Abstract
The phytohormone abscisic acid (ABA) plays a role in stresses that alter plant water status and may also regulate root gravitropism and hydrotropism. ABA also exists in the aquatic algal progenitors of land plants, but other than its involvement in stress responses, its physiological role in these microorganisms remains elusive. We show that exogenous ABA significantly altered the HCO3− uptake of Chamydomonas reinhardtii in a light-intensity-dependent manner. In high light ABA enhanced HCO3− uptake, while under low light uptake was diminished. In the dark, ABA induced a negative geotropic movement of the algae to an extent dependent on the time of sampling during the light/dark cycle. The algae also showed a differential, light-dependent directional taxis response to a fixed ABA source, moving horizontally towards the source in the light and away in the dark. We conclude that light and ABA signal competitively in order for algae to position themselves in the water column to minimise photo-oxidative stress and optimise photosynthetic efficiency. We suggest that the development of this response mechanism in motile algae may have been an important step in the evolution of terrestrial plants and that its retention therein strongly implicates ABA in the regulation of their relevant tropisms.
Subject terms: Microbiology, Tropism
Introduction
Photosynthetic green algae evolved 1 to 1.5 billion years ago when a eukaryotic heterotroph encapsulated a cyanobacterium which ultimately formed a plastid1. Numerous lineages diverged2, including the chlorophytes and charophytes, the progenitors of terrestrial plants which appeared ≈500 million years ago3. The aquatic to terrestrial transition must have posed a significant challenge to algae and environmental differences required the adaptive evolution of protective mechanisms enabling them to become high-light and desiccation tolerant and sessile at the water surface. It is likely that such mechanisms derived from those which had already evolved to ensure their survival. For example, Chlamydomonas sp. alter their depth depending on light level and quality to attenuate photo-oxidative stress4. As such mechanisms became more effective, the depth to which algae needed to descend presumably reduced, enabling them to thrive closer into the shoreline.
Algal genomes encode rudimentary synthesis and signalling pathways for the majority of phytohormones, including ABA5. In terrestrial plants ABA plays a role in seed dormancy and germination, stomatal movements and in other responses to numerous stresses that alter their water status6. ABA may also negatively regulate root gravitropism7 and root hydrotropism8, but the underlying signalling mechanisms remain unclear. However, its algal role remains poorly defined with only a few papers suggesting its involvement in stress responses such as those resulting from desiccation9 and salinity10 and in the initiation of the cell cycle11,12. ABA treatment has also been shown to increase lipid accumulation in Chlorella13.
Within the complexity of multicellular, multi-organ, terrestrial plants it has proved difficult experimentally to isolate the many potential roles of ABA away from its major stress-related role in preventing water loss. ABA loss of function mutants exhibit pleiotropic phenotypes and simply applying ABA to plants induces an immediate stress response. Thus, properly defining a role for ABA in a photosynthetic progenitor of land plants where water stress is not normally an issue, the green algae may give valuable insight into its other roles. Therefore, the aim of this study was to define a specific role for ABA in the motile green alga, Chlamydomonas reinhardtii, providing evidence that this phytohormone mediates the light-dependent diurnal rhythm of up and down gravitaxis of the algae in the water column.
Results
ABA altered the photosynthetic efficiency of C. reinhardtii CC-1021
Reasoning that high-light-induced photo-oxidation likely poses the main threat to photosynthetic algae, here the initial investigation focused on the effect of ABA on the photosynthetic efficiency of C. reinhardtii by measuring the ability of treated algae to deplete their growing media of dissolved HCO3− under different light levels. Alginate-bead-encapsulated C. reinhardtii with different concentrations of ABA were incubated under different light levels in media containing bicarbonate indicator. Under relatively high-light illumination (223.2 µmoles photons m−2 s−1), 50 µM ABA significantly (P < 0.05 by Tukey Kramer’s test) increased mean HCO3− depletion of the media by the algae by ≈20% compared to the control algae under the same light levels. Conversely, at low-light (7.59 µmoles photons m−2 s−1) 50 µM ABA significantly reduced the mean HCO3− depletion of the media by the algae by ≈14.5% (Fig. 1A). Lower ABA doses had an intermediate effect. At 32 µmoles photons m−2 s−1 ABA had no significant effect on the mean HCO3− depletion of the media by the algae regardless of the dose used. When grown at 45 µmoles photons m−2 s−1 and sampled 1 h into the photoperiod, subsequent exposure of the algae to high light conditions at 375 µmoles photons m−2 s−1 increased their mean endogenous ABA content by 1.4 fold (Fig. 1B), but not in a statistically significant manner.
Figure 1.
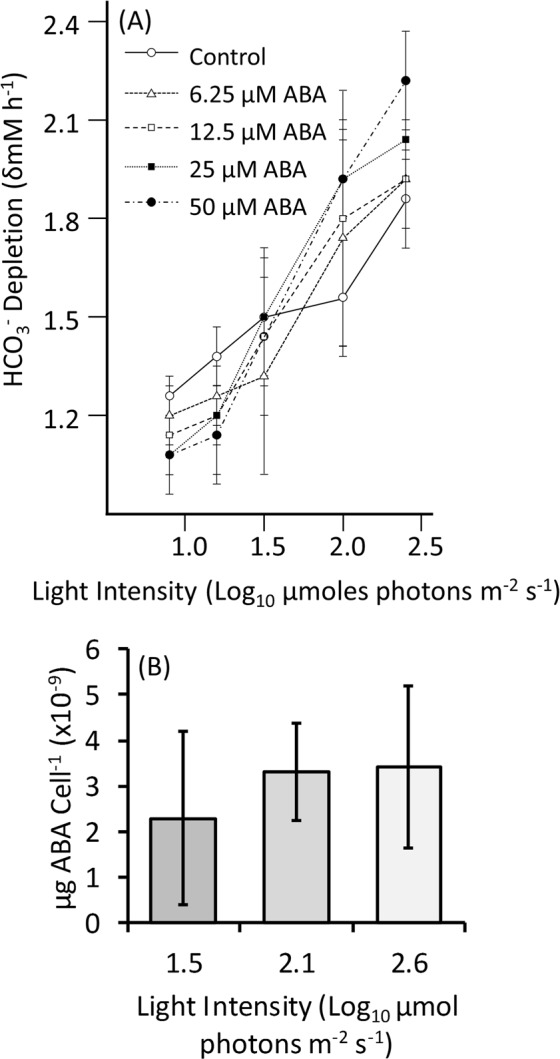
Exogenous abscisic (ABA) acid differentially altered HCO3− uptake by C. reinhardtii in a light-intensity-dependent manner. Mid log phase (A750 = 0.3) algal cultures were immobilised in alginate gel beads containing the concentrations of ABA indicated and in (A) were incubated for 1 h under different light intensities (7.6 to 223.2 µmoles photons m−2 s−1) with bicarbonate indicator buffer in TAP media. Depletion of HCO3− in the media was monitored as the change in absorbance in the indicator buffer at 550 nm after the incubation period with conversion to the change in the concentration of HCO3− in the TAP media by reference to a standard calibration. Shown are the mean δHCO3− (mM) h−1 of n = 5 replicates with errors as the 95% confidence intervals around the means in each case. In (B) mid log phase (A750 = 0.3) algal cultures were sampled 1 h into the photoperiod and subsequently exposed for 1 h to the different light intensities indicated. Algal cells were pelleted from n = 3 replicate 25 mL aliquots of culture. Pellets were extracted and assessed for ABA content by competitive ELISA (MyBioSource Inc.). Data are shown as the mean ABA content cell−1 with error bars shown as +/− the 95% confidence interval around the mean in each case.
