Abstract
There are few effective antimicrobial agents against Balantidium coli infection. The effect of paromomycin sulfate (PS) against B. coli was confirmed in this study of 596 captive cynomolgus monkeys. In several trials, the minimum dose and duration of oral administration of PS were 25 mg/day for 5 + 5 days, with a 2-day withdrawal interval. To facilitate daily PS administration, pumpkin cakes supplemented with PS were made, which not only resulted in precise effects but also increased the efficiency of preparation and administration of PS by the animal care staff. No cysts or trophozoites were detected at 14 or 16 days after the last treatments. There were no obvious differences in blood and biochemical parameters between before and after administration of PS. These results indicate that PS is effective for elimination of B. coli without hematological side effects. These data could contribute to the control of microbiological pathogens during veterinary care and colony management in primate facilities.
Keywords: Balantidium coli, cynomolgus monkey, paromomycin sulfate
Introduction
Balantidium coli is the largest protozoan in the phylum Ciliophora and has a simple life cycle from cyst to trophozoite [11]. B. coli infects host animals that ingest it via consumption of contaminated food and water and settles in the large intestine of not only humans but also swine, canine, and murine hosts, as well as macaque species, e.g., rhesus and cynomolgus monkeys, in the laboratory [6, 8, 11]. Most species infected with B. coli are asymptomatic carriers and do not develop severe symptoms, although immunodeficiency increases the risk of B. coli infection, which often results in severe diarrhea [6, 7]. Both symptomatic animals and asymptomatic carriers excrete cysts within their feces, which become a source of infection for other hosts [11].
Metronidazole and tetracyclines are often used for treatment of B. coli infection [11]. However, B. coli in cattle is resistant to metronidazole [2]. Paromomycin sulfate (PS) is an effective agent against entamoebiasis caused by Entamoeba histolytica that became commercially available in Japan in 2013 [1, 4, 10]. Older literature mentions the use of PS for the treatment of B. coli infection in gorillas [12]. Because PS has been reported to have an antibiotic effect for a wide variety of protozoa species [3], we expected that it could be an effective treatment for B. coli infection in laboratory cynomolgus monkeys.
We recently experienced an outbreak of B. coli among captive nonhuman primates in our research facility. However, there is no current data for a suitable antimicrobial regimen against B. coli. Therefore, the aim of this study was to determine a precise dose and duration for PS treatment of B. coli to prevent future B. coli outbreaks among laboratory cynomolgus monkeys (Macaca fascicularis) based on our experience with an outbreak of B. coli.
Materials and Methods
Background
This study was conducted as a preventive measure for veterinary care of cynomolgus monkeys in our facility. We noticed B. coli infection in two monkeys in a room (room number [Rn] 1) while performing regular health check-ups. However, oral administration of metronidazole (Flagyl, Shionogi & Co., Ltd., Osaka, Japan) at 62.5 mg one time / day / head from days 1 to 14 was not efficacious against B. coli infection in either monkey, with recurrence occurring at days 21 and 28, respectively (Table 1). Consequently, an epidemiological survey was initiated over concerns about the spread of B. coli infection because B. coli was detected in all of the animal breeding rooms at that time. Therefore, only B. coli-positive monkeys were enrolled in three trials to determine a precise protocol for the administration of PS and to monitor subsequent hematological side effects. On the other hand, individual administration of PS to positive monkeys resulted in recurrence of B. coli infection during the first to third trials. To avoid reinfection, all monkeys, including those negative for B. coli infection, received a convenient PS treatment in a fourth trial.
Table 1. Detection of B. coli after metronidazole administration for 14 days.
| Duration (days) | Sex | Age (Years) |
0a | 14b | 21c | 28c | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Animal ID | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | ||
| 62.5 mg | 1 | M | 5.2 | + | − | − | − | − | − | + | − |
| 2 | M | 4.9 | + | + | + | − | + | − | + | − | |
M, male; +, positive; −, negative; trop, trophozoite. aFirst day of administration, bLast day of administration, cReccurence.
