Abstract
Inflammation-related animal model is necessary to better understanding the association of inflammation with tumorigenesis. Although mouse models of inflammation-related lung tumorigenesis on A/J mice strain have been set up in previous study, there is no report on the model on C57BL/6J mice. In this study, C57BL/6J mice were randomly divided into two groups and instilled with benzo(a)pyrene [B(a)p] plus lipopolysaccharide (LPS) with different treatments. Mice in Group I were instilled intratracheally with B(a)p (1 mg/mouse) and LPS (5 µg/mouse), once a week for 4 times, on Tuesday and Friday, respectively [the week of the last time of B(a)p treatment named Week 0]. At Week 4, mice continued to be treated with LPS, once every four weeks for 5 times. Mice in Group II were exposed to B(a)p (1 mg/mouse, once a week for 4 times) and 3 weeks later instilled intratracheally with LPS (2.5 µg/mouse) once every three weeks for 5 times. At Week 30, the incidence, number, size and histopathology of lung tumor in two models were compared. The tumor incidence (96.97%) and mean tumor count (13.0 ± 12.4) of mice in Group II were significantly increased compared with those in Group I (69.23%, 4.9 ± 5.1), respectively. In addition, smaller tumors (≤1 mm) were more abundant in Group II than Group I. Histopathological examination found the tumors induced by B(a)p plus LPS in Group II were more advanced tumors. In conclusion, a better mouse model of inflammation-related lung tumorigenesis induced by B(a)p plus LPS in C57BL/6J mice was set up successfully.
Keywords: benzo(a)pyrene, inflammation, lipopolysaccharide, lung tumorigenesis, mouse model
Introduction
Lung cancer is the leading cause of cancer-related mortality, which accounts for one-quarter of all cancer deaths, and the 5-year survival rate of all stages combined is only 18% [8]. Traditional risk factors of lung cancer are tobacco use, genetics, occupational exposure or environmental pollution [2]. Recent evidence indicates that inflammatory microenvironment has been highlighted as an enhanced factor in lung tumorigenesis [7]. Epidemiological studies have provided convincing evidence showing that pulmonary inflammatory diseases, including pneumococcal pneumonia and chronic obstructive pulmonary disease, increased the risk of lung cancer [1, 5]. Thus, an inflammation-related animal model is required to explore the molecular events involved in inflammation-related lung tumorigenesis.
In recent years, there have been reports about mouse models of inflammation-related lung tumorigenesis [6, 9]. For instance, T. Melkamu et al. developed a mouse model for inflammation-related lung cancer through chronic exposure of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and lipopolysaccharide (LPS) in A/J mice [6]. Despite the advances made with mouse models of inflammation-related lung tumorigenesis, these mouse models have a common limitation about mouse strain, which only provide methods on A/J mice. To our knowledge, A/J mice strain is sensitive to lung cancer induction by carcinogen and is widely used to model cancer. C57BL/6J mice strain, a common inbred strain of laboratory mouse, is commonly used as a background strain of gene-editing mice. Additionally, there is no related reported yet about the mouse model of inflammation-related lung tumorigenesis on C57BL/6J mice strain. Therefore, it is very necessary to establish a mouse model of inflammation-related lung tumorigenesis on C57BL/6J mice, although which are not susceptible to tumors.
In this study, C57BL/6J mice were instilled with benzo(a)pyrene [B(a)p] plus LPS with two different treatments, two models were compared and a better one was chosen to set up a stable mouse model of inflammation-related lung cancer, which will provide a good platform and contribute to explore the molecular events involved in inflammation-related lung tumorigenesis better.
Materials and Methods
Animals
The heterozygous littermates of NLRP3+/− mice were obtained as gift generously from Professor Aihua Zhang in Nanjing Children’s Hospital and then housed in the SPF grade animal room in the College of Public Health of Zhengzhou University, Henan, China. The heterozygous littermates were bred to generate homozygous NLRP3−/− mice which were used in another study and littermate pure C57BL/6J mice. All C57BL/6J mice used in this study were genotyped. Mice were raised in stainless steel cages under standard conditions and allowed food and water ad libitum. The temperature was maintained at 22°C, and the lights began from 08:00 to 20:00. All experimental procedures were approved by the Life Science Institutional Review Board of Zhengzhou University and performed strictly in accordance with the Guideline of Zhengzhou University for Animal Experiments.