ABA and light signal together to position C. reinhardtii CC-1021 vertically in the water column
Since Chlamydomonas are known to respond to changing light levels by exhibiting either positive or negative phototaxis with subsequent adaptation4,14 and since ABA treatment differentially altered the photosynthetic efficiency of the algae in a light dependent manner, thus indicating an ABA/light signalling interaction, it was reasoned that ABA signalling could be involved in determining their vertical position in the water column. C. reinhardtii were thus sampled at different time points over a 24 h cycle and fully dispersed samples incubated in upright measuring cylinders +/−50 µM ABA under either overhead, high-light illumination (319.8 µmoles photons m−2 s−1) or in the dark for 50 min (Figs. 2 and 3).
Figure 2.
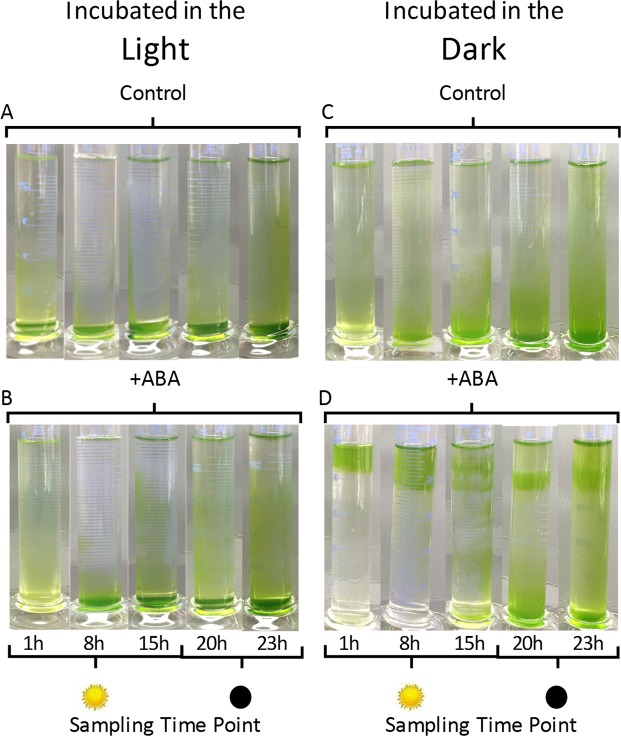
Exogenous abscisic acid (ABA) induced a negative geotropic movement response in C. reinhardtii CC-1021. Algal cultures were grown under a 16 h photoperiod to the mid log phase (A750 = 0.3) growth stage and were sampled at the time points indicated over a 24 h period where the 16 h photoperiod commenced at time = 0 h. Fully dispersed algal samples were placed in measuring cylinders +/−50 µM ABA to form a 10 cm vertical water column and for 50 min were either illuminated from above with high-light (319.8 µmoles photons m−2 s−1) (A,B) or placed in the dark (C,D). Shown is a representative image of n = 5 replicate experiments indicating the positions attained by the algae immediately following the period of incubation in either the light or dark.
Figure 3.
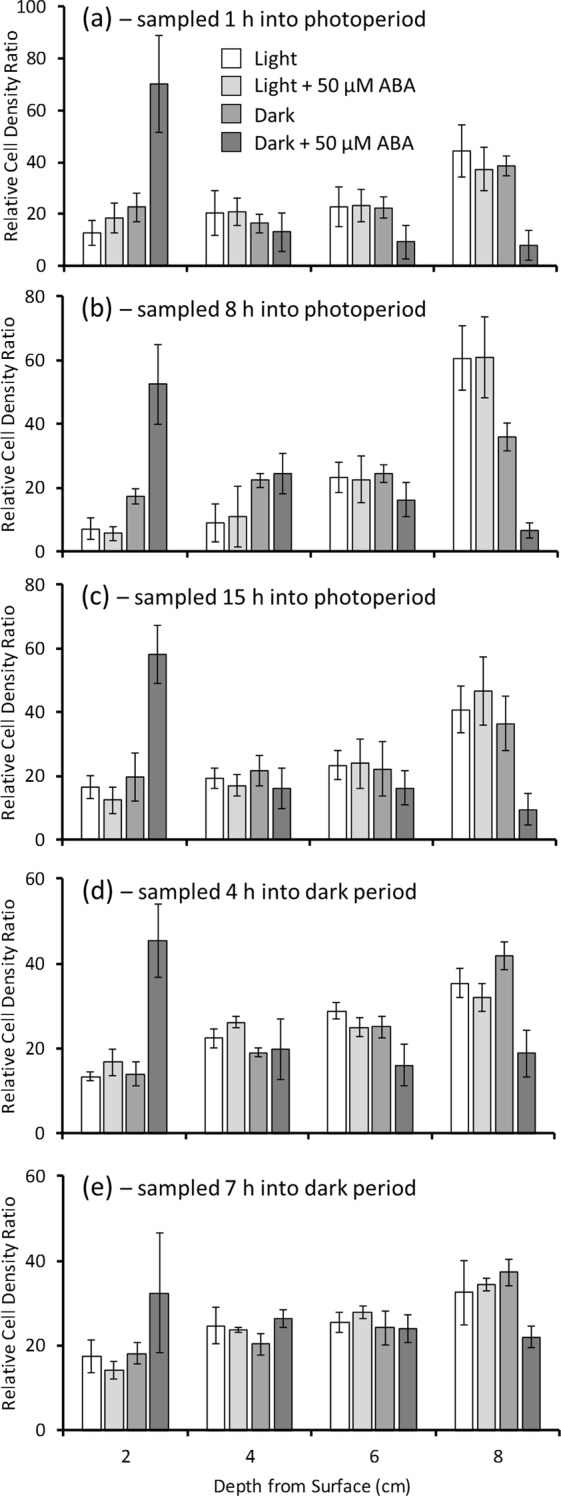
Exogenous abscisic acid (ABA) induced a negative geotropic movement response in C. reinhardtii CC-1021. Algal cultures were grown under a 16 h photoperiod to the mid log phase (A750 = 0.3) growth stage and were sampled at (a) 1 h, (b) 8 h, (c) 15 h, (d) 20 h and (e) 23 h during a 24 h period where the 16 h photoperiod commenced at time = 0 h. Fully dispersed algal samples were placed in measuring cylinders +/−50 µM ABA to form 10 cm vertical culture columns and for 50 min were either illuminated from above with high-light (319.8 µmoles photons m−2 s−1) or placed in the dark. After incubation the A750 at the depths indicated was measured and the relative cell density ratios at each depth were determined within each treatment. Shown are the mean data of n = 5 replicate experiments +/− the 95% confidence intervals around the mean in each case.
Control algae with overhead illumination retreated downwards to an extent dependent on their sampling point in the 24 h light/dark cycle (Figs. 2A and 3). Those sampled mid photoperiod retreated significantly (P < 0.05 by χ2 test of goodness of fit) further downwards than those sampled earlier and towards the end of the photoperiod. Those sampled in the dark period and then illuminated from above retreated downwards less than those sampled at any time point in the photoperiod, with those sampled at the end of the dark period tending to remain more dispersed when returned to the light, but not to a significant extent (P > 0.05 by χ2 test of independence). ABA treatment marginally reduced this negative phototactic response to high-light at some time points, but not at others (Figs 2B and 3). Algae sampled 1 h into the photoperiod and light-incubated with 50 µM ABA remained more dispersed than the controls, but not to a statistically significant extent. When sampled in the middle of the photoperiod ABA had no significant effect, while algae sampled at the end of the photoperiod and in the middle of the dark period showed reduced downward movement when treated with ABA, but again, not to a significant extent. There was no observed difference in the position attained by the algae sampled at the end of the dark period when placed in the light regardless of ABA treatment.
