Abstract
High-density lipoprotein (HDL) function rather than level may better predict cardiovascular disease (CVD). However, the contribution of the impaired antioxidant function of HDL that is associated with increased HDL lipid peroxidation (HDLox) to the development of clinical CVD remains unclear. We have investigated the association between serum HDLox with incident CVD outcomes in Mashhad cohort. Three-hundred and thirty individuals who had a median follow-up period of 7 years were recruited as part of the cohort. The primary end point was cardiovascular event, including myocardial infarction, stable angina, unstable angina, or coronary revascularization. In both univariate/multivariate analyses adjusted for traditional CVD risk factors, HDLox was an independent risk factor for CVD (odds ratio, 1.62; 95% confidence interval, 1.41–1.86; p < 0.001). For every increase in HDLox by 0.1 unit, there was an increase in CVD risk by 1.62-fold. In an adjusted analysis, there was a >2.5-fold increase in cardiovascular risk in individuals with HDLox higher than cutoff point of 1.06 compared to those with lower scores, suggesting HDLox > 1.06 is related to the impaired HDL oxidant function and in turn exposed to elevated risk ofCVD outcomes (hazard ratio, 2.72; 95% CI, 1.88–3.94). Higher HDLox is a surrogate measure of reduced HDL antioxidant function that positively associated with cardiovascular events in a population-based cohort.
Keywords: antioxidant, HDL function, HDLox, high-density lipoprotein, inflammation
1. INTRODUCTION
Cardiovascular disease (CVD) is a major cause of morbidity and mortality, globally. The prevalence of CVD is increasing in developing countries as a result of lifestyle, lack of education, and public awareness of its risk factors, particularly obesity and diabetes mellitus (Azarpazhooh et al., 2010). The pathogenesis of CVD is multifactorial (Smith et al., 2010). Reduced levels of high-density lipoprotein cholesterol (HDL-C) has been used as a predictor of CVD in several epidemiological studies (Desforges, Gordon, & Rifkind, 1989; Rubins et al., 1999). HDL has diverse antiatherogenic properties including reverse cholesterol transport, antioxidant, anti-inflammatory, antithrombotic, endothelial protection (Holzer et al., 2013; McPherson, Young, McKibben, & McEneny, 2007). However, apart from the REVEAL study (The HPS3/TIMI55–REVEAL Collaborative Group, 2017), drugs that raise HDL-C (niacin and the cholesteryl ester transfer protein inhibitors) have not been shown to impact cardiovascular clinical end points (The HPS2-THRIVE Collaborative Group, 2014; Hovingh et al., 2015). In the setting of inflammation and atherogenesis, changes in the lipid and protein content of HDL and oxidation of HDL (HDLox) may lead to the formation of HDL particles that have increased lipid peroxide content and may be dysfunctional and proatherogenic (Navab et al., 2006). HDL function rather than HDL-C levels may be a more important predictor for CVD (Navab et al., 2005; Navab, Reddy, Van Lenten, Anantharamaiah, & Fogelman, 2009; Vílchez, Martínez-Ruiz, Sancho-Rodríguez, Martínez-Hernández, & Noguera-Velasco, 2014). Because of the complexity of HDL metabolism, HDL function has been difficult to assess in humans (Khera et al., 2011; Patel, Khera, Jafri, Wilensky, & Rader, 2011). Cell-based assays of HDL-C efflux capacity have been used in epidemiological and clinical population-based studies (e.g., the Dallas Heart Study) as a measure of antiatherogenic HDL function and as a predictor of CVD risk (Khera et al., 2011; Rohatgi et al., 2014; Vílchez et al., 2014). However, cholesterol efflux assays (Khera et al., 2011) have several limitations; standardization is difficult and there is significant heterogeneity with regard to types of cells and type of readout that is reported (Movva & Rader, 2008). Thus, although the cholesterol efflux assay has provided valuable insights into the role of HDL function in CVD, it is unlikely that this assay with its intrinsic experimental variability and methodological limitations will be routinely used in the clinic. Cell-free assays may give more robust measurements of HDL function compared with cell-based assays. We have developed a cell-free fluorometric (based on the Amplex Red Cholesterol Assay Kit [Invitrogen, Life Technologies, Grand Island, NY]) high throughput method that measures HDL-associated lipid peroxidation (HDLox) that offers a reproducible and rapid means of determining a surrogate measure of HDL function (Kelesidis et al., 2014). Oxidation of HDL may impair HDL’s antioxidant and antiatherogenic properties (Kelesidis et al., 2011, 2016; Navab, Reddy, Van Lenten, & Fogelman, 2011). Reduced antioxidant HDL function, as assessed by biochemical assays, has been associated with CVD (Kelesidis et al., 2014; Patel et al., 2011). In HIV-positive patients on potent antiretroviral therapy (ART), the readout from this assay correlates with measures of subclinical atherosclerosis such as carotid intima media thickness (Kelesidis et al., 2014) and calcium artery score (Zanni et al., 2014). Importantly, the readout from this assay has also been reported to be associated with established proinflammatory and proatherogenic phenotypes of HDL (Kelesidis et al., 2014) and was able to predict the direct ex vivo proatherogenic potential of dysfunctional HDL (its ability to promote monocyte-derived foam cell formation [MDFCF], a key event in early atherogenesis; Angelovich et al., 2017). HDL isolated from HIV+ patients with an otherwise low CVD risk profile, who were taking potent ART [HIV(+)HDL] had reduced antioxidant function and increased lipid peroxide content (HDLox) and promoted ex vivo MDFCF to a greater extent than HDL isolated from healthy individuals (33.0% vs. 26.2% foam cells; Angelovich et al., 2017). There is limited data regarding how different measures of HDL function (such as impaired antioxidant function) other than the impaired HDL-C efflux may contribute to clinical CVD.To our knowledge, the association of HDLox with clinical CVD has not previously been assessed in a large cohort with available clinical outcomes of CVD. The aim of the current study was to evaluate the association between serum HDL lipid peroxide content (HDLox) with CVD risk factors and clinical CVD outcomes in a population recruited from the Mashhad stroke and heart atherosclerotic disorders (MASHAD) cohort.
