Abstract
Sarcopenia, the progressive loss of muscle mass and strength, is one of the major health issues in older adults, given its high prevalence accompanied by huge clinical and socioeconomic implications. Age-related changes in skeletal muscle can be attributed to mechanisms both directly and indirectly related to muscle homeostasis. Indeed, a wide spectrum of age-related modifications in the organism was shown to play a key role in the pathogenesis of sarcopenia. Not surprisingly, sarcopenia has sometimes been indicated as a syndrome stemming from the aging process, and not as univocal standalone disease. Due to the multidimensionality of sarcopenia, a single biomarker approach is not enough to explain the biology of this condition. The aim of this review is to suggest innovative and promising sarcopenia markers investigating the link between skeletal muscle and brain. Indeed, as a neurological origin of sarcopenia has been hypothesized, a new perspective on sarcopenia biomarkers may focus on the dysfunction of the neuromuscular junctions (NMJs). The core SNARE synaptosomal-associated protein of 25 kDa (SNAP25) accumulates in the plasma membrane of nerve terminals at NMJs and regulates exocytosis at peripheral and central synapses. Interestingly, mice studies have shown that SNAP25 affects the neuromuscular function. SNARE complex and, in particular, SNAP25 may represent a promising pathway to explore the molecular and cellular mechanisms regulating muscular homeostasis and concur at profiling the sarcopenia biological background.
Keywords: aging, sarcopenia, biomarkers, neuromuscular junction, SNAP25
Aging and Sarcopenia
The global increase of human life expectancy and the rapid aging of the population represent emerging major sociodemographic phenomena.
The aging phenotype is heterogeneous and individuals of the same chronological age may dramatically differ in their health status from each other. In this context, over the past decades, the concept of frailty has been used to define age-related condition characterized by increased vulnerability of physiological systems to stressors, exposing individuals to increased risk of negative outcomes (1).
The physical manifestation of frailty is typically described as dominated by the presence of sarcopenia, one of the most notable changes in body composition being defined by the loss of skeletal muscle mass and strength (2).
The prevalence of sarcopenia in persons aged 60–70 years old is reported to be between 5 and 13%, but it increases to 11–50% in people older than 80. Starting since the age of 40 years, individuals lose 1–2% of muscle per year. At the age of 70 years, 25–30% of skeletal muscle mass is lost, and muscle strength even more pronouncedly is declined by up to 40% (3).
In older persons, the etiology of sarcopenia is multi-factorial. Under normal circumstances, physiologic muscle mass and function are related to a dynamic balance between positive and negative regulators of muscle growth. The central nervous, immune, and endocrine systems coordinate this balance. The sarcopenia onset and progression depend on a combination of mechanisms, typically involved in the aging process, whose combination results in the disruption of the normal physiology of skeletal muscle (4).
Among the age-related changes in body composition occurring at the level of the muscle fiber structure, modifications of its contractile properties and abnormalities of neuromuscular junctions (NMJs) are particularly evident. In particular, sarcopenia shows a muscle fiber distribution with a predominance of type I fibers correlated to an atrophy of type II fibers (5), and an accumulation of adipose tissue both around and between muscle fibers (6). Notably an altered protein metabolism and a dysregulated autophagy leading to muscle atrophy play a role as well in this phenomenon. The picture is even more complex because mechanisms indirectly related to muscle homeostasis also participate in the pathogenesis of sarcopenia (i.e., hormonal status, inflammatory status, insulin resistance, telomere shorting, and oxidative stress).