Algae of control samples, taken during the photoperiod and placed in the dark, moved downwards to an extent depending on the duration of illumination already received (Fig. 2C). Algae sampled after 1 h of the photoperiod remained dispersed during their subsequent dark incubation, while those sampled at 8 and 15 h into the photoperiod showed a progressive downward movement. Algae sampled during the dark period at 20 h into the 24 h cycle and maintained in the dark moved towards the bottom, while those sampled at 23 h were more dispersed. However, overall the distribution of the algae when incubated in the dark was not significantly (P > 0.05 by χ2 tests of independence) different regardless of the sampling time point. Intriguingly, when samples were treated with ABA and placed in the dark the algae moved upwards significantly (P < 0.05 by χ2 tests of goodness of fit) to form discrete bands near to the surface (Fig. 2D). The depth at which the ABA-treated algae banded depended on their sampling time over the 24 h cycle. Those sampled after the first hour of the photoperiod banded at 0 to 2 cm from the surface. Those sampled later into the photoperiod still exhibited a significant negative geotropism, but banded at two increased depths. Cells sampled towards the end of the photoperiod and into the dark period were less responsive to ABA treatment in the dark, although a proportion of the cells still moved upwards to form a discrete band 2 to 4 cm from the surface.
To determine that the effect of ABA in the dark-incubated algae resulted from active swimming and not from altered buoyancy two independent mutants, CC-477 (BLD1 gene mutation that lacks functional intra-flagellar transport protein 52 and is unable to assemble flagella) and CC-2492 (loss of function mutation of the flagellar outer dynein arm light chain 2 (DLC2) gene) of C. reinhardtii that lacked functional flagella were sampled at the time point, 1 h into the photoperiod, when the CC-1021 strain of the algae had appeared most ABA responsive and were similarly incubated +/−50 µM ABA in the dark (Fig. 4). In the absence of ABA the dark-incubated algal cells of either mutant settled downwards such that the A750 at increasing depths were significantly (P < 0.05 by Tukey Kramer’s test) higher than at shallower depths. The addition of 50 µM ABA in the dark had no significant (P > 0.05 by χ2 tests of independence) effect on this distribution of the algal cells of either mutant suggesting that the presence of functional flagella was required for the ABA-induced upwards movement in the dark observed with the wild type C. reinhardtii strain CC-1021.
Figure 4.
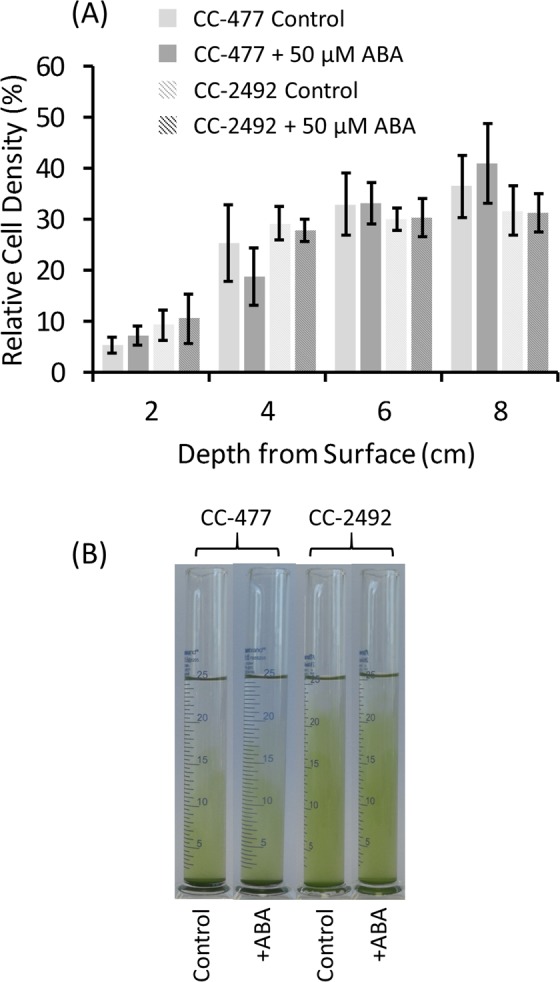
In the absence of light exogenous abscisic acid (ABA) failed to induce a negative geotropic movement in the C. reinhardtii mutant strains CC-477 and CC-2492 that lack functional flagella. Algal cultures of the mutant strains were grown under a 16 h photoperiod to the mid log phase (A750 = 0.3) growth stage and were sampled 1 h into the photoperiod. Fully dispersed algal samples were placed in measuring cylinders +/−50 µM ABA to form 10 cm vertical culture columns and for 50 min were incubated in the dark. After incubation the A750 at the depths indicated was measured and the relative cell density ratios at each depth were determined within each treatment. Shown in (A) are the means of data from n = 4 replicate experiments for each mutant +/− the 95% confidence intervals around the means in each case. Shown in (B) is a representative image of the positions attained by the algae immediately following the period of incubation.
NAA, H2O2 and ACC did not affect the vertical positioning of C. reinhardtii CC-1021 in either the light or dark
Since the ABA-induced upward movement of the algae in complete darkness suggested that the signalling of this phytohormone may have been acting gravitropically, it was deemed prudent to ascertain whether the main gravitropic phytohormone, auxin, had a similar effect on Chlamydomonas. Thus, mid log phase growth algae were sampled mid photoperiod, treated with the cell-permeable, synthetic auxin 1-naphthaleneacetic acid (NAA) and were incubated as for the ABA experiments above (Fig. 5a). Compared to the untreated control algae, NAA at 50 µM had no significant (P > 0.05 by χ2 test of independence) effect on the vertical positioning of the algae in the water column when they were incubated in either the light or the dark. When the algae were incubated in the light there was some indication that NAA had a small effect and although the algae still generally retreated downwards away from the high-light, the proportion remaining at shallower depths was increased with the auxin treatment, but overall the distribution of the algae with and without NAA was not statistically significantly different.
Figure 5.