2 |. METHODS
2.1 |. Study design
The MASHAD study is a cohort study within a representative population from northeastern Iran. The study started in 2007 and will continue until 2020. Eligible participants 35–65 years old without clinical coronary artery disease (CAD), stroke, and peripheral arterial disease were recruited. Eligibility was determined based on brief medical interviews, physical examination, and review by cardiologists. Medical examination was included as angiography, computerized tomography (CT) angiography, stress echocardiography, and exercise tolerance testing and were adjudicated by an electrophysiologist and two interventional cardiologists. Assessments included determination of demographic (age, sex, educational level), anthropometric (weight, height, body mass index, waist circumference), and CVD risk factors (smoking status, lipid profile, fasting blood sugar, blood pressure measurements, history of diabetes, and history of use of medications for hypertension and diabetes). Population characteristics and study design have been previously published (Ghayour-Mobarhan et al., 2015). The primary end point was cardiovascular event, including myocardial infarction, stable angina, unstable angina, or coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting). Two independent cardiologists blinded to the results of all experiments adjudicated all the clinical end points. The study was approved by Ethics Committee of Mashhad University of Medical Sciences, and informed written consent was obtained from all participants.
2.2 |. Reagents
The assay was conducted using reagents from Amplex Red Cholesterol Assay Kit (Invitrogen, Life Technologies, Grand Island, NY), phosphate-buffered saline (BioShop, Burlington, ON, Canada), polyethylene glycol (PEG) molecular weight (MW) 6000 (Sigma-Aldrich, St. Louis, MO), deionized water, catalase enzyme (Sigma-Aldrich). Black 96-well plates were from SPL Life Science (Pocheon,South Korea). Serum HDL-C (mg/dl) was quantified by the autoanalyzer (Eppendorf, Hamburg, Germany).
2.3 |. Determination of HDLox using the Amplex Red Assay
To prepare ApoB-depleted serum by PEG precipitation, 40 μl PEG was added to 100 μl serum of each sample (1:2.5) followed by 30 min incubation at room temperature and centrifugation at 1,000 rpm (4°C). HDLox was assessed by previously validated cell-free fluorometric assay that quantifies HDLox (Kelesidis et al., 2014). In brief, 50 μl of ApoB-depleted serum was added to wells of a 96-well plates in triplicate. The fluorescence readout of Amplex Red conversion to resorufin was determined in the dark in each well every 5 min over 1 hr at wavelengths of 530/590 nm using a plate reader (BioTek, New York, NY). To minimize experimental variability, ApoB-depleted sera from participants in the control group were pooled and were used as an experimental control in each plate as previously described (Kelesidis et al., 2016; Kelesidis, Tran et al., 2017; Zanni et al., 2014). Mean fluorescence from each sample was normalized using the mean fluorescent readout of the pooled control and HDL-C using the following calculation: “normalized” oxidized HDL (nHDLox) = [HDLox_-sample × 40 (mg/dl)]/[HDLox_control × HDLC sample (mg/dl)], where 40 mg/dl represents HDL-C of the pooled serum control (Kelesidis, Oda, et al., 2017). The negative control was 1× reaction buffer and positive control included 20 mM hydrogen peroxide (H2O2) working solution. In the absence of cholesterol oxidase and in the presence of titrated amounts of catalase that quenches auto-oxidation of buffers (to minimize background of the negative control), the oxidation of the fluorochrome Amplex Red allows the measurement of hydroperoxides present in HDL (Kelesidis et al., 2014).
2.4 |. Statistical analysis
All statistical analyses were performed using SPSS software, version 22. Results for normal and nonnormally distributed data were reported as mean ± SD and median and interquartile range, respectively. In our study, all variables have normal distribution except for triglyceride (TG) and high-sensitive C-reactive protein (hs-CRP). Baseline and follow-up characteristics of participants with and without CVD were compared by Student’s t test for normal distributed parameters, χ2 for categorical data, and Mann–Whitney test for variables with a skewed distribution.
Demographic and biochemical variables were compared based on determined cutoff point for HDLox in our population using receiver operating characteristic (ROC) curve analysis. ROC curve analysis was performed using MedCalc Statistical Software version 16.8 (MedCalc Software bvba, Belgium) to determine the optimal cutpoint value and evaluate the discriminatory ability of HDLox to determine diseased and nondiseased subjects, and also compare “baseline serum HDL,” “changes in serum HDL-C over time (until development of clinical CVD),” “baseline HDLox,” and “changes in serum HDL over time (until development of clinical CVD)” as predictors of clinical CVD disease. The association between HDLox and CAD was assessed using logistic regression analysis after adjustment for potential confounders. We also used paired t tests to evaluate HDLox and lipid profile before and after development of CVD in the same individual who was healthy at baseline and developed CVD at follow-up. Cox proportional hazards models were applied to investigate the association between HDLox and the time of primary end points. Hazard ratio for two models of HDL-C level versus HDLox was evaluated.
The proportional hazards assumption was used for all models. Significance was considered as p values less than 0.05.
3 |. RESULTS
3.1 |. Baseline characteristics of study participants
One hundred and thirty-nine participants without clinical CVD at baseline who then developed clinical CVD over 7 years of follow-up (CVD group) and 191 healthy controls were evaluated in our study. The population at baseline and follow-up was homogenous in terms of gender. Our results showed that the mean age of all participants was 53.3 at baseline.
3.2 |. Assessment of HDLox in study participants
The mean baseline HDLox was 0.77 ± 0.24 (range of 0.26–2.46) for the studied participants (n = 330). Based on our results, the mean HDLox was higher in the CVD group at baseline (0.81 ± 0.25) and at 7 years follow-up (1.30 ± 0.28) compared with the control group(0.73 ± 0.24 and 0.90 ± 0.27, respectively; p < 0.001; Table 1). HDLox was similar for both sexes. HDLox increased by a mean of 78% over 7 years in the CVD groups (0.81 ± 0.25 at baseline to 1.30 ± 0.28 at 7 years; p < 0.001) but not in the control group.
