Abstract
Intratumoral genetic heterogeneity and the role of metabolic reprogramming in renal cell carcinoma have been extensively documented. However, the distribution of these metabolic changes within the tissue has not been explored. We report on the first-in-human in vivo non-invasive metabolic interrogation of renal cell carcinoma using hyperpolarized carbon-13 (13C) MRI and describe the validation of in vivo lactate metabolic heterogeneity against multi regional ex vivo mass spectrometry. hyperpolarized carbon-13 (13C)-MRI provides an in vivo assessment of metabolism and provides a novel opportunity to safely and non-invasively assess cancer heterogeneity.
Introduction
Intratumoral genetic heterogeneity in renal cell carcinoma (RCC) has provided important insights into the evolutionary pathway of RCC tumorigenesis.1 However, routine analysis of genetic intratumoral heterogeneity has yet to translate usefully into clinical practice as it requires specialized multiregional tumor sampling, complex computational analysis and sequencing platforms.
Metabolic reprogramming is a feature common to many solid tumors. Increased glucose uptake, glycolysis and reduced oxidative phosphorylation, known as the Warburg effect,2 have been reported in RCC.3 Hyperpolarized carbon-13 (13C) MRI (HP-MRI) is a novel non-ionizing imaging technique that allows non-invasive real-time analysis of metabolic pathways in vivo.4 Hyperpolarization using dissolution-dynamic nuclear polarization (DNP) technology provides unprecedented sensitivity for the detection of metabolism of 13C-labeled substrates such as pyruvate, fumarate and glucose in vivo.4 For example, following administration of 1-[13C] pyruvate, a number of studies have reported on the detection of 1-[13C] lactate via the reaction catalyzed by lactate dehydrogenase (LDH). This technique has recently been successfully translated into the clinical domain and is a promising tool for disease characterization and therapeutic response monitoring in prostate and brain tumors.5–8
Here, we report on the first-in-human non-invasive metabolic interrogation of RCC using HP-MRI and describe the validation of in vivo lactate metabolic heterogeneity imaged using HP-MRI against multiregional ex vivo mass spectrometry.
Methods and materials
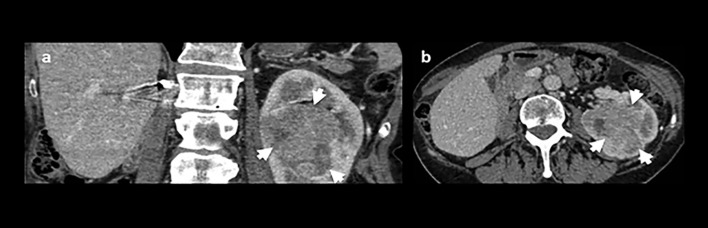
A 72-year-old female with a history of a previous right radical nephrectomy (17 years earlier) for clear cell RCC had an incidental finding of a 6.8 × 5.1 × 6.1 cm mass on the left kidney (Figure 1) confirmed as clear cell RCC.
Figure 1.
Contrast-enhanced CT of the abdomen at the level of left kidney. (a) Coronal and (b) axial slices showing an incidental finding of a 6.8 × 5.1 × 6.1 cm mass in the left kidney (arrows). There were no radiological signs of metastatic disease on CT of chest, abdomen and pelvis.
Histopathological analysis of laparoscopic radical nephrectomy specimen confirmed ISUP/WHO Grade two clear cell RCC (staging pT3a).
The patient provided written informed consent for HP-MRI (Research Ethics Committee (REC) reference number 17/LO/0431) (ClinicalTrials.gov Identifier: NCT03687645 ) and for tissue based assays (REC reference number 16/WS/0039).