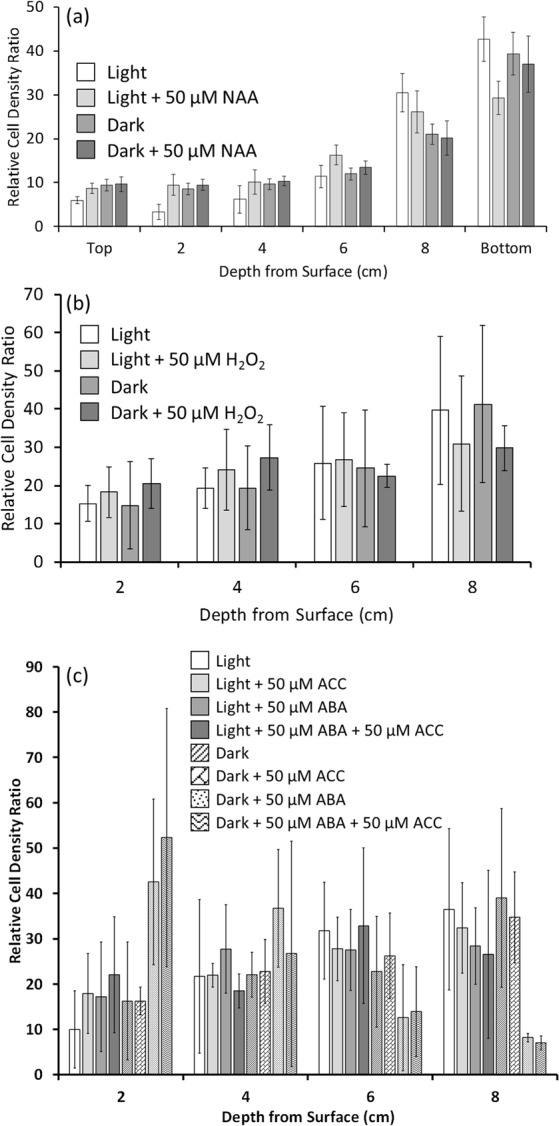
Exogenous 1-naphthaleneacetic acid (NAA), 1-aminocyclopropane-1-carboxylic acid (ACC) and hydrogen peroxide (H2O2) did not significantly affect the vertical positioning of C. reinhardtii CC-1021 in the dark. Algal cultures were grown under a 16 h photoperiod to the mid log phase (A750 = 0.3) growth stage and were sampled 8 h into the 16 h photoperiod. Fully dispersed algal samples were placed in measuring cylinders +/− either 50 µM (a) NAA, (b) H2O2 or (c) ACC, ABA and ACC + ABA to form 10 cm vertical culture columns and for 50 min were either illuminated from above with high-light (319.8 µmoles photons m−2 s−1) or placed in the dark. After incubation the A750 at the depths indicated were measured and the relative cell density ratios for each depth were determined within each treatment. Shown are the mean data of either n = 5 (a) or n = 3 (b,c) replicate experiments +/− the 95% confidence intervals around the means in each case.
In ABA responses such as those which occur in plant guard cells that affect stomatal movements, downstream hydrogen peroxide synthesis and signalling has been shown to be required. Here, when C. reinhardtii were sampled at mid photoperiod, treated with 50 µM H2O2 and incubated as for the ABA experiments above there was no significant (P > 0.05 by χ2 test of independence) effect on the vertical positioning of the algae in the water column when they were incubated in either the light or the dark (Fig. 5b).
Ethylene is antagonistic to a number of plant ABA responses. Here, algae were sampled mid photoperiod and treated with either the precursor of ethylene synthesis, 1-aminocyclopropane-1-carboxylic acid (ACC), either alone or with ACC and ABA together and were incubated as for the ABA experiments above. In either case, ACC treatment had no significant (P > 0.05 by χ2 test of independence) effect on the vertical positioning of the algae in the water column when they were incubated in either the light or the dark and no significant (P > 0.05 by χ2 test of independence) effect on the distribution of the algae induced by ABA (Fig. 5c).
C. reinhardtii CC-1021 exhibited differential, light-dependent taxis to a fixed source of exogenous ABA
In the dark, ABA induced the algae to abandon their usual run and tumble manner of locomotion15 and to swim upwards. The question was, how did cells employ the ABA signal to do this? It was reasoned that the directional response of algae to the ABA signal could be light reversible. Thus, light-permeable acrylic troughs in which had been placed at one end an agarose gel plug containing 1 mM ABA were filled with fully dispersed suspensions of algae and then immediately placed horizontally either with surrounding illumination or in the dark for 1 h. In the light algae moved towards the ABA gel plug, while conversely, in the dark they swam away (Fig. 6). The use of lower concentrations (0.5 and 0.25 mM) of ABA in the gel plug resulted in a similar, but intermediate response (data not shown).
Figure 6.
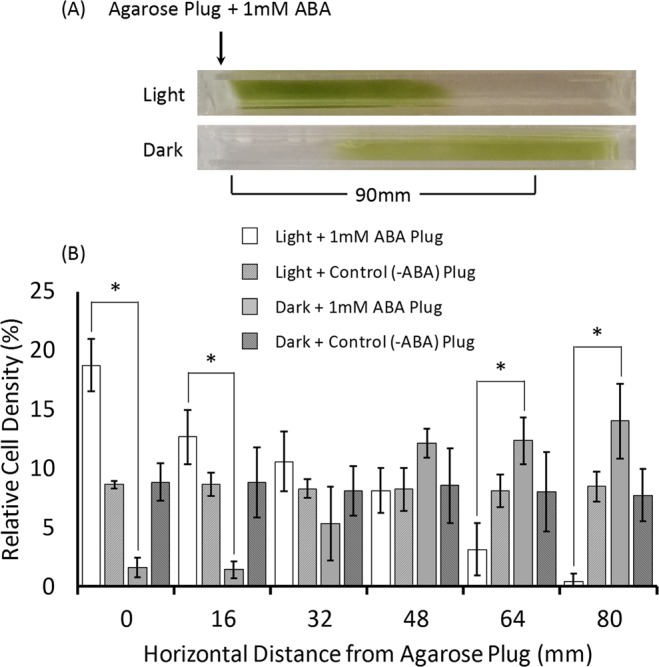
C. reinhardtii CC1021 showed opposite taxis to a fixed source of exogenous abscisic acid (ABA) in the light and dark. Algal cultures were grown under a 16 h photoperiod to the mid log phase (A750 = 0.3) growth stage and were sampled mid photoperiod. Fully dispersed samples of the algal culture were placed in open top 1.5 × 1.5 × 12 cm light-permeable acrylic troughs with a plug of agarose containing 1 mM ABA at the end indicated and were immediately placed horizontally either in the light (45 µmoles photons m−2 s−1) or the dark for 50 min. After incubation samples were simultaneously taken at incremental distances from the agar plug and their A750 measured. Shown in (A) is a representative image indicating the positions attained by the algae immediately following the incubation period. The treatment-dependent relative cell densities at increasing distances from the agar plugs is shown in (B). Significant (P < 0.05 by χ2 tests of goodness of fit) differences in the proportion of algal cells found at various distances from the ABA-containing agar plug between the light and dark incubations of the algae are indicated(*).
Endogenous ABA levels were diurnally altered in C. reinhardtii CC-1021
Overall, the results suggest that in the algae ABA signalled for an upward movement, mediated by light levels and potentially the circadian rhythm. To understand the possible contribution of circadian regulation, endogenous ABA levels of the algae were measured over the 24 h cycle (Fig. 7). Mean ABA levels varied between 2.2 × 10−9 to 4.3 × 10−9 µg cell−1, which assuming a mean Chlamydomonas reinhardtii cell volume16 of 270 µm3 corresponds to an endogenous concentration range of 30.8 to 60.3 nM. During the first 10 h of the photoperiod ABA levels increased by 1.8x to a maximum then declined significantly (P < 0.05 by 2 sample t-test) to a minimum at 13 h. Thereafter, ABA levels significantly (P < 0.05 by 2 sample t-test) increased again reaching near their maximum 2 h into the dark period, then remained stable until the end of the 24 h period. The time at which the level of ABA was lowest was that at which they began to form multiple bands when placed in the dark with ABA.
Figure 7.
