Abstract
The BTBR T+ Itpr3tf/J (BTBR) mouse strain is a widely used model of autism spectrum disorder (ASD). The BTBR mice display behavior consistent with the three diagnostic categories of ASD. However, the behavioral phenotypes of the BTBR mice in a long-term group housing setting are largely unknown because conventional behavioral tests for ASD model mice are designed for use under simplified artificial conditions over a short observation period. In this study, we applied a newly developed assay system, the Multiple Animal Positioning System (MAPS), to quantify behaviors under group housing conditions over four days of continuous observation. Using MAPS, we showed that in a group housing condition, the BTBR mice exhibited lower activity levels in the dark phase and alteration of social behavior in comparison with the C57BL/6J mice. The phenotypes of the BTBR mice were affected by co-housing with the C57BL/6J mice for four days, but the influence was weak and limited. Our results by MAPS differ from those obtained using conventional behavioral tests. The present study demonstrated that MAPS would be useful for evaluating the usual/natural behaviors of various animal models in detail and under more ethological conditions.
Keywords: autism spectrum disorder, behavior, mouse model, social housing
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired social interaction and communication and repetitive behaviors [1, 32, 34]. To elucidate the biological basis of ASD and develop treatments for its symptoms, not only clinical research, but also experimental basic research using animal models, is required. The inbred BTBR T+ Itpr3tf/J (also known as BTBR T+ tf/J) mouse strain is a widely used model of ASD. The BTBR T+ Itpr3tf/J strain is derived from the inbred strain BTBR (Black and Tan BRachyury). L.C. Dunn developed the BTBR T+ Itpr3tf/J from stock obtained from Dobrovolskaia-Zavadskaia, and inserted tufted (Itpr3tf) as a marker [14]. The BTBR T+ Itpr3tf/J (hereinafter referred to as BTBR) mice carry a deletion of the Disc1 (disrupted in schizophrenia 1) gene, which is a candidate gene for schizophrenia and other major mental disorders [6, 9, 11]. BTBR mice lack a corpus callosum, show a reduction in hippocampal commissure [31], and display behaviors consistent with the three diagnostic categories of ASD [1]. Previous studies using conventional behavioral tests have indicated that the BTBR mice exhibit reduced social interactions, unusual vocalizations, and increased repetitive behaviors in comparison with other inbred strains [2, 5, 20, 23, 27, 28]. However, conventional behavioral tests for ASD model mice are designed for use under simplified artificial conditions over a short observation period (i.e., several minutes). Consequently, the behavioral phenotypes of the BTBR mice in group housing settings over a long-term observation period are largely unknown.
There are some reports of long-term behavioral analyses among multiple animals in natural group settings. Moy et al. showed that the BTBR mice built nests from nesting materials, slept together in huddles, and did not display any unusual levels of activity or fighting during the home-cage observation periods [23]. On the other hand, Pobbe et al. reported that the BTBR mice showed reductions in all interactive behaviors and an increase in self-grooming and time spent alone in comparison with the C57BL/6J (B6) mice [25]. The inconsistent results among these studies can be attributed to the timing and/or period of observation used for behavioral scoring. The traditional human observation-based approaches are limited in the number of possible observation periods per day because they require tremendous amounts of work and time to conduct. To solve the problem of the temporal coverage of observation periods and produce a high-throughput analysis system, we recently developed a video-based behavioral analysis system for multiple small animals, called the Multiple Animal Positioning System (MAPS), to monitor behaviors of mouse models over a long-term period under group housing conditions [8]. The first aim of the present study was to characterize the behaviors of the BTBR mice for several days under group housing conditions using MAPS.
In our previous study, MAPS revealed that mice subjected to social isolation after weaning exhibit impaired social proximity in adulthood. The phenotype of these socially isolated mice was partially rescued by cohabitation with group-housed mice [8]. Furthermore, we showed that social isolation after weaning causes alterations in prefrontal cortex function and myelination that do not recover following re-socialization with socially isolated mice, but that can be restored by re-socialization with group-housed mice [17, 19]. These findings indicate that the housing condition of the mice that socially isolated mice re-socialize with is a key determinant for restoring behavioral and neurobiological phenotypes. In the BTBR mice, Yang et al. reported that cross-rearing with the B6 mice after weaning significantly resolved sociability deficits [37]. Recently, we showed that the BTBR mice exhibit thinner myelin in the medial prefrontal cortex (mPFC) and that co-housing with the B6 mice after weaning increased the myelin thickness in the mPFC of the BTBR mice [18]. Therefore, the second aim of the present study was to validate if co-housing with the B6 mice can change the behavior of the BTBR mice.
Materials and Methods
Animal experiments
The Animal Care Committee of Nara Medical University approved the animal experimental protocols used in this study. Mice were housed in the animal facility at a temperature of 22–25°C and humidity of 40–60% on a 12/12 h light/dark cycle (lights on: 8:00–20:00) with free access to food and water. Breeding pairs of the C57BL/6JJcl (B6) and BTBR T+ Itpr3tf/J (JAX stock No. 002282; BTBR) inbred strains of mice were purchased from CLEA Japan (Tokyo, Japan) and Charles River Lab Japan (Yokohama, Japan), respectively, and bred in the animal facilities at Nara Medical University. The day of birth was defined as postnatal day 0 (PD 0). Male pups were weaned at PD 21 and group-housed with same-sex siblings (2–5 pups/cage) until the experiments were conducted.