Specimens
For trials 1–3, fecal specimens from 596 cynomolgus monkeys were collected from six animal breeding rooms. Fecal samples from 10 of 94 monkeys in Rn 1 (42 females and 52 males), 11 of 95 monkeys in Rn 2 (73 females and 22 males), and 15 of 407 monkeys in Rns 3–6 (363 females and 44 males) tested positive for B. coli infection. These three groups were used for trials 1–3, respectively. All positive monkeys are listed in Tables 2, Tables 3, 4. Six months after trials 1–3, analysis of 582 fecal specimens collected from monkeys in Rns 1–6 showed that 22 monkeys were positive for B. coli infection. All monkeys positive and negative for B. coli were enrolled in trial 4. The indoor environment of all animal rooms utilized for these trials at the Research Center for Animal Life Science, Shiga University of Medical Science, was maintained at 25 ± 2°C with 50 ± 5% humidity.
Table 2. Detection of B. coli after paromomycin sulfate (PS) administration in trial 1.
| Duration (days) | Sex | Age (Years) |
0 | 7a | 14b | 28c | 35 | 42 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Doses | Animal ID | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | ||
| 25 mg | 3 | M | 7.8 | + | + | − | − | − | − | − | − | − | − | − | − |
| 4 | M | 7.7 | + | + | − | − | + | − | − | − | − | − | − | − | |
| 5 | M | 6.2 | − | + | − | − | + | − | − | − | − | − | − | − | |
| 50 mg | 6 | M | 9.8 | + | + | − | − | + | − | − | − | − | − | − | − |
| 7 | M | 6.2 | − | + | − | − | + | + | − | − | − | − | − | − | |
| 8 | M | 7.5 | − | + | − | − | − | − | − | − | − | − | − | − | |
| 125 mg | 9 | M | 12.9 | + | − | − | − | + | − | − | − | − | − | − | − |
| 10 | M | 6.5 | + | − | − | − | + | − | − | − | − | − | − | − | |
| 11 | M | 9.7 | + | − | − | − | + | − | − | − | − | − | − | − | |
| 12 | F | 10.4 | + | − | − | − | − | − | − | − | − | − | − | − | |
F, female; M, male; +, positive; −, negative; Trop, trophozoite. aLast day of first dministration (7 days), bReccurence, cLast day of second administration (14 days).
Table 3. Detection of B. coli by paromomycin sulfate (PS) treatment in trial 2.
| Duration (days) | Sex | Age (Years) |
0 | 14a | 21 | 28 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose / durationof administration | Animal ID | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | ||
| 25 mg / 5 + 5 days | 13 | F | Unknown | + | − | − | − | − | − | − | − |
| 14 | M | 9.5 | − | + | − | − | − | − | − | − | |
| 15 | M | 6.8 | + | − | − | − | − | − | − | − | |
| 50 mg / 5 + 5 days | 16 | M | 13.7 | + | − | − | − | − | − | − | − |
| 17 | M | 4.7 | + | − | − | − | − | − | − | − | |
| 18 | M | 4.6 | + | − | − | − | − | − | − | − | |
| 25 mg / 14 days | 19 | F | 14 | + | − | − | − | − | − | − | − |
| 20 | F | 12.5 | + | − | − | − | − | − | − | − | |
| 21 | M | 5.6 | − | + | − | − | − | − | − | − | |
| 50 mg / 14 days | 22 | M | 8.3 | + | − | − | − | − | − | − | − |
| 23 | F | 6.5 | + | − | − | − | − | − | − | − | |
F, female; M, male; +, positive; −, negative; Trop, trophozoite. a2 days after 5 + 5 days of administration, or last day of 14 days of administration.