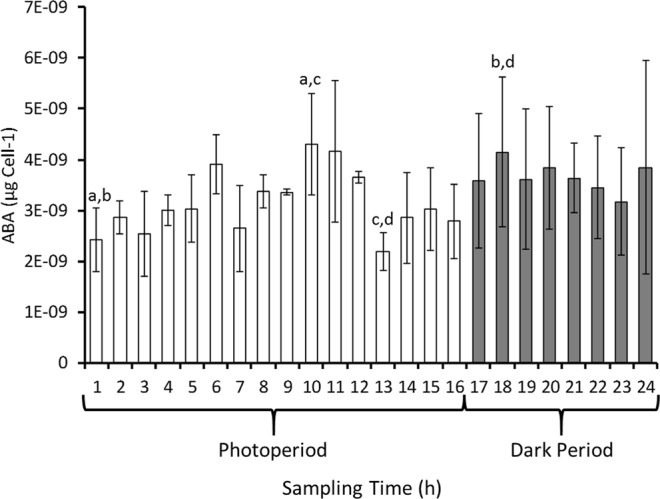
Endogenous abscisic acid (ABA) levels in C. reinhardtii CC-1021. Algal cultures were grown under a 16 h photoperiod to the mid log phase (A750 = 0.3) growth stage and were sampled at the time points indicated over a subsequent 24 h period where the 16 h photoperiod commenced at time = 0 h. Algal cells were pelleted from n = 3 replicate 25 mL aliquots of culture. At each sampling point the A750 of the cultures were determined to assess cell numbers. Pellets were extracted and assessed for ABA content by competitive ELISA (MyBioSource Inc.). Data are shown as the mean ABA content cell−1 with error bars shown as +/− the 95% confidence interval around the mean in each case. Selected significant (P < 0.05 by 2 sample t-test) pairwise differences in mean ABA levels are indicated(a to d).
Discussion
ABA is involved in plant responses to high-light17, which reduces photo-oxidative damage to PSII, and has been shown to increase algal mass under stress18. The results presented here suggest that a high-light and ABA signalling interaction increased algal photosynthetic efficiency in terms of HCO3− uptake. Why ABA reduced algal HCO3− uptake under low light is unclear, but perhaps, as in terrestrial plants19, it initiated PSII repair mechanisms under these conditions which then reduced overall photosynthetic efficiency. Previous studies have suggested that ABA may protect Chlamydomonas against photoinhibition20 and the results presented here would be consistent with such findings. What is interesting is the differential photosynthetic response to ABA under different light levels and the fact that, at particular light intensities, ABA had no significant effect. This suggests that ABA signalling and downstream responses in Chlamydomonas may be modulated by light and it may prove interesting to reassess this response under different light wavelengths.
It was clear that ABA and light signalled competitively to position the algae at a depth that was likely optimal for photosynthesis and that, in high-light, ABA treatment provided some protection. The lack of ABA response in the dark in the two independent flagella mutants would suggest that the ABA-induced upward movement observed in the wild type strain under similar conditions resulted from active swimming and not from a general alteration of cell buoyancy. Why ABA-treated, dark-incubated algae banded at multiple depths in specific samples is unclear, but it may indicate a difference in the sensitivity of the immature and adult zoospores to either light or ABA. The dark-incubated, ABA-treated algae banded at a lower depth when sampled 7 h into the dark period than when sampled at 1 h into the photoperiod, which suggests the influence of a circadian rhythm on the ABA response of the algae. In higher plants, many ABA-mediated responses appear to be regulated in such a circadian fashion21. Here, the endogenous concentrations of ABA detected in the algae were similar to those reported in other studies22,23. However, albeit statistically significant, the maximum 1.8 fold change in endogenous ABA levels of the algae over a 24 h period were not dramatic and considerably less than that which occurs in plants following stress treatments24. Here, exposure of the algae to a short period of high light intensity also only resulted in a marginal increase in ABA levels. Thus, it may be that the effect of light signalling persisted into the dark period such that the algae were only fully ABA-responsive with regard to their upward movement when biochemically prepared for exposure to the light of the early photoperiod, i.e. just before dawn. Additionally, as in plants25, it may be that ABA compartmentalisation, transport and redistribution are an important facet of its signalling capability in Chlamydomonas. The alga does possess potential homologues of genes encoding such plant ABA transporters as Arabidopsis AtABCG40 (PDR12)26, AtABCG2527 and AIT128, but with only partial Chlamydomonas sequences available and varying degrees of sequence similarity (tBLASTn: AtABCG25 vs. XM_001690681 - 38.9% identity over 78% of query sequence; PDR12 vs. XM_001697838 - 39.8% identity over 95% of query sequence; AIT vs. XM_001692259 - 26.2% identity over 37% of query sequence) it will be important to assess whether the encoded potential protein homologues have similar functions.
The differential, light-dependent taxis response of the algae to a fixed ABA source suggested that the orientation of the algae in response to the ABA signal and their resulting direction of movement depended on the presence or absence of light. Chlamydomonas use rhodopsin-initiated Ca2+ currents to regulate flagella movements and thus, their orientation to light and phototactic responses29. Ca2+ signalling is also known to regulate the gravitaxis of the alga Euglena30. In higher plants many ABA responses similarly involve Ca2+ signalling31. For example, the opening and closing of fully-hydrated Arabidopsis stomata in the day/night, light/dark cycle is an ABA-regulated process32 involving downstream Ca2+ movement and signalling31. It will be interesting to see if the case is similar in motile algae such as Chlamydomonas.
The observation that Chlamydomonas cells move towards ABA in the light and away in the dark, taken together with the ABA-induced, negative geotropic response in the dark, strongly indicates that ABA plays a role in the gravitropic/gravitaxis response of this alga that is modulated by exposure to light. How the alga perceives ABA is then a major question as there are no orthologues of the genes encoding the plant cytosol/nuclear localised PYR/PYL/RCAR receptor family33 in the Chlamydomonas genome5. Potential orthologues of genes encoding the G-protein coupled receptor (GPCR) protein, GCR234 and GPCR-types, GTG1 and GTG235, can be identified in the genome of this alga. However, it is still unclear whether or not, as has been proposed, these genes encode proteins that act as ABA receptors36–38. Thus, it will be important to determine the mechanisms by which algae such as Chlamydomonas perceive ABA and mechanistically how the response to its perception interacts with light signalling to determine both their orientation and direction of movement.
This study was unable to provide any evidence that either NAA, ethylene or hydrogen peroxide had any substantial effect on the vertical positioning of Chlamydomonas in the water column in either the light or dark. Green algae have been shown to contain and metabolise auxins39 and exogenous applications of indoleacetic acid (IAA) and NAA have been shown to improve their growth rate18 and to induce tolerance to salinity and temperature40,41. Here, exogenous NAA had a small, but not significant, effect in the light and reduced the extent to which the algae retreated from a high-light source. However, this effect was not substantial and there was no significant effect in the dark. In plants phototropism in response to blue light is regulated by the bacterial two component sensor-like, plasma membrane proteins, Phototropin 1 and 2 (Phot1 and Phot2)42, which interact with members of the NHP3 protein family, inducing their dephosphorylation43. The downstream signalling events are less clear, but the end result is the redistribution of auxin and differential growth between the shaded and illuminated sides of the plant organ. Phototropin also exists in Chlamydomonas and has been shown to be involved in the phototactic responses of this alga44. However, the NPH3 proteins appear to be specific to terrestrial plants and there are no obvious orthologues of the genes encoding these proteins in the Chlamydomonas genome. Indeed, the majority of the auxin signalling components of terrestrial plants appear to be absent in this alga5. Thus, this study would currently suggest that the phototactic responses of Chlamydomonas that occur via phototropin signalling and the gravitaxis response of the alga to ABA in the dark are not linked to the activity of auxin. It will, therefore, be important to identify the downstream protein targets of this photoreceptor in Chlamydomonas and to demonstrate how their activity is modulated by ABA.
