Abstract
Hippocampal episodic memory is fundamentally relational, comprising links between events and the spatiotemporal contexts in which they occurred. Such relations are also important over shorter timescales, during online perception. For example, how do we assess the relative spatial positions of objects, their temporal order, or the relationship between their features? Here, we investigate the role of the hippocampus in online relational processing by manipulating attention to different kinds of relations. While undergoing fMRI, participants viewed two images in rapid succession on each trial and performed one of three relational tasks, judging the images’ relative: spatial positions, temporal onsets, or sizes. Additionally, they sometimes judged whether one image was tilted, irrespective of the other. This served as a baseline item task with no demands on relational processing. The hippocampus showed reliable deactivation when participants attended to relational vs. item information. Attention to temporal relations was associated with the most robust deactivation. One interpretation of such deactivation is that it reflects hippo-campal disengagement. If true, there should be reduced information content and noisier activity patterns for the temporal vs. other tasks. Instead, multivariate pattern analysis revealed more stable hippocampal representations in the temporal task. This increased pattern similarity was not simply a reflection of lower univariate activity. Thus, the hippocampus differentiates between relational and item processing even during online perception, and its representations of temporal relations are particularly robust. These findings suggest that the relational computations of the hippocampus extend beyond long-term memory, enabling rapid extraction of relational information in perception.
Keywords: attention, medial temporal lobe, relational representations, representational similarity analysis
1 |. INTRODUCTION
Our perception of the world is not merely a collection of the myriad items in the environment. We do not perceive items in isolation, but rather in terms of their relationship to other items and the context in which they occur. Such relational discriminations are ubiquitous in everyday life. When placing two paintings side-by-side on a wall, we might make fine spatial discriminations to determine whether one is shifted vertically with respect to the other. Knowledge of which of two cars arrived first at an intersection might determine which of them has the right of way. When deciding which piece of fruit to buy at a grocery store, we might find it useful to compare their sizes.
Although relational attention is a key component of visual perception, studies of attention have traditionally focused on perception of individual features or locations (Kastner & Ungerleider, 2000; Maunsell & Treue, 2006). As a consequence, the neural substrates of relational judgments in online visual perception have been largely unexplored (although see, Franconeri, Scimeca, Roth, Helseth, & Kahn, 2012; Michal, Uttal, Shah, & Franconeri, 2016). One candidate system for supporting relational attention is the hippocampus, a structure traditionally studied for its role in long-term memory, and particularly for relational forms of long-term memory (Eichenbaum & Cohen, 2014).
One possibility is that the scope of relational processing in the hippocampus is limited to long-term memory, insofar as some have argued that the hippocampus is a dedicated memory system (Squire, Stark, & Clark, 2004). Alternatively, the hippocampus may perform relational computations in a more general way across many domains of cognition (Aly & Turk-Browne, 2018; Olsen, Moses, Riggs, & Ryan, 2012; Yonelinas, 2013), and may therefore also be involved in relational perception. In the current study, we addressed this question by comparing BOLD activity in the hippocampus when attention is directed to individual items versus to the relations between items. If the hippocampus plays a general role in relational processing, even on the short timescale of perception, its activity should be modulated by the demand to attend to items versus relations.
A second question of interest concerns the types of relational representations the hippocampus might support. Studies implicating the hippocampus in relational memory have largely focused on spatial and temporal processing (Eichenbaum, 2017). For example, the hippo-campus is necessary for allocentric spatial navigation (Burgess, Maguire, & O’Keefe, 2002), and contains “place cells” that fire when an animal is in a specific location in the environment (Ekstrom et al., 2003; O’Keefe & Dostrovsky, 1971). The hippocampus also contains “spatial view cells,” which respond to locations that an animal is looking at, even in the absence of navigation (Rolls & Wirth, 2018). These findings inspired proposals that the hippocampus is important for memory of spatial context (Burgess, Becker, King, & O’Keefe, 2001; Davachi, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007) and for the construction of spatially coherent scenes (Maguire & Mullally, 2013).
Hippocampal activity also represents the temporal order of experience (Barnett, O’Neil, Watson, & Lee, 2014; Eichenbaum & Cohen, 2014; Kesner & Hunsaker, 2010; Manns, Howard, & Eichenbaum, 2007; Paz et al., 2010; Ranganath, 2019; Sakon, Naya, Wirth, & Suzuki,2014). For example, during quiet wakefulness and sleep, hippocampal place cells fire in the same sequential order as in previous navigation episodes (Carr, Jadhav, & Frank, 2011). Indeed, some hippocampal cells (“time cells”) fire during successive moments in a temporal delay, keeping a record of elapsed time (MacDonald, Lepage, Eden, & Eichenbaum, 2011; Pastalkova, Itskov, Amarasingham, & Buzsaki, 2008). These studies in rodents converge with work in humans that demonstrates a critical role of the hippocampus in memory for temporal sequences (see Davachi & DuBrow, 2015; Ranganath & Hsieh, 2016).
Although the spatial and temporal representations of the hippo-campus have received the most attention, there is also evidence that the hippocampus is critical for other kinds of relational memories as well (Eichenbaum, 2004; Konkel & Cohen, 2009; Konkel, Warren, Duff, Tranel, & Cohen, 2008; McKenzie et al., 2016). For example, Konkel et al. (2008) presented triplets of novel visual objects to patients with hippocampal damage. The object triplets were presented in a particular spatial arrangement and appeared in a particular order. The object triplets were first presented in a study phase, and memory for them was subsequently tested. There were three kinds of memory tests, which assessed memory for different kinds of relations between the triplets: spatial, sequential, and associative relations. In the spatial test, patients were shown the objects again and asked to report whether the objects occurred in the same locations during the study phase. In the sequential test, they were to report whether the items were presented in the same sequential order during the study phase. In the associative test, they were to report whether the items were all shown together during the study phase. Patients with damage to the hippocampus performed at chance in all three tasks, suggesting that the hippocampus is critical for relational memory beyond the spatial and temporal domains. Motivated by these studies, we incorporated multiple relational tasks in the current work: in addition to tasks requiring judgments of spatial and temporal relations, we include a task assessing size relations to test the specificity of relational processing in the hippocampus. Importantly, our current study tested online relational attention, unlike the Konkel et al., study, which was a test of relational long-term memory.
We collected high-resolution structural and functional MRI data in order to examine the role of different hippocampal subfields in online relational processing. We segmented the hippocampus into subiculum, CA1, and a combined region of interest for CA2, CA3, and dentate gyrus (which cannot be separated at the resolution of our fMRI scans). Below, we describe our predictions for these subfield regions of interest.
First, hippocampal subfield CA1 has been linked to both spatial and temporal processing (Eichenbaum, 2014). For example, CA1 activity codes for the position of items and their spatial context (McKenzie et al., 2014), and tracks changes in the locations of perceived items versus their remembered positions in memory (Duncan, Ketz, Inati, & Davachi, 2012). CA1 activity patterns are also modulated by spatial attention (Aly & Turk-Browne, 2016a, 2016b). Moreover, time cells were first discovered in CA1 (MacDonald et al., 2011; Pastalkova et al., 2008); such cells may be important for discriminating the passage of time on the order of seconds or less, as needed in the online temporal attention task in the current study. Indeed, CA1 is necessary for discriminating between memories that were experienced close to one another in time (Gilbert, Kesner, & Lee, 2001). We therefore predicted that CA1 would be modulated by both spatial and temporal attention.
In contrast to CA1, the literature is mixed on the role of CA3 in processing temporal information, with some (Farovik, Dupont, & Eichenbaum, 2010; Kesner & Hunsaker, 2010; Salz et al., 2016) but not all (Makin et al., 2012) studies linking this region to the representation of time or sequences. There is evidence that neural activity in CA2 codes for the passage of time, though this might be on the order of hours to days (Mankin, Diehl, Sparks, Leutgeb, & Leutgeb,2015). Finally, to our knowledge, no studies have examined the dentate gyrus for potential time cells, but lesions to the dentate gyrus do not impair the ability to make fine temporal discriminations in memory (Gilbert et al., 2001). Because of the mixed evidence on seconds-level timing in CA2, CA3, and dentate gyrus, we made no a priori predictions about whether our combined CA2/3/DG region of interest would be modulated by temporal attention. We did, however, expect this region to be modulated by spatial attention (e.g., Aly & Turk-Browne, 2016a, 2016b).