Table 4. Detection of B. coli by paromomycin sulfate (PS) treatment in trial 3.
| Duration (days) | Sex | Age (Years) |
0 | 14a | 21 | 28 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose / durationof administration | Animal ID | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | ||
| 25 mg / 10 days | 24 | F | Unknown | + | + | − | − | − | − | − | − |
| 25 | F | 12.2 | − | + | − | − | − | − | − | − | |
| 26 | F | 10.3 | − | + | − | − | − | − | − | − | |
| 27 | F | 7.5 | − | + | − | − | − | − | − | − | |
| 28 | F | 9.7 | − | + | − | − | − | − | − | − | |
| 29 | F | 9.2 | + | − | − | − | − | − | − | − | |
| 30 | F | 9.2 | + | + | − | − | − | − | − | − | |
| 31 | M | 6.2 | + | + | − | − | − | − | − | − | |
| 32 | F | 5.9 | + | + | − | − | − | − | − | − | |
| 33 | F | 8.2 | − | + | − | − | − | − | − | − | |
| 34 | F | 6 | + | − | − | − | − | − | − | − | |
| 35 | F | 5.1 | + | − | − | − | − | − | − | − | |
| 36 | F | 5.1 | + | − | − | − | − | − | − | − | |
| 37 | F | 4.9 | − | + | − | − | − | − | − | − | |
| 38 | F | 3.3 | + | − | − | − | − | − | − | − | |
F, female; M, male; +, positive; −, negative; Trop, trophozoite. a2 days after 5 + 5 days of administration.
Schedule of trials
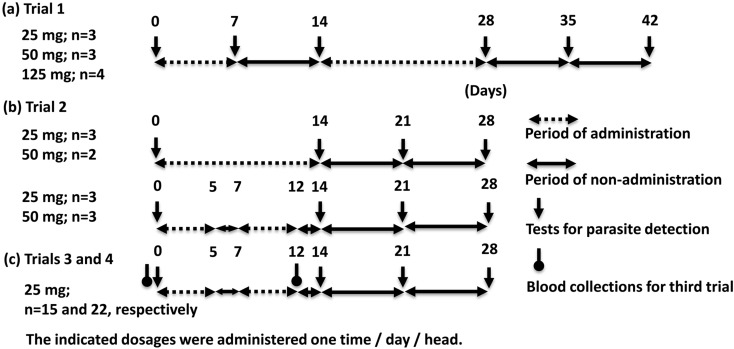
The schedules for all the trials are summarized in Fig. 1. Fecal specimens in trial 1 were collected for B. coli detection on days 0 and 7 during the first treatment, day 14 as the last day of the first treatment, day 28 as the last day of the second treatment, and days 35 and 42 (Fig. 1). Fecal specimens in trials 2 to 4 were collected on days 0, 14, 21, and 28 (Fig. 1). Ten B. coli-positive monkeys in Rn 1 were enrolled in trial 1 to determine the effective duration of PS administration. Each of the positive monkeys received oral PS (Ameparomo, Pfizer Japan Inc., Tokyo, Japan) within a small piece of banana at a dosage of either 25, 50, or 125 mg one time / day / head for 7 days (days 1 to 7). However, B. coli infection recurred on day 14. Consequently, PS was administered for an additional 14 days (days 15 to 28) at the same doses (Fig. 1-a and Table 2).
Fig. 1.
Time course of treatments with paromomycin sulfate (PS), examinations for B. coli detections, and blood collections in the first (a), second (b), and third and fourth (c) trials.
Trial 2 was conducted using 11 B. coli-positive monkeys in Rn 2 to determine the precise minimum dose and duration of PS. Each monkey received PS within pieces of banana with the following combinations of doses and durations: 50 or 25 mg one time/day/head for 5 + 5 days with a 2-day withdrawal interval (days 1 to 5 plus 8 to 12) or 14 days (days 1 to 14), respectively (Fig. 1-b and Table 3).
