Abstract
In the present study, language performance on standardized assessments (e.g., overall verbal performance, receptive and expressive vocabulary) and spontaneous language produced in play was compared between preschool-aged boys with autism spectrum disorder (nASD, n = 25) and boys with fragile X syndrome (FXS, n = 16). At the group-level, we observed weaknesses in the language skills of boys with nASD relative to those with FXS (e.g., when considering raw score performance, standard score performance relative to nonverbal cognitive skills, frequency of talk in play), after controlling for nonverbal IQ and ASD symptom severity. Moreover, although individually most children in both groups demonstrated language delays relative to CA-expectations, language delays relative to nonverbal level-expectations were more common in boys with nASD.
Keywords: Autism Spectrum Disorder, Fragile X Syndrome, Language/Communication
INTRODUCTION
Current estimates indicate that autism spectrum disorder (ASD) affects 1 in 59 children in the United States, making it one of the most commonly occurring neurodevelopmental disorders (Baio et al., 2018). Importantly, ASD is characterized by considerable heterogeneity at every level of description, likely reflecting a complex interplay of both biological and environmental risk factors (Pelphrey, Shultz, Hudac, & Vander Wyk, 2011; Uljarević, Baranek, Vivanti, Hedley, Hundry, & Lane, 2017). Although ASD arises predominantly from unknown causes (Baio, 2012), recent work has led to the identification of multiple etiological and pathophysiological substrates underling this disorder, with an estimated 10 – 20% of individuals with ASD having an identified genetic etiology (e.g., Betancur, 2011; Abrams & Geschwind, 2008; Jeste & Geschwind, 2014). In terms of phenotypic presentation, there is tremendous variation among individuals with ASD in the areas of adaptive functioning skills, cognitive and linguistic levels, and presence/severity of psychiatric comorbidities (e.g., Jones & Klin, 2009).
Moreover, considerable heterogeneity is observed across individuals with ASD in the characteristics considered core to the ASD phenotype. Consider, for instance, the fact that only a proportion of the behaviors implicated in the Diagnostic and Statistical Manual of Mental Disorders – 5th edition (DSM-5; APA, 2013) are required to meet ASD criteria in the restricted, repetitive behavior domain (APA, 2013). As another example, “abnormalities in eye contact,” as well as “a total lack of facial expressions and nonverbal communication,” are direct examples from the DSM-5 indicating the range of ways in which a person can present with a “deficit in nonverbal communicative behaviors used for social interaction (APA, 2013).” Research focused on characterizing this phenotypic variation associated with the ASD phenotype is vital to elucidate how best to maximize a child’s potential for cognitive and functional success.
In the present study, we explored the similarities and differences in the language skills of boys with ASD, for whom a specific genetic etiology has not been identified (herein referred to as nonsyndromic ASD (nASD), and boys with fragile X syndrome (FXS), the most common monogenetic cause of ASD, during the preschool years. Because development reflects complex interactions across multiple factors, direct comparisons between the phenotypes of neurodevelopmental disorders with similar behavioral features will provide insight into common and different mechanisms shaping children’s development. Thus, direct comparisons of the nASD and FXS phenotypes can be used to clarify the nature of language difficulties observed in individuals with these disorders and provide insight into the vulnerabilities associated with language development more generally. Additionally, these types of comparisons will contribute critical information for intervention planning for both groups of children and will provide additional insight into the similarities and differences between the nASD and FXS phenotypes more generally.
Language Difficulties Associated with nASD
Using the diagnostic criteria presented in the DSM-5, individuals diagnosed with nASD demonstrate two fundamental features: (1) clinically significant and persistent impairments in social communication and social interaction skills and (2) a tendency to engage in restricted, repetitive patterns of behavior, interests, or activities (APA, 2013). One of the major changes in the DSM-5 (APA, 2013), relative to its predecessor the DSM-IV-TR (APA, 2000), involved removing “language and communication” difficulties as a primary, independent domain and instead, integrating social interaction difficulties and communication difficulties into a single, conjoined area of difficulty. Indeed, it is true that language difficulties are closely intertwined with social difficulties; however, this does not mean these skills reflect the same construct or negate the importance of investigations of language development in children with nASD. Although there are some individuals with nASD who demonstrate structural language skills (e.g., semantics, syntax) that are consistent with their chronological age (CA) expectations, most individuals with nASD have difficulty acquiring language (e.g., Boucher, 2012; Kjelgaard & Tager-Flusberg, 2001; Klinger et al., 2002; Weismer, Lord, & Esler, 2010). Moreover, the nature of language difficulties in children with nASD extend beyond a difficulty in using language for social purposes.
It is currently estimated that at least half of children with nASD who acquire spoken language demonstrate difficulties relative to CA- and /or nonverbal cognitive level expectations in structural language skills such as vocabulary and grammar during the school-age years or later (e.g., Boucher, 2012; Kjelgaard & Tager-Flusberg, 2001; Klinger et al., 2002). Furthermore, it is currently estimated that around 30% of children with nASD still demonstrate limited spoken language skills by the time they reach the school-age years (e.g., Tager-Flusberg & Kasari, 2013). Indeed, even though there is considerable variation in the development of language among children with nASD, language delays are often the first recognizable symptom of developmental difficulties for children with nASD (e.g., Boucher, 2012; Tager-Flusberg et al., 2009; Talbott et al., 2015). To this regard, results from retrospective studies have identified weaknesses in language and gesture use as early as 12 months of age (e.g., Colgan et al., 2006; Werner et al., 2000). For children with nASD who acquire functional speech, first words emerge around 24 months of age or later (versus 12 months for TD children) and phrases emerge around 48 months of age or later (versus 18 – 23 months for TD children; Mitchell et al., 2006; Rose et al., 2016; Tager-Flusberg, Paul, & Lord, 2005; Weismer & Kover, 2015). Finally, prospective studies of infants at high risk for nASD (i.e., infants who have an older sibling with an nASD diagnosis) have documented language delays apparent within the first year of life for infant siblings who are later diagnosed with nASD themselves (e.g., Mitchell et al., 2006; Ozonoff et al., 2010; Paul et al., 2011; Rozga et al., 2011).
There is a paucity of work considering structural language performance in nASD relative to children with other neurodevelopmental disorders or developmental delays. Multiple investigators have reported a subgroup of children with nASD as having language difficulties resembling those observed for children with specific language impairment (SLI). This has led some researchers to posit shared underpinnings between these two disorders (e.g., Tager-Flusberg & Joseph, 2003; Tomblin, 2011); however, this hypothesis remains controversial, with other researchers noting linguistic differences across the two conditions and arguing that the similarities observed are superficial (e.g., Taylor et al., 2014; Williams et al., 2008). In addition, results from early research considering the language skills of children with nASD relative to children with other developmental delays suggest more severe language difficulties in nASD relative to other populations (e.g., Lord, Pickles, DiLavore, & Shulman, 1996; Sigman & Ruskin, 1999; Weismer, Lord, & Esler, 2010). For example, Weismer, Lord, & Esler (2010), considered the early language patterns of toddlers with nASD (n = 257) to toddlers with developmental delay (n = 69). Although considerable variability was observed, toddlers with nASD were found to demonstrate significant delays in language performance based on CA-level expectations. Furthermore, with respect to nonverbal cognitive level, language in toddlers with nASD was significantly delayed. In contrast, the toddlers with DD demonstrated language skills that were roughly commensurate with their nonverbal cognitive levels.
Despite the frequent occurrence of language difficulties, much remains to be understood regarding the development of structural language skills in children with nASD. This is likely, in part, because the significant heterogeneity in the language skills amongst individuals with nASD increases the complexity of understanding language skills both clinically and theoretically. Disentangling this complexity in language development should be a key focus for current research. Structural language skills facilitate achievements in other developmental domains, such as the abilities to socialize or to develop cognitively (Homer & Tamis-LeMonda, 2005; Vygotsky, 1986; Waxman & Markow, 1995), and are central to a wide range of positive long-term outcomes (e.g., independent living, maintaining employment, establishing and sustaining interpersonal relationships; Clegg, Hollis, Mawhood, & Rutter, 2005; Hartley et al., 2011). Thus, understanding language skills in individuals with nASD not only can elucidate the nature of language development of children, but also can inform treatment planning, which over time can improve adult outcomes. In this study, we use a direct comparison of early language skills in boys with nASD and boys with FXS during the preschool period to help clarify the nature of language difficulties associated with nASD.
The FXS-ASD comorbidity
FXS is a neurodevelopmental disorder resulting from a trinucleotide (CGG) expansion in the FMR1 gene, which is located on the X chromosome (Oostra & Willemson, 2003). FXS is both the most common single-gene cause of ASD and the leading inherited cause of intellectual disability. The trinucleotide expansion observed in FXS leads to the significant reduction or complete absence of the protein, FMRP (fragile X mental retardation protein), which is known to be involved in experience dependent learning and neural plasticity (Bassell & Warren, 2008). The behavioral features associated with the FXS phenotype are more pronounced in males, because of the protective presence of an unaffected X chromosome, which continues to produce FMRP, and the process of X inactivation in females (Loesch et al., 2004; Tassone et al., 1999; Ligsay & Hagerman, 2016; Stembalska et al., 2016). Most males with FXS present with intellectual disability (Hessl et al., 2009). Not surprisingly, language difficulties are also common in males with FXS. As is observed for most children with nASD, early expressive language is delayed relative to CA-expectations in males with FXS (e.g., Kover, McCary, Ingram, Hatton, & Roberts, 2015; Roberts, Mirrett, & Burchinal, 2001), and estimates suggest that up to 30% of individuals with FXS still demonstrate limited spoken language skills into adolescents (e.g., Levy, Gottesman, Borochowitz, Frydman, & Sagi, 2006). Language delays are also observed relative to achievements in nonverbal cognition, although data suggests that this varies as a function of language domain and the developmental period considered (see Abbeduto et al., 2017 for review).