The subiculum plays an important role in spatial processing and navigation (Boccara et al., 2010; Dalton & Maguire, 2017; Hodgetts et al., 2017; Lever, Burton, Jeewajee, O’Keefe, & Burgess, 2009; Taube, Muller, & Ranck, 1990), so we expected it to be modulated by spatial attention (e.g., Aly & Turk-Browne, 2016a, 2016b). To our knowledge, there is very little work on temporal processing signals in subiculum, but there is evidence that subiculum activity patterns are shaped by temporal regularities in experienced events (Schapiro, Kustner, & Turk-Browne, 2012). Thus, it is possible that subiculum will also be modulated by attention to temporal relations.
If the hippocampus plays a role in processing all forms of relations– not just spatial and temporal–we would additionally expect modulation by attention to size relations. We did not have predictions about subfield dissociations for the size task, given the lack of past work exploring this form of relational processing in the hippocampus.
To assess if modulation by online relational attention was specific to the hippocampus, we also examined regions of interest in the surrounding medial temporal lobe (MTL) cortex: parahippocampal cortex (PHC), perirhinal cortex (PRC), and entorhinal cortex (ERC). PHC has been consistently linked to the processing of spatial (Diana, Yonelinas, & Ranganath, 2007; Epstein & Kanwisher, 1998) and temporal (Turk-Browne, Simon, & Sederberg, 2012) context, so we predicted that it would be modulated by attention to both spatial and temporal relations. PRC has been consistently linked to the processing of items (Diana et al., 2007), although recent studies have also linked it to representations of spatial (Bos et al., 2017) and temporal (Naya & Suzuki, 2011) context. Thus, it is possible that PRC will be modulated by attention to both spatial and temporal relations as well. Finally, like CA1, entorhinal cortex (ERC) codes for both spatial and temporal information (Eichenbaum, 2014; Hafting, Fyhn, Molden, Moser, & Moser, 2005; Kraus et al., 2015; Tsao et al., 2018), so we predicted that this region would be modulated by both spatial and temporal attention.
Although we predict that MTL cortex will be modulated by relational attention, there is an alternative possibility. It has been argued that the hippocampus is unique in forming flexible, relational representations between items (Eichenbaum, Otto, & Cohen, 1994). If so, then MTL cortex might only be modulated by attention to items and not modulated by attention to spatial, temporal, or size relations.
To summarize, our approach allows us to test the type and ubiquity of relational representations in the medial temporal lobe: whether such representations exist on the order of seconds during the time-course of perception, how broadly relational computations are applied beyond the spatial and temporal domains, and whether online relational representations are limited to the hippocampus or are also properties of MTL cortex.
2 |. METHODS
2.1 |. Participants
Fifteen participants (8 female, ages 22–33), with normal or corrected-to-normal vision participated for monetary compensation. The study was approved by the Princeton University Institutional Review Board and all participants provided informed consent.
2.2 |. Stimuli
Stimuli were grayscale images of faces and scenes, equated for luminance. Faces had neutral expressions; half were male and half were female. Half of the scenes were indoor scenes and half were outdoor scenes. Stimuli were presented on a projector screen at the back of the MRI scanner bore and were viewed through a mirror attached to the head coil. We selected stimuli from a pool of 96 faces and 96 scenes, each of which was presented once per run.
Stimuli could be presented within a range of spatial positions on the screen. The center of the reference image on each trial was randomly chosen to be between 0 and 20 pixels to the left or right of fixation. The reference images also ranged in size on each trial, starting at 81 × 81 pixels and varying up to 10 pixels smaller or larger. Varying the overall position and size of each image increased demands on relational processing (see below), because the relational tasks could not be performed by attending to only one of the images (e.g., by looking for an established larger size if only a single “large” and a single “small” image size had been used). In other words, this variation in absolute position and size was the baseline for relative differences between the two images that served as the basis of the relational tasks, requiring a focus on relative rather than absolute properties.
2.3 |. Procedure
On each trial, participants were presented with a face and a scene, one above the other (Figure 1). The two items were presented so that one of them was to the left of the other, one appeared on the screen first, and one of them was smaller than the other. Correspondingly, three relational attention tasks were possible: spatial, timing, and size. In addition, each item could, independently of the other, be tilted clockwise or counterclockwise, which enabled an item attention task that did not require relational processing. After the presentation of each pair, participants were shown a response cue (either black or gray) that pointed up or down, indicating which item should be used as the reference for the task judgment. In the spatial task, participants indicated whether the cued item was to the left of the other item. In the timing task, they indicated whether the cued item appeared first. In the size task, they indicated whether the cued item was smaller than the other. In the item task, they indicated whether the cued item was tilted or not. A post-cueing design was used, instead of a precuing design, because if a cue was presented prior to the onset of a trial, participants would be able to perform the item task by attending to the cued item alone. With a post-cue design, participants had to attend to both items for the relational and the item tasks.
FIGURE 1.
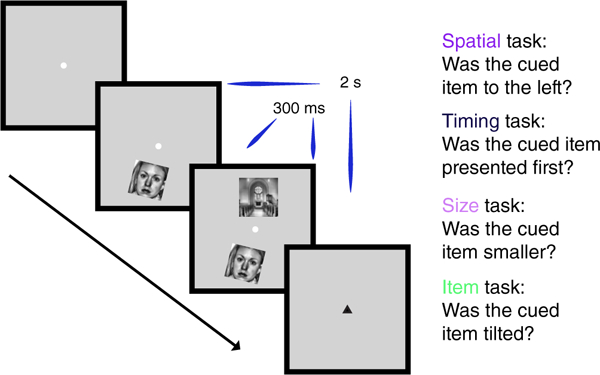
Experimental design. On every trial, participants were presented with a face and a scene, one above and one below fixation. One image was to the left of the other, one appeared on the screen first, and one was smaller than the other. In addition, each item could independently be tilted clockwise or counterclockwise. Participants were cued before every block of eight trials with the name of the task they were to perform on that block: One of the three possible relational attention tasks (spatial, timing, and size) or the item task. After the presentation of each image pair, participants were shown a response post-cue (either black or gray) that pointed up or down, indicating which item should be used as the reference for the task judgment. Trials lasted 2 s in duration, though the two images were shown on the screen for only 300 ms [Color figure can be viewed at wileyonlinelibrary.com]
We included an additional task, in which participants indicated whether the post-cue was black or not, to provide another potential baseline. However, the item task provides a tighter control: as in the relational tasks, the item task required that participants attend to both images presented on each trial (because of the post-cue), with the key difference being that the images could be processed separately and did not need to be judged against each other. Thus, we used the item task as the control against which to examine hippocampal modulation by the relational tasks.
These tasks were completed in a block design, with instructions given prior to each block via on-screen message: “spatial,” “timing,” “size,” “item,” or “cue.”
2.4 |. Pre-scan behavioral session
To prevent neural differences across tasks from being confounded by differences in task difficulty, we used a staircasing procedure to equate performance across the tasks as much as possible. Each participant completed a behavioral session the day before the scan to staircase performance to 75% accuracy. To reach this performance threshold for each task, we made trial-by-trial adjustments to the relational parameters: spatial separation, temporal delay, and size differences between images for the relational tasks; the degree of tilt of the images for the item task; and shade of gray of the cue for the cue task. A separate staircase was run for each task.
All participants completed one block of 60 trials of each task with initial parameters (Table 1). Participants then completed 4–5 stair-cased runs. Each run contained one block of 64 trials for each of the 5 tasks, with the order of blocks counterbalanced across runs and with the five image dimensions fully counterbalanced within block (vertical position, category [face or scene], spatial position, size, and tilt of cued item). When participants responded correctly 4 trials in a row, we increased difficulty by one step. If they responded incorrectly on a trial, we decreased difficulty by one step. Participants controlled the onset of trials by pressing a button to continue to the next one.
TABLE 1.