Trial 3 was conducted with 15 B. coli positive monkeys in Rns 3–6 to confirm the differences in blood and biochemical parameters between before and after administration. Each monkey received PS at 25 mg one time/day/head for 5 + 5 days with a 2-days withdrawal interval (days 1 to 5 plus 8 to 12). Blood was collected before and after PS administration, namely on days 0 and 12 (Fig. 1-c and Table 4).
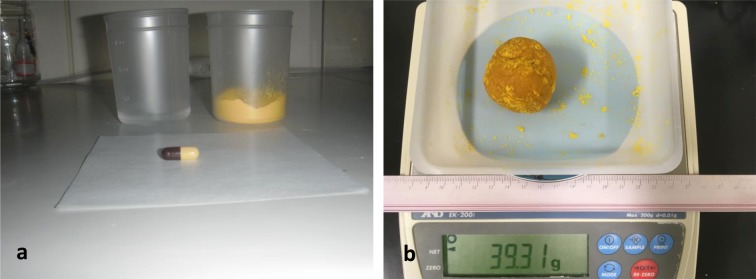
Trial 4 was conducted with monkeys in all rooms (Rns 1–6). The schedule of PS administration was the same as that for trial 3, although the mode of PS administration was changed from that in trials 1–3 (Fig. 1-c). Briefly, the contents of a PS capsule (250 mg of PS) and 20 g of pumpkin powder (Kawanishi Foods, Sapporo, Japan) were dissolved and kneaded in 20 ml of tap water to produce a 40 g pumpkin cake that was divided into 4 g sections that each contained 25 mg PS for 10 cynomolgus monkeys (Fig. 3). Then, all 582 monkeys were treated with the 4 g pumpkin cakes, regardless of whether they were positive or negative for B. coli infection.
Fig. 3.
The preparation of pumpkin cakes within 250 mg paromomycin sulfate (PS) for 10 cynomolgus monkeys. The ingredients included one capsule of PS containing 250 mg of PS, 20 ml of tap water, and 20 g of pumpkin powder (a). The ingredients were mixed in a cup or bowl. The pumpkin cake weighed about 40 g (b) and was divided into 4 g sections for 10 cynomolgus monkeys.
B. coli detection
One gram of fecal material was dissolved in 5 ml saline, and 1 drop (approximately 20 µl) was transferred by micropipette to a glass slide and covered with an 18 × 18 mm glass cover plate for the direct observation method. For the concentration method [13], a 1 g sample was dissolved in 2 to 3 ml of saline, filtered through gauze, and adjusted to a 10 ml volume by adding 10% formalin and 2 ml of ethyl acetate. The mixtures were shaken well and centrifuged at 600 g for 10 min. Then, the supernatant was removed, and the sediment was resuspended with the small amount of remaining solution. One drop (about 20 µl) was transferred using a micropipette to a glass slide and covered with an 18 × 18 mm glass cover plate. Slides prepared by both methods were observed under a microscope and graded as negative or positive.
Blood and blood biochemical examinations
Fifteen blood specimens collected in trial 3 were used for biochemical analysis. The numbers of white blood cells, red blood cells, and platelets, as well as the concentration of hemoglobin, were measured using a Celltac alpha MEK-6450 hematology analyzer (Nihon Kohden, Tokyo, Japan). Total bilirubin, aspartate transaminase, alanine aminotransferase, total cholesterol, triglycerides, blood urea nitrogen (BUN), glucose, albumin, total protein, sodium (Na), potassium (K), calcium (Ca), and chlorine (Cl) concentrations were measured using the SPOTCHEM D-02 Dry Clinical Chemistry Analyzer (SD-4810, Arkray, Inc., Tokyo, Japan).
Statistical analyses
Differences in the results of blood and blood biochemical examinations between before and after administration for 15 from a trial 3 were compared using the paired t-test. A P value of <0.05 was considered statistically significant.
