Abstract
Interventional radiologists have multiple points of involvement in the treatment and management of patients with pancreatic adenocarcinoma. There is increasing interest in cytoreductive treatment of the primary tumor as well as metastatic disease via arterial and ablative techniques. The focus of this article is on the current evidence for pancreatic irreversible electroporation. For patients undergoing curative surgery or terminal palliation, interventional radiology can manage complications and symptoms. In this article, therapeutic and palliative options in this group of patients including postoperative embolization, biliary drainage, and stent placement are reviewed.
Keywords: pancreatic adenocarcinoma, irreversible electroporation, radioembolization, biliary drainage, embolization, interventional radiology
Pancreatic adenocarcinoma accounts for only 3% of cancers, but this highly aggressive disease is the fourth leading cause of cancer-related death. 1 High mortality rates are related to advanced stage at presentation with greater than 50% of newly diagnosed patients presenting with distant metastases 2 . Resection offers the only chance of cure; therefore, determining surgical eligibility is the most important initial objective. Treatment options for pancreatic cancer include a combination of surgery, chemotherapy, and radiation. However, less than 15% of patients are candidates for resection at diagnosis and 94% of patients will die within 5 years of diagnosis. 3 4 Given the multiple therapeutic teams involved in care, patients with pancreatic cancer should be managed by a multidisciplinary team. Outcomes remain disappointing for patients with presumed resectable disease as recurrence is frustratingly common. Adjuvant chemotherapy is now routinely given postoperatively as it improves median survival. 5 6 7
All treatment for patients with metastatic disease is palliative. A meta-analysis of published findings from clinical trials of patients with disseminated disease showed improvement in survival among patients who receive chemotherapy. For many years, gemcitabine was the treatment of choice. 8 The PRODIGE trial demonstrated superior disease-free, metastatic-free, and overall survival with FOLFIRINOX compared with gemcitabine. 9 Additionally, the MPACT study demonstrated that the addition of nab-paclitaxel to gemcitabine improved overall survival compared with gemcitabine alone. 10
Cytoreduction
Primary Tumor
Stage III pancreatic cancer involves a major blood vessel without distant metastasis. Based on the degree of vascular involvement, stage III cancers are divided into two categories: locally advanced pancreatic cancer (LAPC) and borderline resectable pancreatic cancer (BRPC). LAPC is confined regionally without evidence of metastatic disease but with either greater than 180-degree encasement of either the celiac/hepatic artery or with superior mesenteric vein (SMV) or portal vein (PV) occlusion without a technical option for reconstruction. LAPC is considered inoperable due to technical difficulty in achieving negative margins at surgical resection. Less than 40% of patients with LAPC who receive neoadjuvant chemotherapy achieve adequate tumor regression to undergo curative intent resection. 11 Tumors that blur the distinction between resectable and LAPC are deemed BRPC. These tumors abut the SMA, result in severe impingement of the SMV or PV, encase the gastroduodenal artery (GDA), or invade the colon/mesocolon. This subgroup accounts for 5 to 10% of pancreatic carcinoma patients and has disease too advanced to achieve a negative margin with immediate surgery but may be able to achieve a clear surgical margin after neoadjuvant therapy. The true benefit of neoadjuvant therapy in BRPC patients remains incompletely characterized as there are no randomized phase III trials to date. 12 13
Thermal ablation using microwave, cryoablation, or radiofrequency has been used sparingly to treat patients with LAPC secondary to morbidity and mortality related to thermal injury to relevant structures resulting in pancreatitis, duodenal perforation, pancreatic leak, and bleeding. 14 The central retroperitoneal location of the pancreas as well as the mesenteric vascular involvement poses a challenge to ablative techniques including decreased efficacy due to heat sink and the risk for vascular damage or thrombosis. Irreversible electroporation (IRE) is a predominantly nonthermal ablative technique which differs from the aforementioned strategies since IRE induces cellular apoptosis with preservation of the extracellular matrix. IRE delivers targeted millisecond, high-voltage electrical pulses that induce cell membrane permeability by generating defects on a nanoscale in the lipid bilayer. This causes irreversible permeabilization of the cell membrane resulting in cellular apoptosis. 15 16 17 The ability of IRE to permanently damage soft tissues and leave structural matrices and blood vessels intact makes IRE a potentially appealing option for patients with LAPC and vascular encasement.
NanoKnife (Angiodynamics, Queensbury, NY) is the only commercially available IRE device at this time. The procedure is performed under general anesthesia to allow for complete muscle paralysis and cardiac monitoring ( Fig. 1 ). The 19-gauge probes are placed either percutaneously via CT guidance or at open surgery in pairs arranged 1.5 to 2.0 cm apart to bracket the tumor. The generator delivers short, high-voltage pulses across the electrode pairs in synchronization with the R-wave on the electrocardiogram to prevent arrhythmias. IRE is indicated for tumors less than 4.0 cm in axial diameter and inclusion of 5 mm of normal tissue is recommended to ensure a sufficient ablation margin. 18
Fig. 1.
