Abstract
In addition to being a major source of cancer-related pain, metastatic osseous lesions are frequently at risk for pathologic fracture and its accompanying morbidity. While bony metastases are commonly thought of as occurring within the vertebral column, over 80% are found outside the spine. Percutaneous interventional treatment options for nonspinal metastases offer a broad array of minimally invasive, image-guided procedures that are rapidly effective, reduce the need for opioids, and often work in complementary fashion with adjunct treatments in radiation oncology, orthopaedic surgery, and/or medical oncology. This article presents an approach to assess extraspinal metastases, reviews available interventional techniques in use today, and offers example cases as an introductory primer to the thought process used for selecting the appropriate interventional treatment.
Keywords: interventional radiology, bone metastasis, osseous, malignancy, cementoplasty, ablation
Bone represents the third most common site of cancer metastases after the lung and liver. 1 Between 20 and 30% of patients with any advanced malignancy eventually develop osteolytic metastases and approximately half of all patients suffer from intractable bone pain. 2 In addition to being a major source of cancer-related pain, these lesions are frequently at risk for pathologic fracture and its accompanying morbidity. While bony metastases are commonly thought of as occurring within the vertebral column, over 80% are found outside the spine. 3
Percutaneous interventional treatments offer a broad array of minimally invasive, image-guided procedures that are rapidly effective, reduce the need for opioids, and often work in complementary fashion with adjunct treatments in radiation oncology, orthopaedic surgery, and/or medical oncology. This article presents an approach to assess extraspinal lytic metastases, reviews available interventional techniques in use today, and offers example cases as an introductory primer to the thought process used for selecting the appropriate interventional treatment.
General Principles
The presence of bone metastases is a primary source of morbidity for patients and can result in fracture, pain, and/or decreased quality of life. 2 A multidisciplinary approach in the management of patients with osseous metastatic disease and early referral to pain palliation has shown to significantly improve both survival and quality-of-life metrics. At present, radiation therapy is considered the mainstay treatment for the palliation of bony metastases. Although radiotherapy has been shown to be an effective treatment for local control of osseous metastases, its effectiveness in providing timely pain palliation has certain limitations. Up to 20 to 30% of patients treated with external beam radiation report receiving no pain relief at all. Complete pain responses are seen in only up to 25% of patients. 4 5 6 7 8 In addition, the time to maximal pain reduction for radiotherapy occurs 12 to 20 weeks after the completion of treatment—a period that can often meet or exceed a patient's life expectancy. 9 Prior to radiotherapy, patients are prescribed opioids as part of the World Health Organization's analgesic ladder. The well-known drawbacks of constipation, nausea, sedation, dependence, and tachyphylaxis also make opioids a suboptimal analgesic strategy. 10
As pain response is the primary endpoint for most studies related to the treatment of bone metastases, percutaneous therapies offer many added potential benefits. In contrast to external radiation, interventional treatments such as percutaneous ablation and mechanical reinforcement can achieve pain relief in 24 hours to 1 week—frequently in patients whose pain has proven resistant to alternative therapies. Pain reduction is achieved in 70 to 96% of patients as compared with 50 to 80% reported with radiotherapy. 11 In addition, and in contrast to radiation therapy, ablation combined with cementoplasty or percutaneous stabilization (PS) results in tumor cytoreduction and reinforcement of the treated bone. Functional improvement is achieved in nearly 80% of patients and there is limited evidence to suggest that cementoplasty with PS reduces the risk of pathologic fracture in the long bones. 12 13 14
Interventional Treatments
Ablation, cementoplasty, and PS represent methods in which interventional radiologists can offer patients a minimally invasive, rapid, and well-tolerated solution in the treatment of painful bony metastases. More importantly, these interventional procedures do not preclude a patient from concurrent or subsequent radiation therapy, chemotherapy, or orthopaedic surgery. In some instances, interventional treatments may be chosen to allow for quicker initiation of concurrent therapy, since traditional surgical postoperative periods require longer recovery intervals prior to initiation of chemotherapy. Nonetheless, multidisciplinary review at tumor board and consensus is needed prior to choosing any interventional procedure outlined below to ensure that the procedure does not interfere with any other planned treatments.
Interventional approaches to painful bony metastases include thermal ablation that causes cell death through extreme heating or cooling.