Behavioral experiments using MAPS
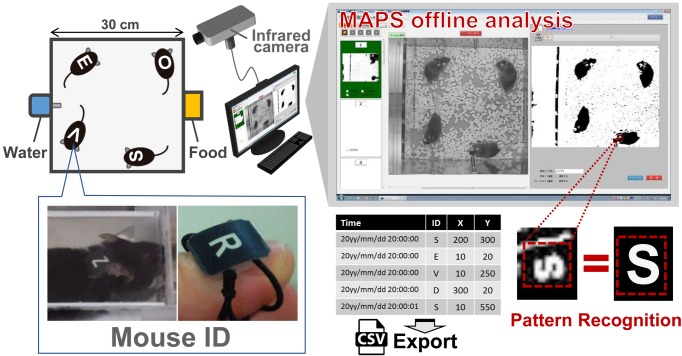
To quantify behavior under group housing conditions, we applied our newly developed assay system, the Multiple Animal Positioning System (MAPS) [8], which is now distributed by O’Hara & Co., Ltd. (Tokyo, Japan). MAPS is a video analysis-based system that can automatically obtain precise individual mouse localization in X-Y coordinates under group housing conditions. MAPS takes infrared images (1 frame per s; fps) under infrared illumination and performs automated pattern matching-based ID identification. In experiments using MAPS, a mouse ID occasionally disappears from the image during long-term group housing (e.g., when mice overlap or their bodies are tilted). When MAPS loses a mouse ID, the lost X-Y coordinate is supplemented with previous data from the coordinates at which the ID was last identified.
Before the behavioral experiments, each mouse was marked with a unique symbol (mouse ID; Fig. 1) on its back using an elastic string while under chloral hydrate anesthesia (400 mg/kg, intraperitoneally). The mouse was given a period of time to habituate to the ID, which continued until the mice huddled together. For the behavioral tests, four adult male mice that had not previously been exposed to one another were placed in an experimental cage (30 × 30 × 25 cm; Fig. 1) from 14:00 to 15:00 on Day 1 and observed continuously for 4 days by MAPS. In the experiments, three combinations of mice, i.e., four B6 mice (B6-only), four BTBR mice (BTBR-only), and two B6 mice and two BTBR mice (B6-BTBR-mix), were used. Behavioral experiments began when the mice were 8–24 weeks old. Age-matched animals were used within an experimental cage for both the B6 and the BTBR mice (Supplementary Table 1 and Supplementary Fig. 1).
Fig. 1.
Schematic of the Multiple Animal Positioning System (MAPS). Adult male mice were individually tagged with a mouse ID (‘uniform number’) on their backs. An infrared camera took an image each second; the software analyzed the images using a pattern recognition technique. The software recognized the ID of each mouse and recorded the X-Y coordinates. If the ID of a mouse could not be recognized, the coordinates of when the ID was last recognized were used. The coordinate data were exported as .csv files and used for analyzing the behavior of each mouse.
Data collection for MAPS experiments
The positioning data from each mouse were exported in comma-separated values (.csv) form from MAPS. Data processing was carried out using Microsoft R Open 3.4.3 (https://mran.microsoft.com/open).
Locomotor activity: Locomotor activity was measured as the total distance traveled (m). In the novel environment, the total distance traveled was calculated during the first 60 min after the introduction of the mice to MAPS. In the familiar environment, the total distance traveled was quantified each hour or 12 h after the beginning of the dark phase (20:00) on Day 1.
Social behavior: We defined a social interaction area for each mouse as a circle 60 mm in radius surrounding the center of the mouse ID. ‘Alone’ was defined as a lack of other mice in the social interaction area. The ‘percent alone’ was calculated as the ratio of time that a mouse spent alone. The ‘duration of social interaction’ was defined as the total time when inter-individual distance was less than 60 mm, i.e., the time that two individuals stayed within the social interaction area.
Conventional behavioral experiments
We performed three conventional behavioral tests: the open-field test, the object investigation test, and the social investigation test (Supplementary Fig. 5). Behavioral experiments began when the mice were eight weeks old. All behavioral indexes were analyzed using TopScanLite 2.00 (CleverSys Inc., Reston, VA, USA).
Open-field test: Mice were tested for 20 min in an open-field apparatus. The apparatus consisted of a square arena (39 × 39 × 34 cm), with a light gray floor and wall. At the beginning of the test a mouse was gently placed in a corner square. Total distance traveled (pixel) was calculated for every 5 min time period. The center area was defined as the center 50% of the apparatus. The ‘time spent in center area (%)’ was calculated as the ratio of time that a mouse spent in the center area in each 5 min time period.
Object investigation test: To examine behavioral responses to a novel object, the day after the open-field test mice were tested for 10 min in the open-field apparatus in the presence of an object stimulus, which was a clear Plexiglas cylinder (Mouse Cylinder SIOT, O’Hara & Co., Ltd.) with a white cover made by a 3D printer (Dreamer, FlashForge Corporation, Zhejiang, China) (Supplementary Fig. 5). The floor of the apparatus was hypothetically divided into nine square sections. The cylinder was placed in one corner section, defined as the object corner. At the beginning of the test a mouse was gently placed in a corner next to the object corner. The ‘time spent in the object corner (%)’ was calculated as the ratio of time that a mouse spent in the object corner. This object investigation test also serves as an acclimation phase for the social investigation test.
