Abstract
Thymol is generally recognized as a safe substance by the FDA and has been widely used in the pharmaceutical, food, and cosmetic industries. Pharmacokinetic (PK) studies of thymol have been previously conducted for oral administration, but there has been no PK study for inhalation administration or intravenous (IV) injection. This study aims at exploring and comparing the inhalation and IV PK profile of thymol in a mouse model. The inhalation PK for mouse model was corrected with fur/skin absorption. Thirty‐two male CD‐1 mice were randomized into two study arms, Arm‐A for intravenous (n = 16) and Arm‐B for inhalation (n = 16). The amount of thymol in the mouse serum was measured for Arm‐A and for Arm‐B at the highest dose. Furthermore, 48 mice were utilized for fur/skin absorption of thymol. In total, 320 mouse serum samples for thymol were analyzed by LC/MS method. After inhalation, the peak concentration of thymol in mouse serum was 42.3 ng/mL (Cmax) and occurred at 2 minutes (tmax). The AUC of the inhaled thymol at 0‐60 minutes (AUC0‐60) was 464 ng/mL/min. From 10‐60 minutes post‐dose, the PK inhalation curve appeared to be higher than that for the IV injection. This is likely attributed to the effect of absorption of thymol through the fur/skin of mice. After an adjustment by fur/skin absorption, the PK profile for net inhalation closely matched the two‐compartment model. In fact, the bioavailability for the net inhalation of thymol was 74% and 77% relative to that for IV injection per AUC0‐60min and AUC0‐infinite, respectively.
Keywords: bioavailability, epinephrine HFA, inhalation, intravenous injection, mice, thymol
1. INTRODUCTION
Primatene® Mist (epinephrine HFA) is a proposed replacement for the previous over‐the‐counter asthma metered‐dose inhaler (MDI), Primatene Mist® CFC.1 The newly formulated epinephrine HFA MDI utilizes thymol as its antioxidant, an inactive ingredient not found in the previous Primatene Mist® CFC. According to the Code of Federal Regulations: “Thymol is an essential oil that is extracted from thyme, mandarine, and tangerine oils and is FDA approved when used as a synthetic flavoring (21 CFR 172.515), a preservative and indirect food additive of adhesives (21 CFR 175.105). The source plant (thyme), from which thymol is extracted, is generally recognized as a safe substance (GRASS) by FDA (21 CFR 182.10, 21 CFR 182.2).”2
Currently, thymol presents a wide range of functional possibilities in the pharmaceutical, food, and cosmetic industries.3 It has also been used as an additive in an inhalation anesthetic.4 For centuries, thymol has been shown to have various pharmacological properties including antioxidant, free radical scavenging, anti‐inflammatory, analgesic, antispasmodic, antibacterial, antifungal, antiseptic, and antitumor activities.3, 5, 6 Previous reports have studied the pharmacokinetics of thymol following oral administration.5 However, a pharmacokinetic study of thymol has not been previously reported for inhalation administration or intravenous (IV) injection. This study aims at exploring the PK curve of thymol after inhalation and IV injection in the mouse model.
2. MATERIALS AND METHODS
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies.7
2.1. Test animals
Outbred CD‐1 (cluster of differentiation 1) male mice with an approximate age of 8 weeks were purchased from ENVIGO (formerly Harlan laboratories). At the time of testing, mice were at a weight range of 31‐37 g. The procedures for receipt, identification, and care of the animals are in accordance with the lab facility standard operating procedures (SOPs). Prior to testing, the protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). The routine diet for mice was 2016 Teklad Global 16% Protein Rodent Diet, purchased from ENVIGO. No dietary ingredients were expected to interfere with the outcome of the studies.
In this study, a total of 32 male CD‐1 mice were randomized into two study arms, Arm‐A for intravenous (n = 16) and Arm‐B for inhalation (n = 16).
2.2. Test articles
The study article used for intravenous injection is 0.1 mg/mL thymol in saline solution. Pure thymol is first dissolved in ethanol to prepare a 5% (w/v) solution. The solution was diluted 500 times with 0.9% saline to prepare 0.1 mg/mL thymol in saline.