Heating Techniques
-
I. Radiofrequency ablation (RFA) is the most studied ablative technique. 4 15 16 17 RFA uses electrical energy to heat tumor tissue to approximately 90°C. RFA systems come in two designs, monopolar and bipolar. Both systems work on the same principle. In monopolar systems, the RFA needle that is inserted into the tumor acts as the negative electrode (the cathode) and grounding pads act as the positive electrode (the anode). A generator produces a rapidly alternating current that is conducted from the needle, through the tissues, and to the grounding pads. This rapidly alternating current excites ions in the tumor tissue, causing heating through friction, and thus cell death. Because the needle has a small cross-sectional area, it creates a very high-energy flux and high heat nearby. The current spreads through the tissues and the circuit is closed when it reaches the large cross-sectional area of the ground pads which have a vastly reduced energy flux. 18 In bipolar needles, the device tip includes both the anode and cathode (active and returning electrode) in the same probe. Single RFA needles generate a spherical zone of heat that can ablate areas 4 cm or smaller. 18 Multiple bipolar needles can be combined to ablate larger areas.
RFA is highly effective at treating intraosseous lytic lesions, but the heat it generates does not penetrate cortical bone well. This can make it a suboptimal choice in the treatment of lytic lesions with a significant soft-tissue component outside of intact cortex. 19 In addition, patients with metal implants or pacemakers should not undergo RF monopolar ablation as the implant or pacer may act as a ground and cause thermal injury or pacemaker malfunction. RFA performed near large blood vessels is subject to the “heat sink” effect whereby flowing blood channels heat away from the intended ablation zone, resulting in unablated residual disease. RFA can also produce charring of tissues around the needle and this charred tissue possesses increased impedance, which interrupts the flow of electricity and interferes with tumoricidal heating. 20 Finally, the zone of ablation produced by RFA is not visible on computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound in real time, requiring detailed understanding of the tumor anatomy and probe performance specifications prior to the procedure. 20
II. In microwave ablation (MWA), an oscillating electromagnetic microwave field causes rapid realignment of polar molecules (predominantly water) and the rapid motion of these molecules generates frictional heat that kills cells. 19 Microwave energy causes uniform tissue heating that is not disrupted by cortical bone and is less susceptible to the heat sink effect than RFA. In addition, MWA is not contraindicated in patients with metal implants. However, MWA ablation zones are not purely spherical—they are often modified teardrop shapes depending on the make and model of probe used—complicating procedural technique and demanding greater operator experience to perform MWA. 21 Similar to RFA, the zone of ablation in MWA is not visible on CT, MRI, or ultrasound in real time.
Cooling Techniques
I. Cryoablation (CA) kills tumor cells through cycles of rapid freezing and gradual thawing. The Joule-Thompson effect—which dictates that certain gases exhibit cooling after being forced through a porous plug or valve—is used to rapidly cool a cryoprobe needle. As the gas—most often argon—moves from an internal feed line into an internal expansion chamber, the needle tip falls to a temperature of −160°C or colder. 22 Tumoricidal effects are achieved when the surrounding tissue is rapidly cooled to between −40°C and −60°C. 22 CA is advantageous because it naturally produces a cold-related anesthetic effect, which allows for reduced sedation and anesthesia. Like MWA, CA is not affected by bone cortex and freezes lesions with soft tissue and bone components easily. CA also permits precise ablation as the ice ball can be seen in real time via MRI/CT/ultrasound because the frozen tissue possesses lower density than the surrounding tissue. 22 In addition, multiple cryoprobes can be used simultaneously, allowing for the ablation of large or irregular tumors in one session. One distinct advantage of CA is its relative preservation of soft tissue and neuronal architecture. Drawbacks of CA include the length of the procedure (two rounds of freezing for ∼10 minutes and thawing for ∼5 minutes are required), cost of the multiple probes that are needed, risk of bleeding as disrupted blood vessels thaw (this is less of a risk in heating ablation as RFA/MWA cauterize as they heat), and delayed cementoplasty (the frozen tissue must be allowed to rewarm to body temperature so as not to interfere with polymethyl methacrylate [PMMA] polymerization).
Mechanical Reinforcement
I. Cementoplasty (or osteoplasty) involves the injection of PMMA into lytic lesions. This treatment offers limited tumoricidal action within approximately 3 mm of the injected cement via the transient exothermic hardening of PMMA, but it does not achieve controlled ablation. 23 24 PMMA performs well for resisting compressive mechanical forces, but it is much weaker than bone when subjected to torsion. 25
II. PS with pins, screws, or a combination of these materials to reinforce bone weakened by metastases has been described only in small retrospective and prospective series in the IR literature. 13 26 27 These options are typically utilized in locations that experience torsional forces, for example, the femoral neck and the humerus. In these early studies, PS has demonstrated reduced fracture rates, reduced pain, and improved function. 11 Some PS techniques, particularly those in the femoral neck, are similar to orthopaedic surgery and entail the insertion of orthopaedic screws across lytic lesions in the neck with or without accompanying cement. Other techniques involve cementoplasty augmented by needle “rebars.” 14 More experimental techniques in diaphyseal bone employ cement-filled catheters or flexible nails which are inserted into the medullae of long bones. 14 28
Treatment Considerations and Scoring Classifications
The techniques described earlier are versatile and allow for treatment of lytic lesions throughout the musculoskeletal system. Determining if a patient is an optimal candidate for IR therapy is a multidisciplinary effort that must involve the patient's medical oncologist, a consulting orthopaedic oncologic surgeon, radiation oncologist, interventional radiologist, and the patient. 12 29 At a minimum, the team must consider the following:
Prior management —Has the patient failed conservative management, e.g., bisphosphonates, chemotherapy, opioids, radiotherapy?
