Abstract
BAY 94-9027 is an extended-half-life, recombinant factor VIII (rFVIII) product conjugated with a 60-kDa branched polyethylene glycol (PEG) molecule indicated for use in previously treated patients (aged ≥ 12 years) with hemophilia A. This randomized, open-label, two-way crossover study compared the pharmacokinetics (PK) of BAY 94-9027 and rFVIII Fc fusion protein (rFVIIIFc) in patients with hemophilia A. Patients aged 18–65 years with FVIII < 1% and ≥ 150 exposure days to FVIII were randomized to receive intravenous single-dose BAY 94-9027 60 IU/kg followed by rFVIIIFc 60 IU/kg or vice versa, with ≥ 7-day wash-out between doses. FVIII activity was measured by one-stage assay. PK parameters, including area under the curve from time 0 to the last data point (AUClast, primary parameter), half-life, and clearance were calculated. Eighteen patients were randomized and treated. No adverse events were observed. In the analysis set excluding one outlier, geometric mean (coefficient of variation [%CV, 95% confidence interval {CI}]) AUClast was significantly higher for BAY 94-9027 versus rFVIIIFc (2940 [37.8, 2440–3550] IU h/dL versus 2360 [31.8, 2010–2770] IU h/dL, p = 0.0001). A population PK model was developed to simulate time to reach FVIII threshold levels; median time to 1 IU/dL was approximately 13 h longer for BAY 94-9027 versus rFVIIIFc after a single infusion of 60 IU/kg. In conclusion, BAY 94-9027 had a superior PK profile versus rFVIIIFc. ClinicalTrials.gov: NCT03364998.
Electronic supplementary material
The online version of this article (10.1007/s00277-019-03747-2) contains supplementary material, which is available to authorized users.
Keywords: Pharmacokinetics, Extended half-life, Hemophilia A, PEGylated, Head-to-head study, Population pharmacokinetics
Introduction
Prophylaxis with factor VIII (FVIII) is the standard treatment for patients with severe hemophilia A (FVIII < 1%) [1]. It aims to reduce bleeding frequency and, ultimately, prevent the development of chronic arthropathy [2–4]. However, prophylaxis regimens typically require frequent intravenous infusions, which can lead to suboptimal adherence and breakthrough bleeding [5]. Although the appropriate level of FVIII to prevent bleeding in individual patients varies depending on the individual’s pharmacokinetics (PK), bleeding phenotype, activity level, and other variables [6–8], an increased time with low FVIII levels is considered an important determinant of breakthrough bleeding during prophylaxis [9].
Extended-half-life (EHL) recombinant FVIII (rFVIII) products with improved PK profiles compared with standard-half-life (SHL) products have the potential to maintain FVIII levels above threshold levels for longer periods of time, which may result in better bleed protection and, consequently, less joint damage [10]. PK parameters, including incremental recovery, half-life (t½), area under the curve (AUC), and clearance (CL) are considered important surrogate efficacy endpoints for new FVIII products [11, 12]. EHL rFVIII products should have a minimum t½ extension ratio of 1.3 to provide a reduction in dosing frequency from three times per week to two times per week compared with SHL rFVIII products while maintaining the same minimum FVIII threshold level [13]. Such prophylaxis regimens that allow for less frequent infusions may also improve adherence [14].
BAY 94-9027 (Jivi®, Bayer AG, Germany) is a B-domain-deleted rFVIII product that has been site-specifically PEGylated with a single 60-kDa (dual-branched) polyethylene glycol (PEG) molecule to improve its PK [15]. In previously treated adults with severe hemophilia A, BAY 94-9027 demonstrated a longer t½ and greater dose-normalized area under the curve from time 0 to infinity (AUCnorm) compared with sucrose-formulated rFVIII (Online Resource: Supplementary Table 1) [16, 17]. Subsequently, in the PROTECT VIII study and its extension, BAY 94-9027 was efficacious in the prevention of bleeds in previously treated adults and adolescents [18, 19]. These positive results led to the approval of BAY 94-9027 by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA) and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan for use in previously treated adults and adolescents (aged ≥ 12 years) with hemophilia A at dosing intervals of up to every 5 days (FDA) and every 7 days (EMA and PMDA) [20–22]. Population PK (popPK) evaluation of FVIII activity–time profiles following BAY 94-9027 dosing have shown that the PK of BAY 94-9027 is adequately described by a one-compartment model with linear elimination [23].
Recombinant FVIII Fc fusion protein (rFVIIIFc; Elocta®/Eloctate®; Biogen, Cambridge, MA, USA) is another EHL rFVIII product approved for routine prophylaxis for all age groups with dosing intervals of up to every 5 days [24]. In the A-LONG study, rFVIIIFc demonstrated a longer t½ and AUCnorm compared with conventional rFVIII (Advate®; Baxter, Deerfield, IL, USA) in previously treated patients aged ≥ 12 years with severe hemophilia A (Online Resource: Supplementary Table 1) [25]. The safety and efficacy of recombinant FVIIIFc has also been demonstrated for the prevention and treatment of bleeding episodes in studies of patients with severe hemophilia A [25, 26]. A two-compartment model with linear elimination has been reported to adequately describe the popPK of rFVIIIFc [27].
To date, no head-to-head comparison of the PK of EHL rFVIII products in patients with hemophilia A has been performed. The objective of the current study was to directly compare the PK profiles of BAY 94-9027 and rFVIIIFc. Concentration data collected using the one-stage assay were used to develop a popPK model for BAY 94-9027 and rFVIIIFc to simulate time to reach FVIII threshold levels.