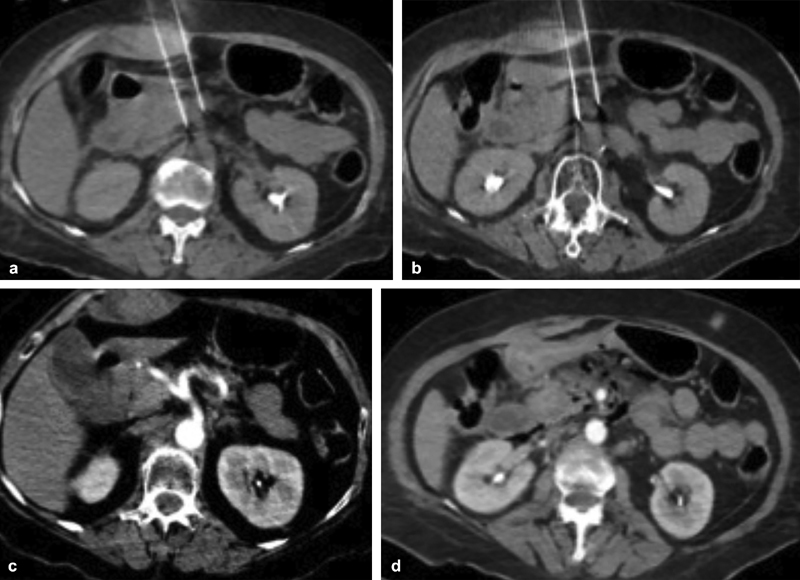
Unenhanced computed tomographic images obtained during pancreatic irreversible electroporation. Note two probes traversing bowel and terminating in close proximity to branches of the celiac axis ( a ) and the superior mesenteric artery ( b ). Contrast-enhanced images obtained immediately following removal of the probes demonstrates normal enhancement of the celiac axis ( c ) and the superior mesenteric artery ( d ) without vascular injury.
Numerous studies have demonstrated the relative safety of IRE when used in close proximity to vessels due to the lack of effect on the extracellular matrix. 19 Currently, open surgical probe placement for IRE is more commonly used than percutaneous placement. The open surgical approach avoids the challenges of overlying viscera during placement of the probes and provides the opportunity to evaluate for metastatic disease, potentially saving the patient from undergoing a futile procedure. However, given the high perioperative morbidity associated with open surgery, the percutaneous approach may be preferable in patients with low performance status. In a large systematic review, Moris et al reported that significant toxicities were more common following open or laparoscopic IRE and the percutaneous approach conveyed lower complication rates. 20 Complications of IRE, frequently classified according to the Clavien-Dindo classification system, have been described. Common low-grade complications include anorexia, dehydration, gastritis, nausea, vomiting, and pancreatitis. Severe complications include upper gastrointestinal bleeding, pulmonary embolus, and bowel perforation. In the PANFIRE study of 25 cases, 11 patients developed grades I to II and 9 developed grade III complications. 21 Huang et al reported three major complications in 70 patients treated with laparoscopic IRE: a bleeding pseudoaneurysm, a pancreatic fistula, and an intra-abdominal abscess. 22 Narayanan and colleagues reported the following complications following percutaneous IRE: 23% of patients experienced abdominal pain, 14% developed pancreatitis, 16% developed a hematoma, 2% developed duodenal stenosis, and 2% developed PV thrombosis. 23 These results demonstrate that IRE is safe with a relatively low morbidity rate and most studies demonstrate few grade IV or V complications.
Evaluating the therapeutic response at imaging of any nonsurgical treatment for pancreatic cancer is a challenge since therapies may not result in regression of the malignant fibrous stroma. IRE shares these limitations, as expected posttreatment imaging changes are largely unknown. Animal models using contrast-enhanced CT demonstrate a well-demarcated hypodense ablation zone following IRE. These findings have not been validated in human studies. The desmoplastic changes surrounding pancreatic tumors may increase the challenges in identifying and quantifying the ablation zone on CT. 24 Early posttreatment contrast-enhanced CT scans frequently demonstrate edema and fat stranding, making the ablation zone appear larger than what is actually achieved. 18 This finding can be incorrectly assumed to represent progression of disease. Stabilization in size of the ablation zone and the development of granulation tissue typically occurs 6 to 8 weeks after IRE; therefore, any increase in volume after this period, enhancement, or new vascular involvement may well represent recurrence. The limitations of imaging surveillance in the posttreatment period is best highlighted by Katz et al in 2012, where 122 patients with borderline resectable disease underwent various neoadjuvant therapies and imaging response was recorded according to RECIST criteria. 25 Using RECIST, 12% of patients demonstrated partial response, 69% had stable disease, and 19% had progressive disease. Of the 85 patients who underwent pancreatectomy, 81 received R0 resections. 25 This study validates the poor correlation of established imaging response criteria with pathologic response in pancreatic cancer.
The absolute survival benefit of pancreatic IRE remains to be determined. One of the earliest prospective studies evaluated the use of IRE in the setting of unresectable cancer. This study found that IRE in conjunction with chemotherapy or radiotherapy increased local progression-free survival, distant progression-free survival, and overall survival when compared with patients with stage III disease treated with chemotherapy or radiotherapy alone. 26 In 2015, Martin et al published a 200-patient registry where patients received chemotherapy or chemoradiation prior to IRE alone (150 patients) or IRE and resection (50 patients). Median overall survival in this heterogeneous group was 24.9 months. 27 In a registry review of 152 patients with LAPC, 144 and 42 patients had neoadjuvant chemotherapy and radiation, respectively. IRE was associated with a 13% major complication rate and 2% mortality. Median time to progression, progression-free survival, and overall survival were 27.3, 22.8, and 30.7 months, respectively. 28 The IMPALA study was not as positive. This single-center prospective cohort study included LAPC patients undergoing resection or IRE after neoadjuvant therapy. In this prospective trial, the median overall survival in patients receiving IRE was similar to patients receiving palliative care and was less than those undergoing resection. 29 The relative and ultimate role of IRE for pancreatic adenocarcinoma remains unknown.
Metastatic Disease
Palliative systemic chemotherapy outcomes have improved over the last decade. 9 10 30 For patients with dwindling systemic options and adequate performance status, ablation or intra-arterial therapy may be performed. Tumor ablation is performed primarily in the setting of oligometastatic disease with tumor diameters of 3 cm or less ( Fig. 2 ). For larger tumors or more diffuse hepatic metastases, arterial therapy can potentially be used ( Fig. 3 ). Importantly, in patients with a disrupted sphincter of Oddi from either stent placement or surgery, antibiotic prophylaxis with moxifloxacin is appropriate to limit the risk of postprocedural infection ( Fig. 4 ) related to the colonized bile ducts. 31 Kim et al recently published multicenter outcomes in 33 patients with metastatic pancreatic adenocarcinoma treated with Yttrium-90 radioembolization. 32 The median survival following radioembolization was 8.1 months and grade III or greater complications were reported in 15% of patients, some of which may have been related to previous chemotherapy (aphthous ulcer formation and neuropathy).