Similarly, green algae are known to synthesise ACC and make ethylene45 and possess many recognisable components of the ethylene signal transduction pathway found in terrestrial plants5,46. Ethylene can be antagonistic to ABA responses in plants, e.g. during seed germination47 and shoot growth48,49. However, here, neither was there a significant effect of the exogenous application of ACC on the vertical positioning of the algae in the water column, nor any significant antagonistic effect on the observed ABA response in either the light or dark. Signalling via the second messenger, H2O2, has long been known to be required for the proper development of some plant ABA responses. For example, H2O2 synthesis by NADPH oxidases and its signalling are required for the ABA-induced guard cell movements that result in stomatal closure and its exogenous application also results in reduced stomatal apertures50,51. Here, such exogenous application of H2O2 did not mimic the effects of ABA and there was no significant effect on the position of the algae in the water column in either the light or the dark. Thus, it may be that many of the interactive ABA signalling processes that occur with the known phytohormones have, to a greater extent, evolved more recently in terrestrial plants.
These observations potentially provide insight into the early evolution of land plants and the ancient role of ABA in their development. Motility may have offered a survival advantage as algae evolved the protective biochemistry required for their transition to a terrestrial environment. During the diurnal variation of light intensity, motile algae may have moved up and down at the shoreline using light and ABA signalling to adjust their depth. As they adapted to high-light and desiccation, their ability to remain at the surface likely increased enabling them to become sessile and fixed in their light orientation. Such cells probably retained their core ABA signalling mechanisms while expanding downstream responses and interactions with other hormone signalling pathways as they developed multicellularity. Interestingly, the etiolation/de-etiolation response in plants appears to involve the expression of many ABA-related genes52 and plants grow upwards more in the pre-dawn hours than during the day53, suggesting retention of the algal ABA response described here. Thus, the results presented here suggest that ABA may be directly involved in plant tropisms that underpin such directional growth in the dark. Evidence that plant orientation results as much from phototropism54 and hydrotropism55 as from gravitropism is increasing and experiments conducted in micro-gravity have helped separate the contributions made by these tropisms in determining plant orientation and morphology56. ABA signalling may modulate plant phototropic and hydrotropic responses and future studies may further elucidate the interactive algal ABA and thus, terrestrial plant, molecular signalling mechanisms that operate to optimise their biochemistry in a changing environment.
Materials and Methods
Culturing of Chlamydomonas reinhardtii
All strains of C. reinhardtii were obtained from the Chlamydomonas Resource Centre, University of Minnesota, USA. Wild type C. reinhardtii57 strain CC-1021 mt+ and mutant strains CC-477 bld1-1 and CC-2492 pf13A mt+ that lacked functional flagella were aseptically maintained at 23 °C under a 16 h photoperiod (45 µmoles photons m−2 s−1) on 1% (w/v) agar plates containing Tris-acetate/phosphate (TAP) media58 containing Hutner’s trace elements59. Final composition of the TAP media was 20 mM Tris-Acetate pH 7.0, 0.62 mM K2HPO4, 0.41 mM KH2PO4, 7.5 mM NH4Cl, 0.41 mM MgSO4, 0.34 mM CaCl2, 1.3 mM EDTA-disodium salt, 77 µM ZnSO4, 0.18 mM H3BO3, 26 µM MnCl2, 6.8 µM CoCl2, 6.3 µM CuSO4, 0.89 µM (NH4)6Mo7O24, 18 µM FeSO4, 0.3 mM KOH.
For liquid culture C. reinhardtii were inoculated from agar plates and were similarly grown in TAP media with rotational shaking (130 rpm) at 23 °C under a 16 h photoperiod (45 µmoles photons m−2 s−1). Growth of the algae in liquid cultures was monitored spectrophotometrically by following the δA750. The presence of predominantly motile zoospores and cell counts relating to A750 measurements were established microscopically and using a haemocytometer respectively.
Effect of abscisic acid (ABA) on HCO3− uptake by C. reinhardtii under different light levels
A 1.0 L culture of C. reinhardtii strain CC-1021 mt+ was grown until the mid-log phase (A750 = 0.3) and the cells were collected by centrifugation (200 × g, 5 min, 23 °C). The cell pellet was re-suspended in 50 mL of TAP media before mixing with 50 mL of 3% (w/v) sodium alginate and the suspension was subdivided before the addition of (+)ABA (Sigma, analytical reagent grade) to final concentrations of between zero and 50 µM. The alginate algal cell suspensions were immediately dropped through the nozzles of individual 25 mL syringes from a height of 40 cm into an excess volume of 0.18 M CaCl2 to form spherical 2 mm diameter gel beads of encapsulated algae either with or without ABA. Replicate (n = 5 per combination of ABA concentration and light level) 1 g aliquots of beads were then placed in sealed thin walled glass vials containing 19 mL of TAP media with 1 mL of freshly prepared bicarbonate indicator buffer (0.47 mM thymol blue, 0.27 mM cresol red, 100 mM NaHCO3, 1.1 M KCl) with no head space and were immediately placed in a light tunnel at distances from a photosynthetic light source (minus UV) that exposed the samples to a range of light intensities between 7.6 and 223.2 µmoles photons m−2 s−1. Samples were incubated at 23 °C for 1 h and the δA550 of the indicator buffer relative to time zero were recorded as an indication of the relative HCO3− depletion of the media. The δA550 values were compared to a standard calibration to determine the changes in the concentration of HCO3− in the TAP media effected by the algae within each of the treatment vials.
Effect of phytohormones on the positioning of C. reinhardtii in the water column
Mid-log phase cultures of algae (A750 = 0.3), grown with a 16 h photoperiod (45 µmoles photons m−2 s−1) at 23 °C, were sampled at various time points (1, 8, 15, 20 and 23 h) during a 24 h cycle with the beginning of the photoperiod starting at time zero. Fully dispersed aliquots of the culture were transferred to 25 mL measuring cylinders to form 10 cm deep columns of algal cell suspensions. For phytohormone treatments, either (+) ABA from a 100 mM stock in 100% (v/v) ethanol, 1-naphthaleneacetic acid (NAA) from a 5.4 mM stock in 10 mM KOH, 1-aminocyclopropane-1-carboxylic acid from a 100 mM stock in water or hydrogen peroxide (H2O2) from a 100 mM stock in water were added to a final concentration of 50 µM. For control treatments an equivalent amount of stock solvent minus the phytohormone was added in each case. The cylinders were then either illuminated from above with high light (319.8 µmoles photons m−2 s−1) or were placed in the dark for 50 min at 23 °C. After incubation the positions of the algae were recorded photographically and the A750 of the cultures immediately measured at various depths from the surface of the water columns.
Taxis of C. reinhardtii in response to a fixed ABA source in the light and dark
Mid-log phase cultures of the algae (A750 = 0.3), grown with a 16 h photoperiod, were sampled 8 h into the photoperiod and replicate (n = 5) fully dispersed 25 mL aliquots were dispensed into individual rectangular (1.5 × 1.5 × 12 cm) open-topped, light-permeable, acrylic troughs into which had been placed, at one end, agarose plugs containing various concentrations of (+) ABA between zero and 1 mM. The troughs were then immediately placed horizontally either in surrounding illumination (45 µmoles photons m−2 s−1) or in the dark and were incubated at 23 °C for 1 h. After incubation the positions of the algae in the tubes were recorded photographically and 200 µL samples simultaneously taken at incrementally increasing distances from the agarose plugs and their A750 measured.