Methods
Study design
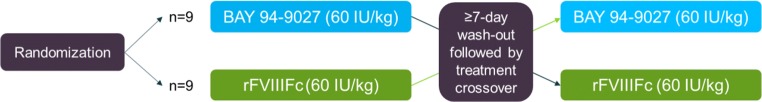
This was a single-center, randomized, open-label, single-dose, two-way crossover study (ClinicalTrials.gov identifier: NCT03364998) (Fig. 1). The primary objective was to compare the PK of BAY 94-9027 and rFVIIIFc. After a wash-out period (specified as ≥ 3 days or ≥ 5 days for SHL or EHL FVIII products, respectively), patients were randomized 1:1 to receive a single infusion of 60 IU/kg BAY 94-9027 or 60 IU/kg rFVIIIFc, followed by crossover to a single infusion of the other treatment, with ≥ 7-day wash-out between doses. The maximum wash-out time between treatments was 28 days. Both products were administered as 10-min intravenous infusions.
Fig. 1.
Study design
Vial strength was not determined in this study. One batch was used for each study drug. Study drug doses were based on the nominal value on the label of the vial. The exact volume needed for the administration of 60 IU/kg was calculated by multiplying the weight of the patient by 60. This total amount (IU) was withdrawn in a single pooling syringe using the required number of vials. The excess vial content was discarded to ensure that all subjects received a 60 IU/kg dose.
The study was approved by the institutional review board at the single site and was carried out in compliance with the protocol, the principles of the Declaration of Helsinki, and Good Clinical Practice guidelines. All patients gave written informed consent before initiation of any study-related procedures.
Patients
Eligible patients were men aged 18–65 years with severe hemophilia A (FVIII <1 IU/dL) previously treated with any FVIII product for ≥ 150 exposure days (EDs). Patients also had to have a body mass index of 18–29.9 kg/m2 and have been able to stop FVIII treatment to complete the wash-out period before study entry and between treatments. Key exclusion criteria included the presence or history of an FVIII inhibitor (≥ 0.6 Bethesda units/mL), diagnosis of any bleeding disorder other than hemophilia A, platelet count < 75,000/mm3, HIV positive with a CD4 count of < 200/mm3, creatinine > 2 times the upper limit of normal (ULN) or alanine aminotransferase or aspartate aminotransferase > 5 times the ULN.
PK assessments
Plasma samples were collected pre-dose and 0.25, 0.5, 1, 3, 6, 8, 24, 48, 72, 96, and 120 h after infusion of each drug. FVIII coagulant activity (FVIII:C) was measured using the same one-stage clotting assay as follows. Plasma concentrations of BAY 94-9027 and rFVIIIFc were determined by a turbidimetric assay with the SynthaSil reagent and activated partial thromboplastin time (APTT) measured on the ACL Advance System against a calibration curve of standard human plasma. The calibration range of the procedure for both BAY 94-9027 and rFVIIIFc was 1 IU/dL (lower limit of quantitation [LLOQ]) to 80 IU/dL (upper limit of quantitation [ULOQ]). Samples above the calibration range were diluted with FVIII-deficient plasma from human donors with congenital FVIII deficiency.
The following PK parameters were assessed using non-compartmental analysis (NCA) (WinNonlin® software, version 5.3; Pharsight, Mountain View, CA, USA): AUC from time 0 to the last data point (AUClast; primary parameter); AUC; maximum concentration (Cmax); t½; CL; mean residence time (MRT); volume of distribution at steady state (Vss); and incremental recovery.
Population PK model
To evaluate differences in the PK of both EHL products in the specific study population, a single integrated PopPK model for BAY 94-9027 and rFVIIIFc was developed with product as the covariate. The analysis was conducted using the nonlinear mixed-effect modeling approach, as implemented in NONMEM® (version 7.4.1; ICON, Hanover, MD, USA). As a starting point, a structural model for each product was selected based on standard diagnostic tools, such as raw-data inspection, goodness of fit, and precision of parameter estimates. Potential candidates as suggested by previous analysis were one- or two-compartment models parameterized in terms of CL, central volume (Vc) and, for the two-compartment model, peripheral volume (Vp) and intercompartmental clearance (Q), with covariate effects of von Willebrand factor (VWF) and lean body weight (LBW) on CL and LBW on Vc. Residual (unexplained) variability was described using a combined (proportional and additive) error model. Data below the LLOQ were accounted for using the M3 method [28]. In the next step, an integrated model was developed by combining the two structural models and subsequently refining the model by testing whether BAY 94-9027 and rFVIIIFc have statistically significant differences in PK parameters (e.g., CL) using the likelihood ratio test (LRT) and a p value of 0.01. Because of the small study size, no additional covariate search was conducted. Additional model refinement consisted of an iterative outlier removal procedure and optimization of the inter-individual variability components of the model. The model was qualified using standard model diagnostic tools, such as uncertainty in parameter estimates, plausibility of estimates (comparison with published information), goodness-of-fit plots, and visual predictive checks.
The popPK model was used to determine individual PK estimates and simulate the time to reach FVIII threshold levels of 1, 3, 5, and 10 IU/dL after a single dose of 60 IU/kg BAY 94-9027 or rFVIIIFc for the study population.
Safety
Safety was assessed by means of clinical and laboratory evaluation at study visits and the recording of adverse events.