Inflammation-related lung tumorigenesis mouse models
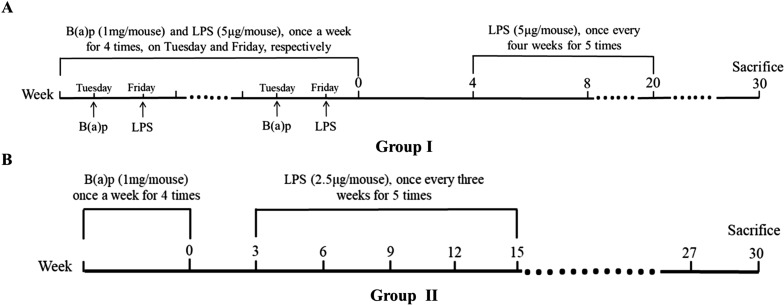
The models for inflammation-related lung cancer in the mouse were induced by instilled intratracheally with B(a)p and LPS. Seventy C57BL/6J mice were randomly divided into two treatment groups (n=35 for each group) and treated as shown in Fig. 1. In detail, mice in group I were instilled intratracheally with B(a)p (Sigma St. Louis, MO, USA) (1 mg/mouse, dissolved in 50 µl tricaprylin solvent) and LPS (Sigma) (5 µg/mouse, dissolved in 50 µl saline), once a week for 4 times, on Tuesday and Friday, respectively. At Week 4 [the week of the last time of B(a)p treatment named Week 0], mice continued to be treated with LPS, once every four weeks for 5 times. Each of mice in group II was exposed to B(a)p (1mg/mouse, once a week for 4 times) and 3 weeks later instilled intratracheally with LPS (2.5 µg/mouse) once every three weeks for 5 times. All instillations were performed under anesthesia with isoflurane (Sigma). The mice were anaesthesia by pentobarbital sodium (100 mg/kg) at Week 30. The lungs were harvested, and visible tumors on the surface of the lung were counted and the size of lung tumors was accessed using a straightedge. The left lobes of the lungs were fixed in 4% paraformaldehyde for histopathological studies and the right lobes were stored at −80°C for subsequent assays.
Fig. 1.
Experimental design of the study. Seventy C57BL/6J mice were randomly divided into two treatment groups (n=35 for each group). (A) Group I: mice were instilled intratracheally with B(a)p (1 mg/mouse) and lipopolysaccharide (LPS) (5 µg/mouse), once a week for 4 times, on Tuesday and Friday, respectively. At Week 4, mice continued to be treated with LPS, once every four weeks for 5 times. (B) Group II: mice were exposed to B(a)p (1 mg/mouse, once a week for 4 times) and 3 weeks later instilled intratracheally with LPS (2.5 µg/mouse) once every three weeks for 5 times. Thirty weeks after the week of the last dose of B(a)p, mice were anaesthesia by pentobarbital sodium.
Histopathological analysis of lung tissues
The fixed lung tissues were embedded in paraffin. After embedded, the lung sections of 5 µm in thickness were stained with hematoxylin and eosin (HE). The types of lung cancer were accessed and tumor grading was evaluated based on predetermined criteria from Cancer Research UK (https://www.cancerresearchuk.org/about-cancer/lung-cancer/stages-types-grades/stages-grades). In detail, each tumor was given a score of 1 to 4. Grade 1: The cells look very like normal cells. They tend to be slow growing and are less likely to spread than higher grade cancer cells. They are called low grade. Grade 2: The cells look more abnormal and are more likely to spread. This grade is also called moderately well differentiated or moderate grade. Grades 3 and 4: The cells look very abnormal and not like normal cells. They tend to grow quickly and are more likely to spread. They are called poorly differentiated or high grade.
Statistical analysis
Data were expressed as mean ± SD. Wilcoxon rank sum test was used for the comparison of tumor incidence. Since the number of tumors, pathological tumor nests and tumor grade were normal distribution, two-tailed Student’s t-test was carried out for the comparisons using SPSS21.0 (IBM, NC). A two-tailed P value <0.05 was considered statistically significant.
Results
Effects of LPS on B(a)p-induced lung tumorigenesis
To establish a stable mouse model of inflammation-related lung cancer, we compared the mortality of mice, tumor incidence, multiplicity and size in two different treatment with B(a)p plus LPS tumor bioassays. As shown in Table 1, deaths of mice were found in Group I and the mortality was 25.71%, but there was no death in Group II. In addition, the mice in Group II significantly increased tumor incidence (96.97%) compared with Group I (69.23%) (P<0.05). Similarly, the mice in Group I induced 4.9 ± 5.1 tumors/mice, whereas the mice in Group II significantly increased the lung tumor multiplicity to 13.0 ± 12.4 tumors/mice (P<0.05). The various size classes of lung tumors were also shown in Table 1. Smaller tumors (≤1 mm) were more abundant in Group II than Group I (P<0.05), but there were no different significantly difference in larger tumors (>1 mm) between Group I and Group II (P>0.05). Taken together, the results indicate that the mice in the model of Group II could better induce lung tumorigenesis compared with Group I.