Task parameters for the staircasing procedure
| Task | Parameter | Initial value | Step size | Lower bound | Upper bound | Reference variability |
|---|---|---|---|---|---|---|
| Spatial | Horizontal offset | 30 px | 0.5 px | 0 px | 150 px | −20 to 20 px from center |
| Timing | Temporal delay | 0.20 s | 0.0167 s | 0 s | 0.2335 s | N/A |
| Size | Size difference | 30 px | 0.5 px | 0 px | 30 px | 151–171 px |
| Item | Degree of tilt | 30° | 1° | 0° | 45° | N/A |
| Cue | Color of cue | 100 | 1 | 0 | 110 | N/A |
The first column indicates the tasks that participants performed. The second indicates the relevant parameter under manipulation. The third contains the initial values of each of the parameters (e.g., images were horizontally offset by 30 px with respect to each other). Step size refers to the amount by which we changed the parameter of interest during the staircasing procedure (e.g., whenever participants performed four trials of the spatial task correctly in a row, we decreased the horizontal offset by 0.5 pixels). The parameters were not able to go lower than the lower bound (5th column) or above the upper bound (6th column). For the spatial and size tasks, the relevant parameter (position and size, respectively) of the reference image was varied to avoid the possibility that participants could perform the task by attending to only one of the items. The last column shows the variability possible in the reference image. The value for color indicates whether the cue was black (a value of 0) or some shade of gray (a value between 1 and 110).
The final parameters from each participant’s staircasing session (average parameters in Table A1, Appendix A) were then set as the parameters for their fMRI session, with the aim of equating performance across tasks in the scanner as best possible. However, these environments differed, with staircasing conducted on a laptop in a testing room and the fMRI stimuli projected on a screen from a different computer and viewed with a mirror. In particular, the rear-projection display system during fMRI had worse perceived contrast, and so the luminance of the cue had to be adjusted slightly.
2.5 |. fMRI session
2.5.1 |. Attention tasks
Runs of the attention tasks consisted of an on-off block design, with twelve 16-s blocks of attention tasks (“on”) interleaved with 8-s blocks of fixation (“off”). Task blocks consisted of eight trials in which a face and scene (identity determined pseudorandomly) appeared above and below fixation. Trial onsets (i.e., the onset of the first item) were time-locked to the repetition time (TR = 2 s) and triggered by the scanner. The duration of the stimulus that appeared onscreen first was 300 ms. As determined from the staircasing session, there was a spatial offset, temporal delay, and size difference between the images on every trial, and each image could be tilted. Also, as in the behavioral session, we drew from a range of reference spatial positions and image sizes so that the relational tasks could not be performed by only attending to one of the images. Each run contained 12 blocks: 4 blocks of the item task, 4 of the cue task, and 4 of one of the relational tasks. This led to three run types–spatial runs, timing runs, and size runs– depending on which relational task was performed. In total, participants completed 384 trials of the cue task and item task, and 128 trials of each of the relational tasks. Participants completed all four runs for a particular relational task consecutively. The order of runs was counterbalanced across participants and the order of blocks within a run was counterbalanced within participants.
2.5.2 |. Localizer run
Participants completed a category localizer with alternating blocks of individual faces or scenes. Participants responded with a button box to indicate whether faces were male or female and whether scenes were indoor or outdoor. The structure and timing of the blocks followed the attention task runs (2 s trials, 8 trials per block, 16 s task/8 s fixation, 12 blocks per run). The order of blocks was counterbalanced across subjects. Data from this run were not used in the current study.
2.6 |. fMRI methods
2.6.1 |. Data acquisition
MRI data were acquired with a 3 T Siemens Skyra scanner. Functional images were collected with a gradient-echo EPI sequence (TR = 2,000 ms; TE = 37 ms; FA = 71°; matrix = 128 × 128). Each of 149 volumes contained 27 slices (1.5 mm isotropic) perpendicular to the long axis of the hippocampus. The partial-volume images were optimized for hippocampal imaging, and therefore excluded parts of occipital, parietal, and frontal cortices. A high-resolution 3D T1-weighted MPRAGE scan was collected for registration. A high-resolution T2-weighted turbo spin-echo scan (60 slices; 0.4 × 0.4 mm in-plane; 1.5 mm thickness) was collected for manual segmentation of hippo-campal subfields and MTL cortex.
2.6.2 |. Preprocessing
The fMRI data were analyzed with FSL and MATLAB. The first five volumes of each run were discarded for T1 equilibration. All images were skull-stripped to improve registration. The images were preprocessed with motion correction (MCFLIRT), slice-time correction, spatial smoothing (5 mm FWHM), high-pass filtering (144 s cutoff), and FILM prewhitening.
2.6.3 |. Region of interest segmentation
Manual segmentation of hippocampal subfields and MTL cortex were conducted using published criteria (Aly & Turk-Browne, 2016b; Duvernoy, 2005; Insausti, 1993; Insausti et al., 1998; Meuller & Weiner, 2009; Pruessner et al., 2002; Yushkevich et al., 2010). We segmented these regions of interest (ROIs) on the T2-weighted scans of each participant. MTL ROIs were entorhinal cortex (ERC), perirhinal cortex (PRC), and parahippocampal cortex (PHC). Hippocampal subfield ROIs were subiculum (SUB), CA1, and a combined region for CA2, CA3, and dentate gyrus (CA2/3/DG). All ROIs were traced on coronal slices along the entire length of the hippocampus, using FSLview. Sample segmentations, for one anterior slice and one posterior slice, are shown in Figure 2.
FIGURE 2.
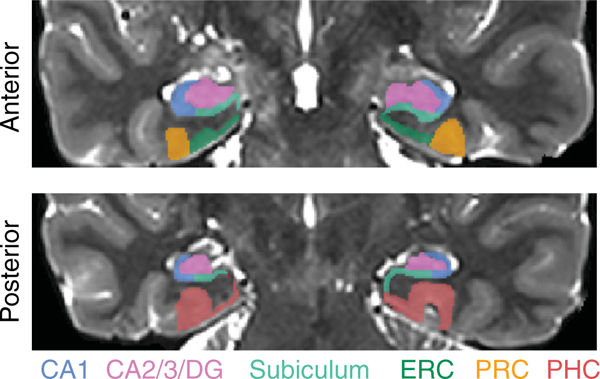
Regions of interest. Example segmentation from one participant is shown for one anterior and one posterior slice. Regions of interest were hand-drawn on individual-participant T2 images. The hippocampal regions of interest were subiculum, CA1, and a combined region of interest for CA2, CA3, and dentate gyrus (CA2/3/ DG). The medial temporal lobe cortex regions of interest were entorhinal cortex [ERC], perirhinal cortex [PRC], and parahippocampal cortex [PHC] [Color figure can be viewed at wileyonlinelibrary.com]
The anterior border of PRC was defined as the most anterior slice in which the collateral sulcus (CS) was visible. The posterior border of PRC was the last slice in which the hippocampal head was visible (Poppenk, Evensmoen, Moscovitch, & Nadel, 2013). The lateral border was at the base of the lateral bank of CS. The medial border depended on whether or not ERC was present. For slices without ERC, the border of PRC coincided with the amygdala. For slices with ERC, the medial border was found halfway up the medial bank of CS. PHC was traced from the first slice of the hippocampal body to the last slice of the hippocampal tail. The lateral border of PHC was perpendicular to the lateral bank of the collateral sulcus. The medial border was the border with SUB, perpendicular to the gray matter bend. The anterior border of ERC was found one slice anterior to the start of the frontotemporal junction. The posterior border was the last slice with the hippocampal head. The lateral border was PRC, and the medial border was the border with SUB, perpendicular to the gray matter bend. CA1, CA2/3/DG, and SUB were traced on all slices in which the hippocampal formation was visible. The medial border of the subiculum was ERC in slices containing the hippocampal head and PHC in slices containing the body and tail. At its most anterior slice, the subiculum comprised the entire ventral aspect of the hippocampus (Duvernoy, 2005); the lateral boundary (with CA1) gradually moved medially until, at the body of the hippocampus, the lateral boundary was at the medial edge of the hippocampus at the point where it pinches into a tear shape. CA1 curved around the lateral edge of the hippocampus and bordered CA2/3 at the dorsal aspect of the hippocampus. The boundary between CA1 and CA2/3/DG was determined by the thickness of CA1 on that slice–usually the upper and lateral 2–3 rows of voxels in the hippocampal formation.
2.6.4 |. Univariate analysis
We estimated stimulus-evoked BOLD responses with a general linear model (GLM) containing block regressors convolved with a canonical hemodynamic response function (HRF), which captured the mean evoked response across blocks. Each run was modeled separately in first-level analyses. The four runs of the same condition were then combined in second-level analyses. For each condition, we registered the parameter estimate images to the participant’s T2 image, converted the parameter estimates to percent signal change, and extracted the average percent signal change over all voxels in each hippocampal and MTL ROI. We then performed random effects t-tests across participants. To isolate signals related to relational processing specifically, we compared evoked activity for each relational task versus the item task.