Statistical analysis
For statistical analysis of the PK parameters obtained by NCA, a log-normal distribution of the parameters was assumed [29]. Log-transformed parameters were analyzed using analysis of variance (ANOVA), including sequence, patient (sequence), period, and treatment effects. Based on these analyses, point estimates (least square means) and confidence intervals (CIs, 90% and 95%) for the BAY 94-9027:rFVIIIFc ratio were calculated by retransformation of the logarithmic data using intra-individual SD of the ANOVA. The lower limit of the 90% CI for the ratio exceeding 0.8 would indicate that BAY 94–9027 is non-inferior to rFVIIIFc; the lower limit of the 95% CI for the ratio exceeding 1.0 would indicate that BAY 94-9027 is superior to rFVIIIFc. Safety analyses were descriptive.
Results
A total of 18 patients were randomized and received single doses of BAY 94-9027 and rFVIIIFc; the demographics and baseline characteristics of the patients are provided in Table 1. The mean age of patients was 36.0 years, all were white, and none had previously received EHL products.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Analysis set A (N = 18) | Analysis set B (N = 17) |
|---|---|---|
| Age, years | ||
| Median (range) | 34 (22–65) | 34 (22–65) |
| Mean (SD) | 36.0 (11.7) | 36.1 (12.1) |
| Race, n (%) | ||
| White | 18 (100) | 17 (100) |
| BMI, kg/m2 | ||
| Median (range) | 25.5 (18.6–29.7) | 25.0 (18.6–29.7) |
| Mean (SD) | 24.8 (3.7) | 24.7 (3.8) |
BMI, body mass index; SD, standard deviation
Using data from all 18 patients (analysis set A), the geometric mean (%CV) for AUClast was 2660 (60.6) IU h/dL for BAY 94-9027 and 2410 (32.1) IU h/dL for rFVIIIFc. The least square mean (90% CIs) for the BAY 94-9027:rFVIIIFc ratio was 1.10 (0.88–1.39), meeting the prespecified criteria for non-inferiority of BAY 94-9027 versus rFVIIIFc; superiority criteria were not met (95% CI 0.84–1.46; p = 0.46). Fifteen patients had a least square mean BAY 94-9027:rFVIIIFc ratio of > 1.0.
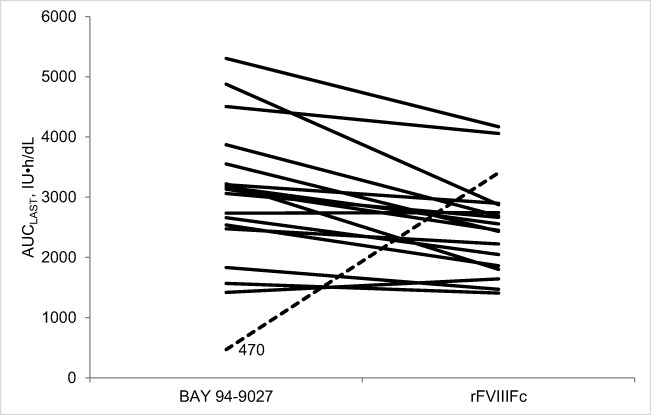
Examination of the individual patient AUClast values after a single infusion of 60 IU/kg BAY 94-9027 or 60 IU/kg rFVIIIFc (Fig. 2), however, showed that one 34-year-old patient had an AUClast of 470 IU h/dL for BAY 94-9027, considerably lower than the geometric mean of 2660 IU h/dL for BAY 94-9027 for all patients. This patient was the only one in the study to have pre-existing anti-PEG IgM (low titer 1:8) prior to administration of BAY 94-9027. For these reasons, this patient was determined to be an outlier and was therefore excluded from further analyses of the PK results (performed on the remaining 17 patients [analysis set B]).
Fig. 2.
Individual patient AUClast values after a single infusion of 60 IU/kg BAY 94-9027 or 60 IU/kg rFVIIIFc (N = 18). One patient (dashed line) had an AUClast of 470 IU h/dL for BAY 94-9027, considerably lower than the geometric mean of 2660 IU h/dL for BAY 94-9027 for all patients
Using analysis set B, the geometric mean (%CV, 95% CI) for AUClast was significantly higher for BAY 94-9027 (2940 [37.8, 2440–3550] IU h/dL) versus rFVIIIFc (2360 [31.8, 2010–2770] IU h/dL, p = 0.0001, Table 2). Similar results were obtained for AUC (Table 2). CL was significantly reduced for BAY 94-9027 versus rFVIIIFc (0.0200 [38.3, 0.0165–0.0241] dL/h/kg versus 0.0250 [32.2, 0.0213–0.0294] dL/h/kg, p = 0.0001, Table 2). The geometric mean [%CV, 95% CI] t½ was significantly longer for BAY 94-9027 versus rFVIIIFc (16.3 [34.1, 13.7–19.3] versus 15.2 [33.1, 12.9–17.9] h, p < 0.05, Table 2). Additional PK parameters are shown in Table 2.
Table 2.
