Abstract
Metabolic syndrome is characterized by a constellation of comorbidities that predispose individuals to an increased risk of developing cardiovascular pathologies as well as type 2 diabetes mellitus (T2DM)1. The gut microbiota is considered as a new key contributor involved in the onset of obesity-related disorders2. In humans, studies have provided evidence for a negative correlation between Akkermansia muciniphila abundance and overweight, obesity, untreated T2DM, or hypertension3–8. As the administration of A.muciniphila has never been investigated in humans, we conducted a randomized double-blind placebo-controlled pilot study in overweight/obese insulin resistant volunteers, 40 were enroled and 32 completed the trial. The primary endpoints were on safety, tolerability and metabolic parameters (i.e., insulin resistance, circulating lipids, visceral adiposity, body mass). The secondary outcomes were the gut barrier function (i.e., plasma lipopolysacharrides (LPS) and gut microbiota composition. In this single-center study, we demonstrated that daily oral supplementation of 1010 bacteria either alive or pasteurized A.muciniphila for 3 months was safe and well tolerated. Compared to the Placebo, pasteurized A.muciniphila improved insulin sensitivity (+28.62±7.02%, P=0.002), reduced insulinemia (-34.08±7.12%, P=0.006) and plasma total cholesterol (-8.68±2.38%, P=0.02). Pasteurized A.muciniphila supplementation slightly decreased body weight (-2.27±0.92kg, P=0.091) as compared to the Placebo group, and fat mass (-1.37±0.82kg, P=0.092) and hip circumference (-2.63±1.14cm, P = 0.091) as compared to baseline. After 3 months of supplementation, A.muciniphila reduced the levels of relevant blood markers of liver dysfunction and inflammation while the overall gut microbiome structure was unaffected. In conclusion, this proof-of-concept study (NCT02637115) shows that the intervention was safe and well-tolerated and that the supplementation with A.muciniphila improves several metabolic paramaters.
To overcome the pandemic worldwide evolution of cardiometabolic diseases, research has increasingly focused its attention on interventions targeting the gut microbiota2. Among commensal bacteria residing in the intestine, A.muciniphila has attracted growing interest for its health-promoting effects9. In rodents, treatment with A.muciniphila reduces obesity and related disorders such as glucose intolerance, insulin resistance, steatosis and gut permeability10–12. Recently, in rodents, we serendipitously discovered that pasteurization of A.muciniphila enhanced its beneficial properties on adiposity, insulin resistance and glucose tolerance11. However, translational evaluation of A.muciniphila for human investigation was hampered by the requirement for animal-derived compounds in the growth medium used to culture this bacterium. We circumvented this major issue by developing a synthetic medium compatible with human administration11.
The main objectives of this exploratory study were (1) to evaluate the feasibility, the safety and the tolerance of A.muciniphila supplementation, and (2) to explore for the first time the metabolic effects of A.muciniphila supplementation in humans. The study was designed as an exploratory and proof-of-concept study for a first supplementation in humans. The primary outcomes were on safety, tolerability (i.e., hepatic function, renal function, inflammation) and metabolic parameters (i.e., insulin resistance, circulating lipids, visceral adiposity, body mass index). The secondary outcomes were the gut barrier function (i.e., plasma lipopolysacharrides (LPS)/metabolic endotoxemia), gut microbiota composition and metabolites. In 2017, the first reported preliminary human data from this study and obtained on 5 volunteers per group suggested that treatment with either placebo, two doses of alive A.muciniphila (low dose 109 bacteria per day or high dose 1010 bacteria per day), or pasteurized A.muciniphila (1010 bacteria per day) was safe in individuals with excess body weight, as no changes in safety parameters or reported adverse events were observed after 15 days of daily administration11.
Here, we further extend this randomized double-blind placebo-controlled proof-of-concept and feasibility study using the daily oral administration for 3 months of A.muciniphila, either Alive or Pasteurized and compared their effects at the highest dose tested that is at 1010 bacteria per day, in individuals exhibiting excess body weight (overweight or obese), insulin resistance and a metabolic syndrome.
Individuals were enrolled and underwent randomization to receive either a placebo (Placebo), live A.muciniphila (Alive, 1010 bacteria per day), or pasteurized A.muciniphila (Pasteurized, 1010 bacteria per day) as supplement for 3 months, with the specific advice to keep their normal dietary intake and physical activity during the study period (Flow chart in Extended Data Fig. 1). Although the subjects were randomized, we found that before starting the supplementation (i.e., T0) the subjects that would receive the pasteurized cells exhibited significantly higher levels of insulin and lower insulin sensitivity than those in the Placebo group (Extended Data Table 1). For safety assessment, an early visit was scheduled after 15 days of supplementation. We found that both safety and tolerability were similar between the two groups receiving the different forms of A.muciniphila as compared to the Placebo (Extended Data Table 2 and 3), excepting a higher white blood cells (WBC) count in the Placebo and the treated groups (Extended Data Table 2). We further followed safety and tolerability parameters until 3 months and did not observe any adverse events (Extended Data Table 4 and 5). In addition, the compliance was higher than 99% in all groups (Extended Data Table 5).
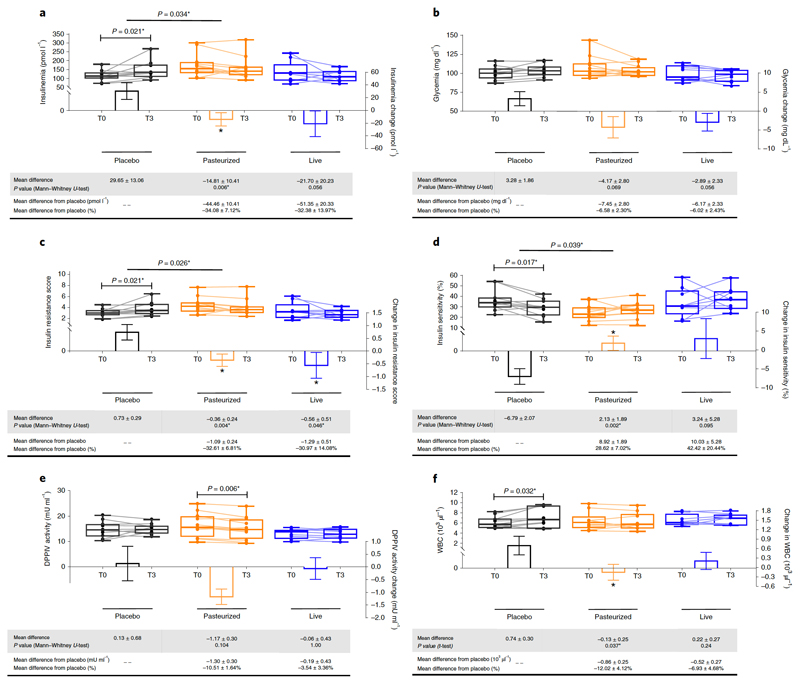
After 3 months, the Placebo group exhibited a significant increase of fasting plasma insulin (P<0.05, T3 versus T0, Fig. 1a), contrary to participants receiving both forms of A.muciniphila in whom reduced plasma insulin levels (by about 30%) were observed as compared to the Placebo (Fig. 1a). This effect was significant between the Pasteurized A.muciniphila and the Placebo group (Fig. 1a). Fasting glycemia was not affected (Fig. 1b), however, the subjects were not highly hyperglycemic at baseline (Extended Data Table 1).
Figure 1. Changes in parameters related to glucose metabolism and WBC.
(a) Insulinemia, (b) Glycemia, (c) Insulin Resistance score, (d) Insulin Sensitivity, (e) DDPIV activity (f) and With blood cell count. Differential values (MD and MD from placebo) are expressed as mean + SEM, either as raw data or as percentages. Bars represent mean change from baseline value per group, with their standard errors. Mann-Whitney tests or unpaired T-tests were performed to compare differential values of both treated groups versus the Placebo group (inter-group changes), according to the distribution. The respective P value are indicated in the table and when the test is significative, bars are marked by * symbol. Lines represent raw values before and after 3 months of supplementation. Distribution of values within each group for each timing is illustrated by a box and whisker. In the box plots, the line in the middle of the box is plotted at the median, the inferior and superior limit of the box correspond to the 25th and the 75th percentiles respectively. The whiskers correspond to the minimum and maximum value. Wilcoxon matched-pairs signed ranks tests or Paired T-tests were performed to verify changes from baseline (intra-group changes), according to the distribution. When the difference is significative, a capped line is marked above the concerned group with the corresponding P value. Changes between 0 and 3 months across the 3 groups were analyzed with Kruskal-Wallis or One-way ANOVA according to the distribution and group-wise comparisons were performed using Bonferroni and Tuckey’s adjustment for multiple testing, respectively. When the difference is significative, a line is marked above the concerned groups with the corresponding P value. DPPIV, dipeptidyl peptidase-IV ; MD, Mean Difference; WBC, white blood cells. Placebo, n = 11; Pasteurized, n =12; Alive, n=9 for all parameters except for WBC : Placebo, n = 11; Pasteurized, n =11; Alive, n=8. All tests were performed in two-tailed. *P < 0.05.