For the inhalation, 0.5% thymol in HFA MDI was used. This article contains the same ingredients as epinephrine HFA MDI, except there is no epinephrine active ingredient. In addition, the thymol concentration is 0.5%, which is 50 times the content in the epinephrine HFA MDI formulation.
2.3. Drug administration
2.3.1. Intravenous (IV) injection
In Arm‐A, 0.1 mg/mL thymol in saline was directly injected as a bolus into the mouse's tail vein at a volume of 0.16 mL within 2‐3 seconds. The 16 mice were lightly anesthetized by isoflurane before injection. The total amount of thymol injected was equivalent to 16 mcg, or 0.48 mg/kg considering an average mouse body weight of 33.6 g.
2.3.2. Inhalation administration
In Arm‐B, a total of 16 mice were mounted into a specially designed stainless steel 21.5 L breathing tank. Fifteen sprays of the test article containing 0.5% thymol in HFA were delivered into the tank. In order to ensure consistent thymol concentration, a stirring fan installed inside the tank was set at 400 RPM and was started before the first spray of the test article. Thirty seconds after the last spray (t = 0 min), two groups of eight mice were mounted into the inhalation chamber, allowing them to breathe from inside the chamber for 10 minutes (Figure 1).
Figure 1.
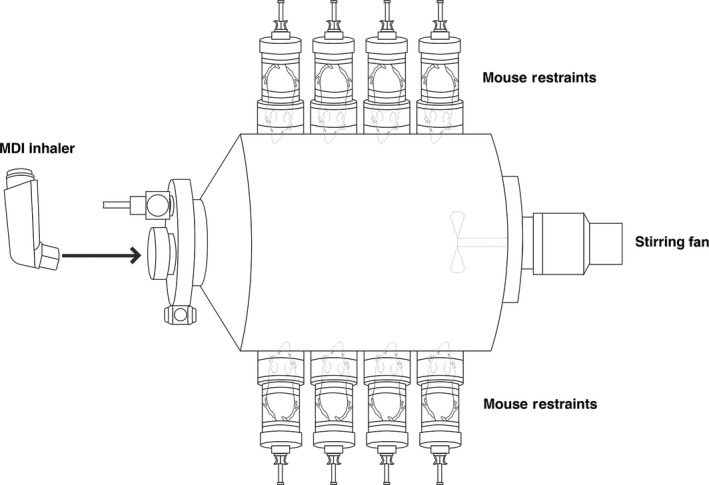
Mice mounted to the tank during treatment of inhalation
The total amount of thymol inhaled was calculated based on the (a) actual concentration of the thymol in the air in the tank, which was measured by a validated LC‐MS to be approximately 69.0 mcg/L, (b) the mouse breathing time (10 minute), and (c) the mouse breath volume per minute of 22.5 mL/min (based on the typical mouse breath frequency of 150 breath/min and mouse tidal volume of 0.15 mL/breath).8 Therefore, the total amount of thymol inhaled in this single‐dose study was calculated to be 15.5 mcg or 0.47 mg/kg. The dose of thymol used in both arms was approximately the same. It is worth mentioning that a thymol concentration of 0.5% is about 50 times the amount of thymol in the epinephrine HFA formulation.
2.4. Fur‐skin absorption
In order to determine the absorption of thymol through fur and skin during the inhalation treatment, an additional experiment was performed to measure the absorption of thymol by fur and skin without thymol inhalation. The mice were placed in the same breathing tank described above but in a reversed position with their tails and body facing the tank (Figure 2). In this setting, both the fur and skin of the mice were exposed to thymol at the same concentration (69.0 mcg/L) and duration (10 minutes). Mice were allowed to breathe fresh air outside of the tank during this trial to prevent thymol inhalation. It is expected that the PK data for mice fur/skin absorption will be very low, as most are near to or lower than quantitative limit (QL) of 5 ng/mL so that the data for fur/skin are highly fluctuating. In order to obtain evaluable data for mouse PK by fur/skin absorption, 24 × 2 = 48 mice were tested in the fur/skin PK investigational study. It should be noted that the dose for fur/skin absorption cannot be directly determined, and was estimated per AUC relative to inhalation.
