Abstract
The robotic surgical system has been applied to various types of pancreatic surgery. However, controversies exist regarding a variety of factors including the safety, feasibility, efficacy, and cost-effectiveness of robotic surgery. This study aimed to evaluate the current status of robotic pancreatic surgery and put forth experts’ consensus and recommendations to promote its development. Based on the WHO Handbook for Guideline Development, a Consensus Steering Group* and a Consensus Development Group were established to determine the topics, prepare evidence-based documents, and generate recommendations. The GRADE Grid method and Delphi vote were used to formulate the recommendations. A total of 19 topics were analyzed. The first 16 recommendations were generated by GRADE using an evidence-based method (EBM) and focused on the safety, feasibility, indication, techniques, certification of the robotic surgeon, and cost-effectiveness of robotic pancreatic surgery. The remaining three recommendations were based on literature review and expert panel opinion due to insufficient EBM results. Since the current amount of evidence was low/meager as evaluated by the GRADE method, further randomized controlled trials (RCTs) are needed in the future to validate these recommendations.
Keywords: Robotic surgery, consensus statement, pancreatectomy, pancreaticoduodenectomy, pancreatic enucleation
Introduction
With advances in technology and techniques, minimally invasive surgery has now become the standard of care in almost every fields in general surgery, including pancreatic surgery (1-3). In 1994, Gagner et al. first reported laparoscopic pancreaticoduodenectomy (4); since then, the laparoscopic technique has been used in all kinds of pancreatic surgery. However, the disadvantages of laparoscopic surgery, including two-dimensional images, poor hand-eye coordination, the limited degree of freedom of movement of the laparoscopic instruments, and fulcrum effect, prevent it from being widely adopted by pancreatic surgeons (5-7). According to the previously published expert consensus, laparoscopic pancreatic surgery is still in its developmental and exploration stage (8,9). Techniques employed among hospitals vary; therefore, laparoscopic pancreatic surgery remains challenging. The advent of the robotic surgical system, which was developed to address the laparoscopic disadvantages, has helped to make minimally invasive pancreatic surgery much more accessible to surgeons (10,11). Since the first report of robotic distal pancreatectomy (RDP) in 2001, pancreaticoduodenectomy, central pancreatectomy, total pancreatectomy, pancreas tumor enucleation, and Appleby operation have been performed using the robotic system (12-21), and this appears to indicate a promising future for the robotic system (22).
Until now, most studies related to robotic pancreatic surgery are published as case reports or case series study (14,15,19,23-31), and there is a limited number of comparative studies or high-quality randomized controlled trials (RCT). Meanwhile, the different status of robotic pancreatic surgery existed in various hospitals, which slows the progress of robotic surgery. Moreover, some controversies exist regarding safety, feasibility, indication and/or contraindication, technique, prevention of complication, certification of the robotic surgeon, and cost-effectiveness of robotic surgery.
To assess the role of robotic pancreatic surgery and improve patient safety, we identified a group of well-known robotic pancreatic surgeons (based on their reported total number of pancreatic cases and publications) and invited them to provide clinical statements related to robotic surgery. We searched the online databases for published articles related to robotic pancreatic surgery; with evidence-based methods (EBMs), all evidence was graded using the GRADE system and upgraded or downgraded after integrating experts’ opinions until a final consensus was reached.
Methods
We referred to the WHO Handbook for Guideline Development and established the Consensus Steering Group*, consisting of 6 experts in the field from all around the world, with the following missions: (I) to approve the use of PICOs (population, intervention, comparator, outcomes); (II) to supervise the literature search and systematic reviews; (III) to check the grade of the evidence; (IV) to draft the final recommendations using a modified Delphi approach; and (V) to approve the publication of the consensus. The Consensus Development Group is a multidisciplinary group of 20 experts with the following missions: (I) to define the scope of the consensus, draft the PICOs; (II) to grade the quality of the evidence; (III) to draft preliminary recommendations; (IV) to write the draft consensus; and (V) to publish and promote the consensus. The Consensus Secretary Group is responsible for conducting systematic reviews and investigation of patients’ views and preferences, along with the Chinese GRADE Center, for providing methodological support.
We have held four meetings until now on questions focusing on hepato-pancreato-biliary (HPB) minimally invasive surgery, in Shanghai (2016.01), Chongqing (2016.05), Lanzhou (2017.10), and Hong Kong (2018.10), involving more than 50 clinical experts. Finally, we formulated sixteen PICO questions for the consensus. Published articles and conference abstracts were identified from PubMed, Embase, the Cochrane Library, and three Chinese literature databases (CNKI, WanFang, and CBM). Additionally, we used the GRADE approach to rate the quality of evidence and the strength of recommendation. The experts in the Consensus Development Group voted on the proposals according to the quality of evidence, patients’ views and preferences, and economic evaluation. The GRADE Grid method and Delphi vote were used to formulate the recommendations. Three rounds of voting were conducted. When 70% of the experts approved a proposal, a consensus was assumed to have been reached.
The formulated recommendations were submitted to 25 experts, who have a broad clinical experience in HPB minimally invasive surgery. The external reviewers were not involved in the development of the consensus. The Consensus Steering Group* discussed the external reviews in a meeting and revised the recommendations based on this feedback. The Consensus Steering Group* plans to update the guideline again before 2022. A flow chart describes the process of a consensus development (Figure 1).
Figure 1.
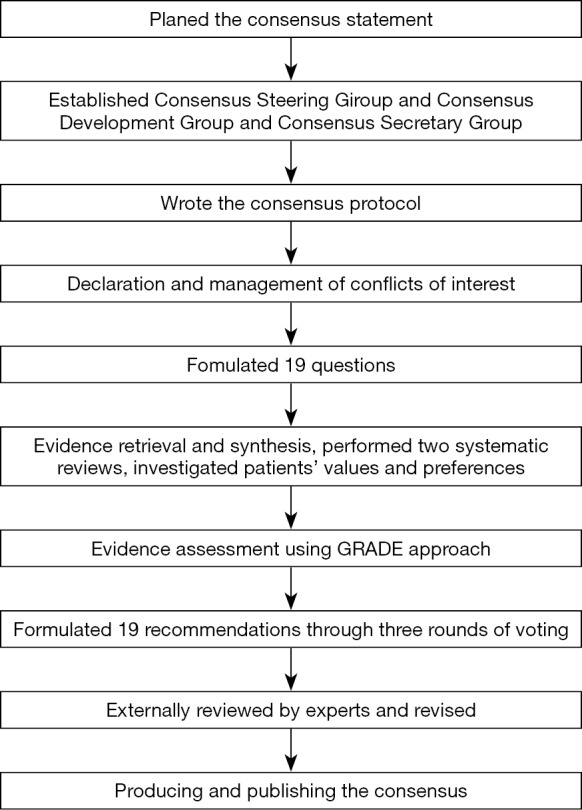
The flow chart illustrates the process of consensus development.
Results
Recommendation 1: RDP is associated with similar lymph nodes harvest number and equivalent margin status as that with laparoscopic distal pancreatectomy (LDP) in case of malignant disease
Level of evidence: low. Level of recommendation: strong (Grade 1C).
The principle of radical tumor resection is R0 resection and complete regional lymphadenectomy (32,33). According to a systemic review on RDP and LDP, in cases of distal pancreatic malignancy, compared with LDP, RDP showed no significant difference in the number of lymph nodes harvested (MD =2.21; 95% CI: −1.29, 5.72) and positive margin rate (OR =−0.10; 95% CI: −0.28, 0.08) (34). Liu et al. (29) designed a comparative study to analyze the perioperative outcome and long-term prognosis of robotic distal pancreatosplenectomy versus laparoscopic distal pancreatosplenectomy in patients with pancreatic cancer using the PSM method, and each arm had 35 cases. The study showed no significant difference in R0 resection rates (100% vs. 97.1%), several harvested lymph nodes (11 vs. 10.5), positive harvested lymph node ratios, and disease-free survival (16 vs. 16 months) and overall survival (27 vs. 25 months) between the two groups. For late-stage pancreatic body-tail malignancy, we can adopt robotic DP with celiac axis resection (RDP-CAR) for treatment. Ocuin et al. (35) reported the oncological outcomes of 19 open and 11 RDP-CAR. Both operations were associated with high R0 resection rates (82% vs. 79%), and there was no difference in the median overall survival for pancreatic cancer between the two groups (33 vs. 40 months).