Results
Administration of metronidazole: Before all PS administration trials, we treated two positive monkeys in Rn 1 with metronidazole for 14 days (days 1 to 14). However, this treatment was not efficacious against B. coli infection. B. coli cysts were found on the last day of the treatment (day 14) and after treatment (days 21 and 28) in one monkey, and they recurred in the other monkey on day 28 (Table 1).
Trial 1
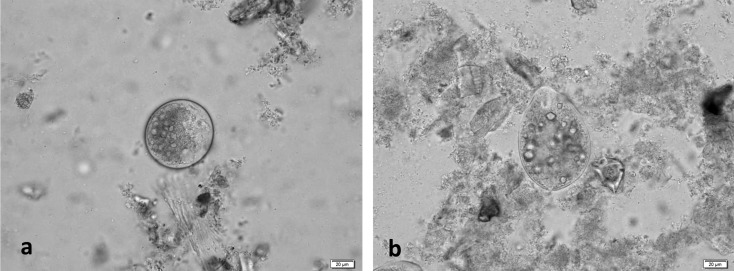
B. coli cysts and trophozoites (Fig. 2) were detected in the fecal samples of 10 of 94 monkeys in Rn 1 (Table 2). Initially, the 10 B. coli-positive monkeys were treated with PS at 25, 50, and 125 mg for 7 days (days 1 to 7), which resulted in the elimination of B. coli trophozoites and cysts on day 7 (Fig. 1-a and Table 2). However, B. coli recurred on day 14. Subsequently, PS was administered to each monkey for 14 days (days 15 to 28) at the same doses, which resulted in the elimination of B. coli on days 28, 35, and 42 (Table 2).
Fig. 2.
Microscopic observations of cysts (a) and trophozoites (b) of B. coli (Case 6 in trial 1).
Trial 2
B. coli was detected in the fecal samples of 11 of 95 monkeys in Rn 2 (Fig. 2). The profiles of the detected cysts and trophozoites before and after administration are shown in Table 3. Since PS administration for 7 days was not effective in trial 1, the dosage was increased to 25 or 50 mg for 5 + 5 days with a 2-day withdrawal interval or 14 days (Fig 1-b). As shown in Table 3, B. coli was not detected in the fecal samples of the treated monkeys on days 14, 21, and 28 (Table 3).
Trial 3
B. coli was found in the fecal samples of 15 of 405 monkeys in Rns 3–6 (Fig. 2). The profiles of the detected cysts and trophozoites are listed in Table 4. No B. coli cysts or trophozoites were found in the fecal samples of the treated monkeys on days 14, 21, and 28 (Table 4). There were no statistically significant differences in the blood biochemical parameters between before and after administration, with the exception of BUN, Na, Ca, and Cl concentrations, although the difference in their averages were within or around the reference values of blood and blood biochemical parameters (Table 5) [9].
Table 5. Blood and blood chemical features before and after administration of paromomycin sulfate (PS).
