutic approach for AF.
ACKNOWLEDGEMENTS
The custom-made PLN antibody for STED was kindly provided by Zhenhui Chen (Krannert Institute of Cardiology, Indiana University School of Medicine, USA).
SOURCES OF FUNDING
This work is supported by 3R01HL089598–07S2 (K.M.A), Basic Research Fellowship from the European Society of Cardiology (D.Y.C.), Netherlands Organization for Scientific Research (NWO) via the Roadmap Initiative Proteins@Work (project 184.032.201; A.J.R.H.), European Union Horizon 2020 program FET-OPEN project MSmed (project 686547; A.J.R.H.), Deutsches Zentrum Fur Herz-Kreislauf-Forschung (German Centre for Cardiovascular Research; S.E.L.), Deutsche Forschungsgemeinschaft (German Research Foundation) Do 769/4–1 and Collaborative Research Centers 1002 (S.E.L.) and 1190 (S.E.L., C.L., and H.L.), NIH grants R01-HL131517 (D.D.), R01-HL089598, R01-HL091947, HL134824, and R01-HL147108 (X.H.T.W.). Resources accessed through the Mouse ES Cell and GEM cores were supported by National Institutes of Health-National Cancer Institute grant CA125123 to Dan L. Duncan Cancer Center.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- AF
Atrial fibrillation
- Ca2+
Calcium
- KO
Knockout
- PLN
Phospholamban
- PP1
Protein phosphatase 1
- PP1c
Protein phosphatase 1 catalytic subunit
- PPP1R3A
Protein phosphatase 1 regulatory subunit type 3A
- R-subunit
Regulatory subunit
- RyR2
Ryanodine receptor type-2
- SERCA2a
Sarco/endoplasmic reticulum calcium ATPase-2a
- SR
Sarcoplasmic reticulum
- WT
Wild-type
Footnotes
DISCLOSURES
XHTW is a founding partner of Elex Biotech, a start-up company that developed drug molecules to target ryanodine receptors for treatment of cardiac arrhythmias. Other authors have no conflicts related to this study.
REFERENCES
- 1.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S, Document R. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan GA, Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int J Cardiol Heart Vasc. 2018;21:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijman J, Guichard JB, Dobrev D, Nattel S. Translational Challenges in Atrial Fibrillation. Circ Res. 2018;122:752–773. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. [DOI] [PubMed] [Google Scholar]
- 6.Brandenburg S, Kohl T, Williams GS, Gusev K, Wagner E, Rog-Zielinska EA, Hebisch E, Dura M, Didie M, Gotthardt M, Nikolaev VO, Hasenfuss G, Kohl P, Ward CW, Lederer WJ, Lehnart SE. Axial tubule junctions control rapid calcium signaling in atria. J Clin Invest. 2016;126:3999–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XH. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 11.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. [DOI] [PubMed] [Google Scholar]
- 14.Backx PH. Complexity, confusion and controversy continue complicating the contribution of RyR2 channel phosphorylation to heart function. J Physiol. 2014;592:1911–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrev D, Wehrens XH. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houser SR. Role of RyR2 phosphorylation in heart failure and arrhythmias: protein kinase A-mediated hyperphosphorylation of the ryanodine receptor at serine 2808 does not alter cardiac contractility or cause heart failure and arrhythmias. Circ Res. 2014;114:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. [DOI] [PubMed] [Google Scholar]
- 18.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. [DOI] [PubMed] [Google Scholar]
- 19.Chiang DY, Lebesgue N, Beavers DL, Alsina KM, Damen JM, Voigt N, Dobrev D, Wehrens XH, Scholten A. Alterations in the interactome of serine/threonine protein phosphatase type-1 in atrial fibrillation patients. J Am Coll Cardiol. 2015;65:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang DY, Alsina KM, Corradini E, Fitzpatrick M, Ni L, Lahiri SK, Reynolds J, Pan X, Scott L Jr., Heck AJR, Wehrens XH. Rearrangement of the Protein Phosphatase 1 Interactome During Heart Failure Progression. Circulation. 2018;138:1569–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heijman J, Ghezelbash S, Wehrens XH, Dobrev D. Serine/Threonine Phosphatases in Atrial Fibrillation. J Mol Cell Cardiol. 2017;103:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang DY, Li N, Wang Q, Alsina KM, Quick AP, Reynolds JO, Wang G, Skapura D, Voigt N, Dobrev D, Wehrens XH. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res. 2014;103:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vafiadaki E, Arvanitis DA, Sanoudou D, Kranias EG. Identification of a protein phosphatase-1/phospholamban complex that is regulated by cAMP-dependent phosphorylation. PLoS One. 2013;8:e80867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessels HJ, Vogel RO, van den Heuvel L, Smeitink JA, Rodenburg RJ, Nijtmans LG, Farhoud MH. LC-MS/MS as an alternative for SDS-PAGE in blue native analysis of protein complexes. Proteomics. 2009;9:4221–4228. [DOI] [PubMed] [Google Scholar]