Recommendation 2: RDP is as safe and feasible as laparoscopic DP. The intraoperative blood loss, length of hospital stay (LOS), overall postoperative complication rate, perioperative mortality, and the rate of postoperative pancreatic fistula (POPF) of RDP is comparable to that of LDP; moreover, RDP has a longer operative time, less intraoperative blood loss, less LOS compared to that with open distal pancreatectomy (ODP); whereas RDP has similar overall complication rate, perioperative mortality, and POPF rate compared to that of ODP
The level of evidence: moderate. Level of recommendation: strong (Grade 1B).
DP involves only resection procedures without pancreatic-intestinal reconstruction, so its difficulty is relatively low among various pancreatectomies. Currently, LDP has already become a standard practice for the treatment of neoplasms located in the pancreatic body and tail. It has been reported that patients can benefit from less blood loss and shortened length of hospital stay after LDP as compared to after open surgery (8). In 2001, Melvin et al. (22) presented the first RDP at the Society of American Gastrointestinal and Endoscopic Surgeons conference; since then, only a few case reports related to the RDP have been published (36-40).
Since 2010, a large number of case series emerged gradually: Giulianotti et al. (41) reported 46 cases of RDP. Among them, the spleen-preserving rate was 50%, which included 17 malignancies, six pancreatic ductal adenocarcinoma (PDAC), seven neuroendocrine neoplasms, two mucinous cystadenocarcinomas, and two pancreatic metastases. Shakir et al. (42) reported 100 RDPs for 30 PDAC, 35 pancreatic neuroendocrine neoplasms, five metastatic tumors, and 30 cases of other pathologies, including chronic pancreatitis, benign, and borderline cystic neoplasms. With increasing experience and improving techniques in robotic pancreatic surgery, indications for RDP have expanded quickly. The most common indications for RDP include benign, borderline and malignant distal pancreatic tumor size of less than 10 cm, and without major vessel or adjacent organ involvement (13,28,29,34,43,44). For cases that needed adjacent organ resection or reconstruction of the superior mesenteric vein (SMV)/portal vein (PV) to achieve R0 resection, only a few studies supported the feasibility of RDP; therefore, the majority excepting highly-skilled surgeons consider extended RDP is still prohibitive (28,42,45).
According to the analysis of the American College of Surgeons National Surgical Quality Improvement Program and National Cancer Database, compared to LDP, RDP has longer operation time (46) and less conversion rate, but there was no significant difference in their perioperative outcomes (44,47,48). Moreover, when compared to ODP, RDP had longer operation time, lesser blood loss, and lesser postoperative bleeding (46).
The meta-analysis by Xu et al. (34) that included three RCTs between 2010 and 2015, comparing RDP and LDP in the United states, Italy, France, Korea, Japan, Singapore, and China, revealed that RDP had less conversion rate (OR =0.52; 95% CI: 0.34, 0.78), but there was no significant difference in the operative time (WMD =24.98 min; 95% CI: −8.63, 58.59), blood loss (WMD =36.46 mL; 95% CI: −35.4, 108.31), transfusion rate (OR =1.02; 95% CI: 0.71, 1.46), postoperative overall complication (OR =0.93; 95% CI: 0.61, 1.4), overall POPF rate (OR =1.02; 95% CI: 0.71, 1.46), type B/C POPF rate (OR =1.07; 95% CI: 0.64, 1.79), and length of hospital stay (WMD =−0.95 days; 95% CI: −2.83, 0.92). Zhao et al. (13) reviewed four non-RCTs that compared RDP with ODP and included literatures published from the United States and Spain between 2010 and 2017. RDP was associated with lesser blood transfusion (OR =0.25; 95% CI: 0.11, 0.44), lower complications (OR =0.68; 95% CI: 0.51, 0.91), and shorter hospital stay (OR =−2.97 days; 95% CI: −4.75, −1.2) than ODP. There was no significant difference between the two groups in POPF (OR =1.13; 95% CI: 0.79, 1.62) and mortality rates (OR =0.42; 95% CI: 0.09, 1.87).
Recommendation 3: there is no significant difference concerning the spleen-preserving rate between RDP and LDP; however, RDP has a higher splenic vessel-preserving rate, which renders RDP more suitable for spleen-preserving DP with splenic vessel preservation
Level of evidence: low. Level of recommendation: strong (Grade 1C).
A previous study had shown that spleen resection could increase postoperative infection and long-term cardiovascular complications (49). Therefore, if there is no tumor involvement in the spleen or splenic vessel and radical lymphadenectomy is not required, spleen-preserving DP should be performed. Currently, there are two kinds of spleen-preserving DP: Kimura method that preserves the splenic vessels and Warshaw method that does not preserve the splenic vessels (50). The advantage of the Kimura method is the low incidence of postoperative gastric varices, low spleen infarction rate, and low rate of secondary splenectomy (51). However, the Kimura method requires a complicated operation technique because it is technically difficult to isolate the splenic artery and vein, and uncontrolled bleeding may be encountered if adhesions exist. The robotic surgical system with delicate manipulation enables effective and efficient hemostasis of splenic vessels (52). The meta-analysis compared the spleen-preserving rate of RDP and LDP, and it revealed that the spleen-preserving rates were comparable between RDP and LDP (OR =1.97; 95% CI: 0.58, 6.65); however, the splenic vessel conservation rate was significantly higher in the RDP group (OR =4.71; 95% CI: 1.77, 12.56) (34). A propensity-score matching (PSM) study of Liu (53) comparing RDP (102 cases) and LDP (102 cases) from 2011 to 2015 suggested that RDP improved spleen and splenic vessel preservation rates in patients with non-malignant tumors with a diameter of 3-5 cm (95.5% vs. 52.4%, P=0.001; 59.1% vs. 19.0%, P=0.007). However, current studies were mostly retrospective, and selection bias was unpreventable. Therefore, RCTs are needed to prove the effect of RDP.
Recommendation 4: about 10–20 consecutive RDP cases are needed for an experienced laparoscopic pancreatic surgeon to surpass the RDP learning curve and reach the optimal operation time
Level of evidence: low. Level of recommendation: low (Grade 2D).
When a new operative procedure is developed, the research of its learning curve could be helpful to instruct the application of the new procedure. Currently, there are few large-volume robotic pancreatic surgery hospitals, and reports regarding the learning curve are limited. Furthermore, the learning curve inevitably depends on the experience of open and laparoscopic surgery of each surgeon. For objective analysis, we need to choose a rational evaluation index in the analysis of the learning curve, which can truly reflect the progression in operation technique. The most common index is operation time.
Regarding RDP, the University of Pittsburgh analyzed the learning curve of 100 patients who underwent RDP; the operative time shows rapid reductions from initial operative time of 331 min after the first 20 and 40 cases to 266 min and 210 min, respectively (P<0.0001).
Moreover, the likelihood of readmission was significantly lower after the first 40 cases (P=0.04), and they concluded that RDP outcomes were optimized after 40 cases (42). Notably, three attending surgeons participated in this study; therefore, the learning curve is presumed to be shortened with a single surgeon.
Meanwhile, Napoli et al. (54) has extensive experience in laparoscopic pancreatic surgery and analyzed 55 consecutive cases of RDP he had performed. He showed that after ten operations, the operation time decreased significantly (421.1 vs. 248.9 min; P<0.0001). According to published articles and expert opinions, considering surgeons with extensive experience of laparoscopic pancreatic surgery, the learning curve for RDP may involve 10–20 operations to reach a considerable operative time. Before surpassing the learning curve, to alleviate the operation difficulty and possible surgical risks, the indication of surgery should be carefully identified, i.e., small tumor size, minimal inflammation, and relatively lean patients.
Recommendation 5: radical antegrade modular pancreatosplenectomy (RAMPS) has a higher R0 resection rate and lymph node harvest number compared to that with conventional DP; however, regarding long-term outcomes, there is insufficient evidence to support RAMPS; robotic RAMPS is feasible and may be applied at surgeons’ discretion
Level of evidence: very low. Level of recommendation: weak (Grade 2D).
Strasberg et al. (55) reported the first RAMPS in 2003 for increasing the R0 resection rate and extent of lymph node dissection to improve patient’s survival. A meta-analysis published in 2017 revealed that RAMPS achieves higher R0 resection rates (OR =2.19; 95% CI: 1.16, 4.13; P=0.02) and more harvested lymph nodes (WMD =7.06; 95% CI: 4.52, 9.60; P<0.01) than conventional DP, but there was no significant difference in disease-free survival and recurrence rate (56). The earliest robotic RAMPS was published in 2000 (57); since then, the University of Yonsei (58,59) reported four cases of robotic RAMPS including three distal PDAC and one invasive intraductal papillary mucinous neoplasm (IPMN), and the average number of harvested lymph nodes was 8.5 (range, 2–23). All cases achieved R0 resection without tumor recurrence and mortality within two years, which preliminarily showed the feasibility of robotic RAMPS. Compared to laparoscopic surgery, the application of robotic surgery can lower the difficulty of pancreatic vessel skeletonization and regional lymphadenectomy (60). In general, the use of robotic RAMPS mainly depends on the surgeon’s preference (36).