Production of hyperpolarized 1-[13C] pyruvate
Hyperpolarized 1-[13C] pyruvate solution was filled and assembled under aseptic conditions then produced using a DNP polarizer (SPINlab, GE Healthcare, Milwaukee, Wisconsin) and a sterilized fluid path.9 The filled sterilized fluid path was loaded into the hyperpolarizer and the sample, consisting of 1.47 g 1-[13C] pyruvic acid (GMP Precursor from Sigma Aldrich, Vienna, Austria) doped with 15 mM AH111501 electron paramagnetic agent, underwent microwave irradiation for approximately 2 h to achieve a polarization of 31.9%. The sample was then dissolved in 38 ml of sterile water and neutralized with 17.5 g sterile trometamol buffer solution (333 mM Tris and 600 mM NaOH) in 19 ml of sterile water. Release criteria for sterile hyperpolarized solution for injection are tabulated in Table 1.
Table 1.
Release criteria for final product
| Release criteria for sterile hyperpolarized solution in Medrad syringe for injection | |
| Appearance | Clear colorless solution with a slightly green tinge and free from visible particles |
| Validations satisfactory for | Sterility: Complies with Ph. Eur. Endotoxins: Complies with Ph.Eur. |
| Physical & chemical parameters | Based on UCSF Limits |
| 13C nuclear polarization | Not Less Than 10.0%a |
| Pyruvate | 220–280 mM |
| Residual AH111501 | Not more than 3.0 µM |
| pH (i) QC module (ii)pH strips ∆ (i) & (ii) |
6.5–8.5 6.5–8.5 ≤1.0 pH unit |
| Drug product temperature | 25.0 – 37.0o Cb |
| Drug product volume | >38 ml |
| Compliance with TSE regulations | |
TSE, transmissible spongiform encephalopathies.
Polarization at the start of dissolution. UCSF limit NLT 15%
Temperature at the time of analysis
Imaging set-up
The patient was positioned in a clinical 3 T integrated PET-MR scanner (Siemens Biograph mMR, Enlargen, Germany) in the supine position with an intravenous (i.v.) catheter placed in the left arm. A specialized custom-design clamshell 13C transmit coil was used with two (anterior and posterior) 7-channel 1H/13C receive surface phase array coils (RAPID Biomedical GmbH, Rimpar, Germany) for signal excitation and reception of 13C signals respectively.
The i.v. line was connected to an automatic dual chamber power injector with the first chamber (chamber A) programmed to deliver hyperpolarized 1-[13C] pyruvate solution at a rate of 5 ml s−1. The second chamber (chamber B) was pre-loaded and programmed to deliver 20 ml of normal saline flush immediately after hyperpolarized solution injection.
Anatomical localization of the renal tumor was performed on axial and coronal T2 weighted imaging. A turbo spin echo sequence was utilized with the following parameters: repetition time = 5400 ms, effective echo time = 111 ms, slice thickness = 3 mm, number of slices = 30, Field of view (axial) = 203 mm x 460 mm, field of view (coronal) = 369 mm x 460 mm, echo train length = 15, number of signal averaging = 1.
Following localization of the tumor, a central axial imaging slice was planned under the direction of a board certified radiologist for subsequent 13C imaging.
Hyperpolarized MRI
40 ml of hyperpolarized 1-[13C] pyruvate was injected at a rate of 5 ml s−1 followed by a flush of 20 ml of normal saline at 3 ml s−1. Repeated 13C chemical shift imaging (CSI) measurements were performed (repetition time = 80 ms, time of echo = 3 ms, flip angle = 10°, bandwidth = 10,000 Hz, field of view = 120 mm x 120 mm, slice thickness = 30 mm, acquisition matrix = 16 × 16), commencing at a delay of 25 s following start of injection to allow for sufficient hyperpolarized solution delivery to the kidney. Single-slice CSI images were acquired every 20 s for a total of 12 repetitions.
The CSI data were analyzed offline (MATLAB 2016; MathWorks Inc., Natick, MA). The individual free induction decays across the CSI grid were apodized with an exponential 10 Hz filter in the time domain and then Fourier transformed. The areas of spectral peaks were calculated to produce the metabolic maps. Metabolic maps of the 1-[13C] labeled lactate and pyruvate spectral areas and lactate/pyruvate ratio were generated for the first acquired CSI dataset (demonstrating the highest formation of 1-[13C] lactate). The 1-[13C] lactate signal was not measurable on subsequent CSI data.