PK parameters following single-dose administrations of BAY 94-9027 and rFVIIIFc (analysis set B, excluding outlier; N = 17)
| Parameter | Geometric mean (%CV) (95% CI) | Geometric least square mean ratioa (95% CI) | p value | |
|---|---|---|---|---|
| BAY 94-9027 | rFVIIIFc | |||
| AUC (IU h/dL) |
3010 (38.3) (2490–3640) |
2400 (32.2) (2040–2820) |
1.26 (1.14–1.38) |
0.0001 |
| AUClast (IU h/dL) |
2940 (37.8) (2440–3550) |
2360 (31.8) (2010–2770) |
1.25 (1.14–1.37) |
0.0001 |
| CL (dL/h/kg) |
0.0200 (38.3) (0.0165–0.0241) |
0.0250 (32.2) (0.0213–0.0294) |
0.80 (0.72–0.87) |
0.0001 |
| Cmax (IU/dL) |
150 (26.0) (131–171) |
194 (64.1) (143–262) |
0.76 (0.60–0.97) |
< 0.05 |
| MRTIV (h) |
23.2 (35.3) (19.4–27.6) |
19.9 (38.4) (16.4–24.1) |
1.17 (1.08–1.26) |
< 0.001 |
| t½ (h) |
16.3 (34.1) (13.7–19.3) |
15.2 (33.1) (12.9–17.9) |
1.07 (1.00–1.15) |
< 0.05 |
| VSS (dL/kg) |
0.462 (15.2) (0.428–0.500) |
0.497 (22.5) (0.444–0.558) |
0.93 (0.86–1.00) |
0.06 |
| Incremental recovery (kg/dL) |
2.26 (16.5) (2.08–2.46) |
3.09 (66.0) (2.27–4.20) |
0.72 (0.55–0.94) |
< 0.05 |
aRatio of BAY 94-9027:rFVIIIFc
AUC, area under the curve from time 0 to infinity; AUClast, AUC from time 0 to the last data point; CL, clearance; Cmax, maximum concentration; MRTIV, mean residence time after intravenous injection; t½, half-life; Vss, volume of distribution at steady state
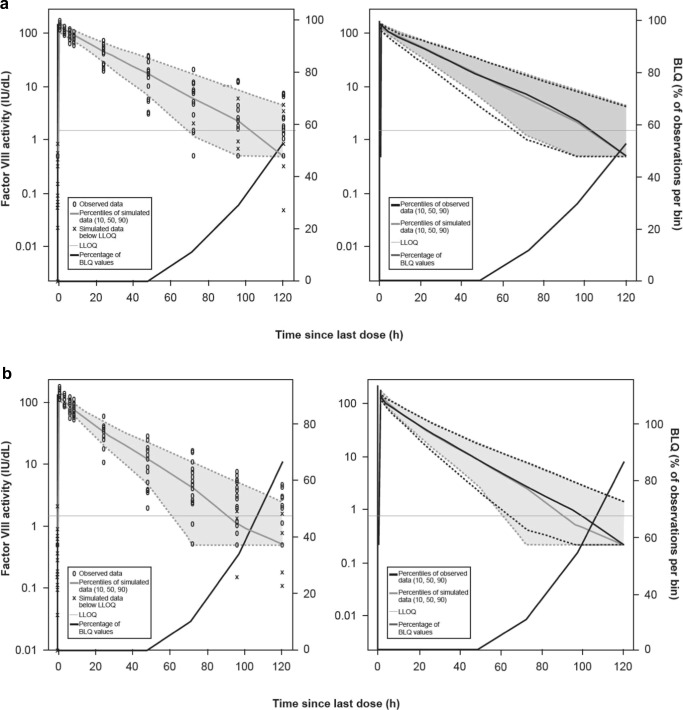
The PK profile for BAY 94-9027 for the outlier patient was excluded from the development of the popPK model. No peripheral distribution compartment could be identified for BAY 94-9027 (relative standard error [RSE] of Q > 180%) and PK of BAY 94-9027 was described by a one-compartment model (technically, the PK of BAY 94-9027 was described by a two-compartment model fixing Q to a very small value [0.001]), while a two-compartment model was used for rFVIIIFc. Further, to minimize the potential bias introduced by implausible values (e.g., due to uncertainty of the assay or deviations in the sampling timepoint), single data points (ten measurements for BAY 94-9027 and 16 measurements for rFVIIIFc) were determined to be outliers and removed during model development. These single data points had a conditional weighted residual value (CWRES) of < −2.5 or > 2.5 (obtained using individual Bayesian post hoc parameter estimates) corresponding to a probability of occurrence under the respective model of < 1%. During this process, the estimate of the residual error was nearly halved to 29.7 %CV; this indicated that these data points were influential outliers and should be removed from the analysis. Compared with rFVIIIFc, the CL of BAY 94-9027 was significantly reduced by approximately 20% (95% CI, − 14.2 to − 26.9%). While all patients (excluding the outlier) had a lower CL for BAY 94-9027 compared with rFVIIIFc, the magnitude varied considerably between the subjects (%CV, 46%). The parameter estimates of the popPK model are shown in Table 3. Visual predictive checks showed good agreement between the popPK model and the observed data in that a statistically significant difference in CL could be detected between treatments (Fig. 3). The model parameters and results are consistent with previous popPK analyses [23, 27].
Table 3.