More recently, a neurological origin of sarcopenia has been postulated (7). Since the integrity of the neurophysiological functions plays a pivotal role for the maintenance of the skeletal muscle in older persons (8), it has been hypothesized that a brain-muscle link could underlie of the sarcopenia development. The assumption that the muscle contractile activity is regulated by the central nervous system (CNS) at the NMJs suggests that the functionality of the NMJs is indispensable for the maintenance of both motor nerve and muscle fibers (9). NMJs have the characteristic structural features of chemical synapses acting as interface between nervous and skeletal muscular system. Age-related alterations in the nervous system play pivotal a role in the musculoskeletal impairment and the decline of the muscle power generation (10). Such age-related remodeling includes loss of motor neurons and transformations of motor units through collateral reinnervation (11), impairment of neuromuscular activation (12), and uncoordinated patterns of intermuscular neural activation (13).
Under these premises, it is evident that sarcopenia is characterized by a peculiar multidimensionality and a consequent heterogeneity. For this reason, the “one-fits-all” approach, where a single process is able to explain the clinical manifestation, may be inadequate for capturing the inner biological foundations of this condition. Thus, a single biomarker is not enough to explain the biology of this complex age-related condition and cannot distinguish sarcopenia from a mere condition of advanced age. Only considering a multidimensional strategy to discover biomarkers of sarcopenia it will be possible to isolate the real fingerprints of this disease.
This review aims to summarize the available literature on sarcopenia biomarkers and to propose innovative and promising ones. Under this perspective, we extended the investigation of the non-muscle-specific sarcopenia biomarkers deepening the analysis of the molecular pathway related to the brain-muscle link.
Biomarkers of Sarcopenia
In recent years, many studies on sarcopenia have identified numerous biochemical biomarkers. These biomarkers could be divided in “muscle-specific” and “non-muscle-specific.”
Established Biomarker of Sarcopenia
Muscle-Specific Biomarkers
The muscle-specific biomarkers should ideally be able to evaluate both muscle mass and strength loss, the two dimensions of sarcopenia.
Several techniques are used to quantify muscle mass. The measurement of muscle or fat free mass is usually obtained by using imaging assessments as the dual energy X-ray absorptiometry, the computerized tomography, the magnetic resonance imaging, or bioelectrical impedance analysis. However, the current lack of a univocal operational definition and the absence of a clear “gold standard” assessment method make the diagnostic problem still problematic.
Multiple tests of physical performance are available, including the gait speed, the Short Physical Performance Battery (SPPB), the 6-min walk test, and the stair climb power test. Unfortunately, each of them has own characteristics and only captures specific aspects of the muscle functioning, thus concurring at the heterogeneous set of possibilities in the measurement of sarcopenia.
In the last years, a number of muscle-specific biomarkers of sarcopenia have been identified. Recently, several studies have raised doubts about the specificity of these markers in detecting sarcopenia.
Among the “muscle-specific” biochemical markers there are several linked to muscle turnover. Procollagen type III N-terminal peptide (P3NP) is a fragment released by the proteolytic cleavage during collagen synthesis in the muscle. It represents an interesting marker for the analysis of skeletal muscle remodeling. Evidence shows that serum P3NP concentrations reflect the muscle mass (14). However, P3NP levels are altered also in autoimmune diseases as psoriasis, psoriatric arthritis, and rheumatoid arthritis (15).
The peptides deriving from the collagen type VI turnover, as a type VI collagen N-terminal globular domain epitope (IC6) and MMP-generated degradation fragment of collagen 6 (C6M) could be potential markers for the muscle loss.
Collagen type VI has been proposed as a biomarker of muscular tissue damage (16). This protein is important for the preservation of muscle trophism; in fact, genetic defects of this protein are linked to very serious muscular diseases. Nevertheless, these molecules were found to be altered also in serum of cancer patients (17).
The 3-methylhistidine (3MH) results from the methylation of histidine residues of actin and myosin, and induces proteolysis of myofibrils; evidence showed that it could be implicated in the sarcopenia pathophysiology (18). It must be taken into consideration that 3MH is strongly influenced by dietary habits and meat consumption (19).
