Abstract
This study examines the long-term OS of relapsed AML patients who were enrolled to 9 successive ECOG-ACRIN trials for newly diagnosed AML, during 1984–2008. The objectives were to examine whether there is a trend of improvement in the survival of relapsed AML patients in the more recent studies and to search for prognostic factors that are associated with long-term OS after relapse. A total of 3012 patients were enrolled, 1779 (59.1%) achieved CR1 and of these, 58.9% relapsed. The median follow-up was 9.7 years. The median OS from relapse was 0.5 years and the 5-year OS was 10 (±1)%. These results were similar even for the most recent studies. A multivariate model showed that age, cytogenetics at diagnosis, duration of CR1 and undergoing allogeneic transplantation were significantly associated with OS from relapse. Even among patients who relapsed with better prognostic factors; age < 40 and CR1 > 12 months, there was no significant OS difference between the studies. In conclusion, this large cohort appears to confirm that the survival of AML patients postrelapse continues to be dismal and has not improved during the past quarter of a century.
1 |. INTRODUCTION
Relapse after achieving a complete remission (CR) in acute myeloid leukemia (AML) portends for a poor prognosis and allogeneic hematopoietic cell transplantation (Allo-HCT) after achieving a second remission likely offers the only possible chance for cure. While approximately 40%−55% of patients who experience disease relapse may achieve a second CR, only 10%−20% have a chance of long-term survival.1–3 This study examines the long-term overall survival (OS) of AML patients (not including acute promyelocytic leukemia) who experienced leukemia relapse after achieving first CR (CR1). It is based on nine successive trials for newly diagnosed AML patients (E3483, E3489, PC486, E3993, E4995, E1490, E3997, E1900, and E3999), conducted by the ECOG-ACRIN Cancer Research Group between March 1984 to November 2008. Recognizing the many changes that have taken place on time in the diagnosis, prognosis and cytogenetic analysis, the objectives of this study were to examine whether there is a trend of change in the survival of relapsed AML patients in the more recent studies and to search for prognostic factors that are associated with long-term OS after relapse.
2 |. METHODS
2.1 |. Patient population
Between 1984 and 2008, 3012 patients, aged 15 years and older, who had untreated AML were enrolled on 9 consecutive, phase II and III, ECOG-ACRIN-led clinical trials.4–12 The treatment protocols, their activation dates and accrual numbers are summarized in Table 1.
TABLE 1.
AML protocols included in the analysis
| Protocol Number |
Phase | Induction | Consolidation | Maintenance | Activation-termination dates |
Final accrual (Pts included) |
|---|---|---|---|---|---|---|
| E34834 | III | 1 or 2 courses of: dauno 60 mg/m2/d, days 1–3; ARAC 25 mg/m2 IV push followed by continuous IV 200 mg/m2/d, days 1–5; and PO 6TG 100 mg/m2 × 2/d, days 1–5 (DAT) | Age< 41 + H LA-matched sibling => alloBMT. Others were initially randomized to 1 of 3 arms: a) observation,b) maintenance ore) 1 course of consolidation. After interim analysis, the observation arm was closed. Consolidation therapy: IV ARAC 3 g/m2 over 1 hr × 2/d, days 1–6,followed by IV amsacrine 100 mg/m2/d, days 7–9. |