Research to date has pointed to several behavioral similarities between the nASD and FXS phenotypes. To begin, nearly all males with FXS display behavioral features that are akin to those typically associated with the nASD phenotype. Examples of these behavioral features include perseverative and noncontingent speech (e.g., Belser & Sudhalter, 2001; Martin, Roberts, Helm-Estabrooks, Sideris, Vanderbilt, & Moskowitz, 2012; Sudhalter & Belser, 2001; Murphy& Abbeduto; 2007); restricted and repetitive behaviors, such as hand/finger and other complex mannerisms (e.g., Oakes et al., 2016); and unusual eye contact (e.g., Cohen et al., 1988; Watson et al., 2008). The occurrence of these symptoms is frequent and severe enough to warrant a comorbid diagnosis of ASD in as many as 60% of males with FXS, when utilizing gold standard diagnostic instruments (e.g., Budimirovic and Kaufmann, 2011; Clifford et al., 2007; Harris et al., 2008; Kaufmann et al., 2004, 2008, 2017; Klusek, Martin, & Losh, 2014; McDuffie et al., 2010). There has been considerable variability, however, in these estimates across studies (Demark, Feldman, & Holden). Other behavioral similarities between the FXS and nASD phenotypes include increased risk of challenging behaviors (Hall, Barnett, & Hustyi, 2016; Chandler et al., 2016), hyperactivity/inattention (e.g., Baumgardner et al., 1995; Thurman et al., 2014), and anxiety (e.g., Cordiero et al., 2011; de Bruin, Ferdinand, Meester, Nijs, & Verheij, 2007; Gevik, Eldevik, Fjæran-Granum, & Sponheim, 2011; Leyfer et al., 2006).
Given these similarities, it seems to follow that neurobiological similarities may also be observed across these phenotypes. To this regard, recent studies (Issofov et al., 2012) have noted that a significant number of the ASD susceptibility genes identified to date are controlled by FMRP, such as SH3 and multiple Ankyrin repeat domains (SHANK) and mammalian target of kinase (PAK). Abnormalities in the GABAergic signaling system have also been implicated in both FXS and nASD (Coghlan et al., 2012), which can interfere with synaptic development and plasticity (e.g., Iossifov et al., 2014; Steinberg & Webber, 2013). Moreover, the actions of many of the ASD susceptibility genes identified to date are controlled by FMRP, further reinforcing the links between FXS and nASD.
In conjunction with these commonalities, developmentally important phenotypic differences have been noted between nASD and FXS (Abbeduto et al., 2014). First, at the group level, the severity of ASD observed in FXS is generally milder than is observed in individuals with nASD, even when only considering children with FXS who meet diagnostic criteria for ASD (e.g., McDuffie et al., 2015; Wolff et al., 2012). Additionally, there is also evidence to suggest between-group differences in the behavioral features correlated with ASD symptom severity (Thurman et al., 2015). Thus, there may be notable differences in the paths by which the similarities observed across these two conditions come to develop. To this regard, there is a growing body of literature suggesting differences in the developmental mechanisms underlying behavioral features commonly observed in both phenotypes but not central to a diagnosis of ASD, such as anxiety and language development (Thurman et al., 2014; Thurman et al., 2015).
Comparisons of Language Skills between Nonsyndromic ASD and Fragile X Syndrome
Early delays in communication are apparent in both children with nASD and children with FXS (Abbeduto et al., 2007; De Giacomo & Fombonne, 1998) and continue to be an area of challenge across the lifespan, at least in individuals with co-occurring intellectual disability (Hartley et al., 2011; Howlin et al., 2014). Moreover, both conditions are characterized by difficulties initiating and responding to the social cues of their interactive partners as well as by increased risk for the presence of maladaptive behaviors that have the potential to negatively impact learning (e.g., Abbeduto, McDuffie, & Thurman, 2014; Thurman, McDuffie, Hagerman, & Abbeduto, 2014).
To date, few studies have been done directly comparing structural skills and/or profiles between children with nASD and children with FXS. In the first study, McDuffie et al. (2013) examined fast-mapping (a word learning process in which children rapidly infer a correspondence between a novel label and a speaker’s intended referent) using a paradigm in which the examiner used a variety of attention-directing cues to direct and maintain the child’s attention to the novel object while the examiner was labelling it. The authors found that boys with FXS demonstrated better performance than did boys with nASD, despite having lower levels of nonverbal cognitive ability, when considering the mean number of correct word learning trials and standardized measures of both receptive and expressive vocabulary ability. That said, the number of boys who successfully learned all the target words did not differ across the samples. Finally, for boys with FXS, but not nASD, performance on the word learning task was significantly associated with concurrent receptive and expressive vocabulary performance as well as nonverbal cognitive ability. In general, results from McDuffie et al., provide some insight into lexical skills in both boys with nASD and boys with FXS. However, it is important to note that the ASD severity scores for the boys with FXS were significantly lower than those for the boys with nASD. Because the authors did not factor this difference into the between-group comparisons conducted; it is not clear whether the differences observed reflect word learning difference or just differences in ASD severity levels.
Thurman et al. (2017), in a partially overlapping sample to those reported by McDuffie et al., more explicitly explored the similarities and differences in the lexical and grammatical skill of boys with FXS (n = 51) and boys with nASD (n = 36) between 4 and 10 years of age who presented with nonverbal IQ scores less than or equal to 85. Results from this study identified important differences between the two conditions in terms of both the language profile associated with each condition and the factors associated language performance. More specifically, Thurman et al. observed vocabulary skills to be a strength, relative to nonverbal cognitive level performance, in boys with FXS. For boys with nASD, vocabulary performance was consistent with nonverbal cognitive performance levels. This pattern of performance remained even after restricting the FXS sample to only those participants who also met criteria for a comorbid diagnosis of ASD. Further analyses done revealed a lexical weakness, when using raw-scores (due to floor effects), for boys with nASD, relative to boys with FXS after controlling for the effects of CA, nonverbal IQ, and severity of ASD symptomatology. Moreover, investigators were able to account for a smaller proportion of the variance in lexical performance for boys with nASD than for the boys with FXS. Finally, differences were observed across the two groups in terms of the predictors of language performance. For boys with nASD nonverbal cognition was found to be the strongest predictor of lexical performance; in contrast, CA was the strongest predictor of lexical performance for boys with FXS. In addition, severity of ASD symptomatology was found to be a significant predictor of lexical performance for boys with FXS but not boy with nASD. It remains unclear however, if this difference is observable in younger children. Because research suggests that language acquisition prior to the age of 5 years is associated with better outcomes for individuals with nASD (e.g., Venter, Lord, and Schopler, 1992), this is an important next step to take.
Recently, Sterling (2018) conducted a comparison of grammar skills between boys with nASD (mean CA = 13.40, SD = 2.00) and boys with FXS with a comorbid diagnosis of ASD (FXS+ASD; mean CA = 12.12 SD = 2.17) who were matched on MLU (ASD: mean = 4.60; FXS+ASD: mean = 3.78; p = .272; d = .37; variance ratio = 0.58). Sterling found that boys with nASD performed significantly better than did boys with FXS+ASD on the norm-referenced assessment of the use of the auxiliary verb “to be.” In addition, consideration of effect sizes demonstrated better performance by the participants with nASD relative to the boys with FXS+ASD on past tense probes on an assessment of sentence imitation skills and on a standardized assessment considering the use of the auxiliary verb “to do.” Importantly, although the two groups were matched on mean length of utterance, nonverbal cognitive skills were significantly stronger for boys with nASD (M = 71.22, SD = 19.88) than for the boys with FXS+ASD (M = 48.89, SD = 8.09). Thus, this data also suggests, broadly, that individuals with FXS may have an advantage in language learning relative to those with nASD relative to their nonverbal cognitive skill level; that is, language abilities seem to fall behind level of nonverbal cognitive ability for those with nASD more so than for those with FXS.
Present Study
In the present study, we sought to expand our understanding of language performance in nASD by considering the similarities and differences in the language skills of boys with nASD and of boys with FXS during the preschool period, a period during which many children with developmental delays are transitioning across the early stages of spoken language development. More specifically, we used multiple analytic approaches to consider whether previous findings of a language weakness in nASD relative to boys with FXS was observable during the preschool years. In addition, we considered children’s language performance on standardized tests that provide an omnibus measure of language ability and measures of both receptive and expressive vocabulary specifically. Moreover, we consider children’s spontaneous use of single-word and multi-word utterances in a less-structured, naturalistic play interaction with a caregiver.
To begin, we conducted preliminary analyses to determine whether prior findings by Thurman et al. (2017) indicating a weakness, at the group-level, in boy with nASD relative to boys with FXS in raw score language performance after controlling for the effects of CA, nonverbal IQ and ASD symptom severity could be replicated during the preschool years. We then considered the following primary aims:
To determine whether boys with nASD demonstrated a weakness, at the group-level, relative to boys with FXS in standard score language performance after controlling for the effects of CA, nonverbal IQ and ASD symptom severity. We hypothesized that boys with nASD would indeed demonstrate a weakness in language performance relative to boys with FXS.
To determine whether boys with nASD demonstrate a weakness, at the group-level in language performance relative to boys with FXS when considering the amount of difference between standard score performance between the nonverbal cognitive and verbal domains. We hypothesized that boys with nASD would indeed demonstrate a weakness in language performance relative to boys with FXS.
To evaluate whether a weakness in language performance, relative to nonverbal cognitive performance or CA, was, at the individual-level, more commonly observed in boys with nASD than in boys with FXS. We hypothesized that a greater proportion of boys with nASD than boys with FXS would be characterized as having weakness in the language than boys with FXS.