| Items | Unit | Reference value [9] | Before (n=15) | After (n=15) | P value | ||
|---|---|---|---|---|---|---|---|
| Average | SE | Average | SE | ||||
| WBC | (102/mm3) | 35–151 | 96.7 | 7.47 | 92.7 | 8.20 | 0.6727 |
| RBC | (104/mm3) | 470–738 | 553.9 | 28.19 | 562.8 | 21.39 | 0.7686 |
| HGB | (g/dl) | 9.1–13.9 | 13.0 | 0.66 | 13.3 | 0.47 | 0.7722 |
| Ht | (%) | 30.8–46.0 | 36.4 | 1.77 | 37.2 | 1.19 | 0.7402 |
| Plate | (102/mm3) | 14.3–49.1 | 40.9 | 3.36 | 37.3 | 2.62 | 0.3059 |
| T. Bil | (mg/dl) | < 0.5 | 0.7 | 0.04 | 0.7 | 0.07 | 0.8154 |
| AST | (IU/l) | 23–78 | 18.5 | 1.81 | 22.7 | 4.21 | 0.2961 |
| ALT | (IU/l) | 14–155 | 28.9 | 4.25 | 35.4 | 6.12 | 0.3254 |
| T.Chol | (mg/dl) | 86–246 | 137.9 | 9.79 | 139.9 | 9.49 | 0.8040 |
| TG | (mg/dl) | 8–85 | 60.2 | 4.08 | 63.9 | 9.90 | 0.7030 |
| BUN | (mg/dl) | 9.8–26.9 | 20.9 | 0.92 | 17.9 | 1.45 | 0.0237 |
| Glu | (mg/dl) | 24–74 | 55.1 | 2.28 | 53.1 | 2.93 | 0.6217 |
| Alb | (g/dl) | 3.3–4.7 | 3.9 | 0.12 | 4.1 | 0.14 | 0.1003 |
| T. Pro | (g/dl) | 6.3–8.4 | 7.8 | 0.27 | 7.4 | 0.33 | 0.3520 |
| Na | (mEq/l) | 147–151 | 142.1 | 1.28 | 147.3 | 1.26 | 0.0093 |
| K | (mEq/l) | 3.1–4.4 | 5.6 | 0.18 | 5.9 | 0.27 | 0.2417 |
| Ca | (mg/dl) | 7.8–9.8 | 10.0 | 0.13 | 10.6 | 0.19 | 0.0063 |
| Cl | (mEq/l) | 106–114 | 109.3 | 1.01 | 112.0 | 0.65 | 0.0103 |
WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; Ht, hematocrit; Plate, platelet; T. Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine transaminase; T. Chol, total cholesterol; TG, triglyceride; BUN, blood urea nitrogen; Glu, glucose; Alb, albumin; T. Pro, total protein; Na, sodium; K, potassium; Ca, calcium; Cl, Chloride.
Trial 4
B. coli was detected in the fecal samples of 22 of 582 monkeys in Rns 1–6 (Table 6). Eleven monkeys refused to eat the pumpkin cakes, so 25 mg of PS was administered within pieces of banana, as in trials 1–3. No B. coli cysts or trophozoites were detected in the fecal samples of monkeys that ate the pumpkin cakes containing 25 mg of PS on days 14, 21, and 28 (Table 6).
Table 6. Detection of B. coli by paromomycin sulfate (PS) treatment in trial 4.
| Duration (days) | Sex | Age (Years) |
0 | 14a | 21 | 28 | Reccurence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose / durationof administration | Animal ID | Cyst | Trop | Cyst | Trop | Cyst | Trop | Cyst | Trop | |||
| 25 mg / 5 + 5 days by pumpkin cake | 4 | M | 8.2 | + | + | − | − | − | − | − | − | Yes |
| 6 | M | 10.3 | + | − | − | − | − | − | − | − | Yes | |
| 18 | M | 5.1 | + | + | − | − | − | − | − | − | Yes | |
| 25 | F | 12.7 | + | + | − | − | − | − | − | − | Yes | |
| 27 | F | 8 | + | + | − | − | − | − | − | − | Yes | |
| 28 | F | 10.2 | + | + | − | − | − | − | − | − | Yes | |
| 36 | F | 5.6 | − | + | − | − | − | − | − | − | Yes | |
| 39 | F | 10.9 | + | + | − | − | − | − | − | − | ||
| 40 | F | 11 | + | + | − | − | − | − | − | − | ||
| 41 | M | 6.6 | + | − | − | − | − | − | − | − | ||
| 42 | M | 6.2 | − | + | − | − | − | − | − | − | ||
| 43 | M | 7.4 | − | + | − | − | − | − | − | − | ||
| 44 | F | 4.8 | − | + | − | − | − | − | − | − | ||
| 45 | F | 5.4 | + | + | − | − | − | − | − | − | ||
| 46 | F | 6.7 | − | + | − | − | − | − | − | − | ||
| 47 | F | 4.8 | + | + | − | − | − | − | − | − | ||
| 48 | F | 4 | + | + | − | − | − | − | − | − | ||
| 49 | F | 3.5 | + | + | − | − | − | − | − | − | ||
| 50 | F | 5.2 | − | + | − | − | − | − | − | − | ||
| 51 | F | 2.6 | + | + | − | − | − | − | − | − | ||
| 52 | F | 2.6 | + | + | − | − | − | − | − | − | ||
| 53 | F | 2.4 | + | + | − | − | − | − | − | − | ||
F, female; M, male; +, positive; −, negative; trop, trophozoite. a2 days after 5 + 5 days of administration.