Skeletal muscle-specific isoform of troponin T (sTnT) may be considerate a biomarker of sarcopenia, since high troponin levels are an expression of muscle wasting. Abreu et al. (20) showed that serum levels of cardiac troponin T (cTnT) decrease in relation to a physical performance improvement, opening new hypotheses about this molecule as marker of muscle damage. It is well-known that cTnT is a biomarker of cardiac dysfunctions as well as diabetes, pulmonary arterial hypertension, and chronic kidney disease (21).
The fact that creatine is related to the skeletal muscle state should also not underestimated. Urinary creatine excretion is correlated with the skeletal muscle mass of total body, and increasing evidences demonstrate that creatine supplementation results in positive effects on muscle strength (22). However, urinary creatine excretion is also altered in other organism dysfunctions as testicular damage (23).
Non-muscle-specific Biomarkers
A lot of non-muscle-specific biomarkers were found to be related to sarcopenia pathology. However, these markers are also involved in mechanisms underlying the aging process and could be modified by environmental factors as lifestyle, comorbidity, diseases, and exposure to drugs. In particular:
Markers of inflammatory system. An imbalance between pro- and anti-inflammatory pathways, responsible for a smoldering low-grade inflammation (the so-called “inflamm-aging” phenomenon) characterizes aging (24). In this situation increased levels of pro-inflammatory cytokines and reduced serum level of anti-inflammatory cytokines are observed. Inflamm-aging could contribute to sarcopenia exerting pro-sarcopenic effects through the inhibition of muscle regeneration (25). Indeed, in older adults, up-regulated levels of C-reactive protein, interleukin-6, interleukin-8, tumor necrosis factor-α, interferon-γ, granulocyte-monocyte colony-stimulating factor, and high-temperature requirement serine protease A1 are related to muscle mass, strength and physical function impairment (26). Moreover, high circulating extracellular heat shock protein 72, a mediator of inflammatory system, results in low muscle mass, weak grip strength, and slow walking speed (27).
Markers of oxidative damage. Oxidative stress and the accumulation of reactive oxygen species increase with aging, and are likely to play an active role in triggering sarcopenia. If free radicals are not eliminated by the anti-oxidant agents, they cause severe damage on muscle cells. Evidence shows that serum concentrations of protein carbonyls, well-known markers of oxidative stress, are related with grip strength. Advanced glycation end products (AGEs) are bioactive compounds formed by non-enzymatic glycation of DNA, proteins, and lipids. Elevated serum levels of AGEs are linked to muscle strength impairment (28), however altered expression of AGEs is related also to diabetes (29).
-
Markers of nutritional status. In older persons the physical performance impairment is associated to anemia with low hemoglobin or low serum albumin and/or selenium (30).
Leptin seems to affect the skeletal muscle, through the modulation of lipolysis and insulin sensitivity. Considering that the muscle is the major user of glucose, sarcopenia may represent a risk factor for the development of insulin resistance (31). Evidences showed an increase of circulating levels of leptin in sarcopenia due to a reduction of leptin receptors along with a loss of muscle mass.
In elder people, positive relationships between plasma levels of uric acid and handgrip (32) and between magnesium levels and indexes of muscle performance were observed (33).
Moreover, the skeletal muscle health is linked to vitamin D. Vitamin D affects muscle cell contractility, proliferation, and differentiation (34). Vitamin D insufficiency results in muscle deficits and vitamin D supplementation has beneficial effects. Further, the identification of vitamin D receptor in skeletal muscle provides evidence for its direct effect on sarcopenia (35).
Markers of endocrine system. Loss of muscle mass is also caused by the reduced capacity for synthesizing proteins and repairing muscle damage which implies a progressive switch from anabolic to catabolic metabolism. It was found that defects in muscle protein homeostasis might be associated to changes in circulating levels of hormones. Studies showed that sex hormones, including testosterone and dehydroepiandrosterone sulfate (DHEAS), whose levels decrease during age, have a role in the sarcopenia onset (36). Testosterone has an anabolic, anti-catabolic, and anti-inflammatory effect on muscle, stimulating cell activation, proliferation, survival, and differentiation, thus playing a role in maintenance of muscle homeostasis (37). DHEAS may affect muscle performance and its age-associated decline is an important determinant of muscle mass and strength loss in older people (38). Sarcopenia is characterized also by a decline of the growth hormone (GH) and the insulin-like growth factor 1 (IGF-1). The actions of GH are mediated by IGF-1, an anabolic hormone, which stimulates muscle growth and regeneration. Giovannini et al. (39) demostrated that IGF-1 administration increases the rate of skeletal muscle functional recovery after injury.