Figure 2.
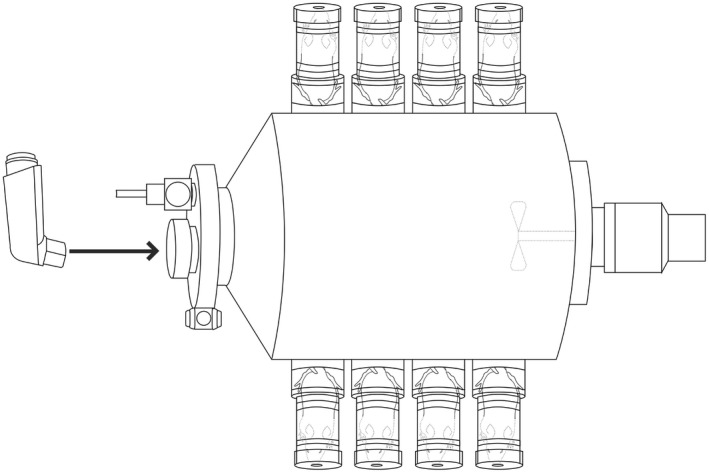
Mice positioned to breathe fresh air outside the tank with the fur/skin exposed to thymol
2.5. PK sampling
Following either injection or inhalation, mouse blood was collected from retro‐orbital sinus under anesthesia (isoflurane) at baseline and 2, 5, 10, 20, 30, and 60 minutes post‐dose. Due to the small body weight of mice, and the limited volume of mouse blood that can be collected, the mice in each study arm were further divided into different subgroups. Specifically, PK samples of 50% of mice (n = 8) in Arm‐A and Arm‐B were collected at baseline, 2, 10, and 30 minutes. The samples of the other 50% (n = 8) in each arm were collected at baseline 5, 20, and 60 minutes. The combination of PK data from subgroups for each arm would provide a global PK profile of thymol in mice. For the fur/skin absorption study, the same sampling points (n = 24) were conducted.
2.6. Determination of thymol in mouse serum
A solid‐phase extraction and LC/MS method was developed and validated to quantify the concentration of thymol in mouse serum. Eugenol, a member of the phenylpropanoid class of chemical compounds, is used as an internal standard for serum samples and was extracted by a solid‐phase extraction technique using a Waters Oasis HLB 96‐well μElution plate. The extracts were then subjected to high‐performance liquid chromatography on a Phenomenex Kinetex C18 30 × 2.1 mm column and eluted by a gradient program of mobile phase A (0.1% Formic Acid in deionized water) and B (0.1% Formic Acid in methanol) with a flow rate of 0.3 mL/min. The chromatographic runtime was 7 min per injection. Compounds were determined by Thermo TQS Quantiva triple quadrupole mass spectrometer equipped with a heated electrospray ionization source in MS/MS mode.
The analytical method was validated using a Quantitative limit of 5 ng/mL and a Linearity range of 5 to 5000 ng/mL. Six levels of calibration standards were included in each calibration curve. The correlation coefficient was found to be 0.9933‐0.9998 and the method precision CV was found to be 6.8%‐9.1%. Furthermore, the method accuracy was 93%‐97% and the mean recovery for thymol was 96% and 92% for the internal standard.
2.7. Pharmacokinetic analysis for thymol in mouse model
To determine the thymol present in mouse serum from intravenous delivery, inhalation, and fur/skin absorption, the following PK models10 were used for PK parameter estimation:
-
1
IV Study: One‐compartment model with first‐order output, after bolus injection
| (1) |
where C(t) is the concentration of the drug in serum at time t, D is the dose of the drug administration, Vd is the apparent volume of distribution, and Ke is the total elimination constant.
-
2
A one‐compartment model with a first‐order input and a first‐order output was used to evaluate the PK model for thymol in mouse serum. The model is defined by the following equation:
| (2) |
where C(t), D, Vd, and Ke have the same definition as given in Equation (1) above and Ka is the absorption constant.