Fig. 2.
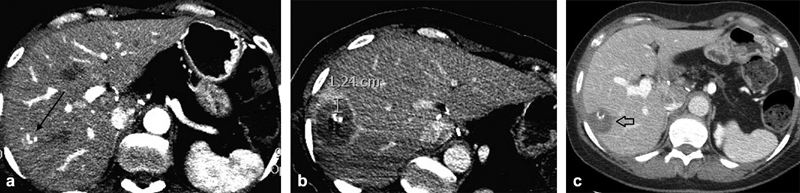
A 54-year-old woman underwent a Whipple procedure for pancreatic carcinoma and developed a solitary right liver metastasis 3 months after surgery (arrow, a ). The tumor was treated with microwave ablation and immediate postablation scan ( b ) demonstrated a minimum 1 cm margin. At 1-month follow-up imaging, the ablation zone ( c , arrow) contracted without evidence of residual tumor. This approach allowed the patient to remain off chemotherapy until developing a greater metastatic burden.
Fig. 3.
A 73-year-old woman with oligometastatic disease from pancreatic cancer (arrows, a ). C-arm computed tomography at mapping angiography confirmed supply to the two tumors from the right hepatic artery (arrows, b ). Follow-up scanning after treatment demonstrated a partial response in the treated masses (arrows, c ).
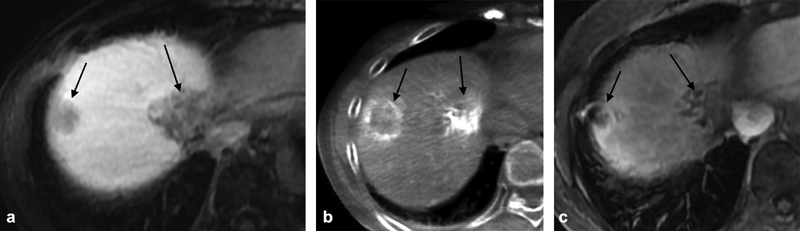
Fig. 4.
A 60-year-old man with a large left lobe metastasis from acinar tumor of the pancreas ( a ). He had previously undergone a Whipple procedure. Radioembolization was planned but at mapping his lung, shunt fraction was 37% ( b ). He was treated instead with bland embolization and developed an abscess which filled the necrotic tumor bed ( c , arrow). As the tube continued to have high daily output, contrast injection was performed which delineated a biliary fistula (arrow, d ). This patient was premedicated with moxifloxacin but developed an abscess despite prophylaxis.
Palliative Biliary Interventions in Nonresectable Patients
Although endoscopy plays a large role in biliary decompression, interventional radiology is commonly involved in treating patients who have had previous surgery with Roux-en-Y anastomosis or in primary tumors when endoscopic stent placement is unsuccessful. Biliary drainage can increase neoadjuvant chemotherapy options and relieve symptoms related to hyperbilirubinemia. 33
The approach for percutaneous transhepatic biliary drainage (PTBD) is based on a combination of operator preference and patient anatomy. Patients with obstruction distal to the confluence of the right and left hepatic ducts typically require only unilateral biliary drainage for successful decompression. 34 In this scenario, there are potential advantages to starting on either the left or right side. The left approach is performed via a subxiphoid approach, limiting the postprocedure pain associated with intercostal puncture associated with right-sided access. The anterior drain position allows for easier self-care. The right-sided approach decreases the complexity of angles to manipulate catheters and wires across the occlusion and also decreases operator radiation exposure. Anatomically, the right lobe is typically larger than the left and will allow for more rapid decompression.
Neoadjuvant gemcitabine and irinotecan (part of FOLFIRINOX) are both extensively metabolized by the liver, a potential issue for patients presenting with biliary obstruction. 35 Hyperbilirubinemia increases the risk for hepatotoxicity with these regimens. 35 36 37 38 Thornton et al assessed the efficacy of total serum bilirubin reduction via PTBD and which factors were most significant in achieving the clinical goal of normalization (defined as serum bilirubin ≤ 1) in 148 patients. 37 A baseline bilirubin of 9 mg/dL or less was more likely to normalize (55 vs. 22%, p < 0.001). Patients with complete liver drainage had a much higher probability of reaching bilirubin normalization (hazard ratio, 2.73; 95% confidence interval [CI], 1.56–4.78; p < 0.001) than those with undrained ducts. At 100 days after intervention, the cumulative incidence of bilirubin normalization was 49% for complete drainage versus only 17% for incomplete drainage. Patients with elevated international normalized ratio were much less likely to obtain bilirubin levels less than 2 mg/dL ( p = 0.002).
Stent Placement
Following biliary drainage, a permanent implant to maintain duct patency helps maintain patients' quality of life ( Fig. 5 ). The first available devices were plastic endoprostheses, but these were limited by high rates of occlusion and migration. 39 The use of metal stents increased potential luminal diameter, raising patency rates and decreasing need for reintervention. 33 Metal stent patency at 6 months ranges from 43 to 81%. 40
Fig. 5.