Recommendation 6: there is insufficient evidence to support the view that the cost of RDP is higher than that of LDP or ODP; the cost-effectiveness of RDP should be synthetically evaluated based on several factors, including overall healthcare expense, patient’s psychological benefit, and social benefit
Level of evidence: very low. Level of recommendation: weak (Grade 2D).
The cost of robotic pancreatic surgery may differ for some degree among countries or regions due to the medical policy or insurance premium (43,52). The most common issue about cost-effectiveness analysis related to RDP is simply on the total operation cost and admission fee. Although a study revealed that compared to LDP or open DP, the operative cost of RDP is higher, the total admission cost was lower owing to the shortened length of hospital stay (61,62). One report from Spain (43) mentioned that the mean operative cost was slightly higher in the RDP group than in the LDP group by almost 200€ per patient, but the mean hospitalization cost was almost 400€ higher in the LDP group, and the mean total costs were similar between the two groups (9,198.64€ vs. 9,399.74€; P>0.5). One systematic review published in 2018 mentioned that the operative cost was almost double in the RADP group than in the LDP group (MD =2,350 USD; 95% CI: 1,165.62, 3,534.78) (34). In this systematic review, a study in Italy (45) in 2014 only mentioned the operative instrument fee instead of the mean total cost. Another study (63) included in this systematic review was published in 2010; due to its earlier publish date, the value of cost analysis may not be applied now. Therefore, the evidence of this systematic review was probably of low level; further cost-effectiveness studies will be needed to evaluate the cost associated with robotic surgery.
When we try to analyze the cost-effectiveness of robotic pancreatic surgery, we should take into account patient’s psychological benefit, availability of the medical resource, and predictive survival rate of the patient, instead of only the medical costs. For example, Anderson et al. (64) stated that patients undergoing minimally invasive DP were more likely to receive adjuvant chemotherapy, which means that the prognosis might be better for this patient.
Recommendation 7: for malignant tumors, robotic pancreaticoduodenectomy (RPD) is associated with higher R0 resection rate but similar lymph node harvest number compared to that with OPD
Level of evidence: moderate. Level of recommendation: strong (Grade 1B).
Surgical resection margin status and the number of the harvested lymph node are the most important indicators for curability of the malignancy and are related to the patient’s long-term prognosis. A recent meta-analysis revealed that RPD was associated with lower positive margin rate than OPD (OR =0.29; 95% CI: 0.15, 0.56), although the lymph node harvest number has no significant difference (WMD =1.82; 95% CI: −0.85, 4.48) (13). However, studies included in this meta-analysis had heterogeneous harvested lymph node (I2=81%), in which Zureikat et al. (65) reported that the average number of harvested lymph node was 27.5 and 19 (P<0.001) in RPD and OPD, respectively, and Bao et al. (66) reported 15 and 20 (P=0.004) in RPD and OPD, respectively. This may reflect the effect of the learning curve and an individual’s proficiency. Wang et al. (67) revealed similar R0 resection rate between the RPD and OPD groups (96.6% vs. 94.3%, P=0.363); however, the RPD group has more harvested lymph nodes than the OPD group (15 vs. 13, P=0.001). The 1-, 2- and 3-year overall survival rates before PSM in each group were 98.9%, 96.2%, 93.2%, and 95.5%, 90.0%, and 85.2%, respectively. The prognosis of RPD is better than that of OPD (P=0.009); however, after pathologic grading and PSM, the RPD group showed no significant difference in the 1-, 2- and 3-year overall survival rates when compared to the OPD group. Meanwhile, two small scale studies using the same method for comparing RPD and OPD revealed no difference between RPD and OPD regarding the R0 resection rate and lymph node harvested number (68,69).
Recommendation 8: RPD can be employed for benign or malignant disease in the region of the pancreas head and duodenum; for large benign tumors, advanced stage malignancies, or conditions that need resection and reconstruction of the involved vessels, RPD should be discreetly performed by surgeons who have surpassed the RPD learning curve
Level of evidence: moderate. Level of recommendation: strong (Grade 1B).
The pancreaticoduodenectomy indicates resection of multiple organs, complicated reconstruction with extensive dissection. A literature review showed that RPD is mainly indicated for pancreatic malignancy. Giulianotti et al. (41,70) reported the first RDP in 2003 and subsequently reported 60 cases of RPD in 2010 for 26 PDAC of the pancreatic head and 15 adenocarcinomas of the ampulla. A multi-institutional study demonstrated 211 cases of RPD, of which 70 cases (33.18%) were PDAC (65). Jung et al. reported 192 RPDs between 2011 and 2016, of which 163 cases (84.9%) were a malignancy, including 87 cases (45.3%) of PDAC (71). Chen et al. (72) reported a prospective matched study between 2010 and 2013 analyzing RPD versus OPD, with 60 RPD including 19 pancreatic adenocarcinomas, three distal common bile duct (CBD) adenocarcinomas, eight duodenal adenocarcinomas, eight ampullary adenocarcinomas, and the largest tumor size 9.8 cm. Wang et al. (67) reported a comparative study between 2012 and 2017 comparing RPD versus OPD, and they reported a total of 118 RPD cases, including 29 pancreatic head adenocarcinomas, 46 ampullary adenocarcinomas, seven distal CBD adenocarcinomas, five duodenal adenocarcinomas, six other malignancies, and 25 benign lesions.
Clinically, the difficulty of performing PD will be significantly increased by large tumors or advanced oncological stages, especially when resection/reconstruction of vessels is required, and the conversion rate will be higher in such severe cases than usual. However, these circumstances may not be an absolute contraindication for RPD (73-76). It could be performed only by those experienced high-volume pancreatic surgeons who have passed the learning curve. One retrospective NSQIP review from 2014 to 2015 revealed that the proportion of vessel resection and malignancy in RPD was less than that of LPD or OPD, although the baseline characteristics between RP, LPD, and ODP were comparable, which might be attributed to a large number of operations performed in the pre-learning curve cohort (77). More complex operations could be performed in the post-learning curve phase.
Recommendation 9: RPD is associated with lesser blood loss and longer operative time in contrast to that with OPD; there is no difference in the intraoperative transfusion rate between RPD and OPD
Level of evidence: moderate. Level of recommendation: strong (Grade 1B).
Operative time, intraoperative blood loss, and transfusion rate are indexes of surgical safety and feasibility. Wang et al. (67) compared the modified Blumgart pancreaticojejunostomy reconstruction by RPD with OPD using PSM method. There were 87 cases in each group, and the study revealed longer operative time (420 vs. 360 min, P<0.001) and less blood loss (120 vs. 250 mL, P=0.001) in the RPD group. A systematic review in 2018 included 11 comparative studies on RPD and OPD, which included seven reports from the United States and three from China for a total of 530 RPD cases and 1,228 OPD cases. This systematic review concluded that RPD is associated with longer operative time (WMD =88.69 min; 95% CI: 33.38, 138.99) and lesser blood loss than OPD (WMD =−197.02 mL; 95% CI: −313.42, −80.61), although the blood transfusion rate was not significantly different between the two groups (OR =0.79; 95% CI: 0.58, 1.07) (13). Because the definition of operative time differed in each study, and the reliability of the conclusion from the review was low. Currently, there is no other RCT comparing RPD and OPD.
Recommendation 10: RPD has comparable perioperative mortality, overall postoperative complication rate, and the rate of POPF as that with OPD; however, RPD has shorter LOS than that with OPD
Level of evidence: moderate. Level of recommendation: strong (Grade 1B).