-
3
The two‐compartment model was also used to assess the PK profile of thymol in this study.
| (3) |
where α and β are elimination constants for the two compartments; Ka is the absorption constant; and A, B, and C are the related constants for the three terms.
The evaluation using the PK model for C(t) based on the above equations for thymol administered by IV and thymol administered by high‐dose inhalation, as well as fur/skin absorption, were calculated as shown in the following section.
In this study, the following PK parameters were evaluated:
Cmax – the maximum value of the thymol concentration in mice serum;
AUC0‐60min – the area under the curve of thymol concentration vs time between zero and 60 minutes post‐treatment;
AUC0‐infinity – the area under the curve of thymol concentration vs time between zero and the infinite time post‐treatment;
tmax – time needed to reach Cmax;
Vd – the apparent volume of distribution;
t1/2 – half‐life for total elimination;
Clt – the clearance of elimination, Clt = VdKe was used.
Ka – absorption constant;
Ke – elimination constant for one‐compartment model; and
α and β – elimination constants for two‐compartment model.
2.8. PK non‐linear mixed effect (NLME) analysis with software WinNonLin
Standard Phoenix/WinNonLin software was used to perform Nonlinear Mixed Effect (NLME) modeling for population PK analysis in both one‐compartment and two‐compartment models. Using the QRPEM (Quasi‐Random Parametric Expectation Maximization) Algorithm, the best model was chosen based on the lowest Akaike Information Criterion (AIC). Additionally, Chi‐square evaluation was performed to calculate the Goodness of Fit (GOF), which is defined below.11
| (4) |
where x(ti) is the serum concentration of thymol under the model at the time of ti; and is the population average of serum concentration of thymol at the time of ti.
2.9. Net inhalation: adjustment of inhalation PK data from fur/skin absorption
During the 10‐minute breathing for inhalation study, the fur/skin of mice was also exposed to gas phase thymol. The PK profile for “net inhalation” should be the PK data of “apparent inhalation” adjusted based on the contribution of the fur/skin absorption seen here.
| (5) |
where CjX(t) is the thymol concentration for j‐compartment model in treatment X at time of t. Additionally, C fur/skin(t) is the thymol concentration for fur/skin absorption at time of t and factor λ = 0 − 1, which will be determined by PK modeling analysis based on lowest GOF according to Equation (4).
3. RESULTS
3.1. Comparison of PK data of thymol in IV, inhalation, and fur/skin administration
Three hundred and twenty (320) thymol PK samples of mouse serum were analyzed for IV, inhalation, and fur/skin delivery routes. The obtained data were summarized in Table 1. Thymol PK curves of each delivery route are presented in Figure 3, where the error bar denotes “standard error.”
Table 1.
Experimental serum concentrations of thymol administrated by IV, inhalation, and fur/skin in mouse model
| Delivery route | IV | Oral "Inhalation" | Fur/skin absorption |
|---|---|---|---|
| Delivery method | 0.16 mL of 0.1 mg/mL thymol, in bolus | 15 Sprays of 0.5% thymol into 21.5 L Tank, Breathing for 10 minutes | the same as the "Inhalation", but breathing outside air |
| Dose, µg/treatment | 16 | 15.5 | 10.9 |
| Dose, mg/kg mouse | 0.48 | 0.47 | 0.33 |
| # of mice treateda | 8 × 2 | 8 × 2 | 24 × 2 |
| Thymol concentration, in ng/mL | Mean ± SEb | Mean ± SEb | Mean ± SEb |
| 0 minutes | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2 minutes | 76.6 ± 6.0 | 39.2 ± 8.6 | 3.4 ± 0.4 |
| 5 minutes | 41.4 ± 5.3 | 19.2 ± 2.1 | 4.0 ± 0.4 |
| 10 minutes | 13.6 ± 1.0 | 16.0 ± 3.6 | 5.5 ± 0.5 |
| 20 minutes | 3.7 ± 1.1 | 6.9 ± 1.3 | 7.5 ± 1.2 |
| 30 minutes | 2.6 ± 0.8 | 6.4 ± 1.5 | 7.3 ± 0.9 |
| 60 minutes | 0.2 ± 0.1 | 3.9 ± 2.2 | 5.9 ± 0.8 |
Due to limited volume of mouse blood that can be collected, the mice in each study arm was divided into two different subgroups to provide a global PK profile: samples from one subgroup (n = 8 for IV and inhalation, and n = 24 for fur/skin absorption) were collected at 0, 2, 10, and 30 minutes; another subgroup (n = 8) were collected at 0, 5, 20, and 60 minutes.