Patient fitness —Is the patient a poor surgical candidate or simply opposed to surgery?
Intention of therapy, i.e., palliative versus curative —How many lesions are afflicting the patient? If there are fewer than three to five lesions (oligometastatic) and an ablative zone 1 cm larger than the lesion(s) can be achieved, can interventional therapies attempt curative intervention? 30
Architecture of the lesion —Is the lesion sclerotic, lytic, or permeative? Is the cortex destroyed in addition to cancellous bone? Is the bone weight bearing? In what direction are the normal biomechanical forces? What is the fracture risk to the patient? This discussion may utilize scoring systems such as the Mirels or Harrington scoring systems or the greater than 30 mm of axial cortical involvement or greater than 50% of circumferential involvement described by Van der Linden .
Nearby critical structures—Primarily joints and nerves . Is precise visualization of the ablative zone required to safely perform the procedure?
Given the variability of lesions that may occur and extraspinal bones that can be involved, no clear guidelines or algorithms exist for treatment. When choosing a percutaneous therapy, each patient should be approached in an individual, case-by-case manner. Certain scoring classifications can also be utilized to help guide decisions at multidisciplinary tumor boards and should be familiar to the interventional radiologist.
The Mirels scoring system (see Table 1 ) is the most commonly used scoring system employed in assessing the risk of pathologic fracture and need for prophylactic fixation at a site of bony metastasis in long bones. 31 The system evaluates the risk of fracture through radiological and clinical factors, such as location (upper limb, lower limb, and peritrochanteric), radiographic appearance (lytic, blastic, or mixed), size of the lesion (<1/3 of cortical thickness, 1/3–2/3 of cortical thickness, and >2/3 of cortical thickness), and accompanying pain (mild, moderate, and functional). Each parameter is scored from 1 to 3, resulting in a total score from a minimum of 4 to a maximum of 12.
Table 1. Mirels' score.
| +1 | +2 | +3 | |
|---|---|---|---|
| Site | Upper limb | Lower limb | Peritrochanteric |
| Pain | Mild (<4/10) | Moderate (≥4/10) | Functional impairment |
| Blastic vs. lytic vs. mixed | Blastic | Mixed | Lytic |
| Degree of cortical bone involvement | <1/3 on axial cross-sectional imaging | 1/3–2/3 on axial cross-sectional imaging | >2/3 on axial cross-sectional imaging |
| Score ≤7: <5% risk of fracture | Score 8: 15% risk of fracture | Score ≥9: high risk of fracture; stabilization recommended |
Source: Adapted from Cazzato et al. 30
The initial validation study retrospectively analyzed 78 metastatic long bone lesions and found that 27 had fractured at 6 months. 29 Increasing Mirels' scores were correlated with increasing fracture risk: lesions with scores ≤7 had a low risk (5%), a score of 8 had 15% risk of fracture, and lesions with scores ≥9 had very significant risk (33%). Scores less than 7 have traditionally been treated with irradiation. Scores of 8 can be treated with conservative or interventional methods based on clinical judgement. Scores of 9 or higher are treated with prophylactic fixation.
The current interventional radiology literature describes using ablation, cementoplasty, or a combination of the two when treating patients with extraspinal, non–weight-bearing lesions of long bones which have Mirels' scores of 7 or less. 2 4 30 Weight-bearing lesions, or lesions that have scores of 9 or above, are universally treated with cementoplasty, and are often additionally treated with PS and ablation. 11 14 32 33 34
The Harrington classifications were developed several years before the Mirels system to guide surgical therapy of metastases and defects within the acetabulum. They are not designed to quantify fracture risk, rather they describe known defects in the acetabular structure. Interventional radiologists may use this system to treat lesions in groups I to III with a combination of cementoplasty, RFA, and osteosynthesis with orthopaedic screws, while group IV almost always requires surgical reconstruction. 34
Harrington groups 35 :
Group I : minimal involvement of the acetabulum in subchondral bone.
Group II : the medial wall of the acetabulum is destroyed, but the roof and the lateral wall are still preserved.
Group III : extensive osteolysis of the medial wall, the roof, and the lateral rim of the acetabulum.
Group IV : complete acetabular collapse.