The short-term outcomes reflect the safety and injury rates from surgery. Kowalsky et al. (78) used multivariate analysis for the prediction of perioperative outcomes and revealed that RPD is a predictive factor for a shortened length of hospital stay (OR =0.33; 95% CI, 0.16, 0.67). Wang et al. (67) reported no significant difference in overall postoperative complication, type B/C POPF, postoperative bleeding, and postoperative hospital stay between RPD and OPD. Napoli et al. (79) grouped RPD and OPD patients using the risk factor of POPF. They found no significant difference in the postoperative morbidity and mortality between RPD and OPD. In the stratified analysis, RPD was associated with higher rates of grade B/C POPF (OR =2.80; 95% CI: 1.01, 7.78) and with equivalent incidence of grade C POPF in the intermediate-risk group, whereas the rate of grade B/C POPF was comparable (OR =0.20; 95% CI: 0.01, 4.17) after either procedure in the high-risk group. Besides, a comparative study revealed that RPD can decrease type B/C POPF rate (OR =0.34; P<0.001) and surgical site infection rate (OR =0.3; P<0.001) compared to OPD. However, for patients with body mass index more than 30 cm/m2, RPD has shorter operative time (381 vs. 428 min), decreased blood loss (250 vs. 500 mL), and less surgical site infection rate (19% vs. 44%, P=0.001) than OPD, but there was no significant difference in the surgical margin positive rate, severe complications, postoperative hospital stays, and 30-day mortality rates between the two groups (80).
A recent meta-analysis revealed no significant difference in the postoperative complication rate (OR =0.67; 95% CI: 0.47, 0.95), POPF rate (OR =1.20; 95% CI: 0.88, 1.63), and perioperative mortality rate (OR =0.92; 95% CI: 0.48, 1.77) between RPD and OPD, but RPD was associated with shorter postoperative hospital stay than OPD (WMD =−2.55 day; 95% CI: −5.21, 0.12) (13). These data suggest that compared with OPD, RPD has comparative safety but less trauma and faster recovery.
Recommendation 11: for a surgeon with extensive experience in laparoscopic pancreatectomy, the operative time will decrease significantly after 40 consecutive cases of RPD
Level of evidence: very low. Level of recommendation: weak (Grade 2D).
Napoli et al. (81) reported a single surgeon’s learning curve for RPD in which the operative time decreased from the 33rd case (564±101.7 vs. 484.1±77.9 min, P=0.0005), and the readmission rate decreased from the 40th case (20.0% vs. 3.3%, P=0.04). The University of Pittsburgh reported 200 consecutive RPD cases and revealed that the blood loss and conversion rate would be improved after 20 RPD cases, the POPF rate will be improved after 40 cases, and the operative time will be shortened after 80 cases. Therefore, a study concluded that the learning curve for RPD is attained within 80 cases (82). A total of four attending surgeons participated in this study. All initial 80 cases required two attending surgeons for the duration of the procedure to ensure patient safety, and after the initial 80 cases, only the dissection of uncinate process or other complex and high-risk procedures required two surgeons. When choosing the operative time as the evaluation index, the two studies showed distinct results, which suggested that more surgeons are involved in the cohort, the more cases are needed to surpass the learning curve. Moreover, previous experience of OPD and LDP should also be considered in the analysis of the learning curve (83). For surgeons with extensive experience with laparoscopic surgery, it is reasonable that about 40 cases of RPD are needed to decrease the operative time significantly.
Recommendation 12: hybrid technique (laparoscopic/robotic) can be used in PD; for surgeons with extensive experience in laparoscopic surgery, a hybrid method can be utilized during the transition to total RPD
Level of evidence: very low. Level of recommendation: weak (Grade 2D).
Currently, the approach of RPD in most reports are full RPD instead of laparoscopic/robotic hybrid PD, which involves the use of traditional laparoscopy for dissection and resection following reconstruction by the robotic system. This hybrid method combines the advantage of traditional laparoscopy, such as quick movement, faster change of instruments, and the benefit of the robotic systems, such as stable platform, articulated instruments, and excellent visualization (17,84-87). At present, a study reported the largest number of laparoscopic/robotic hybrid PD of 132 cases and revealed the following: the operative time decreased from 527 min in the first 50 cases to 350 min in the last 50 cases, the conversion rate dropped from 11% in the first 20 cases to 4.5% in the previous 11 cases (87). For the whole cohort, the R0 resection rate was 87.7%, the median number of harvested lymph nodes was 19, the reoperation rate was 3%, and the 30-day mortality rate was 1.5%. Other comparative studies (88-90) revealed that the perioperative outcome was similar. Kim et al. (30) compared 51 cases of laparoscopic/robotic hybrid PD to 186 OPD cases between 2015 and 2017 and reported that the operative time (335.6 vs. 330.1 min), postoperative complication rate (15.7% vs. 21.0%), and POPF rate (6.0% vs. 12.0%) were similar between the two groups, but the length of hospital stay was shorter (1.6 vs. 15.3 days, P=0.001) and the pain score was lower (3.7 vs. 4.1, P=0.008) in the hybrid group. Current studies showed the feasibility of laparoscopic/robotic hybrid PD, which can be a zone of transition for well-experienced laparoscopic surgeons who dedicated to performing the RPD. However, some reports mentioned that there was no need for hand-assisted or robotic-assisted LPD surgery (91).
Recommendation 13: RPD with resection/reconstruction of PV/SMV is technically demanding and is not recommended for surgeons to perform in their preliminary stages of training
Level of evidence: very low. Level of recommendation: weak (Grade 2D).
The most and maybe the best potential treatment method for pancreatic malignancy is curative surgery. According to the National Comprehensive Cancer Network guideline, despite vessel involvement in pancreatic malignancy, if a surgeon can confidently resect and reconstruct the involved vessel completely and safely, the surgery may still be feasible (92). Studies related to resection/reconstruction of SMV/PV in RPD are scarce (93-96). In 2011, Giulianotti et al. (76) reported two cases of locally advanced pancreatic head tumor resected by RPD combined with PV resection and reconstruction. The operation course was smooth with an operative time of 430 min and blood loss of 175 mL, and all two cases had R0 resection. Kauffmann et al. (20) reported nine cases of RPD with resection and reconstruction of the RPD-SMV/PV, and they concluded that RPD-SMV/PV required longer operative time, was associated with a higher median estimated blood loss, and required blood transfusions, but the incidence and severity of postoperative complications were not increased. RPD-SMV/PV was associated with a higher mean number of examined lymph nodes, but the rate of positive margin was the same (20). There were other case reports (72,74,76) related to the resection and reconstruction of major vessels in RPD. The rare publication of this operation reflects its demanding techniques; therefore, surgeons in the preliminary phase of RPD are not recommended to perform RPD-SMV/PV resection and reconstruction.
Recommendation 14: robotic central pancreatectomy is safe and feasible for benign and borderline tumors in the neck and proximal body of the pancreas. The distal pancreatic stump of at least 5 cm should be retained as suggested
Level of evidence: low. Level of recommendation: weak (Grade 2C).
Central pancreatectomy usually refers to the parenchyma-sparing pancreatectomy which intends to preserve more exocrine and endocrine functions of the remnant pancreas. Giulianotti et al. (97) reported three cases of central pancreatectomy performed between 2004 and 2005 including two serious cystadenomas and one mucinous cystadenoma, with the largest tumor size of 3.5 cm. First, laparoscopy for initial exploration and pancreas dissection following robotic pancreas resection and reconstruction was performed and then gradually turned to purely robotic central pancreatectomy (98-101). Chen et al. (102) reported an RCT comparing robotic central pancreatectomy and open central pancreatectomy; in the robotic group, there were seven IPMNs, nine solid pseudopapillary tumors (SPT), four neuroendocrine tumors (NET), and 27 mucinous cystadenomas and benign epithelial cyst, and the median tumor size was 2.9 (IQR, 2–3.4) cm. Although pancreatic malignancy is not suitable for central pancreatectomy, for isolated pancreatic metastatic tumor, i.e., pancreatic metastasis from renal cell cancer, robotic central pancreatectomy is still feasible (103). To preserve the function of the distal pancreas, the remnant of the pancreas tail should be at least 5 cm (102).
Recommendation 15: there is insufficient evidence to support the view that short-term outcomes of robotic central pancreatectomy are better than that of open central pancreatectomy
Level of evidence: very low. Level of recommendation: weak (Grade 2D).
Currently, there are only a few small case series studies related to robotic central pancreatectomy. Kang et al. (98) compared five robotic central pancreatectomies (two cases of laparoscopic/robotic hybrid central pancreatectomy) to 10 open central pancreatectomies. The operative time of robotic central pancreatectomy is longer than that of open central pancreatectomy (286.5±90.2 vs. 432.0±65.7 min, P=0.013), but the intraoperative blood loss in the robotic cohort is less (432.0±65.7 vs. 286.5±90.2 mL, P=0.013). Cheng et al. (100) analyzed perioperative outcomes of seven robotic central pancreatectomies and 36 open central pancreatectomies and found that the postoperative morbidity (85.7% vs. 50%, P=0.112), POPF rate (71.4% vs. 41.7%, P=0.222), and postoperative hospital stays (21 vs. 18 days, P=0.587) were not significantly different between the two groups, whereas patients who underwent robotic surgery had a faster gastrointestinal function recovery (2 vs. 4 days, P=0.001). Chen et al. (102) reported an RCT comparing robotic and laparoscopic central pancreatectomy. Compared to open surgery, robotic central pancreatectomy showed higher length of hospital stay (15.6 vs. 21.7 days, P=0.002), operative time (160 vs. 193 min, P=0.002), blood loss (50 vs. 200 mL, P<0.001), type B/C POPF (18 vs. 36%, P=0.043), postoperative activity period (3.1 vs. 4.6 days, P<0.001), and recovery of gastrointestinal function (3.5 vs. 5 days, P<0.001).