Social investigation test: To examine behavioral responses to a social stimulus, four hours after the object investigation test, mice were tested for 10 min in the open-field apparatus. Social investigation behaviors were assessed by exposure to a cylinder containing an unfamiliar adult male mouse that was the same strain as the subject mouse. The cylinder was placed in one corner of the open field apparatus (social corner). At the beginning of the test a mouse was gently placed in a corner next to the social corner. The ‘time spent in the social corner (%)’ was calculated as the ratio of time that a mouse spent in the social corner.
Statistical analysis
Values are expressed as the mean ± SE. Data were analyzed using the Welch two-sample t-test and a one-way ANOVA. Tukey’s test was used for post hoc multiple comparisons. Spearman correlation was used for correlation analyses. Statistical significance was set at P<0.05. Graph generation and statistical analyses were carried out using GraphPad Prism 7.0.4 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Decreased locomotor activity during the dark phase in the BTBR mice
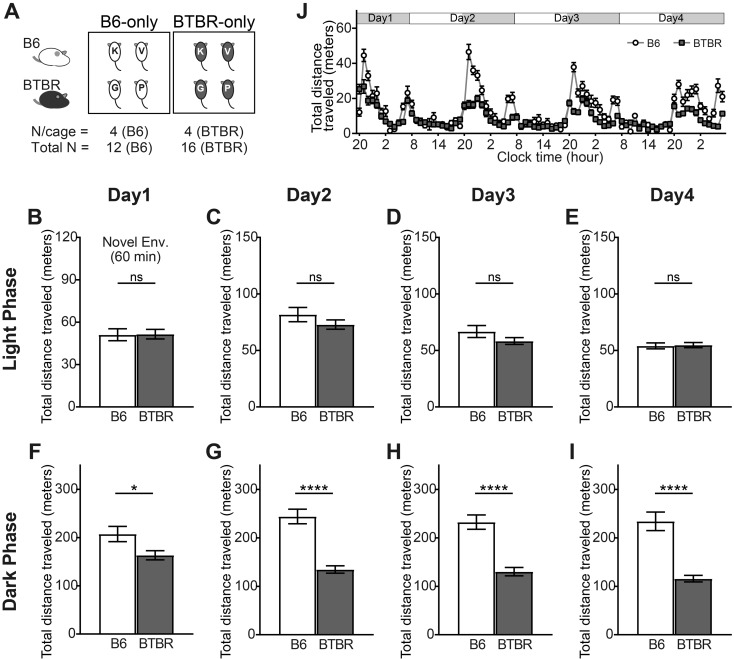
Four adult male mice that had not previously been exposed to one another were introduced in one of the experimental MAPS cages between the hours of 14:00 and 15:00 during the light phase of Day 1. The behavior of the mice was recorded continuously for 4 days using MAPS (Fig. 1). The first experiment was designed to detect the behavioral differences between the B6 and BTBR mice for each of two combinations of mice, i.e., four B6 mice (B6-only) and four BTBR mice (BTBR-only) (Fig. 2A). The locomotor activity of the BTBR mice was comparable to that of the B6 mice in the novel environment after the introduction of the mice to MAPS (Fig. 2B) and during the light phase on Days 2–4 (Figs. 2C–E). In contrast, the BTBR mice exhibited significantly decreased locomotor activity in comparison with the B6 mice during the dark phase on Days 1–4 (Figs. 2F–I). However, the locomotor activity of the BTBR mice peaked just after the beginning of the dark phase (Fig. 2J), suggesting that the BTBR mice maintained a normal circadian rhythm.
Fig. 2.
Activity of B6 and BTBR mice in the B6-only and BTBR-only housing conditions. (A) Experimental design for the B6-only and BTBR-only housing conditions. Four B6 or four BTBR mice (all previously unexposed to one another) were housed per chamber (B6: total n=12; BTBR total: n=16). (B–I) The total distance traveled in the first 60 min after introducing the mice to Multiple Animal Positioning System (MAPS) (novel environment); (B) and during the light and dark phases on Days 1–4 (C–I). Data are shown as the mean ± SE and were analyzed using the Welch two-sample t-test. *P<0.05, ****P<0.0001. (J) The total distance traveled was plotted every 60 min starting at the beginning of the dark phase (20:00) on Day 1. Data are presented as mean ± SE.