Parameter estimates of the popPK model
| Parameter | Value | RSE (%) | 5% CI | 95% CI |
|---|---|---|---|---|
| CL (dL/h) | 1.57 | 10.5 | 1.25 | 1.89 |
| Vc of distribution (dL) | 28.3 | 3.59 | 26.3 | 30.3 |
| Q (dL/h)a | 0.69 | 20.2 | 0.42 | 0.96 |
| Vp of distribution (dL)a | 6.02 | 14.5 | 4.31 | 7.72 |
| Effect of LBW on CL | 1.03 | 32.9 | 0.364 | 1.69 |
| Effect of LBW on Vc of distribution | 1.10 | 15.5 | 0.765 | 1.43 |
| Relative reduction of CL for BAY 94-9027 compared with rFVIIIFcb | − 0.21 | 14.0 | − 0.26 | − 0.15 |
| Inter-individual variability in CL (variance [%CV]) | 0.11 (33.3) | 28.5 | 0.05 | 0.16 |
| Inter-individual variability in Vc of distribution (variance [%CV]) | 0.01 (11.1) | 34.2 | 0.004 | 0.02 |
| Inter-individual variability in change in CL for BAY 94-9027 compared with rFVIIIFc (variance [%CV]) | 0.20 (46.4) | 51.4 | − 0.002 | 0.39 |
| Residual error, additive component (variance) | 0.296 | 18.2 | 0.190 | 0.40 |
| Residual error, proportional component (variance [%CV]) | 0.09 (29.7) | 6.63 | 0.08 | 0.10 |
aOnly applies for rFVIIIFc
bCL (BAY 94-9027) = CL (rFVIIIFc) × (1+ relative reduction in CL)
CL, clearance; %CV, coefficient of variation; LBW, low body weight; Q, intercompartmental CL; RSE, relative standard error; Vc, central volume; Vp, peripheral volume
Fig. 3.
Visual predictive checks on FVIII level–time profiles in the integrated popPK model for BAY 94-9027 (a) and rFVIIIFc (b) BLQ, below the limit of quantification; LLOQ, lower limit of quantitation
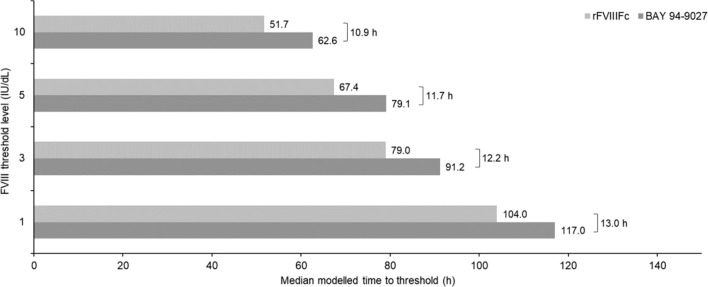
The popPK model was used to derive individual PK estimates and simulate time to reach FVIII threshold levels of 1, 3, 5, and 10 IU/dL after a single infusion of 60 IU/kg BAY 94-9027 or 60 IU/kg rFVIIIFc. For analysis set B (N = 17), median time to an FVIII level of 1 IU/dL was 13 h longer for BAY 94-9027 versus rFVIIIFc (approximately 12.5%). Times to reach 3, 5, and 10 IU/dL thresholds were 12.5, 11.7, and 10.9 h longer, respectively, for BAY 94-9027 versus rFVIIIFc (Fig. 4).
Fig. 4.
Modeled median time to FVIII threshold level after a single infusion of 60 IU/kg BAY 94-9027 or 60 IU/kg rFVIIIFc (analysis set B, excluding outlier; N = 17)
No adverse events were reported during the study.
Discussion
This is the first randomized head-to-head study performed to directly compare the PK of BAY 94-9027 and rFVIIIFc following a single 60 IU/kg infusion in patients with hemophilia A. The results demonstrated that BAY 94-9027 has improved PK parameters compared with rFVIIIFc; the mean AUClast was 25% higher and CL was 20% lower for BAY 94-9027 compared with rFVIIIFc.
The main strength of our study was the crossover design. Both products have previously been shown to have improved PK versus SHL rFVIII products [16, 17, 25]. The reported half-lives of the products based on registrational studies are 17.4 h for BAY 94-9027 [21] and 19.0 h for rFVIIIFc [30]. Supplementary Table 1 also describes PK parameters for these products based on published data. However, indirect comparisons of PK data from registrational studies do not allow for an accurate assessment of how the products compare owing to variation in the type of assay and calibration standard used and the characteristics of the patient populations. For example, one factor that influences PK is FVIII CL, which is highly inversely correlated with VWF levels in individual patients [31]. These issues reinforce the importance of our comparative crossover methodology, in which PK parameters were evaluated using the same assay in the same population of patients, allowing for direct comparison of the two products.
The clinical implication of our study is related to the concept that EHL rFVIII products can be used to extend the dosing interval [32] or provide higher FVIII levels for longer periods [33]. In this context, simulations using the popPK model showed that median time to a threshold level of 1 IU/dL FVIII was 13 h longer for BAY 94-9027 versus rFVIIIFc after a single infusion of 60 IU/kg. This increase in the time above threshold may thereby provide improved bleeding protection [9, 12]. However, only prospective studies can precisely assess the effects of improved PK on bleeding and individualized PK-based prophylaxis with BAY 94-9027.
One patient exhibited a lower AUClast value for BAY 94-9027 than the other patients and was the only one found to have pre-existing anti-PEG IgM; he was therefore determined to be an outlier and was excluded from subsequent PK analyses. Pre-existing anti-PEG and anti-drug IgM have also been reported with BAX 855 and N8-GP, two other PEGylated FVIII products, and non-PEG therapeutics, such as biologic tumor necrosis factor (TNF) inhibitors [34–38]. Increased clearance, resulting in a reduced AUClast, of a drug secondary to pre-existing anti-PEG antibodies has been reported with other PEGylated therapeutics (e.g., PEG-asparaginase) [39].
