Abstract
The Omentum is a large flat adipose tissue layer nestling on the surface of the intra-peritoneal organs. Besides fat storage, omentum has key biological functions in immune-regulation and tissue regeneration.
Omentum biological properties include neovascularization, haemostasis, tissue healing and regeneration and as an in vivo incubator for cells and tissue cultivation. Some of these properties have long been noted in surgical practice and used empirically in several procedures.
In this review article, the author tries to highlight the omentum biological properties and their application in regenerative surgery procedures. Further, he has started a process of standardisation of basic biological principles to pave the way for future surgical practice.
Keywords: Omentum, Regenerative surgery, Tissue regeneration
1. Introduction
The Omentum either Epiploon [epipleen in Greek means floating] or Greater Omentum is a large flat adipose tissue layer covered by visceral peritoneum that hangs down from the greater curve of the stomach floating on the surface of the intra-peritoneal organs, mostly small and large bowel.
The Lesser Omentum connects the lesser curvature of the stomach and the proximal duodenum to the liver and defines the lesser sac anteriorly. Its gastro-hepatic ligament contains the left gastric vessels with their lymph nodes. The hepato-duodenal ligament (the gate for the Winslow foramen) contains the portal vein, the hepatic artery, the extra-hepatic bile duct, and the hepatic lymph nodes group [1].
The first scientific report of the omentum derives from the ancient Egyptians who, when embalming human bodies, used to assess and catalogue their dead bodies by looking at the variants in what we recognise today as the omentum [2].
Galeno (128-199 AD) deemed that the physiologic role of the omentum was to maintain warmth in the intestines. This belief came from the observation of a gladiator who had an omental resection after a stab injury and, after then, complained greatly for the rest of his life of feeling cold [3].
Since the beginning of the twentieth century, abdominal infection control and wound isolation were well-recognised functions of the omentum. The British surgeon Rutherford Morrison in his publication “Introduction to Surgery” (1910) called it “the policeman of the abdomen”.
2. Omentum looks like tissue but functions as an organ
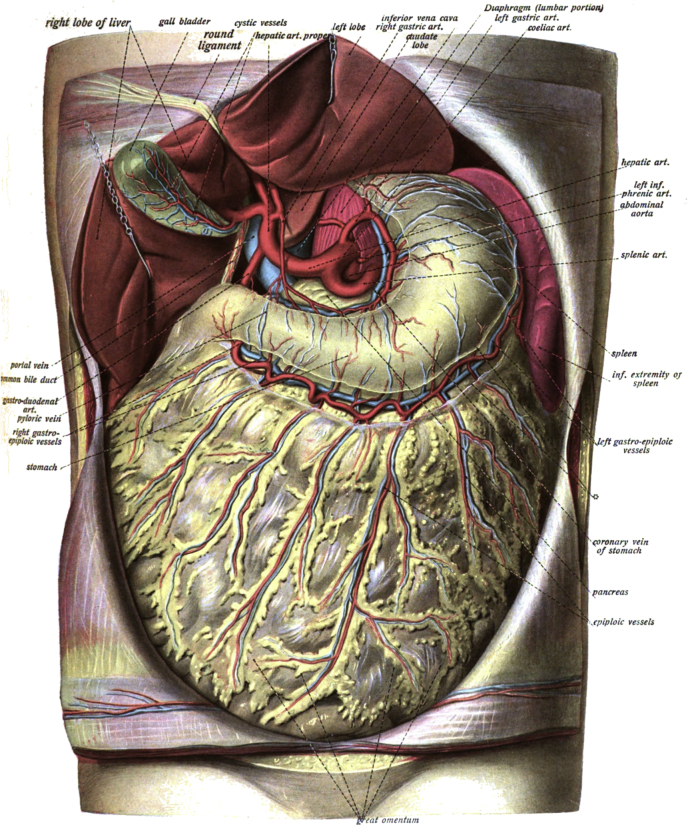
The omentum consists of a double sheet of the peritoneum, folded on itself so that it has four mesothelial layers. Two anterior layers wrapping the omentum descend from the greater curvature of the stomach and the proximal part of the duodenum. They pass in front of the bowel, sometimes reaching the pelvis, before turning back on themselves, and rising up as far as the transverse colon, where they separate and enclose that part of the intestine defining the gastro-colic ligament (Fig. 1).
Fig. 1.
Dr. Johannes Sobotta - Atlas and Text-book of Human Anatomy Volume III Vascular System, Lymphatic system, Nervous system and Sense Organs 1908.
The size of the omentum varies greatly from 300 gr to 2000 gr with a surface area of 300 cm2–1500 cm2.
The left border of the greater omentum is continuous with the gastro-splenic ligament; the right border extends to the beginning of the D2.
Authors generally consider most of the ligaments in the upper gastrointestinal tract as part of the omentum. These include the gastro-phrenic ligament, the gastro-colic ligament and the gastro-splenic ligament.
The spleno-renal ligament, which connects the left kidney to the spleen, can be considered part of the greater omentum. In this part of the abdomen the peritoneal cavity comes into contact with the lesser sac. The last part of the splenic artery, the vein and the tail of the pancreas pass through this ligament [4].
The right and left gastroepiploic arteries provide the blood supply to the omentum. The right gastroepiploic artery is, in most of the cases, a branch of the gastroduodenal artery. The left gastroepiploic artery is the largest branch of the splenic artery. Both arteries ultimately derive from the celiac trunk. The right and left gastroepiploic arteries anastomose within the two layers of the greater omentum along the greater curvature of the stomach.
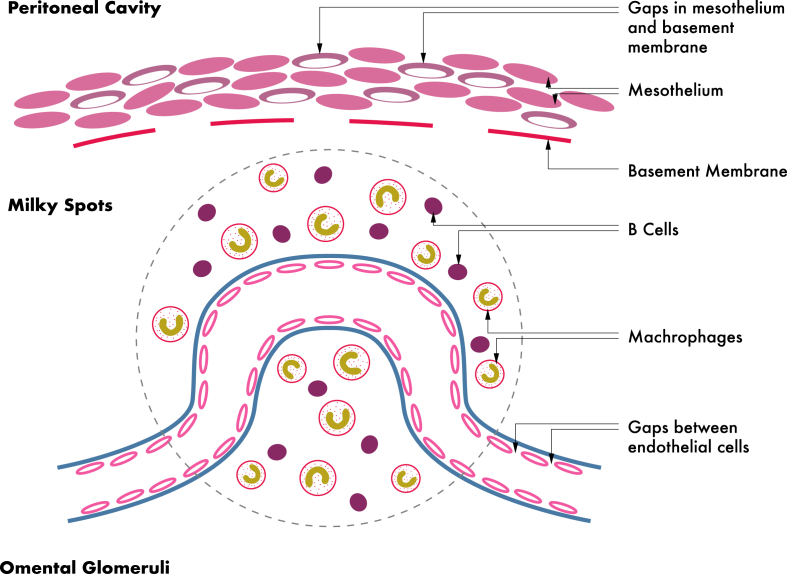
The lining of the omentum is composed of two layers of mesothelial cells. The trabecular connective stroma tissue contains omentum adipose cells, fibroblasts, pericytes, and leukocytes. The last being aggregate in the perivascular area to form the features named “milky spots”.
The omentum has a rich vascular supply in which capillaries form numerous characteristic spiral loops that are called omental glomeruli due to their similarity to renal glomeruli. These capillary beds lie directly under the mesothelium [5].
3. Omentum functions as an organ
An organ as defined by most Biology Dictionaries is “a self-contained group of tissues that performs specific functions in an organism”.
Surprisingly, the omentum is a very mobile organ; it moves around the peritoneal cavity dealing with infections and contaminants. It also deals with the control of the inflammation, promotes revascularization and tissue regeneration [6], [7].
3.1. Key biological functions
3.1.1. Fat storage
Omentum can store large amounts of adipose tissue. There are substantial biological differences from the omentum (OAT) and the subcutaneous adipose tissue (SAT). Lipolysis activity has different metabolic paths, e.g. omental cells are more sensitive to catecholamine than SAT and there are differences in insulin receptors and sensitivity as well [8].
Furthermore, the omentum adipose tissue (OAT) plays a basic role in the local immunologic response. OAT also contains a population of multipotent-mesenchymal stem cells (MSCs) that facilitate endometrial tumour growth more effectively than MSCs from SAT [9].