Tissue handling
After macroscopic pathology review, multiregional tissue samples were collected within 30 min of nephrectomy. A 1 cm thick axial slice of kidney was selected at the level of the renal hilum (visually matched to the MRI CSI imaging slice) and regional sampling was labelled sequentially (10 samples of tumor, and 5 samples of adjacent non-tumorous kidney tissue). Samples were placed in cryo-vials and immediately snap frozen with liquid nitrogen. Samples were formalin fixed, processed in paraffin and stained with hematoxylin & eosin after sectioning for microscopic confirmation of presence or absence of malignancy.
Lactate measurement by liquid chromatography-mass spectrometry
Frozen tissue samples were weighed into Precellys tubes prefilled with ceramic beads (Stretton Scientific Ltd., Derbyshire, UK), and an exact volume of extraction solution (30% acetonitrile, 50% methanol and 20% water) was added to obtain 40 mg specimen per mL of extraction solution. Samples were lyzed using a Precellys®24 tissue homogeniser (Bertin Corp, Rockville, MD. 5500 rpm 15 s x 2) and then centrifuged (16,162 x g for 10 min at 4°C). The supernatant was transferred to glass vials (Microsolv Technology Corp., Leland, NC) and stored at −80°C until LC-MS analysis.
Samples were randomized in order to avoid bias due to machine drift and the operator was blind to the HP-MRI assessment. LC-MS analyses were performed on a Q Exactive mass spectrometer (Thermo Fisher Scientific) mass spectrometer coupled to an Ultimate 3000 RSLC system (Dionex). The liquid chromatography system was fitted a ZIC-pHILIC column (150 × 2.1 mm) and respective guard (20 × 2.1 mm) (all Merck Millipore, Germany), and metabolites were eluted with the previously described gradient.10 The mass spectrometer was operated in full MS and polarity switching mode. The acquired spectra were analyzed using XCalibur Quan Browser software (Thermo Fisher Scientific). Absolute quantification of lactate was performed by interpolation of the corresponding standard curve obtained from serial dilutions of a commercially available standard (Sigma Aldrich, Vienna, Austria) running with the same batch of samples.
Results
1-[13C] Lactate signal was observed on the first, and 1-[13C] pyruvate signal on the first and second acquisition of the CSI temporal series.
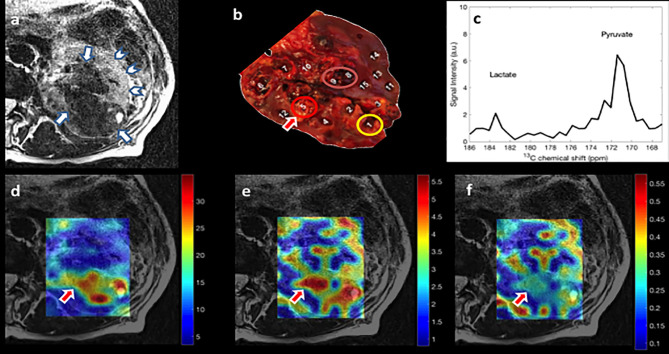
The 1-[13C] pyruvate signal (Figure 2d) provides a measure of blood flow, whilst the 1-[13C] lactate signal demonstrates metabolism (Figure 2e).9
Figure 2.
(a) Axial T2 weighted turbo spin echo MRI through kidney. Renal tumor (arrows) and normal-appearing renal tissue (arrow head) are shown. (b) 1 cm thick axial slice of kidney at the level of the renal hilum and 15 tissue samples (1–10 tumor samples and 11–15 non-tumor samples). Images from 13C CSI first acquisition (c–f). (c) Corresponding spectra of the arrowed region on (b) (region 5). The spectrum shows prominent signals from lactate (left) and pyruvate (right). (d) Interpolated hyperpolarized 1-[13C] pyruvate map overlaid on T2 weighted turbo spin echo MRI. (e) Interpolated hyperpolarized 1-[13C] lactate map overlaid on T2 weighted turbo spin echo MRI. (f) Interpolated hyperpolarized 1-[13C] lactate/pyruvate map overlaid on T2 weighted turbo spin echo MRI. Bottom row depicts intratumoral heterogeneity of; pyruvate delivery (d), pyruvate to lactate metabolic conversion (e) and the ratio of lactate to pyruvate map (f) (a.u.). CSI, chemical shift imaging.