Altered social behavior in the BTBR mice under a group housing condition
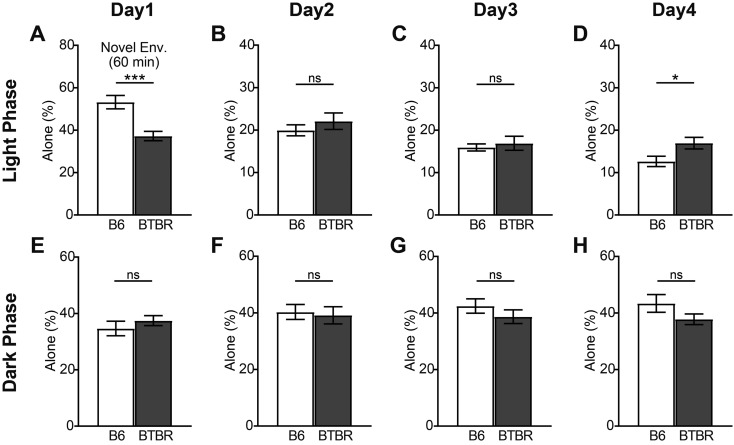
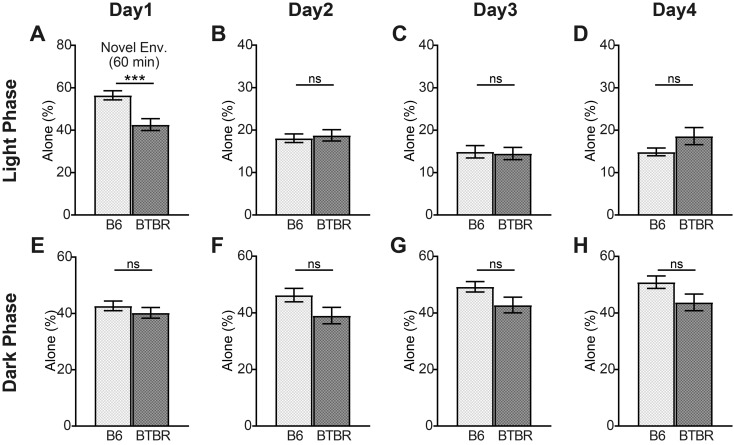
Next, we quantified the time spent alone as an index of social behavior in the B6-only and BTBR-only housing conditions (Fig. 3). In the novel environment, the ratio of time that the BTBR mice spent alone was lower than that of the B6 mice (Fig. 3A). Thereafter, we did not observe any significant differences in the amount of time spent alone until Day 4. The B6 mice showed a tendency to gradually decrease the amount of time spent alone during the light phase. The pattern was reversed during the dark phase, with an increase in time spent alone observed (Supplementary Fig. 2). In contrast, the BTBR mice did not show continually changes in the amount of time spent alone in the light and dark phases (Supplementary Fig. 2). Therefore, the difference between the two mice groups increases with time, and might become significant on the fourth day (Fig. 3D).
Fig. 3.
The amount of time B6 and BTBR mice spent alone in the B6-only and BTBR-only housing conditions. The ratio of time that each mouse spent alone in the first 60 min after introduction to Multiple Animal Positioning System (MAPS) (novel environment) (A); and during the light and dark phases on Days 1–4 (B–H). Data are shown as mean ± SE and were analyzed using a Welch two-sample t-test. *P<0.05, ***P<0.001.
Effects of co-housing with the B6 mice on the behavior of the BTBR mice
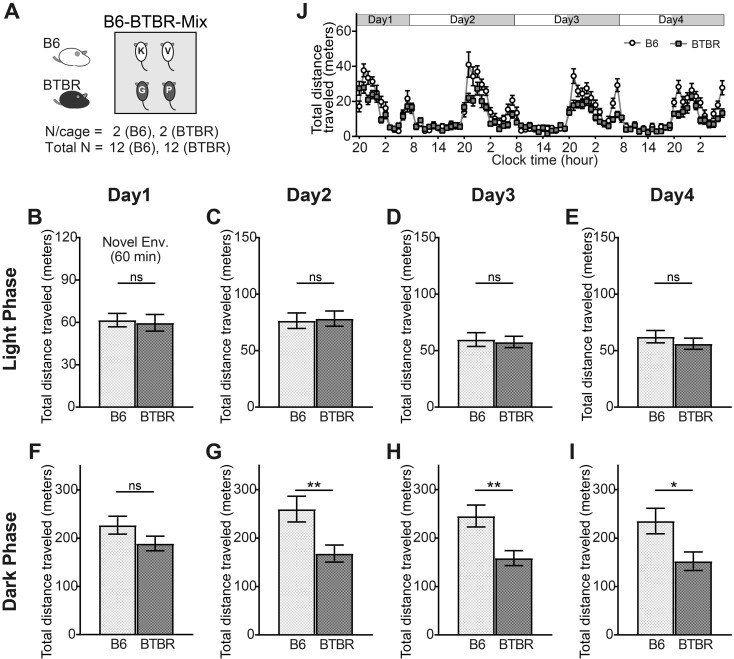
To validate whether the behavior of the BTBR mice was affected by the properties of their cage mates, we examined behavior in the B6-BTBR-mix housing condition, in which two B6 and two BTBR mice were placed in the same experimental cage (Fig. 4A). The lower activity levels of the BTBR mice during the dark phase were maintained in the B6-BTBR-mix condition, except on Day 1 (Figs. 4F–I). There were no significant differences in the level of activity between groups during the light phase (Figs. 4B–E). Additionally, the circadian rhythm of the locomotor activity of both strains of mice remained normal under the B6-BTBR-mix condition (Fig. 4J). The ratio of time that the BTBR mice spent alone significantly decreased in the novel environment (Fig. 5A). The significant differences in the amount of time spent alone during the light phase on Day 4 that were found in the B6-only and BTBR-only conditions (Fig. 3D) disappeared in the B6-BTBR-mix condition (Fig. 5D). These results suggest that there was an effect of co-housing with B6 mice on the behavior of the BTBR mice, but that the influence was weak and limited.
Fig. 4.
Activity of B6 and BTBR mice in the B6-BTBR-mix housing condition. (A) Experimental design for the B6-BTBR-mix housing condition. Two B6 and two BTBR mice (all previously unexposed to one another) were housed per chamber (B6: total n=12; BTBR: total n=12). (B–I) The total distance traveled in the first 60 min after introducing the mice to Multiple Animal Positioning System (MAPS) (novel environment) (B); and during the light and dark phases on Days 1–4 (C–I). Data are shown as mean ± SE and were analyzed using a Welch two-sample t-test. *P<0.05, **P<0.01. (J) The total distance traveled was plotted every 60 min starting at the beginning of the dark phase (20:00) on Day 1. Data are shown as mean ± SE.