3.1.2. Immune-regulation
The basic functional immunologic units are called milky spots (Fig. 2). The cells clusters of milky spots derive from the mononuclear phagocyte system and include macrophages (70%), B-lymphocytes (10%), T-lymphocytes (10%), mast cells, and stromal cells. They are arranged around the omental glomeruli that lie directly beneath the mesothelium. These structures are supported by a delicate network of reticular fibres which, constitute the framework of the organ [10], [11].
Fig. 2.
Omental Glomeruli: structure and function.
Both the endothelium lining, the omental glomeruli and the mesothelium overlying the milky spots are specially adapted to facilitate transmigration of leukocytes and for the interchange of interstitial fluid. The endothelial cells lining the blood vessels in the milky spots contain fenestrations. Similarly, there are intercellular openings between the mesothelial cells overlying milky spots, and there is not basal lamina in the submesothelial connective tissue [12], [13].
In experimental models, macrophage precursors in the milky spots appear to be different to and independent of precursors derived from the bone marrow. After being activated they become dendritic in shape, migrate to the mesothelial surface and acquire marked phagocytic abilities [14].
The omentum contains large numbers of B and T lymphocytes which are usually located around the periarteriolar vessels. The omentum appears to be a primary site of B-lymphocyte development. These B-lymphocytes are predominantly CD5+ B, and are common in the omentum as well as in the peritoneum. However, they are infrequently found in the blood, spleen and lymph nodes. Further, conventional B and T-lymphocytes are not found in the omentum. The CD5+B lymphocytes develop in the omental milky spots independently from the thymus or bone marrow. Therefore, the fetal omentum, like the fetal liver and bone marrow, acts as a primary site of B-lymphocytes development and may be considered as a sort of “intestinal thymus”. The function of the CD5+ B lymphocytes is still unclear [10], [15], [16].
Mesothelial cells lining the peritoneal cavity and endothelial cells lining blood vessels share the same mesodermal origin. Human omental microvascular endothelial (HOME) and mesothelial (MESO) cells share many phenotypic properties. They have been used in experimental models for seeding synthetic vascular grafts and line the luminal surface with a new endothelium showing less thrombogenic sensitivity thus preventing stenosis [17].
3.1.2.1. Omentum activation - infection and wound isolation
The omentum is commonly found wrapped around areas of infection and injury. It has well known properties to constrain the spread of intra-peritoneal infections by moving to the infection site and isolating it from the nearby healthy areas.
Therefore, the omentum has been recognised as having an important role in the immune defence, specifically in the peritoneal cavity. It plays this role by adhering to sites of inflammation, absorbing bacteria and other contaminants, and providing leukocytes for the local immune response [18].
There is still a great debate on omental migration capabilities.
In 1926, Florey H. conducted a series of experiments and concluded that there was no intrinsic omental movement, but rather passive movement. This motility resulted from the peristalsis of the gut and the action of the diaphragm [19].
The motility of the omentum towards the source of inflammation has been more recently associated to changes in the peritoneal fluid flow and consequent passive dislocation of the omentum to seal the affected area. This mechanism would allow the omentum to move around the abdominal cavity and adhere to foreign bodies and inflamed areas.
An active migration of the omentum on the site of the inflammation has been hypothesised to account for the massive migration of macrophages from the milky spot to the site of infection observed in cases of peritonitis.
The omentum has an established clinical property to pursue and contain the site of injury. A striking feature of the omentum is that its volume expands in response to foreign particles and inflammation. It makes a large number of immunomodulatory cells along with cells having stem cell properties in a process called Omentum Activation.
Several experimental studies have demonstrated that intra-peritoneal introduction of foreign particles can induce a dramatic increase in the omentum volume due primarily to growth factor activation and expansion of the stromal cell population [20], [21].
Activated omentum stromal cells (OCs) become a rich source for growth factors including fibroblast growth factor (b-FGF) and vascular endothelial growth factor (VEGF). They also express adult stem cell markers including SDF-1α, CXCR4, WT-1, as well as pluripotent embryonic stem cell markers, Nanog, Oct-4, and SSEA-1 [20], [22].
The activated omentum contains at least three distinct groups of cells that can facilitate regeneration of damaged tissue: immunomodulatory CD45+ Gr1+ MDSCs; CD45− cells that have the ability to suppress Th17 cells; and CD45−CD34+ MSCs-type [10].
T cells play critical roles in both the acute inflammation process and the resulting chronic fibrosis. In experimental models omentum works suppressing the T cells activation and proliferation possibly through the CD45− cells that inhibit Th17 cells.
In addition, in experimental models, omentum stromal cells (OCs) reduce the levels of pro-inflammatory cytokines such as IL-6 and IL-12 p40 [10].
3.1.2.2. Omentum activation in the acute peritonitis battlefield
In experimental peritonitis model, the omentum was the only abdominal organ that showed an increase in blood flow during peritonitis and abdominal sepsis [23].
The activated omentum adheres on and seals off areas of contamination, such as in acute appendicitis or diverticulitis. The omentum can rapidly produce a fibrin layer and stick to the contaminated area.
Over the course of a few days, the fibrin begins to organise. Firstly via neovascularization, then fibroblasts migration and collagen production ending with formation of dense adhesions [23], [24].
At the same time, the omentum starts the absorption and clearance of bacteria and necrotic material from the site of inflammation. The omentum, in commune with the diaphragmatic stomata has the ability to absorb particles/debrides from the peritoneal cavity. But unlike the stomata, the omentum activates the milky spots, which contain macrophages working in team with B-lymphocytes. The macrophages, engage in phagocytosis attack particles and bacteria, finally clearing the peritoneal cavity [25].
In experimental models reproducing peritonitis, the omentum appears to be the principal organ by which firstly macrophages and then neutrophils migrate into the peritoneal cavity. Due the high permeability of the milky spots there is a rapid exposure of the resident macrophages to the intra-peritoneal stimulants. The milky spots provide the correct microenvironment and growth factors [macrophage colony stimulating factors: GM-CSF and M-CSF] for macrophage maturation, differentiation and proliferation [26], [27], [28].
The omentum also facilitates the migration and proliferation of neutrophils from the circulation through the granulocyte colony stimulating factor (G-CSF). Because of the permeability of the milky spots, there is direct exposure of the post-capillary venules to the antigens coming from the peritoneal cavity. The neutrophils are then recruited from the circulation and migrate via the post-capillary venules in the glomerular loop and then via the mesothelial stomata into the peritoneal cavity [24], [29].
The omentectomy seems to impair the peritoneal defence mechanisms in experimental models such as in humans. One surgical retrospective analysis compared a group of 406 patients having had omentectomy with proctocolectomy and ileoanal anastomosis with a group of 239 patients underwent to a similar procedure without omentectomy. Results showed that the omentectomy group had a significantly higher incidence of postoperative sepsis then the group sparing omentum.
In addition, there was no difference between the two groups in the incidence of postoperative small bowel obstruction due to adhesions [30], [31].
3.1.3. Omentum: and tissue regeneration
Omental transposition to injured organs has been used in surgery for its healing capacity for more than a century. However, the mechanism by the omentum generates the healing process on injured tissues is not entirely understood and effective empiric procedures are still common in daily surgical practice.
In order to properly analyse the omentum regenerative properties, we need to first re-examine its activation process.
In the previous sections we have described that the Omentum becomes immunologically active when exposed to inflammation-infections factors or foreign bodies.
Likewise, the activated omentum shows increased stem cell markers and angiogenic growth factors. These regenerative and neoangiogenic attitudes have been well recognised and reported in literature.
Although the regenerative capacity of the omentum to promote wound healing and its anti-inflammatory pathways are well known, the biological understanding of the mechanisms is quite poor.
Recent data suggests that the omentum contains various clusters of cells that accumulate in the activated omentum with differing roles in mitigating inflammation and supporting tissue regeneration [10].
Literature shows in the omentum the presence of various cells with stem cell roles. Experimental models suggest that omentum contains pluripotent stem cells able to differentiate into nerve cells, adipocytes, and hepatocytes [21], [22], [32], [33].
It has been found that activated omentum contains cells (MSCs-type) with stem cell functions among the CD45−CD34+ subsets. This MSCs clusters have the potential to differentiate into bone, fat and cartilage [34], [35].
Recent studies have confirmed that MSCs not only differentiate into cells of mesodermal lineage, but also cells of endodermal and ectodermal lineages, including cardiomyocytes, lung epithelial cells, hepatocytes, neurons, and pancreatic islets [36], [37], [38], [39], [40], [41], [42], [43], [44], [45].
MSCs were originally identified from bone marrow but cells with similar characteristics have been later recognised and isolated from various mesodermal lineage tissue. They are stored in clusters ready to be activated [46].