In an analysis of the risk factors for pathologic femoral fractures, Van der Linden et al noted that axial cortical involvement greater than 30 mm (30% fracture rate) and circumferential cortical involvement greater than 50% (35% fracture rate) were predictive of fracture. 36
Harrington also authored a study analyzing risk factors for femoral fracture and found that cortical bone destruction greater than 50%, a lesion larger than 2.5 cm, a pathological avulsion fracture of the lesser trochanter, and persisting stress pain despite irradiation were all predictive of fracture. 37
Cases
The following series of cases demonstrate the method by which patients with osseous metastatic disease were ultimately treated with percutaneous interventional techniques. The cases and their accompanying discussion highlight the current lack of an algorithmic approach to this disease process and stress the need for multidisciplinary care in the management of such patients.
Case 1 (see Fig. 1 )
Fig. 1.
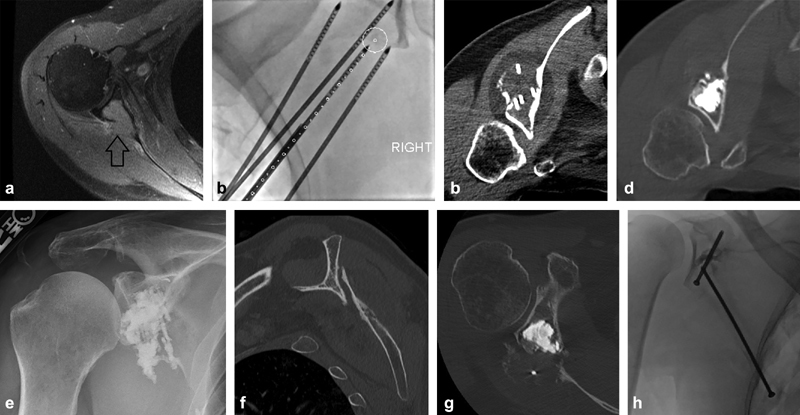
A 54-year-old man with a history of metastatic renal cell carcinoma presenting with right shoulder pain. Initial imaging demonstrated a right scapular lesion with posterior cortical destruction and invasion into the glenoid body but no definite fracture extension into the joint (arrow) ( a ). He received palliative radiation therapy, but was admitted to the hospital with persistent severe shoulder pain 1 month later. Open surgical repair was not offered as first-line therapy given the expected surgical morbidity and required interruption in ongoing systemic therapies in the setting of progressive disease. Goals of therapy included pain palliation, increased shoulder mobility, prevention of disease progression into the glenoid cortex and articular surface, and reinforcement of the glenoid to prevent stress fracture. Cryoablation was chosen for maximal visualization of the ablation zone and minimal damage to the adjacent joint. Four probes were placed using needle guidance software to optimize tumor coverage ( b ). Following cryoablation ( c ), a “hot” thaw was performed with active heating of the ablation probe tips for greater than 15 minutes to melt the ice and facilitate cement deposition. The glenoid cavity was then filled with polymethyl methacrylate under intermittent CT imaging to ensure no extravasation into the joint ( d ). Pain and function were significantly improved postoperatively ( e ). Unfortunately, he developed recurrent pain 2 months later and CT scan demonstrated a scapular body fracture with severe displacement and over-ride ( f ), despite the glenoid remaining well reinforced ( g ). Nonoperative management was chosen due to the expected morbidity associated with any operation and the patient's desire to avoid an extensive operation. For lesions isolated to the glenoid, osteoplasty alone is usually clinically sufficient to resist the compressive forces in this area with arm abduction; however, there was significant tumor destruction extending down into the midscapular body, and this fracture likely occurred due to a combination of overuse during the healing phase and multidirectional stress forces in the area of fracture. The addition of a scapular body screw ( h ), despite its inherent challenges, may have provided superior stabilization in this setting.
A 54-year-old man with metastatic renal cell carcinoma.
Prior management : systemic therapy, radiation therapy.
Patient fitness : ambulating with a walker due to lower extremity pain.
Intention of therapy : pain palliation, increased mobility, local tumor control, structural reinforcement of the glenoid.
Architecture of the lesion : destructive glenoid and scapular body lesion. No significant fracture displacement.
Nearby critical structures : joint space, suprascapular nerve.
Case 2 (see Fig. 2 )
Fig. 2.