Heterogeneous 1-[13C] pyruvate signal was seen across the RCC, reflective of variability of blood flow (Figure 2d). Heterogeneous formation of 1-[13C] lactate is also evident (Figure 2e). Moreover, areas of high 1-[13C] lactate formation did not always conform to areas of high blood flow as highlighted by the lactate/pyruvate ratio map (Figure 2f).
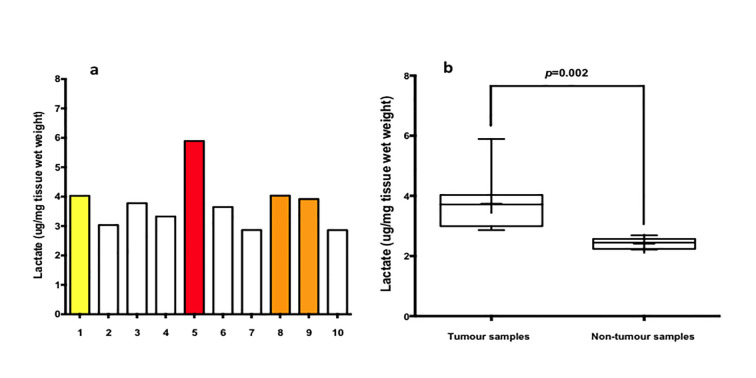
LC-MS analysis confirmed heterogeneous lactate levels within tumor samples (Figure 2b, samples 1–10), as depicted on the bar chart in Figure 3. The highest level of lactate by mass spectrometry (Figure 3—red bar) was found in region 5, corresponding to the region displaying the highest 1-[13C] lactate signal in the HP-MRI scan (Figure 2—red arrow). Further 1-[13C] lactate formation was observed at imaging corresponding to samples 8–9 (Figure 3a—orange bars) and sample 1 (Figure 3a—yellow bar).
Figure 3.
(a) Bar chart of liquid chromatography–mass spectrometry analysis. The graph depicts liquid chromatography–mass spectrometry analysis of the multiregional samples from the tumor. The highest level of lactate accumulation was found in region 5 (red bar) in consistence with the findings of hyperpolarized 1-[13C] lactate map (Figure 2e, red arrow). Colored bars correspond to colored circles in Figure 2b. (b) Box and whisker plot of liquid chromatography–mass spectrometry analysis of the multiregional samples from tumor and non-tumor areas. The median and interquartile range of the weight of lactate per sampled area was 3.7 μg/mg of tissue wet weight and 0.89 for tumor samples and 2.4 μg/mg of tissue wet weight and 0.18 for non-tumor samples respectively (p = 0.002). The boundaries of the box show 25th and 75th percentiles, and the line within the box is the median. Whiskers show 10th and 90th percentiles.
Overall, we observed heterogeneity of HP-MRI 1-[13C] lactate signal generally conforming to the heterogeneity found at mass spectrometry metabolic analysis.
Discussion and conclusion
In this study, we report on the first-in-human in vivo non-invasive metabolic assessment of RCC using 1-[13C] pyruvate HP-MRI and validate the results with ex vivo multi regional LC-MS analysis.
HP-MRI using dissolution DNP produces a dramatic signal enhancement, allowing real-time imaging of metabolic pathways.4 Until now, the clinical application of HP-MRI in malignancies has been limited to prostate cancer5,6 and brain tumors.7,8 To the best of our knowledge this study represents two novel factors: it is the first-in-human 1-[13C] pyruvate HP-MRI study in RCC, and it is the first HP-MRI study with tissue assay validation.