TABLE 1.
HDLox characteristics in study population
| Variable | Baseline | Follow-up | HDLox changes over time | Raw data of HDLox | Adjustment of HDLox by pooled control | Normalized by HDL-C (nHDLox) | nHDLox across cutoff point | |
|---|---|---|---|---|---|---|---|---|
| <1.06 | >1.06 | |||||||
| HDLox | 0.77 ± 0.24 | 1.1 ±0.27 | 81.6 ±97.9 | 3,136.6 ±547.3 | 0.94 ±0.19 | 1.17 ±0.34 | 0.82 ±0.17 | 1.35 ±0.25 |
| p < 0.001 | p < 0.001 | |||||||
Note. HDL-C: high-density lipoprotein cholesterol; HDLox, HDL lipid peroxidation.
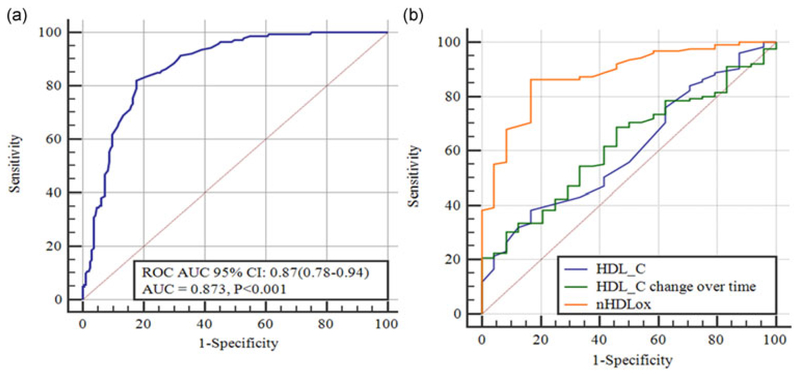
Furthermore, we have attempted to determine the optimal cutoff value of nHDLox for assessing the risk of incident CVD (Figure 1a). The area under the ROC curve (AUC) showed the ability of nHDLox score to distinguish between those with high and low levels of HDLox, verified by the AUC of 0.87 (95% CI: 0.78–0.94). The AUC can be considered as the probability that individuals with high scores of HDLox would have increased risk of CVD than those with lower nHDLox, being useful for CVD prevention aims.
FIGURE 1.
(a) ROC curve for determining cutoff value to predict cardiovascular event risk (n = 330). The ROC AUC with 95% CI was shown in the box. (b) A model comprising high-density lipoprotein, HDL-C changes in cohort study, and HDLox to show the comparison of AUC of CVD patients. AUC: area under the ROC curve; CI: confidence interval; CVD: cardiovascular disease; HDL-C: high-density lipoprotein cholesterol;HDLox: HDL-associated lipid peroxidation; ROC: receiver operating characteristic [Color figure can be viewed at wileyonlinelibrary.com]
The optimal nHDLox cutoff point in our participants using Youden index was 1.06, with a high sensitivity and specificity (sensitivity and specificity were reported as 83.3 and 82.1, respectively). ROC curve analysis was used to evaluate the discriminatory ability of nHDLox to correctly pick diseased and nondiseased subjects, and also compare the efficacy of “serum HDL-C,” “changes in serum HDL-C” with “nHDLox” so as to assess the CVD disease (Figure 1b). Since AUC is a measure of the overall performance of a diagnostic test, we compared the overall diagnostic performance of different potential predictors of CVD by comparing their AUCs. Based on our data, HDL-ox has a better overall diagnostic performance than baseline serum HDL-C, or its changes over time.
3.3|. General characteristics of studied population in terms of HDLox
In further analysis, we categorized the whole population based on the cutoff values of HDLox. Table 2 illustrates that individuals with HDLox score higher than cutoff (>1.06) possessed higher levels of CVD conventional risk variables, as well as higher prevalence of CVD. So that serum TG, fasting blood glucose, and serum HDL-C were also higher with high HDLox.
TABLE 2.
Baseline characteristics across the threshold of HDLox (n = 330)
| Variables | Lower than cutoff (<1.06) | Higher than cutoff (>1.06) | p Value |
|---|---|---|---|
| Anthropometries | |||
| Age (years) | 51.74 ±8.3 | 53.31 ±7.17 | 0.09 |
| Sex (females), n (%) | 105 (59.7%) | 76 (49%) | 0.053 |
| Weight (kg) | 69.44 ±12.5 | 73.67 ±12.7 | 0.005 |
| BMI (kg/m2) | 27.5 ±4.7 | 28 ±4.5 | 0.3 |
| PAB (HK) | 38.3 ± 36.6 | 59.9 ±40.2 | 0.3 |
| Smoking, n (%) | |||
| Nonsmoker | 18 (13.3%) | 23 (15.6%) | 0.25 |
| Current smoker | 23 (17%) | 35 (23.8%) | |
| Blood pressure | |||
| BSP (mmHg) | 128.2 ± 20.7 | 129.2 ±21.3 | 0.68 |
| BDP (mmHg) | 82.3 ± 12 | 82.8 ±11 | 0.72 |
| Lipid profile | |||
| Cholesterol (mg/dl) | 198 ± 36 | 196.8 ± 43 | 0.77 |
| TG (mg/dl) | 115 (87–159) | 141 (103–214) | <0.001 |
| HDL-C (mg/dl) | 48.15 ± 14.6 | 40.35 ±11 | <0.001 |
| LDL (mg/dl) | 119.9 ±35.27 | 116.9 + 38 | 0.49 |
| Blood glucose | |||
| FBG (mg/dl) | 97.44 ± 39.6 | 112.44 ±61 | 0.01 |
| Inflammation | |||
| hs-CRP (mg/dl) | 1.8 (1.14–3.37) | 1.96 (1.07–4.9) | 0.4 |
Data reported as mean ± SD except for TG and hs-CRP.
