Abstract
As one of the most important aquatic fish, Micropterus salmoides suffers lethal and epidemic disease caused by rhabdovirus at the juvenile stage. In this study, a new strain of M. salmoides rhabdovirus (MSRV) was isolated from Yuhang, Zhejiang Province, China, and named MSRV-YH01. The virus infected the grass carp ovary (GCO) cell line and displayed virion particles with atypical bullet shape, 300–500 nm in length and 100–200 nm in diameter under transmission electron microscopy. The complete genome sequence of this isolate was determined to include 11 526 nucleotides and to encode five classical structural proteins. The construction of the phylogenetic tree indicated that this new isolate is clustered into the Vesiculovirus genus and most closely related to the Siniperca chuatsi rhabdovirus. To explore the potential for a vaccine against MSRV, a glycoprotein (1–458 amino acid residues) of MSRV-YH01 was successfully amplified and cloned into the plasmid pFastBac1. The high-purity recombinant bacmid-glycoprotein was obtained from DH10Bac through screening and identification. Based on polymerase chain reaction (PCR), western blot, and immunofluorescence assay, recombinant virus, including the MSRV-YH01 glycoprotein gene, was produced by transfection of SF9 cells using the pFastBac1-gE2, and then repeatedly amplified to express the glycoprotein protein. We anticipate that this recombinant bacmid system could be used to challenge the silkworm and develop a corresponding oral vaccine for fish.
Keywords: Micropterus salmoides, Rhabdovirus, Glycoprotein, Baculovirus system
1. Introduction
As a commercial freshwater species, largemouth bass (Micropterus salmoides) has been introduced to various countries including China and cultured widely (Lorenzoni et al., 2002; Gao and Chen, 2018; Zhang LJ et al., 2018). In recent years, the annual production of M. salmoides has increased dramatically to reach 456 888 metric tons in China in 2017 (Zhang et al., 2018). Unfortunately, both wild and cultured M. salmoides encounter lethal and epidemic diseases caused by viruses, parasites, and bacteria due to immoderate culture and environmental contamination (Bauer, 2013; Ma et al., 2013; Ho et al., 2018). Among these diseases, the frequent outbreak of M. salmoides rhabdovirus (MSRV) is of particular concern because it is lethal to juveniles (Gao and Chen, 2018). MSRV was first discovered by Ma in April 2011 at a farm in Zhongshan City, Guangdong Province, China (Ma et al., 2013). Mortality amongst juveniles (2.5–3.5 cm in length) was 40% (Ma et al., 2013; Fu et al., 2017). The lack of a particular therapy against MSRV highlights the urgency and significance of investigating the infection mechanism of this virus.
The rhabdovirus family of traditional single-stranded RNA viruses, is a virulent cause of serious aquatic disease in marine and freshwater fish all over the world. Clinical symptoms of rhabdovirus infection include septicemia, necrotic ulceration, ascites, and multi-organ hemorrhage associated with high morbidity and mortality (Hoffmann et al., 2005; Galinier et al., 2012). In 1974, the first rhabdovirus in fish was isolated from Anguilla rostrate and tentatively designated as an eel virus American (EVA) (Sano et al., 1976; Galinier et al., 2012). By 2018 the International Committee on Taxonomy of Viruses (ICTV) had recognized eighteen rhabdovirus genera of fish including perch rhabdovirus (PRV), Siniperca chuatsi rhabdovirus (SCRV), and snakehead rhabdovirus (SHRV) (Amarasinghe et al., 2017). The formerly identified fish rhabdoviruses are classified into the genera of Vesiculovirus and Novirhabdovirus (Galinier et al., 2012). Two new strains, namely trout rhabdovirus 903/87 (TRV-903/87) and sea trout rhabdovirus 28/97 (STRV-28/97), are unassigned species (Hoffmann et al., 2005). Most rhabdoviruses have a negative-strand RNA genome that encodes at least five common open reading frames (ORFs): nucleoprotein (N), phosphoprotein (P), matrixprotein (M), glycoprotein (G), and polymerase (L). Novirhabdovirus has been demonstrated to be a distinct rhabdovirus group possessing an additional gene encoding a nonstructural, nonvirion protein (NV) (Hoffmann et al., 2005). Among these viral proteins, glycoprotein is the major structural protein responsible for forming virion spikes (Compans et al., 1970; Ksenofontov et al., 2008). Glycoprotein also functions in a similar way to the influenza virus M2 protein channel by allowing the release of the infectious nucleic acid from the viral coat (Compans et al., 1970; Ksenofontov et al., 2008). For pattern recognition of rhabdovirus in fish, glycoprotein is theoretically triggered by interferon in both immune and non-immune cells depending on a canonical integrin-binding site containing peptide region. Thus, glycoprotein is frequently used to construct anti-viral vaccine to enhance a specific immune response system (Lorenzen et al., 1998; Chen et al., 2012; Fu et al., 2017).
There is no commercially available prokaryotic expression system, including protein glycosylation, correct folding, and formation of disulfide bonds, for the manufacture of highly bioactive glycoprotein (Zhang S et al., 2018). In this study, a new strain of MSRV was isolated from juvenile M. salmoides with severe symptoms of MSRV infection collected from a fingerling factory. We determined the entire genome sequence of this virus, and a taxonomic classification was simultaneously proposed following analysis of its phylogenetic relationship with previously discovered rhabdoviruses. We then synthesized virus glycoprotein by the baculovirus expression vector (BEV) system to produce commercial vaccine against this virus.
2. Materials and methods
2.1. Fish sample collections
Moribund largemouth bass (1.5–4.0 cm in length, n=50) were collected from the M. salmoides fingerling factory in Yuhang, Zhejiang Province, China in 2017. Fish were placed in plastic bags containing crushed ice and brought to the laboratory within 4 h. Fish were later placed in 10.0 mL centrifuge tubes containing RNAlater (ThermoFisher, USA) and stored at −80 °C for the complete genome sequence determination. For virus isolation, fresh fish were directly stored at −80 °C.
2.2. Virus isolation
Virus was isolated from the tissue homogenate of diseased larva fish. The grass carp ovary (GCO) cell line is established in our laboratory, and grows in M199 medium with 10% (v/v) fetal bovine serum (FBS; GIBCO, USA) at 24 °C. After being centrifuged and filtered through a sterile 0.22-µm filter, the viral filtrate was propagated in GCO as described previously (Ma et al., 2013). Cell cultures were then examined daily for cytopathic effect (CPE).