Recommendation 16: robotic pancreatic enucleation can be applied in superficial benign tumors; the safe distance from tumor margin to the main pancreatic duct (MPD) should be at least 2 mm
Level of evidence: low. Level of recommendation: weak (Grade 2C).
Pancreatic enucleation is also a kind of parenchyma-sparing pancreas surgery. The possibility of enucleation was related to the tumor size and the distance from the MPD. The indication for robotic pancreatic enucleation was the same as that in laparoscopic pancreatic enucleation, such as superficial and borderline pancreas tumor, tumor size less than 2 cm, and the distance between the tumor and the MPD was at least 2 mm. Currently, only a few reports are related to robotic enucleation. A research (103) reported 26 cases of robotic pancreatic enucleation, and the average tumor size was 23±12 mm (range, 7–40 mm), and the distance from the MPD was at least 1–2 mm according to the preoperative magnetic resonance image. Tian et al. (104) reported one PSM study related to robotic and open pancreatic enucleation, and each group included 60 NET cases with tumor size of less than 2 cm and the distance from the MPD was more than 2 mm. The study revealed that robotic enucleation showed shorter operative time (117 vs. 150 mins, P<0.001), less blood loss (32.5 vs. 80.0 mL, P=0.008), and the same POPF rate. Jin et al. (105) compared 31 cases of robotic pancreatic enucleation and 25 cases of open pancreatic enucleation for eight IPMNs, 13 NET, four mucinous cystadenomas, five SPTs, and one HCC with pancreatic metastasis in the robotic group, with tumor size was 2.0 mm (IQR, 1.5–2.6 mm), and they reported that tumor size, location, and pathology in the two groups were comparable and that robotic surgery could shorten the operative time (100 vs. 140 min, P=0.009) and decrease the intraoperative blood loss (39 vs. 100 mL, P=0.001) compared to open surgery in pancreatic enucleation. The rates of a significant complication and grade B/C POPF are similar between the two groups.
Recommendation 17: various techniques could be used for pancreatic-enterostomy in RPD, and the most commonly used technique is pancreaticojejunostomy; surgeons can choose a suitable method of pancreaticojejunostomy at their discretion
The risk of POPF was related to the texture of pancreas, MPD diameter, pathological type, and intraoperative blood loss (106,107). The pancreatojejunostomy (PJ) reconstruction method was not an independent factor for POPF (19,108). One study published in 2017 reported 5,316 PD cases performed by 62 surgeons from 17 medical centers, of which 5,040 (94.8%) were PJ reconstruction and 276 (5.2%) were pancreaticogastrostomy (PG) reconstruction. After adjusting for confounding factors, the report showed no significant difference in the POPF rate between the two techniques (109).
Similar to OPD, most pancreas reconstruction method in RPD was PJ reconstruction, which was the most critical step in PD operation, especially when the pancreatic texture was soft, and the MPD was non-dilated. Giulianotti et al. (110,111) reported that end-to-side PJ anastomosis could be performed if the MPD >3 mm and reinforced by suturing the intestinal seromuscular layer to the pancreas parenchyma; when the MPD was less than three mm, the POPF rate was high after PJ reconstruction and the PG reconstruction was recommended. Another method for PJ reconstruction was the invagination method. Instead of performing duct-to-mucosa anastomosis, this method can decrease the difficulty of anastomosis, but its safety and effectiveness evidence is still lacking (12).
Recommendation 18: when the main pancreatic duct is accidentally injured during robotic pancreatic enucleation, a salvage pancreatectomy or pancreatic-enterostomy can be performed; one stage repair of the main pancreatic duct is feasible, but its safety needs to be evaluated with further study
MPD injury can be a complication of pancreatic enucleation when there is no safe distance between the tumor and MPD (104,105). Preoperative image evaluation and intraoperative ultrasonography are essential for the prevention of MPD injury (112). A study reported that preoperative pancreatic duct stent implantation was useful for prevention and early detection of MPD injury, but the risk for secondary pancreatitis remains a concern (113,114). Clinically, several salvage surgeries are available as treatment of MPD injury during pancreatic enucleation, such as DP, central pancreatectomy, PJ anastomosis, or even PD surgery. Alternatively, some surgeons try to use pancreatic duct stent or end-to-end pancreatic stump anastomosis to reconstruct the continuity of the MPD. This one-stage reconstruction method can decrease the complication of salvage surgery and preserve the normal anatomic structure, but further study will be needed for evaluation of safety and long-term outcome (115,116).
Recommendation 19: the surgical concept of RPD is different from that of OPD due to different view angles during surgery, lack of tactile feedback, and more dependence on the operative instruments in robotic surgery
The surgical concept of RPD differs from that of OPD because of varying surgical visual angles, lack of tactile feedback, and high dependence on the operative instruments in robotic surgery. Compared to OPD in which the view angle was from the anterior to posterior direction, the view angle in RPD was different because of its caudal to cranial viewing angle; thus, steps in the operative procedure are quite different between these two operations. For example, in OPD, the peritoneal reflection is usually opened as a first step on the right side of the duodenum to perform the Kocher maneuver, but in RPD, the right colon and hepatic flexure are usually mobilized first, and then the Kocher maneuver is performed from the inferior to superior direction. In OPD, the uncinate process is separated from the PV/SMV via an anterior to posterior approach, but in RPD, the dissection sequence is from inferior to superior instead. Moreover, owing to the reverse Trendelenburg position, especially in obese patients, the transverse colon was challenging to overturn, which hampered the mobilization of the proximal jejunum in the left of the Treitz ligament and inferior to the transverse mesocolon. In RPD, the proximal jejunum is mobilized by a superior transverse mesocolon approach from the right side of the Treitz ligament (72,110). Similar to traditional laparoscopy, robotic surgery lacks hand haptic feedback, and visual feedback is utilized instead of surgeons’ hands to identify tumor location or vessel pulsation (117). Therefore, a preoperative imaging study is essential, as it cannot only evaluate the tumor resectability but also reveal major vessel variation.
Meanwhile, the suturing ability in RPD was better than that in LPD, but still much less straightforward than that in OPD. Therefore, to reduce the instruments exchange procedure and enhance the efficiency, the vessels are usually occluded with clips in RPD instead of suturing or ligation. Moreover, automatic linear staplers are typically applied for the transection and reconstruction of the gastrointestinal tract in RPD and suture materials that easier for continuous suturing are used for hepatico-jejunum and/or PJ reconstruction (19,73,118,119).
Acknowledgments
We thank Dr. Chih-Yuan Wang in the translation of the consensus statement. We thank all the external reviewers for giving responses to the consensus.
Funding: This work was supported by the National Key Research and Development Program of China (grand number 2017YFC0110405) and the National Natural Science Foundation of China (grant number 81500499).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Members of the Consensus Steering Group: Rong Liu, Yupei Zhao, Go Wakabayashi, Chinnusamy Palanivelu, Allan Tsung, Kehu Yang. Members of the Consensus Development Group: Brian K. P. Goh, Charing Ching-Ning Chong, Chang Moo Kang, Chenghong Peng, Eli Kakiashvili, Ho-Seong Han, Hong-Jin Kim, Jin He, Jae Hoon Lee, Kyoichi Takaori, Marco Vito Marino, Shen-Nien Wang, Tiankang Guo, Thilo Hackert, Ting-Shuo Huang, Yiengpruksawan Anusak, Yuman Fong, Yuichi Nagakawa, Yi-Ming Shyr, Yao-Ming Wu. Members of the Consensus Secretary Group: Guodong Zhao, Zizheng Wang, Kongyuan Wei, Wenbo Tang.