To determine whether the correlates of language performance (i.e., CA, NVIQ, and severity of ASD symptomatology in the social affective and restricted and repetitive behavior domains) are similar between boys with nASD and boys with FXS. We hypothesized that there may be differences in the developmental factors predicting language abilities between these two conditions.
METHOD
Participants
Participants were 16 males with FXS and 25 males with nASD between the ages of 3.50 and 5.50 years of age who were drawn from a longitudinal study examining early language abilities in preschoolers with nASD or FXS. Participants were recruited nationally through the help of the [removed for blinding]. Due to the higher prevalence rate, participants with nASD were more likely to reside locally than were those with FXS. Participants were also recruited using parent listservs, social media sites, advertisements by the National Fragile X Foundation, and through clinics and preschools specialized in working with children with neurodevelopmental disorders. The following inclusion criteria, based on parent report, were utilized in the larger project: (a) male aged 36 – 66 months at Time 1, (b) children for whom English is the primary language of exposure; (e) no sensory or physical impairments that would limit participation in project activities; and (f) must have no medical conditions that precludes participation (e.g., severe and frequent seizures) that prevent them from meeting the demands of the testing protocol. In addition, participants reported by parents to be of above average intellectual ability (parent report or prior documentation of IQ greater than 110 or greater) were not included in the present project as nearly all males with FXS demonstrate cognitive skills in the low average to intellectual disability range. Approval from the Institutional Review Board, as well as parental informed consent, was obtained.
Documentation of a diagnosis of FMR1 full mutation (i.e., >200 CGG repeats, with or without mosaicism) was required and obtained for all participants with FXS. For participants with nASD, families provided documentation of an existing community diagnosis of ASD. This diagnosis was confirmed through administration of the Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord, Rutter, DiLavore, Risi, Gotham, & Bishop, 2012). Project staff who administered the ADOS had completed research reliability training.
Descriptive statistics for the samples are presented in Table 1. Participants with FXS and participants with nASD differed significantly in terms of nonverbal IQ (U = 94.00, z = 2.83, p = .004, r = .46, s2ratio = 1.41). Analyses indicated that the two groups did not differ significantly on CA (U = 183.00, z = −0.45, p = .65, r = .05, s2ratio = 1.09) or on overall ASD symptom severity (U = 132.50, z = 1.27, p = .21, r = .24, s2ratio = 0.61). Direct assessment of ASD symptom severity was available for 14 of the 16 males with FXS; these results indicated that all but 2 of those assessed earned an ASD severity score in the ASD classification range. For participants with FXS, the racial/ethnic composition of the sample was 25% Hispanic, with 6% Black/African American, 67% Caucasian, and 25% Multi-racial. For the participants with nASD, the racial/ethnic composition of the sample was 32% Hispanic, with 4% Asian, 8% Black/African-American, 64% Caucasian, 20% Multi-racial, and 4% preferring not to answer. Data regarding highest education reported in each household are presented in Table 2.
Table 1.
Descriptive statistics (Mean, Standard Deviation, and Range) for participant groups
| Fragile X Syndrome (n = 16) | Nonsyndromic ASD (n = 25) | |
|---|---|---|
| Chronological Age | 4.53 (0.17,3.82–5.50) | 4.60 (0.14,3.50–5.56) |
| Nonverbal IQ1 | 56.63 (17.29,30–91) | 76.12 (20.55,30–113) |
| Autism Symptom Severity2 | 5.86 (2.21, 2–9) 3 | 6.84 (1.72,4–10) |
Differential Ability Scales –2nd Edition, Special Nonverbal Composite,
Autism Diagnostic Observation Schedule −2 Comparison Score,
ADOS-2 data missing for 2 participants with FXS.
Table 2.
Descriptive statistics regarding highest caregiver education level as a function of diagnostic group.
| Fragile X Syndrome (n = 16) | Nonsyndromic ASD (n = 25) | |
|---|---|---|
| Prefer Not to Answer | 12.5% | 20% |
| Graduated high school/GED | 0% | 4% |
| Some college/technical school | 18.8% | 8% |
| Graduated – Associates/technical college degree | 12.5% | 20% |
| Graduated – B.A./B.S. | 0% | 20% |
| Some graduate work completed | 6.3% | 8% |
| Graduated – M.A/M.S. or higher graduate degree | 50% | 20% |
Measures
Differential Ability Scales – II Upper Level Early Years (DAS-II; Elliott, 2007a, b).
The DAS-II Upper Level Early Years provides an assessment of general intellectual functioning for children aged 3 ½ - 8 years and was designed to provide specific information about an individual’s strengths and weaknesses across a wide range of intellectual activities. This battery has six core subtests measures verbal skills (i.e., verbal comprehension and naming vocabulary), nonverbal reasoning skills (i.e., picture similarities and matrices), and nonverbal spatial skills (i.e., pattern construction and copying). The DAS-II yields standard scores (SSs) with a general-population mean of 100, and SD of 15. The DAS-II also yields T-scores for each subtest, with a general-population mean of 50 and SD of 10. The Special Nonverbal Composite, which reflects nonverbal cognition using both nonverbal reasoning and nonverbal spatial subtests was used to assess overall nonverbal IQ. Performance on the verbal subtests as well as the overall Verbal Cluster standard score was also considered in analyses.
Peabody Picture Vocabulary Test – 4th edition (PPVT-4; Dunn & Dunn, 2007).
The PPVT-4 is an individually administered measure of receptive vocabulary for children and adults aged 2 ½ to 90 years and older. The general population mean for the SS is 100, with a SD of 15 and a floor of 20. Administration of the Version A and Version B were alternated across participants in each group; thus, approximately half of the participants in each group received Version A and half of the participants received Version B of this measure.
Expressive Vocabulary Test – 2nd edition (EVT-2; Williams, 2007).
The EVT-2 is an individually administered measure of expressive vocabulary for children and adults aged 2 ½ to 90 years and older. The mean for the general population SS is 100, with a SD of 15 and a floor of 20 beginning at age 4 years 4 months. The EVT-2 was co-normed with the PPVT-4 to provide a thorough evaluation of both receptive and expressive vocabulary attainment. Administration of the Version A and Version B were alternated across participants in each group; thus, approximately half of the participants in each group received Version A and half of the participants received Version B of this measure.
Play Session - Language Indicator Score (modification of Expressive Communication Indicator reported by Luze et al., 2001).
Children participated in a 20-minute semi-structured play session with a caregiver (Communication Play Protocol; Adamson et al., 2009). These samples were used as the context for coding child communication acts based on the coding procedures outlined by the Expressive Communication Indicator (ECI; Carta et al., 2010). In this coding system, four key communicative elements are considered representing prelinguistic language domain (gestures, vocalizations) and spoken language domain (single word and multiple word utterances). This method has been shown to be a reliable (reported 90% interobserver agreement) metric for typically developing children from birth to three years of age (Luze et al., 2001). Moreover, this method of assessing communication skills has been found to be significantly associated with both direct and parent report measures of child communicative ability, demonstrates sensitivity to change over time, and has been shown to be a useful method of assessing communication in children with developmental disabilities (e.g., Greenwood, Carta, Walker, Hughes, & Weathers, 2006; Greenwood, Walker, Buzhardt, 2010; Luze et al., 2001; Yoder et al., 2009).
Of primary interest for this report were the two key communicative elements representing the spoken language domain (i.e., occurrence of single word and multiple word utterances). Trained observers coded the Play Sessions using the Behavioral Observation Research Interactive Software (Friard & Gamba, 2016). Coders segmented all participant talk into C-units, which is preferred when assessing spoken language skills as it provides a more objective criteria, relative to utterances, and avoids overestimating language ability(Abbeduto et al., 2014). C-units are defined as an independent clause and any of its modifiers, which can include dependent clauses (Abbeduto et al., 2014). The weighted frequency of single word (1 point) and multiword (2 points) utterances within the play sessions were computed to generate an overall Language Indicator Score, indicative of overall spoken language skills. Intraclass Correlation Coefficient (ICC) estimates and their 95% confidence intervals were computed, for 12% of the sample, based on a mean-rating (k=2), absolute-agreement, 2-way mixed effects model. Results indicate excellent reliability for the language indicator score (ICC = .99; 95% confidence interval, .97 – 1.00), which is the metric considered in analyses.
Autism Diagnostic Observation Schedule-2.
The ADOS-2 (Lord, Rutter, DiLavore, & Risi, 2007) is a semi-structured play-based interaction in which a trained examiner creates specific interactive contexts to observe reciprocal social interaction skills as well as the presence of repetitive behaviors. One of four ADOS modules is administered based upon the participant’s expressive language level. In the current project, participants received modules 1 or 2. The Comparison Score, an indicator of overall ASD severity level, as well as the domain severity scores (Hus, Gotham, & Lord, 2014) were utilized in analyses. Within the present study, 28 Module 1s (10 FXS, 17 nASD) and 12 Module 2s (4 FXS, 8 nASD) were administered. Examiners who had achieved standard research reliability training standards on the ADOS-2 administered ADOS-2 sessions in the present project. Ten percent of the administrations were randomly selected to assess reliability. Mean agreement for the algorithm items was 94.30%.
Analysis
Preliminary analyses were conducted to determine if prior findings reported by Thurman et al. (2015) indicating a strength in raw score language performance by the participants with FXS relative to their peers with nASD was observable during the preschool years, after controlling for the effects of CA, nonverbal IQ, and ASD symptom severity. Floor effects prevented the authors from using preferred standard score metrics. To consider the aims of the present study, multiple analytic approaches were then used in the present project to consider the similarities and differences in standard score language performance between preschool-aged boys with nASD and boys with FXS, with analyses focusing at both the group-level and the individual level.