Table 1. B(a)p plus lipopolysaccharide (LPS) induced lung tumorigenesis in C57BL/6J mice.
| Group | No. of mice | Lung tumor | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Deaths | Mortality | Tumor incidence | Tumor/mouse (Mean ± SD) | Tumor size | |||
| ≤1 mm | >1 mm | |||||||
| I | Male | 17 | 3 | 18% | 64% | 4.8 ± 5.0 | 3.2 ± 2.5 | 1.0 ± 1.7 |
| Female | 18 | 6 | 33% | 75% | 5.2 ± 5.8 | 3.3 ± 2.1 | 2.7 ± 3.8 | |
| Total | 35 | 9 | 26% | 69% | 4.9 ± 5.1 | 3.3 ± 2.2 | 1.6 ± 2.6 | |
| II | Male | 17 | 0 | 0 | 100% | 13.0 ± 9.5 | 9.2 ± 8.1 | 3.1 ± 3.4 |
| Female | 18 | 0 | 0 | 94% | 13.0 ± 15.1 | 8.8 ± 12.6 | 3.4 ± 4.0 | |
| Total | 35 | 0 | 0 | 97%* | 13.0 ± 12.4* | 9.0 ± 10.8* | 3.3 ± 3.7 | |
*P<0.05, vs. Group I.
Pathological alterations in the lungs of mice exposed to B(a)p plus LPS
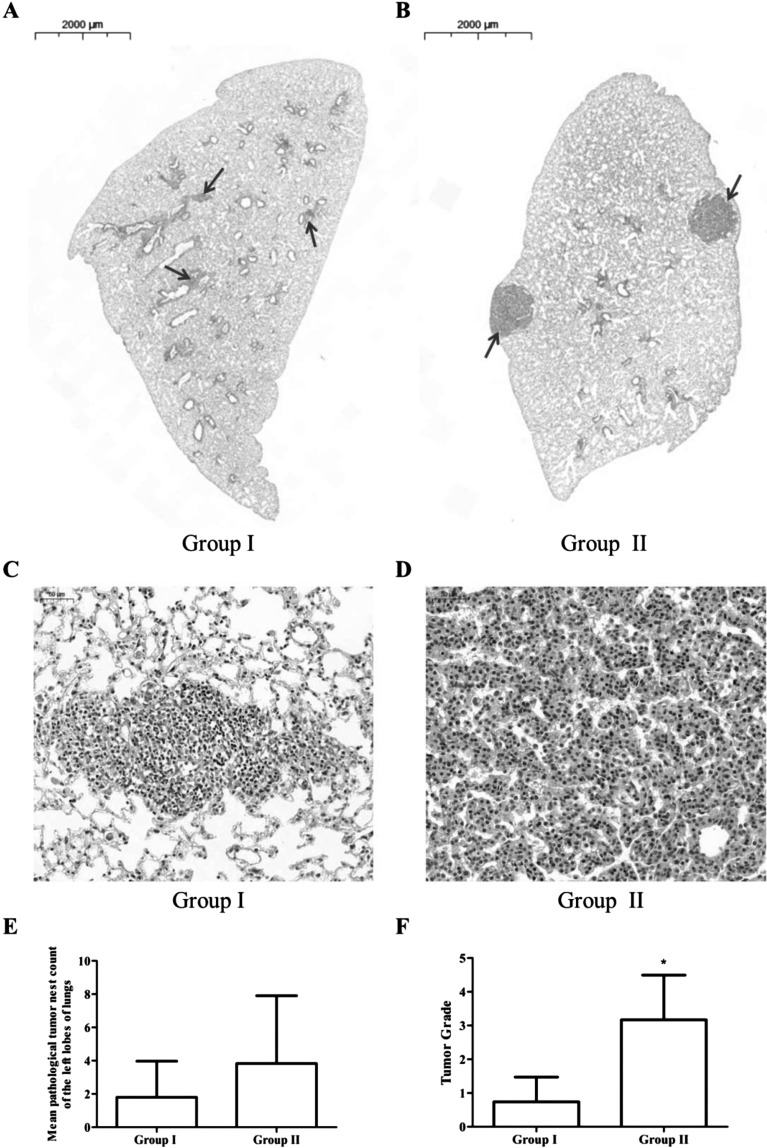
The histology of lung tissues of mice exposure to B(a)p plus LPS was assessed by HE staining (Fig. 2). As depicted in Fig. 2A and 2B, the number and size of pathological tumor nests were lower in mice of Group I compared with those in Group II. In addition, a similar trend was observed on the average number of pathological tumor nests between Group I and Group II, but the reduction was not significant (P>0.05) (Fig. 2E).