Measurements of endogenous ABA in C. reinhardtii
Replicate (n = 3) samples of known A750 and thus, known cell density, were taken at hourly intervals from a mid-log phase culture of C. reinhardtii grown under a 16 h photoperiod (45 µmoles photons m−2 s−1) at 23 °C. Aliquots (25 mL) of the culture were centrifuged to collect the algal cells and pellets were then immediately frozen in liquid N2 and freeze-dried. The freeze-dried pellets were then extracted and ABA contents determined using a competitive enzyme-linked immunosorbent assay60 kit (MyBioSource Inc., San Diego, CA, USA) as per the manufacturer’s instructions. Freeze dried algal pellets were extracted with 200 µL of kit sample extraction buffer for 16 h at 4 °C with rotational shaking at 60 rpm in the dark. This ratio of freeze dried algal cells to sample extraction buffer volume ensured that the final amounts of ABA measured in the test samples fell within the optimal detection range of the ELISA assay. Sample extracts were subsequently centrifuged (13,000 × g, 15 min 4 °C) and 50 µL aliquots of the clear supernatants were applied to individual wells of the ABA-coated ELISA plates. Aliquots (50 µL) of (+) ABA standards (0 to 10 µg mL−1) were similarly applied to adjacent wells. Next, 50 µL of anti-ABA antibody solution was added to each well, mixed and incubated at 37 °C for 30 min. Wells of the ELISA plate were subsequently aspirated and washed 3 times before the addition of 100 µL of horseradish peroxidase (HRP) second antibody conjugate and further incubation at 37 °C for 30 min. The ELISA plate wells were again aspirated and washed 5 times before the addition of 90 µL of TMB chromogenic substrate with incubation in the dark for 20 min at 37 °C. HRP activity was halted by the addition of 50 µL of stop solution and the fluorescence of individual wells of the ELISA plate was then measured at 450 nM using a FLUOstar Omega microplate reader (BMG Labtech). The amount of ABA in the test samples was then determined by comparison of the sample 450 nm readings with the curve generated from those of the standards applied to the ELISA plate. The amounts of ABA determined in each test sample were then divided by the number of algal cells extracted in each case and the results expressed as µg ABA algal cell−1.
Data handling and statistical analysis
Where appropriate, groups of data were assessed for normality by Shapiro Wilk tests and for between group homogeneity of variances around the means by Bartlett’s tests. For HCO3− depletion data, overall between data group comparisons of means were made by one and two way ANOVAs and post hoc pairwise mean comparisons by Tukey Kramer tests. For the algal movement data, comparisons of the relative distributions of the algae in response to the treatments were undertaken using χ2 tests of independence with post hoc χ2 tests of goodness of fit. Endogenous ABA data was analysed by one way ANOVA and selected pairwise comparisons by 2 sample t-tests. All tests were performed at α = 0.05 level of confidence.
Acknowledgements
We wish to acknowledge Dr. Man-Kim Cheung for valuable technical assistance. LA-H was funded by the Ministry of Higher Education, Al Baha University, Saudi Arabia. AG and RD were funded by the University of the West of England, Bristol, UK.
Author Contributions
L.A.-H., A.G. and R.D. performed the experimental work. H.M. provided substantial technical expertise. M.L. and I.W. supervised the research. All authors wrote the manuscript.
Data Availability
The data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leliaert F, et al. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012;31:1–46. doi: 10.1080/07352689.2011.615705. [DOI] [Google Scholar]
- 2.Yoon HS, Hackett YD, Ciniglia C, Pinto G, Bhattacharya D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 2004;21:809–818. doi: 10.1093/molbev/msh075. [DOI] [PubMed] [Google Scholar]
- 3.Delwiche CF, Cooper ED. The evolutionary origin of a terrestrial flora. Curr. Biol. 2015;25:899–910. doi: 10.1016/j.cub.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Hegemann, P. & Berthold, P. Sensory photoreceptors and light control of flagellar activity in The Chlamydomonas Sourcebook: Cell Motility and Behavior, Vol. 3 (ed. Whitman, G.) 395–429 (Acad. Press, Elsevier, London, UK, 2009).
- 5.Wang C, Yang L, Li S-S, Han G-Z. Insights into the origin and evolution of the plant hormone signalling machinery. Plant Physiol. 2015;167:872–886. doi: 10.1104/pp.114.247403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vishwakarma, K. et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 8, 161, fpls.2017.00161 (2017). [DOI] [PMC free article] [PubMed]
- 7.Han W, Rong H, Zhan H, Wang MH. Abscisic acid is a negative regulator of root gravitropism in Arabidopsis thaliana. Biochem. Biophys. Res. Comm. 2009;78:695–700. doi: 10.1016/j.bbrc.2008.11.080. [DOI] [PubMed] [Google Scholar]
- 8.Moriwaki T, Miyazawa Y, Fujii N, Takahashi H. Light and abscisic acid signalling are integrated by MIZ1 gene expression and regulate hydrotropic response in roots of Arabidopsis thaliana. Plant, Cell Environ. 2012;35:1359–1368. doi: 10.1111/j.1365-3040.2012.02493.x. [DOI] [PubMed] [Google Scholar]
- 9.Holzinger A, Becker B. Desiccation tolerance in the streptophyte green alga Klebsormidium: the role of phytohormones. Comm. Integ. Biol. 2015;8:e1059978. doi: 10.1080/19420889.2015.1059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan AK, Rose PD. Abscisic acid metabolism in salt-stressed cells of Dunaliella salina: possible interrelationship with beta-carotene accumulation. Plant Physiol. 1991;97:798–803. doi: 10.1104/pp.97.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Ando H, Hanaoka M, Tanaka K. Abscisic acid participates in the control of cell cycle initiation through heme homeostasis in the unicellular red alga Cyanidioschyzon merolae. Plant Cell Physiol. 2016;57:953–960. doi: 10.1093/pcp/pcw054. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi, Y. & Tanaka, K. Transcriptional regulation of tetrapyrrole biosynthetic genes explains abscisic acid-induced heme accumulation in the unicellular red alga Cyanidioschyzon merolae. Front. Plant Sci. 7, 1300, fpls.2016.01300 (2016). [DOI] [PMC free article] [PubMed]
- 13.Wu G, et al. The effects of abscisic acid, salicylic acid and jasmonic acid on lipid accumulation in two freshwater Chlorella strains. J. Gen. Appl. Microbiol. 2018;64:42–49. doi: 10.2323/jgam.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Mayer AM. Chlamydomonas: Adaptation phenomena in phototaxis. Nat. 1968;217:875–876. doi: 10.1038/217875b0. [DOI] [Google Scholar]
- 15.Bennett, R. R. & Golestanian, R. A steering mechanism for phototaxis in Chlamydomonas. J. Roy. Soc. Inter. 12, 20141164; rsif.2014.1164 (2015). [DOI] [PMC free article] [PubMed]
- 16.Weiss D, Schneider G, Niemann B. Computed tomography of cryogenic biological specimens based on X-ray microscopic images. Ultramicro. 2000;84:185–197. doi: 10.1016/S0304-3991(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 17.Galvez-Valdivieso G, et al. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell. 2009;21:2143–2162. doi: 10.1105/tpc.108.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park W-K, et al. Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl. Biochem. Biotech. 2013;171:1128–1142. doi: 10.1007/s12010-013-0386-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang Fubiao, Liu Jianchao, Chen Minxue, Zhou Lujian, Li Zhaowei, Zhao Qian, Pan Gang, Zaidi Syed-Hassan-Raza, Cheng Fangmin. Involvement of Abscisic Acid in PSII Photodamage and D1 Protein Turnover for Light-Induced Premature Senescence of Rice Flag Leaves. PLOS ONE. 2016;11(8):e0161203. doi: 10.1371/journal.pone.0161203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saradhi PP, et al. Protection against the photoinduced inactivation of the photosystem II complex by abscisic acid. Plant, Cell Environ. 2000;23:711–718. doi: 10.1046/j.1365-3040.2000.00579.x. [DOI] [Google Scholar]
- 21.Seung D, Risopatron JPM, Jones BJ, Marc J. Circadian clock-dependent gating in ABA signalling networks. Protoplasma. 2012;249:445–457. doi: 10.1007/s00709-011-0304-3. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch R, Hartung W, Gimmler H. Abscisic acid content of algae under stress. Bot. Acta. 1989;102:326–334. doi: 10.1111/j.1438-8677.1989.tb00113.x. [DOI] [Google Scholar]
- 23.Hartung W, Gimmler H. A stress physiological role for abscisic acid (ABA) in lower plants. Prog. in Bot. 1994;55:157–173. doi: 10.1007/978-3-642-78568-9_9. [DOI] [Google Scholar]
- 24.Hartung W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010;37:806–812. doi: 10.1071/FP10058. [DOI] [Google Scholar]
- 25.Li, B. & Jia, W. ABA Transport and distribution in relation to its function in plants. In: Zhang, D. P. (ed.) Abscisic acid: metabolism, transport and signaling. Springer, Dordrecht (2014).