References
- 1.Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg 2011;35:1469-77. 10.1007/s00268-011-1051-5 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection—2,804 patients. Ann Surg 2009;250:831-41. 10.1097/SLA.0b013e3181b0c4df [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302:1557-64. 10.1001/jama.2009.1451 [DOI] [PubMed] [Google Scholar]
- 4.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. 10.1007/BF00642443 [DOI] [PubMed] [Google Scholar]
- 5.Liang S, Hameed U, Jayaraman S. Laparoscopic pancreatectomy: indications and outcomes. World J Gastroenterol 2014;20:14246. 10.3748/wjg.v20.i39.14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006;244:931. 10.1097/01.sla.0000246856.03918.9a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heemskerk J, Zandbergen R, Maessen JG, et al. Advantages of advanced laparoscopic systems. Surg Endosc 2006;20:730-3. 10.1007/s00464-005-0456-3 [DOI] [PubMed] [Google Scholar]
- 8.Edwin B, Sahakyan MA, Abu Hilal M, et al. Laparoscopic surgery for pancreatic neoplasms: the European association for endoscopic surgery clinical consensus conference. Surg Endosc 2017;31:2023-41. 10.1007/s00464-017-5414-3 [DOI] [PubMed] [Google Scholar]
- 9.Szold A, Bergamaschi R, Broeders I, et al. European Association of Endoscopic Surgeons (EAES) consensus statement on the use of robotics in general surgery. Surg Endosc 2015;29:253-88. 10.1007/s00464-014-3916-9 [DOI] [PubMed] [Google Scholar]
- 10.Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14. 10.1097/01.sla.0000103020.19595.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diana M, Marescaux J. Robotic surgery. Br J Surg 2015;102:e15-e28. 10.1002/bjs.9711 [DOI] [PubMed] [Google Scholar]
- 12.Liu R, Zhao GD, Tang WB, et al. A single-team experience with robotic pancreatic surgery in 1010 cases. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Liu C, Li S, et al. Safety and efficacy for robot-assisted versus open pancreaticoduodenectomy and distal pancreatectomy: A systematic review and meta-analysis. Surg Oncol 2018;27:468-78. 10.1016/j.suronc.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Cai Y, Li Y, et al. Robotic Pancreaticoduodenectomy: Single-Surgeon Initial Experience. Indian J Surg 2018;80:42-7. 10.1007/s12262-016-1555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra F, Checcacci P, Vegni A, et al. Surgical and oncological outcomes of our first 59 cases of robotic pancreaticoduodenectomy. J Visc Surg 2019;156:185-90. 10.1016/j.jviscsurg.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 16.Piedimonte S, Wang Y, Bergman S, et al. Early experience with robotic pancreatic surgery in a Canadian institution. Can J Surg 2015;58:394-401. 10.1503/cjs.003815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji W, Ding K, Kao X, et al. Robotic and laparoscopic hybrid pancreaticoduodenectomy: surgical techniques and early outcomes. Chin Med J (Engl) 2014;127:3027-9. [PubMed] [Google Scholar]
- 18.Zeh HJ, Zureikat AH, Secrest A, et al. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol 2012;19:864-70. 10.1245/s10434-011-2045-0 [DOI] [PubMed] [Google Scholar]
- 19.Watkins AA, Kent TS, Gooding WE, et al. Multicenter outcomes of robotic reconstruction during the early learning curve for minimally-invasive pancreaticoduodenectomy. HPB (Oxford) 2018;20:155-65. 10.1016/j.hpb.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 20.Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 2016;401:1111-22. 10.1007/s00423-016-1499-8 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TK, Zenati MS, Boone BA, et al. Robotic pancreaticoduodenectomy in the presence of aberrant or anomalous hepatic arterial anatomy: safety and oncologic outcomes. HPB (Oxford) 2015;17:594-9. 10.1111/hpb.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. 10.1007/s00464-001-8192-9 [DOI] [PubMed] [Google Scholar]
- 23.Wang SE, Shyr BU, Chen SC, et al. Robotic distal pancreatectomy: Comparison of spleen-preservation by the Warshaw technique and splenectomy. Int J Med Robot 2018:e1922. 10.1002/rcs.1922 [DOI] [PubMed] [Google Scholar]
- 24.Lalli R, Merritt N, Schlachta CM, et al. Robotic-assisted, spleen-preserving distal pancreatectomy for a solid pseudopapillary tumour in a pediatric patient: a case report and review of the literature. J Robot Surg 2019;13:325-9. 10.1007/s11701-018-0835-0 [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Jin JB, Zhan Q, et al. Robot-assisted duodenum-preserving pancreatic head resection with pancreaticogastrostomy for benign or premalignant pancreatic head lesions: a single-centre experience. Int J Med Robot 2018;14:e1903. 10.1002/rcs.1903 [DOI] [PubMed] [Google Scholar]
- 26.Goh BKP, Low TY, Lee SY, et al. Initial experience with robotic pancreatic surgery in Singapore: single institution experience with 30 consecutive cases. ANZ J Surg 2019;89:206-10. 10.1111/ans.14673 [DOI] [PubMed] [Google Scholar]
- 27.Coratti A, Di Marino M, Coratti F, et al. Initial Experience With Robotic Pancreatic Surgery: Technical Feasibility and Oncological Implications. Surg Laparosc Endosc Percutan Tech 2016;26:31-7. 10.1097/SLE.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 28.Souche R, Herrero A, Bourel G, et al. Robotic versus laparoscopic distal pancreatectomy: a French prospective single-center experience and cost-effectiveness analysis. Surg Endosc 2018;32:3562-9. 10.1007/s00464-018-6080-9 [DOI] [PubMed] [Google Scholar]
- 29.Qu L, Zhiming Z, Xianglong T, et al. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: A retrospective propensity score-matched study. Int J Surg 2018;55:81-6. 10.1016/j.ijsu.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Han Y, Kang JS, et al. Comparison of surgical outcomes between open and robot-assisted minimally invasive pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2018;25:142-9. 10.1002/jhbp.522 [DOI] [PubMed] [Google Scholar]
- 31.McMillan MT, Zureikat AH, Hogg ME, et al. A Propensity Score-Matched Analysis of Robotic vs Open Pancreatoduodenectomy on Incidence of Pancreatic Fistula. JAMA Surg 2017;152:327-35. 10.1001/jamasurg.2016.4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection. Ann Surg 2013;257:731-6. 10.1097/SLA.0b013e318263da2f [DOI] [PubMed] [Google Scholar]
- 33.Ashfaq A, Pockaj BA, Gray RJ, et al. Nodal counts and lymph node ratio impact survival after distal pancreatectomy for pancreatic adenocarcinoma. J Gastrointest Surg 2014;18:1929-35. 10.1007/s11605-014-2566-5 [DOI] [PubMed] [Google Scholar]
- 34.Xu SB, Jia CK, Wang JR, et al. Do patients benefit more from robot assisted approach than conventional laparoscopic distal pancreatectomy? A meta-analysis of perioperative and economic outcomes. J Formos Med Assoc 2019;118:268-78. [DOI] [PubMed] [Google Scholar]
- 35.Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford) 2016;18:835-42. 10.1016/j.hpb.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talamini MA, Chapman S, Horgan S, et al. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 2003;17:1521-4. 10.1007/s00464-002-8853-3 [DOI] [PubMed] [Google Scholar]
- 37.Horgan S, Galvani C, Gorodner V, et al. Robotic distal pancreatectomy and nephrectomy for living donor pancreas-kidney transplantation. Transplantation 2007;84:934-6. 10.1097/01.tp.0000284585.12633.06 [DOI] [PubMed] [Google Scholar]
- 38.Giulianotti PC, Kuechle J, Salehi P, et al. Robotic-assisted laparoscopic distal pancreatectomy of a redo case combined with autologous islet transplantation for chronic pancreatitis. Pancreas 2009;38:105-7. 10.1097/MPA.0b013e31816b3100 [DOI] [PubMed] [Google Scholar]
- 39.Tomulescu V, Stanciulea O, Balescu I, et al. First year experience of robotic-assisted laparoscopic surgery with 153 cases in a general surgery department: indications, technique and results. Chirurgia (Bucur) 2009;104:141-50. [PubMed] [Google Scholar]
- 40.Vasilescu C, Sgarbura O, Tudor S, et al. Robotic spleen-preserving distal pancreatectomy. A case report. Acta Chir Belg 2009;109:396-9. 10.1080/00015458.2009.11680446 [DOI] [PubMed] [Google Scholar]