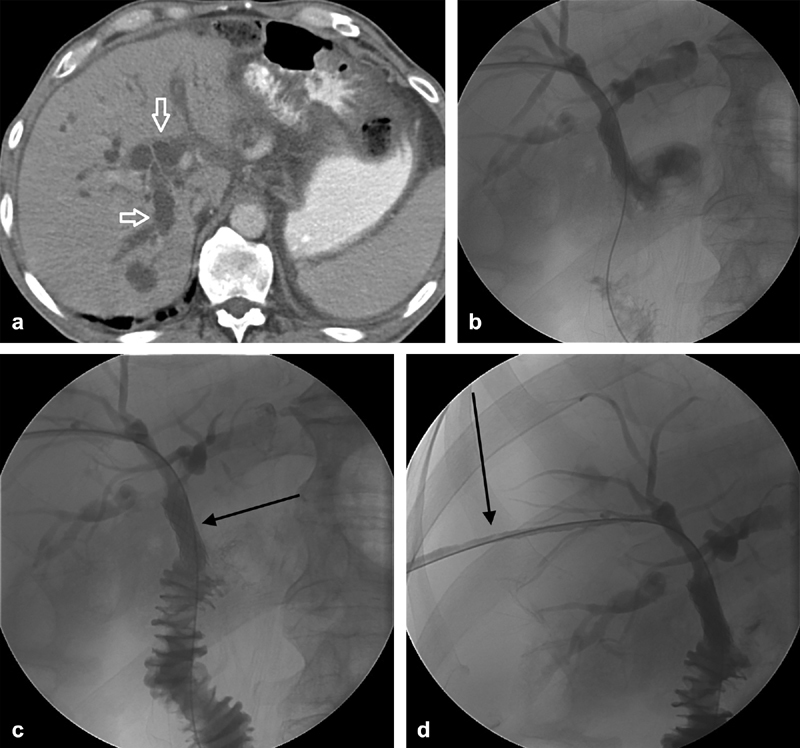
A 63-year-old man who had previously undergone a Whipple procedure with a Roux-en-Y anastomosis developed recurrence at the resection margin with biliary dilation ( a , arrows). Percutaneous cholangiography and drainage were performed ( b ) followed by metal stent placement ( c , arrow) at the anastomosis. Follow-up cholangiography documented stent patency and a mature bilicutaneous tract (arrow, d ) and the tube was removed.
Covered stents have improved patency. Randomized prospective trials comparing covered versus bare metal stents demonstrated superior patency rates with covered stents. Isayama et al reviewed 112 patients and demonstrated stent occlusion in 8 patients (14%) after a mean of 304 days in the covered group, compared with 21 patients (38%) at a mean of 166 days in the bare group. 41 Cumulative patency of covered stents was also significantly higher than that of uncovered stents ( p = 0.0066). Krokidis et al demonstrated covered stents significantly improved primary patency rates compared with bare stents ( p = 0.007). 42 Covered stents are more prone to migration. In a prospective randomized trial, zero uncovered and eight (12%) covered stents migrated ( p = 0.0061). 43
Complications of Percutaneous Transhepatic Biliary Drainage and Stent Placement
Interventional radiology can manage the vast majority of procedural complications. 33 One of the most common immediate complications is hemobilia. Most cases are related to drain sideholes crossing a PV branch. These patients can be managed by drain repositioning or upsizing. The drainage of bright red blood and/or hemodynamic instability should generate heightened index of suspicion of an arterial bleed. 44 Fidelman et al reported arterial injury in 2.2% of drainage procedures ( Fig. 6 ). Onset can be delayed weeks to months following drainage due to tamponade by the drainage catheter. 45 At angiography, both the catheter and groin should be sterilely prepped. The bleeding site may initially be obscured due to tamponade from the catheter. Repeat angiography after catheter removal over a guidewire may help identify the injured branch and facilitate embolization.
Fig. 6.
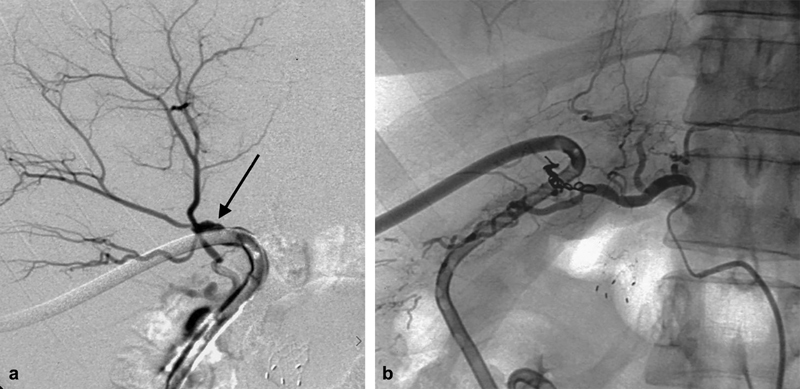
A 52-year-old woman status post biliary drainage for pancreatic malignancy reported bright red blood in her drainage bag. Initial cholangiography revealed communication with the hepatic artery (arrow, a ). Emergent embolization was performed and final angiography demonstrated no further communication ( b ).