At the group level, we used two approaches to examine between group differences in standard score language performance and language performance when playing with a caregiver. We predicted that males with FXS would demonstrate a verbal advantage relative to males with nASD; thus, one-tail analyses were conducted. In the first set of analyses (Aim 1), we conducted a series of multiple regression analyses to evaluate whether boys with FXS demonstrated an advantage relative to boys with nASD after controlling for the effects of CA, nonverbal IQ, and overall ASD symptom severity. In the second set of analyses (Aim 2), between-group comparisons were conducted utilizing differences scores representing the Nonverbal IQ standard score performance (DAS-II SNC SS) minus standard score performance on each standardized language measure (i.e., DAS-II Verbal SS, PPVT-4 SS, EVT-2 SS).
At the individual level, we sought to compare the proportion of children in each diagnostic group classified as demonstrating a verbal weakness relative to both CA and to nonverbal cognitive level (Aim 3). To achieve this goal, we computed a difference score representing the differences between children’s DAS-II Nonverbal Reasoning standard score and DAS-II Verbal standard score. These values were then compared to the DAS-II norming tables, which specify the magnitude of a between-cluster standard score difference required for statistical significance. Using this information, we identified the children for whom their SS performance on the Verbal domain was significantly weaker than their standard score performance on the Nonverbal Reasoning domain. To consider the delays relative to CA-expectations, the proportion of children in each diagnostic group who earned a SS ≤ 75 on the DAS-II, PPVT-4, and EVT-2 were considered.
Finally, we considered the correlates of within-syndrome variation in language performance for children in both diagnostic groups (Aim 4). For both diagnostic groups, we used Pearson correlation coefficients to consider the concurrent relations between language performance and CA, NVIQ, and ASD symptomatology (i.e., severity of both social affective symptomatology and restricted and repetitive behaviors).
RESULTS
Descriptive statistics for performance on the standardized language measures and for the Play Session - Language Indicator Score were computed and are presented in Table 3.
Table 3.
Descriptive statistics (Mean, Standard Deviation, and Range) for language measures as a function participant group
| Fragile X Syndrome (n = 16) | Nonsyndromic ASD (n = 25) | |
|---|---|---|
| DAS-II Verbal SS | 63.13 (20.84,30–92) | 69.92 (20.52,30–100) |
| PPVT-4 SS1 | 67.56 (23.66, 25 – 101) | 73.00 (22.97, 20 – 105) |
| EVT-2 SS2 | 62.93 (24.19,20–92) | 73.32 (25.17,20–110) |
| ECI-LI | 170.44 (152.95,0–540) | 156.12 (111.78,0–362) |
PPVT-4 data missing for 1 participant with ASD,
EVT-2 data missing for 1 participant with FXS
Preliminary Analyses
Preliminary analyses were conducted to determine if, in our younger sample of boys with nASD or FXS, we could replicate prior findings reported by Thurman et al. (2015) indicating a strength in raw score-level language performance by the participants with FXS relative to their peers with nASD. We considered whether diagnostic group was a significant predictor of raw score level performance on multiple standardized measures of language ability. All regression models were significant with diagnostic group emerging as a significant unique predictor for DAS-II Verbal Comprehension ability score (p = .02, η2partial = .11), DAS-II Naming Vocabulary ability score (p = .03, η2partial = .10), PPVT-4 growth score (p = .02, η2partial = .13), and EVT-2 growth score (p = .014, η2partial = .09). In all models, scores for boys with FXS were significantly higher than scores for boys with nASD after controlling for CA, nonverbal IQ, and ASD symptom severity (regression model data not presented here but available from the authors).
Diagnostic Group as a Predictor of Language Performance
First, we conducted a series of multiple regression analyses to evaluate whether preschool-aged boys with nASD demonstrated a disadvantage relative to boys with FXS in standard score language performance after controlling for the effects of CA, nonverbal IQ, and overall ASD symptom severity (Table 4). The regression models considering performance on the DAS-II Verbal Comprehension subtest, F(4, 38) = 12.07, p < .001, with an R2adjusted value of .54, and DAS-II Naming Vocabulary subtests, F(4, 38) = 10.65, p < .001, with an R2adjusted value of .50, were significant. Results indicated that boys with FXS earned T-scores that were approximately 5 points higher than boys with nASD, for both Verbal Comprehension (p = .07, one-tailed) and Naming Vocabulary (p = .09, one-tailed). These numbers suggest that diagnostic group accounted for approximately 5 – 6% of the variance in language performance independently, which was not enough to meet criterion for a significant difference when CA, nonverbal IQ, and ASD symptom severity were held constant. In this, and all other analyses, the same pattern of findings were observed when regression models included only the males with FXS who earned ASD severity scores in the clinical range.
Table 4.
Linear regression analyses evaluating between-group differences in language performance
| B (unstandardized) | SEB | β | p value | η2 partial | |
|---|---|---|---|---|---|
| DAS-II Verbal Comprehension T score | |||||
| Chronological Age | −0.27 | 1.94 | −0.15 | 0.89 | .001 |
| Nonverbal IQ | 0.43 | 0.08 | 0.79 | <.001* | .479* |
| Autism Symptom Severity | −0.58 | 0.79 | −0.09 | 0.47 | .015 |
| Diagnostic Group | 4.97 | 3.35 | 0.20 | 0.07 | .061 |
| DAS-II Naming Vocabulary T score | |||||
| Chronological Age | −1.58 | 2.24 | −0.08 | 0.49 | .014 |
| Nonverbal IQ | .50 | 0.09 | 0.83 | <0.001* | .489* |
| Autism Symptom Severity | 0.70 | 0.91 | 0.10 | 0.45 | .017 |
| Diagnostic Group | 5.28 | 3.86 | 0.20 | 0.09 | .052 |
| PPVT-4 Standard Score | |||||
| Chronological Age | 0.49 | 3.85 | 0.01 | 0.90 | .000 |
| Nonverbal IQ | 0.92 | 0.16 | 0.85 | <0.001* | .508* |
| Autism Symptom Severity | −0.45 | 1.57 | −0.37 | 0.77 | .002 |
| Diagnostic Group | 12.29 | 6.81 | 0.26 | 0.04* | .090* |
| EVT-2 Standard Score | |||||
| Chronological Age | 1.11 | 3.90 | 0.03 | 0.78 | .002 |
| Nonverbal IQ | 1.01 | 0.15 | 0.87 | <0.001 | .561* |
| Autism Symptom Severity | 0.13 | 1.59 | 0.01 | 0.94 | .000 |
| Diagnostic Group | 9.88 | 6.72 | 0.19 | 0.08 | .060 |
| Play Session - Language Indicator Score | |||||
| Chronological Age | 45.69 | 22.83 | .24 | .05 | .105 |
| Nonverbal IQ | 4.66 | 0.90 | .78 | <0.001* | .441* |
| Autism Symptom Severity | −1.44 | 9.30 | −.022 | .88 | .001 |
| Diagnostic Group | 113.59 | 39.34 | .427 | .004* | .197* |
p < .05
The regression model assessing receptive vocabulary performance as measured by standard scores from the PPVT-4 was also significant (Table 4; F(4, 37) = 12.72, p < .001, R2adjusted = .56). In this model, diagnostic group was a significant predictor even when CA, nonverbal IQ, and ASD symptom severity were included in the model (Table 4). Diagnostic group accounted for 9% of the variance in PPVT-4 standard scores uniquely, with boys with FXS having scores approximately 12 points higher than boys with nASD (p = .04), when CA, nonverbal IQ and ASD symptom severity were held constant. Finally, on the EVT-2 (Table 4), the combination of variables considered in the present project did significant predict standard score performance, F(4, 38) = 14.84, p < .001, with an R2adjusted value of .59. We observed that the boys with FXS demonstrating an approximate 9-point standard score advantage, with diagnostic group accounting for 6% of the variance in EVT-2 standard scores uniquely, however this was not enough to meet criterion for a significant difference, when CA, nonverbal IQ, and ASD symptom severity were held constant (p = .07, one-tailed).
In addition, we considered whether preschool-aged boys with FXS demonstrated an advantage relative to boys with nASD on Play Session-Language Indicator score after controlling for the effects of CA, nonverbal IQ, and overall ASD symptom severity (Table 4). Results demonstrated that the model was indeed significant (Table 4; F(4, 38) = 9.31, p < .001, R2adjusted = .47). Moreover, diagnostic group accounted for approximately 20 % of the variance in Play Session-Language Indicator scores uniquely, with boys with FXS demonstrating scores approximately 100 points higher than boys with nASD (p = .004, one-tailed) when CA, nonverbal IQ, and ASD symptom severity were held constant. Follow-up analyses were conducted to unpack this finding by considering between-group differences in the production of both single word and multiple word utterances. Results of these analyses demonstrate the same pattern of performance with diagnostic group accounted for 15.84% of the variance in number of single word utterances (unstandardized B = 37.93, SEB = 14.98, β = .41, η2partial =.214, p = .008, one-tailed) and 13.47% of the variance in the number of multiple word utterances produced (unstandardized B = 37.83, SEB = 16.46, β = .37, η2partial =.214, p = .01, one-tailed), when the influences of CA, Nonverbal IQ, and ASD severity were held constant.
Across all models, Nonverbal IQ was consistently observed to be the strongest independent predictor of language performance, accounting for 40 – 50% of the variance in models. CA was observed to be an independent predictor of the Play Session-Language Indicator score and the total number of utterances produced in the play session. ASD severity was not observed to be a significant unique predictor of language performance on any of the models considered.