SE, Standard error = where, s is standard deviation, n is number of mice in the treatment.
Figure 3.
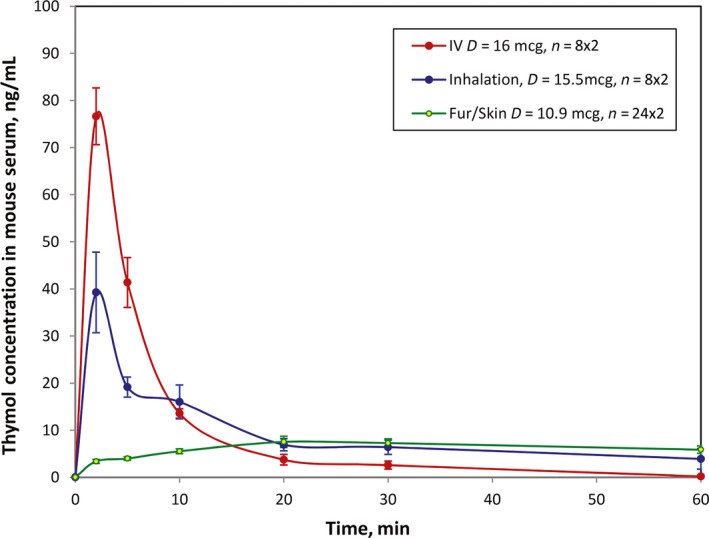
Thymol serum concentration curve vs time in mouse model by experiment
Overall, the PK exposure of thymol for intravenous injection was higher than that of the inhalation. Cmax occurred at 2 minutes in both IV and inhalation administration routes and was found to be 76.6 ng/mL and 39.2 ng/mL, respectively. During the first 10 minutes, the IV thymol curve was higher than the inhalation curve. However, at 10‐20 minutes after administration, the IV thymol PK curve became lower than that of the inhalation curve. From 20 to 60 minutes, the inhalation curve continued to be higher and indicated a sustained release pattern characterized by an almost flat curve.
3.2. Results of the PK parameters obtained by model analysis
The experimental PK data using the PK models were calculated by NLME with WinNonLin software. The obtained PK parameters are provided in Table 2.
Table 2.
PK parameters for thymol PK studies by IV, inhalation, and fur/skin delivery
| Delivery route | Intravenous | Inhalation | Fur/skin | Bioavailability for net inhalation | |
|---|---|---|---|---|---|
| Apparent | Net, λ = 0.25 | ||||
| Dose, μg | 16.0 | 15.5 | 15.5 | 10.4 | |
| PK model applied | 1‐Compartment | 2‐Compartment | 2‐Compartment | 1‐Compartment | |
| Reference equations | Equation ((1)) | Equation ((3)) | Equation ((5)) | Equation ((2)) | |
| PK parameters | |||||
| Cmax, ng/mL | 78.3 | 53.9 | 42.3 | 8.09 | 54% |
| AUC 0‐60 min, ng/mL*min | 626.7 | 529.7 | 464.3 | 356.9 | 74% |
| AUC 0‐infinity, ng/mL*min | 626.7 | 575.6 | 484.2 | 459.9 | 77% |
| t max, min | 2.00 | 2.00 | 2.00 | 20.0 | |
| t 1/2, min | 3.43 | 3.24 | 4.73 | 19.5 | |
| V d, for first compartment, mL | 136.4 | 125.9 | 64.7 | 647.5 | |
| for second compartment, mL | ‐ | 283.3 | 75.4 | ‐ | |
| Clt for first compartment, mL/min | 27.6 | 26.9 | 9.48 | 23.0 | |
| Clt for second compartment, mL/min | ‐ | 52.1 | 14.6 | ‐ | |
| Absorption constant, K a, min‐1 | ‐ | 1.79 | 1.61 | 0.064 | |
| Elimination constant, K e, min‐1 | 0.20 | 0.21 | 0.15 | 0.036 | |
| α, min−1 | ‐ | 0.76 | 0.51 | ‐ | |
| β, min−1 | ‐ | 0.052 | 0.056 | ‐ | |
| Goodness of Fit, see Equation ((4)) | 1.8 | 4.8 | 2.3 | 0.7 | |
3.3. Thymol PK profile for IV delivery
As expected, Thymol PK for IV delivery fits one‐compartment model with first‐order output. Due to the fact that IV delivery was administrated as a bolus, the IV PK profile meets instantaneous input, as described by Equation (1), with a GOF of 1.8.