Interventional Radiology Management of Postoperative Complications following Pancreaticoduodenectomy
Pancreatic Leak
The most common postoperative complication following pancreatic resection is the development of an abdominal fluid collection or abscess resulting from a pancreatic, biliary, or enteric leak. The incidence of such fluid collections ranges from 9 to 34% depending on the extent of pancreatic resection. 46 47 These fluid collections may be managed conservatively, though interventional radiology is consulted if the clinical status of the patient worsens or surgically placed drains do not adequately manage the collection. A large portion of postoperative fluid collections/abscesses are caused by pancreatic fistulas. Postoperative pancreatic fistula (POPF) represents a failure of healing/sealing of a pancreatic-enteric anastomosis or parenchymal leak not directly related to an anastomosis. 48 Clinical findings include abdominal pain, distension, delayed gastric emptying, fever, white blood cell count greater than 10,000, and increasing C-reactive protein. The presence of a POPF is defined as any fluid collection with an amylase three times or greater than that of serum on postoperative day 3 or more. 48 According to the International Study Group on Pancreatic Fistulas (ISGPF), POPFs are graded A, B, or C according to the clinical impact on the patient's hospital course. 49 Grade B POPF involves radiology simply adjusting existing drain placement, whereas grade C involves new image-guided drain placement or relaparotomy. Large-bore (up to 24F) catheters may be needed for purulent drainage and/or viscous pancreatic contents. 33 Undrained pancreatic fluid collections containing pancreatic enzymes can erode into blood vessels resulting in post-pancreatectomy hemorrhage (PPH). Baker et al reported that percutaneous drainage of abdominal fluid collections secondary to POPF was the most beneficial adjunctive therapy with 94% of fistula patients being treated successfully with only minimally invasive procedures. 49 Sohn et al have reported that only 4.8% of patients undergoing postoperative abscess drainage later required reexploration. 50
Biliary Leak
Postoperative biliary complications, specifically those directly related to hepaticoenterostomy, remain a major cause of morbidity to patients. The incidence of bile leakage after hepaticojejunostomy ranges from 0.4 to 8%. 51 Similar to pancreatic leaks, the International Study Group of Liver Surgery (ISGLS) defines bile leakage as bilirubin concentration in drain fluid that is at least three times the serum bilirubin concentration on or after postoperative day 3 or the need for additional intervention in associated bilomas. 52 Postoperative bile leakage is graded A, B, or C based on impact on management: grades B and C involve active intervention without and with relaparotomy, respectively. Interventional radiology assists in the treatment of postoperative bile leakage by performing percutaneous transhepatic cholangiography (PTC) and/or PTBD. PTBD decreases pressure within the bile ducts, assisting healing at the disrupted anastomosis. 49 In a nondilated biliary system, clinical success is feasible in 65 to 75% of attempts. 34 These drainages are more challenging ( Fig. 7 ) due to the diminutive nature of nondilated ducts and rapid intraductal contrast drainage in the absence of a downstream obstruction. 33 In the series published by Baker et al, no patients who underwent PTC/PTBD required relaparotomy for treatment of biliary complications. However, 56% of patients with PTBD also required percutaneous drainage of additional intra-abdominal fluid collections. 49
Fig. 7.
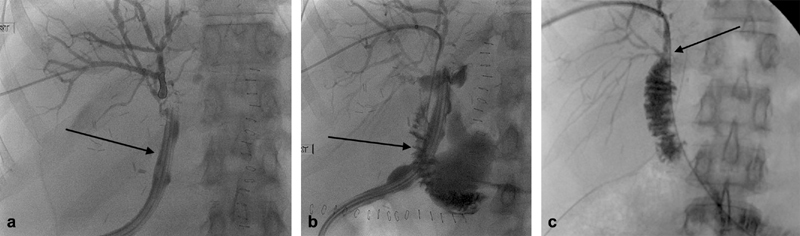
A 44-year-old woman with bilious output from her surgical drain following a Whipple. The ducts were nondilated, and after access was successfully achieved, cholangiography demonstrated primary output into the bowel via the drain (arrow, a ). The draining jejunal limb was able to be accessed (arrow, b ) and the leak healed after several weeks of drainage (arrow, c ).
Postpancreatectomy Hemorrhage