Our study has some potential limitations. First, as a single chromogenic (two-stage) assay that could accurately measure FVIII activity of both BAY 94-9027 and FVIIIFc could not be identified, the same one-stage assay was used to assess FVIII activity for both products. The one-stage assay has been shown to give consistent results between PEGylated and non-PEGylated rFVIII [40], and it was found to accurately measure both products in the current study, with values within 20% for both products when analyzed against a plasma standard. However, the chromogenic assay measured values 40–60% higher than expected for rFVIIIFc and could not be validated. Therefore, the chromogenic assay was not used in the study. Second, NCA methods were used to compare the PK parameters, thereby providing a comparison that was unaffected by assumptions regarding the distribution of FVIII [41]. The popPK-model-based analysis, however, showed that a one-compartment model adequately described BAY 94-9027 but not rFVIIIFc, which was taken into account when simulating individual time-to-threshold values. Last, only patients aged 18–65 years were enrolled in this study. However, no major differences in the PK characteristics of BAY 94-9027 have been seen between adults and adolescents [17]. By contrast, the t½ of rFVIIIFc is decreased in adolescents aged 12–17 years compared with adults (aged ≥ 18 years) [42]. Taken together, these data suggest that the improved PK characteristics of BAY 94-9027 versus rFVIIIFc observed in adults in this study are likely to be seen also in adolescents.
In conclusion, BAY 94-9027 had an extended t½, a higher AUC (based on direct measurement), and longer median time to > 1 IU/dL FVIII (based on popPK modeling) compared with rFVIIIFc following a single infusion in patients with severe hemophilia A. Real-world data may provide an insight into whether these PK advantages provide additional bleeding protection.
Electronic supplementary material
(DOCX 61 kb)
Acknowledgments
The authors thank the following personnel from Bayer: Snejana Krassova (medical affairs), Lisa Michaels (clinical development), Yvonne Katterle (bioanalytics), Jean Allen, Hristo Hristov and Gani Ivanov (study management), Camila Linardi (clinical pharmacology), and Joel Krasnow (pharmacovigilance). The authors also thank Peter Vis from LAP&P Consultants BV (Leiden, the Netherlands) for assistance with the popPK analysis.
Author contributions
Anita Shah provided substantial contribution to the study design, implementation, and data analysis. Alexander Solms also provided substantial contribution to the data analysis. Maurice Ahsman supported the popPK and modeling simulation. The statistical analyses were performed by Sara Weigmann. Erik Berntorp, Andreas Tiede, Alfonso Iorio and Maria Elisa Mancuso provided considerable input into the data interpretation. Toshko Lissitchkov and his colleague Tihomir Zhivkov were the lead and sub-investigators, respectively. All authors provided input into drafting and revision of this manuscript, and all agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was funded by Bayer AG, Leverkusen, Germany. Medical writing assistance was provided by Sorcha Wahlkvist of Sudler Medical Communications, fully funded by Bayer.
Data availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal.
Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was provided by the patients, and the protocol was approved by the single site’s independent Ethics Committee/Institutional Review Board.
Conflict of interest
Anita Shah, Alexander Solms, and Sara Wiegmann are employees of Bayer. Maurice Ahsman is an employee of LAP&P Consultants BV, working for Bayer. Anita Shah and Alexander Solms are also shareholders of Bayer. Erik Berntorp is a consultant for Bayer, LFB, Octapharma, Roche, and Shire; is a Speaker Bureau member for Bayer; and has received research support from Bayer, CSL Behring, Shire, and Sobi/Bioverativ. Andreas Tiede has received research grants and personal fees for lectures and consultancy from Alnylam, Bayer, Biogen Idec, Biotest, Boehringer Ingelheim, Chugai, CSL Behring, Daiichi Sankyo, Leo Pharma, Novo Nordisk, Octapharma, Pfizer, Portola, Roche, Shire, and Sobi. Alfonso Iorio’s institution has received grants/research support from Bayer, CSL, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, and Shire. Maria Elisa Mancuso is a consultant for Bayer, CSL Behring, Novo Nordisk, Roche, Pfizer, Baxalta/Shire, Kedrion, and Catalyst, and a Speaker Bureau member for Bayer, CSL Behring, Novo Nordisk, Roche, Baxalta/Shire, Biotest, and Octapharma. Toshko Lissitchkov is a shareholder of Bayer, Sobi, Octapharma, Roche, Sanofi, and Shire; has received grants/research support from Bayer, Octapharma, and Sanofi; is a consultant for Bayer, Sobi, Roche, and Shire; is a Speaker Bureau member for Roche and Shire; and has received from the company financial support as a principal investigator of clinical trials. Tihomir Zhivkov has nothing to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, Ludlam CA, Mahlangu JN, Mulder K, Poon MC, Street A, Treatment Guidelines Working Group on Behalf of The World Federation Of H Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 2.Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM, Group ES A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT study) J Thromb Haemost. 2011;9(4):700–710. doi: 10.1111/j.1538-7836.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 3.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco-Johnson ML, Funk S, Jacobson L, Valentino LA, Hoots WK, Buchanan GR, DiMichele D, Recht M, Brown D, Leissinger C, Bleak S, Cohen A, Mathew P, Matsunaga A, Medeiros D, Nugent D, Thomas GA, Thompson AA, McRedmond K, Soucie JM, Austin H, Evatt BL. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 4.Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK (2011) Clotting factor concentrates given to prevent bleeding and bleeding-related complications in people with hemophilia A or B. Cochrane Database Syst Rev (9):CD003429. 10.1002/14651858.CD003429.pub4 [DOI] [PubMed]