Markers related to growth factors. Myostatin and the growth differentiation factor-15 (GDF-15), members of the transforming growth factor-β (TGF-β) superfamily, are negative regulators of skeletal muscle myogenesis and inhibitors of muscle growth. Interestingly, it was found that older myostatin-null mice show resistance to the sarcopenic phenotype (40) and the use of antibodies against myostatin lead to an improvement of muscle mass and grip strength (41). Follistatin, a myostatin inhibitor, seems to be an interesting tool in evaluating the muscle damage (42). Another sarcopenia biomarker is brain-derived neurotrophic factor (BDNF), which induces the production of growth factors associated with differentiation, plasticity, and neuronal growth. In skeletal muscle, BDNF is involved in the regulation and survival of motoneurons, in the development and differentiation of myoblasts, in the interaction between immune cells and muscle cells (43). However, myostatin and follistatin are altered in osteoarthritis (44, 45), GDF-15 in cardiovascular disease (46), and BDNF in mood disorders (47) and deregulated heart autonomic system (48).
Markers of NMJ dysfunction. In the skeletal muscle, NMJs are implicated in the transduction of the action potentials, and their dysfunction can lead to a gradual alteration of this process. The structural features of NMJs are similar to chemical synapses and many molecules involved in the formation of the NMJs have an essential role in their maintenance (49). Agrin is a heparan sulfate proteoglycan synthesized at the levels of motor neurons, transported along axons and released into the synaptic basal lamina of the NMJs. Here agrin stimulates the assembly of the postsynaptic apparatus, including the clustering of acetylcholine receptors and the stabilization of presynaptic structures. Agrin is fundamental for the formation and stabilization of NMJs (50). The proteolytic cleavage of agrin at the NMJs by neurotrypsin produces a C-terminal 22-kDa fragment (CAF), which is released into the circulation and can be detected in human serum. Elevated serum CAF levels resulting from NMJs disassembly and denervation are associated with sarcopenia (51) and kidney dysfunction (52).
Promising Biomarker of Sarcopenia
Core SNARE Synaptosomal-Associated Protein of 25 kDa (SNAP25)
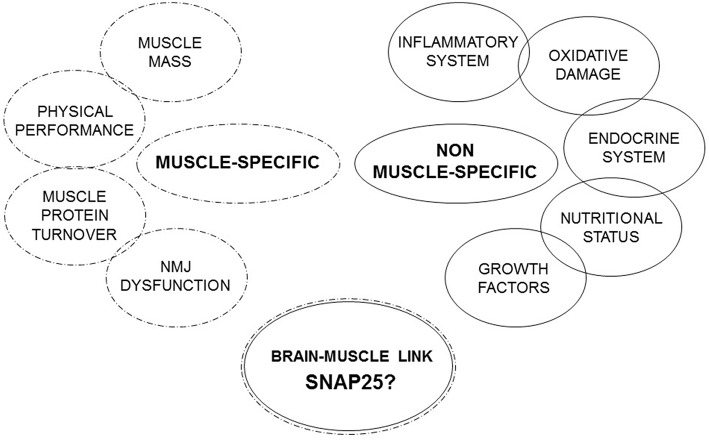
Under these premises, a single biomarker approach is not enough to explain the real fingerprints of sarcopenia and no single established sarcopenia biomarker is specific for this condition. Therefore, in this review we propose to focus on molecules that spanning different systems and organs studying the link between brain and muscle (Figure 1).
