Abstract
Cholangiocarcinoma is a heterogeneous and target-rich disease with differences in actionable targets. Intrahepatic and extrahepatic types of cholangiocarcinoma differ significantly in clinical presentation and underlying genetic aberrations. Research has shown that extrahepatic cholangiocarcinoma is more likely to be associated with ERBB2 (HER2) genetic aberrations. Various anti-HER2 clinical trials, case reports and other molecular studies show that HER2 is a real target in cholangiocarcinoma; however, anti-HER2 agents are still not approved for routine administration. Here, we show in a metastatic cholangiocarcinoma with ERBB2 amplification identified on liquid biopsy (circulating tumor DNA (ctDNA) testing), a dramatic response to now over 12 months of dual-anti-HER2 therapy. We also summarize the current literature on anti-HER2 therapy for cholangiocarcinoma. This would likely become another treatment option for this target-rich disease.
Subject terms: Targeted therapies, Biliary tract cancer, Cancer genetics, Tumour heterogeneity, Tumour biomarkers
Introduction
Cholangiocarcinoma (CCA) is a lethal tumor arising from the epithelium of the bile ducts that most often presents at an advanced stage. A total of 10–20% of primary hepatic malignancies are CCA, which is the second most common hepatobiliary malignancy.1 Risk factors for development of CCA include cigarette smoking, heavy alcohol use, primary sclerosing cholangitis, viral hepatitis B and C, and genetic diseases such as Lynch syndrome.2
CCA, including gallbladder cancer, is a heterogeneous group of diseases. Multiple aberrations can be seen, including ERBB2, microsatellite instability high (MSI-H), IDH1, BRAF, FGFR fusion, BRCA/DNA-repair related, MET amplification, NTRK fusion, TP53, KRAS, ARID1A, MCL1, PBRM1, SMAD4, FBXW7, and CDKN2A.3–10 As in most cancers, these aberrations are not actionable; however, cholangiocarcinoma is a very “target rich” disease; ERBB2, MSI-H, IDH1, BRAF, FGFR fusion, MET amplification, and NTRK fusion5,6,8,9,11 may be susceptible to approved or off-label or presence of drugs treatments through clinical trials that are showing promise. Tumor-agnostic FDA-approved immunotherapy for MSI-H tumors and larotrectinib for NTRK-fusion tumors are showing promise.9,12 A number of published cases and open clinical trials with early results have demonstrated activity in IDH1, BRAF-mutant, MET-amplified, ERBB2-amplified, and FGFR- fusion tumors.6,7,13,14
The ERBB2 gene encodes for the protein HER2 or HER2/neu, which is a tyrosine kinase family receptor. Breast, stomach and esophageal cancers have well-established associations with ERBB2 genetic aberrations; and are approved for anti-HER2.15,16 However, reports have also shown the finding of HER2 aberrations in CCA and urinary bladder cancers.17 These are interestingly seen more with extrahepatic cholangiocarcinomas and gallbladder cancers as opposed to intrahepatic cholangiocarcinomas.
We present a 71-year-old female diagnosed with metastatic CCA with ERBB2 (HER2) 3+ amplification determined by circulating tumor DNA (ctDNA) testing (“liquid biopsy”) and confirmed by tissue-based testing. She was treated with dual HER2-directed therapy (trastuzumab/pertuzumab), and responded very well with regression of tumor on imaging as well as normalization of liver function tests and tumor marker levels including improvement in her overall clinical status. Serial ctDNA testing (Table 1) alongside standard of care imaging continues to show ongoing durable control >12 months into therapy. Other case reports and studies highlighting the association of HER2 and CCA are also presented. The patient provided written informed consent to report this case.
Table 1.