We also measured insulin sensitivity indexes and insulin resistance (HOMA method), and found that insulin sensitivity was significantly reduced at T3 in the Placebo group (Fig. 1c and d). Conversely, both forms of A.muciniphila improved this parameter. Indeed, Pasteurized A.muciniphila markedly and significantly improved insulin sensitivity index by about 30% as compared to the Placebo group (Fig. 1c and d) and Alive A.muciniphila significantly improved the insulin resistance score (Fig. 1c). HbA1c was not modified by the supplementation with A.muciniphila (Extended Data Table 4), however, this may be explained by the fact that the subjects were not diabetic and had normal HbA1c at baseline (Extended Data Table 1).
Besides its impact on incretins and glucose metabolism, the activity of the enzyme dipeptidyl peptidase-IV (DPP-IV) is thought to be involved in modulating inflammation. Indeed, several studies have shown a lower inflammatory tone upon the use of DPP-IV inhibitors, thereby suggesting that this enzyme may contribute to improve glucose metabolism and cardiometabolic risk by other mechanisms than incretins levels modulation13–15. Here, we found that Pasteurized A.muciniphila significantly lowered DPP-IV activity at the end of the 3 months period as compared to baseline (Fig. 1e). This parameter remained stable in both the Placebo and the Alive A.muciniphila groups. Consistent with the hypothesis that this enzyme may contribute to improve glucose metabolism and cardiometabolic risk by other mechanisms than incretins levels modulation, we did not find any significant changes in plasma GLP-1 levels (Extended Data Fig. 2).
WBC counts are elevated in obesity16, and numerous very large cohort studies and meta-analysis have clearly linked elevated WBC counts with glucose intolerance or with the risk of developing type 2 diabetes17. More recently WBC counts were proposed as predictors for the incident of T2DM in obese subjects18–20. Therefore, in accordance with these observations, we measured WBC counts in the groups. Interestingly, we found that WBC remained significantly increased as compared to baseline and week 2 in the Placebo group (Extended Data Table 2 and Fig. 1f) whereas Pasteurized A.muciniphila supplementation completely abolished this effect, resulting in significantly lower WBC counts in the Pasteurized A.muciniphila group as compared to the Placebo group (Fig. 1f). The magnitude of the differences between either T0 versus T3 or versus the Placebo group (i.e., 866 cells/µl) is highly significant since a difference comprised between 300 to 1000 cells/µl is considered as clinically relevant17–20.
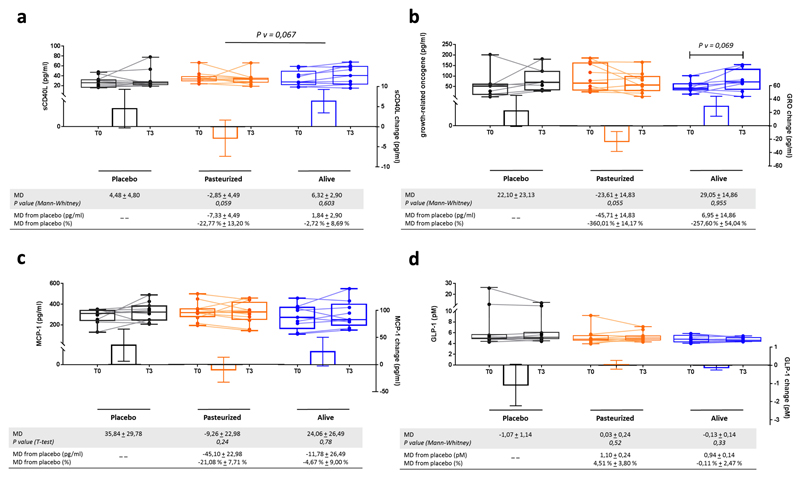
Although C-reactive protein (CRP) was not significantly changed (Extended Data Table 4), we measured other markers associated with cardiometabolic risks. We found lower sCD40L levels (Soluble CD40 Ligand) in the Pasteurized versus the Placebo group but this effect did not reach significance (P=0.059) (Extended Data Fig.2a). The chemokine GRO (growth-regulated oncogene/CXCL1) decreased in the Pasteurized A.muciniphila group at T3 versus T0 and versus the Placebo group (P=0.055) whereas MCP-1 (monocyte chemoattractant protein-1) decreased by 21% versus Placebo but did not reach significance (Extended Data Fig. 2b and c).
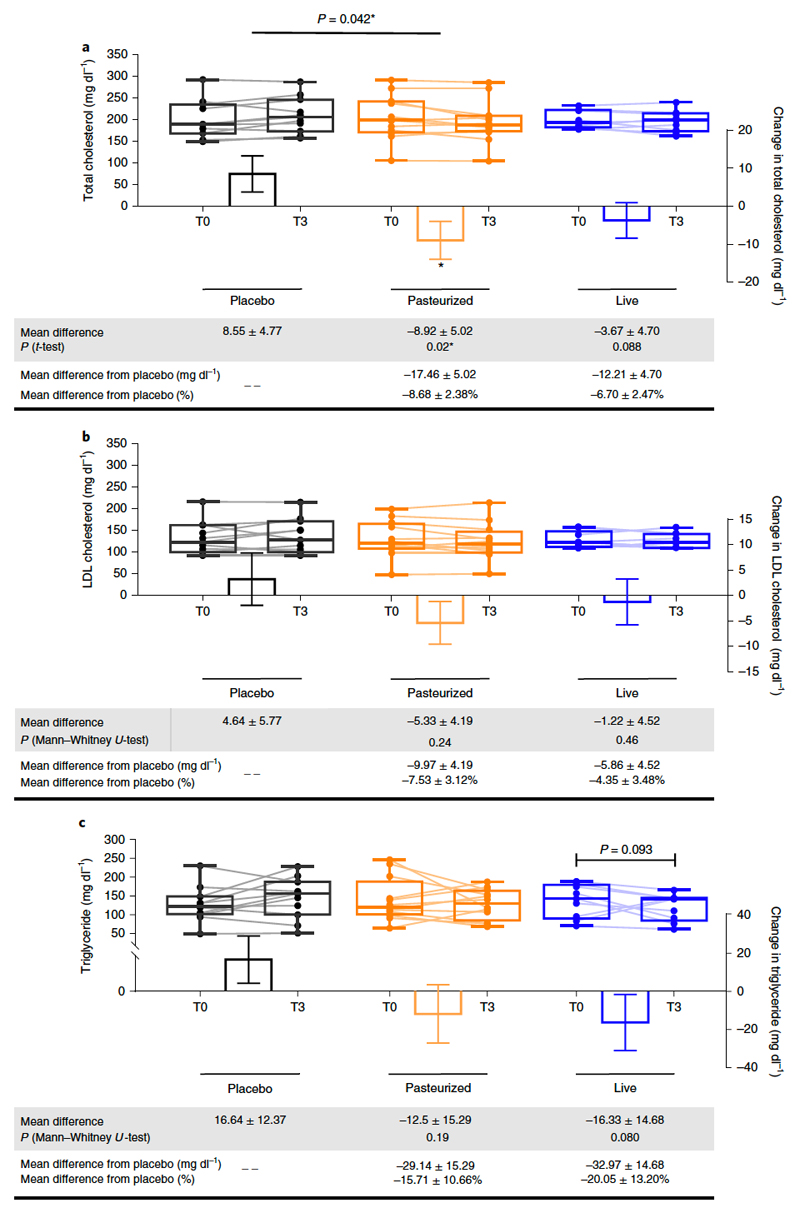
Recent studies showed that A.muciniphila gavage reduces plasma cholesterol in rodents11,21 and can also prevent the development of atherosclerosis22. We found that administration of Pasteurized A.muciniphila significantly decreased total cholesterol by 8.68% compared to the Placebo (Fig. 2a), whereas LDL cholesterol was -7.53% lower and triglycerides -15.71% lower but did not reach significance (Fig. 2b and c). Interestingly, the magnitude of the effects observed on lipids was equivalent to that induced by dietary supplementation with phytosterols according to a recent meta-analysis 23.
Figure 2. Changes in parameters related to lipid metabolism.
(a) Total cholesterol, (b) LDL-cholesterol and (c) Triglycerides. Differential values (MD and MD from placebo) are expressed as mean ± SEM, either as raw data or as percentages. Bars represent mean change from baseline value per group, with their standard errors. Mann-Whitney tests or unpaired T-tests were performed to compare differential values of both treated groups versus the Placebo group (inter-group changes), according to the distribution. The respective P value are indicated in the table and when the test is significative, bars are marked by * symbol. Lines represent raw values before and 3 months after receiving treatment. Distribution of values within each group for each timing is illustrated by a box and whisker. In the box plots, the line in the middle of the box is plotted at the median, the inferior and superior limit of the box correspond to the 25th and the 75th percentiles respectively. The whiskers correspond to the minimum and maximum value. Wilcoxon matched-pairs signed ranks tests or Paired T-tests were performed to verify changes from baseline (intra-group changes), according to the distribution,when drawn, the capped line above the concerned group shows the corresponding P-value. When the difference is significative, a capped line is marked above the concerned group. Changes between 0 and 3 months across the 3 groups were analyzed with Kruskal-Wallis or One-way ANOVA according to the distribution and group-wise comparisons were performed using Bonferroni and Tuckey’s adjustment for multiple testing, respectively. When the difference is significative, a line is marked above the concerned groups with the corresponding P value. MD, Mean Difference. Placebo, n = 11; Pasteurized, n =12; Alive, n=9 for all parameters. All tests were performed in two-tailed. *P < 0.05.