- 5.Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686. doi: 10.2147/PPA.S139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlova A, Oldenburg J. Defining severity of hemophilia: more than factor levels. Semin Thromb Hemost. 2013;39(7):702–710. doi: 10.1055/s-0033-1354426. [DOI] [PubMed] [Google Scholar]
- 7.Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia. 2014;20(5):607–615. doi: 10.1111/hae.12438. [DOI] [PubMed] [Google Scholar]
- 8.Iorio A, Iserman E, Blanchette V, Dolan G, Escuriola Ettingshausen C, Hermans C, Negrier C, Oldenburg J, Reininger A, Rodriguez-Merchan C, Spannagl M, Valentino LA, Young G, Steinitz-Trost KN, Gringeri A. Target plasma factor levels for personalized treatment in haemophilia: a Delphi consensus statement. Haemophilia. 2017;23(3):e170–e179. doi: 10.1111/hae.13215. [DOI] [PubMed] [Google Scholar]
- 9.Collins PW, Blanchette VS, Fischer K, Bjorkman S, Oh M, Fritsch S, Schroth P, Spotts G, Astermark J, Ewenstein B, r AHFPFMSG Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7(3):413–420. doi: 10.1111/j.1538-7836.2008.03270.x. [DOI] [PubMed] [Google Scholar]
- 10.Mahlangu J, Young G, Hermans C, Blanchette V, Berntorp E, Santagostino E. Defining extended half-life rFVIII-A critical review of the evidence. Haemophilia. 2018;24(3):348–358. doi: 10.1111/hae.13438. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency (2016) Guideline on the clinical investigation of recombinant and human plasma-derived factor VIII products. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500201773.pdf. Accessed 07 Jan 2019
- 12.Valentino LA, Pipe SW, Collins PW, Blanchette VS, Berntorp E, Fischer K, Ewenstein BM, Oh M, Spotts G. Association of peak factor VIII levels and area under the curve with bleeding in patients with haemophilia A on every third day pharmacokinetic-guided prophylaxis. Haemophilia. 2016;22(4):514–520. doi: 10.1111/hae.12905. [DOI] [PubMed] [Google Scholar]
- 13.Hermans C, Mahlangu J, Booth J, Schutz H, Santagostino E, Young G, Lee HY, Steinitz-Trost KN, Blanchette V, Berntorp E. Pharmacokinetic modelling and validation of the half-life extension needed to reduce the burden of infusions compared with standard factor VIII. Haemophilia. 2018;24(3):376–384. doi: 10.1111/hae.13483. [DOI] [PubMed] [Google Scholar]
- 14.De Moerloose P, Urbancik W, Van Den Berg HM, Richards M. A survey of adherence to haemophilia therapy in six European countries: results and recommendations. Haemophilia. 2008;14(5):931–938. doi: 10.1111/j.1365-2516.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 15.Mei B, Pan C, Jiang H, Tjandra H, Strauss J, Chen Y, Liu T, Zhang X, Severs J, Newgren J, Chen J, Gu JM, Subramanyam B, Fournel MA, Pierce GF, Murphy JE. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116(2):270–279. doi: 10.1182/blood-2009-11-254755. [DOI] [PubMed] [Google Scholar]
- 16.Coyle TE, Reding MT, Lin JC, Michaels LA, Shah A, Powell J. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost. 2014;12(4):488–496. doi: 10.1111/jth.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah A, Coyle T, Lalezari S, Fischer K, Kohlstaedde B, Delesen H, Radke S, Michaels LA. BAY 94-9027, a PEGylated recombinant factor VIII, exhibits a prolonged half-life and higher area under the curve in patients with severe haemophilia A: comprehensive pharmacokinetic assessment from clinical studies. Haemophilia. 2018;24(5):733–740. doi: 10.1111/hae.13561. [DOI] [PubMed] [Google Scholar]
- 18.Reding MT, Ng HJ, Poulsen LH, Eyster ME, Pabinger I, Shin HJ, Walsch R, Lederman M, Wang M, Hardtke M, Michaels LA. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J Thromb Haemost. 2017;15(3):411–419. doi: 10.1111/jth.13597. [DOI] [PubMed] [Google Scholar]
- 19.Reding MT, Ng HJ, Tseneklidou-Stoefer D, Linardi C, Laelzari S (2018) Safety of long-term prophylaxis with BAY 94-9027: interim results of >5 years of treatment in the PROTECT VIII extension trial. Haemophilia 24(S5):W-P-001 (404)
- 20.US Food and Drug Administration (2018) Jivi® Antihemophilic factor (recombinant) PEGylated-aucl prescribing information. https://www.fda.gov/downloads/BiologicsBloodVaccines/UCM618979.pdf. Accessed Jan 2019
- 21.European Medicines Agency (2018) Jivi SmPC. https://www.ema.europa.eu/documents/product-information/jivi-epar-product-information_en.pdf. Accessed Jan 2018
- 22.Pharmaceuticals and Medical Devices Agency (2018) New drugs approved in September 2018. https://www.pmda.go.jp/files/000227117.pdf. Accessed January 2019