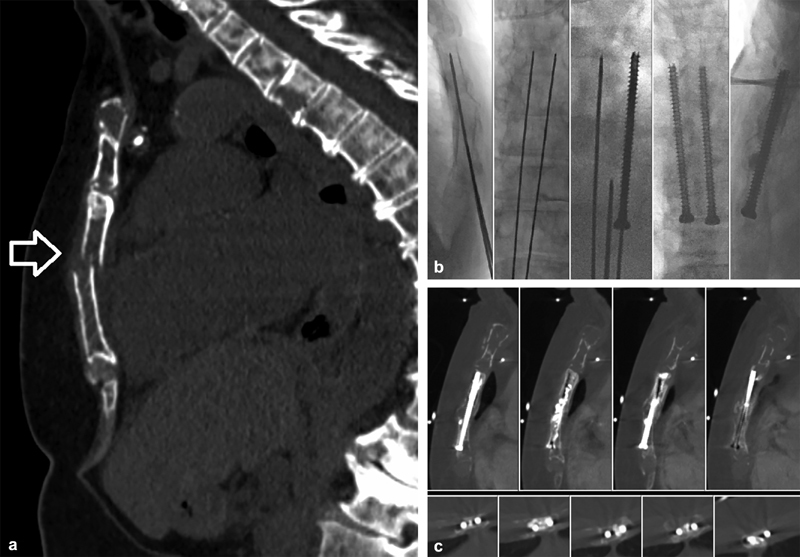
A 69-year-old woman with a history of CKD and multiple myeloma status post stem cell transplant with relapsed and progressive disease presenting with severe sternal pain. At the time of presentation, she was experiencing excruciating pain of 10/10 when using her walker for ambulation despite an oral analgesic regimen of MS Contin 15 mg q12h and 6–8 additional 5 mg oxycodone pills daily. CT imaging demonstrated diffuse lytic disease throughout the sternum with a single mildly displaced, mildly angulated, axially oriented pathologic fracture through the mid sternum (arrow) ( a ). The goal of therapy was pain palliation and increased mobility. Open surgical plating was considered but not offered due to her poor functional status, need for ongoing systemic therapy, diffuse sternal bone loss, and therapy-induced thrombocytopenia. The biomechanical forces exerted on the sternum are complex and include a high degree of rotational and oblique forces. Without completely intact cortex to transmit these forces, cementoplasty alone, even if complete sternal fill is achieved, will quickly fail under these noncompressive forces. The axial fracture orientation had disrupted the craniocaudal cortical buttress, and therefore, we elected to place craniocaudal screws, tangential to the fracture line, to recreate the vertical sternal rigidity. Importantly, the minimal degree of angulation and displacement allowed for effective fracture reduction with minimal manipulation of the bone access trocars. Two parallel 4.5-mm cannulated screws were utilized to prevent any rotational movement that could occur around a single screw axis ( b ). Osteoplasty with polymethyl methacrylate (PMMA) was performed subsequently to stabilize the lytic cavity. Ablation was not performed with the following considerations: her disease was widely metastatic; reasonable local control of myeloma can often be achieved with adequate cement fill; and ablation could cause further injury to the sternum, heart, and, lungs and may hinder progressive healing. Achieving adequate fixation with such severe bone loss is challenging and was addressed in this case by using fully threaded screws to maximize anchoring with the PMMA. Her sternal pain was negligible and ambulation improved at 3 weeks postprocedure. Over the next 2 years, she experienced no recurrent pain or construct failure on imaging ( c ), at which time she died from her progressive disease.
A 71-year-old woman with multiple myeloma and sternal pain when using her walker.
Prior management : systemic therapy.
Patient fitness : painful ambulation with assistive device.
Intention of therapy : pain palliation, increased mobility.
Architecture of the lesion : diffuse lytic disease, single transverse sternal fracture, minimally displaced.
Nearby critical structures : heart, lungs, skin.
Case 3 (see Fig. 3 )
Fig. 3.
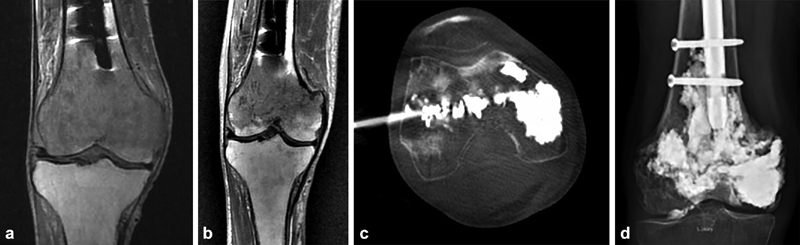
A 69-year-old man with a history of lymphoma status post remote prophylactic intramedullary nail placement in the right femur presenting with progressive right knee pain. MRI demonstrated new diffuse infiltrative disease within the distal femoral condyles with preservation of the joint space and no definite cortical fracture ( a ). He underwent radiation therapy totaling 20 Gy in addition to ongoing systemic therapy but had persistent right knee pain 3 months later. Repeat MRI at that time demonstrated some tumor response, but a new minimally compacted pathologic fracture was best seen along the medial metaphysis, again with relative preservation of the joint space ( b ). A total knee replacement was considered but was deferred in favor of a minimally invasive approach due to the patient's poor overall functional status, previous instrumentation of the femur, and relatively preserved joint, realizing that the same surgical reconstruction could still be performed later if necessary. In this case, the fracture is transverse, almost completely perpendicular to the major vertical force vector transmitted through the knee with weight bearing, and without any significant angulation or displacement. Moreover, there is no clear approach to placing reinforcing screws tangential to the fracture line. Lymphoma, like myeloma, is often easily displaced by polymethyl methacrylate, allowing for extensive cementation. Additionally, imaging did not demonstrate a large fracture cleft extending into the joint that might lead to cement preferentially extravasating into the joint space. With these considerations, we elected to perform robust osteoplasty alone, striving for continual, robust cement deposition from the distal portion of the intramedullary nail to the subarticular bone, with the goal of providing just enough stabilization to allow the natural healing process to occur. Osteoplasty was performed through two 11-gauge bone trocars from a medial and lateral approach with cement delivery through a coaxial cement delivery cannula ( c ). Ablation was not performed because of this tumor's sensitivity to radiation therapy, difficulty in encompassing the whole lesion, potential injury to the intact knee cartilage and healthy bone, and relative ease of cement fill. The procedure was terminated when a small amount of cement was seen outside the bone within the anterior joint space. Intermittent CT imaging was utilized over fluoroscopy to more quickly and accurately identify cement extravasation. His pain significantly improved postprocedure and eventually subsided completely. He continues to walk without an assistive device one and a half years from stabilization with stable radiographic findings ( d ).