- 26.Kang J, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuromori T, et al. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanno Y, et al. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc. Natl Acad. Sci. USA. 2012;109:9653–9658. doi: 10.1073/pnas.1203567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harz H, Hegemann P. Rhodopsin-regulated calcium currents in Chlamydomonas. Nat. 1991;351:489–491. doi: 10.1038/351489a0. [DOI] [Google Scholar]
- 30.Häder, D. P. & Hemmersbach, R. Gravitaxis in Euglena in Euglena: Biochemistry, Cell and Molecular Biology. Advances in Experimental Medicine and Biology, Vol 979 (eds Schwartzbach, S. & Shigeoka, S.) 237–266 (Springer, 2017). [DOI] [PubMed]
- 31.Kudla J, et al. Advances and current challenges in calcium signalling. New Phytol. 2018;218:414–431. doi: 10.1111/nph.14966. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro DM, et al. Differential requirement for NO during ABA-induced stomatal closure in turgid and wilted leaves. Plant, Cell Environ. 2008;32:46–57. doi: 10.1111/j.1365-3040.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- 33.Fujii H, et al. In vitro reconstitution of an abscisic acid signalling pathway. Nat. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, et al. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Sci. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 35.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Christmann A, Grill E. Are GTGs ABA’s biggest fans? Cell. 2009;136:21–23. doi: 10.1016/j.cell.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 37.Risk JM, Day CL, Macknight RC. Reevaluation of abscisic acid-binding assays shows that G-Protein-Coupled Receptor 2 does not bind abscisic acid. Plant Physiol. 2009;150:6–11. doi: 10.1104/pp.109.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo J, Yang X, Weston DJ, Chen J-G. Abscisic Acid Receptors: Past, Present and Future. J. Integ. Plant Biol. 2011;53:469–479. doi: 10.1111/j.1744-7909.2011.01044.x. [DOI] [PubMed] [Google Scholar]
- 39.Žižková E, et al. Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Annal. Bot. 2017;119:151–166. doi: 10.1093/aob/mcw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowak J, Sonaike B, Lawson GW. Auxin induced stress tolerance in algae. Environ. Pollut. 1988;51:213–218. doi: 10.1016/0269-7491(88)90262-X. [DOI] [PubMed] [Google Scholar]
- 41.Piotrowska-Niczyporuk A, Bajguz A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae) Plant Growth Regul. 2014;73:57–66. doi: 10.1007/s10725-013-9867-7. [DOI] [Google Scholar]
- 42.Kutschera U, Briggs WR. Root phototropism: from dogma to the mechanism of blue light perception. Planta. 2012;235:443–452. doi: 10.1007/s00425-012-1597-y. [DOI] [PubMed] [Google Scholar]
- 43.Pedmale UV, Liscum E. Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J. Biol. Chem. 2007;282:19992–20001. doi: 10.1074/jbc.M702551200. [DOI] [PubMed] [Google Scholar]
- 44.Trippens J, et al. Phototropin influence on eyespot development and regulation of phototactic behavior in Chlamydomonas reinhardtii. Plant Cell. 2012;24:4687–4702. doi: 10.1105/tpc.112.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Booker MA, DeLong A. Producing the ethylene signal: Regulation and diversification of ethylene biosynthetic enzymes. Plant Physiol. 2015;169:42–50. doi: 10.1104/pp.15.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ju, C. et al. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants1, 14004, nplants14004 (2015). [DOI] [PubMed]
- 47.Zhou L, Jang JC, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. PNAS USA. 1998;95:10294–10299. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J. Exp. Bot. 2000;51:1575–1584. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- 49.LeNoble ME, Spollen WG, Sharp RE. Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 2004;55:237–245. doi: 10.1093/jxb/erh031. [DOI] [PubMed] [Google Scholar]
- 50.Desikan R, et al. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- 51.Wang P, Song CP. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 2008;178:703–718. doi: 10.1111/j.1469-8137.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 52.Humplík Jan F., Bergougnoux Véronique, Jandová Michaela, Šimura Jan, Pěnčík Aleš, Tomanec Ondřej, Rolčík Jakub, Novák Ondřej, Fellner Martin. Endogenous Abscisic Acid Promotes Hypocotyl Growth and Affects Endoreduplication during Dark-Induced Growth in Tomato (Solanum lycopersicum L.) PLOS ONE. 2015;10(2):e0117793. doi: 10.1371/journal.pone.0117793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seluzicki A, Burko Y, Chory J. Dancing in the dark: darkness as a signal in plants. Plant, Cell Environ. 2017;40:2487–2501. doi: 10.1111/pce.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vandenbrink, J. P., Kiss, J. Z., Herranz, R. & Medina, F. J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 5, 563, fpls.2014.00563 (2014). [DOI] [PMC free article] [PubMed]
- 55.Shkolnik D, Fromm H. The Cholodny-Went theory does not explain hydrotropism. Plant Sci. 2016;252:400–403. doi: 10.1016/j.plantsci.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Poulet L, Fontaine J-P, Dussap C-G. Plant’s response to space environment: a comprehensive review including mechanistic modelling for future space gardeners. Bot. Lett. 2016;163:337–347. doi: 10.1080/23818107.2016.1194228. [DOI] [Google Scholar]
- 57.Dangeard P-A. Recherches sur les algues inférieures. Annal. Sci. Nat. Bot. 1888;7:105–175. [Google Scholar]
- 58.Gorman DS, Levine RP. Cytochrome F and plastocyanin - Their sequence in photosynthetic electron transport chain of Chlamydomonas reinhardi. PNAS USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hutner SH, Provasoli L, Schatz A, Haskins CP. Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc. Am. Phil. Soc. 1950;94:152–170. [Google Scholar]
- 60.Walker-Simmons, M. K., Rose, P. A., Hogge L. R. & Abrams S. R. Abscisic Acid. In: Tucker G. A., Roberts J. A. (eds) Plant Hormone Protocols. Methods in Molecular Biology 141. Humana Press (2000). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.