BDP: blood diastolic pressure; BMI: body mass index; BSP: blood systolic pressure; FBG: fasting blood glucose; HDL-C: high-density lipoprotein cholesterol; HDLox: HDL-associated lipid peroxidation; ROC: receiver operating characteristic; hs-CRP: high-sensitive C-reactive protein; LDL: low density lipoprotein; PAB: pro-oxidant antioxidant balance; TG: triglyceride.
3.4 |. Association of HDLox with CVD risk factors and CVD risk
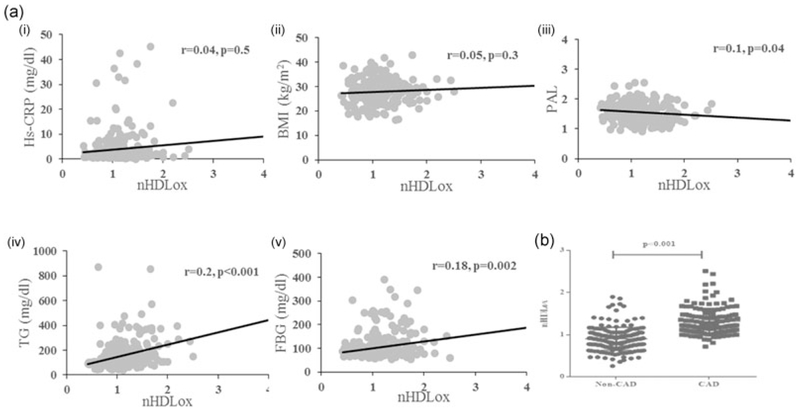
We assessed the correlation between HDLox with different anthropometrics and metabolic measures. A significant negative correlation between HDLox and HDL-C concentration was found and revealed that increasing circulating HDL-C may be associated with a decline in HDLox (Pearson’s correlation coefficient of 0.27; p ≤ 0.001). Scatter plot analyses showed significant correlations between HDLox and the levels of HDL-C (r = 0.35; data not shown). Moreover, data indicated significant and positive correlations between HDLox with serum TG (r = 0.2, p = 0.001), and fasting blood glucose (r = 0.13; p = 0.002), while it had a negative correlation with physical activity level (r = −0.1; p = 0.04; Figure 2A). Our results showed that HDLox was associated with incident CVD (p < 0.001; Figure 2B).
FIGURE 2.
(a) HDLox is significantly associated with various anthropometric and metabolic parameters in our cohort. The values from HDLox of individuals are plotted against (i) hs-CRP, (ii) BMI, (ii) PAL, (iv) TG, and (v) FBG. (b) The individuals suffered from CVD possess significantly higher HDLox (1.35 ± 0.25) compared with the healthy subjects (0.82 ± 0.17; p < 0.001). BMI: body mass index; CVD: cardiovascular disease; FBG: fasting blood glucose; HDLox: HDL-associated lipid peroxidation; hs-CRP: high-sensitive C-reactive protein; PAL: physical activity level; TG: triglyceride
Similarly, the association of nHDLox with hs-CRP as an inflammatory risk factor (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.002–1.09; p = 0.04), and physical activity (OR, 1.04; 95% CI, 1.002–1.09; p = 0.04) contributing in CVD development were analyzed. However, after adjustment of traditional CVD risk factors, these associations were not remained significant. In both univariate and multivariate models, the association of TG and also serum FBG with HDLox was also demonstrated. Based on this, a serum HDLox above the cutoff was associated with 0.3% increase in serum TG (OR,1.003; 95% CI, 1.001–1.006; p = 0.04). Accordingly, higher HDLox was statistically associated with higher FBG (OR, 1.006; 95% CI,1.001–1.01; p = 0.01; Table S1).
In line with these analyses, we examined the associations of three potential predictors of CVD named (a) serum HDL-C, (b) HDLox, and (c) the changes in serum HDL-C after 7 years with the risk of CVD development and also we compared unadjusted HDLox and nHDLox (normalized by HDL-C; Table 3). Data showed that neither serum HDL nor its changes over time were associated with the risk of CVD, while univariate analysis showed that with increasing nHDLox by means of 0.1 unit, the risk of incident CVD increased approximately more than 1.5-fold (OR, 1.55; 95% CI, 1.38–1.73). After adjustment for age, sex, BMI, smoking, cholesterol, hypertension, and diabetes, this association remained significant (OR, 1.62; 95% CI, 1.41–1.86). HDLox is reported to be a stronger indicator of CVD, as compared to HDL-C level (p < 0.001). The association of HDLox values that adjusted by pooled control and incident CVD risk (OR, 1.36; 95% CI, 1.19–1.55) and also nHDLox with risk of CVD (OR, 1.62; 95% CI 1.41–1.86) demonstrated that before adjustment and/or by adding potential confounders of CVD to the models, these associations were significant (p < 0.001; Table 3).
TABLE 3.
The association of serum “HDL-C,” “HDLox,” and “changes in HDL-C over time” with CVD risk
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | B | OR (95% Cl) | p Value | B | OR (95% Cl) | p Value |
| HDL-C (mg/dl) | ‒0.04 | 0.95 (0.88–1.02) | 0.2 | ‒0.07 | 0.92 (0.82–1.04) | 0.2 |
| Changes in HDL-C over time (%) | ‒0.16 | 0.98 (0.96–1) | 0.1 | ‒0.01 | 0.98 (0.95–1) | 0.1 |
| Adjustment of HDLox by pooled control | 0.25 | 1.30 (1.15–1.44) | <0.001 | 0.3 | 1.36 (1.19–1.55) | <0.001 |
| nHDLox (normalized by HDL-C) | 0.43 | 1.55 (1.38–1.73) | <0.001 | 0.5 | 1.62 (1.41–1.86) | < 0.001 |
Note. The odds ratio was adjusted for age, sex, BMI, smoking, total cholesterol, hypertension, and diabetes.
BMI: body mass index; CVD: cardiovascular disease; HDL-C: high-density lipoprotein cholesterol; HDLox: HDL lipid peroxidation.