PO 6TG 40 mg/m2 ×2/d, for 4 days, followed by SC ARAC 60 mg/m2 on the 5th day. Duration: 2 yr. |
Mar 1984 -Jan 1988 | 534 (445) |
| PC4865 | II | 1 or 2 courses of DAT | Patients in CR:a) age<41 & HLA identical sibling => alloBMTb) all others => autoBMT | Apr 1987-Apr 1990 | 123 (98) | |
| E34896 | III | 1 or 2 courses of idarubicin 12 mg/m2/d, days 1–3, and ARAC 25 mg/m2 IV push followed by continuous IV 100 mg/m2/d, days 1–7 | Idarubicin 12 mg/m2/d, days 1–2, and ARAC 25 mg/m2 IV push followed by continuous IV 100 mg/m2/d, days l-5a) Patients with a HLA-matched orsingle- mismatched family member => alloBMT -All others were randomized to:b) autoBMT ore) A single course of IV ARAC 3 g/m2 over 3 hr × 2/d, days 1–6 | Feb 1990-Feb 1995 | 808 (753) | |
| E14907 | III | 1 or 2 courses of dauno 60 mg/m2/d, days 1–3, ARAC 25 mg/m2 IV push followed by continuous IV 100 mg/m2/d, days 1–7 + GM-CSF vs. placebo from dll | A single course of IV ARAC 1.5 g/m2 over 1 hrx2/d, days 1–6 + GM-CSF vs. placebo from dll | Sep 1990-Nov 1992 | 124 (115) | |
| E39938 | III | GM-CSF vs. placebo as priming, ARAC continuous IV 100 mg/m2/d, days 1–7 Patients were randomized to either: a) dauno 45 mg/m2/d, days 1–3 (DA) orb) mitox 12 mg/m2/d, days 1–3 (MA) ore) idarubicin 12 mg/m2/d, days 1–3 (IA). |
Age<70: IV ARAC 1.5 g/m2 over 1 hr × 2/d, days 1–6 + GM-CSF from d5 Age>70: IV ARAC 1.5 g/m2 over 1 hr × 2/d, days 1–3 + GM-CSF from d5 | Apr 1993-Feb 1997 | 362 (343) | |
| E499512 | II | 2 cycles of: dauno 45 mg/m2/d, days 1–3, ARAC continuous IV 100 mg/m2/d, days 1–7 and ARAC 2 g/m2 over 75–90 min × 2/d, days 8–10 | Age< 51 + HLA-matched sibling: alloPBSCT Others—2 courses of: ARAC 3 g/m2 over 3 hr × 2/d, days 1, 3, 5 and then autoPBSCT |
Aug 1996-Feb 1997 | 66 (59) | |
| E39979 | II | Dauno 45 mg/m2/d, days 1–3, ARAC continuous IV 100 mg/m2/d, days 1–7 and ARAC 2 g/m2 over 60–90 min × 2/d, days 8–10 + rhlL-11 and GM-CSF from days 11–12 | 2 courses of: ARAC 3 g/m2 over 3 hr × 2/d, days 1, 3, 5 + rhlL-11 and GM-CSF from d6 | June 1998-Apr 1999 | 36 (35) | |
| E399910 | III | 1 or 2 courses of dauno 45 mg/m2/d, days 1–3, ARAC continuous IV 100 mg/m2/d, days 1–7 and zosuquidar vs. placebo |
|
Jul 2002-Sep 2005 | 449 (421) | |
| E190011 | III | Dauno 45 vs. 90 mg/m2/d, days 1–3, ARAC continuous IV 100 mg/m2/d, days 1–7 | Unfavorable/intermediate risk cytogenetic profile or a WBC> 100 000/μL at diagnosis + HLA-matched sibling => allo- HCT. All the others:
|
Dec 2002-Nov 2008 | 657 (644) |
Abbreviations: allo, allogeneic; ARAC, cytosine arabinoside; auto,autologous; BMT, bone marrow transplant; d, day; dauno, daunorubicin; GM-CSF, granulocyte-macrophage colony-stimulating factor; GO, gemtuzumab ozogamicin; HCT, hematopoietic cell transplantation; IV,intravenous; mitox, mitoxantrone; PB, peripheral blood; PO, per os; 6TG, 6-thioguanine; SC, subcutaneous.
Acute promyelocytic leukemia patients were included in the older AML studies but are excluded from this analysis due to the unique behavior and treatment of this disease. Each protocol was approved by the Institutional Review Boards at each respective institution. All patients signed a written informed consent.