When comparing the major postoperative complications of pancreatic resection, PPH has the lowest incidence (<10%), but highest mortality (up to 38%). 46 53 Yekebas et al concluded that PPH alone was a predictor of increased mortality, as 16% of patients with PPH died compared with 2.3% without PPH. 54 The International Study Group of Pancreatic Surgery grading system (A, B, or C) for PPH is based on three factors: onset, location, and severity. 46 Time of onset is broken up into early and late PPH. Early PPH occurs less than 24 hours postoperatively and is often caused by stump insufficiency due to technical failure of GDA ligation or perioperative coagulopathy. Late PPH occurs greater than 24 hours postoperatively. Late PPH is caused by complications from the operation, including the following: intra-abdominal abscess, ulceration at the anastomosis, erosion of a vessel secondary to pancreatic fistula, or a pseudoaneurysm. The location of PPH is classified as either intraluminal (ILH) or intra-abdominal/extraluminal hemorrhage (ELH). Findings of ILH include hematemesis, bloody nasogastric tube aspirate, and melena. ELH can present with hemorrhage via surgical drains. Initial management of ILH is usually endoscopic, while ELH is more commonly managed by interventional radiology. It should be noted that a scenario of false ELH can occur secondary to ILH with coexisting anastomotic disruption. The severity of PPH is classified as mild (<3 g/dL hemoglobin decrease with no clinical decompensation) or severe (decrease in hemoglobin > 4 g/dL and/or need for reintervention). 46
Interventional radiologist participates in the diagnosis and treatment of PPH via embolization or potentially endovascular-covered stent placement ( Fig. 8 ). Prior to angiography, computed tomographic angiography (CTA) is typically obtained which characterizes the cause, site, and nature of bleeding. Unenhanced, arterial, and venous phases in the CTA are imperative to accurately localize the source of bleeding. 53 PPH can be preceded by a sentinel bleed that usually presents as low-volume bleeding without hemodynamic instability. This clinical scenario is an indication for immediate angiography, as there is a 76% incidence of PPH within 14 to 85 hours from the sentinel bleed. 54 55 The most common site of major PPH is the GDA stump. However, the common hepatic artery (CHA), superior mesenteric artery (SMA) branches, and splenic artery/vein can all be a source of PPH. Even in the setting of an unidentifiable source on CTA, catheter angiography of the celiac axis and SMA is a reasonable next step, given the high associated mortality. Vasospasm and vessel irregularity are indirect signs of bleeding sources and empiric embolization is often reasonable. 53 Embolization as a definitive therapy has a success rate ranging from 77 to 88%. 33 Covered stents are used for defects in the common hepatic or superior mesenteric arteries to preserve distal flow ( Fig. 9 ). Involvement of the CHA or other major large blood vessels can result in more rapid extravasation with associated morbidity and mortality. If a pseudoaneurysm persists after embolization, direct thrombin injection from a percutaneous approach may be attempted if the site is appropriately accessible. 56 The use of a multidisciplinary approach to treat PPH can greatly reduce patient morbidity and improve outcomes. In fact, a series published by Sanjay et al showed no patient required a relook laparotomy for uncontrolled bleeding after interventional radiology was successful in stenting or embolization. This is of great significance, considering relaparotomy in this patient population has a mortality rate of 13 to 60%. 57
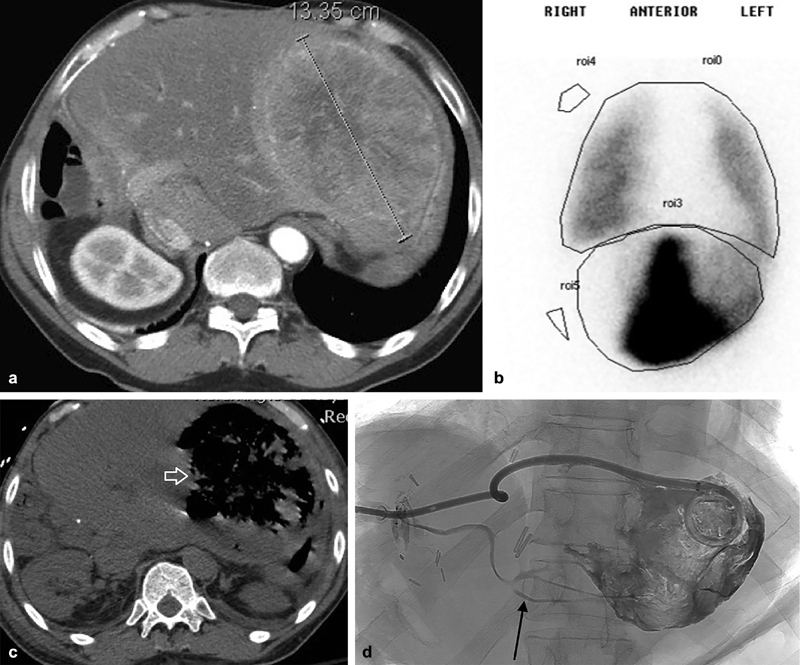
Fig. 8.
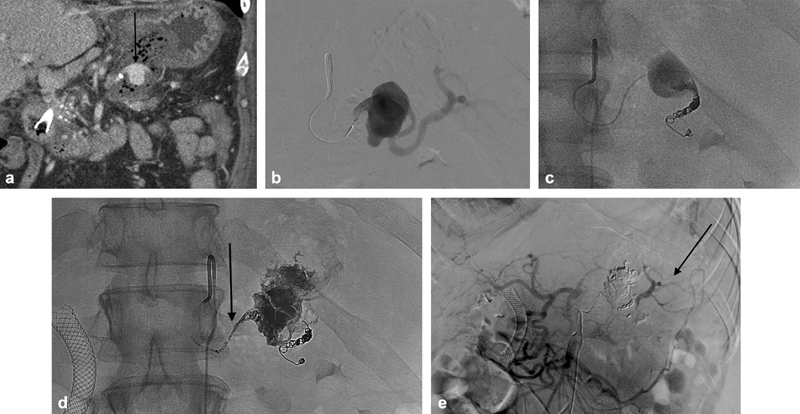
A 55-year-old man with postsurgical pancreatitis resulting in hematocrit drop and sanguineous output via his surgical drain. Computed tomography revealed a sizable pseudoaneurysm in the splenic artery (arrow, a ). Angiography ( b ) demonstrated significant outflow and the distal splenic artery was catheterized and coiled ( c ). Ethyl vinyl copolymer was injected to fill the pseudoaneurysm and feeding artery (arrow, d ). Final angiography was performed via the superior mesenteric artery and demonstrated occlusion of the pseudoaneurysm with downstream perfusion of the spleen (arrow, e ) via collaterals.
Fig. 9.
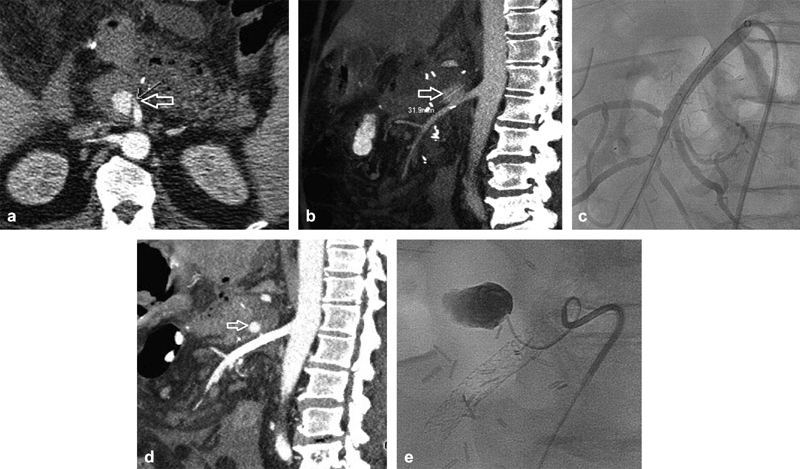
An 81-year-old man following a Whipple with pancreatic leak and a pseudoaneurysm arising from the superior aspect of the proximal superior mesenteric artery (arrow, a, b ). This was crossed and a balloon expandable covered stent was placed ( c ). Follow-up imaging demonstrated persistent filling (arrow, d ) and a type 1 endoleak was suspected. A microcatheter was advanced alongside the covered stent ( e ) and ethyl vinyl copolymer was injected. The proximal stent graft was dilated and the leak resolved.