Unlike the multiregional sampling used for tissue-based assessment of genetic and metabolic heterogeneity, HP-MRI provides a non-invasive technique that can be repeated over time and thus could be used for longitudinal tumor monitoring.6
It has been previously shown that the pyruvate signal build-up precedes the lactate signal build-up in the time course data of pre-clinical animal models, consistent with the notion that the pyruvate signal reflects its delivery to the tissue and cells whilst the lactate signal reflects pyruvate-to-lactate conversion catalyzed by lactate dehydrogenase in the glycolytic pathway.11
In our patient, the regional distribution of pyruvate and lactate differed markedly across the RCC, suggesting that blood flow and metabolism were not integrally linked within the cancer.
One possible explanation for our observation is that higher levels of LDH are found in areas of tumor which exhibit hypoxia.12 Tumor hypoxia can be caused by high levels of metabolic activity or through relative decrease in effective blood flow.13 Hence low 1-[13C] pyruvate signal, reflecting limited blood flow, may occur together with high 1-[13C] lactate signal, reflecting increased metabolic activity.
We also observed heterogeneity of in vivo 1-[13C] lactate signal across the tumor confirmed by ex vivo mass spectrometry. Genomic, microstructural and macrostructural heterogeneity has been noted in cancers including RCC.1,14,15 Our results confirm heterogeneous metabolic activity is also present within RCC. Future work linking genomic, metabolic, microstructural and macrostructural changes is needed to explore if RCC metabolic heterogeneity relates to genomic heterogeneity; and, whether metabolic heterogeneity is a result of micro/macrostructural heterogeneity or conversely results in RCC micro/macrostructural heterogeneity.
The CSI sequence used within this study enabled single slice imaging with limited temporal resolution. New sequences are being developed for hyperpolarized 13C metabolic imaging allowing full anatomical coverage and improved temporal resolution.16–18
HP-MRI has a variety of potential clinical applications. For example, knowledge of RCC metabolic activity may provide a prognostic tool to help treatment stratification, such as whether active surveillance or urgent surgery is warranted. Temporal changes in metabolism could be used to monitor patients on active surveillance, and to provide an assessment of treatment response. Indeed, identifying areas of higher metabolic activity within solid tumors in general could help guide target biopsy and themselves act as targets for novel focal therapies.
Learning points
Metabolic reprogramming is a feature common to many solid tumors with increased glucose uptake, glycolysis and reduced oxidative phosphorylation (Warburg effect) reported in RCC
Hyperpolarized carbon-13 (13C) MRI is a novel non-ionizing imaging technique that allows non-invasive real-time analysis of metabolic pathways in vivo.
We report on the first-in-human in vivo non-invasive metabolic assessment of RCC using 1-[13C] pyruvate HP-MRI and validate the results with ex vivo multiregional liquid chromatography–mass spectrometry analysis.
The regional distribution of pyruvate and lactate differed markedly across the RCC, suggesting that blood flow and metabolism were not integrally linked within the cancer
Knowledge of RCC metabolic activity using hyperpolarized carbon-13 (13C) MRI may provide a future prognostic tool to help treatment stratification.
Footnotes
Maxine Tran and Arash Latifoltojar have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Maxine Tran, Email: m.tran@ucl.ac.uk.
Arash Latifoltojar, Email: arash.latifoltojar.10@ucl.ac.uk.
Joana B. Neves, Email: joana.b.neves@ucl.ac.uk.
Marianthi-Vasiliki Papoutsaki, Email: v.papoutsaki@ucl.ac.uk.
Fiona Gong, Email: f.gong@ucl.ac.uk.
Arnaud Comment, Email: arnaud.comment@cruk.cam.ac.uk.
Ana S. H. Costa, Email: ash51@mrc-cu.cam.ac.uk.
Matthias Glaser, Email: matthias.glaser@ucl.ac.uk.
My-Anh Tran-Dang, Email: my-anh.tran-dang@nhs.net.
Soha El Sheikh, Email: s.elsheikh@nhs.net.
Wivijin Piga, Email: wivijinpiga2@gmail.com.
Alan Bainbridge, Email: alan.bainbridge1@nhs.net.
Anna Barnes, Email: anna.p.barnes@ucl.ac.uk.
Tim Young, Email: ty272@mrc-cu.cam.ac.uk.