Serial circulating tumor DNA evaluation in our patient
| Amplification or mutation | Amplification or %cfDNA | Amplification or %cfDNA | Amplification or %cfDNA | Amplification or %cfDNA | Amplification or %cfDNA | Amplification or %cfDNA |
|---|---|---|---|---|---|---|
| Jan 2018 | Mar 2018 | May 2018 | Jun 2018 | Aug 2018 | Oct 2018 | |
| Treatment | Gemcitabine + Carboplatin chemotherapy | Dual-anti-HER2 blockade (Pertuzumab + Trastuzumab) | ||||
| ERBB2 (HER2) amplification |
+++ Plasma Copy Number: 68.5 |
+++ Plasma Copy Number: 70.6 |
+++ Plasma Copy Number: 52.6 |
+++ Plasma Copy Number: 46.0 |
+++ Plasma Copy Number: 48.3 |
+++ Plasma Copy Number: 6.4 |
| Myc amplification |
++ Plasma Copy Number: 3.3 |
++ Plasma Copy Number: 2.7 |
++ Plasma Copy Number: 3.1 |
++ Plasma Copy Number: 3.3 |
++ Plasma Copy Number: 2.7 |
NDa |
| Tumor markers | ||||||
| CA-19-9 (units/ml) | 99 | 462 | 366 | 154 | 90 | 57 |
| Clonal aberrations | ||||||
| TP53 Y220C | ND | 60.7% | 35.6% | 33.0 % | 32.9% | 2.1% |
| Subclonal aberrations | ||||||
| TP53 P190S | ND | 0.1% | ND | ND | ND | ND |
| NTRK1 P453P | ND | ND | ND | 0.08% | ND | ND |
| MYC P177P | ND | ND | ND | 0.1% | ND | 0.2% |
| PDGFRA E298D | ND | ND | ND | ND | ND | 0.2% |
| FGFR2 S24F | ND | ND | ND | ND | ND | 0.1% |
ND not detectable
Results
Case presentation
A 71-year-old female presented in December 2017 after diagnosis of metastatic CCA. Ultrasound demonstrated innumerable liver lesions, which on confirmed on follow-up computed tomography and magnetic resonance imaging showing multiple liver lesions consistent with CCA with intrahepatic metastases. The patient was also noted to have metastatic periportal and aortocaval adenopathy.
The patient was not a candidate for surgical intervention due to bilobar disease with innumerable liver lesions. Platinum-based chemotherapy was recommended. She was started on a combination carboplatin and gemcitabine (not cisplatin due to age and sensorineural hearing loss). A baseline ctDNA test was obtained as part of the standard of care at our institution for patients with gastrointestinal malignancies, and in particular cholangiocarcinoma, given the target-rich nature of the disease. Testing is performed through commercially available platforms. Guardant360 testing showed ERBB2 (HER2) amplification 3+ and was confirmed through tumor tissue-based immunohistochemistry as well as genetic testing through the commercially available platform by Tempus confirming this (Fig. 1). Given the liver-predominant nature of the disease, upfront Y90-radioembolization was also planned. However, within 2 months, the patient had rapid progression of disease with rising tumor markers, rising ctDNA levels, derangement in liver function tests and decline in clinical condition.
Fig. 1.
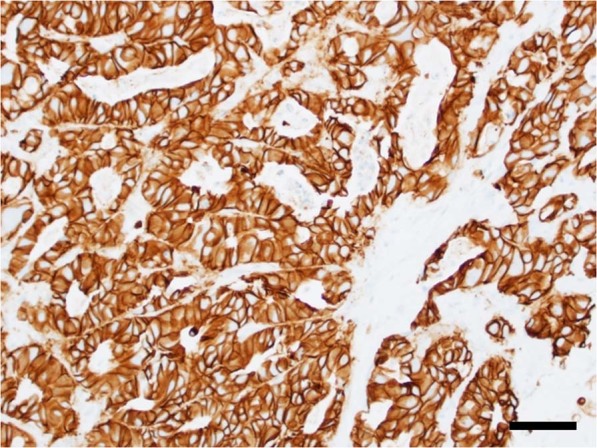
Tumor with intense HER2/Neu 3+ positivity on immunohistochemistry (scale 50 μm)
We initially considered the patient’s eligibility for the MyPathway Study which has an arm for dual-anti-HER2 therapy.18 However, due to her rapid decline was deemed ineligible. Best supportive/palliative care vs. off-label anti-HER2 therapy was discussed. Given the patient’s excellent overall baseline performance status, we began off-label treatment with anti-HER2 pertuzumab/trastuzumab combination therapy.