Fig. 5.
The amount of time B6 and BTBR mice spent alone in the B6-BTBR-mix housing condition. The ratio of time that each mouse spent alone in the first 60 min after introduction to Multiple Animal Positioning System (MAPS) (novel environment) (A); and during the light and dark phases on Days 1–4 (B–H). Data are shown as mean ± SE and were analyzed using a Welch two-sample t-test. ***P<0.001.
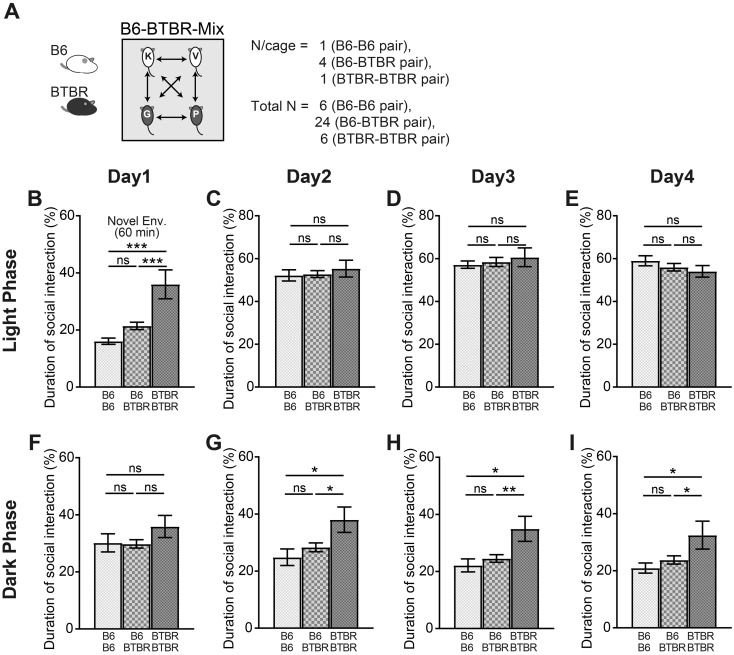
Social interaction preference in the B6-BTBR-mix condition
In the B6-BTBR-mix housing condition, the amount of time spent alone by the BTBR mice was comparable to that of the B6 mice, with the exception of in a novel environment (Fig. 5). We also examined whether individual animals exhibited a social preference by analyzing the time spent with B6 or BTBR mice (Fig. 6A). In a novel environment, the BTBR-BTBR mice pair showed a significant increase in the duration of social interaction compared to the B6-BTBR mice pair, whereas no significant difference in the duration of social interaction was observed between the B6-B6 mice pair and B6-BTBR mice pair (Fig. 6B). During the dark phase, with the exception of Day 1, the BTBR-BTBR mice pair exhibited a significant increase in the duration of social interaction compared to the B6-BTBR mice pair; no difference was found between the B6-B6 mice pair and B6-BTBR mice pair (Figs. 6F–I). In contrast, during the light phases on Days 2–4, there were no differences in the durations of social interaction among all mice pairs (Figs. 6C–E).
Fig. 6.
The amount of time B6 and BTBR mice spent in social interaction in the B6-BTBR-mix housing condition. (A) The experimental subject is a pair of mice (B6-B6 pair, B6-BTBR pair, or BTBR-BTBR pair) in this case, rather than an individual mouse (B6-B6 pair: total n=6; B6-BTBR pair: total n=24; BTBR-BTBR pair: total n=6) (B–I). The amount of time spent in social interaction between each mice pair in the first 60 min after introduction to Multiple Animal Positioning System (MAPS) (novel environment) (B); and during the light and dark phases on Days 1–4 (C–I). Data are shown as mean ± SE and were analyzed using one-way ANOVA and Tukey’s multiple comparison tests. *P<0.05, **P<0.01, ***P<0.001.
Correlation analyses of mouse age and behavioral phenotypes
As shown above, the BTBR mice exhibited lower activity levels in the dark phase and alterations in their social behavior in comparison with the B6 mice. As we used a wide range of animal ages in this study (8–24 weeks, Supplementary Fig. 1), we investigated whether the phenotypes of the BTBR mice were affected by the age of the mouse using data from all three housing conditions (B6-only, BTBR-only, and B6-BTBR-mix housing conditions). There was no significant correlation between mouse age (weeks) and total distance traveled (meters) in the B6 mice during the dark phase on Days 1–4 (Supplementary Fig. 3A and Supplementary Table 2). Likewise, in the BTBR mice, no significant correlation between mouse age (weeks) and total distance traveled (meters) was detected during the dark phase, except on Day 2 (Supplementary Fig. 3B and Supplementary Table 2). Furthermore, there was no significant correlation between mouse age (weeks) and time spent alone (%) in the B6 mice during the light phase. Similarly, no significant difference was found in the BTBR mice during the light phase, except on Day 2 (Supplementary Fig. 4 and Supplementary Table 3). The significant correlation on Day 2 in the BTBR mice cannot explain the phenotype of BTBR mice observed in this study. In the comparison of age between groups, there were no significant differences between the BTBR and B6 mice in the B6-only and BTBR-only housing conditions (Supplementary Fig. 1A), or the B6-BTBR-mix housing conditions (Supplementary Fig. 1B).