2.2 |. Cytogenetic risk classification
Cytogenetic risk was classified as “favorable,” “intermediate,” “unfavorable,” or “undetermined” after central review by the ECOG-ACRIN Cytogenetics Committee, according to the definitions established by the Southwest Oncology Group (SWOG) and ECOG-ACRIN.13 The favorable risk category included patients with inv(16)/t(16;16)/del(16q) or t(8;21), with or without other chromosome abnormalities. The intermediate risk category included patients characterized by +8; -Y; +6; del(9q); del(12p) or normal karyotype. The unfavorable risk category was defined by the presence of one or more of −5/del(5q); −7/del(7q); inv(3q)/t(3;3); deletion 20q or 21q; translocation involving 11q23, t(6;9); t(9;22); deletion 17p or complex karyotype, defined as 3 or more chromosomal abnormalities.
Minimal cytogenetic information was available for patients enrolled on the following earlier protocols: E1490, E3483, and PC486, consistent with the less than optimal cytogenetic data during this period. All cytogenetic data that will be presented in this manuscript are from time of diagnosis and not from time of relapse.
2.3 |. Hematopoietic cell transplantation (HCT)
Among the 9 studies, HCT was part of the treatment regimen in 5 studies (E3483, E3489, PC486, E4995, E1900) and patients were classified according to the type of transplant received. In most cases, HCT was performed as part of protocol treatment. However, if a patient’s record had a definitive comment indicating that the patient received HCT off-protocol, i.e., not part of the prescribed therapy, this information was applied. All other patients were classified as “no transplant”.
2.4 |. Statistical analysis
OS was defined as time from first relapse to death from any cause. Kaplan-Meier estimates were used to estimate the event-time distributions for OS. Multivariate model stratified on protocol were used to examine whether the following factors are prognostic for OS from relapse: age, gender, cytogenetic risk, ECOG performance status, WBC count, platelets, hemoglobin, marrow blasts, peripheral blood blasts, duration of CR1 and whether patients received transplant while in CR1. If > 10% of patients had a missing value for a particular covariate, an indicator for whether or not the covariate was missing was indicated in the multivariate mode. All P values were based on 2-sided tests.
3 |. RESULTS
A total of 3012 patients were enrolled in the 9 studies. Among those 3012 patients enrolled, 1779 (59.1%) achieved CR1 and of these, 58.9% (1048 patients) relapsed. The median follow-up on the relapse patients was 9.7 years. The median age at diagnosis of the relapsed patients was 50 (range: 16–84) years and 51% were males. The median duration of CR1 was 7.2 months. The characteristics at disease diagnosis of all relapsed patients and of patients younger vs. older than 55 years are summarized in Table 2. Fifty six percent of the patients had cytogenetic results; 11.8% had favorable cytogenetics, 52.1%—intermediate, 28.6%—unfavorable and 7.5% had indeterminate baseline results. 195 patients (18.6% of all relapsed patients) had undergone either an autologous (140 patients) or an allogeneic HCT (55 patients) while in CR1.
TABLE 2.
Baseline characteristics of the 1048 relapsed patients