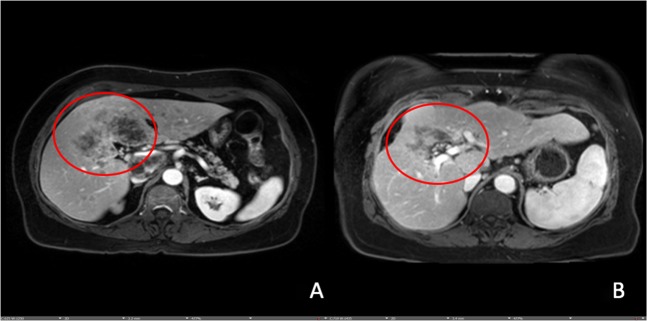
Immediate and rapid improvement of tumor markers was noted. After just one treatment, the patient’s liver function tests improved; most notably, the dominant TP53 mutation reduced from 60.7 to 2.1% (Table 1). Clinical variables continued to improve rapidly through and continues to do so now over a year into treatment. Scans also showed excellent ongoing durable response (Fig. 2a, b).
Fig. 2.
MRI pre- and post-treatment with decreased size and enhancement of lesions in liver
Discussion
CCA is classified into intrahepatic and extrahepatic types based on anatomic location. These are clinically different from each other in terms of presentation, and more importantly the type and frequency of genetic aberrations. Generally, intrahepatic CCA is associated with IDH1, FGFR fusion and BRCA/DNA-repair gene alterations, while extrahepatic CCA is more frequently associated with ERBB2 genetic aberrations.19 A number of these aberrations are potentially actionable in terms of FDA-approved therapies and/or availability of clinical trials or off-label therapies. For a rare disease like CAA, even the latter are pertinent. There are several well-established risk factors related to the development of CCA. However, it has been reported that only 10% of intrahepatic CCA is associated with these risk factors and the remaining 90% are sporadic.20
What is unique about our case is that the HER2 aberration was detected on circulating tumor DNA testing (“liquid biopsy”). This was later confirmed on tissue-based testing (Fig. 1). This would not have been known otherwise. Furthermore, for a patient with refractory cholangiocarcinoma that progresses on frontline therapy, prognosis in such situations is in the order of weeks–months. The fact that patient continues to have durable control 12 months and beyond is very encouraging and unprecedented. Progression-free survival of second-line chemotherapeutic regimens is in the order of 4 months on average.
Several studies have described the association of CCA subgroups with various aberrations (Table 2). Churi et al. examined 75 CCA patients for tumoral genetic differences. They identified TP53, KRAS, ARID1A, IDH1, MCL1, and PBRM1 mutations in 35%, 24%, 20%, 18%, 16%, and 11% of intrahepatic CCA patients, respectively. TP53, KRAS, ERBB2, SMAD4, FBXW7, and CDKN2A were found in 45%, 40%, 25%, 25%, 15%, and 15% of extrahepatic CCA patients, respectively.19 The finding of a higher proportion of ERBB2 mutations in extrahepatic CCA and gallbladder cancer suggests that these patients are more likely to carry it as opposed to intrahepatic CCA. Yoshikawa et al. measured HER2/neu expression in 236 cases of CCA by immunohistochemistry. They reported a 0.9% and 8.5% positive HER2/neu expression rate in intrahepatic and extrahepatic CCAs, respectively.21 In a study by Lee et al., HER2 and HER3 overexpression was observed in 6% and 39%, respectively, in patients with extrahepatic CCA, with HER3 overexpression associated with worse survival.22 A systemic review and meta-analysis by Galdy et al. showed a stronger rate of HER2 expression in extrahepatic CCA (~20%) vs. intrahepatic CCA (<5%).23 Summary of the studies showing HER2 aberrations in CCA and treatment outcomes where available are presented in Table 2.
Table 2.