Figure 1.
Muscle-specific and non-muscle-specific factors involved in sarcopenia.
The core SNARE synaptosomal-associated protein of 25 kDa (SNAP25) could represent a promising pathway. SNAP25 plays a central role in the release of neurotransmitters, inhibiting the voltage-gated calcium channels (VGCC) function and reducing responsiveness to depolarization. SNAP25 accumulates in the plasma membrane of nerve terminals at NMJs, consistent with its SNARE protein role in regulating exocytosis at peripheral and central synapses. In CNS, SNAP25 participates with syntaxin-1 and synaptobrevin/VAMP2 (53) in the regulation of synaptic vesicle exocytosis. SNAP25 has two isoforms, SNAP25a and SNAP25b, resulting from alternative splicing of the exon 5 of the SNAP25 gene, which are differentially expressed during the development. The reduction of this protein levels has been reported to negatively affect synaptic function (54) and to be related even to age-related diseases (i.e., Alzheimer's disease and diabetes) (55, 56). SNAP25 modulates VGCCs, it negatively controls neuronal calcium responsiveness to depolarization through VGCC inhibition (57). Consistently, silencing endogenous SNAP25 in glutamatergic neurons results in increased VGCC activity.
SNAP25 is synthesized in mouse motor nerve endings and the level of SNAP25 mRNA affects neurotransmitter exocytosis (58). Furthermore, neuromuscular transmission is compromised in the presence of a mutation in SNAP25b gene that inhibits synaptic vesicle exocytosis (59). Notably, adult SNAP25+/– mice express low protein levels and are characterized by a decrease of neuromuscular strength (60), suggesting that reduced levels of this protein negatively affect neuromuscular function. These observations seem to be supported by recent data showing that zebrafish embryos model motor impairment, increased motility and neurotransmitter secretion were associated with an over-expression of SNAP25 (61).
Conclusions and Future Perspectives
Even if sarcopenia and aging share common molecular and cellular mechanisms, a definitive pathophysiological framework of these conditions is still absent. There is, thus, the need of employing a more comprehensive approach to study the age-related changes of body composition leading to sarcopenia. In particular, it is necessary to recognize the inadequacies of previous paradigms, too often investigating sarcopenia as a standalone disease, independently of the aging process.
Under this perspective it is necessary to deepen the study of the brain-muscle link. NMJ seems to be the most innovative and attractive marker of sarcopenia to study. This aspect represents an important conceptual advance, because till now research on sarcopenia has mainly focused on the study of muscular factors and less on the possible role of neurological mechanisms in its onset and development. Therefore, the SNARE complex and, in particular SNAP25, may represent a promising pathway to explore the molecular and cellular mechanisms regulating muscular homeostasis and NMJs. In conclusion, the proposed SNAP25 pathway could represent a new piece enriching the panel of the established biomarkers of sarcopenia (Figure 1).
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all participants of the S.A.M.B.A. project: Mario Clerici (IRCCS Fondazione Don Gnocchi, University of Milan, Milan, Italy); Franca Guerini, Roberta Mancuso and Fabio Trecate (IRCCS Fondazione Don Gnocchi, Milan, Italy); Matteo Cesari, Daniela Mari and Beatrice Arosio (IRCCS Fondazione Ca' Granda, Ospedale Maggiore Policlinico, University of Milan, Milan, Italy); Cecilia Gelfi, Mara Biasin and Giuseppina Bernardelli (University of Milan, Italy); Elisabetta Menna and Mariaelvina Sala (CNR Neuroscience Institute, Milan, Italy).
Footnotes
Funding. This work was supported by CARIPLO Foundation Italy (2017-0622).