Fig. 2.
Pathological alterations in the left lobes of lungs of C57BL/6J mice exposed to B(a)p plus lipopolysaccharide (LPS). (A and B): The pulmonary morphological alterations in the cross-section of the left lobes of lungs of C57BL/6J mice exposed to B(a)p plus LPS with different treatment were evaluated by HE staining; (C and D): Pathological tumor nests in cross-section of the left lobes of lungs in the two groups with amplification (200×); (E): The number of pathological tumor nests in cross-section of the left lobes of lungs in two groups; (F): Tumor grades in B(a)p plus LPS exposure mice. Data were expressed as mean±SD. *P<0.05, vs. Group I.
To evaluate the stage of every tumor in each mouse, tumors were graded in a blinded manner on a scale of 1 to 4, and grade 4 indicated the most advanced tumor phenotype. The non-small cell lung cancer was induced by B(a) p plus LPS exposure and tumor grading was shown in Fig. 2F. The grade of lung tumors in Group II were significantly higher than that in Group I (P<0.05), suggesting the tumors induced by B(a)p plus LPS of mice in Group II were more advanced tumor.
Discussion
In this study, we found C57BL/6J mice exposed to two different treatments with B(a)p plus LPS could induce lung tumorigenesis, but there were significant differences on tumor incidence, mean tumor count and tumor size of visible tumors in mice between these two groups. We used 1mg/mouse B(a)p, once a week for 4 times, for animals experiments, which was based on the previous reported study [3]. Moreover, two different exposure methods with B(a)p plus LPS mainly were the different LPS treatment. It has been reported that A/J mice were administered weekly intranasal instillation of LPS at a dose of 5 µg/mouse after the last dose of NNK for a total of 22 weeks, which enhanced NNK-induced lung tumorigenesis [6]. In another study about inflammation-related lung squamous cell carcinoma, A/J mice were instilled intranasally with LPS at a dose of 4 µg/mouse weekly and N-nitroso-trischloroethylurea (NTCU) on Mondays and Wednesdays, respectively, throughout the study [9]. To set up the model of inflammation-related lung tumorigenesis on C57BL/6J mice, mice were instilled intratracheally with B(a)p and LPS at a dose of 5 µg/mouse, once a week for 4 times, on Tuesday and Friday, respectively. However, mice died during the period of treatment and pulmonary abscess was observed in nine mice. It is inconsistent with earlier reported [6, 9], different tolerance to LPS in the different mice strains or the synergistic effect of B(a)p and LPS may be the reasons. Therefore, we adjusted the way of LPS exposure from once a week to once every four weeks for 5 times after the last dose of B(a)p. In addition, the dose of LPS was adjusted to 2.5 µg/mouse at the same time, and B(a)p pretreated-mice were then exposed to LPS once every three weeks for 5 times after the last dose of B(a)p. There was no mice death after adjusting the LPS treatment both Group I and Group II. Interesting, the lung tumor incidence, multiplicity and size of the mice in Group I were decreased compared with those in Group II, which may be attributed to pulmonary burden induced by the synergistic effect of B(a)p and LPS. The lung injury may be induced during the early period of treatment in Group I, which is unfavorable to lung tumorigenesis. The exact mechanism involved in the difference between these models of lung cancer induced by B(a)p plus LPS, however, is unclear and will be investigated further.