2.6.5 |. Multivariate pattern similarity analysis
We combined the first-level analyses for even and odd runs of each type (spatial, timing, size) in a second-level analysis. We then registered the parameter estimate images to the participant’s T2-weighted anatomical image and extracted the parameter estimate for each voxel of every ROI for each of the tasks. To calculate pattern similarity for each task and ROI, we reshaped into vectors the across-voxel activity patterns in each ROI. The pattern similarity score for that task and ROI was the correlation between the vectors from even and odd runs. For example, pattern similarity for the timing task was the Pearson correlation between the mean pattern of activity across voxels for the timing task during odd runs and the mean pattern of activity across voxels for the timing task during even runs. For each participant, task, and ROI, we averaged the pattern similarity measures across the left and right hemispheres of the brain–we did not predict hemispheric differences and therefore this reduced the number of statistical comparisons. Because the item task was included in all three relational run types, we calculated pattern similarity for the item task in each run type separately, and then averaged across run types, resulting in an overall item pattern similarity score. As described above for univariate analyses, to isolate information related to relational processing, we compared pattern similarity for each relational task to the item task.
2.6.6 |. Multivariate-univariate dependence analysis
Univariate and multivariate measures are not necessarily independent (Coutanche, 2013; Davis et al., 2014). Indeed, univariate effects (i.e., modulation of overall activity levels) can often drive multivariate ones (i.e., similarity of activity patterns). We therefore quantified the relationship between univariate and pattern similarity measures to assess whether attentional modulation effects in each measure were related or distinct, using an approach known as “multivariate-univariate dependence” (MUD) analysis (Aly & Turk-Browne, 2016b).
The MUD analysis consists of computing the contribution of each voxel to pattern similarity and then calculating the Pearson correlation between these contribution scores and voxels’ level of activity. This quantifies and describes the relationship between univariate activity and pattern similarity: a positive correlation indicates that voxels with the highest activity contribute most to pattern similarity, a negative correlation indicates that voxels with the lowest activity contribute most to pattern similarity, and a zero correlation indicates that a balance of activation and deactivation leads to a stable pattern.
To implement the MUD analysis, we used the same vectors of parameter estimates that we extracted for the pattern similarity analyses. For each participant, ROI, and task, we first normalized the parameter estimates by subtracting the mean and dividing by the root sum-of-squares. We then computed, for each voxel in an ROI, the product of these normalized values from even and odd runs. These products provide a voxel-specific measure of multivariate “influence”–the extent to which a voxel contributed to the pattern similarity measure for that task. Voxels with a positive product (i.e., two positive values or two negative values) contribute to positive pattern similarity, whereas voxels with a negative product (i.e., one positive and one negative value) contribute to negative pattern similarity. Moreover, the magnitude of the product is proportional to the contribution–the larger the product in absolute terms, the greater the “influence.” The sum of these normalized products across voxels is equivalent to the Pearson correlation, hence the relationship between the sign/magnitude of the product and the contribution to pattern similarity.
For each voxel, we also obtained the mean level of univariate activity for each task. Finally, we correlated the multivariate influence scores (i.e., the normalized products) with univariate activity across voxels, for each ROI and task. A reliable correlation across participants (whether positive or negative) would suggest that univariate activity and pattern similarity at least partly capture similar information in the data (see Aly & Turk-Browne, 2016b, for simulations that demonstrate the efficacy of this approach).
3. |. RESULTS
3.1 |. Behavior
We examined reaction times (RTs) and accuracy for each task of interest: the item task, and the spatial, timing, and size relational tasks (Table 2). Inverse efficiency (RT/accuracy; Townsend & Ashby, 1978), a measure of behavior that accounts for speed/accuracy tradeoffs, was matched across relational tasks (F[2,28] = 0.51, p = 0.60). Because behavioral performance was not different between the relational tasks, a task-difficulty explanation of hippocampal and MTL activity differences between these tasks (see below) is unlikely. That said, the lack of a behavioral difference between the relational tasks is a null effect that we cannot overly interpret, as failure to demonstrate a difference is not strong evidence for equality.
TABLE 2.
Behavioral performance
| Task | Accuracy (%) | RT (seconds) | Inverse efficiency |
|---|---|---|---|
| Spatial | 74.86 (7.33) | 0.95 (0.16) | 0.0128 |
| Timing | 78.18 (12.70) | 0.98 (0.16) | 0.0128 |
| Size | 71.67(11.15) | 0.94 (0.16) | 0.0133 |
| Item | 86.00 (6.40) | 0.89 (0.16) | 0.0104 |
Means are shown with standard deviation in parentheses.
Despite our efforts to balance performance across all tasks, the item task resulted in better performance than the relational tasks (all pairwise comparisons of inverse efficiency, p < 0.01). Nevertheless, the item task served as a common baseline across relational tasks and so this cannot explain neural differences between relational tasks.
3.2 |. Evoked univariate activity
Compared with the item task, the timing task led to deactivation in all hippocampal and MTL cortical ROIs (Figure 3; PHC: t[14] = −6.23, p = 0.000022; PRC: t[14] = −5.61, p = 0.000064; ERC: t[14] = −4.88, p = 0.00024; SUB: t[14] = −5.28, p = 0.00011; CA1: t(14) = −6.20, p = 0.000023; CA2/3/DG: t(14) = −5.76, p = 0.000049). The spatial task was associated with deactivation relative to the item task in the MTL cortical ROIs (PHC: t(14) = −3.17, p = 0.0068; PRC: t(14) = −3.13, p = 0.0073; ERC: t(14) = −2.54, p = 0.024) but not the hippocampal ROIs (SUB: t(14) = −1.07, p = 0.30; CA1: t(14) = −1.76, p = 0.10; CA2/3/DG: t(14) = −1.84, p = 0.09). There were no differences in univariate activity between the size task and the item task in any ROI (PHC: t(14) = 0.50, p = 0.63; PRC: t(14) = −0.20, p = 0.85; ERC: t(14) = 0.42, p = 0.68; SUB: t(14) = 0.95, p = 0.36; CA1: t(14) = 0.47, p = 0.65; CA2/3/DG: t(14) = 1.26, p = 0.23).
FIGURE 3.
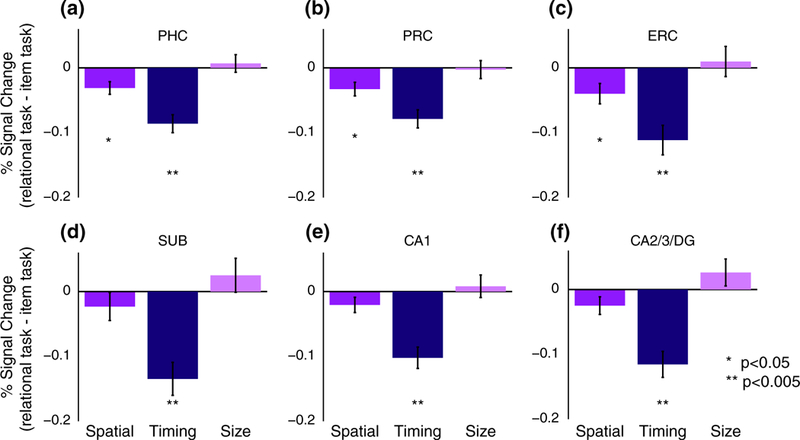
Univariate evoked activity. Percent signal change for the spatial, timing, and size relational tasks, relative to activity for the item task, in each MTL cortical ROI (a: Parahippocampal cortex [PHC]; b: Perirhinal cortex [PRC]; c: Entorhinal cortex [ERC]) and each hippocampal ROI (d: Subiculum [SUB]; e: CA1; f: CA2/3/DG). Error bars reflect ±1 SEM across subjects [Color figure can be viewed at wileyonlinelibrary.com]
Comparing the relational tasks directly, each of the hippocampal and MTL cortical ROIs showed significant differences (i.e., main effect of relational task, PHC: F(2,28) = 16.68, p = 0.000017; PRC: F(2,28) = 8.73, p = 0.0011; ERC: F(2,28) = 8.308, p = 0.0015; SUB: F(2,28) = 12.58, p = 0.00013; CA1: F(2,28) = 14.28, p = 0.000053; CA2/3/DG: F(2,28) = 16.18, p = 0.000021). Follow-up t-tests showed that the timing task was associated with stronger deactivation compared with both the spatial and size tasks in all ROIs (all ps < 0.005).