The recent demonstration of the contemporary localization of the MSCs and MDSCs (myeloid derived suppressor cells) in the omentum has been significant. This evidence can explain the omentum anti-inflammatory and immunomodulatory properties and also the tissue regeneration and wound healing capacity. MDSCs proliferate in the activated omentum and regulate inflammatory and immune responses, meanwhile MSCs activation operates as the main cellular source for tissue repairing activity [10].
4. Omentum basic biological principles can pave the way for future surgical practice
We have seen above that omentum has unique biological properties including neovascularization, haemostasis, tissue healing and regeneration and as an in vivo incubator for cell and tissue cultivation. Some of these properties have long been noted in surgical practice.
4.1. Neovascularization
The fundamental property of the omentum is to promote neoangiogenesis in structures to which it is applied, this effect can also extend to adjacent structures. Some researchers have found that the human omental microvascular endothelial cells (HOME cells) express some angiogenic peptides such as b-FGF and VEGF; these are key factors to induce neovascularization [47].
This process of neoangiogenesis allows the omentum to provide vascular support and blood supplementation to adjacent tissues and to promote the healing process in ischaemic and inflamed tissue [24], [48].
Omentum adipose tissue (OAT) has unique biological properties that do not exist in any other fat tissue.
An angiogenic lipid-soluble factor distinctive from VEGF and b-FGF has been demonstrated by Goldsmith et al. in 1985; then applied in an experimental study to speed the process of bone healing [49], [50], [51].
4.2. Tissue healing and regeneration
Literature has revealed that the omentum is a great source of various growth factors, neurotropic and haemostatic factors and inflammatory mediators.
In addition, it contains pluripotent stem cells that can differentiate into a variety of cell types [3], [52].
The recent demonstration of the presence of the MSCs and MDSCs together in the omentum partially explains its tissue regeneration and wound healing capacity [10].
In addition, other authors have discovered that omentum MSCs-like not only differentiate into cells of mesodermal lineage, but also to cells of endodermal and ectodermal lineages, including cardiomyocytes, lung epithelial cells, hepatocytes, neurons, and pancreatic islets [36], [37], [38], [39], [40], [41], [42], [43], [44], [45].
The omentum supports early post-natal haematopoiesis and lymphopoiesis and in experimental models, this ability continues throughout the rest of life [53].
Furthermore, activated omentum stromal cells (OCs) produces stromal cell derived factor -SDF-1α. It is a strong chemotactic factor, which attracts different stem cells to the injury site to promote tissue repair [53].
4.3. In vivo incubator for cells and tissue cultivation
Considering all the above, it is highly probable that the omentum could operate as a site to expand, maintain survival and function of cells, organs and tissue.
There has been a longstanding surgical practice to rescue splenic function after total splenectomy by implanting small pieces of spleen “spleen chips” in the omentum. Histo-physiology studies have proven that these implanted spleen chips not only survive but thrive [54].
In diabetes type 1 cases, Literature has reported that pancreatic islets have been injected into the portal vein with the aim to implant them into the liver. However, Literature has reported a very modest survival rate of these islets. The reasons for the low survival rate have been supposed to be poor blood supplementation and hostile extra-celluar microenvironmental factors [55].
Experimental studies in rats indicated that implanting pancreatic islets in an omental pouch resulted in impressive clinical improvements with normalization of blood diabetes parameters [56], [57].
A clinical trial of islets transplantation into an omental pouch is currently in course promoted by the University of Alberta [ClinicalTrials.gov Identifier: NCT02821026]. The trial tests this new procedure in people with difficult to control Type 1 diabetes. “Insulin producing cells (islets) are isolated from a pancreas of a deceased organ donor. After the cells are carefully prepared, the islets are transplanted into patient's omentum pouch. These transplanted islets may produce insulin for the patient. Patient may be able to reduce or eliminate the need for insulin injections for an unknown period of time”.
In other experimental models supported by tissue engineering with biodegradable scaffolds and 3D shaped scaffolds, omentum has demonstrated to be the best in vivo environment to expand cultivated hepatocytes to regenerate liver [58].
Kadir B et al. have used rabbit model to expand in vivo cartilage tissue. They reported that the transplantation of the cartilage grafts into the omentum mesothelium enhanced the chondrocyte counts and volumes compared with others in vivo culture. Authors recommended the mesothelium as an effective in vivo culture medium for osteo-chondral tissue growth [59].
Therefore, it is reasonable to suppose that the omentum is capable of supporting free structures such as the trachea, oesophagus and other tissue. These structures can be then used for reconstructive purposes [60], [61], [62], [63].
In the last few years the omentum has become the favourable implantation place for growing embryonic tissue to maturity.
Specifically, it has been seen in experimental models that embryonic kidney and pancreas implanted into an omental pouch grow up and complete the natural maturation process developing adult normal organs. This impressive result is mainly due to the blood supplementation and optimal microenvironment, provided by the omentum hosting the organ. The neoangiogenesis and immune-regulation facilities explain why the omentum can be the main in vivo incubator to grow mature organs from embryonic ones [64], [65], [66], [67].
4.4. Basic surgical procedures to use the omentum in clinical practice
The omental transposition is the most commune surgical manoeuvre for positioning the omentum on the injured organ or tissue. This move can be a simple placing and fixation of the transposed omentum or an omental flap (omentoplasty) can be prepared to elongate and better position the omentum on the receiving site.
The omental flap is prepared by dividing and sparing the gastroepiploic vessels from the great curve of the stomach to maintain intact the flap blood supply. The advantage of the technique is that the flap can be moved distant from the omentum and thereby reach places in the chest, pelvis and base of the trunk that are otherwise impossible to reach.
In case the omentum needs to be transposed in a remote position such as head and neck a microsurgical free omental graft can be shaped to reach the implantation site.
Omentum fractions can be taken and implanted directly on the receiving site, either after centrifugation or cultivation. In these cases a surgeon can consider the use of appropriate scaffolds to facilitate the survival, differentiation and proliferation of the transplanted cells. The preparation of the receiving site with different growth factors can be useful in vivo to facilitate the development of specific tissue.
The omentum can be folded as a pouch to hold in vivo biomaterial, cells, tissue and organs and to favour their growth and integration. An omentum pouch can facilitate tissue survival and growth, expedite cell cultivation and embryonic organ maturation.
5. Omentum: current use in surgery
5.1. General surgery
Some of the omentum properties such as migration, adherence and sealing off contaminated areas are very well known in course of complicated colonic diverticulitis and appendicitis; and they are easily recognised in the process of integration of intra-abdominal foreign bodies such as drains or dialysis catheters.
Using the same principles, omentum has been applied in gastrointestinal surgery to wrap around the sites of the anastomosis and so reinforce the joining, and to prevent leakage.
The omentoplasty technique divides the right or left gastroepiploic arteries up to the vasa recta along the greater curvature of the stomach to mobilize the omentum pedicle from the transverse colon and making a vascularized omental pedicle flap. The flap can be lengthened to various extensions to fill empty spaces, protect the intraperitoneal cavity from leakage and cover bowel anastomosis. Vascularized omental pedicle flap can be used to prevent the small bowel from falling down into the pelvis after major colorectal resections such as abdominoperineal resection, thus preventing radiation enteritis due to adjuvant radiotherapy for advanced rectal carcinoma [68].
Numerous studies evaluated the use of the omentum to support gastrointestinal anastomoses. In some experimental studies, there have been conflicting results as to whether reinforcing a compromised (i.e., ischaemic or technically inadequate) anastomosis with a well-vascularized omentum would have reduced the leak rate. Results have been controversial, however, there have not been observed any significant improvement in fatal leak rates using a transposed omentum [2], [69], [70].
Merad F et al. in a prospective randomized trial have assessed the omentoplasty to wrap the anastomosis and prevent anastomotic leak after colorectal resections. 705 patients underwent elective bowel resection and were divided into four groups: ileo- or colo-colonic anastomosis, supraperitoneal colorectal anastomosis, infraperitoneal colorectal anastomosis, and ileo- or colo-anal anastomosis. Patients were randomized to have either omental wrapping of the anastomosis or no wrapping. When comparing the omental reinforcement group with the control, there was no significant difference in either anastomotic leakage (4.7% vs 5.2%) or deaths (4.9% vs 4.2%). The Authors concluded that omental reinforcement of colorectal anastomosis was not clearly beneficial to prevent anastomotic leakage [71].
Nonetheless, another study showed completely the opposite.
Tocchi A et al. reported favourable results with omentoplasty in a trial of 112 patients who underwent colorectal resection. Only 3.8% of patients who had omentoplasty developed anastomotic leaks, compared with 11.8% of patients who underwent anastomosis without omental flap [72].