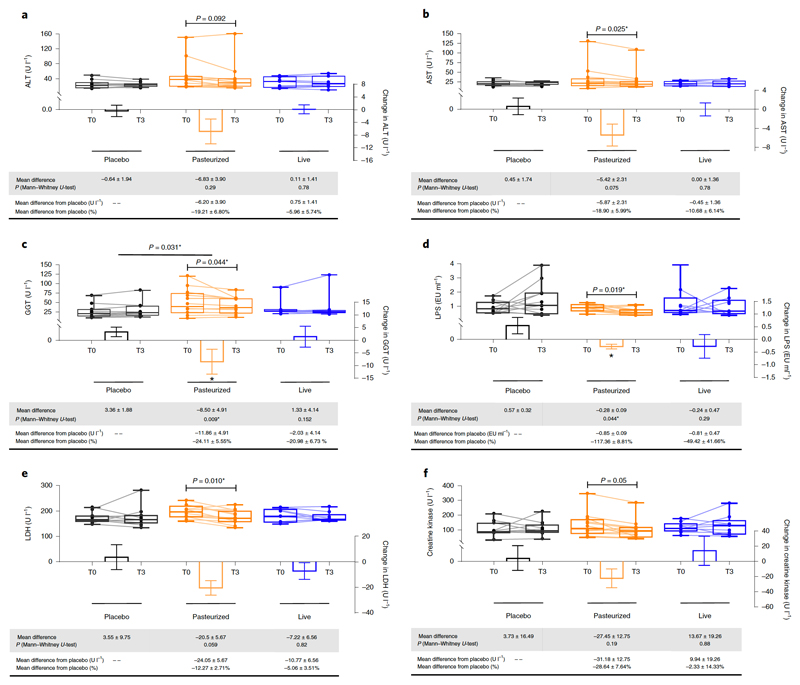
Numerous large cohort studies have linked raised activity of hepatic enzymes such as gamma-glutamyl transferase (γGT), aspartate-aminotransferase (AST) and alanine-aminotransferase (ALT) to adverse changes in glucose and lipid metabolisms, to the extent that those enzymes are considered inflammatory markers and risk factors for the development of insulin resistance and incident T2DM 24–27. In rodents, several studies12,28–30 have reported that supplementation with A.muciniphila reduces γGT, AST and ALT levels as well as hepatic steatosis. Strikingly, Pasteurized A.muciniphila significantly reduced both γGT and AST levels after 3 months as compared to baseline (Fig. 3b and c), but not ALT (Fig. 3a). Particularly, γGT levels were markedly and significantly decreased by about 24% in Pasteurized A.muciniphila as compared to T3 levels observed in the Placebo (P=0.009). None of these parameters were affected by supplementation with Alive A.muciniphila (Fig. 3a-c).
Figure 3. Changes in hepatic and general enzymes.
(a) Alanine aminotransferase activity (ALT), (b) Aspartate aminotransferase activity (AST), (c) ɣ-Glutamyltransferase (GGT), (d) Lipopolysaccharides (LPS), (e) Lactate Dehydrogenase (LDH) and (f) Creatine Kinase (CK). Differential values (MD and MD from placebo) are expressed as mean ± SEM, either as raw data or as percentages. Bars represent mean change from baseline value per group, with their standard errors. Mann-Whitney tests were performed to compare differential values of both treated groups versus the Placebo group (inter-group changes), according to the distribution. The respective P value are indicated in the table and when the test is significative, bars are marked by * symbol. Lines represent raw values before and after 3 months of supplementation. Distribution of values within each group for each timing is illustrated by a box and whisker. In the box plots, the line in the middle of the box is plotted at the median, the inferior and superior limit of the box correspond to the 25th and the 75th percentiles respectively. The whiskers correspond to the minimum and maximum value. Wilcoxon matched-pairs signed ranks tests were performed to verify changes from baseline (intra-group changes), according to the distribution. When the difference is significative, a capped line is marked above the concerned group with the corresponding P value. Kruskal-Wallis analysis were used to compare changes between 0 and 3 months across the 3 groups according to the distribution. All group-wise comparisons were performed using Bonferroni’s adjustment for multiple testing. When the difference is significative, a line is marked above the concerned groups with the corresponding P value. MD, Mean Difference. Placebo, n = 11; Pasteurized, n =12; Alive, n=9 for all parameters except for Creatine Kinase : Placebo, n = 11; Pasteurized, n =11; Alive, n=8. All tests were performed in two-tailed. *P < 0.05.
To further explore the potential mechanisms underlying the reduction of γGT and AST, we focused on plasma LPS. Indeed, numerous data obtained in humans suggest that translocation of endotoxins contributes to liver injury31–33 as well as insulin resistance32,34. Moreover, we and others have shown that A.muciniphila reinforces the gut barrier function and eventually reduces plasma LPS10,11,22,29. Therefore, we measured plasma LPS before and after A.muciniphila supplementation. Pasteurized A.muciniphila significantly decreases LPS as compared to baseline, but also as compared to the Placebo group at T3 (Fig. 3d). Thus, we speculate that such significant findings could be involved in the favorable metabolic changes observed, such as improved glucose metabolism and hepatic inflammatory markers and decreased WBC count. It is worth nothing that Pasteurized A.muciniphila supplementation decreases serum lactate dehydrogenase (LDH) and creatine kinase (CK) levels at T3 versus T0, two enzymes considered as valid markers of whole-body tissue damage and muscle-specific injury, respectively (Fig. 3e and f).
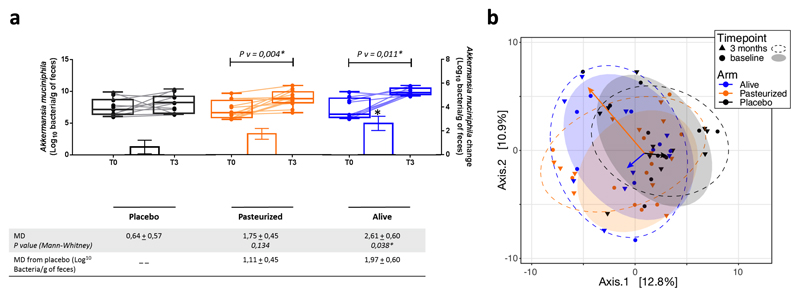
As the gut microbiota has been linked with metabolism and cardiometabolic risk factors2,35,36, and A.muciniphila with improved metabolic parameters36,37 we measured the levels of A.muciniphila at baseline and after supplementation (Extended Data Fig. 3a). First, we found that the abundance of A.muciniphila was similar between groups at baseline, whereas the supplementation significantly increased by 1.7 to 2.6 Log the quantity of A.muciniphila recovered in the feces of Pasteurized A.muciniphila and Alive A.muciniphila groups, respectively (Extended Data Fig. 3a). Interestingly, baseline characterization of the fecal microbiome was performed on the 3 groups and showed that there was no significant difference between groups at baseline (permutational MANOVA, R2=0.066, p-value=0.51, Extended Data Fig. 3b). Moreover, at the end of the intervention, the difference in gut microbiome composition between the 3 groups was slightly higher than at baseline while still non-significant (permutational MANOVA, R2=0.075, p-value=0.18, Extended Data Fig. 3b). We evaluated the alteration in microbiota composition from baseline to endpoint (pairing per individual) and found that none of the treatments induced significant community-wide compositional change, although treatment with alive bacteria had a slightly higher impact (partial dbRDA, adjusted R2=0.03, p-value=0.095), than Pasteurized (partial dbRDA, adjusted R2=0.02, p-value=0.14), and Placebo the lowest (partial dbRDA, adjusted R2=0.01, p-value=0.66). Therefore, these results demonstrate that the supplementation with either Pasteurized A.muciniphila or Alive A.muciniphila did not affect the overall structure of the gut microbiome. This finding is also in line with previous data obtained in rodents, showing that the gut microbiome from mice supplemented with Alive A.muciniphila was not significantly modified10.
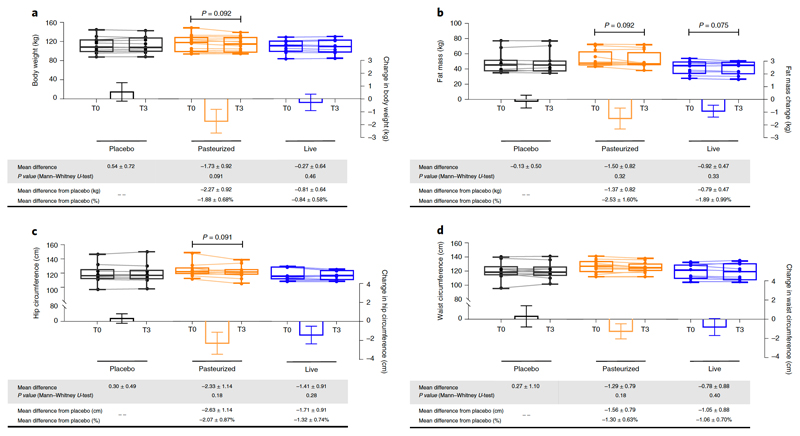
We also observed that Pasteurized A.muciniphila administration slightly decreased body weight by about -2.27 kg (P=0.09), fat mass by about -1.37 kg (P=0.09) and hip circumference by -2.63 cm (P=0.09) (Fig. 4a-c) as compared to the placebo group. Waist circumference was decreased by about 1.56 cm, but this change did not reach statistical significance (Fig. 4d). These differences are all of clinical relevance in the context of metabolic disorders and we may not rule out that the improvement of the different metabolic parameters is associated with the impact of the supplementation on body weight, fat mass, and hip circumference.