Diagnostic Group as a Predictor of Nonverbal-Verbal Performance Difference Scores
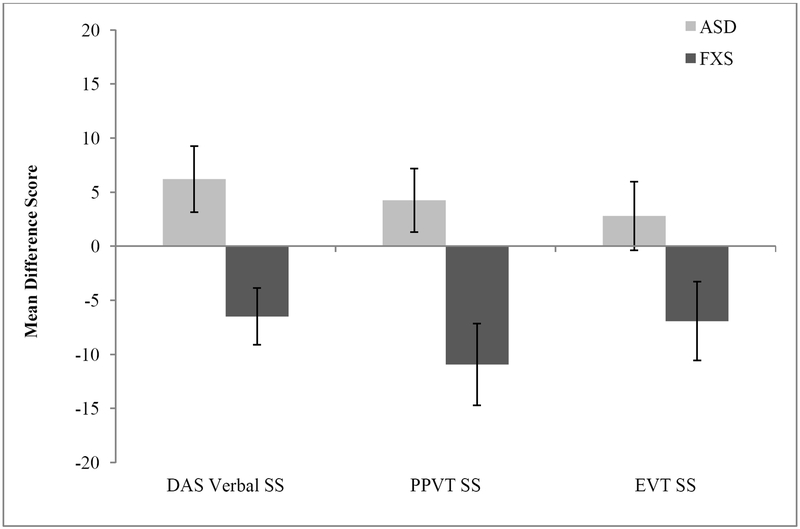
Between-group comparisons were then conducted utilizing scores representing the differences between overall Nonverbal IQ standard score performance and standard score performance on each standardized language measure (i.e., DAS-II Nonverbal IQ minus DAS-II Verbal SS, DAS-II Nonverbal IQ minus PPVT-4 SS, DAS-II Nonverbal IQ minus EVT-2 SS). For the DAS-II Verbal SS (U = 90.50, z = −2.93, p = .002, one-tailed), the PPVT-4 SS (U = 89.00, z = −2.85, p = .002, one-tailed), and EVT-2 SS (U = 120.00, z = −1.89, p = .03, one-tailed) boys with FXS were observed to demonstrate better language skills relative to overall nonverbal IQ than boys with nASD. More specifically, as seen in Figure 1, boys with FXS demonstrated a mean Nonverbal IQ SS score that was 6.5 points lower than DAS-II Verbal SS, 10.94 points lower than PPVT-4 SSs, and 6.9 points lower than EVT-2 SSs on average. In contrast, boys with nASD demonstrated a mean Nonverbal IQ SS that was 6.2 points higher than their mean DAS-II Verbal SS, 4.25 points higher than mean PPVT-4 SSs, and 2.8 points higher than mean EVT-2 SSs on average.
Figure 1.
Differences scores reflecting Nonverbal SS Performance (DAS SNC) minus Verbal SS Performance (DAS Verbal SS, PPVT SS, and EVT SS) as a function of diagnostic group.
Follow-up analyses were conducted, in the form of a series of multiple regression analyses, to evaluate whether to determine if these between group differences remained after controlling for the effects of overall ASD symptom severity. Models for differences scores utilizing both the DAS-II Verbal SS, F(2,38) = .426, p = .02, r2adj = .15, and the PPVT-4 SS, F(2,37) = 3.98, p = .03, r2adj = .14, were significant. Moreover, in both of these models, diagnostic group was a significant predictor even when ASD symptom severity was included in the model. More specifically, for the DAS-II Verbal SS, diagnostic group accounted for 19% of the variance in the difference scores, with boys with FXS a DAS-II Verbal SS advantage of 13.84 points relative to Nonverbal IQ SS performance (represented by a score of −13.84 when computing “Nonverbal IQ SS performance minus DAS-II Verbal SS performance”). For the PPVT-4 SS, diagnostic group accounted for 17% of the variance in the difference scores, with boys with FXS a DAS-II Verbal SS advantage of 14.47 points relative to Nonverbal IQ SS performance (represented by a score of −14.47 when computing “Nonverbal IQ SS performance minus DAS-II Verbal SS performance”). Finally, for the EVT-2 SS, the overall model did not meet criterion for a significant prediction of language performance, F(2,38) = 1.65, p = .21, r2adj = .03.
Individual Level Performance as a Function of Diagnostic Group
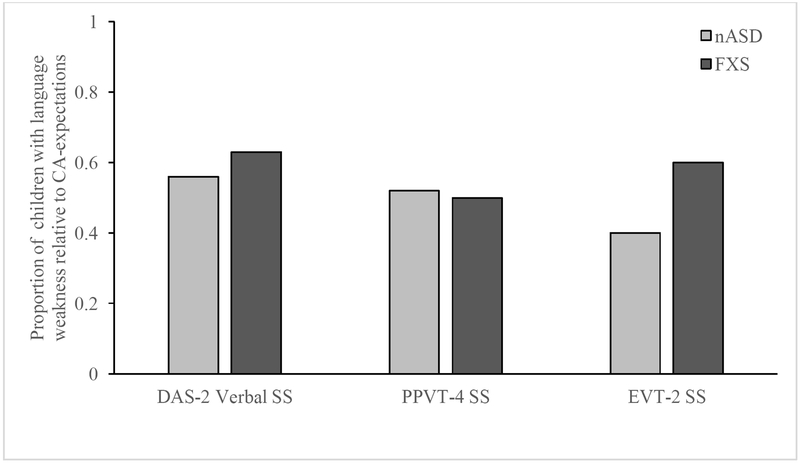
Language performance for both boys with nASD and boys with FXS and were also considered at the individual level. First, we considered the proportion of participants in each diagnostic who demonstrated a delay relative to CA-expectations, defined as a standard score ≤ 75. As seen in Figure 2, the proportions varied slightly as a function of assessment, but generally was centered around 50% of the sample for both diagnostic groups.
Figure 2.
Proportion of children, as a function of diagnostic group, for whom language scores were 2 or more standard deviations (70 +/−5) below norming sample mean (100).
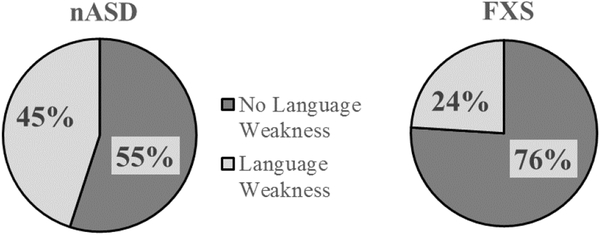
Finally, using the guidelines provided in the DAS-II norming tables, which allows for the classification of a significant difference between standard score performance on the cluster domains (Verbal, Nonverbal Reasoning, and Spatial). As seen in Figure 3, when comparing the percent of participants classified as having a verbal weakness, a larger proportion of boys with nASD were classified as demonstrating a verbal weakness, indicated by the SS profile in which Verbal SS performance was significantly lower than Nonverbal Reasoning SS performance, a larger proportion of boys with nASD were classified as having a verbal weakness. Follow-up analyses were conducted for the boys with nASD to consider whether those classified as having or not having a language weakness differed from one another on other developmental measures. The two groups did not differ on CA, nonverbal reasoning, or ASD severity (see Table 5).
Figure 3.
DAS-II profile performance demonstrating percent of sample, as a function of diagnostic group, for whom DAS-II Verbal SS performance was statistically weaker than DAS-II Nonverbal Reasoning SS.
Table 5.
Descriptive statistics for boys with nASD as a function of verbal weakness classification.
| nASD − No Verbal Weakness (M, SD) | nASD + Verbal Weakness (M, SD, Range) | |
|---|---|---|
| CA | 4.71 (0.67) | 4.43 (0.74) |
| Nonverbal Reasoning | 75.40 (22.61) | 77.20 (18.11) |
| ASD Severity | 7.13 (1.77) | 6.40 (1.65) |
Correlates of Within-Syndrome Variation in Language Abilities
Finally, we considered the concurrent correlations between language performance and other child characteristics; specifically, CA, NVIQ, and ASD symptomatology. For both boys with nASD and boys with FXS, NVIQ was significantly correlated with language performance across all measures (see Table 6). In addition, for boys with FXS significant correlations were observed between ASD symptomatology in the form of severity of restricted and repetitive behaviors, and DAS-II Verbal Comprehension ability scores, PPVT-4 raw scores, and EVT-2 raw scores. These associations were not significant for boys with nASD; in fact, for DAS-II Verbal Comprehension ability scores (z = −2.63, p = .004) and EVT-2 growth scores (z = −2.32, p = .01), a significant difference in correlation strength was observed between the two diagnostic groups. Finally, the correlations between CA and ASD symptomatology in the form of social affective symptoms did not reach criterion for a significant association with language performance in either diagnostic group (see Table 6).
Table 6.
Pearson correlation coefficients considering correlates of language performance as a function of diagnostic group
| CA | NVIQ | SA severity | RRB Severity | |
|---|---|---|---|---|
| nASD | ||||
| DAS-II Verbal Comprehension raw score | .02 | .68** | −.20 | −.01 |
| DAS II Naming Vocabulary raw score | .09 | 71** | −.22 | −.10 |
| PPVT-4 growth score | .17 | .76** | −.21 | −.28 |
| EVT-2 growth score | .23 | 74** | −.06 | −.07 |
| Play Session - Language Indicator score | .16 | .68** | −.18 | −.28 |
| FXS | ||||
| DAS-II Verbal Comprehension raw score | .32 | 72** | −.41 | . 73** |
| DAS II Naming Vocabulary raw score | .08 | 75** | −.25 | −.37 |
| PPVT-4 growth score | .18 | 72** | −.40 | 57** |
| EVT-2 growth score | .39 | .69** | −.41 | −.75** |
| Play Session - Language Indicator score | .14 | 72** | −.29 | −.45 |
DISCUSSION
Although no longer considered a primary, independent symptom domain of ASD, most individuals with nASD have difficulty acquiring language. Difficulties in structural language skills (e.g., semantics, syntax) can negatively impact achievements in other domains (e.g., nonverbal cognition) as well as a wide range of long-term positive outcomes, such as independent living, maintenance of employment, establishment of interpersonal relationships (e.g., Vygotsky, 1986; Waxman & Markow, 1995; Clegg, Hollis, Mawhood, & Rutter, 2005; Hartley et al., 2011). In fact, results of longitudinal studies of adult outcomes of nASD have found the most consistent predictor of adult independence to be structural language skills, particularly the development of “meaningful” spoken language before 5 years of age (Billstedt et al., 2007; Eaves & Ho, 2008; Howlin et al., 2004; Lord & Bailey, 2002). Thus, understanding language skills in individuals with nASD, particularly prior to the age of 5 years, not only can elucidate the nature of language development of children, but also can inform treatment planning, which over time can improve adult outcomes.