- 23.Ahsman M, Vis P, Shah A, Garmann D, Solms A (2018) Predictable and reliable individualized pharmacokinetic profiling with BAY 94-9027: integrated population pharmacokinetics analysis. Haemophilia 24(S5):T-P-092 (372)
- 24.Swedish Orphan Biovitrum AB (2016) Elocta® (recombinant human coagulation factor VIII, Fc fusion protein). Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003964/WC500198642.pdf. Accessed 07 Jan 2019
- 25.Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, Hanabusa H, Gupta N, Kulkarni R, Fogarty P, Perry D, Shapiro A, Pasi KJ, Apte S, Nestorov I, Jiang H, Li S, Neelakantan S, Cristiano LM, Goyal J, Sommer JM, Dumont JA, Dodd N, Nugent K, Vigliani G, Luk A, Brennan A, Pierce GF, Investigators AL. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325. doi: 10.1182/blood-2013-10-529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young G, Mahlangu J, Kulkarni R, Nolan B, Liesner R, Pasi J, Barnes C, Neelakantan S, Gambino G, Cristiano LM, Pierce GF, Allen G. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967–977. doi: 10.1111/jth.12911. [DOI] [PubMed] [Google Scholar]
- 27.Nestorov I, Neelakantan S, Ludden TM, Li S, Jiang H, Rogge M. Population pharmacokinetics of recombinant factor VIII Fc fusion protein. Clin Pharmacol Drug Dev. 2015;4(3):163–174. doi: 10.1002/cpdd.167. [DOI] [PubMed] [Google Scholar]
- 28.Garmann D, McLeay S, Shah A, Vis P, Maas Enriquez M, Ploeger BA. Population pharmacokinetic characterization of BAY 81-8973, a full-length recombinant factor VIII: lessons learned - importance of including samples with factor VIII levels below the quantitation limit. Haemophilia. 2017;23(4):528–537. doi: 10.1111/hae.13192. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency (2010) Guideline on the Investigation of Bioequivalence https://www.ema.europa.eu/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf. Accessed Jan 2019 [DOI] [PubMed]
- 30.European Medicines Agency (2015) Eloctate SmPC
- 31.Lalezari S, Martinowitz U, Windyga J, Enriquez MM, Delesen H, Schwartz L, Scharrer I. Correlation between endogenous VWF:Ag and PK parameters and bleeding frequency in severe haemophilia A subjects during three-times-weekly prophylaxis with rFVIII-FS. Haemophilia. 2014;20(1):e15–e22. doi: 10.1111/hae.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimenez-Yuste V, Auerswald G, Benson G, Lambert T, Morfini M, Remor E, Salek SZ. Achieving and maintaining an optimal trough level for prophylaxis in haemophilia: the past, the present and the future. Blood Transfus. 2014;12(3):314–319. doi: 10.2450/2014.0298-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dargaud Y, Delavenne X, Hart DP, Meunier S, Mismetti P. Individualized PK-based prophylaxis in severe haemophilia. Haemophilia. 2018;24(Suppl 2):3–17. doi: 10.1111/hae.13397. [DOI] [PubMed] [Google Scholar]
- 34.Felis-Giemza A, Moots RJ. Measurement of anti-drug antibodies to biologic drugs. Rheumatology (Oxford) 2015;54(11):1941–1943. doi: 10.1093/rheumatology/kev279. [DOI] [PubMed] [Google Scholar]
- 35.Konkle BA, Stasyshyn O, Chowdary P, Bevan DH, Mant T, Shima M, Engl W, Dyck-Jones J, Fuerlinger M, Patrone L, Ewenstein B, Abbuehl B. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–1085. doi: 10.1182/blood-2015-03-630897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai WC, Al-Maini MH, Pavelka K, Mahgoub E, Kotak S, Korth-Bradley J, Pedersen R, Mele L, Shen Q, Vlahos B. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. PLoS One. 2017;12(4):e0175207. doi: 10.1371/journal.pone.0175207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullins ES, Stasyshyn O, Alvarez-Roman MT, Osman D, Liesner R, Engl W, Sharkhawy M, Abbuehl BE. Extended half-life pegylated, full-length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemophilia. 2017;23(2):238–246. doi: 10.1111/hae.13119. [DOI] [PubMed] [Google Scholar]
- 38.Meunier S, Alamelu J, Ehrenforth S, Hanabusa H, Abdul Karim F, Kavakli K, Khodaie M, Staber J, Stasyshyn O, Yee DL, Rageliene L. Safety and efficacy of a glycoPEGylated rFVIII (turoctocog alpha pegol, N8-GP) in paediatric patients with severe haemophilia A. Thromb Haemost. 2017;117(9):1705–1713. doi: 10.1160/TH17-03-0166. [DOI] [PubMed] [Google Scholar]
- 39.Rau RE, Dreyer Z, Choi MR, Liang W, Skowronski R, Allamneni KP, Devidas M, Raetz EA, Adamson PC, Blaney SM, Loh ML, Hunger SP. Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: a report from the children’s oncology group. Pediatr Blood Cancer. 2018;65(3):e26873. doi: 10.1002/pbc.26873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Church N, Leong L, Katterle Y, Ulbrich HF, Noerenberg I, Kitchen S, Michaels LA. Factor VIII activity of BAY 94-9027 is accurately measured with most commonly used assays: results from an international laboratory study. Haemophilia. 2018;24(5):823–832. doi: 10.1111/hae.13564. [DOI] [PubMed] [Google Scholar]
- 41.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. doi: 10.1007/978-1-62703-050-2_16. [DOI] [PubMed] [Google Scholar]
- 42.Chowdary P, Fosbury E, Riddell A, Mathias M. Therapeutic and routine prophylactic properties of rFactor VIII Fc (efraloctocog alfa, Eloctate((R))) in hemophilia A. J Blood Med. 2016;7:187–198. doi: 10.2147/JBM.S80814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 61 kb)
Data Availability Statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal.
Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.