Figure 4. Changes in anthropometric parameters.
(a) Body weight, (b) Fat mass, (c) Hip circumference and (d) Waist circumference. Differential values (MD and MD from placebo) are expressed as mean ± SEM, either as raw data or as percentages. Bars represent mean change from baseline value per group, with their standard errors. Mann-Whitney tests were performed to compare differential values of both treated groups versus the Placebo group (inter-group changes), according to the distribution. The respective P value are indicated in the table. Lines represent raw values before and after 3 months of supplementation. Distribution of values within each group for each timing is illustrated by a box and whisker. In the box plots, the line in the middle of the box is plotted at the median, the inferior and superior limit of the box correspond to the 25th and the 75th percentiles respectively. The whiskers correspond to the minimum and maximum value. Wilcoxon matched-pairs signed ranks tests were performed to verify changes from baseline (intra-group changes), according to the distribution and, when drawn, the capped line above the concerned group shows the corresponding P-value. Kruskal-Wallis analysis was used to compare changes between 0 and 3 months across the 3 groups according to the distribution. All group-wise comparisons were performed using Bonferroni’s adjustment for multiple testing. MD, Mean Difference. Placebo, n = 11; Pasteurized, n =12; Alive, n=9 for all parameters except for Hip and Waist: Placebo, n = 10; Pasteurized, n =12; Alive, n=9. All tests were performed in two-tailed. MD, Mean Difference. All tests were performed in two-tailed.
It is important to note that there are several limitations to our study. Although the majority of the primary outcomes were reached we did not find significant changes in visceral adiposity and body mass index. However, we did not use specific and accurate methods such as dual-energy X-ray absorptiometry to precisely estimate the quantity of visceral versus subcutaneous fat mass. Also, this pilot and exploratory study enrolled a small number of subjects, meaning that the study was not powered to deliver definitive conclusions on the enpoints related to metabolic parameters. Also, the physical activity level and the precise calories intake were not determined by dedicated measures. However, all the groups were blindly investigated, therefore we may argue that the confounding factors were likely equally distributed between the different groups. Finally, we observed comparable apparent worsening of the phenotype of Placebo group over time as observed in other studies38–40.
In conclusion, this proof-of-concept prospective study shows the feasibility to culture and administer A.muciniphila to humans. Our data unequivocally show that such administration of a daily dose as high as of 1010 cells of A.muciniphila, is safe during a longer-term (i.e., 3 months).
This study provides a promising signal for the development of future clinical intrerventions with appropriate design to confirm and extend our findings showing the safety and the impact of oral supplementation with A.muciniphila in overweight or obese insulin-resistant individuals.
Online Methods
Subjects and study design
This study was designed as parallel-groups, randomized, placebo-controlled pilot study. Between December 2015 and December 2017, thirty-two overweight/obese subjects (BMI > 25 kg m-2) aged between 18 and 70 years were voluntary enrolled to participate. Eligible participants had been diagnosed with metabolic syndrome following the NCEP ATP III definition (at least three of the five following criteria: fasting glycemia > 100 mg/dl, blood pressure ≥ 130/85 mmHg or antihypertensive treatment, fasting triglyceridemia ≥ 150 mg/dl, HDL cholesterol <40 mg/dl for males, <50 mg/dl for females, and/or waist circumference >102 cm for males, >88 cm for females) and whose insulin sensitivity was <75%41,42, evaluated by HOMA-modelling of insulin sensitivity (HOMA Calculator© the University of Oxford). Written informed consent was obtained from each participant and the study protocol was approved by the Commission d’Ethique Biomédicale Hospitalo-facultaire of the UCLouvain/Université catholique de Louvain (Brussels, Belgium) (members of the ethics committee: Maloteaux J-M., Maes M., Schamps G., Duveiller V., Liesse M-C., Berliere M., Horsmans Y., Reding R., Rennotte M-T., de Barsy T., Desmedt M., Otte J-B., van den Hove-Vandenbroucke M-F., Orban T., Coen J-C., Evrard P., Gaziaux E., Massion J., Van Helleputte E., Coupez C., Dooms M., Vandeuren C., Terlinden G., de Pierpont P.). The study was registered at https://clinicaltrials.gov as NCT02637115.
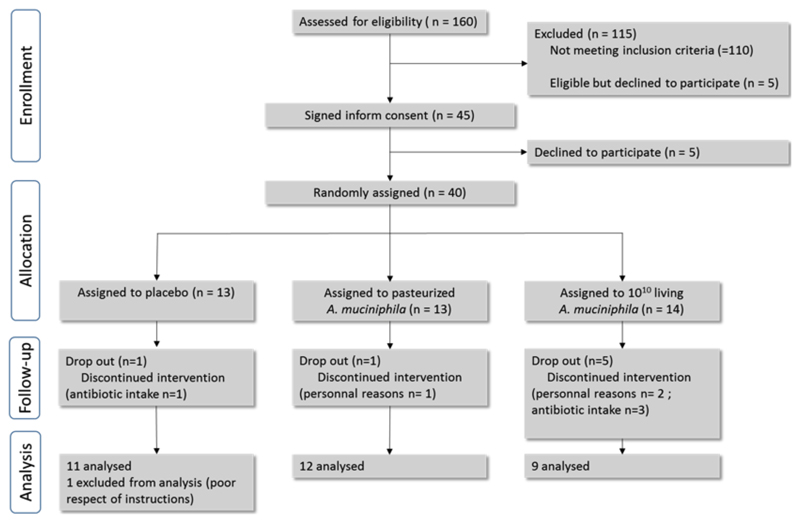
Participants were recruited at the Cliniques Universitaires Saint-Luc located in Brussels, Belgium. A total of 160 subjects aged 18–70 years were screened. Forty-five overweight or obese individuals with insulin resistance and a metabolic syndrome were eligible for inclusion. Among this group, 5 declined to participate. Therefore 40 individuals were enrolled and received either a placebo (Placebo), live A.muciniphila (Alive, 1010 bacteria per day), or pasteurized A.muciniphila (Pasteurized, 1010 bacteria per day) as supplement for 3 months, with the specific advice to keep their normal dietary intake and physical activity during the study period (Flow chart in Extended Data Fig. 1). To prevent any viability or shelf life issues, the A.mucniphila cells were delivered to the subjects in frozen form in glycerol. The Placebo contained the same amount of glycerol. The viable count of the A.muciniphila cells delivered to the subjects did not change during the entire intervention (data not shown).
Out of the 40 subjects, seven had to be excluded before completion of the study : 1 in the Placebo group, 1 in the Pasteurized group and five in the Alive group, with a total of 3 early termination due to personal reasons (i.e., mainly because of the difficulty to attend the nine scheduled visits at the hospital) and 4 due to untimely use of antibiotics during the study. One additional subject in the Placebo group was excluded from the analysis for protocol violation. This resulted in a total of 32 subjects: a Placebo group of 11 subjects, a Pasteurized group of 12 subjects and an Alive group of 9 subjects who completed the 3-months supplementation. Subjects were allocated to one of the treatment arms following a randomized block design with a block size of 8. The Microsoft Excel randomization function was used to generate the allocation sequence. Subjects and physicians were both blinded to the treatment allocation. Beside the placebo (an equivalent volume of sterile PBS containing glycerol), participants were assigned to ingest either 1010 cells of live A.muciniphila (Alive), or 1010 cells pasteurized A.muciniphila (Pasteurized) in PBS containing glycerol daily for 3 months. Packaging were given to the subjects every two weeks during follow-up visits, with the instructions to take one dose every morning on an empty stomach. Participants were instructed to keep the packages in the freezer compartment of a home refrigerator until time of consuming. A temperature sensor was also provided to all participants in order to monitor the temperature during transport and home storage (at -20°C). The anaerobic fermentation, concentration and packaging of the bacteria and placebo were performed according to the HACCP quality system using the food-grade medium level as described previously11. Pasteurization consisted of heat treatment at 70°C for 30 minutes of fresh A.muciniphila.
The exclusion criteria were: presence of acute or chronic progressive or chronic unstabilized diseases, alcohol consumption (> 2 glasses/day), previous bariatric surgery, any surgery in the 3 months before the study or planned in the 6 months after enrolling, pregnancy or pregnancy planned in the 6 months after enrolling, regular physical activity (> 30 min of sports 3 times a week), consumption of dietary supplements (omega-3 fatty acids, probiotics, prebiotics, plant stanols/sterols) in the month prior the study, inflammatory bowel disease or irritable bowel syndrome, diabetic gastrointestinal autonomic neuropathy (such as gastroparesis or reduced gastrointestinal motility), consumption of more than 30 g of dietary fibers per day, consumption of vegetarian or unusual diets, lactose intolerance or milk protein allergy, gluten intolerance, current treatment with medications influencing parameters of interest (glucose-lowering drugs such as metformin, DPP-4 inhibitors, GLP-1 receptor agonists, acarbose, sulfonylureas, glinides, thiazolidinediones, SGLT2 inhibitors, insulin, lactulose, consumption of antibiotics in the 2 months prior or during the study, glucocorticoids, immunosuppressive agents, statins, fibrates, orlistat, cholestyramine, or ezetimibe) and baseline glycated hemoglobin (HbA1c) > 7.5%.