- 41.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. 10.1007/s00464-009-0825-4 [DOI] [PubMed] [Google Scholar]
- 42.Shakir M, Boone BA, Polanco PM, et al. The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. HPB (Oxford) 2015;17:580-6. 10.1111/hpb.12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ielpo B, Duran H, Diaz E, et al. Robotic versus laparoscopic distal pancreatectomy: A comparative study of clinical outcomes and costs analysis. Int J Surg 2017;48:300-4. 10.1016/j.ijsu.2017.10.075 [DOI] [PubMed] [Google Scholar]
- 44.Raoof M, Nota C, Melstrom LG, et al. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: Analysis of the National Cancer Database. J Surg Oncol 2018;118:651-6. 10.1002/jso.25170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butturini G, Damoli I, Crepaz L, et al. A prospective non-randomised single-center study comparing laparoscopic and robotic distal pancreatectomy. Surg Endosc 2015;29:3163-70. 10.1007/s00464-014-4043-3 [DOI] [PubMed] [Google Scholar]
- 46.Xourafas D, Ashley SW, Clancy TE. Comparison of Perioperative Outcomes between Open, Laparoscopic, and Robotic Distal Pancreatectomy: an Analysis of 1815 Patients from the ACS-NSQIP Procedure-Targeted Pancreatectomy Database. J Gastrointest Surg 2017;21:1442-52. 10.1007/s11605-017-3463-5 [DOI] [PubMed] [Google Scholar]
- 47.Zureikat AH, Borrebach J, Pitt HA, et al. Minimally invasive hepatopancreatobiliary surgery in North America: an ACS-NSQIP analysis of predictors of conversion for laparoscopic and robotic pancreatectomy and hepatectomy. HPB (Oxford) 2017;19:595-602. 10.1016/j.hpb.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 48.Stiles ZE, Dickson PV, Deneve JL, et al. The impact of unplanned conversion to an open procedure during minimally invasive pancreatectomy. J Surg Res 2018;227:168-77. 10.1016/j.jss.2018.02.028 [DOI] [PubMed] [Google Scholar]
- 49.Weledji EP. Benefits and risks of splenectomy. Int J Surg 2014;12:113-9. 10.1016/j.ijsu.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 50.Hwang HK, Kang CM, Chung YE, et al. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc 2013;27:774-81. 10.1007/s00464-012-2551-6 [DOI] [PubMed] [Google Scholar]
- 51.Del Chiaro M, Moretto C, Croce C, et al. Robotic pancreatectomies: A single institution experience. Pancreatology 2009;9:463-4. [Google Scholar]
- 52.Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. 10.1007/s00464-010-1504-1 [DOI] [PubMed] [Google Scholar]
- 53.Liu R, Liu Q, Zhao ZM, et al. Robotic versus laparoscopic distal pancreatectomy: A propensity score-matched study. J Surg Oncol 2017;116:461-9. 10.1002/jso.24676 [DOI] [PubMed] [Google Scholar]
- 54.Napoli N, Kauffmann EF, Perrone VG, et al. The learning curve in robotic distal pancreatectomy. Updates Surg 2015;67:257-64. 10.1007/s13304-015-0299-y [DOI] [PubMed] [Google Scholar]
- 55.Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery 2003;133:521-7. 10.1067/msy.2003.146 [DOI] [PubMed] [Google Scholar]
- 56.Cao F, Li J, Li A, et al. Radical antegrade modular pancreatosplenectomy versus standard procedure in the treatment of left-sided pancreatic cancer: A systemic review and meta-analysis. BMC Surgery 2017;17:67. 10.1186/s12893-017-0259-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc 2010;24:1533-41. 10.1007/s00464-009-0806-7 [DOI] [PubMed] [Google Scholar]
- 58.Choi SH, Kang CM, Hwang HK, et al. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg 2012;16:868-9. 10.1007/s11605-012-1825-6 [DOI] [PubMed] [Google Scholar]
- 59.Lee SH, Kang CM, Hwang HK, et al. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc 2014;28:2848-55. 10.1007/s00464-014-3537-3 [DOI] [PubMed] [Google Scholar]
- 60.Royall NA, Walsh RM. Robotic distal pancreatectomy and splenectomy: rationale and technical considerations. J Vis Surg 2017;3:135. 10.21037/jovs.2017.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker EH, Ross SW, Seshadri R, et al. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot 2016;12:554-60. 10.1002/rcs.1688 [DOI] [PubMed] [Google Scholar]
- 62.Fisher AV, Fernandes-Taylor S, Schumacher JR, et al. Analysis of 90-day cost for open versus minimally invasive distal pancreatectomy. HPB (Oxford) 2019;21:60-6. 10.1016/j.hpb.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 63.Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148:814-23. 10.1016/j.surg.2010.07.027 [DOI] [PubMed] [Google Scholar]
- 64.Anderson KL, Jr, Adam MA, Thomas S, et al. Impact of minimally invasive vs. open distal pancreatectomy on use of adjuvant chemoradiation for pancreatic adenocarcinoma. Am J Surg 2017;213:601-5. 10.1016/j.amjsurg.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 65.Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. 10.1097/SLA.0000000000001869 [DOI] [PubMed] [Google Scholar]
- 66.Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg 2014;18:682-9. 10.1007/s11605-013-2410-3 [DOI] [PubMed] [Google Scholar]
- 67.Wang SE, Shyr BU, Chen SC, et al. Comparison between robotic and open pancreaticoduodenectomy with modified Blumgart pancreaticojejunostomy: A propensity score-matched study. Surgery 2018;164:1162-7. 10.1016/j.surg.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 68.Ielpo B, Caruso R, Duran H, et al. Robotic versus standard open pancreatectomy: a propensity score-matched analysis comparison. Updates Surg 2019;71:137-44. 10.1007/s13304-018-0529-1 [DOI] [PubMed] [Google Scholar]
- 69.Kauffmann EF, Napoli N, Menonna F, et al. A propensity score-matched analysis of robotic versus open pancreatoduodenectomy for pancreatic cancer based on margin status. Surg Endosc 2019;33:234-42. 10.1007/s00464-018-6301-2 [DOI] [PubMed] [Google Scholar]
- 70.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: Personal experience in a large community hospital. Arch Surg 2003;138:777-84. 10.1001/archsurg.138.7.777 [DOI] [PubMed] [Google Scholar]
- 71.Jung JP, Zenati MS, Dhir M, et al. Use of Video Review to Investigate Technical Factors That May Be Associated With Delayed Gastric Emptying After Pancreaticoduodenectomy. JAMA Surg 2018;153:918-27. 10.1001/jamasurg.2018.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen S, Chen JZ, Zhan Q, et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc 2015;29:3698-711. 10.1007/s00464-015-4140-y [DOI] [PubMed] [Google Scholar]
- 73.Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. 10.1002/bjs.9135 [DOI] [PubMed] [Google Scholar]
- 74.Allan BJ, Novak SM, Hogg ME, et al. Robotic vascular resections during Whipple procedure. J Vis Surg 2018;4:13. 10.21037/jovs.2017.12.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Q, Tang W, Zhou R, et al. Robotic distal pancreatectomy: experience in a high-volume center. Ann Pancreat Cancer 2018;1:15 10.21037/apc.2018.03.01 [DOI] [Google Scholar]
- 76.Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 2011;40:1264-70. 10.1097/MPA.0b013e318220e3a4 [DOI] [PubMed] [Google Scholar]
- 77.Zimmerman AM, Roye DG, Charpentier KP. A comparison of outcomes between open, laparoscopic and robotic pancreaticoduodenectomy. HPB (Oxford) 2018;20:364-9. 10.1016/j.hpb.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 78.Kowalsky SJ, Zenati MS, Steve J, et al. A Combination of Robotic Approach and ERAS Pathway Optimizes Outcomes and Cost for Pancreatoduodenectomy. Ann Surg 2019;269:1138-45. 10.1097/SLA.0000000000002707 [DOI] [PubMed] [Google Scholar]
- 79.Napoli N, Kauffmann EF, Menonna F, et al. Robotic versus open pancreatoduodenectomy: a propensity score-matched analysis based on factors predictive of postoperative pancreatic fistula. Surg Endosc 2018;32:1234-47. 10.1007/s00464-017-5798-0 [DOI] [PubMed] [Google Scholar]