3.4. Thymol PK profile for absorption through the fur and skin of mice
The PK curve for fur/skin absorption shows a type of sustained absorption in Figure 3 where 20 minutes after administration, the thymol level in serum for fur/skin is higher than both IV and inhalation delivery. The PK profile for fur/skin absorption of thymol fits the one‐compartment model with first‐order input and first‐order output, as described in Equation (2), with a GOF of 0.7.
A small absorption constant (Ka = 0.064) found during fur/skin administration of the mouse model indicates the sustained characteristic of thymol on the fur/skin of mice.
3.5. Thymol PK profile for “net inhalation” delivery
The higher PK data found in the inhalation curve from 10 to 60 minutes post‐dose may be due to the contribution from thymol absorbed through the fur/skin during the dosing.
Thymol Mouse PK Curves are essentially for “inhalation ‐ λ × skin/fur” which is a combined contribution from “skin/fur only” and “net Inhalation.” PK NLME analysis by Phoenix/WinNonLin for λ = 0, 0.25, 0.50, and 0.75 were conducted. When λ = .25, GOF has a minimum value of 2.3. In Table 2, the PK parameters for “net inhalation” (λ = .25) are also provided. More information on the “net inhalation” PK calculation regarding λ is provided in Figure S1.
The relatively small value of λ could be a result of multiple factors. (a) In the fur/skin absorption study (see Figure 2), the mouse tail is directly exposed under thymol vapor. Mouse tails are naked and likely to have a higher absorption with thymol. (b) Additionally, in the inhalation study (see Figure 1), the mouse tail is far away from the thymol vapor and exposed to outside fresh air. The combination of these two factors causes a smaller adjustment factor λ = .25.
The bioavailability for the net inhalation of thymol (after a correction for skin absorption of thymol) was 74% and 77% relative to that for IV injection per AUC0‐60 min and AUC0‐infinite, respectively, as shown in Table 2.
3.6. Thymol PK profile based on PK models for IV, net inhalation, and fur/skin delivery
Based on the obtained PK parameters, C(t) was obtained based on PK models for (a) IV, (b) net inhalation, and (c) fur/tail absorption. The C(t) obtained per the PK model and experimental data are summarized in Table S1.
The PK curves obtained by PK models are comparable with the experimental data obtained from the actual PK study in Figure 4. The figure shows that the PK models are suitable for the mouse thymol PK profile by IV injection, net inhalation (after a correction for fur/skin absorption of thymol with λ = .25), and fur/skin delivery routes.
Figure 4.