At baseline and at the end of the intervention, anthropometrics measurements were assessed including body weight (kg) and body mass index (BMI in kg m-2). Waist and hip circumference (cm) were measured using a flexible tape. Fat mass (kg) was assessed using electric bioimpedance analysis (Body Composition Analyzer, type BC-418 MA, TANITA). Blood samples were collected at baseline, and at the end of the intervention, after an overnight fast (8 hours minimum). Based on the analytes of interest, different tubes were used: sodium fluoride-coated tubes for fasting glycemia and insulinemia, lithium-heparin-coated tubes for enzymatic activities, LPS-free heparin sulfate coated tubes for LPS measurement (BD Vacutainer, NH sodium heparin, 368480). One part of blood tubes was directly sent to the hospital laboratory for the following blood analyses: fasting glycemia, insulinemia, HbA1c (%), total cholesterol, LDL-cholesterol (calculated), HDL-cholesterol, triglycerides, gamma glutamyl-transferase (γGT), alanine amino-transferase (ALT), aspartate amino-transferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), and white blood cell count (WBC). The other tubes were brought to the research laboratory and kept on ice. Plasma was immediately isolated from whole blood by centrifugation at 4200 g for 10 min at 4°C and stored at -80°C for further analyses.
For safety purposes, the participants were asked to come back to the study hospital two weeks after the beginning of the intervention for blood sampling and clinical examination, allowing comparison of clinical parameters with baseline values. Blood sample analysis included C-reactive protein (CRP), urea, creatinine, glomerular filtration rate (GFR), AST, ALT, γGT, LDH, CK, various coagulation parameters and the hematologic profile. Forty participants were included in the analysis of the safety at two weeks. Same measurements were performed at 3 months for the thirty-two subjects who completed the intervention.
Compliance and presence of undesired effects were monitored every two weeks during follow-up visits based on questionnaire to fill. For this, we listed in the provided document the undesired events most likely to occur during the study. We also invited subjects to point out any other undesired event that newly emerged or worsened. The list of undesired effects included nausea, flatulence, bloating, cramps, borborygmi, and gastric reflux. If event(s) occurred, participants had to specify the number of days during which the effect(s) manifested itself (themselves). Each adverse event was calculated as the percentage of occurrence on the total number of days of intervention. Compliance was also assessed according to subject’s daily records and returned packaging counts. The compliance was calculated as percentage of number of days where packages were actually ingested on the total number of days of intervention. The participants were instructed to maintain their usual diets, level of physical activity, current treatment and current lifestyles throughout the intervention period. Quality control tests were also applied during the protocol, in fact, each subject received a 2-weeks supply of bacteria (14 bags + 1 bag in case of difficulties for attending the hospital visit and having to interrupt the supplementation). Thus, the subjects came at the clinic every 2 weeks to receive a novel supply containing 2 weeks of bacteria or placebo (15 bags 14+1). At the hospital, bags were stored and kept at -80°C before delivery to subjects. During transport from laboratory to home and then in a refrigerator at home, we provided each participant with a device (TempTale4, Sensitech) for monitoring the temperature throughout the study, including at home, in order to detect any potential temperature deviation over the period of supplementation. In addition, we randomly tested the viability of Akkermansia (for the alive group) by culturing the content of the bags maintained not only at -80°C but also at -20°C, thereby validating the viability of cells at -20°C.
Biochemical analyses
Insulin sensitivity and insulin resistance were both analyzed by Homeostasis model assessment (HOMA). This test consists of taking three blood samples at 5 minutes intervals for each individual. The insulinemia and glycemia was determined for each sample and the mean values were then introduced in the software HOMA calculator 2 (v2.3.3) available from http://www.dtu.ox.ac.uk/homacalculator/ to estimate insulin sensitivity (%) and insulin resistance.43,44
Insulinemia was evaluated by immunoanalysis. Glycemia was assessed by enzymatic test (hexokinase) with UV detection (Cobas 8000 - Roche Diagnostics). HbA1c (%) was determined by high-performance liquid chromatography (G8 - Tosoh). C-reactive protein was assessed by immunoturbidimetry (Cobas 8000 - Roche Diagnostics). Total cholesterol, HDL cholesterol, triglycerides and γGT were dosed by enzymatic colorimetric test (Cobas 8000 - Roche Diagnostics). LDL cholesterol concentrations were estimated using Friedwald’s formula. AST and ALT were assessed by enzymatic dosage (IFCC) without activation by pyridoxal phosphate (Cobas 8000 - Roche Diagnostics). Kinetic enzymatic test was performed to evaluate urea, and creatinine was assessed by kinetic staining test (Jaffé method) (Cobas 8000 - Roche Diagnostics). The GFR was estimated according to the CKD-EPI equation. The parameters related to muscle function (CK and LDH) were assessed by UV test (Cobas 8000 - Roche Diagnostics). All these tests were performed at the hospital laboratory.
Blood LPS endotoxin activity was measured using Endosafe-MCS (Charles River Laboratories, Lyon, France) based on the Limulus amaebocyte Lysate (LAL) kinetic chromogenic methodology that measures color intensity directly related to the endotoxin concentration in a sample. Plasma was diluted 1/50 to 1/100 with endotoxin-free buffer (Charles River Laboratories) to minimize interferences in the reaction and heated for 15 min at 70°C. Each sample was diluted with endotoxin-free LAL reagent water (Charles River Laboratories) and treated in duplicate. Two spikes for each sample were included in the determination. All samples were validated for recovery and coefficient of variation. The lower limit of detection was 0.005 EU/ml.
GRO, sCD14L, and MCP-1 were assessed in blood sample in duplicate using a Bio-Plex Multiplex kit (Human Cytokine/Chemokine Magnetic Bead Panel, EMD Millipore, Brussels, Belgium) and measured by using Luminex technology (Bio-Rad Bioplex; Bio-Rad) following the manufacturer’s instructions. Active plasma GLP-1 levels were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) (EMD Millipore, Brussels, Belgium).
DPP-IV activity was assessed by quantifying the production of para-nitroanilide (PNA) from Glycine-Proline-PNA (Sigma, St. Louis, MO, USA) using a standard curve of free PNA. For this, plasma samples were incubated for 30 minutes with Gly-Pro-PNA at 37°C and enzymatic activity was measured by kinetic analysis (380 nm) (SpectraMax M2; Molecular Devices, SanJose, CA, USA).
Faecal microbiome analysis
A.muciniphila was quantified with qPCR as described in Everard et al.10 Each assay was performed in duplicate in the same run. The cycle threshold of each sample was then compared with a standard curve (performed in triplicate) made by diluting genomic DNA (fivefold serial dilution) (DSMZ).
Faecal microbiota taxonomic composition was determined by DNA extraction of faecal samples stored frozen (-80C) and library preparation for dual-index 16S rRNA gene sequencing as described in Vandeputte et al45. Demultiplexing of the sequencing data was performed using LotuS55 (version 1.565), followed by quality control and sequence variants matrix building using the DADA246 pipeline (version 1.6.0) with taxonomic annotation by RDP classifier 47 (version 2.12) with default parameters. Statistical analyses of microbiota composition were performed in R using packages vegan48 and CoDaSeq49. As recommended for microbiota compositional data analysis, the abundance matrix was centre log-ratio (clr) transformed (CoDaSeq:codaseq.clr), using the minimum proportional abundance detected for each taxon for imputation of zeroes. Samples with > 10000 reads (N= 63 samples and genera with relative abundance > 0.001 (N=99) were included in the data analysis. Differences in microbiota profiles between treatment arms at baseline and at endpoint were evaluated by permutational MANOVA. Alteration of the microbiota from baseline to endpoint was evaluated per treatment arm and pairing by participant by performing a distance-based redundancy analysis (partial dbRDA, clr-transformed matrix, euclidean distance) by using timepoint as an explanatory variable while partialling-out intra-invidual similarity.
Faecal microbiota dissimilarity between samples was represented by principal coordinates analysis at genus-level using Aitchison distance (Euclidean distance with clr-transformed matrix) using the phyloseq, vegan and ggplot2 R packages. Confidence ellipses for each of the 6 sample groups (corresponding to the 3 different treatment arms at baseline or at endpoint) were drawn at 0.80 confidence level assuming a Student’s t-distribution. The intervention effect is symbolized by colored arrows, with direction and length corresponding to the shift in group centroid from baseline to endpoint for each treatment arm. Arrows lengths were multiplied by 5 for visual clarity and the 3 arrows were re-centered to the centroid of all baseline samples (3 arms confounded).