Anastomotic leak is the most feared complication in upper GI surgery for cancer of the cardias and oesophagus. The leak rate is higher in cervical anastomoses, while it is virtually always fatal when it occurs in the intra-thoracic anastomoses. Some Authors recommend omental flaps to protect anastomosis, prevent leak or delimitate micro perforation and improve infection control [73].
Omentum is routinely used for the treatment of benign perforated gastric ulcer. Ulcer excision and omental patch closure are as effective as gastrectomy. Likewise, in perforated duodenal ulcers, the Graham omental patch closure is a well known and commonly performed effective procedure [74].
The omental patch used for closing gastro-duodenal defects promotes the wound healing through the combined effects of neovascularization, granulation, scaffolding and fibrosis in order to regenerate a normal duodenal wall on the perforated site [75].
The omentum has been used to fill cavities, to prevent intra-abdominal collections due to its fluid absorption and haemostatic capacities and eventually to promote healing through neoangiogenesis and tissue repairing.
Di Giorgio A et al. have proposed a new procedure for the abdominal rectopexy using an omental flap to support and lift the prolapsed rectum and to refashion the ano-rectal angle. The technique does not use any synthetic meshes avoiding the severe complications related to the mesh technique [76].
Some surgeons have applied transposed omentum to improve the haemostasis during liver resections and to line the bed of hydatid cysts in the liver [77].
In paediatric splenic trauma, spleen-wrapping omentoplasty has been associated with splenorrhaphy or partial splenectomy as an effective spleen-saving procedure [77].
Omentum has been used to cover defects and to repair injured abdominal wall from the chest up to the perineum [77].
5.2. Neurosurgery
The omentum flap transposition on the brain surface generates neoangiogenesis resulting in numerous neovascular connections between these two structures. The omentum improves the healing of brain injury through neoangiogenesis thereby increasing the brain blood flow and oxygen level. Furthermore, omental neurotransmitters (dopamine, noradrenaline and acetylcholine) and neurotropic factors such as nerve growth factor and gangliosides help to restore the neurologic functions [82].
These omental properties have been surgically practised by Rafael et al.; in two cases affected by temporal lobe epilepsy, they positioned the omental tissue directly on the epileptic focus and this resulting in reduction of seizures. Hypo-perfusion and metabolic stress of the epileptic foci were normalized and neuronal hyper-excitability was reduced [83].
Goldsmith HS, applied brain omental graft to patients with chronic ischemic cerebrovascular diseases and Alzheimer reporting some cognitive and memory improvements [84], [85].
Intracranial omentum transplantation can alleviate symptoms in patients with cerebrovascular moyamoya disease. It is a rare, progressive cerebrovascular disorder caused by blocked arteries at the base of the brain in an area called the basal ganglia.
In a series of 30 children with moyamoya disease omental transplantation improved neovascularization in either the anterior or the posterior cerebral artery territory. All patients showed significant clinical neurological improvements [86], [87].
Normington EY et al., showed in a case report that the omentum microsurgical free graft can be transposed to the subarachnoid space to attempt the closure of Cerebrospinal fluid fistulas (CSF) [88].
There are some published reports in favour of omental transplantation in acute and chronic spinal cord injury. The omentum has been used to support the regeneration of neurons across a freshly transected spinal cord in experimental models. The same has been observed in at least one patient, resulting in the unexpected recovery of limb function [89], [90].
5.3. Heart and chest surgery
It has been seen that the omentum is an extraordinary source of angiogenic factors like VEGF and FGF-2. Specifically, CD34 + cell population of the human omentum could be responsible for the observed clinical benefits of omental transplantation by promoting angiogenesis and synthesizing angiogenic growth factors to facilitate revascularization of injured tissue [91].
Hybrid surgical angiogenesis has been proposed to regenerate infarcted myocardium tissue. Omentum transplantation together with cell patch cardiomyoplasty (transplantation of skeletal myoblasts from autologous rectus muscle into the infarcted myocardium area) and in conjunction with coronary artery bypass may stimulate myo-angiogenesis in the ischemic scar tissue, resulting in a regeneration of the myocardium tissue and improvement of functional capacity [92].
There have been several attempts to use omental flaps to patch and dress the mediastinum in patients with sternal wound infection and mediastinitis secondary to cardiac surgery. These reports are not adequately controlled. Nevertheless, Authors comment that omental flaps are associated with fewer septic complications than pectorals major flaps, and are connected with higher rates of healing and lower mortality when compared with simple debridement [93], [94], [95].
In chest surgery, omentum has been widely used to fill up dead-septic space in chronic empyema, for chest wall reconstruction and to seal a leaking bronchus stump after pneumonectomy. Omentoplasty can also prevent bronchial dehiscence following lung transplantation [96].
5.4. Vascular surgery
In surgical practice the omentum is often wrapped around aortic reconstructions to protect from graft infection and aorto-enteric fistula formation.
Omental flaps have been proven to be a valid treatment option for patients with Buerger's disease. Literature shows marked improvement of intermittent claudication and pain, the healing of ischemic ulcers and delaying of progression of gangrene in lower and upper extremities [97], [98].
In the arterial ileo-femoral graft reconstruction the availability of soft tissue covering the surgical access can sometimes be challenging, especially when there was a wide debridement of the nearby soft tissues following a previous graft infection and pseudo-aneurism. In this situation, the omentum transposed and distributed over the femoral area can help to close the defect, to protect the arterial graft reconstruction and providing a surface to facilitate the skin regrowth [99].
5.5. Plastic surgery
In plastic surgery omental flaps transposed through the neck have been used in conjunction with skin graft, to repair full thickness defects of the cheek in the so-called “omental sandwich” technique [100].
The omentum prepared as microsurgical free graft has been applied to reconstruct various head wounds and defects, such as in wide temporo-parietal region wounds involving total ear reconstruction [101].
Locally advanced breast cancer with chest wall involvement has been treated with debulking chest surgery and omental flap transposition as a palliative procedure in the attempt to control local disease and alleviate symptoms [102].
Omental flaps were applied for massive abdominal wall reconstruction to cover the bridging synthetic mesh, prevent infections and facilitating the process of mesh integration into the host tissue [103].
Omentum flap has shown to prevent the formation of lymphedema after ileo-inguinal radical lymph node dissection by improving the draining of the lymphatic fluids [104].
Matsuda et al. proposed transplantation of “fragment omental tissue and preadipocytes” for soft tissue augmentation. The suggestion was based on the high vascularization of the omentum and the capacity to produce large amounts of vascular endothelial growth factor (VEGF) [105].
Similarly, there have been reports on the use of the omentum as a free graft for the treatment of chronic ulcers, progressive hemifacial atrophies, and chronic wounds. The transferred omentum appears to maintain its own volume and biology in the receiving area maintaining wound regenerative properties and the capacity to filling up the soft tissue defects [106].
5.6. Orthopaedic surgery
Literature reports some anecdotic experience such as of Knazozovicky that in 1929 use successfully the transposed omentum as autologous implant for an arthroplasty [53].
Omentoplasty is recognised as one of the most effective treatments for septic lesions of costal cartilage and sternum. The radiation osteomyelitis refers to the process of necrosis of the bony structures in irradiated sites. Omental flap associated with skin grafting has been successfully applied for the treatment of the full thickness necrotic infection on the anterior chest wall secondary to mastectomy and postoperative radiotherapy [78].
The omentum can be used as sliding protecting support to prevent adhesions of nerves and tendons after extensive forearm tenolysis. The omental graft has helped to impede infection, protect the exposed nerves and tendons and direct the wound healing [79].
Omental microsurgical free graft transplantation has been applied in the management of brachial plexus injury to cover the wound site, protect nerves and improve pain control [80].
Goitz RJ et al. have successfully used omental flap to recover median nerve injured after multiple operations for a recalcitrant carpal tunnel syndrome and untreatable median neuritis [81].
5.7. Urogenital surgery
Laparoscopic extraperitoneal tunnelling and omental wrapping of the peritoneal dialysis catheters improve some symptoms and complications like pain due to catheter migration and peri-catheter leakage [107].
Omental wrapping and stent insertion have been the combined procedure for the prevention and treatment of urinary fistulas as in the case of urinary tract injury or ureteral iatrogenic lesions.
In some circumstances where the kidney blood perfusion is affected, omentum has been proposed to provide alternative blood supply to the kidney and rescue renal function [108].