2.3. Transmission electron micrograph assay
GCO cells were inoculated with this rhabdovirus for 3 d and then centrifuged (5000 r/min for 10 min) to collect a cell pellet for transmission electron micrograph (TEM) observation. The cell pellet was fixed with 2.5% (0.025 g/mL) glutaraldehyde buffered in 0.1 mol/L phosphate-buffered saline (PBS, pH 7.4) for 2 h and post-fixed in 1% (v/v) osmium tetroxide solution (pH 7.4) for 1 h. Fixed pellet was subsequently dehydrated with ethanol and acetone and embedded in Epon 812 (SPI, USA). Ultrathin sections were sliced using ultra 45° diamond knives (Diatome, Switzerland) and ultramicrotome (Leica, Germany), and double-stained with uranyl acetate and lead citrate following observation on a Tecnai G2 20 TWIN (FEI, the Netherlands).
2.4. Genome sequence analysis
The virus-infected GCO cells were frozen and thawed twice and then centrifuged at 5000 r/min for 10 min to discard cell debris. Total RNA was extracted from the prepared supernatant using RNAiso Plus reagent (TaKaRa, Japan). The first-strand complementary DNA (cDNA) was produced using the moloney murine leukemia virus (M-MLV) reverse transcriptase system (Promega, USA). To obtain the entire viral genome sequence, distinct fragments were cloned with eight pairs of primers (Table 1) designed according to the known fish rhabdoviruses (SCRV, GenBank number DQ399789.1). Polymerase chain reaction (PCR) amplification was carried out with an initial denaturing step at 95 °C for 10 min, followed by 35 cycles of amplification as follows: 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 90 s. Finally, an elongation step of 10 min at 72 °C was performed. The 3' rapid amplification of cDNA ends (3'-RACE) first-strand cDNA synthesis was performed using the SMART RACE cDNA amplification kit (Clontech, Japan). Gene-specific primer (MSRV-3-R) and 10×Universal Primer Mix (included in the SMART RACE kit) were used for the 3'-RACE and the PCR was performed with the SeqAmp™ DNA Polymerase system (Clontech, Japan) following the manufacturer’s instructions. The amplification product was electrophoresed and sequenced (Sanya, China).
Table 1.
Primers and primer sequences
| Primer name | Sequence (5'→3') |
| MSRV-3-R | GATTACGCCAAGCTTGGACAACCT CACAGAGATAGCGGACC |
| MSRV-N-F | ATGGAAMACCAAATCATCARGAGA |
| MSRV-N-R | TCACAAAGCTTGGTGCTTCAGCAG |
| MSRV-NG-F | CTGATGACCAAGACGAGGAAGGAG |
| MSRV-NG-R | TGAAGTCTTATCGGAACAAACAGC |
| MSRV-G-F | ATGAAATYRATCATTGCACYTACG |
| MSRV-G-R | TTAGGGAACAAATTGATACTGCT |
| MSRV-GL-F | GTCAGACGACTCCTCAAATGA |
| MSRV-GL-R | ATCTTGGAAGTTCGAGAAGGC |
| MSRV-L-1F | ATGGATTATTCTCARGAATATGT |
| MSRV-L-1R | CTGACCAGGGAATCTGTGATTCCA |
| MSRV-L-2F | CCAGAGAGGAAGAGCCATATTTAG |
| MSRV-L-2R | TTATTCCGTCCAYGCAGCYTCTCT |
| MSRV-5-F | AGTAGAGAGTGGGATAGCCTCAGTC |
| MSRV-5-R | ACGAGAAAAACAAACACAGTCTCT GA |
| rtMSRV-GF | AGCAGCATCACCAGCCACATC |
| rtMSRV-GR | CGTCCGTCGCTTGACTCAATT |
F: forward; R: reverse
2.5. Phylogenetic analyses
The available rhabdovirus genomes from fish and other animals were downloaded from GenBank for the phylogenetic analyses. Genome sequences were aligned using ClustalW and the alignments were subsequently imported in MEGA Version 7. A neighbor-joining (NJ) Kimura 2-parameter model was carried out with 1000 data bootstrap replications for the phylogenetic tree construction.
2.6. Recombinant bacmid construction of glycoprotein
Synthesized cDNA first strand (Section 2.4) was used as the template for the expression of the optimizing codon sequence of glycoprotein (1–458 amino acid residues). The optimizing codon sequence was then amplified using the specific primers 8294-1-F1 (CTGCTGTGTTAATTTTGCTCTTGGTCCGATTTTCGGAGCCTTACCCGCTGTTTGTTCCG) and 8294-1-R1 (GATGGTGGTGGTGGTGGCTGCCGC TCACTCCAGTTCCCACC) (the underlined section being the amplifying sequence). The PCR reaction was carried out as follows: denaturing at 94 °C for 5 min followed by 35 amplification cycles (94 °C for 40 s, 58 °C for 40 s, and 72 °C for 90 s) and finally extension at 72 °C for 10 min. The target glycoprotein was amplified from the first-round PCR product using primers containing EcoRI and HindIII sites (underlined): 8294-1-F2 (CGGTCCGAAGCGCGCGGAATTCGCCGCCACCATGGTAAGAACTGCTGTGTTAATTTTGC) and 8294-1-R2 (CCTCTAGTACTTCTCGACAAGCTTTCAGTGATGGTGGTGGTGGTGGCTGCC). The purified amplified PCR product (1–458 amino acid residues) without transmembrane domain (460–481 amino acid residues, FIHTIVYLVILCGIILLLYRCL) was digested by EcoRI and HindIII, and further inserted into the EcoRI and HindIII double-digested site of the pFastBac1 vector containing 6×His-tag (GSHHHHHH) at the C-terminal. The constructed plasmid was designated as pFastBac1-gE2. DH10Bac competent cells were transformed with pFastBac1-gE2, and the single colonies were inoculated into Luria-Bertani (LB) medium. Recombinant plasmid was extracted from competent cells using a plasmid DNA extract kit according to the product instructions (Tiangen, China) and confirmed by PCR.
2.7. Recombinant virus amplification and target protein identification
SF9 cells were cultured in SF-900II serum free medium (SFM; with 10% FBS) without CO2 and the transformation was performed in a lipid-DNA complex (100 μL A solution containing 2 μg recombinant bacmids, 100 μL B solution containing SF-900II with 6% (0.06 g/mL) Cellfectin). Transfected SF9 cells were cultured at 28 °C for one week. The cells were then freeze-thawed and centrifuged to collect supernatant and cell debris.