The specific aims of the present study, therefore, considered comparisons between the language skills of preschool-aged boys with nASD to preschool-aged boys with FXS. Not only are both conditions associated with difficulties acquiring language, they are also both associated with difficulties navigating reciprocal social interactions and at increased risk for behavioral difficulties likely to influence language learning negatively. Thus, direct comparisons of the language skills between nASD and FXS will inform our understanding of the factors shaping language development in these groups, which can be used to inform treatment planning. Moreover, this line of research will elucidate the similarities and differences between the nASD and FXS phenotypes more generally.
Comparisons of Language Performance between nASD and FXS
Results from the present study demonstrated that, even though structural language difficulties are not considered central to a diagnosis of ASD, preschool-aged boys with nASD demonstrated a weakness in language skills relative to their male peers with FXS. These findings are consistent with prior work considering language skills across these two neurodevelopmental disorders (e.g., Sterling, 2018; Thurman et al., 2017). The prior work done in this area has focused on school-age boys with nASD or FXS, most of whom demonstrating phrase level speech or higher. In contrast, most (70%) of the children in the present study were at an expressive language level consistent with the use of the ADOS-2 Module 1, which is designed for children who have speech abilities ranging from no speech at all up to and including the use of simple, but not consistent, phrases. Thus, the present study was unique in comparing language skills between boys with nASD and boys with FXS in the preschool years, a period of considerable variation in spoken language skills/level.
Preliminary analyses considering DAS-II Verbal Comprehension ability scores, DAS-II Naming Vocabulary ability scores, PPVT-4 growth scores, and EVT-2 growth scores demonstrated a verbal advantage for boys with FXS relative to boys with nASD across all metrics considered after controlling for between-group differences in CA, nonverbal IQ, and ASD symptom severity. Across these models, we found that diagnostic group accounted for 9 – 13% of unique variance in scores for these measures.
Results from primary analyses demonstrate significant differences in PPVT-4 SS performance between the two groups, favoring the FXS sample, and nonsignificant trends for the same pattern of findings on all other standard score measures. It is important to note that with our small sample sizes, we would expect statistical power to be sufficient to detect only large effects; thus, our findings indicating nonsignificant trends would be more likely to reach criterion for a statistical difference in studies with larger samples. Nonetheless, overall, standard scores ranged from 5 – 10 points lower for boys with nASD than boys with FXS; diagnostic group accounted for 9% of unique variance in PPVT-4 SSs and 6% of unique variance in SSs for all other measures considered. Collectively, we see that during the preschool period, significant differences in raw score performance on standardized measures of language are observed between boys with nASD and boys with FXS, with these between-group raw score differences likely to contribute to small differences in standard score performance.
Moreover, when considering language performance measured during a play session with the caregiver, weighted scores for boys with nASD were 100 points lower than those for boys with FXS, which would be equivalent to producing 100 fewer single-word utterances or 50 fewer multiple-word utterances during the 20-minute sample. With this difference, diagnostic group accounted for approximately 20% of unique variance in language scores collected in the play session. Again, this was found after controlling for the effects of CA, nonverbal IQ, and ASD symptom severity.
Results did show that the standard score profile of language performance relative to nonverbal cognitive skills significantly differed between boys with nASD and boys with FXS. On all three language measures considered, boys with nASD, as a group, demonstrated verbal performance standard scores that lagged nonverbal standard score performance. In contrast, boys with FXS demonstrated the opposite profile, with nonverbal standard score performance lagging verbal standard score performance. Across all analyses the same pattern of findings were observed when the FXS sample was restricted to only boys with ASD severity scores in the clinical range.
We also found that, when considering language performance individually for both groups, most children demonstrated CA-level delays on the DAS-II (nASD: 56% versus FXS: 62.5%), PPVT-4 (nASD: 52% versus 50%), and EVT-2 (nASD: 40% versus 60%). However, our data suggests that boys with nASD are at greater risk than are boys with FXS of having a language weakness relative to their nonverbal cognitive ability, with 45% of our boys with nASD, versus 24% of our boys with FXS, earning a verbal standard score that was significantly lower than their Nonverbal Reasoning standard score on the DAS-II.
Correlates of Language Performance
Finally, we considered the correlates of language performance for both boys with nASD and boys with FXS. It should be acknowledged that the correlations are rather preliminary, given that with the small sample size we would expect statistical power to be sufficient to detect only large effects (see Cohen, 1988 or Looney 2018). That said, our data suggest that there are likely both similarities and differences across the two groups. For both boys with nASD and boys with FXS, despite the between group differences in overall nonverbal cognitive ability, and despite differences the language performance relative to nonverbal cognitive ability, language performance was strongly associated with advancements in nonverbal cognition. In contrast, our data suggests differences in the relation between severity of repetitive behaviors and some areas of language performance between the two groups. In our sample of boys with FXS, but not in boys with nASD, severity of restricted and repetitive behaviors was strongly and negatively associated with multiple measures of language performance. For boys with nASD, no significant associations were observed between the language measures and severity of restricted and repetitive behaviors. Moreover, we found that the magnitude of the association between repetitive behaviors and language performance when considering raw score performance on the DAS-2 Verbal Comprehension subtest and growth scores on the EVT-2 significantly differed.
Although preliminary, this finding is consistent with prior reports of a between-group difference in the relation between language performance and restricted and repetitive behaviors (Thurman et al., 2015). Importantly, there is literature supporting a negative relationship between repetitive behaviors and language skills in young children with nASD (e.g., Paul, Chawarska, Cicchetti, Volkmar, 2008; Ray-Subramian & Weismer, 2012). Ray-Subramian & Weismer (2012), found that, in toddler with nASD, in addition to a significant relation between language and restricted and repetitive behaviors, gains in language skills predicted decreases in RRBs. The differences observed across projects may be due to the present study’s use of the ADOS-2 RRB severity scores, which was specifically designed to limit the influence of developmental characteristics on ASD severity scores (Hus et al., 2014). Thus, it may be that the attempt to limit the influence of developmental characteristics on ADOS-2 severity scores was not generalizable to participants with FXS and/or that the negative association between language skills and severity restricted and repetitive behaviors is much stronger in FXS than it is in nASD.
Clinical Implications
In combination with findings from previous work (e.g., Thurman et al., 2017; Sterling, 2018; Weismer, Lord, & Esler, 2010; Rose, Trembath, Keen, & Paynter, 2016), our findings suggest that children with nASD are at greater risk for delays in language skills relative to their nonverbal cognitive abilities than children with FXS. Moreover, our findings indicate that most preschoolers with nASD demonstrate significant delays in languages skills relative to their CA and/or nonverbal cognitive level and, therefore, will require speech/language intervention to stimulate development in these domains, in addition to the standard recommendations for intervention targeting social communication skills or pragmatic language. For boys with FXS, even though only 24% of the sample was observed to demonstrate a language delay relative to their nonverbal cognitive level, most boys were found to demonstrate delays relative to CA-expectations. Research done by Roberts, Mirrett, & Burchinal (2001) suggested that during early development, for every month in CA, children with FXS gain .39 months in expressive language and .49 months in receptive language. Thus, boys with FXS are also in need of speech/language intervention to stimulate development in these domains.
In addition, our findings are important in demonstrating the need to consider the behavioral similarities and differences observed between the nASD and FXS phenotypes and even across children with the same condition more deeply. Even though neurobiological and behavioral similarities are observed across these two conditions, findings from the present study, and prior work in this area, suggests that there are differences in the factors shaping language development between nASD and FXS. Even though the present study controlled for differences in ASD severity between the two groups, children with nASD may still be disadvantaged relative to those with FXS in terms of reciprocal social interaction skills that are likely to facilitate and support word learning. For example, McDuffie et al., (2015) found that even when matching individuals with nASD or FXS on overall severity of ASD symptomatology, boys with nASD still tended to demonstrate more severely affected scores on all social affective symptoms considered than did boys with FXS. Though not largely discrepant, these differences over time may still negatively influence word learning opportunities for boys with nASD. In addition, our data and previous work in this area suggests that nonverbal cognition may play a key role in language development for boys with nASD. Thus, identifying the specific cognitive skill and mechanisms supporting successful language learning in nASD may be critical for supporting optimal outcomes for this population.
It is not surprising that there is a growing body of research demonstrating differences in the mechanisms supporting development between boys with nASD and boys with FXS; neurodevelopmental disorders represent multi-system disorders being shaped by a variety of attributes across development. Disentangling this complexity, in the relations between biological, genetic, environmental, and developmental factors, has the potential to inform our understanding of the developmental mechanisms underlying both nASD and FXS, as well as development more generally. More specifically, understanding the mechanisms leading to and from language development in boys with nASD and boys with FXS will likely help us identify the factors predicting treatment response across these conditions.