Discussion
There is some information about the use of PS for the treatment of B. coli infection in gorillas and cattle [2, 12]. Since the US Food and Drug Administration has not approved PS for human use, metronidazole and tetracycline are often used for treatment of human B. coli infections [4]. As indicated by the results in Table 1, the use of metronidazole could lead to the recurrence of B. coli cysts due to the remaining metronidazole-resistant cysts in the lamina propria of the large intestine. Together, the results of this study clarified that PS is more effective than metronidazole for treatment of B. coli infection.
For treatment of human E. histolytica infection, the manufacturer of PS recommends a precise dose of 500 mg at three times per day for 10 days (total, 15,000 mg) [1]. Because the body weight of a cynomolgus monkey is about 1/20 of that of an average human, the expected dose for a cynomolgus monkey by body weight conversion should be a total of 750 mg. However, a total of 250 mg PS for 10 days administration (5 + 5 days with a 2-day withdrawal interval) was sufficiently effective for treatment of B. coli infection in cynomolgus monkeys. However, B. coli may be more sensitive to PS than E. histolytica, and Pentatrichomonas hominis and Giardia spp. were detected in several monkeys by microscopic observation after treatment (data not shown). Therefore, the minimum dose in this study should be adopted for the treatment of B. coli infection in cynomolgus monkeys.
There is little information about the pharmacokinetics of PS by the oral route. The drug safety information for Ameparomo states that PS directly inhibits the function of the 30S ribosome during protein translation, without absorption by the gastrointestinal tract [5, 11]. PS is given orally three times per day for human cases of B. coli infection. However, it is difficult to administer a medication three times per day to monkeys. The times of daily administration are associated with the duration of PS in the feces of the large intestine. In our facility, most monkeys evacuate feces one time each day in the morning. Therefore, once daily administration of PS could be sufficiently effective to control infection in both B. coli-positive and B. coli-negative cynomolgus monkeys. Some monkeys developed diarrhea due to the antibacterial effect of PS. However, quick excretion of PS by frequent defecation could be improved by increasing the frequency of administration and the dose of PS.
On the other hand, it is also an insurmountable task to administer a drug to about 600 animals each day. Use of a pumpkin cake containing PS lessened the difficulty of PS administration for the technical staff. Also, in trials 1–3, PS was administered to each positive monkey by adding the drug to pieces of banana. Preparation of 15 pieces of banana with the required dose of PS for 15 cynomolgus monkeys for one person required about 30 min. In trial 4, the preparation of 600 pumpkin cakes containing the appropriate concentration of PS required about 3 h. Hence, this method is feasible for administration of PS to many animals within a relatively short period and is suitable for use in laboratories housing a large number of primates.
Cynomolgus monkeys in Japan are mostly imported from East and South Asia. Some reproductive facilities maintain cynomolgus monkeys in incomplete in-house buildings. Therefore, these monkeys are at a risk of infection via the consumption of drainage water contaminated with B. coli cysts. Even if the carrier monkeys are asymptomatic, it is important to verify the absence of B. coli infection while under quarantine. When monkeys are moved to research facilities in Japan, B. coli cysts may be transported to new facilities due to latent infection in the large intestine. In addition, stress conditions in each facility, e.g., phenomena related to living conditions or experimentation with immunosuppressive agents, may induce the B. coli recurrence.