To confirm the positive recombinant baculovirus, the acquired supernatants and cell debris were used for DNA and protein extraction. The DNA was extracted using the DNA extraction kit (Qiagen, Germany) according to the operating instructions and the PCR was conducted as described in Section 2.4. To determine the target protein, the supernatant and cell debris were lysed using radio-immunoprecipitation assay (RIPA) lysis buffer and then centrifuged at 12 000 r/min for 10 min to collect the total protein in the supernatant. The western blot analysis was performed using polyclonal mouse-anti-His first antibody (Servicebio, China) and horseradish peroxidase-conjugated goat-anti-mouse second antibody (ThermoFisher, USA).
2.8. Dynamics of glycoprotein synthesis in the SF9 cells
The SF9 cell suspension was adjusted to give a cell density of 4×105 cells/mL, and 2.0 mL of the suspension was then pipetted into 6-well plates. The cell supernatant (200 μL) with recombinant baculovirus and control plasmid was pipetted into each well, respectively. After challenge for 1, 2, 3, 4, and 5 d, the samples from the experimental and control groups were freeze-thawed for PCR detection and western blot analysis of glycoprotein. The first cDNA strain was synthesized using PrimeScript™ RT Master Mix (Perfect Real Time) according to the protocol (TaKaRa, Japan). Fluorescent quantitative real-time PCR (qRT-PCR) was then performed using the LightCycler® 480 SYBR Green I Master (Roche, Switzerland) according to the operating procedure. Primer sequences specific to the recombinant baculovirus (rtMSRV-GF, rtMSRV-GR) are provided in Table 1. The virus copies were calculated according to the standard curve (the qRT-PCR to detect the viral genome had been constructed in our lab but the data have not been published). Another sample was simultaneously fixed with 4% (0.04 g/mL) formaldehyde for immunofluorescence assay according to our previous procedure (Lv et al., 2015). The dilutions of mouse-anti-His polyclonal first antibody (Servicebio, China) and goat-anti-mouse second antibody (Servicebio, China) were determined to be 1:200 and 1:400 in PBS, respectively. The sections were stained with 4',6-diamidino-2-phenylindole (DAPI) and then observed with fluorescein isothiocyanate (FITC) and DAPI emission filters using confocal laser scanning microscopy (NIKON DS-U3, Japan).
3. Results
3.1. Identification of MSRV-infected fish
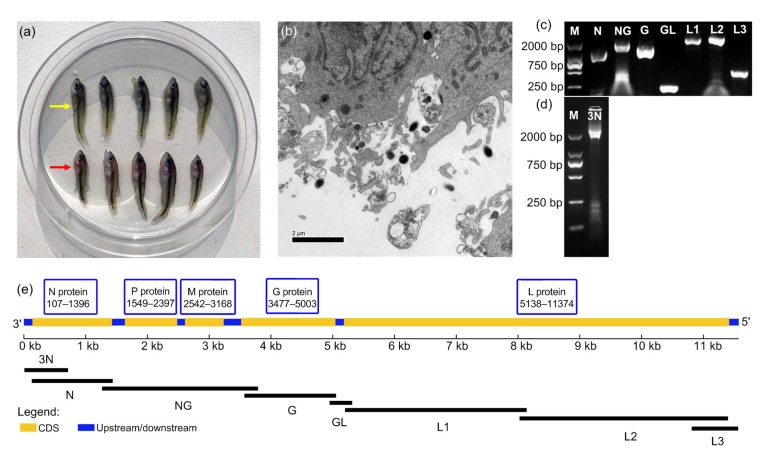
Distinct from formerly isolated MSRV, the newly isolated MSRV was designated as MSRV-YH01. Affected fingerlings exhibited corkscrew and irregular swimming in both natural and artificial conditions. As shown in Fig. 1a, internal hemorrhage was clearly observed in organs including the gills and liver of infected fish. The virus filtrate isolated from the diseased fish was inoculated onto a GCO cell monolayer and the CPE was successfully observed (data not shown). TEM observation revealed that MSRV-YH01 virions have an atypical bullet-shaped morphology and viral capsid (Fig. 1b). The MSRV virions ranged from 300 to 500 nm in length and 120 to 200 nm in diameter (n=10). The control sample was not included due to the lack of other rhabdovirus samples.
Fig. 1.
Isolation and identification of MSRV-YH01
(a) Clinical signs of M. salmoides infected with MSRV (red arrow) as compared with the healthy M. salmoides (yellow arrow). (b) The TEM observation of virus virions inoculated in GCO cells. (c) The seven overlapping fragments cloned by RT-PCR. (d) The fragment cloned by 3'-RACE amplification. (e) Putative gene on MSRV-YH01 genome. N: nucleoprotein; P: phosphoprotein; M: matrixprotein; G: glycoprotein; L: polymerase; CDS: coding sequence; M: DNA marker
3.2. Entire genome sequence determination of MSRV-YH01
The entire genome sequence of MSRV-YH01 was amplified into eight overlapping fragments (Figs. 1c and 1d) and deposited in GenBank with accession number MK397811. It comprised 11 526 nucleotides and encoded five rhabdovirus structural
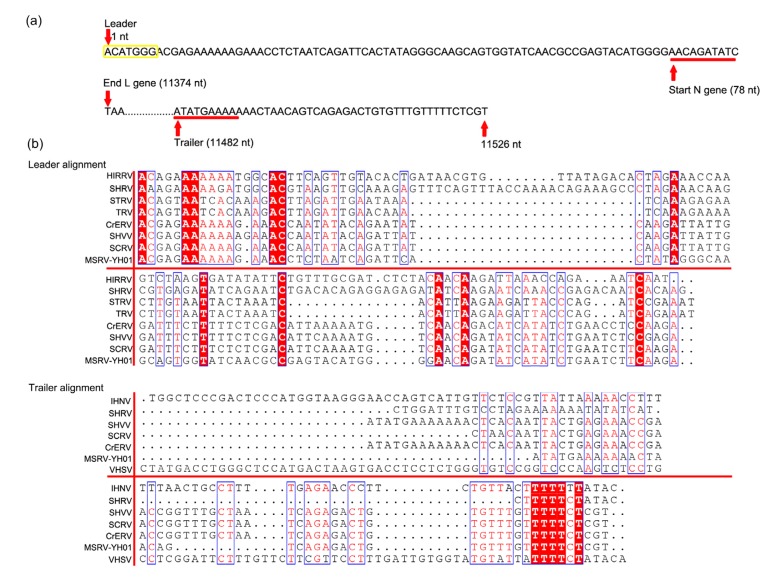
proteins including N, P, M, G, and L proteins (Fig. 1e). The 3' leader and 5' trailer regions of MSRV-YH01 were composed of 77 and 45 nucleotides, respectively. The putative polyadenylation signal (ACATGGGA CGAGAAAAAA) was included in the 3' leader region (Fig. 2). The 3' leader region of MSRV shared low homology at the genetic level compared with other fish rhabdoviruses (Fig. 2). The deduced sequences of N, P, M, G, and L proteins comprised 430, 283, 209, 509, and 2079 amino acids, respectively. The theoretical masses of these proteins were calculated to be 47.5, 31.1, 23.1, 56.4, and 238.0 kDa (Table 2).