It is important to note that this pattern of results cannot be attributed to our finding that the item task was easier than the relational tasks. For example, one alternative explanation for the timing task deactivation relative to the item task is that more difficult tasks lead to greater suppression of “default mode” processing in the MTL (Greicius, Supekar, Menon, & Dougherty, 2009). However, if we were simply observing default-mode suppression for more difficult tasks, we should have observed more deactivation for all relational tasks versus the item task, because the item task was easier than all relational tasks. This is not the pattern that we observed, particularly for the size task, which often showed numerically higher activity levels compared with the item task. Furthermore, after correcting for multiple comparisons, there were no relationships between univariate activity and behavioral performance across participants, further arguing against the notion that deactivation reflects difficulty with the task (although this is a null effect that should not be over-interpreted). Below, we explore another form of the disengagement hypothesis in more detail.
3.3 |. Multivariate pattern similarity
Does hippocampal deactivation for the timing task reflect disengagement? As noted above, this cannot be based on task difficulty per se, given the lack of reliable deactivation for the other relational tasks in the hippocampus. However, perhaps the timing task engages other brain regions, which in turn reduce the need for active hippocampal processing. If the hippocampus is disengaged during the timing task, then representations in the hippocampus should not contain task-related information and should instead be governed by noise or other idiosyncratic, task-irrelevant processing. Accordingly, activity patterns in the hippocampus should be unreliable across repetitions of the timing task and show reduced pattern similarity. Alternatively, reduced mean activity could reflect a sharper, sparser representation of attended information resulting from demands on relational processing supported by the hippocampus, which would in turn be associated with stable patterns of activity (Aly & Turk-Browne, 2016a, 2016b; Kok, Jehee, & de Lange, 2012).
To assess these alternatives, we performed pattern similarity analyses, with a special interest in the timing task because it was the only task associated with robust deactivation in the hippocampus (Figure 4). These analyses revealed more stable patterns of activity during the timing task relative to the item task in SUB (t(14) = 2.28, p = 0.038) and CA1 (t(14) = 3.22, p = 0.0062), but no other ROIs (PHC: t(14) = 0.64, p = 0.53; PRC: t(14) = 1.40, p = 0.18; ERC: t (14) = 1.50, p = 0.15; CA2/3/DG: t(14) = 1.48, p = 0.16).
FIGURE 4.
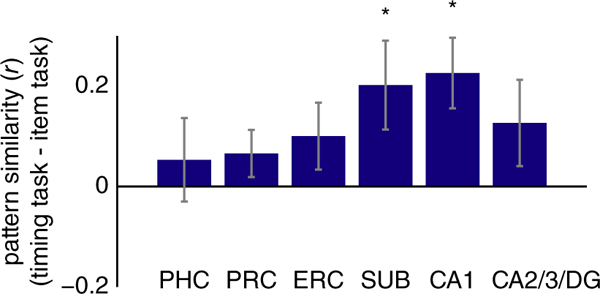
Multivariate pattern similarity for the timing task. Pearson correlation between activity patterns in each ROI for odd versus even runs of the timing task, relative to the correlation between activity patterns for the item task. Subiculum [SUB] and CA1 showed greater pattern similarity for temporal attention versus item attention. Error bars reflect ±1SEM across subjects. * p < 0.05 [Color figure can be viewed at wileyonlinelibrary.com]
In contrast, there were no differences between pattern similarity for the spatial task versus item task or the size task versus item task in any hippocampal or MTL cortical ROI (Spatial vs. Item: PHC: t(14) = 0.20, p = 0.84; PRC: t(14) = 0.57, p = 0.58; ERC: t(14) = 0.98, p = 0.34; SUB: t(14) = −0.10, p = 0.92; CA1: t(14) = 0.80, p = 0.44; CA2/3/DG: t(14) = −0.57, p = 0.57; Size vs. Item: PHC: t(14) = 0.47, p = 0.65; PRC: t(14) = −0.74, p = 0.47; ERC: t(14) = −1.76, p = 0.10; SUB: t(14) = 0.17, p = 0.86; CA1: t(14) = 2.06, p = 0.06; CA2/3/DG: t(14) = 0.59, p = 0.57).
These data suggest that reduced hippocampal activity (in subiculum and CA1) in the timing task may reflect sharpening of representations rather than disengagement from the task. The subiculum effect, however, was weak and did not survive correction for multiple comparisons across regions (FDR corrected p = 0.114). In contrast, the CA1 effect is robust even after correcting for multiple comparisons (FDR corrected p = 0.037). Because we were interested in the timing task in particular (given the univariate results), we corrected for multiple comparisons across regions of interest, but not across the relational tasks.
3.4 |. Multivariate-univariate dependence analysis
Subiculum and CA1 showed lower levels of activity for the timing task (Figure 3d,e) but higher pattern similarity for the timing task (Figure 4). Is the increase in pattern similarity a result of univariate deactivation in these ROIs (see Coutanche, 2013)? For example, is the stability of the activity pattern simply a consequence of some voxels consistently deactivating in the timing task, or does the pattern reflect information that is not captured in terms of a mean response? To address this question, we conducted a MUD analysis to quantify the relationship between univariate activity in each voxel and its contribution to pattern similarity (Figure 5; Aly & Turk-Browne, 2016b). Insofar as deactivation is responsible for pattern similarity, we should observe a negative relationship in the MUD analysis: voxels with the lowest activity levels should be the largest contributors to pattern similarity. However, there was no reliable relationship between voxels’ univariate activity and their contribution to pattern similarity for the timing task in SUB (t(14) = 0.81, p = 0.43) or CA1 (t(14) = 0.61, p = 0.55).
FIGURE 5.
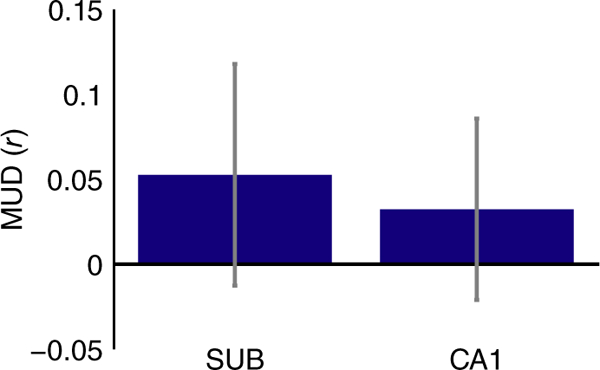
MUD analysis for the timing task. The contribution of each voxel to pattern similarity was estimated by normalizing BOLD activity over voxels within each ROI, separately for the timing task in even and odd runs, and computing pairwise products across runs. To estimate multivariate-univariate dependence, these products were then correlated with the average univariate activity in the timing task for each voxel. Neither SUB nor CA1 showed a relationship between the two measures, suggesting that deactivated voxels were not solely responsible for increased pattern similarity. Error bars reflect ±1SEM across subjects [Color figure can be viewed at wileyonlinelibrary.com]
The absence of a correlation between univariate activity and contributions to pattern similarity across voxels suggests that it is neither high nor low univariate activity that is driving pattern stability: instead, the elevated pattern similarity in the timing task reflects a balance of voxel activation and deactivation that together underlie the stable pattern (see simulations in Aly & Turk-Browne, 2016b, for a demonstration). However, this interpretation rests on a null result (the absence of a relationship between univariate activity and pattern similarity); thus, a Type II error cannot be ruled out. Future studies using the MUD analysis will be important for characterizing when the hippocampus does, and does not, show dependencies between univariate activity and multivariate pattern similarity.
4 |. DISCUSSION
Attention has been studied primarily in terms of individual features and locations, but our experience of the world is fundamentally relational, consisting of representations of items and their associations to other items and the global context. We examined the neural substrates of relational attention, focusing on the hippocampus because of its critical role in relational forms of long-term memory (Cohen et al., 1999; Eichenbaum & Cohen, 2014; Ryan, Althoff, Whitlow, & Cohen, 2000). According to some theories (Squire et al., 2004), the hippocampus is a dedicated memory system, and thus its role in relational processing should be limited to relational memory. Alternatively, the relational computations of the hippocampus might support a more general function, and contribute to relational processing across domains of cognition from perception to long-term memory (Aly & Turk-Browne, 2018; Shohamy & Turk-Browne, 2013; Yonelinas,2013). In the current study, we tested the hypothesis that the hippo-campus would be recruited by relational attention even during online perceptual processing, with no demands on long-term memory. More-over, we tested whether the hippocampus is specialized for some types of relational processing (e.g., spatial or temporal), or plays a broader role in other types of relations as well (e.g., relative size).