Behavioral phenotypes of the BTBR mice, assessed by conventional behavioral tests
Our findings obtained by MAPS under a long-term group housing condition are very different from those observed in previous studies using the conventional open-field test and three-chamber test [20, 23, 35,36,37]. To investigate these differences, we assessed the behavioral phenotypes of the BTBR mice by conventional behavioral tests (Supplementary Fig. 5). The open-field test results showed that BTBR mice exhibited higher locomotor activity than the B6 mice, consistent with previous studies (Supplementary Fig. 6A). Interestingly, locomotor activity of the BTBR mice peaked just after the beginning of the open-field test and then gradually decreased over time, eventually reaching a level comparable with that of the B6 mice (Supplementary Fig. 6A, 6C, 6D). No difference in time spent in the center was found between the B6 and BTBR mice (Supplementary Fig. 6B). These results suggest that the higher activity of the BTBR mice measured by the open-field test is an instantaneous and transient phenotype.
We next examined the behavioral responses of the BTBR mice to novel object and social stimuli. The BTBR mice showed a significant increase in the time spent in the object corner compared to the B6 mice (Supplementary Fig. 7A). In contrast, no difference in the time spent in the social corner was observed between the BTBR and B6 mice (Supplementary Fig. 7B). A comparison of the responses to the object and social stimuli in each group showed that the B6 mice spent significantly more time in the social corner than in the object corner, whereas the BTBR mice spent a comparable amount of time in both corners (Supplementary Fig. 7C).
Discussion
This study investigated the behavioral phenotypes of the BTBR mice continuously for 4 days under group housing conditions using MAPS, a recently developed video-based behavioral analysis system [8]. MAPS revealed that the BTBR mice exhibited significantly less locomotor activity than the B6 mice during the dark phase. Using a computer vision system capable of capturing the behavior of a single mouse in the home-cage environment, Jhuang et al. reported that the total resting time of the BTBR mice was significantly longer than that of the B6 mice [10]. The results obtained from the long-term observation system were inconsistent with previous reports of studies that analyzed behavior using conventional behavioral tests (i.e., open-field test), which showed higher levels of locomotor activity in the BTBR mice [20, 23, 36, 37]. However, our results also show that BTBR mice exhibit hyperactivity in the conventional open-field test, suggesting that inconsistent results between the studies using MAPS (long-term observation system) and studies using a conventional open-field test are not due to differences in the experimental environment between the facilities. These results demonstrate that the analysis of an animal model using conventional behavioral tests under simplified artificial conditions and over short observation durations cannot evaluate the more precise and ethological aspects of the animal model.
The BTBR mouse strain is a widely used model of ASD because of the animals’ reduced sociability, which is consistent with the most important features of the ASD phenotype [2,3,4,5, 7, 10, 20, 22, 23, 25,26,27, 30, 33, 35,36,37,38]. In this study, we quantified the time spent alone as an index of social behavior and showed that the ratio of time that the BTBR mice spent alone was significantly lower than that of the B6 mice in the novel environment. However, the amount of time spent alone increased during the light phase on Day 4 in the B6-only and BTBR-only housing conditions. Although it is difficult to explain the bidirectional change in social behavior that occurred in the BTBR mice, it is possible that the B6 mice changed their behavior according to the change in environment while the behavior of the BTBR mice remained constant. This explanation is inferred from our finding that B6 mice showing a tendency to gradually decrease the amount of time spent alone during the light phase and BTBR showing no continually change, resulting the difference between the two mice groups increases with time. This suggests that the BTBR mice are indifferent to alterations in their surrounding environment and act at their own pace. This behavioral inflexibility of the BTBR mice is consistent with the clinical symptoms of ASD [1].
Then, we examined if co-housing with B6 mice could change the behavior of the BTBR mice. Our previous study revealed that the phenotype of the socially isolated-reared mice after weaning was partially rescued by co-housing with group-housed/reared mice [8]. In contrast, the BTBR mice exhibited a robust phenotype and the influence of co-housing with the B6 mice on this phenotype was weak and limited. However, it is noteworthy that the housing of the BTBR mice with the B6 mice occurred after the animals reached adulthood (8–24 weeks of age) and for only 4 days in this study. It would be interesting to determine if co-housing with the B6 mice for a longer period starting at a younger age would lead to different alterations in the behavioral phenotype of the BTBR mice, particularly because we previously showed that the thinner myelin found in the mPFC of the BTBR mice was rescued by co-housing with B6 mice after weaning [18]. That is, the extent of mPFC myelination may be dependent on social experience with cage mates in early life. Several studies have demonstrated that myelin is required to exert proper brain function [21, 24], and that mPFC myelination plays an important role in the social behavior of mice [15, 16, 19].
Furthermore, BTBR mice exhibited a tendency to spend more time with other BTBR mice than with the B6 mice during the active period (novel environment and dark phase) in the B6-BTBR-mix housing condition. During the dark phase, the BTBR mice showed lower activity than the B6 mice; it is possible that mice with lower activity levels would be more likely to spend more time together. However, since the activity of the BTBR mice was comparable to that of the B6 mice in the novel environment, we cannot exclude the possibility that BTBR mice prefer to spend time with the same strain. This possibility may be related to human studies which recently reported an atypical form of empathy in individuals with ASD toward others with ASD [12, 13].