Statistical analysis
Normal distribution of continuous variables, expressed as raw data or as the difference between the two main time points (T0 and T3 months), was tested using the Shapiro–Wilk test. Box plots and Q-Q plots appearance were also taken into account. All the following statistical tests were chosen in accordance with the normality tests. For all parameters, and within each group, the intervention effect was calculated by subtracting the value obtained at T0 from the value obtained at T3 months for each participant. We designed the differential value obtained “mean difference” (MD). The “MD from placebo” was then calculated by subtracting the MD calculated for the Placebo group from the MD calculated for the active group. “MD from placebo” was expressed as raw value and as percentage. Unpaired-t-test or non-parametric Mann-Whitney test was performed to assess the significance of differences between MDs of the two treated group versus MDs of the Placebo group. Following the distribution, either paired T-test or non-parametric two-tailed Wilcoxon matched-pairs signed ranks test were performed to identify differences between T0 and T3 months within each group. One-way ANOVA or Kruskal-Wallis tests were used for the comparison of baseline parameters and the differential values across the 3 groups, according to the distribution and the P values adjusted by using Bonferroni correction. For baseline characteristics, mean and standard deviations were used to present the raw data of the normal variables, while median and interquartile range were used to report non-normal variables. Data of safety tables were expressed as means and standard deviations. Data presented in figures were expressed as means and standard errors of the mean. Statistical analyses were conducted using the SPSS software (version: 23.0 SPSS, INC). All tests were two-tailed and significance was set at P < 0.05. Graphics were drawn using GraphPad Prism software (version 7.0, GraphPad Software, San Diego, CA, USA).
Extended Data
Extended data figure 1. Flow chart of the interventional study.
Diagram of the participant selection procedure, with the following information: number of individuals enrolled at each step of the study progress; number of individuals included in the final analysis; details of events that led to a reduction in the group size.
Extended data figure 2. Changes in inflammatory parameters and GLP-1.
(a) Soluble CD40 Ligand (sCD40L), (b) Growth-related oncogene (GRO/CXCL1), (c) Monocyte Chemoattractant Protein-1 (MCP-1) and (d) Glucagon-like peptide-1 (GLP-1).
Differential values (MD and MD from placebo) are expressed as mean ± SEM, either as raw data or as percentages. Bars represent mean change from baseline value per group, with their standard errors. Mann-Whitney tests or unpaired T-tests were performed to compare differential values of both treated groups versus the Placebo group (inter-group changes), according to the distribution. The respective P value are indicated in the table. Lines represent raw values before and after 3 months of supplementation. Distribution of values within each group for each timing is illustrated by a box and whisker. In the box plots, the line in the middle of the box is plotted at the median, the inferior and superior limit of the box correspond to the 25th and the 75th percentiles respectively. Wilcoxon matched-pairs signed ranks tests or Paired T-tests were performed to verify changes from baseline (intra-group changes), according to the distribution and, when drawn, the capped line above the concerned group shows the corresponding P-value. Changes between 0 and 3 months across the 3 groups were analyzed with Kruskal-Wallis or One-way ANOVA according to the distribution and group-wise comparisons were performed using Bonferroni and Tuckey’s adjustment for multiple testing, respectively. MD, Mean Difference. Placebo, n = 11; Pasteurized, n =12; Alive, n=9 for all parameters except for GRO: Placebo, n = 7; Pasteurized, n =10; Alive, n=8. All tests were performed in two-tailed.
Extended data figure 3. Changes in faecal microbiome.
(a) Akkermansia muciniphila abundance in feces evaluated by Q-PCR. Differential values (MD and MD from placebo) are expressed as mean ± SEM as raw. Bars represent mean change from baseline value per group, with their standard errors. Mann-Whitney tests were performed to compare differential values of both treated groups versus the Placebo group (inter-group changes), according to the distribution. The respective P value are indicated in the table. Lines represent raw values before and after 3 months of supplementation. Distribution of values within each group for each timing is illustrated by a box and whisker. In the box plots, the line in the middle of the box is plotted at the median, the inferior and superior limit of the box correspond to the 25th and the 75th percentiles respectively. Wilcoxon matched-pairs signed ranks tests were performed to verify changes from baseline (intra-group changes), according to the distribution. When the difference is significative, a capped line is marked above the concerned group with the corresponding P value. Kruskal-Wallis analysis were used to compare changes between 0 and 3 months across the 3 groups, according to the distribution. MD, Mean Difference. Placebo, n = 11; Pasteurized, n =12; Alive, n=9. All tests were performed in two-tailed. *P < 0.05.
(b) Visualization of the participants’ faecal microbiota composition at baseline and endpoint of the intervention. Faecal microbiota dissimilarity between samples is represented by principal coordinates analysis (genus-level Aitchison distance), with 6 sample groups - corresponding to the 3 different treatment arms at baseline or at endpoint represented by confidence ellipses (80% confidence interval). Intervention effects are symbolized by colored arrows, with direction and length corresponding to the shift in group centroid coordinates from baseline to endpoint for each treatment arm (re-scaled x4 and re-centered at baseline global centroid). Placebo, n = 11; Pasteurized, n =12; Alive, n=9.
Extended data table 1. Baseline Participant Characteristics.
| Parameters | Placebo (n = 11) | Pasteurized (n=12) | Alive (n = 9) | Ptotal | P1 | P2 |
|---|---|---|---|---|---|---|
| Age (years) | 49,45 ± 9,67 | 52,75 ± 7,16 | 52,89 ± 8,59 | 0,58a | 0,36 | 0,42 |
| Sex (nb (%)) | ||||||
| Male | 5 (45) | 4 (33) | 6 (67) | |||
| Female | 6 (55) | 8 (67) | 3 (33) | |||
| Height (cm) | 173,05 ± 9,32 | 170,54 ± 8,8 | 172,11 ± 10,15 | 0,81a | 0,52 | 0,83 |
| Body Weight (kg) | 112,45 ± 16,74 | 115,91 ± 17,23 | 109,03 ± 13,96 | 0,63a | 0,63 | 0,63 |
| BMI | 37,63 ± 5,82 | 39,81 ± 4,77 | 36,82 ± 3,68 | 0,35a | 0,34 | 0,72 |
| Waist (cm) | 120,2 ± 12,51 | 126,46 ± 8,75 | 120,06 ± 10,43 | 0,29a | 0,19 | 0,97 |
| Hip (cm) | 118,8 (12,4) | 123,46 (7,8) | 117,83 (16,5) | 0,22b | 0,13 | 0,65 |
| Waist-hip ratio | 1,02 ± 0,11 | 1,03 ± 0,09 | 1,02 ± 0,10 | 0,97a | 0,82 | 0,94 |
| Systolic blood pressure (mm Hg) | 150 (12) | 150,00 (20) | 140,00 (19) | 0,15b | 0,38 | 0,18 |
| Diastolic blood pressure (mm Hg) | 90,00 (9) | 102,50 (19) | 100 (19) | 0,08b | 0,02 | 0,44 |
| Fasting Blood glucose (mg/dl) | 100,33 (11,67) | 103,00 (15,08) | 94,33 (18,5) | 0,34b | 0,24 | 0,82 |
| Glycated hemoglobin A1c (%) | 5,53 ± 0,36 | 5,77 ± 0,42 | 5,63 ± 0,35 | 0,33a | 0,11 | 0,52 |
| Insulinemia (pmol/L) | 111,36 (28,83) | 155,83 (57,01) | 128,7 (86,42) | 0,030b * | 0,014 * | 0,47 |
| % insulin sensibility | 34,00 (8,5) | 23,75 (9,17) | 30,8 (21,65) | 0,025b * | 0,007 * | 0,5 |
| Total Cholesterol (mg/dl) | 200,73 ± 43,19 | 203,00 ± 51,34 | 198,67 ± 20,47 | 0,97a | 0,91 | 0,9 |
| LDL Cholesterol (mg/dl) | 131,63 ± 37,64 | 129,92 ± 41,10 | 128,78 ± 18,63 | 0,98a | 0,92 | 0,84 |
| HDL Cholesterol (mg/dl) | 42 (7) | 44,5 (12,25) | 43 (18,5) | 0,70b | 0,41 | 0,82 |
| Ratio (tot cholesterol/HDL) | 4,65 ± 1,05 | 4,59 ± 1,14 | 4,84 ± 0,92 | 0,86a | 0,91 | 0,61 |
| Aspartate aminotransferase activity (U/L) | 17,00 (8) | 23 (17,75) | 20 (11,5) | 0,26b | 0,12 | 0,49 |
| Alanine aminotransferase activity (U/L) | 21 (14) | 37,5 (26,25) | 30,78 (28,5) | 0,14b | 0,42 | 0,34 |
| ɣ-Glutamyltransferase activity (U/L) | 21 (17) | 40,5 (51) | 27 (5) | 0,084b | 0,45 | 0,13 |
| Lactate dehydrogenase activity (UI/L) | 166 (21) | 197,5 (41,75) | 180 (50) | 0,082b | 0,023 | 0,46 |
| Creatine kinase (U/L) | 90 (64) | 96 (100) | 111 (48,50) | 0,68b | 0,48 | 0,33 |
| Triglycerides (mg/dl) | 127,45 ± 46,49 | 139,83 ± 47,79 | 135,56 ± 44,13 | 0,84a | 0,58 | 0,69 |
| DDPIV activity (mU/ml) | 14,72 (4,45) | 15,58 (7,67) | 13,89 (3,13) | 0,12b | 0,36 | 0,18 |
ptotal: aP-values from one-way ANOVA except for Sex; bP-values from Kruskal-Wallis.
P1-2 = P-values for comparison of Placebo groups versus Pasteurized group (P1) and versus Alive group (P2) with the two-tailed Mann-Whitney U test or two-tailed unpaired T-test according to the distribution, with p > 0,017 due to the Bonferroni adjustment. Baseline values are presented as mean + SD for normal variables while non-normal variables are presented as Median (inter-quartile range).