| Continues variables | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age <55 | Age ≥ 55 | Total | ||||||||||
| N | Median | Min | Max | N | Median | Min | Max | N | Median | Min | Max | |
| Age | 638 | 39.0 | 16.0 | 54.0 | 408 | 63.0 | 55.0 | 84.0 | 1046 | 50.0 | 16.0 | 84.0 |
| Peripheral WBC count (×109/L) | 640 | 17.4 | 0.4 | 396.0 | 408 | 5.4 | 0.5 | 270.0 | 1048 | 11.7 | 0.4 | 396.0 |
| Peripheral Blasts (%) | 615 | 39.0 | 0.0 | 99.0 | 386 | 15.0 | 0.0 | 99.0 | 1001 | 30.0 | 0.0 | 99.0 |
| Hemoglobin (g/dL) | 633 | 9.1 | 0.2 | 15.4 | 405 | 9.1 | 4.0 | 15.1 | 1038 | 9.1 | 15.4 | |
| Peripheral platelet count (×109/L) | 638 | 51.5 | 1.0 | 999.0 | 407 | 56.0 | 5.0 | 512.0 | 1045 | 55.0 | 999.0 | |
| Marrow Blasts (%) | 572 | 70.0 | 1.0 | 100.0 | 387 | 62.0 | 3.0 | 100.0 | 959 | 67.0 | 1.0 | 100.0 |
| Duration of CR (months) | 640 | 7.8 | 0.2 | 131.8 | 408 | 7.1 | 0.3 | 102.7 | 1048 | 7.5 | 0.2 | 131.8 |
| Categorial variables | ||||||
|---|---|---|---|---|---|---|
| Qualitative variables | Age <55 | Age ≥ 55 | Total | |||
| N | % | N | % | N | % | |
| Gender | ||||||
| Male | 319 | 49.8 | 214 | 52.5 | 533 | 50.9 |
| Female | 319 | 49.8 | 194 | 47.5 | 513 | 49.0 |
| Missing | 2 | 0.3 | 2 | 0.2 | ||
| Performance status | ||||||
| 0 | 260 | 40.6 | 148 | 36.3 | 408 | 38.9 |
| 1 | 306 | 47.8 | 207 | 50.7 | 513 | 49.0 |
| 2 | 58 | 9.1 | 44 | 10.8 | 102 | 9.7 |
| 3 | 11 | 1.7 | 6 | 1.5 | 17 | 1.6 |
| 4 | 2 | 0.3 | 2 | 0.2 | ||
| Missing | 3 | 0.5 | 3 | 0.7 | 6 | 0.6 |
| Cytogenetics | ||||||
| Favorable | 58 | 9.1 | 11 | 2.7 | 69 | 6.6 |
| Intermediate | 183 | 28.6 | 123 | 30.1 | 306 | 29.2 |
| Unfavorable | 93 | 14.5 | 75 | 18.4 | 168 | 16.0 |
| Indeterminate | 23 | 3.6 | 21 | 5.1 | 44 | 4.2 |
| Unknown/Unacceptable | 283 | 44.2 | 178 | 43.6 | 461 | 44.0 |
| On study Transplantation after CR1 | ||||||
| No transplant | 467 | 73.0 | 386 | 94.6 | 853 | 81.4 |
| Allogeneic-HCT | 54 | 8.4 | 1 | 0.2 | 55 | 5.2 |
| Autologous-HCT | 119 | 18.6 | 21 | 5.1 | 140 | 13.4 |
The median OS from relapse was 0.5 years. The 2- and 5-year OS were 16(±1)% and 10(±1)%, respectively. These results were similar even for the most recent studies (E1900 and E3999) as the median OS rates were 0.6 and 0.4 years, respectively and the 5-year OS was 12 (±2) and 5 (±2)%, respectively (Table 3).
TABLE 3.
OS from relapse by study
| Study | Total N | N of CR | N of relapse | Median OS (years) | 2-year OSa ± SE | 5-year OSa ± SE |
|---|---|---|---|---|---|---|
| Studies enrolling patients with age ≤ 55 or 60 years old | ||||||
| E3483 | 475 | 295 | 187 | 0.5 | 12 ± 2 | 6 ± 2 |
| PC486 | 101 | 72 | 38 | 0.4 | 21 ± 7 | 5 ± 4 |
| E3489 | 763 | 508 | 281 | 0.6 | 18 ± 2 | 14 ± 2 |
| E4995 | 65 | 46 | 16 | 0.3 | 6 ± 6 | 6 ± 6 |
| E3997 | 35 | 20 | 14 | 0.9 | 36 ± 13 | 29 ± 12 |
| E1900 | 652 | 425 | 221 | 0.6 | 22 ± 3 | 12 ± 2 |
| Studies enrolling patients with age > 55 or 60 years old | ||||||
| E1490 | 117 | 65 | 44 | 0.3 | 7 ± 4 | 7 ± 4 |
| E3993 | 356 | 149 | 123 | 0.4 | 13 ± 3 | 6 ± 2 |
| E3999 | 448 | 199 | 124 | 0.4 | 10 ± 3 | 5 ± 2 |
| All patients | ||||||
| All patients | 3012 | 1779 | 1048 | 0.5 | 16 ± 1 | 10 ± 1 |
Abbreviations: CR, complete response; HCT, hematopoietic cell transplantation; N, number; WBC, white blood cell
OS was defined as time from first disease relapse to death from any cause.