Limitations and Conclusions
There are certain limitations that are worth noting in the present study. First, although we were able include a more inclusive group of children with nASD than in previous studies (NVIQ up to 110), our nASD sample did not represent the full range of cognitive skills for children with nASD. Thus, there is need for continued examination of the full range of cognitive skills for children with nASD relative other neurodevelopmental disorders. Second, the sample size is relatively small, although it is on par with most other studies in the field of research that includes a sample of participants with FXS. Third, we have included only boys in the present study, due to the higher rates of males affected by both neurodevelopmental disorders and the fact that FXS is an X-linked disorder, affecting girls differently than boys. It is important for this work to be extended to consider the same relations and questions in girls with nASD and FXS. Fourth, the present project utilized a cross-sectional approach during a developmental period associated with considerable language growth. Longitudinal examinations of the similarities and differences in language acquisition processes associated with these neurodevelopmental disorders are needed. Finally, the present project considered a small number of predictors likely to influence language development in these populations. Thus, additional predictors warrant consideration, such as factors known to support language acquisition (e.g., joint attention, imitation) and phenotypic features that potentially interfere with learning (e.g., attention, challenging behaviors).
In conclusion, taken together, the data from the present project provide additional support (1) language is an area of weakness for many children with nASD and (2) that there may be differences in the factors influencing language development between nASD and FXS that are likely to influence either treatment response or the active ingredients needed within the intervention approach to support optimal language outcomes in these populations. Language delays can create a developmental cascade that has significant long-term implications across multiple domains and levels. For example, language facilitates cognitive development in that it provides a tool that can be used to organize information, refine/clarify categorical boundaries, and efficiently access and use information from others (e.g., Donald, 1991; Vygotsky, 1986; Waxman & Markow, 1995). In addition, language skills by the age of 5 years have been shown to be a strong predictor of long-term success for individuals with nASD. Research has also demonstrated that early language difficulties also shapes the child’s surrounding environment. For example, as discussed by Iverson & Wozniak (2016), there is a bi-directional developmental cascade that unfolds across time in the interplay between production of early communicative acts (e.g., use of gaze, production of vocalizations, gestures, spoken language) and the responses of the child’s social partners (e.g., caregivers) that supports a child’s growth toward increased communicative complexity. Finally, we know that these early difficulties are also shaping, and in turn being shaped by, the child’s neurobiological development (e.g., Dawson, 2008). It is therefore vital that we continue assess language skills, relative to both nonverbal cognitive performance and CA and recommend speech/language intervention accordingly.
In addition, information from the present project adds to our understanding of the FXSASD association. A notable number of similarities have been observed across the nASD and FXS phenotypes. That said, there is also a growing body of research suggesting important ASD symptom-level differences and differences in the factors associated with ASD severity. To clarify the nature of the relationship between these two conditions and provide insight into the complex developmental mechanisms leading to their phenotypic weaknesses investigations we must expand the scope of our empirical investigations. More specifically, research is need that both considers more broadly the ways in which the nASD and FXS phenotypes are similar and different. More work is needed, however, that considers these phenotypes across a variety of different domains as well as different developmental periods. In the present project, we see that in addition to differences in ASD symptomatology, it appears that the mechanisms underlying language development may also be different between those with nASD and those with FXS. Such between-group comparisons will likely help clarify the factors influencing heterogeneity within and across both conditions. In addition, as we consider development in both nASD and FXS, it is important that we take a deeper look into the mechanisms underlying the similarities and differences across these conditions. For example, given the findings from the present project, it is important to look deeper into the factors shaping language development in both nASD and FXS and understand how these mechanisms compare across these conditions. This deeper understanding is likely to elucidate the links between biomedical and behavioral attributes, thereby supporting the development of intervention methods that can be used to improve the quality of life outcomes in both nASD and in FXS.
Acknowledgements
This research was supported by grants R03 DC014543 from the National Institute of Deafness and Communication Disorders, U54 HD079125 from the National Institute of Child Health and Human Development, UL1 TR000002 from the National Center for Advancing Translational Sciences. We wish to thank the children and their families for their participation in this study. We also thank Cynde Josol, Amy Banasik, Theofilos Mazidzoglou, and Claudine Anglo for assisting with data collection; Karina Gonzalez and Vivian Nguyen for coordinating all study visits; and Danielle Harvey for assisting with data analyses. Angela John Thurman has received financial support to develop and implement outcome measures for clinical trials from Fulcrum Therapeutics. No other authors have financial disclosures to make.
Footnotes
Conflict of Interest: Author A has received financial support to develop outcome measures for clinical trial studies from Fulcrum Therapeutics. Authors B declares no conflict of interest.
References
- Abbeduto L, Brady N, & Kover ST (2007). Language development and fragile X syndrome: Profiles, syndrome-specificity, and within-syndrome differences. Developmental Disabilities Research Reviews, 13), 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, McDuffie A, & Thurman AJ (2014). The fragile X syndrome–autism comorbidity: what do we really know? Frontiers in Genetics, 5, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams BS, & Geschwind DH (2008). Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews Genetics, 9, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson LB, Bakeman R, Deckner DF, & Romski M (2009). Joint engagement and the emergence of language in children with autism and Down syndrome. Journal of Autism and Developmental Disorders, 39, 84– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th edn). Arlington: American Psychiatric Publishing. [Google Scholar]
- American Psychiatric Association, & American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association. [Google Scholar]
- Baio J (2012). Autism and developmental disabilities monitoring network surveillance year 2008 principal investigators; centers for disease control and prevention. prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveillence Summaries, 61, 1–19. [PubMed] [Google Scholar]
- Bassell GJ, & Warren ST (2008). Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron,60, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardner TL, Reiss AL, Freund LS, & Abrams MT (1995). Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics, 95(5), 744–752. [PubMed] [Google Scholar]
- Belser RC, & Sudhalter V (2001). Conversational characteristics of children with fragile X syndrome: Repetitive speech. American Journal on Mental Retardation, 106, 28–38. [DOI] [PubMed] [Google Scholar]
- Betancur C (2011). Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain research, 1380, 42–77. [DOI] [PubMed] [Google Scholar]
- Billstedt E, Carina Gillberg I, & Gillberg C (2007). Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. Journal of Child Psychology and Psychiatry, 48, 1102–1110. [DOI] [PubMed] [Google Scholar]
- Boucher J (2012). Research review: structural language in autistic spectrum disorder–characteristics and causes. Journal of Child Psychology and Psychiatry, 53, 219–233. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, & Kaufmann WE (2011). What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience, 33, 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Howlin P, Simonoff E, O’Sullivan T, Tseng E, Kennedy J, … Baird G (2016). Emotional and behavioural problems in young children with autism spectrum disorder. Developmental Medicine & Child Neurology, 58, 202–208. [DOI] [PubMed] [Google Scholar]
- Clegg J, Hollis C, Mawhood L, & Rutter M (2005). Developmental language disorders–a follow-up in later adult life. Cognitive, language and psychosocial outcomes. Journal of Child Psychology and Psychiatry, 46, 128–149. [DOI] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, & Loesch DZ (2007). Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders, 37, 738–747. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, & Nutt DJ (2012). GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neuroscience & Biobehavioral Reviews, 36, 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioural sciences Hillsdale. NJ: Lawrence Earlbaum Associates. [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, … & Brown WT (1988). Social gaze, social avoidance, and repetitive behavior in fragile X males: a controlled study. American Journal on Mental Retardation, 92, 436–446. [PubMed] [Google Scholar]
- Colgan SE, Lanter E, McComish C, Watson LR, Crais ER, & Baranek GT (2006). Analysis of social interaction gestures in infants with autism. Child Neuropsychology, 12, 307–319. [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders, 3, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G (2008). Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology, 20, 775–803. [DOI] [PubMed] [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, de Nijs PF, & Verheij F (2007). High rates of psychiatric co-morbidity in PDD-NOS. Journal of Autism and Developmental Disorders, 37, 877–886. [DOI] [PubMed] [Google Scholar]
- De Giacomo A, & Fombonne E (1998). Parental recognition of developmental abnormalities in autism. European Child & Adolescent Psychiatry, 7(3), 131–136. [DOI] [PubMed] [Google Scholar]
- Donald M (1991). Origins of the Modern Mind: Three Stages in the Evolution of Culture and Cognition. Cambridge: Harvard University Press. [Google Scholar]
- Dunn LM, & Dunn DM (2007). PPVT-4: Peabody picture vocabulary test. Pearson Assessments. [Google Scholar]
- Eaves LC, & Ho HH (2008). Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders, 38, 739–747. [DOI] [PubMed] [Google Scholar]
- Elliot CD (2007a). Differential Abilities Scale—Second Edition (DAS-II). San Antonio, TX: PsychCorp. [Google Scholar]
- Elliott CD (2007b). Differential Ability Scales – Second Edition: Introductory and Technical Handbook. Minneapolis, MN: PsychCorp. [Google Scholar]
- Friard O, & Gamba M (2016). BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution, 7, 1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Gjevik E, Eldevik S, Fjæran-Granum T, & Sponheim E (2011). Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders, 41, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CR, Carta JJ, Walker D, Hughes K, & Weathers M (2006). Preliminary investigations of the application of the Early Communication Indicator (ECI) for infants and toddlers. Journal of Early Intervention, 28, 178–196. [Google Scholar]
- Greenwood C, Walker D Buzhardt J (2010). The Early Communication Indicator (ECI) for Infants and Toddlers: Growth Norms from Two States. Journal of Early Intervention, 32, 5, 310–334. [Google Scholar]