Significant difference between groups value (P < 0.05 for Ptotal ; P < 0.017 for P1 & P2)
Extended data table 2. Safety parameters measured in all groups at baseline and after two weeks of treatment.
| Placebo | Pasteurized Akk -1010 | Alive Akk -1010 | ||||
|---|---|---|---|---|---|---|
| Safety parameters | Baseline | Safety | Baseline | Safety | Baseline | Safety |
| Systolic blood pressure (mm Hg) | 145,92 ± 13,94 | 134,92 ± 18,53 | 150,38 ± 12,52 | 134,85 ± 19,20* | 138,00 ± 17,97 | 138,21 ± 16,84 |
| Diastolic blood pressure (mm Hg) | 94,92 ± 13,97 | 90,31 ± 9,89 | 101,92 ± 11,28 | 91,77 ± 17,19 | 95,57 ± 19,29 | 92,43 ± 12,49 |
| C-reactive protein (mg/dl) | 5,23 ± 5,38 | 4,78 ± 3,14 | 7,77 ± 9,12 | 9,46 ± 11,33 | 4,07 ± 4,43 | 5,48 ± 5,860 |
| White Blood cells (103/μl) | 6,00 ± 1,08 | 6,78 ± 1,48* | 6,47 ± 1,66 | 7,33 ± 1,54* | 6,74 ± 1,28 | 7,5 ± 1,53* |
| Prothrombin time (sec) | 11,48 ± 0,69 | 11,35 ± 0,62 | 11,66 ± 0,89 | 11,5 ± 0,83 | 11,47 ± 0,67 | 11,38 ± 0,70 |
| Alanine aminotransferase activity (U/L) | 23,38 ± 10,4 | 24,54 ± 10,94 | 47,62 ± 38,01 | 44,85 ± 35,59 | 33,43 ± 12,89 | 35,43 ±13,44 |
| Aspartate aminotransferase activity (U/L) | 18,92 ± 5,78 | 18,77 ± 4,80 | 32,92 ± 31,45 | 35,23 ± 41,2 | 22,07 ± 7,08 | 23,64 ± 7,06 |
| γ-Glutamyltransferase activity (U/L) | 25,92 ± 16,59 | 29,38 ± 24,77 | 50,85 ± 32,93 | 47,85 ± 27,82 | 39,64 ± 21,1 | 40,86 ± 20,08 |
| Urea (mg/dl) | 33,69 ± 7,76 | 30,77 ± 6,76 | 30,38 ± 3,10 | 35,69 ± 13,38 | 32,21 ± 6,71 | 32,64 ± 7,33 |
| Creatinine (mg/dl) | 0,84 ± 0,15 | 0,83 ± 0,17 | 0,82 ± 0,13 | 0,84 ± 0,14 | 0,86 ± 0,14 | 0,90 ± 0,11 |
| Glomerular filtration rate (ml min-1 1,73m-2) | 84,15±16,09 | 86 ± 13,99 | 85,69 ± 14,13 | 82,92 ± 12,28 | 88,79 ± 15,84 | 82,14 ± 12,98 |
| Creatine kinase activity (U/L) | 114,3 ± 59,88 | 100,9 ± 58,96 | 152 ± 81,47 | 123,1 ± 63,19 | 118,4 ± 50,57 | 165 ± 175,8 |
| Lactate dehydrogenase activity (UI/L) | 177,4 ± 23,01 | 170,2 ± 21,46 | 193,9 ± 21,01 | 189,5 ± 39,34 | 190,9 ± 45,51 | 189,7 ± 32,22 |
This table includes all participants that proceeded to the safety visit: Placebo n=13, Pasteurized n=13, Alive n=14. Data are expressed as mean ± SD. Non-parametric two-tailed Wilcoxon matched-pairs signed ranks tests were performed to verify changes from baseline in each group.
Significant difference from baseline value (P < 0.05)
Extended data table 3. Proportion of subjects experiencing self-reported adverse effects after two weeks of treatment.
| Placebo | Pasteurized Akk - 1010 |
Alive Akk - 1010 | |
|---|---|---|---|
| Nausea | 2/13 | 1/13 | 0/14 |
| Flatulence | 3/13 | 3/13 | 1/14 |
| Bloating | 2/13 | 0/13 | 1/14 |
| Cramps | 4/13 | 2/13 | 1/14 |
| Borborygmi | 3/13 | 1/13 | 3/14 |
| Gastric reflux | 2/13 | 1/13 | 0/14 |
Extended data table 4. Safety parameters measured in all groups at baseline and at the end of the intervention.
| Placebo | Pasteurized Akk -1010 | Alive Akk -1010 | ||||
|---|---|---|---|---|---|---|
| Safety parameters | Baseline | 12 weeks | Baseline | 12 weeks | Baseline | 12 weeks |
| Systolic blood pressure (mm Hg) | 146,7 ± 15,60 | 129,00 ± 18,43* | 151,08 ± 12,81 | 132,67 ± 11,79* | 133,38 ± 12,41 | 124,63 ± 16,72* |
| Diastolic blood pressure (mm Hg) | 91,00 ± 9,08 | 81,70 ± 9,04* | 102,08 ± 11,77 | 83,92 ±10,38* | 92,25 ± 9,51 | 78,50 ± 13,03* |
| HbA1c (%) | 5,53 ± 0,36 | 5,61 ± 0,30 | 5,77 ± 0,42 | 5,72 ± 0,37 | 5,63 ± 0,35 | 5,62 ± 0,33 |
| C-reactive protein (mg/dl) | 5,00 ± 5,51 | 5,31 ± 6,10 | 8,08 ± 9,45 | 8,84 ± 10,71 | 3,89 ± 4,08 | 3,78 ± 3,77 |
| White Blood cells (103/μl) | 6,14 ± 1,13 | 6,87 ± 1,81* | 6,37 ± 2,43 | 6,24 ± 1,56 | 6,52 ± 2,40 | 6,74 ± 2,46 |
| Prothrombin time (sec) | 11,61 ± 0,69 | 11,37 ± 0,71 | 11,64 ± 4,61 | 11,61 ± 3,42 | 11,5 ± 0,73 | 11,61 ± 0,69 |
| Alanine aminotransferase activity (U/L) | 24 ± 11,26 | 23,36 ± 6,76 | 46,75 ± 39,56 | 39,92 ± 39,76 | 30,78 ± 13,84 | 30,89 ± 15,11 |
| Aspartate aminotransferase activity (U/L) | 18,82 ± 6,32 | 19,27 ± 3,64 | 33,33 ± 32,82 | 27,92 ± 26,21* | 20,33 ± 5,66 | 20,33 ± 7,75 |
| γ-Glutamyltransferase activity (U/L) | 26,18 ± 17,93 | 29,55 ± 20,20 | 50,83 ± 34,39 | 42,33 ± 22,58* | 34,11 ± 21,22 | 35,44 ± 33,00 |
| Urea (mg/dl) | 32,73 ± 7,99 | 32,36 ± 7,47 | 30,42 ± 3,23 | 33,42 ± 6,46 | 32,44 ± 6,58 | 33,56 ± 8,43 |
| Creatinine (mg/dl) | 0,84 ± 0,16 | 0,84 ± 0,18 | 0,81 ± 0,13 | 0,78 ± 0,09 | 0,83 ± 0,13 | 0,88 ± 0,11 |
| Glomerular filtration rate (ml min-1 1,73m-2) | 86,27 ± 16,69 | 87,36 ± 18,50 | 84,00 ± 13,31 | 88,67 ± 11,60 | 91,44 ± 14,48 | 86,33 ± 12,99 |
| Creatine kinase activity (U/L) | 106,36 ± 48,71 | 110,09 ± 48,00 | 133,54 ± 85,66 | 106,09 ± 72,75* | 117,89 ± 35,11 | 131,56 ± 70,39 |
| Lactate dehydrogenase activity (Ul/L) | 173,46 ± 20,90 | 177,00 ± 38,99 | 197,17 ± 25,43 | 176,67 ± 27,13* | 184,00 ± 24,98 | 176,78 ± 18,69 |
This table includes all participants that completed the study: Placebo n=11, Pasteurized n=12, Alive n=9. Data are expressed as mean ± SD. Non-parametric two-tailed Wilcoxon matched-pairs signed ranks tests were performed to verify changes from baseline in each group.
Significant difference from baseline value (P < 0.05)
Extended data table 5. Frequency of occurrence of adverse events during the whole intervention period.
| Frequency % | Placebo | Pasteurized Akk - 1010 |
Alive Akk
- 1010 |
Ptotal | P1 | P2 |
|---|---|---|---|---|---|---|
| Nausea | 0,792 | 1,341 | 0,468 | 0,196 | 0,726 | 0,653 |
| Flatulence | 0,907 | 2,788 | 0,709 | 0,319 | 0,436 | 0,798 |
| Bloating | 1,204 | 0,185 | 0,356 | 0,636 | 0,176 | 0,331 |
| Cramps | 2,104 | 0,477 | 0,96 | 0,375 | 0,065 | 0,256 |
| Borborygmi | 0,593 | 0,651 | 1,194 | 0,603 | 0,928 | 0,249 |
| Gastric reflux | 0,798 | 2,480 | 0,121 | 0,652 | 0,499 | 0,257 |
| Compliance % | 99,4 | 99,08 | 99,76 | 0,676 | 0,56 | 0,3 |
Values are expressed as the percentage of events occurring for adverse events (frequency) and as the percentage of days where the packages were correctly taken (compliance).
ptotal : P-values from one-way ANOVA.