HER2/Neu (ERBB2) expression in cholangiocarcinoma and response to therapy
| Study | N/year | Patients | Therapy used | Outcome (response/duration—months) |
|---|---|---|---|---|
| Javle et al. (MyPathway Clinical trial—NCT02091141)18 | 11 patients/ 2017 | Refractory metastatic biliary cancer with HER2 amplification/overexpression (8 patients) or putative activating mutations by gene sequencing (3 patients),18 FISH, or IHC | Pertuzumab + trastuzumab until disease progression or unacceptable toxicity | 4 patients had partial responses (PR) and 3 had stable disease (SD) for >4 months |
| Javle et al.27 | 2015 | Gallbladder cancer—9 patients CCA—5 patients | Trastuzumab, lapatinib, or pertuzumab | 1 CR, 4 PR and 4 SD in gallbladder cases; 1 mixed response on lapatinib with HER2 mutation. Duration of response 8–160+ weeks (median 40 weeks); 3 still on therapy. 1 HER2 amplification was acquired post-FGFR fusion therapy. No responses in CCA cases |
| Nam et al.32 | 2016 | 3 patients with gallbladder cancer | All 3 patients received trastuzumab alongside single- or doublet chemotherapy | 2 PR; 1 SD. Duration of response 12–34 weeks. |
| Subbaiah et al.33 | 2013 | 1 patient with gallbladder cancer | Phase-1 study: anti-HER-2/neu antibody trastuzumab and the HER-2/neu tyrosine kinase inhibitor lapatinib, and then with trastuzumab and erlotinib | SD Duration of response 10.8 months |
| Churi et al.19 | 2014 | 2 patients with HER2/neu mutations | Trastuzumab and lapatinib, respectively | No responses observed |
| Lee et al.22 | 2012 | Overexpression of HER2 and HER3 was observed in 6 % (13/224) and 39 % (90/230) of EHCCs, respectively | No treatment data provided | – |
| Mckay et al.34 | 2011 | Expression data only; only CC patients; no gallbladder cancer patients. 19 cases were positive per IHC (10 were scored as +2 and one as +3) | No treatment data provided | – |
| Sorscher et al.30 | 2013 | Gallbladder cancer—1 patient HER2/neu+++ | Trastuzumab + weekly paclitaxel (chemo later discontinued) | Marked partial response (5.4 cm → 1.3 cm). Duration of response ongoing (at least 6 months) |
| Jung et al.28 | 2017 | 3/84 patients with HER2/neu+ distal extrahepatic CC (3.6%) | No treatment data provided | – |
| Yoshikawa et al.21 | 2007 | HER2/neu expression 0.9% in intrahepatic CC and 8.5% in extrahepatic CC | No treatment data provided | – |
In terms of this particular assay’s ability to detect ERBB2 (HER2/neu) amplification, data were presented by the group from M.D. Anderson Cancer Center at the American Society of Clinical Oncology Conference (ASCO) 2018.24,25 Bardelli and colleagues validated the performance of the Guardant360 ctDNA assay retrospectively in a cohort of HER2/neu amplified metastatic colorectal cancer patients treated in the landmark HERACLES study (phase 2 study of lapatinib plus trastuzumab). The group investigated 48 plasma samples from 29 patients. CtDNA was identified in 47–48 samples; ERBB2 amplification was confirmed in 46 of these 47 (97.9%) samples. The authors reported that a threshold of ≥3 copies of ERBB2 in circulation allowed identification of 94% of fluorescence in situ hybridisation-positive patients. This threshold also correlated with clinical response.24,25 These results have been validated in other tumor types and cohorts of patients.25,26 Liquid biopsy, therefore, appears to be a reliable and valid tool to detect ERBB2 (Her2/neu), which is indeed an actionable finding across many tumor types including cholangiocarcinoma.
HER2/neu blockade has shown favorable results in cancers carrying HER2 aberrations. Javle et al. investigated the response of anti-HER2 therapy in 8 patients with HER2 mutated gallbladder cancer and showed an overall improvement in terms of disease stability (n = 3), partial response (n = 4), or complete response (n = 1) in their entire patient cohort.27 In another study, the researchers studied HER2/neu expression in extrahepatic CCA patients (n = 84) and reported that anticancer therapy targeting HER2 receptors may be a reasonable option for these patients.28 Our patient has responded very well to anti-HER2 drugs and showed an improvement in symptoms, lab results and shrinkage in tumor size. This implicates the use of targeted therapy as a favorable option in CCA alongside other patients who received anti-HER2-based therapy (Table 2).
CCA diagnosis at an advanced stage has very poor outcomes and is often considered incurable. Valle et al.29 reported a study of metastatic CCA patients (n = 410), and reported median overall survival of 11.7 months and 8.1 months in a cisplatin–gemcitabine group and gemcitabine group, respectively. In the second-line setting, outcomes are extremely poor (progression-free survival, 3–4 months; overall survival, 6 months). Compared to historical reports, outcomes in patients receiving anti-HER2 therapy in HER2/neu+ CCA is by far superior and very encouraging (Table 2).30,31
Our patient at one point was given a choice of hospice and was not eligible for any of the trials due to deranged liver function tests. But now on off-label dual-anti-HER2 therapy continues to be on treatment with excellent performance status without any chemotherapy for more than 12 months at present.