We found strong deactivation throughout the hippocampus when participants attended to temporal relations, as compared with attending to items. This reduction in univariate activity was accompanied by an increase in multivariate pattern similarity in the timing task relative to the item task. These results echo other findings showing that reductions in activity can be accompanied by increases in information content in patterns of activity (Aly & Turk-Browne, 2016a, 2016b; Bell, Summerfield, Morin, Malecek, & Ungerleider, 2016; Kok et al., 2012), and raises the possibility of sparse, sharper representations in the hippocampus when attention is directed to temporal relations. Further analyses indicated that higher pattern similarity in the timing task was not simply a consequence of lower levels of activity: instead, the stable patterns of activity in the timing task were a result of a balance of activation and deactivation. Finally, the selectivity of this pattern of results to temporal attention is unlikely to be due to differences in task difficulty, because all relational tasks were matched in behavioral performance.
We also found that entorhinal, perirhinal, and parahippocampal cortices were all modulated by both spatial and temporal relational attention. However, these regions were deactivated for relational versus item attention, and did not show more stable patterns of activity for relational versus item attention. Thus, the current study cannot rule out that these regions were simply disengaged during the relational attention tasks, and this finding requires further investigation.
4.1 |. What is represented in the hippocampus during online attention?
What is the content of the sharpened representations in the hippo-campus during temporal attention? One possibility is that these stable activity patterns represent a specific, yet abstract, attentional state. Another possibility is that these stable activity patterns do not represent an abstract attentional set, but rather represent precise information about the components of stimuli that are attended on the temporal attention trials (e.g., a short-term representation of whether the upper vs. lower part of the screen changed first). Our current results cannot adjudicate between these possibilities, because both components (the abstract attentional set, and the precise features that are attended) are inherent aspects of the attention task. Indeed, it is difficult to conceive of any study design that can separate the brain’s representation of an abstract attentional state from its representation of the attended features, because the attended features are a key aspect of defining the attentional state in the first place. What can be concluded, however, is that the patterns of activity in the hippocampus for temporal attention code for the commonalities of that attentional state across different trials that vary in terms of the visual images presented and their precise timing.
Are the results of the current study merely reflecting relational long-term memory in the hippocampus? We think that this is unlikely for several reasons. For example, one might argue that participants are incidentally encoding the stimuli into memory. While this is certainly possible, that is not enough to explain our results. If memory were the driving force behind the differential hippocampal modulation we observed across tasks, then there must have been different amounts of incidental encoding in these tasks–but there is no reason why that should be true. Even if different amounts of incidental encoding were occurring across tasks, our dissociation between univariate activity and pattern similarity in the hippocampus complicates the interpretation. Specifically, greater pattern similarity (in the timing task) and greater univariate activity (in the other tasks) have both been linked to better memory encoding in the hippocampus (Carr, Engel, & Knowlton, 2013; Wolosin, Zeithamova, & Preston, 2013).
Thus, our results cannot be accounted for by appealing to long-term memory, and instead concur with recent neuropsychological and neuroimaging studies highlighting a role for the hippocampus in online processing without demands on long-term memory, including visual perception and attention tasks (e.g., Aly, Ranganath, & Yonelinas, 2013; Aly & Turk-Browne, 2016b; Lee, Yeung, & Barense, 2012; Warren, Duff, Tranel, & Cohen, 2011; Zeidman & Maguire, 2016; Zeidman, Mullally, & Maguire, 2015). These findings challenge the traditional perspective of the hippocampus as a dedicated declarative memory system (Squire et al., 2004; Squire & Wixted, 2011), and high-light the reach of the hippocampus to attention and perception.
4.2 |. Space and time in the hippocampus
Along with long-term memory, studies of the hippocampus have also focused extensively on its role in representing space (Bird & Burgess, 2008; Bussey & Saksida, 2005; Kumaran & Maguire, 2005; Nadel, 1991; O’Keefe & Nadel, 1978). Recently, however, there has been increased focus on the importance of the hippocampus for temporal processing–both its contribution to the temporal organization of memories (Davachi & DuBrow, 2015; Eichenbaum, 2013; Howard & Eichenbaum, 2013; Hsieh, Gruber, Jenkins, & Ranganath, 2014; Jenkins & Ranganath, 2010; Paz et al., 2010; Schapiro, Turk-Browne, Norman, & Botvinick, 2016; Staresina & Davachi, 2009), as well as the perception of time (Barnett et al., 2014; Palombo, Keane, & Verfaellie, 2016). For example, the hippocampus contains “time cells” whose successive activity signals the passage of time (Eichenbaum, 2014; MacDonald et al., 2011; Pastalkova et al., 2008). Beyond space and time, some studies support the view that the hippocampus engages in relational processing irrespective of content (Eichenbaum, 2004; Hannula, Tranel, & Cohen, 2006; Konkel et al., 2008; McKenzie et al., 2016; Schiller et al., 2015). Most previous work with fMRI, however, has focused on one aspect of relational processing (e.g., spatial relations) and thus has not been in a position to test these different perspectives. By comparing multiple relations in the same experiment (see Konkel et al., 2008 for a similar approach in a patient study of memory), our findings were able to highlight a special role for the hippocampus in the online processing of temporal relations.
We observed reliable deactivation throughout all hippocampal subfields during attention to temporal relations. However, pattern similarity was higher during temporal attention in subiculum and CA1, but not CA2/3/DG. One might have expected stable activity patterns for temporal attention in CA2/3/DG, given that CA3, like CA1, contains time cells (Salz et al., 2016), and is implicated in temporal and sequential processing more generally, including on the order of seconds (Farovik et al., 2010; Kesner & Hunsaker, 2010). However, our region of interest also includes CA2 and dentate gyrus, which have not yet (to our knowledge) been implicated in seconds-level timing (see Mankin et al., 2015 for timing on the order of hours to days; also see Gilbert et al., 2001). Thus, the lack of pattern stability for temporal attention in CA2/3/DG is inconclusive and requires further investigation with studies that can separately examine CA2, CA3, and DG.
Likewise, the lack of an effect for spatial relational attention in the hippocampus is unexpected given extensive evidence that the hippocampus is involved in spatial processing (Eichenbaum & Cohen, 2014), including spatial relational attention (Aly & Turk-Browne, 2016a, 2016b). One possibility is that the hippocampus is involved in fine relational discriminations (e.g., Aly et al., 2013; Barnett et al.,2014) but is not required when such discriminations can be solved on the basis of individual featural comparisons (Baxter, 2009; Bussey & Saksida, 2005). However, we designed the tasks so that such feature- level comparisons would not be sufficient to support performance–that is, the jitter in spatial location and size was meant to ensure that attention to, and comparison of, both items was required to do the relational tasks. Thus, another possibility is that the hippocampus only becomes involved in spatial processing given sufficiently complex or “high-resolution” task demands (Aly et al., 2013; Yonelinas, 2013). For example, the hippocampus is engaged by the demand to attend to, and find similarities or differences in, spatial layouts of complex, naturalistic scenes (Aly et al., 2013; Aly & Turk-Browne, 2016a, 2016b; Lee et al., 2012). It is possible that our spatial relational task does not sufficiently tax the abstract and flexible spatial representations of the hippocampus, and can be solved on the basis of relational representations elsewhere.
This raises an important question for future research: Does the hippocampus become involved at the same level of complexity when assessing relations in the spatial and temporal domains? Or do some types of relations require the hippocampus at an earlier level of complexity than others? These are difficult problems to solve, but these limitations point to a need in the field: a need to define what is “complex” or “high resolution” with enough precision that these definitions can be used to generate testable hypotheses about when the hippo-campus should, and should not, be involved in any given task.
4.3 |. Transformation of relational representations from perception to memory
In studies of long-term memory encoding and retrieval, the hippocampus shows greater univariate activity for relational memory versus item memory (see Davachi, 2006 for review). This contrasts with our current findings, in which the hippocampus showed less univariate activity for relational versus item attention. An open question is why the direction of the relational versus item effect switches from perception to memory. There are at least three possibilities.
The first possibility is that there are two different relational computations in the hippocampus. One supports in-the-moment attention and perception, is sharply tuned, and is associated with reductions in univariate activity. The other supports long-term memory, is more integrative, and is associated with enhancements in univariate activity.