Previous studies have reported impaired sociability in BTBR mice using the conventional three chamber test [20, 23, 28, 29, 35, 37] and the visible burrow system (VBS) [4, 25]. However, the impairment of sociability in BTBR mice measured by MAPS seems to be more uncertain than previous studies. In addition, conventional behavioral tests in this study showed that the BTBR mice exhibited an increased response to a novel object compared to the B6 mice, whereas, no difference was observed in their responses to a social stimulus. These results suggest that the impairment of sociability in BTBR mice might not be severe, but the response to the object is stronger. Our results suggest that sociability in the BTBR mice is impaired because the BTBR mice are more attracted to objects in the conventional three chamber test. In the very large experimental cage like VBS, which comprises a burrow/tunnel system and an adjacent open surface area, the lower activity of the BTBR mice might lead to a decrease in the number of approaches and an increase in time spent alone because they cannot find cage mates, which may be interpreted as an impairment of sociability. Behavioral tests include a number of confounding factors, so it is important to evaluate and consider the phenotype of the animal model by comprehensively interpreting the results of various types of tests.
As mentioned above, MAPS would be useful for evaluating the usual/natural behaviors of animal models in a long-term group housing setting. However, there are some limitations of MAPS. Especially, MAPS takes images at 1 fps, a time resolution that makes it difficult to assess fine-scale behavioral interactions, such as an approach or chasing/following behaviors, which are evaluated in human observation-based approaches. It is necessary to improve the time resolution of MAPS for analyzing fine-scale and detailed behavioral interactions in future studies.
In conclusion, we demonstrated the behavioral phenotypes of the BTBR mice, i.e., lower activity levels during the dark phase, alteration of social behavior, and inflexible behavior, under a group housing condition under long-term observation using MAPS. MAPS is a useful tool for the detailed and ethological evaluation of the behavioral phenotypes of animal models.
Supplementary Material
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (16H06403, 16H06400 to M.M., 17H06060 to M.N., and 17H07032 to N.E.). We thank Mamiko Okuda for technical assistance. The English language review by editage (https://www.editage.jp/) was greatly appreciated.
References
- 1.American Psychiatric Association2013. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- 2.Babineau B.A., Yang M., Berman R.F., Crawley J.N.2013. Low home cage social behaviors in BTBR T+tf/J mice during juvenile development. Physiol. Behav. 114-115: 49–54. doi: 10.1016/j.physbeh.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard D.C., Defensor E.B., Meyza K.Z., Pobbe R.L., Pearson B.L., Bolivar V.J., Blanchard R.J.2012. BTBR T+tf/J mice: autism-relevant behaviors and reduced fractone-associated heparan sulfate. Neurosci. Biobehav. Rev. 36: 285–296. doi: 10.1016/j.neubiorev.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bove M., Ike K., Eldering A., Buwalda B., de Boer S.F., Morgese M.G., Schiavone S., Cuomo V., Trabace L., Kas M.J.H.2018. The Visible Burrow System: A behavioral paradigm to assess sociability and social withdrawal in BTBR and C57BL/6J mice strains. Behav. Brain Res. 344: 9–19. doi: 10.1016/j.bbr.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Chadman K., Guariglia S.2012. The BTBR T+ tf/J (BTBR) mouse model of autism. Autism.S1: 9. [Google Scholar]
- 6.Clapcote S.J., Roder J.C.2006. Deletion polymorphism of Disc1 is common to all 129 mouse substrains: implications for gene-targeting studies of brain function. Genetics 173: 2407–2410. doi: 10.1534/genetics.106.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defensor E.B., Pearson B.L., Pobbe R.L., Bolivar V.J., Blanchard D.C., Blanchard R.J.2011. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav. Brain Res. 217: 302–308. doi: 10.1016/j.bbr.2010.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo N., Ujita W., Fujiwara M., Miyauchi H., Mishima H., Makino Y., Hashimoto L., Oyama H., Makinodan M., Nishi M., Tohyama C., Kakeyama M.2018. Multiple animal positioning system shows that socially-reared mice influence the social proximity of isolation-reared cagemates. Commun Biol 1: 225. doi: 10.1038/s42003-018-0213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennah W., Thomson P., Peltonen L., Porteous D.2006. Genes and schizophrenia: beyond schizophrenia: the role of DISC1 in major mental illness. Schizophr. Bull. 32: 409–416. doi: 10.1093/schbul/sbj079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhuang H., Garrote E., Mutch J., Yu X., Khilnani V., Poggio T., Steele A.D., Serre T.2010. Automated home-cage behavioural phenotyping of mice. Nat. Commun. 1: 68. doi: 10.1038/ncomms1064 [DOI] [PubMed] [Google Scholar]
- 11.Kilpinen H., Ylisaukko-Oja T., Hennah W., Palo O.M., Varilo T., Vanhala R., Nieminen-von Wendt T., von Wendt L., Paunio T., Peltonen L.2008. Association of DISC1 with autism and Asperger syndrome. Mol. Psychiatry 13: 187–196. doi: 10.1038/sj.mp.4002031 [DOI] [PubMed] [Google Scholar]
- 12.Komeda H.2015. Similarity hypothesis: understanding of others with autism spectrum disorders by individuals with autism spectrum disorders. Front. Hum. Neurosci. 9: 124. doi: 10.3389/fnhum.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komeda H., Kosaka H., Saito D.N., Mano Y., Jung M., Fujii T., Yanaka H.T., Munesue T., Ishitobi M., Sato M., Okazawa H.2015. Autistic empathy toward autistic others. Soc. Cogn. Affect. Neurosci. 10: 145–152. doi: 10.1093/scan/nsu126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Jackson Laboratory Mouse Strain Datasheet − 002282. Available from https://www.jax.org/strain/002282.