3.5 |. Association of HDLox with cardiovascular events
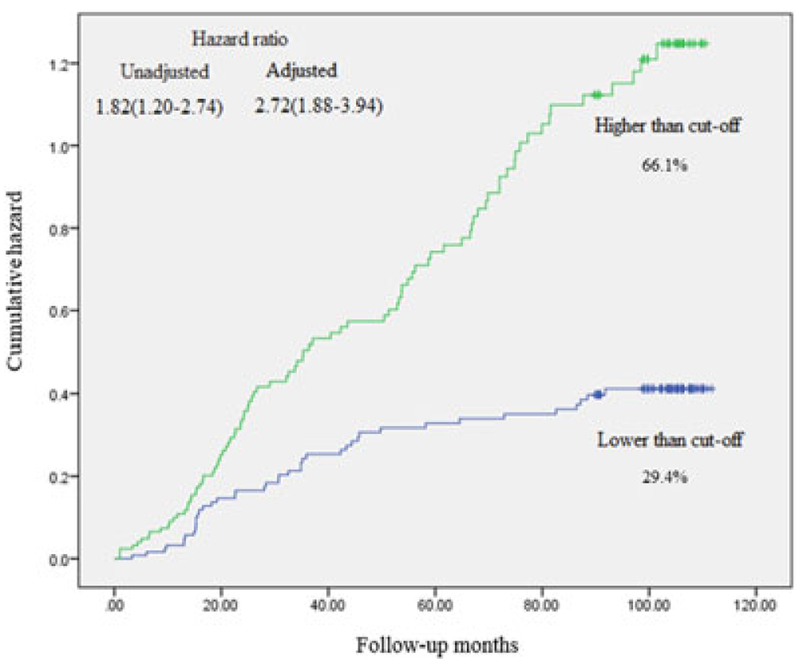
Hundred and thirty-nine participants were confirmed to have a primary CVD over follow-up period of 7 years. Kaplan–Meier curve and hazard ratios are illustrated for HDLox cutoff point (lower HDLox as the reference), derived from Cox proportional hazards models. Adjusted models included traditional risk factors for CVD and HDL-C level (Figure 3).
FIGURE 3.
Kaplan-Meier curve and hazard ratios for HDLox cutoff point (lower HDLox as the reference), derived from Cox proportional hazards models. The hazard ratio was adjusted for age, sex, smoking status, body mass index, total cholesterol level, presence or absence of diabetes, and hypertension. HDLox: HDL lipid peroxidation [Color figure can be viewed at wileyonlinelibrary.com]
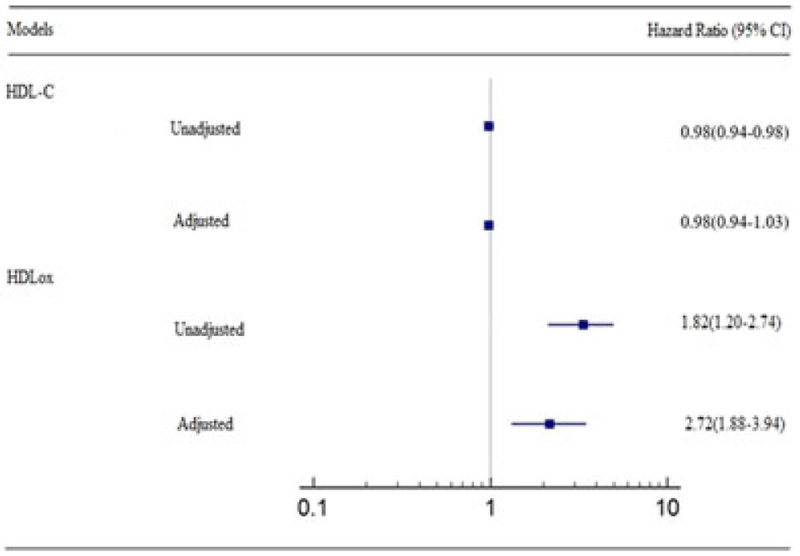
There was an increased risk of CVD in participants with higher than HDL-ox cutoff point as compared to those with lower one. Furthermore, after adjustment for potential confounders, the result remained significant (hazard ratio, 2.72; 95% CI, 1.88–3.94). However, we could not find any significant association between serum HDL-C level in terms of CVD risk in an adjusted model (hazard ratio, 0.98; 95% CI, 0.94–1.03; Figure 4).
FIGURE 4.
Cardiovascular disease events and hazard ratios based on two models of HDL-C level and HDLox. Hazard ratios and 95% confidence intervals, based on Cox proportional hazards models are illustrated. A total of 139 participants possessed a primary end point of cardiovascular disease including myocardial infarction, stable angina, unstable angina, or coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting). The hazard ratio was adjusted for age, sex, smoking status, body mass index, total cholesterol, diabetes, and hypertension. HDL: high-density lipoprotein; HDLox: HDL lipid peroxidation [Color figure can be viewed at wileyonlinelibrary.com]
The positive associations have also been found between HDLox and the primary end point of CVD (Table 4). After adjustment for age, sex, BMI, smoking, total cholesterol, presence or absence of diabetes, and hypertension, the associations also remained significant. Cox proportional hazards regression analysis revealed that age, physical activity, hypertension, and diabetes were associated with risk of the study endpoints by both univariate and multivariate models (Table S2).
TABLE 4.
Hazard ratios in cardiovascular events according to HDLox cutoff point
| HDLox | |||||
|---|---|---|---|---|---|
| End point | All participants | Lower than cutoff | higher than cutoff | Unadjusted hazard ratioa | Adjusted hazard ratiob |
| Atherosclerotic cardiovascular disease | 139 | 24 | 115 | 2.72 (1.88–3.94) | 1.82 (1.21–2.74) |
| Myocardial infarction | 13 | 3 | 10 | 4.82 (1.31–17.73) | 3.12 (0.7–13.6) |
| Stable angina | 48 | 10 | 38 | 8.20 (3.67–18.33) | 6.09 (2.55–14.52) |
| Unstable angina | 16 | 3 | 13 | 5 (1.4–17.82) | 4.08 (1.04–16.05) |
| Coronary revascularization | 62 | 8 | 54 | 7.51 (3.52–16.01) | 5.11 (2.20–11.62) |
Note. HDLox: HDL lipid peroxidation.