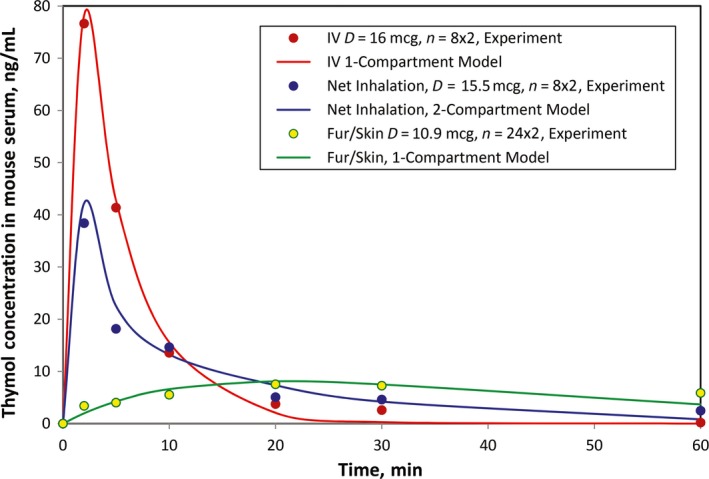
Thymol serum concentration curve vs time in mouse model: experiment vs PK models
4. DISCUSSION
The major purpose of this study was to obtain the PK information for thymol by inhalation. IV delivery was also studied to provide a basic PK profile, and fur/skin absorption was studied to provide a basis for adjustment to obtain a PK profile for “net inhalation.” IV delivery for the mouse model shows a one‐compartment model with instantaneous input and first‐order output. Fur/skin absorption for the mouse model shows a one‐compartment model with first‐order input and first‐order output. However, the inhalation study of thymol shows a two‐comparment model, which is consistent with the thymol delivery route during inhalation from lung to central circle system.
The pharmacokinetic results confirmed the systemic exposure from both inhalation and IV injection of thymol in mice. Based on NLME analysis of population PK data, the peak concentration of net inhaled thymol in mouse serum was approximately 42.3 ng/mL (Cmax) and occurred at 2 minutes (tmax) after inhalation. The AUC of inhaled thymol from 0‐60 minutes (AUC 0‐60 min) was 464 ng/mL × min which was 74% relative to thymol administrated by IV (Table 2). The elimination half‐life was approximately 3.43 and 4.73 minutes for IV and net inhalation routes, respectively.
Notably, the concentration used in this PK study for inhaled thymol, 0.47 mg/Kg, is about 50 times the amount of thymol used in the epinephrine HFA MDI formulation. By comparison, the LD50 (median lethal dose) for IV administration in mice is 100 mg/kg12, 13 which is over 200 times greater than the thymol used in this PK study and over 10 000 times greater than the amount in epinephrine HFA MDI. Therefore, the observed bioavailability of inhaled thymol in this study was far below the toxic levels.
As demonstrated above, the higher PK curve from the inhalation route relative to that of the intravenous route from 10 to 60 minutes is most likely attributable to the effect of absorption of thymol through the skin and fur of mice. It is also noteworthy that mice have a very rapid breathing rate (150 breaths/min).8 Lung scintigraphy studies in humans have shown that the faster the inspiratory flow, the lesser the deposition of the respirable fraction of an orally administered therapeutic aerosol in the lower respiratory tract.14, 15 Therefore, in addition to deposition on the fur and skin of the mice with subsequent absorption into the circulation, most of the dose delivered to the mice may have been deposited in the nose and, to a lesser extent, in the oral cavity and nasopharynx which was then swallowed with slow absorption from the GI tract.
Furthermore, using the mouse PK data obtained from this study, the human serum thymol concentration with epinephrine HFA use can be estimated. The maximum recommended daily dose of epinephrine HFA is eight inhalations; and the maximum daily dose of thymol in humans from epinephrine HFA is 36.6 mcg/day or 0.73 mcg/kg/day, assuming a body weight of 50 kg for consumers who are at least 12 years old. Therefore, the relative daily thymol dose in mice is approximately 644 times (470/0.73) higher than the maximum daily exposure to thymol in humans when epinephrine HFA is used. From the mouse PK data, the estimated Cmax of thymol in humans when administering epinephrine HFA at the maximum daily dose is approximately 0.07 ng/mL (Table 3), which is nominal, with a possibly faster tmax (~2 minutes). Moreover, since thymol is rapidly eliminated from the circulation with the elimination half‐life of 3.9 minutes for the mouse model, chronic inhalation of epinephrine HFA would not pose any safety concerns.
Table 3.