A 69-year-old male/female with lytic lymphoma metastases to the femoral condyles.
Prior management : systemic therapy, radiation therapy.
Patient fitness : ambulatory with assistive device.
Intention of therapy : pain palliation, prevention of fracture progression, preservation of ambulation.
Architecture of the lesion : permeative and lytic lesion in a weight-bearing bone.
Nearby critical structures : joint space. Cement leakage into the joint impairs function, destroy cartilage, and requires surgical removal.
Case 4 (see Fig. 4 )
Fig. 4.
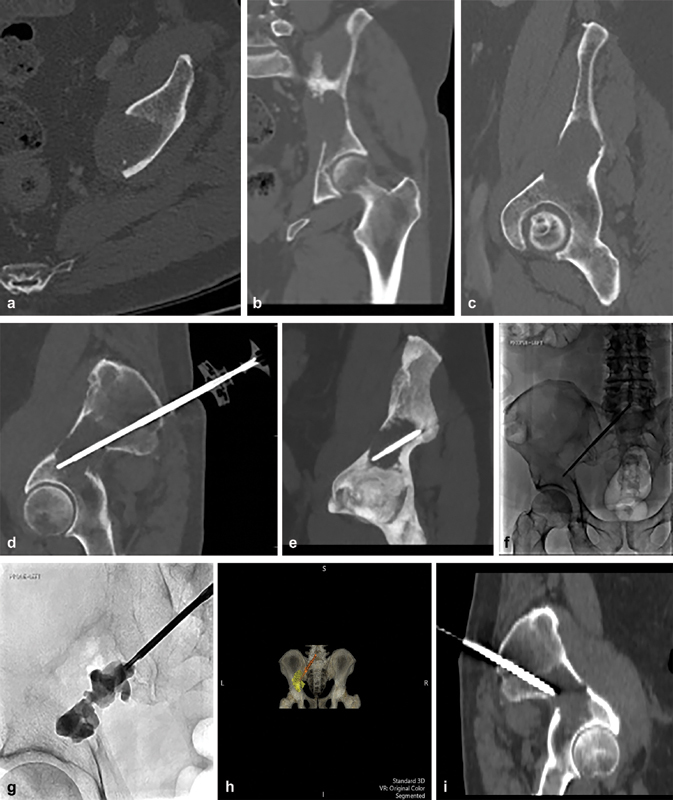
( a–c ) CT axial, coronal, and in plane views of left periacetabular lesion within the ilium demonstrating complete cortical erosion of the medial wall of the ilium. ( d, e ) CT of polymethyl methacrylate (PMMA) injection trochar positioned in the lesion following RFA. ( f, g ) Fluoroscopic images showing injection of PMMA. ( h ) 4D CT showing a posterior view of the pelvis, the trochar, and the lesion filled with PMMA. ( i ) Sagittal view of the pelvis demonstrating the course of the screw.
A 68-year-old male with hepatocellular carcinoma (HCC) and left hip lesion.
Prior management : five rounds of radiation to the left iliac bone.
Patient fitness : able to ambulate with pain.
Intention of therapy : cure of a single oligometastatic focus and mechanical stabilization.
Architecture of the lesion : Harrington 1.
Nearby critical structures : sciatic nerve; a posterior approach with the RFA probe must be superior enough to avoid the nerve as it courses deep to the piriformis. Joint space; cement leakage into the joint impairs function, destroys cartilage, and may require surgical removal.