References
- 1.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. (2016) 31:3–10. 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. (2014) 43:748–59. 10.1093/ageing/afu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandervoot AA, Symons TB. Functional and metabolic consequences of sarcopenia. Can J Appl Physiol. (2001) 26:90–101. 10.1139/h01-007 [DOI] [PubMed] [Google Scholar]
- 4.Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med. (2018) 5:61. 10.3389/fmed.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. (1979) 46:451–6. 10.1152/jappl.1979.46.3.451 [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. (2001) 90:2157–65. 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 7.Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res. (2013) 2013:791679. 10.1155/2013/791679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauretani F, Meschi T, Ticinesi A, Maggio M. “Brain-muscle loop” in the fragility of older persons: from pathophysiology to new organizing models. Aging Clin Exp Res. (2017) 29:1305–11. 10.1007/s40520-017-0729-4 [DOI] [PubMed] [Google Scholar]
- 9.Shigemoto K, Kubo S, Mori S, Yamada S, Akiyoshi T, Miyazaki T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr Gerontol Int. (2010) 10 (Suppl. 1):S137–47. 10.1111/j.1447-0594.2010.00608.x [DOI] [PubMed] [Google Scholar]
- 10.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. (2010) 20:49–64. 10.1111/j.1600-0838.2009.01084.x [DOI] [PubMed] [Google Scholar]
- 11.Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. (1997) 127 (Suppl. 5):1011S–3S. 10.1093/jn/127.5.1011S [DOI] [PubMed] [Google Scholar]
- 12.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol Ser A Biol Sci Med Sci. (2011) 66:115–21. 10.1093/gerona/glq153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, et al. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol Ser A Biol Sci Med Sci. (1998) 53:B415–23. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin S, He EJ, Kawakubo M, Schroeder ET, Yarasheski K, Opiteck GJ, et al. N-terminal propeptide of type III procollagen as a biomarker of anabolic response to recombinant human GH and testosterone. J Clin Endocrinol Metab. (2009) 94:4224–33. 10.1210/jc.2009-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Voort EAM, Wakkee M, Veldt-Kok P, Darwish Murad S, Nijsten T. Enhanced liver fibrosis test in patients with psoriasis, psoriatic arthritis and rheumatoid arthritis: a cross-sectional comparison with procollagen-3 N-terminal peptide (P3NP). Br J Dermatol. (2017) 176:1599–606. 10.1111/bjd.15220 [DOI] [PubMed] [Google Scholar]
- 16.Nedergaard A, Sun S, Karsdal MA, Henriksen K, Kjaer M, Lou Y, et al. Type VI collagen turnover-related peptides-novel serological biomarkers of muscle mass and anabolic response to loading in young men. J Cachexia Sarcopenia Muscle. (2013) 4:267–75. 10.1007/s13539-013-0114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willumsen N, Bager C, Karsdal MA. Matrix metalloprotease generated fragments of type VI collagen have serum biomarker potential in cancer—a proof of concept study. Transl Oncol. (2019) 12:693–8. 10.1016/j.tranon.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young VR, Munro HN. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Feder Proc. (1978) 37:2291–300. [PubMed] [Google Scholar]
- 19.Kochlik B, Gerbracht C, Grune T, Weber D. The influence of dietary habits and meat consumption on plasma 3-methylhistidine-A potential marker for muscle protein turnover. Mol Nutr Food Res. (2018) 62:e1701062. 10.1002/mnfr.201701062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abreu EL, Cheng AL, Kelly PJ, Chertoff K, Brotto L, Griffith E, et al. Skeletal muscle troponin as a novel biomarker to enhance assessment of the impact of strength training on fall prevention in the older adults. Nurs Res. (2014) 63:75–82. 10.1097/NNR.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 21.Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. (2017) 113:1708–18. 10.1093/cvr/cvx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. (2010) 14:155–9. 10.1007/s12603-009-0124-8 [DOI] [PubMed] [Google Scholar]