- Hall SS, Barnett RP, & Hustyi KM (2016). Problem behaviour in adolescent boys with fragile X syndrome: relative prevalence, frequency and severity. Journal of Intellectual Disability Research, 60, 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, … & Hagerman RJ (2008). Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation, 113(6), 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, & Bailey DB Jr (2011). Exploring the adult life of men and women with fragile X syndrome: results from a national survey. American Journal of Intellectual and Developmental Disabilities, 116, 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Nguyen DV, Green C, Chavez A, Tassone F, Hagerman RJ, … & Hall S (2009). A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. Journal of Neurodevelopmental Disorders, 1, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, & Rutter M (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, 45, 212–229. [DOI] [PubMed] [Google Scholar]
- Howlin P, Savage S, Moss P, Tempier A, & Rutter M (2014). Cognitive and language skills in adults with autism: a 40-year follow-up. Journal of Child Psychology and Psychiatry, 55, 49–58. [DOI] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44, 2400–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O’roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, … & Smith JD (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515(7526), 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, & Wilson RK (2012). De novo gene disruptions in children on the autism spectrum. Neuron, 74, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, & Wozniak RH (2016). Transitions to intentional and symbolic communication in typical development and in autism spectrum disorder In Keen D, Meadan H, Brady NC, & Halle JW (Eds) Prelinguistic and minimally verbal communicators on the autism spectrum (pp. 51–72). Springer, Singapore. [Google Scholar]
- Jeste SS, & Geschwind DH (2014). Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nature Reviews Neurology, 10, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A, Jones W, & Klin A (2009). Heterogeneity and homogeneity across the autism spectrum: the role of development. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 471–473. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Capone GT, Clarke M, Budimirovic DB, & Tarsy D (2008). Autism in Genetic Intellectual Disability In A. W. & Zimmerman (Eds.), Autism - Current Theories and Evidence (pp. 81–108). Totowa, NJ: Humana Press. [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, … & Stanard P (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A, 129, 225–234. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, … & Brown WT (2017). Autism spectrum disorder in fragile X syndrome: cooccurring conditions and current treatment. Pediatrics, 139(Supplement 3), S194–S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard MM, & Tager-Flusberg H (2001). An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes, 16, 287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger GF, Dawson G, & Renner P (2002). Autistic Disorder In Mash EJ & Barkley RA (Eds.), Child Psychopathology, Second Edition (pp. 409–454). New York: Guilford Press. [Google Scholar]
- Klusek J, Martin GE, & Losh M (2014). A comparison of pragmatic language in boys with autism and fragile X syndrome. Journal of Speech, Language, and Hearing Research, 57(5), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, McCary LM, Ingram AM, Hatton DD, & Roberts JE (2015). Language development in infants and toddlers with fragile X syndrome: change over time and the role of attention. American Journal on Intellectual and Developmental Disabilities, 120, 125–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Gottesman R, Borochowitz Z, Frydman M, & Sagi M (2006). Language in boys with fragile X syndrome. Journal of Child Language, 33, 125–144. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, … & Lainhart JE (2006). Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36, 849–861. [DOI] [PubMed] [Google Scholar]
- Ligsay A, & Hagerman RJ (2016). Review of targeted treatments in fragile X syndrome. Intractable & Rare Diseases Research, 5, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, & Hagerman RJ (2004). Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews, 10, 31–41. [DOI] [PubMed] [Google Scholar]
- Looney SW (2018). Practical issues in sample size determination for correlation coefficient inference. SM Journal of Biometrics and Biostatistics, 3, 1027. [Google Scholar]
- Lord C, & Bailey A (2002). Autism spectrum disorders In Rutter M, & Taylor E (Eds.), Child and adolescent psychiatry (pp. 664–681). Oxford: Blackwell Scientific. [Google Scholar]
- Lord C, Pickles A, Dilavore PC, & Shulman C (1996, June). Longitudinal studies of young children referred for possible autism. In biannual meetings of the International Society for Research in Child and Adolescent Psychopathology. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–Second edition (ADOS-2). Los Angeles: Western Psychological Services. [Google Scholar]
- Luze GJ, Linebarger DL, Greenwood CR, & Carta JJ (2001). Developing a general outcome measure of growth in the expressive communication of infants and toddlers. School Psychology Review, 30, 383– 406. [Google Scholar]
- Martin GE, Roberts JE, Helm-Estabrooks N, Sideris J, Vanderbilt J, & Moskowitz L (2012). Perseveration in the connected speech of boys with fragile X syndrome with and without autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities, 117, 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, & Brown WT (2010). Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. American Journal on Intellectual and Developmental Disabilities, 115, 307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Kover ST, Hagerman R, & Abbeduto L (2013). Investigating word learning in fragile X syndrome: A fast-mapping study. Journal of Autism and Developmental Disorders, 43, 1676–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, & Abbeduto L (2015). Symptoms of autism in males with fragile X syndrome: A comparison to nonsyndromic ASD using current ADIR scores. Journal of Autism and Developmental Disorders, 45, 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, & Bryson S (2006). Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics, 27, S69–S78 [DOI] [PubMed] [Google Scholar]
- Murphy MM, Abbeduto L, Schroeder S, & Serlin R (2007). Contribution of social and information-processing factors to eye-gaze avoidance in fragile X syndrome. American Journal on Mental Retardation, 112, 349–360. [DOI] [PubMed] [Google Scholar]
- Oakes A, Thurman AJ, McDuffie A, Bullard LM, Hagerman RJ, & Abbeduto L (2016). Characterising repetitive behaviours in young boys with fragile X syndrome. Journal of Intellectual Disability Research, 60, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, & Willemsen R (2003). A fragile balance: FMR1 expression levels. Human Molecular Genetics, 12(suppl_2), R249–R257. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … & Steinfeld MB (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 256–266. [PMC free article] [PubMed] [Google Scholar]
- Paul R, Chawarska K, Cicchetti D, & Volkmar F (2008). Language outcomes of toddlers with autism spectrum disorders: A two year follow-up. Autism Research, 1, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, & Klin A (2011). Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry, 52, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, & Vander Wyk BC (2011). Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 52, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray-Subramanian CE, & Weismer SE (2012). Receptive and expressive language as predictors of restricted and repetitive behaviors in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 2113–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, & Burchinal M (2001). Receptive and expressive communication development of young males with fragile X syndrome. American Journal on Mental Retardation, 106, 216–230. [DOI] [PubMed] [Google Scholar]
- Rose V, Trembath D, Keen D, & Paynter J (2016). The proportion of minimally verbal children with autism spectrum disorder in a community-based early intervention programme. Journal of Intellectual Disability Research, 60, 464–477. [DOI] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, & Sigman M (2011). Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders, 41, 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Ruskin E, Arbelle S, Corona R, Dissanayake C, Espinosa M, … & Robinson BF (1999). Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development, i–139. [DOI] [PubMed] [Google Scholar]
- Steinberg J, & Webber C (2013). The roles of FMRP-regulated genes in autism spectrum disorder: single-and multiple-hit genetic etiologies. The American Journal of Human Genetics, 93, 825–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stembalska A, Laczmanska I, Gil J, & Pesz KA (2016). Fragile X syndrome in females-a familial case report and review of the literature. Developmental Period Medicine, 20, 99–104. [PubMed] [Google Scholar]
- Sterling A (2018). Grammar in Boys with Idiopathic Autism Spectrum Disorder and Boys with Fragile X Syndrome Plus Autism Spectrum Disorder. Journal of Speech, Language, and Hearing Research, 61, 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, & Joseph RM (2003). Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society B: Biological Sciences, 358(1430), 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, & Kasari C (2013). Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Research, 6, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, & Lord C (2005). Language and communication in autism. In Handbook of Autism and Pervasive Developmental Disorders, 1, 335–364. [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, et al. (2009). Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research, 52, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, & Tager-Flusberg H (2015). Maternal gesture use and language development in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Adolph KE, Homer B, & Tamis-LeMonda CS (2005). Social referencing in infant motor action In Homer BD, Tamis-LeMonda CS (Ed.) The Development of Social Cognition and Communication (pp. 145–164)/ Mahwah, NJ.. [Google Scholar]
- Tassone F, Hagerman RJ, Iklé DN, Dyer PN, Lampe M, Willemsen R, … & Taylor AK (1999). FMRP expression as a potential prognostic indicator in fragile X syndrome. American Journal of Medical Genetics Part A, 84, 250–261. [PubMed] [Google Scholar]
- Taylor LJ, Maybery MT, Grayndler L, & Whitehouse AJ (2014). Evidence for distinct cognitive profiles in autism spectrum disorders and specific language impairment. Journal of Autism and Developmental Disorders, 44, 19–30. [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman RJ, Josol CK, & Abbeduto L (2017). Language skills of males with fragile X syndrome or nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman R, & Abbeduto L (2014). Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities, 35, 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, & Abbeduto L (2015a) Autism symptomatology in boys with fragile X syndrome: A cross-sectional developmental trajectories comparison with nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 2816–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin B (2011). Co-morbidity of autism and SLI: kinds, kin and complexity. International Journal of Language & Communication Disorders, 46, 127–137. [DOI] [PubMed] [Google Scholar]
- Uljarević M, Baranek G, Vivanti G, Hedley D, Hudry K, & Lane A (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research, 10, 703–710. [DOI] [PubMed] [Google Scholar]
- Vygotsky LS (1986). Thought and language. Cambridge: MIT [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, & Reiss AL (2008). Aberrant brain activation during gaze processing in boys with fragile X syndrome. Archives of General Psychiatry, 65, 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SR, & Markow DB (1995). Words as invitations to form categories: Evidence from 12-to 13-month-old infants. Cognitive Psychology, 29, 257–302. [DOI] [PubMed] [Google Scholar]
- Weismer SE, Lord C, & Esler A (2010). Early language patterns of toddlers on the autism spectrum compared to toddlers with developmental delay. Journal of Autism and Developmental disorders, 40, 1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Dawson G, Osterling J, & Dinno N (2000). Brief report: Recognition of autism spectrum disorder before one year of age: A retrospective study based on home videotapes. Journal of Autism and Developmental Disorders, 30, 157–162. [DOI] [PubMed] [Google Scholar]
- Williams D, Botting N, & Boucher J (2008). Language in autism and specific language impairment: Where are the links? Psychological Bulletin, 134(6), 944. [DOI] [PubMed] [Google Scholar]
- Williams KT (2007). EVT-2: Expressive vocabulary test. Pearson Assessments. [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, & Piven J (2012). Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]