CR, complete response; N, number; OS, overall survival; SE, standard error.
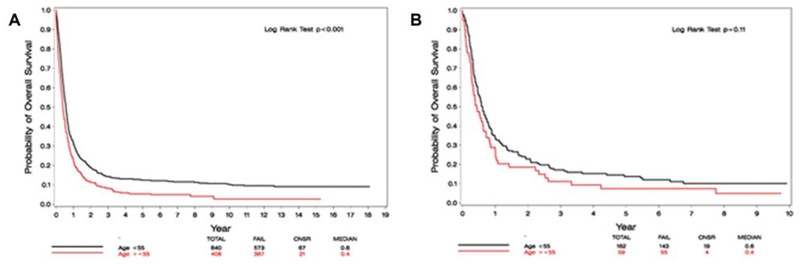
By age stratification (< or ≥ 55 year), OS after relapse was significantly better among patients <55 years in the entire cohort (5-year OS was 13(±1)% versus 5(±1)%, respectively. HR: 0.72; 95% CI [0.63, 0.82]; P < .001); this difference was not significant in the most recent study—E1900 (HR: 0.78; 95% CI [0.57, 1.06], P = .11) (Figure 1). Separately analyzing studies enrolling patients younger than 55 or 60 years (E3483, E3489, PC486, E4995, E3997, E1900) compared with those enrolling older patients (E3993, E1490, E3999) did not reveal any significant difference between the studies throughout the years (P = .07 and P = .59, respectively, Figure 2).
FIGURE 1.
(A) OS from relapse by age—the entire cohort. (B) OS from relapse by age—the most recent study (E1900)
FIGURE 2.
(A). OS from relapse of studies enrolling patients with age ≤ 55 or 60 years (continues variables). (B). OS from relapse of studies enrolling patients with age > 55 or 60 years (categorial variables)
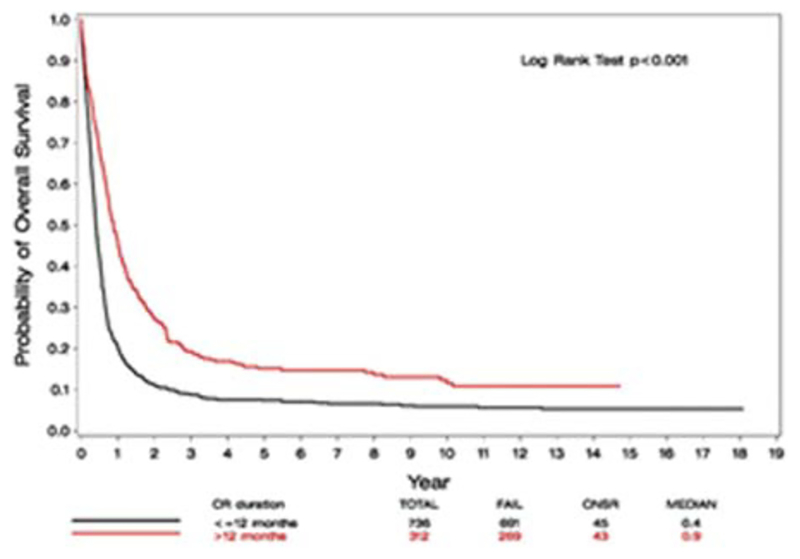
Among the 1048 relapsed patients, 587 patients had cytogenetic risk group data available. Among relapsed patients <55 years, those with unfavorable cytogenetics had the poorest prognosis (median OS of 0.4 years and 5-year OS of 7(±3)%). Those with favorable and intermediate cytogenetics had similar OS with 0.7 and 0.6 years median OS and 5-year OS of 16(±5)% and 17(±3)%, respectively. Among patients >55 years, the median OS was 1.4, 0.4 and 0.3 years and the 2-year OS was 38 (±1), 12 (±3), and 9 (±3), according to cytogenetic risk group (favorable, intermediate and high risk, respectively). Despite the modest differences between groups, cytogenetics were found, by univariate analysis, to be significantly associated with OS, in both age groups of < and ≥55 (P = .005 and P = .001, respectively). Regarding the duration of CR1, a cut-off of 12 months was found to be associated with OS. The median OS from relapse of patients with CR1 duration ≤12 months was 0.4 years, compared to 0.9 years of those with CR1 > 12 months (HR: 0.58; 95% CI [0.50, 0.67]; P < .001, Figure 3). Undergoing allo or auto HCT after achieving CR1 was not found, by univariate analysis, to be associated with survival, compared to not undergoing HCT (allo vs. no transplant HR: 1.17; 95% CI [0.88, 1.55]; P = .27; auto vs. no transplant HR: 0.90; 95% CI [0.74, 1.08]; P = .25).