- 23.Nahas K, le Net JL, Provost JP, Tomaszewski KE. An investigation of urinary creatine excretion as a potential marker for testicular damage. Hum Exp Toxicol. (1993) 12:173–6. 10.1177/096032719301200214 [DOI] [PubMed] [Google Scholar]
- 24.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Ser A Biol Sci Med Sci. (2014) 69 (Suppl. 1):S4–9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 25.Franckhauser S, Elias I, Rotter Sopasakis V, Ferre T, Nagaev I, Andersson CX, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. (2008) 51:1306–16. 10.1007/s00125-008-0998-8 [DOI] [PubMed] [Google Scholar]
- 26.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol Ser A Biol Sci Med Sci. (2004) 59:242–8. 10.1093/gerona/59.3.m242 [DOI] [PubMed] [Google Scholar]
- 27.Ogawa K, Kim HK, Shimizu T, Abe S, Shiga Y, Calderwood SK. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones. (2012) 17:349–59. 10.1007/s12192-011-0310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalal M, Ferrucci L, Sun K, Beck J, Fried LP, Semba RD. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J Gerontol Ser A Biol Sci Med Sci. (2009) 64:132–7. 10.1093/gerona/gln018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyer F, Vidot JB, Dubourg AG, Rondeau P, Essop MF, Bourdon E. Oxidative stress and adipocyte biology: focus on the role of AGEs. Oxid Med Cell Longev. (2015) 2015:534873. 10.1155/2015/534873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauretani F, Semba RD, Bandinelli S, Ray AL, Guralnik JM, Ferrucci L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: the InCHIANTI Study. Am J Clin Nutr. (2007) 86:347–52. 10.1093/ajcn/86.2.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. (2008) 18:388–95. 10.1016/j.numecd.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 32.Macchi C, Molino-Lova R, Polcaro P, Guarducci L, Lauretani F, Cecchi F, et al. Higher circulating levels of uric acid are prospectively associated with better muscle function in older persons. Mechanisms Ageing Dev. (2008) 129:522–7. 10.1016/j.mad.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominguez LJ, Barbagallo M, Lauretani F, Bandinelli S, Bos A, Corsi AM, et al. Magnesium and muscle performance in older persons: the InCHIANTI study. Am J Clin Nutr. (2006) 84:419–26. 10.1093/ajcn/84.1.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman LP. Transcriptional targets of the vitamin D3 receptor-mediating cell cycle arrest and differentiation. J Nutr. (1999) 129 (Suppl. 2):581S−6S. 10.1093/jn/129.2.581S [DOI] [PubMed] [Google Scholar]
- 35.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. (2001) 33:19–24. [DOI] [PubMed] [Google Scholar]
- 36.Sakuma K, Yamaguchi A. Sarcopenia and age-related endocrine function. Int J Endocrinol. (2012) 2012:127362. 10.1155/2012/127362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. (2005) 122:659–67. 10.1016/j.cell.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 38.Greenlund LJ, Nair KS. Sarcopenia–consequences, mechanisms, and potential therapies. Mechanisms Ageing Dev. (2003) 124:287–99. 10.1016/s0047-6374(02)00196-3 [DOI] [PubMed] [Google Scholar]
- 39.Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C. Modulation of GH/IGF-1 axis: potential strategies to counteract sarcopenia in older adults. Mechanisms Ageing Dev. (2008) 129:593–601. 10.1016/j.mad.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M. Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol. (2006) 209:866–73. 10.1002/jcp.20778 [DOI] [PubMed] [Google Scholar]