For rare, target-rich diseases like CCA, there is a need to consider approval and it is encouraging to see some recent tumor-agnostic approvals, such as immunotherapy for MSI-H and larotrectinib for NTRK-fusion cancers.9,12 We believe our case and recent single-arm phase 2 study outcomes warrant consideration toward approval of anti-HER2 agents (trastuzumab/pertuzumab) in patients with CCA with ERBB2 amplification who have very limited options and poor prognosis.
Furthermore, liquid biopsy testing for HER2 is not routinely performed at most institutions. Our patient’s positive finding would have been missed. In target-rich diseases such as CCA, where tissue acquisition is a challenge, liquid biopsy provides a viable alternative for both diagnostic and monitoring techniques.
In conclusion, we were able to identify ERBB2 (HER2/neu) amplification on liquid biopsy in a patient with CCA and found anti-HER2 therapy as an effective treatment strategy. This will have implications for other patients with CCA in terms of identification of this target and considerations toward anti-HER2 therapy on- or off-trial.
Methods
The patient provided written informed consent for her case to be presented. Separate IRB/FDA approval not required for off-label use of the anti-HER2 therapy or reporting of the case.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Author contribution
P.M.K. conceived the idea, and is the patient’s primary oncologist alongside A.R. B.Y. drafted the initial draft alongside comprehensive literature review by F.S. and S.K. Additional details provided by A.R. P.M.K. revised the draft before an additional round of revisions by all authors. This was approved by all authors before submission to the journal. P.M.K., S.K., and A.R. revised the paper. All authors again approved of the final changes before resubmission.
Data availability
The authors declare that data supporting the findings of this study are available within the paper. Commercially available platforms were employed with results shown in the figures and tables included.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies the paper on the npj Precision Oncology website (10.1038/s41698-019-0091-4).
References
- 1.Jusakul A, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7:1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Zheng, Nie Shen-Dan, Jiang Bo, Wang Jun, Lv Pin. Risk factors for extrahepatic cholangiocarcinoma. European Journal of Cancer Prevention. 2019;28(4):254–257. doi: 10.1097/CEJ.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 3.Gbolahan O, Hashemi-Sadraei N, O'Neil B. Prolonged response to anti-PD-1 antibody therapy in chemotherapy-refractory cholangiocarcinoma with high tumor mutational burden. J. Natl Compr. Cancer Netw. 2019;17:644–648. doi: 10.6004/jnccn.2019.7304. [DOI] [PubMed] [Google Scholar]
- 4.Winkelmann Ria, Schneider Markus, Hartmann Sylvia, Schnitzbauer Andreas, Zeuzem Stefan, Peveling-Oberhag Jan, Hansmann Martin, Walter Dirk. Microsatellite Instability Occurs Rarely in Patients with Cholangiocarcinoma: A Retrospective Study from a German Tertiary Care Hospital. International Journal of Molecular Sciences. 2018;19(5):1421. doi: 10.3390/ijms19051421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farshidfar F, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal Lipika, Shi Lei, Liu Leah Y., Fece de la Cruz Ferran, Lennerz Jochen K., Raghavan Srivatsan, Leschiner Ignaty, Elagina Liudmila, Siravegna Giulia, Ng Raymond W.S., Vu Phuong, Patra Krushna C., Saha Supriya K., Uppot Raul N., Arellano Ron, Reyes Stephanie, Sagara Takeshi, Otsuki Sachie, Nadres Brandon, Shahzade Heather A., Dey-Guha Ipsita, Fetter Isobel J., Baiev Islam, Van Seventer Emily E., Murphy Janet E., Ferrone Cristina R., Tanabe Kenneth K., Deshpande Vikram, Harding James J., Yaeger Rona, Kelley Robin K., Bardelli Alberto, Iafrate A. John, Hahn William C., Benes Cyril H., Ting David T., Hirai Hiroshi, Getz Gad, Juric Dejan, Zhu Andrew X., Corcoran Ryan B., Bardeesy Nabeel. TAS-120 Overcomes Resistance to ATP-Competitive FGFR Inhibitors in Patients with FGFR2 Fusion–Positive Intrahepatic Cholangiocarcinoma. Cancer Discovery. 2019;9(8):1064–1079. doi: 10.1158/2159-8290.CD-19-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J. Gastrointest. Oncol. 2016;7:E98–E102. doi: 10.21037/jgo.2016.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drilon A, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churi CR, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS ONE. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal L, et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer. 2017;123:1979–1988. doi: 10.1002/cncr.30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golub D, et al. Mutant Isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front. Oncol. 2019;9:417. doi: 10.3389/fonc.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meric-Bernstam F, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 16.Kumler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat. Rev. 2014;40:259–270. doi: 10.1016/j.ctrv.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Latif Z, et al. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br. J. Cancer. 2003;89:1305–1309. doi: 10.1038/sj.bjc.6601245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javle MM, et al. Herbert Hurwitz Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: preliminary data from MyPathway. J. Clin. Oncol. 2017;35:402. doi: 10.1200/JCO.2017.35.4_suppl.402. [DOI] [Google Scholar]
- 19.Churi Chaitanya R., Shroff Rachna, Wang Ying, Rashid Asif, Kang HyunSeon C., Weatherly Jacqueline, Zuo Mingxin, Zinner Ralph, Hong David, Meric-Bernstam Funda, Janku Filip, Crane Christopher H., Mishra Lopa, Vauthey Jean-Nicholas, Wolff Robert A., Mills Gordon, Javle Milind. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE. 2014;9(12):e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur. J. Gastroenterol. Hepatol. 2007;19:615–617. doi: 10.1097/MEG.0b013e328224b935. [DOI] [PubMed] [Google Scholar]
- 21.Yoshikawa D, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer. 2008;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HJ, Chung J-Y, Hewitt SM, Yu E, Hong S-M. HER3 overexpression is a prognostic indicator of extrahepatic cholangiocarcinoma. Virchows Arch. 2012;461:521–530. doi: 10.1007/s00428-012-1321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galdy S, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastas. Rev. 2017;36:141–157. doi: 10.1007/s10555-016-9645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardelli Alberto, Siravegna Giulia, Sartore-Bianchi Andrea, Nagy Rebecca J, Mussolin Benedetta, Cassingena Andrea, Martino Cosimo, Lonardi Sara, Zagonel Vittorina, Leone Francesco, Amatu Alessio, Trusolino Livio, Odegaard Justin, Lanman Richard B., Marsoni Silvia, Siena Salvatore. Plasma HER2 (ERBB2) copy number to predict response to HER2-targeted therapy in metastatic colorectal cancer. Journal of Clinical Oncology. 2018;36(15_suppl):3506–3506. doi: 10.1200/JCO.2018.36.15_suppl.3506. [DOI] [Google Scholar]
- 25.Siravegna G, et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin. Cancer Res. 2019;25:3046–3053. doi: 10.1158/1078-0432.CCR-18-3389. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, et al. Detection of ERBB2 (HER2) gene amplification events in cell-free DNA and response to anti-HER2 agents in a large asian cancer patient cohort. Front. Oncol. 2019;9:212. doi: 10.3389/fonc.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javle M, et al. HER2/neu-directed therapy for biliary tract cancer. J. Hematol. Oncol. 2015;8:58. doi: 10.1186/s13045-015-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung MJ, et al. Gene copy number variation and protein overexpression of EGFR and HER2 in distal extrahepatic cholangiocarcinoma. Pathology. 2017;49:582–588. doi: 10.1016/j.pathol.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 30.Sorscher S. Marked radiographic response of a HER-2-overexpressing biliary cancer to trastuzumab. Cancer Manag. Res. 2013;9:1–3. doi: 10.2147/CMAR.S55091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornaro L, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J. Exp. Clin. Cancer Res. 2015;34:156. doi: 10.1186/s13046-015-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam AR, et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget. 2016;7:58007–58021. doi: 10.18632/oncotarget.11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbiah IM, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget. 2013;4:156–165. doi: 10.18632/oncotarget.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKay SC, et al. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB. 2011;13:309–319. doi: 10.1111/j.1477-2574.2010.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the paper. Commercially available platforms were employed with results shown in the figures and tables included.