FIGURE 3.
OS from relapse, by duration of CR1 ≤/>12 months
In a multivariate model including all patients (Supporting Information Table S1), younger age (HR: 1.018; 95% CI [1.011, 1.026]; P < .001), favorable/intermediate cytogenetics at baseline (favorable vs. unfavorable (HR: 0.661; 95% CI [0.477, 0.915]; P = .013); intermediate vs. unfavorable (HR: 0.742; 95% CI [0.596, 0.925]; P = .008)), duration of CR1 above 12 months (HR: 0.557; 95% CI [0.473, 0.655]; P < .001) and not receiving allo-HCT after CR1 (HR: 1.484; 95% CI [1.075, 2.048]; P = .016) were significantly associated with better OS from relapse. White blood cell (WBC) count, hemoglobin, platelet count, percentage of marrow or peripheral blood blasts and undergoing auto-HCT after CR1 were not associated significantly with OS.
Gene mutation data were only available for part of the patients in E1900. Out of 652 patients enrolled on this study, 221 patients achieved CR and then relapsed. Among those with gene mutation data available, 32.8% had DNMT3A mutation, 25.1% had FLT-ITD mutation and 27% had NPM1 mutation. A multivariate analysis which included these 3 gene mutations (FLT3-ITD, DNMT3A, and NPM1) revealed that only FLT3-ITD had statistically significant impact on prognosis.
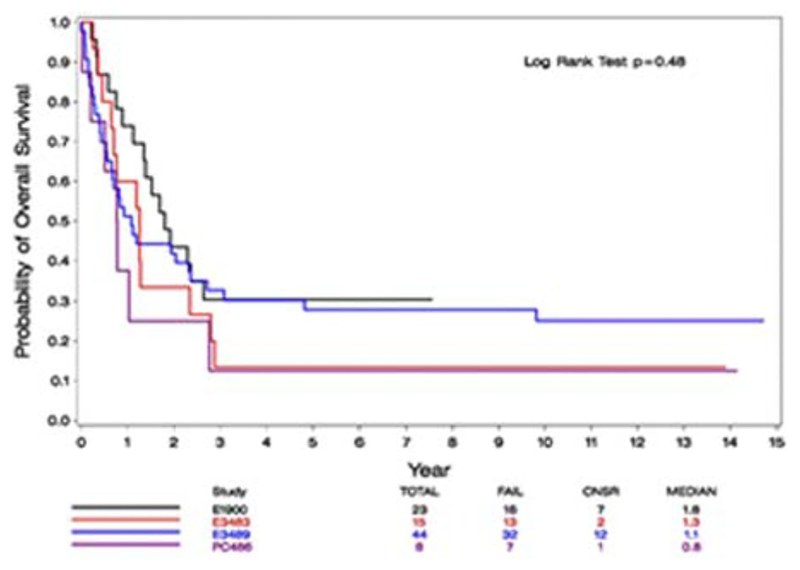
Finally, we focused on patients who relapsed with better prognostic factors; age younger than 40 and CR1 duration of more than 12 months. A comparison of OS of these patients among the different studies during the years revealed no statistically significant difference (P = .48, Figure 4).
FIGURE 4.