A second possibility is that the same set of relational computations in the hippocampus is expressed in different ways in perception and memory: the initial representation is sharp and sparse, but over time is transformed to a more integrated representation. We believe that this second possibility is unlikely, because the hippocampus shows greater univariate activity for relational versus item encoding, arguing against the emergence of a univariate activity enhancement over the time course of consolidation.
A final possibility lies somewhere in between: the hippocampus has a common set of relational computations, but these computations are differently taxed during perception and memory. In attention and perception tasks, stimuli are often repeated many times, presented for a short duration, and only have to be processed enough to accomplish the task in the immediate moment. In memory tasks, a stimulus might be shown only once, and elaborative processing is helpful during both encoding (to create a distinct memory representation) and retrieval (to bring to mind associated details). These task demands might prioritize a finely-tuned, sharp, and short-lasting representation during attention/perception tasks, and a richer, more integrated, longer-lasting representation during memory tasks.
Importantly, this viewpoint suggests that it is not perception versus memory per se that produces these differences in the properties of hippocampal representations, but the demands of the typical tasks used to study these cognitive processes (see Aly & Yonelinas, 2012, for a similar perspective). This perspective predicts that making a perception (or attention) task more like a memory task would yield greater hippocampal activity for relational versus item perception (or attention). This might be accomplished by more closely matching perception tasks to the encoding phase of long-term memory studies; for example, by showing stimuli only once each, and for a longer duration. Including a long-term memory test following the perception task would enable separate examination of hippocampal effects related to memory encoding versus those related to perceptual processing (e.g., as in Lee, Brodersen,& Rudebeck, 2013). These and other approaches will be useful for characterizing relational representations in the hippocampus during perception and memory, and determining whether a single set of underlying computations supports both.
5 |. CONCLUSIONS
Attention gates what we perceive and remember, and yet we know relatively little about how attention modulates neural activity in the hippocampus. Recent work has made important progress in elucidating how the hippocampus is modulated by the focus of attention (Aly & Turk-Browne, 2016a, 2016b, 2017), in line with the current findings. We provide evidence that the hippocampus is differentially involved in relational and item attention, even during online visual perception. Attention to temporal relations reduces hippocampal activity and increases hippocampal pattern stability, with balanced activation and deactivation producing a sharpened representation. These findings show that the relational computations of the hippocampus can extend beyond long-term memory, enabling the rapid online extraction of relational information during visual perception.
Acknowledgments
Funding information
National Eye Institute, Grant/Award Number:R01-EY021755
APPENDIX A
TABLE A1.
Mean and standard deviation of final parameters from each participant’s staircasing session
| Horizontal offset (pixels) |
Temporal delay (seconds) |
Size difference (pixels) | Degree of tilt (angle) |
Color of Cue (shading: 0 = black; 110 = gray) |
|
|---|---|---|---|---|---|
| Mean | 5.73 | 0.11 | 4.8 | 3.53 | 33.6 |
| Standard deviation | 2.63 | 0.13 | 2.18 | 0.99 | 20.5 |
REFERENCES
- Aly M, Ranganath C, & Yonelinas AP (2013). Detecting changes in scenes: The hippocampus is critical for strength-based perception. Neuron, 78, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, & Turk-Browne NB (2016a). Attention promotes episodic encoding by stabilizing hippocampal representations. Proceedings of the National Academy of Sciences of the United States of America, 113, E420–E429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, & Turk-Browne NB (2016b). Attention stabilizes representations in the human hippocampus. Cerebral Cortex, 26, 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, & Turk-Browne NB (2017). How hippocampal memory shapes, and is shaped by, attention In Hannula DE & Duff MC (Eds.), The hippocampus from cells to systems (pp. 369–403). Switzerland: Springer International Publishing AG. [Google Scholar]
- Aly M, & Turk-Browne NB (2018). Flexible weighting of diverse inputs makes hippocampal function malleable. Neuroscience Letters, 680, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, & Yonelinas AP (2012). Bridging consciousness and cognition in memory and perception: Evidence for both state and strength processes. PLoS One, 7(1), e30231. 10.1371/journal.pone.0030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AJ, O’Neil EB, Watson HC, & Lee AC (2014). The human hippocampus is sensitive to the durations of events and intervals within a sequence. Neuropsychologia, 64, 1–12. [DOI] [PubMed] [Google Scholar]
- Baxter MG (2009). Involvement of medial temporal lobe structures in memory and perception. Neuron, 61, 667–677. [DOI] [PubMed] [Google Scholar]
- Bell AH, Summerfield C, Morin EL, Malecek NJ, & Ungerleider LG (2016). Encoding of stimulus probability in macaque inferior temporal cortex. Current Biology, 26, 2280–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, & Burgess N (2008). The hippocampus and memory: Insights from spatial processing. Nature Reviews Neuroscience, 9,182–194. [DOI] [PubMed] [Google Scholar]
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, & Moser M-B (2010). Grid cells in pre-and parasubiculum. Nature Neuroscience, 13, 987–994. [DOI] [PubMed] [Google Scholar]
- Bos JJ, Vinck M, van Mourik-Donga LA, Jackson JC, Witter MP, & Pennartz CMA (2017). Perirhinal firing patterns are sustained across large spatial segments of the task environment. Nature Communications, 8, 15602–15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Becker S, King JA, & O’Keefe J (2001). Memory for events and their spatial context: Models and experiments. Philosophical Transactions of the Royal Society of London B, 356, 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, & O’Keefe J (2002). The human hippocampus and spatial and episodic memory. Neuron, 35, 625–641. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, & Saksida LM (2005). Object memory and perception in the medial temporal lobe: An alternative approach. Current Opinion in Neurobiology, 15, 730–737. [DOI] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, & Frank LM (2011). Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nature Neuroscience, 14, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Engel SA, & Knowlton BJ (2013). Top-down modulation of hippocampal encoding activity as measured by high-resolution functional MRI. Neuropsychologia, 51, 1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, & Nash C (1999). Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus, 9, 83–98. [DOI] [PubMed] [Google Scholar]
- Coutanche MN (2013). Distinguishing multi-voxel patterns and mean activation: Why, how, and what does it tell us? Cognitive Affective & Behavioral Neuroscience, 13, 667–673. [DOI] [PubMed] [Google Scholar]
- Dalton MA, & Maguire EA (2017). The pre/parasubiculum: A hippocampal hub for scene-based cognition? Current Opinion in Behavioral Sciences, 17, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Davachi L, & DuBrow S (2015). How the hippocampus preserves order: The role of prediction and context. Trends in Cognitive Sciences, 19, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T, LaRocque KF, Mumford JA, Norman KA, Wagner AD, & Poldrack RA (2014). What do differences between multi-voxel and univariate analysis mean? How subject-, voxel-, and trial-level variance impact fMRI analysis. Neuroimage, 97, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences, 11, 379–386. [DOI] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, & Davachi L (2012). Evidence for area CA1 as a match/mismatch detector: A high-resolution fMRI study of the human hippocampus. Hippocampus, 22, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM (2005). The human hippocampus: Functional anatomy, vascularization and serial sections with MRI (3rd ed.). Berlin; New York: Springer. [Google Scholar]
- Eichenbaum H (2004). Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 44, 109–120. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2013). Memory on time. Trends in Cognitive Sciences, 17, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2014). Time cells in the hippocampus: A new dimension for mapping memories. Nature Reviews Neuroscience, 15, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Time (and space) in the hippocampus. Current Opinion in Behavioral Sciences, 17, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, & Cohen NJ (2014). Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron, 83, 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, & Cohen NJ (1994). Two functional components of the hippocampal memory system. Behavioral and Brain Sciences, 17, 449–472. [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, & Fried I (2003). Cellular networks underlying human spatial navigation. Nature, 425, 184–188. [DOI] [PubMed] [Google Scholar]