P1-2: P-values for comparison of Placebo groups versus Pasteurized group (P1) and versus Alive group (P2) with the two-tailed unpaired T-test, with p > 0,017 due to the Bonferroni adjustment.
Acknowledgements
We wish to thank A. Barrois for excellent technical assistance. We also wish to sincerely thank the volunteers who participated in this study. We are very grateful to M. Buysschaert for his continuous support and helpful criticism during the preparation of this project. PDC is a senior research associate at FRS-FNRS (Fonds de la Recherche Scientifique), Belgium. AE is a research associated at FRS-FNRS, Belgium. CDr was supported by a FIRST Spin-Off grant from the Walloon Region (convention 1410053). Research in the Wageningen lab of WMdV was partially supported by ERC Advanced Grant 250172 (Microbes Inside), the SIAM Gravity Grant 024.002.002 and the Spinoza Award of the Netherlands Organization for Scientific Research. PDC is the recipient of grants from FNRS (convention J.0084.15, convention 3.4579.11), PDR (Projet de Recherche, convention: T.0138.14), FRFS-WELBIO (WELBIO-CR-2017C-02). This work is supported by the Funds Baillet Latour (Grant for Medical Research 2015), a Prize of the Banque Transatlantique Belgium, a FIRST Spin-Off grant (FSO) from the Walloon Region, Belgium (convention 1410053). PDC is a recipient of POC ERC grant 2016 (European Research Council, Microbes4U_713547) and ERC Starting Grant 2013 (Starting grant 336452-ENIGMO). P.D.C. and J.R. are recipients of a grant from the FNRS and FWO (EOS program no. 30770923).
Main funding bodies : The Funds Baillet Latour (Grant for Medical Research 2015), the Walloon Region of Belgium (FIRST Spin-Off grant, convention 1410053), The European Research Council (POC ERC grant 2016-Microbes4U_713547, ERC Starting Grant 2013-336452-ENIGMO, ERC Advanced Grant 250172-Microbes Inside) and The Prize “Banque Transatlantique Belgium”.
Footnotes
Author contributions
P.D.C. conceived the project. J.-P.T., M.P.H., A.L., D.M., A.E., C. Depommier, C. Druart, H.P., M.V.H., W.M.d.V. and P.D.C. designed the clinical study. P.D.C. supervised the clinical part of the study and W.M.d.V. contributed to the microbial culturing of A. muciniphila. P.D.C., A.E., C. Depommier, C. Druart, M.d.B., J.-P.T., A.L., D.M. and M.P.H. performed the clinical part of the study. N.M.D. contributed to interpretation of the results. S.V.-S., G.F. and J.R. performed the fecal microbiome sequencing and analysis. P.D.C., A.E. and C. Depommier performed the experiments and interpreted all the results. P.D.C., A.E. and C. Depommier generated the figures and tables. P.D.C. and C. Depommier wrote the manuscript. All authors discussed the results and approved the manuscript.
Competing financial interests
A.E., C.D., H.P., P.D.C. and W.M.d.V. are inventors of patent applications (PCT/EP2013/073972; PCT/EP2016/071327, PCT/EP2016/060033 filed in European Patent Office (EP), Australia (AU), Brazil (BR), Canada (CA), China (CN), Eurasian Patent Organization (EA), Israel (IL), India (IN), Hong Kong (HK), Japan (JP), South Korea (KR), Mexico (MX), New Zealand (NZ), and the United States (US)) dealing with the use of A.muciniphila and its components in the context of obesity and related disorders. P.D.C. and W.M.d.V. are co-founders of A-Mansia Biotech SA.
Data availability
The data that support the findings of this study are available. All the figures are provided with individual values in order to have a direct access to the raw data. The 16S sequencing datasets generated during the current study are available in the EGA repository (European Genome-Phenome Archive, https://ega-archive.org/, accession number EGAS00001003585).
References
- 1.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 2.Cani PD, et al. Microbial regulation of organismal energy homeostasis. Nature Metabolism. 2019;1:34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 3.Brahe LK, et al. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutrition & diabetes. 2015;5:e159. doi: 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yassour M, et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016;8:17. doi: 10.1186/s13073-016-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu R, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 8.Dao MC, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, de Vos WM. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Frontiers in microbiology. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National academy of sciences of the United States of America. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plovier H, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 12.Everard A, et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nature communications. 2019;10:457. doi: 10.1038/s41467-018-08051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkin SL, et al. Effect of dipeptidyl peptidase-4 inhibitors on circulating tumor necrosis factor-alpha concentrations: A systematic review and meta-analysis of controlled trials. J Diabetes Complications. 2017;31:1458–1464. doi: 10.1016/j.jdiacomp.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Akoumianakis I, Antoniades C. Dipeptidyl peptidase IV inhibitors as novel regulators of vascular disease. Vascul Pharmacol. 2017;96-98:1–4. doi: 10.1016/j.vph.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Olivares M, et al. The Potential Role of the Dipeptidyl Peptidase-4-Like Activity From the Gut Microbiota on the Host Health. Frontiers in microbiology. 2018;9:1900. doi: 10.3389/fmicb.2018.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veronelli A, et al. White blood cells in obesity and diabetes: effects of weight loss and normalization of glucose metabolism. Diabetes Care. 2004;27:2501–2502. doi: 10.2337/diacare.27.10.2501. [DOI] [PubMed] [Google Scholar]
- 17.Ohshita K, et al. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care. 2004;27:491–496. doi: 10.2337/diacare.27.2.491. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes research and clinical practice. 2018;141:140–147. doi: 10.1016/j.diabres.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, et al. White blood cell subtypes and risk of type 2 diabetes. J Diabetes Complications. 2017;31:31–37. doi: 10.1016/j.jdiacomp.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Twig G, et al. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care. 2013;36:276–282. doi: 10.2337/dc11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen J, et al. Low-Density Lipoprotein Receptor Signaling Mediates the Triglyceride-Lowering Action of Akkermansia muciniphila in Genetic-Induced Hyperlipidemia. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:1448–1456. doi: 10.1161/ATVBAHA.116.307597. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 23.Ras RT, et al. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations--a meta-analysis of randomized controlled studies. Atherosclerosis. 2013;230:336–346. doi: 10.1016/j.atherosclerosis.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care. 2005;28:2913–2918. doi: 10.2337/diacare.28.12.2913. [DOI] [PubMed] [Google Scholar]
- 25.Rantala AO, et al. Gamma-glutamyl transpeptidase and the metabolic syndrome. J Intern Med. 2000;248:230–238. doi: 10.1046/j.1365-2796.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 26.Lim JS, Lee DH, Park JY, Jin SH, Jacobs DR., Jr A strong interaction between serum gamma-glutamyltransferase and obesity on the risk of prevalent type 2 diabetes: results from the Third National Health and Nutrition Examination Survey. Clin Chem. 2007;53:1092–1098. doi: 10.1373/clinchem.2006.079814. [DOI] [PubMed] [Google Scholar]
- 27.Fraser A, et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanninen A, et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67:1445–1453. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- 29.Grander C, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2017 doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 30.Wu W, et al. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Frontiers in microbiology. 2017;8:1804. doi: 10.3389/fmicb.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harte AL, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 33.Miele L, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 34.Lassenius MI, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–1815. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allin KH, et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61:810–820. doi: 10.1007/s00125-018-4550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 37.Dao MC, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 38.Scherbaum WA, et al. Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycaemia*. Diabetes, obesity & metabolism. 2008;10:675–682. doi: 10.1111/j.1463-1326.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosenstock J, Rigby SP, Ford DM, Tao B, Chou HS. The glucose and lipid effects of colesevelam as monotherapy in drug-naive type 2 diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2014;46:348–353. doi: 10.1055/s-0033-1358759. [DOI] [PubMed] [Google Scholar]
- 40.Kim SG, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014;9:e92843. doi: 10.1371/journal.pone.0092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werner M, Tonjes A, Stumvoll M, Thiery J, Kratzsch J. Assay-dependent variability of serum insulin levels during oral glucose tolerance test: influence on reference intervals for insulin and on cut-off values for insulin sensitivity indices. Clin Chem Lab Med. 2008;46:240–246. doi: 10.1515/CCLM.2008.020. [DOI] [PubMed] [Google Scholar]
- 42.Borza D, et al. Influence of assay-dependent variability of serum insulin levels on insulin sensitivity indices. Clin Chem Lab Med. 2008;46:1655–1656. doi: 10.1515/CCLM.2008.315. [DOI] [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 44.Dennis JM, et al. Precision Medicine in Type 2 Diabetes: Clinical Markers of Insulin Resistance Are Associated With Altered Short- and Long-term Glycemic Response to DPP-4 Inhibitor Therapy. Diabetes Care. 2018;41:705–712. doi: 10.2337/dc17-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandeputte D, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 46.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nature methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oksanen JB, et al. vegan: Community Ecology Package. R package version 2.5-3. 2018 [Google Scholar]
- 49.Gloor GB, Reid G. Compositional analysis: a valid approach to analyze microbiome high-throughput sequencing data. Can J Microbiol. 2016;62:692–703. doi: 10.1139/cjm-2015-0821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available. All the figures are provided with individual values in order to have a direct access to the raw data. The 16S sequencing datasets generated during the current study are available in the EGA repository (European Genome-Phenome Archive, https://ega-archive.org/, accession number EGAS00001003585).