Hazard ratios and 95% confidence intervals for HDLox in higher HDLox cutoff compared to the lower scores for the given end points, obtained from Cox proportional hazards models. Atherosclerotic cardiovascular disease included myocardial infarction, stable angina, unstable angina, or coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting).
The hazard ratio was adjusted for age, sex, body mass index, total cholesterol, presence or absence of diabetes, and hypertension.
4 |. DISCUSSION
Using data from MASHAD cohort study, we showed that patients with a verified diagnosis of clinical CVD had higher HDLox values compared to those without CVD.
We found a positive association between serum HDLox and risk of CVD outcomes. Even after normalizing by HDL-C as a potential confounder, this association was also found to be significant. Overall, for every increase in nHDLox by 0.1 unit, there was an increase in CVD risk by 1.62-fold. Following adjustment for traditional CVD risk factors, the association between HDLox and risk of CVD development remained significant. HDL-C was not a predictor for CVD in our cohort. Interestingly, we observed that none of the traditional risk factors including serum HDL changed statistically over the following of 7 years of our study; however, HDLox remarkably went up by 81.6 ± 97.9% in participants who were healthy at baseline, but suffered CVD at follow-up (p < 0.001). Our findings clarify that determining the cutoff point according to the HDLox provided evidence of a consistent relationship between HDLox and clinical CVD. HDLox may be an important surrogate measure of HDL dysfunction and a major predictor of clinical CVD risk.
Moreover, further analysis showed that in this population baseline concentration of HDL-C was not associated with cardiovascular events in both unadjusted and adjusted analysis. Contrarily, in an adjusted model comprising conventional CVD risk factors, more than 2.5-fold increase in cardiovascular risk was observed in higher scores of HDLox cutoff, in contrast to the lower scores. Therefore, although no association was found between HDL-C and our study endpoints, positive associations were existed among HDLox and end point of CVD comprising myocardial infarction, stable angina, unstable angina, or coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting).
Serum HDL has several functions that can now be measured by means of cholesterol efflux capacity (cell-based; Sankaranarayanan et al., 2011) and antioxidant activity (cell free; Kelesidis et al., 2011; Rohatgi et al., 2014). Although cholesterol efflux measurement using cell-based methods have currently been utilized for quantifying HDL function (Saleheen et al., 2015; Zhang, Cai, Peterson, Kris-Etherton, & Heuvel, 2011), several limitations such as heterogeneity of using cells, TGs as confounding factors as well as standardization deficit may be important and make difficulties in large-scale studies (Annema et al., 2016; Sen et al., 2017). Other cell-free assays such as the HDL-apoA-I exchange (HAE; Kelesidis, Oda et al., 2017) are technically challenging and cannot be used in the clinic. Our data support the use of the biochemical cell-free Amplex Red assay of HDLox as an important measure of HDL function in the setting of clinical CVD. The utility of the fluorometric biological assay provided a reliable, low-cost, and convenient way to measure HDL function. This assay may prevent known limitations of cell-based HDL functionality measurements (Kelesidis et al., 2012; Morgantini et al., 2014).
Several aspects of HDL function remain unclear (Tsompanidi, Brinkmeier, Fotiadou, Giakoumi, & Kypreos, 2010). To our knowledge, the current study is among the first studies to demonstrate that patients with verified diagnosis of clinical CVD have higher HDLox values compared to those without clinical CVD. Patel et al. (2011) demonstrated that subjects with acute coronary syndrome have impaired HDL with less anti-inflammatory capacity compared with controls or subjects with stable CAD. In this study a biochemical cell-free fluorometric assay was used to determine the high-density lipoprotein inflammatory index (HII), which measures the ability of HDL to mitigate oxidation of low-density lipoprotein. However, this study was cross-sectional and development of clinical CVD was not assessed in relationship to HDL function. In our prospective study with available clinical CVD outcomes we also determined that high levels of serum HDLox values were associated with high risk of incident CVD. In addition, although, antioxidant activity of HDL with regard to its atheroprotective impact can be determined by fluorometric cell free measurements of the HII (Kelesidis et al., 2011, 2012; Navab et al., 2001), assessment of HII has several methodological limitations that preclude its use in the clinic.
The role of oxidation in initiation and development of atherosclerosis is well documented (Mackness & Durrington, 1995). Lipid oxidation plays a prominent role in inflammation of the artery wall. Plasma lipid peroxide content as well as oxidation of low-density lipoprotein is reported to be enhanced in systemic inflammation (Girotti, 1998) and metabolic syndrome (Furukawa et al., 2017). HDL is modified in inflammatory conditions, contains high levels of lipid peroxides, and has impaired functions such as the reverse cholesterol transport. Clinical studies have reported that dysfunctional HDL is found in conditions that are associated with oxidative stress such as human immunodeficiency virus infection (HIV; Kelesidis et al., 2014). Decreased antioxidant function of HDL is related to increased HDLox values (Sen et al., 2017). HDL involves in translocation of lipid peroxides and impaired HDL function is also pertinent to lipid peroxide content of HDL (Navab et al., 2006). Therefore, quantifying the amount of HDLox can accurately be used as a measure of HDL function. The impact of HDLox on CVD has been assessed in atherosclerosis animal model (Kelesidis et al., 2014) and in in vitro (Angelovich et al., 2017) and in vivo studies (Kelesidis et al., 2014; Kelesidis, Oda et al., 2017; Sen et al., 2017).