- 41.Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. (2013) 7:352–60. 10.1097/SPC.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curcio F, Ferro G, Basile C, Liguori I, Parrella P, Pirozzi F, et al. Biomarkers in sarcopenia: a multifactorial approach. Exp Gerontol. (2016) 85:1–8. 10.1016/j.exger.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 43.Papathanassoglou ED, Miltiadous P, Karanikola MN. May BDNF be implicated in the exercise-mediated regulation of inflammation? Critical review and synthesis of evidence. Biol Res Nurs. (2015) 17:521–39. 10.1177/1099800414555411 [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Shao Y, Lin C, Zeng C, Fang H, Pan J, et al. Myostatin serum concentrations are correlated with the severity of knee osteoarthritis. J Clin Lab Anal. (2017) 31:22094. 10.1002/jcla.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthritis Cartilage. (2012) 20:1451–64. 10.1016/j.joca.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 46.Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. (2017) 63:140–51. 10.1373/clinchem.2016.255174 [DOI] [PubMed] [Google Scholar]
- 47.Peng S, Li W, Lv L, Zhang Z, Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med. (2018) 26:127–36. [PubMed] [Google Scholar]
- 48.Pius-Sadowska E, Machalinski B. BDNF—a key player in cardiovascular system. J Mol Cell Cardiol. (2017) 110:54–60. 10.1016/j.yjmcc.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci. (2014) 6:208. 10.3389/fnagi.2014.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. (2010) 137:1017–33. 10.1242/dev.038711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol. (2016) 79:31–6. 10.1016/j.exger.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 52.Arampatzis S, Chalikias G, Devetzis V, Konstantinides S, Huynh-Do U, Tziakas D. C-terminal fragment of agrin (CAF) levels predict acute kidney injury after acute myocardial infarction. BMC Nephrol. (2017) 18:202. 10.1186/s12882-017-0611-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montecucco C, Schiavo G, Pantano S. SNARE complexes and neuroexocytosis: how many, how close? Trends Biochem Sci. (2005) 30:367–72. 10.1016/j.tibs.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 54.Antonucci F, Corradini I, Morini R, Fossati G, Menna E, Pozzi D, et al. Reduced SNAP-25 alters short-term plasticity at developing glutamatergic synapses. EMBO Rep. (2013) 14:645–51. 10.1038/embor.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Daghri NM, Costa AS, Alokail MS, Zanzottera M, Alenad AM, Mohammed AK, et al. Synaptosomal protein of 25 kDa (Snap25) polymorphisms associated with glycemic parameters in type 2 diabetes patients. J Diab Res. (2016) 2016:8943092. 10.1155/2016/8943092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerini FR, Agliardi C, Sironi M, Arosio B, Calabrese E, Zanzottera M, et al. Possible association between SNAP-25 single nucleotide polymorphisms and alterations of categorical fluency and functional MRI parameters in Alzheimer's disease. J Alzheimer Dis. (2014) 42:1015–28. 10.3233/JAD-140057 [DOI] [PubMed] [Google Scholar]
- 57.Pozzi D, Condliffe S, Bozzi Y, Chikhladze M, Grumelli C, Proux-Gillardeaux V, et al. Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels. Proc Natl Acad Sci USA. (2008) 105:323–8. 10.1073/pnas.0706211105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Islamov RR, Samigullin DV, Rizvanov AA, Bondarenko NI, Nikolskiy EE. Synaptosome-associated protein 25 (SNAP25) synthesis in terminal buttons of mouse motor neuron. Doklady Biochem Biophys. (2015) 464:272–4. 10.1134/S1607672915050026 [DOI] [PubMed] [Google Scholar]
- 59.Shen XM, Selcen D, Brengman J, Engel AG. Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology. (2014) 83:2247–55. 10.1212/WNL.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corradini I, Donzelli A, Antonucci F, Welzl H, Loos M, Martucci R, et al. Epileptiform activity and cognitive deficits in SNAP-25+/− mice are normalized by antiepileptic drugs. Cereb Cortex. (2014) 24:364–76. 10.1093/cercor/bhs316 [DOI] [PubMed] [Google Scholar]
- 61.McKee AG, Loscher JS, O'Sullivan NC, Chadderton N, Palfi A, Batti L, et al. AAV-mediated chronic over-expression of SNAP-25 in adult rat dorsal hippocampus impairs memory-associated synaptic plasticity. J Neurochem. (2010) 112:991–1004. 10.1111/j.1471-4159.2009.06516.x [DOI] [PubMed] [Google Scholar]