OS from relapse of patients with age < 40 and CR1 > 12 months, by study
4 |. DISCUSSION
The first goal of this study was to examine whether in more recent studies there is an improvement in the survival of AML patients whose disease relapses after achieving a first remission. Our analysis demonstrates that there has been no change in the poor prognosis of relapsed AML patients over the last 25 years. Allogeneic HCT is the only curative option for relapsed AML patients, but only 40%−55% of the patients achieve a second CR and only about 50%−70% of those who achieve a second CR are candidates for transplant (approximately 20%−30% of relapsed patients). Despite improvement in the technique of allogeneic HCT and in supportive care, the mortality associated with this procedure may reach 20%−30% and about the same proportion of patients who survive the transplant will eventually relapse.14 Thus, the 5-year OS of relapsed AML patients remains approximately 10%, depending on several prognostic factors. Based on this cohort of more than 3000 patients, age, cytogenetics at baseline, duration of CR1 (≤ vs. > 12 months) and undergoing allo-HCT were significantly associated with OS from relapse. The importance of duration of CR1 previously has been reported in relatively small studies.3,15–18 Duration of CR1 also was included as one of 4 prognostic factors in a prognostic index that was built based on trials of the Dutch-Belgian Hemato-Oncology Cooperative Group and the Swiss Group for Clinical Cancer Research Collaborative Group (HOVON-SAKK) trials.1 Similarly, age and cytogenetics at diagnosis were found to correlate with OS, both in that prognostic index and in our cohort. These confirmatory data, although previously reported, may be important when considering the very long term follow-up of the patients in this study. It is interesting that although age >55 years was found to be a poor prognostic factor for postrelapse OS in the entire cohort of the 9 studies considered, this did not apply to the most recent study, E1900. E1900 enrolled patients up to age 60 years and there was no difference in outcome between patients ≤ or > 55 years old. Nevertheless, age as a continuous factor was found to be associated with postrelapse OS. Debate in the literature continues regarding the impact of undergoing transplant in CR1 on OS postrelapse. According to the current study, the HOVON-SAKK Prognostic Index1 as well as the UK Medical Research Council (MRC)2 and the German-Austrian AML Study Group (AMLSG)19 data, patients who underwent transplant in CR1 have a worse prognosis post relapse compared to those who did not. In contrast, data of University of Washington,3 demonstrate no impact of transplantation in CR1 on OS among relapsed patients. A possible limitation of our study regarding the impact of transplantation on postrelapse OS is that transplantation was part of the protocol in 5 studies but transplants done off-protocol were only incompletely recorded. The impact of cytogenetics at diagnosis on patients postrelapse has been previously reported.18,20
While we realize the limitations of this study, such as the absence of data regarding treatment at relapse, achievement of CR2 as well as disease free survival from CR2, and the incompleteness of cytogenetic data, it is the most striking finding of this analysis that the prognosis of relapsed AML has not improved over the last 25 years.
It should be emphasized that the lack of improvement in the survival of relapsed patients is true even in patients with relatively better prognostic factors; the OS of patients younger than age 40 with CR1 duration of more than 1 year was not improved with recent studies, despite the introduction of some therapeutic modifications and targeted agents (e.g., higher anthracycline doses and gemtuzumab ozogamicin). Long-term survival was observed in less than 10% of patients. These were patients who survived the relapse, achieved a second CR and then successfully underwent an allogeneic transplant. These data are crucial when considering postremission strategies, and suggest that offering a therapy most likely to lead to cure in CR1 is the preferred option. Nowadays, new strategies are being incorporated into AML treatment, as FLT3 inhibitors, IDH inhibitors and venetoclax. These agents were not available during the time period of the study and may change the future results of relapsed AML patients.
In conclusion, this study of a very large cohort of ECOG-ACRIN patients, appears to confirm that, unfortunately, the survival of AML patients postrelapse continues to be dismal and has not improved during the past quarter of a century. In addition, the current study demonstrates the prognostic impact of age, duration of CR1, cytogenetics at diagnosis and undergoing allo-HCT at first CR on the long term OS postrelapse.