- 25.Wessels HJ, Vogel RO, Lightowlers RN, Spelbrink JN, Rodenburg RJ, van den Heuvel LP, van Gool AJ, Gloerich J, Smeitink JA, Nijtmans LG. Analysis of 953 human proteins from a mitochondrial HEK293 fraction by complexome profiling. PLoS One. 2013;8:e68340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang DY, Kongchan N, Beavers DL, Alsina KM, Voigt N, Neilson JR, Jakob H, Martin JF, Dobrev D, Wehrens XH, Li N. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol. 2014;7:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010. pii: 1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heide H, Bleier L, Steger M, Ackermann J, Drose S, Schwamb B, Zornig M, Reichert AS, Koch I, Wittig I, Brandt U. Complexome profiling identifies TMEM126B as a component of the mitochondrial complex I assembly complex. Cell Metab. 2012;16:538–549. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero-Castillo S, Baertling F, Kownatzki D, Wessels HJ, Arnold S, Brandt U, Nijtmans L. The Assembly Pathway of Mitochondrial Respiratory Chain Complex I. Cell Metab. 2017;25:128–139. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard MJ, Dent P, Smythe C, Cohen P. Targetting of protein phosphatase 1 to the sarcoplasmic reticulum of rabbit skeletal muscle by a protein that is very similar or identical to the G subunit that directs the enzyme to glycogen. Eur J Biochem. 1990;189:243–249. [DOI] [PubMed] [Google Scholar]
- 32.Berrebi-Bertrand I, Souchet M, Camelin JC, Laville MP, Calmels T, Bril A. Biophysical interaction between phospholamban and protein phosphatase 1 regulatory subunit GM. FEBS Lett. 1998;439:224–230. [DOI] [PubMed] [Google Scholar]
- 33.Savage DB, Zhai L, Ravikumar B, Choi CS, Snaar JE, McGuire AC, Wou SE, Medina-Gomez G, Kim S, Bock CB, Segvich DM, Solanky B, Deelchand D, Vidal-Puig A, Wareham NJ, Shulman GI, Karpe F, Taylor R, Pederson BA, Roach PJ, O’Rahilly S, DePaoli-Roach AA. A prevalent variant in PPP1R3A impairs glycogen synthesis and reduces muscle glycogen content in humans and mice. PLoS Med. 2008;5:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittig I, Beckhaus T, Wumaier Z, Karas M, Schagger H. Mass estimation of native proteins by blue native electrophoresis: principles and practical hints. Mol Cell Proteomics. 2010;9:2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landstrom AP, Dobrev D, Wehrens XHT. Calcium Signaling and Cardiac Arrhythmias. Circ Res. 2017;120:1969–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heijman J, Ghezelbash S, Wehrens XH, Dobrev D. Serine/Threonine Phosphatases in Atrial Fibrillation. J Mol Cell Cardiol. 2017;103:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. [DOI] [PubMed] [Google Scholar]
- 38.Brandenburg S, Pawlowitz J, Fakuade FE, Kownatzki-Danger D, Kohl T, Mitronova GY, Scardigli M, Neef J, Schmidt C, Wiedmann F, Pavone FS, Sacconi L, Kutschka I, Sossalla S, Moser T, Voigt N, Lehnart SE. Axial Tubule Junctions Activate Atrial Ca(2+) Release Across Species. Front Physiol. 2018;9:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steenaart NA, Ganim JR, Di Salvo J, Kranias EG. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch Biochem Biophys. 1992;293:17–24. [DOI] [PubMed] [Google Scholar]
- 40.Akaike T, Du N, Lu G, Minamisawa S, Wang Y, Ruan H. A Sarcoplasmic Reticulum Localized Protein Phosphatase Regulates Phospholamban Phosphorylation and Promotes Ischemia Reperfusion Injury in the Heart. JACC Basic Transl Sci. 2017;2:160–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker MA, Williams GSB, Kohl T, Lehnart SE, Jafri MS, Greenstein JL, Lederer WJ, Winslow RL. Superresolution modeling of calcium release in the heart. Biophys J. 2014;107:3018–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker MA, Kohl T, Lehnart SE, Greenstein JL, Lederer WJ, Winslow RL. On the Adjacency Matrix of RyR2 Cluster Structures. PLoS Comput Biol. 2015;11:e1004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer-Roxlau S, Lammle S, Opitz A, Kunzel S, Joos JP, Neef S, Sekeres K, Sossalla S, Schondube F, Alexiou K, Maier LS, Dobrev D, Guan K, Weber S, El-Armouche A. Differential regulation of protein phosphatase 1 (PP1) isoforms in human heart failure and atrial fibrillation. Basic Res Cardiol. 2017;112:43. [DOI] [PubMed] [Google Scholar]
- 44.Greiser M, Halaszovich CR, Frechen D, Boknik P, Ravens U, Dobrev D, Luckhoff A, Schotten U. Pharmacological evidence for altered src kinase regulation of I (Ca,L) in patients with chronic atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:383–392. [DOI] [PubMed] [Google Scholar]
- 45.Christ T, Boknik P, Wohrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. [DOI] [PubMed] [Google Scholar]
- 46.Delibegovic M, Armstrong CG, Dobbie L, Watt PW, Smith AJ, Cohen PT. Disruption of the striated muscle glycogen targeting subunit PPP1R3A of protein phosphatase 1 leads to increased weight gain, fat deposition, and development of insulin resistance. Diabetes. 2003;52:596–604. [DOI] [PubMed] [Google Scholar]
- 47.Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casado V, Ciruela F, Lluis C, Franco R, Cinca J, Hove-Madsen L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J. 2011;32:721–729. [DOI] [PubMed] [Google Scholar]
- 48.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR-Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. [DOI] [PubMed] [Google Scholar]
- 49.Christ T, Rozmaritsa N, Engel A, Berk E, Knaut M, Metzner K, Canteras M, Ravens U, Kaumann A. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc Natl Acad Sci U S A. 2014;111:11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molina CE, Abu-Taha IH, Wang Q, Rosello-Diez E, Kamler M, Nattel S, Ravens U, Wehrens XHT, Hove-Madsen L, Heijman J, Dobrev D. Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients With and Without Atrial Fibrillation. Front Physiol. 2018;9:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.