Patient was initially treated with transarterial radioembolization as a subsequent bridge to orthotopic liver transplant. Twelve months following transplant, the patient began reporting left hip pain and a positron emission tomography scan revealed a solitary hypermetabolic focus (pathologically shown to be HCC) in the left iliac bone extending toward the acetabular roof. Despite five rounds of radiation therapy, he continued to experience pain and interval growth of his lesion. Given these findings, an interventional approach was sought for ablation and cementoplasty.
This lesion was treated in a hybrid CT-fluoroscopy suite to allow for CT-guided probe placement and live fluoroscopic PMMA injection. Multiplanar images demonstrate the lytic lesion on axial and coronal images ( Fig. 4a ). Multiplanar oblique images formatted into the plane of the RFA probe and cement trocar are shown. Fluoroscopic images demonstrating the injection of PMMA. A 4D reconstruction of the pelvis along with the trocar and cement within the right ilium.
Conclusion
Percutaneous techniques offered in interventional radiology for the treatment of lytic metastases are safe, effective, and complementary with the other specialties involved in the care of complex oncology patients. These treatments may offer quicker and more durable pain relief than other therapies and allow for quicker initiation of other medical treatments. They may also be used for both palliative and curative indications. Understanding the modalities available to the interventionalist and close collaboration with surgical, medical, and radiation oncology specialties are important for choosing the right treatment for patients with painful extraspinal bone metastases.
Footnotes
Conflict of Interest None.
References
- 1.Macedo F, Ladeira K, Pinho F et al. Bone metastases: an overview. Oncol Rev. 2017;11(01):321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munk P L, Rashid F, Heran M K et al. Combined cementoplasty and radiofrequency ablation in the treatment of painful neoplastic lesions of bone. J Vasc Interv Radiol. 2009;20(07):903–911. doi: 10.1016/j.jvir.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Kakhki V R, Anvari K, Sadeghi R, Mahmoudian A S, Torabian-Kakhki M. Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur. 2013;16(02):66–69. doi: 10.5603/NMR.2013.0037. [DOI] [PubMed] [Google Scholar]
- 4.Goetz M P, Callstrom M R, Charboneau J W et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22(02):300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert H A, Kagan A R, Nussbaum H et al. Evaluation of radiation therapy for bone metastases: pain relief and quality of life. AJR Am J Roentgenol. 1977;129(06):1095–1096. doi: 10.2214/ajr.129.6.1095. [DOI] [PubMed] [Google Scholar]
- 6.Hoskin P J. Palliation of bone metastases. Eur J Cancer. 1991;27(08):950–951. doi: 10.1016/0277-5379(91)90254-b. [DOI] [PubMed] [Google Scholar]
- 7.Janjan N A.Radiation for bone metastases: conventional techniques and the role of systemic radiopharmaceuticals Cancer 199780(8, Suppl):1628–1645. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi S, Hoshi H, Iida T. Reirradiation with local-field radiotherapy for painful bone metastases. Radiat Med. 2002;20(05):231–236. [PubMed] [Google Scholar]
- 9.Motamedi K, Levine B D, Bukata S V, Genshaft S. Percutaneous image-guided musculoskeletal tumor treatments. AJR Am J Roentgenol. 2016;207(03):517–525. doi: 10.2214/AJR.16.16170. [DOI] [PubMed] [Google Scholar]
- 10.WHO's Cancer Pain Ladder for Adults.World Health Organization, World Health Organization, November 27, 2013Available at:www.who.int/cancer/palliative/painladder/en/. Accessed June 13, 2019
- 11.Cazzato R L, Palussière J, Buy X et al. Percutaneous long bone cementoplasty for palliation of malignant lesions of the limbs: a systematic review. Cardiovasc Intervent Radiol. 2015;38(06):1563–1572. doi: 10.1007/s00270-015-1082-7. [DOI] [PubMed] [Google Scholar]
- 12.Mesko N W, Joshua L M, Steven A L. Minimally invasive techniques for pain palliation in extraspinal bone metastases. Curr Orthop Pract. 2016;27(06):686–695. [Google Scholar]
- 13.Mavrovi E, Pialat J B, Beji H, Kalenderian A C, Vaz G, Richioud B. Percutaneous osteosynthesis and cementoplasty for stabilization of malignant pathologic fractures of the proximal femur. Diagn Interv Imaging. 2017;98(06):483–489. doi: 10.1016/j.diii.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Kelekis A, Filippiadis D, Anselmetti G et al. Percutaneous augmented peripheral osteoplasty in long bones of oncologic patients for pain reduction and prevention of impeding pathologic fracture: the Rebar concept. Cardiovasc Intervent Radiol. 2016;39(01):90–96. doi: 10.1007/s00270-015-1138-8. [DOI] [PubMed] [Google Scholar]