Mokhort et al. have proposed the omentovesicopexy for the treatment of neurogenic dysfunctions of the urinary bladder. In this technique, according to the Authors, the omentum provides re-innervation and revascularization of the neurogenic bladder re-establishing a partial functional recovery [109].
Kamey Y et al. in a case report have used a composite gastric seromuscular and omental pedicle flap to repair combined defects of the urethra, scrotum and abdominal wall in a patient with Fournier's gangrene. This technique has been described as successful to provide effective closure of perineal complex defects including the repair of the urethra [110].
Omentotesticulopexy has been recommended for the treatment of high-undescended testes with the purpose of improving the vascular supply and reduce the rate of testicular atrophy and sterility [111].
5.8. Gynaecological surgery
Patsner B et al. reviewed the use of omental flap to prevent postoperative complications such as pelvic collection, abscess or intestinal obstruction after radical abdominal hysterectomy [112].
Likewise, in a case report, an omental flap was prepared and allocated into the pelvis up to the perineum for a perineal reconstruction after radical vulvectomy [113].
Kusiak JF proposed and successfully used an omental cylinder flap lined with a split-thickness skin graft to reconstruct a neovagina following pelvic exenteration for gynaecological cancer [114].
Obstetric trauma, radiotherapy and radical gynaecological surgeries can result in different vesicovagina iatrogenic fistulae. The omentum has been transferred in a variety of different procedures in the attempted of treatment of these challenging fistulae [115].
6. Debate and proposals
In the last decades the surgical discussion has been centred mostly on technologic advancements.
Laparoscopy, minimally invasive procedures and robotics have been recognised to perfectly replicate, sometimes even better, the performance of open surgery. Furthermore, important short-term measures such as postoperative time of recovery, pain control, reduction of hospital stay and return to normal activities, have improved significantly.
However, long term results have not improved significantly over those of previous decades.
Long-term results can improve only when the cause of disease has been addressed. Therefore, degenerative chronic disease, recurrence, morbidity and survivals rate (e.g. advanced cancer) have, in general, achieved minimal improvements.
There appears to be a widening gap between biology research, biotechnology advancements and surgical practice. One of the main causes seems to be the poor communication between scientists investigating regenerative procedures and surgeons in clinical practice. This has led to difficulty in turning insights into clinical effective surgical procedures.
The result is that only a small part of the enormous amount of research performed in the last few years in biotechnologies, cells and tissue engineering, stem cell treatments, tissue growth factors and self-repairing techniques have been taken up in clinical practice.
Regenerative surgery might positively impact the complex problem of aging related disease and tissue-organ deterioration.
Chronic degenerative diseases are a heavy cost burden on both public and private health sectors. Often in daily practice, patients are offered only symptoms control and pain management treatments.
A more effective communication between biomedical researchers and surgeons should be promoted to expand understanding and delivery of potentially curative treatments, which may be applied through the regenerative surgery. This would be beneficial for both current and future aging generations and improve cost/effectiveness.
New educational and training programs for the future surgeons should be introduced to fill the gap. Next surgeons generations shall be expert in new technologies alongside biotechnologies, cells and tissue engineering, stem cells treatments, tissue mediators and self-repairing techniques.
Omentum is an organ that shows unique properties in biology and surgery. It can be seen as a very active team player working in connection with all other organs and tissue in its vicinity. When activated, it expands its volume and blood flow, it grows different type of cells and produces a variety of molecules working in concert to protect and regenerate injured tissue.
In Surgery, the omentum has demonstrated enormous versatility in different sectors and appears robust and relatively simple to use in surgical practice. Further, it facilitates some procedures of cell and tissue transplantation and hosts progenitors cells, embryonic organs and various tissue. Many, and perhaps most of these effects can beneficially be applied in surgical practice.
General Surgeons have always struggled with leakage complications following bowel anastomosis. Tension, torsion and mostly poor vascularization of the anastomosis have always been recognised as major contributing factors for the anastomotic leak.
As reported in the general surgery section omentum anastomotic wrapping seems unclear to induce significant improvements in the leak rate.
Otherwise, surgeons know that omental patch procedure is very effective to treat perforated peptic ulcers.
It seems evident that in colorectal anastomosis we can apply omentum to cover an anastomosis already sealed by the suture without exposing the open wound in direct contact with the omentum.
In the Graham patch procedure, the omentum is applied to an open wound; therefore, there is direct contact with blood and tissue factors coming from the gastroduodenal debride ulcers. Hence, the “wound effect” looks critical for the local activation of the omentum.
The proposal is to interpose a thin omentum patch; either fragmented omentum or better an omental thin flap into the stapled line. This may promotes omental neovascularization and tissue regeneration to facilitate the definite healing of the anastomosis.
An experimental model would easily determine whether this surgical hypothesis is correct.
In Orthopaedics, omentum arthroplasty using omentum fragments or tiny patches to be allocated over the capsule bursa tissue may be considered to stimulate local MSCs and tissue recovery. Local stimulation of MSCs has already been proven effective to regenerate capsule bursa tissue and neo-cartilage formation in clinical research [116], [117], [118].
Conflicts of interest
The author declares that he does not have financial or nonfinancial competing interests.
Acknowledgments
The author sincerely appreciates Ms Jordana Lyden-Swift for drafting images.
The author is grateful to Thomas Richard Swift for the linguistic revision of the manuscript and for his friendship.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.07.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Drake Richard, Vogl A. Wayne, Mitchell Adam. Churchill Livingstone/Elsevier; Philadelphia, PA: 2010. Gray's anatomy for students. [Print] [Google Scholar]
- 2.McLachlin A.D., Denton D.W. Omental protection of intestinal anastomoses. Am J Surg. 1973;125:134–140. doi: 10.1016/0002-9610(73)90018-4. [DOI] [PubMed] [Google Scholar]
- 3.Goldsmith H.S. Springer-Verlag; New York: 1990. The omentum: research and clinical applications. [Google Scholar]
- 4.Chung Kyung Won. Lippincott Williams & Wilkins; Hagerstown, MD: 2005. Gross anatomy (Board review) p. 205. [Google Scholar]
- 5.Ackermann P.C., De Wet P.D., Loots G.P. Microcirculation of the rat omentum studied by means of corrosion casts. Acta Anat (Basel) 1991;140:146–149. doi: 10.1159/000147051. [DOI] [PubMed] [Google Scholar]
- 6.Collins D., Hogan A.M., O'Shea D., Winter D.C. The omentum: anatomical, metabolic, and surgical aspects. J Gastrointest Surg. 2009;13:1138–1146. doi: 10.1007/s11605-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 7.Liebermann-Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin N Am. 2000;80:275–293. doi: 10.1016/s0039-6109(05)70406-0. [DOI] [PubMed] [Google Scholar]