We have attempted to address the limited understanding of HDL function in the setting of CVD by exploring the associations of HDLox with covariates that may affect HDL function among the CVD and the control group. According to our findings, the association between TG and high-sensitive C reactive protein with HDLox, have revealed that subjects with high TG or high levels of hs-CRP possessed increased values of HDLox, exposing to high risk of CVD. Recent studies have reported an association of HDLox with plasma markers of inflammation including IL-6 and hs-CRP over time in HIV-infected individuals, which are related to accelerate CVD risk and also mortality (Kelesidis et al., 2016). As previously demonstrated, cholesteryl ester, free cholesterol, and also TG have inverse relationship with antioxidant function of HDL (Camont et al., 2013). A study that involved HIV-1-infected men also suggested the correlation between increased HDLox and declined HAE, lower albumin, and apoA-I and higher BMI (Kelesidis, Oda et al., 2017). We did not find a significant association between HDLox and BMI in our study. Obesity and dietary fat can modulate ABCA1-dependent efflux, HDL-mediated activation of endothelial nitric oxide synthase, and HDL function (Wesnigk et al., 2016). However this association may be more important in states of increased oxidative stress such as chronic HIV-1 infection (Kelesidis, Oda et al., 2017). HDLox was positively associated with anthropometric factors including BMI, hs-CRP, waist circumference, fasting glucose, leptin, amylin, and TGs. Furthermore, negative significant correlation has been found among HDLox and physical activity, HDL concentration, insulin resistance, and adiponectin (Kelesidis et al., 2014).
To our knowledge, this cohort study is the first one to assess HDLox and its optimal threshold to determine dysfunctional HDL and consequently predict the risk of CVD.
The limitation of our study is that other risk factors such as albumin that may affect HDL functionality were not included in our study.
5 |. CONCLUSION
We have found that HDLox was positively associated with cardiovascular events in a representative population-based cohort. HDLox threshold can be used to identify subjects who have an elevated risk of CVD.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grant awarded (ID: 941684) by the Mashhad University of Medical Sciences. The authors gratefully acknowledge the work of Dr. Arian (Mashhad University of Medical Sciences) for providing catalase.
Funding information
Mashhad University of Medical Sciences, Grant/Award Number: 941684
Abbreviations:
- ABCA1
ATP binding cassette transporter A1
- ACS
acute coronary syndrome; apoB, apolipoprotein B
- ART
antiretroviral therapy
- AUC
area under the ROC curve
- BMI
body mass index
- CAD
coronary artery disease
- CETP
cholesteryl ester transfer protein
- CVD
cardiovascular disease
- ETT
stress echocardiography and exercise tolerance testing
- FBG
fasting blood glucose
- HAE
HDL-apoA-I exchange
- HDL-C
high-density lipoprotein cholesterol
- HDL
high-density lipoprotein
- HDLox
HDL lipid peroxidation
- HII
high-density lipoprotein inflammatory index
- HIV
human immunodeficiency virus
- hs-CRP
high-sensitive C-reactive protein
- LDL
low-density lipoprotein
- MASHAD
Mashhad stroke and heart atherosclerotic disorders
- MDFCF
monocyte-derived foam cell formation
- OR
odds ratio
- PEG
polyethylene glycol
- ROC
receiver operating characteristic
- TG
triglyceride.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
How to cite this article: Samadi S, Mehramiz M, Kelesidis T, et al. High-density lipoprotein lipid peroxidation as a molecular signature of the risk for developing cardiovascular disease: Results from MASHAD cohort. J Cell Physiol. 2019; 1–10. https://doi.org/10.1002/jcp.28276
REFERENCES
- Angelovich TA, Hearps AC, Oda MN, Borja MS, Huynh D, Homann S, … Kelesidis T (2017). Dysfunctional high-density lipoprotein from HIV+ individuals promotes monocyte-derived foam cell formation in vitro. AIDS, 31(17), 2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annema W, Dikkers A, De Boer JF, Van Greevenbroek MMJ, Van Der Kallen CJH, Schalkwijk CG, … Tietge UJF (2016). Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: The CODAM study. Scientific Reports, 6, 27367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarpazhooh M, Etemadi M, Donnan G, Mokhber N, Majdi M, Ghayour-Mobarhan M, & Panahandeh M (2010). Excessive incidence of stroke in Iran: Evidence from the Mashhad stroke incidence study (msis), a population-based study of stroke. International Journal of Stroke, 5, 207. [DOI] [PubMed] [Google Scholar]
- Camont L, Lhomme M, Rached F, Le Goff W, Nègre-Salvayre A, Salvayre R, … Kontush A (2013). Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipidssignificance: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arteriosclerosis,Thrombosis, and Vascular Biology, 33(12), 2715–2723. [DOI] [PubMed] [Google Scholar]
- Desforges JF, Gordon DJ, & Rifkind BM (1989). High-density lipoprotein–The clinical implications of recent studies. New England Journal of Medicine, 321(19), 1311–1316. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, … Shimomura I (2017). Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation, 114(12), 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayour-Mobarhan M, Moohebati M, Esmaily H, Ebrahimi M, Parizadeh SMR, Heidari-Bakavoli AR, … Azarpazhooh MR (2015). Mashhad stroke and heart atherosclerotic disorder (MA-SHAD) study: Design, baseline characteristics and 10-year cardiovascular risk estimation. International Journal of Public Health, 60(5), 561–572. [DOI] [PubMed] [Google Scholar]
- Girotti AW (1998). Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of Lipid Research, 39(8), 1529–1542. [PubMed] [Google Scholar]
- The HPS2-THRIVE Collaborative Group (2014). Effects of extendedrelease niacin with laropiprant in high-risk patients. New England Journal of Medicine, 2014(371), 203–212. [DOI] [PubMed] [Google Scholar]
- The HPS3/TIMI55-REVEAL Collaborative Group (2017). Effects of anacetrapib in patients with atherosclerotic vascular disease. New England Journal of Medicine, 377(13), 1217–1227. [DOI] [PubMed] [Google Scholar]
- Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, & Marsche G (2013). Aging affects high-density lipoprotein composition and function. Biochimica et Biophysica Acta (BBA), 1831(9), 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovingh GK, Kastelein JJP, Van Deventer SJH, Round P, Ford J, Saleheen D, … Barter PJ (2015). Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): A randomised, double-blind, placebo-controlled phase 2 trial. The Lancet, 386(9992), 452–460. [DOI] [PubMed] [Google Scholar]