- 15.Callstrom M R, Charboneau J W, Goetz M P et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224(01):87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- 16.Carrafiello G, Laganà D, Ianniello A et al. Radiofrequency thermal ablation for pain control in patients with single painful bone metastasis from hepatocellular carcinoma. Eur J Radiol. 2009;71(02):363–368. doi: 10.1016/j.ejrad.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Dupuy D E, Liu D, Hartfeil D et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116(04):989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenette J P, Lopez M J, Kim E, Dupuy D E. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation--a multicenter American College of Radiology Imaging Network Study. Radiology. 2013;268(03):907–915. doi: 10.1148/radiol.13122398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinshaw J L, Lubner M G, Ziemlewicz T J, Lee F T, Jr, Brace C L. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34(05):1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatli S, Tapan U, Morrison P R, Silverman S G. Radiofrequency ablation: technique and clinical applications. Diagn Interv Radiol. 2011;18(05):508–516. doi: 10.4261/1305-3825.DIR.5168-11.1. [DOI] [PubMed] [Google Scholar]
- 21.Pusceddu C, Sotgia B, Fele R M, Ballicu N, Melis L. Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol. 2016;39(01):74–80. doi: 10.1007/s00270-015-1151-y. [DOI] [PubMed] [Google Scholar]
- 22.Gardner C S, Ensor J E, Ahrar K et al. Cryoablation of bone metastases from renal cell carcinoma for local tumor control. J Bone Joint Surg Am. 2017;99(22):1916–1926. doi: 10.2106/JBJS.16.01182. [DOI] [PubMed] [Google Scholar]
- 23.Cazzato R L, Garnon J, Shaygi B et al. Percutaneous consolidation of bone metastases: strategies and techniques. Insights Imaging. 2019;10(01):14. doi: 10.1186/s13244-019-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol. 2010;27(02):124–136. doi: 10.1055/s-0030-1253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basile A, Giuliano G, Scuderi V et al. Cementoplasty in the management of painful extraspinal bone metastases: our experience. Radiol Med (Torino) 2008;113(07):1018–1028. doi: 10.1007/s11547-008-0314-1. [DOI] [PubMed] [Google Scholar]
- 26.Pusceddu C, Fancellu A, Ballicu N, Fele R M, Sotgia B, Melis L. CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture. Skeletal Radiol. 2017;46(04):539–545. doi: 10.1007/s00256-017-2584-y. [DOI] [PubMed] [Google Scholar]
- 27.Liu X W, Jin P, Liu K et al. Comparison of percutaneous long bone cementoplasty with or without embedding a cement-filled catheter for painful long bone metastases with impending fracture. Eur Radiol. 2017;27(01):120–127. doi: 10.1007/s00330-016-4347-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y I, Kang H G, Kim T S, Kim S K, Kim J H, Kim H S. Palliative percutaneous stabilization of lower extremity for bone metastasis using flexible nails and bone cement. Surg Oncol. 2014;23(04):192–198. doi: 10.1016/j.suronc.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Angelini A, Trovarelli G, Ruggieri P. Metastases to the pelvis: algorithm of treatment. Management of Bone Metastases. 2018:103–113. [Google Scholar]
- 30.Cazzato R L, Buy X, Grasso R F et al. Interventional radiologist's perspective on the management of bone metastatic disease. Eur J Surg Oncol. 2015;41(08):967–974. doi: 10.1016/j.ejso.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Piccioli A, Spinelli M S, Maccauro G. Impending fracture: a difficult diagnosis. Injury. 2014;45 06:S138–S141. doi: 10.1016/j.injury.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2012;35(06):1428–1432. doi: 10.1007/s00270-011-0330-8. [DOI] [PubMed] [Google Scholar]
- 33.Deib G, Deldar B, Hui F, Barr J S, Khan M A. Percutaneous microwave ablation and cementoplasty: clinical utility in the treatment of painful extraspinal osseous metastatic disease and myeloma. AJR Am J Roentgenol. 2019:1–8. doi: 10.2214/AJR.18.20386. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A C, Hirsch J A, Chaudhry Z A et al. Evaluating the safety and effectiveness of percutaneous acetabuloplasty. J Neurointerv Surg. 2012;4(02):134–138. doi: 10.1136/jnis.2011.004879. [DOI] [PubMed] [Google Scholar]
- 35.Harrington K D. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(04):653–664. [PubMed] [Google Scholar]
- 36.Van der Linden Y M, Dijkstra P D, Kroon H M et al. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br. 2004;86(04):566–573. [PubMed] [Google Scholar]
- 37.Harrington K D. New trends in the management of lower extremity metastases. Clin Orthop Relat Res. 1982;(169):53–61. [PubMed] [Google Scholar]