- 8.Arner P. Differences in lipolyses between human subcutaneous and omental adipose tissue. Ann Med. 1995 Aug;27(4):435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- 9.Klopp Ann H., Zhang Yan, Solley Travis, Amaya- Manzanares Felipe, Marini Frank, Andreeff Michael. Omental Adipose Tissue–Derived Stromal Cells Promote Vascularization and Growth of Endometrial Tumors. Clin Cancer Res. 2012;18(3):771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah Shivanee, Lowery Erin, Braun Rudolf K., Martin Alicia, Huang Nick, Medina Melissa. Cellular basis of tissue regeneration by omentum. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijffels J.F., Hendrickx R.J., Steenbergen J.J., Eestermans I.L., Beelen R.H. Milky spots in the mouse omentum may play an important role in the origin of peritoneal macrophages. Res Immunol. 1992;143:401–409. doi: 10.1016/s0923-2494(05)80072-0. [DOI] [PubMed] [Google Scholar]
- 12.Mironov V.A., Gusev S.A., Baradi A.F. Mesothelial stomata overlying omental milky spots: scanning electron microscopic study. Cell Tissue Res. 1979;201:327–330. doi: 10.1007/BF00235068. [DOI] [PubMed] [Google Scholar]
- 13.Krist L.F., Eestermans I.L., Steenbergen J.J., Hoefsmit E.C., Cuesta M.A., Meyer S. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 14.Dux K. Proliferative activity of macrophages in the greater omentum of the mouse in relation to the early postnatal development of the vascular structures. J Leukoc Biol. 1986;40:445–458. doi: 10.1002/jlb.40.4.445. [DOI] [PubMed] [Google Scholar]
- 15.Kantor A.B., Herzenberg L.A. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 16.Solvason N., Kearney J.F. The human fetal omentum: a site of B cell generation. J Exp Med. 1992;175:397–404. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung-Welch N., Patton W.F., Shepro D., Cambria R.P. Two-stage isolation procedure for obtaining homogenous populations of microvascular endothelial and mesothelial cells from human omentum. Microvasc Res. 1997;54:121–134. doi: 10.1006/mvre.1997.2039. [DOI] [PubMed] [Google Scholar]
- 18.Hall J.C., Heel K.A., Papadimitriou J.M., Platell C. The pathobiology of peritonitis. Gastroenterology. 1998;114:185–196. doi: 10.1016/s0016-5085(98)70646-8. [DOI] [PubMed] [Google Scholar]
- 19.Florey H. The nature of the movements of the omentum. J Pathol. 1926;29(1):97–106. [Google Scholar]
- 20.Litbarg N.O., Gudehithlu K.P., Sethupathi P., Arruda J.A., Dunea G., Singh A.K. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007;328:487–497. doi: 10.1007/s00441-006-0356-4. [DOI] [PubMed] [Google Scholar]
- 21.Singh A.K., Patel J., Litbarg N.O., Gudehithlu K.P., Sethupathi P., Arruda J.A. Stromal cells cultured from omentum express pluripotent markers, produce high amounts of VEGF, and engraft to injured sites. Cell Tissue Res. 2008;332:81–88. doi: 10.1007/s00441-007-0560-x. [DOI] [PubMed] [Google Scholar]
- 22.Singh Ashok K., Pancholi Nishit, Patel Jilpa, Litbarg Natalia O., Gudehithlu Krishnamurthy P., Sethupathi Perianna. Omentum facilitates liver regeneration. World J Gastroenterol. 2009;15:1057–1064. doi: 10.3748/wjg.15.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron Platell, Cooper Deborah, Papadimitriou John M., Hall John C. The omentum. World J Gastroenterol. Apr 15, 2000;6(2):169–176. doi: 10.3748/wjg.v6.i2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konturek S.J., Brzozowski T., Majka I., Pawlik W., Stachura J. Omentum and basic fibroblast growth factor in healing of chronic gastric ulcerations in rats. Dig Dis Sci. 1994;39:1064–1071. doi: 10.1007/BF02087559. [DOI] [PubMed] [Google Scholar]
- 25.Shipley P.G., Cunningham R.S. Studies on the absorption from serous cavities: 1. The omentum as a factor in absorption from the peritoneal cavity. Am J Physiol. 1916;40:75–81. [Google Scholar]
- 26.Shimotsuma M., Simpson-Morgan M.W., Takahashi T., Hagiwara A. Activation of omental milky spots and milky spot macrophages by intraperitoneal administration of a streptococcal preparation, OK-432. Cancer Res. 1992;52:5400–5402. [PubMed] [Google Scholar]
- 27.Pinho M. de FB., Hurtado S.P., El-Cheikh M.C., Rossi M.I., Dutra H.S., Borojeecic R. Myelopoiesis in the omentum of normal mice and during abdominal inflammatory process. Cell Tissue Res. 2002;308:87–96. doi: 10.1007/s00441-002-0550-y. [DOI] [PubMed] [Google Scholar]
- 28.Pinho M. de FB., Hurtado S.P., El-Cheikh M.C., Borojeecic R. Haemopoietic progenitors in the adult mouse omentum: permanent production of B lymphocytes and monocytes. Cell Tissue Res. 2005;319:91–102. doi: 10.1007/s00441-004-0998-z. [DOI] [PubMed] [Google Scholar]
- 29.Fukatsu K., Saito H., Han I., Yasuhara H., Lin M.T., Inoue T. The greater omentum is the primary site of neutrophil exudation in peritonitis. J Am Coll Surg. 1996;183:450–456. [PubMed] [Google Scholar]
- 30.Ambroze W.L., Wolff B.G., Kelly K.A., Beart R.W., Dozois R.R., Ilstrup D.M. Let sleeping dogs lie: role of the omentum in the ileal pouch-anal anastomosis procedure. Dis Colon Rectum. 1991;34:563. doi: 10.1007/BF02049895. 56. [DOI] [PubMed] [Google Scholar]
- 31.Pothinam S., Sirinavasatian P., Lumbiganon P. Febrile and infectious morbidity after abdominal hysterectomy at Srinagarind Hospital. J Med Assoc Thail. 1992;75:178–183. [PubMed] [Google Scholar]
- 32.Rajesh R.V., Heo G.-N., Park M.-R., Nam J.-S., Kim N.-K. Proteomic analysis of bovine omental, subcutaneous and intramuscular preadipocytes during in vitro adipogenic differentiation. Comp Biochem Physiol Genom Proteonom. 2010;5:234–244. doi: 10.1016/j.cbd.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi R., Azizi S., Delirezh N., Hobbenaghi R., Amini K. Comparison of beneficial effects of undifferentiated cultured bone marrow stromal cells and omental adipose-derived nucleated cell fractions on sciatic nerve regeneration. Muscle Nerve. 2011;43:157–163. doi: 10.1002/mus.21895. [DOI] [PubMed] [Google Scholar]
- 34.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 35.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D.S. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 36.Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Investig. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 38.Wang G., Bunnell B.A., Painter R.G., Quiniones B.C., Tom S., Lanson N.A., Jr Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spees J.L., Olson S.D., Ylostalo J., Lynch P.J., Smith J., Perry A. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz R.E., Reyes M., Koodie L., Jiang Y., Blackstad M., Lund T. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Investig. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato Y., Araki H., Kato J., Nakamura K., Kawano Y., Kobune M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 43.Munoz J.R., Stoutenger B.R., Robinson A.P., Spees J.L., Prockop D.J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriscot C., de Fraipont F., Richard M.J., Marchand M., Savatier P., Bosco D. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23:594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 45.Oh S.H., Muzzonigro T.M., Bae S.H., LaPlante J.M., Hatch H.M., Petersen B.E. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Investig. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 46.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 47.Bikfalvi A., Alterio J., Inyang A.L., Dupuy E., Laurent M., Hartmann M.P. Basic fibroblast growth factor expression in human omental microvascular endothelial cells and the effect of phorbol ester. J Cell Physiol. 1990;144:151–158. doi: 10.1002/jcp.1041440120. [DOI] [PubMed] [Google Scholar]
- 48.Weber T., Hanisch E., Baum R.P., Seufert R.M. Late results of heterotopic autotransplantation of splenic tissue into the greater omentum. World J Surg. 1998;22:883–889. doi: 10.1007/s002689900487. [DOI] [PubMed] [Google Scholar]
- 49.Goldsmith H.S., Griffith A.L., Kupferman A., Catsimpoolas N. Lipid angiogenic factor from omentum. Jama. 1984;252:2034–2036. [PubMed] [Google Scholar]