- 15.Liu J., Dietz K., DeLoyht J.M., Pedre X., Kelkar D., Kaur J., Vialou V., Lobo M.K., Dietz D.M., Nestler E.J., Dupree J., Casaccia P.2012. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15: 1621–1623. doi: 10.1038/nn.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Dupree J.L., Gacias M., Frawley R., Sikder T., Naik P., Casaccia P.2016. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 36: 957–962. doi: 10.1523/JNEUROSCI.3608-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makinodan M., Ikawa D., Yamamuro K., Yamashita Y., Toritsuka M., Kimoto S., Yamauchi T., Okumura K., Komori T., Fukami S.I., Yoshino H., Kanba S., Wanaka A., Kishimoto T.2017. Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci. Rep. 7: 5481. doi: 10.1038/s41598-017-05632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makinodan M., Okumura K., Ikawa D., Yamashita Y., Yamamuro K., Toritsuka M., Kimoto S., Yamauchi T., Komori T., Kayashima Y., Yoshino H., Wanaka A., Kishimoto T.2017. Effects of cross-rearing with social peers on myelination in the medial prefrontal cortex of a mouse model with autism spectrum disorder. Heliyon 3: e00468. doi: 10.1016/j.heliyon.2017.e00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makinodan M., Rosen K.M., Ito S., Corfas G.2012. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337: 1357–1360. doi: 10.1126/science.1220845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarlane H.G., Kusek G.K., Yang M., Phoenix J.L., Bolivar V.J., Crawley J.N.2008. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 7: 152–163. doi: 10.1111/j.1601-183X.2007.00330.x [DOI] [PubMed] [Google Scholar]
- 21.McKenzie I.A., Ohayon D., Li H., de Faria J.P., Emery B., Tohyama K., Richardson W.D.2014. Motor skill learning requires active central myelination. Science 346: 318–322. doi: 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyza K.Z., Defensor E.B., Jensen A.L., Corley M.J., Pearson B.L., Pobbe R.L., Bolivar V.J., Blanchard D.C., Blanchard R.J.2013. The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behav. Brain Res. 251: 25–34. doi: 10.1016/j.bbr.2012.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moy S.S., Nadler J.J., Young N.B., Perez A., Holloway L.P., Barbaro R.P., Barbaro J.R., Wilson L.M., Threadgill D.W., Lauder J.M., Magnuson T.R., Crawley J.N.2007. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 176: 4–20. doi: 10.1016/j.bbr.2006.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nave K.A.2010. Myelination and support of axonal integrity by glia. Nature 468: 244–252. doi: 10.1038/nature09614 [DOI] [PubMed] [Google Scholar]
- 25.Pobbe R.L., Pearson B.L., Defensor E.B., Bolivar V.J., Blanchard D.C., Blanchard R.J.2010. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav. Brain Res. 214: 443–449. doi: 10.1016/j.bbr.2010.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scattoni M.L., Martire A., Cartocci G., Ferrante A., Ricceri L.2013. Reduced social interaction, behavioural flexibility and BDNF signalling in the BTBR T+ tf/J strain, a mouse model of autism. Behav. Brain Res. 251: 35–40. doi: 10.1016/j.bbr.2012.12.028 [DOI] [PubMed] [Google Scholar]
- 27.Scattoni M.L., Ricceri L., Crawley J.N.2011. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 10: 44–56. doi: 10.1111/j.1601-183X.2010.00623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverman J.L., Tolu S.S., Barkan C.L., Crawley J.N.2010. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 35: 976–989. doi: 10.1038/npp.2009.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman J.L., Yang M., Lord C., Crawley J.N.2010. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11: 490–502. doi: 10.1038/nrn2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman J.L., Yang M., Turner S.M., Katz A.M., Bell D.B., Koenig J.I., Crawley J.N.2010. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience 171: 1197–1208. doi: 10.1016/j.neuroscience.2010.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlsten D., Metten P., Crabbe J.C.2003. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 971: 47–54. doi: 10.1016/S0006-8993(03)02354-0 [DOI] [PubMed] [Google Scholar]
- 32.Waye M.M.Y., Cheng H.Y.2018. Genetics and epigenetics of autism: A Review. Psychiatry Clin. Neurosci. 72: 228–244. doi: 10.1111/pcn.12606 [DOI] [PubMed] [Google Scholar]
- 33.Wöhr M., Roullet F.I., Crawley J.N.2011. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 10: 35–43. doi: 10.1111/j.1601-183X.2010.00582.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamasue H.2016. Promising evidence and remaining issues regarding the clinical application of oxytocin in autism spectrum disorders. Psychiatry Clin. Neurosci. 70: 89–99. doi: 10.1111/pcn.12364 [DOI] [PubMed] [Google Scholar]
- 35.Yang M., Abrams D.N., Zhang J.Y., Weber M.D., Katz A.M., Clarke A.M., Silverman J.L., Crawley J.N.2012. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol. Behav. 107: 649–662. doi: 10.1016/j.physbeh.2011.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M., Clarke A.M., Crawley J.N.2009. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur. J. Neurosci. 29: 1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M., Perry K., Weber M.D., Katz A.M., Crawley J.N.2011. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 4: 17–27. doi: 10.1002/aur.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M., Scattoni M.L., Zhodzishsky V., Chen T., Caldwell H., Young W.S., McFarlane H.G., Crawley J.N.2007. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front. Behav. Neurosci. 1: 1. doi: 10.3389/neuro.08.001.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.