Fig. 2.
Homologous sequence alignment of 3'-leader and 5'-trailer of MSRV-YH01
(a) Nucleotide sequences of the 3'-leader and 5'-trailer regions. The N gene transcription initiation and 5'-trailer transcription initiation were underlined. (b) Alignment of the available 3'-leader and 5'-trailer sequences assigned fish rhabdovirus with identified fish rhabdovirus. HIRRV (NC_005093.1, hirame rhabdovirus), SHRV (NC_000903.1, snakehead rhabdovirus), STRV (AF434992.1, sea trout rhabdovirus), TRV (AF434991.1, trout rhabdovirus), CrERV (MH319839.1, Chinese rice-field eel rhabdovirus), SHVV (KP876483.1, hybrid snakehead virus), SCRV (DQ399789.1, Siniperca chuatsi rhabdovirus), IHNV (L40883.1, infectious hematopoietic necrosis virus), and VHSV (GQ385941.1, viral hemorrhagic septicemia virus)
Table 2.
Predicted genes and putative proteins of MSRV-YH01
| Gene | Gene length (nt) | Number of amino acids (aa) | Theoretical MW (kDa) | Theoretical protein pI |
| N | 1290 | 430 | 47.5 | 5.52 |
| P | 849 | 283 | 31.1 | 4.50 |
| M | 627 | 209 | 23.1 | 7.70 |
| G | 1527 | 509 | 56.4 | 6.44 |
| L | 6237 | 2079 | 238.0 | 8.13 |
nt: nucleotides; aa: amino acids; MW: molecular weight; pI: isoelectric point
3.3. Phylogenetic analyses of MSRV
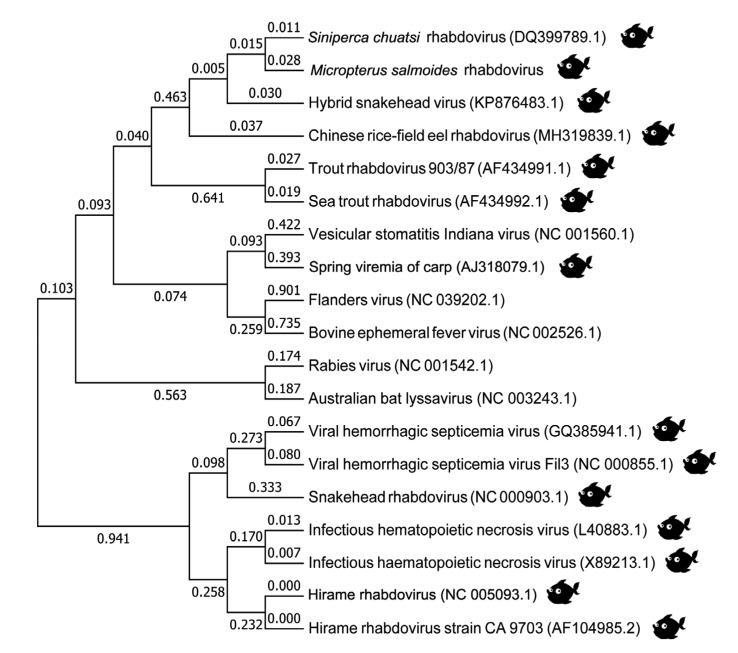
To investigate the phylogenetic relationship of MSRV-YH01 with previously reported rhabdovirus, the entire genome sequence was aligned with other fish and animal rhabdoviruses available in GenBank. The NJ tree derived from analysis of the complete gene sequence showed that MSRV-YH01 is closely related to SCRV from China (Fig. 3). Other fish rhabdoviruses including hybrid snakehead virus (SHVV), Chinese rice-field eel rhabdovirus (CrERV), and STRV were more closely related to MSRV-YH01 than rhabdoviruses isolated from other animals.
Fig. 3.
Phylogenetic analysis of MSRV-YH01 with representative animal rhabdoviruses
3.4. Identification of the plasmid construction of pFastBac1-gE2
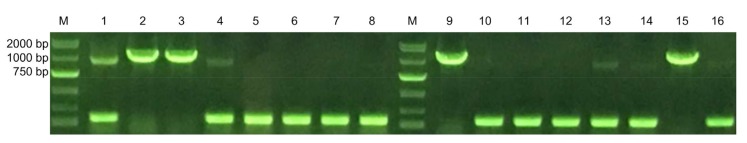
The recombinant plasmid pFastBac1-gE2 was transferred into Escherichia coli DH10Bac. Positive recombinational colonies with white color were amplified with specific primers. As displayed in Fig. 4, the positive recombinants were identified when a target 1386 bp sequence was detected, suggesting that the glycoprotein had been successfully recombined into the pFastBac1-gE2 system.
Fig. 4.
Construction and identification of the pFastBac1-gE2
Screening of positive transformations by colony PCR. 1–16: PCR products. M: DNA marker
3.5. Identification of the expression of glycoprotein in recombinant baculovirus
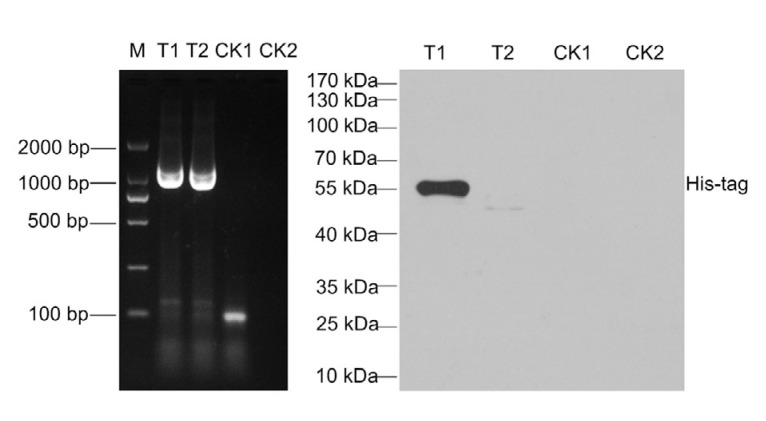
The SF9 cell was infected with the recombinant baculovirus and harvested after challenge for 72 h. Detection of a target 1386 bp region in the supernatant and cell debris was a positive sign of successful infection. No target sequence was amplified in the control group (Fig. 5). Using western blot assay, the target protein labeled with His-tag was determined with a specific band of 55 kDa in the sediment of cell lysis (Fig. 5). No corresponding band was observed in the cell culture supernatant and control cells.