Estimated thymol PK parameters in humans from epinephrine HFA use
| Items | Mice, this study (Experimental data) | Human, primatene mist (Estimation) |
|---|---|---|
| Dose of thymol | ||
| Maximum daily dose | 15 sprays of 0.5% thymol, breathing for 10 minutes | Maximum daily dose 8 sprays of primatene mist |
| Thymol daily dose, mcg/kg/day | 470 | 0.73 |
| Relative daily dose: Mouse vs human | 641 | 1 |
| PK parameters | ||
| C max, ng/mL | 42.3 | 0.07 |
| t max, min | 2 | ~2 |
| AUC0‐60 min, ng/mL × min | 464 | 0.7 |
DISCLOSURE
This study was financially supported by Amphastar Pharmaceuticals, Inc Dr Tashkin reports personal fees from Amphastar Pharmaceuticals, outside the submitted work. Kevin Xie, Dr Mary Luo and Dr Jack Zhang are employees of by Amphastar Pharmaceuticals, Inc at the time of the study and manuscript preparation.
AUTHOR CONTRIBUTION
Dr Jack Zhang conceived the study and was in charge of overall direction and planning. Dr Jack Zhang and Dr Mary Luo designed the method and the computational framework and analyzed the data. Dr Jack Zhang and Dr Kevin Xie carried out the implementation. Dr Mary Luo wrote the manuscript with input from all authors especially from Dr Donald P. Tashkin. Dr Donald P. Tashkin provided critical feedback and helped shape the research, analysis, and manuscript. All authors discussed the results and contributed to the final manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
ACKNOWLEDGEMENTS
The authors thank Lucy Peng of Non‐Clinical Division of Amphastar New Drug Research Center, Ellen Feng and Joe Lu of Amphastar Information Technology and Service Division for their Support of this work. The authors thank Pao Hean and Eric Wong for their preparation of this manuscript.
Xie K, Tashkin DP, Luo MZ, Zhang JY. Pharmacokinetic study of thymol after intravenous injection and high‐dose inhalation in mouse model. Pharmacol Res Perspect. 2019;e00515 10.1002/prp2.515
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. FDA Briefing Document for a Joint Meeting of the Nonprescription Drugs and the Pulmonary and Allergy Drugs Advisory Committees. Epinephrine Metered Dose Inhaler. 2014. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/NonprescriptionDrugsAdvisoryCommittee/UCM386675.pdf. Accessed Jun 9, 2018.
- 2. Code of Federal Regulations, Title 21, Vol 3 Rev. April 1, 2015. 21CFR182.10.
- 3. Salehi B, Mishra AP, Shukla I, et al. Thymol, thyme, and other plant sources: health and potential uses. Phytother Res. 2018;32:1688‐1706. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . WHO model prescribing information: drugs used in anaesthesia. 1989;22‐24. http://apps.who.int/medicinedocs/en/d/Jh2929e/4.4.html.
- 5. Nagoor Meeran MF, Javed H, Al Taee H, Azimullah S, Ojha SK. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and and pharmaceutical development. Front Pharmacol. 2017;8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchese A, Orhan IE, Daglia M, et al. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402‐414. [DOI] [PubMed] [Google Scholar]
- 7. Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2018;123:233–235. [DOI] [PubMed] [Google Scholar]
- 8. Gad SC, Chengelis CP. Animal models in toxicology. New York, NY: Marcel Dekker; 1992. [Google Scholar]
- 9. Boroujerdi M. Pharmacokinetics, Principles and Applications. New York: McGRAW‐Hill; 1995:117, 158, and 268. [Google Scholar]
- 10. Walpole RE, Myers RH, Myers SL, Ye K. Probability & Statistics for Engineers and Scientists, 9th edn Boston: Pearson; 2017:371. [Google Scholar]
- 11. Thymol . Toxnet. Toxicology data network. https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+89-83-8. Accessed Jun 10, 2018.
- 12. Lewis RJ. Sax's Dangerous Properties of Industrial Materials. 11th edn Hoboken, NJ: Wiley‐Interscience, Wiley & Sons, Inc.; 2004:3463. [Google Scholar]
- 13. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 2003;56:600‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2‐agonist particle size. Am J Respir Crit Care Med. 2005;172:1497‐1504. [DOI] [PubMed] [Google Scholar]
- 15. Lavorini F, Fontana GA, Usmani OS. New inhaler devices ‐ the good, the bad and the ugly. Respiration. 2014;88:3‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