Supplementary Material
ACKNOWLEDGMENT
We thank Martin M. Oken, MD, and Peter A. Cassileth, MD (deceased), for their significant contributions to this manuscript.
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA180795, CA180791, CA189859, CA180790, CA180853, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
CONFLICT OF INTEREST
No conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- [1].Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–1978. [DOI] [PubMed] [Google Scholar]
- [2].Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013;31(10):1293–1301. [DOI] [PubMed] [Google Scholar]
- [3].Araki D, Othus M, Walter RB, et al. Effect of allogeneic hematopoietic cell transplantation in first complete remission on post-relapse complete remission rate and survival in acute myeloid leukemia. Haematologica. 2015;100(7):e254–e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cassileth PA, Lynch E, Hines JD, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood. 1992;79(8):1924–1930. [PubMed] [Google Scholar]
- [5].Cassileth PA, Andersen J, Lazarus HM, et al. Autologous bone marrow transplant in acute myeloid leukemia in first remission. J Clin Oncol. 1993;11(2):314–319. [DOI] [PubMed] [Google Scholar]
- [6].Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649–1656. [DOI] [PubMed] [Google Scholar]
- [7].Rowe JM, Andersen JW, Mazza JJ, et al. A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (> 55 to 70 years of age) with acute myelogenous leukemia: a study of the Eastern Cooperative Oncology Group (E1490). Blood. 1995;86(2):457–462. [PubMed] [Google Scholar]
- [8].Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood. 2004;103(2):479–485. [DOI] [PubMed] [Google Scholar]
- [9].Cripe LD, Rader K, Tallman MS, et al. Phase II trial of subcutaneous recombinant human interleukin 11 with subcutaneous recombinant human granulocyte-macrophage colony stimulating factor in patients with acute myeloid leukemia (AML) receiving high-dose cytarabine during induction: ECOG 3997. Leuk Res. 2006;30(7):823–827. [DOI] [PubMed] [Google Scholar]
- [10].Cripe LD, Uno H, Paietta EM, et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood. 2010;116(20):4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cassileth PA, Lee SJ, Litzow MR, et al. Intensified induction chemotherapy in adult acute myeloid leukemia followed by high-dose chemotherapy and autologous peripheral blood stem cell transplantation: an Eastern Cooperative Oncology Group trial (E4995). Leuk Lymphoma. 2005;46(1):55–61. [DOI] [PubMed] [Google Scholar]
- [13].Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- [14].Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121(7):1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kantarjian HM, Keating MJ, Walters RS, McCredie KB, Freireich EJ. The characteristics and outcome of patients with late relapse acute myelogenous leukemia. J Clin Oncol. 1988;6(2):232–238. [DOI] [PubMed] [Google Scholar]
- [16].Keating MJ, Kantarjian H, Smith TL, et al. Response to salvage therapy and survival after relapse in acute myelogenous leukemia. J Clin Oncol. 1989;7(8):1071–1080. [DOI] [PubMed] [Google Scholar]
- [17].Uhlman DL, Bloomfield CD, Hurd DD, Peterson BA. Prognostic factors at relapse for adults with acute myeloid leukemia. Am J Hematol. 1990;33(2):110–116. [DOI] [PubMed] [Google Scholar]
- [18].Thalhammer F, Geissler K, Jager U, et al. Duration of second complete remission in patients with acute myeloid leukemia treated with chemotherapy: a retrospective single-center study. Ann Hematol. 1996;72(4):216–222. [DOI] [PubMed] [Google Scholar]
- [19].Schlenk RF, Frech P, Weber D, et al. Impact of pretreatment characteristics and salvage strategy on outcome in patients with relapsed acute myeloid leukemia. Leukemia. 2017;31(5):1217–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kern W, Schoch C, Haferlach T, et al. Multivariate analysis of prognostic factors in patients with refractory and relapsed acute myeloid leukemia undergoing sequential high-dose cytosine arabinoside and mitoxantrone (S-HAM) salvage therapy: relevance of cytogenetic abnormalities. Leukemia. 2000;14(2):226–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.