Conclusion
Interventional radiology plays an integral role in managing surgical complications following attempted curative resection of pancreatic adenocarcinoma. There may be further growth opportunities in CT-guided IRE based on the lower toxicity compared with open ablation, although the optimal utilization of this technology needs further research. Arterial and ablative approaches have a role in treating appropriately selected patients with liver metastases or oligometastatic disease elsewhere.
Footnotes
Conflicts of Interest D.B.B. receives research support from Sirtex Medical and is a consultant for BTG, Astra-Zeneca, and Becton-Dickinson/Bard.
The other authors have no conflicts to disclose.
References
- 1.Cancer Facts & Figures2018. | American Cancer Society. Available at:https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. Accessed April 9, 2019
- 2.Gilbert J W, Wolpin B, Clancy T et al. Borderline resectable pancreatic cancer: conceptual evolution and current approach to image-based classification. Ann Oncol. 2017;28(09):2067–2076. doi: 10.1093/annonc/mdx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammel P, Huguet F, van Laethem J L et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 4.Rossi M L, Rehman A A, Gondi C S. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20(32):11142–11159. doi: 10.3748/wjg.v20.i32.11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neoptolemos J P, Stocken D D, Friess H et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Post S, Neuhaus P et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(03):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 7.Regine W F, Winter K A, Abrams R A et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(09):1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 8.Burris H A, III, Moore M J, Andersen J et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(06):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff D D, Ervin T, Arena F P et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Onofrio M, Barbi E, Girelli R et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: an overview. World J Gastroenterol. 2010;16(28):3478–3483. doi: 10.3748/wjg.v16.i28.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varadhachary G R, Tamm E P, Abbruzzese J L et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(08):1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.de Liguori Carino N, O'Reilly D A, Siriwardena A K et al. Irreversible Electroporation in pancreatic ductal adenocarcinoma: Is there a role in conjunction with conventional treatment? Eur J Surg Oncol. 2018;44(10):1486–1493. doi: 10.1016/j.ejso.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 14.Pezzilli R, Serra C, Ricci C et al. Radiofrequency ablation for advanced ductal pancreatic carcinoma: is this approach beneficial for our patients? A systematic review. Pancreas. 2011;40(01):163–165. doi: 10.1097/MPA.0b013e3181eab751. [DOI] [PubMed] [Google Scholar]
- 15.Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007;6(04):255–260. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 16.Lee E W, Thai S, Kee S T. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010;4 01:S99–S104. doi: 10.5009/gnl.2010.4.S1.S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower M, Sherwood L, Li Y, Martin R. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol. 2011;104(01):22–28. doi: 10.1002/jso.21899. [DOI] [PubMed] [Google Scholar]
- 18.Rashid M F, Hecht E M, Steinman J A, Kluger M D. Irreversible electroporation of pancreatic adenocarcinoma: a primer for the radiologist. Abdom Radiol (NY) 2018;43(02):457–466. doi: 10.1007/s00261-017-1349-3. [DOI] [PubMed] [Google Scholar]
- 19.Martin R C, II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215(03):361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Moris D, Machairas N, Tsilimigras D I et al. Systematic review of surgical and percutaneous irreversible electroporation in the treatment of locally advanced pancreatic cancer. Ann Surg Oncol. 2019;26(06):1657–1668. doi: 10.1245/s10434-019-07261-7. [DOI] [PubMed] [Google Scholar]
- 21.Scheffer H J, Vroomen L G, de Jong M C et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology. 2017;282(02):585–597. doi: 10.1148/radiol.2016152835. [DOI] [PubMed] [Google Scholar]
- 22.Huang K W, Yang P C, Pua U et al. The efficacy of combination of induction chemotherapy and irreversible electroporation ablation for patients with locally advanced pancreatic adenocarcinoma. J Surg Oncol. 2018;118(01):31–36. doi: 10.1002/jso.25110. [DOI] [PubMed] [Google Scholar]
- 23.Narayanan G, Hosein P J, Beulaygue I C et al. Percutaneous image-guided irreversible elecroporation for treatment of locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol. 2017;28:342–348. doi: 10.1016/j.jvir.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Akinwande O, Ahmad S S, Van Meter T, Schulz B, Martin R C. CT findings of patients treated with irreversible electroporation for locally advanced pancreatic cancer. J Oncol. 2015;2015:680319. doi: 10.1155/2015/680319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz M H, Fleming J B, Bhosale P et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 26.Martin R C, II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20 03:S443–S449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 27.Martin R C, II, Kwon D, Chalikonda Set al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy Ann Surg 201526203486–494., discussion 492–494 [DOI] [PubMed] [Google Scholar]
- 28.Holland M M, Bhutiani N, Kruse E Jet al. A prospective, multi-institution assessment of irreversible electroporation for treatment of locally advanced pancreatic adenocarcinoma: initial outcomes from the AHPBA pancreatic registryHPB (Oxford)2019. Doi: 10.1016/j.hpb.2018.12.004. [Epub ahead of print] [DOI] [PubMed]
- 29.Vogel J A, Rombouts S J, de Rooij T et al. Induction chemotherapy followed by resection or irreversible electroporation in locally advanced pancreatic cancer (IMPALA): a prospective cohort study. Ann Surg Oncol. 2017;24(09):2734–2743. doi: 10.1245/s10434-017-5900-9. [DOI] [PubMed] [Google Scholar]