- Kelesidis T, Currier JS, Huynh D, Meriwether D, Charles-Schoeman C, Reddy ST, … Yang OO (2011). A biochemical fluorometric method for assessing the oxidative properties of HDL. Journal of Lipid Research, 52(12), 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Jackson N, McComsey GA, Wang X, Elashoff D, Dube MP, … Currier JS (2016). Oxidized lipoproteins are associated with markers of inflammation and immune activation in HIV-1 infection. AIDS, 30(17), 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Oda MN, Borja MS, Yee Y, Ng KF, Huynh D, … Currier JS (2017). Predictors of impaired HDL function in HIV-1 infected compared to uninfected individuals. Journal of Acquired Immune Deficiency Syndromes, 75(3), 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Reddy ST, Huynh D, Meriwether D, Fogelman AM, Navab M, & Yang OO (2012). Effects of lipid-probe interactions in biochemical fluorometric methods that assess HDL redox activity. Lipids in health and disease, 11(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Roberts CK, Huynh D, Martínez-Maza O, Currier JS,Reddy ST, & Yang OO (2014). A high throughput biochemical fluorometric method for measuring lipid peroxidation in HDL. PLoS One, 9(11), e111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Tran TTT, Brown TT, Moser C, Ribaudo HJ, Dube MP, … Currier JS (2017). Changes in plasma levels of oxidized lipoproteins and lipoprotein subfractions with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy and associations with common carotid artery intima-media thickness: ACTG 5260s. Antiviral herapy, 22(2), 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, … Rader DJ (2011). Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. New England Journal of Medicine, 364(2), 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackness MI, & Durrington PN (1995). HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis, 115(2), 243–253. [DOI] [PubMed] [Google Scholar]
- McPherson PAC, Young IS, McKibben B, & McEneny J (2007). High density lipoprotein subfractions: Isolation, composition, and their duplicitous role in oxidation. Journal of Lipid Research, 48(1), 86–95. [DOI] [PubMed] [Google Scholar]
- Morgantini C, Meriwether D, Baldi S, Venturi E, Pinnola S, Wagner AC, … Reddy ST (2014). HDL lipid composition is profoundly altered in patients with type 2 diabetes and atherosclerotic vascular disease. Nutrition, Metabolism, and Cardiovascular Diseases, 24(6), 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movva R, & Rader DJ (2008). Laboratory assessment of HDL heterogeneity and function. Clinical Chemistry, 54(5), 788–800. [DOI] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, & Fogelman AM (2006). Mechanisms of disease: Proatherogenic HDL–an evolving field. Nature Reviews Endocrinology, 2(9), 504–511. [DOI] [PubMed] [Google Scholar]
- Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Hama S, … Fogelman AM (2005). The double jeopardy of HDL. Annals of Medicine, 37(3), 173–178. [DOI] [PubMed] [Google Scholar]
- Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, & Fogelman AM (2001). A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. Journal of Lipid Research, 42(8), 1308–1317. [PubMed] [Google Scholar]
- Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, & Fogelman AM (2009). The role of dysfunctional HDL in atherosclerosis. Journal of Lipid Research, 50(Suppl), S145–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Reddy ST, Van Lenten BJ, & Fogelman AM (2011). HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nature Reviews Cardiology, 8(4), 222–232. [DOI] [PubMed] [Google Scholar]
- Patel PJ, Khera AV, Jafri K, Wilensky RL, & Rader DJ (2011). The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. Journal of the American College of Cardiology, 58(20), 2068–2075. [DOI] [PubMed] [Google Scholar]
- Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, … Shaul PW (2014). HDL cholesterol efflux capacity and incident cardiovascular events. New England Journal of Medicine, 371(25), 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, … Wittes J (1999). Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. New England Journal of Medicine, 341(6), 410–418. [DOI] [PubMed] [Google Scholar]
- Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, … Rader DJ (2015). Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. The Lancet Diabetes and Endocrinology, 3(7), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, & Rothblat GH (2011). A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. Journal of Lipid Research, 52(12), 2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen SR, Nguyen HCX, Angelovich TA, Hearps AC, Huynh D, Jaworowski A, & Kelesidis T (2017). Cell-free biochemical fluorometric enzymatic assay for high-throughput measurement of lipid peroxidation in high density lipoprotein. Journal of Visualized Experiments, (128), e56325, 10.3791/56325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Chen W, Kähönen M, Kettunen J, Lehtimäki T, Peltonen L, … Murray SS (2010). Longitudinal genome-wide association of cardiovascular disease risk factors in the Bogalusa heart study. PLoS Genetics, 6(9), e1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsompanidi EM, Brinkmeier MS, Fotiadou EH, Giakoumi SM, & Kypreos KE (2010). HDL biogenesis and functions: Role of HDL quality and quantity in atherosclerosis. Atherosclerosis, 208(1), 3–9. [DOI] [PubMed] [Google Scholar]
- Vílchez JA, Martínez-Ruiz A, Sancho-Rodríguez N, Martínez-Hernández P, & Noguera-Velasco JA (2014). The real role of prediagnostic high-density lipoprotein cholesterol and the cancer risk: A concise review. European Journal of Clinical Investigation, 44(1), 103–114. [DOI] [PubMed] [Google Scholar]
- Wesnigk J, Bruyndonckx L, Hoymans VY, De Guchtenaere A, Fischer T, Schuler G, … Adams V (2016). Impact of lifestyle intervention on HDL-induced eNOS activation and cholesterol efflux capacity in obese adolescent. Cardiology Research andPractice, 2016, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni MV, Kelesidis T, Fitzgerald ML, Lo J, Abbara S, Wai B, … Grinspoon SK (2014). HDL redox activity is increased in HIV-infected men in association with macrophage activation and noncalcified coronary atherosclerotic plaque. Antiviral Therapy, 19(8), 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cai S, Peterson BR, Kris-Etherton PM, & Heuvel JPV (2011). Development of a cell-based, high-throughput screening assay for cholesterol efflux using a fluorescent mimic of cholesterol. ASSAY and Drug Development Technologies, 9(2), 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.