In the present study, a suitable dose and duration of PS treatment for B. coli infection was determined. PS was surprisingly effective against B. coli when compared with our experience with metronidazole, although it was not sufficiently effective against B. coli infection in the first experiment (Table 1). Pumpkin cakes supplemented with PS were originally developed for use in this study to decrease labor of the animal care staff. This method could also be applied to other species of intestinal protozoa by changing the treatment dose and duration. This is the first scientific report of the use of PS for B. coli, a pathogen other than E. histolytica in a captive colony of nonhuman primates.
Conflict of Interest
The authors declare that there are no conflicts of interest associated with this work.
References
- 1.Ameparomo cupsule 250 mg, statement of virtues. 2012. http://database.japic.or.jp/pdf/newPINS/00061344.pdf. (in Japanese).
- 2.Bilal C.Q., Khan M.S., Avais M., Ijaz M., Khan J.A.2009. Prevalence and chemotherapy of Balantidium coli in cattle in the River Ravi region, Lahore (Pakistan). Vet. Parasitol. 163: 15–17. doi: 10.1016/j.vetpar.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 3.Davidson R.N., den Boer M., Ritmeijer K.2009. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 103: 653–660. doi: 10.1016/j.trstmh.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 4.Drugs com. https://www.drugs.com/monograph/paromomycin-sulfate.html.
- 5.Kanyok T.P., Reddy M.V., Chinnaswamy J., Danziger L.H., Gangadharam P.R.1994. In vivo activity of paromomycin against susceptible and multidrug-resistant Mycobacterium tuberculosis and M. avium complex strains. Antimicrob. Agents Chemother. 38: 170–173. doi: 10.1128/AAC.38.2.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn E.M., Mätz-Rensing K., Stahl-Hennig C., Makoschey B., Hunsmann G., Kaup F.J.1997. Intestinal manifestations of experimental SIV-infection in rhesus monkeys (Macaca mulatta): a histological and ultrastructural study. Zentralbl. Veterinärmed. B. 44: 501–512. [DOI] [PubMed] [Google Scholar]
- 7.Mansfield K.G., Lin K.C., Newman J., Schauer D., MacKey J., Lackner A.A., Carville A.2001. Identification of enteropathogenic Escherichia coli in simian immunodeficiency virus-infected infant and adult rhesus macaques. J. Clin. Microbiol. 39: 971–976. doi: 10.1128/JCM.39.3.971-976.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakauchi K., Nakajima H., Sakakibara I.1990. Detection of Balantidium coli from evacuated feces in cynomolgus monkeys (Macaca fascicularis). Nippon Juigaku Zasshi 52: 1323–1324. doi: 10.1292/jvms1939.52.1323 [DOI] [PubMed] [Google Scholar]
- 9.Ohto K.2006. Changes of hematological and blood biochemi- cal markers in rearing monkeys. pp. 53–58, 134–136. In: The TPRC Handbook on the Care and Management of the Laboratory Cynomolgus Monkeys (Yoshida, T. and Fujimoto, K. eds.), Springer Japan, Tokyo (in Japanese). [Google Scholar]
- 10.PMDA (Pharmaceutical and Medical Devices Agency, Japan) report. New Drugs Approved in FY 2012. http://www.pmda.go.jp/files/000153273.pdf.
- 11.Schuster F.L., Ramirez-Avila L.2008. Current world status of Balantidium coli. Clin. Microbiol. Rev. 21: 626–638. doi: 10.1128/CMR.00021-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teare J.A., Loomis M.R.1982. Epizootic of balantidiasis in lowland gorillas. J. Am. Vet. Med. Assoc. 181: 1345–1347. [PubMed] [Google Scholar]
- 13.Young K.H., Bullock S.L., Melvin D.M., Spruill C.L.1979. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 10: 852–853. [DOI] [PMC free article] [PubMed] [Google Scholar]