- 50.Goldsmith H.S. Forefront Publishing; Wilton CT: 2000. The Omentum. Application to brain and spinal cord. [Google Scholar]
- 51.Nottebaert M., Lane J.M., Juhn A., Burstein A., Schneider R., Klein C. Omental angiogenic lipid fraction and bone repair. An experimental study in the rat. J Orthop Res. 1989;7:157–169. doi: 10.1002/jor.1100070202. [DOI] [PubMed] [Google Scholar]
- 52.Logmans A., Schoenmakers C.H., Haensel S.M., Koolhoven I., Trimbos J.B., van Lent M. High tissue factor concentration in the omentum, a possible cause of its hemostatic properties. Eur J Clin Investig. 1996;26:82–83. doi: 10.1046/j.1365-2362.1996.107247.x. [DOI] [PubMed] [Google Scholar]
- 53.Vernik J., Singh A.K. Omentum : power to heal and regenerate. Int J Artif Organs. 2007;30(2):95–99. doi: 10.1177/039139880703000203. [DOI] [PubMed] [Google Scholar]
- 54.Marques R.G., Petroianu A., Coelho J.M., Portela M.C. Regeneration of splenic autotransplants. Ann Hematol. 2002;81:622–626. doi: 10.1007/s00277-002-0564-2. [DOI] [PubMed] [Google Scholar]
- 55.Gaglia J.L., Shapiro A.M., Weir G.C. Islet transplantation: progress and challenge (review) Arch Med Res. 2005;36:273–280. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Kin T., Korbutt G.S., Rajotte R.V. Survival of islets in omental pouch. Am J Transplant. 2003;3:281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 57.Rogers S.A., Chen F., Talcott M., Hammerman M.R. Islet cell engraftment and control of diabetes in rats after transplantation of pig pancreatic anlagen. Am J Physiol Endocrinol Metab. 2004;286:E502–E509. doi: 10.1152/ajpendo.00445.2003. [DOI] [PubMed] [Google Scholar]
- 58.Lee H., cusick R.A., Utsunomiya H., Ma P.X., Langer R., Vacanti J.P. Effect of implantation site on hepatocytes heterotopically transplanted on biodegradable polymer schaffolds. Tissue Eng. 2003;9:1227–1232. doi: 10.1089/10763270360728134. [DOI] [PubMed] [Google Scholar]
- 59.Buyukdogana Kadir, Dorala Mahmut Nedim, Bilgec Onur, Turhanb Egemen, Hurib Gazi, Sargon Mustafa Fevzi. Peritoneum and omentum are natural reservoirs for chondrocytes of osteochondral autografts: a comparative animal study. Acta Orthop Traumatol Turc. 2016;50(5):539–543. doi: 10.1016/j.aott.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., Xu P., Chen H., Yang Z., Zhang Q. Improvement of tracheal autograft survival with transplantation into the greater omentum. Ann Thorac Surg. 1995;60:1592–1596. doi: 10.1016/0003-4975(95)00839-x. [DOI] [PubMed] [Google Scholar]
- 61.Chung E.J., Ju H.W., Yeon Y.K., Lee J.S., Lee Y.J., Seo Y.B. Development of an omentum-cultured oesophageal scaffold reinforced by a 3D-printed ring: feasibility of an in vivo bioreactor. Artif Cells Nanomed Biotechnol. 2018;46(Suppl. 1):885–895. doi: 10.1080/21691401.2018.1439039. [DOI] [PubMed] [Google Scholar]
- 62.Chamorro M., Carceller F., Llanos C., Rodríguez-Alvariño A., Colmenero C., Burgueño M. The effect of omental wrapping on nerve graft regeneration. Br J Plast Surg. 1993;46:426–429. doi: 10.1016/0007-1226(93)90050-l. [DOI] [PubMed] [Google Scholar]
- 63.Kin S., Kim J., Shin J., Kil K., Kim H., Kim J. Use of omentum as an in vivo cell culture system in tissue engineering. Am Soc Artif Intern Organs J. 2004;50:464–467. doi: 10.1097/01.mat.0000138016.83837.8a. [DOI] [PubMed] [Google Scholar]
- 64.Rogers S.A., Lowell J.A., Hammerman N.A., Hammerman M.R. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54:27–37. doi: 10.1046/j.1523-1755.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 65.Rogers S.A., Liapis H., Hammerman M.R. Transplantation of metanephroi across the major histocompatibility complex in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:132–136. doi: 10.1152/ajpregu.2001.280.1.R132. [DOI] [PubMed] [Google Scholar]
- 66.Rogers A., Liapis H., Hammerman M.R. Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transpl Immunol. 2005;14:67–75. doi: 10.1016/j.trim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Hammerman M.R. Renal organogenesis from transplanted metanephric primordia. J Am Soc Nephrol. 2004;15:1126–1132. doi: 10.1097/01.asn.0000106020.64930.64. [DOI] [PubMed] [Google Scholar]
- 68.De Broux E., Parc Y., Rondelli F., Dehni N., Tiret E., Parc R. Sutured perineal omentoplasty after abdominoperineal resection for adenocarcinoma of the lower rectum. Dis Colon Rectum. 2005;48:476–481. doi: 10.1007/s10350-004-0784-8. [DOI] [PubMed] [Google Scholar]
- 69.Adams W., Ctercteko G., Bilous M. Effect of an omental wrap on the healing and vascularity of compromised intestinal anastomoses. Dis Colon Rectum. 1992;35:731–738. doi: 10.1007/BF02050320. [DOI] [PubMed] [Google Scholar]
- 70.Carter D.C., Jenkins D.H., Whitfield H.N. Omental reinforcement of intestinal anastomoses. An experimental study in the rabbit. Br J Surg. 1972;59:129–133. doi: 10.1002/bjs.1800590214. [DOI] [PubMed] [Google Scholar]
- 71.Merad F., Hay J.M., Fingerhut A., Flamant Y., Molkhou J.M., Laborde Y. Omentoplasty in the prevention of anastomotic leakage after colonic or rectal resection: a prospective randomized study in 712 patients. French Associations for Surgical Research. Ann Surg. 1998;227:179–186. doi: 10.1097/00000658-199802000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tocchi A., Mazzoni G., Lepre L., Costa G., Liotta G., Agostini N. Prospective evaluation of omentoplasty in preventing leakage of colorectal anastomosis. Dis Colon Rectum. 2000;43(7):951–955. doi: 10.1007/BF02237357. [DOI] [PubMed] [Google Scholar]
- 73.Thakur B., Zhang C.S., Tan Z.B. Omentoplasty versus no omentoplasty for esophagogastrostomy after surgery for cancer of cardia and esophagus. Indian J Cancer. 2004;41:167–169. [PubMed] [Google Scholar]
- 74.Madiba T.E., Nair R., Mulaudzi T.V., Thomson S.R. Perforated gastric ulcer - reappraisal of surgical options. S Afr J Surg. 2005;43:58–60. [PubMed] [Google Scholar]
- 75.Raj B.R., Subbu K., Manoharan G. Omental plug closure of large duodenal defects- an experimental study. Trop Gastroenterol. 1997;18:180–182. [PubMed] [Google Scholar]
- 76.Di Giorgio A., Biacchi D., Sibio S., Accarpio F., Sinibaldi G., Petrella L. Abdominal rectopexy for complete rectal prolapse: preliminary results of a new technique. Int J Colorectal Dis. 2005;20:180–189. doi: 10.1007/s00384-004-0650-0. [DOI] [PubMed] [Google Scholar]
- 77.Liebermann D.M., Kaufmann M. Utilization of the greater omentum in surgery: a historical review. Neth J Surg. 1991;43:136–144. [PubMed] [Google Scholar]
- 78.Sato M., Tanaka F., Wada H. Treatment of necrotic infection on the anterior chest wall secondary to mastectomy and postoperative radiotherapy by the application of omentum and mesh skin grafting. Surg Today. 2002;32:261–263. doi: 10.1007/s005950200031. [DOI] [PubMed] [Google Scholar]
- 79.Ueda K., Harashina T., Harada T., Oba S., Nagasaka S. Omentum as gliding material after extensive forearm tenolysis. Br J Plast Surg. 1993;46:590–593. doi: 10.1016/0007-1226(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 80.Ciuce C., Seddiq F., Fodor M., Constantinescu D., Todoran M., Andercou A. Omental free-tissue transfer: indications and results from personal experience. Microsurgery. 2003;23:198–205. doi: 10.1002/micr.10130. [DOI] [PubMed] [Google Scholar]
- 81.Goitz R.J., Steichen J.B. Microvascular omental transfer for the treatment of severe recurrent median neuritis of the wrist: a long-term follow-up. Plast Reconstr Surg. 2005;115:163–171. [PubMed] [Google Scholar]
- 82.Goldsmith H.S., Chen W.F., Duckett S.W. Brain vascularization by intact omentum. Arch Surg. 1973;106:695–698. doi: 10.1001/archsurg.1973.01350170061015. [DOI] [PubMed] [Google Scholar]
- 83.Rafael H., Mego R., Moromizato P., Garcia W. Omental transplantation for temporal lobe epilepsy: report of two cases. Neurol India. 2002;50:71–74. [PubMed] [Google Scholar]
- 84.Goldsmith H.S. Omental transposition to the brain for Alzeheimer's disease. Ann NY Acad Sci. 1997;826:323–336. doi: 10.1111/j.1749-6632.1997.tb48483.x. [DOI] [PubMed] [Google Scholar]