Fig. 5.
Identification of protein expression in recombinant baculovirus inoculated in SF9 cells by PCR and western blot assay
T1 and T2 indicate the samples collected from the supernatant and cell bricks inoculated with recombinant baculovirus, respectively. CK1 and CK2 indicate the samples collected from the supernatant and cell bricks inoculated with control plasmid, respectively. M indicates DNA marker
3.6. Titer identification of recombinant baculovirus
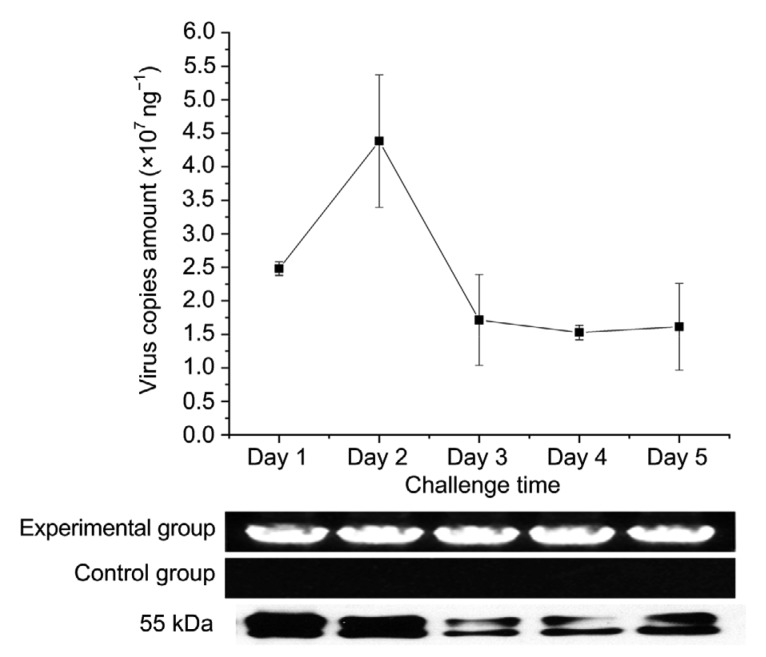
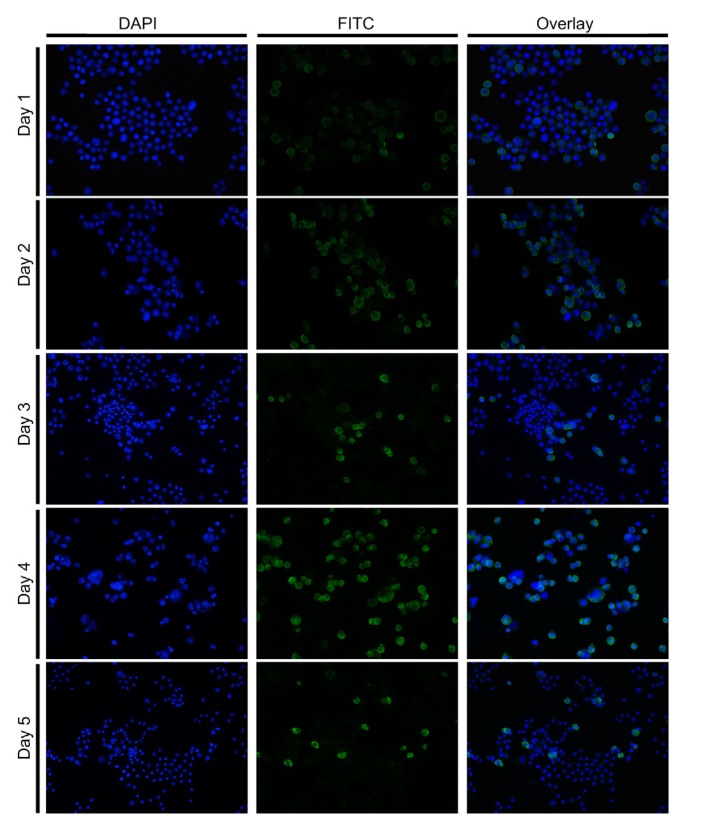
The recombinant baculovirus titer and expression of glycoprotein were detected within 5 d of infection. The recombinant baculovirus was positively observed in the experimental group by means of a target 1386 bp region, while no target sequence was detected in the control group. Absolute quantification PCR assays were run for virus titer detection. The mean titer of recombinant baculovirus was determined to be 2.5×107 copies/ng at 1 d post infection (dpi) increasing to 4.4×107 copies/ng at 2 dpi (Fig. 6) and then decreasing from Days 3 to 5. The expression of glycoprotein was also detected within 5 d of infection with a boost at Days 1 and 2. Indirect immunofluorescence assay, however, showed an increase in expressing glycoprotein from Days 1 up to 5 (Fig. 7).
Fig. 6.
Real-time PCR, PCR, and western blot assay of glycoprotein in SF9 cells within 5 d infection with recombinant baculovirus
Data are expressed as mean±standard deviation (SD), n=3
Fig. 7.