C57BL/6J mice strain is characterized by well breeding, long-lived and low susceptibility to tumors. On the other hand, C57BL/6J mice strain not only is the most widely used mouse strain for use as models of human disease, but also is commonly used as a background strain for gene-editing mice. A number of studies have used knockout mice on a C57BL/6J background to demonstrate underlying molecular mechanisms involved in related disease. For example, NLRP3 inflammasome-deficient mice on a C57BL/6J genetic background, including NLRP3−/−, ASC−/− and Casp1−/− mice, were used to illuminate the critical role of NLRP3 inflammasome in colitis-associated colorectal tumor [10]. In another study, it was shown that nuclear factor 2 erythroid related factor 2 (Nrf2) deletion did not enhance the effects of B(a)p on ovarian follicle in mice on a C57BL/6J genetic background [4]. In our study, we successfully set up a mouse model of inflammation-related lung tumorigenesis induced by B(a)p plus LPS in C57BL/6J mice, which will conducive to provide a better study strategy to demonstrate the critical role of pulmonary inflammation in lung tumorigenesis through developing a knockout mouse model on a C57BL/6J genetic background.
In conclusion, our study set up a stable mouse model of inflammation-related lung tumorigenesis induced by B(a)p plus LPS in C57BL/6J mice, which will contribute to explore the related molecular mechanisms involved in inflammation-related lung tumorigenesis better.
Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81402712); and the outstanding youth grant of Zhengzhou University (No.1421329082); and the training grant of Zhengzhou University (2017ZDGGJS039); and the grant from Henan Department of Education (No.14A330001); and the grant from Henan Department of Science and Technology, China (No. 162102310319); and the grant of Medical Science Research Foundation of Henan Province (No. 2018020477).
References
- 1.Bozinovski S., Vlahos R., Anthony D., McQualter J., Anderson G., Irving L., Steinfort D.2016. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br. J. Pharmacol. 173: 635–648. doi: 10.1111/bph.13198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong Q.Y., Wu G.M., Qian G.S., Hu C.P., Zhou J.Y., Chen L.A., Li W.M., Li S.Y., Wang K., Wang Q., Zhang X.J., Li J., Gong X., Bai C.X., Lung Cancer Group of Chinese Thoracic Society; Chinese Alliance Against Lung Cancer2015. Prevention and management of lung cancer in China. Cancer 121:(Suppl 17): 3080–3088. doi: 10.1002/cncr.29584 [DOI] [PubMed] [Google Scholar]
- 3.Huang H., Pan X., Jin H., Li Y., Zhang L., Yang C., Liu P., Liu Y., Chen L., Li J., Zhu J., Zeng X., Fu K., Chen G., Gao J., Huang C.2015. PHLPP2 Downregulation Contributes to Lung Carcinogenesis Following B[a]P/B[a]PDE Exposure. Clin. Cancer Res. 21: 3783–3793. doi: 10.1158/1078-0432.CCR-14-2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim J., Ortiz L., Nakamura B.N., Hoang Y.D., Banuelos J., Flores V.N., Chan J.Y., Luderer U.2015. Effects of deletion of the transcription factor Nrf2 and benzo [a]pyrene treatment on ovarian follicles and ovarian surface epithelial cells in mice. Reprod. Toxicol. 58: 24–32. doi: 10.1016/j.reprotox.2015.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin T.Y., Huang W.Y., Lin J.C., Lin C.L., Sung F.C., Kao C.H., Yeh J.J.2014. Increased lung cancer risk among patients with pneumococcal pneumonia: a nationwide population-based cohort study. Lung 192: 159–165. doi: 10.1007/s00408-013-9523-z [DOI] [PubMed] [Google Scholar]
- 6.Melkamu T., Qian X., Upadhyaya P., O’Sullivan M.G., Kassie F.2013. Lipopolysaccharide enhances mouse lung tumorigenesis: a model for inflammation-driven lung cancer. Vet. Pathol. 50: 895–902. doi: 10.1177/0300985813476061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi L., Wang L., Hou J., Zhu B., Min Z., Zhang M., Song D., Cheng Y., Wang X.2015. Targeting roles of inflammatory microenvironment in lung cancer and metastasis. Cancer Metastasis Rev. 34: 319–331. doi: 10.1007/s10555-015-9570-4 [DOI] [PubMed] [Google Scholar]
- 8.Siegel R.L., Miller K.D., Jemal A.2018. Cancer statistics, 2018. CA Cancer J. Clin. 68: 7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 9.Song J.M., Qian X., Teferi F., Pan J., Wang Y., Kassie F.2015. Dietary diindolylmethane suppresses inflammation-driven lung squamous cell carcinoma in mice. Cancer Prev. Res. (Phila.) 8: 77–85. doi: 10.1158/1940-6207.CAPR-14-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaki M.H., Vogel P., Body-Malapel M., Lamkanfi M., Kanneganti T.D.2010. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 185: 4912–4920. doi: 10.4049/jimmunol.1002046 [DOI] [PMC free article] [PubMed] [Google Scholar]