- 30.Young R, Mainwaring P, Clingan P et al. nab-Paclitaxel plus gemcitabine in metastatic pancreatic adenocarcinoma: Australian subset analyses of the phase III MPACT trial. Asia Pac J Clin Oncol. 2018;14(05):e325–e331. doi: 10.1111/ajco.12999. [DOI] [PubMed] [Google Scholar]
- 31.Khan W, Sullivan K L, McCann J W et al. Moxifloxacin prophylaxis for chemoembolization or embolization in patients with previous biliary interventions: a pilot study. AJR Am J Roentgenol. 2011;197(02):W343-5. doi: 10.2214/AJR.10.6019. [DOI] [PubMed] [Google Scholar]
- 32.Kim A Y, Frantz S, Brower J, Akhter N. Radioembolization with Yttrium-90 microspheres for the treatment of liver metastases of pancreatic adenocarcinoma: a multicenter analysis. J Vasc Interv Radiol. 2019;30(03):298–30400. doi: 10.1016/j.jvir.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Brown D B, Narayanan G. Interventional radiology and the pancreatic cancer patient. Cancer J. 2012;18(06):591–601. doi: 10.1097/PPO.0b013e3182745bee. [DOI] [PubMed] [Google Scholar]
- 34.Saad W E, Wallace M J, Wojak J C, Kundu S, Cardella J F. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21(06):789–795. doi: 10.1016/j.jvir.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Tempero M A, Malafa M P, Al-Hawary M et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(08):1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 36.Shibata T, Ebata T, Fujita K et al. Optimal dose of gemcitabine for the treatment of biliary tract or pancreatic cancer in patients with liver dysfunction. Cancer Sci. 2016;107(02):168–172. doi: 10.1111/cas.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton R H, Ulrich R, Hsu M et al. Outcomes of patients undergoing percutaneous biliary drainage to reduce bilirubin for administration of chemotherapy. J Vasc Interv Radiol. 2012;23(01):89–95. doi: 10.1016/j.jvir.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Eklund J W, Trifilio S, Mulcahy M F.Chemotherapy dosing in the setting of liver dysfunction Oncology (Williston Park) 200519081057–1063., discussion 1063–1064, 1069 [PubMed] [Google Scholar]
- 39.Lammer J, Hausegger K A, Flückiger F et al. Common bile duct obstruction due to malignancy: treatment with plastic versus metal stents. Radiology. 1996;201(01):167–172. doi: 10.1148/radiology.201.1.8816539. [DOI] [PubMed] [Google Scholar]
- 40.Bezzi M, Zolovkins A, Cantisani V et al. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol. 2002;13(06):581–589. doi: 10.1016/s1051-0443(07)61651-0. [DOI] [PubMed] [Google Scholar]
- 41.Isayama H, Komatsu Y, Tsujino T et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53(05):729–734. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krokidis M, Fanelli F, Orgera G et al. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovasc Intervent Radiol. 2011;34(02):352–361. doi: 10.1007/s00270-010-9880-4. [DOI] [PubMed] [Google Scholar]
- 43.Telford J J, Carr-Locke D L, Baron T H et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72(05):907–914. doi: 10.1016/j.gie.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Venkatanarasimha N, Damodharan K, Gogna A et al. Diagnosis and management of complications from percutaneous biliary tract interventions-erratum. Radiographics. 2017;37(03):1004. doi: 10.1148/rg.2017174004. [DOI] [PubMed] [Google Scholar]
- 45.Fidelman N, Bloom A I, Kerlan R K, Jr et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology. 2008;247(03):880–886. doi: 10.1148/radiol.2473070529. [DOI] [PubMed] [Google Scholar]
- 46.Wente M N, Veit J A, Bassi C et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(01):20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Cronin C G, Gervais D A, Castillo C F-D, Mueller P R, Arellano R S. Interventional radiology in the management of abdominal collections after distal pancreatectomy: a retrospective review. AJR Am J Roentgenol. 2011;197(01):241–246. doi: 10.2214/AJR.10.5447. [DOI] [PubMed] [Google Scholar]
- 48.Bassi C, Dervenis C, Butturini G et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(01):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Baker T A, Aaron J M, Borge M, Pierce K, Shoup M, Aranha G V.Role of interventional radiology in the management of complications after pancreaticoduodenectomy Am J Surg 200819503386–390., discussion 390 [DOI] [PubMed] [Google Scholar]
- 50.Sohn T A, Yeo C J, Cameron J L et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg. 2003;7(02):209–219. doi: 10.1016/s1091-255x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 51.de Castro S M, Kuhlmann K F, Busch O Ret al. Incidence and management of biliary leakage after hepaticojejunostomy J Gastrointest Surg 20059081163–1171., discussion 1171–1173 [DOI] [PubMed] [Google Scholar]
- 52.Koch M, Garden O J, Padbury R et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(05):680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Puppala S, Patel J, McPherson S, Nicholson A, Kessel D. Hemorrhagic complications after Whipple surgery: imaging and radiologic intervention. AJR Am J Roentgenol. 2011;196(01):192–197. doi: 10.2214/AJR.10.4727. [DOI] [PubMed] [Google Scholar]
- 54.Yekebas E F, Wolfram L, Cataldegirmen G et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246(02):269–280. doi: 10.1097/01.sla.0000262953.77735.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tien Y W, Wu Y M, Liu K L, Ho C M, Lee P H. Angiography is indicated for every sentinel bleed after pancreaticoduodenectomy. Ann Surg Oncol. 2008;15(07):1855–1861. doi: 10.1245/s10434-008-9894-1. [DOI] [PubMed] [Google Scholar]
- 56.Wallace M J, Choi E, McRae S, Madoff D C, Ahrar K, Pisters P. Superior mesenteric artery pseudoaneurysm following pancreaticoduodenectomy: management by endovascular stent-graft placement and transluminal thrombin injection. Cardiovasc Intervent Radiol. 2007;30(03):518–522. doi: 10.1007/s00270-006-0109-5. [DOI] [PubMed] [Google Scholar]
- 57.Sanjay P, Kellner M, Tait I S. The role of interventional radiology in the management of surgical complications after pancreatoduodenectomy. HPB (Oxford) 2012;14(12):812–817. doi: 10.1111/j.1477-2574.2012.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