Temporal expression of glycoprotein in SF9 cells challenged with recombinant baculovirus
The cells and fusional glycoprotein were observed with FITC and DAPI emission filters using confocal laser scanning microscopy post stimulation
4. Discussion
Among the more than 60 virus species that cause disease in fish, the viruses that belong to the Rhabdoviridae family are verified to be the most prevalent and virulent. In this study, a novel strain of rhabdovirus was isolated from seriously diseased M. salmoides in Yuhang, Zhejiang Province, China. Wild and cultivated fish from the following species are found to be infected with rhabdovirus: Chinese perch (S. chuatsi), largemouth bass (M. salmoides), snakehead fish (Channa maculata), Japanese flounder (Paralichthys olivaceus), black seabream (Milio macrocephalus), and marbled sand goby (Oxyeleotris marmor) (Luo et al., 2013; Fu et al., 2017; Zhang LJ et al., 2018). In contrast to most marine or freshwater rhabdovirus, MSRV displays age specificity in which it can induce serious disease solely in juvenile fish. Previous research has reported that the typical clinical symptoms (such as severe internal hemorrhaging) of rhabdovirus-infected fish, like C. maculata and Ophicephalus striatus, are observed (Luo et al., 2013; Zeng et al., 2013). In this research, M. salmoides fingerling fish with similar internal hemorrhage in gills and liver were sampled. Infected fish fry exhibited irregular swimming, corkscrew and crooked body. The severe symptoms caused by MSRV infection in the wild were observed in the artificial infection experiment (data not shown). Formerly, Ma et al. (2013) isolated a strain of SCRV from M. salmoides at a farm in Guangdong Province of China, which induced 40% mortality of juvenile fish. In 2015, Fu et al. (2017) isolated a novel strain of MSRV from the juvenile M. salmoides exhibiting clinical manifestation including punctate hemorrhages on their body surface and fins at Guangdong Province, China. Lei et al. (2015) also identified a rhabdovirus of M. salmoides that caused high death rates. Consistent with these earlier results, the new strain of MSRV isolated in this study also induced high mortality (nearly 90% death rate) in the epidemic fingerling factory.
The MSRV was not restricted to a specific cell line. In addition to causing CPE in epithelioma papillosum of carp (EPC), Chinese perch brain (CPB), fathead minnow (FHM), and largemouth bass skin (LBS) cell lines (Dorson et al., 1984; Fu et al., 2017; Gao and Chen, 2018), MSRV-YH01 successfully infected the GCO cell line and resulted in obvious CPE. Unfortunately, we did not see the representative bullet-shaped morphology of rhabdovirus by TEM. The MSRV-YH01 virions ranged from 300 to 500 nm in length and 100 to 200 nm in diameter, which was much bigger than the morphology of rhabdovirus reported before (Castric et al., 1984; Assenberg et al., 2010). Viral capsid was clearly seen in the virion surface of MSRV-YH01. Based on RACE and RT-PCR, the complete genome sequence of MSRV-YH01 was assembled and demonstrated to include 11 526 nucleotides. It shares the distinct feature with rhabdovirus that the entire ORF encodes five structural proteins. The 3'-leader region of MSRV-YH01 comprises 77 first nucleotides of the genome starting with trinucleotide ACA, which is different from former research. The 3'-leader sequence starts with the trinucleotide ACG in all identified viruses including Ephemerovirus, Vesiculovirus, and Lyssavirus (Walker et al., 1994). The alignment of the available 3'-leader sequence demonstrates partial conservation on nucleotides, proving its role in viral mRNA transcription (Barr et al., 2002). Nevertheless, it appeared clearly that the leader sequence of MSRV-YH01 was longer than those of other Vesiculovirus with an additional seven nucleotides detected (ACATGGG). The leader region transcribed with a plus and minus sequence from viral mRNA might be responsible for an unknown function (Barr et al., 2002).
Until now, the Rhabdoviridae family has been identified as comprising six recognized genera: Ephemerovirus, Lyssavirus, Novirhabdovirus, Vesiculovirus, Cytorhabdovirus, and Nucleorhabdovirus (Hoffmann et al., 2005; Sakai et al., 2015; Zhang LJ et al., 2018). Although previous research had reported the successful isolation of the MSRV strain, our research was the first time to determine the complete nucleotide sequence of MSRV-YH01. Rather than construct the phylogenetic tree by the multiple alignment of the highly-conserved N protein, we used the multiple alignment of the complete nucleotide sequence to give us a better understanding of the relationship between MSRV-YH01, fish rhabdovirus, and rhabdovirus discovered in other animals. The MSRV-YH01 genus was found to be grouped together with the closest relationship being with SCRV. Thus MSRV-YH01 is predicted to be clustered into one monophyletic lineage belonging to the Vesiculovirus. Previous studies also demonstrated the genetic relatedness of several fish rhabdoviruses to Vesiculovirus, including the eel virus Europe X (EVEX), pike fry rhabdovirus (PFRV), spring viremia carp virus (SVCV), EVA, ulcerative disease rhabdovirus (UDRV), and SCRV (Lorenzen et al., 1998; Chen et al., 2012). In addition, this result was consistent with previous research that rhabdovirus isolated from two distant aquatic regions not connected by waterways exhibits high identity (Ma et al., 2013). The discovery of MSRV in distant regions including Guangdong, Zhejiang, and Guangxi Provinces of China therefore represents a threat to cultured and wild M. salmoides globally through the transportation of virus-infected juvenile fish. Simultaneous virus mutation is highly likely, making it hard to prevent viral disease. Interestingly, vesicular stomatitis Indiana virus displayed a closer relationship to SVCV than to other fish rhabdoviruses, lending weight to the conjuncture of virus mutation and transportation between different kinds of animal.
As the preferred means to protect animals from pathogens, vaccination has been explored and applied worldwide. Rhabdovirus glycoproteins have been successfully explored as DNA vaccines to protect fish against viruses including infectious hematopoietic necrosis virus (IHNV), viral haemorrhagic septicaemia rhabdovirus (VHSV), hirame rhabdovirus (HIRRV), SHRV, SVCV, and SCRV (Anderson et al., 1996; Lorenzen et al., 1998; Kim et al., 2000; Seo et al., 2006; Emmenegger and Kurath, 2008; Chen et al., 2012; Alonso and Leong, 2013). The insect baculovirus expression system is widely used for the synthesis of recombinant proteins due to its biological functions including translation modification and polyhedron promoter. The overall purpose of this part was to produce recombinant forms, high-quality MSRV glycoprotein for subunit vaccines. The glycoprotein ORF of MSRV-YH01 was cloned into the pFastBac1 plasmid initiated by the polyhedron promoter containing a 6×His-tag upstream of the cloning site. Recombinant baculovirus was identified by PCR detection of the DNA extracted from the supernatant and cell debris. Fusional MSRV glycoprotein was successfully expressed by the recombinant baculovirus with a target 1386 bp region and approximately 55 kDa protein detected by PCR and western blot, respectively. The corresponding band was not observed in the cell culture supernatant suggesting that the protein was not a secretory expression. In accordance with the qRT-PCR, the fusional MSRV glycoprotein was found to be highly expressed on Days 1 and 2, followed by a decrease. In contrast, indirect immunofluorescence assay also detected increased expression of MSRV glycoprotein which picked up at Day 4. As semi-adherent cells, SF9 cells grow in suspension and are beneficial for expanding production. The optimized cell amount continued with a high level of expression of MSRV glycoprotein. Similar protein expression systems have been constructed for the expression of specific proteins, such as the recombinant glycoprotein of western equine encephalitis virus, GP5 glycoprotein of porcine reproductive and respiratory syndrome virus (PRRSV), E envelope glycoprotein of Japanese encephalitis virus, Suid Herpesvirus-1 glycoprotein E, and other viral proteins, and the recombinant proteins displayed virion-like bioactivity (Grabowska et al., 2009; Toth et al., 2011; Xu et al., 2011, 2012; Serena et al., 2013). The silkworm-BEV system could become a valuable tool for producing high-quality recombinant glycoproteins. Used as a substitute for fish meal protein, the highly expressed recombinant protein in baculovirus-infected silkworm could be considered in the development of a new oral vaccine (Zhang et al., 2011).