Hassan Jeraj, Email: hjeraj@googlemail.com.
Ramla Awais, Email: r.awais@ucl.ac.uk.
Sola Adeleke, Email: s.adeleke@ucl.ac.uk.
Christopher Holt, Email: christopher.holt1@nhs.net.
James O’Callaghan, Email: james.ocallaghan@alumni.ucl.ac.uk.
Frazer Twyman, Email: f.twyman@ucl.ac.uk.
David Atkinson, Email: d.atkinson@ucl.ac.uk.
Christian Frezza, Email: cf366@mrc-cu.cam.ac.uk.
Erik Årstad, Email: e.arstad@ucl.ac.uk.
David Gadian, Email: d.gadian@ucl.ac.uk.
Mark Emberton, Email: m.emberton@ucl.ac.uk.
Shonit Punwani, Email: shonit.punwani@gmail.com.
REFERENCES
- 1.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. . Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med Overseas Ed 2012; 366: 883–92. doi: 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science 1956; 123: 309–14. doi: 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 3.Courtney KD, Bezwada D, Mashimo T, Pichumani K, Vemireddy V, Funk AM, et al. . Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell Metab 2018; 28: 793–800. doi: 10.1016/j.cmet.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comment A, Merritt ME. Hyperpolarized magnetic resonance as a sensitive detector of metabolic function. Biochemistry 2014; 53: 7333–57. doi: 10.1021/bi501225t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, et al. . Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med 2013; 5: 198ra108. doi: 10.1126/scitranslmed.3006070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal R, Vigneron DB, Kurhanewicz J. Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur Urol 2017; 72: 1028–9. doi: 10.1016/j.eururo.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park I, Larson PEZ, Gordon JW, Carvajal L, Chen H-Y, Bok R, et al. . Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med 2018; 80: 864–73. doi: 10.1002/mrm.27077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miloushev VZ, Granlund KL, Boltyanskiy R, Lyashchenko SK, DeAngelis LM, Mellinghoff IK, et al. . Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Production in Brain Tumor Patients. Cancer Res 2018; 78: 3755–60. doi: 10.1158/0008-5472.CAN-18-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardenkjaer-Larsen JH, Leach AM, Clarke N, Urbahn J, Anderson D, Skloss TW. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed 2011; 24: 927–32. doi: 10.1002/nbm.1682 [DOI] [PubMed] [Google Scholar]
- 10.Mackay GM, Zheng L, van den Broek NJF, Gottlieb E. Analysis of cell metabolism using LC-MS and isotope tracers. Methods Enzymol 2015; 561: 171–96. doi: 10.1016/bs.mie.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 11.Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, et al. . Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res 2008; 68: 8607–15. doi: 10.1158/0008-5472.CAN-08-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukacova S, Sørensen BS, Alsner J, Overgaard J, Horsman MR. The impact of hypoxia on the activity of lactate dehydrogenase in two different pre-clinical tumour models. Acta Oncol 2008; 47: 941–7. doi: 10.1080/02841860701644086 [DOI] [PubMed] [Google Scholar]
- 13.Eales KL, Hollinshead KER, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016; 5: e190. doi: 10.1038/oncsis.2015.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malandrino A, Mak M, Kamm RD, Moeendarbary E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extreme Mech Lett 2018; 21: 25–34. doi: 10.1016/j.eml.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 1805; 2010: 105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH, et al. . Hyperpolarized 13C MRI: path to clinical translation in oncology. Neoplasia 2019; 21: 1–16. doi: 10.1016/j.neo.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Hesketh RL, Wright AJ, Brindle KM. Hyperpolarized 13C spectroscopic imaging using single‐shot 3D sequences with unpaired adiabatic refocusing pulses NMR Biomed. 2018; 31: e4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty BJ, Lau JYC, Chen AP, Cunningham CH. Dual-Echo EPI sequence for integrated distortion correction in 3D time-resolved hyperpolarized 13C MRI. Magn Reson Med 2018; 79: 643–53. doi: 10.1002/mrm.26698 [DOI] [PubMed] [Google Scholar]