- Epstein R, & Kanwisher N (1998). A cortical representation of the local visual environment. Nature, 392, 598–601. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, & Eichenbaum H (2010). Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learning & Memory, 17, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconeri SL, Scimeca JM, Roth JC, Helseth SA, & Kahn LE (2012). Flexible visual processing of spatial relationships. Cognition, 122, 210–227. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, & Lee I (2001). Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus, 11, 626–636. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, & Dougherty RF (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex, 19, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, & Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436, 801–806. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, & Cohen NJ (2006). The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience, 26, 8352–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts CJ, Voets NL, Thomas AG, Clare S, Lawrence AD, & Graham KS (2017). Ultra-high-field fMRI reveals a role for the subiculum in scene perceptual discrimination. Journal of Neuroscience, 37, 3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, & Eichenbaum H (2013). The hippocampus, time, and memory across scales. Journal of Experimental Psychology: General, 142, 1211–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, & Ranganath C (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron, 81, 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R (1993). Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus, 3, 19–26. [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P Pitkanen A (1998). MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology, 19, 659–671. [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, & Ranganath C (2010). Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. Journal of Neuroscience, 30,15558–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, & Ungerleider LG (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–341. [DOI] [PubMed] [Google Scholar]
- Kesner RP, & Hunsaker MR (2010). The temporal attributes of memory. Behavioral Brain Research, 215, 299–309. [DOI] [PubMed] [Google Scholar]
- Kumaran D, & Maguire EA (2005). The human hippocampus: Cognitive maps or relational memory. Journal of Neuroscience, 25, 7254–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok P, Jehee JFM, & de Lange FP (2012). Less is more: Expectation sharpens representations in the primary visual cortex. Neuron, 75,265–270. [DOI] [PubMed] [Google Scholar]
- Konkel A, & Cohen NJ (2009). Relational memory and the hippocampus: Representations and methods. Frontiers in Neuroscience, 3,166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, & Cohen NJ (2008). Hippocampal amnesia impairs all manner of relational memory. Frontiers in Human Neuroscience, 15, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Brandon MP, Robinson RJ, Connerney MA, Hasselmo ME, & Eichenbaum H (2015). During running in place, grid cells integrate elapsed time and distance run. Neuron, 88, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Yeung L-K, & Barense MD (2012). The hippocampus and visual perception. Frontiers in Human Neuroscience, 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, & Burgess N (2009). Boundary vector cells in the subiculum of the hippocampal formation. Journal of Neuroscience, 29, 9771–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Brodersen KH, & Rudebeck SR (2013). Disentangling spatial perception and spatial memory in the hippocampus: A univariate and multivariate pattern analysis fMRI study. Journal of Cognitive Neuroscience, 25, 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, & Mullally SL (2013). The hippocampus: A manifesto for change. Journal of Experimental Psychology: General, 142, 1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, & Eichenbaum H (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 71, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, & Leutgeb JK.(2015) Hippocampal CA2 activity patterns change over itme to a larger extent than between spatial contexts. Neuron, 85, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, & Leutgeb JK (2012). Neuronal code for extended time in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 109, 19462–19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, & Eichenbaum H (2007). Gradual changes in hippocampal activity support remembering the order of events. Neuron, 56, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, & Treue S (2006). Feature-based attention in visual cortex. Trends in Neurosciences, 29, 317–322. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Frank A, Kinsky NR, Porter B, Riviere PD, & Eichenbaum H (2014). Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron, 83, 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Keene CS, Farovik A, Bladon J, Place R, & Komorowski EH(2016).Representation of memories in the cortical-hippocampal system: Results from the application of population similarity analyses. Neurobiology of Learning and Memory, 134, 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuller SG, & Weiner MW (2009). Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus, 19, 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal AL, Uttal D, Shah P, & Franconeri SL (2016). Visual routines for extracting magnitude relations. Psychonomic Bulletin and Review, 23, 1802–1809. [DOI] [PubMed] [Google Scholar]
- Nadel L (1991). The hippocampus and space revisited. Hippocampus, 1, 221–229. [DOI] [PubMed] [Google Scholar]
- Naya Y, & Suzuki WA (2011). Integrating what and when across the primate medial temporal lobe. Science, 333, 773–776. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Dostrovsky J (1971). The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34, 171–175. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The hippocampus as a cognitive map. Oxford: Oxford University Press. [Google Scholar]
- Olsen RK, Moses SN, Riggs L, & Ryan JD (2012). The hippocampus supports multiple cognitive processes through relational binding and comparison. Frontiers in Human Neuroscience, 6, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo DJ, Keane MM, & Verfaellie M (2016). Does the hippocampus keep track of time? Hippocampus, 26, 372–379. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, & Buzsaki G (2008). Internally generated cell assembly sequences in the rat hippocampus. Science, 321, 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, & Fried I (2010). A neural substrate in the human hippocampus for linking successive events. Proceedings of the National Academy of Sciences of the United States of America, 107, 6046–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, & Nadel L (2013). Long- axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17, 230–240. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A…Evans AC (2002). Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: Considering the variability of the collateral sulcus. Cerebral Cortex, 12, 1342–1353. [DOI] [PubMed] [Google Scholar]
- Ranganath C (2019). Time, memory, and the legacy of Howard Eichenbaum. Hippocampus, 2018, 23007 10.1002/hipo.23007 [DOI] [PubMed] [Google Scholar]
- Ranganath C, & Hsieh L-T (2016). The hippocampus: A special place for time. Annals of the New York Academy of Sciences, 1369, 93–110. [DOI] [PubMed] [Google Scholar]
- Rolls ET, & Wirth S (2018). Spatial representations in the primate hippocampus, and their functions in memory and navigation. Progress in Neurobiology, 171, 90–113. 10.1016/j.pneurobio.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, & Cohen NJ (2000). Amnesia is a deficit in relational memory. Psychological Science, 11, 454–461. [DOI] [PubMed] [Google Scholar]
- Sakon JJ, Naya Y, Wirth S, & Suzuki W (2014). Context-dependent incremental timing cells in the primate hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 111, 18351–18356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz DM, Tiganj Z, Khasnabish S, Kohley A, Sheehan D, Howard MW, & Eichenbaum H (2016). Time cells in hippocampal area CA3. Journal of Neuroscience, 36, 7476–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Kustner LV, & Turk-Browne NB (2012). Shaping of object representations in the human medial temporal lobe based on temporal regularities. Current Biology, 22, 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Turk-Browne NB, Norman KA, & Botvinick MM(2016). Statistical learning of temporal community structure in the hippocampus. Hippocampus, 26, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Eichenbaum H, Buffalo EA, Davachi L, Foster DJ, Leutgeb S, & Ranganath C (2015). Memory and space: Towards an understanding of the cognitive map. Journal of Neuroscience, 35,13904–13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, & Turk-Browne NB (2013). Mechanisms for widespread hippocampal involvement in cognition. Journal of Experimental Psychology: General, 142, 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, & Clark RE (2004). The medial temporal lobe. Annual Review of Neuroscience, 27, 279–306. [DOI] [PubMed] [Google Scholar]
- Squire LR, & Wixted JT (2011). The cognitive neuroscience of human memory since HM. Annual Review of Neuroscience, 34, 259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2009). Mind the gap: Binding experiences across space and time in the human hippocampus. Neuron, 63,267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, & Ranck JB (1990). Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. Journal of Neuroscience, 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JT, & Ashby FG (1978). Methods of modeling capacity in simple processing systems In Castellan J & Restle F (Eds.), Cognitive theory (Vol. 3, pp. 200–239). Hillsdale (NJ): Erlbaum. [Google Scholar]
- Turk-Browne NB, Simon MG, & Sederberg PB (2012). Scene representations in parahippocampal cortex depend on temporal context. Journal of Neuroscience, 32, 7202–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser M-B, & Moser EI (2018). Integrating time from experience in the lateral entorhinal cortex. Nature, 561, 57–62. [DOI] [PubMed] [Google Scholar]
- Warren DE, Duff M, Tranel D, & Cohen NJ (2011). Observing degradation of visual representations over short intervals when medial temporal lobe is damaged. Journal of Cognitve Neuroscience, 23, 3862–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, & Preston AR (2013). Distributed hippocampal patterns that discriminate reward context are associated with enhanced associative binding. Journal of Experimental Psychology: General, 142, 1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research, 254, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Wang H, Pluta J, Das SR, Craige C, Avants BB Meuller S (2010). Nearly automatic segmentation of hippocampal sub-fields in in vivo focal T2-weighted MRI. Neuroimage, 53, 1208–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, & Maguire EA (2016). Anterior hippocampus: The anatomy of perception, imagination, and episodic memory. Nature Reviews Neuroscience, 17,173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Mullally SL, & Maguire EA (2015). Constructing, perceiving, and maintaining scenes: Hippocampal activity and connectivity. Cerebral Cortex, 25, 3836–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]