In conclusion, the new isolated MSRV-YH01 from Zhejiang Province induces serious disease in M. salmoides, with infected fish displaying serious clinical features including abnormal swimming and multi-organ hemorrhage. Based on genome analysis, the entire genome sequence of MSRV-YH01 was found to include five classical structural proteins of rhabdovirus, and the phylogenetic tree indicated that this new MSRV isolate was clustered into the SCRV and belonged to Vesiculovirus genus. Although the recombinant fusional protein of MSRV-YH01 glycoprotein was highly expressed in BEV system, more research is needed to verify its potential role as an oral vaccine.
Acknowledgments
We thank the Huzhou Fisheries Technical Extension Station (Huzhou, China) for the provision of ill M. salmoides.
Footnotes
Project supported by the Zhejiang Key R&D Program (No. 2018C02033) and the Zhejiang Provincial Science and Technology Program (No. 2019YSZX0002), China
Contributors: Wei-da SHI, Xiao-ying HANG, and Ying-lei WU collected the ill fish. Li LIU designed the project. Sun-jian LYU, Xue-mei YUAN, and Hai-qi ZHANG performed the experiments. Sun-jian LYU performed data analysis and wrote this paper. All authors read and approved the final manuscript. Therefore, all authors have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Sun-jian LYU, Xue-mei YUAN, Hai-qi ZHANG, Wei-da SHI, Xiao-ying HANG, Li LIU, and Ying-lei WU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Alonso M, Leong JAC. Licensed DNA vaccines against infectious hematopoietic necrosis virus (IHNV) Recent Pat DNA Gene Seq. 2013;7(1):62–65. doi: 10.2174/1872215611307010009. [DOI] [PubMed] [Google Scholar]
- 2.Amarasinghe GK, Bào Y, Basler CF, et al. Taxonomy of the order Mononegavirales: update 2017. Arch Virol. 2017;162(8):2493–2504. doi: 10.1007/s00705-017-3311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson ED, Mourich DV, Fahrenkrug SC, et al. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol. 1996;5(2):114–122. [PubMed] [Google Scholar]
- 4.Assenberg R, Delmas O, Morin B, et al. Genomics and structure/function studies of Rhabdoviridae proteins involved in replication and transcription. Antiviral Res. 2010;87(2):149–161. doi: 10.1016/j.antiviral.2010.02.322. [DOI] [PubMed] [Google Scholar]
- 5.Barr JN, Whelan SPJ, Wertz GW. Transcriptional control of the RNA-dependent RNA polymerase of vesicular stomatitis virus. Biochim Biophys Acta. 2002;1577(2):337–353. doi: 10.1016/S0167-4781(02)00462-1. [DOI] [PubMed] [Google Scholar]
- 6.Bauer EF. Cascades of enemy release: impacts of an invasive species (Neogobius melanostomus) on the parasite communities of two native predators (Micropterus dolomieu and Micropterus salmoides). MS T. University of New York College of Environmental Science and Forestry, Syracuse, New York, USA; 2013. [Google Scholar]
- 7.Castric J, Rasschaert D, Bernard J. Evidence of lyssaviruses among rhabdovirus isolates from the European EEL Anguilla Anguilla . Ann Inst Pasteur/Virol. 1984;135(1):35–55. doi: 10.1016/S0769-2617(84)80038-6. [DOI] [Google Scholar]
- 8.Chen ZY, Lei XY, Zhang QY. The antiviral defense mechanisms in mandarin fish induced by DNA vaccination against a rhabdovirus. Vet Microbiol. 2012;157(3-4):264–275. doi: 10.1016/j.vetmic.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Compans RW, Klenk HD, Caliguiri LA, et al. Influenza virus proteins: I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- 10.Dorson M, Torchy C, Chilmonczyk S, et al. A rhabdovirus pathogenic for perch, Perca fluviatilis L.: isolation and preliminary study. J Fish Dis. 1984;7(3):241–245. doi: 10.1111/j.1365-2761.1984.tb00929.x. [DOI] [Google Scholar]
- 11.Emmenegger EJ, Kurath G. DNA vaccine protects ornamental koi (Cyprinus carpio koi) against North American spring viremia of carp virus. Vaccine. 2008;26(50):6415–6421. doi: 10.1016/j.vaccine.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Fu XZ, Lin Q, Liang HR, et al. The biological features and genetic diversity of novel fish rhabdovirus isolates in China. Arch Virol. 2017;162(9):2829–2834. doi: 10.1007/s00705-017-3416-z. [DOI] [PubMed] [Google Scholar]
- 13.Galinier R, van Beurden S, Amilhat E, et al. Complete genomic sequence and taxonomic position of eel virus European X (EVEX), a rhabdovirus of European eel. Virus Res. 2012;166(1-2):1–12. doi: 10.1016/j.virusres.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Gao EB, Chen GF. Micropterus salmoides rhabdovirus (MSRV) infection induced apoptosis and activated interferon signaling pathway in largemouth bass skin cells. Fish Shellfish Immunol. 2018;76:161–166. doi: 10.1016/j.fsi.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Grabowska AK, Lipińska AD, Rohde J, et al. New baculovirus recombinants expressing Pseudorabies virus (PRV) glycoproteins protect mice against lethal challenge infection. Vaccine. 2009;27(27):3584–3591. doi: 10.1016/j.vaccine.2009.03.067. [DOI] [PubMed] [Google Scholar]
- 16.Ho PY, Chen YC, Maekawa S, et al. Efficacy of recombinant protein vaccines for protection against Nocardia seriolae infection in the largemouth bass Micropterus salmoides . Fish Shellfish Immunol. 2018;78:35–41. doi: 10.1016/j.fsi.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann B, Beer M, Schütze H, et al. Fish rhabdoviruses: molecular epidemiology and evolution. In: Fu ZF , editor. The World of Rhabdoviruses. Springer, Berlin, Heidelberg; 2005. pp. 81–117. [DOI] [PubMed] [Google Scholar]