- 85.Rafael H., Mego R., Moromizato P., Espinoza M. Omental transplantation for Alzheimer's disease. Neurol India. 2000;48:319–321. [PubMed] [Google Scholar]
- 86.Karasawa J., Touho H., Ohnishi H., Miyamoto S., Kikuchi H. Cerebral revascularization using omental transplantation for childhood moyamoya disease. J Neurosurg. 1993;79:192–196. doi: 10.3171/jns.1993.79.2.0192. [DOI] [PubMed] [Google Scholar]
- 87.Yoshioka N., Tominaga S., Suzuki Y., Yamazato K., Hirano S., Nonaka K. Cerebral revascularization using omentum and muscle free flap for ischemic cerebrovascular disease. Surg Neurol. 1998;49:58–65. doi: 10.1016/s0090-3019(97)00122-5. [DOI] [PubMed] [Google Scholar]
- 88.Normington E.Y., Papay F.A., Yetman R.J. Treatment of recurrent cerebrospinal fluid rhinorrhea with a free vascularised omental flap: a case report. Plast Reconstr Surg. 1996;98:514–519. doi: 10.1097/00006534-199609000-00025. [DOI] [PubMed] [Google Scholar]
- 89.Goldsmith H.S. Brain and spinal cord revascularization by omental transposition. Neurol Res. 1994;16:159–162. doi: 10.1080/01616412.1994.11740218. [DOI] [PubMed] [Google Scholar]
- 90.Duffill J., Buckley J., Lang D., Neil-Dwyer G., Mcginn F., Wade D. Prospective study of omental transposition in patients with chronic spinal injury. J Neurol Neurosurg Psychiatry. 2001;71:73–80. doi: 10.1136/jnnp.71.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia-Gomez I., Goldsmith H.S., Angulo J., Prados A., Lopez-Hervas P., Cuevas B. Angiogenic capacity of human omental stem cells. Neurol Res. 2005;27:807–811. doi: 10.1179/016164105X63674. [DOI] [PubMed] [Google Scholar]
- 92.Kanamori T., Watanabe G., Yasuda T., Nagamine H., Kamiya H., Koshida Y. Hybrid surgical angiogenesis: omentopexy can enhance myocardial angiogenesis induced by cell therapy. Ann Thorac Surg. 2006;81:160–167. doi: 10.1016/j.athoracsur.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Yasuura K., Okamoto H., Morita S., Ogawa Y., Sawazaki M., Seki A. Results of omental flap transposition for deep sternal wound infection after cardiovascular surgery. Ann Surg. 1998;227:455–459. doi: 10.1097/00000658-199803000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D'Udekem Y., Lengele B., Noirhomme P., El Khoury G., Vanwijck R., Rubay J.E. Radical debridement and omental transposition for post sternotomy mediastinitis. Cardiovasc Surg. 1998;6:415–418. doi: 10.1016/s0967-2109(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 95.López-Monjardin H., de-la-Peña-Salcedo A., Mendoza-Muñoz M., López-Yáñez-de-la-Peña A., Palacio-López E., López-García A. Omentum flap versus pectoralis major flap in the treatment of mediastinitis. Plast Reconstr Surg. 1998;101:1481–1485. doi: 10.1097/00006534-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Levashev Y.N., Akopov A.L., Mosin I.V. The possibilities of greater omentum usage in thoracic surgery. Eur J Cardiothorac Surg. 1999;15:465–468. doi: 10.1016/s1010-7940(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 97.Talwar S., Choudhary S.K. Omentopexy for limb salvage in Buerger's disease: indications, technique and results. J Postgrad Med. 2001;47:137–142. [PubMed] [Google Scholar]
- 98.Ala-Kulju K., Virkkula L. Use of omental pedicle for treatment of Buerger's disease affecting the upper extremities. A modified technique. VASA. 1990;19:330–333. [PubMed] [Google Scholar]
- 99.Mendes D., Kahn M., Ibrahim I.M., Sussman B., Fox R., Dardik H. Omental protection of autogenous arterial reconstruction following femoral prosthetic graft infection. J Vasc Surg. 1985;2:603–606. doi: 10.1067/mva.1985.avs0020603. [DOI] [PubMed] [Google Scholar]
- 100.Harashina T., Imai T., Wada M. The omental sandwich reconstruction for a full-thickness cheek defect. Plast Reconstr Surg. 1979;64:411–415. [PubMed] [Google Scholar]
- 101.Park C., Roh T.S., Chi H.S. Total ear reconstruction in the devascularized temporoparietal region: use of omental free flap. Plast Reconstr Surg. 2003;111:1391–1397. doi: 10.1097/01.PRS.0000049113.27514.B2. [DOI] [PubMed] [Google Scholar]
- 102.Henderson M.A., Burt J.D., Jenner D., Crookes P., Bennett R.C. Radical surgery with omental flap for uncontrolled locally recurrent breast cancer. ANZ J Surg. 2001;71:675–679. doi: 10.1046/j.0004-8682.2001.02234.x. [DOI] [PubMed] [Google Scholar]
- 103.Wong C.H., Tan B.K., Koong H.N., Lim C.H., Chia S.J., Song C. Use of omentum flap as additional soft tissue cover for abdominal wall defects reconstructed with Gore-Tex. Plast Reconstr Surg. 2005;116:1715–1720. doi: 10.1097/01.prs.0000185664.33079.5d. [DOI] [PubMed] [Google Scholar]
- 104.Benoit L., Boichot C., Cheynel N., Arnould L., Chauffert B., Cuisenier J. Preventing lymphedema and morbidity with an omentum flap after ilioinguinal lymphnode dissection. Ann Surg Oncol. 2005;12:793–799. doi: 10.1245/ASO.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 105.Masuda T., Furue M., Matsuda T. Novel strategy for soft tissue augmentation based on transplantation of fragmented omentum and preadipocytes. Tissue Eng. 2004;10:1672–1683. doi: 10.1089/ten.2004.10.1672. [DOI] [PubMed] [Google Scholar]
- 106.Ohtsuka H., Shioya N. The fate of free omental transfers. Br J Plast Surg. 1985;38:478–482. doi: 10.1016/0007-1226(85)90003-7. [DOI] [PubMed] [Google Scholar]
- 107.Ogunc G. Minilaparoscopic extraperitoneal tunneling with omentopexy: a new technique for CAPD catheter placement. Perit Dial Int. 2005;25:551–555. [PubMed] [Google Scholar]
- 108.Dalela D., Gupta V.P., Goel A., Singh K.M. Omental wrap around the renal pedicle: an adjunctive step to minimize morbidity and recurrence after lymphorenal disconnection for chyluria. BJU Int. 2004;94:673–674. doi: 10.1111/j.1464-410X.2004.05021.x. [DOI] [PubMed] [Google Scholar]
- 109.Mokhort V.A., Makarov V.N. Omentovesicopexy with transposition of the bladder into the abdominal cavity in the treatment of neurogenic bladder. Urol Nefrol. 1990;4:20–24. [PubMed] [Google Scholar]
- 110.Kamei Y., Aoyama H., Yokoo K., Fujii K., Kondo C., Sato T. Composite gastric seromuscular and omental pedicle flap for urethral and scrotal reconstruction after Fournier's gangrene. Ann Plast Surg. 1994;33:565–568. doi: 10.1097/00000637-199411000-00018. [DOI] [PubMed] [Google Scholar]
- 111.Shoshany G., Shofty R., Livne E., Hayari L., Mordechovitz D. Testicular neovascularization by “omentotesticulopexy”: a possible adjuvant in the surgical correction of high undescended testes. J Pediatr Surg. 1996;31:1229–1232. doi: 10.1016/s0022-3468(96)90239-0. [DOI] [PubMed] [Google Scholar]
- 112.Patsner B., Hackett T.E. Use of the omental J-flap for prevention of postoperative complications following radical abdominal hysterectomy: report of 140 cases and literature review. Gynecol Oncol. 1997;65:405–407. doi: 10.1006/gyno.1997.4700. [DOI] [PubMed] [Google Scholar]
- 113.Shylasree T.S., Karandikar S., Freites O., Mcgregor I., Carr N.D. Omentopexy for reconstruction of the perineum following a radical vulvectomy: a case report. Int J Gynecol Cancer. 2004;14:1122–1125. doi: 10.1111/j.1048-891X.2004.14611.x. [DOI] [PubMed] [Google Scholar]
- 114.Kusiak J.F., Rosenblum N.G. Neovaginal reconstruction after exenteration using an omental flap and split-thickness skin graft. Plast Reconstr Surg. 1996;97:775–781. doi: 10.1097/00006534-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 115.Alagumuthu M., BhupatiB Das, SibaP Pattanayak, Mangual Rasananda. The omentum: a unique organ of exceptional versatility. Indian J Surg. May-June, 2006;68(No. 3):136. 14. [Google Scholar]
- 116.Di Nicola V., Di Nicola R. Self-repair in degenerative joint disease. Curr Aging Sci. 2012;5(3) doi: 10.2174/1874609811205030015. [DOI] [PubMed] [Google Scholar]
- 117.Di Nicola V., Pierpaoli W. Biological baseline of joint self-repair procedures. Curr Aging Sci. 2013;6(2) doi: 10.2174/18746098112059990029. [DOI] [PubMed] [Google Scholar]
- 118.Di Nicola V., Di Pietrantonio M. MRI in degenerative joint disease (DJD): a proposal for imaging standardization in regenerative medicine. SM Musculoskelet Disord. 2016;1(2):10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.