- 80.Girgis MD, Zenati MS, Steve J, et al. Robotic approach mitigates perioperative morbidity in obese patients following pancreaticoduodenectomy. HPB (Oxford) 2017;19:93-8. 10.1016/j.hpb.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 81.Napoli N, Kauffmann EF, Palmeri M, et al. The Learning Curve in Robotic Pancreaticoduodenectomy. Dig Surg 2016;33:299-307. 10.1159/000445015 [DOI] [PubMed] [Google Scholar]
- 82.Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. 10.1001/jamasurg.2015.17 [DOI] [PubMed] [Google Scholar]
- 83.Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg 2010;145:634-40. 10.1001/archsurg.2010.118 [DOI] [PubMed] [Google Scholar]
- 84.Walsh RM, Chalikonda S, How I. Do It: Hybrid Laparoscopic and Robotic Pancreaticoduodenectomy. J Gastrointest Surg 2016;20:1650-7. 10.1007/s11605-016-3170-7 [DOI] [PubMed] [Google Scholar]
- 85.Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas 2010;39:160-4. 10.1097/MPA.0b013e3181bd604e [DOI] [PubMed] [Google Scholar]
- 86.Kim H, Kim JR, Han Y, et al. Early experience of laparoscopic and robotic hybrid pancreaticoduodenectomy. Int J Med Robot 2017. doi: . 10.1002/rcs.1814 [DOI] [PubMed] [Google Scholar]
- 87.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. 10.1097/SLA.0b013e3182a4e87c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg 2012;10:475-9. 10.1016/j.ijsu.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 89.Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 2012;26:2397-402. 10.1007/s00464-012-2207-6 [DOI] [PubMed] [Google Scholar]
- 90.Zhou NX, Chen JZ, Liu Q, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot 2011;7:131-7. 10.1002/rcs.380 [DOI] [PubMed] [Google Scholar]
- 91.Fernandes E, Giulianotti PC. Robotic-assisted pancreatic surgery. J Hepatobiliary Pancreat Sci 2013;20:583-9. 10.1007/s00534-013-0615-1 [DOI] [PubMed] [Google Scholar]
- 92.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:1028-61. 10.6004/jnccn.2017.0131 [DOI] [PubMed] [Google Scholar]
- 93.Wang WL, Ye S, Yan S, et al. Pancreaticoduodenectomy with portal vein/superior mesenteric vein resection for patients with pancreatic cancer with venous invasion. Hepatobiliary Pancreat Dis Int 2015;14:429-35. 10.1016/S1499-3872(15)60400-3 [DOI] [PubMed] [Google Scholar]
- 94.Zhang XM, Fan H, Kou JT, et al. Resection of portal and/or superior mesenteric vein and reconstruction by using allogeneic vein for pT3 pancreatic cancer. J Gastroenterol Hepatol 2016;31:1498-503. 10.1111/jgh.13299 [DOI] [PubMed] [Google Scholar]
- 95.Gage MM, Reames BN, Ejaz A, et al. Pancreaticoduodenectomy with en bloc vein resection for locally advanced pancreatic cancer: a case series without venous reconstruction. Chin Clin Oncol 2018;7:7. 10.21037/cco.2018.01.01 [DOI] [PubMed] [Google Scholar]
- 96.Kasumova GG, Conway WC, Tseng JF. The Role of Venous and Arterial Resection in Pancreatic Cancer Surgery. Ann Surg Oncol 2018;25:51-8. 10.1245/s10434-016-5676-3 [DOI] [PubMed] [Google Scholar]
- 97.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic middle pancreatectomy. J Laparoendosc Adv Surg Tech A 2010;20:135-9. 10.1089/lap.2009.0296 [DOI] [PubMed] [Google Scholar]
- 98.Kang CM, Kim DH, Lee WJ, et al. Initial experiences using robot-assisted central pancreatectomy with pancreaticogastrostomy: a potential way to advanced laparoscopic pancreatectomy. Surg Endosc 2011;25:1101-6. 10.1007/s00464-010-1324-3 [DOI] [PubMed] [Google Scholar]
- 99.Kim DH, Kang CM, Lee WJ, et al. Robotic central pancreatectomy with pancreaticogastrostomy (transgastric approach) in a solid pseudopapillary tumor of the pancreas. Hepatogastroenterology 2011;58:1805-8. [DOI] [PubMed] [Google Scholar]
- 100.Cheng K, Shen B, Peng C, et al. Initial experiences in robot-assisted middle pancreatectomy. HPB (Oxford) 2013;15:315-21. 10.1111/j.1477-2574.2012.00605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Addeo P, Langella S, Arru L, et al. Robotic middle pancreatectomy with the double pursestring invaginated pancreaticogastrostomy (with video). J Visc Surg 2016;153:475-6. 10.1016/j.jviscsurg.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 102.Chen S, Zhan Q, Jin JB, et al. Robot-assisted laparoscopic versus open middle pancreatectomy: short-term results of a randomized controlled trial. Surg Endosc 2017;31:962-71. 10.1007/s00464-016-5046-z [DOI] [PubMed] [Google Scholar]
- 103.Sperti C, Beltrame V, Milanetto AC, et al. Parenchyma-sparing pancreatectomies for benign or border-line tumors of the pancreas. World J Gastrointest Oncol 2010;2:272-81. 10.4251/wjgo.v2.i6.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian F, Hong XF, Wu WM, et al. Propensity score-matched analysis of robotic versus open surgical enucleation for small pancreatic neuroendocrine tumours. Br J Surg 2016;103:1358-64. 10.1002/bjs.10220 [DOI] [PubMed] [Google Scholar]
- 105.Jin JB, Qin K, Li H, et al. Robotic enucleation for benign or borderline tumours of the pancreas: a retrospective analysis and comparison from a high-volume centre in Asia. World J Surg 2016;40:3009-20. 10.1007/s00268-016-3655-2 [DOI] [PubMed] [Google Scholar]
- 106.Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. 10.1016/j.jamcollsurg.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 107.Pratt WB, Callery MP, Vollmer CM., Jr Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008;32:419-28. 10.1007/s00268-007-9388-5 [DOI] [PubMed] [Google Scholar]
- 108.Memeo R, Sangiuolo F, de Blasi V, et al. Robotic pancreaticoduodenectomy and distal pancreatectomy: State of the art. J Visc Surg 2016;153:353-9. 10.1016/j.jviscsurg.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 109.Ecker BL, McMillan MT, Maggino L, et al. Pancreatogastrostomy Vs. Pancreatojejunostomy: a Risk-Stratified Analysis of 5316 Pancreatoduodenectomies. J Gastrointest Surg 2018;22:68-76. 10.1007/s11605-017-3547-2 [DOI] [PubMed] [Google Scholar]
- 110.Giulianotti PC, Mangano A, Bustos RE, et al. Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique: Lessons learned since the first worldwide RPD performed in the year 2001. Surg Endosc 2018;32:4329-36. 10.1007/s00464-018-6228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giulianotti PC, Gonzalez-Heredia R, Esposito S, et al. Trans-gastric pancreaticogastrostomy reconstruction after pylorus-preserving robotic Whipple: a proposal for a standardized technique. Surg Endosc 2018;32:2169-74. 10.1007/s00464-017-5916-z [DOI] [PubMed] [Google Scholar]
- 112.Strobel O, Cherrez A, Hinz U, et al. Risk of pancreatic fistula after enucleation of pancreatic tumours. Br J Surg 2015;102:1258-66. 10.1002/bjs.9843 [DOI] [PubMed] [Google Scholar]
- 113.Song KB, Kim SC, Hwang DW, et al. Enucleation for benign or low-grade malignant lesions of the pancreas: Single-center experience with 65 consecutive patients. Surgery 2015;158:1203-10. 10.1016/j.surg.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 114.Misawa T, Imazu H, Fujiwara Y, et al. Efficacy of nasopancreatic stenting prior to laparoscopic enucleation of pancreatic neuroendocrine tumor. Asian J Endosc Surg 2013;6:140-2. 10.1111/ases.12006 [DOI] [PubMed] [Google Scholar]
- 115.Lu WJ, Cai HL, Ye MD, et al. Enucleation of non-invasive tumors in the proximal pancreas: indications and outcomes compared with standard resections. J Zhejiang Univ Sci B 2017;18:906-16. 10.1631/jzus.B1600597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu R, Wang ZZ, Gao YX, et al. Application of End-to-end Anastomosis in Robotic Central Pancreatectomy. J Vis Exp 2018. doi: . 10.3791/57495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milone L, Daskalaki D, Wang X, et al. State of the art of robotic pancreatic surgery. World J Surg 2013;37:2761-70. 10.1007/s00268-013-2275-3 [DOI] [PubMed] [Google Scholar]
- 118.Horiguchi A, Uyama I, Miyakawa S. Robot-assisted laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2011;18:287-91. 10.1007/s00534-010-0325-x [DOI] [PubMed] [Google Scholar]
- 119.Napoli N, Kauffmann EF, Menonna F, et al. Indications, technique, and results of robotic pancreatoduodenectomy. Updates Surg 2016;68:295-305. 10.1007/s13304-016-0387-7 [DOI] [PubMed] [Google Scholar]