- 18.Kim CH, Johnson MC, Drennan JD, et al. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J Virol. 2000;74(15):7048–7054. doi: 10.1128/JVI.74.15.7048-7054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ksenofontov AL, Badun GA, Fedorova NV, et al. Quantitation of the glycoprotein spike area on the surface of enveloped viruses. Mol Biol. 2008;42(6):973–975. doi: 10.1134/s0026893308060204. [DOI] [PubMed] [Google Scholar]
- 20.Lei Y, Qi RR, Cui LB, et al. Diagnosis of rhabdovirus disease in juvenile largemouth bass Micropterus salmonides . J Dalian Fish Univ. 2015;30(3):305–308. (in Chinese) [Google Scholar]
- 21.Lorenzen N, Lorenzen E, Einer-Jensen K, et al. Protective immunity to VHS in rainbow trout (Oncorhynchus mykiss, Walbaum) following DNA vaccination. Fish Shellfish Immunol. 1998;8(4):261–270. doi: 10.1006/fsim.1997.0134. [DOI] [Google Scholar]
- 22.Lorenzoni M, Dörr AJM, Erra R, et al. Growth and reproduction of largemouth bass (Micropterus salmoides Lacépède, 1802) in Lake Trasimeno (Umbria, Italy) Fish Res. 2002;56(1):89–95. doi: 10.1016/S0165-7836(01)00309-5. [DOI] [Google Scholar]
- 23.Luo X, Deng GC, Zhao CC, et al. Isolation and preliminary identification of rhabdovirus from Channa maculata cultured in pond. Acta Hydrobiol Sin. 2013;37(4):620–625. doi: 10.7541/2013.72. (in Chinese) [DOI] [Google Scholar]
- 24.Lv SJ, Lu BJ, Xu JH, et al. Immune response of peroxinectin of Chinese mitten crab Eriocheir sinensis to exterior stimulation. Dev Comp Immunol. 2015;51(1):56–64. doi: 10.1016/j.dci.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Ma DM, Deng GC, Bai JJ, et al. A strain of Siniperca chuatsi rhabdovirus causes high mortality among cultured largemouth bass in south China. J Aquat Anim Health. 2013;25(3):197–204. doi: 10.1080/08997659.2013.799613. [DOI] [PubMed] [Google Scholar]
- 26.Sakai K, Hagiwara K, Omatsu T, et al. Isolation and characterization of a novel rhabdovirus from a wild boar (Sus scrofa) in Japan. Vet Microbiol. 2015;179(3-4):197–203. doi: 10.1016/j.vetmic.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Sano T, Nishimura T, Okamoto N, et al. Isolation of rhabdovirus from European eels (Anguilla anguilla) at Japanese port of entry. Fish Health News. 1976;5:5–6. [Google Scholar]
- 28.Seo JY, Kim KH, Kim SG, et al. Protection of flounder against hirame rhabdovirus (HIRRV) with a DNA vaccine containing the glycoprotein gene. Vaccine. 2006;24(7):1009–1015. doi: 10.1016/j.vaccine.2005.07.109. [DOI] [PubMed] [Google Scholar]
- 29.Serena MS, Geisler C, Metz GE, et al. Expression and purification of Suid Herpesvirus-1 glycoprotein E in the baculovirus system and its use to diagnose Aujeszky’s disease in infected pigs. Protein Expr Purif. 2013;90(1):1–8. doi: 10.1016/j.pep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toth AM, Geisler C, Aumiller JJ, et al. Factors affecting recombinant Western equine encephalitis virus glycoprotein production in the baculovirus system. Protein Expr Purif. 2011;80(2):274–282. doi: 10.1016/j.pep.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker PJ, Wang YH, Cowley JA, et al. Structural and antigenic analysis of the nucleoprotein of bovine ephemeral fever rhabdovirus. J Gen Virol. 1994;75(8):1889–1899. doi: 10.1099/0022-1317-75-8-1889. [DOI] [PubMed] [Google Scholar]
- 32.Xu XG, Wang ZS, Zhang Q, et al. Baculovirus surface display of E envelope glycoprotein of Japanese encephalitis virus and its immunogenicity of the displayed proteins in mouse and swine models. Vaccine. 2011;29(4):636–643. doi: 10.1016/j.vaccine.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Xu XG, Wang ZS, Zhang Q, et al. Baculovirus as a PRRSV and PCV2 bivalent vaccine vector: baculovirus virions displaying simultaneously GP5 glycoprotein of PRRSV and capsid protein of PCV2. J Virol Methods. 2012;179(2):359–366. doi: 10.1016/j.jviromet.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Zeng WW, Wang Q, Wang YY. Isolation and characterization of a rhabdovirus from snakehead fish (Ophicephalus striatus) J Fish China. 2013;37(9):1416–1424. doi: 10.3724/SP.J.1231.2013.38588. (in Chinese) [DOI] [Google Scholar]
- 35.Zhang LJ, Li NQ, Lin Q, et al. An avirulent Micropterus salmoides rhabdovirus vaccine candidate protects Chinese perch against rhabdovirus infection. Fish Shellfish Immunol. 2018;77:474–480. doi: 10.1016/j.fsi.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Huang YH, Zhu JL, et al. Expression of hNeuritin protein in a baculovirus expression system and the analysis of its activity. Gene. 2018;647:129–135. doi: 10.1016/j.gene.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Zhang XL, Xiao F, Li SM, et al. China Fishery Statistical Yearbook 2018. China Agriculture Press, Beijing, China; 2018. p. 33. (in Chinese) [Google Scholar]
- 38.Zhang ZY, You Y, Meng FL, et al. A primary research of using silkworm larvae as substitutes of feed proteins. J Changsha Univ. 2011;25(2):68–69. doi: 10.3969/j.issn.1008-4681.2011.02.025. (in Chinese) [DOI] [Google Scholar]